Abstract
Bending inherently planar π-cores consisting of only six-membered rings has traditionally been challenging because a powerful transformation is required to compensate for the significant strain energy associated with bending. Herein, we demonstrate that sulfur extrusion can achieve substantial molecular bending of a perylene structure to form a substructure of a Vögtle belt, a proposed yet hitherto elusive carbon nanotube fragment. Bent perylene bisimide (PBI) derivatives were synthesized through a double-sulfur-extrusion reaction from the corresponding sulfur-containing V-shaped precursors with an internal alkyl tether. The effect of bending the inherently planar PBI core, which is a recent topic of interest for the design of advanced organic electronic and optoelectronic materials, was investigated systematically. Increasing the curvature leads to a red shift in the absorption and emission spectra, while the fluorescence quantum yields remain high. This stands in contrast with the nonemissive features of previously reported nonplanar PBI derivatives based on conjugative tethers. Detailed photophysical measurements indicated that the increasing curvature with shorter alkyl tethers (i) slightly facilitates intersystem crossing and (ii) significantly suppresses the internal conversion in the excited state of the present bent PBI derivatives. The latter characteristics originate from the restricted dynamic motion associated with the charge-transfer (CT) character between the core chromophores and the N-aryl units.
Introduction
Recent progress in organic synthesis has led to the development of various nonplanar π-conjugated molecules, which had remained elusive targets until several decades ago due to the intrinsically planar nature of sp2-hybridized carbons.1−13 To date, there have been numerous reports on bowl-shaped and warped π-systems in which the incorporation of non-six-membered rings induces positive or negative Gaussian curvatures.14−21 However, examples of the coercive bending of inherently planar π-cores that consist of only six-membered rings, which have zero Gaussian curvature, remain limited because the synthesis of these contorted π-systems requires a powerful transformation to compensate for the significant strain energy associated with their curvature.
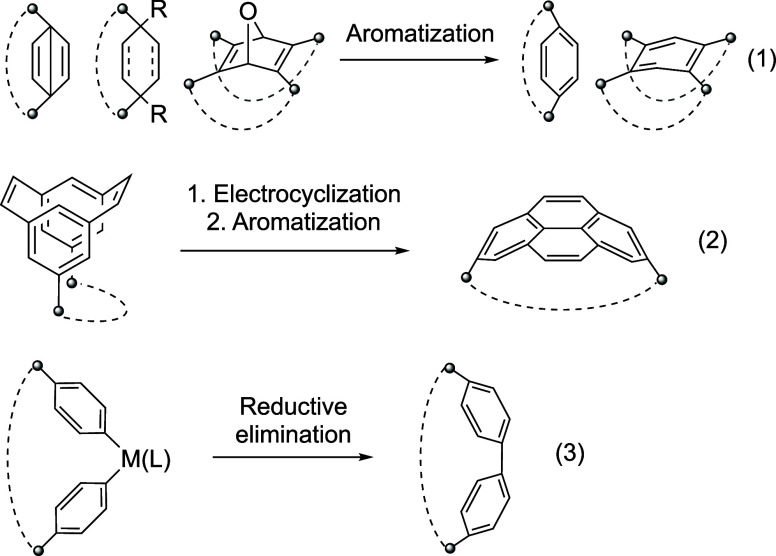
Representative strategies for bending π-systems are summarized in Figure 1. [n]Paracyclophane, the simplest model of a curved π-system, has been synthesized from the Dewar benzene precursor via aromatization (eq 1).22−24 Bodwell and co-workers have reported the synthesis of π-extended cyclophanes such as pyrenophane and teropyrenophane via the electrocyclization of the corresponding cyclophanediene precursors and subsequent aromatization (eq 2).25−29 Cyclo[n]paraphenylenes have been prepared using two approaches: preconstruction of the main framework using sp3-hybridized carbon atoms (eq 1)30,31 and reductive elimination from less-strained organometallic intermediates (eq 3).32,33 The reductive-elimination strategy has also been employed to synthesize carbon nanobelts.34 Recently, zigzag carbon nanobelts have been synthesized via the reductive aromatization of an oxanorbornadiene segment as the key step (eq 1).35,36
Figure 1.
Strategies for the syntheses of bent π-systems.
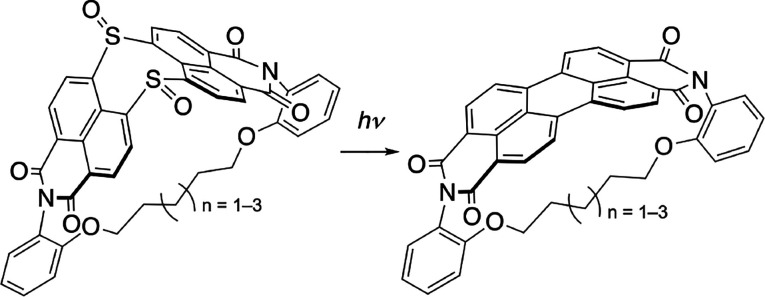
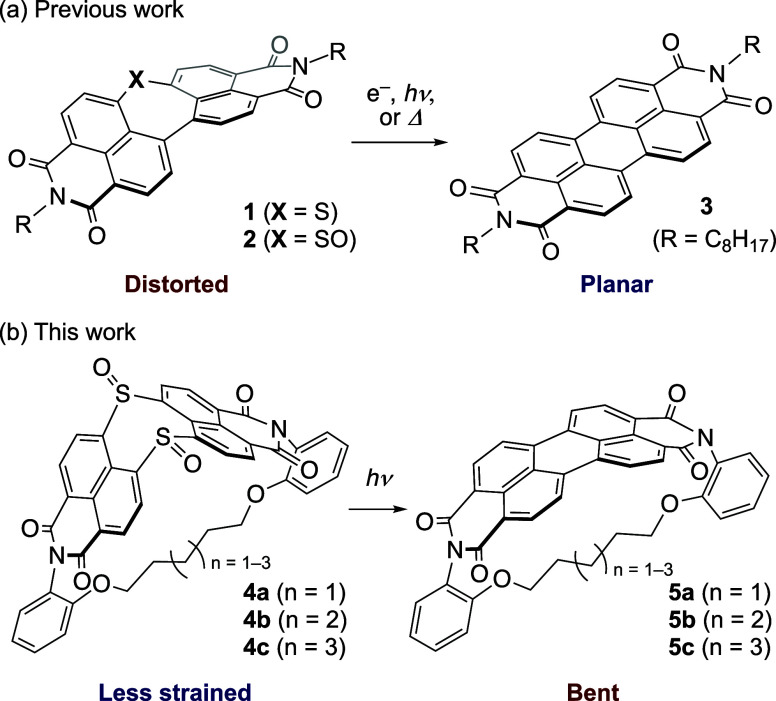
Our group has recently demonstrated that dinaphtho[1,8-bc:1′,8′-ef]thiepine bisimide (DNTBI) 1 and its sulfoxide (2) undergo sulfur extrusion upon electron injection, heating, or photoirradiation to yield PBI 3 almost quantitatively (Figure 2a).37,38 This transformation is accompanied by the release of strain from the distorted precursors. Here, we demonstrate that PBI derivatives with two inserted sulfoxide units undergo a double-sulfur-extrusion reaction (Figure 2b). This reaction was applied to bend an inherently planar PBI core; i.e., the photoirradiation of alkyl-tethered precursors 4a–4c afforded the corresponding (perylene bisimide)phanes (PBIphanes) 5a–5c. The curved perylene unit represents a fragment of a Vögtle belt, which is a proposed yet hitherto elusive carbon nanotube fragment.39 The molecular design of 5a–5c was inspired by Bodwell’s pyrenophanes.7,27−29 While ring-contraction via sulfur extrusion from a C(sp3)–S–C(sp3) unit is a representative strategy to create cyclophanes,22 the synthesis of strained molecules by sulfur extrusion from a C(sp2)–S–C(sp2) unit remains so far unprecedented.40
Figure 2.
Synthesis of PBIs via sulfur-extrusion reactions from the corresponding precursors with inserted sulfur/sulfoxide units.
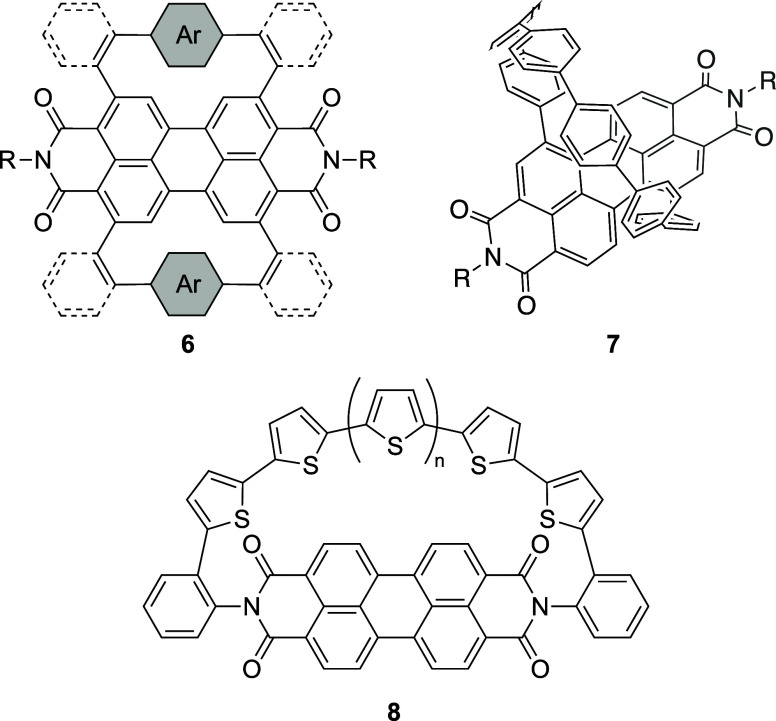
Figure 3 presents the previously reported PBIphanes 6–8. Xiao and co-workers have reported 6, which adopts contorted structures induced by π-conjugative linkers.41 Oligoparaphenylene-tethered PBIphane 7(42) and oligothiophene-tethered PBIphanes 8(43) adopt twisted and planar PBI cores, respectively. Our PBIphanes 5a–5c contain nonconjugative alkyl tethers to induce the curvature of the π-system. Hence, 5a–5c should be more suitable candidates to explore the effects of the bending of inherently planar PBIs44−47 on the chemical and physical properties of the π-conjugated cores, which has been a recent topic of high interest for the design of advanced organic electronic and optoelectronic materials.48−50 Twisted PBI derivatives with alkyl tethers have been studied by Würthner and co-workers.51,52
Figure 3.
Structures of previously reported PBIphanes 6, 7, and 8.
Results and Discussion
Synthesis and Sulfur-Extrusion Reactions of Model Compounds
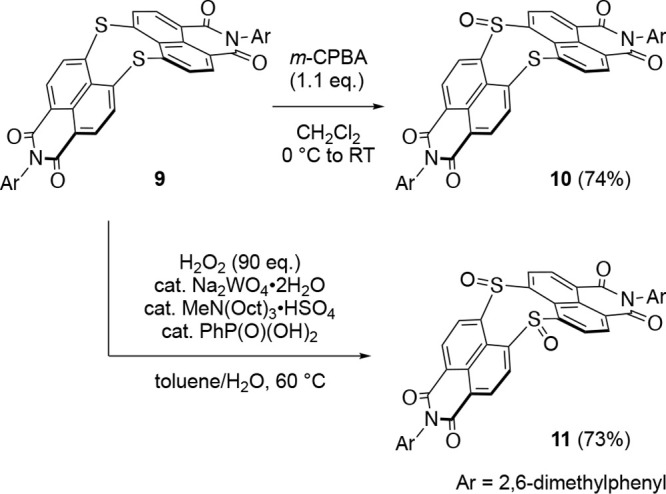
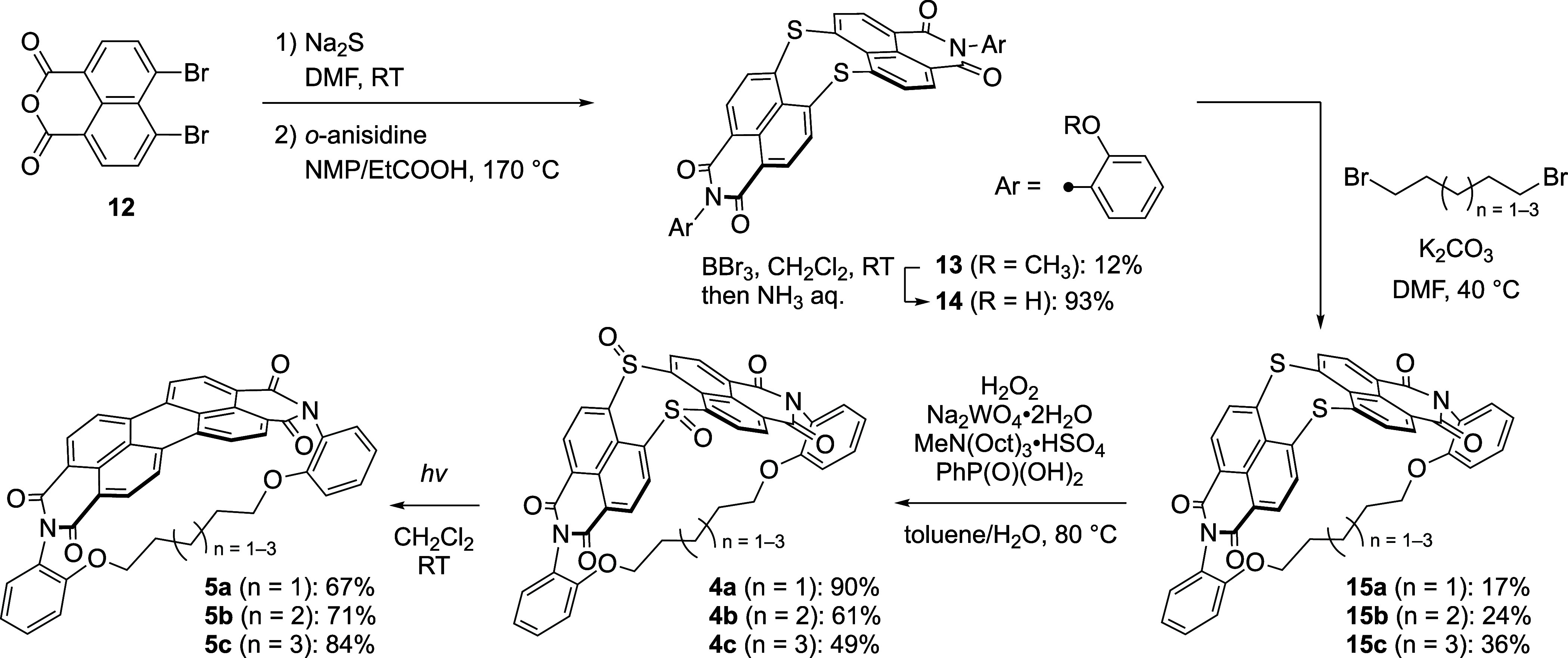
Dinaphtho[1,8-bc:1′,8′-fg][1,5]dithiocine bisimide (DNDTBI) 9(53) was subjected to oxidation with m-chloroperbenzoic acid (m-CPBA, 1.1 equiv) to afford the corresponding sulfoxide 10 in 74% yield (Scheme 1). The tungsten-catalyzed oxidation54 of 9 with hydrogen peroxide provided disulfoxide 11 in 73% yield.
Scheme 1. Synthesis of Sulfoxide Derivatives of DNDTBI.
The solid-state structures of 10 and 11 were unambiguously determined by using single-crystal X-ray diffraction analysis (Figure 4). In the crystal, 10 and 11 adopt a V-shaped structure similar to that of 9. The interplanar angles between the two naphthalene monoimide units gradually decrease in the order 9 (113°) > 10 (100°) > 11 (94°) due to the smaller bond angle of sulfoxide compared to that of sulfide. The distances between the ortho-substituents of the N-aryl groups of 9, 10, and 11 are 11.0, 9.2–9.8, and 8.2 Å, respectively. These values are comparable to the O–O distance of 1,6-hexanediol (8.7 Å) and longer than that of 1,5-pentanediol (7.3 Å).
Figure 4.
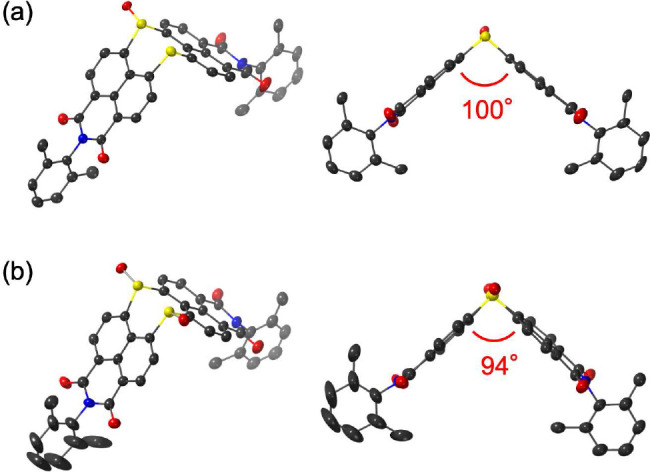
X-ray crystal structures of (a) 10 and (b) 11 with thermal ellipsoids at 50% probability; all hydrogen atoms have been omitted for the sake of clarity.
The UV/vis absorption spectra of 9–11 are shown in Figure 5. DNDTBI 9 exhibits a broad absorption band at 450 nm, which was assigned to the charge-transfer (CT) transition from the central sulfur atoms to the naphthalene monoimide units.53 Accordingly, monosulfoxide 10 shows hypsochromically shifted absorption with a shoulder peak tailing to 460 nm. Furthermore, the CT transition completely disappeared in the absorption spectrum of 11, with the remaining sharp absorption bands tailing at 420 nm.
Figure 5.
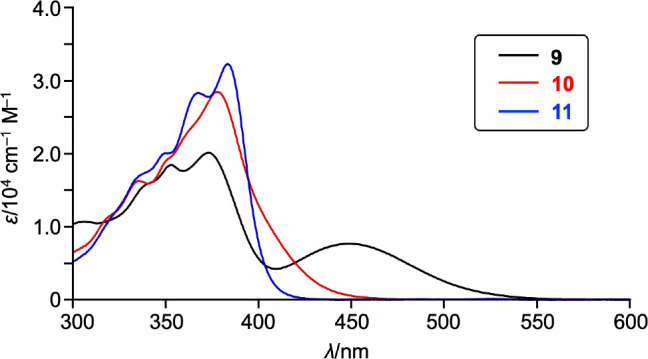
UV/vis absorption spectra of 9–11 in CH2Cl2; λ, wavelength; ε, extinction coefficient.
Subsequently, we examined the photoinduced sulfur-extrusion reactions of 9–11 in CH2Cl2 (Figure 6). Photoirradiation was performed using a high-pressure mercury lamp equipped with a sharp cutoff filter (λ > 380 nm). Compound 9 underwent the sulfur-extrusion reaction, albeit sluggishly, to afford PBI 3′ in only 19% yield even after an extended photoirradiation period of 3 h. In contrast, the sulfur-extrusion reaction of 10 afforded PBI 3′ almost quantitatively within 900 s. Notably, a broad absorption band appeared at 370–450 nm during the irradiation of 10. This feature resembles the spectrum of DNTBI 1 rather than that of its sulfoxide 2. These results suggest that the sulfoxide unit rather than the sulfide unit of 10 was removed preferentially, which is in agreement with the observed trend for 1 and 2, i.e., the sulfur-extrusion reaction of 2 proceeded more rapidly than that of 1.38 Furthermore, the sulfur-extrusion reaction of 11 was faster than that of 10, reaching completion to give 3′ in approximately 30 s. After 15 min of photoirradiation, the absorption spectrum is almost identical to that of PBI 3′, indicating nearly quantitative conversion. The lack of isosbestic points can be attributed to the formation of DNTBI sulfoxide 2 as an intermediate.
Figure 6.
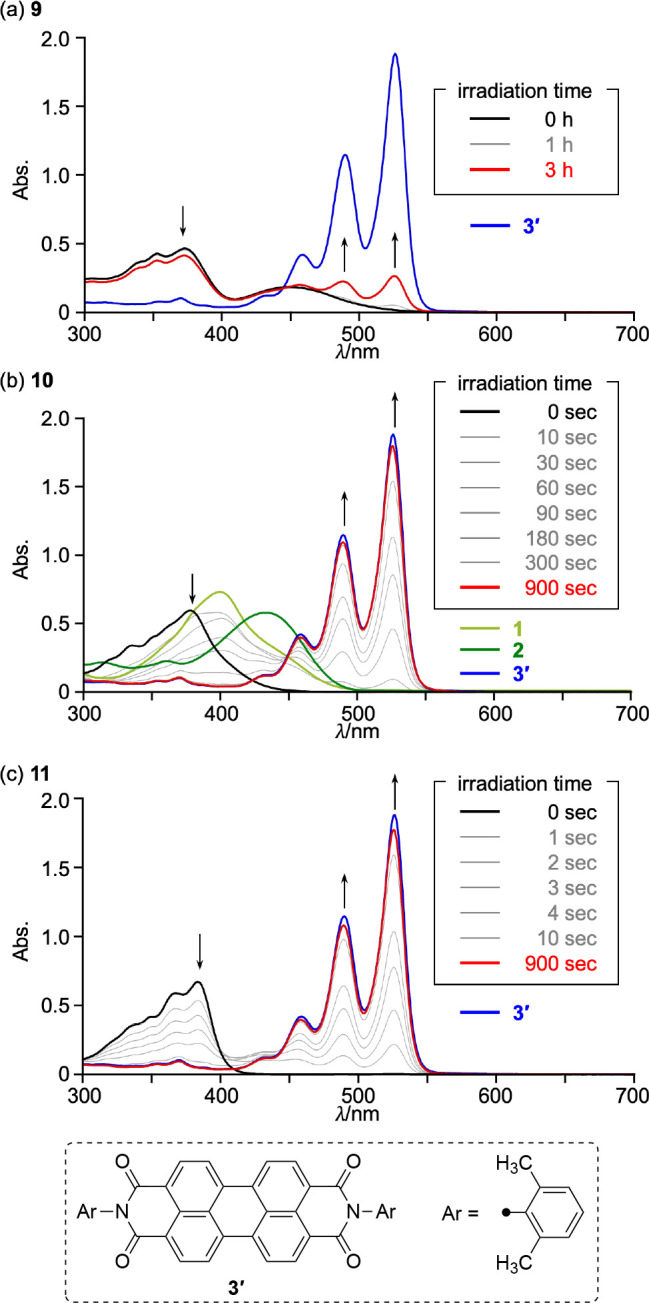
Changes in the UV/vis absorption spectra of (a) 9, (b) 10, and (c) 11 upon photoirradiation in CH2Cl2. A high-pressure mercury lamp equipped with a sharp cutoff filter (λ > 380 nm) was employed for the photoirradiation; [1] = [2] = [3′] = [9]0 = [10]0 = [11]0 = 2.1 × 10–5 M–1.
We also examined the sulfur-extrusion reaction of 11 via electron injection or heating. The cyclic voltammograms of 11 in CH2Cl2 (Figure S39) feature an irreversible reduction wave at −1.38 V in the forward direction. Upon backsweeping, two peaks were observed at −0.97 and −1.16 V, which are identical to those of PBI 3′. Furthermore, spectroelectrochemical measurements of 11 revealed that the absorption spectrum of 11 after electrochemical reduction is identical to that of the radical anion of PBI 3′ (Figure S40). These results indicate that electron injection triggers the extrusion of sulfur from 11, as was observed for the previously reported DNTBI sulfoxide 2. On the other hand, the thermogravimetric analysis (TGA) profile of 11 exhibits no characteristic mass decrease due to sulfur extrusion while DNTBI sulfoxide 2 underwent sulfur extrusion upon heating (Figure S42).
Synthesis of PBIphanes
The synthesis of PBIphanes 5a–5c is shown in Scheme 2. In our previous procedure, a 4,5-dibromonaphthalene monoimide derivative was subjected to a nucleophilic aromatic substitution (SNAr) reaction with Na2S to afford DNTBI 9.53 Here, we attempted to treat 4,5-dibromo-1,8-naphthalic anhydride 12 with o-anisidine; however, this resulted in an undesired competing SNAr reaction at the bromo substituents. We thus conducted the initial SNAr reaction of 12 with Na2S and subsequent treatment with o-anisidine afforded o-anisyl DNTBI 13 in 12% yield. The aryl oxygen groups of 13 were deprotected with BBr3 to furnish 2-hydroxyphenyl DNTBI 14 in 93% yield. Treatment of 14 with 1,5-dibromopentane, 1,6-dibromohexane, or 1,7-dibromoheptane provided the corresponding internally tethered products 15a, 15b, and 15c in 17, 24, and 36% yield, respectively. The sulfide units of 15a, 15b, and 15c were converted to sulfoxides through tungsten-catalyzed oxidation to give 4a, 4b, and 4c in 90, 61, and 49% yield, respectively. Finally, photoirradiation of 4a, 4b, and 4c with a high-pressure mercury lamp afforded the desired PBIphanes 5a, 5b, and 5c in 67, 71, and 84% yield, respectively.
Scheme 2. Synthesis of PBIphanes 5a–5c.
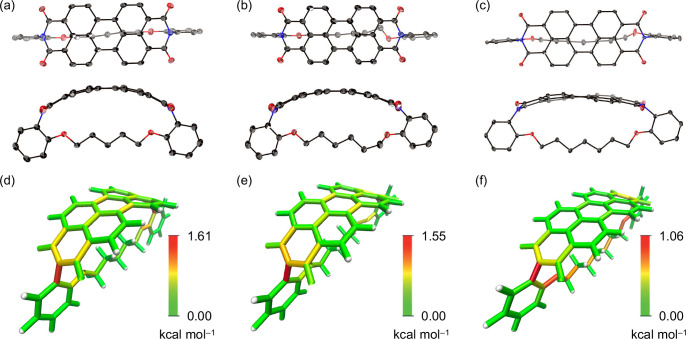
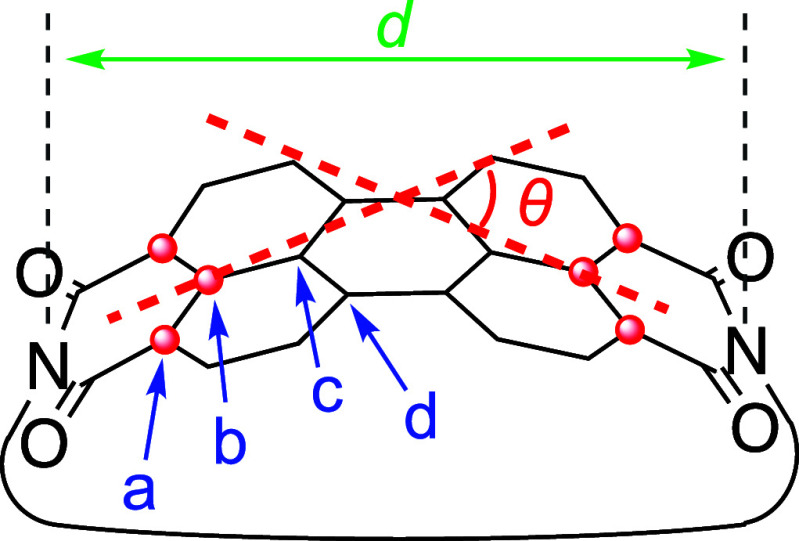
The structures of PBIphanes 5a–5c were unambiguously determined by X-ray diffraction analysis (Figure 7a–c). Structural parameters are summarized in Table 1. In the single crystal, PBIphanes 5a, 5b, and 5c adopt bent structures with nitrogen–nitrogen distances, d, of 10.55, 10.78, and 11.15 Å, respectively. These nitrogen–nitrogen distances are significantly shorter than that of planar PBI 3 (d = 11.3 Å)55 and comparable to those of previously reported contorted PBIphanes 6 (d = 10.25–11.36 Å). The end-to-end bend angles, θ, are 33.8° (5a), 29.4° (5b), and 19.4° (5c). In the X-ray crystal structure of 5b, one methylene unit neighboring an oxygen atom adopts a gauche configuration, which can be attributed to the mismatch due to having an even-numbered alkyl chain as the tethering unit. The π-orbital-axis-vector (POAV) angles,56 which provide a measure of the degree of orbital hybridization based on structural aspects, were calculated based on the crystal structures of 5a–5c (Table 1), in which the obtained POAV angles at the equivalent carbons were averaged. The POAV angles increase in the order 5c < 5b < 5a, indicating that increased curvature decreases the s-character of the constituent carbon atoms. The deviation from planarity is most pronounced at the imide-substituted carbon atoms (position a).
Figure 7.
X-ray crystal structures (top: top view; bottom: side view) of (a) 5a, (b) 5b, and (c) 5c with thermal ellipsoids at 50% probability; all hydrogen atoms are omitted for clarity. Visualized local strains in (d) 5a, (e) 5b, and (f) 5c were calculated at the B3LYP/6-31G(d) level.
Table 1. Structural Parameters and Calculated Strain Energies for 5a–5c.
| da/Å | θb/° | POAV angle/° | Ec/kcal mol–1 | ||||
|---|---|---|---|---|---|---|---|
| a | b | c | d | ||||
| 5a | 10.55 | 33.8 | 3.2 | 1.2 | 1.2 | 2.2 | 22.9 |
| 5b | 10.78 | 29.4 | 2.6 | 1.2 | 1.5 | 2.0 | 19.7 |
| 5c | 11.15 | 19.4 | 2.0 | 0.6 | 0.7 | 1.0 | 17.4 |
Nitrogen–nitrogen distance.
End-to-end bend angle.
Strain energy.
The strain energies in 5a, 5b, and 5c were investigated using hypothetical homodesmotic reactions at the B3LYP/6-31G(d) level and were calculated to be 22.9, 19.7, and 13.6 kcal mol–1, respectively (Figure S45). The local strain energies were also visualized using the StrainVis tool (Figure 7d–f).57 The PBIphanes 5a, 5b, and 5c exhibit the greatest local strain at the C–N bond of the N-aryl substituents, which increases with decreasing length of the alkyl linker (5a: 1.61 kcal mol–1; 5b: 1.55 kcal mol–1; 5c: 1.06 kcal mol–1). In the perylene core, the strain is mainly localized at the peripheral bonds around the naphthalene segments, as was also observed in a computational simulation of a Vögtle belt.57 The C–O bonds in 5a–5c also contribute substantially to the accommodation of the local strain.
The solubility value of 5a in CHCl3 (0.11 mg/mL) is higher than those of 5b (0.027 mg/mL) and 5c (0.045 mg/mL), due to the large molecular curvature. However, these solubility values of PBIphanes 5a–5c are smaller than that of planar N-(2-methoxyphenyl)-substituted PBI 3″ (0.38 mg/mL), which exhibits high solubility due to the existence of rotamers. The relatively low solubility of PBIphanes 5a–5c is attributable to the restricted rotation of their N-substituents.
Aromaticity of PBIphanes
In the 1H NMR spectra of 5a–5c and untethered planar PBI 3″ in tetrachloroethane-d2, the signals of the aromatic protons showed an upfield shift with increasing curvature (5a: 8.64 and 8.62 ppm; 5b: 8.67 and 8.65 ppm; 5c: 8.71 and 8.69 ppm; 3″: 8.74 and 8.72 ppm). DFT calculations reproduced this trend (Table S3). Nevertheless, the nucleus-independent chemical shift (NICS) values at the carbonyl-substituted six-membered rings of 5a–5c and 3″, which represent an averaged value among four rings, are almost comparable (5a: −6.44 ppm; 5b: −6.44 ppm; 5c: −6.52 ppm; 3″: −6.61 ppm). Consequently, we conclude that (i) increasing the molecular curvature in PBI has a negligible effect on the aromaticity and (ii) the observed upfield shifts of the aromatic proton signals originate from the change in orientation toward the neighboring carbonyl group or aromatic ring.
Electrochemical Properties of PBIphanes
The cyclic voltammograms and differential pulse voltammograms of 5a–5c and 3″ were measured in CH2Cl2 by using 0.1 M Bu4NPF6 as the supporting electrolyte and Ag/AgNO3 as the reference electrode (Figure S41). The ferrocene/ferrocenium couple (Fc/Fc+) was used as an internal reference. For 5a–5c and 3″, two reversible reduction waves (5a: −1.03 and −1.27 V; 5b: −1.01 and −1.24 V; 5c: −1.00 and −1.23 V; and 3″: −1.03 and −1.22 V) were observed. These results indicate that the sensitivity of the CV measurements is insufficient to distinguish the subtle effect of molecular bending on the electron-accepting abilities.
Photophysical Properties of PBIphanes
The UV/vis absorption and emission spectra of PBI 3″ and PBIphanes 5a–5c are shown in Figure 8, and their photophysical parameters are summarized in Table 2. Increasing the structural curvature of the PBI cores results in a red shift of the longest-wavelength absorption peaks from 527 nm (3″) to 544 nm (5a), together with decreased extinction coefficients. This trend contrasts with those of pyrenophane and teropyrenophane, in which increased structural curvature leads to blue-shifted absorption spectra.26,28 These results firmly corroborate the recent argument that the symmetry of the local orbitals in the K-region (bonding or antibonding) governs the (de)stabilization of the HOMO and LUMO.50 The shortest-wavelength emission peaks also exhibited a bathochromic shift from 535 nm (3″) to 555 nm (5a) with increasing curvature. The PBIphanes exhibited intense emission with quantum yields, ΦF, of 0.84–0.88, which are larger than that of planar PBI 3″ (ΦF = 0.76). It is worth noting here that the previously reported π-tethered contorted PBI derivatives 6 exhibit behavior that is different from that of the present PBIphanes 5a–5c: (i) negligible correlation between the absorption wavelength and molecular bending and (ii) weak emission (ΦF = 0.01–0.32)41; these differences highlight the fact that the electronic properties of 6 are substantially altered by the π-delocalization onto the tethering π-linkers.
Figure 8.
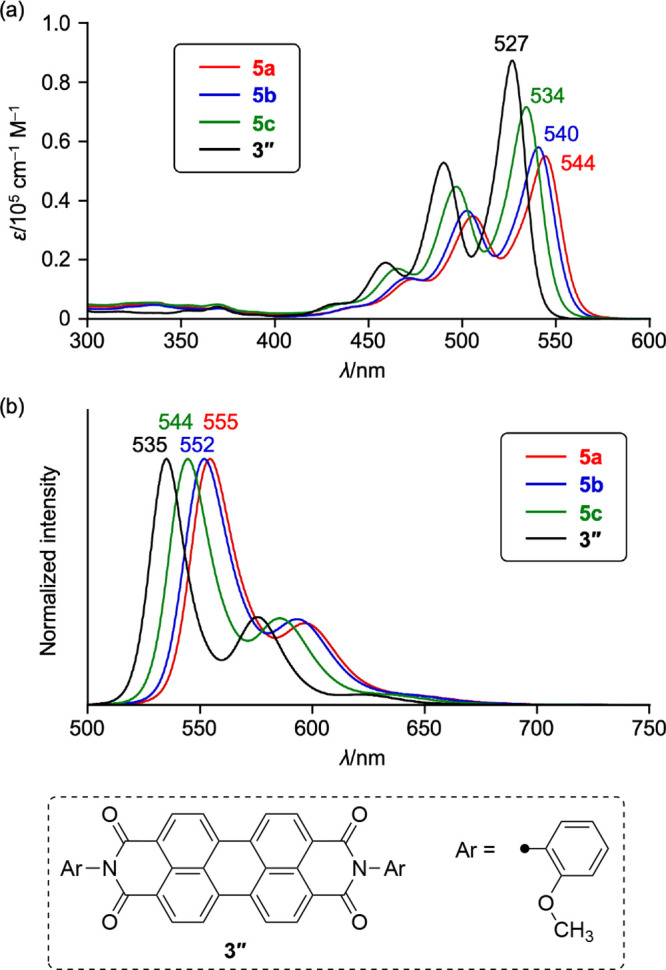
UV/vis (a) absorption and (b) emission spectra of 3″, 5a, 5b, and 5c in CHCl3; λ, wavelength; and ε, extinction coefficient.
Table 2. Photophysical Parameters of 3″ and 5a–5c.
| Δνa/cm–1 | ΦFb | ΦISCc | τFd/ns | kre/108 s–1 | knrf/108 s–1 | |
|---|---|---|---|---|---|---|
| 5a | 364 | 0.88 | 4.9 | 1.8 | 0.24 | |
| 5b | 403 | 0.84 | 0.001 | 4.6 | 1.8 | 0.35 |
| 5c | 344 | 0.84 | 0.002 | 4.1 | 2.0 | 0.39 |
| 3″ | 284 | 0.76 | 3.6 | 2.1 | 0.67 |
Stokes shift.
Fluorescence quantum yield.
ISC quantum yield.
Fluorescence lifetime.
Radiative decay constant.
Nonradiative decay constant.
The full widths at half-maximum (fwhm’s) of the 0–0 absorption bands of 5a, 5b, 5c, and 3″ calculated from the Gauss fitting are 659, 686, 661, and 601 cm–1, respectively. The Stokes shifts of 5a, 5b, 5c, and 3″ are 364, 403, 344, and 284 cm–1, respectively. The larger fwhm and Stokes shift values of PBIphanes 5a–5c compared to those of planar PBI 3″ were attributed to their nonplanar structures. Interestingly, the fwhm and Stokes shift values of 5b are larger than those of 5a and 5c, suggesting that the mismatch of its even-numbered alkyl chain increases the structural flexibility.
The photophysical parameters of 5a–5c and 3 are summarized in Table 2. Their radiative decay rates, kr, decrease slightly from 2.1 × 108 to 1.8 × 108 s–1 with increasing structural curvature. TD-DFT calculations at the CAM-B3LYP/6-31G(d) level indicated that the oscillator strength of the S0–S1 transitions also decreases in the same order (3: 0.96 > 5c: 0.78 > 5b: 0.70 > 5a: 0.67) (Figure S46), which is in agreement with their extinction coefficients. These results can be explained by the decreased HOMO–LUMO overlap with increasing curvature. The increase in structural curvature also decreases the nonradiative decay rates, knr, from 0.67 × 108 to 0.24 × 108 s–1. Transition-absorption-spectroscopy measurements indicated that the photoexcitation of 5b and 5c affords long-lived species with lifetimes of 86 and 125 μs, which were assigned to the triplet excited states (Figure S47). The intersystem crossing (ISC) quantum yield values, ΦISC, of 5b and 5c are 0.1 and 0.2%, respectively (Figure S48). These ΦISC values are greater than the reported triplet yield of N,N′-bis(2,5-di-tert-butylphenyl)-substituted PBI 3‴ (ΦISC < 10–4),58 indicating that the molecular bending promotes ISC.59 However, the ΦISC values are in general very low, indicating that the nonradiative deactivation of the bent PBIs 5a–5c mainly proceeds through internal conversion (IC). Using the reported fluorescence yield and decay of PBI 3‴, its IC rate constant was estimated to be knr = 3 × 106 s–1, which is approximately one-tenth of those of 5a–5c. TD-DFT calculations of 5a–5c also indicated the charge-transfer (CT) character between the core chromophores and the aryl units as the second excited state (S2) in the S0-optimized geometries (Figure S46). Thus, the disordered motion in the alkyl linker is anticipated to cause a vibronic admixture between S1 and S2 to promote the IC processes to the S0 states via CT character. Indeed, the ΦF values in toluene (3″: 87%; 5a: 90%; 5b: 86%; 5c:87%) are larger than those in CHCl3. Furthermore, the fact that the knr value of 5c is greater than those of 5a and 5b strongly supports this hypothesis, because the flexibility of the alkyl chain linker increases with increasing length, allowing the orbital hybridization between HOMO and HOMO–1 as shown in Figure S46. In contrast, shorter linkages restrict the aforementioned orbital overlap due to the more rigid geometry. However, the knr value in 5a is greater than that in 3‴, indicating that the alkyl linkages essentially induce vibronic coupling for the nonradiative processes.
Conclusions
We have described the synthesis and properties of bent PBI derivatives 5a–5c, which feature an internal alkyl chain tethering the two N-aryl substituents. These PBIphanes were synthesized via sulfur-extrusion reactions from corresponding disulfoxide precursors 4a–4c. X-ray diffraction analysis of 5a–5c demonstrated that their degree of curvature increases with decreasing length of the alkyl tether. The strain energy was evaluated to be as high as 22.9 kcal mol–1 (5a) based on homodesmotic reactions. The 1H NMR analysis of 5a–5c indicated that the aromatic proton signals were shifted upfield by approximately 0.1 ppm with increasing molecular curvature. This shift is due to the change in orientation toward the neighboring carbonyl group or aromatic ring rather than a change in aromaticity. Electrochemical measurements of 5a–5c indicated that increasing the molecular curvature in PBI has a negligible effect on electron affinity. In sharp contrast, the increased curvature leads to a red shift of the absorption and emission spectra (by ∼20 nm), while the high fluorescence quantum yields (up to 88%) are maintained. This behavior contrasts with the nonemissive features of previously reported contorted π-extended PBI derivatives 6. Detailed photophysical measurements of 5a–5c indicated that the nonradiative deactivation of the S1 state via IC is suppressed by the restricted dynamic motion associated with the CT character between the core chromophores and the N-aryl units. Our results demonstrate that the double-sulfur-extrusion reactions enable the construction of curved perylene structures and the effect that the structural curvature exerts on the structural, electrochemical, and photophysical properties of these PBIs. These findings can be expected to find applications in the design of advanced organic materials.
Acknowledgments
This work was supported by JSPS KAKENHI grants JP20H05862 (H.S. and N.F.), JP20H05863 (H.S.), JP20H05867 (N.F.), JP22K14663 (N.F.), and JP23H03947 (N.F.), as well as JST, PRESTO grant JPMJPR21Q7 (N.F.). This paper is dedicated to Prof. Atsuhiro Osuka on the occasion of his 70th birthday.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.4c05358.
Experimental details and spectral data for all new compounds (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Gingras M. One Hundred Years of Helicene Chemistry. Part 3: Applications and Properties of Carbohelicenes. Chem. Soc. Rev. 2013, 42, 1051–1095. 10.1039/C2CS35134J. [DOI] [PubMed] [Google Scholar]
- Scott L. T. Methods for the Chemical Synthesis of Fullerenes. Angew. Chem., Int. Ed. 2004, 43, 4994–5007. 10.1002/anie.200400661. [DOI] [PubMed] [Google Scholar]
- Kawase T.; Kurata H. Ball-, Bowl-, and Belt-Shaped Conjugated Systems and Their Complexing Abilities: Exploration of the Concave–Convex π–π Interaction. Chem. Rev. 2006, 106, 5250–5273. 10.1021/cr0509657. [DOI] [PubMed] [Google Scholar]
- Wu Y.-T.; Siegel J. S. Aromatic Molecular-Bowl Hydrocarbons: Synthetic Derivatives, Their Structures, and Physical Properties. Chem. Rev. 2006, 106, 4843–4867. 10.1021/cr050554q. [DOI] [PubMed] [Google Scholar]
- Pascal R. A. Twisted Acenes. Chem. Rev. 2006, 106, 4809–4819. 10.1021/cr050550l. [DOI] [PubMed] [Google Scholar]
- Shen Y.; Chen C.-F. Helicenes: Synthesis and Applications. Chem. Rev. 2012, 112, 1463–1535. 10.1021/cr200087r. [DOI] [PubMed] [Google Scholar]
- Bodwell G. J. Extraordinary Transformations to Achieve the Synthesis of Remarkable Aromatic Compounds. Chem. Rec. 2014, 14, 547–567. 10.1002/tcr.201402034. [DOI] [PubMed] [Google Scholar]
- Miao Q. Heptagons in Aromatics: From Monocyclic to Polycyclic. Chem. Rec. 2015, 15, 1156–1159. 10.1002/tcr.201510009. [DOI] [PubMed] [Google Scholar]
- Segawa Y.; Yagi A.; Matsui K.; Itami K. Design and Synthesis of Carbon Nanotube Segments. Angew. Chem., Int. Ed. 2016, 55, 5136–5158. 10.1002/anie.201508384. [DOI] [PubMed] [Google Scholar]
- Hiroto S. Innovative Synthesis and Functions of Curved π-Conjugated Molecules. Bull. Chem. Soc. Jpn. 2018, 91, 829–838. 10.1246/bcsj.20170435. [DOI] [Google Scholar]
- Saito M.; Shinokubo H.; Sakurai H. Figuration of Bowl-shaped π-Conjugated Molecules: Properties and Functions. Mater. Chem. Front. 2018, 2, 635–661. 10.1039/C7QM00593H. [DOI] [Google Scholar]
- Márquez I. R.; Castro-Fernández S.; Millán A.; Campaña A. G. Synthesis of Distorted Nanographenes Containing Seven- and Eight-membered Carbocycles. Chem. Commun. 2018, 54, 6705–6718. 10.1039/C8CC02325E. [DOI] [PubMed] [Google Scholar]
- Majewski M. A.; Stępień M. Bowls, Hoops, and Saddles: Synthetic Approaches to Curved Aromatic Molecules. Angew. Chem., Int. Ed. 2019, 58, 86–116. 10.1002/anie.201807004. [DOI] [PubMed] [Google Scholar]
- Barth W. E.; Lawton R. G. Dibenzo[ghi,mno]fluoranthene. J. Am. Chem. Soc. 1966, 88, 380–381. 10.1021/ja00954a049. [DOI] [Google Scholar]
- Scott L. T.; Jackson E. A.; Zhang Q.; Steinberg B. D.; Bancu M.; Li B. A Short, Rigid, Structurally Pure Carbon Nanotube by Stepwise Chemical Synthesis. J. Am. Chem. Soc. 2012, 134, 107–110. 10.1021/ja209461g. [DOI] [PubMed] [Google Scholar]
- Feng C.-N.; Kuo M.-Y.; Wu Y.-T. Synthesis, Structural Analysis, and Properties of [8]Circulenes. Angew. Chem., Int. Ed. 2013, 52, 7791–7794. 10.1002/anie.201303875. [DOI] [PubMed] [Google Scholar]
- Sakamoto Y.; Suzuki T. Tetrabenzo[8]circulene: Aromatic Saddles from Negatively Curved Graphene. J. Am. Chem. Soc. 2013, 135, 14074–14077. 10.1021/ja407842z. [DOI] [PubMed] [Google Scholar]
- Yamamoto K.; Harada T.; Okamoto Y.; Chikamatsu H.; Nakazaki M.; Kai Y.; Nakao T.; Tanaka M.; Harada S.; Kasai N. Synthesis and Molecular Structure of [7]Circulene. J. Am. Chem. Soc. 1988, 110, 3578–3584. 10.1021/ja00219a036. [DOI] [Google Scholar]
- Luo J.; Xu X.; Mao R.; Miao Q. Curved Polycyclic Aromatic Molecules That Are π-Isoelectronic to Hexabenzocoronene. J. Am. Chem. Soc. 2012, 134, 13796–13803. 10.1021/ja3054354. [DOI] [PubMed] [Google Scholar]
- Kawasumi K.; Zhang Q.; Segawa Y.; Scott L. T.; Itami K. A Grossly Warped Nanographene and the Consequences of Multiple Odd-membered-ring Defects. Nat. Chem. 2013, 5, 739–744. 10.1038/nchem.1704. [DOI] [PubMed] [Google Scholar]
- Fukui N.; Kim T.; Kim D.; Osuka A. Porphyrin Arch-Tapes: Synthesis, Contorted Structures, and Full Conjugation. J. Am. Chem. Soc. 2017, 139, 9075–9088. 10.1021/jacs.7b05332. [DOI] [PubMed] [Google Scholar]
- Kane V. V.; De Wolf W. H.; Bickelhaupth F. Synthesis of Small Cyclophanes. Tetrahedron 1994, 50, 4575–4622. 10.1016/S0040-4020(01)85002-X. [DOI] [Google Scholar]
- Jenneskens L. W.; de Kanter F. J. J.; Kraakman P. A.; Turkenburg L. A. M.; Koolhaas W. E.; de Wolf W. H.; Bickelhaupt F.; Tobe Y.; Kakiuchi K.; Odaira Y. [5]Paracyclophane. J. Am. Chem. Soc. 1985, 107, 3716–3717. 10.1021/ja00298a051. [DOI] [Google Scholar]
- Tobe Y.; Kawaguchi M.; Kakiuchi K.; Naemura K. [2.2]Orthoparacyclophane: The Last and Most Strained [2.2] Cyclophane. J. Am. Chem. Soc. 1993, 115, 1173–1174. 10.1021/ja00056a066. [DOI] [Google Scholar]
- Bodwell G. J.; Bridson J. N.; Houghton T. J.; Kennedy J. W. J.; Mannion M. R. 1,8-Dioxa[8](2,7)pyrenophane, a Severely Distorted Polycyclic Aromatic Hydrocarbon. Angew. Chem., Int. Ed. 1996, 35, 1320–1321. 10.1002/anie.199613201. [DOI] [Google Scholar]
- Bodwell G. J.; Bridson J. N.; Cyrañski M. K.; Kennedy J. W. J.; Krygowski T. M.; Mannion M. R.; Miller D. O. Nonplanar Aromatic Compounds. 8.1 Synthesis, Crystal Structures, and Aromaticity Investigations of the 1,n-Dioxa[n](2,7)pyrenophanes. How Does Bending Affect the Cyclic π-Electron Delocalization of the Pyrene System?. J. Org. Chem. 2003, 68, 2089–2098. 10.1021/jo0206059. [DOI] [PubMed] [Google Scholar]
- Merner B. L.; Dawe L. N.; Bodwell G. J. 1,1,8,8-Tetramethyl[8](2,11)teropyrenophane: Half of an Aromatic Belt and a Segment of an (8,8) Single-Walled Carbon Nanotube. Angew. Chem., Int. Ed. 2009, 48, 5487–5491. 10.1002/anie.200806363. [DOI] [PubMed] [Google Scholar]
- Merner B. L.; Unikela K. S.; Dawe L. N.; Thompson D. W.; Bodwell G. J. 1,1,n,n-Tetramethyl[n](2,11)teropyrenophanes (n = 7–9): A Series of Armchair SWCNT Segments. Chem. Commun. 2013, 49, 5930–5932. 10.1039/c3cc43268h. [DOI] [PubMed] [Google Scholar]
- Unikela K. S.; Roemmele T. L.; Houska V.; McGrath K. E.; Tobin D. M.; Dawe L. N.; Boeré R. T.; Bodwell G. J. Gram-Scale Synthesis and Highly Regioselective Bromination of 1,1,9,9-Tetramethyl[9](2,11)teropyrenophane. Angew. Chem., Int. Ed. 2018, 57, 1707–1711. 10.1002/anie.201713067. [DOI] [PubMed] [Google Scholar]
- Jasti R.; Bhattacharjee J.; Neaton J. B.; Bertozzi C. R. Synthesis, Characterization, and Theory of [9]-, [12]-, and [18]Cycloparaphenylene: Carbon Nanohoop Structures. J. Am. Chem. Soc. 2008, 130, 17646–17647. 10.1021/ja807126u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaba H.; Omachi H.; Yamamoto Y.; Bouffard J.; Itami K. Selective Synthesis of [12]Cycloparaphenylene. Angew. Chem., Int. Ed. 2009, 48, 6112–6116. 10.1002/anie.200902617. [DOI] [PubMed] [Google Scholar]
- Yamago S.; Watanabe Y.; Iwamoto T. Synthesis of [8]Cycloparaphenylene from a Square-Shaped Tetranuclear Platinum Complex. Angew. Chem., Int. Ed. 2010, 49, 757–759. 10.1002/anie.200905659. [DOI] [PubMed] [Google Scholar]
- Tsuchido Y.; Abe R.; Ide T.; Osakada K. A Macrocyclic Gold(I)–Biphenylene Complex: Triangular Molecular Structure with Twisted Au2(diphosphine) Corners and Reductive Elimination of [6]Cycloparaphenylene. Angew. Chem., Int. Ed. 2020, 59, 22928–22932. 10.1002/anie.202005482. [DOI] [PubMed] [Google Scholar]
- Povie G.; Segawa Y.; Nishihara T.; Miyauchi Y.; Itami K. Synthesis of a Carbon Nanobelt. Science 2017, 356, 172–175. 10.1126/science.aam8158. [DOI] [PubMed] [Google Scholar]
- Cheung K. Y.; Watanabe K.; Segawa Y.; Itami K. Synthesis of a Zigzag Carbon Nanobelt. Nat. Chem. 2021, 13, 255–259. 10.1038/s41557-020-00627-5. [DOI] [PubMed] [Google Scholar]
- Han Y.; Dong S.; Shao J.; Fan W.; Chi C. Synthesis of a Sidewall Fragment of a (12,0) Carbon Nanobelt. Angew. Chem., Int. Ed. 2021, 60, 2658–2662. 10.1002/anie.202012651. [DOI] [PubMed] [Google Scholar]
- Hayakawa S.; Matsuo K.; Yamada H.; Fukui N.; Shinokubo H. Dinaphthothiepine Bisimide and Its Sulfoxide: Soluble Precursors for Perylene Bisimide. J. Am. Chem. Soc. 2020, 142, 11663–11668. 10.1021/jacs.0c04096. [DOI] [PubMed] [Google Scholar]
- Tanaka Y.; Matsuo K.; Yamada H.; Fukui N.; Shinokubo H. Gram-Scale Diversity-Oriented Synthesis of Dinaphthothiepine Bisimides as Soluble Precursors for Perylene Bisimides. Eur. J. Org. Chem. 2022, 2022, e202200770 10.1002/ejoc.202200770. [DOI] [Google Scholar]
- Vögtle F.; Schröder A.; Karbach D. Strategy for the Synthesis of Tube-Shaped Molecules. Angew. Chem., Int. Ed. Engl. 1991, 30, 575–577. 10.1002/anie.199105751. [DOI] [Google Scholar]
- Lewis S. E. Cycloparaphenylenes and Related Nanohoops. Chem. Soc. Rev. 2015, 44, 2221–2304. 10.1039/C4CS00366G. [DOI] [PubMed] [Google Scholar]
- Liu T.; Yang J.; Geyer F.; Conrad-Burton F. S.; Hernández Sánchez R.; Li H.; Zhu X.; Nuckolls C. P.; Steigerwald M. L.; Xiao S. Stringing the Perylene Diimide Bow. Angew. Chem., Int. Ed. 2020, 59, 14303–14307. 10.1002/anie.202004989. [DOI] [PubMed] [Google Scholar]
- Li A.; Zhang X.; Wang S.; Wei K.; Du P. Synthesis and Physical Properties of a Perylene Diimide-Embedded Chiral Conjugated Macrocycle. Org. Lett. 2023, 25, 1183–1187. 10.1021/acs.orglett.3c00152. [DOI] [PubMed] [Google Scholar]
- Bold K.; Stolte M.; Shoyama K.; Krause A.; Schmiedel A.; Holzapfel M.; Lambert C.; Würthner F. Macrocyclic Donor-Acceptor Dyads Composed of Oligothiophene Half-Cycles and Perylene Bisimides. Chem. – Eur. J. 2022, 28, e202200355 10.1002/chem.202200355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Würthner F. Perylene Bisimide Dyes as Versatile Building Blocks for Functional Supramolecular Architectures. Chem. Commun. 2004, 1564–1579. 10.1039/B401630K. [DOI] [PubMed] [Google Scholar]
- Weil T.; Vosch T.; Hofkens J.; Peneva K.; Müllen K. The Rylene Colorant Family—Tailored Nanoemitters for Photonics Research and Applications. Angew. Chem., Int. Ed. 2010, 49, 9068–9093. 10.1002/anie.200902532. [DOI] [PubMed] [Google Scholar]
- Zhan X.; Facchetti A.; Barlow S.; Marks T. J.; Ratner M. A.; Wasielewski M. R.; Marder S. R. Rylene and Related Diimides for Organic Electronics. Adv. Mater. 2011, 23, 268–284. 10.1002/adma.201001402. [DOI] [PubMed] [Google Scholar]
- Würthner F.; Saha-Möller C.; Fimmel B.; Ogi S.; Leowanawat P.; Schmidt D. Perylene Bisimide Dye Assemblies as Archetype Functional Supramolecular Materials. Chem. Rev. 2016, 116, 962–1052. 10.1021/acs.chemrev.5b00188. [DOI] [PubMed] [Google Scholar]
- Conrad-Burton F. S.; Liu T.; Geyer F.; Costantini R.; Schlaus A. P.; Spencer M. S.; Wang J.; Hernández Sánchez R.; Zhang B.; Xu Q.; Steigerwald M. L.; Xiao S.; Li H.; Nuckolls C. P.; Zhu X. Controlling Singlet Fission by Molecular Contortion. J. Am. Chem. Soc. 2019, 141, 13143–13147. 10.1021/jacs.9b05357. [DOI] [PubMed] [Google Scholar]
- Schaack C.; Evans A. M.; Ng F.; Steigerwald M. L.; Nuckolls C. High-Performance Organic Electronic Materials by Contorting Perylene Diimides. J. Am. Chem. Soc. 2022, 144, 42–51. 10.1021/jacs.1c11544. [DOI] [PubMed] [Google Scholar]
- Cruz C. M.; Walsh J. C.; Juríček M. Bending Pyrenacenes to Fill Gaps in Singlet-Fission-Based Solar Cells. Org. Mater. 2022, 4, 163–169. 10.1055/a-1939-6110. [DOI] [Google Scholar]
- Osswald P.; Würthner F. Conformational Effects of Bay Substituents on Optical, Electrochemical and Dynamic Properties of Perylene Bisimides: Macrocyclic Derivatives as Effective Probes. Chem.—Eur. J. 2007, 13, 7395–7409. 10.1002/chem.200700601. [DOI] [PubMed] [Google Scholar]
- Weh M.; Rühe J.; Herbert B.; Krause A.-M.; Würthner F. Deracemization of Carbohelicenes by a Chiral Perylene Bisimide Cyclophane Template Catalyst. Angew. Chem., Int. Ed. 2021, 60, 15323–15327. 10.1002/anie.202104591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y.; Tajima K.; Fukui N.; Shinokubo H. Dinaphtho[1,8-bc:1′,8′-fg][1,5]dithiocine Bisimide. Asian J. Org. Chem. 2021, 10, 541–544. 10.1002/ajoc.202000722. [DOI] [Google Scholar]
- Sato K.; Hyodo M.; Aoki M.; Zheng X.-Q.; Noyori R. Oxidation of Sulfides to Sulfoxides and Sulfones with 30% Hydrogen Peroxide under Organic Solvent- and Halogen-Free Conditions. Tetrahedron 2001, 57, 2469–2476. 10.1016/S0040-4020(01)00068-0. [DOI] [Google Scholar]
- Briseno A. L.; Mannsfeld S. C. B.; Reese C.; Hancock J. M.; Xiong Y.; Jenekhe S. A.; Bao Z.; Xia Y. Perylenediimide Nanowires and Their Use in Fabricating Field-Effect Transistors and Complementary Inverters. Nano Lett. 2007, 7, 2847–2853. 10.1021/nl071495u. [DOI] [PubMed] [Google Scholar]
- Haddon R. C. Rehybridization and π-Orbital Overlap in Nonplanar Conjugated Organic Molecules: π-Orbital Axis Vector (POAV) Analysis and Three-dimensional Hückel Molecular Orbital (3D-HMO) Theory. J. Am. Chem. Soc. 1987, 109, 1676–1685. 10.1021/ja00240a013. [DOI] [Google Scholar]
- Colwell C. E.; Price T. W.; Stauch T.; Jasti R. Strain Visualization for Strained Molecules. Chem. Sci. 2020, 11, 3923–3930. 10.1039/D0SC00629G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford W. E.; Kamat P. V. Photochemistry of 3,4,9,10-perylenetetracarboxylic dianhydride dyes. 3. Singlet and triplet excited-state properties of the bis(2,5-di-tert-butylphenyl)imide derivative. J. Phys. Chem. 1987, 91, 6373–6380. 10.1021/j100309a012. [DOI] [Google Scholar]
- Nagarajan K.; Mallia A. R.; Muraleedharan K.; Hariharan M. Enhanced Intersystem Crossing in Core-twisted Aromatics. Chem. Sci. 2017, 8, 1776–1782. 10.1039/C6SC05126J. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.