Abstract
To thrive in challenging environments, individuals must pursue rewards while avoiding threats. Aberrant threat responses are characteristic of anxiety disorders. Extensive studies in animals and humans have identified the central extended amygdala (EAc)—which includes the central nucleus of the amygdala (Ce) and the bed nucleus of the stria terminalis (BST)—as a conserved substrate for defensive behavior. These studies suggest the EAc influences defensive responding and assembles fearful and anxious states. One impact of these investigations has been the proliferation of a view that the EAc is fundamentally a defensive substrate. Yet mechanistic work in animals has implicated the EAc in numerous appetitive and consummatory processes, yielding fresh insights into the microcircuitry of emotion-relevant response selection across various contexts. Coupled with the EAc’s centrality in a conserved network of brain regions that encode multisensory environmental and interoceptive information, these findings allude to a broader role for the EAc as an arbiter of survival-relevant tradeoffs for action selection. Determining how the EAc optimizes these tradeoffs promises to improve our understanding of common psychiatric illnesses like anxiety, depression, alcohol- and substance-use disorders, and anhedonia.
Keywords: Fear, Anxiety, Defensive Behavior, Reward, Consummatory, Action Selection, Extended Amygdala, Bed Nucleus of the Stria Terminalis, Central Nucleus of the Amygdala, BST, BNST, Ce, CeA
Optimizing Survival-Relevant Tradeoffs in a Challenging World
The natural world is an unforgiving place, where opportunities to acquire resources, reproduce, and explore must be balanced against ubiquitous threats like predation, starvation, and injury (Blanchard et al., 2011; Blanchard & Blanchard, 2008; Mobbs et al., 2009, 2015). An animal that grazes with reckless abandon might enjoy the short-term benefits of better nutrition, but it’s more likely than its vigilant conspecifics to be injured or killed by a predator (Cooper & Blumstein, 2015; Evans & Stempel et al., 2018). Conversely, an animal that tends to forgo its meals and flee at the faintest sign of danger might avoid predators in the short term, but it will eventually suffer malnourishment. Survival-relevant tradeoffs like these pervade the natural world (Fig. 1A, left), and the central nervous system evolved to manage them. The human brain also manages emotion-relevant tradeoffs, for example the decision of whether to socialize with others or avoid them (Fig. 1A, right). While some trepidation in approaching others can be adaptive, an extreme bias toward avoidance can be maladaptive and characteristic of anxiety-related psychopathology (Fox & Kalin, 2014; Shackman et al., 2016). Importantly, the same avoidant behavior could result from any of several biases in the response-selection process (Fig. 1B). How might this selection process be organized in the brain to promote survival in a world of innumerable possibilities? We posit that the brain dynamically integrates sensations, memories, homeostatic signals, preferences, expectations, and other factors into n-dimensional feature space where weighted environmental and interoceptive evidence (i.e., E*WkT) for survival- and emotion-relevant responses can be represented as values (i.e., V[Rk]) and compared (Fig. 1C). The brain must then resolve the feature space to select and trigger adaptive physiology, cognition, and behavior that promote survival and optimize well-being by striking the best balance between risks and rewards.
Figure 1.
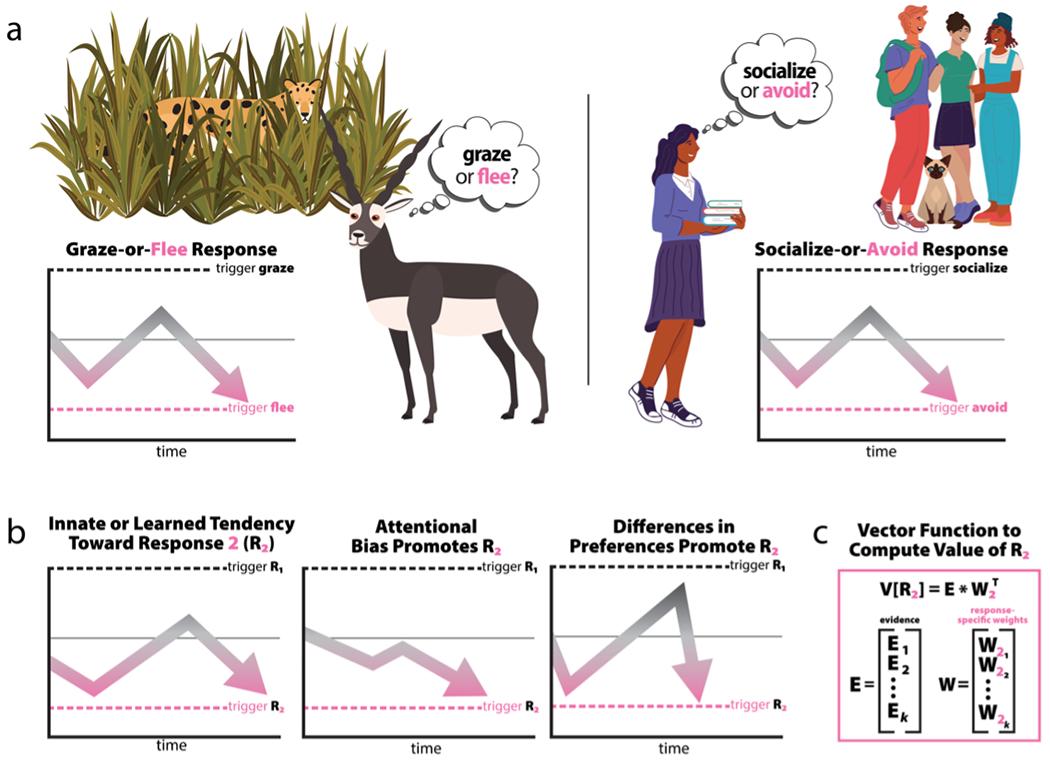
In nature and society, behavior is characterized by risk-vs-reward tradeoffs. a) Adaptive responses are selected from competing options. A hungry gazelle detects a predator and must select between grazing and freezing (left)—neither of which is inherently maladaptive. This selection process can be defined as a function of the value of each response, i.e., f(V[R1], V[R2]). While we are agnostic about the specific computations underlying the tradeoffs inherent to response selection, these choices can be conceptualized with simplified drift-diffusion models (DDM; Ratcliff & McKoon, 2008) in which responses are triggered as accumulating evidence surpasses a decision threshold, represented here as dashed lines bisected by a grey line indicating the indifference point. In humans, the systems that underlie these survival-relevant selection processes can select emotion-relevant responses (right). b) Different underlying processes can trigger the same response. Even with a simplified two-option DDM—which has been useful for characterizing multi-alternative valuation decisions (Krajbich & Rangel, 2011)—different underlying processes can bias individuals toward the same response: an innate or learned tendency toward one response over another (left), an attentional bias that leads to disproportionate accumulation of evidence in favor of one response over another (middle), or differences in the valuation of evidence between responses (right) illustrate sources of bias toward response R2. c) Response selection as a computational process in an n-dimensional feature space. The value of any response (e.g., V[R2]) can be conceptualized as the product of all available evidence (e.g., [E1, E2… Ek]) times the context-specific weight afforded to each piece of evidence (e.g., [W21, W22… W2k]T). In the case of our gazelle, E1 might represent predator proximity, and W22 the gazelle’s sensitivity to predator proximity in the context of escape decisions. Of note, the weights may comprise a sparse matrix; that is, many pieces of evidence may have no (or little) bearing on a specific response.
What constitutes “adaptive” depends on the feature-space inputs, which are unique to individuals at a given moment: Grazing for a few extra seconds as a predator approaches might be adaptive if an animal is especially hungry, if the quality of its food source is high, if its surroundings favor last-second escapes, if nearby conspecifics diffuse the likelihood of being attacked, if the predator is frail or immature, and so on. As the value of each input waxes and wanes, perturbations in the feature space nudge the probability of action selection toward one response or another. Input from a plethora of brain regions shapes the feature space. To avoid “paralysis by analysis” in the management of these high-stakes tradeoffs, specific substrates must integrate across this feature space to rapidly select the most adaptive response. The EAc is well-positioned for this role.
Evidence for an Evolutionarily-Conserved Role for the EAc in Defensive Behavior
In the crucible of natural selection, the primacy of survival has spurred the evolution of defensive adaptations across phylogeny. In mammals, the EAc is a conserved neural substrate that responds to both innate and learned threats. Situated at the center of a distributed network of brain regions that promote fitness in challenging contexts (Fox et al., 2015b; Mobbs et al., 2015), the EAc receives robust direct and indirect input from contextual, sensory, regulatory, and evaluative regions (de Olmos & Heimer, 1999; Swanson & Petrovich, 1998). Two of its major subcomponents—the Ce and the BST—form a functionally coupled circuit (Oler et al., 2012; Oler & Tromp et al., 2017; Tillman et al., 2018; Gorka et al., 2018; Avery et al., 2014) and exhibit similar patterns of gene expression (Bupesh et al., 2011; Fox et al., 2015b; Lein et al., 2007), neurochemistry (Gray & Magnuson, 1992), cellular composition (McDonald, 1982, 1983), and structural connectivity (Fox et al., 2015; Oler & Tromp et al., 2017; Roy et al., 2009). Direct projections from the EAc to effector regions like the periaqueductal gray (PAG) and parvocellular reticular formation (PCRt) trigger selected responses (Han et al., 2017; Tovote et al., 2016). The EAc is therefore well-positioned to synthesize environmental and interoceptive inputs into a meaningful gestalt, rapidly select optimal defensive responses, and launch those responses into action (Fox et al., 2015b; Mobbs et al., 2015).
Neuroimaging studies of threat-anticipation tasks in humans, and human neuroimaging papers that use the words “fear” and “anxiety,” often report significant activation in the Ce and BST (Fig. 2A, top, adapted from Fox & Shackman, 2019, p. 60, Figure 2; Hur et al., 2020; Shackman & Fox, 2016; Somerville et al., 2013; Hur et al., in press). In nonhuman primate (NHP) neuroimaging studies, individual differences in rhesus (Macaca mulatta) neuroendocrine and behavioral reactivity to potential threats are associated with increased [18F]fludeoxyglucose (FDG) metabolism in the Ce and BST (Fig. 2A, bottom; Fox et al., 2008; Fox et al., 2015a; Oler et al., 2010), as well as increased functional connectivity between these regions (Fox et al., 2018; Oler & Tromp et al., 2017). Loss-of-function studies tell a similar story and induce a “threat blind” phenotype in NHPs: rhesus monkeys with gross amygdala lesions, which include the Ce, exhibit more affiliative and sexual behaviors toward intact conspecifics (Emery et al., 2001; Machado & Bachevalier, 2008), are more likely to consume unpalatable foods (Machado et al., 2010), and more readily interact with novel and potentially dangerous objects (Bliss-Moreau et al., 2010, 2011; but see Charbonneau et al., 2021). Similarly, rhesus monkeys with spatially-precise Ce lesions show blunted freezing in response to potential threats and are quicker than intact conspecifics to reach past a snake and retrieve food rewards (Kalin et al., 2004). In rodents, decades of fear-conditioning and threat-related studies have built a foundation for investigating threat processing and have been instrumental to the formulation, testing, and refinement of hypotheses regarding individual responses of the Ce and BST to phasic and sustained threats (Davis et al., 2010; Walker et al., 2009; Walker & Davis, 2008; Marcinkiewcz et al., 2016; Tovote et al., 2016; Perusini & Fanselow, 2015). Despite long-standing evidence of its role in non-defensive processes (e.g., Aggleton, 2000; Baxter & Murray, 2002; Whalen & Phelps, 2009), the sheer volume of studies implicating the EAc in threat processing could lead one to conclude that it is fundamentally a defensive substrate.
Figure 2.
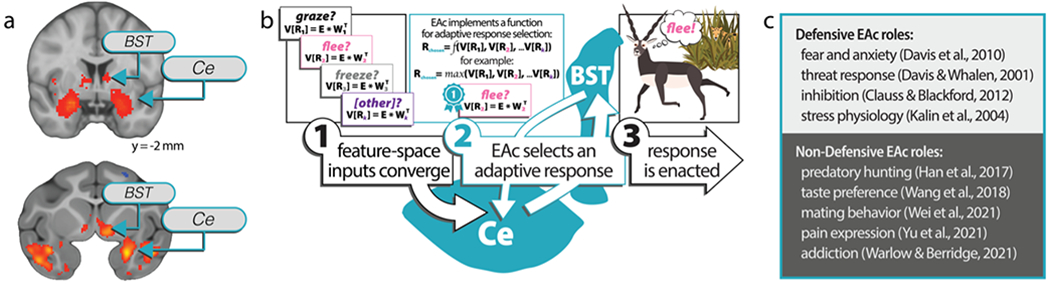
The EAc selects defensive and non-defensive responses. a) Studies of humans and rhesus monkeys implicate the EAc in uncertain threat response. As reported in Shackman and Fox, 2016, a Neurosynth-enabled (Yarkoni et al., 2011) automated meta-analysis of “fear” and “anxiety” neuroimaging studies in humans reveals Ce and BST activation (top), and large-scale (N=592) nonhuman primate neuroimaging studies of response to uncertain threat (Fox et al., 2015a) show that rhesus anxious temperament predicts elevated EAc metabolism during exposure to an uncertain threat represented by an unfamiliar human intruder (bottom). b) Feature-space model of EAc-implemented function for selecting between graze (R1), flee (R2), and freeze (R3) responses based on the weighted valuations of those responses in each context. In this simplified three-choice model, 1) feature-space inputs encoding salient, weighted environmental and interoceptive evidence converge on the EAc; 2) the EAc represents and resolves the feature space through an unknown selection function (shown here as a placeholder function to represent what is almost certainly a more complicated process; see Krajbich & Rangel, 2011) to guide survival-relevant and emotion-relevant tradeoffs for action selection and adaptive physiology; and 3) instructions to enact the winning response are pushed downstream to effector regions capable of triggering changes in physiology, cognition, and behavior. c) An illustrative list of defensive and non-defensive EAc roles highlights the EAc’s involvement in diverse response sets. Of note, we use the terms “defensive” and “non-defensive” to be inclusive of physiological, cognitive, and behavioral changes, as well as the phenomenological states that elicit EAc involvement.
Rodent Studies Uncover the EAc’s Diverse Roles in Survival-Related Response Selection
In the past decade, methodological advances have endowed researchers with tools that enable cell type-specific targeting, millisecond-resolution observation, and bidirectional control of neural populations (Chen et al., 2013; Deisseroth, 2011; Resendez & Stuber, 2015; Roth, 2016; Fox & Shackman, 2019). Coupled with well-validated fear-conditioning and threat-related assays, these methods are elucidating the mechanisms that subserve threat processing and have uncovered intermingled populations of EAc neurons that function as substrates for the selection of defensive responses. For example, cell type-specific manipulations within the mouse Ce have identified a competitive inhibitory microcircuit—comprised of intermingled corticotropin releasing hormone-positive (CRH+) and somatostatin-positive (SST+) neurons—that rapidly selects between fleeing and freezing (Fadok et al., 2017; Fig. 3A). Similarly, distinct cell types have been implicated in competitive responses to learned vs. unlearned threats (Isosaka et al., 2015). Other studies have characterized a lateral Ce (CeL) microcircuit that gates conditioned freezing through projections to the medial Ce (CeM; Botta et al., 2015; Ciocchi et al., 2010; Haubensak et al., 2010). This work shows that “CeLoff” neurons—which express the anxiety-associated genetic marker protein kinase C-delta (PKCδ+)—form a reciprocal inhibitory microcircuit with intermingled “CeLon”/PKCδ-negative (PKCδ−) neurons. Threat conditioning increases the basal firing rate of the “CeLoff”/PKCδ+ population, leading to stronger local inhibition of CeL-CeM projections and increased threat generalization—a transdiagnostic feature of anxiety disorders (Lissek et al., 2010, 2014; Morey et al., 2020). These findings dovetail with work NHPs demonstrating that levels of the transcript encoding for PKCδ in the CeL is associated with individual differences in threat responding (Kovner et al., 2020).
Figure 3.
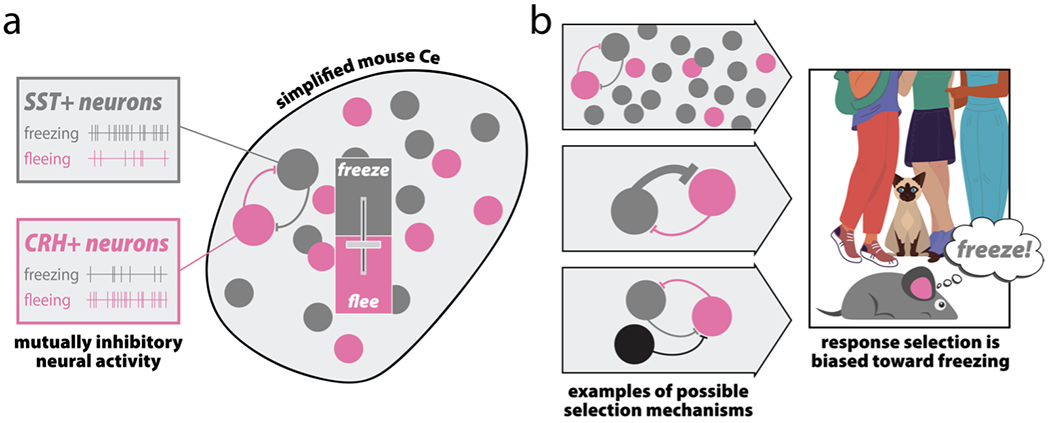
Genetically dissociable microcircuits provide a substrate for response selection through the implementation of a selection function (e.g., f(V[R1], V[R2], …V[Rk]; see Fig. 2B). a) Mutually inhibitory neural activity in the mouse Ce. A competitive inhibitory microcircuit composed of intermingled, competing populations of SST+ and CRH+ neurons select between freezing and fleeing responses, respectively (adapted from Fadok et al., 2017). The activity of either population generates strong inhibitory postsynaptic currents that suppress the other population, thereby serving as a rapid, winner-take-all mechanism for selecting between active and passive threat response. b) Possible mechanisms for response selection. Several distinct mechanisms could dispositionally bias an individual toward passive threat response (i.e., maladaptive freezing), characteristic of behavioral inhibition (Roelofs, 2017; Roelofs, Hagenaars, & Stins, 2010); for example: a preponderance of SST+ neurons (top), disproportionately strong SST+ to CRH+ projections (middle), or the presence of a third population of neurons that co-inhibits CRH+ neurons (bottom). Importantly, while we have highlighted the SST+ and CRH+ microcircuit in the mouse Ce, it is likely that imbalances in other microcircuits—for example, the aforementioned “CeLon”/PKCδ− and “CeLoff”/PKCδ+ microcircuit—could drive similar tendencies. We hypothesize similar alterations in other EAc regions, such as the BST.
To understand how EAc alterations promote pathological anxiety, we need to carefully consider its broader role in arbitrating survival-relevant tradeoffs (i.e., its role in selecting adaptive behavior as a function of V[Ri], or f(V[R1], V[R2]; see Fig. 2B, C). For instance, researchers have shown that chemogenetic inhibition of CeL PKCδ+ neurons—the same cells that appear to play a mechanistic role in threat generalization—induces risky feeding behavior in mice, as measured by the consumption of bitter tastants that control animals tend to reject (Cai et al., 2014; Ponserre et al., 2020). Other studies have shown that gustatory cortical projections to the Ce encode—and, when manipulated, can even reverse—the hedonic value of bitter tastants (Wang et al., 2018). These studies of consummatory behavior are especially interesting in the context of survival-relevant tradeoffs, since aversion to bitterness is an evolved safeguard against the consumption of toxic substances (Bachmanov et al., 2014). Intriguingly, they also hint toward the versatility of the EAc’s microcircuitry; that is, the ability of some populations of neurons to bidirectionally control divergent survival behaviors (e.g., eating, threat reactivity) depending on the current context (and experimental probe/assay). Context-dependent repurposing of microcircuits would be an efficient solution to the demands of flexibly responding to the innumerable feature-space perturbations that increase or decrease the adaptiveness of specific emotion-relevant responses. For example, while it may be generally maladaptive to graze while predators are nearby, specific constraints—such as life-threatening malnutrition—may reshape the feature space so radically that grazing becomes the optimal response. In this case, perhaps “CeLon”/PKCδ− neurons suppress “CeLoff”/PKCδ+ neurons to reduce threat responding and promote risky feeding, triggering a “Hail Mary” response as an alternative to imminent death. It is also possible, however, that this appearance of multifunctionality could arise as a product of within-cell-type heterogeneity, and that intermingled groups of ostensibly specific EAc neurons might be further functionally dissociable (e.g., see Zeng, 2022).
An increasing number of studies remind us that the EAc is not a solely defensive substrate. For example, researchers investigating the neural substrates of predatory hunting have begun to dissect the Ce’s involvement in prey pursuit and capture. By stimulating the axon terminals of intermingled populations of Ce neurons in mice, Han and colleagues (2017) identified parallel pathways that control appetitive locomotion and consummatory behaviors: a Ce-PAG pathway motivates prey pursuit, while a Ce-PCRt motivates prey consumption. Even in sated animals, activating the Ce-PAG pathway triggers immediate predatory hunting of live or artificial prey, whereas activating the Ce-PCRt pathway triggers immediate biting attacks against these targets, as well as fictive feeding in the absence of prey. Fascinatingly, activating the Ce-PCRt pathway does not trigger attacks against other mice, indicating that it is not an indiscriminate “rage circuit,” but rather a context-specific circuit for food consumption. In the Kash Lab, researchers investigating the molecular substrates of binge eating discovered a population of prepronociceptin (Pnoc)-expressing neurons in the Ce that project food-palatability information to the ventral BST, parabrachial nucleus (PBN), and nucleus of the solitary tract (Hardaway et al., 2019). Activation of Ce Pnoc neurons was sufficient to motivate real-time place preference—a widely used index of reward value. Notably, however, the consequences of manipulating these neurons were specific to reward: inhibiting these cells failed to induce anxious behavior in the open field test, elevated plus maze, or other anxiogenic assays. Other work demonstrates that even the Ce’s putatively “escape-related” CRH+ neurons can motivate reward seeking in specific contexts. For example, mice will optogenetically self-stimulate “escape-related” CRH+ Ce cells, suggesting an increase in appetitive motivation or hedonic pleasure (Kim et al., 2017). Self-stimulation of these cells has also been shown to increase the amount of effort that rats will expend to obtain sucrose rewards, implying a role in incentive motivation (Baumgartner et al., 2021). Other studies have implicated the EAc in a spectrum of roles ranging from mating behaviors (Wei et al., 2021) and social interaction (Flanigan & Kash, 2020), to binge drinking (Rinker et al., 2017) and nociception (Yu et al., 2021).
The BST, like the Ce, is enriched with distinct neuron populations that mediate several physiological and behavioral features of defensive responding (Kim et al., 2013), making it a priority target for dissecting the mechanisms of anxiety disorders. Moreover, an expanding literature on sex dimorphism in this region hints at its relevance to the profound sex differences in the prevalence of anxiety disorders, which are about twice as common in women compared to men (Lebow & Chen, 2016; Bandelow & Michaelis, 2015). Although less is known about the BST’s role in reward and another non-defensive processes, it boasts deep molecular heterogeneity, and its neurons express a range of neuropeptide markers that enable fine-grained modulation of physiological and behavioral survival-related tradeoffs (Gungor & Paré, 2016; Giardino et al., 2018). In mice, for instance, parallel circuits comprised of genetically distinct, lateral hypothalamus (LH)-projecting BST neurons are differentially involved in promoting defensive and appetitive behaviors: one circuit, comprised of CRH+ neurons, promotes avoidance, whereas the other, comprised of cholecystokinin-positive (CCK+) neurons, promotes feeding and mate approach (Giardino et al., 2018). Intriguingly, the latter population may play a key role in addiction (Giardino & Pomrenze, 2021) and appears to interact with estradiol-2 in the presence of cocaine and opioids to reinforce drug-seeking behavior (Ma & Giardino, 2022). This not only highlights the involvement of the BST in non-defensive responding, but also underscores the importance of studying sex as a biological variable in neuroscientific research.
These findings motivate our view that distinct alterations across or within several EAc circuits could give rise to nearly indistinguishable clinical phenotypes, for instance by increasing avoidance (Giardino et al., 2018), dampening incentive motivation (Mahler & Berridge, 2012; Warlow & Berridge, 2021; Baumgartner et al., 2021), shaping hedonic values (Wang et al., 2018), moderating reward-reinforcement signaling (Hardaway et al., 2019), or some combination thereof. Taken together, recent studies of predation and reward demonstrate that the EAc plays a critical role in both aversive and appetitive survival-related functions—and that the functional “identity” of specific neuron populations is highly context dependent. On balance, these observations render views of the EAc’s specificity to threat processing untenable and require us to fundamentally reconsider what the EAc is doing in threatening contexts.
Biological Degeneracy Ensures Partial Redundancy for EAc-Mediated Processes
The EAc does not have a monopoly on selecting between survival-relevant tradeoffs. For instance, in a laboratory paradigm used to induce panic via the inhalation of carbon dioxide (CO2)-enriched air, even patients with focal bilateral amygdala damage can mount adaptive panic responses (Khalsa et al., 2016). And in freely-behaving mice, a feed-forward excitatory circuit projecting from the dorsomedial superior colliculus (dMSC) to the PAG encodes threat levels and initiates rapid escapes in response to threat stimuli that are parametrically modulated for saliency (Evans & Stempel et al., 2018). Redundancies and “emergency brakes” are to be expected, since evolution favors biological degeneracy—that is, “the ability of elements that are structurally different to perform the same function or yield the same output” (Edelman & Gally, 2001, p. 13,763)—over single points of failure. This is consistent with survival as a core determinant of brain evolution across phylogeny. Still, the EAc is uniquely poised to function as an arbiter for survival-relevant tradeoffs. It integrates a wealth of information from myriad regions necessary to encode a survival-relevant feature space (i.e., by computing f(V[R1], V[R2], …V[Rk]), forms numerous microcircuits capable of rapidly selecting between competing physiological and behavioral responses, and projects to regions that can trigger those responses. Importantly, it is precisely these physiological and behavioral tradeoffs that are shared between survival- and emotion-relevant responses. Therefore, we expect the function of the EAc in survival to be particularly relevant for understanding pathological anxiety and other psychiatric illnesses characterized by prominent alterations in emotion and motivation (e.g., depression, alcohol- and substance-use disorders, anhedonia). While the EAc is not required to mount innate, largely reflexive responses like those we’ve described here, it seems to be involved in processing both learned (Li, 2019; Fadok et al., 2017; Sanford et al., 2017; Yu, 2017) and unlearned (Isosaka et al., 2015) threats. The Ce exhibits activity-dependent synaptic plasticity (Samson & Paré, 2005), and our work in nonhuman primates suggests that it represents the contributions of learning and experience to the risk of developing anxiety disorders (Holley & Campos et al., 2022; Fox et al., 2015a).
Characterizing Response-Selection Mechanisms in the EAc
We hypothesize that the EAc encodes an n-dimensional feature space, where multiple inputs from across the brain converge to form an integrated view that enables adaptive responding to both threats and opportunities. The EAc is hypothesized to play a critical role in normative fear and anxiety (Davis et al., 2010; Fox et al., 2015b; Fox & Shackman, 2019), as well as anxiety-related psychopathology (Avery et al., 2016; Clauss, 2019; Morey et al., 2020; Shackman & Fox, 2021). Although scores of studies document the relationship between alterations in the EAc and differences in threat processing, an expanding mechanistic literature reminds us that the EAc is not threat-specific, and that it guides survival-relevant response selection more broadly. Importantly, lesion studies that find preferential deficits in threat responding do not imply that this region is uninvolved in triggering other responses. The historical tendency to focus on threat processing could reflect experimental biases, or some underlying threat-bias in the EAc’s response selection mechanisms. To better understand response selection mechanisms within the EAc must be thoroughly characterized. It is possible, for instance, for dissimilar mechanisms to have the same net effect, thereby promoting a somewhat uniform anxious phenotype via distinct EAc alterations. In fact, we expect this to be the case, and to contribute to the challenges in the pharmacological treatment of anxiety disorders (Garakani et al., 2020; Koen & Stein, 2011). For example, a competitive microcircuit that selects between two mutually exclusive behaviors, such as the Ce CRH+/SST+ microcircuit that selects between fleeing and freezing (Fadok, 2017), could feature any of several alterations that would dispositionally bias an individual toward one behavior over another. A maladaptive bias toward defensive freezing, which is thought to underlie temperamental behavioral inhibition and the risk to develop anxiety-related psychopathology (Fox & Kalin, 2014), could be driven by 1) a preponderance of SST+ neurons (Fig. 3C, top), 2) disproportionately strong inhibitory SST+ projections onto CRH+ neurons (Fig. 3C, middle), or 3) the tendency of a third population of neurons to inhibit CRH+ neurons (Fig. 3C, bottom). Similar outcomes could arise via alterations in the aforementioned “CeLon”/PKCδ− and “CeLoff”/PKCδ+ microcircuit. These illustrative mechanisms might respond differently—or not at all—to a common intervention, underscoring a major barrier to the development of one-size-fits-all treatments for psychopathology.
The implications of this within-region cell-type heterogeneity present a challenge for the neuroimaging community. A voxel, the smallest unit of spatial resolution in functional magnetic resonance imaging (fMRI), may represent the activation of hundreds of thousands of neurons. Because of this, blood oxygen level-dependent signal (BOLD) responses collected from intermingled neuron populations that form competitive microcircuits for response selection might look identical in the scanner even when subjects exhibit opposite responses to a given stimulus. But because this heterogeneity is unlikely to be uniform across voxels, it can also lend to the development of hypotheses that move beyond univariate relationships between regional signals and fear/anxiety measures. For example, based on findings from mice, we might hypothesize that the voxels of the basal and lateral regions of the amygdala each contain some mixture of reward- and threat-sensitive cells. With this hypothesis in mind, we might not expect to see differences in activation across these amygdala voxels in a straightforward test of reward vs threat. However, we might expect multivoxel pattern analysis (MVPA; Norman et al., 2006) to reveal dissociable patterns of activation that are characteristic of reward or threat processing, because each voxel has a different mixture of cell types. By parametrically modulating reward or threat information, we may be able to detect changes in patterns—not in any one voxel, but across voxels. Such research could extend MVPA’s many contributions (e.g., Chang et al., 2015; Frick et al., 2014; Liu et al., 2015; Norman et al., 2006; Woo et al., 2017) by evaluating hypotheses that posit a conserved organization of reward- and threat-sensitive cells across species. This approach could also be coupled with pharmacological methods: By combining perturbations of the EAc’s feature space (i.e., by modulating reward or threat evidence) with drugs believed to target a subset of cell types, we should be able to test hypotheses derived from rodent literature concerning the relationship between specific cell types and the function of the EAc. Such drugs may be useful for these studies, even if they have side-effects or lack clinical efficacy, and include those that target specific serotonin (Sharp & Barnes, 2020), oxytocin (Quintana et al., 2021), and CRH receptors (Zorrilla & Koob, 2010), among others (e.g., neuropeptide Y, cannabinoids, vasopressin, substance P, etc.). These examples illustrate the types of approaches we expect to enable key advances in precision psychiatric diagnostics and treatment in the years to come. Creative study design centered on cross-modality approaches like these are needed to help blunt the enormous public-health burden of anxiety disorders (Beddington et al., 2008) and improve the effectiveness and availability of treatment to the untold millions who suffer their effects (Bandelow & Michaelis, 2015; U.S. Burden of Disease Collaborators, 2018).
Toward an Improved Understanding of Common Psychiatric Disorders
In a seminal review, Rangel, Camerer, and Montague (2008) laid out the processes necessary for action selection to take place in the brain, noting that each process is experimentally tractable: (1) representation of a problem, (2) assignment of values to possible options, (3) selection and implementation of a winning option, (4) evaluation of the outcome, and (5) feedback to enable learning and refinement. These functions are not unique to a single brain region. Here, we have argued that the EAc integrates salient environmental and interoceptive features in an n-dimensional space (akin to steps 1 and 2, above), and guides adaptive responses to challenges and opportunities alike by resolving that feature space to select winning strategies (akin to step 3, above). Although it lies beyond the scope of our mini-review, recent work suggests that the EAc is well-suited to perform steps (4) and (5), for example, via inputs from the PBN (Palmiter, 2018) and ventral tegmental area (Li, 2019), respectively. Moreover, the EAc is differentiated from other brain systems involved in action selection by its direct projections to the effector regions that can induce specific aspects of an emotional response, including species-typical physiological changes (e.g., cardiorespiratory and skin-conductance responses) and behaviors (escape, pursuit, freezing, etc.).
The EAc is uniquely poised to perform survival- and emotion-relevant action selection, and so it is a priority target for understanding psychiatric disorders characterized by prominent alterations in emotion or motivation. However, our expanding knowledge of its neuron populations and their multifunctional, context-dependent involvement in defensive and non-defensive processes should give us pause as we carefully rethink what these findings mean for the study of mental illness. This may require a conceptual reframing of how EAc alterations contribute to pathophysiology. Here, we have proposed approaching survival- and emotion-relevant tradeoffs (Fig. 1) as the outputs of an n-dimensional feature space that is encoded and resolved by the EAc (Fig. 2B). This computational approach to understanding survival- and emotion-relevant response selection in the EAc is intended to complement and integrate with other theories of how the brain implements these tradeoffs (e.g., Mobbs et al., 2015; Perusini & Fanselow, 2015; LeDoux & Pine, 2015; etc.).
A major implication of this conceptual reframing is that the same disordered phenotype could arise from alterations in distinct cellular/molecular substrates, which presents challenges for the development of effective treatments. Because the mechanism(s) that the EAc uses to compare feature vectors for survival- and emotion-relevant decisions are presently unknown (Fig. 2B, middle), investigations that parametrically modulate feature-space inputs will be especially valuable in elucidating the mechanisms that select between the EAc’s response sets—and, when imbalanced, contribute to maladaptive responding (Fig. 3B). Importantly, there are likely to be many-to-one and one-to-many relationships between biological dysregulation and psychopathology. As outlined in Figure 3, multiple, distinct biological mechanisms within the EAc could lead to the same output. (Similarly—although not discussed in detail here—a common CeL alteration might differentially bias physiological, cognitive, and behavioral outputs via distinct alterations in downstream mechanisms, for example in regions innervated by CeM outputs). A current challenge for human research is to develop and test hypotheses derived from animal studies to understand the role of the EAc in human psychopathology. Here, NHP studies of the EAc’s non-defensive functions (e.g., Parkinson, 2001) will be instrumental in understanding how mechanistic discoveries in rodents relate to the disordered emotion-relevant responses common to clinical populations. A focused effort toward characterizing how the EAc’s feature-space inputs are encoded and what the comparison process for response selection entails will enable targeted manipulations of specific cells, genes, and molecules and uncover clinical entry points in the development of new interventions for a range of common psychiatric disorders.
Acknowledgements
This work was supported by NIH Grants R01MH121735, R21MH129851 to ASF, and the California National Primate Research Center (P51OD011107).
References:
- Aggleton JP (2000). The amygdala: A functional analysis. Oxford University Press, Oxford, UK. [Google Scholar]
- Avery SN, Clauss JA, & Blackford JU (2016). The Human BNST: Functional Role in Anxiety and Addiction. Neuropsychopharmacology, 41(1), 126–141. 10.1038/npp.2015.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery SN, Clauss JA, Winder DG, Woodward N, Heckers S, & Blackford JU (2014). BNST neurocircuitry in humans. NeuroImage, 91, 311–323. 10.1016/j.neuroimage.2014.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov AA, Bosak NP, Lin C, Matsumoto I, Ohmoto M, Reed DR, & Nelson TM (2014). Genetics of Taste Receptors. Current Pharmaceutical Design, 20(16), 2669–2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandelow B, Michaelis S (2015) Epidemiology of anxiety disorders in the 21st century. Dialogues in Clinical Neuroscience, 17(3), 327–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner HM, Schulkin J, & Berridge KC (2021). Activating Corticotropin-Releasing Factor Systems in the Nucleus Accumbens, Amygdala, and Bed Nucleus of Stria Terminalis: Incentive Motivation or Aversive Motivation? Biological Psychiatry, 89(12), 1162–1175. 10.1016/j.biopsych.2021.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter MG, & Murray EA (2002). The amygdala and reward. Nature Reviews Neuroscience, 3(7), 563–573. 10.1038/nrn875 [DOI] [PubMed] [Google Scholar]
- Blanchard DC, & Blanchard RJ (2008). Chapter 2.4 Defensive behaviors, fear, and anxiety. In D. C. B. Robert. Blanchard Guy Griebel and David Nutt (Ed.), Handbook of Behavioral Neuroscience: Vol. Volume 17 (pp. 63–79). Elsevier. http://www.sciencedirect.com/science/article/pii/S1569733907000057 [Google Scholar]
- Blanchard DC, Griebel G, Pobbe R, & Blanchard RJ (2011). Risk assessment as an evolved threat detection and analysis process. Neuroscience and Biobehavioral Reviews, 35(4), 991–998. 10.1016/j.neubiorev.2010.10.016 [DOI] [PubMed] [Google Scholar]
- Beddington J, Cooper CL, Field J, Goswami U, Huppert FA, Jenkins R, Jones HS, Kirkwood TB, Sahakian BJ, Thomas SM (2008) The mental wealth of nations. Nature, 455(7216), 1057–60. doi: 10.1038/4551057a [DOI] [PubMed] [Google Scholar]
- Bliss-Moreau E, Toscano JE, Bauman M, Mason WA, & Amaral DG (2010). Neonatal Amygdala or Hippocampus Lesions Influence Responsiveness to Objects. Developmental Psychobiology, 52(5), 487–503. 10.1002/dev.20451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss-Moreau E, Toscano JE, Bauman M, Mason WA, & Amaral DG (2011). Neonatal Amygdala Lesions Alter Responsiveness to Objects in Juvenile Macaques. Neuroscience, 178, 123–132. 10.1016/j.neuroscience.2010.12.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botta P, Demmou L, Kasugai Y, Markovic M, Xu C, Fadok JP, Lu T, Poe MM, Xu L, Cook JM, Rudolph U, Sah P, Ferraguti F, & Lüthi A (2015). Regulating anxiety with extrasynaptic inhibition. Nature Neuroscience, 18(10), 1493–1500. 10.1038/nn.4102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bupesh M, Abellán A, & Medina L (2011). Genetic and experimental evidence supports the continuum of the central extended amygdala and a mutiple embryonic origin of its principal neurons. The Journal of Comparative Neurology, 519(17), 3507–3531. 10.1002/cne.22719 [DOI] [PubMed] [Google Scholar]
- Cai H, Haubensak W, Anthony T, & Anderson DJ (2014). Central amygdala PKC-δ+ neurons mediate the influence of multiple anorexigenic signals. Nature Neuroscience, 17(9), 1240–1248. 10.1038/nn.3767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang LJ, Gianaros PJ, Manuck SB, Krishnan A, & Wager TD (2015). A Sensitive and Specific Neural Signature for Picture-Induced Negative Affect. PLoS Biology, 13(6), e1002180. 10.1371/journal.pbio.1002180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbonneau JA, Bennett JL, & Bliss-Moreau E (2021). Amygdala or hippocampus damage only minimally impacts affective responding to threat. Behavioral Neuroscience. 10.1037/bne0000491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T-W, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V, Looger LL, Svoboda K, & Kim DS (2013). Ultra-sensitive fluorescent proteins for imaging neuronal activity. Nature, 499(7458), 295–300. 10.1038/nature12354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J-S, & Kim JJ (2010). Amygdala regulates risk of predation in rats foraging in a dynamic fear environment. Proceedings of the National Academy of Sciences, 107(50), 21773–21777. 10.1073/pnas.1010079108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciocchi S, Herry C, Grenier F, Wolff SBE, Letzkus JJ, Vlachos I, Ehrlich I, Sprengel R, Deisseroth K, Stadler MB, Müller C, & Lüthi A (2010). Encoding of conditioned fear in central amygdala inhibitory circuits. Nature, 468(7321), 277–282. 10.1038/nature09559 [DOI] [PubMed] [Google Scholar]
- Clauss J. (2019). Extending the neurocircuitry of behavioural inhibition: A role for the bed nucleus of the stria terminalis in risk for anxiety disorders. General Psychiatry, 32(6), e100137. 10.1136/gpsych-2019-100137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J, William E, & Blumstein DT. (Eds.). (2015). Escaping From Predators: An Integrative View of Escape Decisions. Cambridge University Press. 10.1017/CBO9781107447189 [DOI] [Google Scholar]
- Davis M, Walker DL, Miles L, & Grillon C (2010). Phasic vs Sustained Fear in Rats and Humans: Role of the Extended Amygdala in Fear vs Anxiety. Neuropsychopharmacology, 35(1), 105–135. 10.1038/npp.2009.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Olmos JS, & Heimer L (1999). The concepts of the ventral striatopallidal system and extended amygdala. Annals of the New York Academy of Sciences, 877, 1–32. [DOI] [PubMed] [Google Scholar]
- Deisseroth K (2011). Optogenetics. Nature Methods, 8(1), 26–29. 10.1038/nmeth.f.324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman GM, & Gally JA (2001). Degeneracy and complexity in biological systems. Proceedings of the National Academy of Sciences of the United States of America, 98(24), 13763–13768. 10.1073/pnas.231499798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery NJ, Capitanio JP, Mason WA, Machado CJ, Mendoza SP, & Amaral DG (2001). The effects of bilateral lesions of the amygdala on dyadic social interactions in rhesus monkeys (Macaca mulatta). Behavioral Neuroscience, 115(3), 515–544. [PubMed] [Google Scholar]
- Evans DA, Stempel AV, Vale R, Ruehle S, Lefler Y, & Branco T (2018). A synaptic threshold mechanism for computing escape decisions. Nature, 558(7711), 590–594. 10.1038/s41586-018-0244-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadok JP, Krabbe S, Markovic M, Courtin J, Xu C, Massi L, Botta P, Bylund K, Müller C, Kovacevic A, Tovote P, & Lüthi A (2017). A competitive inhibitory circuit for selection of active and passive fear responses. Nature, 542(7639), 96–100. 10.1038/nature21047 [DOI] [PubMed] [Google Scholar]
- Flanigan ME, & Kash TL (n.d.). Coordination of social behaviors by the bed nucleus of the stria terminalis. European Journal of Neuroscience, n/a(n/a). 10.1111/ejn.14991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AS, & Kalin NH (2014). A translational neuroscience approach to understanding the development of social anxiety disorder and its pathophysiology. The American Journal of Psychiatry, 171(11), 1162–1173. 10.1176/appi.ajp.2014.14040449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AS, Oler JA, Birn RM, Shackman AJ, Alexander AL, & Kalin NH (2018). Functional Connectivity within the Primate Extended Amygdala Is Heritable and Associated with Early-Life Anxious Temperament. Journal of Neuroscience, 38(35), 7611–7621. 10.1523/JNEUROSCI.0102-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AS, Oler JA, Shackman AJ, Shelton SE, Raveendran M, McKay DR, Converse AK, Alexander A, Davidson RJ, Blangero J, Rogers J, & Kalin NH (2015a). Intergenerational neural mediators of early-life anxious temperament. Proceedings of the National Academy of Sciences of the United States of America, 112(29), 9118–9122. 10.1073/pnas.1508593112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AS, & Shackman AJ (2019). The central extended amygdala in fear and anxiety: Closing the gap between mechanistic and neuroimaging research. Neuroscience Letters, 693, 58–67. 10.1016/j.neulet.2017.11.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AS, Oler JA, Tromp DPM, Fudge JL, & Kalin NH (2015b). Extending the amygdala in theories of threat processing. Trends in Neurosciences, 38(5), 319–329. 10.1016/j.tins.2015.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AS, Shelton SE, Oakes TR, Davidson RJ, & Kalin NH (2008). Trait-Like Brain Activity during Adolescence Predicts Anxious Temperament in Primates. PLoS ONE, 3(7). 10.1371/journal.pone.0002570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick A, Gingnell M, Marquand AF, Howner K, Fischer H, Kristiansson M, Williams SCR, Fredrikson M, & Furmark T (2014). Classifying social anxiety disorder using multivoxel pattern analyses of brain function and structure. Behavioural Brain Research, 259, 330–335. 10.1016/j.bbr.2013.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garakani A, Murrough JW, Freire RC, Thom RP, Larkin K, Buono FD, & Iosifescu DV (2020). Pharmacotherapy of Anxiety Disorders: Current and Emerging Treatment Options. Frontiers in Psychiatry, 11. https://www.frontiersin.org/article/10.3389/fpsyt.2020.595584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardino WJ, Eban-Rothschild A, Christoffel DJ, Li SB, Malenka RC, & de Lecea L (2018). Parallel circuits from the bed nuclei of stria terminalis to the lateral hypothalamus drive opposing emotional states. Nature Neuroscience, 21(8), 1084–1095. 10.1038/s41593-018-0198-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardino WJ, & Pomrenze MB (2021). Extended amygdala neuropeptide circuitry of emotional arousal: Waking up on the wrong side of the bed nuclei of stria terminalis. Frontiers in Behavioral Neuroscience, 15, 613025. 10.3389/fnbeh.2021.613025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka AX, Torrisi S, Shackman AJ, Grillon C, & Ernst M (2018). Intrinsic functional connectivity of the central nucleus of the amygdala and bed nucleus of the stria terminalis. NeuroImage, 168, 392–402. 10.1016/j.neuroimage.2017.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray TS, & Magnuson DJ (1992). Peptide immunoreactive neurons in the amygdala and the bed nucleus of the stria terminalis project to the midbrain central gray in the rat. Peptides, 13(3), 451–460. 10.1016/0196-9781(92)90074-d [DOI] [PubMed] [Google Scholar]
- Grillon C (2008). Models and mechanisms of anxiety: Evidence from startle studies. Psychopharmacology, 199(3), 421–437. 10.1007/s00213-007-1019-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gungor NZ, & Paré D (2016). Functional heterogeneity in the bed nucleus of the stria terminalis. The Journal of Neuroscience, 36(31), 8038–8049. 10.1523/JNEUROSCI.0856-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han W, Tellez LA, Rangel MJ, Motta SC, Zhang X, Perez IO, Canteras NS, Shammah-Lagnado SJ, van den Pol AN, & de Araujo IE (2017). Integrated Control of Predatory Hunting by the Central Nucleus of the Amygdala. Cell, 168(1–2), 311–324.e18. 10.1016/j.cell.2016.12.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardaway JA, Halladay LR, Mazzone CM, Pati D, Bloodgood DW, Kim M, Jensen J, DiBerto JF, Boyt KM, Shiddapur A, Erfani A, Hon OJ, Neira S, Stanhope CM, Sugam JA, Saddoris MP, Tipton G, McElligott Z, Jhou TC, … Kash TL (2019). Central Amygdala Prepronociceptin-Expressing Neurons Mediate Palatable Food Consumption and Reward. Neuron, 102(5), 1037–1052.e7. 10.1016/j.neuron.2019.03.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R, Biag J, Dong H-W, Deisseroth K, Callaway EM, Fanselow MS, Lüthi A, & Anderson DJ (2010). Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature, 468(7321), 270–276. 10.1038/nature09553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley D, Campos LJ, Zhang Y, Capitanio JP, & Fox AS (2022). Rhesus nervous temperament predicts peri-adolescent central amygdala metabolism and behavioral inhibition measured by a machine-learning approach. BioRxiv 10.1101/2022.07.26.501512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur J, Kuhn M, Grogans SE, Anderson AS, Islam S, Kim HC, Tillman RM, Fox AS, Smith JF, DeYoung KA, Shackman AJ (in press). Anxiety-related fronto-cortical activity is associated with dampened stressor reactivity in the real world. Psychological Science. [coordinate table on bioRxiv; maps on NeuroVault] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur J, Smith JF, DeYoung KA, Anderson AS, Kuang J, Kim HC, Tillman RM, Kuhn M, Fox AS, & Shackman AJ (2020). Anxiety and the Neurobiology of Temporally Uncertain Threat Anticipation. The Journal of Neuroscience, 40(41), 7949–7964. 10.1523/JNEUROSCI.0704-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isosaka T, Matsuo T, Yamaguchi T, Funabiki K, Nakanishi S, Kobayakawa R, & Kobayakawa K (2015). Htr2a-Expressing Cells in the Central Amygdala Control the Hierarchy between Innate and Learned Fear. Cell, 163(5), 1153–1164. 10.1016/j.cell.2015.10.047 [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, & Davidson RJ (2004). The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 24(24), 5506–5515. 10.1523/JNEUROSCI.0292-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalsa SS, Feinstein JS, Li W, Feusner JD, Adolphs R, & Hurlemann R (2016). Panic Anxiety in Humans with Bilateral Amygdala Lesions: Pharmacological Induction via Cardiorespiratory Interoceptive Pathways. The Journal of Neuroscience, 36(12), 3559–3566. 10.1523/JNEUROSCI.4109-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Zhang X, Muralidhar S, LeBlanc SA, & Tonegawa S (2017). Basolateral to central amygdala neural circuits for appetitive behaviors. Neuron, 93(6), 1464–1479.e5. 10.1016/j.neuron.2017.02.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Adhikari A, Lee SY, Marshel JH, Kim CK, Mallory CS, Lo M, Pak S, Mattis J, Lim BK, Malenka RC, Warden MR, Neve R, Tye KM, & Deisseroth K (2013). Diverging neural pathways assemble a behavioural state from separable features in anxiety. Nature, 496(7444), 219–223. 10.1038/nature12018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koen N, & Stein DJ (2011). Pharmacotherapy of anxiety disorders: A critical review. Dialogues in Clinical Neuroscience, 13(4), 423–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovner R, Souaiaia T, Fox AS, French DA, Goss CE, Roseboom PH, Oler JA, Riedel MK, Fekete EM, Fudge JL, Knowles JA, & Kalin NH (2020). Transcriptional Profiling of Primate Central Nucleus of the Amygdala Neurons to Understand the Molecular Underpinnings of Early-Life Anxious Temperament. Biological Psychiatry. 10.1016/j.biopsych.2020.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajbich I, & Rangel A (2011). Multialternative drift-diffusion model predicts the relationship between visual fixations and choice in value-based decisions. Proceedings of the National Academy of Sciences of the United States of America, 108(33), 13852–13857. 10.1073/pnas.1101328108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, & Pine DS (2016). Using neuroscience to help understand fear and anxiety: A two-system framework. The American Journal of Psychiatry, 173(11), 1083–1093. 10.1176/appi.ajp.2016.16030353 [DOI] [PubMed] [Google Scholar]
- Lebow MA, & Chen A (2016). Overshadowed by the amygdala: The bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Molecular Psychiatry, 21(4), 450–463. 10.1038/mp.2016.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, Chen L, Chen L, Chen T-M, Chin MC, Chong J, Crook BE, Czaplinska A, Dang CN, Datta S, … Jones AR (2007). Genome-wide atlas of gene expression in the adult mouse brain. Nature, 445(7124), 168–176. 10.1038/nature05453 [DOI] [PubMed] [Google Scholar]
- Li B (2019). Central amygdala cells for learning and expressing aversive emotional memories. Current Opinion in Behavioral Sciences, 26, 40–45. 10.1016/j.cobeha.2018.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Kaczkurkin AN, Rabin S, Geraci M, Pine DS, & Grillon C (2014). Generalized Anxiety Disorder is Associated with Overgeneralization of Classically Conditioned-Fear. Biological Psychiatry, 75(11), 909–915. 10.1016/j.biopsych.2013.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Rabin S, Heller RE, Lukenbaugh D, Geraci M, Pine DS, & Grillon C (2010). Overgeneralization of Conditioned Fear as a Pathogenic Marker of Panic Disorder. The American Journal of Psychiatry, 167(1), 47–55. 10.1176/appi.ajp.2009.09030410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Guo W, Fouche J-P, Wang Y, Wang W, Ding J, Zeng L, Qiu C, Gong Q, Zhang W, & Chen H (2015). Multivariate classification of social anxiety disorder using whole brain functional connectivity. Brain Structure & Function, 220(1), 101–115. 10.1007/s00429-013-0641-4 [DOI] [PubMed] [Google Scholar]
- Ma Y, & Giardino WJ (2022). Neural circuit mechanisms of the cholecystokinin (CCK) neuropeptide system in addiction. Addiction Neuroscience, 3, 1–6. 10.1016/j.addicn.2022.100024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado CJ, & Bachevalier J (2008). Behavioral and hormonal reactivity to threat: Effects of selective amygdala, hippocampal or orbital frontal lesions in monkeys. Psychoneuroendocrinology, 33(7), 926–941. 10.1016/j.psyneuen.2008.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado CJ, Emery NJ, Mason WA, & Amaral DG (2010). Selective changes in foraging behavior following bilateral neurotoxic amygdala lesions in rhesus monkeys. Behavioral Neuroscience, 124(6), 761–772. 10.1037/a0021560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, & Berridge KC (2012). What and when to “want”? Amygdala-based focusing of incentive salience upon sugar and sex. Psychopharmacology, 221(3), 407–426. 10.1007/s00213-011-2588-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcinkiewcz CA, Mazzone CM, D’Agostino G, Halladay LR, Hardaway JA, DiBerto JF, Navarro M, Burnham N, Cristiano C, Dorrier CE, Tipton GJ, Ramakrishnan C, Kozicz T, Deisseroth K, Thiele TE, McElligott ZA, Holmes A, Heisler LK, & Kash TL (2016). Serotonin engages an anxiety and fear-promoting circuit in the extended amygdala. Nature, 537(7618), 97–101. 10.1038/nature19318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ (1982). Cytoarchitecture of the central amygdaloid nucleus of the rat. The Journal of Comparative Neurology, 208(4), 401–418. 10.1002/cne.902080409 [DOI] [PubMed] [Google Scholar]
- McDonald AJ (1983). Neurons of the bed nucleus of the stria terminalis: A golgi study in the rat. Brain Research Bulletin, 10(1), 111–120. [DOI] [PubMed] [Google Scholar]
- Mobbs D, Hagan CC, Dalgleish T, Silston B, & Prévost C (2015). The ecology of human fear: Survival optimization and the nervous system. Frontiers in Neuroscience, 9. 10.3389/fnins.2015.00055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D, Marchant JL, Hassabis D, Seymour B, Tan G, Gray M, Petrovic P, Dolan RJ, & Frith CD (2009). From Threat to Fear: The Neural Organization of Defensive Fear Systems in Humans. The Journal of Neuroscience, 29(39), 12236–12243. 10.1523/JNEUROSCI.2378-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey RA, Haswell CC, Stjepanović D, Dunsmoor JE, & LaBar KS (2020). Neural correlates of conceptual-level fear generalization in posttraumatic stress disorder. Neuropsychopharmacology, 45(8), 1380–1389. 10.1038/s41386-020-0661-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman KA, Polyn SM, Detre GJ, & Haxby JV (2006). Beyond mind-reading: Multi-voxel pattern analysis of fMRI data. Trends in Cognitive Sciences, 10(9), 424–430. 10.1016/j.tics.2006.07.005 [DOI] [PubMed] [Google Scholar]
- Oler JA, Birn RM, Patriat R, Fox AS, Shelton SE, Burghy CA, Stodola DE, Essex MJ, Davidson RJ, & Kalin NH (2012). Evidence for coordinated functional activity within the extended amygdala of non-human and human primates. NeuroImage, 61(4), 1059–1066. 10.1016/j.neuroimage.2012.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oler JA, Fox AS, Shelton SE, Rogers J, Dyer TD, Davidson RJ, Shelledy W, Oakes TR, Blangero J, & Kalin NH (2010). Amygdalar and hippocampal substrates of anxious temperament differ in their heritability. Nature, 466(7308), 864–868. 10.1038/nature09282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oler JA, Tromp DPM, Fox AS, Kovner R, Davidson RJ, Alexander AL, McFarlin DR, Birn RM, E Berg B, deCampo DM, Kalin NH, & Fudge JL (2017). Connectivity between the central nucleus of the amygdala and the bed nucleus of the stria terminalis in the non-human primate: Neuronal tract tracing and developmental neuroimaging studies. Brain Structure & Function, 222(1), 21–39. 10.1007/s00429-016-1198-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter RD (2018). The parabrachial nucleus: CGRP neurons function as a general alarm. Trends in Neurosciences, 41(5), 280–293. 10.1016/j.tins.2018.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson JA, Crofts HS, McGuigan M, Tomic DL, Everitt BJ, & Roberts AC (2001). The role of the primate amygdala in conditioned reinforcement. The Journal of Neuroscience, 21(19), 7770–7780. 10.1523/JNEUROSCI.21-19-07770.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perusini JN, & Fanselow MS (2015). Neurobehavioral perspectives on the distinction between fear and anxiety. Learning & Memory, 22(9), 417–425. 10.1101/lm.039180.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponserre M, Fermani F, & Klein R (2020). Encoding of environmental cues in central amygdala neurons during foraging (p. 2020.09.28.313056). 10.1101/2020.09.28.313056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana DS, Lischke A, Grace S, Scheele D, Ma Y, & Becker B (2021). Advances in the field of intranasal oxytocin research: Lessons learned and future directions for clinical research. Molecular Psychiatry, 26(1), 80–91. 10.1038/s41380-020-00864-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel A, Camerer C, & Montague PR (2008). A framework for studying the neurobiology of value-based decision making. Nature Reviews. Neuroscience, 9(7), 545–556. 10.1038/nrn2357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff R, & McKoon G (2008). The diffusion decision model: Theory and data for two-choice decision tasks. Neural Computation, 20(4), 873–922. 10.1162/neco.2008.12-06-420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resendez SL, & Stuber GD (2015). In vivo Calcium Imaging to Illuminate Neurocircuit Activity Dynamics Underlying Naturalistic Behavior. Neuropsychopharmacology, 40(1), 238–239. 10.1038/npp.2014.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinker JA, Marshall SA, Mazzone CM, Lowery-Gionta EG, Gulati V, Pleil KE, Kash TL, Navarro M, & Thiele TE (2017). Extended Amygdala to Ventral Tegmental Area Corticotropin-Releasing Factor Circuit Controls Binge Ethanol Intake. Biological Psychiatry, 81(11), 930–940. 10.1016/j.biopsych.2016.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelofs K (2017). Freeze for action: neurobiological mechanisms in animal and human freezing. Philosophical Transactions of the Royal Society of London. Series B, Biological sciences, 372(1718), 20160206. 10.1098/rstb.2016.0206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelofs K, Hagenaars MA, & Stins J (2010). Facing freeze: social threat induces bodily freeze in humans. Psychological Science, 21(11), 1575–1581. 10.1177/0956797610384746 [DOI] [PubMed] [Google Scholar]
- Roth BL (2016). DREADDs for Neuroscientists. Neuron, 89(4), 683–694. 10.1016/j.neuron.2016.01.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy AK, Shehzad Z, Margulies DS, Kelly AMC, Uddin LQ, Gotimer K, Biswal BB, Castellanos FX, & Milham MP (2009). Functional connectivity of the human amygdala using resting state fMRI. NeuroImage, 45(2), 614–626. 10.1016/j.neuroimage.2008.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson RD, & Paré D (2005). Activity-dependent synaptic plasticity in the central nucleus of the amygdala. The Journal of Neuroscience, 25(7), 1847–1855. 10.1523/JNEUROSCI.3713-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford CA, Soden ME, Baird MA, Miller SM, Schulkin J, Palmiter RD, Clark M, & Zweifel LS (2017). A central amygdala CRF circuit facilitates learning about weak threats. Neuron, 93(1), 164–178. 10.1016/j.neuron.2016.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, & Fox AS (2021). Two decades of anxiety neuroimaging research: New insights and a look to the future. The American Journal of Psychiatry, 178(2), 106–109. 10.1176/appi.ajp.2020.20121733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, & Fox AS. (2016). Contributions of the Central Extended Amygdala to Fear and Anxiety. The Journal of Neuroscience, 36(31), 8050–8063. 10.1523/JNEUROSCI.0982-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Tromp DPM, Stockbridge MD, Kaplan CM, Tillman RM, & Fox AS (2016). Dispositional negativity: An integrative psychological and neurobiological perspective. Psychological Bulletin, 142(12), 1275–1314. 10.1037/bul0000073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp T, & Barnes NM (2020). Central 5-HT receptors and their function; present and future. Neuropharmacology, 177, 108155. 10.1016/j.neuropharm.2020.108155 [DOI] [PubMed] [Google Scholar]
- Somerville LH, Wagner DD, Wig GS, Moran JM, Whalen PJ, & Kelley WM (2013). Interactions between transient and sustained neural signals support the generation and regulation of anxious emotion. Cerebral Cortex (New York, N.Y.: 1991), 23(1), 49–60. 10.1093/cercor/bhr373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, & Petrovich GD (1998). What is the amygdala? Trends in Neurosciences, 21(8), 323–331. 10.1016/s0166-2236(98)01265-x [DOI] [PubMed] [Google Scholar]
- Tillman RM, Stockbridge MD, Nacewicz BM, Torrisi S, Fox AS, Smith JF, & Shackman AJ (2018). Intrinsic functional connectivity of the central extended amygdala. Human Brain Mapping, 39(3), 1291–1312. 10.1002/hbm.23917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovote P, Esposito MS, Botta P, Chaudun F, Fadok JP, Markovic M, Wolff SBE, Ramakrishnan C, Fenno L, Deisseroth K, Herry C, Arber S, & Lüthi A (2016). Midbrain circuits for defensive behaviour. Nature, 534(7606), 206–212. 10.1038/nature17996 [DOI] [PubMed] [Google Scholar]
- U.S. Burden of Disease Collaborators: Mokdad AH, Ballestros K, Echko M, Glenn S, Olsen HE, et al. (2018). The state of US Health, 1990-2016: Burden of diseases, injuries, and risk factors among U.S. states. JAMA, 319(14):1444–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, & Frye CA (2007). The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nature Protocols, 2(2), 322–328. 10.1038/nprot.2007.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, & Davis M (2008). Role of the extended amygdala in short-duration versus sustained fear: A tribute to Dr. Lennart Heimer. Brain Structure & Function, 213(1–2), 29–42. 10.1007/s00429-008-0183-3 [DOI] [PubMed] [Google Scholar]
- Walker DL, Miles LA, & Davis M (2009). Selective Participation of the Bed Nucleus of the Stria Terminalis and CRF in Sustained Anxiety-Like versus Phasic Fear-Like Responses. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 33(8), 1291–1308. 10.1016/j.pnpbp.2009.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Gillis-Smith S, Peng Y, Zhang J, Chen X, Daniel Salzman C, Ryba NJP, & Zuker CS (2018). The coding of valence and identity in the mammalian taste system. Nature, 558(7708), 127–131. 10.1038/s41586-018-0165-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warlow SM, & Berridge KC (2021). Incentive motivation: ‘wanting’ roles of central amygdala circuitry. Behavioural Brain Research, 411, 113376. 10.1016/j.bbr.2021.113376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei D, Talwar V, & Lin D (2021). Neural circuits of social behaviors: Innate yet flexible. Neuron. 10.1016/j.neuron.2021.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen PJ, & Phelps EA (2009). The human amygdala. Guilford Press, New York City, NY. [Google Scholar]
- Woo C-W, Chang LJ, Lindquist MA, & Wager TD (2017). Building better biomarkers: Brain models in translational neuroimaging. Nature Neuroscience, 20(3), 365–377. 10.1038/nn.4478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, & Wager TD (2011). Large-scale automated synthesis of human functional neuroimaging data. Nature Methods, 8(8), 665–670. 10.1038/nmeth.1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K, Ahrens S, Zhang X, Schiff H, Ramakrishnan C, Fenno L, Deisseroth K, Zhao F, Luo MH, Gong L, He M, Zhou P, Paninski L, & Li B (2017). The central amygdala controls learning in the lateral amygdala. Nature Neuroscience, 20(12), 1680–1685. 10.1038/s41593-017-0009-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Pati D, Pina MM, Schmidt KT, Boyt KM, Hunker AC, Zweifel LS, McElligott ZA, & Kash TL (2021). Periaqueductal gray/dorsal raphe dopamine neurons contribute to sex differences in pain-related behaviors. Neuron, 109(8), 1365–1380.e5. 10.1016/j.neuron.2021.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H. (2022). What is a cell type and how to define it? Cell, 185(18), 2739–2755, 10.1016/j.cell.2022.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP, & Koob GF (2010). Progress in corticotropin-releasing factor-1 antagonist development. Drug Discovery Today, 15(9-10), 371–383. 10.1016/j.drudis.2010.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]


