Version Changes
Revised. Amendments from Version 2
More specific details on the study's duration, inclusion requirements, and microscope observations are included in the most recent version. To compare with placental malaria, we have included the figure of histological examinations of normal placental tissue in this version.
Abstract
Background
Malaria in pregnancy leads to placental malaria. The primary pathogenesis of the complex fetal implications in placental malaria is tissue hypoxia due to sequestrations of Plasmodium falciparum-infected erythrocytes in the placenta. However, the pathomechanism of placental Plasmodium vivax infection has not been thoroughly investigated. Hypoxia-inducible factor-1α (HIF-1α) is a key transcriptional mediator of the response to hypoxic conditions, which interacts with the change and imbalances of many chemical mediators, including angiogenic factors, leading to fetal growth abnormality.
Methods
This study was conducted cross-sectionally in Maumere, Sikka Regency, East Nusa Tenggara Province, previously known as one of the malaria endemic areas with a high incidence of low birth weight (LBW) cases. This study collected peripheral and umbilical blood samples and placental tissues from mothers who delivered their babies with LBW at the TC Hiller Regional Hospital. All of the blood samples were examined for parasites by microscopic and PCR techniques, while the plasma levels of VEGF, PlGF, VEGFR-1, VEGFR-2, and HIF-1α were determined using ELISA. The sequestration of infected erythrocytes and hemozoin was determined from placental histological slides, and the expression of placenta angiogenic factors was observed using the immunofluorescent technique.
Results
In this study, 33 cases had complete data to be analyzed. Of them, 19 samples were diagnosed as vivax malaria and none of falciparum malaria. There were significant differences in Δ 10th percentile growth curve of baby's body weights and also all angiogenic factors in placental tissues {VEGF, PlGF, and VEGFR-1, VEGFR-2, and HIF-1α} between those infected and not infected cases (p<0.05), but not for VEGF and VEGFR-2 in the plasma.
Conclusion
This study indicated that Plasmodium vivax sequestration may promote LBW through alterations and imbalances in angiogenic factors led by HIF-1α.
Keywords: Placental malaria, Plasmodium vivax, LBW, HIF1-α, angiogenesis factors
Introduction
Malaria is still a major public health problem and the main cause of disease and death in developing countries 1 . Young children and pregnant women, especially in the 1 st and 2 nd trimesters, are the populations most vulnerable to malaria infection 1 . In malaria-endemic areas, at least 25% of pregnant women are infected with malaria, which accounts for 20% of maternal deaths 2, 3 . Rijken et al. reported that around 75 million pregnant women in the Asia-Pacific region were exposed to Plasmodium vivax in 2007 2 . In 2019, for 33 countries in the World Health Organisation (WHO) African Region with moderate to high malaria transmission, it was estimated that 12 million (35%) of 33 million pregnancies were exposed to Plasmodium falciparum malaria 4 . Globally, malaria causes more than 10,000 maternal deaths and 200,000 neonatal deaths annually, most of which are due to low birth weight (LBW) 4– 6 . Pregnant women living in stable transmission areas are at risk for placental malaria, and pregnant women living in unstable areas have three times the risk of developing severe malaria than non-pregnant adult women living in the same areas 2, 3 .
Indonesia is one of the countries in Southeast Asia with a relatively high number of malaria cases. In 2016, there were 218,480 positive cases of malaria, which decreased by almost half of the positive malaria cases in 2012 7 . The malaria parasites found were Plasmodium falciparum (62%) and Plasmodium vivax (37%). Based on WHO reported malaria cases by health facilities data in Indonesia (2016), there were 218,450 confirmed cases of malaria with a death rate of 161 cases 8 . Papua, West Papua, and East Nusa Tenggara (NTT) are the three provinces with high malaria-endemic rates. Three regencies in NTT, which are highly endemic areas for malaria, are Sikka Regency, Lembata Regency, and Ngada Regency. Sikka Regency is located on Flores Island, borders East Flores Regency and Ende Regency. It covers 7,436.10 km 2 and had a population of 315,477, according to a 2010 survey 9 . In 2008, 87,622 clinical malaria cases were reported in Sikka Regency 10 . A previous study conducted on 92 babies born with low birth weight in T.C. Hillers Regional Hospital, Maumere, Sikka Regency, between December 2012 and December 2013 reported that 39 (42.4%) infants had congenital malaria, caused by Plasmodium falciparum, Plasmodium vivax, or mixed infection, of which 19 (48.7%) infants were asymptomatic, while the rest had sepsis, jaundice, and prematurity 11 . This finding might emphasize that Plasmodium vivax also causes comparable effects, as Plasmodium falciparum has been linked to low birth weight and gestational malaria cases. Furthermore, the prevalence of instances among pregnant mothers may differ depending on the particular research location or endemic region in question.
Placental malaria is characterized by the accumulation of parasite-infected erythrocytes, mononuclear cells, and malaria pigment (hemozoin) in the placental blood vessels 12 . Most reports of placental malaria are caused by infection with Plasmodium falciparum, which sequesters in the syncytiotrophoblast. This sequestration occurs by expressing Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) surface protein, which specifically mediates the cytoadhesion of Plasmodium falciparum-infected erythrocytes to placental chondroitin sulfate A (CSA) located in the syncytiotrophoblast 6, 13, 14 . This cytoadhesion and sequestration prevent the escape of circulating adult parasites, thereby avoiding the mechanism of clearance by complement and spleen. During the occurrence of placenta malaria, the Th1/Th2 balance shifts to the Th1 pathway, and there is an increase in the production of the pro-inflammatory cytokines, interferon-ϒ (IFN-ϒ) and tumor necrosis factor-α (TNF-α). This inflammatory response causes changes in the structure and function of the placenta, which are associated with poor pregnancy outcomes such as maternal anemia, prematurity, stunted fetal growth, and LBW 15 . Placental malaria is a major cause of stunted fetal growth with poor pregnancy outcomes in the form of babies born with LBW 16 .
Placenta malaria will evoke complement activation, both systemically and at the maternal-fetal interface in the placenta 17 . There is an increase in the complement of anaphylatoxin C5a in circulating blood and placental tissue in mothers with placental malaria 5 . Previous in vitro studies showed that Plasmodium falciparum glycosylphosphatidylinositol (PfGPI), together with C5a, increased pro-inflammatory milieu at the maternal-fetal interface in the form of increased cytokines and chemokines derived from monocytes 18 . One of the consequences is the occurrence of dysregulation of angiogenic factors 19 , which causes stunted fetal growth 5, 6, 20, 21 . The local oxygen environment during pregnancy is one of the essential regulatory factors of angiogenesis. The main pathway of oxygen regulation of gene expression is hypoxia-inducible factor-1α (HIF-1α). Under hypoxic conditions, HIF-1α accumulation upregulates VEGF, which is a major proangiogenic factor directly. VEGF activity will induce the expression of vascular endothelial growth factor receptor-1 (VEGFR-1/Flt-1) and vascular endothelial growth factor receptor-2 (VEGFR-2/KDR). During hypoxia, VEGF will bind to receptors and stimulate capillary growth. Hypoxia-inducible factor-1α (HIF-1α) also increases the soluble anti-angiogenic factor Flt-1 (sFlt-1) 22, 23 . High placental HIF-1α expression after the 1 st trimester of pregnancy is an abnormal condition that describes the occurrence of placental hypoxia due to inadequate placentation. Increased placental HIF-1α expression will cause an increase in placental sFlt-1 levels, which will be released into the maternal circulation. Soluble Flt-1 (sFlt-1) strongly binds free VEGF and PlGF, decreasing placental angiogenic activity. This condition is found in pregnancies with pre-eclampsia and IUGR 24– 26 and in placental malaria 27 . Increased placental HIF-1α expression is also found in maternal anemia 28 .
Carvalho et al., for the first time, demonstrated that Plasmodium vivax-infected erythrocytes (Pv-iEs) can perform ex vivo cytoadhesion on human lung endothelial cells (HLECs), Saimiri brain endothelial cells (SBECs), and placental cryosections both under static and flow conditions. This cytoadhesion is smaller than that of Plasmodium falciparum-infected erythrocytes (Pf-iEs) and is partly mediated by the VIR protein encoded by the Plasmodium vivax variant (vir) gene 29 . Fernandez-Becerra et al. showed in their study that spleen-dependent Plasmodium vivax genes encode immunogenic proteins during infection, so it can be said that the spleen is an organ that plays a major role in expressing parasitic proteins involved in cytoadhesion 30 . Pv-iEs binding to endothelial cells is mediated by glycosaminoglycans, namely chondroitin sulfate A (CSA) and hyaluronic acid (HA) 29, 31 . Although all stages of the Plasmodium vivax were found in peripheral blood, only a few mature forms of schizonts were found. It is impossible to establish whether the disproportionate Plasmodium vivax parasitemia results from its sequestration in specific organs 32 .
Data on the number of pregnancies infected with Plasmodium vivax have not been widely published. The mechanism and clinical implications of Plasmodium vivax infection in pregnancy are still unknown, possibly because it is considered to cause a milder clinical effect than Plasmodium falciparum infection 33 . However, many studies have reported that vivax malaria in pregnancy is more associated with low birth weight and maternal anemia 34, 35 and may not be affected by parity because women living in areas with low malaria endemicity have low immunity to malaria 36 . In this study, an in vivo study was conducted on pregnant women infected with malaria, emphasizing placental malaria. It aimed to determine the effect of placental and maternal plasma angiogenesis factors on the pathomechanism of LBW through HIF-1α regulation. This study used a peripheral blood sample to determine gestational malaria and a cord blood sample to determine placental malaria. Placental samples were also examined to identify the placenta's pathological properties and diagnose placental malaria. It is the first study of placental malaria angiogenesis factors in Indonesia using human placenta samples.
Methods
Ethics approval and consent
This research was conducted according to the guidelines of the Declaration of Helsinki and approved by the Faculty of Medicine Universitas Brawijaya Ethics Committee number 307/EC/KEPK/11/2018. Written informed consent, consisting of consent stating the baby's data to be used, was obtained from all subjects before they participated in this study.
Characteristics of research participants
This study used a cross-sectional research design in T.C. Hillers Regional Hospital, Maumere, Sikka Regency, East Nusa Tenggara Province, a malaria-endemic area in Indonesia. This study used a purposive sampling technique in managing research participants. Thus, all of the subjects which met the inclusion criteria were included. This study was conducted for 10 months and the subjects were pregnant women who came to T.C. Hillers Regional Hospital in 2018 and met the inclusion criteria. The inclusion criteria were pregnant women who gave birth to babies with low birth weight (LBW) (< 2500 g) and small for gestational age (SGA) (birth weight < 10th percentile for gestational age at birth). Women who were primigravidae (being pregnant for the first time) and multigravidae (being pregnant more than once) were included in this study. Subjects were divided into two groups. They were (i) the case group, pregnant women who met the inclusion criteria and the malaria diagnosis criteria, and (ii) the control group, pregnant women who met the inclusion criteria and did not meet the malaria diagnosis criteria. All the participants were in term pregnancy, and the gestational age ranged from 37 to 42 weeks 37 . Malaria cases were determined based on the discovery of Plasmodium parasites on examination of thin or thick blood smears and/or the discovery of Plasmodium DNA in maternal blood and or umbilical cord blood by polymerase chain reaction (PCR) examination, and or the discovery of Plasmodium parasites or hemozoin in the placenta.
Diagnosis of placental malaria
The diagnosis of malaria case in this study was confirmed by the discovery of either the asexual form of Plasmodium on examination of thick or thin blood smears derived from maternal blood and or umbilical cord blood and Plasmodium DNA obtained by polymerase chain reaction (PCR) examination and finding of placental malaria, which is determined by placental tissue histopathological examination based on infected erythrocyte sequestration, monocyte infiltration, and hemozoin deposition 38 . Hemozoin is a brown pigment crystal that forms in the digestive vacuole of Plasmodium as a product of hemoglobin catabolism 39 . Hemozoin was seen as greenish-black or yellowish-brown granules using a light microscope and deposited intra or extra erythrocytes. Observations were made using a light microscope with 1000x magnification in 100 fields of view. Identification of infected erythrocytes was carried out by observing 100 fields of view. Hemozoin deposition was identified by observing 100 fields, and then the total number of hemozoin was calculated by dividing all fields by 100. The count includes hemozoin found within and outside infected erythrocytes, hemozoin attached to connective tissue, in the intervillous space (free hemozoin), or hemozoin inside free macrophages or macrophages covered by fibrin in the intervillous space.
Definition of low birth weight
Low birth weight is a birth weight of less than 2500 g 37 . In this study, what is meant by low birth weight is the difference between birth weight and the 10th percentile of the mean birth weight curve for boys and girls by gestational age from the reference curves of birth weight, length, and head circumference for gestational ages in Yogyakarta, Indonesia 40 .
Maternal peripheral blood sample preparation
In total, 10 milliliters of maternal venous blood were drawn from the median vein and put into a vacutainer containing anticoagulant (BD Vacutainer EDTA Tubes, 366643) for making blood smear preparations, plasma, and dried blood spots on filter paper. Microscopic examination was carried out on blood smear preparations. Dried blood spots were then used for PCR examination to confirm malaria diagnosis. Plasma was stored at 20°C and sent to Malang City (in collaboration with the Prodia Laboratory) in less than 48 hours. Using the ELISA method, these samples were then used to examine plasma levels of VEGF, PlGF, VEGFR-1 (sFlt-1), VEGFR-2, and HIF-1α.
Umbilical artery blood sample preparation
In total, five milliliters of blood were drawn from the neonates' umbilical arteries and collected in an anticoagulant tube (BD Vacutainer EDTA Tubes, 367863) for hemoglobin and leukocyte count testing. Blood smears were made to identify malaria parasites, as well as dried blood spots on filter paper for PCR analysis.
Parasitemia examination
The blood sample was dropped on an object glass, where a thin smear was made and dried. Furthermore, the smears were fixed evenly using absolute methanol and dried. The smears were stained with Giemsa solution (a mixture of Giemsa stain (Merck, HX612241) and Giemsa buffer (Bioanalytica, Indonesia) with a ratio of 1:9), then rinsed and dried. The thin smear slides were examined microscopically at 100 times magnification under the objective lens with immersion oil on at least 100 visual fields for each examination. To calculate the degree of parasitemia, 1000x magnification was carried out using a light microscope (Olympus Biological Microscope, CX23), counting the number of erythrocytes infected with malaria parasites per 1000 erythrocytes 11 .
Placental sampling for histopathological preparation
Samples obtained from the central part of the gross placental anatomy with a size of 2 cm and fixation with 10% neutral buffer formalin were then embedded into paraffin for immunofluorescence examination to determine the expression of VEGF, PlGF, VEGFR-1, VEGFR-2, and HIF-1α, the sequestration of infected erythrocytes and or hemozoin, as well as placental parasitemia. Placental histology preparations were made by cutting tissue that had previously been fixed with 10% neutral buffer formalin and inserted into a tissue cassette. Then, the samples were put into a basket and processed using a tissue processor tool (Thermo Scientific Microm STP 120 Spin Tissue Processor). The process of casting into paraffin blocks was done using a tissue embedding tool (Sakura Tissue-Tek TEC 5 Embedding Station). The paraffin blocks were cooled in a freezer before cutting with a microtome (Leica, RM2245). Then, the tissue was put into an incubator (Memmert, UN30) for 30 minutes in a 70–80°C temperature setting to maximize further deparaffination. Furthermore, after the tissue was removed from the incubator, deparaffination and Hematoxylin-Eosin staining were performed using the Tissue Tex DRS 2000 Multiple Slide Stainer tool.
Immunofluorescence preparation
Immunofluorescence examination procedure on placental tissue was used to measure the placental expression of variables VEGF (anti-VEGFA antibody, SC 7269 FITC), PlGF (anti-PLGF antibody, SC 518003), VEGFR-1 (anti-VEGFR-1 antibody, SC 271789 PE), VEGFR-2 (anti-VEGFR-2 antibody, SC 6251 FITC), and HIF-1α (anti-HIF-1α antibody (SC 13515 PE). The immunofluorescence procedure was performed by single staining for HIF-1α and double staining for other variables. The immunofluorescence images were observed using a fluorescence microscope Olympus IX71 with 400x magnification. The results of the immunofluorescence images were then quantified using ImageJ 1.52p Fiji software. The parameter used was integrated density (IntDent), defined with the sum of the pixel value in selection (RawIntDen) multiplied by area in scaled units, then divided by area in pixel (RawInden * (Area in scaled units) / (Area in pixels)).
Nested Polymerase Chain Reaction (PCR) examination to detect Plasmodium falciparum and Plasmodium vivax DNA
According to the manufacturer's instructions, DNA samples were obtained from blood sample extraction using PureLinkTM Genomic DNA Kits (Invitrogen, Carlsbad, California, USA). After purification, all samples were stored at -20°C till ready for the nested PCR. For the amplification of Plasmodium genus sequences, outer primer pairs (rPLU1 and rPLU5) were used. In the second reaction, two inner primers were used to detect P. falciparum (rFal1-rFal2) and P. vivax (rVIV1-rVIV2). PCR was performed using a Go Tag® Green Master Mix (Promega, Madison, Wisconsin, USA). 1 l template was treated with the following conditions: 1 l of each primer (10 M), 12.5 l of PCR master mix, and 9.5 l of double-distilled H 2O 2. The second nest reactions were carried out similarly using different primers. Sterile distilled water was used as a control 11 .
Enzyme-Linked Immunosorbent Assay (ELISA)
ELISA examination was performed to measure plasma levels of VEGF (human VEGF ELISA kit, Biolegend, Catalog No. 446507), PlGF (human PGF ELISA kit, bt-Lab, Catalog No. EO138Hu), VEGFR-1(sFlit1) (human VEGFR-1 ELISA kit, Invitrogen, REF#BMS268-3), VEGFR-2 (human VEGFR-1 ELISA kit, Invitrogen, REF#BMS2019), and HIF-1α (human HIF-1α ELISA kit, Invitrogen, REF#EHIF1A). The technique used was a quantitative sandwich enzyme immunoassay with the following procedure. The ELISA's standard was pipetted into a microplate that had been pre-coated with a specific antibody to the antigen according to the manufactured ELISA Kit. Each sample was pipetted into each well so the antigen would be bound to the immobilized antibody. Unbound materials were discarded. Then, a biotin-conjugated antibody specific to the antigen was added to the wells and washed. Afterwards, avidin-conjugated Horseradish Peroxidase (HRP) was added and washed to remove unbound materials. Staining was done by adding a solution substrate, incubating for 30 minutes, and adding a stop solution to stop the reaction. The preparations were read with a microplate reader (ZENIX-320).
Neonatal examination
The obstetrician examined newborns for birth weight, length, head circumference, mid-upper arm circumference, chest circumference, Apgar score, placental size, and placental weight. The birth weight was measured immediately after birth using calibrated weighing scales. The birth length, head circumference, mid-upper arm circumference, and chest circumference were measured using a measuring tape. Apgar score was noted at the first minute of birth. The placental size was measured using a ruler, and the weight was measured using calibrated weighing scales.
Statistical analysis
All statistical analyses were conducted using the SPSS 23 Statistic Program for Windows. The techniques for analyzing data include the normality test using the Shapiro-Wilk test to determine the normal data distribution. Comparative analysis between the case and control groups was carried out using a one-way ANOVA test for normally distributed data and the Kruskal Wallis test for non-normally distributed data to assess dysregulation of angiogenesis in the pathomechanism of low birth weight due to malaria-inhibited fetal growth in the placenta. The correlation test used was a Pearson test for normally distributed data and Spearman's rho test for non-normally distributed data to assess the correlation between the variables observed in the study. Path analysis was used to determine the conceptual research framework, i.e., whether the authors' concepts are relevant to the research findings. Data analysis was carried out with a confidence level of 95% and a degree of significance by p≤0.05.
Results
Subject characteristics
A total of 33 subjects met the inclusion criteria of this study Table 1. This study showed no statistically significant differences between the case and control groups in maternal age, gestational age, and maternal leukocyte count. In the case group, the age distribution of pregnant women is slightly lower than the age distribution of pregnant women in the control group, but this is not statistically different. Statistically significant differences were only found in maternal hemoglobin levels in the case group (p=0,00<α), lower than in the control group. The subject characteristics and distributions are shown in Table 1.
Table 1. Subject characteristics and distributions.
| Characteristics | Controls (N=14)
mean ± SD (min-max) |
Cases (N=19)
mean ± SD (min-max) |
p-value |
|---|---|---|---|
| Age (year) | 32.21±6.25 (20–38) | 27.68±7.21 (19–39) | 0.08 |
| Gestational Age (Weeks) | 38.64±0.84 (38–40) | 39.47±1.39 (38–42) | 0.08 |
| Haemoglobin Level (g dL -1) | 11.10±1.20 (10.01–13.16) | 9.88±0.62 (9.03–11.28) | 0.00 |
| Leukocyte Number (10 3 µL -1) | 7796.79±2385.25 (4560–13890) | 7622.11±2317.69 (4900–14200) | 0.68 |
Notes: All data were analyzed with the Mann-Whitney test. The case group was pregnant mothers who were diagnosed with malaria (+); The control group was normal pregnant mothers/malaria (-) SD = standard deviation.
The laboratory tests were carried out to identify the control and case groups, as shown in Table 2.
Table 2. Laboratory examination results.
| No | Examination | Controls
(N=14) |
Cases
(N=19) |
Notes |
|---|---|---|---|---|
| 1 | Thin/Thick Peripheral
Blood Smear |
26 | 7 | P. vivax |
| 2 | Blood PCR from Mother | 21 | 12 | P. vivax |
| 3 | Cord Blood PCR | 31 | 2 | P. vivax |
| 4 | Placental Histopathological
Examination |
14 | 19 | IE/hemozoin (+) |
Notes: IE: infected erythrocytes; PCR=polymerase chain reaction.
Plasmodium vivax was identified in all subjects of the case group, and no Plasmodium falciparum nor mixed infection was found. Histopathological examination of placental tissue from the case group showed sequestration of infected erythrocytes and/or hemozoin, followed by monocyte infiltration in the intervillous space in all samples. Two of the 19 malaria cases were identified from cord blood PCR. The examination of maternal peripheral blood smear can at least identify parasites compared to PCR and histopathological examination of the placenta, which is about 36.84%. The blood smears from the malaria case group are shown in Figure 1. Histopathological examination of placental tissue, which showed placental malaria compared to normal placental tissue, are shown in Figure 2.
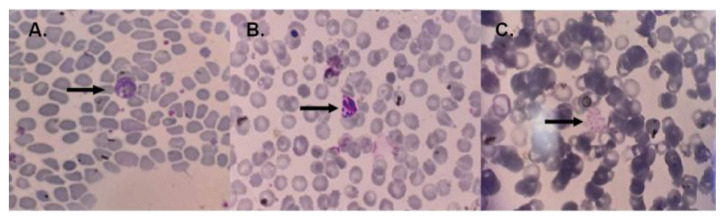
Figure 1. Blood smears from the malaria case group.
In panel A, the arrow indicates the amoeboid form of Plasmodium vivax, panel B shows young schizonts of Plasmodium vivax, and panel C displays the mature schizont of Plasmodium vivax. The image size is the only modification made; no changes to brightness or contrast have been made. The examination was performed by using a light microscope (Olympus Biological Microscope, CX23) with 1000x magnification. The figures were captured using a mobile device, which could explain the absence of a scale bar.
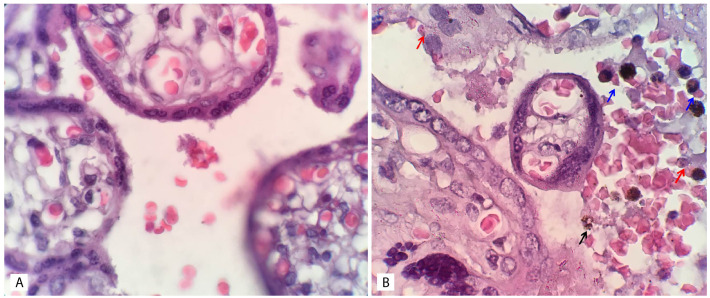
Figure 2. Histopathological examination of placental tissue.
A: Normal placental tissue. B: Placental malaria. The red arrow indicates infected erythrocyte sequestration, the blue arrow indicates monocyte infiltration, and the black arrow indicates free hemozoin in the intervillous space. The image size is the only modification made; no changes to brightness or contrast have been made. The examination was performed by using a light microscope (Olympus Biological Microscope, CX23) with 1000x magnification. The figures were captured using a mobile device, which could explain the absence of a scale bar.
The characteristics of the babies in this study were recorded, as shown in Table 3. There was a significant difference (p=0,00<α) in the mean Δ 10th percentile growth curve of birth weight between the case group (413.16±460,33 g) and the control group (-42.86±129.88 g). However, there was no significant difference (p=0.33>α) in the mean birth weight (g) between the case group (2189.47 ±182.06 g) and the control group (2239.29 ±190.85 g). The mean value of birth weight in the case group appears to be significantly smaller than the mean in the control group. It can be said that pregnant women with malaria are likely to give birth to a baby with a smaller birth weight than a baby born to a non-malaria pregnant woman.
Table 3. The results of the comparison test of baby characteristics.
| Variables | Controls
Mean ± deviation |
Cases
Mean ± deviation |
p-value |
|---|---|---|---|
| Δ 10 th percentile growth curve (g) | -42.86±129.88 | 413.16±460.33 | 0.00 ** |
| Baby birthweight (g) | 2239.29 ±190.85 | 2189.47 ±182.06 | 0.33 ** |
| Body length (cm) | 41.86±2.91 | 42.95±3.58 | 0.45 ** |
| Head circumference (cm) | 27.21±1.05 | 27.47±1.26 | 0.50 ** |
| Upper arm circumference (cm) | 6.21±0.70 | 6.05±0.71 | 0.50 ** |
| Chest circumference (cm) | 20.71±1.33 | 21.00±1.89 | 0.53 ** |
| APGAR score | 9.14±0.36 | 9.16±0.60 | 0.85 ** |
| Placental size (cm 3) | 404.86±44.84 | 392.16±40.08 | 0.42 * |
| Placental weight (g) | 457.14±43.22 | 450.00±57.73 | 0.52 ** |
| Hemoglobin level (g dL -1) | 16.35±2.30 | 14.96±2.49 | 0.11 * |
| Leukocyte number (10 3 µL -1) | 10.24±2.11 | 12.70±2.81 | 0.01 * |
Note: *) comparative results on the free sample t-test **) comparative results on the Mann-Whitney test
Association of angiogenic factors expression with birth weight
VEGF in the placenta of the case group was lower (64,404±28,942) than the control group (226,693±39,025). In both the case and control groups, VEGF expression was more abundant in the trophoblast cells of the placental villi than in the intervillous space. PlGF expression in the placenta of the case group was lower (22,814,440±9,497,663) than the control group (84,693,238±29,981,727). PlGF expression in the case group was abundant in trophoblast cells of the placental villi, whereas it was primarily found in the intervillous space in the control group. PlGF expression in the placenta of the case group was significantly lower than that of the control group.
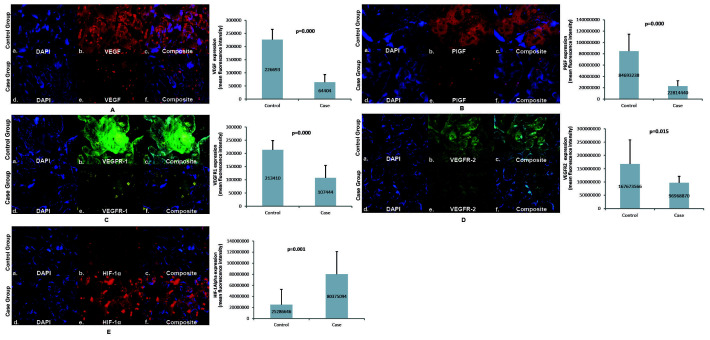
The expression of VEGFR-1 in the placenta of the case group was lower (107,444±46,696) than the control group (213,410±35,251). VEGFR-1 expression was more abundant in trophoblast cells in the placental villi than in the intervillous space in the case and control groups. The expression of VEGFR-1 in the case group's placenta was significantly lower than that of the control group. VEGFR-2 expression in the placenta of the case group was lower (96,968,870±24,300,623) than the control group (167,673,566±90,824,566). VEGFR-2 expression was abundant in trophoblast cells in the placental villi in the case and control groups. The expression of VEGFR-2 in the case group's placenta was significantly lower than that of the control group. The expression of HIF-1α in the placenta of the case group was higher (80,375,094±40,647,360) than the control group (25,286,646±27,238,953). In the case and control groups, HIF-1α expression was more commonly found in the trophoblast cells of the placental villi. HIF-1α expression in the placenta of the case group was significantly higher than that of the control group, as seen in Figure 3.
Figure 3. Angiogenic factors expression in placenta.
Panel A to E shows the angiogenic expression in the placenta using immunofluorescence staining of VEGF, PlGF, VEGFR-1, VEGFR-2, and HIF-1α, respectively, with its semiquantitative analysis; VEGF: vascular endothelial growth factor; PlGF: placental growth factor; VEGFR-1: vascular endothelial growth factor receptor-1; VEGFR-2: vascular endothelial growth factor receptor-2; HIF-1α: hypoxia-inducible factor-1α. The examination was conducted using a fluorescence microscope Olympus IX71 with 400x magnification. The immunofluorescence figures were captured using a mobile device, which could explain the absence of a scale bar.
The path analysis test results showed a significant direct effect (p=0.04) on HIF-1α expression on VEGF expression with an effect coefficient of -0.55. Likewise, there was a significant direct effect (p=0.03) of HIF-1α expression on PlGF expression with an effect coefficient of -0.60. VEGF expression had no direct effect on VEGFR-1 expression (p=0.89) but had a direct effect on VEGFR-2 expression (p=0.00) with a coefficient of effect of 0.75. There was a significant direct effect (p=0.00) on PlGF expression on VEGFR-1 expression, with an effect coefficient of 0.94. VEGFR-1 expression had no direct effect on birth weight (p=0.46), but VEGFR-2 expression had a significant direct effect (p=0.02) on birth weight with an effect coefficient of 0.74.
Comparison test of angiogenic factor in the placenta and maternal plasma
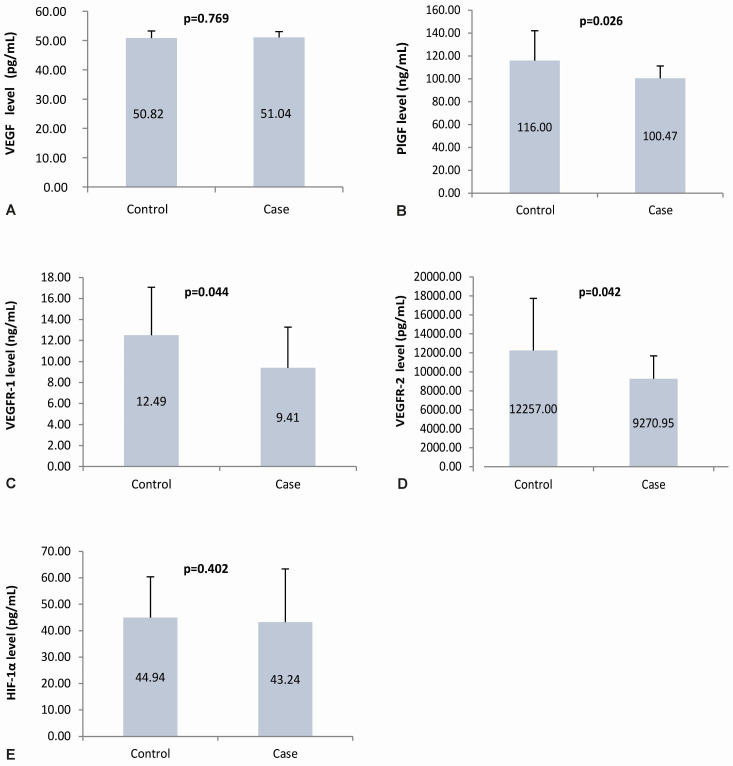
The mean maternal plasma VEGF levels in the case and control groups were almost the same; statistical results showed no statistically significant difference (p=0.77). The mean maternal plasma PlGF, VEGFR-1, and VEGFR-2 levels in the case group were lower than in the control group. Statistically, there is a significant difference (p=0.03, 0.04, 0.04, respectively). Similar to the VEGF plasma level, the HIF-1α plasma levels in the case and control groups had values that were not much different, which showed no significant difference (p=0.40). The angiogenic factor levels in the case and control group are presented in Figure 4.
Figure 4. Angiogenic factors level in maternal plasma.
Panel A to E shows the angiogenic factors level in the maternal plasma using ELISA of VEGF, PlGF, VEGFR-1, VEGFR-2, and HIF-1α, respectively; VEGF: vascular endothelial growth factor; PlGF: placental growth factor; VEGFR-1: vascular endothelial growth factor receptor-1; VEGFR-2: vascular endothelial growth factor receptor-2; HIF-1α: hypoxia-inducible factor-1α.
Comparison test of the placenta and maternal plasma data
There was a significant difference in the mean expression of VEGF, PlGF, VEGFR-1, and VEGFR-2 placenta between the case and control groups. The mean value of placental VEGF, PlGF, VEGFR-1, and VEGFR-2 expression in the case group was lower than the mean in the control group. In addition, there was a significant difference in the mean placental HIF-1α expression between the case and the control groups. However, in contrast to other angiogenic factors, the mean placental HIF-1α expression in the case group was higher than the mean placental HIF-1α expression in the control group. The comparison of angiogenic factor expression in the placenta is shown in Table 4.
Table 4. The comparison test of angiogenic factors in the placenta and maternal plasma between the case and control group.
| Variable | Controls (n=14)
Mean ± SD |
Cases (n=19)
Mean ± SD |
p-value | |||
|---|---|---|---|---|---|---|
| Placenta
(Mean fluorescence intensity) |
Plasma | Placenta
(Mean fluorescence intensity) |
Plasma | Placenta | Plasma | |
| VEGF | 226,693 ± 39,025 | 50.82 ± 2.41
(pg/mL) |
64,404 ± 28,942 | 51.04 ± 1.99
(pg/mL) |
0.00 * | 0.77 * |
| PlGF | 84,693,238 ± 29,981,727 | 116.00 ± 26.19
(ng/mL) |
22,814,440 ± 9,497,663 | 100.47 ± 10.72
(ng/mL) |
0.00 * | 0.03 * |
| VEGFR-1 | 213,410 ± 35,251 | 12.49 ± 4.57
(ng/mL) |
107,444 ± 46,695 | 9.41 ± 3.86
(ng/mL) |
0.00 * | 0.04 * |
| VEGFR-2 | 167,673,566 ± 90,824,566 | 12257 ± 5482
(pg/mL) |
96,968,870 ± 24,300,623 | 9271 ± 2419
(pg/mL) |
0.02 ** | 0.04 * |
| HIF-1α | 25,286,646.1±27,238,953 | 44.94 ± 15.49
(pg/mL) |
80,375,094 ± 40,647,360 | 43.24 ± 20.19
(pg/mL) |
0.00 ** | 0.40 ** |
Notes: *) T-test **) Mann-Whitney test SD=standard deviation.
There was no significant difference in the mean VEGF plasma levels between the case and control groups. The mean value of VEGF plasma levels in the case group was slightly higher than the average VEGF level in the control group. However, this increase was not statistically significant. Similar to VEGF, the mean plasma levels of HIF-1α between case and control groups did not show a significant difference. However, HIF-1α in the case group was slightly lower than the mean HIF-1α expression in the control group. The plasma levels of PlGF, VEGFR-1, and VEGFR-2 between the case and control groups showed significant differences. The mean levels of PlGF, VEGFR-1, and VEGFR-2 in the case group were lower than in the control group. The comparison of maternal plasma angiogenic factor levels is shown in Table 4.
Discussion
All malaria-positive samples in this study were identified using maternal peripheral blood smear examination or PCR examination caused by Plasmodium vivax ( Table 2). Microscopic examination of blood smears only detected 7 of the 19 positive samples (36.84%). On the other hand, 12 blood samples were detected positively by PCR examination (63.16%). The results showed that PCR is more sensitive than blood smear examination. Malaria detection using microscopic examination could only detect Plasmodium at 20 parasites/μL levels. It was influenced by the experience of the observer and the quality of staining blood smears. PCR is the most sensitive method because it can detect 2–6 parasites/μL 41– 44 . It was surprising that all the histopathological preparations of 19 samples showed pigment/hemozoin depositions with or without infected erythrocytes or monocytes accumulation, indicating that all of the samples had already been infected as active acute, active chronic, and past infection 38, 45– 47 . In this study, all positive samples did not show signs and symptoms of malaria, so all positive samples were subclinical infections.
Investigation of acute infection through maternal peripheral blood smear examination cannot fully describe the history of maternal exposure to malaria during pregnancy. The absence of parasitemia on peripheral blood smear examination does not always represent a malaria-free placental infection 46 . In this study, acute, chronic, and past malaria infection in pregnancy may be related to the adverse consequences of placental histopathological changes. In cases of subclinical malaria, placental histopathological changes might be found due to most of the sub-populations of parasites sequestered in several organs that serve as reservoirs of the parasite, such as the spleen and placenta, causing other inflammatory reactions 42, 46, 48, 49 .
Characteristics of subjects and their distribution
Characteristics of mothers. This study showed significant differences in the mean hemoglobin levels in women infected with Plasmodium vivax during pregnancy compared to those not infected. At the same time, gestational age, maternal age, and leukocyte count showed no significant difference. The average hemoglobin level of pregnant women infected with malaria was 1.22 g/dL lower than the control group (mean ± standard deviation [SD]; 9.88±0.62 vs 11.10±1.20, p=0.00). This finding aligned with several previous studies, which also showed that vivax malaria infection is associated with maternal anemia, abortion, premature birth, congenital malaria, and other severe complications 36, 50– 54 . Single Plasmodium vivax infection during pregnancy had a significant association with a twofold increased risk of maternal anemia compared to the group of mothers who did not experience malaria infection 36, 55 . The occurrence of maternal anemia is closely correlated with nutritional status 36 . However, one of the other risk factors that can increase anemia in pregnant women with Plasmodium vivax infection is young age pregnancy 56 . In this study, the mean age of pregnant women in the case group was lower than the control group (mean ± SD; 27.68 ± 7.21 vs. 32.21 ± 6.25, p = 0.08); there was no significant difference. This factor may be considered one of the critical risk factors in the subsequent study involving many subjects.
The parity distribution in the case group was mainly primiparity (11/19; 57.9%), while in the control group, the highest number was mothers with a third parity (6/14; 42.9%). This result has a distribution pattern similar to Bardaji et al.. In their study, most malaria pregnant women were primigravida, and malaria prevalence tended to decrease as gravidity increased 35 . In a study of Karen women in Thailand involving a comparable number of participants, Plasmodium vivax was more common in primigravida than in multigravida, and this was associated with anemia and an increased risk of LBW 51 .
Both case and control groups showed no significant difference (mean ± SD: 39.47±1.39 vs. 38.64±0.84, p=0.078) in gestational age, but all participants gave birth at term. In the study of Bardaji et al., it was found that the average gestational age was 38.6 weeks, with 12.6% of all participants giving birth prematurely (<37 weeks) 35 . From the results of their study, it cannot be concluded that there is a related relationship because it requires a joint analysis with other factors such as the severity of malaria infection, the onset of malaria infection, and pregnancy outcomes, which in this case includes the baby's birth weight. As in this study, all infants were born at term but had a significant effect of small for gestational age with malaria infection status in the form of the chronic active or previous history of malaria.
Characteristics of babies. This study showed a significant difference in the mean Δ 10th percentile growth curve of birth weight between the case groups. However, there were no significant differences in the average birth weight of the baby, the length of the baby's birth weight, head circumference, upper arm circumference, chest circumference, Apgar score, placental weight, placenta size, and hemoglobin levels. However, the baby's mean hemoglobin level in the case group was 1.39 g/dL lower than the control group. The result was consistent with a previous study, which reported that a single episode of P. vivax infection during pregnancy was associated with a significant reduction in neonatal birth weight and maternal hemoglobin levels 33 . The results of the meta-analysis study also stated that there was an increased risk of 1-2 times the occurrence of LBW in pregnant women infected with malaria compared to those who were not infected 57 .
Meanwhile, babies in both the case and control groups were included in LBW criteria, so it is unknown whether the leading cause of LBW in the case group is influenced by the main factor of Plasmodium vivax infection, as in the previous studies 33, 56 . According to Bardaji et al., there is no direct relationship between Plasmodium vivax infection and an increased risk of LBW, but they stated that Plasmodium vivax infection during pregnancy is closely associated with maternal anemia, where this condition may harm the health of the newborn 35 . In addition, maternal anemia is a risk factor for the occurrence of LBW with an odds ratio (OR) value of 1.23 (95% confidence interval [CI]: 1.06–1.43) 58 .
The results showed significant differences in the LBW leukocyte count parameters in the case and control groups (mean ± SD: 12.70 × 10 3±2.81/μL vs. 10.24 × 10 3±2.11/μL, p<0.05). In general, the leukocyte count values in newborns can range from 10,000–26,000/μL, which is also highly dependent on the time of sampling 59 . Meanwhile, the results of the leukocyte count in newborns at Bantul Hospital, Indonesia, in 195 infants showed an average leukocyte count of 14.62 × 10 3±3.49/μL 60 . The leukocyte count in newborns in the case and control groups showed values within normal limits, with the leukocyte count in the case group significantly higher than the control group. Several factors can increase the leukocyte count in newborns, including infection, inflammation, medication history, and stress 61– 63 .
Sequestration of hemozoin, infected erythrocytes, and monocytes in histopathology result
Sequestration is the attachment of infected erythrocytes to host cells associated with severe malaria, such as cerebral malaria and malaria in pregnancy. Sequestration occurs in the capillaries and post-capillary venules of specific organs such as the brain, lungs, and placenta. Sequestration correlates with mechanical obstruction of blood flow in the microvasculature and the activation of vascular endothelial cells, leading to pathological outcomes. Some parasitic and host proteins as ligands and receptors for sequestration have been identified and explored further 64 .
The sequestration phenomenon occurred in Plasmodium falciparum infection 45, 65, 66 . Interestingly, none of the subjects in this study were infected with Plasmodium falciparum. However, hemozoin depositions were found in all 19 positive samples, while Plasmodium vivax-infected erythrocyte sequestrations were found in some samples. The result indicates that the Plasmodium vivax could cause sequestration of infected erythrocytes. Submicroscopic placental infection of Plasmodium vivax confirmed by placental histopathology means that Plasmodium vivax can be selectively sequestered in the placenta even at low densities in peripheral blood 67 . The finding of Plasmodium vivax-infected erythrocytes sequestration was also mentioned by Carvalho et al. through an ex vivo study, which found that Plasmodium vivax-infected erythrocytes could adhere to the placental tissue. This study also demonstrated the cytoadherence of Plasmodium vivax-infected erythrocytes on endothelial cells by in vitro approach. It was known to have the same strength as cytoadherence of Plasmodium falciparum-infected erythrocytes 29 . Toda et al. found that Plasmodium vivax-infected reticulocytes could bind to human spleen fibroblasts (hSF). The binding involves the transcription factor of the NF-kB signaling pathway by plasma-derived extracellular vesicles that act as cargo for the Plasmodium vivax parasite. This process facilitates the expression of ICAM-1 on the surface of hSF as a receptor for Plasmodium vivax sequestration 49 . Other evidence supporting placental malaria due to other non-falciparum Plasmodium species is a submicroscopic infection of Plasmodium malariae and Plasmodium ovale in the placenta confirmed by PCR of placental blood samples. Plasmodium malariae infection was detected higher in placental blood samples than in peripheral blood samples, indicating a possibility of Plasmodium malariae binding affinity to the placenta. However, the study of non- falciparum placental infection was not supported by histopathological examination of the placenta 68 .
Malaria infection causes changes in the placenta structure in the form of hemozoin deposition and an increase in monocyte infiltration in the intervillous space, which interferes with maternal-fetal circulation 46 . Sequestration of infected erythrocytes, hemozoin deposition, and monocyte infiltration in placental histopathology are the main signs of placental malaria 12, 69, 70 . The pathomechanism of sequestration of infected erythrocytes in the placenta is clearly described in Plasmodium falciparum infection. However, Plasmodium vivax, which has been considered to cause a mild latent infection than Plasmodium falciparum, has also been reported to cause placental malaria. Histopathological changes of placental malaria due to Plasmodium falciparum can show a different representation of placental malaria due to Plasmodium vivax. However, these differences have not yet been delineated 46 . The histopathological appearance of the placenta due to Plasmodium vivax infection is similar to that of Plasmodium falciparum. Plasmodium vivax has also demonstrated the sequestration mechanism of infected erythrocytes in placental tissue structures 47, 71 . These findings are consistent with the results of this study.
Several mechanisms can explain the Plasmodium vivax-infected erythrocytes sequestration phenomenon. First, the Plasmodium vivax-infected erythrocyte sequestration can occur under low shear stress conditions in the placental intervillous space and causes a local inflammatory process of the placenta 31 . Second, Plasmodium vivax lacks surface proteins, such as Plasmodium falciparum Erythrocyte Membrane Protein-1 (PfEMP-1) called Variant Surface Antigen-2-CSA (VAR2CSA) for adhesion and sequestration of CSA in the placental intervillous space 72, 73 . However, the Plasmodium vivax genome contains a subtelomeric multigene family called VIR proteins. VIR proteins can mediate the adhesion of infected erythrocytes to ICAM-1 by VIR14 and the CSA by VIR2 and VIR24. The bindings are speculative and require further research 71, 72, 74 . The discovery of VIR binding in ICAM-1 and CSA proved the presence of Plasmodium vivax sequestration in the previous study 29 . The third mechanism that can explain the sequestration of Plasmodium vivax is the formation of rosettes by interacting with the Glycophorin C receptor present on normal erythrocytes, which has the adhesive power as in Plasmodium falciparum. However, further research is needed to determine the specific mechanism of rosette formation 71, 75, 76 .
On histopathological examination of the placenta, it was found that pigment/hemozoin depositions were trapped in the fibrin or freely circulating in the intervillous space and monocyte infiltration in all 19 positive samples. This finding indicates that the mother had experienced malaria infection during her pregnancy 45, 77 . The presence of pigment/hemozoin and monocyte depositions in the placenta suggests malaria infection does not occur in late pregnancy. The infection process has occurred long enough to cause deposition and accumulation of inflammatory cells in the placenta acquired early in pregnancy or before pregnancy 67, 68 . However, the time required for placental malaria's appearance is unknown and requires further research.
The placental expression and plasma level of VEGF, PlGF, VEGFR-1, VEGFR-2, and HIF-1α in placenta malaria
Vascular Endothelial Growth Factor (VEGF). In this study, VEGF expression in the placenta of the case group was lower than in the control group. In the case and control groups, VEGF expression was more abundant in trophoblast cells in the placental villi than in the intervillous space. VEGF expression in the case group placenta was significantly lower than in the control group (p=0.00). Expression of VEGF placental IUGR was inconsistent between several studies that have been carried out. The results of this study are consistent with several studies, which reported a decrease in VEGF expression accompanied by increased placental PlGF expression of IUGR 78, 79 . Several studies reported an increase in the expression of VEGF, VEGFA, bFGF, and eNOS in the IUGR placenta due to placental hypoxia 80– 82 . However, another study concluded no decrease in VEGF expression of IUGR compared to normal placentas 83, 84 . In addition, VEGF expression and plasma VEGF levels could not predict late-onset pre-eclampsia, small gestational age (SGA), or premature delivery 85 .
Plasma VEGF levels in the case group were lower and not significantly different from the control group in this study (p=0.77). The findings were consistent with several studies, which found that maternal plasma VEGF levels of IUGR and SGA did not show significant differences compared to pre-eclampsia 86 or normal pregnancy 87 . Moreover, Tang et al. reported that maternal plasma VEGF levels in pregnancies with pre-eclampsia were lower than in normal pregnancies and were associated with impaired fetal growth 88 . However, Boras et al. found that maternal free plasma levels of VEGF and sFlt-1 were higher in IUGR than in normal pregnancies 89 .
Besides regulating development and vascular remodeling during placentation, VEGF, PlGF, and Angiopoietin are also involved in trophoblast invasion. Failure of trophoblast invasion and remodeling of the spiral arteries causes placental hypoxia, which involves pregnancy complications such as pre-eclampsia and IUGR 90 , causing the fetus to experience hypoxia. At low oxygen concentrations, there should be an increase in VEGF expression. The mechanism that can explain the decrease in placental VEGF expression in this study is increased soluble VEGFR-1 (sFlt-1) in the placental intervillous space. sFlt-1 is a form of the VEGFR-1 gene physiologically secreted by the placenta. sFlt-1 physiologically binds to free VEGF and PlGF with solid affinity and inhibits their binding to VEGFR-1 and VEGFR-2 receptors, decreasing placental VEGF and PlGF expression. HIF-1α regulates placental sFlt-1 expression; under conditions of placental hypoxia, there will be an increase in placental sFlt-1 expression 91, 92 . The sFlt-1 expression was also reported to increase in IUGR placentas 93, 94 . An in vivo study in transgenic mice by Vogtmann et al. found that an increase in placental sFlt1 would disrupt the vascular endothelial growth factor signaling pathway, resulting in decreased expression of VEGFA, VEGFB, and PlGF and also increased the expression of band and mRNA of Caspase-9 95 . Placental hypoxia will increase placental VEGF expression 90, 91 ; however, the source of circulating VEGF is not only from the placenta but also from other organs 96 , which can explain why the plasma levels of VEGF in the case group and the control group were not different.
This study's finding of decreased placental VEGF expression can describe early-onset IUGR because placental hypoxia correlated with placental HIF-1α expression. However, histo-morphological studies of placental villi were not performed to confirm the dominance of branching angiogenesis in placental villi. Early-onset IUGR in this study can be caused by malaria infection before pregnancy or early pregnancy 45 , as evidenced in this study.
Placental Growth Factor (PlGF). The placenta is the only organ that produces PlGF 96 . PlGF significantly functions in the activation, proliferation, and migration of endothelial cells and trophoblast invasion into the maternal spiral arteries 97 . In this study, the expression of PlGF in the placenta in the case group was lower than in the control group (p=0.00). In the case group, PlGF expression was more abundant in trophoblast cells in the placental villi, whereas in the control group, it was primarily found in the intervillous space. Studies on PlGF expression in placental IUGR have also shown inconsistent results. The results of this study support previous studies that reported that placental PlGF expression in severe IUGR was significantly lower than in controls, and inhibition of PlGF/Flt-1 signalling will interfere with trophoblast proliferation and migration 97– 99 . However, Alahakoon et al. found an increase in PlGF and KDR expression in IUGR and pre-eclampsia placentas 84 . It explained that hyperoxia conditions in IUGR due to impaired oxygen extraction to the fetus increase PlGF expression and decrease placental VEGF expression, with the consequence of reduced capillary branching and terminal villi resulting in placental dysfunction 90, 91, 100, 101 .
Placental hypoxia conditions that occur early in pregnancy decrease placental PlGF production by the syncytiotrophoblast, so PlGF expression and PlGF levels in maternal circulation decrease 90, 91, 98 . In this study, the plasma levels of PlGF in the case group were significantly lower than in the control group (p=0.03). Previous studies have also shown that plasma or plasma PlGF levels are lower in pregnancies with IUGR 93, 102, 103 , pregnancies with pre-eclampsia 95, 104– 106 , and pregnancies with pre-eclampsia and IUGR 86, 88, 94, 107– 113 . Decreased maternal plasma PlGF levels in pregnancy complications due to defective placentation are associated with increased production of sFlt-1 in the ischemic placenta, which is then secreted into the maternal circulation 102, 104 . Furthermore, significantly lower PlGF levels in the case group indicate that the causes of IUGR in the two groups may be different. Malaria infection in the placenta causes the activation of complement C5a, which will stimulate the accumulation of monocytes to release sFlt-1 18 , explaining that the plasma levels of PlGF in the case group were significantly lower than the control group.
Vascular Endothelial Growth Factor Receptor (VEGFR)-1 and VEGFR-2. This study showed that VEGFR-1 expression in the placenta was lower in the case group than in the control group (p=0.00). VEGFR-1 expression was more abundant in trophoblast cells in the placental villi than in the intervillous space, both in the case and control group. VEGFR-1 is a receptor for VEGF and PlGF. Helske et al. found that VEGFR-1 expression was increased in preeclamptic and IUGR placentas compared with normal placentas. The upregulated expression is not exactly found on all preeclamptic samples but may be associated with hypoxia and abnormal function of the placenta 114 . However, the down-regulation of both receptors was also reported during mid-pregnancy with IUGR 115 .
In line with VEGFR-1, the expression of VEGFR-2 in the case group's placenta was lower than in the control group (p=0.02). In the case and control groups, VEGFR-2 expression was more abundant in trophoblast cells in the placental villi. VEGFR-2 is a significant receptor for VEGF and plays a role in endothelial proliferation and normal vascular formation. In the placenta, the role of VEGFR-2 is to change trophoblasts into intravascular trophoblasts and form spiral arteries. VEGFR-2 is expressed in low amounts under hypoxic conditions because oxygen levels regulate VEGFR-2 expression. Placental VEGFR-2 and sFlt-1 expression was also reported to decrease in cases of pre-eclampsia 93, 116 .
In this study, plasma levels of VEGFR-1 in the case group were lower and significantly different from the control group (p=0.04). Anna et al. found that serum VEGFR-1 concentrations in women with IUGR were decreased, along with a decrease in PlGF. At low oxygen concentrations, PlGF and VEGFR-1 levels decrease 117 . Besides that, serum levels of VEGFR-2 in the case group were lower and significantly different from the control group (p=0.04). Chaiworapongsa et al. found soluble VEGFR-2 (sVEGFR2) concentration increased in women with preeclamptic pregnancies and interpreted that sVEGFR-2 in maternal plasma could reflect endothelial cell function 118 .
Hypoxia-Inducible Factor-1α (HIF-1α). This study revealed that the expression of HIF-1α in the case group's placenta was higher than in the control group (p=0.00). In the case group and the control group, HIF-1α expression was more commonly found in trophoblast cells in the placental villi. There are few studies in humans examining HIF-1α expression in the placenta of pregnancies with complications such as pre-eclampsia, IUGR, and SGA. The results of this study support an in vivo study on the IUGR mice model conducted by Robb et al., who found an increase in HIF-1α expression in the placenta 119 .
In this study, plasma levels of HIF-1α in the case group were lower and not significantly different from the control group (p=0.40). So far, studies of HIF-1α have been mainly carried out in placental tissue, as in the discussion on placental HIF-1α expression above. Ashur-Fabian et al. found elevated HIF-1α and p21 mRNA expression in all pregnancy plasma samples with hypoxia and IUGR and recommended them as markers for hypoxic pregnancy and/or IUGR 120 . In pregnancy, HIF-1α is expressed in the placenta early in normal pregnancy and throughout pregnancy under hypoxic conditions 121 . Those explain no statistically significant difference in plasma HIF-1α levels in the case and control groups.
Under normoxic conditions, HIF-1α will undergo rapid degradation so that it is considered inactive. HIF-1α will regulate genes that control cell growth, differentiation, and metabolism at low oxygen levels. The active form of HIF-1α continuously exposed to cultured cells inhibited trophoblast differentiation. In placental IUGR, impaired invasion and remodeling of the spiral arteries result in placental ischemia throughout pregnancy because of the increased expression of HIF-1α. The increase in placental HIF-1α expression in this study represents early-onset IUGR. However, this study has not determined whether placental hypoperfusion causes hypoxia as the main factor driving the increase in HIF-1α because other factors, such as reactive oxygen species (ROS) and inflammation induced by nuclear factor kappa (NF-κB), can also modulate HIF-1α accumulation.
Association between low birth weight and VEGF, PlGF, VEGFR-1, VEGFR-2, HIF-1α expression in placenta
Path analysis revealed the effect of placental angiogenesis and HIF-1α transcription factors on LBW. This analysis showed that the signaling pathway from HIF-1α to placental VEGF expression, then from placental VEGF expression to placental VEGFR-2 expression, and from placental VEGFR-2 expression to LBW showed statistically significant results. In contrast, HIF-1α could not cause a direct effect on LBW (p=0.88). Placental VEGF expression significantly affected placental VEGFR-1 expression. The signaling pathway of HIF-1α significantly affected placental PlGF expression, and then placental PlGF expression significantly affected placental VEGFR-1 expression, but this pathway did not significantly affect the incidence of LBW (p=0.50).
VEGF and VEGFR regulate vasculogenesis during early embryogenesis and angiogenesis in later stages of pregnancy. VEGF binds to VEGFR-1/Flt-1 and VEGFR-2/KDR, which is essential in physiological and pathological angiogenesis 122 . PlGF binds to the VEGFR-1 receptor and plays a role in angiogenesis in the later stages of pregnancy 123 . VEGFR-2 is the primary receptor mediating the proangiogenic effects of VEGF 124 . sFlt1, the free form of Flt-1, can bind strongly to VEGFA, PlGF, and VEGFB. Because the trophoblast lies between the maternal and fetal vascular systems, it is thought that sFlt1 functions as a separator between the maternal and fetal circulations in the placenta by suppressing excessive angiogenesis and abnormal vascular permeability. Therefore, sFlt1 levels are maintained in the suitable range under physiological conditions. The increased expression of sFLT-1, triggered by hypoxic conditions, will bind to VEGF, causing disturbances in vasculogenesis and angiogenesis, leading to obstetric complications. Several studies have reported overexpression of sFlt1 and decreased expression of VEGFA in preeclamptic patients 125 .
In placental malaria infection, Plasmodium-infected erythrocytes accumulate in the intervillous space of the placenta. Several histopathological characteristics of placental malaria, including thickening of the basement membrane and infiltration of monocytes in the intervillous space of the placenta (intervillositis), can increase the resistance to oxygen transport across the placenta. Accumulation of inflammatory cells and infected erythrocytes can cause placental hypoxia due to oxygen consumption by these cells and decreased blood perfusion due to reduced effective surface area for feto-maternal exchange. Such placental dysfunction can result in stunted fetal growth, characterized by low birth weight babies 27 .
Increased expression of HIF-1α is a marker of placental hypoxia, which should increase the expression of VEGF, VEGFR-1, and VEGFR-2. Decreased VEGFR, VEGFR-1, and VEGFR-2 expression is possible through increased sFlt-1 binding to VEGF with strong affinity. sFlt-1 of the placenta increases its expression under hypoxic conditions 91, 92 . Further studies are needed to prove this.
The pathway analysis results indicate a dysregulation of the angiogenic factor VEGF and its receptor VEGFR-2 due to the regulation of HIF-1α in placental malaria on the incidence of LBW. It can be interpreted that the occurrence of LBW in placental malaria is due to the influence of angiogenesis factors VEGF and VEGFR-2, which are regulated by HIF-1α and are more significant than LBW in non-malaria cases.
Association between low birth weight and hemoglobin, PlGF levels, VEGFR-1 levels, and VEGFR-2 levels in plasma
The effect of plasma angiogenesis and HIF-1α transcription factors on the incidence of LBW was observed using binary logistic regression as bivariate analysis. Based on the observational test of data in maternal plasma, four parameters were significantly different between the case group and the control group: hemoglobin, PlGF levels, VEGFR-1 levels, and VEGFR-2 levels. It showed that hemoglobin levels were the most significant (p=0.01) in influencing the incidence of LBW, followed by plasma levels of PlGF (p=0.02) and plasma levels of VEGFR-1 (p=0.05). The plasma levels of VEGFR-2 did not affect the incidence of LBW (p=0.13).
The hematological effect of Plasmodium vivax malaria is anemia with its consequent increased morbidity and mortality and more frequent blood transfusions. Although the parasitemia of Plasmodium vivax malaria is lower than that of Plasmodium falciparum malaria, it can cause severe anemia, as in Plasmodium falciparum malaria. Anemia in Plasmodium vivax malaria can be explained by two mechanisms. First, production/transfer of infected and uninfected erythrocytes are more in Plasmodium vivax malaria, with 34 uninfected erythrocytes for one infected erythrocyte (in Plasmodium falciparum malaria, the ratio is 8:1). Second, Plasmodium vivax-infected erythrocytes undergo more rapid deformity limiting erythrocytes that are expelled through the microvessels of the spleen during the 'spleen clearance' phase. In addition to these two mechanisms, activation of the immune system due to Plasmodium vivax infection increases the detection and removal of abnormal infected and uninfected erythrocytes 55 .
In this study, maternal anemia significantly affected the incidence of LBW. Several publications also reported the same thing. Plasmodium vivax malaria infection in pregnant women in Bolivia was more at risk of anemia and giving birth to low birth weight babies than pregnant women who were not infected 36 as well as in a multicenter study in an area of low malaria transmission 35 and Mangaluru, India 126 .
Based on the above discussion, in this study, it can be concluded that angiogenesis factors and HIF-1α transcription factors in the placenta or local environment play a more critical role in the incidence of low birth weight than those in the systemic. Those indicated that there is dysregulation of placental angiogenesis factors VEGF, PlGF, and their receptors VEGFR-1 and VEGFR-2, which is triggered by placental hypoxia conditions (marked by increased placental HIF-1α expression) during placental Plasmodium vivax infection. Further research with an increased sample size is needed to reveal this conclusion.
Acknowledgments
The authors would like to acknowledge and thank the Chairman of dr. T.C. Hiller Regional Hospital, Public Health Office of Sikka Regency, and Public Health Office of Nusa Tenggara Timur (NTT), Indonesia, for the permission and substantial support to this research. We also thank Tarina Widaningrum S.Si., MP. Ami Maghfironi, S.Si., and Wahyuda Ngatiril Lady S.Si., for their assistance in carrying out laboratory work.
Funding Statement
This work was supported by the PNBP Faculty of Medicine Universitas Brawijaya and Universitas Brawijaya (Contract Number 30/SK/UN10.7/PN/BPPM/2017).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 3; peer review: 1 approved
Data availability
Underlying data
Figshare: HIF-1α REGULATED PATHOMECHANISM OF LOW BIRTH WEIGHT THROUGH ANGIOGENESIS FACTORS IN PLACENTAL Plasmodium vivax INFECTION. https://doi.org/10.6084/m9.figshare.16577468 127 .
This project contains the following underlying data:
-
-
Raw Data.xlsx (ELISA Graph and Raw Data, Immunofluorescence Graph and Raw Data, Subject Characteristics, and Baby's Characteristics Data)
-
-
Figure 1A (Blood Smear Figure 1A)
-
-
Figure 1B (Blood Smear Figure 1B)
-
-
Figure 1C (Blood Smear Figure 1C)
-
-
Figure 2A (VEGF immunofluorescence)
-
-
Figure 2B (PlGF immunofluorescence)
-
-
Figure 2C (VEGFR-1 immunofluorescence)
-
-
Figure 2D (VEGFR-2 immunofluorescence)
-
-
Figure 2E (HIF-α immunofluorescence)
-
-
Figure 3 (Angiogenic Factor Expression Graph)
-
-
Figure 4 (Angiogenic Factor Level in Maternal Plasma Graph)
-
-
PCR Images 1–4 (Raw Gel Images of PCR test from maternal (1–3) and cord (4) blood samples)
Data are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
References
- 1. Centers for Disease Control and Prevention (CDC): Malaria's Impact Worldwide. CDC,2021. Reference Source
- 2. Rijken MJ, McGready R, Boel ME, et al. : Malaria in pregnancy in the Asia-Pacific region. Lancet Infect Dis. 2012;12(1):75–88. 10.1016/S1473-3099(11)70315-2 [DOI] [PubMed] [Google Scholar]
- 3. Kovacs SD, Rijken MJ, Stergachis A: Treating severe malaria in pregnancy: a review of the evidence. Drug Saf. 2015;38(2):165–81. 10.1007/s40264-014-0261-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. WHO: World Malaria Report 2020. WHO,2020;73:1–4. Reference Source [Google Scholar]
- 5. Conroy AL, Silver KL, Zhong K, et al. : Complement activation and the resulting placental vascular insufficiency drives fetal growth restriction associated with placental malaria. Cell Host Microbe. 2013;13(2):215–26. 10.1016/j.chom.2013.01.010 [DOI] [PubMed] [Google Scholar]
- 6. Sharma L, Shukla G: Placental malaria: a new insight into the pathophysiology. Front Med (Lausanne). 2017;4:117. 10.3389/fmed.2017.00117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ministry of Health of the Republic of Indonesia: Malaria Elimination in Indonesia.2017.
- 8. WHO Report: World Malaria Report 2017. World Health Organization,2017. Reference Source
- 9. Sikka Regency Government: Profile of Sikka Regency.2017. Reference Source
- 10. Kazwaini M, Laumalay HM, Prasetyawan FS, et al. : SISTEM SURVEILANS MALARIA DI PROVINSI NUSA TENGGARA TIMUR.2011. Reference Source
- 11. Fitri LE, Jahja NE, Huwae IR, et al. : Congenital malaria in newborns selected for low birth-weight, anemia, and other possible symptoms in maumere, Indonesia. Korean J Parasitol. 2014;52(6):639–44. 10.3347/kjp.2014.52.6.639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Muehlenbachs A, Nabasumba C, McGready R, et al. : Artemether-lumefantrine to treat malaria in pregnancy is associated with reduced placental haemozoin deposition compared to quinine in a randomized controlled trial. Malar J. 2012;11(1): 150. 10.1186/1475-2875-11-150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pehrson C, Mathiesen L, Heno KK, et al. : Adhesion of Plasmodium falciparum infected erythrocytes in ex vivo perfused placental tissue: a novel model of placental malaria. Malar J. 2016;15(1): 292. 10.1186/s12936-016-1342-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ayres Pereira M, Mandel Clausen T, Pehrson C, et al. : Placental sequestration of Plasmodium falciparum malaria parasites is mediated by the interaction between VAR2CSA and chondroitin sulfate A on syndecan-1. PLoS Pathog. 2016;12(8): e1005831. 10.1371/journal.ppat.1005831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Talundzic E: Plasticity and diversity of the Plasmodium falciparum placental malaria antigen VAR2CSA. University of Georgia,2013. Reference Source [Google Scholar]
- 16. Schlaudecker EP, Munoz FM, Bardají A, et al. : Small for gestational age: Case definition & guidelines for data collection, analysis, and presentation of maternal immunization safety data. Vaccine. 2017;35(48 Pt A):6518–6528. 10.1016/j.vaccine.2017.01.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ngai M, Weckman AM, Erice C, et al. : Malaria in pregnancy and adverse birth outcomes: new mechanisms and therapeutic opportunities. Trends Parasitol. 2020;36(2):127–37. 10.1016/j.pt.2019.12.005 [DOI] [PubMed] [Google Scholar]
- 18. Conroy A, Serghides L, Finney C, et al. : C5a enhances dysregulated inflammatory and angiogenic responses to malaria in vitro: potential implications for placental malaria. PLoS One. 2009;4(3): e4953. 10.1371/journal.pone.0004953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McDonald CR, Tran V, Kain KC: Complement activation in placental malaria. Front Microbiol. 2015;6:1460. 10.3389/fmicb.2015.01460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weckman AM, Ngai M, Wright J, et al. : The impact of infection in pregnancy on placental vascular development and adverse birth outcomes. Front Microbiol. 2019;10:1924. 10.3389/fmicb.2019.01924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Singh PP, Bhandari S, Sharma RK, et al. : Association of angiopoietin dysregulation in placental malaria with adverse birth outcomes. Dis Markers. 2020;2020: 6163487. 10.1155/2020/6163487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nevo O, Soleymanlou N, Wu Y, et al. : Increased expression of sFlt-1 in in vivo and in vitro models of human placental hypoxia is mediated by HIF-1. Am J Physiol Regul Integr Comp Physiol. 2006;291(4):1085–93. 10.1152/ajpregu.00794.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rath G, Aggarwal R, Jawanjal P, et al. : HIF-1 alpha and placental growth factor in pregnancies complicated with pre-eclampsia: a qualitative and quantitative analysis. J Clin Lab Anal. 2016;30(1):75–83. 10.1002/jcla.21819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tal R, Shaish A, Barshack I, et al. : Effects of hypoxia-inducible factor-1alpha overexpression in pregnant mice: possible implications for pre-eclampsia and intrauterine growth restriction. Am J Pathol. 2010;177(6):2950–62. 10.2353/ajpath.2010.090800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen DB, Zheng J: Regulation of placental angiogenesis. Microcirculation. 2014;21(1):15–25. 10.1111/micc.12093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zimna A, Kurpisz M: Hypoxia-Inducible factor-1 in physiological and pathophysiological angiogenesis: applications and therapies. Biomed Res Int. 2015;2015: 549412. 10.1155/2015/549412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Boeuf P, Tan A, Romagosa C, et al. : Placental hypoxia during placental malaria. J Infect Dis. 2008;197(5):757–65. 10.1086/526521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Michalitsi V, Dafopoulos K, Gourounti K, et al. : Hypoxia-inducible factor-1α (HIF-1α) expression in placentae of women with iron deficiency anemia and β-thalassemia trait. J Matern Neonatal Med. 2015;28(4):470–4. 10.3109/14767058.2014.921672 [DOI] [PubMed] [Google Scholar]
- 29. Carvalho BO, Lopes SCP, Nogueira PA, et al. : On the cytoadhesion of Plasmodium vivax-infected erythrocytes. J Infect Dis. 2010;202(4):638–47. 10.1086/654815 [DOI] [PubMed] [Google Scholar]
- 30. Fernandez-Becerra C, Bernabeu M, Castellanos A, et al. : Plasmodium vivax spleen-dependent genes encode antigens associated with cytoadhesion and clinical protection. Proc Natl Acad Sci U S A. 2020;117(23):13056–65. 10.1073/pnas.1920596117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chotivanich K, Udomsangpetch R, Suwanarusk R, et al. : Plasmodium vivax adherence to placental glycosaminoglycans. PLoS One. 2012;7(4): e34509. 10.1371/journal.pone.0034509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Costa FTM, Avril M, Nogueira PA, et al. : Cytoadhesion of Plasmodium falciparum-infected erythrocytes and the infected placenta: a two-way pathway. Braz J Med Biol Res. 2006;39(12):1525–36. 10.1590/s0100-879x2006001200003 [DOI] [PubMed] [Google Scholar]
- 33. Pincelli A, Neves PAR, Lourenço BH, et al. : The Hidden Burden of Plasmodium vivax malaria in pregnancy in the Amazon: an observational study in Northwestern Brazil. Am J Trop Med Hyg. 2018;99(1):73–83. 10.4269/ajtmh.18-0135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rayis DA, Ahmed MA, Omer EM, et al. : Plasmodium vivax malaria among pregnant women in Eastern Sudan. Asian Pacific J Trop Dis. 2016;6(6):421–3. 10.1016/S2222-1808(15)61058-1 [DOI] [Google Scholar]
- 35. Bardají A, Martínez-Espinosa FE, Arévalo-Herrera M, et al. : Burden and impact of Plasmodium vivax in pregnancy: a multi-centre prospective observational study. PLoS Negl Trop Dis. 2017;11(6):1–22. 10.1371/journal.pntd.0005606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brutus L, Santalla J, Schneider D, et al. : Plasmodium vivax malaria during pregnancy, Bolivia. Emerg Infect Dis. 2013;19(10):1605–11. 10.3201/eid1910.130308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. American College of Obstetricians and Gynecologists: Practice bulletin no. 134: fetal growth restriction. Obs Gynecol. 2013;121(5):1122–33. 10.1097/01.AOG.0000429658.85846.f9 [DOI] [PubMed] [Google Scholar]
- 38. Rogerson SJ, Hviid L, Duffy PE, et al. : Malaria in pregnancy: pathogenesis and immunity. Lancet Infect Dis. 2007;7(2):105–17. 10.1016/S1473-3099(07)70022-1 [DOI] [PubMed] [Google Scholar]
- 39. Olivier M, Van Den Ham K, Shio MT, et al. : Malarial pigment hemozoin and the innate inflammatory response. Front Immunol. 2014;5:25–10. 10.3389/fimmu.2014.00025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Haksari EL, Lafeber HN, Hakimi M, et al. : Reference curves of birth weight, length, and head circumference for gestational ages in Yogyakarta, Indonesia. BMC Pediatr. 2016;16(1): 188. 10.1186/s12887-016-0728-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Moody A: Rapid diagnostic tests for malaria parasites. Clin Microbiol Rev. 2002;15(1):66–78. 10.1128/CMR.15.1.66-78.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Harris I, Sharrock WW, Bain LM, et al. : A large proportion of asymptomatic Plasmodium infections with low and sub-microscopic parasite densities in the low transmission setting of Temotu Province, Solomon Islands: challenges for malaria diagnostics in an elimination setting. Malar J. 2010;9(1): 254. 10.1186/1475-2875-9-254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Golassa L, Enweji N, Erko B, et al. : Detection of a substantial number of sub-microscopic Plasmodium falciparum infections by polymerase chain reaction: a potential threat to malaria control and diagnosis in Ethiopia. Malar J. 2013;12(1): 352. 10.1186/1475-2875-12-352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Berzosa P, De Lucio A, Romay-Barja M, et al. : Comparison of three diagnostic methods (microscopy, RDT, and PCR) for the detection of malaria parasites in representative samples from equatorial Guinea. Malar J. 2018;17(1): 333. 10.1186/s12936-018-2481-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rogerson SJ, Pollina E, Getachew A, et al. : Placental monocyte infiltrates in response to Plasmodium falciparum malaria infection and their association with adverse pregnancy outcomes. Am J Trop Med Hyg. 2003;68(1):115–9. 10.4269/ajtmh.2003.68.1.0680115 [DOI] [PubMed] [Google Scholar]
- 46. Parekh FK, Davison BB, Gamboa D, et al. : Placental histopathologic changes associated with subclinical malaria infection and its impact on the fetal environment. Am J Trop Med Hyg. 2010;83(5):973–80. 10.4269/ajtmh.2010.09-0445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. López-Guzmán C, Carmona-Fonseca J: Submicroscopic placental malaria: Histopathology and expression of physiological process mediators. Rev Peru Med Exp Salud Publica. 2020;37(2):220–8. 10.17843/rpmesp.2020.372.4759 [DOI] [PubMed] [Google Scholar]
- 48. Chaikitgosiyakul S, Rijken MJ, Muehlenbachs A, et al. : A morphometric and histological study of placental malaria shows significant changes to villous architecture in both Plasmodium falciparum and Plasmodium vivax infection. Malar J. 2014;13(1):1–13. 10.1186/1475-2875-13-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Toda H, Diaz-Varela M, Segui-Barber J, et al. : Plasma-derived extracellular vesicles from Plasmodium vivax patients signal spleen fibroblasts via NF-kB facilitating parasite cytoadherence. Nat Commun. 2020;11(1): 2761. 10.1038/s41467-020-16337-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rivera AJ, Rivera LL, Dubon JM, et al. : Effect of Plasmodium vivax malaria on perinatal health. Rev Honduras Pediátrica. 1993;16:7–10. [Google Scholar]
- 51. Nosten F, Rogerson SJ, Beeson JG, et al. : Malaria in pregnancy and the endemicity spectrum: what can we learn? Trends Parasitol. 2004;20(9):425–32. 10.1016/j.pt.2004.06.007 [DOI] [PubMed] [Google Scholar]
- 52. Kochar DK, Saxena V, Singh N, et al. : Plasmodium vivax malaria. Emerg Infect Dis. 2005;11(1):132–4. 10.3201/eid1101.040519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rodriguez-Morales AJ, Sanchez E, Vargas M, et al. : Pregnancy outcomes associated with Plasmodium vivax malaria in Northeastern Venezuela. Am J Trop Med Hyg. 2006;74(5):755–7. 10.4269/ajtmh.2006.74.755 [DOI] [PubMed] [Google Scholar]
- 54. Carvalho BO, Matsuda JS, Luz SL, et al. : Gestational malaria associated to Plasmodium vivax and Plasmodium falciparum placental mixed-infection followed by foetal loss: a case report from an unstable transmission area in Brazil. Malar J. 2011;10(1): 178. 10.1186/1475-2875-10-178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Douglas NM, Anstey NM, Buffet PA, et al. : The anaemia of Plasmodium vivax malaria. Malar J. 2012;11: 135. 10.1186/1475-2875-11-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Poespoprodjo JR, Fobia W, Kenangalem E, et al. : Adverse pregnancy outcomes in an area where multidrug-resistant Plasmodium vivax and Plasmodium falciparum infections are endemic. Clin Infect Dis. 2008;46(9):1374–81. 10.1086/586743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Anggara FY, Rahardjo SS, Murti B: Meta-Analysis: The Effect of Malaria Infection on the Incidence of Low Birth Weight. J Matern Child Heal. 2020;5(5):549–62. Reference Source [Google Scholar]
- 58. Figueiredo ACMG, Gomes-Filho IS, Silva RB, et al. : Maternal anemia and low birth weight: a systematic review and meta-analysis. Nutrients. 2018;10(5):601. 10.3390/nu10050601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Milcic TL: The complete blood count. Neonatal Netw. 2010;29(2):109–15. 10.1891/0730-0832.29.2.109 [DOI] [PubMed] [Google Scholar]
- 60. Hariningrum A: Rentang Nilai Hitung Darah Lengkap Bayi Baru Lahir di RSU PKU Muhammadiyah Bantul. Universitas Gadjah Mada,2014. Reference Source [Google Scholar]
- 61. Manroe BL, Weinberg AG, Rosenfeld CR, et al. : The neonatal blood count in health and disease. I. Reference values for neutrophilic cells. J Pediatr. 1979;95(1):89–98. 10.1016/s0022-3476(79)80096-7 [DOI] [PubMed] [Google Scholar]
- 62. Weinberg AG, Rosenfeld CR, Manroe BL, et al. : Neonatal blood cell count in health and disease. II. Values for lymphocytes, monocytes, and eosinophils. J Pediatr. 1985;106(3):462–6. 10.1016/s0022-3476(85)80681-8 [DOI] [PubMed] [Google Scholar]
- 63. De Winter JP, Van Bel F: The effect of glucocorticosteroids on the neonatal blood count. Acta Paediatr Scand. 1991;80(2):159–62. 10.1111/j.1651-2227.1991.tb11827.x [DOI] [PubMed] [Google Scholar]
- 64. Franke-Fayard B, Fonager J, Braks A, et al. : Sequestration and tissue accumulation of human malaria parasites: can we learn anything from rodent models of malaria? PLoS Pathog. 2010;6(9): e1001032. 10.1371/journal.ppat.1001032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ismail MR, Ordi J, Menendez C, et al. : Placental pathology in malaria: a histological, immunohistochemical, and quantitative study. Hum Pathol. 2000;31(1):85–93. 10.1016/s0046-8177(00)80203-8 [DOI] [PubMed] [Google Scholar]
- 66. Suguitan AL, Jr, Leke RG, Fouda G, et al. : Changes in the levels of chemokines and cytokines in the placentas of women with Plasmodium falciparum malaria. J Infect Dis. 2003;188(7):1074–82. 10.1086/378500 [DOI] [PubMed] [Google Scholar]
- 67. Mayor A, Bardají A, Felger I, et al. : Placental infection with plasmodium vivax: a histopathological and molecular study. J Infect Dis. 2012;206(12):1904–10. 10.1093/infdis/jis614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Doritchamou JYA, Akuffo RA, Moussiliou A, et al. : Submicroscopic placental infection by non- falciparum Plasmodium spp. PLoS Negl Trop Dis. 2018;12(2): e0006279. 10.1371/journal.pntd.0006279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Carmona-Fonseca J, Arango E, Maestre A: Placental malaria in Colombia: histopathologic findings in Plasmodium vivax and P. falciparum infections. Am J Trop Med Hyg. 2013;88(6):1093–101. 10.4269/ajtmh.12-0363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Souza RM, Ataíde R, Dombrowski JG, et al. : Placental histopathological changes associated with Plasmodium vivax infection during pregnancy. PLoS Negl Trop Dis. 2013;7(2): e2071. 10.1371/journal.pntd.0002071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Totino PR, Lopes SC: Insights into the cytoadherence phenomenon of Plasmodium vivax: the putative role of phosphatidylserine. Front Immunol. 2017;8: 1148. 10.3389/fimmu.2017.01148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Requena P, Rui E, Padilla N, et al. : Plasmodium vivax VIR proteins are targets of naturally-acquired antibody and T cell immune responses to malaria in pregnant women. PLoS Negl Trop Dis. 2016;10(10): e0005009. 10.1371/journal.pntd.0005009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zakama AK, Ozarslan N, Gaw SL: Placental malaria. Curr Trop Med Rep. 2020;7(4):162–171. 10.1007/s40475-020-00213-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bernabeu M, Lopez FJ, Ferrer M, et al. : Functional analysis of Plasmodium vivax VIR proteins reveals different subcellular localizations and cytoadherence to the ICAM-1 endothelial receptor. Cell Microbiol. 2012;14(3):386–400. 10.1111/j.1462-5822.2011.01726.x [DOI] [PubMed] [Google Scholar]
- 75. Marín-Menéndez A, Bardají A, Martínez-Espinosa FE, et al. : Rosetting in plasmodium vivax: a cytoadhesion phenotype associated with anaemia. PLoS Negl Trop Dis. 2013;7(4): e2155. 10.1371/journal.pntd.0002155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Albrecht L, Lopes SCP, da Silva ABIE, et al. : Rosettes integrity protects Plasmodium vivax of being phagocytized. Sci Rep. 2020;10(1): 16706. 10.1038/s41598-020-73713-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Mens PF, Bojtor EC, Schallig HDFH: Molecular interactions in the placenta during malaria infection. Eur J Obstet Gynecol Reprod Biol. 2010;152(2):126–32. 10.1016/j.ejogrb.2010.05.013 [DOI] [PubMed] [Google Scholar]
- 78. Khaliq A, Li XF, Shams M, et al. : Localization of placenta Growth Factor (PIGF) in human term placenta. Growth Factors. 1996;13(3–4):243–50. 10.3109/08977199609003225 [DOI] [PubMed] [Google Scholar]
- 79. Lyall F, Young A, Boswell F, et al. : Placental expression of vascular endothelial growth factor in placentae from pregnancies complicated by pre-eclampsia and intrauterine growth restriction does not support placental hypoxia at delivery. Placenta. 1997;18(4):269–76. 10.1016/s0143-4004(97)80061-6 [DOI] [PubMed] [Google Scholar]
- 80. Chen Q, Schlichtherle M, Wahlgren M: Molecular aspects of severe malaria. Clin Microbiol Rev. 2000;13(3):439–50. 10.1128/CMR.13.3.439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kinzler W, Kizhner O, Peltier M: 402: Role of angiogenesis-related genes in fetal growth restriction. Am J Obstet Gynecol. 2008;199(6):S122. 10.1016/j.ajog.2008.09.431 [DOI] [Google Scholar]
- 82. Szentpéteri I, Rab A, Kornya L, et al. : Gene expression patterns of Vascular Endothelial Growth Factor (VEGF-A) in human placenta from pregnancies with intrauterine growth restriction. J Matern Fetal Neonatal Med. 2013;26(10):984–9. 10.3109/14767058.2013.766702 [DOI] [PubMed] [Google Scholar]
- 83. Tse JY, Lao TT, Chan CC, et al. : Expression of Vascular Endothelial Growth Factor in third-trimester placentas is not increased in growth-restricted fetuses. J Soc Gynecol Investig. 2001;8(2):77–82. 10.1177/107155760100800203 [DOI] [PubMed] [Google Scholar]
- 84. Alahakoon TI, Zhang W, Arbuckle S, et al. : Reduced angiogenic factor expression in intrauterine fetal growth restriction using semiquantitative immunohistochemistry and digital image analysis. J Obstet Gynaecol Res. 2018;44(5):861–72. 10.1111/jog.13592 [DOI] [PubMed] [Google Scholar]
- 85. Andraweera PH, Dekker GA, Roberts CT: The Vascular Endothelial Growth Factor family in adverse pregnancy outcomes. Hum Reprod Update. 2012;18(4):436–57. 10.1093/humupd/dms011 [DOI] [PubMed] [Google Scholar]
- 86. Afzal I: Master of philosophy the levels of angiogenic and anti-angiogenic molecule concentrations in pregnancy based disorders in the maternal and fetal.2012. [Google Scholar]
- 87. Wallner W, Sengenberger R, Strick R, et al. : Angiogenic growth factors in maternal and fetal serum in pregnancies complicated by intrauterine growth restriction. Clin Sci (Lond). 2007;112(1):51–7. 10.1042/CS20060161 [DOI] [PubMed] [Google Scholar]
- 88. Tang Y, Ye W, Liu X, et al. : VEGF and sFLT-1 in serum of PIH patients and effects on the foetus. Exp Ther Med. 2019;17(3):2123–8. 10.3892/etm.2019.7184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Borras D, Perales-Puchalt A, Ruiz Sacedón N, et al. : Angiogenic growth factors in maternal and fetal serum in pregnancies complicated with intrauterine growth restriction. J Obstet Gynaecol. 2014;34(3):218–20. 10.3109/01443615.2013.834304 [DOI] [PubMed] [Google Scholar]
- 90. Gourvas V, Dalpa E, Vrachnis N, et al. : Placental angiogenesis and fetal growth restriction. From Preconception to Postpartum.2012;179. 10.5772/37879 [DOI] [Google Scholar]
- 91. Arroyo JA, Winn VD: Vasculogenesis and angiogenesis in the IUGR placenta. Semin Perinatol. Elsevier,2008;32(3):172–7. 10.1053/j.semperi.2008.02.006 [DOI] [PubMed] [Google Scholar]
- 92. Tang L, He G, Liu X, et al. : Progress in the understanding of the etiology and predictability of fetal growth restriction. Reproduction. 2017;153(6):R227–40. 10.1530/REP-16-0287 [DOI] [PubMed] [Google Scholar]
- 93. Nevo O, Many A, Xu J, et al. : Placental expression of soluble fms-like tyrosine kinase 1 is increased in singletons and twin pregnancies with Intrauterine Growth Restriction. J Clin Endocrinol Metab. 2008;93(1):285–92. 10.1210/jc.2007-1042 [DOI] [PubMed] [Google Scholar]
- 94. Tsatsaris V, Goffin F, Munaut C, et al. : Overexpression of the soluble Vascular Endothelial Growth Factor receptor in preeclamptic patients: pathophysiological consequences. J Clin Endocrinol Metab. 2003;88(11):5555–63. 10.1210/jc.2003-030528 [DOI] [PubMed] [Google Scholar]
- 95. Vogtmann R, Kühnel E, Dicke N, et al. : Human sFLT1 leads to severe changes in placental differentiation and vascularization in a transgenic hsFLT1/rtTA FGR mouse model. Front Endocrinol (Lausanne). 2019;10:165. 10.3389/fendo.2019.00165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Cerdeira AS, Karumanchi SA: Angiogenic factors in preeclampsia and related disorders. Cold Spring Harb Perspect Med. 2012;2(11): a006585. 10.1101/cshperspect.a006585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Joó JG, Rigó J, Jr, Börzsönyi B, et al. : Placental gene expression of the Placental Growth Factor (PlGF) in intrauterine growth restriction. J Matern Fetal Neonatal Med. 2017;30(12):1471–5. 10.1080/14767058.2016.1219993 [DOI] [PubMed] [Google Scholar]
- 98. Wu WB, Xu YY, Cheng WW, et al. : Decreased PGF may contribute to trophoblast dysfunction in fetal growth restriction. Reproduction. 2017;154(3):319–29. 10.1530/REP-17-0253 [DOI] [PubMed] [Google Scholar]
- 99. Ravikumar G, Mukhopadhyay A, Mani C, et al. : Placental expression of angiogenesis-related genes and their receptors in IUGR pregnancies: correlation with fetoplacental and maternal parameters. J Matern Fetal Neonatal Med. 2020;33(23):3954–61. 10.1080/14767058.2019.1593362 [DOI] [PubMed] [Google Scholar]
- 100. Ahmed A, Perkins J: Angiogenesis and intrauterine growth restriction. Baillieres Best Pract Res Clin Obstet Gynaecol. 2000;14(6):981–98. 10.1053/beog.2000.0139 [DOI] [PubMed] [Google Scholar]
- 101. Burton GJ, Charnock-Jones DS, Jauniaux E: Regulation of vascular growth and function in the human placenta. Reproduction. 2009;138(6):895–902. 10.1530/REP-09-0092 [DOI] [PubMed] [Google Scholar]
- 102. Benton SJ, McCowan LM, Heazell AEP, et al. : Placental Growth Factor as a marker of fetal growth restriction caused by placental dysfunction. Placenta. 2016;42:1–8. 10.1016/j.placenta.2016.03.010 [DOI] [PubMed] [Google Scholar]
- 103. Komwilaisak R, Tangkiratichai P: Maternal serum angiogenic growth factors in intrauterine growth restriction versus normal pregnancies. J Med Assoc Thai. 2017;100(2):119–24. [PubMed] [Google Scholar]
- 104. Maynard SE, Venkatesha S, Thadhani R, et al. : Soluble Fms-like Tyrosine Kinase 1 and endothelial dysfunction in the pathogenesis of Pre-eclampsia. Pediatr Res. 2005;57(5 Pt 2):1R–7R. 10.1203/01.PDR.0000159567.85157.B7 [DOI] [PubMed] [Google Scholar]
- 105. Vrachnis N, Kalampokas E, Sifakis S, et al. : Placental Growth Factor (PlGF): a key to optimizing fetal growth. J Matern Fetal Neonatal Med. 2013;26(10):995–1002. 10.3109/14767058.2013.766694 [DOI] [PubMed] [Google Scholar]
- 106. Rădulescu C, Bacârea A, Huțanu A, et al. : Placental growth factor, soluble fms-like tyrosine kinase 1, soluble endoglin, IL-6, and IL-16 as biomarkers in Preeclampsia. Mediators Inflamm. 2016;2016: 3027363. 10.1155/2016/3027363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Bersinger NA, Ødegård RA: Serum levels of macrophage colony stimulating, vascular endothelial, and Placenta Growth Factor in relation to later clinical onset of pre-eclampsia and a small-for-gestational age birth. Am J Reprod Immunol. 2005;54(2):77–83. 10.1111/j.1600-0897.2005.00290.x [DOI] [PubMed] [Google Scholar]
- 108. Karumanchi SA, Epstein FH: Placental ischemia and soluble fms-like tyrosine kinase 1: cause or consequence of pre-eclampsia? Kidney Int. 2007;71(10):959–61. 10.1038/sj.ki.5002281 [DOI] [PubMed] [Google Scholar]
- 109. Romero R, Nien JK, Espinoza J, et al. : A longitudinal study of angiogenic (Placental Growth Factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop pre-eclampsia and deliver a small for gestational age neonate. J Matern Fetal Neonatal Med. 2008;21(1):9–23. 10.1080/14767050701830480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Ghosh SK, Raheja S, Tuli A, et al. : Can maternal serum Placental Growth Factor estimation in early second trimester predict the occurrence of early onset pre-eclampsia and/or early onset intrauterine growth restriction? a prospective cohort study. J Obstet Gynaecol Res. 2013;39(5):881–90. 10.1111/jog.12006 [DOI] [PubMed] [Google Scholar]
- 111. Laskowska M, Laskowska K, Oleszczuk J: aVEGF-A and its Soluble Receptor Type 1 (sVEGFR-1, sFlt-1) Concentrations in Pregnancies with Intrauterine Growth Restriction in the Presence or Absence of Preeclampsia. Res J Pharm Biol Chem Sci. 2015;6(2):319–25. Reference Source [Google Scholar]
- 112. Chang YS, Chen CN, Jeng SF, et al. : The sFlt-1/PlGF ratio as a predictor for poor pregnancy and neonatal outcomes. Pediatr Neonatol. 2017;58(6):529–33. 10.1016/j.pedneo.2016.10.005 [DOI] [PubMed] [Google Scholar]
- 113. Naimi AA, Schmidt-fittschen M, Herzeg A, et al. : Is there any association between the angiogenic factors sflt-1 / plgf and intrauterine growth restriction in patients with preeclamsia? J Womens Health Gyn. 2019;6(3):1–7. Reference Source [Google Scholar]
- 114. Helske S, Vuorela P, Carpén O, et al. : Expression of Vascular Endothelial Growth Factor Receptors 1, 2 and 3 in placentas from normal and complicated pregnancies. Mol Hum Reprod. 2001;7(2):205–10. 10.1093/molehr/7.2.205 [DOI] [PubMed] [Google Scholar]
- 115. Regnault TR, de Vrijer B, Galan HL, et al. : The relationship between transplacental O 2 diffusion and placental expression of PlGF, VEGF and their receptors in a placental insufficiency model of Fetal Growth Restriction. J Physiol. 2003;550(Pt 2):641–56. 10.1113/jphysiol.2003.039511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Nevo O, Lee DK, Caniggia I: Attenuation of VEGFR-2 expression by sFlt-1 and low oxygen in human placenta. PLoS One. 2013;8(11): e81176. 10.1371/journal.pone.0081176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Semczuk-Sikora A, Krzyzanowski A, Stachowicz N, et al. : [Maternal serum concentration of angiogenic factors: PIGF, VEGF and VEGFR-1 and placental volume in pregnancies complicated by intrauterine growth restriction]. Ginekol Pol. 2007;78(10):783–6. [PubMed] [Google Scholar]
- 118. Chaiworapongsa T, Romero R, Gotsch F, et al. : Low maternal concentrations of soluble vascular endothelial growth factor receptor-2 in pre-eclampsia and small for gestational age. J Matern Fetal Neonatal Med. 2008;21(1):41–52. 10.1080/14767050701831397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Robb KP, Cotechini T, Allaire C, et al. : Inflammation-induced Fetal Growth Restriction in rats is associated with increased placental HIF-1α accumulation. PLoS One. 2017;12(4): e0175805. 10.1371/journal.pone.0175805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Ashur-Fabian O, Yerushalmi GM, Mazaki-Tovi S, et al. : Cell free expression of hif1α and p21 in maternal peripheral blood as a marker for pre-eclampsia and fetal growth restriction. PLoS One. 2012;7(5): e37273. 10.1371/journal.pone.0037273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Pringle KG, Kind KL, Sferruzzi-Perri AN, et al. : Beyond oxygen: complex regulation and activity of hypoxia inducible factors in pregnancy. Hum Reprod Update. 2010;16(4):415–31. 10.1093/humupd/dmp046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Shibuya M: Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) signaling in angiogenesis: a crucial target for anti- and pro-angiogenic therapies. Genes Cancer. 2011;2(12):1097–105. 10.1177/1947601911423031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Wang Y, Zhao S: Vascular biology of the placenta. In: Colloquium Series on Integrated Systems Physiology: From Molecule to Function.Morgan & Claypool Life Sciences,2010;2(1):1–98. 10.4199/C00016ED1V01Y201008ISP009 [DOI] [PubMed] [Google Scholar]
- 124. Otrock ZK, Mahfouz RA, Makarem JA, et al. : Understanding the biology of angiogenesis: review of the most important molecular mechanisms. Blood Cells Mol Dis. 2007;39(2):212–20. 10.1016/j.bcmd.2007.04.001 [DOI] [PubMed] [Google Scholar]
- 125. Kappou D, Sifakis S, Konstantinidou A, et al. : Role of the angiopoietin/Tie system in pregnancy (Review). Exp Ther Med. 2015;9(4):1091–6. 10.3892/etm.2015.2280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Chandrashekar VN, Punnath K, Dayanand KK, et al. : Malarial anemia among pregnant women in the south-western coastal city of Mangaluru in India. Informatics Med Unlocked. 2019;15: 100159. 10.1016/j.imu.2019.02.003 [DOI] [Google Scholar]
- 127. Prasetyorini N, Erwan NE, Sardjono TW, et al. : HIF-1α regulated pathomechanism of low birth weight through angiogenesis factors in placental Plasmodium vivax Infection. figshare. Journal contribution,2021. 10.6084/m9.figshare.16577468.v4 [DOI] [Google Scholar]