Abstract
Modern medicine has been shaken by the surge of psychedelic science that proposes a new approach to mitigate mental disorders, such as depression and post-traumatic stress disorder. Clinical trials to investigate whether psychedelic substances can treat psychiatric conditions are now underway, yet less discussion gravitates around their use in neurological disorders due to brain injury. One suggested implementation of brain-complexity enhancing psychedelics is to treat people with post-comatose disorders of consciousness (DoC). In this article, we discuss the rationale of this endeavour, examining possible outcomes of such experiments by postulating the existence of an optimal level of complexity. We consider the possible counterintuitive effects of both psychedelics and DoC on the functional connectivity of the default mode network and its possible impact on selfhood. We also elaborate on the role of computational modelling in providing complementary information to experimental studies, both contributing to our understanding of the treatment mechanisms and providing a path towards personalized medicine. Finally, we update the discourse surrounding the ethical considerations, encompassing clinical and scientific values.
Keywords: brain complexity, whole-brain model (WBM), disorders of consciousness, treatment, psychedelics, criticality
Introduction
Psychedelic drugs include several substances which are commonly divided into two categories: typical and atypical psychedelics. Typical psychedelics act upon the 5-HT2A receptor, such as psilocybin, lysergic acid diethylamide (LSD), and N, N-Dimethyltryptamine (DMT), and atypical psychedelics act primarily through other neurotransmitter systems, such as the glutamatergic N-methyl-D-aspartate (NMDA) receptor antagonist ketamine (Vollenweider and Preller 2020). In the twentieth century, scientific research into psychedelic drugs flourished through several lines of investigation, including their potential to be employed as an adjunct psychotherapeutic tool in treating psychiatric disorders (Rhead et al. 1977) where this research informed clinical practice (Pahnke 1963, Grof et al. 1973, Savage and Mccabe 1973). This scientific prosperity was halted after the prohibition of psychedelics in the USA in 1970 which quickly spread worldwide, as these substances were labelled as having no medical use or benefit. This has been suggested by some to be the largest censorship in the history of science (Nutt et al. 2020). Nevertheless, in the last decade, a resurgence of scientific interest in these substances is re-establishing these ‘non-specific amplifiers’ of experience (Grof, 1979) as holding great potential in psychiatry, (Carhart-Harris and Goodwin 2017, Nutt 2019, Vollenweider and Preller 2020) where several clinical trials utilising psychedelic drugs to treat a number of affective psychiatric disorders have yielded promising results (Gasser et al. 2014, Griffiths et al. 2016, Danforth et al. 2018, Carhart-Harris et al. 2021, Mitchell et al. 2021, 2023, Spriggs et al. 2021, Bogenschutz et al. 2022, Daws et al. 2022). In these applications, the experience-distorting properties of psychedelic drugs are thought to be critical to their therapeutic efficacy (Yaden and Griffiths 2020). However, an opinion paper in 2019 by Scott and Carhart-Harris (Scott and Carhart-Harris 2019) proposed a different scientific rationale by suggesting to use psychedelic drugs to treat post-comatose disorders of consciousness (DoC). People with DoC represent a group of severely brain-injured patients with varying capacities of awareness, as well as a pathologically low brain complexity. DoC is an umbrella term that encompasses different clinical entities: the unresponsive wakefulness syndrome (UWS) is characterised by the presence of wakefulness and reflex actions yet an absence of awareness (Laureys et al. 2010); the minimally conscious state (MCS) is characterised by lack of functional communication but an increased level of awareness through non-reflex behaviours (e.g. visual pursuit, respond to command) (Giacino et al. 2002). Additional recent investigations have demonstrated that some UWS patients (called MCS* or patients with cognitive-motor dissociation, CMD) show covert consciousness with neuroimaging even if no overtly conscious behaviour is displayed at the bedside (Owen et al. 2006, Monti et al. 2010, Schiff 2015, Thibaut et al. 2021). When a patient regains communication, they no longer occupy a DoC and are considered as emerged from MCS (EMCS). For recent reviews on DoC, consult Zasler et al. (2019), Alnagger et al. (2023), and Sanz et al. (2023). Rather than leveraging the impact of psychedelic drugs on experience, as in their psychotherapeutic utilisation, the approach outlined by Scott and Carhart-Harris (Scott and Carhart-Harris 2019) focuses on the direct mechanisms of psychedelic drugs to increase the complexity of networks in the brains of people with DoC. Here we update the scientific discourse on this original rationale and set our vision for its potential implementation. We also discuss its impact on the science of consciousness and comment on possible ethical concerns.
Brain complexity and consciousness
In the field of the neuroscience of consciousness, there is a consensus over the link between brain complexity and consciousness (Sarasso et al. 2021). There are prominent theories that directly draw a relationship between the two, such as the Integrated Information Theory (IIT) (Tononi 2008, Oizumi et al. 2014) or the entropic brain hypothesis (Carhart-Harris et al. 2014, Carhart-Harris 2018). Brain complexity refers to the irreducibility of brain activity that emerges from the combination of integration and differentiation. Operationally, it can be measured in various ways. One of the simplest is via Lempel-Ziv Complexity (LZC), which gauges the repetitiveness of brain activity after binarisation. LZC decreases in anaesthesia (Schartner et al. 2015) and DoC (Altlntop et al. 2022, Liu et al. 2023), and changes over sleep stages (Andrillon et al. 2016, Aamodt et al. 2023). Another related measure is the perturbational complexity index (PCI), which computes the complexity of the response of the brain to either an external perturbation such as a transcranial magnetic stimulation (TMS) pulse (Casali et al. 2013) or an intracortical stimulation (Comolatti et al. 2019), and that decreases during general anaesthesia, sleep, and DoC (Casali et al. 2013, Casarotto et al. 2015, Rosanova et al. 2018, Comolatti et al. 2019). New promising measures, such as the explainable consciousness indicator (ECI), provide more than a single number (i.e. one for wakefulness and one for awareness) (Lee et al. 2022). While most of these measures (e.g. PCI) are inspired by specific theoretical frameworks (e.g. IIT), they are not direct measures of any theoretical construct within the theories themselves. For the specific case of PCI, we invite the interested reader to consult Mediano et al. (2022) and Leung and Tsuchiya (2023) for a larger discussion. Finally, (intrinsic) information (Barbosa et al. 2020), used in the latest version of IIT (Albantakis et al. 2023), is not yet measured by LZC or PCI. For a larger discussion about complexity measures and how to interpret them, see Box 1.
Box 1: Complexity and the need for a conscious-ometer.
One of the objectives of the neuroscience of consciousness is to scientifically study and measure consciousness, to respond to questions like: which states in humans are conscious and which are not? Are infants conscious, and from what age? Are other systems (biological or not) conscious?
As such, there has been a huge intellectual investment in creating a hypothetical ‘conscious-ometer’ (Hunt et al. 2022), an instrument that is able to index consciousness in a similar fashion a thermometer tracks temperature. Among other approaches, measures of complexity have been largely explored. While there is an overall accordance in measurements of complexity, they differ one with the other regarding how they are quantified, and what we can infer from them. Here, we will focus on complexity measures extracted from magnetoencephalography (MEG) or electroencephalography (EEG), either alone or coupled with a stimulation. For the sake of succinctness, we will not discuss other indexes that can be derived from functional magnetic resonance imaging (fMRI) (Luppi et al. 2023).
One of the first indexes to be utilized was LZC, which quantifies the repetitiveness in a string series. LZC is a value that ranges from 0 to 1 after normalization, where 0 stands for complete repetitiveness and 1 to a random signal (Schartner et al. 2015). LZC is still commonly employed today and current interpretations of LZC focus on quantifying the various states the brain can occupy, suggesting that higher LZC equates to a higher number of (phenomenological) states that one can experience. In theory, LZC can be considered continuous, and direct comparisons (assuming the same set-up) have a clear interpretation: if A has higher LZC than B, then A could have a richer phenomenology. One strong limitation of LZC is that it can be maximal with high levels of noise (i.e. white noise), as it measures directly differentiation rather than integration (Sarasso et al. 2021). Note that new versions of LZC, such as the permutational LZC, try to overcome this limitation by avoiding binarization but investigating motifs that are features of the signal that repeat over time [for an updated version of this work, see Bai et al. (2015) and Liu et al. (2023)].
PCI is a measurement that is only possible when there is an active perturbation of ongoing neural activity (measured with EEG), either by TMS or by intracortical stimulation. The original version of PCI is called PCILZ (PCI Lempel-Ziv) (Casali et al. 2013) and it is characterised by the application of LZC on the matrix of significant sources of the TMS-evoked potential. The advantage compared to LZC is that it is calculated on evoked data that has been baselined, decreasing the effect of noise, and directly appealing to the causal power of the stimulation. No investigation has so far explored whether PCI is continuous, specifically investigating whether it could have any value from 0 to 1, or if it has a specific distribution, i.e. some values are more likely to happen compared to others. The creation of the threshold (i.e. 0.31) leads to a strong dichotomic interpretation of PCI: is the person conscious or not? This interpretation has a huge clinical value, guiding management of patients with DoC. While it was created to differentiate global states (conscious vs unconscious), it has also been used to make comparisons between different local states (see the work by Darmani and colleagues) (Darmani et al. 2021). A new recent version of PCI, PCIst (PCI State Transition) (Comolatti et al. 2019) does not rely on the source reconstruction, but acts directly on the electrode level. Despite the fact that PCIst has not been as extensively tested as PCILZ, it holds promise due to the generalizability to intracortical recordings. Interpretation of PCIst is still to be explored. However, in theory, a direct comparison between different values should be possible, as it is characterized by non-zero values with no upper limit.
Whether consciousness is unidimensional or not is a topic that deserves a discussion in itself. Both LZC and PCI provide a single number, which might be problematic according to some since consciousness is unlikely to be unidimensional (Walter 2021). Novel measurements such as the ECI (Lee et al. 2022) attempt to resolve this problem. ECI utilises a machine learning classifier to give one dichotomic value (high or low) on two dimensions (awareness and arousal) that are commonly used in the clinical setting. ECI measures the probability (from 0 to 1) of having awareness and arousal, using 0.5 as threshold. Interpretation of single values over the dimensions is currently difficult, but it at least assumes a more comprehensive framing of consciousness. This might lead to the evolution of novel indexes that englobe other relevant dimensions such as ‘connectedness’ (Martial et al. 2020).
In healthy subjects, brain complexity and consciousness strongly covary: when a person is conscious they report having a subjective experience and their brain complexity is high; conversely, when someone has no experience, brain complexity is low (Casali et al. 2013). The observation is robust for both spontaneous reversible states such as dreamless sleep (Massimini et al. 2005, Schartner et al. 2017) and induced reversible unconsciousness states like dreamless general anaesthesia with no report under propofol, xenon, and midazolam (Sarasso et al. 2015). Analogous to this, brain complexity is low in unconscious DoC patients (i.e. UWS) (Casali et al. 2013, Casarotto et al. 2015). Since DoC patients are unable to report (by whatever means), there is little to no information about the phenomenology of these patients (Fingelkurts and Fingelkurts 2023). When observing the difference in complexity between UWS and MCS, the latter has higher complexity compared to the former reflecting the ‘minimal’ conserved level of consciousness (Casarotto et al. 2015). For PCI, this facilitated the creation of a theoretical threshold to differentiate the two, effectively separating conscious and unconscious states (Casarotto et al. 2015). Currently, no other thresholds are available for other measures such as LZC, possibly due to an absence of standardised methodology in preprocessing the data. Remarkably, during the psychedelic state, both LZC and analogous fMRI-measurements are higher than in wakefulness (Schartner et al. 2017, Viol et al. 2017). Consequently, if consciousness and complexity are inherently connected, as it is currently thought, in principle, administering a psychedelic to patients with DoC could ameliorate their consciousness via increasing complexity. This might manifest through new behaviours, (delayed) reports of richer mental imagery, or merely through episodes of disconnected consciousness (a state of consciousness that has no link with the environment, such the one experienced in dreaming). We should note that these two latter cases might not be detected, at least during the moment when they are experienced, but could be reported a posteriori. If psychedelics were an effective treatment for DoC, it might completely change the management of this fragile population for whom limited therapeutic options are currently available (Thibaut et al. 2019).
Psychedelics impart transient distortions of perceptions and at high doses in optimal settings can result in experiences often termed ‘mystical experiences’ characterised by sense of unity and interconnectedness, which can result in profound psychological changes (Hirschfeld and Schmidt 2021). Until now, the utility of psychedelics to treat psychiatric disorders has mainly hinged upon this impact on subjective experience, as the antidepressive potency of the treatment that has been shown to be linked to the self-reported importance of the mystical experience (Griffiths et al. 2016, Ross et al. 2016). Notwithstanding, there is current discussion whether the positive clinical changes after exposure to psychedelic drugs can be solely attributed to the subjective experience (Yaden and Griffiths 2020) or to its underlying neurophysiological effects (Olson 2021). Additionally, there are several nuanced positions within each perspective, i.e. a change of the model of the Self (Letheby 2021), or a modification of metaphysical beliefs (Timmermann et al. 2021). While we consider this debate to be useful for the current use of psychedelics, we deem it secondary in the novel interests of psychedelics for brain-injured patients (Gosseries and Martial 2020, Khan et al. 2021). Here, we are presenting a novel use case for psychedelics in that we are utilising the mechanistic capabilities of the drugs to directly increase brain complexity.
Complexity: is there an optimal value?
If we assume that psychedelics increase complexity per se, then we would expect an amelioration of consciousness in DoC, ‘regardless’ of the diagnosis. If anything, unresponsive patients, who have the lowest complexity and no signs of consciousness, have the highest probability to display changes in conscious behaviours or mental state. This is not to say that MCS patients could not show significant improvements, but only that functional or enhanced behaviours might be cognitively more demanding than showing any conscious sign whatsoever. Such outcomes would reconcile the anthology of findings, solidifying the fundamental relationship between brain complexity and consciousness. Additionally, it would provide insights into the minimal biological requirement to have any psychedelic effect.
One possibility to consider is that an increase in complexity might not always be functional. During the psychedelic state, several cognitive functions are impaired, including working memory and attentional resources (Quednow et al. 2012, Bayne and Carter 2018, Healy 2021). Therefore, whilst brain complexity seems to increase under the effect of psychedelics (Schartner et al. 2017, Viol et al. 2017), we could hardly consider this to be a functional state one could permanently inhabit. We here speculate that there might be an optimal point for complexity, around which consciousness is high and functional; going past this point of optimality will impair cognition. The proposition of an optimal point for emergence (and loss of) consciousness has been already explored by theories concerning entropy (Carhart-Harris et al. 2014, Carhart-Harris 2018) and brain criticality (Tagliazucchi and Chialvo 2013, Kim and Lee 2019), but it has not focused specifically on an optimal point of complexity for consciousness and cognition. Along these lines, we firstly refer to the literature exploring criticality.
Criticality is a collective, naturally observed behaviour, thought to be one of the main ways that nature produces complexity (Chialvo 2010, Tagliazucchi and Chialvo 2013, O’Byrne and Jerbi 2022). The regime around which the critical point is situated denotes the transition zone between ordered and disordered states and, when disrupted, neurological conditions can emerge [e.g. epilepsy (Du et al. 2019, Amin Moosavi and Truccolo 2023, Burrows et al. 2023)]. There is substantial and growing evidence that during ordinary conscious states the activity of the brain occupies critical, or marginally sub-critical dynamics, just below a local maxima of criticality, at every macroscale, as shown by in vivo (Dahmen et al. 2019, Ma et al. 2019), EEG (Linkenkaer-Hansen et al. 2001, and fMRI, Kitzbichler et al. 2009) recordings. At states of diminished or loss of consciousness including DoC and anaesthesia, the dynamical working point of the brain has been shown to be further from criticality, occupying more stable states. Whereas, during non-ordinary states of consciousness such as the psychedelic state, the brain dynamics reside in a hyper-entropic, super-critical zone (for an updated review of empirical studies about criticality of altered states of consciousness, consult Gervais et al. 2023). Note however that recent investigations have argued that some DoC might inhabit a ‘super’-critical state, suggesting that drugs pushing towards sub-criticality should be more efficacious in those cases (Maschke et al. 2022, Gervais et al. 2023). Criticality has been associated with maximal environmental sensitivity and internal repertoire of available states (O’Byrne and Jerbi 2022). This enables flexible thinking, yet is thought to result in inefficient cognition. Evidence from focused attention tasks and focused meditation show a slight shift towards sub-criticality which is thought to narrow the set of possible responses, decreasing the generalised environmental sensitivity, whilst increasing the response reliability (Fagerholm et al. 2015, Gollo 2017, Ponce-Alvarez et al. 2018, Irrmischer et al. 2018a, 2018b). Interestingly, the super-critical psychedelic state where LZC seems to be biologically maximised is associated with the peak subjective experience, ego dissolution, and feelings of connectedness with the environment (Carhart-Harris 2018, Carhart-Harris et al. 2019). Therefore, extrapolating these results to states in which environmental sensitivity and awareness is maximized such as the psychedelic state, suggests brains that are highly sensitive to perturbation, thus closer to criticality, may not be optimised for cognitive-based task demands. Whist speculative, this suggests that there may exist a point at which complexity is optimal for task-related cognitive effort which resides in a slightly subcritical zone. In other words, we might imagine that given a (biological) structure, the landscape of possible states that it can dwell is discretely defined as the (values of) complexity that the system can produce. Thus, this might similarly limit how cognition is shaped. In this context, when referring to cognition we mean, loosely speaking, to the ‘ensemble’ of cognitive functions such as memory, attentional resources, learning, decision-making, and language (see Supplementary Figure S1 for a visual representation). Note that given the residual architecture of the brain structure following injury, we might speculate that some patients already are in the cognitively optimal point of sub-criticality for their particular structural attributes. If that was the case, any augmentation of their brain complexity would push the dynamics into a more chaotic regime, closer towards to criticality or beyond to a super-critical state, which may be detrimental for their cognition. Perhaps psychedelics could increase environmental awareness in DoC patients, yet even so, their cognition may be inhibited further from their pathological setpoint due to the non-optimal complexity for their impaired structural connectivity (Fig. 1). While the role of criticality has been explicitly addressed by some theories [i.e. entropic brain (Carhart-Harris 2018)], it has been unexplored by others [i.e. IIT (Albantakis et al. 2023)]. Only recently new experimental investigations are pinpointing the relationship between criticality and other measures, such as PCI (Maschke et al. 2023) or surrogates of integrated information (Kim and Lee 2019). Future work should define if the suggested link between an optimal level of complexity, cognition, and consciousness, based on the residual connectivity, is substantial or not.
Figure 1.
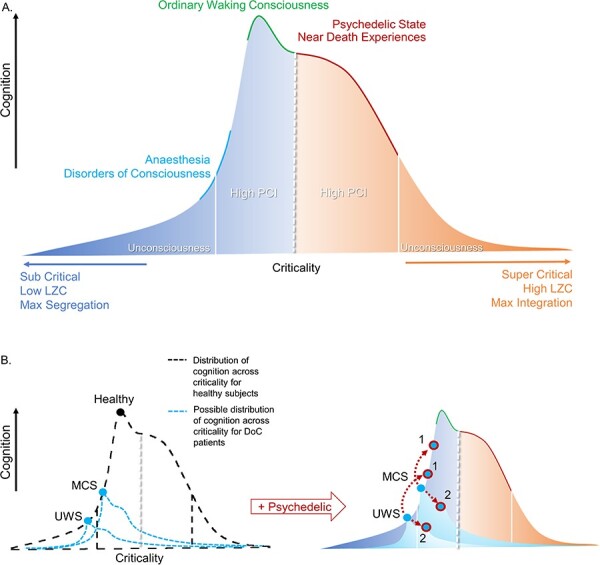
DoC = disorders of consciousness; PCI = perturbational complexity index; LZC = Lempel-Ziv complexity. Colours: orange = super-critical area; dark blue = sub-critical area.
(a) Global states without perturbation. Speculative schematic showing a landscape of states of consciousness and their cognitive capacities in accordance with the criticality of underlying networks in the brain. At each pole, both sub-critical and super-critical, a threshold represented by white lines represents unconsciousness and low PCI. Normal waking consciousness occupies a slightly sub-critical zone, where cognition is optimised at the local maximum. Psychedelic states and some other non-ordinary states of consciousness occupy a super-critical zone where cognition is forfeited for maximal sensitivity to internal and/or external stimuli. DoC and anaesthesia occupy a sub-critical zone that spans over unconscious states and states in which we are unsure as to the level of residual consciousness. (b) Pharmacological perturbation of DoC patients. Left, distribution of criticality for healthy subjects in black. In blue are possible distributions of cognition across levels of criticality for example UWS and MCS patients. The injured structural connectivity of DoC patients could result in deviations of the distribution of possible cognition across levels of criticality from that of healthy subjects. The circles represent the current level of cognition at a given moment. Right, upper. A speculative schematic prediction of an example UWS and MCS patient (baseline, light blue point) that receives a psychedelic to augment the complexity (blue points, circled in red). We display two possible scenarios. In Scenario 1, the patient follows the same distribution of healthy subjects, and their consciousness and related cognition increases (higher cognition, higher red outlined blue dot). Scenario 2 illustrates the idea that due to the damaged structural connectivity of the patients, the distribution of possible states changes so that the augmentation in complexity would be detrimental to residual cognitive capacities, as they already would occupy the point of optimal complexity for their given structure. Here, the point shifts from the local maximum of possible cognition (lower cognition, lower red outlined blue dot)
There is no medicine that achieves a 100% efficacy, and it is likely that not all patients would respond to a psychedelic treatment, even if the theoretical prerequisites were correct. In a scenario where psychedelics increase complexity in MCS patients but not in UWS patients, it would suggest that psychedelics can increase complexity in patients who are already conscious [considering MCS as conscious, as suggested by Giacino et al. (2002)]. This reduces to the idea of the difference between ‘resonance versus creation’: do psychedelics need an already complex system to lead to increased complexity, or are they causally linked with an increased complexity per se? Even if we assume that psychedelics would increase complexity in DoC, would they need a (partially) complex system such the brain of a MCS patient, or could they be effective in restoring complexity itself in a ‘non-complex brain’ like the one of a UWS patient? Note that this interpretation is possible even if there were some UWS patients (i.e. behaviourally unresponsive at the bedside) who responded to the treatment, since we have recently understood that a significant percentage of UWS patients are actually MCS*/CMD patients (Owen et al. 2006, Monti et al. 2010, Thibaut et al. 2021). In theory, MCS*/CMD patients should respond to the treatment as MCS patients, but as this category of patients has only recently been characterised, the effects of any treatment on them should be examined in more detail. On the same line, the effects of the drug on EMCS patients are likely to be analogous to the one of healthy participants, meaning with an increase of complexity without necessarily a richer behavioural repertoire.
The initial interest of psychedelics as a treatment for DoC stems from the effects on brain complexity (Schartner et al. 2017, Viol et al. 2017, Scott and Carhart-Harris 2019, Gosseries and Martial 2020). Nevertheless, two recent investigations have shown that psilocybin and sub-anaesthetic doses of ketamine increase spontaneous (LZC) but not evoked-complexity (PCI) in healthy participants (Farnes et al. 2020, Ort et al. 2023). Does the absence of an effect on PCI, arguably the gold-standard of complexity-measurements in DoC, jeopardise the general idea of using psychedelics in this population? We do not think so. In part due to evidence provided by one study (Farnes et al. 2020), which suggests that LZC might be an index of richness of subjective experience, while PCI one of the presence of consciousness. Secondly, it is worth noting that the within-subject comparison in these two studies was done against wakefulness, when complexity was already high. As such, this could have caused the absence of difference in PCI, as this would be a sheer marker of the presence of phenomenological consciousness. This is crucially in opposition to the circumstance of administering psychedelic drugs to people with DoC, in particular UWS patients, who have ‘low’ brain complexity as baseline. For a larger discussion about complexity measures and how they inform us about consciousness, we refer to Box 1.
Comparing the effects between healthy participants and patients provides an initial guide to the expected outcomes in the clinical population. However, patients have a different brain architecture that might impact the effectiveness of the drugs in unexpected ways. One example is the effect on the default mode network (DMN), which has been considered to be crucial to sustain consciousness (thus, complex activity) in healthy participants, yet is affected by psychedelics and associated with alterations in the sense of Self.
Contribution of the ‘Self’: the role of the default mode network in diminished selfhood
Both the study of psychedelics and the science of DoC present significant considerations regarding one’s sense of Self. Defining the Self is notoriously difficult and perhaps is best described as a hypernym encompassing one’s functional capacities for several metacognitive phenomena including Self awareness, sense of agency, theory of mind, and reality testing (Seth 2013, Lebedev et al. 2015). Notwithstanding this inherent complexity, the DMN is commonly associated with several functional roles involved in one’s sense of Self (Lebedev et al. 2015) including supporting a bistable system of ‘internal-external’ awareness (Vanhaudenhuyse et al. 2011). Psychedelic drugs can impart a feeling of dissolving of one’s sense of Self, termed ego dissolution. Ego dissolution has been linked to decreased coupling of the DMN and the medial temporal lobe, in addition to altering the connectivity between the DMN and other resting state networks (i.e. salience network or dorsal attentional network) (Carhart-Harris et al. 2012, Stoliker et al. 2023). There is also a dose-dependent effect of psychedelics on the functional connectivity of the DMN, such that higher doses lead to more disintegrated network functioning (Madsen et al. 2021). Interestingly, patients with DoC, a population with a questionable level of selfhood, have impaired functioning of the DMN at rest. In fact, MCS patients have stronger DMN connectivity compared to UWS patients (as with other networks, such as the auditory network) (Demertzi et al. 2015), while anticorrelation between DMN and positive-task networks appears only in EMCS patients (Di Perri et al. 2016). In other words, DMN is impaired in patients with lower levels of consciousness. This brings forth an interesting consideration: how would psychedelic drugs, agents which dissolve one’s feeling of selfhood, act in patients who either have a misrepresentation of the self-model or lack the model outright? Or, taking this paradox in neuroscientific terms: how could psychedelic substances, which impair the connectivity of the DMN, be beneficial to DoC patients, when the severity of their condition is directly linked to the DMN disorganisation? As suggested by Letherby’s work, perhaps selfhood is a concept that emerges from a number of hierarchical predictive models, which requires the integration and unification of cognitive processing across levels and domains (Letheby and Gerrans 2017). Therefore, a patient who responds to a psychedelic drug treatment and has an episode of transiently increased consciousness would likely have difficulties with their phenomenological representation of the Self-model, perhaps to an even greater extent than their baseline state. This would be due to the dysfunctional residual levels of selfhood as a result of their condition, in combination with the pharmacological perturbation of their self-model. In fact, it is worth noticing that psychedelics are effective for disorders affecting both the representation of the Self and the DMN, such as major depression (Carhart-Harris et al. 2021, Daws et al. 2022), recently considered as a problem of consciousness itself (Whiteley 2021). While there might be future experiments utilising fMRI on DoC undergoing psychedelics, this might be primarily explored by modelling. In theory, this could reconcile the apparent contradiction between the positive effects on consciousness predicted by the M/EEG literature and complexity, with the possible theoretical negative one suggested by some fMRI works.
While we have here outlined an informed guess on a relevant network that might be informative in the exploration of the effects of psychedelics on patients with DoC, there is likely several other potential regions, networks, or characteristic brain dynamics that might be informative. In this regard, computational modelling offers a unique opportunity to unveil key biomarkers that could have been missed considering only the effect of psychedelics in healthy participants.
The role of computational modelling
Scott and Carhart-Harris’ paper (Scott and Carhart-Harris 2019) mentions the utility of animal models in investigating new treatment paradigms, which is undoubtedly a tried and tested method. With animal modelling, we would minimise the risks for distress in humans that may arise from the psychedelic-elicited shift in subjective experience. One suggestion along this line would be to explore whether administering a psychedelic drug can increase the consciousness of animals during pharmacologically induced (e.g. general anaesthesia) or pathological (e.g. DoC-like) low conscious states. Despite some limitations inherent to animal modelling, these experiments would provide a valuable starting point, testing the potential efficacy of such an approach and allowing the investigation of its possible neurophysiological mechanisms.
Besides animal models, computational modelling could provide a useful path of scientific exploration and clinical application, particularly through the use of whole brain computational models (WBM) (Cofré et al. 2020, Whiteley 2021). This approach might be the sole practical way to perform experiments before the safety profile of these drugs is clarified. By creating in silico models of DoC patients, we could simulate psychedelic perturbations upon these patient models, and explore from the bottom up the mechanisms of psychedelic-induced increases in complexity and its relationship to brain structure and function. WBMs incorporate empirical structural and functional data, grounded by a theoretical delineation of local brain activity, which can take several forms (Fig. 2) (Cofré et al. 2020). The interactions between such local dynamics along an empirical structural connectivity can simulate a number of useful data depending on the type of model, including the fMRI blood oxygen-level dependent (BOLD) signal. The parameters of the model are then optimised to minimise the distance between specific characteristics (e.g. functional connectivity) of the simulated and empirical functional data to obtain the most realistic simulations. Whilst we do not yet directly have access to fMRI data of patients with DoC under psychedelic drugs, in theory, this could be approximated through several approaches which ultimately induce parameter changes on patient models. Some biologically realistic models can facilitate the simulation of 5-HT2A receptor stimulation by including positron emission tomography (PET)-derived receptor density maps as parameters within the model. A 2018 study (Deco et al. 2018b) simulated the effects of a pharmacological perturbation of LSD on the global dynamics of brains of healthy subjects via receptor density maps. Such simulations on models built on patients’ injured structural connectomes could assess the resultant global brain dynamics (following a simulated treatment) in becoming more similar to dynamics of healthy brains, through the characterisation of modelling-based measures. Although aspirational, this research could have considerable clinical applications, via the prediction of the efficacy of such treatments on these empirically based models formed by individual DoC patients. This would enable a stratification of responders, who have positive expected changes due to the medication, and non-responders, patients for whom the drug may not be effective. Finding ‘predictive biomarkers’ based on individual brain signatures could inform if a drug has the highest changes of working effectively a priori. This would improve efficiency and reduce material and labour costs in terms of drugs and personnel to perform ineffective and/or difficult experiments, in addition to reducing possible adverse effects due to high medicalisation, effectively providing a cheaper and more ethical health care.
Figure 2.
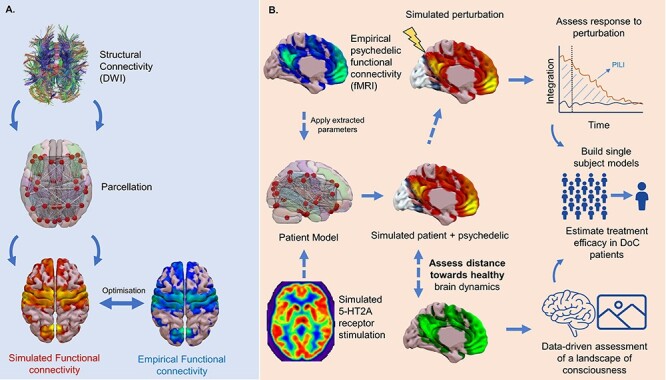
DoC = disorders of consciousness, PET = Positron emission tomography; 5-HT2A receptor= Serotonin 2A receptor; PILI= perturbative integration latency index; DWI = diffusion weighed imaging.
Illustration of the potential contribution of whole brain computational modelling. (a) The construction of a whole brain computational model from empirical structural connectivity derived from DWI. The model parameters are then optimised to achieve the best fit between simulated and empirical connectivity. (b) Parameters from models optimised on healthy controls under psychedelics could be extracted and applied to patient models to achieve a simulated psychedelic perturbation. Alternatively, neurotransmitter information from PET could be added to a dynamic mean field model to simulate receptor stimulation (i.e. serotonin-2A, 5-HT2A). The resulting dynamics could then be investigated through in silico perturbation protocols or through being compared to healthy subjects to assess the descriptive distance from the brains of healthy controls. Such techniques could build towards having single subject models through which psychedelic treatment could be initially assessed
WBMs could also assist experiments in an auxiliary fashion. A number of in silico based perturbation methods have been recently developed. For example, akin to a digital PCI, a perturbative integration latency index (PILI) (Deco et al. 2018a) can be calculated by measuring the latency return to the baseline state of integration after a modelled perturbation. By considering both the resultant amplitude of stimulation and the time taken to return to baseline, PILI measures the instability of a system. Unstable dynamics are related to more chaotic interactions, consistent with what would be expected from a system at a higher complexity. This measure has shown to be indicative of a particular state of consciousness: in fact, PILI was shown to be higher in a WBM of LSD compared to placebo (Jobst et al. 2021). Similar in silico perturbation protocols could be applied to models of individual DoC patients in both the baseline state and simulated psychedelic state. This could surpass experimental measures like the PCI by not only determining the level of consciousness of a particular subject, but also to help to understand the dependencies between complexity, brain structure, and function in (non-)responders. As discussed, this might be crucial for having a better view on the role of regions and networks (e.g. DMN).
Understanding the mechanisms of action of psychedelics might be informative not only to pinpoint drugs’ effects per se, but to frame conscious states as a whole. In fact, while there have been several attempts to create a taxonomy of consciousness [i.e. as a combination of wakefulness and awareness (Laureys 2005) or multidimensional (Walter 2021)], very few have explicitly tried to place the psychedelic experience in their organisation [for an example, see Martial et al. (2020)]. This is not to say that there are no theories that address how we experience the psychedelic state, but rather that there are only a few that normalise them in a coherent, unified, space. Only recently the field of psychedelic science has started merging with the neuroscience of consciousness at large, and with the science of DoC specifically. We consider this to be of paramount importance to account for similarities and differences with other (non-ordinary) states of consciousness (Timmermann et al. 2023), like near-death experiences (Timmermann et al. 2018, Martial et al. 2019, Sweeney et al. 2022, Fritz et al. 2024) or meditation (Smigielski et al. 2019). Nevertheless, even when such a comparison is made, most of the time it is focused on the phenomenological profile of experiences, rather than the brain signatures. If this permits us to have an easily accessible comparison, it might overlook some underlying similarities that are evident from the brain activity [see Moujaes et al. (2024) for an example].
Recently, deep convoluted neural networks such as variational autoencoders in conjunction with WBM have been employed to allow the visualisation of brain imaging data within a reduced dimensional space [see Perl et al. (2020, 2023) or Escrichs et al. (2022)]. These latent space representations may reveal data-driven relationships between psychedelic states and others, which can go on to inform theoretical frameworks, in this way forming a virtuous cycle of collaboration between theory and experimentation, computation, and clinical work (Luppi et al. 2021). Therefore, in tandem with experimental work, modelling experiments can importantly provide a mechanistic understanding of the effects of psychedelics on global brain dynamics, identify good potential candidate patients, and further develop the theoretically based framework to inform future clinical trials, and ultimately personalised interventions (Luppi et al. 2023).
Modelling has recently contributed to our understanding of drugs mechanisms and effects (Luppi et al. 2023, Singleton et al. 2022), and provides an inexpensive and available tool to perform experiments that are experimentally difficult or impossible. This might be a crucial first step before launching in vivo experiments, whose ethicality continues to be discussed.
Ethical considerations and the question of a good outcome
In addition to growing excitement around the possibility of employing psychedelics as a treatment for DoC, there have also been some ethical concerns regarding the validity and necessity of such experiments (Peterson et al. 2019). DoC is a fragile population that deserves special scrutiny, and in agreement with the concerns originally raised (Scott and Carhart-Harris 2019) and the follow-up paper focusing on the ethics of such experiments (Peterson et al. 2019), before launching such experimentation there are several considerations that should be weighted to ensure the most beneficial and safe setting for the patients. In fact, some side effects that might be trivial for healthy participants, (i.e. nausea and vomiting) could be dangerous for patients with DoC (i.e. need aspiration). Additionally, the effect of psychedelics on epileptogenic threshold has not been studied yet, and several patients of DoC suffer from some sort of epilepsy (Lejeune et al. 2021). Finally, DoC have a high medical burden, making possible interaction with drugs given as standard care to be investigated. Nevertheless, given the general safety profile of the typical psychedelics (Nutt et al. 2010), it is the phenomenological aspect of psychedelics that has been more discussed. The associated mystical experiences from high doses of psychedelic drugs are generally euphoric, pleasant experiences in which one often reports feelings of oceanic boundlessness, the dissolving of one’s sense of Self with a sense of connection to the environment (Griffiths et al. 2006). Despite this, psychedelic drug administration has the potential to result in unpleasant experiences, sometimes associated with feelings of anxiety, dysphoria, and confusion, commonly referred to as a ‘bad trip’ (Strassman 1984, Barrett et al. 2016). This raises the cogent question of how to deal with such challenging experiences in a population with no capacity to report them. One possibility is to not endure any experimental procedure if there is any risk of inducing such negative experiences. It is worth noticing that while there are no conclusive data about the prevalence of challenging experiences in different settings and populations, the risk of these in clinical settings is very low, where the preparations of both the environment and patient’s expectations are controlled (Johnson et al. 2008, Carbonaro et al. 2016, Simonsson et al. 2023) [but see Gashi et al. (2021) for a report in recreational use]. A recent paper has detailed the importance of set and setting in the use of psychedelics in DoC, highlighting the challenges of preparing a patient for the treatment (Rankaduwa and Owen 2023). Another possibility one could propose is to utilise a psychedelic-like analogue that is non-psychedelic in terms of its impact on experience. A concerted effort is underway to synthetise non-psychoactive analogous of ibogaine (Cameron et al. 2021) and LSD (Kaplan et al. 2022, Lewis et al. 2023) with the same neuroplastic profile increasing neurogenesis and/or spinogenesis (Ly et al. 2018, Vargas et al. 2023). At the time of writing, as far as we are aware, only one Phase I Trial with a non-hallucinogenic analogous is on the way for human use, beside some anecdotal reports online. Despite such ongoing efforts, it is unknown whether a non-psychedelic analogue would increase brain complexity whilst leaving subjective experience unaltered. The existence alone of such substance would have huge implications on the nature of the relationship between complexity and consciousness, as well as understanding if clinical changes could be attributed to the subjective experience (Yaden and Griffiths 2020) or to its underlying neurophysiological effects (Olson 2021). Another option could be to utilise other agents that can halt any experiential alterations: a novel study has shown that ketanserin, a 5-HT2A antagonist, can drastically decrease the intensity and the duration of LSD experiences (Becker et al. 2023). Although again, the question arises of how brain complexity would be impacted after blocking the alterations of subjective experience.
Considering the current knowledge on DoC, we could ask to what extent a patient could experience anything at all. That is to say, could a UWS patient who is deemed to be unconscious, with no phenomenological experience, be impacted by a challenging one? Here, perhaps the clinical perspective and scientific perspective dissociate; we must consider what represents a successful outcome, both clinically and scientifically. If it were the case that even a single UWS patient who, as a result of psychedelic drug administration, underwent (and reported) a challenging experience, it would mean that their consciousness would have been transiently augmented. Even though this experience could have induced a degree of suffering for the individual, such episodes of augmented consciousness could allow a patient to interact (at least partly) with the environment and the individuals present at bedside. While hard to quantify, some philosophers would consider recovering (even temporarily) consciousness per se to be beneficial to the individual (Levy 2014, Lee 2019). Whether or not to consider an augmentation of consciousness that allows a patient to understand their condition, even if for a transient period, a desirable outcome is surely a question that would yield different individual responses. The opportunity of communication or even conscious perception between patients and relatives/caregivers, in such a debilitating disorder, might be precious and enriching for both parts. Note that a priori chances of having a treatment that forces consciousness upon an unconscious patient are low, since so far the majority of tested interventions have higher efficacy for MCS patients than for UWS ones (Thibaut et al. 2019).
Psychedelics are currently investigated for the positive neuroplastic effects, which has led several authors to start naming them as psychoplastogen (Ly et al. 2018, Dunlap et al. 2020, Vargas et al. 2023). As such, it should be considered that DoC patients might benefit from the plastic effects alone, counterbalancing the brain damage causing the deficits in consciousness. This is not to say that any plasticity-based re-organisation has a net positive value, as demonstrated by epileptic foci that emerge after stroke or phenomena like the phantom limb, but simply stating that it might be advantageous in a population that has a dramatic need for functional (and structural) replacement due to a massive loss of neural tissue. While secondary to the aim of this work, we consider it pertinent to explore the impact of nature, dose, and administration mode of psychedelics to maximise functional plasticity, especially in view of a possible translation to brain-damaged patients.
Finally, one cannot discuss the benefit of psychedelics whilst ignoring the torn issue of their illegality. While this might change in the future years, as hinted by the recent evolution of the legal framework surrounding 3,4-Methylenedioxymethamphetamine (MDMA)(-assisted psychotherapy), we should recognise that there are no possible changes of the treatment of DoC if there is no parallel political and juridical advancement. As such, even few cases that positively respond to psychedelics could galvanise a paradigm shift that would speed up the research lines previously detailed (i.e. creation of non-hallucinogenic analogues).
Conclusion
Through augmenting the complexity of networks in the brains of people suffering from DoC, psychedelics could potentially unlock a new treatment paradigm to treat these devastating disorders. Significant ethical concerns regarding the ability to report negative experiences arise, although once properly contextualised and considering the potential benefit to patients, we believe they ultimately dissolve. These initial experiments have potential to impact clinical domains and the science of consciousness by empirically investigating the links between complexity, cognition, and consciousness; whilst there exists the possibility that consciousness could arise as a function of complexity modulation, expectations should be managed and the definition of what constitutes a good outcome should be considered. Additionally, the possible existence of an optimal ‘complexity point’ tempers the excitement of drugs that in general increase brain complexity, as much as the possible counter-intuitive (desirable?) effects on DMN. Computational modelling could also provide an important contribution by testing treatment efficacy in silico and further investigating the links between brain structure, function, and complexity (see Box 2). As the zeitgeist is establishing psychedelics as useful tools in psychiatry, we believe that they hold great potential when applied to neurological conditions, both clinically as a treatment and scientifically as a probe for consciousness.
Box 2: Take home messages.
In this article we have examined some underexplored topics surrounding the rationale and the contribution of using psychedelics as treatment for DoC. We consider that such experiments would help elucidating the following points:
‘There could exist an optimal amount of complexity for consciousness and cognition’. While it is undoubtful that there is a strong link between those, it is unclear whether there is an optimal level or amount of complexity, both phenomenologically and functionally. If there is, this would likely be caused by the individual structural substrate of a brain (or system). In other words, given a specific brain structure, such as a person with DoC or other brain difference, increasing complexity could lead to impaired cognition and possibly consciousness.
‘DMN disorganisation and effects on the Self due to psychedelics in DoC is a compelling area to explore’. Consciousness and Self are deeply bounded, and a discussion on one ought to include the other. DoC have impaired network organisation, including of the DMN that is linked with their condition. Psychedelics are known to alter the normal functional organisation of the brain, decreasing integrity within resting state networks (in particular of the DMN) and increasing connectivity between them. Given the functional disintegration of the DMN that is induced by both DoC and psychedelics, understanding how psychedelics affect the DMN of DoC patients (which is already functionally impaired) could be crucial to understanding the phenomenological effects of this treatment.
‘Modelling is a promising avenue for identifying potential biomarkers of treatment efficacy and defining the landscape of consciousness’. Several groups have now demonstrated the utility of modelling to frame consciousness at large. Additionally, in the strive for personalised medicine, in silico biomarkers have a pivotal importance to create individualised predictive biomarkers for appropriate care.
Supplementary Material
Contributor Information
Paolo Cardone, Coma Science Group, GIGA-Consciousness, University of Liège, Avenue de l’hôpital 11, Liège 4000, Belgium; Centre du Cerveau2, University Hospital of Liège, Avenue de l’hôpital 11, Liège 4000, Belgium.
Naji Alnagger, Coma Science Group, GIGA-Consciousness, University of Liège, Avenue de l’hôpital 11, Liège 4000, Belgium; Centre du Cerveau2, University Hospital of Liège, Avenue de l’hôpital 11, Liège 4000, Belgium.
Jitka Annen, Coma Science Group, GIGA-Consciousness, University of Liège, Avenue de l’hôpital 11, Liège 4000, Belgium; Centre du Cerveau2, University Hospital of Liège, Avenue de l’hôpital 11, Liège 4000, Belgium; Department of Data Analysis, University of Ghent, Henri Dunantlaan 1, Ghent 9000, Belgium.
Aminata Bicego, Sensation and Perception Research Group, GIGA-Consciousness, University of Liège, Avenue de l’hôpital 11, Liège 4000, Belgium.
Olivia Gosseries, Coma Science Group, GIGA-Consciousness, University of Liège, Avenue de l’hôpital 11, Liège 4000, Belgium; Centre du Cerveau2, University Hospital of Liège, Avenue de l’hôpital 11, Liège 4000, Belgium; Sensation and Perception Research Group, GIGA-Consciousness, University of Liège, Avenue de l’hôpital 11, Liège 4000, Belgium.
Charlotte Martial, Coma Science Group, GIGA-Consciousness, University of Liège, Avenue de l’hôpital 11, Liège 4000, Belgium; Centre du Cerveau2, University Hospital of Liège, Avenue de l’hôpital 11, Liège 4000, Belgium.
Supplementary data
Supplementary data is available at Neuroscience of Consciousness online.
Conflict of interest
None declared.
Funding
The study was supported by the University and University Hospital of Liège, the Léon Frederique foundation, the Belgian National Funds for Scientific Research (F.R.S-FNRS), the ERA-Net FLAG-ERA JTC2021 project ModelDXConsciousness (Human Brain Project Partnering Project), FNRS project MIS F.4521.23, FNRS project No PDR/BEJ T.0134.21, the University of Liège Conseil Sectoriel de la Recherche, the BIAL Foundation, AstraZeneca Foundation, the Generet funds and the King Baudouin foundation, the James McDonnell Foundation, Mind Science Foundation, Mind Care Foundation, IAP research network P7/06 of the Belgian Government (Belgian Science Policy) and Wallonia as part of a 474 program of the BioWin Health Cluster framework, and the GIGA Doctoral School for Healthy Sciences (University of Liège). P.C. and N.A. are research fellows, and O.G. is a research associate at the F.R.S-FNRS. J.A. is postdoctoral fellow funded (1265522N) by the Fund for Scientific Research-Flanders (FWO).
Data Availability Statement
No new data were generated or analysed in support of this research.
References
- Aamodt A, Sevenius Nilsen A, Markhus R et al. EEG Lempel-Ziv complexity varies with sleep stage, but does not seem to track dream experience. Front Hum Neurosci 2023;16:883. 10.3389/fnhum.2022.987714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albantakis L, Barbosa L, Findlay G et al. Integrated information theory (IIT) 4.0: formulating the properties of phenomenal existence in physical terms. PLoS Comput Biol 2023;19:e1011465. 10.1371/journal.pcbi.1011465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alnagger N, Cardone P, Martial C et al. The current and future contribution of neuroimaging to the understanding of disorders of consciousness. Presse Med 2023;52:104163. 10.1016/j.lpm.2022.104163 [DOI] [PubMed] [Google Scholar]
- Altlntop ÇG, Latifoǧlu F, Akln AK et al. Classification of depth of coma using complexity measures and nonlinear features of electroencephalogram signals. Int J Neural Syst 2022;32:2250018. 10.1142/S0129065722500186 [DOI] [PubMed] [Google Scholar]
- Amin Moosavi S, Truccolo W. Criticality in probabilistic models of spreading dynamics in brain networks: epileptic seizures. PLoS Comput Biol 2023;19:e1010852. 10.1371/journal.pcbi.1010852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrillon T, Poulsen AT, Hansen LK et al. Neural markers of responsiveness to the environment in human sleep. J Neurosci 2016;36:6583–96. 10.1523/JNEUROSCI.0902-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y, Liang Z, Li X. A permutation Lempel-Ziv complexity measure for EEG analysis. Biomed Signal Process Control 2015;19:102–14. 10.1016/j.bspc.2015.04.002 [DOI] [Google Scholar]
- Barbosa LS, Marshall W, Streipert S et al. A measure for intrinsic information. Sci Rep 2020;10:1–9. 10.1038/s41598-020-75943-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett FS, Bradstreet MP, Leoutsakos JMS et al. The challenging experience questionnaire: characterization of challenging experiences with psilocybin mushrooms. J Psychopharmacol 2016;30:1279–95. 10.1177/0269881116678781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayne T, Carter O. Dimensions of consciousness and the psychedelic state. Neurosci Conscious 2018;2018:niy008. 10.1093/nc/niy008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker AM, Klaiber A, Holze F et al. Ketanserin reverses the acute response to LSD in a randomized, double-blind, placebo-controlled, crossover study in healthy participants. Int J Neuropsychopharmacol 2023;26:97–106. 10.1093/ijnp/pyac075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogenschutz MP, Ross S, Bhatt S et al. Percentage of heavy drinking days following psilocybin-assisted psychotherapy vs placebo in the treatment of adult patients with alcohol use disorder: a randomized clinical trial. JAMA Psychiatry 2022;79:953–62. 10.1001/jamapsychiatry.2022.2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows DRW, Diana G, Pimpel B et al. Microscale neuronal activity collectively drives chaotic and inflexible dynamics at the macroscale in seizures. J Neurosci 2023;43:3259–83. 10.1523/JNEUROSCI.0171-22.2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron LP, Tombari RJ, Lu J et al. A non-hallucinogenic psychedelic analogue with therapeutic potential. Nature 2021;589:474–9. 10.1038/s41586-020-3008-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonaro TM, Bradstreet MP, Barrett FS et al. Survey study of challenging experiences after ingesting psilocybin mushrooms: acute and enduring positive and negative consequences. J Psychopharmacol 2016;30:1268–78. 10.1177/0269881116662634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL. The entropic brain - revisited. Neuropharmacology 2018;142:167–78. 10.1016/j.neuropharm.2018.03.010 [DOI] [PubMed] [Google Scholar]
- Carhart-Harris RL, Erritzoe D, Williams T et al. Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proc Natl Acad Sci U S A 2012;109:2138–43. 10.1073/pnas.1119598109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Friston KJ, Barker EL. REBUS and the anarchic brain: toward a unified model of the brain action of psychedelics. Pharmacol Rev 2019;71:316–44. 10.1124/pr.118.017160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris R, Giribaldi B, Watts R et al. Trial of psilocybin versus escitalopram for depression. N Engl J Med 2021;384:1402–11. 10.1056/NEJMoa2032994 [DOI] [PubMed] [Google Scholar]
- Carhart-Harris RL, Goodwin GM. The therapeutic potential of psychedelic drugs: past, present, and future. Neuropsychopharmacology 2017;42:2105–13. 10.1038/npp.2017.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Leech R, Hellyer PJ et al. The entropic brain: a theory of conscious states informed by neuroimaging research with psychedelic drugs. Front Hum Neurosci 2014;8:20. 10.3389/fnhum.2014.00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casali AG, Gosseries O, Rosanova M et al. A theoretically based index of consciousness independent of sensory processing and behavior. Sci Transl Med 2013;5:198ra105. 10.1126/scitranslmed.3006294 [DOI] [PubMed] [Google Scholar]
- Casarotto S, Comanducci A, Rosanova M et al. Stratification of unresponsive patients by an independently validated index of brain complexity. Ann Neurol 2016;80:718–29. 10.1002/ana.24779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chialvo DR. Emergent complex neural dynamics. Nat Phys 2010;6:744–50. 10.1038/nphys1803 [DOI] [Google Scholar]
- Cofré R, Herzog R, Mediano PAM et al. Whole-brain models to explore altered states of consciousness from the bottom up. Brain Sci 2020;10:1–29. 10.3390/brainsci10090626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comolatti R, Pigorini A, Casarotto S et al. A fast and general method to empirically estimate the complexity of brain responses to transcranial and intracranial stimulations. Brain Stimul 2019;12:1280–9. 10.1016/j.brs.2019.05.013 [DOI] [PubMed] [Google Scholar]
- Dahmen D, Grün S, Diesmann M et al. Second type of criticality in the brain uncovers rich multiple-neuron dynamics. Proc Natl Acad Sci U S A 2019;116:13051–60. 10.1073/pnas.1818972116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danforth AL, Grob CS, Struble C et al. Reduction in social anxiety after MDMA-assisted psychotherapy with autistic adults: a randomized, double-blind, placebo-controlled pilot study. Psychopharmacology (Berl) 2018;235:3137–48. 10.1007/s00213-018-5010-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmani G, Nieminen JO, Bergmann TO et al. A degraded state of consciousness in healthy awake humans? Brain Stimul 2021;14:710–2. 10.1016/j.brs.2021.04.012 [DOI] [PubMed] [Google Scholar]
- Daws RE, Timmermann C, Giribaldi B et al. Increased global integration in the brain after psilocybin therapy for depression. Nat Med 2022;28:844–51. 10.1038/s41591-022-01744-z [DOI] [PubMed] [Google Scholar]
- Deco G, Cabral J, Saenger VM et al. Perturbation of whole-brain dynamics in silico reveals mechanistic differences between brain states. Neuroimage 2018a;169:46–56. 10.1016/j.neuroimage.2017.12.009 [DOI] [PubMed] [Google Scholar]
- Deco G, Cruzat J, Cabral J et al. Whole-brain multimodal neuroimaging model using serotonin receptor maps explains non-linear functional effects of LSD. Curr Biol 2018b;28:3065–3074.e6. 10.1016/j.cub.2018.07.083 [DOI] [PubMed] [Google Scholar]
- Demertzi A, Antonopoulos G, Heine L et al. Intrinsic functional connectivity differentiates minimally conscious from unresponsive patients. Brain 2015;138:2619–31. 10.1093/brain/awv169 [DOI] [PubMed] [Google Scholar]
- Di Perri C, Bahri MA, Amico E et al. Neural correlates of consciousness in patients who have emerged from a minimally conscious state: a cross-sectional multimodal imaging study. Lancet Neurol 2016;15:830–42. 10.1016/S1474-4422(16)00111-3 [DOI] [PubMed] [Google Scholar]
- Du J, Vegh V, Reutens DC. Small changes in synaptic gain lead to seizure-like activity in neuronal network at criticality. Sci Rep 2019;9:1097. 10.1038/s41598-018-37646-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap LE et al. Identification of psychoplastogenic N,N-dimethylaminoisotryptamine (isoDMT) analogues through structure−activity relationship studies. Cite This: J Med Chem 2020;63:1142–55. 10.1021/acs.jmedchem.9b01404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escrichs A, Perl YS, Uribe C et al. Unifying turbulent dynamics framework distinguishes different brain states. Commun Biol 2022;5:1–13. 10.1038/s42003-022-03576-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerholm ED, Lorenz R, Scott G et al. Cascades and cognitive state: focused attention incurs subcritical dynamics. J Neurosci 2015;35:4626–34. 10.1523/JNEUROSCI.3694-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnes N, Juel BE, Nilsen AS et al. Increased signal diversity/complexity of spontaneous EEG, but not evoked EEG responses, in ketamine-induced psychedelic state in humans. PLoS One 2020;15:e0242056. 10.1371/journal.pone.0242056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingelkurts AA, Fingelkurts AA. Contemplating on the nature of selfhood in DoC patients: neurophenomenological perspective. J Integr Neurosci 2023;22:23. 10.31083/j.jin2201023 [DOI] [PubMed] [Google Scholar]
- Fritz P, Lejeune N, Cardone P et al. Bridging the gap: (a)typical psychedelic and near-death experience insights. Curr Opin Behav Sci 2024;55:101349. 10.1016/j.cobeha.2023.101349 [DOI] [Google Scholar]
- Gashi L, Sandberg S, Pedersen W. Making “bad trips” good: how users of psychedelics narratively transform challenging trips into valuable experiences. Int J Drug Policy 2021;87:102997. 10.1016/j.drugpo.2020.102997 [DOI] [PubMed] [Google Scholar]
- Gasser P, Holstein D, Michel Y et al. Safety and efficacy of lysergic acid diethylamide-assisted psychotherapy for anxiety associated with life-threatening diseases. J Nerv Ment Dis 2014;202:513–20. 10.1097/NMD.0000000000000113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervais C, Boucher LP, Villar GM et al. A scoping review for building a criticality-based conceptual framework of altered states of consciousness. Front Syst Neurosci 2023;17:1085902. 10.3389/fnsys.2023.1085902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacino JT, Ashwal S, Childs N et al. The minimally conscious state. Neurol Rev 2002;64:350–1. 10.1212/WNL.58.3.349 [DOI] [Google Scholar]
- Gollo LL. Coexistence of critical sensitivity and subcritical specificity can yield optimal population coding. J R Soc Interface 2017;14:20170207. 10.1098/rsif.2017.0207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosseries O, Martial C. The use of psychedelics in the treatment of disorders of consciousness. Alius Bulletin 2020;4 10.34700/gv0w-y052. [DOI] [Google Scholar]
- Griffiths RR, Johnson MW, Carducci MA et al. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: a randomized double-blind trial. J Psychopharmacol 2016;30:1181–97. 10.1177/0269881116675513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Richards WA, McCann U et al. Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacology (Berl) 2006;187:268–83. 10.1007/s00213-006-0457-5 [DOI] [PubMed] [Google Scholar]
- Grof S. Realms of the Human Unconscious: Observations from LSD Research. London: Souvenir Press, 1979. [Google Scholar]
- Grof S, Goodman LE, Richards WA et al. LSD assisted psychotherapy in patients with terminal cancer. Int Pharmacopsychiatry 1973;8:129–44. 10.1159/000467984 [DOI] [PubMed] [Google Scholar]
- Healy CJ. The acute effects of classic psychedelics on memory in humans. Psychopharmacology (Berl) 2021;238:639–53. 10.1007/s00213-020-05756-w [DOI] [PubMed] [Google Scholar]
- Hirschfeld T, Schmidt TT. Dose–response relationships of psilocybin-induced subjective experiences in humans. J Psychopharmacol 2021;35:384–97. 10.1177/0269881121992676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt T, Ericson M, Schooler J. Where’s my consciousness-ometer? How to test for the presence and complexity of consciousness. Perspectives Psychol Sci 2022;17:1150–65. 10.1177/17456916211029942 [DOI] [PubMed] [Google Scholar]
- Irrmischer M, Houtman SJ, Mansvelder HD et al. Controlling the temporal structure of brain oscillations by focused attention meditation. Hum Brain Mapp 2018a;39:1825–38. 10.1002/hbm.23971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irrmischer M, Poil S, Mansvelder HD et al. Strong long‐range temporal correlations of beta/gamma oscillations are associated with poor sustained visual attention performance. Eur J Neurosci 2018b;48:2674–83. 10.1111/ejn.13672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobst BM, Atasoy S, Ponce-Alvarez A et al. Increased sensitivity to strong perturbations in a whole-brain model of LSD. Neuroimage 2021;230:117809. 10.1016/j.neuroimage.2021.117809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Richards WA, Griffiths RR. Human hallucinogen research: guidelines for safety. J Psychopharmacol 2008;22:603–20. 10.1177/0269881108093587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan AL, Confair DN, Kim K et al. Bespoke library docking for 5-HT2A receptor agonists with antidepressant activity. Nature 2022;610:582–91. 10.1038/s41586-022-05258-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SM, Carter GT, Aggarwal SK et al. Psychedelics for brain injury: a mini-review. Front Neurol 2021;12:1260. 10.3389/fneur.2021.685085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Lee UC. Criticality as a determinant of integrated information Φ in human brain networks. Entropy 2019;21:981. 10.3390/e21100981 [DOI] [Google Scholar]
- Kitzbichler MG, Smith ML, Christensen SR et al. Broadband criticality of human brain network synchronization. PLoS Comput Biol 2009;5:e1000314. 10.1371/journal.pcbi.1000314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laureys S. The neural correlate of (un)awareness: lessons from the vegetative state. Trends Cognit Sci 2005;9:556–9. 10.1016/j.tics.2005.10.010 [DOI] [PubMed] [Google Scholar]
- Laureys S, Celesia GG, Cohadon F et al. Unresponsive wakefulness syndrome: a new name for the vegetative state or apallic syndrome. BMC Med 2010;8:2–5. 10.1186/1741-7015-8-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebedev AV, Lövdén M, Rosenthal G et al. Finding the self by losing the self: neural correlates of ego-dissolution under psilocybin. Hum Brain Mapp 2015;36:3137–53. 10.1002/hbm.22833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AY. Is consciousness intrinsically valuable? Philos Stud 2019;176:655–71. 10.1007/s11098-018-1032-8 [DOI] [Google Scholar]
- Lee M, Sanz LR, Barra A et al. Quantifying arousal and awareness in altered states of consciousness using interpretable deep learning. Nat Commun 2022;13:1–14. 10.1038/s41467-022-28451-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejeune N, Zasler N, Formisano R et al. Epilepsy in prolonged disorders of consciousness: a systematic review. Brain Inj 2021;35:1485–95. 10.1080/02699052.2021.1973104 [DOI] [PubMed] [Google Scholar]
- Letheby C. Philosophy of Psychedelics 2021. 10.1093/med/9780198843122.001.0001 [DOI] [Google Scholar]
- Letheby C, Gerrans P. Self unbound: ego dissolution in psychedelic experience. Neurosci Conscious 2017;3. 10.1093/nc/nix016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung A, Tsuchiya N. Separating weak integrated information theory into inspired and aspirational approaches. Neurosci Conscious 2023;2023:niad012. 10.1093/nc/niad012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy N. The value of consciousness. J Conscious Stud 2014;21:127–38. [PMC free article] [PubMed] [Google Scholar]
- Lewis V, Bonniwell EM, Lanham JK et al. A non-hallucinogenic LSD analog with therapeutic potential for mood disorders. Cell Rep 2023;42:112203. 10.1016/j.celrep.2023.112203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkenkaer-Hansen K, Nikouline VV, Palva JM et al. Long-range temporal correlations and scaling behavior in human brain oscillations. J Neurosci 2001;21:1370–7. 10.1523/JNEUROSCI.21-04-01370.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zeng W, Pan N et al. EEG complexity correlates with residual consciousness level of disorders of consciousness. BMC Neurol 2023;23:140. 10.1186/s12883-023-03167-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luppi AI, Cabral J, Cofre R et al. Computational modelling in disorders of consciousness: closing 1 the gap towards personalised models for restoring 2 consciousness. NeuroImage 2023;275:120162. 10.1016/j.neuroimage.2023.120162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luppi AI, Cain J, Spindler LRB et al. Mechanisms underlying disorders of consciousness: bridging gaps to move toward an integrated translational science. Neurocrit Care 2021;35:37–54. 10.1007/s12028-021-01281-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luppi AI, Hansen JY, Adapa R et al. In vivo mapping of pharmacologically induced functional reorganization onto the human brain’s neurotransmitter landscape. Sci Adv 2023;9. 10.1126/sciadv.adf8332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luppi AI, Vohryzek J, Kringelbach ML et al. Distributed harmonic patterns of structure-function dependence orchestrate human consciousness. Commun Biol 2023;6:117. 10.1038/s42003-023-04474-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly C, Greb AC, Cameron LP et al. Psychedelics promote structural and functional neural plasticity. Cell Rep 2018;23:3170–82. 10.1016/j.celrep.2018.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen MK, Stenbæk DS, Arvidsson A et al. Psilocybin-induced changes in brain network integrity and segregation correlate with plasma psilocin level and psychedelic experience. Eur. Neuropsychopharmacol. 2021;50:121–32. 10.1016/j.euroneuro.2021.06.001 [DOI] [PubMed] [Google Scholar]
- Martial C, Cassol H, Charland-Verville V et al. Neurochemical models of near-death experiences: a large-scale study based on the semantic similarity of written reports. Conscious Cogn 2019;69:52–69. 10.1016/j.concog.2019.01.011 [DOI] [PubMed] [Google Scholar]
- Martial C, Cassol H, Laureys S et al. Near-death experience as a probe to explore (disconnected) consciousness. Trends Cogn Sci 2020;24:173–83. 10.1016/j.tics.2019.12.010 [DOI] [PubMed] [Google Scholar]
- Maschke C, Duclos C, Blain-Moraes S. Paradoxical markers of conscious levels: effects of propofol on patients in disorders of consciousness. Front Hum Neurosci 2022;16:690. 10.3389/fnhum.2022.992649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maschke C, O’Byrne J, Colombo MA et al. Criticality of resting-state EEG predicts perturbational complexity and level of consciousness during anesthesia. bioRxiv 2023. 10.1101/2023.10.26.564247 [DOI] [Google Scholar]
- Massimini M, Ferrarelli F, Huber R et al. Breakdown of cortical effective connectivity during sleep. Science (1979) 2005;309:2228–33. 10.1126/science.1117256 [DOI] [PubMed] [Google Scholar]
- Ma Z, Turrigiano GG, Wessel R et al. Cortical circuit dynamics are homeostatically tuned to criticality in vivo. Neuron 2019;104:655–664.e4. 10.1016/j.neuron.2019.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mediano PAM, Rosas FE, Bor D et al. The strength of weak integrated information theory. Trends Cognit Sci 2022;26:646–55. 10.1016/j.tics.2022.04.008 [DOI] [PubMed] [Google Scholar]
- Mitchell JM, Bogenschutz M, Lilienstein A et al. MDMA-assisted therapy for severe PTSD: a randomized, double-blind, placebo-controlled phase 3 study. Nat Med 2021;27:1025–33. 10.1038/s41591-021-01336-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JM, Ot’alora G. M, van der Kolk B et al. MDMA-assisted therapy for moderate to severe PTSD: a randomized, placebo-controlled phase 3 trial. Nat Med 2023;29:2473–80. 10.1038/s41591-023-02565-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti MM, Vanhaudenhuyse A, Coleman MR et al. Willful modulation of brain activity in disorders of consciousness. N Engl J Med 2010;362:579–89. 10.1056/NEJMoa0905370 [DOI] [PubMed] [Google Scholar]
- Moujaes F et al. Comparing neural correlates of consciousness: from psychedelics to hypnosis and meditation. Biol Psychiatry Cogn Neurosci Neuroimaging 2024;9:533–43. 10.1016/j.bpsc.2023.07.003 [DOI] [PubMed] [Google Scholar]
- Nutt D. Psychedelic drugs-a new era in psychiatry? Dialogues Clin Neurosci 2019;21:139–47. 10.31887/DCNS.2019.21.2/dnutt [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt D, Erritzoe D, Carhart-Harris R. Psychedelic psychiatry’s brave new world. Cell 2020;181:24–8. 10.1016/j.cell.2020.03.020 [DOI] [PubMed] [Google Scholar]
- Nutt DJ, King LA, Phillips LD. Drug harms in the UK: a multicriteria decision analysis. Lancet 2010;376:1558–65. 10.1016/S0140-6736(10)61462-6 [DOI] [PubMed] [Google Scholar]
- O’Byrne J, Jerbi K. How critical is brain criticality? Trends Neurosci 2022;45:820–37. 10.1016/j.tins.2022.08.007 [DOI] [PubMed] [Google Scholar]
- Oizumi M, Albantakis L, Tononi G. From the phenomenology to the mechanisms of consciousness: integrated information theory 3.0. PLoS Comput Biol 2014;10:e1003588. 10.1371/journal.pcbi.1003588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson DE. The subjective effects of psychedelics may not be necessary for their enduring therapeutic effects. ACS Pharmacol Transl Sci 2021;4:563–7. 10.1021/acsptsci.0c00192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ort A, Smallridge JW, Sarasso S et al. TMS-EEG and resting-state EEG applied to altered states of consciousness: oscillations, complexity, and phenomenology. iScience 2023;26:106589. 10.1016/j.isci.2023.106589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, Coleman MR, Boly M et al. Detecting awareness in the vegetative state. Science (1979) 2006;313:1402. 10.1126/science.1130197 [DOI] [PubMed] [Google Scholar]
- Pahnke WN. Drugs and mysticism: an analysis of the relationship between psychedelic drugs and the mystical consciousness. Harvard University, 1963. [Google Scholar]
- Perl YS, Bocaccio H, Pérez-Ipiña I et al. Generative embeddings of brain collective dynamics using variational autoencoders. Phys Rev Lett 2020;125:238101. 10.1103/PhysRevLett.125.238101 [DOI] [PubMed] [Google Scholar]
- Perl YS, Pallavicini C, Piccinini J et al. Low-dimensional organization of global brain states of reduced consciousness. Cell Rep 2023;42:112491. 10.1016/j.celrep.2023.112491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson A, Tagliazucchi E, Weijer C. The ethics of psychedelic research in disorders of consciousness. Neurosci Conscious 2019;2019:1–8. 10.1093/nc/niz013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponce-Alvarez A, Jouary A, Privat M et al. Whole-brain neuronal activity displays crackling noise dynamics. Neuron 2018;100:1446–1459.e6. 10.1016/j.neuron.2018.10.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quednow BB, Kometer M, Geyer MA et al. Psilocybin-induced deficits in automatic and controlled inhibition are attenuated by ketanserin in healthy human volunteers. Neuropsychopharmacology 2012;37:630–40. 10.1038/npp.2011.228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankaduwa S, Owen AM. Psychedelics, entropic brain theory, and the taxonomy of conscious states: a summary of debates and perspectives. Neurosci Conscious 2023;2023:niad001. 10.1093/nc/niad001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhead JC, Soskin RA, Turek I et al. Psychedelic drug (DPT)-assisted psychotherapy with alcoholics: a controlled study. J Psychoactive Drugs 1977;9:287–300. 10.1080/02791072.1977.10472060 [DOI] [Google Scholar]
- Rosanova M, Fecchio M, Casarotto S et al. Sleep-like cortical OFF-periods disrupt causality and complexity in the brain of unresponsive wakefulness syndrome patients. Nat Commun 2018;9:4427. 10.1038/s41467-018-06871-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross S, Bossis A, Guss J et al. Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: a randomized controlled trial. J Psychopharmacol 2016;30:1165–80. 10.1177/0269881116675512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz LRD, Laureys S, Gosseries O. Towards modern post-coma care based on neuroscientific evidence. Int J Clin Health Psychol 2023;23:100370. 10.1016/j.ijchp.2023.100370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarasso S, Boly M, Napolitani M et al. Consciousness and complexity during unresponsiveness induced by propofol, xenon, and ketamine. Curr Biol 2015;25:3099–105. 10.1016/j.cub.2015.10.014 [DOI] [PubMed] [Google Scholar]
- Sarasso S, Casali AG, Casarotto S et al. Consciousness and complexity: a consilience of evidence. Neurosci Conscious 2021;2021:1–24. 10.1093/nc/niab023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage C, Mccabe OL. Residential psychedelic (LSD) therapy for the narcotic addict. A controlled study. Arch Gen Psychiatry 1973;28:808–14. 10.1001/archpsyc.1973.01750360040005 [DOI] [PubMed] [Google Scholar]
- Schartner MM, Carhart-Harris RL, Barrett AB et al. Increased spontaneous MEG signal diversity for psychoactive doses of ketamine, LSD and psilocybin. Sci Rep 2017;7:46421. 10.1038/srep46421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schartner MM, Pigorini A, Gibbs SA et al. Global and local complexity of intracranial EEG decreases during NREM sleep. Neurosci Conscious 2017;2017:1–12. 10.1093/nc/niw022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schartner M, Seth A, Noirhomme Q et al. Complexity of multi-dimensional spontaneous EEG decreases during propofol induced general anaesthesia. PLoS One 2015;10:e0133532. 10.1371/journal.pone.0133532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff ND. Cognitive motor dissociation following severe brain injuries. JAMA Neurol 2015;72:1413–5. 10.1001/jamaneurol.2015.2899 [DOI] [PubMed] [Google Scholar]
- Scott G, Carhart-Harris RL. Psychedelics as a treatment for disorders of consciousness. Neurosci Conscious 2019;2019:1–8. 10.1093/nc/niz003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth AK. Interoceptive inference, emotion, and the embodied self. Trends Cognit Sci 2013;17:565–73. 10.1016/j.tics.2013.09.007 [DOI] [PubMed] [Google Scholar]
- Simonsson O, Hendricks PS, Chambers R et al. Prevalence and associations of challenging, difficult or distressing experiences using classic psychedelics. J Affect Disord 2023;326:105–10. 10.1016/j.jad.2023.01.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton SP, Luppi AI, Carhart-Harris RL et al. Receptor-informed network control theory links LSD and psilocybin to a flattening of the brain’s control energy landscape. Nat Commun 2022;13:1–13. 10.1038/s41467-022-33578-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smigielski L, Scheidegger M, Kometer M et al. Psilocybin-assisted mindfulness training modulates self-consciousness and brain default mode network connectivity with lasting effects. Neuroimage 2019;196:207–15. 10.1016/j.neuroimage.2019.04.009 [DOI] [PubMed] [Google Scholar]
- Spriggs MJ, Douglass HM, Park RJ et al. Study protocol for “psilocybin as a treatment for anorexia nervosa: a pilot study”. Front Psychiatry 2021;12:1770. 10.3389/fpsyt.2021.735523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoliker D, Novelli L, Vollenweider FX et al. Effective connectivity of functionally anticorrelated networks under lysergic acid diethylamide. Biol Psychiatry 2023;93:224–32. 10.1016/j.biopsych.2022.07.013 [DOI] [PubMed] [Google Scholar]
- Strassman RJ. Adverse reactions to psychedelic drugs. A review of the literature. J Nerv Ment Dis 1984;172:577–95. 10.1097/00005053-198410000-00001 [DOI] [PubMed] [Google Scholar]
- Sweeney MM, Nayak S, Hurwitz ES et al. Comparison of psychedelic and near-death or other non-ordinary experiences in changing attitudes about death and dying. PLoS One 2022;17:e0271926. 10.1371/journal.pone.0271926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliazucchi E, Chialvo DR. Brain complexity born out of criticality. AIP Conf Proc 2013;1510:4–13 10.1063/1.4776495. [DOI] [Google Scholar]
- Thibaut A, Panda R, Annen J et al. Preservation of brain activity in unresponsive patients identifies MCS star. Ann Neurol 2021;90:89–100. 10.1002/ana.26095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibaut A, Schiff N, Giacino J et al. Therapeutic interventions in patients with prolonged disorders of consciousness. Lancet Neurol 2019;18:600–14. 10.1016/S1474-4422(19)30031-6 [DOI] [PubMed] [Google Scholar]
- Timmermann C, Bauer PR, Gosseries O et al. A neurophenomenological approach to non-ordinary states of consciousness: hypnosis, meditation, and psychedelics. Trends Cogn Sci 2023;27:139–59. 10.1016/j.tics.2022.11.006 [DOI] [PubMed] [Google Scholar]
- Timmermann C, Kettner H, Letheby C et al. Psychedelics alter metaphysical beliefs. Sci Rep 2021;11. 10.1038/s41598-021-01209-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmermann C, Roseman L, Williams L et al. DMT models the near-death experience. Front Psychol 2018;9:1–12. 10.3389/fpsyg.2018.01424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G. Consciousness as integrated information: a provisional manifesto. Biol Bull 2008;215:216–42. 10.2307/25470707 [DOI] [PubMed] [Google Scholar]
- Vanhaudenhuyse A, Demertzi A, Schabus M et al. Two distinct neuronal networks mediate the awareness of environment and of self. J Cogn Neurosci 2011;23:570–8. 10.1162/jocn.2010.21488 [DOI] [PubMed] [Google Scholar]
- Vargas MV, Dunlap LE, Dong C et al. Psychedelics promote neuroplasticity through the activation of intracellular 5-HT2A receptors. Science (1979) 2023;379:700–6. 10.1126/science.adf0435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viol A, Palhano-Fontes F, Onias H et al. Shannon entropy of brain functional complex networks under the influence of the psychedelic Ayahuasca. Sci Rep 2017;7:1–13. 10.1038/s41598-017-06854-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollenweider FX, Preller KH. Psychedelic drugs: neurobiology and potential for treatment of psychiatric disorders. Nat Rev Neurosci 2020;21:611–24. 10.1038/s41583-020-0367-2 [DOI] [PubMed] [Google Scholar]
- Walter J. Consciousness as a multidimensional phenomenon: implications for the assessment of disorders of consciousness. Neurosci Conscious 2021;2021:1–15. 10.1093/nc/niab047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteley C. Depression as a disorder of consciousness. Br J Philos Sci 2021. 10.1086/716838 [DOI] [Google Scholar]
- Yaden DB, Griffiths RR. The subjective effects of psychedelics are necessary for their enduring therapeutic effects. ACS Pharmacol Transl Sci 2020;4:568–72. 10.1021/acsptsci.0c00194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zasler ND, Aloisi M, Contrada M et al. Disorders of consciousness terminology: history, evolution and future directions. Brain Inj 2019;33:1684–9. 10.1080/02699052.2019.1656821 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No new data were generated or analysed in support of this research.


