Abstract
Background
Mobile health technology's impact on cardiovascular risk factor control is not fully understood. This study evaluates the association between interaction with a mobile health application and change in cardiovascular risk factors.
Methods and Results
Participants with hypertension with or without dyslipidemia enrolled in a workplace‐deployed mobile health application‐based cardiovascular risk self‐management program between January 2018 and December 2022. Retrospective evaluation explored the influence of application engagement on change in blood pressure (BP), total cholesterol (TC), low‐density lipoprotein cholesterol (LDL‐C), and weight. Multiple regression analyses examined the influence of guideline‐based, nonpharmacological lifestyle‐based digital coaching on outcomes adjusting for confounders. Of 102 475 participants, 49.1% were women. Median age was 53 (interquartile range, 43–61) years, BP was 134 (interquartile range, 124–144)/84 (interquartile range, 78–91) mm Hg, TC was 183 (interquartile range, 155–212) mg/dL, LDL‐C was 106 (82–131) mg/dL, and body mass index was 30 (26–35) kg/m2. At 2 years, participants with baseline systolic BP ≥140 mm Hg reduced systolic BP by 18.6 (SEM, 0.3) mm Hg. At follow up, participants with baseline TC ≥240 mg/dL reduced TC by 65.7 (SEM, 4.6) mg/dL, participants with baseline LDL‐C≥160 mg/dL reduced LDL‐C by 66.6 (SEM, 6.2) mg/dL, and participants with baseline body mass index ≥30 kg/m2 lost 12.0 (SEM, 0.3) pounds, or 5.1% of body weight. Interaction with digital coaching was associated with greater reduction in all outcomes.
Conclusions
A mobile health application‐based cardiovascular risk self‐management program was associated with favorable reductions in BP, TC, LDL‐C, and weight, highlighting the potential use of this technology in comprehensive cardiovascular risk factor control.
Keywords: artificial intelligence, blood pressure, cardiovascular diseases, cholesterol, heart disease risk factors, mobile health, weight loss
Subject Categories: Cardiovascular Disease, Diet and Nutrition, Exercise, Lifestyle, Risk Factors
Nonstandard Abbreviations and Acronyms
- DHI
digital health intervention
- mHealth
mobile health
- TC
total cholesterol
Research Perspective.
What Is New?
In this real‐world study of a mobile health application‐based cardiovascular risk self‐management program, fully automated lifestyle‐based digital coaching was associated with reduction in BP, total cholesterol, low‐density lipoprotein cholesterol, and weight.
These results highlight the potential use of mobile health technology in cardiovascular risk control and cardiovascular disease prevention.
What Question Should Be Addressed Next?
Further research is needed to examine the effectiveness of mobile health platforms on these and additional clinical outcomes in additional populations.
High blood pressure (BP) or hypertension is the most common, costly, but modifiable major risk factor for the development of cardiovascular disease (CVD), including heart failure and stroke, as well as premature death, both in the United States and globally. 1 This silent but pervasive condition affects nearly half (47%) of the US adult population. 2 Hypertension prevalence is highest among non‐Hispanic Black men and women, and education (specifically a college education) appears to be protective. 3 In 2019, the estimated direct and indirect cost associated with hypertension was $52.2 billion, with projections expected to triple by 2030. 2 Recognizing the clinical and economic burden of this issue, the US Department of Health and Human Services has designated hypertension as a top‐priority health indicator, issuing a national call to action aimed at reducing hypertension diagnoses and increasing control of BP among adults with established hypertension by 2030. 4
Hypertension rarely occurs in isolation and often coexists with ≥1 risk‐enhancing CVD comorbidities such as overweight/obesity, dyslipidemia, metabolic syndrome, and diabetes. 5 Global cardiovascular risk factor optimization through shared lifestyle and pharmacologic interventions markedly reduces CVD‐related morbidity and death. 6 , 7 , 8 Indeed, a recent American Heart Association scientific statement reinforces lifestyle modification as a critical component of first‐line treatment for individuals with mild to moderately elevated BP and blood cholesterol. 9 Despite well‐established guidelines, significant gaps persist in addressing global cardiovascular health and represent a significant public health challenge. Equally important is the need to bridge these gaps by promoting equitable access to care that empowers individuals with the tools and knowledge required to enable self‐management of comprehensive cardiovascular risk factor optimization. In this evolving landscape, mobile health (mHealth) technology emerges as a transformative solution to address these challenges. Given that 85% of US adults now own smartphones, mHealth interventions have the potential to play an important role in facilitating the prevention, management, and control of CVD risk factors. 10 , 11 , 12 Yet still, despite the ubiquity of smartphone‐based mHealth interventions, many lack an evidence‐based foundation and are often confined to addressing single risk factors or conditions, serving as what is commonly termed “point solutions.” 13
The current study aims to fill this gap by evaluating an mHealth application‐based digital health intervention (DHI) designed to support comprehensive cardiovascular risk factor modification. Briefly, this innovative DHI harnesses user‐generated data from a connected BP monitor, data from connected electronic health records, self‐reported metrics, and artificial intelligence capabilities to deliver personalized and evidence‐based lifestyle digital coaching for the self‐management of cardiovascular risk. Now, several years after its large‐scale implementation, the present study sets out to examine the impact of this mHealth application on comprehensive cardiovascular risk self‐management, specifically its influence on BP, total cholesterol (TC), low‐density lipoprotein cholesterol (LDL‐C), and weight within a large, geographically diverse population of adults with hypertension with or without dyslipidemia. As a secondary aim, the impact of digital coaching engagement on these primary outcome measures will be assessed.
Methods
Study Population
This study deployed a retrospective cohort, pre‐post observational design. Self‐measured and self‐reported data derived from the DHI (eg, Hello Heart) were deidentified, aggregated, and analyzed to determine the impact of the DHI on primary outcomes of interest. All study participants were aged ≥18 years, and enrollees of Hello Heart through their (or their spouse's/domestic partner's) employer‐based health plan. Individuals whose benefit structure includes access to Hello Heart were eligible to enroll in the program if they had hypertension, defined as prior diagnosis of hypertension and/or a medical or pharmacy insurance claim relating to hypertension, with or without dyslipidemia. All potentially eligible users were approached using mailed postcards, onsite enrollment communications, email, or the employer's benefits package online portal communications. Enrollment and participation in the program were voluntary. All DHI users self‐enlist by agreeing to the terms of service and privacy policy specifying research use of deidentified and securely encrypted data.
The study included U.S.‐based Hello Heart users who enrolled between January 1, 2018 and December 31, 2022. All study participants submitted/shared at least 2 BP measurements, at least 2 cholesterol readings, at least 2 weight measurements during the study period defined below (Figure S1). Potential participants were excluded if they did not meet the inclusion criteria.
This study was reviewed by the Western Institutional Review Board–Copernicus Group Institutional Review Board (tracking ID: 20226635) and determined to be exempt under 45 CFR 46.104(d)(4). In addition, a waiver of Health Insurance Portability and Accountability Act authorization for the use and disclosure of aggregated, deidentified data was obtained. No compensation was provided. The study adhered with the Strengthening the Reporting of Observational Studies in Epidemiology cohort reporting guidelines.
Intervention Description
The cardiovascular risk self‐management program included a smartphone application paired with a US Food and Drug Administration–cleared Bluetooth‐enabled BP monitor (Zewa UAM‐910BT, Zewa UAM‐905HH, or A&D UA‐651BLE). Participants track their BP, heart rate, cholesterol levels, activity levels, and weight via their personal smartphones. The application incorporates medication adherence reminders and clinically based digital coaching to drive lifestyle changes using algorithms based on individual usage patterns. The program incorporates mHealth best practices including ease of use, gamification, artificial intelligence, straightforward comprehension, and clarity to maximize user engagement. 14 , 15 The application organized medical data in a centralized mobile platform, and a subset of participants remotely connected their electronic health records to automatically populate clinical, laboratory, and medication use data. The user interface was available in English and Spanish.
Digital Coaching
The program involved on‐demand digital coaching derived from guidelines‐based recommendations for nonpharmacological interventions to promote healthy lifestyle, dietary habits, physical activity, stress reduction, and medication adherence. Artificial intelligence–based algorithms create a highly personalized user experience by considering participant characteristics (eg, sex), participant usage patterns (eg, which features of the application the participant interacts with), and participant feedback (eg, participants' ability to rate insights as “helpful” or “unhelpful”). Based on these inputs, the algorithm creates a personalized application experience, for example, prioritizing digital coaching tips that are similar to ones rated favorably by the user. This process is ongoing, continually improving the personalized insights it provides. The application also uses the Hook Model methodology, which creates habits of application use with a simple looping method of trigger, action, variable reward, and ongoing investment in the application. The digital coaching consisted of the items below.
Feedback After Each Reading
The application provided feedback after each BP or cholesterol reading that was added to the application, either automatically or via manual input by the participant. It informed participants in simple language what each reading meant and whether their readings were in the desired range. It also encouraged them to continue monitoring and taking their medications regularly. For critically high BP readings (>180/120 mm Hg), the application took participants through a repeat BP and symptom check process and encouraged them to contact a health care professional on the basis of the BP and presence of symptoms, consistent with clinical guidelines.
Daily Insights
Participants received daily easy‐to‐follow lifestyle coaching insights based on their individual characteristics (see above under Digital Coaching). These insights were based on medical guidelines and information from sources such as the Centers for Disease Control and Prevention and the American Heart Association, and included lifestyle, dietary (eg, Dietary Approaches to Stop Hypertension diet) and exercise‐related suggestions that may help in lowering BP, cholesterol, and weight (Figure S2). Insights are based on the Transtheoretical Model for health behavior change with messaging to support users in any of the 6 stages of change. 16 The digital coaching also reinforces self‐efficacy with insights to support intrinsic motivation for behavior change. A senior content writer with an advanced degree in public health developed these coaching insights, and their accuracy for information was confirmed by board‐certified physicians in internal medicine and/or cardiology.
Medication Tracking
Participants could add their medications in the application (including drug name, dosage, and frequency) and could set reminders to take them.
Correlation Insights
The application provided participants with insights showing the potential impact of their actions on their health to provide a directional feedback loop and encourage participants to improve and maintain healthy habits. For participants who logged weight and physical activity, the application provided correlation on longitudinal change in their weight or physical activity and change in their BP or cholesterol levels over time. For example, a recent increase in a participant's physical activity or a reduction in weight and its correlation with reduction in BP, if any, or a recent reduction in physical activity or weight gain and its correlation with an increase in BP, if any, was provided to participants (Figure S3).
For participants who added their medications in the application, the application showed a correlation between the initiation of a medication and change in BP over time (Figure S4).
Measurements
Participants self‐reported demographic data at the time of downloading the application including age, sex, geographic location, and relationship (employee or spouse/domestic partner). Comorbidities including diabetes, depression, and anxiety were self‐reported by participants in the application.
BP was self‐measured by participants using the Bluetooth‐enabled BP monitors noted above and using the same BP monitor throughout the evaluation period. Participants were included in the BP cohort if they had at least 2 BP readings available, including 1 at baseline and 1 at any of the follow‐up time points. Baseline BP was calculated as median systolic and diastolic BP during the first week of BP measurement after program enlistment. Follow‐up BP was calculated as weighted mean systolic and diastolic BP at 12 weeks (BP measurements for weeks 11–12), 1 year (BP measurements for weeks 48–55) and 2 years (BP measurements for weeks 96–111) as described previously. 17 Participants enrolled in and used the program at different times; hence, not all participants provided data for all time points. Participants engaged beyond the predefined follow‐up time points were not included in the hypertension cohort analysis unless they also collected BP measurements during the prespecified follow‐up time period. Change in systolic and diastolic BP was calculated as follow‐up BP minus baseline BP values. BP readings with systolic or diastolic BP values <30 mm Hg or >300 mm Hg were excluded.
For the majority of participants in the cholesterol cohort, TC and LDL‐C test results were directly imported into the application from electronic health records connected to the application. Participants could also enter their cholesterol results manually in the application. Participants were included in the cholesterol cohort if they had at least 2 cholesterol test results available that were at least 30 days apart, including a baseline cholesterol value defined as a TC or LDL‐C test result available up to 6 months before or 1 month after the registration in the program. Follow‐up cholesterol values were defined as the most recent TC or LDL‐C test result available for the participant during the study period. Change in TC or LDL‐C values was calculated as follow‐up TC or LDL‐C value minus baseline TC or LDL‐C value.
Participants self‐reported weight into the application. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Participants were included in the weight cohort if they had at least 2 weight measures available that were at least 30 days apart during the study period, including a baseline weight measure defined as weight measurement entered up to 1 month before or 1 month after the registration in the program. Follow‐up weight measure was defined as the most recent weight measurement available for the participant. Change in weight was calculated as follow‐up weight minus baseline weight. Baseline BMI was categorized as (1) normal, baseline BMI<25 kg/m2; (2) overweight, baseline BMI 25 to <30 kg/m2; and (3) obese, baseline BMI ≥30 kg/m2.
Objectively measured physical activity levels derived from participants' phone or wearable devices were automatically pushed to the application. The cumulative number of daily insights read, cumulative number of correlation insights read, and cumulative number of measurements added at each time point were calculated for each participant. These variables were used as a measure of interaction with digital coaching for that participant. Interaction with medication tracking was assessed by identifying participants who listed or imported their medications in the application.
Statistical Analysis
All participants who enrolled during the study duration and who met inclusion criteria were included. Participants were included in each of the BP, cholesterol, weight cohorts depending on the data available for each participant, and individual participants could be in multiple cohorts.
To study the association between interaction with digital coaching and change in systolic and diastolic BP, 1 mixed‐effects linear regression model was fit to systolic or diastolic BP readings at 0, 12, 52, and 104 weeks. Fixed‐effects program engagement variables included the number of BP readings, the number of daily insights viewed, the number of correlation insights viewed, as well as binary indicator variables for step activity, weight, and medication feature usage. Count variables were natural‐log transformed to reduce skew.
A mixed‐effects linear regression model was used to study the association between interaction with digital coaching and TC and LDL‐C. Fixed‐effects program engagement variables included the number of cholesterol tests and a binary indicator variable for cholesterol medication listed in the application. To control for variation in the duration between cholesterol measurements, cholesterol measurements were calculated as measurements per month and number of insights read were calculated as number per day.
A mixed‐effects linear regression model was used to study the association between interaction with digital coaching and weight. Number of weight measurements was included as a fixed‐effect program engagement variable. To control for variation in the duration between weight measurements, number of weight measurements per day and number of insights read per day were calculated.
All models included age, age squared, and sex as fixed effects. Age and age squared were scaled to capture their effects per every 1 year increase in age. Furthermore, all models used the raw scores as repeated outcomes and included a random intercept for employer name as well as participant. Interaction terms between each covariate and time were added to estimate the effect of each covariate on rate of change of the outcomes. Time was modeled as a categorical variable for BP models given that measurements were at consistent intervals, whereas time was modeled as a continuous variable for the cholesterol and weight models since the measurement of the outcomes were highly variable with respect to the baseline. Variance estimation employed restricted maximum likelihood estimation, while an unstructured covariance matrix was used for all models. The Bayesian information criterion was used for variable selection, selecting the model with the lowest Bayesian information criterion and the one with the most evidence of parsimony. 18 Continuous variables are presented as mean (SEM) or median (interquartile range [IQR]); categorical variables are presented as percentages. Statistical analyses were performed using R version 4.2.0 (R Foundation for Statistical Computing, Vienna, Austria). Statistical significance was defined at P<0.005. 19 In accordance with privacy and commercial use agreements, the data sets generated during and/or analyzed in the present study are not publicly available.
Results
Baseline Characteristics
Table 1 represents the baseline characteristics by study cohorts. A total of 102 475 participants met the inclusion criteria. The median age for the population was 53 (IQR, 43–61) years, and of the 83 647 with sex data available, 41 039 (49.1%) were women. Baseline systolic and diastolic BP were 134 (IQR, 124–144) mm Hg and 84 (IQR, 78–91) mm Hg, respectively. Baseline TC was 183 (IQR, 155–212) mg/dL, baseline LDL‐C was 106 (IQR, 82–131) mg/dL, baseline weight was 195 (IQR, 166–228) pounds, and baseline BMI was 30 (IQR, 26–35) kg/m2.
Table 1.
Baseline Characteristics for Study Population by Cohort
| Characteristics | Total population (N=102 475) | BP cohort (n=41 794) | Cholesterol cohort (n=1837) | Weight cohort (n=16 402) |
|---|---|---|---|---|
| Age, y, median (IQR) | 53 (43–61) | 56 (47–62) | 57 (49–64) | 55 (47–62) |
| Sex, n (%) | ||||
| Female | 41 039 (49.1) | 16 520 (47.2) | 804 (46.7) | 6954 (47.4) |
| Male | 42 608 (50.9) | 18 496 (52.8) | 919 (53.3) | 7721 (52.6) |
| Comorbidities, n (%) | ||||
| Diabetes | 7191 (7.0) | 3205 (7.7) | 258 (14.0) | 1448 (8.8) |
| Depression | 8547 (8.3) | 2897 (6.9) | 196 (10.7) | 1719 (10.5) |
| Anxiety | 15 544 (15.2) | 5355 (12.8) | 290 (15.8) | 2777 (16.9) |
| Baseline systolic BP, mm Hg, median (IQR) | 134 (124–144) | 133 (124–143) | 133 (125–143) | 132 (124–143) |
| Baseline diastolic BP, mm Hg, median (IQR) | 84 (78–91) | 83 (77–90) | 83 (77–89) | 83 (77–90) |
| Baseline total cholesterol, mg/dL | 183 (155–212) | 181 (154–210) | 180 (153–211) | 182 (156–211) |
| Baseline LDL‐ C, mg/dL | 106 (82–131) | 104 (81–129) | 102 (81–129) | 105 (83–131) |
| Baseline weight, pounds | 195 (166–228) | 194 (166–225) | 197 (170–230) | 198 (169–230) |
| Baseline BMI, kg/m2 | 30.0 (26.0–35.0) | 29.0 (26.0–34.0) | 30.3 (26.5–35.2) | 30.1 (26.6–34.7) |
BMI indicates body mass index; BP, blood pressure; IQR, interquartile range; and LDL‐C, low‐density lipoprotein cholesterol.
BP Cohort
Table 2 presents BP change over time for participants stratified by baseline BP. In participants with baseline systolic BP of 140 mm Hg or higher, at 1 year the median systolic BP was reduced for 4797 of 6012 participants (79.8%) with a mean reduction of 17.1 (SEM, 0.2) mm Hg in systolic BP and 9.4 (SEM, 0.1) mm Hg in diastolic BP. In participants with baseline systolic BP of 140 mm Hg or higher at 2 years, the median systolic BP was reduced for 2471 of 3037 participants (81.4%), with a mean reduction of 18.6 (SEM, 0.3) mm Hg in systolic BP and 9.9 (SEM, 0.2) mm Hg in diastolic BP.
Table 2.
BP Reduction Over Time by Baseline BP
| Time from enrollment | Participants at each time point, n* | Participants who reduced median systolic BP, n (%)* | Mean reduction in systolic BP (SEM)† | Mean reduction in diastolic BP (SEM)† |
|---|---|---|---|---|
| Baseline systolic BP between 120 mm Hg to 129 mm Hg | ||||
| Baseline | 10 253 | … | … | … |
| 12 wk | 7645 | 3566 (46.6) | −8.36 (0.1) | −4.10 (0.1) |
| 1 y | 4879 | 2164 (44.4) | −7.94 (0.1) | −4.51 (0.1) |
| 2 y | 3013 | 1261 (41.9) | −7.62 (0.2) | −4.48 (0.2) |
| Baseline systolic BP between 130 mm Hg and 139 mm Hg | ||||
| Baseline | 11 610 | … | … | … |
| 12 wk | 8976 | 5258 (58.7) | −9.56 (0.1) | −5.26 (0.1) |
| 1 y | 5350 | 3206 (59.9) | −10.0 (0.1) | −5.68 (0.1) |
| 2 y | 2885 | 1768 (61.3) | −10.1 (0.2) | −5.91 (0.2) |
| Baseline systolic BP ≥140 mm Hg | ||||
| Baseline | 14 055 | … | … | … |
| 12 wk | 10 940 | 8373 (76.5) | −15.6 (0.1) | −8.51 (0.1) |
| 1 y | 6012 | 4797 (79.8) | −17.1 (0.2) | −9.42 (0.1) |
| 2 y | 3037 | 2471 (81.4) | −18.6 (0.3) | −9.93 (0.2) |
BP indicates blood pressure.
Number of participants at each time represent participants who had application activity during the weeks listed. Since participants enrolled in the program at different times during the study period, duration of follow‐up was not the same for all participants and not all participants provided data for all time points.
Reduction was calculated in participants who reduced systolic BP.
Table S1 and Figure 1 present results from the mixed‐effects linear regression analysis to identify variables associated with change in systolic BP. At 12 weeks, the log‐transformed number of correlation insights read and log‐transformed number of BP measurements were associated with a reduction in systolic BP. At 1 year, male sex, log‐transformed number of correlation insights read, and log‐transformed number of BP measurements were associated with a reduction in systolic BP. At 2 years, male sex, log‐transformed number of correlation insights read, and log‐transformed number of BP measurements were associated with a reduction in systolic BP.
Figure 1. Variables associated with change in systolic BP.
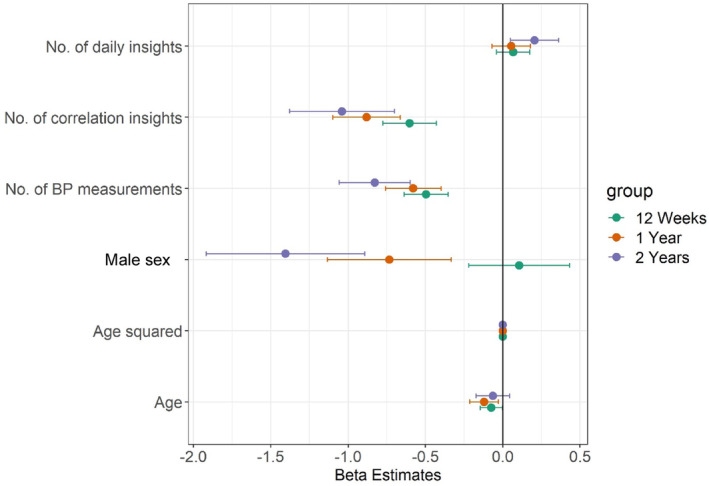
Dots represent estimates, and bars represent 95% CIs. BP indicates blood pressure.
Table S2 and Figure 2 present results from the mixed‐effects linear regression analysis to identify variables associated with change in diastolic BP. At 12 weeks, the log‐transformed number of correlation insights read and log‐transformed number of BP measurements were associated with a reduction in diastolic BP. At 1 year, male sex, log‐transformed number of correlation insights read, and log‐transformed number of BP measurements were associated with a reduction in diastolic BP. At 2 years, male sex, log‐transformed number of correlation insights read, and log‐transformed number of BP measurements were associated with a reduction in diastolic BP.
Figure 2. Variables associated with change in diastolic BP.
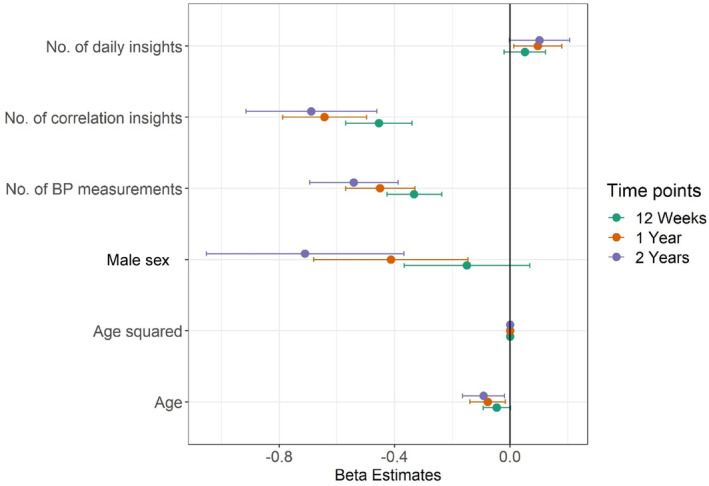
Dots represent estimates, and bars represent 95% CIs. BP indicates blood pressure.
Cholesterol Cohort
A total of 1837 participants were included in the cholesterol cohort. Median duration between baseline and follow‐up cholesterol tests was 13.0 (IQR, 8.4–24.0) months. Table 3 presents TC reduction over time for participants by baseline TC. In participants with baseline TC ≥240 mg/dL, 83.5% had reduced TC, with a mean reduction of 65.7 (SEM, 4.6) mg/dL.
Table 3.
Reduction in TC by Baseline TC
| Baseline TC, mg/dL | Number of users reduced TC (%) | Baseline TC, mean (SEM) | Follow‐up TC, mean (SEM) | Mean reduction (SEM) | Percent reduction, mean (SEM) |
|---|---|---|---|---|---|
| Overall | 669 (53.4) | 183 (1.2) | 177 (1.2) | −29.0 (1.1) | −14.1 (0.5) |
| TC <200 | 386 (45.2) | 160 (0.9) | 162 (1.1) | −19.4 (0.9) | −11.7 (0.5) |
| TC 200 to 239 | 192 (66.4) | 218 (0.7) | 204 (2.0) | −30.9 (1.9) | −14.1 (0.9) |
| TC ≥240 | 91 (83.5) | 266 (2.4) | 214 (4.7) | −65.7 (4.6) | −24.3 (1.6) |
Negative value represents reduction in total cholesterol. Baseline TC, follow‐up TC, and mean reduction values are reported in units of mg/dL. The cholesterol cohort was composed predominantly (>90%) of users who imported cholesterol values from a connected electronic health record. Hello Heart has since launched an easier way to connect electronic health records. TC indicates total cholesterol.
Table S3 and Figure 3 present results from the mixed‐effects linear regression analysis to identify variables associated with change in TC. Rate of cholesterol measurement and cholesterol medication listed in the application were significantly associated with a reduction in TC levels.
Figure 3. Estimates for variables associated with change in total cholesterol.
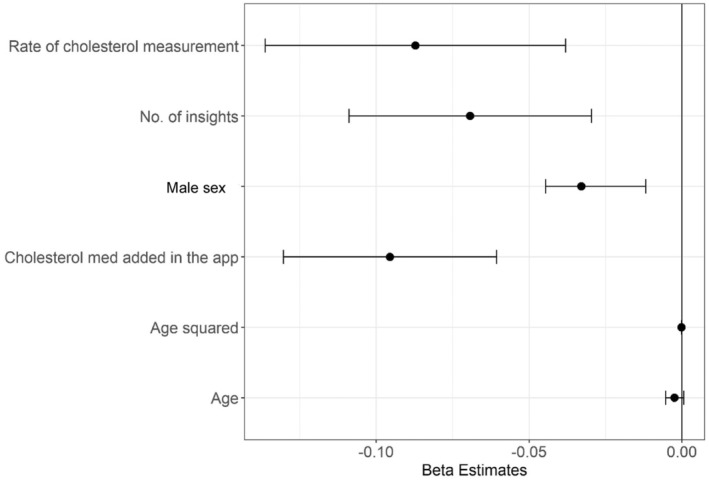
Dots represent estimates, and bars represent 95% CIs. App indicates application; and med, medication.
Table 4 presents LDL‐C reduction over time for participants by baseline LDL‐C. In participants with LDL ≥160 mg/dL at baseline, 79.8% reduced LDL‐C with a mean reduction of 66.6 (SEM, 6.2) mg/dL.
Table 4.
Reduction in LDL‐C by Baseline LDL‐C
| Baseline LDL‐C, mg/dL | Number of users reduced LDL‐C, n (%) | Baseline LDL‐C, mean (SEM) | Follow up LDL‐C, mean (SEM) | Mean reduction (SEM) | Percent reduction, mean (SEM) |
|---|---|---|---|---|---|
| Overall | 665 (52.6) | 106 (1.0) | 101 (1.0) | −26.1 (1.2) | −20.8 (0.7) |
| LDL‐C <100 | 255 (42.9) | 75.9 (0.7) | 80.6 (1.1) | −13.4 (0.7) | −17.3 (0.9) |
| LDL‐C 100–159 | 331 (58) | 125 (0.7) | 117 (1.3) | −26.2 (1.2) | −20.3 (0.9) |
| LDL‐C 160 to–189 | 54 (79.4) | 172 (1.1) | 133 (4.8) | −52.3 (4.7) | −30.1 (2.7) |
| LDL‐C ≥190 | 25 (80.6) | 214 (7.0) | 140 (11.4) | −97.6 (15.3) | −42.9 (4.8) |
| LDL‐C ≥160 | 79 (79.8) | 185 (3.0) | 135 (4.8) | −66.6 (6.2) | −34.2 (2.5) |
Negative value represents reduction in LDL‐C. Baseline LDL‐C, follow‐up LDL‐C, and mean reduction values are reported in units of mg/dL. A total of 22.2% of users achieved reduction to LDL‐C <70 mg/dL. The cholesterol cohort was composed predominantly (>90%) of users who imported cholesterol values from a connected electronic health record. Hello Heart has since launched an easier way to connect electronic health records. LDL‐C indicates low‐density lipoprotein cholesterol.
Table S4 and Figure 4 present results from the mixed‐effects linear regression analysis to identify variables associated with change in LDL‐C. The rate of cholesterol measurement was significantly associated with a reduction in LDL‐C levels.
Figure 4. Estimates for variables associated with change in LDL cholesterol.
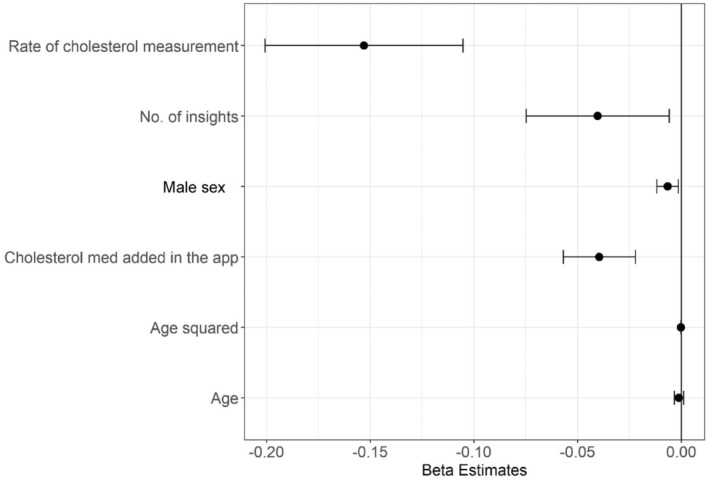
Dots represent estimates, and bars represent 95% CIs. App indicates application; and med, medication.
Weight Cohort
A total of 16 402 participants were included in the weight cohort. The median duration between baseline and follow‐up weight measurement was 7.2 (IQR, 3.2–15.0) months. Table 5 presents weight reduction over time for participants by baseline BMI. In participants with obese BMI (≥ 30 kg/m2) at baseline, 64.0% reduced weight with a mean reduction of 12.0 (SEM, 0.3) pounds, corresponding to 5.1% mean reduction.
Table 5.
Reduction in Weight in Pounds by Baseline BMI Category
| Category | Number of users reduced weight | Baseline weight, mean (SEM) | Follow‐up weight, mean (SEM) | Reduction in weight, mean (SEM) | Percent reduction, mean (SEM) |
|---|---|---|---|---|---|
| Overall | 6126 (59.3) | 203 (0.5) | 200 (0.5) | −9.37 (0.2) | −4.39 (0.1) |
| Healthy (BMI <25 kg/m2) | 750 (47.6) | 147 (0.5) | 149 (0.6) | −4.54 (0.1) | −3.11 (0.1) |
| Overweight (BMI 25 to <30 kg/m2) | 2056 (57.8) | 182 (0.4) | 181 (0.4) | −6.86 (0.2) | −3.78 (0.1) |
| Obese (BMI ≥30 kg/m2) | 3320 (64.0) | 234 (0.6) | 229 (0.6) | −12.0 (0.3) | −5.05 (0.1) |
Negative value represents reduction in weight; Healthy=baseline BMI<25 kg/m2; overweight=baseline BMI 25 to <30 kg/m2; obese=baseline BMI ≥30 kg/m2 Baseline weight, follow‐up weight, and mean reduction values are reported in units of pounds. Among participants who started with BMI ≥30 kg/m2, 11.1% reduced to BMI<30 kg/m2. Among participants who started with BMI ≥27 kg/m2, 7.3% reduced to BMI<27 kg/m2. BMI indicates body mass index.
Table S5 and Figure 5 present results from the mixed‐effects linear regression analysis to identify variables associated with change in weight. Rate of insights read and rate of weight measurements were significantly associated with reduction in weight, while male sex was significantly associated with increase in weight.
Figure 5. Estimates for variables associated with change in weight.
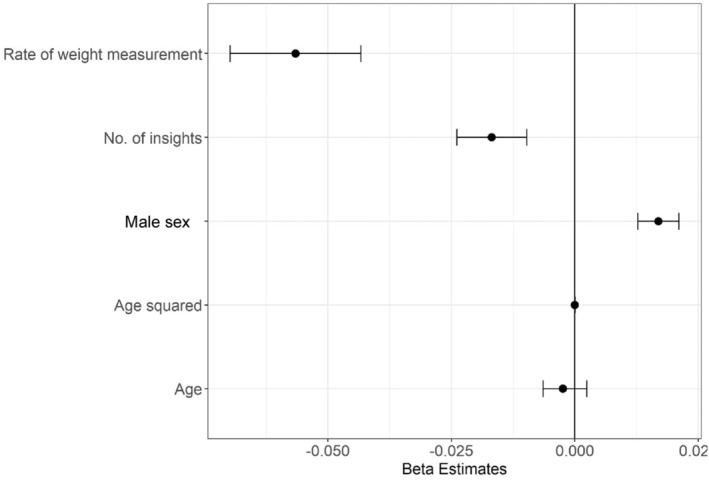
Dots represent estimates, and bars represent 95% CIs.
Discussion
In a large cohort of individuals with suboptimal cardiovascular risk factors and enrolled in a mHealth application‐based self‐management program, this study showed the following key results: (1) There were clinically meaningful reductions in systolic BP, diastolic BP, TC, LDL‐C, and weight; and (2) interaction with lifestyle‐based, automated digital coaching was associated with a greater reduction in BP, TC, LDL‐C, and weight.
Hypertension, dyslipidemia, and obesity are major risk factors for CVD and cardiovascular death, yet controlling these risk factors remains challenging. Only 1 in 4 adults with hypertension have their BP under control, only slightly more than half of adults who would benefit from a statin for cholesterol management are taking one, and nearly three quarters of adults are overweight or obese. 20 , 21 , 22 Effective control of these risk factors using lifestyle, dietary, and pharmacologic interventions is shown to reduce the incidence of CVD and associated morbidity and death. 6 , 7 , 8
Prior studies assessing the role of mHealth application‐based behavioral coaching in risk factor management have reported mixed results. A systematic review by Tucker et al on self‐monitoring of BP in hypertension found that self‐monitoring of BP alone was not associated with BP reduction, but self‐monitoring in conjunction with cointerventions including treatment titration or lifestyle education led to clinically significant BP reduction. 23 A randomized clinical trial assessing the effect of a biweekly telephone‐based health coaching for 6 months on hypertension found no difference in BP reduction between the intervention and control groups. 24 The study found that participants receiving coaching demonstrated a significant increase in physical activity, reduction in sodium intake, and increased frequency in home BP monitoring than the control group. In a separate study assessing the use of artificial intelligence–based digital coaching program in BP control and weight loss, there was an overall reduction in BP associated with engagement with the program; digital coaching was not associated with a reduction in systolic BP but was associated with weight reduction. 25 In the current study, a fully automated, mHealth application‐based cardiovascular risk self‐management program among individuals with hypertension was associated with a reduction in BP, TC, LDL‐C, and weight.
Gazit et al previously reported a significant reduction in BP among a population using the Hello Heart mHealth application‐based BP self‐management program. 17 In that study, 84% of members with baseline BP >140/90 mm Hg reduced BP, sustained up to 3 years, and the mean reduction in systolic BP in that group was 21 mm Hg. In the current study, similar outcomes were observed, with >80% of participants with systolic BP 140 mm Hg or higher observing a mean reduction in systolic BP, with a mean reduction of 18.6 mm Hg at 2 years. Reductions of this magnitude are clinically meaningful, as prior studies have reported that a 5‐mm Hg reduction of systolic BP reduced the risk of major cardiovascular events by about 10%, while a 10‐mm Hg reduction of systolic BP reduced the risk of major cardiovascular events by 20%. 6 , 26 It is notable that this outcome is replicated in the current study in a study population that is much larger in sample size and with recruitment spanning over a longer time period, including the COVID‐19 pandemic, during which other studies have reported an overall worsening trend in BP control. 27 , 28 The BP reduction is maintained despite adding more condition‐specific pathways to support additional cardiovascular risk factors, and in fact, the other outcomes reported in this study only reinforce the pleiotropic benefits of lifestyle modification.
In addition, over a median duration of 13 months, participants in this program observed a significant reduction in TC and LDL‐C. Among users with LDL‐C of 160 to 189 mg/dL, 79.4% reduced LDL‐C, with a mean reduction of 52.3 mg/dL. The reduction was even high among users with LDL‐C≥190 mg/dL, where 80.6% reduced LDL‐C, with a mean reduction of 97.6 mg/dL. Prior studies have reported that for every 38.7 mg/dL (1 mmol/L) decrease in TC, there was a 17.5% reduction in relative risk for all‐cause death; 24.5% reduction in coronary heart disease–related death; and 29.5% reduction in any coronary heart disease event. 29 Similarly impressive reductions are noted in a meta‐analysis of LDL‐C reduction, where for each 38.7‐mg/dL reduction in LDL‐C, there was a 23% reduction in major vascular events, which included cardiovascular deaths, heart attacks, and strokes. 7 Blood et al previously reported reduction in LDL‐C in participants of a large health care system who received remote monitoring and guidelines‐based educational coaching by navigators and pharmacists. 30 To our knowledge, the current study is the first to report a reduction in cholesterol levels in participants enrolled in a fully automated mHealth application‐based cardiovascular risk self‐management program.
An important finding of our study was the association between adding a cholesterol medication in the application and reduction in TC, highlighting the potential role of a medication‐tracking feature in improving medication adherence. Suboptimal statin adherence is a major problem in cholesterol management and has been shown to be associated with greater risk of cardiovascular death. 31 The 2018 cholesterol guideline also emphasized the importance of tracking statin adherence in routine follow‐up visits. 32 In this study, participants could add medications and set reminders to take medications in the application. Further studies on the use of medication tracking using mHealth technology to improve medication adherence are needed to understand its utility in better disease management.
Participants in the program with baseline BMI in the overweight and obese categories achieved significant weight reduction, with a mean weight loss of 6.86 pounds for baseline overweight BMI and 12.0 pounds for baseline obese BMI over a median duration of 7.2 months. This is a clinically meaningful improvement, as prior trials have demonstrated that individuals who lost at least 10% of their body weight had a 21% lower risk of a composite of death from cardiovascular causes, nonfatal acute myocardial infarction, nonfatal stroke, or admission to a hospital for angina. 33 A prior meta‐analysis assessing the relationship between duration of lifestyle interventions on long‐term weight loss found a rate of weight reduction of about 0.9 pounds per month in individuals who received frequent and sustained lifestyle interventions. 34 In the current study, participants received regular and sustained coaching with daily insights on dietary‐ and exercise‐related recommendations and correlation insights that provided feedback on the impact of change in their physical activity level or weight on their BP levels, encouraging them to improve or maintain a healthy lifestyle.
Despite advances in therapeutic interventions that are proven to help in the primary and secondary prevention of CVD, it still remains the leading cause of morbidity and death in the United States. There has been an increasing focus on comprehensive cardiovascular risk factor control including hypertension, dyslipidemia, and obesity. 35 Multiple studies assessing pharmacological and lifestyle interventions have reported substantial reductions in cardiovascular outcomes with comprehensive risk factor control in various populations. 36 , 37 , 38 , 39 This study highlights the potential role of mHealth technology with lifestyle‐based digital coaching in comprehensive cardiovascular risk factor control. Such technology may help individuals to be active participants in managing their own cardiovascular risk by adopting a healthier lifestyle, better monitoring of their risk factors, and better medication adherence. 40 Future studies including randomized control trials assessing the effectiveness of a mobile health technology with lifestyle‐based digital coaching in reducing CVD and cardiovascular death may help us understand its role in the prevention of cardiovascular disease.
The strengths of this study are the large population of participants in the program with >2 years of real‐world follow‐up data. The study population was situated in geographically diverse locations across a variety of blue‐ and white‐collar industries. This study observed comprehensive improvements across a variety of cardiovascular risk factors and was able to examine which features of the mHealth solution were associated with improvements across these risk factors. Finally, the results replicate BP reduction previously noted among a population using the Hello Heart application and build on this with additional findings in cholesterol and weight reduction. This real‐world evidence of comprehensive cardiovascular risk factor improvement with a BP monitor connected to a smartphone application with automated lifestyle coaching demonstrates the potential of this strategy to achieve better cardiovascular outcomes.
Limitations
As this was an observational study, one cannot make a causal inference based on the results. The study population consisted of middle‐aged individuals with employer‐sponsored health insurance; thus, these results cannot be generalized to other populations such as those not employed or those without health insurance. Although the sample size of the study was large, this does not exclude the possibility of selection bias, which would be present if the trends for those who remained engaged with the program were systematically different than those who did not remain engaged. Due to the retrospective nature of data collection, the possibility of other unmeasured factors confounding our results, such as an addition of a new treatment by the health care team, cannot be excluded, although the application encouraged participants to regularly engage with their care team for preventive care. Furthermore, we employed mixed‐effects regression models that assume data are missing at random. Because this is an unverifiable assumption, our results could be biased if this assumption is not achieved in reality. In addition, the possibility of error during BP measurements and/or human error during manual entry of readings cannot be excluded, although the majority of BP and cholesterol readings were automatically imported from a BP monitor or electronic health record, respectively. In addition, data‐cleaning steps were taken to reduce the impact of measurement error, and BP readings were derived by taking a median of measurements taken during the week. Additionally, to address human error during measurement, the application prompted users to verify or recheck if an extreme reading was entered. We could not verify participant medication adherence using external data sources. As such, we could not determine the impact of the initiation or intensification of lipid‐lowering therapy not reported in the application on changes in TC and LDL‐C levels. Finally, diabetes control in participants was not evaluated, as diabetes management was not a part of the program.
Conclusions
In this real‐world study of an mHealth application‐based cardiovascular risk self‐management program, fully automated lifestyle‐based digital coaching was associated with reduction in BP, TC, LDL‐C, and weight. These results highlight the potential use of mHealth technology in cardiovascular risk control and CVD prevention.
Sources of Funding
This study was funded by Hello Heart.
Disclosures
Drs Paz, Pargaonkar, and Gazit and B. J. Roach, M. Meadows, and J. M. Roberts are employed by Hello Heart and receive equity from Hello Heart. Dr Paz is also employed by White Plains Hospital. Drs Zaleski, Craig, and Serra are employed by CVS Health Corporation and hold stock and receive equity from CVS Health. Dr Zaleski is also employed by Hartford Hospital. Dr Dunn is employed by the American Heart Association. Dr Michos has served as a consultant for Amgen, AstraZeneca, Boehringer Ingelheim, Edwards Life Science, Esperion, Medtronic, Merck, New Amsterdam, Novartis, Novo Nordisk, and Pfizer. She is supported by the Amato Fund in Women's Cardiovascular Health Research at Johns Hopkins University and American Heart Association Grants 946 222, 20SFRN35380046, and 20SFRN35490003.
Supporting information
Tables S1–S5.
Figures S1–S4.
Acknowledgments
The authors gratefully acknowledge colleagues and collaborators at Hello Heart, CVS Health, and the American Heart Association, respectively, for their ongoing leadership and digital product support.
This manuscript was sent to Mahasin S. Mujahid, PhD, MS, FAHA, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.033328
For Sources of Funding and Disclosures, see page 12.
References
- 1. Vaduganathan M, Mensah GA, Turco JV, Fuster A, Roth GA. The global burden of cardiovascular diseases and risk: a compass for future health. J Am Coll Cardiol. 2022;80:2361–2371. DOI: 10.1016/j.jacc.2022.11.005 [DOI] [PubMed] [Google Scholar]
- 2. Tsao CW, Aday AW, Almarzooq ZI, Anderson CAM, Arora P, Avery CL, Baker‐Smith CM, Beaton AZ, Boehme AK, Buxton AE, et al. Heart disease and stroke Statistics‐2023 update: a report from the American Heart Association. Circulation. 2023;147:e93–e621. DOI: 10.1161/CIR.0000000000001123 [DOI] [PubMed] [Google Scholar]
- 3. Ostchega Y, Fryar CD, Nwankwo T, Nguyen DT. Hypertension prevalence among adults aged 18 and over: United States, 2017–2018. NCHS Data Brief. 2020;364:1–8. https://www.cdc.gov/nchs/data/databriefs/db364‐h.pdf [PubMed] [Google Scholar]
- 4. Healthy People . Heart disease and stroke. 2030. [Internet]. Accessed October 3, 2023. https://health.gov/healthypeople/objectives‐and‐data/browse‐objectives/heart‐disease‐and‐stroke.
- 5. Schmieder RE, Ruilope LM. Blood pressure control in patients with comorbidities. J Clin Hypertens. 2008;10:624–631. DOI: 10.1111/j.1751-7176.2008.08172.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. The Blood Pressure Lowering Treatment Trialists' Collaboration . Pharmacological blood pressure lowering for primary and secondary prevention of cardiovascular disease across different levels of blood pressure: an individual participant‐level data meta‐analysis. Lancet. 2021;397:1625–1636. DOI: 10.1016/S0140-6736(21)00590-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Silverman MG, Ference BA, Im K, Wiviott SD, Giugliano RP, Grundy SM, Braunwald E, Sabatine MS. Association between lowering LDL‐C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta‐analysis. JAMA. 2016;316:1289–1297. DOI: 10.1001/jama.2016.13985 [DOI] [PubMed] [Google Scholar]
- 8. Ma S, Avenell A, Bolland M, Hudson J, Stewart F, Robertson C, Sharma P, Fraser C, MacLennan G. Effects of weight loss interventions for adults who are obese on mortality, cardiovascular disease, and cancer: systematic review and meta‐analysis. BMJ. 2017;359:j4849. DOI: 10.1136/bmj.j4849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gibbs BB, Hivert M‐F, Jerome GJ, Kraus WE, Rosenkranz SK, Schorr EN, Spartano NL, Lobelo F. Physical activity as a critical component of first‐line treatment for elevated Blood pressure or cholesterol: who, what, and how?: a scientific statement from the American Heart Association. Hypertension. 2021;78:e26–e37. DOI: 10.1161/HYP.0000000000000196 [DOI] [PubMed] [Google Scholar]
- 10. Pew Research Center . Mobile Fact Sheet. [Internet]. Accessed October 3, 2023. https://www.pewresearch.org/internet/fact‐sheet/mobile/.
- 11. Schorr EN, Gepner AD, Dolansky MA, Forman DE, Park LG, Petersen KS, Still CH, Wang TY, Wenger NK. Harnessing mobile health technology for secondary cardiovascular disease prevention in older adults: a scientific statement from the American Heart Association. Circ Cardiovasc Qual Outcomes. 2021;14:e000103. DOI: 10.1161/HCQ.0000000000000103 [DOI] [PubMed] [Google Scholar]
- 12. Shan R, Ding J, Plante TB, Martin SS. Mobile health access and use among individuals with or at risk for cardiovascular disease: 2018 health information National Trends Survey (HINTS). J Am Heart Assoc. 2019;8:e014390. DOI: 10.1161/JAHA.119.014390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tomlinson M, Rotheram‐Borus MJ, Swartz L, Tsai AC. Scaling up mHealth: where is the evidence? PLoS Med. 2013;10:e1001382. DOI: 10.1371/journal.pmed.1001382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Miller AS, Cafazzo JA, Seto E. A game plan: gamification design principles in mHealth applications for chronic disease management. Health Informatics J. 2016;22:184–193. DOI: 10.1177/1460458214537511 [DOI] [PubMed] [Google Scholar]
- 15. Anderson K, Burford O, Emmerton L. Mobile health apps to facilitate self‐care: a qualitative study of user experiences. PLoS One. 2016;11:e0156164. DOI: 10.1371/journal.pone.0156164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Prochaska JO, Velicer WF. The transtheoretical model of health behavior change. Am J Health Promot. 1997;12:38–48. DOI: 10.4278/0890-1171-12.1.38 [DOI] [PubMed] [Google Scholar]
- 17. Gazit T, Gutman M, Beatty AL. Assessment of hypertension control among adults participating in a mobile technology blood pressure self‐management program. JAMA Netw Open. 2021;4:e2127008. DOI: 10.1001/jamanetworkopen.2021.27008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sakamoto Y, Kitagawa G. Akaike Information Criterion Statistics. Kluwer Academic Publishers; 1987. [Google Scholar]
- 19. Johnson VE. Revised standards for statistical evidence. PNAS. 2013;110:19313–19317. DOI: 10.1073/pnas.1313476110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Million Hearts . Estimated hypertension prevalence, treatment, and control among U.S. adults. [Internet]. Accessed October 3, 2023. https://millionhearts.hhs.gov/data‐reports/hypertension‐prevalence.html.
- 21. Wall HK, Ritchey MD, Gillespie C, Omura JD, Jamal A, George MG. Vital signs: prevalence of key cardiovascular disease risk factors for million hearts 2022—United States, 2011–2016. MMWR Morb Mortal Wkly Rep. 2018;67:983–991. DOI: 10.15585/mmwr.mm6735a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. National Center for Chronic Disease Prevention and Health Promotion (NCCDPHP) . Heart disease and stroke. [Internet]. Accessed October 3, 2023. https://www.cdc.gov/chronicdisease/resources/publications/factsheets/heart‐disease‐stroke.htm.
- 23. Tucker KL, Sheppard JP, Stevens R, Bosworth HB, Bove A, Bray EP, Earle K, George J, Godwin M, Green BB, et al. Self‐monitoring of blood pressure in hypertension: a systematic review and individual patient data meta‐analysis. PLoS Med. 2017;14:e1002389. DOI: 10.1371/journal.pmed.1002389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hoppe KK, Smith M, Birstler J, Kim K, Sullivan‐Vedder L, LaMantia JN, Knutson Sinaise MR, Swenson M, Fink J, Haggart R. Effect of a telephone health coaching intervention on hypertension control in young adults: the MyHEART randomized clinical trial. JAMA Netw Open. 2023;6:e2255618. DOI: 10.1001/jamanetworkopen.2022.55618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Branch OH, Rikhy M, Auster‐Gussman LA, Lockwood KG, Graham SA. Relationships between blood pressure reduction, weight loss, and engagement in a digital app–based hypertension care program: observational study. JMIR Form Res. 2022;6:38215. DOI: 10.2196/38215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, Chalmers J, Rodgers A, Rahimi K. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta‐analysis. Lancet. 2016;10022:P957–P967. DOI: 10.1016/S0140-6736(15)01225-8 [DOI] [PubMed] [Google Scholar]
- 27. Gotanda H, Liyanage‐Don N, Moran AE, Krousel‐Wood M, Green JB, Zhang Y, Nuckols TK. Changes in Blood pressure outcomes among hypertensive individuals during the COVID‐19 pandemic: a time series analysis in three US healthcare organizations. Hypertension. 2022;79:2733–2742. DOI: 10.1161/HYPERTENSIONAHA.122.19861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shah NP, Clare RM, Chiswell K, Navar AM, Shah BR, Peterson EDMD. Trends of blood pressure control in the U.S. during the COVID‐19 pandemic. Am Heart J. 2022;247:15–23. DOI: 10.1016/j.ahj.2021.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gould AL, Davies GM, Alemao E, Yin DD, Cook JR. Cholesterol reduction yields clinical benefits: meta‐analysis including recent trials. Clin Ther. 2007;29:778–794. DOI: 10.1016/j.clinthera.2007.05.012 [DOI] [PubMed] [Google Scholar]
- 30. Blood AJ, Cannon CP, Gordon WJ, Mailly C, MacLean T, Subramaniam S, Tucci M, Crossen J, Nichols H, Wagholikar KB, et al. Results of a remotely delivered hypertension and lipid program in more than 10 000 patients across a diverse health care network. JAMA Cardiol. 2023;8:12–21. DOI: 10.1001/jamacardio.2022.4018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rodriguez F, Maron DJ, Knowles JW, Virani SS, Lin S, Heidenreich PA. Association of statin adherence with mortality in patients with atherosclerotic cardiovascular disease. JAMA Cardiol. 2019;4:206–213. DOI: 10.1001/jamacardio.2018.4936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella‐Tommasino J, Forman DE, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/pcna guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2019;139:e1082–e1143. DOI: 10.1161/CIR.0000000000000625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Look AHEAD Research Group , Gregg E, Jakicic J, Blackburn G, Bloomquist P, Bray G, Clark J, Coday M, Curtis J, Egan C. Association of the magnitude of weight loss and changes in physical fitness with long‐term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: a post‐hoc analysis of the look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol. 2016;4:913–921. DOI: 10.1016/S2213-8587(16)30162-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Singh N, Stewart RAH, Benatar JR. Intensity and duration of lifestyle interventions for long‐term weight loss and association with mortality: a meta‐analysis of randomised trials. BMJ Open. 2019;9:e029966. DOI: 10.1136/bmjopen-2019-029966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Joseph JJ, Deedwania P, Acharya T, Aguilar D, Bhatt DL, Chyun DA, Di Palo KE, Golden SH, Sperling LS on behalf of the American Heart Association Diabetes Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Clinical Cardiology; and Council on Hypertension . Comprehensive management of cardiovascular risk factors for adults with type 2 diabetes: a scientific statement from the American Heart Association. Circulation. 2022;145:e722–e759. DOI: 10.1161/CIR.0000000000001040 [DOI] [PubMed] [Google Scholar]
- 36. Bittner V, Bertolet M, Felix RB, Farkouh ME, Goldberg S, Ramanathan KB, Redmon JB, Sperling L, Rutter MK; BARI 2D Study Group . Comprehensive cardiovascular risk factor control improves survival: the BARI 2D trial. J Am Coll Cardiol. 2015;66:765–773. DOI: 10.1016/j.jacc.2015.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jeemon P, Prabhakaran D, Goenka S, Ramakrishnan L, Padmanabhan S, Huffman M, Joshi P, Sivasankaran S, Mohan BVM, Ahmed F, et al. Impact of comprehensive cardiovascular risk reduction programme on risk factor clustering associated with elevated blood pressure in an Indian industrial population. IJMR. 2012;135:485–493. https://journals.lww.com/ijmr/fulltext/2012/35040/impact_of_comprehensive_cardiovascular_risk.7.aspx [PMC free article] [PubMed] [Google Scholar]
- 38. Schwalm JD, McCready T, Lopez‐Jaramillo P, Yusoff K, Attaran A, Lamelas P, Camacho PA, Majid F, Bangdiwala SI, Thabane L, et al. A community‐based comprehensive intervention to reduce cardiovascular risk in hypertension (HOPE 4): a cluster‐randomised controlled trial. Lancet. 2019;394:1231–1242. DOI: 10.1016/S0140-6736(19)31949-X [DOI] [PubMed] [Google Scholar]
- 39. Ijzelenberg W, Hellemans IM, van Tulder MW, Heymans MW, Rauwerda JA, van Rossum AC, Seidell JC. The effect of a comprehensive lifestyle intervention on cardiovascular risk factors in pharmacologically treated patients with stable cardiovascular disease compared to usual care: a randomised controlled trial. BMC Cardiovasc Disord. 2012;12:71. DOI: 10.1186/1471-2261-12-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cheikh‐Moussa K, Mira JJ, Orozco‐Beltran D. Improving engagement among patients with chronic cardiometabolic conditions using mHealth: critical review of reviews. JMIR Mhealth Uhealth. 2020;8:e15446. DOI: 10.2196/15446 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S5.
Figures S1–S4.


