Abstract
In this study, we explore a potential vaccine for human papillomavirus (HPV)-induced tumors, using heat shock protein as an adjuvant, a peptide vaccine for safety, and adeno-associated virus (AAV) as a gene delivery vector. The tumor vaccine was devised by constructing a chimeric gene which contained HPV type 16 E7 cytotoxic T-lymphocyte (CTL) epitope DNA (M. C. Feltkamp, H. L. Smits, M. P. Vierboom, R. P. Minnaar, B. M. de Jongh, J. W. Drijfhout, J. ter Schegget, C. J. Melief, and W. M. Kast, Eur. J. Immunol. 23:2242–2249, 1993) fused with the heat shock protein gene as a tumor vaccine delivered via AAV. Our results demonstrate that this vaccine can eliminate tumor cells in syngeneic animals and induce CD4- and CD8-dependent CTL activity in vitro. Moreover, studies with knockout mice with distinct T-cell deficiencies confirm that CTL-induced tumor protection is CD4 and CD8 dependent. Taken together, the evidence indicates that this chimeric gene delivered by AAV has potential as a cervical cancer vaccine.
Cervical carcinoma remains one of the most common malignancies worldwide, with 500,000 new cases diagnosed each year (5) and 200,000 cervical cancer deaths annually (21). Human papillomavirus type 16 (HPV-16) is the predominant etiologic agent of cervical cancer and carries three transforming oncogenes, E5, E6, and E7 (10, 20, 45). Their products are thus unique tumor antigens and can be ideally used as tumor vaccines (7). Because E6 and E7 oncoproteins are consistently retained and expressed, the E6 and E7 oncogenes become more attractive targets for T-cell-based immunotherapy of cervical cancer. Evidence for the value of HPV antigen-directed immunotherapy against cervical cancer comes from the experimental and natural papillomavirus-associated tumors that can be controlled by immunization with E7 antigen. Previous studies have used different modes of immunization, such as (i) recombinant E7 vaccinia viruses (2, 3, 16, 24, 28, 42), (ii) syngeneic cells transfected with E7 (8, 9), (iii) E7 protein-pulsed dendritic cells (12, 37), (iv) peptides corresponding to a cytotoxic T-lymphocyte (CTL) epitope in E7 with incomplete Freund's adjuvant (13), (v) E7 vaccine based on papillomavirus-like particles (17, 25, 31), and (vi) Salmonella enterica serovar Typhimurium expressing E7 epitope inserted into hepatitis B virus core (26). These studies demonstrate that CTLs are likely to be the most effective immunological effector mechanisms.
Adeno-associated virus (AAV), a single-stranded virus, has been studied as a vector for gene therapy (29, 41). Classified as a defective human parvovirus, AAV has many natural features that are attractive for a human gene therapy vector, such as nonpathogenicity, targeted integrating capability, and a broad host range (human, simian, murine, canine, and avian). In sharp contrast to other viral vectors that have been used in vaccination, such as vaccinia virus or adenovirus, AAV vectors do not express any viral genes. The only viral DNA that must be included in an AAV vector is the 145-bp inverted terminal repeat. Since naked DNA is used for immunization, the only gene expressed by AAV vectors is that for the antigen itself.
Since HPV-16 E7 is a transforming oncoprotein (20), dangerous side effects such as transformation are not anticipated with a protein vaccine. Peptide vaccination with a CTL epitope to prevent the outgrowth of a tumor is a safe and effective immunotherapeutic method (13). However, a peptide vaccine combined with a toxic adjuvant such as incomplete Freund's adjuvant sometimes can lead to rapid tumor growth through specific T-cell tolerance induction (36). Recently, Mycobacterium tuberculosis heat shock protein 70 (hsp70) has been used as an adjuvant-free carrier to stimulate the humoral and cellular response to a full-length human immunodeficiency virus p24 (33) that is covalently linked to hsp. Mycobacterial hsp as an adjuvant has also been reported to enhance the induction of cellular immunity by an ovalbumin peptide vaccine (34).
In this study, we have accordingly taken advantage of the safety of a peptide vaccine with hsp as an adjuvant and AAV as a gene delivery vector. Thus, we devised the tumor vaccine by constructing a chimeric gene containing the HPV-16 E7 CTL epitope (13, 35) and hsp. The efficacy of protection against tumors was investigated through use of the vector AAV against syngeneic tumors. Results show that intramuscular (i.m.) vaccination with this chimeric gene via AAV vector could efficiently eliminate tumor cells, indicating that vaccination with this gene could be a therapeutic treatment for cervical cancer containing HPV-16 E7.
Construction and generation of recombinant AAV (rAAV) encoding E7 CTL epitope fused to hsp DNA.
The DNA fragment containing the HPV-16 E7 amino acid 44 to 62 coding sequence (35) was synthesized by PCR using the plasmid HPV-16 E7/pCEP4 as a template, which contained the sequence nucleotide 540 to 885 of HPV-16. The forward primer used in the PCR was 5′-GATGGTgaattcATGCAAGCAGAACCG-3′, which not only spanned the region covering the initiation codon (boldface) and HPV-16 E7 amino acid 44 to 47 coding sequence but also contained an EcoRI site (lowercase) at the 5′ end. The reverse primer was 5′-AAACCGAAGggatccGTCACACTTGCA-3′, which contained a BamHI site (lowercase) immediately after the HPV-16 E7 amino acid 62 coding sequence.
The plasmid vector pKS70 (33), which was created by subcloning the M. tuberculosis hsp 70 gene into the expression vector pT7-7 at the BamHI and HindIII sites, was a gift from Richard A. Young. The E7 epitope PCR product as described above was transferred into pGEM-T vector (Promega, Madison, Wis.) to generate pGEM-T/E7CTL and then subcloned into pKS70 at EcoRI and BamHI sites to generate pKS70/E7CTL-hsp. The plasmid pKS70/E7CTL-hsp was sequenced by the dideoxynucleotide termination method, and the HPV-16 E7 amino acid 44 to 62 coding sequence was confirmed to be in frame with the hsp70 gene. Then the DNA fragment of E7CTL-hsp was isolated by digesting EcoRI and HindIII from the plasmid pKS70/E7CTL-hsp, subcloned into SK(+) vector (Stratagene), and named pSK/E7CTL-hsp. The SmaI and XhoI DNA fragment from pSK/E7CTL-hsp was transferred into pXX-UF1 (23), which contains the green fluorescent protein gene under the regulation of the human cytomegalovirus immediate-early promoter and flanked by AAV 145-bp inverted terminal repeat sequences. The pXX-UF1 plasmid was digested with PvuII and SalI to remove the green fluorescent protein gene, and then the E7CTL-hsp DNA fragment was placed downstream of the transcriptional regulation of human cytomegalovirus immediate-early promoter and named pXX/E7CTL-hsp.
Preparation of rAAV E7CTL-hsp viral vector was done by cotransfection according to published protocols with modifications (30, 40). Briefly, a total of 49 μg of plasmid DNA (16 μg of pXX/E7CTL-hsp plasmid plus 8 μg of pXX2, which encoded Rep and Cap proteins, and 25 μg of pXX6, which encoded adenovirus gene products) was used to transfect 293 cells in each 15-cm-diameter dish using a modified calcium phosphate precipitation method. Cells from 80 dishes were harvested 48 h posttransfection, then cell mixtures were homogenated, and CsCl was added to a final density of 1.37 g/ml. The virus particles were then purified by CsCl density gradient centrifugation as previously published (39). Titers of rAAV E7CTL-hsp were determined by slot blot analysis to calculate the relative concentration of viral particles. The vector titers were in the range of 1 × 1012 to 5 × 1012 viral particles per ml (data not shown).
Northern blot analysis of chimeric E7 gene expression through AAV vector in 293 cells.
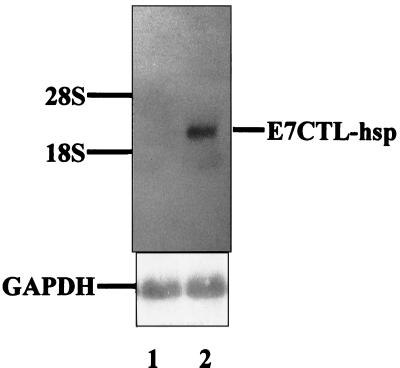
To determine whether the chimerically constructed E7CTL-hsp gene can be expressed via AAV delivered to cells, we injected 109 viral particles of rAAV E7CTL-hsp into 293 cells. Two days later, the cellular RNA was extracted and the expression of the E7CTL-hsp chimeric gene was analyzed by Northern blotting. Figure 1 shows that the chimeric E7CTL-hsp RNA (lane 2) could be expressed in rAAV encoding this chimeric construct but not in the control cells which were infected with rAAV lacZ (lane 1).
FIG. 1.
Northern blot analysis. RNA (20 μg) was fractionated by agarose gel electrophoresis, blotted, and hybridized with 32P-labeled E7CTL-hsp DNA and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA separately. A GAPDH probe was used to ensure equal RNA loading. Lane 1, 293 cells infected with rAAV lacZ; lane 2, 293 cells infected with rAAV E7CTL-hsp.
Efficient transduction of muscle cells with AAV vector.
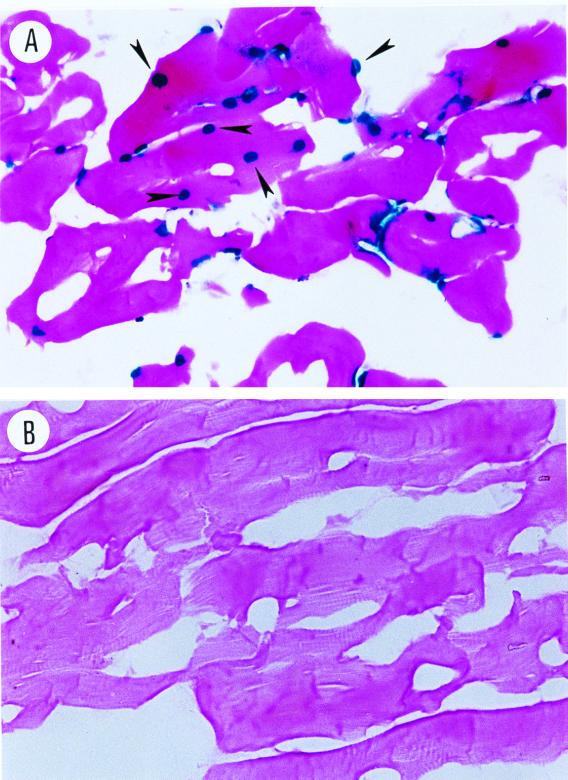
A previous report demonstrated that AAV vector can deliver the gene efficiently into muscle and establish long-term gene transduction (39). In this study, we introduced this chimeric tumor vaccine into muscle by delivery by AAV vector. Hence, we first tested the efficiency of AAV vectors encoding the lacZ reporter gene that harbors a nuclear localization signal under the regulation of the cytomegalovirus immediate-early promoter by delivering the vector to muscle. C57BL/6 (H-2b) mice were anesthetized with 2.5% Avertin intraperitoneally. Thirty microliters of rAAV lacZ (3 × 1010 viral particles) was injected into the hind leg tibialis anterior muscles percutaneously. After 2 weeks, mice were sacrificed, and muscle tissues were cryosectioned and stained for β-galactosidase activity, using X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) as a substrate. Figure 2A shows β-galactosidase nuclear staining within most of the myotubes (marked by arrow), indicating efficient gene transduction by the AAV vector.
FIG. 2.
X-Gal staining of the cryostat sections of the muscle injected with rAAV lacZ. (A) Five-week-old mice were injected with rAAV lacZ (3 × 1010 viral particles) in the tibialis anterior muscle. After 14 days, the cryostat sections were stained with X-Gal and eosin. (B) Control mice without rAAV lacZ injection.
Vaccination with rAAV encoding E7CTL-hsp generates tumor elimination.
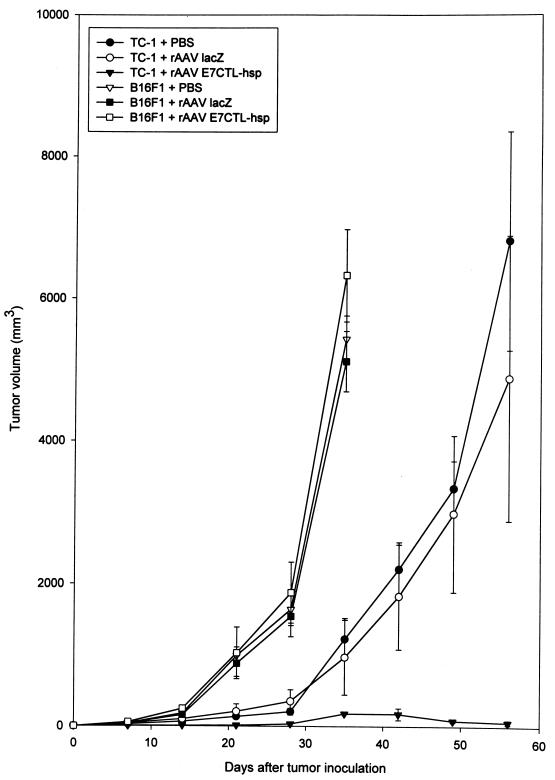
TC-1 was an E7-expressing tumorigenic cell line. It was established from primary lung epithelial cells of C57BL/6 mice immortalized by HPV-16 E6 and E7 and then transformed with an activated ras oncogene (38). To assess treatments of an established tumor, 10 C57BL/6 mice of each group were subcutaneously injected in the left flank with 5 × 104 TC-1 or B16F1 cells. B16F1 was a melanoma cell line derived from C57BL/6 mice (ATCC CRL 6322) and served as non-E7 expression syngeneic cells. After 1 week of tumor cell injection, they were immunized with 5 × 1010 viral particles of rAAV encoding E7CTL-hsp, rAAV lacZ, or normal saline (mock) into the hind leg tibialis anterior muscles. Then the tumor volume was measured once a week. As shown in Fig. 3, vaccination with rAAV E7CTL-hsp had significantly retarded the tumor growth induced by E7 expression cells (TC-1) but not that induced by non-E7 expression cells (B16F1), while inoculation with rAAV lacZ or mock vector had no effect on tumor elimination.
FIG. 3.
In vivo tumor elimination assay. Groups of 10 mice were injected subcutaneously with 5 × 104 TC-1 or B16F1 tumor cells and, after 1 week, were immunized with 5 × 1010 viral particles of rAAV E7CTL-hsp or rAAV lacZ or phosphate-buffered saline (PBS) (mock). The tumor volume was monitored once a week. The data are represented as means and standard errors of each group.
Cellular immune response in mice immunized with rAAV E7CTL-hsp.
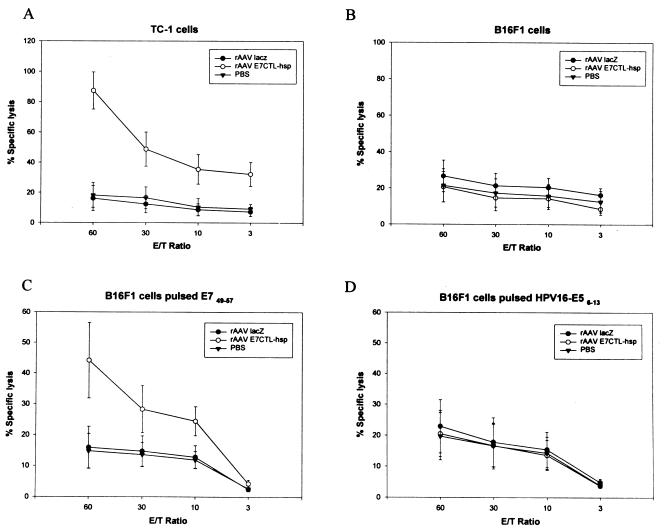
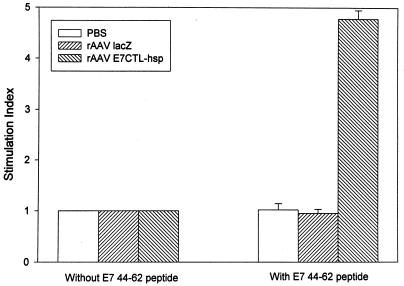
To elucidate the mechanism of protection against TC-1 tumors, we determined whether a CTL response was induced in mice immunized with rAAV encoding E7CTL-hsp. Spleen cells from C57BL/6 mice immunized with rAAV encoding E7CTL-hsp, rAAV lacZ, or phosphate-buffered saline were isolated and stimulated in vitro with E7 amino acid 49 to 57 synthetic peptide, which was identified as a CTL epitope (13). These stimulated spleen cells were then tested for recognition and lysis of 51Cr-labeled target cells including TC-1 (Fig. 4A); B16F1, which was a syngeneic C57BL/6 cell line (Fig. 4B); B16F1 pulsed with E7 peptide 49 to 57 (Fig. 4C); and B16F1 pulsed with nonspecific peptide HPV-16 E5 peptide 6 to 13 (Fig. 4D). As shown in Fig. 4, TC-1 and syngeneic B16F1 cells loaded with E7 49 to 57 peptide were lysed, whereas B16F1 cells alone or B16F1 cells pulsed with nonspecific peptide were not. The CTL response to TC-1 cells was significantly higher than that to B16F1 cells in the rAAV E7CTL-hsp-vaccinated mice by Student's t test (P < 0.05), and the CTL lysis activity for E7 peptide-pulsed B16F1 cells was also higher than that for E5 peptide-pulsed cells (P < 0.05). Moreover, we assayed for stimulation of E7 44 to 62-specific major histocompatibility complex (MHC) class II restricted proliferation response (35). Figure 5 shows that spleen cells from mice vaccinated with rAAV E7CTL-hsp could be stimulated by E7 peptide 44 to 62, but cells from rAAV lacZ-vaccinated and mock-vaccinated mice could not. Also, the spleen cells from mice vaccinated with rAAV E7CTL-hsp could not be stimulated by nonspecific peptide (E5 peptide 6 to 13 [data not shown]). Taken together, these results indicate that vaccination with rAAV encoding E7CTL-hsp induces CD4- and CD8-dependent CTL response to retard tumor growth.
FIG. 4.
HPV-16 E7 49 to 57 specific CTL responses induced by immunization with rAAV E7CTL-hsp. C57BL/6 mice were i.m. immunized with rAAV E7CTL-hsp. Four weeks after the vaccination, the spleens were collected and analyzed for in vitro CTL assay. (A) TC-1 cells; (B) B16F1 cells; (C) B16F1 cells pulsed with E7 peptide 49 to 57, (D) B16F1 cells pulsed with HPV-16 E5 peptide 6 to 13. The data were the averages of five vaccinated mice. E/T, effector/target. PBS, phosphate-buffered saline.
FIG. 5.
Proliferation of rAAV E7CTL-hsp-vaccinated lymphocytes in response to E7 peptide 44 to 62. C57BL/6 mice were i.m. immunized with rAAV E7CTL-hsp, rAAV lacZ, or mock. Four weeks after the vaccination, the spleens were collected and analyzed for T-cell proliferation measured by incorporation of [3H]thymidine. The data were the averages of five vaccinated mice of each group. The error bars represent standard errors. Stimulation index was defined as the ratio of mean counts per minute of incorporated [3H]thymidine for cells with antigen (E7 peptide 44 to 62) to mean counts per minute for cells without any added antigen. PBS, phosphate-buffered saline.
Vaccination-induced tumor protection involves both CD4 and CD8 T cells.
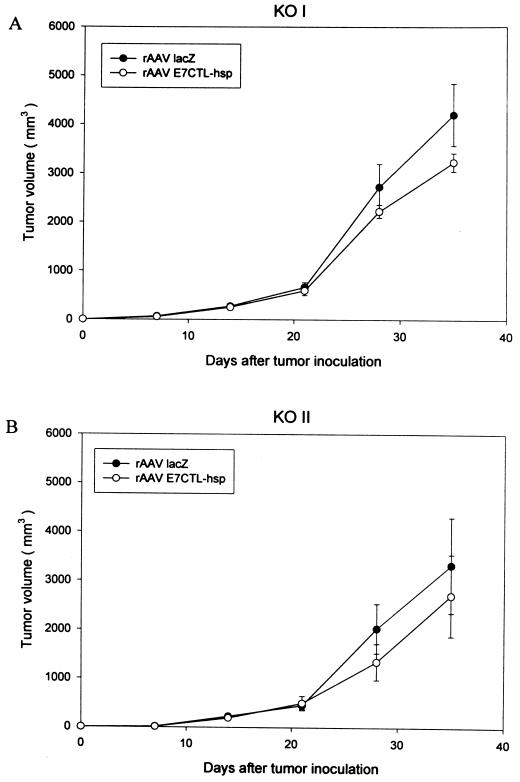
To understand the relative roles of CD4 and CD8 T cells in rAAV E7CTL-hsp vaccine-induced tumor protection, mice deficient in CD4 and CD8 T cells as a result of targeted gene disruption at β2m and MHC-II, respectively, were studied. The source of CD8 and CD4 T-cell-deficient mice was β2m−/− and MHC-II−/− mice on a C57BL/6 background, respectively (18, 22, 44). Breeders for β2m−/− and MHC-II−/− mice were kindly provided by B. J. Fowlkes (National Institutes of Health, Bethesda, Md.), and mice were bred under specific-pathogen-free conditions in the Institute of Molecular Biology Animal Facility of the Academia Sinica. Groups (n = 6) of CD4 (KOII) and CD8 (KOI) T-cell-deficient mice were injected with 5 × 104 TC-1 cells, followed 1 week later by vaccination with rAAV E7CTL-hsp or control rAAV lacZ. The tumor growth of TC-1 cells in the immunocompromised mice (KOI and KOII) was faster than that in the immunocompetent mice (C57BL/6). We monitored the tumor size in KOI and KOII mice for only 5 weeks after TC-1 cell injection into the immunocompromised mice as compared to the 8 weeks of observation for the immunocompetent mice (Fig. 3). Figure 6 shows clearly evident tumor growth in both CD4 and CD8 T-cell-deficient groups. It was not statistically significantly different between the rAAV E7CTL-hsp- and rAAV lacZ-vaccinated mice, implying participation of both CD4 and CD8 T cells in our rAAV E7CTL-hsp vaccine-induced tumor protection.
FIG. 6.
CD4- and CD8-dependent lymphocytes in tumor eradication by rAAV E7CTL-hsp vaccination. KOI (CD8-deficient) (A) and KOII (CD4-deficient) (B) mice were subcutaneously injected with 5 × 104 TC-1 tumor cells. One week later, they were divided into two groups for treatments with 5 × 1010 viral particles of rAAV lacZ or rAAV E7CTL-hsp. Each group consists of six mice. Tumor volume was checked once a week.
As described above, vaccination with E7 by different strategies can inhibit tumor growth (2, 3, 8, 9, 12, 16, 24, 28, 37, 42). The response to tumor inhibition is mediated by CTLs. It has been reported that this CTL response is CD4 and CD8 dependent (35, 38). However, CTL response induced by virus-like-particle-based E7 vaccine is CD8 dependent but CD4 independent (17, 25, 37). Our results demonstrate that E7 CTL epitope fused with hsp as a vaccine can enter the MHC class I and class II processing pathways in antigen-presenting cells and stimulate the production of CD8 CTLs. It may be the reason that M. tuberculosis hsp 70 is an especially powerful antigen containing multiple B- and T-cell epitopes (34). Previously, M. tuberculosis hsp70 has been used as an adjuvant-free carrier to stimulate the humoral and cellular response to a full-length human immunodeficiency virus p24 protein (33) or ovalbumin peptide (34) that is covalently linked to the hsp. The mechanisms by which hsp70 enables covalently linked polypeptide fusion partners to enter into the MHC class I and II antigen-presenting pathway and to elicit CD8 CTLs have been proposed to be (i) hsp70's ability to assist protein folding (15, 43) and to facilitate the translocation of proteins into subcellular compartments (4, 11), (ii) hsp's ability to facilitate the breakdown of intracellular proteins (32), and (iii) the high frequency of T cells directed against mycobacterial hsp70. Our study further supports the idea that hsp can act as an adjuvant to facilitate E7 peptide antigen inducement of CD4- and CD8-dependent CTL responses to E7-containing tumors.
Our study demonstrates that AAV vector, which has all viral coding sequences (96% of genome) removed (1, 14, 39), represents a promising alternative for delivering tumor vaccine (27). The first clinical trial of a live recombinant vaccinia virus expressing the E6 and E7 proteins of HPV-16 and -18 has been evaluated elsewhere (2). For gene therapy, AAV vector may be superior to other viral vectors that have been used in vaccination, such as vaccinia virus and adenovirus, since AAV vectors do not express viral genes. As with immunization with naked DNA, the only expressed gene carried by AAV vectors is the cloned gene itself. Using vaccinia virus or adenovirus as a vector to deliver vaccine may offer some advantages in the stimulation of the immune system; however, the advantages are probably outweighed by the significant risks associated with virus infection, especially for immunodeficient patients (6, 19). In addition, it was previously reported that AAV vector does not induce strong cellular immune responses to the transduced cells, allowing the persistence of gene expression. The gene expression in skeletal muscle transduced by AAV has been shown to persist for more than 1.5 years (39). Although AAV vector can elicit a humoral immune response which results in neutralizing activity after a second administration, repeated dosing appears not to be necessary given that the originally transduced cells can escape a CTL response and persist in the long term. AAV may thus be useful for the viral immunization of humans. Up to now, for vaccination, AAV vectors represent the combination of the best properties of viral and nonviral vectors. Recently, a study of vaccine development targeted at herpes simplex virus infection has demonstrated that AAV-mediated immunization can prime specific CTL and antibody responses (27). Hence, our study provides a potential vaccine for HPV-induced tumors.
In this study, we successfully developed rAAV encoding HPV-16 E7 CTL peptide DNA fused with hsp DNA as a tumor vaccine. It is a potential vaccine for cervical cancer treatment using hsp as a carrier protein and delivery by rAAV vector.
Acknowledgments
We are grateful to Richard A. Young for providing the plasmid pKS70, T. C. Wu for providing TC-1 cells, B. J. Fowlkes for providing β2m−/− and MHC-II−/− mice, and Samuel S. Chen for editing the English.
This work was supported by National Science Council grant NSC 87-2312-B106-003.
REFERENCES
- 1.Afione S A, Conrad C K, Kearns W G, Chunduru S, Adams R, Reynolds T C, Guggino W B, Cutting G R, Carter B J, Flotte T R. In vivo model of adeno-associated virus vector persistence and rescue. J Virol. 1996;70:3235–3241. doi: 10.1128/jvi.70.5.3235-3241.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borysiewicz L K, Fiander A, Nimako M, Man S, Wilkinson G W, Westmoreland D, Evans A S, Adams M, Stacey S N, Boursnell M E, Rutherford E, Hickling J K, Inglis S C. A recombinant vaccinia virus encoding human papillomavirus types 16 and 18, E6 and E7 proteins as immunotherapy for cervical cancer. Lancet. 1996;347:1523–1527. doi: 10.1016/s0140-6736(96)90674-1. [DOI] [PubMed] [Google Scholar]
- 3.Boursnell M E, Rutherford E, Hickling J K, Rollinson E A, Munro A J, Rolley N, McLean C S, Borysiewicz L K, Vousden K, Inglis S C. Construction and characterization of a recombinant vaccinia virus expressing human papillomavirus proteins for immunotherapy of cervical cancer. Vaccine. 1996;14:1485–1494. doi: 10.1016/S0264-410X(96)00117-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brodsky J L. Post-translational protein translocation: not all hsc70s are created equal. Trends Biochem Sci. 1996;21:122–126. [PubMed] [Google Scholar]
- 5.Burghardt E, Webb M J, Monaghan J M, Kindermann G. Surgical gynecological oncology. New York, N.Y: Thieme Medical Publishers; 1993. [Google Scholar]
- 6.Cason J, Khan S A, Best J M. Towards vaccines against human papillomavirus type-16 genital infection. Vaccine. 1993;11:603–611. doi: 10.1016/0264-410x(93)90302-e. [DOI] [PubMed] [Google Scholar]
- 7.Chen C H, Wu T C. Experimental vaccine strategies for cancer immunotherapy. J Biomed Sci. 1998;5:231–252. doi: 10.1007/BF02255855. [DOI] [PubMed] [Google Scholar]
- 8.Chen L, Mizuno M T, Singhal M C, Hu S L, Galloway D A, Hellstrom I, Hellstrom K E. Induction of cytotoxic T lymphocytes specific for a syngeneic tumor expressing the E6 oncoprotein of human papillomavirus type 16. J Immunol. 1992;148:2617–2621. [PubMed] [Google Scholar]
- 9.Chen L P, Thomas E K, Hu S L, Hellstrom I, Hellstrom K E. Human papillomavirus type 16 nucleoprotein E7 is a tumor rejection antigen. Proc Natl Acad Sci USA. 1991;88:110–114. doi: 10.1073/pnas.88.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen S L, Han C P, Tsao Y P, Lee J W, Yin C S. Identification and typing of human papillomavirus in cervical cancers in Taiwan. Cancer. 1993;72:1939–1945. doi: 10.1002/1097-0142(19930915)72:6<1939::aid-cncr2820720624>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 11.Cyr D M, Neupert W. Stress-inducible cellular responses. In: Feige U, Morimoto R I, Yahara I, Polla B S, editors. Stress-inducible cellular responses. Basel, Switzerland: Birkhauser; 1996. pp. 25–40. [Google Scholar]
- 12.De Bruijn M L, Schuurhuis D H, Vierboom M P, Vermeulen H, de Cock K A, Ooms M E, Ressing M E, Toebes M, Franken K L, Drijfhout J W, Ottenhoff T H, Offringa R, Melief C J. Immunization with human papillomavirus type 16 (HPV16) oncoprotein-loaded dendritic cells as well as protein in adjuvant induces MHC class I-restricted protection to HPV16-induced tumor cells. Cancer Res. 1998;58:724–731. [PubMed] [Google Scholar]
- 13.Feltkamp M C, Smits H L, Vierboom M P, Minnaar R P, de Jongh B M, Drijfhout J W, ter Schegget J, Melief C J, Kast W M. Vaccination with cytotoxic T lymphocyte epitope-containing peptide protects against a tumor induced by human papillomavirus type 16-transformed cells. Eur J Immunol. 1993;23:2242–2249. doi: 10.1002/eji.1830230929. [DOI] [PubMed] [Google Scholar]
- 14.Flotte T R, Afione S A, Conrad C, McGrath S A, Solow R, Oka H, Zeitlin P L, Guggino W B, Carter B J. Stable in vivo expression of the cystic fibrosis transmembrane conductance regulator with an adeno-associated virus vector. Proc Natl Acad Sci USA. 1993;90:10613–10617. doi: 10.1073/pnas.90.22.10613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flynn G C, Pohl J, Flocco M T, Rothman J E. Peptide-binding specificity of the molecular chaperone BiP. Nature. 1991;353:726–730. doi: 10.1038/353726a0. [DOI] [PubMed] [Google Scholar]
- 16.Gao L, Chain B, Sinclair C, Crawford L, Zhou J, Morris J, Zhu X, Stauss H. Immune response to human papillomavirus type 16 E6 gene in a live vaccinia vector. J Gen Virol. 1994;75:157–164. doi: 10.1099/0022-1317-75-1-157. [DOI] [PubMed] [Google Scholar]
- 17.Greenstone H L, Nieland J D, de Visser K E, De Bruijn M L, Kirnbauer R, Roden R B, Lowy D R, Kast W M, Schiller J T. Chimeric papillomavirus virus-like particles elicit antitumor immunity against the E7 oncoprotein in an HPV16 tumor model. Proc Natl Acad Sci USA. 1998;95:1800–1805. doi: 10.1073/pnas.95.4.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grusby M J, Johnson R S, Papaioannou V E, Glimcher L H. Depletion of CD4+ T cells in major histocompatibility complex class II-deficient mice. Science. 1991;253:1417–1420. doi: 10.1126/science.1910207. [DOI] [PubMed] [Google Scholar]
- 19.Hagensee M C, Carter J J, Wipf G C, Galloway D A. Immunization of mice with HPV vaccinia virus recombinants generates serum IgG, IgM, and mucosal IgA antibodies. Virology. 1995;206:174–182. doi: 10.1016/s0042-6822(95)80032-8. [DOI] [PubMed] [Google Scholar]
- 20.Howley P M. Role of the human papillomaviruses in human cancer. Cancer Res. 1991;51:5019s–5022s. [PubMed] [Google Scholar]
- 21.International Agency for Research on Cancer. Monograph on cancer. Human papillomaviruses. Geneva, Switzerland: World Health Organization; 1995. [Google Scholar]
- 22.Koller B H, Marrack O, Kappler J W, Smithies O. Normal development of mice deficient in β2M, MHC class I proteins, and CD8+ T cells. Science. 1990;248:1227–1230. doi: 10.1126/science.2112266. [DOI] [PubMed] [Google Scholar]
- 23.Li J, Dressman D, Tsao Y P, Sakamoto A, Hoffman E P, Xiao X. Recombinant AAV vector-mediated sarcoglycan gene transfer in a hamster model for limb girdle muscular dystrophy. Gene Ther. 1999;6:74–82. doi: 10.1038/sj.gt.3300830. [DOI] [PubMed] [Google Scholar]
- 24.Lin K Y, Guarnieri F G, Staveley O C K F, Levitsky H I, August J T, Pardoll D M, Wu T C. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res. 1996;56:21–26. [PubMed] [Google Scholar]
- 25.Liu X S, Abdul-Jabbar I, Qi Y M, Frazer L H, Zhou J. Mucosal immunisation with papillomavirus virus-like particles elicits systemic and mucosal immunity in mice. Virology. 1998;252:39–45. doi: 10.1006/viro.1998.9442. [DOI] [PubMed] [Google Scholar]
- 26.Londono L P, Chatfield S, Tindle R W, Herd K, Gao X M, Frazer I, Dougan G. Immunization of mice using Salmonella typhimurium expressing human papillomavirus type 16 E7 epitopes inserted into hepatitis B virus core antigen. Vaccine. 1996;14:545–552. doi: 10.1016/0264-410x(95)00216-n. [DOI] [PubMed] [Google Scholar]
- 27.Manning W C, Paliard X, Zhou S, Bland M P, Lee A Y, Hong K, Walker C M, Escobedo J A, Dwarki V. Genetic immunization with adeno-associated virus vectors expressing herpes simplex virus type 2 glycoproteins B and D. J Virol. 1997;71:7960–7962. doi: 10.1128/jvi.71.10.7960-7962.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meneguzzi G, Cerni C, Kieny M P, Lathe R. Immunization against human papillomavirus type 16 tumor cells with recombinant vaccinia viruses expressing E6 and E7. Virology. 1991;181:62–69. doi: 10.1016/0042-6822(91)90470-v. [DOI] [PubMed] [Google Scholar]
- 29.Muzyczka N. Use of adeno-associated virus as a general transduction vector for mammalian cells. Curr Top Microbiol Immunol. 1992;158:97–129. doi: 10.1007/978-3-642-75608-5_5. [DOI] [PubMed] [Google Scholar]
- 30.Pan R Y, Xiao X, Chen S L, Li J, Lin L C, Wang H J, Tsao Y P. Disease-inducible transgene expression from a recombinant adeno-associated virus vector in a rat arthritis model. J Virol. 1999;73:3410–3417. doi: 10.1128/jvi.73.4.3410-3417.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schafer K, Muller M, Faath S, Henn A, Osen W, Zentgraf H, Benner A, Gissmann L, Jochmus I. Immune response to human papillomavirus 16 L1E7 chimeric virus-like particles:induction of cytotoxic T cells and specific tumor protection. Int J Cancer. 1999;81:881–888. doi: 10.1002/(sici)1097-0215(19990611)81:6<881::aid-ijc8>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 32.Sherman M Y, Goldberg A L. Stress-inducible cellular responses. In: Feige U, Morimoto R I, Yahara I, Polla B S, editors. Stress-inducible cellular responses. Basel, Switzerland: Birkhauser; 1996. pp. 57–78. [Google Scholar]
- 33.Suzue K, Young R A. Adjuvant-free hsp70 fusion protein system elicits humoral and cellular immune responses to HIV-1 p24. J Immunol. 1996;156:873–879. [PubMed] [Google Scholar]
- 34.Suzue K, Zhou X, Eisen H N, Young R A. Heat shock fusion proteins as vehicles for antigen delivery into the major histocompatibility complex class I presentation pathway. Proc Natl Acad Sci USA. 1997;94:13146–13151. doi: 10.1073/pnas.94.24.13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tindle R W, Fernando G J, Sterling J C, Frazer I H. A “public” T-helper epitope of the E7 transforming protein of human papillomavirus 16 provides cognate help for several E7 B-cell epitopes from cervical cancer-associated human papillomavirus genotypes. Proc Natl Acad Sci USA. 1991;88:5887–5891. doi: 10.1073/pnas.88.13.5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Toes R E, Offringa R, Blom R J, Melief C J, Kast W M. Peptide vaccination can lead to enhanced tumor growth through specific T-cell tolerance induction. Proc Natl Acad Sci USA. 1996;93:7855–7860. doi: 10.1073/pnas.93.15.7855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tuting T, DeLeo A B, Lotze M T, Storkus W J. Genetically modified bone marrow-derived dendritic cells expressing tumor-associated viral or “self” antigens induce antitumor immunity in vivo. Eur J Immunol. 1997;27:2702–2707. doi: 10.1002/eji.1830271033. [DOI] [PubMed] [Google Scholar]
- 38.Wu T C, Guarnieri F G, Staveley O C K F, Viscidi R P, Levitsky H I, Hedrick L, Cho K R, August J T, Pardoll D M. Engineering an intracellular pathway for major histocompatibility complex class II presentation of antigens. Proc Natl Acad Sci USA. 1995;92:11671–11675. doi: 10.1073/pnas.92.25.11671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiao X, Li J, Samulski R J. Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector. J Virol. 1996;70:8098–8108. doi: 10.1128/jvi.70.11.8098-8108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xiao X, Li J, Samulski R J. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J Virol. 1998;72:2224–2232. doi: 10.1128/jvi.72.3.2224-2232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xiao X, deVlaminck W, Monahan J. Adeno-associated virus (AAV) vectors for gene transfer. Adv Drug Delivery Rev. 1993;12:201–215. [Google Scholar]
- 42.Zhu X, Tommasino M, Vousden K, Sadovnikava E, Rappuoli R, Crawford L, Kast M, Melief C J, Beverley P C, Stauss H J. Both immunization with protein and recombinant vaccinia virus can stimulate CTL specific for the E7 protein of human papilloma virus 16 in H-2d mice. Scand J Immunol. 1995;42:557–563. doi: 10.1111/j.1365-3083.1995.tb03696.x. [DOI] [PubMed] [Google Scholar]
- 43.Zhu X, Zhao X, Burkholder W F, Gragerov A, Ogata C M, Gottesman M E, Hendrickson W A. Structural analysis of substrate binding by the molecular chaperone DnaK. Science. 1996;272:1606–1614. doi: 10.1126/science.272.5268.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zijlstra M, Li E, Sajjadi F, Subramani S, Jaenisch R. Germ-line transmission of a disrupted β2-microglobulin gene produced by homologous recombination in embryonic stem cells. Nature. 1989;342:435–438. doi: 10.1038/342435a0. [DOI] [PubMed] [Google Scholar]
- 45.zur Hausen H, de Villiers E M. Human papillomaviruses. Annu Rev Microbiol. 1994;48:427–447. doi: 10.1146/annurev.mi.48.100194.002235. [DOI] [PubMed] [Google Scholar]