Abstract
Purpose
The RAS/MEK signaling pathway is essential in carcinogenesis and frequently altered in non-small-cell lung cancer (NSCLC), notably by KRAS mutations (KRASm) that affect 25%-30% of non-squamous NSCLC. This study aims to explore the impact of KRASm subtypes on disease phenotype and survival outcomes.
Patients and methods
We conducted a retrospective analysis of the French Epidemiological Strategy and Medical Economics database for advanced or metastatic lung cancer from 2011 to 2021. Patient demographics, histology, KRASm status, treatment strategies, and outcomes were assessed.
Results
Of 10 177 assessable patients for KRAS status, 17.6% had KRAS p.G12C mutation, 22.6% had KRAS non-p.G12C mutation, and 59.8% were KRASwt. KRASm patients were more often smokers (96.3%) compared with KRASwt (85.8%). A higher proportion of programmed death-ligand 1 ≥50% was found for KRASm patients: 43.5% versus 38.0% (P < 0.01). KRASm correlated with poorer outcomes. First-line median progression-free survival was shorter in the KRASm than the KRASwt cohort: 4.0 months [95% confidence interval (CI) 3.7-4.3 months] versus 5.1 months (95% CI 4.8-5.3 months), P < 0.001. First-line overall survival was shorter for KRASm than KRASwt patients: 12.6 months (95% CI 11.6-13.6 months) versus 15.4 months (95% CI 14.6-16.2 months), P = 0.012. First-line chemoimmunotherapy offered better overall survival in KRAS p.G12C (48.8 months) compared with KRAS non-p.G12C (24.0 months) and KRASwt (22.5 months) patients. Second-line overall survival with immunotherapy was superior in the KRAS p.G12C subgroup: 12.6 months (95% CI 8.1-18.6 months) compared with 9.4 months (95% CI 8.0-11.4 months) for KRAS non-p.G12C and 9.6 months (8.4-11.0 months) for KRASwt patients.
Conclusion
We highlighted distinct clinical profiles and survival outcomes according to KRASm subtypes. Notably KRAS p.G12C mutations may provide increased sensitivity to immunotherapy, suggesting potential therapeutic implications for sequencing or combination of therapies. Further research on the impact of emerging KRAS specific inhibitors are warranted in real-world cohorts.
Key words: real life data, NSCLC, KRAS mutational status, immunotherapy, prognosis
Highlights
-
•
We present a comprehensive analysis of the predictive and prognostic value of KRAS mutation subtypes in NSCLC.
-
•
NSCLC harboring KRAS mutation have a high proportion of PD-L1 >50% and a more aggressive outcome.
-
•
KRAS p.G12C subtype provides the best efficacy for first-line chemoimmunotherapy compared with non-G12C and KRASwt diseases.
Introduction
The treatment landscape for non-small-cell lung cancer (NSCLC) is rapidly evolving and, following the principles of precision medicine, is becoming increasingly biomarker driven with new targeted therapies used in concert with companion molecular diagnostics.1 NSCLC is associated with several addictive oncogenic driver alterations, e.g. epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK), ROS proto-oncogene 1 (ROS1), B-Raf proto-oncogene (BRAF) and neurotrophic tyrosine receptor kinase (NTRK), which are relevant for selecting the most beneficial regimen.2,3 New NSCLC biomarkers continue to emerge, including rearranged during transfection (RET) fusion, hepatocyte growth factor receptor (MET) exon 14 (ex14) skipping mutations and MET amplification, receptor tyrosine kinase erbB-2 (ERBB2/HER2) mutation, Kirsten rat sarcoma viral oncogene homolog (KRAS) exon 2 p.G12C mutation, neuregulin (NRG1) fusion, and EGFR exon 20 insertions. Additionally, programmed death-ligand 1 (PD-L1) and tumor mutational burden can predict a better response to immune checkpoint inhibitors in NSCLC.
The mitogen-activated protein kinases (MAPK) form a group of proteins involved mainly in cell division, but also in many cellular processes such as apoptosis, cell differentiation, in response to various external signals (growth factors, osmotic stress, cytokines, etc.), in particular via the activation of intracellular signaling pathways.4 The most important of these pathways is the RAS/RAF/MEK/ERK pathway. Its alteration, leading to permanent activation, is a key process in carcinogenesis and can be caused by different processes, including excessive external stimulation (pathological accumulation of growth factors) or activating mutations affecting the different proteins of this cascade.5 The development of specific inhibitors of the different actors involved in this signaling pathway allowed to break the differentiation process and the abnormal multiplication responsible for tumorigenesis.
Historically, the first molecules targeting the RAS/RAF/MEK/ERK pathway targeted proteins located downstream, with anti-BRAF or anti-MEK therapies, particularly in melanoma and digestive cancers. More recently, molecules targeting the RAS/RAF/MEK/ERK pathway have arrived, but more upstream, with the development of therapies targeting the mutated form of the KRAS protein in its G12C form. Approximatively 25%-30% of non-squamous NSCLC harbor a KRAS mutation (KRASm) in Western countries. These mutations mainly affect codons 12, 13, and 61. KRAS mutation is assumed to be associated with a poor prognosis. KRASm have been shown to be associated with shorter disease-free survival and overall survival in resected NSCLC,6 especially in the case of G12C subtype mutation.7 For advanced disease, the presence of a KRASm seems to be related to a shorter overall survival for patients treated with platinum-based chemotherapy.8 Before the era of immunotherapy, the prognostic and predictive values of the KRASm seemed to disappear in second line among patients who then received docetaxel or erlotinib as standard second-line therapies.9 On the contrary, regarding immunotherapy, KRASm tumors seem to have an associated inflammatory phenotype that would increase the sensitivity to immunotherapy.10
Mutations of the KRAS gene are generally mutually exclusive from other oncogene addictions such as EGFR mutations or ALK rearrangement that are eligible for targeted therapy.11,12 Targeting KRASm is therefore a major therapeutic advance.13 So far, two molecules have been approved for the treatment of KRAS p.G12C-mutated NSCLC after failure of platinum-based chemotherapy and immunotherapy targeting programmed cell death protein 1 (PD-1)/PD-L1: sotorasib (AMG510)14,15 and adagrasib (MRTX849).16 Still, the magnitude of benefit has been questioned in the second-line setting.
This non-interventional, retrospective, comparative study was conducted to describe baseline characteristics, treatment strategies, and outcomes of patients with advanced NSCLC according to their KRAS status included in the Epidemiological Strategy and Medical Economics (ESME) data platform for Advanced and Metastatic Lung Cancer (AMLC), before KRAS inhibitor market authorization. Our data may be of significant relevance for the subsequent development and sequencing of KRAS inhibitors in the treatment strategy of patients.
Methods
Study design
The ESME Lung Cancer (ESME LC) database (clinicaltrial.gov NCT03848052) is a centralized, deidentified structured database derived from electronic health records of consecutive adult patients treated for lung cancer at 1 of the 38 health facilities (17 private non-profit comprehensive cancer centers spread over 19 sites, 1 center affiliated to the Unicancer network, and 18 university or general hospitals) participating in the ESME LC program. Patient-, hospitalization-, and pharmacy-related data are collected, including patient demographic characteristics, pathology, treatment strategies, and outcomes.
Unicancer manages the ESME LC data platform in accordance with current best practice guidelines. This platform is supervised by an independent scientific committee, which approved the present work. In compliance with French regulations, the ESME Data Warehouse was authorized by the French data protection authority [initial authorization number DE-2017-397 and subsequent amendment dated 14 October 2019, in accordance with General Data Protection Regulation (GDPR)]. Data are updated on a yearly basis and run into a data management process aimed at ensuring the quality of the data analyzed. No formal dedicated informed consent is required, but all patients have approved the use of their data.
Patient population
In this study, patient selection focused on patients with histologically confirmed advanced NSCLC, diagnosed from 1 January 2011. Patients were either newly diagnosed or relapsed from early-stage disease. To ensure at least 6 months of follow-up for patient outcomes, data were extracted from the ESME LC database on the data cut-off of 2 October 2021.
To better assess the specific impact of KRAS status, we excluded patients who did not have molecular analysis. We also excluded from our cohort those who presented a targetable alteration in one of the known oncogenic drivers (EGFR, ALK, BRAF, MET, ROS1 or HER2).
Data collection
The following data were collected: clinical characteristics [gender, age, Eastern Cooperative Oncology Group (ECOG) performance status (PS), co-morbidities, other cancer history); smoking status; disease characteristics (date and stage of primary tumor diagnostic and time to relapse/advanced disease, histological subtype, location of metastases); KRAS mutational status and other mutations; treatment strategy (number of treatment lines, therapeutic agents, disease evolution over time from index date, patient status at the end of each line, i.e. deceased, alive without progression or alive with progression and treatments received).
Survival status was collected. A treatment line was defined as a given therapeutic strategy delivered for metastatic NSCLC, until progression. The start date of the first-line therapy was defined as the date of the first systemic treatment initiated after diagnosis. Disease progression was defined as the occurrence of a new metastatic site or progression of existing metastases, regional tumoral or lymph node progression, or discontinuation of systemic treatment due to progression or death from any cause.
Statistical analyses
Demographic analyses were carried out on the entire cohort. Treatment and survival outcomes were restricted to the subpopulation with lung cancer diagnosed from 1 January 2015.
Data were summarized by median and range for continuous variables and by frequency and percentage for categorical variables. The number of missing data were presented for each variable, but not considered for the percentage calculations. Comparisons between groups were carried out using the Kruskal–Wallis test for continuous variables and the chi-square test or Fisher’s exact test for categorical variables. Overall survival was defined as the time from advanced disease to the date of death from any cause or end of follow-up at the cut-off date. OSx was defined as the time from the start date of first (OS1) and second (OS2) line of therapy to the date of death from any cause or end of follow-up at the cut-off date. Progression-free survival (PFS) was defined as the time from the start date of first (PFS1) and second (PFS2) line to the date of disease progression, or death from any cause or end of follow-up at the cut-off date. For time to event endpoint (overall survival and PFS), the Kaplan–Meier method was used to estimate median survival and survival rates at different time points. The hazard ratio and associated 95% confidence interval (CI) were estimated using a Cox proportional-hazards model. Survival analyses were only carried out when the available target population included at least 30 patients. Statistical analyses were carried out using SAS version 9.4 or higher (SAS Institute, Inc., Cary, NC).
Results
Study population
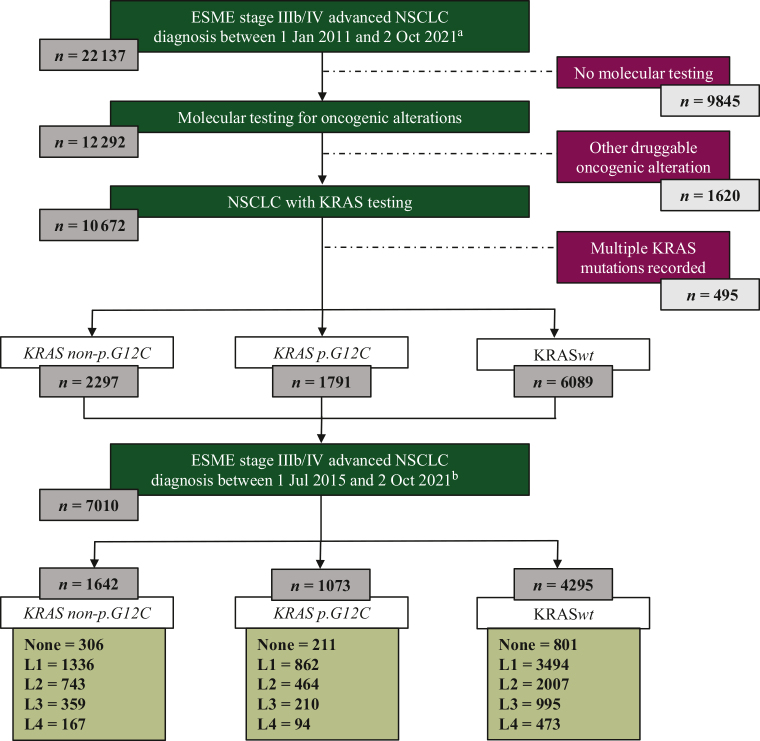
A total of 22 137 patients with advanced NSCLC diagnosed from 1 January 2011 were registered in this observational cohort (Figure 1). Data were extracted from the ESME LC data platform on 2 October 2021. Patients without molecular testing (n = 9845) or with other druggable oncogenic alteration (n = 1620) were excluded. Patients with multiple KRASm recorded were also excluded (n = 495). Three subgroups of patients with demographic data and treatment outcomes were described according to their KRAS mutational status if available before first-line therapy start: KRAS p.G12C for patients with KRAS G12C mutation subtype (n = 1791), KRAS non-p.G12C for patients with KRAS mutation other than G12C (n = 2297), and KRASwt for patient with KRAS wild-type (wt) tumor (n = 6089). Treatment strategies and outcomes were described for patients whose advanced disease was diagnosed from 2015.
Figure 1.
Flow chart. ESME, Epidemiological Strategy and Medical Economics; NSCLC, non-small-cell lung cancer. aCohort used for demographic statistical analyses. bCohort used for analytic statistical analyses.
Patients and disease characteristics
Baseline characteristics for each cohort are summarized in Table 1. According to KRAS status (mutant versus wt): 39.9% versus 35.1% were women (P < 0.01); 3.8% versus 14.2% were non-smokers (P < 0.01). ECOG-PS and stage at NSCLC diagnosis status were similar. KRASm patients had more contralateral lung metastases: 28.9% versus 25.4% (P < 0.01) and bone metastases: 41.6% versus 38.0% (P < 0.01); and less pleural metastases: 6.2% versus 8.1% (P < 0.01) and liver metastases: 13.6% versus 15.4% (P = 0.01) than KRASwt patients. There was no difference regarding the prevalence of brain, meninges, or adrenal metastases. The proportion of patients with PD-L1 tumor proportion score (TPS) ≥50% was superior for KRASm than KRASwt patients: 43.5% versus 38.0% (P < 0.01).
Table 1.
Baseline patient and disease characteristics at advanced NSCLC.
| KRASwt n = 6089 | KRASm n = 4088 | P value | KRASG12A n = 291 | KRASG12C n = 1791 | KRASG12D n = 538 | KRASG12V n = 756 | KRASG13C n = 192 | KRASG13D n = 136 | KRASmother n = 384 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Age | <0.01a | |||||||||
| Median (years) | 64.0 | 63.0 | 63.0 | 62.0 | 63.5 | 63.0 | 62.0 | 63.0 | 63.0 | |
| Gender | <0.01b | |||||||||
| Female | 2140 (35.1%) | 1633 (39.9%) | 106 (36.4%) | 744 (41.5%) | 224 (41.6%) | 305 (40.3%) | 61 (31.8%) | 47 (34.6%) | 146 (37.1%) | |
| Smoking status | <0.01b | |||||||||
| Non-smoker | 831 (14.2%) | 149 (3.8%) | 13 (4.6%) | 26 (1.5%) | 44 (8.5%) | 33 (4.6%) | 3 (1.6%) | 10 (7.5%) | 20 (5.4%) | |
| Smoker | 5030 (85.8%) | 3804 (96.2%) | 269 (95.4%) | 1709 (98.5%) | 471 (91.5%) | 692 (95.4%) | 187 (98.4%) | 123 (92.5%) | 353 (94.6%) | |
| Tobacco consumption | <0.01a | |||||||||
| Median (pack-years) | 34.0 | 40.0 | ||||||||
| ECOG PS | 0.70b | |||||||||
| 0-1 | 1044 (75.4%) | 780 (74.6%) | 55 (80.9%) | 360 (74.4%) | 105 (76.6%) | 120 (75.0%) | 39 (70.9%) | 20 (64.5%) | 81 (73.6%) | |
| ≥2 | 341 (24.6%) | 265 (25.3%) | 13 (19.1%) | 124 (25.6%) | 32 (23.4%) | 40 (25.0%) | 16 (29.1%) | 11 (35.5%) | 29 (26.4%) | |
| Advanced disease modality | 0.46a | |||||||||
| De novo | 5197 (85.4%) | 3467 (84.8%) | 255 (87.6%) | 1517 (84.7%) | 445 (79.7%) | 638 (84.4%) | 171 (89.1%) | 113 (83.1%) | 328 (85.4%) | |
| Recurrent | 892 (14.6%) | 621 (15.2%) | 36 (12.4%) | 274 (15.3%) | 93 (17.3%) | 118 (15.6%) | 21 (10.9%) | 23 (16.9%) | 56 (14.6%) | |
| PD-L1 | <0.01b | |||||||||
| Negative | 262 (12.7%) | 186 (10.7%) | 12 (9.6%) | 70 (9.2%) | 22 (10.0%) | 40 (12.3%) | 9 (12.5%) | 6 (12.0%) | 27 (14.3%) | |
| 1%-49% | 1022 (49.4%) | 799 (45.8%) | 51 (40.8%) | 353 (46.3%) | 108 (49.3%) | 143 (43.9%) | 38 (52.8%) | 24 (48.0%) | 82 (43.4%) | |
| ≥50% | 786 (38.0%) | 759 (43.5%) | 62 (49.6%) | 340 (44.6%) | 89 (40.6%) | 143 (43.9%) | 25 (34.7%) | 20 (40.0%) | 80 (42.3%) | |
| Metastatic site | ||||||||||
| Brain | 1739 (30.9%) | 1247 (32.6%) | 0.11b | 83 (30.5%) | 550 (32.7%) | 164 (32.2%) | 232 (32.9%) | 63 (36.4%) | 43 (32.5%) | 112 (31.3%) |
| Leptomeningeal | 39 (0.6%) | 21 (0.4%) | 0.39b | |||||||
| Liver | 867 (15.4%) | 520 (13.6%) | 0.01b | 38 (14.0%) | 216 (12.8%) | 71 (13.9%) | 96 (13.6%) | 23 (13.3%) | 21 (15.9%) | 55 (15.4%) |
| Controlateral lung | 1432 (25.4%) | 1105 (28.9%) | <0.01b | |||||||
| Bones | 2140 (38.0%) | 1592 (41.6%) | <0.01b | 118 (43.4%) | 678 (40.3%) | 216 (42.4%) | 288 (40.9%) | 75 (43.4%) | 52 (39.4%) | 165 (46.1%) |
| Pleural effusion | 457 (8.1%) | 239 (6.2%) | <0.01b | |||||||
| Adrenal glands | 1248 (22.2%) | 883 (23.1%) | 0.30b |
ECOG PS, Eastern Cooperative Oncology performance status; NSCLC, non-small-cell lung cancer; PD-L1, programmed death-ligand 1.
Kruskal-Wallis rank sum test.
Pearson’s chi-square test.
Among KRASm patients, 291 (7.1%) were KRASG12A; 1791 (43.8%) were KRAS p.G12C; 538 (13.2%) were KRASG12D; 756 (18.5%) were KRASG12V; 192 (4.7%) were KRASG13C; 136 (3.3%) were KRASG13D, and 384 (9.4%) had another KRAS mutation subtype (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2024.103473). According to KRAS mutant subtype (KRAS p.G12C versus KRAS non-p.G12C): 41.5% versus 38.7% were women (P = 0.07); 1.5% versus 5.5% were non-smokers (P < 0.01); ECOG PS and stage at NSCLC diagnosis status were similar. There was no difference in the location of metastatic sites between the two subgroups, except for the adrenal metastases, with a higher prevalence for KRAS non-p.G12C patients (24.7% versus 20.9%; P < 0.01). PD-L1 expression levels (i.e. ≥50%) were similar for KRAS p.G12C patients and KRAS non-p.G12C patients (44.6% versus 42.7%; P = 0.20).
Overall survival according to KRAS mutational status and treatment lines
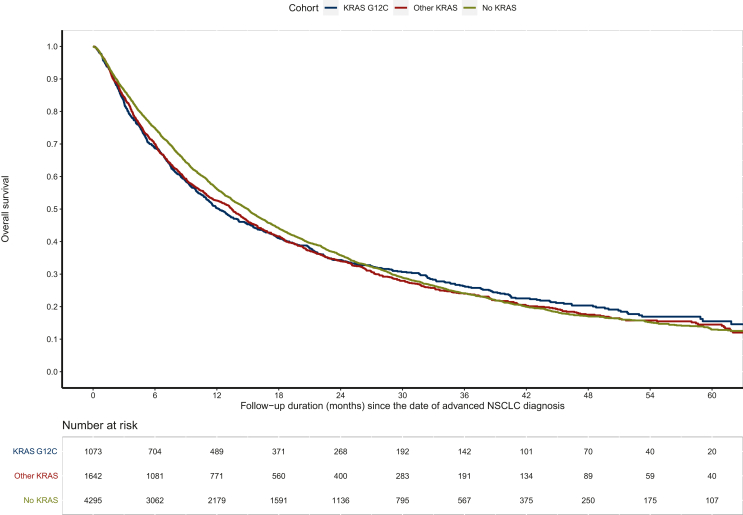
We assessed the impact of KRAS status on overall survival from advanced disease in all 7010 patients included in the ESME database, including patients who received no treatment (Figure 2). In this cohort, 306/1642 KRAS non-p.G12C patients (18.6%), 211/1073 KRAS p.G12C patients (19.7%), and 801/4295 KRASwt patients (18.6%) did not receive any treatment due to the aggressiveness of the disease. Patients with KRASm (G12C or non-G12C, n = 2715) had a non-significant lower overall survival than KRASwt patients (n = 4295): 13.0 months (95% CI 11.9-13.9 months) versus 15.0 months (95% CI 14.1-15.6 months), P = 0.070. According to KRASm, overall survival was similar for G12C mutations and non-G12C mutations: 12.2 months (95% CI 11.0 to 13.9 months) versus 13.4 months (95% CI 12.1 to 14.5 months), P = 0.677).
Figure 2.
Overall survival according to KRAS mutation in patients included in the ESME AMLC database. NSCLC, non-small-cell lung cancer.
For patients who received at least one cancer treatment, KRASm were associated with worse survival. Median overall survival measured from the start of first-line treatment (OS1) was 12.6 months (95% CI 11.6-13.6 months) for KRASm patients versus 15.4 months (95% CI 14.6-16.2 months) for KRASwt patients (P = 0.012). Median OS1 according to KRASm subtype were as follows: 12.3 months (95% CI 10.9-14.7 months) and 12.7 months (95% CI 11.6-13.9 months) for KRAS p.G12C and KRAS non-p.G12C, respectively.
Among patients who were treated with two or more lines of treatment, the median overall survival from second-line therapy (OS2) according to KRASm was as follows: 9.1 months (95% CI 7.2-10.9 months), 8.3 months (95% CI 7.4-9.4 months), and 10.3 months (95% CI 9.3-11.1 months) for KRAS p.G12C, KRAS non-p.G12C, and KRASwt subgroups, respectively. There was no significant difference of OS2 for KRASm versus KRASwt patients (P = 0.064).
Therapeutic strategies for front-line treatment and survival outcomes
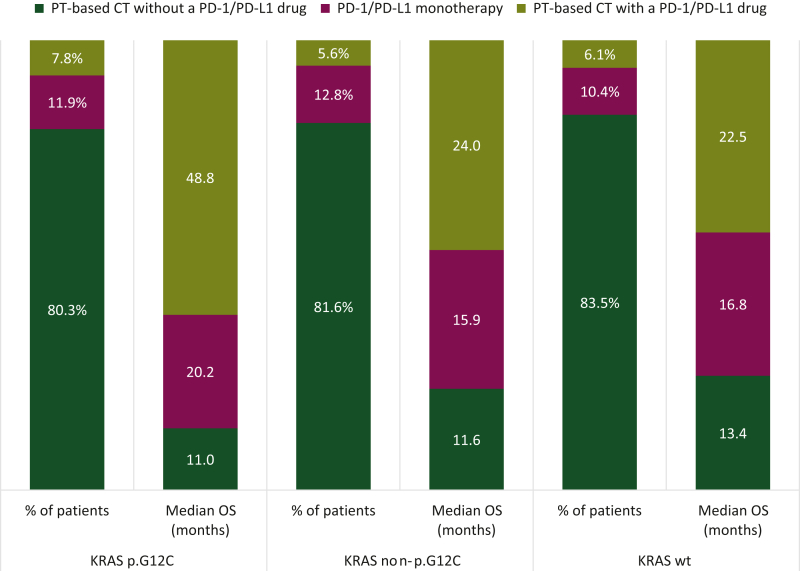
At the time of patient recruitment, the main first-line strategy used for advanced disease was, in decreasing order of frequency: platinum-based chemotherapy without a PD-1/PD-L1 inhibitor (80%-83%), immunotherapy with anti-PD-1/PD-L1 as monotherapy (10%-13%), and chemotherapy associated with anti- PD-1/PD-L1 (6%-8%). For patients who received at least one therapy, overall survival from first-line start was longer for KRASwt (n = 3494) patients than for KRASm (N = 2198) patients (P = 0.012) (Figure 3).
Figure 3.
First-line treatment strategies and overall survival according to KRAS mutation subtype. CT, chemotherapy; OS, overall survival; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; PT, platinum.
In patients treated with chemoimmunotherapy in first line (L1), median OS1 was 48.8 months (95% CI 16.4 months to not reached) for the KRAS p.G12C cohort; 24.0 months (95% CI 15.5-37.1 months) for the KRAS non-p.G12C cohort, and 22.5 months (95% CI 18.9-28.4 months) for the KRASwt cohort. Regarding other therapeutic strategies, single-agent immunotherapy offered better overall survival than platinum-based chemotherapy, whether for KRAS p.G12C [20.2 months (95% CI 14.3-32.3 months) versus 11.0 months (95% CI 9.9-12.9 months)], KRAS non-p.G12C [15.9 months (95% CI 12.2-20.2 months) versus 11.6 months (95% CI 10.0-13.0 months)], and KRASwt [16.8 months (95% CI 13.0-21.3 months) versus 13.4 months (95% CI 12.4-14.2 months)] patients. Whatever the first-line strategy adopted, real-world progression-free survival (PFS1) was shorter in patients harboring KRASm (G12C or non-G12C) than in KRASwt patients: 4.0 months (95% CI 3.7-4.3 months) versus 5.1 months (95% CI 4.8-5.3 months), P < 0.001. Among KRASm patients, PFS1 was similar for KRAS p.G12C and KRAS non-p.G12C patients: 4.3 months (95% CI 3.8-4.7 months) versus 3.8 months (95% CI 3.5-4.2 months), P = 0.268.
Second-line treatment strategies and survival outcomes
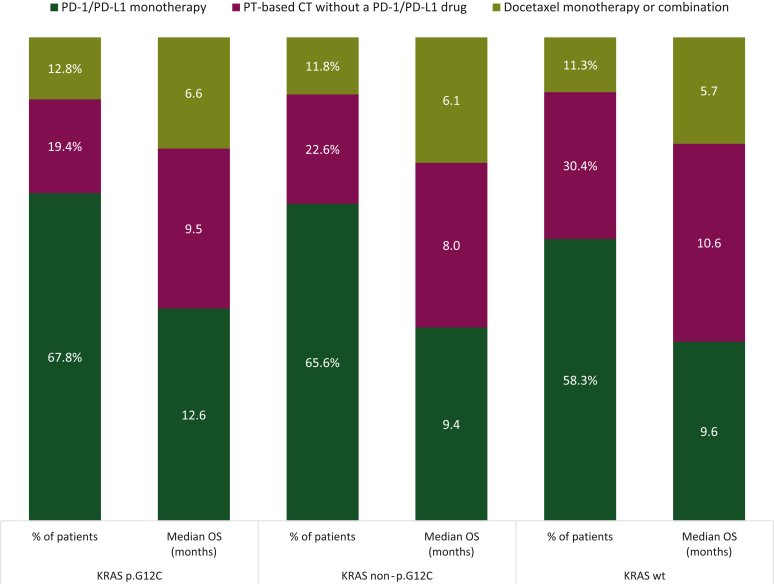
A second line of treatment was administered in 43.2%, 45.2%, and 46.7% of patients in the KRAS p.G12C, KRAS non-p.G12C, and KRASwt subgroups, respectively (Figure 4). Main treatment strategies were as follows in decreasing order of frequency: PD-1/PD-L1 antibodies as monotherapy (58%-68%), platinum-based chemotherapy without a PD-1/PD-L1 drug (19%-30%), docetaxel monotherapy, or combination (11%-13%). For patients who received at least two therapies, overall survival from second line (OS2) was longer for KRAS p.G12C patients than for KRAS non-p.G12C patients (P = 0.003).
Figure 4.
Second-line treatment strategies and overall survival according to KRAS mutation subtype. CT, chemotherapy; OS, overall survival; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; PT, platinum.
The median overall survival from second-line initiation (OS2) of patients treated with immunotherapy was superior in the KRAS p.G12C subgroup: 12.6 months (95% CI 8.1-18.6 months) compared with the other groups: OS2 was 9.4 months (95% CI 8.0-11.4 months) for KRAS non-p.G12C and 9.6 months (8.4-11.0 months) for KRASwt patients. For KRAS p.G12C patients, median OS2 was higher with immunotherapy than with platinum-based chemotherapy [9.5 months (95% CI 6.1-13.4 months)] and docetaxel monotherapy or combination [6.6 months (95% CI 3.9-10.0 months)]. Similarly, for KRAS non-p.G12C patients, OS2 seemed higher with immunotherapy than with platinum-based chemotherapy [8.0 months (95% CI 5.7-12.7 months)] and docetaxel monotherapy or combination [6.1 months (95% CI 4.3-8.0 months)]. For KRASwt patients, OS2 was higher with platinum-based chemotherapy without a PD-1/PD-L1 drug [10.6 months (95% CI 8.9-12.1 months)] than with immunotherapy [9.6 months (95% CI 8.4-11.0 months)] or docetaxel monotherapy or combination [5.7 months (95% CI 4.3-7.7 months)].
Second-line median progression-free survival (PFS2) was 2.3 months (95% CI 2.0-2.8 months), 1.9 months (95% CI 1.7-2.2 months), and 2.8 months (95% CI 2.5-3.0 months) in the KRAS p.G12C, KRASnon-G12C, and KRASwt subgroups, respectively. Overall, there were no significant PFS differences between KRASm and KRASwt patients (P = 0.163). According to KRASm subtypes, PFS2 was significantly higher for KRAS p.G12C than for KRAS non-p.G12C (P = 0.004). PFS2 was significantly higher in KRASwt patients than in KRASnon-G12Cpatients (P = 0.004).
Metastatic localization evolution across treatment lines
We focused on the three most frequent metastatic sites from primary NSCLC outside of the lung: brain, bone, and liver. Across treatment lines, the percentage of patients with brain, liver or bone metastasis increased regardless of KRAS mutation status, i.e. with disease natural history (Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2024.103473). KRASm patients (G12C or non-G12C) had slightly more brain and bone metastases than did KRASwt patients.
The prevalence of brain metastases was approximatively one-third before first-line treatment: 31.2%, 30.7%, and 28.7% for patients in the KRAS p.G12C, KRAS non-p.G12C, and KRASwt subgroups, respectively. Brain metastases were symptomatic in 45.4%, 45.7%, and 44.6% of the patients. Despite the presence of brain metastases, overall survival from L1 was not modified across subgroups. OS1 was 12.9 months (with brain metastases) and 11.4 months (without brain metastases) for KRAS p.G12C patients. OS1 was 13.8 and 14.2 months for KRAS non-p.G12C patients. OS1 was 15.9 and 16.9 months for KRASwt patients. Similar results were observed for PFS1 according to the presence of brain metastases: PFS1 was 4.2 and 3.7 months in KRAS p.G12C patients with and without brain metastases; 3.1 and 4.0 months in KRAS non-p.G12C patients; and 4.6 and 5.1 months in KRASwt patients.
Discussion
To our knowledge, this observational cohort is one of the largest published focusing on the impact of KRAS mutational status of patients with advanced NSCLC.17, 18, 19, 20 It offers a unique and detailed perspective on this population of interest as the targeting of KRAS mutations finally enters clinical routine with the market authorization of sotorasib and adagrasib.
This database is the result of a strong national initiative leading to the production of exhaustive real-life data concerning cancer patients treated in the main French cancer centers and therefore getting closer to real life. Among the 22 137 patients diagnosed with advanced NSCLC and consecutively included in this database during a 10-year period, KRAS status was evaluable for 10 177 patients. Of these, 1791 patients (17.6%) had a KRAS p.G12C mutation, 2297 (22.6%) had a KRAS non-p.G12C mutation, and 6089 (59.8%) had no KRASm. The G12C subtype corresponds to 43.8% of all the KRASm observed. These data are similar to those previously reported.21 Other druggable alterations were excluded from our cohort.
We decided to describe our cohort of patients according to their KRAS mutational status in three subgroups (KRAS p.G12C versus KRAS non-p.G12C versus KRASwt) to evaluate its predictive and prognostic value on patient and disease characteristics and treatment outcomes. Gender and age were similar in the three subgroups. The prevalence of smoking (former or current smoker) was major in patients with KRASm (96.3%), notably G12C (98.5% and 94.5% for KRAS p.G12C and KRAS non-p.G12C patients, respectively) but it remains very frequent in KRASwt patients (85.8%).
The use and analysis of real-life data can naturally be criticized. The prospective nature of the recruitment of patients in our cohort, however, is a strong point. The study of PFS and overall survival of patients in this cohort reinforces the use of real-life data as a research basis or even as a comparative arm, as our data are similar to those published in randomized trials.22, 23, 24, 25 At the beginning of this project, the combination of chemotherapy plus immunotherapy for first-line treatment in advanced NSCLC was not yet a therapeutic standard. In the KRASwt cohort, overall survival was similar to those reported in the KEYNOTE 189 study.26 Regardless of KRAS status, immunotherapy used as monotherapy in first line provided a benefit in overall survival compared with chemotherapy as in the KEYNOTE 024 (pembrolizumab) and Impower 110 (atezolizumab) studies,27,28 and unlike CheckMate 026 (nivolumab).29
Of greater importance, we show that KRASm NSCLC is associated with high expression of PD-L1 (P < 0.01). This observation may explain the excellent overall survival results observed in KRASm patients in our cohort treated by immunotherapy either alone or in combination with chemotherapy in a first-line setting. These results must be interpreted with caution, however, given the small numbers of patients receiving immunotherapy in first-line metastatic treatment line in our cohort due to the inclusion period of our study. As a second line of treatment, most patients received immunotherapy as monotherapy (58%-68%), platinum-based chemotherapy (19%-30%) or docetaxel used alone or in combination (11%-13%). We noticed a significantly higher overall survival in KRAS p.G12C patients (12.6 months) treated with immunotherapy compared with KRAS non-p.G12C (9.4 months) and KRASwt (9.6 months) patients. The benefit of immunotherapy compared with docetaxel independently of PD-L1 expression in terms of overall survival is consistent with that already shown in the CheckMate 057 and OAK studies.25,30
In the next years, the impact on survival outcomes of the use of KRAS p.G12C specific inhibitors, such as sotorasib or adagrasib, will also need to be assessed in large real-life cohorts.31
Ultimately, our data provide some rationale for the subsequent development of KRAS inhibitors in metastatic KRASm NSCLC, in the setting of immunotherapy-based therapies; this may include combination with immunotherapy alone, in PD-L1 ≥50%, or with chemoimmunotherapy whatever the PD-L1 status is, the latter strategy being more intensive in line with the aggressiveness of the disease. These combinations may be limited, however, due to the toxicity of the combination of KRAS inhibitors and immune checkpoint inhibitors, particularly liver toxicity for sotorasib. This may also be used in early-stage NSCLC as a neoadjuvant approach. Meanwhile, sequencing strategies may be assessed, with KRAS inhibitors used as maintenance, or even consolidation or adjuvant setting in non-metastatic NSCLC.
Conclusion
This study confirms the major impact of the presence of the KRAS mutation in NSCLC for both prognostic and predictive purposes. Notably, we highlighted the over-representation of former or current smoker patients in this population, which presented more aggressive forms of NSCLC compared with KRASwt patients (more stage IV at diagnosis, shorter PFS1 and OS1, regardless of the type of treatment). In addition, patients with KRAS p.G12C-mutated NSCLC have an increased sensitivity to immunotherapy in comparison with KRAS non-p.G12C mutations and KRASwt patients.
Acknowledgements
We deeply thank all patients who contributed to the ESME LC database for granting access to their data and allowing this work. We thank the participating sites and each ESME local coordinator for managing the project at the local level (alphabetical order): Institut de Cancérologie de l’Ouest, Angers/Nantes; CHU, Angers; CH Annecy-Genevois, Annecy; Institut Ste Catherine, Avignon; CH Côte Basque, Bayonne; Institut Bergonié, Bordeaux; Centre François Baclesse, Caen; CH Métropole Savoie, Chambéry; Centre Jean Perrin, Clermont-Ferrand; Hôpital Intercommunal de Créteil; Centre Georges-François Leclerc, Dijon; CH Départemental de Vendée, La Roche-sur-Yon; Centre Oscar Lambret, Lille; CHU Dupuytren, Limoges; Centre Léon Bérard, Lyon; CH Est francilien, Marne la Vallée; Hôpital Européen, Marseille; Institut Paoli-Calmettes, Marseille; CH Est francilien, Meaux; Institut de Cancérologie de Montpellier; GHRMSA/Hôpital Emile Muller, Mulhouse; Institut de Cancérologie de Lorraine, Nancy; Centre Antoine Lacassagne, Nice; Institut Jean Godinot, Reims; CHU Pontchaillou, Rennes; Centre Eugène Marquis, Rennes; Centre Henri Becquerel, Rouen; CH, Saint-Quentin; Centre Paul Strauss ICANS, Strasbourg; CH Sainte-Musse, Toulon; Institut Claudius Regaud IUCT-O, Toulouse; CHRU, Tours; CHU, Rouen; Hôpital Intercommunal de Toulon, Toulon La Seyne sur Mer; Hôpital d’instruction des Armées Ste Anne, Toulon; CH Nord-Ouest, Villefranche-sur-Saône; Institut Curie, Paris/Saint-Cloud, Gustave Roussy, Villejuif. We also thank the ESME central coordinating staff, the LC Scientific Group and the ESME Strategic Committee for their ongoing support.
Funding
This work was supported by Unicancer. Unicancer manages independently ESME databases (i.e. data collection, analysis, and publication) and is the sole data controller for data processing. The ESME LC database receives financial support from industrial partners.
Disclosure
QDT received honoraria from Amgen, AstraZeneca, and Sanofi and meeting/travel support from Amgen and Sanofi. CC received grants or contracts, consulting fees, personal/institutional honoraria, and meeting/travel support from AstraZeneca, Amgen, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, GlaxoSmithKline, Lilly, Merck Sharp & Dohme (MSD), Novartis, Pfizer, Roche, Sanofi, and Takeda. TF received personal/institutional honoraria from Janssen, Lilly, and Roche. JRM received grants or contracts from MSD, received payment or honoraria for lectures, presentations, speaker’s bureaus, manuscript writing or educational events from AstraZeneca, Edimark, Janssen, MSD, Sanofi, Roche, and Takeda and meeting/travel support from Ose-Immunotherapeutics. MP received consulting fees from AstraZeneca, Bristol-Myers Squibb, Daiichi Sankyo, Eli Lilly, Eisai, GlaxoSmithKline, Ipsen, Janssen, MSD, Novocure, Pfizer, Roche, and Takeda; payment or honoraria for lectures, presentations, speaker bureau, manuscript writing or educational events from Anheart Therapeutics, AstraZeneca, Bristol-Myers Squibb, Janssen, MSD, Pfizer, Sanofi, and Takeda; payment for expert testimony from AstraZeneca, Bristol-Myers Squibb, Janssen, and Roche; support for attending meetings and/or travel from AstraZeneca, Bristol-Myers Squibb, MSD, Pfizer, Roche, and Takeda; and participated on a data safety monitoring board or advisory board for Pharmamar and Roche. CAV received consulting fees or meeting/travel support from Amgen, AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, Lilly, MSD, Novartis, Pfizer, Roche, Sanofi Aventis, and Takeda; and participated in an advisory board for AbbVie, AstraZeneca, Bristol-Myers Squibb, Janssen, Lilly, MSD, Pfizer, Roche, and Sanofi. GJ received meeting/travel support from Sanofi. SH received personal/institutional honoraria from AstraZeneca, Bristol-Myers Squibb, Roche, Sanofi, and Takeda; and meeting/travel support from Novartis and Sanofi. AS received consulting fees from Exafield and Guidepoint; honoraria from Amgen, AstraZeneca, and MSD; meeting/travel support from Amgen and Roche; and is a board member of the SFFPO. EP received personal/institutional honoraria from Amgen, AstraZeneca, and MSD; meeting/travel support from Takeda and Amgen; and participated in an advisory board for Takeda. NG declared research grants/support from AbbVie, Amgen, AstraZeneca, BeiGene, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo, Gilead, Hoffmann-La Roche, Janssen, LEO Pharma, Lilly, Merk Serono, MSD, Novartis, Sanofi, Sivan; consultative services for AbbVie, Amgen, AstraZeneca, BeiGene, Bristol-Myers Squibb, Daiichi-Sankyo, Gilead, Ipsen Hoffmann-La Roche, Janssen, LEO Pharma, Lilly, MSD, Mirati, Novartis, Pfizer, Pierre Fabre, Sanofi, Takeda; participation on a data safety monitoring board for Hoffmann-La Roche; and employment of a family member with AstraZeneca. PDR received meeting/travel support from Boehringer Ingelheim, Daiichi Sankyo, Summit Therapeutics, Sanofi, and Takeda; and participated in an advisory board for Sanofi and Takeda. All other authors have declared no conflicts of interest.
Data Sharing
The datasets analyzed for the current study were extracted from ESME LC Data Platform. In accordance with ethical and legal requirements related to the ESME data warehouse, individual data from the ESME databases cannot be made available. For any specific demand, please contact the corresponding author. Each demand will be examined on a case-by-case basis by the scientific committee.
Supplementary data
References
- 1.Yang S.R., Schultheis A.M., Yu H., Mandelker D., Ladanyi M., Büttner R. Precision medicine in non-small cell lung cancer: current applications and future directions. Semin Cancer Biol. 2022;84:184–198. doi: 10.1016/j.semcancer.2020.07.009. [DOI] [PubMed] [Google Scholar]
- 2.Yates L.R., Seoane J., Le Tourneau C., et al. The European Society for Medical Oncology (ESMO) precision medicine glossary. Ann Oncol. 2018;29(1):30–35. doi: 10.1093/annonc/mdx707. [DOI] [PubMed] [Google Scholar]
- 3.Skoulidis F., Heymach J.V. Co-occurring genomic alterations in non-small-cell lung cancer biology and therapy. Nat Rev Cancer. 2019;19(9):495–509. doi: 10.1038/s41568-019-0179-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simanshu D.K., Nissley D.V., McCormick F. RAS proteins and their regulators in human disease. Cell. 2017;170(1):17–33. doi: 10.1016/j.cell.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drosten M., Barbacid M. Targeting the MAPK pathway in KRAS-driven tumors. Cancer Cell. 2020;37(4):543–550. doi: 10.1016/j.ccell.2020.03.013. [DOI] [PubMed] [Google Scholar]
- 6.Finn S.P., Addeo A., Dafni U., et al. Prognostic impact of KRAS G12C mutation in patients with NSCLC: results from the European Thoracic Oncology Platform Lungscape Project. J Thorac Oncol. 2021;16(6):990–1002. doi: 10.1016/j.jtho.2021.02.016. [DOI] [PubMed] [Google Scholar]
- 7.Nadal E., Chen G., Prensner J.R., et al. KRAS-G12C mutation is associated with poor outcome in surgically resected lung adenocarcinoma. J Thorac Oncol. 2014;9(10):1513–1522. doi: 10.1097/JTO.0000000000000305. [DOI] [PubMed] [Google Scholar]
- 8.Marabese M., Ganzinelli M., Garassino M.C., et al. KRAS mutations affect prognosis of non-small-cell lung cancer patients treated with first-line platinum containing chemotherapy. Oncotarget. 2015;6(32):34014–34022. doi: 10.18632/oncotarget.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rulli E., Marabese M., Torri V., et al. Value of KRAS as prognostic or predictive marker in NSCLC: results from the TAILOR trial. Ann Oncol. 2015;26(10):2079–2084. doi: 10.1093/annonc/mdv318. [DOI] [PubMed] [Google Scholar]
- 10.Liu C., Zheng S., Jin R., et al. The superior efficacy of anti-PD-1/PD-L1 immunotherapy in KRAS-mutant non-small cell lung cancer that correlates with an inflammatory phenotype and increased immunogenicity. Cancer Lett. 2020;470:95–105. doi: 10.1016/j.canlet.2019.10.027. [DOI] [PubMed] [Google Scholar]
- 11.Gainor J.F., Varghese A.M., Ou S.H.I., et al. ALK rearrangements are mutually exclusive with mutations in EGFR or KRAS : an analysis of 1,683 patients with non–small cell lung cancer. Clin Cancer Res. 2013;19(15):4273–4281. doi: 10.1158/1078-0432.CCR-13-0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karachaliou N., Mayo C., Costa C., Magrí I., et al. KRAS mutations in lung cancer. Clin Lung Cancer. 2013;14(3):205–214. doi: 10.1016/j.cllc.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Huang L., Guo Z., Wang F., Fu L. KRAS mutation: from undruggable to druggable in cancer. Signal Transduct Target Ther. 2021;6(1):386. doi: 10.1038/s41392-021-00780-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blair H.A. Sotorasib: first approval. Drugs. 2021;81(13):1573–1579. doi: 10.1007/s40265-021-01574-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong D.S., Fakih M.G., Strickler J.H., et al. KRAS G12C inhibition with sotorasib in advanced solid tumors. N Engl J Med. 2020;383(13):1207–1217. doi: 10.1056/NEJMoa1917239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ou S.H.I., Jänne P.A., Leal T.A., et al. First-in-Human phase I/IB dose-finding study of adagrasib (MRTX849) in patients with advanced KRASG12C solid tumors (KRYSTAL-1) J Clin Oncol. 2022;40:2530–2538. doi: 10.1200/JCO.21.02752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruppert A.M., Beau-Faller M., Debieuvre D., et al. Outcomes of patients with advanced NSCLC from the Intergroupe Francophone de CancErologie Thoracique Biomarkers France Study by KRAS mutation subtypes. JTO Clin Res Rep. 2020;1(3) doi: 10.1016/j.jtocrr.2020.100052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sebastian M., Eberhardt W.E.E., Hoffknecht P., et al. KRAS G12C-mutated advanced non-small cell lung cancer: a real-world cohort from the German prospective, observational, nation-wide CRISP Registry (AIO-TRK-0315) Lung Cancer. 2021;154:51–61. doi: 10.1016/j.lungcan.2021.02.005. [DOI] [PubMed] [Google Scholar]
- 19.Arbour K.C., Rizvi H., Plodkowski A.J., et al. Treatment outcomes and clinical characteristics of patients with KRAS-G12C–mutant non–small cell lung cancer. Clin Cancer Res. 2021;27(8):2209–2215. doi: 10.1158/1078-0432.CCR-20-4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spira A.I., Tu H., Aggarwal S., et al. A retrospective observational study of the natural history of advanced non–small-cell lung cancer in patients with KRAS p.G12C mutated or wild-type disease. Lung Cancer. 2021;159:1–9. doi: 10.1016/j.lungcan.2021.05.026. [DOI] [PubMed] [Google Scholar]
- 21.Garcia B.N.C., van Kempen L.C., Kuijpers C.C.H.J., Schuuring E., Willems S.M., van der Wekken A.J. Prevalence of KRAS p.(G12C) in stage IV NSCLC patients in the Netherlands; a nation-wide retrospective cohort study. Lung Cancer. 2022;167:1–7. doi: 10.1016/j.lungcan.2022.03.015. [DOI] [PubMed] [Google Scholar]
- 22.Mok T.S.K., Wu Y.L., Kudaba I., et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819–1830. doi: 10.1016/S0140-6736(18)32409-7. [DOI] [PubMed] [Google Scholar]
- 23.Gandhi L., Rodríguez-Abreu D., Gadgeel S., et al. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N Engl J Med. 2018;378(22):2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 24.Brahmer J., Reckamp K.L., Baas P., et al. Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rittmeyer A., Barlesi F., Waterkamp D., et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gadgeel S., Rodríguez-Abreu D., Speranza G., et al. Updated analysis from KEYNOTE-189: pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non-small-cell lung cancer. J Clin Oncol. 2020;38(14):1505–1517. doi: 10.1200/JCO.19.03136. [DOI] [PubMed] [Google Scholar]
- 27.Reck M., Rodríguez-Abreu D., Robinson A.G., et al. Updated analysis of KEYNOTE-024: pembrolizumab versus platinum-based chemotherapy for advanced non–small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol. 2019;37(7):537–546. doi: 10.1200/JCO.18.00149. [DOI] [PubMed] [Google Scholar]
- 28.Herbst R.S., Giaccone G., de Marinis F., et al. Atezolizumab for first-line treatment of PD-L1-selected patients with NSCLC. N Engl J Med. 2020;383(14):1328–1339. doi: 10.1056/NEJMoa1917346. [DOI] [PubMed] [Google Scholar]
- 29.Carbone D.P., Reck M., Paz-Ares L., et al. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med. 2017;376(25):2415–2426. doi: 10.1056/NEJMoa1613493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borghaei H., Paz-Ares L., Horn L., et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wislez M., Mascaux C., Cadranel J., et al. 1400P Real-world effectiveness and safety of sotorasib in patients with KRAS G12C mutated metastatic non-small cell lung cancer (NSCLC): results of the IFCT-2102 lung KG12Ci study. Ann Oncol. 2023;34:S801–S802. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.