Abstract
The role of robotics has grown exponentially. There is an active interest amongst practitioners in the transferability of the potential benefits into plastic and reconstructive surgery; however, many plastic surgeons report lack of widespread implementation, training, or clinical exposure. We report the current evidence base, and surgical opportunities, alongside key barriers, and limitations to overcome, to develop the use of robotics within the field. This systematic review of PubMed, Medline, and Embase has been conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PROSPERO (ID: CRD42024524237). Preclinical, educational, and clinical articles were included, within the scope of plastic and reconstructive surgery. 2, 181, articles were screened; 176 articles met the inclusion criteria across lymph node dissection, flap and microsurgery, vaginoplasty, craniofacial reconstruction, abdominal wall reconstruction and transoral robotic surgery (TOR). A number of benefits have been reported including technical advantages such as better visualisation, improved precision and accuracy, and tremor reduction. Patient benefits include lower rate of complications and quicker recovery; however, there is a longer operative duration in some categories. Cost presents a significant barrier to implementation. Robotic surgery presents an exciting opportunity to improve patient outcomes and surgical ease of use, with feasibility for many subspecialities demonstrated in this review. However, further higher quality comparative research with careful case selection, which is adequately powered, as well as the inclusion of cost-analysis, is necessary to fully understand the true benefit for patient care, and justification for resource utilisation.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11701-024-01987-7.
Keywords: Robotic, Robotic assisted, Plastic and reconstructive surgery
Introduction
The role of robotics has grown exponentially. Robotic surgery, also known as robotic-assisted surgery, allows for complex minimally invasive surgical procedures to be completely or part-performed with a mechanical system consisting of articulating arms, typically controlled at a separate console by the surgeon.
The Da Vinci Surgical Robotic System (Intuitive Surgical, Sunnyvale, CA, USA), has been widely implemented in various surgical specialities, such as general surgery, urology, and gynaecology, within 66 countries. A recent systematic review of laparoscopic and robotic surgery found comparable or improved complication rates with robotic surgery, with reduced recovery time and length of stay [1].
Robotic consoles can offer accuracy, and precision, as well as minimally invasive access to difficult areas, with improved visualisation. Surgeons have better ergonomic performance, with a reduction in mental and physical workload [2]. Additionally, wireless connection broadens opportunities within telesurgery to facilitate remote operating [3].
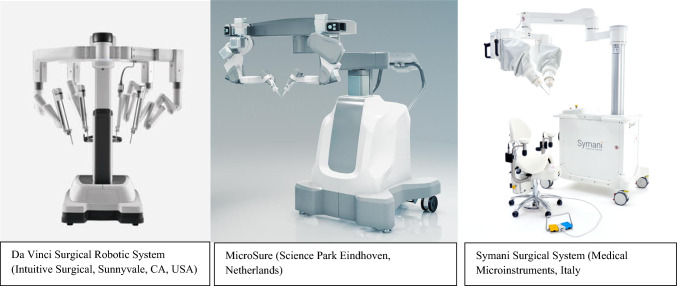
The application of robotic surgery in clinical plastic and reconstructive practice is yet to be well established [4]. There is an active interest amongst practitioners in the transferability of these potential benefits into a speciality that works in collaboration with many surgical disciplines; however, many plastic surgeons report lack widespread implementation or exposure [5]. Whilst Da Vinci Surgical Robotic System (Intuitive Surgical, Sunnyvale, CA, USA) is the most well-known resource, MUSA Microsure (Science Park Eindhoven, Netherlands) and Symani Surgical System (Medical Microinstruments, Italy) are competitors in the market, particularly for use within microsurgery (Fig. 1).
Fig. 1.
Robotic equipment utilised within clinical practice. All three are controlled by a separate master console
Microsurgery is an area which requires high precision, excellent magnified visualisation, and tremor reduction. Whilst robotic surgery may exceed in these domains, the impact of loss of haptic feedback requires investigation. There are other potential barriers within the widespread implementation of robotics and robotic-assisted surgery within plastic and reconstructive surgery such as the financial incurrence and sparce training opportunities [5].
The aims of this systematic review are to assess the feasibility of robotic surgery within plastic and reconstructive surgery and review the barriers and limitations to clinical implementation and training.
Methods
This systematic review has been conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [6]. Methodology was designed a priori, and this review is registered with PROSPERO (ID: CRD42024524237).
A literature search of PubMed, Medline and Embase for publications within the past 10 years was conducted by author L.A. Additional articles found through reference screening were included. Titles and abstracts were screened by two independent authors (B.R and E.B), with discrepancies for inclusion reviewed by a third author independently (L.A). This review includes all study types such as randomised controlled trials (RCT), prospective cohort, retrospective cohort, case series/reports, case–control, cross-sectional studies and preclinical studies.
Eligibility criteria
Articles were accepted for inclusion using the following criteria:
Patients/populations who have undergone robotic surgery for reconstruction or oncological resection, within the scope of plastic and reconstructive surgery.
Adults and children
Articles which described robotic procedures within the scope of plastic and reconstructive surgery
Preclinical and educational studies within the scope of robotic plastic and reconstructive surgery including animal, synthetic and cadaveric models.
Articles were excluded from this review using the following criteria:
Articles pertaining to robotic surgery outside the scope of plastic and reconstructive surgery.
Inguinal hernia repair
Articles not available in English language
Articles published prior to 2013.
Search strategy
Search strategy employed is described below. Key words and subject headings were combined using Boolean logic and refined with consensus from all authors:
Robot* AND
Micro* OR reconstruct* OR flap OR nerve OR anastomosis OR abdominal wall OR pelvic floor OR supermicrosurg* OR head and neck OR oral OR oropharyngeal OR vaginoplasty OR breast OR nasal OR plastic
Data metrics
Data were tabulated into a predetermined Excel spreadsheet by authors LA and E.B [7]. This was subsequently refined following a pilot collection with a random sample of papers. Articles upon paper review which were deemed not suitable for inclusion were discussed with an independent third party (B.L). Data items obtained included article characteristics (title, author, year, journal, impact factor, type of study, multicentre/single centre), demographics (number of participants, gender, age, control), procedure (subspeciality, specific task, robot, ports, location of ports), and outcomes (operative duration, length of Stay, blood loss, peri-operative complications, long-term outcomes, follow-up duration, learning curve, and cost).
Risk of bias
Risk of bias was assessed by authors LA and E.B. RCT’s were reviewed using Cochrane’s risk of bias tool (RoB 2) [8]. Non-randomised trials was assessed using Cochrane’s ROBINS-I tool [9]. The Joan Briggs Institute Critical Appraisal Checklist for Case Series and the Joan Briggs Institute Critical Appraisal Checklist for Case Reports was used to review case series and case reports, respectively. [10, 11] A report of bias is included in the appendices.
Data synthesis
Narrative synthesis, and quantitative analysis was performed where possible. Descriptive analysis of continuous data is represented with ranges, mean values, or overall rate. Categorical data is presented with percentage prevalence. Subcategories are defined by subspeciality and procedure.
Study characteristics were tabulated and compared against planned subgroups to determine their suitability for each synthesis. Nonparametric data were analysed using a Wilcoxon test or an unpaired T test. Forrest plots were constructed, (in subcategories with article number > 5, where possible), using odds ratios for dichotomous and continuous outcomes and heterogeneity tested for using Chi-square and I2 test. Statistical analysis was performed using RevMan Software [12].
Results
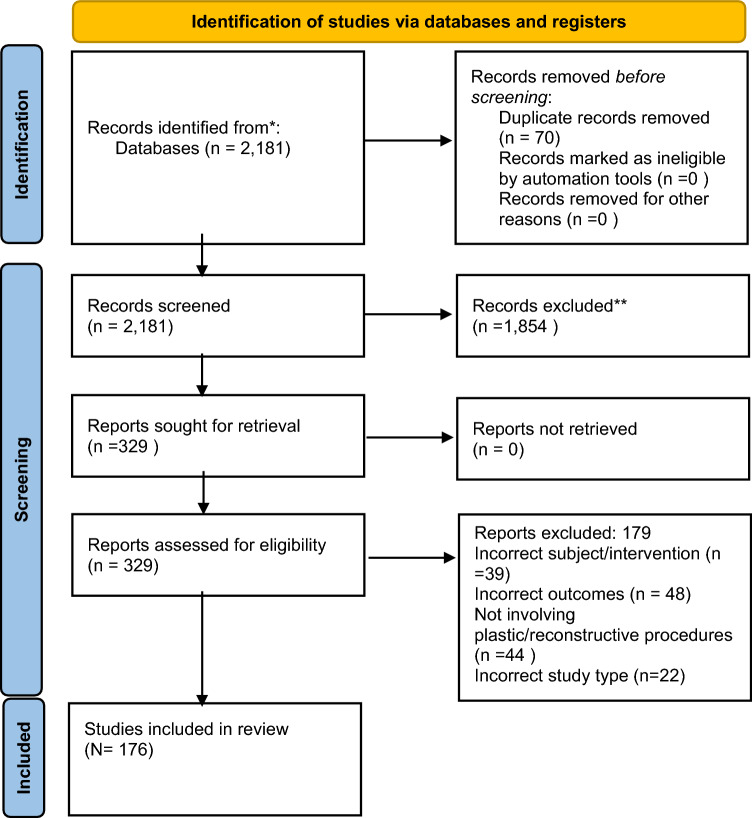
The literature search yielded a total of 2181 articles (Fig. 2). Following abstract screening, a total of 176 articles were included in this systematic review. A total of 149 clinical articles were found (Table 1). A total of 11 preclinical articles were included (Two of which also included clinical data) (Table 2) [13–23]. A total of 18 educational articles were included (Table 2) [24–41].
Fig. 2.
PRISMA flow diagram of the literature search for robotics in plastic and reconstructive surgery
Table 1.
Clinical publications within the scope of robotic plastic and reconstructive surgery (BL blood loss, LOS length of stay)
| Reference | Year | N = | Robot | Specific skill/task | Control procedure | Outcomes | Duration of surgery compared to control | LOS compared to control | Peri-operative complications difference compared to control | Length of f/u (months) | Additional outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lymph Node Dissection = 11 Articles | |||||||||||
| Kim et al | 2013 | 20 | Da Vinci | Neck dissection | Conventional |
Operative time, BL Complications, LOS Number of nodes |
Significantly higher | No difference | No difference | 8 | Higher scar satisfaction with robotic patients |
| Tae et al | 2013 | 11 | Da Vinci | Neck dissection | Conventional |
Operative time Post-operative drainage Cosmetic satisfaction |
Significantly higher | No difference | Higher cosmetic satisfaction with robotic patients | ||
| Kim et al | 2015 | 2 | Da Vinci S | Neck dissection | Endoscopic neck dissection |
Operative time Complications Scarring Lymphoedema |
Higher | No lymphoedema | Excellent cosmetic satisfaction, hidden scar | ||
| Du et al | 2017 | 1 | Da Vinci | Axillary dissection |
Operative time, LOS Complications Number of nodes |
10 | |||||
| Lira et al | 2017 | 6 | da Vinci Si | Neck dissection | Endoscopic and conventional |
Operative time Complications, LOS Number of nodes Disease-free survival |
Higher | Statistically lower | 18.6 | No difference in resection outcomes or disease-free survival | |
| Melly et al | 2017 | 1 | Da Vinci | Axillary dissection |
LOS, Complications |
||||||
| He et al | 2018 | 13 | Da Vinci | Axillary dissection |
Operative time, BL Number of nodes Complications |
16.5 | |||||
| Singh et al | 2018 | 51 | Da Vinci si | Inguinal dissection | Conventional inguinal dissection |
Operative time, LOS, BL Number of Nodes Complications |
Significantly higher (excluding docking time) | Significantly shorter | Significantly lower incidence of major complications, edge necrosis, limb oedema | 40 | |
| Paek et al | 2019 | 28 | Da Vinci | Neck dissection | Conventional neck dissection |
Complications Number of Nodes |
Significantly higher | No difference | No statistical difference | ||
| Lee et al | 2020 | 1 | Da Vinci Xi | Axillary dissection |
Operative time, LOS Complications Number of Nodes |
none | |||||
| Song et al | 2020 | 4 | Da Vinci | Neck dissection |
Operative time Blood Loss Complications |
||||||
| Craniofacial = 2 Articles | |||||||||||
| Lin et al | 2022 | 15 | KUKA robotic arm | Mandibular contouring |
Osteotomy surface position error/plane angle error Operation time BL, LOS, Complications Safety outcomes Patient satisfaction/pain scale/score at 1&6 months |
No statistical difference | No difference | No difference | |||
| Lin et al | 2023 | 15 | KUKA robotic arm | Mandibular contouring | Conventional |
Osteotomy surface position error/plane angle error Operation time BL, LOS, Complications Safety outcomes Patient satisfaction/pain scale/score at 1&6 months |
No difference | No difference | No difference | 6 | |
| Cleft Palate = 1 Article | |||||||||||
| Teblick et al | 2023 | 29 | Da Vinci | Modified Furlow double-opposing Z-palatoplasty | Eustachian Tube Function | Lower hearing thresholds and faster resolution of OME were found | |||||
| Pedicled and Free Flap Harvest = 21 Articles | |||||||||||
| Pederson et al | 2014 | 10 | Da Vinci | RAM |
Operative time Complications |
12 | No recurrence | ||||
| Clemens et al | 2014 | 12 | Da Vinci | Pedicled LD | Conventional |
Operative time, LOS Complications |
7.1 | ||||
| Chung et al | 2015 | 12 | Da Vinci | Pedicled LD |
Operative time, LOS Complications Patient satisfaction |
15.7 | High patient satisfaction with 1–10 Likert scale | ||||
| Lai et al | 2018 | 1 | Da Vinci | Pedicled LD | Complications | 5 | |||||
| Lai et al | 2018 | 2 | Da Vinci Si | Pedicled LD |
Operative time Complications Blood Loss Technical report |
8 | |||||
| Houvenaeghal et al | 2019 | 80 | Da Vinci Si /Xi | Pedicled LD |
Operative time, LOS Complications, BL |
Lack of long dorsal scar | |||||
| Ozkan et al | 2019 | 1 | da Vinci Xi | Omental |
Operative time Complications Flap Survival |
12 | |||||
| Houvenaegel et al | 2020 | 46 | da Vinci Xi/Si | Pedicled LD | Conventional |
Operative time, LOS Complications |
Significantly higher (improved with experience) | No difference | Significantly lower | ||
| Fouarge et al | 2020 | 6 | da Vinci si /Xi | Pedicled LD |
Operative time, LOS Complications Technical report |
||||||
| Frey et al | 2020 | 5 | da Vinci xi | Free omental flap (lymph node transfer) | LOS, Complications | ||||||
| Haverland et al | 2020 | 6 | Da Vinci | RAM (pelvic recon) | Complications | 9.2 | |||||
| Moon et al | 2020 | 21 | da Vinci xi | Pedicled LD |
Operative time Complications |
19.2 | |||||
| Winocour et al | 2020 | 25 | Da Vinci | Pedicled LD | Conventional |
Operative time, LOS Complications Flap Survival |
Significantly higher | Significantly shorter | Higher rate of seroma | 60 | Reduction of opioid requirements but not significant |
| Asaad et al | 2021 | 7 | Da Vinci | RAM (pelvic reconstruction) |
LOS, BL Complications |
||||||
| Day et al | 2021 | 1 | Da Vinci | Pedicled omental | Complications | ||||||
| Joo et al | 2021 | 1 | Da Vinci SP | Pedicled LD |
Histology Operative time Complications PROMS—BREAST Q |
||||||
| Cheon et al | 2022 | 41 | da Vinci Si/Xi/SP | Pedicled LD |
Operative time, LOS Complications |
15.6 | Higher patient satisfaction with robotic group | ||||
| Davila et al | 2022 | 16 | Da Vinci | RAM (pelvic reconstruction) | Conventional |
Operative time, LOS Complications |
No difference | No difference | No difference statistically but lower rate in robotic group | 36 | |
| Hwang et al | 2022 | 3 | da Vinci SP | Pedicled LD |
Operative time Complications |
||||||
| Seon Eo et al | 2023 | 20 | Da Vinci | Pedicled LD | Endoscopic conventional |
Operative time, LOS Opioid requirement Complications |
Significantly higher | No difference | No difference | 18.4 | statistically higher overall patient satisfaction |
| Shin et al | 2023 | 11 | da Vinci si | RFFF harvest |
Operative time Method of anastomosis Flap Survival Complications |
Significantly longer | Notably less scarring with no longitudinal volar incision | ||||
| Hans et al | 2013 | 2 (1 flap inset) | Da Vinci | RFF inset, TOR resection |
Operative time, LOS Resection margins |
8 | |||||
| Flap Inset and Anastomosis (Vessel, Nerve. Lymph) = 16 Articles | |||||||||||
| Song et al | 2013 | 5 | Da Vinci | Flap inset, anastomosis, TOR resection |
Operative time Complications |
12.8 | |||||
| Lai et al | 2014 | 5 | da Vinci Si | RFF inset, venous anastomosis |
Operative time Complications Flap Loss |
7 | |||||
| Miyamato et al | 2014 | 6 | da Vinci S | Nerve graft |
Complications Deltoid function recovery |
10 | 5/6 regained deltoid function | ||||
| Tsai et al | 2017 | 14 | Da Vinci | RFF inset, secondary venous anastomosis | Conventional |
Complications Flap Revision Long Term Outcome (FIGS) |
No difference | 3 | |||
| Lai et al | 2019 | 15 | Da Vinci | RFF anastomosis (vein/artery) | Microscope (conventional) |
Operative duration Complications Vessel Diameter |
Significantly higher | 100% flap survival | 11.5 | ||
| Van Mulken et al | 2020 | 20 | MUSA | LVA | Microscope (conventional) |
Operative time Anastomosis patency Complications Patient satisfaction Postoperative: daily wearing of compressive garment, manual lymph drainage, lymph ICF score, EUL index score |
Significantly higher anastomosis time but showed steep improvement with learning curve | No difference | 3 | No difference in Lymph ICF score | |
| Chang et al | 2021 | 1 | da Vinci Xi | Bilateral sympathetic trunk recon with sural nerve graft (neurosynthesis) |
LOS PROM (questionnaire) |
42 | 70% improvement in symptoms | ||||
| Barbon et al | 2022 | 22 | Symani surgical system | Arterial/venous/nerve/lymph | Microscope (conventional) |
Operative duration Learning curve Suture size Number of sutures Patency |
significantly higher BUT improved in second cohort more than hand-sewn | Steep robotic learning curve to a comparable time with hand sewn anastomosis | |||
| Lindenblatt et al | 2022 | 5 | Symani surgical system | LVA/arterial |
Operative Time Surgical Experience |
||||||
| Van mulken et al | 2022 | 20 | MUSA | LVA | Microscope (conventional) | 1-year outcomes—QOL (Arm circumference, compression garment, manual drainage, arm dermal backflow and patency of anastomosis tested with ICG | 12 | Patient outcomes were comparable with conventional procedure. 42.9% reduction in use of compression garments | |||
| Beier et al | 2023 | 23 | Symani surgical System | Arterial End-to-end/End-to -side anastomosis |
Operative Time Revisions |
||||||
| Besmens et al | 2023 | 6 | Symani Surgical System (and exoscope) | Arterial anastomosis/neurosynthesis |
Operative time Anastomosis patency |
||||||
| Chen et al | 2023 | 23 | da Vinci xi | Nerve anastomosis |
Operative time, LOS Level of nerve injury Length of defect Complications |
24 | Effective in reducing sweating across all sites assessed | ||||
| Innocenti et al | 2023 | 1 | Symani surgical system | Arterial/venous anastomosis ALT |
Surgical Experience Number of sutures Operative Time Complications |
||||||
| Weinzierl et al | 2023 | 8 | Symani surgical system | LVA |
Operative Time Number of sutures Anastomotic Patency |
||||||
| Flap Pedicle Dissection = 8 Articles | |||||||||||
| Gundlapalli et al | 2018 | 1 | Da Vinci | DIEP |
Operative Time Complications Cost |
9 | |||||
| Choi et al | 2021 | 17 | da Vinci sp | DIEP |
Operative time Complication |
||||||
| Bishop et al | 2022 | 21 | Da Vinci | DIEP |
Operative time, LOS Complications |
5 | |||||
| Daar et al | 2022 | 4 | da Vinci xi | DIEP |
Operative time, LOS Complications |
6.31 | |||||
| Dayaratna et al | 2022 | 1 | da Vinci Xi | DIEP submuscular pedicle dissection |
Operative time Complications PROM—BREAST Q |
None, minimal pain, low analgesic requirements | 3 | ||||
| Wittesaele et al | 2022 | 10 | Da Vinci | DIEP |
Operative time, LOS Complications |
1 | |||||
| Tsai et al | 2023 | 13 | Da Vinci | DIEP | Conventional pedicle dissection |
Operative time Complications |
14 | ||||
| Zanaty et al | 2023 | 1 | Da Vinci | Internal thoracic artery harvest |
Surgical Experience Complications |
||||||
| Vaginoplasty = 3 Articles | |||||||||||
| Boztosun et al | 2016 | 1 | Da Vinci Xi | Sigmoid vaginoplasty |
Operative time, LOS Complications Long term outcomes |
6 | More minimally invasive approach | ||||
| Dy et al | 2021 | 47 | 2 groups—Da Vinci SP/Xi | Vaginoplasty—gender affirmation | SP vs Xi da Vinci |
Operative time Complications, BL Neovaginal dimensions |
SP shorter duration | No statistical difference |
Not statistically different lower rate of BO in xi, higher rate of transfusion and vaginal stenosis in SP |
12 |
Few incisions result in better cosmetic benefit single port shorter and doesn’t impede second surgeon |
| Blasdel et al | 2023 | 43 | Da Vinci SP | Vaginoplasty with peritoneal flap recon, in patients with genital hypoplasia | Traditional vaginal canal dissection, peritoneal flap |
Complications PROMs |
12 | ||||
| Nerve Decompression = 1 Article | |||||||||||
| Bruyere et al | 2016 | 1 | Da Vinci Si | Neurolysis of lateral cutaneous nerve of the thigh | LOS, Complications | - | 6 | 100% reduction in pain (0/10 from 5/10) | |||
| Abdominal Wall Reconstruction = 28 Articles | |||||||||||
| Chen et al | 2016 | 39 | Da Vinci | Small ventral hernia mesh repair | Laparoscopic |
Operative time, LOS Complications Defect size |
Significantly longer (65) | No difference | No difference in readmissions, no difference in complication rate | 1.5 | |
| Bittner et al | 2017 | 26 | da Vinci si/xi | Transversus Abdominis release and mesh | Open |
Hermia Characteristics Operative time, LOS Complications |
Significantly higher (287) | Significantly shorter (6) | No difference | 2 | |
| Gonzalez et al | 2017 | 368 | Da Vinci Xi | Ventral wall hernia repair mesh/direct closure |
Operative time, LOS Complications |
||||||
| Jamshidian et al | 2017 | 3 | da Vinci Xi | Spigelian hernia repair with mesh |
Operative time, BL, LOS Complications Opioid Use |
||||||
| Prabhu et al | 2017 | 177 | Intraperitoneal mesh repair | Laparoscopic |
Operative time, LOS Complications |
Significantly higher | Significantly shorter | Statistically lower | Higher rate of bowel injury, and systemic problems in laparoscopic group | ||
| Wang et al | 2017 | 1 | Da Vinci | Stratafix/mesh intercostal hernia repair | LOS, Complications | ||||||
| Warren et al | 2017 | 53 | Da Vinci | Retromuscular/ PP mesh ventral hernia repair | Laparoscopic |
Rate of fascial closure Operative time, LOS Opioid use Complications Cost |
Significantly longer | Significantly shorter | Significantly higher rate of seroma | 2 |
LVHR and RRVHR ($13,943 vs. $19,532; p = 0.07) Robotic cost of procedure higher, however overall cost comparable as shorter length of hospital stay fascial closure achieved more in robotic surgery, extraperitoneal mesh was performed majority of robotic cases |
| Carbonell et al | 2018 | 111 | Da Vinci | Retromuscular mesh hernia repair | Open |
Complications, LOS Readmission/Reoperations |
Significantly longer | Significantly shorter | Higher rate of seroma/sso | 1 | Higher surgical site occurrences were noted with r-RVHR, consisting mostly of seromas not requiring intervention |
| Martin-Del-Campo et al | 2018 | 38 | not specified | Transversus Abdominis release & retromuscular synthetic mesh | Open |
Hernia dimensions Operative time, LOS, BL, Complications |
Significantly higher (211) | Significantly shorter (6) | Systemic complications significantly lower (0 vs 13) | ||
| Muysoms et al | 2018 | 41 | da Vinci xi | Retromuscular TAR mesh umbilical hernia repair |
Learning curve Operative time Complications PROM—EuraHS-QoL |
1 |
Total skin-to-skin operative time decreased through the series Significant improvement in PROMS compared to preop scores |
||||
| Walker et al | 2018 | 142 | Da Vinci | Preperitoneal mesh ventral hernia repair | Laparoscopic |
Operative time, LOS Complications |
Significantly longer | No difference | Significantly lower | 4 | |
| Kudsi et al | 2019 | 1 | da Vinci Xi | Sugarbaker parastomal hernia repair with mesh and TAR | Operative time, LOS, BL, Complications | 3 | |||||
| Kudsi et al | 2020 | 164 | da Vinci xi | Transabdominal vs totally extraperitoneal hernia repair all robotic | Transabdominal vs totally extraperitoneal hernia repair all robotic (all retromuscular) |
Operative time, BL, LOS Complications Pain Readmission |
TEP shorter duration of surgery | No difference | Minor complications statistically higher for TA group, seroma frequency and rate of SSE also higher | Minor complications statistically higher for TA group, seroma frequency and rate of SSE also higher | |
| Olavarria et al | 2020 | 65 | da Vinci xi | Intraperitoneal mesh ventral hernia repair | Laparoscopic |
Operative time, LOS Complications Long term outcomes—Recurrence, QOL, Cost |
Significantly longer | No difference | No differences | 6.4 |
Clinicians had 50 cases as a learning curve prior trial Increased cost for robotics of 90 days of care including surgery ($15 865 (£12 746; €14 125) v $12 955; cost ratio 1.21, 1.07 to 1.38; adjusted absolute cost difference $2767, $910 to $4626; P = 0.004) |
| Mudyanadzo et al | 2020 | 16 | not specified | Ventral incisional hernia repair | Laparoscopic | LOS, Opioid use | No difference | Use of opioids reduced for robotic group | 2 | ||
| Petro et al | 2020 | 39 | da Vinci si/xi | Intraperitoneal ventral hernia mesh repair | Laparoscopic |
Operative time, LOS Long term outcomes—recurrence, NRS 11 score, PROM ISPI, hernia specific QOL Cost |
significantly longer (94) | No difference | No difference | 3 |
Cost of reusables was comparable but higher cost for robotics because of higher operative time Comparable NRS 11 score/PROMS |
| Bergholz et al | 2021 | 1 | Mesh intercostal hernia repair | LOS, Complications | |||||||
| Dhanani et al | 2021 | 65 | da Vinci xi | Intraperitoneal mesh and direct closure repair | Laparoscopic |
Complications Long term outcomes—PROM, functional status, VAS, cosmetic satisfaction |
Not reported | 12 | No difference in PROM outcomes at 1 year | ||
| Rayman et al | 2021 | 3 | da Vinci Si | Transabdominal preperitoneal spigelian hernia repair | Laparoscopic |
Operative time, LOS Complications Post op pain Defect/mesh size |
17 | ||||
| costa et al | 2022 | 18 | Not specified | Intraperitoneal ventral hernia mesh repair | Laparoscopic |
Operative time, LOS Complications Long term outcomes—recurrence, EORTC QLQ-C30 |
Significantly longer | No difference | No difference | 24 | No difference in long-term outcomes |
| Kakela et al | 2022 | 19 | da Vinci xi | Retromuscular TEP mesh | laparoscopic |
Operative time, LOS Long term outcomes.- pain, VAS (M1, 12), SF-36 PROM, hernia recurrence |
Significantly longer | No difference | 12 | Robotic less pain at 1 month and 1 year using VAS all 9 scores for SF-36 favour robotics but not statistically significant for most emotional status and social function improved significantly | |
| Kudsi et al | 2022 | 138 | Da Vinci | Mixed methods ventral hernia repair | Obesity class II vs class III | LOS, Complications | No difference | No difference | 33.6 | Comparison of patient BMI and outcomes in robotics no difference in peri-operative outcomes and intra-operative variables, except higher rate of mesh use in more obese patients | |
| Petro et al | 2022 | 38 | da Vinci si/xi | Intraperitoneal ventral hernia mesh repair | Laparoscopic | LOS, Long term outcomes—pain intensity, PROMIS pain score, HERQless, recurrence, reoperations | 12 | Higher rate of recurrence but better PROM outcomes | |||
| Pereira et al | 2022 | 665 | Lateral abdominal mesh hernia repair | Open |
Operative time, LOS Complications Long-term outcomes—PROM (HerQless, PROMIS pain,) |
Significantly longer | Significantly shorter |
Bowel injury higher in open but not significant SSI/use of epidural significantly lower overall significantly lower rate of complications |
12 | No difference in long-term outcomes | |
| Shimada | 2022 | 1 | da Vinci xi | Retromuscular extraperitoneal mesh ventral hernia repair | Operative time, LOS, BL, Complications | 7 | |||||
| Dhanani et al | 2023 | 65 | da Vinci xi | Intraperitoneal mesh and direct closure repair | Laparoscopic |
Complications Long term outcomes—functional status, VAS, cosmetic satisfaction |
No difference | 24 | Significantly lower rate of revision surgery in robotic group no difference in SSO at 2 years (seroma/haematoma) no difference in PROMS | ||
| Lima et al | 2023 | 1 | Spigelian hernia mesh repair | Complications | 0.25 | ||||||
| Petro et al | 2023 | 100 | Da Vinci | Robotic enhanced view totally extraperitoneal (eTEP) or robotic intraperitoneal onlay mesh midline ventral hernia < 7 cm | TEP vs intraperitoneal mesh |
LOS, Complications Opioid Use Long term—PROM |
Significantly longer for TEMP | No difference between robotic groups | 12 |
Significant difference in HERQLes favouring IPOM (but only 12 months post op) no difference in recurrence at 1 year (51 totally extraperitoneal, 49 intraperitoneal mesh) |
|
| Mastectomy = 18 Articles | |||||||||||
| Sarfati et al | 2017 | 1 | Da Vinci | Nipple-sparing mastectomy | Operative time, LOS | 3 | |||||
| Toesca et al | 2017 | 3 | Da Vinci S | Nipple-sparing mastectomy |
Operative time, LOS Complications Patient satisfaction |
8 |
No complications at 8 months, high cosmetic satisfaction small incision Reduction in operative time from 7–2.5 h over 3 cases |
||||
| Toesca et al | 2017 | 29 | Da Vinci Xi/Si | Nipple-sparing mastectomy |
Learning Curve Operative time Complications, LOS |
8 | |||||
| Lai et al | 2018 | 15 | Da Vinci Si | Nipple-sparing mastectomy |
Learning Curve Operative time Complications, LOS |
6.3 | 100% high satisfaction reported, shorter duration of surgery with more surgical experience | ||||
| Park et al | 2018 | 1 | Da Vinci Xi | Nipple-sparing mastectomy |
Operative time Pathology, LOS Complications |
0 | 12 | ||||
| Rajappa et al | 2018 | 1 | Da Vinci Si | Nipple-sparing mastectomy |
Operative time, LOS Complications |
Not specified | |||||
| Sarfati et al | 2018 | 1 | Da Vinci Xi | Nipple-sparing mastectomy | Complications | ||||||
| Sarfati et al | 2018 | 33 | da Vinci Xi | Nipple-sparing mastectomy (immediate implant recon) |
Operative time Complications PROMS—BREAST Q |
12 | |||||
| Lai et al | 2018 | 2 | da Vinci Si | Nipple-sparing mastectomy & lat dorsi flap harvest |
Operative time, LOS Complications |
0 | 8 | ||||
| Houvenaeghal et al | 2019 | 27 (17 lat dorsi flap) | da vinci Si /Xi | Nipple-sparing mastectomy |
Operative time Learning curve Complications, LOS |
Time of surgery and anaesthesia decreased with learning curve | |||||
| Houvenaeghal et al | 2019 | 80 | Da Vinci Si /Xi | Latissimus dorsi flap robotic ± mastectomy |
Operative time, LOS Complications |
Single incision, lack of long of long dorsal scar | |||||
| Kuo et al | 2019 | 3 | da vinci xi | Nipple-sparing/skin-sparing mastectomy |
Operative time, LOS Complications |
5 | |||||
| Lai et al | 2019 | 22 | Da Vinci | Nipple-sparing mastectomy |
Operative time docking time BL, complications recurrence |
Docking time dropped with more experience | 6.9 ± 3.5 |
All patients reported to be satisfied with outcome US $6000/ use |
|||
| Lai et al | 2019 | 39 | Da Vinci | Nipple-sparing mastectomy |
Operative time Learning curve complications pathology/resection margins |
8.6 | Significantly reduced surgical duration with procedures performed over 1 year | ||||
| Lai et al | 2020 | 54 | Da Vinci Si | Nipple-sparing mastectomy | Conventional |
Operative time, BL Resection margins Complications Cost Long term outcomes -photography, PROMS (cosmesis) |
Significantly longer | Significantly longer | no difference | 14 |
$10,877 robotic vs $5702 conventional—significantly higher Significantly higher patient satisfaction (better scar, better nipple position) |
| Toesca et al | 2021 | 40 | Da Vinci S | Nipple-sparing mastectomy | Conventional |
Operative time, LOS Complications, BL Long term recurrence, survival, PROMs (breast-Q, NAC questionnaire) |
Significantly higher | Significantly shorter | significantly lower (notably nipple ischaemia, skin necrosis, haematoma, seroma, and open group more likely to have > 1 complication | 28.6 |
Significantly higher satisfaction in robotic, and in psychological wellbeing No difference in rate of implant loss |
| Park et al | 2022 | 167 | Da Vinci Si | Nipple-sparing mastectomy | Conventional |
Complications Recurrence |
significantly lower rate of complications in 30 days including nipple necrosis lower rate of Clive dindo classification 3 |
18 | No difference in recurrence | ||
| Moon et al | 2022 | 40 | da Vinci S | Nipple-sparing mastectomy | Conventional |
Complications Pain |
Significantly longer | No difference | Perioperative only | Lower pain reported for robotic group | |
| Transoral Robotic Surgery = 43 Articles | |||||||||||
| Chan et al | 2013 | 4 | Da Vinci | Resection parapharyngeal space neoplasm |
Histopathology Complications, LOS Long term outcomes—function/oral diet |
None | 1–15 months | ||||
| Chia et al | 2013 | 2015 | Oropharyngeal carcinoma (majority t1/t2 staging) | Complications | Low rate of long-term PEG dependency | ||||||
| Durmus et ala | 2013 | 22 | da Vinci S/Si | Resection of cancer of unknown primary oral |
Operative time Complications |
All achieved oral diet D1 RT 100% of patients 0 trachy, 0 gastrostomy | |||||
| Durmus et alb | 2013 | 3 |
Retromolar trigone tumour resection HPV neg |
Operative time, BL Complications |
1–16 months | ||||||
| Hans et al | 2013 | 2 | Da Vinci | T3 hypopharyngeal SCC resection and RFF inset |
Operative time, LOS Complications |
8 | (1 flap inset) | ||||
| Lee et al | 2013 | 27 | Da Vinci | Lateral oropharyngectomy (T1/T3 tonsillar cancer) | Conventional |
Operative time, LOS BL, Complications Long term outcomes—survival, VHI, MDADI |
Significantly shorter than mandibulotomy significantly longer than transoral |
Significantly shorter than mandibulotomy, no difference from transoral | No difference | 20.3 |
Higher disease-free and overall survival compared with control No difference in VHI/MDADI scores |
| Patel et al | 2013 | 47 | Oropharyngeal tumour identification |
Resection margins Complications |
|||||||
| Tsang et al | 2013 | 1 | Da Vinci S | Nasopharyngectomy via lateral palatal flap approach |
Resection margins Operative duration Complications |
6 | |||||
| White et al | 2013 | 64 | Da Vinci | Recurrent oropharyngeal SCC resection T1-4 | Open |
Operative time, LOS Complications, BL Long term—OS, DFS, death |
Significantly shorter | Significantly shorter | Significantly fewer (fistula and oedema and overall) | 24 |
Lower rate of tracheostomy/NG tube with robotic surgery Decreased incidence of positive margins with robotic surgery (significant) 2-year recurrence free significantly higher for robotic |
| Chung et al | 2014 | 641 | Partial pharyngectomy | Conventional | LOS, Complications | Significantly shorter | Significantly lower |
Lower cost for robotic surgery overall. $29,365 vs $20,706 lower rate of tracheostomy and peg |
|||
|
Chung et al (same as above × 3 diff data sets) |
2014 | 147 | Partial glossectomy base of tongue | Conventional | LOS, Complications | Significantly shorter | No difference |
Lower overall cost for robotics $19,091 vs $23,414 open Significantly lower rate of tracheostomy and PEG |
|||
|
Chung et al (same as above × 3 diff data sets) |
2014 | 68 | Partial glossectomy (anterior) | Conventional | LOS, Complications | Significantly longer | No difference (except higher rates of transient dysphagia) |
No difference in total cost ( robotic $22,111 vs open $21,376) Signifiantly lower rate of tracheostomy and PEG |
|||
| Durmus et al | 2014 | 22 | Oral cancer of unknown primary resection |
Operative time Complications Long term outcomes—HCNI PROM |
12 | Patients maintain long-term and highly functional QOL status | |||||
| Ford et al | 2014 | 65 | Da Vinci | OPSCC resection (majority t1/t2) | Conventional |
Operative time Resection Margins long-term—OS, DFS |
36 |
Significantly higher 3-year survival for robotic group recurrence free survival |
|||
| Hammoudi et al | 2014 | 26 | Da Vinci | Primary scc resection (any neck dissections were conventional) | Conventional |
Operative time Resection margins Length of Stay Complications Tracheostomy prevalence Cost |
No difference | Significantly shorter | No difference | 19 |
Significantly fewer tracheostomies (n = 4) SIGNIFICANTLY shorter duration of NG feed No difference in 3-year disease-free survival Significantly lower cost for robotics (higher operative cost ($7781 vs $4375) lower overall cost due to length of stay ($20,885 vs $27,926) |
| Van Loon et al | 2014 | 18 | Da Vinci | T1/T2 OP cancer resection |
Operative time Resection Margins Blood loss Long term outcomes -PROMS (EORTC-C30, H&N35) |
33.7 | 2-year disease-free survival of 86% | ||||
| Almeida et al | 2015 | 410 | Larynx/pharyngeal cancer |
Resection margins Long term outcomes -OS, DFS |
20 | ||||||
| Dabas et al | 2015 | 60 | Da Vinci | Resection oropharyngeal ca and conventional neck dissection (ipsilateral) |
Operative time Blood Loss Complications Long term—functional outcomes |
8 | |||||
| Mercante et al | 2015 | 13 | T1/T2 OP ca without adjuvant Tx |
procedure time, set up time, operative time complications hospital stay blood loss recovery to normal breathing swallowing/removal of NGT |
12 | Long term—QoL, dysphagia score, FESS, penetration aspiration scale, MDADI, VHI-10 @ 6&12 months | |||||
| Mockelmann et al | 2015 | 41 | Oropharyngeal resection T1-4 | Staged vs concomitant neck dissection (21 control with concurrent, and 20 in intervention arm) |
Length of Stay Complications Timing of neck dissection |
Timing of neck dissection didn’t make a difference in outcomes. (immediate vs average of 10 days) | |||||
| Razafindranaly et al | 2015 | 84 | Da Vinci | Supraglottic scc resection |
Resection margins Complications Oral diet/tracheostomy prevalence |
20—tracheostomy temp 64—NG for median of 8 days (0–10) 8—permanent PEG |
14 | ||||
| Smith et al | 2015 | 42 | Da Vinci | oropharyngeal SCC resection and neck dissection (majority t3/4) | CRT ( non-operative) |
Resection margins Complications Long term—OS, DFS |
36 | ||||
| Aubry et al | 2016 | 178 | Da Vinci | Tumour resection |
Length of stay Complications |
||||||
| Fujiwara et al | 2016 | 10 | Da Vinci | OPSCC T1-T2 |
Operative time Length of Stay Complications Resection margins Surgical feasibility Blood Loss Function (swallow) |
||||||
| Granell et al | 2016 | 1 | Da Vinci S HD | Access and resection of parapharyngeal tumour (cavernous haemangioma) |
Resection margins length of Stay Complications |
None described | 12 | ||||
| Duek et al | 2017 | 1 | Da Vinci | Resection parapharyngeal space tumour |
Operative time Complications |
None | 4 | ||||
| Frenkel et al | 2017 | 425 |
H&N resection (333 concurrent neck dissection, 92 staged neck dissection) |
Complications Length of Stay |
Risk adjusted LOS was less for concurrent | neck dissection timing not associated with changes in complications, readmissions, tracheostomy, or gastrostomy | |||||
| Gorphe et al | 2017 | 27 | Da Vinci Xi | TOR resection |
Operative time resection margins complications unplanned tracheostomy/death |
15 temp tracheostomies | |||||
| Lallemant et al | 2017 | 23 | Da Vinci | SCC posterior pharyngeal wall resection |
Resection margins Length of Stay Complications Long term—OS, DFS |
NG feed needed for average of 22 days × 4 PEG (note post -op dysphagia—most likely due to site) |
27 | ||||
| Mahmoud et al | 2017 | 559 | TOR OPSCC vs primary CRT | Primary CRT | Long term outcomes—DFS, OS | 29 | |||||
| Rubek et al | 2017 | 30 | Da Vinci Si HD | Oropharyngeal SCC |
Length of Stay Resection margins Complications |
Tube dependency 4.6 days | 19 | ||||
| Sethia et al | 2017 | 111 | OP cancer resection—TOR vs TOR and adjuvant therapy |
Complications Long term—PROM (HCNI), OS, recurrence |
Reduced rate of PEG at immediately post op 3,6,12 months | 12 | There were no statistically significant differences (P > .05) in aesthetics, social disruption (attitudinal), or speech (attitudinal) at any time point. Also, there were no statistically significant differences (P > .05) for all QOL domains at 12 months | ||||
| Alessandrini et al | 2018 |
8—Xi 8—S |
da vinci Xi vs S |
BOT SCC resection (T1/T2) Da vinci si vs xi |
× 2 robotic groups |
operative time Resection margins complications blood loss post-operative functional outcomes (VAS, LOS, NG) |
Si had statistically longer console time and overall operating time | Significantly shorter for Xi |
Xi significantly shorter NG use and pain scores no complications reported |
Not specified | |
| Doazan et al | 2018 | 122 | Supraglottic SCC | Long term—OS, DFS, recurrence | 42.8 | ||||||
| Li et al | 2018 | 2224 | T1/T2 OPSCC resection | Conventional and TLM |
Resection margins OS |
Significantly shorter | 60 |
TOR not associated with increased survival however there is a lower likelihood of need for CRT " |
|||
| Scott-Wittenborn et al | 2018 | 6 | Da Vinci | Base of tongue/palate unknown primary | ICG intraoperative tumour identification intraoperatively using ICG—unsuccessful | ICG was not beneficial for tumour identification or resection using the da vinci | |||||
| Hardy et al | 2019 | 1 | T3 pharyngeal SCC |
resection margins complications recurrence |
24 | ||||||
| Nichols et al | 2019 | 34 | OPSCC T1-T2 | Radiotherapy |
Complications Long term outcomes -PROMS, recurrence |
25 | Higher rate of neutropenia, hearing loss, and tinnitus in radiotherapy group, with a higher rate of trismus reported within the TOR group | ||||
| Petruzzi et al | 2019 | 1 | Da Vinci Si | Retropharyngeal lymph node dissection | Operative time | ||||||
| Holcomb et al | 2020 | 2 | Da Vinci Si | Salvage oropharyngectomy and submental artery island flap inset |
Operative time, LOS Complications |
4 | |||||
| Kubik et al | 2020 | 23 | Da Vinci | HPV unknown primary—BOT mucosectomy |
Surgical experience Complications Long term—survival, recurrence |
23 | |||||
| Sano et al | 2021 | 68 | Resection | Conventional and TLM | Resection margins | ||||||
| D'Andrea et al | 2022 | 53 | Da vinci Xi | Salvage surgery oropharyngeal (Mostly T2) |
Complications Long term outcomes—PROM (MDADI, EORTC QLQC30/ H&N35 |
24 | The preoperative, 1-year, and 2-year MDADI total scores were 71.4, 64.3, and 57.5, respectively. The preoperative, 1-year, and 2-year QLQ-C30 global scores were 61.2, 59.4, and 80.6, respectively. Decannulation was possible in 97.1% of the tracheotomized patients. The two-year enteral tube dependence was 23.1%. The two-year overall survival, disease-free survival, and local control rates were 59%, 46.1%, and 80.9%, respectively | ||||
| Nichols et al | 2022 | 34 | TOR OPSCC T1-T2 | Radiotherapy |
Long term outcomes recurrence MDADI QLQ-C30 H&N35 VHI-10 FOIS |
45 | MDADI 2 years—84.8, 3 years 83.3. no difference in functional outcome | ||||
| Virgilio et al | 2023 | 139 | OPSCC resection and neck dissection (mostly t1/t2) |
Resection margins tracheostomy/PEG prevalence Long term—recurrence, OS, DFS |
26 | TOR can de-intensify the need for CRT | |||||
Table 2.
Preclinical and educational articles within the scope of robotic plastic and reconstructive surgery
| Topic | First author | Year | Robot | Model/number | Operation performed | Participant | Outcomes reported |
|---|---|---|---|---|---|---|---|
| Preclinical – 11 articles | |||||||
| Lymph node dissection | Lee et al | 2020 | Da Vinci Xi |
Cadaveric N = 2 |
Axillary dissection | Expert surgeon | Safety and feasibility prior to clinical implementation |
| Microsurgery | Feng et al | 2017 | Robotic ENT Microsurgical System |
Chicken N = 7 (each arm) conventional vs Robotic |
End-to-end anastomosis |
6 novices 1 Expert surgeon |
Microvascular tremor scale (based on instrument tip movement) was significantly lower for robotic Comparable duration between conventional and robotic groups Subjective feedback found robotic performance to be more accurate with improved handling and stability |
| Microsurgery | Van Mulken | 2018 | Microsure |
Silicone vessel/Rat N = 8 (Each arm) conventional vs robotic |
Preparation, transection, and anastomosis | Expert surgeon |
Longer time to complete procedure for robotic (27 vs 12 min) 3 events of system reset required |
| Microsurgery | Ballestin et al | 2020 | Symani Surgical System |
Synthetic 1 mm vessel (6 manual & 6 robotic performed by each trainee) |
Microneedle driving, stitch placement, anastomosis | 40 expert surgeon 20 novices |
Improved precision with robot in both groups (suture distances, angulation) Longer time to perform anastomosis (11 vs 6.5 min)- decreased with practice, however experts did not show improvement after 5th attempt |
| Microsurgery | Malzone et al | 2023 | Symani Surgical System |
Rat femoral vessels conventional vs robotic |
End-to-end arterial and venous anastomosis | Not specified |
Rat vessel diameter 1.09 mm average Procedure performance time higher in robotic group Plateau in learning curve at 60 sutures Mean number of sutures/anastomoses = 8 in both manual and robotic groups Equivalent vessel patency with histologically assessed lower tissue damage for robotic |
| Flap | Zhu et al | 2016 | Omega 6, Force Dimension, Nyon |
Sheep mandible N = 6 Conventional vs Robot assisted |
Free fibula flap—osteotomy robot assisted guidance for osteotomy line and bony fixation. Manual harvest and inset |
Expert surgeon | Higher accuracy and improved implant orientation compared to freehand measurement/technique |
| Flap | Manrique et al | 2020 | Da Vinci Xi |
Cadaveric N = 8 |
Bilateral DIEP pedicle dissection (TAPP and TEP approach) | Expert surgeon |
Duration: TEP 56 min, TAPP 65 min Mean pedicle dissection TEP 39 min, TAPP 36 min Demonstrated feasibility, with TEP representing a less invasive technique |
| Abdominal wall | Sanchez et al | 2018 | Da Vinci |
Synthetic training model Laparoscopic vs Robotic N = 14 (1 performed by each surgeon) |
Incisional hernia repair | 14 expert surgeons | Less upper limb disturbance and lower mental effort for robotic group |
| TOR | Chen et al | 2017 | Da Vinci Sp/Si |
Cadaveric N = 4 |
Transoral base of tongue resection | Expert surgeon | Single port system allows for more streamlined workflow |
| TOR | Tay et al | 2018 | Endomaster |
Cadaveric N = 4 |
Radical tonsillectomies | Expert surgeon | Good visualisation, quick docking |
| Miscellaneous | Friedrich et al | 2018 | Da Vinci |
Silicone bench model Manual vs Laparoscopic vs Robotic N = 15 (for each) |
Assessment of Haptic Feedback correctly order silicone with defined rigidity and 5 steel tension springs |
Expert surgeon | Manual model demonstrated higher rate of correctly performed task |
| Education – 18 articles | |||||||
| Suturing | Leijte et al | 2020 | RobotiX Mentor VR simulator | VR simulator | Suturing |
15 Robotic surgeons 26 Laparoscopic surgeons 29 Novices |
Time, economy of movement/error, accuracy, precision assessed through RobotiX Qualitative feedback reported good didactic value for proficiency-based training |
| Suturing | De Groote et al | 2022 | Not specified |
Chicken Proficiency based progression training vs traditional training N = 18 (each arm) |
Suturing/knot tying | 36 novices | Higher rate of competency achieved with PBP group (eLearning until proficiency prior to task completion) |
| Microsurgery | Liverneaux et al | 2013 | Da Vinci | VR simulator, earthworm, rat model | Anastomosis | Surgical trainees | Description of training course involving 3 tier model approach with validated Structured Assessment of Robotic Microsurgery Skills (SARMS) |
| Microsurgery | Perez et al | 2013 | Da Vinci Trainer |
VR exercise N = 49 |
VR exercise |
11 trainees with microsurgery experience 38 trainees without microsurgery experience |
Quantitative assessment: microsurgery trainees achieved better results regarding economy of movement, precision, and force Qualitative feedback: microsurgical trainees reported similar ergonomics between microsurgery and robotics |
| Microsurgery | Alrasheed et al | 2014 | Da Vinci |
3 mm synthetic vessel N = 5 performed by each participant |
End-to-end anastomosis | 10 trainees |
Structured Assessment of Robotic Microsurgical Skills (SARMS) assessed by 4 expert surgeons Operative Time (9-44 min) Decrease in operative time over 5 performed procedures |
| Microsurgery | Selber et al | 2014 | Da Vinci |
Synthetic vessel N = 5 (performed by each participant in each arm) Robotic only |
End-to-end anastomosis | 10 surgical trainees |
All skill and overall performance improved over 5 sessions, and operative time decreased for all Initially steep skill learning curve followed by gradual improvement |
| Microsurgery | Willems et al | 2016 | Da Vinci |
Synthetic vessel N = 80 performed by each participant ( at depths of 0, 10, 20 cm with sidewall angles of 20 and 30 degrees conventional vs robotic |
End-to-end anastomosis | 2 surgical trainees |
OSAT—no difference between manual and robotic longer duration in manual group higher subjective comfort in robotic group robotic group performed better as depth increased |
| Microsurgery | Clarke et al | 2018 |
Rat vessel aorta N = 6 (by each surgeon in each arm) Conventional vs robotic |
End-to-end anastomosis |
14 microsurgeon with no robotic experience 14 robotic surgeons with no microsurgical experience |
Manual Group: 17 min (microsurgeon) 44 min (robotic) Robotic Group: 37.5 min (microsurgeon) 48.5 min (robotic surgeon) Steeper learning curve with microsurgeon Feasible skill acquisition exercise |
|
| Microsurgery | Van Mulken et al | 2018 | Microsure |
2 mm silicone vessels N = 10 (each arm, and by each of the participants) conventional vs robotic |
End-to-end anastomosis | 3 various level trainees |
Anastomosis time manual vs robotic (12.5 vs 35.1 min) Comparable rate of improvement between manual and robotic when assessed with Structured Assessment of Microsurgical Skills Demonstrated steeper learning curve with the robotic group |
| Microsurgery | Yang et al | 2022 | Da Vinci Trainer |
VR exercise N = 60 |
VR exercise |
30 trainees with da Vinci training 30 trainees with Da Vinci training and microsurgery training |
Microsurgery aided memory retention, with steeper learning curves and better skill level |
| Microsurgery | Beier et al | 2023 | Symani Surgical System |
Synthetic 1/2 mm vessels / Chicken N = 10 |
End-to-end anastomosis | Expert surgeons | 4-week training programme in which 10 successful anastomosis was deemed to be sufficient for progression into clinical practice |
| Flap | Louis et al | 2017 | Da Vinci Si |
Porcine N = 3 |
RAM harvest | Expert surgeon |
4 trocars used 80 min average operative time 16 cm average muscle length Demonstrated learning curve reflected in reduced operating time |
| Abdominal wall | Thomaier et al | 2016 | Da Vinci Trainer |
Bench model N = 20 laparoscopic box trainer N = 20 robotic simulation |
Peg transfer tasks | Novices |
Assessment through OSATS, Global Operative Assessment of Laparoscopic Skills (GOALS) and Global Evaluative Assessment of Robotic Skills (GEARS Skill acquisition and retention following time No differences between groups after first training session. Robotic training group demonstrated higher economy of motion, and fewer errors in comparison to laparoscopic, with no significant deterioration over time |
| Abdominal wall | Orlando et al | 2017 | Da Vinci Trainer |
Bench model N = 20 laparoscopic N = 20 robotic simulation |
Peg transfer tasks | Novices |
Assessment through OSATS, GOALS, GEARS Skill acquisition and retention following time No differences between groups after first training session. Robotic training group demonstrated higher economy of motion, and fewer errors in comparison to laparoscopic, with no significant deterioration over time |
| Abdominal wall | Jacob et al | 2017 | Da Vinci Xi |
Porcine N = 1 |
Extended total extraperitoneal dissection | Expert surgeons | Successful completion of abdominal wall dissection |
| Mastectomy | Lee et al | 2021 | Da Vinci Si/Xi |
Cadaveric/animal N = 24 |
Nipple-sparing mastectomy |
2 Plastic surgeons 13 breast surgeons |
Subjective participant feedback indicated positive learning experience |
| TOR | Bur et al | 2017 | Da Vinci |
Synthetic Porcine N = 29 |
Posterior hemi glossectomy |
20 surgical trainees 5 expert Surgeons |
GEARS Faster performance and better technical skill in more senior surgeons Increase in scores and speed of operating over time Good qualitative feedback from trainees as a teaching model |
| TOR | Zhang et al | 2017 | Da Vinci Trainer |
VR Simulator N = 16 |
12 simulated exercises | Novices |
Article validates simulation training in robotic skills, with all novices achieving competency (benchmark 91%) A longer gap between training resulted in a longer time to achieve competency |
Clinical articles were subcategorised by subspeciality (Fig. 3). A total of 11 articles described robotic lymph node dissection [13, 42–51]. A total of 21 articles described robotic pedicled or free flap harvest [52–72]. A total of eight articles described robotic flap pedicle or vessel dissection [73–80]. 16 articles detailed robotic free flap inset or anastomosis (vessel, nerve and lymphovascular) [34, 81–95]. Two articles described robotic craniofacial techniques (mandibular contouring) [96, 97]. One cohort study described a robotic cleft palate surgery [98]. One case report described robotic nerve decompression [99]. Three articles described vaginoplasty/gender reassignment robotic techniques [100–102]. A total of 28 articles described ventral abdominal wall reconstruction and hernia repair [103–130]. A total of 18 articles pertained to robotic mastectomy [56, 72, 131–146]. Finally, a total of 43 articles described transoral robotic surgery (TOR) [81, 147–188].
Fig. 3.
Total number of articles in each subcategory within the scope of robotic plastic and reconstructive surgery
Peri-operative outcomes
Lymph node dissection
Reported length of stay, complications, and recurrence (of disease) are displayed in Table 3. Six articles found the average operative time to be higher for robotic surgery (Table 1). The peri-operative complication rate was found to be comparable, within the reported studies. The average length of stay was shorter for robotic surgery; however, only two articles reported length of stay for conventional lymph node dissection (P = 0.46).
Table 3.
Lymph node dissection length of stay, complications, and rate of recurrence within the literature
| Robotic vs conventional | Control procedure | Number | Length of stay (days) | Complications | Complication rate | Recurrence | Recurrence rate | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Robot | Control | Robot | Control | Robot | Control | Robot | Control | Robot | Control | Robot | Control | ||
| Kim et al., [42] | Endoscopic neck dissection | 2 | 3 | ||||||||||
| Tae et al., [43] | Conventional | 11 | 19 | 3 | 7 | 27.27% | 36.9% | 0 | 2 | 0.00% | 10.53% | ||
| Kim et al., [42] | Conventional | 20 | 33 | 17 | 15.5 | 6 | 9 | 30.00% | 27.27% | 0 | 2 | 0.00% | 6.06% |
| Singh et al., [49] | Conventional inguinal dissection | 51 | 100 | 0 | 0 | 0.00% | |||||||
| Paek et al., [50] | Conventional neck dissection | 28 | 117 | 4.5 | 4.1 | ||||||||
| Total | Total | Average | Total | Average complication rate | Total | Overall rate | Overall rate | ||||||
| 112 | 272 | 10.75 | 10.75 | 9 | 16 | 28.64% | 32.10% | 0 | 4 | 0.00% | 7.69% | ||
| Un- paired single tail T test (5%) | P = 0.46 | P = 0.096 | P = 0.281 | P = 0.00* | P = 0.008* | ||||||||
| Robotic only | Number | Length of stay (Days) | Complications | Complication rate | Recurrence | Recurrence rate |
|---|---|---|---|---|---|---|
| Lira et al., [46] | 6 | 5 | 4 | 66.67% | ||
| Lee et al., [13] | 3 | 8 | 0 | |||
| Du et al., [45] | 1 | 6 | 0 | 0 | 0.00% | |
| He et al., [48] | 13 | 0 | 0 | 0.00% | ||
| Melly et al., [47] | 1 | 3 | ||||
| Song et al., [51] | 4 | 1 | 25.00% | 0 | 0.00% | |
| Total | Average | Total | Average complication rate | Total | Overall rate | |
| 28 | 5.50 | 1 | 25.00% | 4 | 16.67% |
| Overall | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of patients | Average length of stay (Days) | Complications | Average complication rate | Recurrence (total N) | Recurrence rate % | ||||||
| Robot | Control | Robot | Control | Robot | Control | Robot | Control | Robot | Control | Robot | Control |
| 140 | 272 | 7.25 | 9.80 | 10 | 16 | 27.42% | 31.10% | 4 | 4 | 3.77% | 7.69% |
Pedicled and free flap harvest
Peri-operative outcomes regarding pedicled and free flap harvest are reported in Table 4. Average harvest time is higher in the robotic group, although not this was not statistically significant. Average length of stay within comparative studies is lower in the robotic group; however, overall results show a comparable length of stay with conventional surgery. Overall, average complication rates are lower than conventional approaches; however, not statistically significant within comparative studies (P = 0.061).
Table 4.
Peri-operative outcomes of robotic and robotic-assisted pedicled and free flap harvest (RFFF radial forearm free flap, RAM rectus abdominis muscle, LD latissimus dorsi)
| Robotic vs conventional | Control procedure | Number of patients | Length of stay (days) | Complications | Complication rate | Harvest time (min) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Robot | Control | Robot | Control | Robot | Control | Robot | Control | Robot | Control | ||
| Shin et al., [71]. (RFFF) | Conventional | 11 | 11 | 0 | 1 | 0.00% | 9.09% | 107.2 | 67 | ||
| Davila et al., [68] (RAM) | Conventional | 16 | 20 | 10.2 | 11.2 | 5 | 11 | 31.25% | 55.00% | ||
| Winocour et al., [63] (LD) | Conventional | 25 | 27 | 2 | 3 | 4 | 1 | 16.00% | 3.00% | ||
| Houvenaegel et al., [58] (LD) | Conventional | 46 | 59 | 4.3 | 3.86 | 14 | 34 | 30.43% | 57.60% | ||
| Clemens et al., [53] (LD) | Conventional | 12 | 64 | 2.7 | 3.4 | 2 | 24 | 16.67% | 37.50% | 92 | 58 |
| Eo et al., [70] (LD) | Endoscopic/Conventional | 20 | 37 | 10.2 | 10.8 | 5 | 9 | 25.00% | 24.32% | 75.9 | 35.5 |
| Total | Average | Total | Average Complication Rate | Average Harvest Time | |||||||
| 130 | 218 | 5.88 | 6.45 | 30 | 80 | 19.89% | 36.70% | 91.7 | 53.50 | ||
| Un- paired single tail T test | P = 0.415 | P = 0.088 | P = 0.061 | P = 0.057 | |||||||
| Robotic Only | Number of patients | Length of stay | Complications | Complication rate | Harvest time |
|---|---|---|---|---|---|
| Robot | Robot | Robot | Robot | Robot | |
| Ozkan et al., [57] (Omental) | 1 | 12 | 0 | 0.00% | 60 |
| Pederson et al., 2014 (RAM) | 10 | 1 | 10.00% | 60 | |
| Day et al., [65] (Omental) | 1 | 0 | 0.00% | ||
| Lai et al., [72] (LD) | 1 | 0 | 0.00% | ||
| Moon et al., [62] (LD) | 21 | 7 | 4 | 19.05% | 58 |
| Cheon et al., [67] (LD) | 41 | 9 | 17 | 41.46% | 70 |
| Chung et al., [155] (LD) | 12 | 0 | 0.00% | 85.8 | |
| Hwang et al., [69] (LD) | 3 | 0 | 0.00% | 59 | |
| Fouarge et al., [59] (LD) | 6 | 5 | 0 | 0.00% | 110 |
| Joo et al., [66] (LD) | 1 | 6 | 0 | 0.00% | 100 |
| Haverland et al., [61] (RAM) | 6 | 1 | 16.67% | ||
| Asaad et al., [64] (RAM) | 7 | 7 | 1 | 14.29% | |
| Frey et al., [60] (Omental) | 5 | 5.2 | 2 | 40.00% | |
| Total | Average | Total | Average complication rate | Average harvest time | |
| 115 | 7.31 | 26 | 10.88% | 75.35 |
| Overall | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Number of patients | Average length of stay | Total complications | Average complication rate | Average harvest time | |||||
| Robot | Control | Robot | Control | Robot | Control | Robot | Control | Robot | Control |
| 245 | 218 | 6.72 | 6.45 | 56 | 80 | 13.73% | 36.70% | 79.81 | 53.50 |
Microsurgery
Peri-operative outcomes for flap pedicle dissection, flap inset, and microsurgical anastomosis are shown in Table 5. No comparative studies were found for pedicle dissection, with majority of articles pertaining to deep inferior epigastric perforator (DIEP) pedicle dissection. Anastomosis time was found to be longer for robotic surgery; however, docking time was not reported in any studies. There was a comparable rate of overall complications. Only three non-comparative studies reviewed length of stay, with the average being 7.1 days.
Table 5.
Peri-operative outcomes of robotic pedicle dissection and microsurgery. (DIEP deep inferior epigastric perforator)
| Flap Pedicle Dissection | ||||||||
|---|---|---|---|---|---|---|---|---|
| Robotic Only | Number of patients | Length of stay (days) | Complications | Complication rate | Procedure time (mins) | Docking time (mins) | Harvest time (mins) | Console time (mins) |
| Zanaty et al., [80] (Thoracic artery) | 1 | |||||||
| Gundlapalli et al., [73] (DIEP) | 1 | 0 | 0.0% | 480 | 20 | 40 | ||
| Dayaratna et al., [77] (DIEP) | 1 | 0 | 0.0% | 680 | 16 | 92 | 92 | |
| Bishop et al., [75] (DIEP) | 21 | 3.8 | 5 | 23.8% | 425.3 | 44.8 | 44.8 | |
| Daar et al., [75] (DIEP) | 4 | 3.7 | 2 | 50.0% | 717.6 | |||
| Wittesaele et al., [78] (DIEP) | 10 | 4.5 | 1 | 10.0% | 479 | 27.5 | 86 | 86 |
| Tsai et al., [79] (DIEP) | 13 | 1 | 7.7% | 15 | 53 | 53 | ||
| Choi et al., [74] (DIEP) | 17 | 487 | 65 | 65 | ||||
| Total | Average | Total | Average complication rate | Average | Average | Average | Average | |
| 68 | 4 | 9 | 15.3% | 545 | 20 | 63 | 68 | |
| Flap inset/microsurgical anastomosis | ||||||||
|---|---|---|---|---|---|---|---|---|
| Robotic Only | Number of patients | Length of stay | Complications | Complication rate | Procedure time (mins) | Inset time (mins) | Anastomosis time (mins) | Console time (mins) |
| Hans et al., [81] (RFFF inset) | 1 | 14 | 0 | 0% | 310 | 35 | 75 | |
| Song et al., [82] (RFF inset/anastomosis) | 5 | 0 | 0% | 591 | 150 | |||
| Lai et al., [83] (RFF inset, venous anastomosis) | 5 | 0 | 0.0% | 142 | 31 | 40 | ||
| Miyamato et al., [84] (Nerve) | 6 | 0 | 0.0% | |||||
| Chang et al., [88] (Nerve graft) | 1 | 4 | 0 | 0.0% | ||||
| Lindenblatt et al., [90] (LVA/arterial anastomosis) | 5 | |||||||
| Beier et al., [34] (arterial anastomosis) | 23 | 6 | 18.75% | 69 | 69 | |||
| Besmens et al., [92] (arterial anastomosis) | 6 | 33 | 33 | |||||
| Chen et al., [93] (nerve anastomosis) | 23 | 3.2 | 2 | 13.0% | 510 | |||
| Innocenti et al., [94] (arterial/venous anastomosis) | 1 | 0 | 0.0% | 22 | 22 | |||
| Weinzierl et al., [95] (LVA) | 8 | 0 | 0% | 22.6 | 22.6 | |||
| Total | Average | Total | Average complication rate | Average | Average | Average | Average | |
| 76 | 7.07 | 8 | 3.5% | 388 | 33 | 63 | 50 | |
| Flap inset/microsurgical anastomosis | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Robotic Vs Conventional | Control | Number of patients | Length of stay | Complications | Complication rate | Procedure time (mins) | Inset time (mins) | Anastomosis time (mins) | Console time (mins) | |||||||
| Robot | Control | Robot | Control | Robot | Control | Robot | Control | Robot | Control | Robot | Control | Robot | Control | |||
| Tsai et al., [85] (RFF inset, secondary venous anastomosis) | Conventional | 14 | 33 | 2 | 1 | 15.4% | 3.03% | |||||||||
| Lai et al., [86] (arterial/venous anastomosis) | Conventional | 15 | 26 | 1 | 0 | 6.67% | 38 | 28 | 38 | 28 | ||||||
| Van Mulken et al., [87] (LVA) | Conventional | 20 | 12 | 0 | 0 | 0% | 115 | 81 | 25 | 9 | ||||||
| Barbon et al., [89] (arterial/venous/nerve/lymph anastomosis) | Conventional | 22 | 11 | 25.3 | 14.1 | |||||||||||
| Van mulken et al., [91] (LVA) | Conventional | 20 | 12 | |||||||||||||
| Total | Total | Total | Average Complication Rate | Average | Average | Average | ||||||||||
| 91 | 94 | 0 | 3 | 1 | 3.3% | 3.03% | 115 | 81.0 | 32 | 18.5 | 32 | 21.1 | ||||
| Overall (anastomosis/inset) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total number of patients | Average length of stay (days) | Total complications | Average complication rate | Average procedure time | Average inset time | Average anastomosis time | Average console time | ||||||||
| Robot | Control | Robot | Control | Robot | Control | Robot | Control | Robot | Control | Robot | Control | Robot | Control | Robot | Control |
| 167 | 94 | 7.07 | 11 | 1 | 3.40% | 3.03% | 409.01 | 81.00 | 33.00 | 49.95 | 18.50 | 37.56 | 28.00 | ||
Mastectomy
Peri-operative outcomes regarding nipple-sparing mastectomy are shown in Table 6. Operative time was found to be comparable overall; however, this included reconstruction time. Overall length of stay was comparable between open and robotic groups. Overall rate of complication was lower in robotic nipple-sparing mastectomy (P = 0.0007) (Fig. 4).
Table 6.
Peri-operative outcomes in robotic nipple-sparing mastectomy
| Robotic vs Conventional | Control procedure | Number | Length of stay (days) | Complications | Complication rate | Procedure time (resection alone) | Procedure time + implant | Recurrence | Recurrence rate | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Robot | Control | Robot | Control | Robot | Control | Robot | Control | Robot | Control | Robot | Control | Robot | Control | Robot | Control | ||
| Toesca et al., [144] | Conventional | 40 | 40 | 2.3 | 2.4 | 12 | 20 | 30.0% | 50% | 108 | 60 | 138 | 216 | ||||
| Moon et al., [146] | Conventional | 40 | 41 | 9.2 | 7.1 | 6 | 11 | 15.0% | 26.83% | 279 | 207 | ||||||
| Lai et al., [143] | Conventional | 54 | 62 | 7 | 5 | 29 | 38 | 53.7% | 61.29% | 224 | 197 | 0 | 5 | 0.00% | 8.06% | ||
| Park et al., [145] | Conventional | 167 | 334 | 47 | 132 | 28.1% | 39.5 | 0 | 2 | 0.00% | 0.60% | ||||||
| Total | Average | Total | Average complication rate | Average | Average | Total | Overall rate | ||||||||||
| 301 | 477 | 6.17 | 4.83 | 94 | 208 | 31.7% | 44.41% | 108 | 60.0 | 214 | 206.7 | 0 | 7.0 | 0.0% | 1.8% | ||
| Un-paired single tale T test | P = 0.307 | P = 0.198 | P = 0.144 | P = 0.437 | P = 0.072 | P = 0.183 | |||||||||||
| Robotics only | Number | Length of stay (days) | Complications | Complication rate | Procedure time | Procedure time + implant | Recurrence | Recurrence rate | |
|---|---|---|---|---|---|---|---|---|---|
| Robot | Robot | Robot | Robot | Robot | Robot | Robot | Robot | ||
| Kuo et al., [140] | - | 3 | 10 | 94 | 0 | 0.00% | |||
| Sarfati et al., [131] | 1 | 5 | 0 | 0.0% | 150 | 0 | 0.00% | ||
| Lai et al., [134] | - | 2 | 1 | 50.0% | 0 | 0.00% | |||
| Toesca et al., [132] | 3 | 2 | 0 | 0.0% | 285 | ||||
| Rajappa et al., [136] | - | 1 | 2 | 0 | 0.0% | 330 | |||
| Houvenaeghal et al., [56] | 44 | 3 | 26 | 59.1% | 154 | ||||
| Houvenaeghal et al., [139] | - | 80 | 4 | 46 | 57.5% | 305 | |||
| Park et al., [135] | 1 | 0 | 0.0% | 409 | 0 | 0.00% | |||
| Lai et al., [142] | - | 39 | 6.7 | 12 | 30.8% | 257 | |||
| Sarfati et al., [137] | 33 | 0 | 0.0% | 85 | |||||
| Toesca et al., [132] | - | 29 | 2 | 0 | 0.0% | 180 | |||
| Lai et al., [141] | 22 | 0 | 0.0% | 192 | |||||
| Lai et al., [72] | - | 15 | 6.7 | 3 | 20.0% | 282 | |||
| Sarfati et al., [137] | 1 | ||||||||
| Total | Total | Total | Average Complication Rate | Average | Average | Total | Overall Rate | ||
| 274 | 41.4 | 88 | 18.1% | 241 | 220 | 0 | 0.0% |
| Overall | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | Average length of stay | Total complications | Average complication rate | Average procedure time | Average procedure time + implant | Total recurrence | Recurrence rate | ||||||||
| Robot | Control | Robot | Control | Robot | Control | Robot | Control | Robot | Control | Robot | Control | Robot | Control | Robot | Control |
| 575 | 477 | 4.99 | 4.83 | 182 | 208 | 21.5% | 44.41% | 214 | 60 | 214 | 206.7 | 7 | 0.0% | 1.8% | |
Fig. 4.
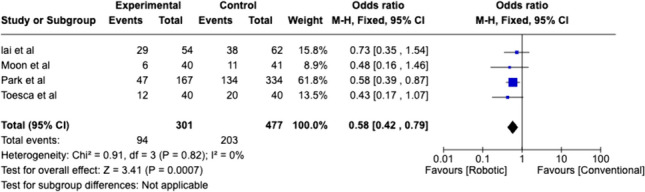
Weighted analysis of comparative studies reviewing complication rate of robotic nipple sparing mastectomy with conventional nipple sparing mastectomy
Abdominal wall
Outcomes regarding abdominal wall reconstruction are collated in Table 7. Separate comparisons are demonstrated between robotic versus laparoscopic, and robotic versus open repair. Weighted analysis of comparative robotic versus laparoscopic studies found high heterogeneity (85%), and favours robotic surgery with reduced complications (P = 0.02) (Fig. 5). Robotic surgery had fewer complications when compared with open surgery (P = 0.0001), with lower heterogeneity (Fig. 6).
Table 7.
Peri-operative outcomes reported in abdominal wall reconstruction. (TAR transversus abdominis release)
| Robotic vs Laparoscopic Hernia Repair | Control Procedure | Number of Patients | Length of Stay (Days) | Complications | Complication Rate | Operative Time (mins) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Robot | Control | Robot | Control | Robot | Control | Robot | Control | Robot | Control | ||
| Costa et al., [122] | Laparoscopic | 18 | 19 | 3.67 | 3.95 | 3 | 2 | 16.67% | 10.53% | 355.6 | 293.5 |
| Chen et al., [103] | Laparoscopic | 39 | 33 | 0.49 | 0.21 | 3 | 3 | 7.69% | 9.09% | 156 | 65 |
| Petro et al., [118] | Laparoscopic | 39 | 36 | 0.5 | 1 | 4 | 4 | 10.26% | 11.11% | 146 | 94 |
| Olavarria et al., [116] | Laparoscopic | 65 | 59 | 0 | 0 | 14 | 11 | 21.54% | 18.64% | 141 | 77 |
| Walker et al., [113] | Laparoscopic | 142 | 75 | 1.4 | 0.7 | 37 | 43 | 26.06% | 57.33% | 116.9 | 98.7 |
| Warren et al., [109] | Laparoscopic | 53 | 103 | 1 | 2 | 36 | 37 | 67.92% | 35.92% | 245 | 122 |
| Kakela et a, [123] | Laparoscopic | 19 | 19 | 0.9 | 0.6 | 0.00% | 0.00% | 135 | 43.6 | ||
| Prabhu et al., [107] | Laparoscopic | 177 | 450 | 0 | 1 | 14 | 84 | 7.91% | 18.67% | N = 47 < 2 h) | N = 31 > 2 h |
| Total | Average | Total | Average Complication Rate | Average | |||||||
| 552 | 794 | 0.995 | 1.18 | 111 | 184 | 19.76% | 20.16% | 185 | 113 | ||
| Un-paired single tail T test | P = 0.382 | P = 0.216 | P = 0.484 | P = 0.069 | |||||||
| Robotic vs Open | Control procedure | Number | Length of stay (days) | Complications | Complication rate | Operative time (mins) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Robot | Control | Robot | Control | Robot | Control | Robot | Control | Robot | Control | ||
| Martin-Del-Campo et al., [111] (TAR) | Open | 38 | 76 | 1.3 | 6 | 0 | 13 | 0.00% | 17.11% | 299 | 211 |
| Pereira et al., [126] | Open | 665 | 665 | 1 | 3 | 79 | 123 | 11.88% | 18.50% | ||
| Bittner et al., [104] (TAR) | Open | 26 | 76 | 3 | 6 | 5 | 34 | 19.23% | 44.74% | 365 | 287 |
| Carbonell et al., [110] | Open | 111 | 222 | 2 | 3 | 47 | 61 | 42.34% | 27.48% | 45% > 240 | |
| Total | Average | Total | Average complication rate | Average | |||||||
| 840 | 1039 | 1.83 | 4.50 | 131 | 231 | 18.36% | 26.95% | 332 | 249 | ||
| Un-paired single tail T test | P = 0.017* | P = 0.220 | P = 0.231 | P = 0.120 | |||||||
| Robotics only | Number of patients | Length of stay (days) | Complications | Complication rate | Operative time (min) |
|---|---|---|---|---|---|
| Robot | Robot | Robot | Robot | Robot | |
| Wang et al., [108] | 1 | 4 | 0 | 0.00% | |
| Jamshidian et al., [106] | 3 | 1 | 0 | 0.00% | 88 |
| Kudsi et al., [114] (TAR) | 1 | 1 | 0 | 0.00% | 302 |
| Muysoms et al., [112] | 41 | 114 | |||
| Shimada et al., [127] | 1 | 5 | 0 | 0.00% | 253 |
| Lima et al., [129] | 1 | 0 | 0.00% | ||
| Bergholz et al., [119] | 1 | 1 | 0 | 0.00% | |
| Gonzalez et al., [105] | 368 | 1 | 44 | 11.96% | 102.1 |
| Total | Average | Total | Average Complication Rate | Average | |
| 417 | 2.17 | 44 | 1.71% | 172 |
| Overall | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total number of patients | Average length of stay (days) | Total complications | Average complication rate | Average operative time (mins) | |||||
| Robot | Control | Robot | Control | Robot | Control | Robot | Control | Robot | Control |
| 1809 | 1833 | 1.57 | 2.29 | 286 | 415 | 12.81% | 22.43% | 201 | 144 |
Fig. 5.
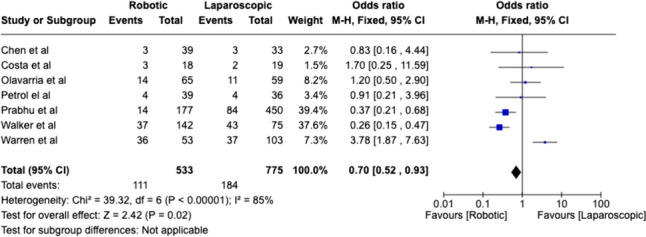
Weighted analysis of comparative studies reviewing complication rate between robotic abdominal wall reconstruction and laparoscopic abdominal wall reconstruction
Fig. 6.
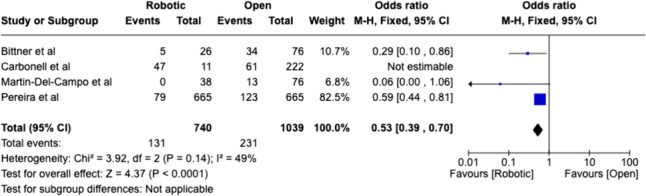
Weighted analysis of comparative studies reviewing complication rates in robotic versus open abdominal wall reconstruction
Length of stay was shorter for robotic surgery in comparison to both groups, however, was only statistically significant for robotic versus open (P = 0.017). Overall operative time was higher for robotic surgery but was not statistically significant within laparoscopic and open subgroups.
Transoral robotic surgery
TOR operative outcomes are reported in Table 8. Length of stay was shorter for robotic surgery; however, this was not statistically significant. A statistically significant lower rate of complications is found for robotic surgery in comparison to open surgery (P = 0.033). Disease-free survival was higher within the robotic cohort; however, this was not found to be statistically significant.
Table 8.
Peri-operative outcomes reported for transoral robotic surgery (TOR) (DFS; disease-free survival)
| Robotic Vs Conventional | Control procedure | Number of patients | Length of stay (Days) | Complications | Complication rate | DFS | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Robot | Control | Robot | Control | Robot | Control | Robot | Control | Robot | Control | ||
| Li et al., [178] | Open | 2224 | 6697 | 4.3 | 5.1 | ||||||
| Chung et al., [54] (anterior partial glossectomy) | Open | 68 | 3915 | 4.8 | 4 | 0 | 131 | 0.0% | 3.3% | ||
| Chung et al., [54] (posterior pharyngectomy) | Open | 641 | 1426 | 3.7 | 5.2 | 21 | 71 | 3.3% | 5.0% | ||
| Chung et al., [54] (posterior partial glossectomy) | Open | 147 | 747 | 3.54 | 5.06 | 14 | 37 | 9.5% | 5.0% | ||
| Sano et al., [185] | Open | 68 | 236 | ||||||||
| Ford et al., [157] | Open | 65 | 65 | 89% | 73% | ||||||
| White et al., [154] | Open | 64 | 64 | 3.8 | 8 | 26 | 55 | 40.6% | 85.9% | 74% | 43% |
| Lee et al., [151] | Open | 27 | 30 | 14.6 | 24.6 | 95.70% | 91.60% | ||||
| Hammoudi et al., [158] | Open | 26 | 26 | 11 | 19 | 1 | 2 | ||||
| Total | Average | Total | Average complication rate | Average rate of DFS | |||||||
| 3330 | 13,206 | 6.53 | 10.14 | 62 | 296 | 13.36% | 24.80% | 86.2% | 69.2% | ||
| Un-paired, single tail T test (5%) | 0.164 | 0.033* | 0.314 | 0.126 | |||||||
| Robotic Vs Radiotherapy | Control procedure | Number of patients | Length of stay (days) | Complications | Complication rate | DFS | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Robot | Control | Robot | Control | Robot | Control | Robot | Control | Robot | Control | ||
| Mahmoud et al., [173] | Radiotherapy | 559 | 1314 | ||||||||
| Nichols et al., [181] | Radiotherapy | 34 | 34 | 94 | 74 | 276.5% | 217.6% | ||||
| Smith et al., [165] | CRT | 42 | 38 | 94% | 85% | ||||||
| Nichols et al., [187] | Radiotherapy | 34 | 34 | 88.20% | |||||||
| Total | Total | Average Complication Rate | Average DFS Rate | ||||||||
| 669 | 1420 | 94 | 74 | 276.47% | 217.65% | 91.1% | 85% | ||||
| Robotic Only | Number | Length of stay (days) | Complications | Complication rate | DFS | Recurrence | Recurrence rate |
|---|---|---|---|---|---|---|---|
| Van Loon et al., [159] | 18 | 4.2 | 2 | 11.1% | 86% | 2 | 11.1% |
| Mercante et al., [162] | 13 | 7 | 4 | 30.8% | |||
| Sethia et al., [175] | 111 | 9 | 8.1% | ||||
| Chan et al., [147] | 4 | 4.25 | 0 | 0.0% | |||
| Chia et al., [148] | 2015 | 205 | 10.2% | ||||
| Durmus et al., [149] | 22 | 0 | 0.0% | ||||
| Durmus et al., [150] | 3 | ||||||
| Hans et al., [81] | 3 | 14 | 0 | 0.0% | 0 | 0.0% | |
| Patel et al., [152] | 47 | 5 | 10.6% | ||||
| Tsang et al., 2013 | 1 | 0 | 0.0% | ||||
| Almeida et al., [160] | 410 | 94.50% | 43 | 10.5% | |||
| Dabas et al., [161] | 60 | 4.15 | 3 | 5.0% | 64% | ||
| Razafindranaly et al., [164] | 84 | 37 | 44.0% | 2 | 2.4% | ||
| Aubry et al., [166] | 178 | 12.6 | 87 | 48.9% | |||
| Fujiwara et al., [167] | 10 | 8 | 0 | 0.0% | 1 | 10.0% | |
| Graneli et al., [168] | 1 | 3 | 0 | 0.0% | 0 | 0.0% | |
| Duek et al., [169] | 1 | 3 | 0 | 0.0% | 0 | 0.0% | |
| Lallemant et al., [172] | 23 | 12.7 | |||||
| Rubek et al., [174] | 30 | 5.3 | 6 | 20.0% | |||
| Doazan et al., [177] | 122 | 90.20% | 14 | 11.5% | |||
| Scott-Wittenbom et al., [179] | 6 | ||||||
| Hardy et al., [180] | 1 | 0 | 0.0% | 0 | 0.0% | ||
| Petruzzi et al., [182] | 1 | 0 | 0.0% | ||||
| Holcomb et al., [183] | 2 | 0 | 0.0% | 1 | 50.0% | ||
| Kubik et al., [184] | 23 | 1 | 4.3% | ||||
| D’Andrea et al., [186] | 53 | 21 | 39.6% | 46.10% | |||
| Virgilio et al., [188] | 139 | 71.30% | |||||
| Durmus et al. [156] | 22 | 0 | 0.0% | ||||
| Frenkel et al., [170] | 425 | 273 | 64.2% | ||||
| Gorphe et al., [171] | 27 | 8 | 29.6% | ||||
| Mockelmann et al., [163] | 41 | 8 | 9 | 22.0% | |||
| Allessandrini et al., [176] | 16 | 6.13 | 0.0% | ||||
| Total | Average | Total | Average complication rate | Average DFS | Average recurrence | Overall recurrence rate | |
| 3912 | 7.10 | 661 | 13.6% | 75.35% | 6.5 | 9.44% |
| Overall | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of patients | Length of stay | Complications | Average complication rate | Average DFS rate | Average recurrence | Overall recurrence rate | |||||||
| Robot | Control | Robot | Control | Robot | Control | Robot | Control | Robot | Control | Robot | Control | Robot | Control |
| 7911 | 14,626 | 6.90 | 10.14 | 817 | 370 | 22.3% | 63.37% | 81.18% | 73.15% | 6.5 | 9.44% | ||
Operative time was variable, and few conclusions can be drawn (Table 1). Lee et al. reported a longer duration compared to transoral resection; however, White et al., found a shorter duration for excision of recurrent oropharyngeal SCC [151, 154]. White et al., also found a better rate of negative margins with robotic surgery [154]. Hammoudi et al. found no difference in procedure duration for resection of primary SCC [158].
Post-operative outcomes
Patient-reported outcomes and long-term outcomes are reported in Table 1. The quality and standard of assessment varied greatly. Patient satisfaction was reported in three (27%) lymph node (neck) dissection articles, all of which found better scores compared to open with regard to cosmesis and scarring [42–44]. Lin et al. found comparable results for patient satisfaction and pain for mandibular contouring [96].
Flap/microsurgery
High patient satisfaction for latissimus dorsi muscle flap harvests were reported in three articles; one cohort study found significantly higher BREAST-Q scores than open [54, 66, 70]. 31% of flap inset or anastomosis articles reported post-operative outcomes other than complications [84, 85, 87, 91, 93]. Van Mulken et al. reported robotic lymphovascular anastomosis to have comparable lymph ICF scores to conventional microsurgery. Miyamoto et al. and Chen et al. reported successful patient outcomes of nerve grafts (sympathetic trunk reconstruction and nerve to deltoid) [84, 93]. Two articles detailing pedicle dissection of DIEP flaps reported favourable outcomes, and no hernias; however, there are no comparative results [73, 77].
Abdominal wall reconstruction
Patient-reported outcome measures (PROMs) were described in 8 (28.6%) articles of abdominal wall reconstruction. Three articles reviewed pain with VAS scores and found no difference (2 RCT’s) or less pain at 1 month/1 year (prospective cohort) [116, 120, 123]. Kakela et al. found comparable PROMs (SF-36) with laparoscopic surgery, with high scores for emotional status and social function for robotic surgery. Three articles found no difference between robotic and laparoscopic surgery in reported patient outcomes, including functional status [120, 122, 126]. One RCT found higher HERqLess scores for robotic versus laparoscopic ventral mesh hernia repair [118].
One RCT compared robotic extraperitoneal versus intraperitoneal onlay mesh (IPOM) for ventral hernia repair and found that IPOM had significantly higher HerQLess scores at 1 year follow-up.
Mastectomy
A total of four (22.2%) of mastectomy articles reported patient qualitative outcomes. Two articles reported high scores for cosmetic satisfaction with minimal scarring, whilst one case control study found significantly higher scores in a cosmetic outcome questionnaire than open surgery, with better scarring and a better position of the nipple–areolar complex [132–134, 143]. One RCT documented significantly higher satisfaction within the BREAST-Q questionnaire for robotic surgery [144].
TOR
Three TOR studies reported a lower rate of tracheostomies in the peri-operative period, as well as a lower requirement and durations of nasogastric feeding/PEG feeding [154, 155, 158].
Two studies found significantly higher 3-year disease-free survival with robotic surgery in HPV negative patients, and comparable rates of survival for HPV positive patients for oropharyngeal SCC primary resection [157, 173]. This was echoed by Lee et al., in which robotic surgery had a higher overall and disease-free survival rate at 2 years for lateral oropharyngectomy as treatment for tonsillar cancer [151]. White et al. found a higher rate of 2-year disease-free survival for open surgery to treat recurrent oropharyngeal SCC (T1-T4) [155].
Two articles evaluated patient outcomes through the Head and Neck Cancer Inventory (HCNI); Durmus et al. reported patients to have highly functional quality of life within their case series of carcinoma of unknown primary resection [156]. Sethia et al. found comparable outcomes for robotic oropharyngeal resection with and without adjuvant therapy [175]. Lee et al. also reported no difference in VHI and MDADI scores between open and robotic lateral oropharyngectomy for tonsillar cancer [151].
Cost
Gundlapalli et al. reported a higher procedural cost for their case report of a robotic-assisted DIEP breast reconstruction of $16,000 versus $14,000. There were no other articles which reported cost within robotic flap harvest or microsurgery.
Lai et al. reported a higher cost for robotic nipple-sparing mastectomy in comparison to conventional treatment of $10, 877 versus $5,702 [143].
Within the subcategory of abdominal wall reconstruction three articles (11%) reported cost. Olavarria et al. found robotic patients had an increased total cost for 90 days of care in comparison to laparoscopic ventral mesh hernia repair in their RCT ($15, 865 robotic versus $12, 955) [116]. In addition to this, a separate RCT found that whilst the cost of reusables was comparable between robotic and laparoscopic ventral hernia repair, the total cost was significantly higher for robotic patients due to the overall operative time (Cost ratio of 1.13 robotic versus laparoscopic 0.97 P = 0.03) [125]. In contrast a retrospective cohort study found whilst the procedure costs were higher for robotic surgery, the overall cost of patient care was shorter because of reduced length of hospital stay (robotic $13, 943 versus $19, 532, P = 0.07) [109].
Two TOR articles reported cost (4.7%). Chung et al. found that overall cost was significantly lower for robotic pharyngectomy ($20,706 versus $29,365) and posterior partial glossectomy ($19, 091 versus $23,414), whilst anterior partial glossectomy demonstrated no difference in the total cost of procedure between TOR and conventional approaches ($22,111 versus $21,376) [155]. Hammoudi et al. reported higher costs for robotic oropharyngeal SCC resection; however, the overall cost accounting for duration of hospital stay was significantly less ($20,885 vs $27,926) [158].
Learning curve
Learning curve was reported in clinical studies as changes in operative time (Table 1). Three abdominal wall reconstruction articles commented that skin-to-skin operating time decreased throughout their cohort [112, 116, 120]. Muysoms et al. analysed operative time for 41 transabdominal retromuscular hernia repairs, and commented that the decrease was largely contributed to by improved efficacy in the dissection aspect of the procedure [112]. Olavarria et al. reported a training exposure of 50 cases, through simulation and cadaveric models, prior to performing ventral hernia repairs was necessary to ensure optimal clinical practice [116]. A total of four mastectomy articles reported operative time to decrease with as clinical exposure increased, including a decrease in docking time [132–134, 139, 142]. Lai et al. achieved an average time for nipple-sparing mastectomy of 100 min, in a series of 39 patients [142].
Van Mulken et al. reported robotic microvascular anastomosis to require a longer time to complete; however, a steep learning curve resulted in a reduction in this [87]. Barbon et al. also reported a steep learning curve for anastomosis with time taken to complete being comparable to hand-sewn operative time, with the quickest robotic anastomosis taking around 10 min (Table 2) [89].
Selber et al. also reported a steep learning curve in surgical trainees over five sessions, followed by gradual improvement [29]. Two training models in microvascular anastomosis reported a plateau in learning curve of robotic anastomosis by expert surgeons on synthetic silicone vessels and rat vessels to be 5 and 8 attempts, respectively [16, 17]. Beier et al. developed a 4-week training programme with synthetic 1 and 2 mm vessels, in which 10 successful anastomosis were deemed to be the benchmark for skill acquisition before progression to clinical practice [34].
Surgical ease of use
Robotic surgery offers several mechanical advantages to aid surgical performance. Many authors commented upon improved visibility with higher 3-dimensional resolution, magnification, and lighting, allowing for depth of field perception and a 360° view of a cavity [54, 132].
The Da Vinci robotic arms have 7° of freedom which allow for higher dexterity and greater range of motion, optimising the user’s ability to dissect the surgical plane and increasing access to difficult anatomical areas [137].
Insufflation was found to be useful attribute for nipple-sparing mastectomy [131, 135, 141]. Through a single small incision approach, Toesca et al. reported easy identification of structures such as intercostal perforators which contribute to nipple–areolar complex survival and flap survival, and better view of the surgical plane [132]. The use of carbon dioxide helped to reduce bleeding and perform better haemostasis [132]. There was a higher surgical challenge with larger ptotic breasts [136]. Motion scaling, and tremor filtration provides high precision and stability; this was also found to be advantageous for flap and microsurgery [77, 132, 137].
The robotic technique of pedicle dissection of the DIEP flap minimizes incision of the anterior rectus muscles and provides improved dexterity and motion; however, due to the space occupation of the robot and the console it may be challenging for two surgical teams to work simultaneously, thus potentially increasing operative duration [73, 77, 80].
Robotic equipment also eliminates haptic feedback; however, users have reported that they were able to compensate effectively for this by relying on visual cues and felt able to complete the vessel and lymphovascular anastomosis without difficulty [83, 85, 90].
Feng et al. reviewed tremor during microsurgery, based on instrument tip movement and found that this was significantly lower in robotic surgery in an ex vivo model [14]. Furthermore, in a simulation model of 1 mm synthetic vessels, robotic anastomosis was performed with greater precision (measured in suture distance and angulation) when compared with manual approaches for 40 expert surgeons and 20 novices [17].
Discussion
This study demonstrates feasibility and safety of robotic surgery within plastic and reconstructive surgery in several subcategories. There are clear benefits to the surgeon, as described above, with improved access to difficult areas, tremor reduction and motion scaling, and improved ergonomic efficiency [2].
These attributes are particularly useful in cavity surgery and could create opportunities to complete challenging procedures which could not be accessed through an open approach due to narrow openings, such as nasopharyngeal resection and microvascular reconstruction, or where there may be a high risk of complications, or prolonged recovery time associated with conventional open approaches.
One example of this is TOR, whereby access and exposure is often obtained through techniques with higher morbidity, such as mandible splitting, leading to specific complications and expectations for recovery outside of the intended resection. Furthermore, although DIEP flap harvest can be regarded as having more superficial access, Tsai et al. found the anterior rectus sheath incision for pedicle dissection to be significantly smaller than conventional approaches, and thus less invasive [79]. It is not yet clear if this translates to reduced hernia occurrence post-operatively.
As interest within microsurgery grows, Da Vinci, and other companies such as Symani Surgical Systems and Microsure, have created an instrument portfolio that is well adapted to this field. Literature shows these tools can perform vessel, nerve and lymphovascular anastomosis with non-inferior outcomes to conventional approaches. Improved surgical ergonomics has allowed end-to-end anastomosis of 1 mm diameter vessels as reported in preclinical studies, with higher ease [16]. Whilst nerve repair can be performed robotically, there is lack of substantial evidence or comparison to conventional approaches. Whilst this approach is more minimally invasive, further research to determine the overall benefit, safety and cost would be beneficial.
Loss of haptic feedback is often considered to be disadvantage of robotic surgery. Surgeons have reported a compensation for this by relying on visual cues which has not impacted their performance. Further research could assess how easily a surgeon may adapt to the loss of true haptic feedback, as well as looking into the incorporation of haptic feedback into robotic instruments.
Single port access is highly advantageous for breast surgery including resection and reconstruction. Quicker docking can reduce operative time and the smaller incision offers a better cosmetic outcome with reduced scarring [101].
The high precision and accuracy of robotic surgery, could improve patient care, reflected in the lower rate of complications reported, reduced blood loss, reduced post-operative pain, as well as the comparable or reduced length of recovery. Whilst operative time is reported to be higher for robotics, many centres have shown a learning curve in adapting to new techniques.
Post-operative outcomes
There is a paucity of data evaluating patient reported outcomes within the literature. Outcomes within case series/case reports were often reported anecdotally, without use of validated or quantitative assessment tools. However, several articles have reported high patient satisfaction with regard to cosmetic outcome and scarring. Robotic neck dissection approach has been performed with a smaller retro-auricular incision.
Furthermore, robotic latissimus dorsi muscle flap harvest and radial forearm flap harvest can offer reduced scarring through a more minimally invasive approach, resulting in absence of long scars, on the back and forearm, respectively. Whilst this is the case, compared to open techniques, insufflation with reduced scarring, can also be achieved with an endoscopic approach. A comparison of the benefits to the surgeon and patient between endoscopic and robotic-assisted technique would be valuable to ascertain the true benefit of robotic assistance in this procedure. Some patients may require incorporation of skin within an LD flap for example in salvage procedures, or delayed reconstruction of the irradiated breast. The quality of coverage at the recipient site may be insufficient to accommodate the optimal reconstructive outcome, with particular importance of the integrity of the lower pole. In these circumstances, robotic surgery may present few advantages for LD flap harvest, and thus patient selection is important.
Patients undergoing robotic nipple-sparing mastectomy and reconstruction have also reported a higher scar satisfaction, with the use of a single incision in the axilla, in which multiple robotic arms can be used. There is a clear benefit to procedures in which access can move towards less invasive approaches, and robotic surgery within breast reconstruction and lymph node dissection are promising avenues for future research.
The rate of hernia recurrence within abdominal wall reconstruction is challenging to ascertain given the variable and often short length of follow-up reported within the literature. The mean length of follow-up within this subcategory is 9 months (0.25–33.6 months).
There is a high variance of histopathology within the transoral robotic surgery subcategory, as well as tumour location, stage of disease, and patient demographics. Few conclusions can be drawn between the comparative studies given the variability. However, the results reported, suggest that TOR results in non-inferior patient outcomes in comparison to conventional approaches.
Cost
Cost is poorly reported within the literature. Cost-analysis of robotic reconstructive procedures to review total cost of patient care would be beneficial in ascertaining the economic barriers that prevent the implementation of robotic within clinical practice in this speciality. Reasons suggested for higher cost include the initial purchase of robotic equipment, and prolonged operative duration utilising resources [118].
However, several articles within abdominal wall reconstruction and TOR, have reviewed the total cost of patient care, and found that the overall financial burden is significantly less than conventional approaches after accounting for length of hospital stay. This could be because of fewer complications, and reduced pain with a minimally invasive approach [109, 155, 158]. Chung et al. also reported reduced requirement of tracheostomies, nasogastric feeding, and percutaneous endoscopic gastrostomy (PEG) feeding, which could account for a decrease in overall consumables cost. Several articles have also described a learning curve throughout their studies, reflected in a shorter operative duration, which could have an impact for cost incurred. The cost of training surgeons, and theatre teams to use robotic equipment should also be accounted for.
Whilst the initial cost may be high for robotic surgery, the overall cost may be offset by the reduction in complication rate, and reduced length of stay. It is important to delineate when and where the cost of robotics, including resource utilisation, is balanced by proven improved patient outcomes in order to implement this effectively in future practice.
Learning curve
All studies which report a learning curve in this review, do so indirectly, as a reduction in operative time [142]. Whilst a reduction in the time taken to perform the procedure can be seen as an improvement in skill acquisition, duration of surgery can be affected by various factors in clinical practice including team efficiency and education. Standardised training for skill acquisition with appropriate measures of assessment in a controlled setting will aid in understanding the number of procedures required to achieve clinical competency in each subspeciality. global evaluative assessment of robotic skills (GEARS), and structured assessment of robotic microsurgery skills (SARMS), have been used as objective quantitative assessment tools in this field.
Training should also encompass theatre staff, as set up time including robot docking, change of arms, and equipment troubleshooting can be optimised to reduce burden and improve patient care [133]. Prolonged operative duration incurs significant resource utilisation including time, cost, equipment, and staff. Barbon et al. was able to demonstrate a steep learning curve in microvascular anastomosis to achieve an anastomotic time which was comparable with conventional approaches [89].
Vierstraete et al. describe the current training pathway of abdominal wall reconstruction and ventral hernia repair and found in their experience of posterior component separation that there was a gradual reduction in operative time until the surgical team reached their ‘comfort zone’ at around 20–25 cases. Depending on the frequency with which this procedure is performed, it may take a long period of time for the surgeon to reach that level of experience [189].
Other limitations
This report shows technical feasibility of robotic surgery; however, many articles are a relatively low level of evidence, with a high prevalence of case reports and case series. This review presents small sample sizes and as such, statistical analysis is likely to be underpowered, impeding ability to present true statistical significance. Whilst this study can suggest non-inferiority of robotic surgery, patient advantages remain to be clearly demonstrated.
There is a lack reported of long-term outcomes and formal PROMs, with variable follow-up duration. Due to large heterogeneity of the data and variance within patient selection, and outcomes reported, particularly within transoral robotic surgery, we have been unable to perform a weighted analysis for most subcategories, which would provide a more powerful comparison.
Conclusions
This literature review demonstrates technical feasibility of robotics in plastic and reconstructive surgery. High cosmetic satisfaction is reported with minimally invasive approaches. Operative time is higher than conventional approaches, although steep learning curves are reported, and this may contribute to a higher initial cost. Overall cost may be offset with improved patient outcomes within TOR and abdominal wall reconstruction; however, further reporting of cost and cost-effectiveness is necessary. Technical advantages can potentially translate to improvements in complication rate, and a faster recovery time, with non-inferior patient outcomes reported, with thoughtful case selection. However clearer evidence to support improved outcomes within the field, particularly in comparison with laparoscopic surgery, is required to justify the financial incurrence and demand on resources. Robotic surgery could play an exciting role within plastic surgery, and future research should focus on robotic training, as well as producing higher quality comparative clinical research, which is adequately powered, to fully understand the true benefit for patient care.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
Authors Contributions are detailed below Design: L.A, B.R, E.B, B.L, S.J, P.B, A.P Literature Search/Screening: L.A, B.R, E.B, B.L Data Collection : L.A, E.B, B.R Data Analysis: L.A, B.R Manuscript Writing : L.A, B.R, S.J, B.L, PB, AP Tables and Figures: S.J, B.L, B.R, LA Risk of Bias: E.B, L.A Editing : L.A, B.R, E.B, B.L, S.J, P.B, A.P All authors have reviewed the final submission.
Funding
There was no funding allocated for this research.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Conflict of interest
There are no conflicts of interest to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kawka M, Fong Y, Gall TMH. Laparoscopic versus robotic abdominal and pelvic surgery: a systematic review of randomised controlled trials. Surg Endosc [Internet]. 2023 Sep 1 [cited 2024 Apr 6];37(9):6672. Available from: https://pubmed.ncbi.nlm.nih.gov/37442833/. Accessed 14 Mar 2024 [DOI] [PMC free article] [PubMed]
- 2.Moore LJ, Wilson MR, McGrath JS, Waine E, Masters RSW, Vine SJ (2015) Surgeons’ display reduced mental effort and workload while performing robotically assisted surgical tasks, when compared to conventional laparoscopy. Surg Endosc 29(9):2553–2560 [DOI] [PubMed] [Google Scholar]
- 3.Struk S, Qassemyar Q, Leymarie N, Honart J-F, Alkhashnam H, De Fremicourt K et al (2018) The ongoing emergence of robotics in plastic and reconstructive surgery. Annales de Chirurgie Plastique Esthétique 63(2):105–112 [DOI] [PubMed] [Google Scholar]
- 4.Dobbs TD, Cundy O, Samarendra H, Khan K, Whitaker IS (2017) A systematic review of the role of robotics in plastic and reconstructive surgery—from inception to the future. Front Surg 15:4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jimenez C, Stanton E, Sung C, Wong AK (2022) Does plastic surgery need a rewiring? A survey and systematic review on robotic-assisted surgery. JPRAS Open 33:76–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. The BMJ 29:372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Microsoft Corporation (2018) Microsoft Excel [Internet]. Available from: https://office.microsoft.com/excel. Accessed 14 Mar 2024
- 8.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I et al (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 28:l4898 [DOI] [PubMed] [Google Scholar]
- 9.Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al (2016) ROBINS-I: a tool for assessing risk of bias in non-randomized. BMJ 355:i4919. 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed]
- 10.Munn Z, Barker T, Moola S, Tufanaru C, Stern C, McArthur A et al (2020) Methodological quality of case series studies: an introduction to the JBI critical appraisal tool. JBI Evidence Synthesis 18(10):2127–2133 [DOI] [PubMed] [Google Scholar]
- 11.Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, Currie M, Qureshi R, Mattis P, Lisy K, Mu P-F (2017) Systematic reviews of etiology and risk. In: Aromataris E, Munn Z (eds) Joanna Briggs Institute reviewer's manual. The Joanna Briggs Institute, 2017. Available from https://reviewersmanual.joannabriggs.org/
- 12.Review Manager (RevMan) [Computer program]. Version 7.2.0. The Cochrane Collaboration, 2024. Available at revman.cochrane.org
- 13.Lee J, Park HS, Lee H, Lee K, Han DH, Lee DW (2020) Axillary lymph node dissection using a robotic surgical system: initial experience. J Surg Oncol 122(6):1252–1256 [DOI] [PubMed] [Google Scholar]
- 14.Feng AL, Razavi CR, Lakshminarayanan P, Ashai Z, Olds K, Balicki M et al (2017) The robotic ENT microsurgery system: a novel robotic platform for microvascular surgery. Laryngoscope 127(11):2495–2500 [DOI] [PubMed] [Google Scholar]
- 15.van Mulken TJM, Schols RM, Qiu SS, Brouwers K, Hoekstra LT, Booi DI et al (2018) Robotic (super) microsurgery: feasibility of a new master-slave platform in an in vivo animal model and future directions. J Surg Oncol 118(5):826–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malzone G, Menichini G, Innocenti M, Ballestín A (2023) Microsurgical robotic system enables the performance of microvascular anastomoses: a randomized in vivo preclinical trial. Sci Rep 13(1):14003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ballestin A, Malzone G, Menichini G, Lucattelli E, Innocenti M, Ballestín A, et al (2022) New Robotic System with Wristed Microinstruments Allows Precise Reconstructive Microsurgery: Preclinical Study. Ann Surg Oncol 29(12):7859–7867 [DOI] [PubMed]
- 18.Zhu J-H, Deng J, Liu X-J, Wang J, Guo Y-X, Guo C-B (2016) Prospects of robot-assisted mandibular reconstruction with fibula flap: comparison with a computer-assisted navigation system and freehand technique. J Reconstr Microsurg 32(9):661–669 [DOI] [PubMed] [Google Scholar]
- 19.Manrique OJ, Bustos SS, Mohan AT, Nguyen M-D, Martinez-Jorge J, Forte AJ et al (2020) Robotic-assisted DIEP flap harvest for autologous breast reconstruction: a comparative feasibility study on a cadaveric model. J Reconstr Microsurg 36(05):362–368 [DOI] [PubMed] [Google Scholar]
- 20.Sánchez A, Rodríguez O, Jara G, Sánchez R, Vegas L, Rosciano J et al (2018) Robot-assisted surgery and incisional hernia: a comparative study of ergonomics in a training model. J Robot Surg 12(3):523–527 [DOI] [PubMed] [Google Scholar]
- 21.Chen MM, Orosco RK, Lim GC, Holsinger FC (2018) Improved transoral dissection of the tongue base with a next-generation robotic surgical system. Laryngoscope 128(1):78–83 [DOI] [PubMed] [Google Scholar]
- 22.Tay G, Tan H, Nguyen TK, Phee SJ, Iyer NG (2018) Use of the EndoMaster robot-assisted surgical system in transoral robotic surgery: A cadaveric study. Int J Med Robot Comput Assist Surg 14(4):e1930 [DOI] [PubMed] [Google Scholar]
- 23.Friedrich DT, Dürselen L, Mayer B, Hacker S, Schall F, Hahn J et al (2018) Features of haptic and tactile feedback in TORS-a comparison of available surgical systems. J Robot Surg 12(1):103–108 [DOI] [PubMed] [Google Scholar]
- 24.Leijte E, de Blaauw I, Rosman C, Botden SMBI (2020) Assessment of validity evidence for the RobotiX robot assisted surgery simulator on advanced suturing tasks. BMC Surg 20(1):183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Groote R, Puliatti S, Amato M, Mazzone E, Rosiello G, Farinha R, et al (2022) Proficiency-based progression training for robotic surgery skills training: a randomized clinical trial. Larcher A Decoene J, Tuyten T, D’Hondt M, Hubert N, Chatzopoulos C, De Troyer B UP, Group JE working group on robot-assisted surgery of the EA of U, the ERUS Education Working, editors. BJU Int 30(4):528–535 [DOI] [PubMed]
- 26.Liverneaux PA, Hendriks S, Selber JC, Parekattil SJ (2013) Robotically assisted microsurgery: development of basic skills course. Arch Plast Surg 40(04):320–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perez M, Perrenot C, Tran N, Hossu G, Felblinger J, Hubert J (2013) Prior experience in micro-surgery may improve the surgeon’s performance in robotic surgical training. Int J Med Robot Comput Assist Surg 9(3):351–358 [DOI] [PubMed] [Google Scholar]
- 28.Alrasheed T, Liu J, Hanasono MM, Butler CE, Selber JC (2014) Robotic Microsurgery: validating an assessment tool and plotting the learning curve. Plast Reconstr Surg 134(4):794–803 [DOI] [PubMed] [Google Scholar]
- 29.Selber J, Alrasheed T (2014) Robotic microsurgical training and evaluation. Semin Plast Surg 28(01):005–010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Willems JIP, Shin AM, Shin DM, Bishop AT, Shin AY (2016) A comparison of robotically assisted microsurgery versus manual microsurgery in challenging situations. Plast Reconstr Surg 137(4):1317–1324 [DOI] [PubMed] [Google Scholar]
- 31.Clarke NS, Price J, Boyd T, Salizzoni S, Zehr KJ, Nieponice A et al (2018) Robotic-assisted microvascular surgery: skill acquisition in a rat model. J Robot Surg 12(2):331–336 [DOI] [PubMed] [Google Scholar]
- 32.van Mulken TJM, Boymans CAEM, Schols RM, Cau R, Schoenmakers FBF, Hoekstra LT et al (2018) Preclinical experience using a new robotic system created for microsurgery. Plast Reconstr Surg 142(5):1367–1376 [DOI] [PubMed] [Google Scholar]
- 33.Yang M, Peng J, Wang X, Lei H, Li X, Yang K (2022) Reinforcing the effect of microsurgery practice during robotic suturing skill acquisition. Int J Medi Robot Comput Assist Surg. 10.1002/rcs.2350 [DOI] [PubMed] [Google Scholar]
- 34.Beier JP, Hackenberg S, Boos AM, Modabber A, Duong Dinh TA, Hölzle F (2023) First series of free flap reconstruction using a dedicated robotic system in a multidisciplinary microsurgical center. Plast Reconstr Surg Glob Open 11(9):e5240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Louis V, Chih-Sheng L, Chevallier D, Selber JC, Xavier F, Liverneaux PA (2018) A porcine model for robotic training harvest of the rectus abdominis muscle. Annales de Chirurgie Plastique Esthétique 63(2):113–116 [DOI] [PubMed] [Google Scholar]
- 36.Thomaier L, Orlando M, Abernethy M, Paka C, Chen CCG (2017) Laparoscopic and robotic skills are transferable in a simulation setting: a randomized controlled trial. Surg Endosc 31(8):3279–3285 [DOI] [PubMed] [Google Scholar]
- 37.Orlando MS, Thomaier L, Abernethy MG, Chen CCG (2017) Retention of laparoscopic and robotic skills among medical students: a randomized controlled trial. Surg Endosc 31(8):3306–3312 [DOI] [PubMed] [Google Scholar]
- 38.Jacob MO, Karatassas A, Hewett P, Guirgis M, Hensman C, Catterwell R (2023) The use of a porcine model to teach advanced abdominal wall dissection techniques. Surg Endosc 37(12):9684–9689 [DOI] [PubMed] [Google Scholar]
- 39.Lee J, Park HS, Lee DW, Song SY, Yu J, Ryu JM et al (2021) From cadaveric and animal studies to the clinical reality of robotic mastectomy: a feasibility report of training program. Sci Rep 11(1):21032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bur AM, Gomez ED, Newman JG, Weinstein GS, O’Malley BW, Rassekh CH et al (2017) Evaluation of high-fidelity simulation as a training tool in transoral robotic surgery. Laryngoscope 127(12):2790–2795 [DOI] [PubMed] [Google Scholar]
- 41.Zhang N, Sumer BD (2013) Transoral robotic surgery: simulation-based standardized training. JAMA Otolaryngol Head Neck Surg 139(11):1111 [DOI] [PubMed] [Google Scholar]
- 42.Kim WS, Byeon HK, Park YM, Ha JG, Kim ES, Koh YW et al (2015) Therapeutic robot-assisted neck dissection via a retroauricular or modified facelift approach in head and neck cancer: a comparative study with conventional transcervical neck dissection. Head Neck 37(2):249–254 [DOI] [PubMed] [Google Scholar]
- 43.Tae K, Ji YB, Song CM, Jeong JH, Cho SH, Lee SH (2014) Robotic selective neck dissection by a postauricular facelift approach: comparison with conventional neck dissection. Otolaryngol Head Neck Surg 150(3):394–400 [DOI] [PubMed] [Google Scholar]
- 44.Kim WS, Jittreetat T, Nam W, Sannikorn P, Choi EC, Koh YW (2015) Reconstruction of the segmental mandibular defect using a retroauricular or modified face-lift incision with an intraoral approach in head and neck cancer. Acta Otolaryngol 135(5):500–506 [DOI] [PubMed] [Google Scholar]
- 45.Du J, Mo H, Fan L, Jiang J (2017) Robot-assisted internal mammary lymph chain excision for breast cancer: a case report. Medicine 96(35):e7894–e7894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lira RB, Chulam TC, de Carvalho GB, Schreuder WH, Koh YW, Choi EC et al (2018) Retroauricular endoscopic and robotic versus conventional neck dissection for oral cancer. J Robot Surg 12(1):117–129 [DOI] [PubMed] [Google Scholar]
- 47.Melly L, Jansens J-L, Kalscheuer G, Belhaj A, Rondelet B (2018) Robotic lymphadenectomy of an internal mammary lymph node metastasis. Acta Chir Belg 118(5):320–321 [DOI] [PubMed] [Google Scholar]
- 48.Qingqing H, Jian Z, Dayong Z, Ziyi F, Luming Z, Peng Z et al (2018) Robot-assisted internal mammary lymph node chain dissection for breast cancer. Clin Breast Cancer 18(4):e441–e445 [DOI] [PubMed] [Google Scholar]
- 49.Singh A, Jaipuria J, Goel A, Shah S, Bhardwaj R, Baidya S et al (2018) Comparing outcomes of robotic and open inguinal lymph node dissection in patients with carcinoma of the penis. J Urol 199(6):1518–1525 [DOI] [PubMed] [Google Scholar]
- 50.Paek SH, Lee HA, Kwon H, Kang KH, Park SJ (2020) Comparison of robot-assisted modified radical neck dissection using a bilateral axillary breast approach with a conventional open procedure after propensity score matching. Surg Endosc 34(2):622–627 [DOI] [PubMed] [Google Scholar]
- 51.Song R-Y, Sohn HJ, Paek SH, Kang KH (2020) The first report of robotic bilateral modified radical neck dissection through the bilateral axillo-breast approach for papillary thyroid carcinoma with bilateral lateral neck metastasis. Surg Laparosc Endosc Percutan Tech 30(3):e18-22 [DOI] [PubMed] [Google Scholar]
- 52.Pedersen J, Song DH, Selber JC (2014) Robotic, intraperitoneal harvest of the rectus abdominis muscle. Plast Reconstr Surg 134(5):1057–1063 [DOI] [PubMed] [Google Scholar]
- 53.Clemens M, Kronowitz S, Selber J (2014) Robotic-assisted latissimus dorsi harvest in delayed-immediate breast reconstruction. Semin Plast Surg 28(01):020–025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chung J-H, You H-J, Kim H-S, Lee B-I, Park S-H, Yoon E-S (2015) A novel technique for robot assisted latissimus dorsi flap harvest. J Plast Reconstr Aesthet Surg 68(7):966–972 [DOI] [PubMed] [Google Scholar]
- 55.Lai H-W, Chen S-T, Lin S-L, Lin Y-L, Wu H-K, Pai S-H et al (2018) Technique for single axillary incision robotic assisted quadrantectomy and immediate partial breast reconstruction with robotic latissimus dorsi flap harvest for breast cancer: a case report. Medicine 97(27):e11373–e11373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Houvenaeghel G, Bannier M, Rua S, Barrou J, Heinemann M, Knight S et al (2019) Robotic breast and reconstructive surgery: 100 procedures in 2-years for 80 patients. Surg Oncol 31:38–45 [DOI] [PubMed] [Google Scholar]
- 57.Ozkan OO, Ozkan OO, Cinpolat A, Arici C, Bektas G, Can Ubur M et al (2019) Robotic harvesting of the omental flap: a case report and mini-review of the use of robots in reconstructive surgery. J Robot Surg 13(4):539–443 [DOI] [PubMed]
- 58.Houvenaeghel G, El Hajj H, Schmitt A, Cohen M, Rua S et al (2020) Robotic-assisted skin sparing mastectomy and immediate reconstruction using latissimus dorsi flap a new effective and safe technique: a comparative study. Surg Oncol 35:406–411 [DOI] [PubMed] [Google Scholar]
- 59.Fouarge A, Cuylits N (2020) From open to robotic-assisted latissimus dorsi muscle flap harvest. Plast Reconstr Surg Glob Open 8(1):e2569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Frey JD, Yu JW, Cohen SM, Zhao LC, Choi M, Levine JP (2020) Robotically assisted omentum flap harvest: a novel, minimally invasive approach for vascularized lymph node transfer. Plast Reconstr Surg Glob Open 8(4):e2505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Haverland R, Rebecca AM, Hammond J, Yi J (2021) A case series of robot-assisted rectus abdominis flap harvest for pelvic reconstruction: a single institution experience. J Minim Invasive Gynecol 28(2):245–248 [DOI] [PubMed] [Google Scholar]
- 62.Moon KC, Yeo HD, Yoon ES, Lee BI, Park SH, Chung JH et al (2020) Robotic-assisted latissimus dorsi muscle flap for autologous chest reconstruction in Poland syndrome. J Plast Reconstr Aesthet Surg 73(8):1506–1513 [DOI] [PubMed] [Google Scholar]
- 63.Winocour S, Tarassoli S, Chu CK, Liu J, Clemens MW, Selber JC (2020) Comparing outcomes of robotically assisted latissimus dorsi harvest to the traditional open approach in breast reconstruction. Plast Reconstr Surg 146(6):1221–1225 [DOI] [PubMed] [Google Scholar]
- 64.Asaad M, Pisters LL, Klein GT, Adelman DM, Oates SD, Butler CE et al (2021) Robotic rectus abdominis muscle flap following robotic extirpative surgery. Plast Reconstr Surg 148(6):1377–1381 [DOI] [PubMed] [Google Scholar]
- 65.Day SJ, Dy B, Nguyen M-D (2021) Robotic omental flap harvest for near-total anterior chest wall coverage: a potential application of robotic techniques in plastic and reconstructive surgery. BMJ Case Rep 14(2):e237887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Joo OY, Song SY, Lew DH, Park HS, Lee DW (2021) Robotic harvest of a latissimus dorsi flap using a single-port surgical robotic system in breast reconstruction. Arch Plast Surg 48(06):577–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cheon JH, Kim HE, Park SH, Yoon ES (2022) Ten-year experience of robotic latissimus muscle flap reconstructive surgery at a single institution. J Plast Reconstr Aesthet Surg 75(10):3664–3672 [DOI] [PubMed] [Google Scholar]
- 68.Davila AA, Goldman J, Kleban S, Lyons M, Brosious J, Bardakcioglu O et al (2022) Reducing complications and expanding use of robotic rectus abdominis muscle harvest for pelvic reconstruction. Plast Reconstr Surg 150(1):190–195 [DOI] [PubMed] [Google Scholar]
- 69.Hwang Y-J, Chung J-H, Lee H-C, Park S-H, Yoon E-S (2022) Single-port transaxillary robot-assisted latissimus dorsi muscle flap reconstruction for poland syndrome: concomitant application of robotic system to contralateral augmentation mammoplasty. Arch Plast Surg 49(03):373–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eo PS, Kim H, Lee JS, Lee J, Park HY, Yang JD (2023) Robot-assisted latissimus dorsi flap harvest for partial breast reconstruction: comparison with endoscopic and conventional approaches. Aesthet Surg J 44(1):38–46 [DOI] [PubMed] [Google Scholar]
- 71.Shin S-W, Kim H, Nam W, Kim HJ, Cha I-H, Koh YW et al (2023) Robot-assisted radial forearm free flap harvesting: a propensity score-matched case–control study. J Robot Surg 17(4):1429–1434 [DOI] [PubMed] [Google Scholar]
- 72.Lai H-W, Lin S-L, Chen S-T, Lin Y-L, Chen D-R, Pai S-S et al (2018) Robotic nipple sparing mastectomy and immediate breast reconstruction with robotic latissimus dorsi flap harvest—technique and preliminary results. J Plast Reconstr Aesthet Surg 71(10):e59-61 [DOI] [PubMed] [Google Scholar]
- 73.Gundlapalli VS, Ogunleye AA, Scott K, Wenzinger E, Ulm JP et al (2018) Robotic-assisted deep inferior epigastric artery perforator flap abdominal harvest for breast reconstruction: a case report. Microsurgery 38(6):702–705 [DOI] [PubMed] [Google Scholar]
- 74.Choi JH, Song SY, Park HS, Kim CH, Kim JY, Lew DH et al (2021) Robotic DIEP flap harvest through a totally extraperitoneal approach using a single-port surgical robotic system. Plast Reconstr Surg 148(2):304–307 [DOI] [PubMed] [Google Scholar]
- 75.Bishop SN, Asaad M, Liu J, Chu CK, Clemens MW, Kapur SS et al (2022) Robotic harvest of the deep inferior epigastric perforator flap for breast reconstruction: a case series. Plast Reconstr Surg 149(5):1073–1077 [DOI] [PubMed] [Google Scholar]
- 76.Daar DA, Anzai LM, Vranis NM, Schulster ML, Frey JD, Jun M et al (2022) Robotic deep inferior epigastric perforator flap harvest in breast reconstruction. Microsurgery 42(4):319–325 [DOI] [PubMed] [Google Scholar]
- 77.Dayaratna N, Ahmadi N, Mak C, Dusseldorp JR (2023) Robotic-assisted deep inferior epigastric perforator (DIEP) flap harvest for breast reconstruction. ANZ J Surg 93(4):1072–1074 [DOI] [PubMed] [Google Scholar]
- 78.Wittesaele W, Vandevoort M (2022) Implementing the Robotic deep inferior epigastric perforator Flap in daily practice: a series of 10 cases. J Plast Reconstr Aesthet Surg 75(8):2577–2583 [DOI] [PubMed] [Google Scholar]
- 79.Tsai C-Y, Kim B-S, Kuo W-L, Liu K-H, Chang TN-J, Cheong DC-F et al (2023) Novel port placement in robot-assisted DIEP flap harvest improves visibility and bilateral DIEP access: early controlled cohort study. Plast Reconstr Surg 152(4):590–595 [DOI] [PubMed] [Google Scholar]
- 80.Zanaty M, Atluri S, Taylor B, Choutka O (2023) Novel use of robotically harvested internal thoracic artery in high-flow cerebral bypass. World Neurosurg 178:52 [DOI] [PubMed] [Google Scholar]
- 81.Hans S, Jouffroy T, Veivers D, Hoffman C, Girod A, Badoual C et al (2013) Transoral robotic-assisted free flap reconstruction after radiation therapy in hypopharyngeal carcinoma: report of two cases. Eur Arch Otorhinolaryngol 270(8):2359–2364 [DOI] [PubMed]
- 82.Song HG, Yun IS, Lee WJ, Lew DH, Rah DK (2013) Robot-assisted free flap in head and neck reconstruction. Arch Plast Surg 40(04):353–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lai C-S, Chen I-C, Liu S-A, Lu C-T, Yen J-H, Song D-Y (2015) Robot-assisted free flap reconstruction of oropharyngeal cancer–a preliminary report. Ann Plast Surg 74:S105–S108 [DOI] [PubMed] [Google Scholar]
- 84.Miyamoto H, Leechavengvongs S, Atik T, Facca S, Liverneaux P (2014) Nerve transfer to the deltoid muscle using the nerve to the long head of the triceps with the da Vinci robot: six cases. J Reconstr Microsurg 30(06):375–380 [DOI] [PubMed] [Google Scholar]
- 85.Tsai Y-C, Liu S-A, Lai C-S, Chen Y-W, Lu C-T, Yen J-H et al (2017) Functional outcomes and complications of robot-assisted free flap oropharyngeal reconstruction. Ann Plast Surg 78(3):S76-82 [DOI] [PubMed] [Google Scholar]
- 86.Lai C, Lu C, Liu S, Tsai Y, Chen Y, Chen I (2019) Robot-assisted microvascular anastomosis in head and neck free flap reconstruction: preliminary experiences and results. Microsurgery 39(8):715–720 [DOI] [PubMed] [Google Scholar]
- 87.van Mulken TJM, Schols RM, Scharmga AMJ, Winkens B, Cau R, Schoenmakers FBF et al (2020) First-in-human robotic supermicrosurgery using a dedicated microsurgical robot for treating breast cancer-related lymphedema: a randomized pilot trial. Nat Commun 11(1):757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chang TN, Daniel BW, Hsu AT, Chen LW, Sung CW, Chuang DC et al (2021) Reversal of thoracic sympathectomy through robot-assisted microsurgical sympathetic trunk reconstruction with sural nerve graft and additional end-to-side coaptation of the intercostal nerves: a case report. Microsurgery 41(8):772–776 [DOI] [PubMed] [Google Scholar]
- 89.Barbon C, Grünherz L, Uyulmaz S, Giovanoli P, Lindenblatt N (2022) Exploring the learning curve of a new robotic microsurgical system for microsurgery. JPRAS Open 34:126–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lindenblatt N, Grünherz L, Wang A, Gousopoulos E, Barbon C, Uyulmaz S et al (2022) Early experience using a new robotic microsurgical system for lymphatic surgery. Plast Reconstr Surg Glob Open 10(1):e4013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.van Mulken TJM, Wolfs JAGN, Qiu SS, Scharmga AMJ, Schols RM, van Weezelenburg MAS et al (2022) One-year outcomes of the first human trial on robot-assisted lymphaticovenous anastomosis for breast cancer-related lymphedema. Plast Reconstr Surg 149(1):151–161 [DOI] [PubMed] [Google Scholar]
- 92.Besmens IS, Politikou O, Giovanoli P, Calcagni M, Lindenblatt N (2024) Robotic microsurgery in extremity reconstruction - experience with a novel robotic system. Surg Innov 31(1):42–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen LW-Y, Chang TN-J, Lee C-P, Sung CW-H, Cheng C, Chang K-H et al (2023) Robotic sympathetic trunk reconstruction for compensatory sweating after thoracic sympathectomy. JTCVS Tech 21:251–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Innocenti M, Malzone G, Menichini G (2023) First-in-human free flap tissue reconstruction using a dedicated microsurgical robotic platform. Plast Reconstr Surg 151(5):1078–1082 [DOI] [PubMed] [Google Scholar]
- 95.Weinzierl A, Barbon C, Gousopoulos E, von Reibnitz D, Giovanoli P, Grünherz L et al (2023) Benefits of robotic-assisted lymphatic microsurgery in deep anatomical planes. JPRAS Open 37:145–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lin L, Sun M, Xu C, Gao Y, Xu H, Yang X et al (2022) Assessment of robot-assisted mandibular contouring surgery in comparison with traditional surgery: a prospective, single-center. Randomized Controlled Trial Aesthet Surg J 42(6):567–579 [DOI] [PubMed] [Google Scholar]
- 97.Lin L, Zhao Z, Han W, Sun M, Zhang Z, Kim BS et al (2023) Advances in robot-assisted surgery for facial bone contouring surgery. J Craniofac Surg 34(2):813–816 [DOI] [PubMed] [Google Scholar]
- 98.Téblick S, Ruymaekers M, de Casteele E, Boudewyns A, Nadjmi N, Teblick S et al (2023) The effect of soft palate reconstruction with the da Vinci robot on middle ear function in children: an observational study. Int J Oral Maxillofac Surg 52(9):931–938 [DOI] [PubMed]
- 99.Bruyere A, Hidalgo Diaz JJ, Vernet P, Salazar Botero S, Facca S, Liverneaux P-A (2016) Technical feasibility of robot-assisted minimally-invasive neurolysis of the lateral cutaneous nerve of thigh: about a case. Ann Chir Plast Esthet 61(6):872–876 [DOI] [PubMed] [Google Scholar]
- 100.Boztosun A, Olgan S (2016) Robotic sigmoid vaginoplasty in an adolescent girl with mayer-rokitansky-kuster-hauser syndrome. Female Pelvic Med Reconstr Surg 22(5):e32–e35 [DOI] [PubMed] [Google Scholar]
- 101.Dy GW, Jun MS, Blasdel G, Bluebond-Langner R, Zhao LC (2021) Outcomes of gender affirming peritoneal flap vaginoplasty using the da vinci single port versus xi robotic systems. Eur Urol 79(5):676–683 [DOI] [PubMed] [Google Scholar]
- 102.Blasdel G, Kloer C, Parker A, Shakir N, Zhao LC, Bluebond-Langner R (2023) Genital hypoplasia before gender-affirming vaginoplasty: does the robotic peritoneal flap method create equivalent vaginal canal outcomes? Plast Reconstr Surg 151(4):867–874 [DOI] [PubMed] [Google Scholar]
- 103.Chen YJ, Huynh D, Nguyen S, Chin E, Divino C, Zhang L (2017) Outcomes of robot-assisted versus laparoscopic repair of small-sized ventral hernias. Surg Endosc 31(3):1275–1279 [DOI] [PubMed] [Google Scholar]
- 104.Bittner JG 4th, Alrefai S, Vy M, Mabe M, Del Prado PAR, Clingempeel NL (2018) Comparative analysis of open and robotic transversus abdominis release for ventral hernia repair. Surg Endosc 32(2):727–734 [DOI] [PubMed] [Google Scholar]
- 105.Gonzalez A, Escobar E, Romero R, Walker G, Mejias J, Gallas M et al (2017) Robotic-assisted ventral hernia repair: a multicenter evaluation of clinical outcomes. Surg Endosc 31(3):1342–1349 [DOI] [PubMed]
- 106.Jamshidian M, Stanek S, Sferra J, Jamil T (2018) Robotic repair of symptomatic Spigelian hernias: a series of three cases and surgical technique review. J Robot Surg 12(3):557–560 [DOI] [PubMed] [Google Scholar]
- 107.Prabhu AS, Dickens EO, Copper CM, Mann JW, Yunis JP, Phillips S et al (2017) Laparoscopic vs robotic intraperitoneal mesh repair for incisional hernia: an Americas Hernia Society Quality Collaborative Analysis. J Am Coll Surg 225(2):285–293 [DOI] [PubMed] [Google Scholar]
- 108.Wang SC, Singh TP (2017) Robotic repair of a large abdominal intercostal hernia: a case report and review of literature. J Robot Surg 11(2):271–274 [DOI] [PubMed] [Google Scholar]
- 109.Warren JA, Cobb WS, Ewing JA, Carbonell AM (2017) Standard laparoscopic versus robotic retromuscular ventral hernia repair. Surg Endosc 31(1):324–332 [DOI] [PubMed] [Google Scholar]
- 110.Carbonell AM, Warren JA, Prabhu AS, Ballecer CD, Janczyk R et al (2018) Reducing length of stay using a robotic-assisted approach for retromuscular ventral hernia repair: a comparative analysis from the americas hernia society quality collaborative. Ann Surg 267(2):210–217 [DOI] [PubMed] [Google Scholar]
- 111.Martin-Del-Campo LA, Weltz AS, Belyansky I, Novitsky YW (2018) Comparative analysis of perioperative outcomes of robotic versus open transversus abdominis release. Surg Endosc 32(2):840–845 [DOI] [PubMed] [Google Scholar]
- 112.Muysoms F, Van Cleven S, Pletinckx P, Ballecer C, Ramaswamy A (2018) Robotic transabdominal retromuscular umbilical prosthetic hernia repair (TARUP): observational study on the operative time during the learning curve. Hernia 22(6):1101–1111 [DOI] [PubMed] [Google Scholar]
- 113.Walker PA, May AC, Cherla DV, Santillan MR et al (2018) Multicenter review of robotic versus laparoscopic ventral hernia repair: is there a role for robotics. Surg Endosc 32(4):1901–1905 [DOI] [PubMed] [Google Scholar]
- 114.Kudsi OY, Gokcal F (2019) Robotic approach to modified Sugarbaker parastomal hernia repair by performing transversus abdominis release—a video vignette. Colorectal Dis 21(7):854–855 [DOI] [PubMed] [Google Scholar]
- 115.Kudsi OY, Chang K, Bou-Ayash N, Gokcal F (2020) Transabdominal (TA) versus totally extraperitoneal (TEP) robotic retromuscular ventral hernia repair: a propensity score matching analysis. Surg Endosc 34(8):3550–3559 [DOI] [PubMed] [Google Scholar]
- 116.Olavarria OA, Bernardi K, Shah SK, Wilson TD, Wei S, Pedroza C et al (2020) Robotic versus laparoscopic ventral hernia repair: multicenter, blinded randomized controlled trial. BMJ 370:m2457–m2457 [DOI] [PMC free article] [PubMed]
- 117.Mudyanadzo TA, Hunter JD 3rd, Rider PF, Richards WO (2020) An evaluation of robotic ventral hernia repair. Am Surg 86(1):e45–e46 [PubMed] [Google Scholar]
- 118.Petro CC, Zolin S, Krpata D, Alkhatib H, Tu C, Rosen MJ et al (2021) Patient-reported outcomes of robotic vs laparoscopic ventral hernia repair with intraperitoneal mesh: the PROVE-IT randomized clinical trial. JAMA Surg 156(1):22–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bergholz D, Obi JR, Grossman R, Rasul TF (2021) Robotic repair of an acquired abdominal intercostal hernia. CRSLS : MIS case reports from SLS. 8(4):e2021.00061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dhanani NH, Olavarria OA, Holihan JL, Shah SK, Wilson TD, Loor MM et al (2021) Robotic versus laparoscopic ventral hernia repair: one-year results from a prospective, multicenter. Blinded Randomized Controlled Trial Ann Surg 273(6):1076–1080 [DOI] [PubMed] [Google Scholar]
- 121.Rayman S, Yuori M, Jacob R, Ephraim K, Mohammad A, Lior S et al (2021) Transabdominal Preperitoneal (TAPP) for the Treatment of Spigelian hernias. JSLS 25(2):e2021.00024 [DOI] [PMC free article] [PubMed]
- 122.Costa TN, Abdalla RZ, Tustumi F, Junior UR, Cecconello I (2023) Robotic-assisted compared with laparoscopic incisional hernia repair following oncologic surgery: short- and long-term outcomes of a randomized controlled trial. J Robot Surg 17(1):99–107 [DOI] [PubMed] [Google Scholar]
- 123.Kakela P, Mustonen K, Rantanen T, Paajanen H, Käkelä P, Mustonen K et al (2023) Robotic versus hybrid assisted ventral hernia repair: a prospective one-year comparative study of clinical outcomes. Acta Chir Belg 123(4):411–417 [DOI] [PubMed]
- 124.Kudsi OY, Gokcal F, Bou-Ayash N, Watters E, Pereira X, Lima DL et al (2022) A comparison of outcomes between class-II and class-III obese patients undergoing robotic ventral hernia repair: a multicenter study. Hernia 26(6):1531–1539 [DOI] [PubMed] [Google Scholar]
- 125.Petro CC, Thomas JD, Tu C, Krpata DM, Beffa LR, Rosen MJ et al (2022) Robotic vs laparoscopic ventral hernia repair with intraperitoneal mesh: 1-year exploratory outcomes of the PROVE-IT randomized clinical trial. J Am Coll Surg 234(6):1160–1165 [DOI] [PubMed] [Google Scholar]
- 126.Pereira X, Lima DL, Huang L-C, Salas-Parra R, Shah P, Malcher F et al (2023) Robotic versus open lateral abdominal hernia repair: a multicenter propensity score matched analysis of perioperative and 1-year outcomes. Hernia 27(2):293–304 [DOI] [PubMed] [Google Scholar]
- 127.Shimada G, Matsubara T, Sanbonmatsu M, Nakabayashi R, Miyachi Y, Taketa T et al (2023) The first case of robotic-assisted transabdominal retrorectus repair for incisional hernia in Japan. Asian J Endosc Surg 16(2):305–311 [DOI] [PubMed] [Google Scholar]
- 128.Dhanani NH, Lyons NB, Olavarria OA, Bernardi K, Holihan JL, Shah SK et al (2023) Robotic versus laparoscopic ventral hernia repair: two-year results from a prospective, multicenter. Blinded Randomized Clinical Trial Ann Surg 278(2):161–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lima DL, Alcabes A, Viscarret V, Nogueira R, Malcher F (2023) Incarcerated epiploic appendix in a spigelian hernia treated by robotic-assisted surgery. CRSLS MIS Case Rep SLS 10(2):e2023.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Petro CC, Maskal SM, Renton DB, Yunis JP, Meara MP, Diaz K et al (2023) Robotic enhanced-view totally extraperitoneal vs intraperitoneal onlay mesh evaluation: 1-year exploratory outcomes of the REVEAL randomized clinical trial. J Am Coll Surg 237(4):614–620 [DOI] [PubMed] [Google Scholar]
- 131.Sarfati B, Honart J-F, Leymarie N, Rimareix F, Al Khashnam H, Kolb F (2017) Robotic da Vinci Xi-assisted nipple-sparing mastectomy: first clinical report. Breast J 24(3):373–376 [DOI] [PubMed] [Google Scholar]
- 132.Toesca A, Peradze N, Galimberti V, Manconi A, Intra M, Gentilini O et al (2017) Robotic nipple-sparing mastectomy and immediate breast reconstruction with implant: first report of surgical technique. Ann Surg 266(2):e28-30 [DOI] [PubMed] [Google Scholar]
- 133.Toesca A, Peradze N, Manconi A, Galimberti V, Intra M, Colleoni M et al (2017) Robotic nipple-sparing mastectomy for the treatment of breast cancer: feasibility and safety study. The Breast 31:51–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lai H-W, Lin S-L, Chen S-T, Chen S-L, Lin Y-L, Chen D-R et al (2018) Robotic nipple-sparing mastectomy and immediate breast reconstruction with gel implant. Plast Reconstr Surg Glob Open 6(6):e1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Park HS, Kim JH, Lee DW, Song SY, Park S, Kim Il S et al (2018) Gasless Robot-Assisted Nipple-Sparing Mastectomy: A Case Report. J Breast Cancer 21(3):334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rajappa SK, Kumar R, Garg S, Ram D (2018) Robotic nipple-sparing mastectomy: the first experience from Indian subcontinent. Breast J 24(6):1114–1115 [DOI] [PubMed] [Google Scholar]
- 137.Sarfati B, Struk S, Leymarie N, Honart J-F, Alkhashnam H, Kolb F et al (2018) Robotic nipple-sparing mastectomy with immediate prosthetic breast reconstruction: surgical technique. Plast Reconstr Surg 142(3):624–627 [DOI] [PubMed] [Google Scholar]
- 138.Sarfati B, Struk S, Leymarie N, Honart J-F, Alkhashnam H, Tran de Fremicourt K et al (2018) Robotic prophylactic nipple-sparing mastectomy with immediate prosthetic breast reconstruction: a prospective study. Ann Surg Oncol 25(9):2579–2586 [DOI] [PubMed] [Google Scholar]
- 139.Houvenaeghel G, Bannier M, Rua S, Barrou J, Heinemann M, Van Troy A et al (2019) Breast cancer robotic nipple sparing mastectomy: evaluation of several surgical procedures and learning curve. World J Surg Oncol 17(1):27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kuo W-L, Huang J-J, Huang Y-T, Chueh L-F, Lee J-T, Tsai H-P et al (2020) Robot-assisted mastectomy followed by immediate autologous microsurgical free flap reconstruction: techniques and feasibility in three different breast cancer surgical scenarios. Clin Breast Cancer 20(1):e1-8 [DOI] [PubMed] [Google Scholar]
- 141.Lai H-W, Chen S-T, Lin S-L, Chen C-J, Lin Y-L, Pai S-H et al (2019) Robotic nipple-sparing mastectomy and immediate breast reconstruction with gel implant: technique, preliminary results and patient-reported cosmetic outcome. Ann Surg Oncol 26(1):42–52 [DOI] [PubMed] [Google Scholar]
- 142.Lai H-W, Wang C-C, Lai Y-C, Chen C-J, Lin S-L, Chen S-T et al (2019) The learning curve of robotic nipple sparing mastectomy for breast cancer: an analysis of consecutive 39 procedures with cumulative sum plot. Eur J Surg Oncol 45(2):125–133 [DOI] [PubMed] [Google Scholar]
- 143.Lai H-W, Chen S-T, Mok CW, Lin Y-J, Wu H-K, Lin S-L et al (2020) Robotic versus conventional nipple sparing mastectomy and immediate gel implant breast reconstruction in the management of breast cancer - a case control comparison study with analysis of clinical outcome, medical cost, and patient-reported cosmetic results. J Plast Reconstr Aesthet Surg 73(8):1514–1525 [DOI] [PubMed] [Google Scholar]
- 144.Toesca A, Sangalli C, Maisonneuve P, Massari G, Girardi A, Baker JL et al (2022) A randomized trial of robotic mastectomy versus open surgery in women with breast cancer or BrCA mutation. Ann Surg 276(1):11–19 [DOI] [PubMed] [Google Scholar]
- 145.Park HS, Lee J, Lai H-W, Park JM, Ryu JM, Lee JE et al (2022) Surgical and oncologic outcomes of robotic and conventional nipple-sparing mastectomy with immediate reconstruction: international multicenter pooled data analysis. Ann Surg Oncol 29(11):6646–6657 [DOI] [PubMed] [Google Scholar]
- 146.Moon J, Lee J, Lee DW, Lee HS, Nam DJ, Kim MJ et al (2021) Postoperative pain assessment of robotic nipple-sparing mastectomy with immediate prepectoral prosthesis breast reconstruction: a comparison with conventional nipple-sparing mastectomy. Int J Med Sci 18(11):2409–2416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chan JYK, Tsang RK, Eisele DW, Richmon JD (2013) Transoral robotic surgery of the parapharyngeal space: a case series and systematic review. Head Neck 37(2):293–298 [DOI] [PubMed] [Google Scholar]
- 148.Chia SH, Gross ND, Richmon JD (2013) Surgeon experience and complications with Transoral Robotic Surgery (TORS). Otolaryngol Head Neck Surg 149(6):885–892 [DOI] [PubMed]
- 149.Durmus K, Rangarajan SV, Old MO, Agrawal A, Teknos TN, Ozer E (2013) Transoral robotic approach to carcinoma of unknown primary. Head Neck 36(6):848–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Durmus K, Apuhan T, Ozer E (2013) Transoral robotic surgery for retromolar trigone tumours. Acta Otorhinolaryngol Ital 33(6):425–427 [PMC free article] [PubMed] [Google Scholar]
- 151.Lee SY, Park YM, Byeon HK, Choi EC, Kim S-H (2014) Comparison of oncologic and functional outcomes after transoral robotic lateral oropharyngectomy versus conventional surgery for T1 to T3 tonsillar cancer. Head Neck 36(8):1138–1145 [DOI] [PubMed] [Google Scholar]
- 152.Patel SA, Magnuson JS, Holsinger FC, Karni RJ, Richmon JD, Gross ND et al (2013) Robotic surgery for primary head and neck squamous cell carcinoma of unknown site. JAMA Otolaryngol Head Neck Surg 139(11):1203–1211 [DOI] [PubMed]
- 153.RK T, WK H, WI W, JY C (2013) Transoral robotic assisted nasopharyngectomy via a lateral palatal flap approach. Laryngoscope 123(9):2180–2183 [DOI] [PubMed]
- 154.White H, Ford S, Bush B, Holsinger FC, Moore E, Ghanem T et al (2013) Salvage surgery for recurrent cancers of the oropharynx: comparing TORS with standard open surgical approaches. JAMA Otolaryngol Head Neck Surg 139(8):773–778 [DOI] [PubMed]
- 155.Chung TK, Rosenthal EL, Magnuson JS, Carroll WR (2015) Transoral robotic surgery for oropharyngeal and tongue cancer in the United States. Laryngoscope 125(1):140–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Durmus K, Patwa HS, Gokozan HN, Kucur C, Teknos TN, Agrawal A et al (2014) Functional and quality-of-life outcomes of transoral robotic surgery for carcinoma of unknown primary. Laryngoscope 124(9):2089–2095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ford SE, Brandwein-Gensler M, Carroll WR, Rosenthal EL, Magnuson JS (2014) Transoral robotic versus open surgical approaches to oropharyngeal squamous cell carcinoma by human papillomavirus status. Otolaryngol Head Neck Surg 151(4):606–611 [DOI] [PubMed] [Google Scholar]
- 158.Hammoudi K, Pinlong E, Kim S, Bakhos D, Morinière S (2015) Transoral robotic surgery versus conventional surgery in treatment for squamous cell carcinoma of the upper aerodigestive tract. Head Neck 37(9):1304–1309 [DOI] [PubMed] [Google Scholar]
- 159.van Loon JWL, Smeele LE, Hilgers FJM, van den Brekel MWM (2015) Outcome of transoral robotic surgery for stage I-II oropharyngeal cancer. Eur Arch Otorhinolaryngol 272(1):175–183 [DOI] [PubMed] [Google Scholar]
- 160.de Almeida JR, Li R, Magnuson JS, Smith RV, Moore E, Lawson G et al (2015) Oncologic outcomes after transoral robotic surgery: a multi-institutional study. JAMA Otolaryngol Head Neck Surg 141(12):1043–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Dabas S, Dewan A, Ranjan R, Dewan AK, Puri A, Shah SH et al (2015) Transoral robotic surgery in management of oropharyngeal cancers: a preliminary experience at a tertiary cancer centre in India. Int J Clin Oncol 20(4):693–700 [DOI] [PubMed] [Google Scholar]
- 162.Mercante G, Masiello A, Sperduti I, Cristalli G, Pellini R et al (2015) Quality of life and functional evaluation in patients with tongue base tumors treated exclusively with transoral robotic surgery: a 1-year follow-up study. J Craniomaxillofac Surg 43(8):1561–1566 [DOI] [PubMed] [Google Scholar]
- 163.Mockelmann N, Busch C-J, Munscher A, Knecht R, Lorincz BB, Möckelmann N et al (2015) Timing of neck dissection in patients undergoing transoral robotic surgery for head and neck cancer. Eur J Surg Oncol 41(6):773–778 [DOI] [PubMed]
- 164.Razafindranaly V, Lallemant B, Aubry K, Moriniere S, Vergez S, De ME et al (2016) Clinical outcomes with transoral robotic surgery for supraglottic squamous cell carcinoma: experience of a French evaluation cooperative subgroup of GETTEC. Head Neck 38:E1097–E1101 [DOI] [PubMed] [Google Scholar]
- 165.Smith RV, Schiff BA, Garg M, Haigentz M (2015) The impact of transoral robotic surgery on the overall treatment of oropharyngeal cancer patients. Laryngoscope 125:S1-15 [DOI] [PubMed] [Google Scholar]
- 166.Aubry K, Vergez S, de Mones E, Moriniere S, Choussy O, Malard O et al (2016) Morbidity and mortality revue of the French group of transoral robotic surgery: a multicentric study. J Robot Surg 10(1):63–67 [DOI] [PubMed] [Google Scholar]
- 167.Fujiwara K, Fukuhara T, Kitano H, Fujii T, Koyama S, Yamasaki A et al (2016) Preliminary study of transoral robotic surgery for pharyngeal cancer in Japan. J Robot Surg 10(1):11–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Granell J, Alonso A, Garrido L, Gutierrez-Fonseca R (2016) Transoral fully robotic dissection of a parapharyngeal hemangioma. J Craniofac Surg 27(7):1806–1807 [DOI] [PubMed] [Google Scholar]
- 169.Duek I, Amit M, Sviri GE, Gil Z (2017) Combined endoscopic transcervical-transoral robotic approach for resection of parapharyngeal space tumors. Head Neck 39(4):786–790 [DOI] [PubMed] [Google Scholar]
- 170.Frenkel CH, Yang J, Zhang M, Altieri MS, Telem DA, Samara GJ (2017) Compared outcomes of concurrent versus staged transoral robotic surgery with neck dissection. Otolaryngol Head Neck Surg 157(5):791–797 [DOI] [PubMed] [Google Scholar]
- 171.Gorphe P, Von Tan J, El Bedoui S, Hartl DM, Auperin A, Qassemyar Q et al (2017) Early assessment of feasibility and technical specificities of transoral robotic surgery using the da Vinci Xi. J Robot Surg 11(4):455–461 [DOI] [PubMed] [Google Scholar]
- 172.Lallemant B, Moriniere S, Ceruse P, Lebalch M, Aubry K, Hans S et al (2017) Transoral robotic surgery for squamous cell carcinomas of the posterior pharyngeal wall. Eur Arch Otorhinolaryngol 274(12):4211–4216 [DOI] [PubMed] [Google Scholar]
- 173.Mahmoud O, Sung K, Civantos FJ, Thomas GR, Samuels MA (2018) Transoral robotic surgery for oropharyngeal squamous cell carcinoma in the era of human papillomavirus. Head Neck 40(4):710–721 [DOI] [PubMed] [Google Scholar]
- 174.Rubek N, Channir HI, Charabi BW, Lajer CB, Kiss K, Nielsen HU et al (2017) Primary transoral robotic surgery with concurrent neck dissection for early stage oropharyngeal squamous cell carcinoma implemented at a Danish head and neck cancer center: a phase II trial on feasibility and tumour margin status. Eur Arch Otorhinolaryngol 274(5):2229–2237 [DOI] [PubMed] [Google Scholar]
- 175.Sethia R, Yumusakhuylu AC, Ozbay I, Diavolitsis V, Brown NV, Zhao S et al (2018) Quality of life outcomes of transoral robotic surgery with or without adjuvant therapy for oropharyngeal cancer. Laryngoscope 128(2):403–411 [DOI] [PubMed] [Google Scholar]
- 176.Alessandrini M, Pavone I, Micarelli A, Caporale C (2018) Transoral robotic surgery for the base of tongue squamous cell carcinoma: a preliminary comparison between da Vinci Xi and Si. J Robot Surg 12(3):417–423 [DOI] [PubMed] [Google Scholar]
- 177.Doazan M, Hans S, Morinière S, Lallemant B, Vergez S, Aubry K et al (2018) Oncologic outcomes with transoral robotic surgery for supraglottic squamous cell carcinoma: results of the French Robotic Surgery Group of GETTEC. Head Neck 40(9):2050–2059 [DOI] [PubMed] [Google Scholar]
- 178.Li H, Torabi SJ, Park HS, Yarbrough WG, Mehra S, Choi R et al (2019) Clinical value of transoral robotic surgery: nationwide results from the first 5 years of adoption. Laryngoscope 129(8):1844–1855 [DOI] [PubMed] [Google Scholar]
- 179.Scott-Wittenborn N, Jackson RS (2018) Intraoperative imaging during minimally invasive transoral robotic surgery using near-infrared light. Am J Otolaryngol 39(2):220–222 [DOI] [PubMed] [Google Scholar]
- 180.Hardy BM, LoSavio P, Al-Khudari S (2020) Transoral robotic resection of recurrent neopharyngeal squamous cell carcinoma. Ear Nose Throat J 99(5):303–304 [DOI] [PubMed] [Google Scholar]
- 181.Nichols AC, Theurer J, Prisman E, Read N, Berthelet E, Tran E et al (2019) Radiotherapy versus transoral robotic surgery and neck dissection for oropharyngeal squamous cell carcinoma (ORATOR): an open-label, phase 2, randomised trial. Lancet Oncol 20(10):1349–1359 [DOI] [PubMed] [Google Scholar]
- 182.Petruzzi G, Zocchi J, Moretto S, Pichi B, Cristalli G, Mercante G et al (2019) Transoral robotic retropharyngeal lymph node dissection in a recurrent head and neck carcinoma. Head Neck 41(11):4051–4053 [DOI] [PubMed] [Google Scholar]
- 183.Holcomb AJ, Richmon JD (2021) Transoral robotic salvage oropharyngectomy with submental artery island flap reconstruction. Head Neck 43(2):E13–E19 [DOI] [PubMed] [Google Scholar]
- 184.Kubik MW, Channir HI, Rubek N, Kim S, Ferris RL, von Buchwald C et al (2021) TORS base-of-tongue mucosectomy in human papilloma virus-negative carcinoma of unknown primary. Laryngoscope 131(1):78–81 [DOI] [PubMed] [Google Scholar]
- 185.Sano D, Shimizu A, Tateya I, Fujiwara K, Mori T, Miyamoto S et al (2021) Treatment outcomes of transoral robotic and non-robotic surgeries to treat oropharyngeal, hypopharyngeal, and supraglottic squamous cell carcinoma: a multi-center retrospective observational study in Japan. Auris Nasus Larynx 48(3):502–510 [DOI] [PubMed] [Google Scholar]
- 186.D’Andrea G, Bordenave L, Nguyen F, Tao Y, Paleri V, Temam SS et al (2022) A prospective longitudinal study of quality of life in robotic-assisted salvage surgery for oropharyngeal cancer. Eur J Surg Oncol 48(6):1243–1250 [DOI] [PubMed]
- 187.Nichols AC, Theurer J, Prisman E, Read N, Berthelet E, Tran E et al (2022) Randomized trial of radiotherapy versus transoral robotic surgery for oropharyngeal squamous cell carcinoma: long-term results of the ORATOR trial. J Clin Oncol 40(8):866–875 [DOI] [PubMed] [Google Scholar]
- 188.De Virgilio A, Pellini R, Cammaroto G, Sgarzani R, De Vito A et al (2023) Trans oral robotic surgery for oropharyngeal cancer: a multi institutional experience. Eur J Surg Oncol 49(9):106945 [DOI] [PubMed] [Google Scholar]
- 189.Vierstraete M, Simons M, Borch K, de Beaux A, East B, Reinpold W et al (2022) Description of the current da Vinci® training pathway for robotic abdominal wall surgery by the European Hernia Society. J Abdom Wall Surg 30:1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.