Abstract
INTRODUCTION
The effects of sleep–wake behavior on perceived fatigability and cognitive abilities when performing daily activities have not been investigated across levels of cognitive reserve (CR).
METHODS
CR Index Questionnaire (CRIq) data were collected and subjected to moderated mediation analysis.
RESULTS
In amnestic mild cognitive impairment (aMCI; n = 41), CR moderated sleep‐related impairments (SRIs), and fatigability at low CR (CRIq < 105.8, p = 0.004) and mean CR (CRIq = 126.9, p = 0.03) but not high CR (CRIq > 145.9, p = 0.65) levels. SRI affected cognitive abilities mediated by fatigability at low CR (p < 0.001) and mean CR (p = 0.003) levels. In healthy controls (n = 13), SRI in fatigability did not alter cognitive abilities across CR levels; controls had higher leisure scores than patients with aMCI (p = 0.003, effect size = 0.93).
DISCUSSION
SRI can amplify impaired cognitive abilities through exacerbation of fatigability in patients with aMCI with below‐mean CR. Therefore, improving sleep–wake regulation and leisure activities may protect against fatigability and cognitive decline.
Highlights
Clinical fatigue and fatigability cannot be alleviated by rest.
Clinical fatigability disrupts daily activities during preclinical Alzheimer's.
High cognitive reserve mitigates sleep–wake disturbance effects.
High cognitive reserve attenuates clinical fatigability effects on daily functioning.
Untreated obstructive sleep apnea potentiates Alzheimer's pathology in the brain.
Keywords: Alzheimer's disease, cognitive ability, cognitive reserve, fatigue, leisure activities, older adults, sleep, sleep fragmentation, sleep–wake behavior, sleep–wake dysregulation, sleep–wake misalignment
1. BACKGROUND
Sleep–wake regulation is crucial for maintaining the integrity of brain structures and functions. 1 Sleep–wake dysregulation manifests as increased nighttime awakenings and sleep fragmentation, 2 reduced slow‐wave deep sleep, 3 <6 hours of sleep, 4 and circadian rhythm misalignment. 5 Chronic and persistent sleep–wake dysregulation impairs the clearance of brain waste products during slow‐wave sleep (non‐REM stage 3), which activates the immune response and downstream neuroinflammation. Clinical fatigue associated with Alzheimer's disease (AD) is a form of central fatigue, defined as a persistent and distressing feeling of a lack of energy, tiredness, or exhaustion not relieved by rest that affects cognition, function, behavior, and mood regulation. 6 Perceived fatigability represents fatigue symptom severity in a given cognitive or physical task in the context of daily activities. 7
A large body of literature demonstrates that individuals with chronic and persistent sleep–wake disturbances have a higher prevalence of amyloid‐β (Aβ) peptide positivity, tau tangles, and proinflammatory cytokines. 8 , 9 For example, the Baltimore Longitudinal Study of Aging found that self‐reported short sleep and poor sleep quality were associated with a greater Aβ burden detected using carbon 11‐labeled Pittsburgh Compound B positron emission tomography. 4 More recent studies have demonstrated bidirectional links between sleep–wake impairments and AD. 10 Therefore, exposure to chronic sleep–wake dysregulation promotes the accumulation of AD pathology, which could start 15 to 20 years before clinical symptoms of amnestic mild cognitive impairment (aMCI) or dementia arise.
RESEARCH IN CONTEXT
Systematic review: We reviewed the literature using traditional sources, meeting abstracts, and presentations. According to the cognitive reserve concept, healthy lifestyle behaviors may mitigate the effects of Alzheimer's pathology on cognition and function; individuals with high cognitive reserve are less susceptible to Alzheimer's pathology. Moreover, central fatigability is a sequela of neuroinflammation associated with Alzheimer's pathology. However, the relationships between sleep–wake behavior, fatigability, and cognitive reserve have not been studied.
Interpretation: Sleep–wake disturbances exacerbated fatigability, which was attributed to declines in cognitive ability. Patients with amnestic mild cognitive impairment with high cognitive reserve and cognitively normal controls showed attenuated effects of sleep–wake disturbances on fatigability and cognitive ability.
Future directions: This study initiated a novel area of research by linking the sleep‐fatigability paradigm with the cognitive reserve framework. Future investigations on the mechanisms behind sleep–wake behavior, fatigability, and Alzheimer's pathology applying the cognitive reserve concept are warranted.
As the disease progresses, the formation of amyloid plaques can damage the suprachiasmatic nucleus (SCN), the timekeeper for the biological circadian clock. 11 As a result, patients with AD may have irregular sleep–wake patterns, called irregular sleep–wake rhythm disorder 12 (ISWRD; International Classification of Diseases, 10th edition diagnosis). 13 Estimates suggest that 25% to 50% of people living with AD have altered sleep–wake rhythms, including sundowning syndrome, which causes a remarkable burden to patients and their families. 14 Nevertheless, early identification and treatment of sleep–wake disturbances may reduce the risk of accumulating AD pathology in the brain, thus delaying the onset of AD.
Advances in medical technology have allowed the use of AD biomarkers to accurately diagnose and determine AD stages in living patients. 15 However, AD biomarkers cannot fully capture a patient's adaptability to the disease (eg, fatigue and coping strategies). The concept of cognitive reserve (CR) provides new perspectives on individual differences in susceptibility to AD depending on brain function and neural compensation, governed by lifelong education, the complexity of work/occupation, and cognitively stimulating leisure activities as proxies. 16 CR plays a critical role in moderating brain function and the clinical expression of cognition, function, and behavior. 16 , 17 Currently, limited therapeutic interventions are available to improve cognition and function once Aβ plaques and pathologic tau accumulate in the brain. However, behavior can be modified to promote healthy lifestyles that can attenuate the negative impact of AD on cognition and brain function, including adjustments to diet, smoking and alcohol consumption cessation, physical activity, cognitively stimulating leisure activities, sleep, and meditation. 18 , 19 Nevertheless, the relationship between sleep–wake behavior and CR has not been studied. 18 Moreover, emerging evidence supports the neuroinflammatory hypothesis of AD, and AD affects cognition, function, and behavior (eg, clinical fatigue). However, the relationship between clinical fatigue and CR has also not been explored.
To fill these gaps, we conducted a study to examine the effects of sleep–wake disturbances on perceived fatigability and cognitive abilities while performing daily activities; these were examined in patients with different levels of CR. We hypothesized that higher CR levels would have a greater ability to attenuate the detrimental effects of sleep‐related impairment (SRI) on fatigability, in part to protect against cognitive decline in older adults without dementia.
2. METHODS
We conducted a comparative study in older adults without depression aged 55 to 90 years who were diagnosed with aMCI or were cognitively normal (CN) to examine the inter‐ and intra‐individual variability of the dynamic relationships among the variables of interest.
2.1. Participants and setting
Participants were recruited from the Memory Disorders Clinic of the New York State Psychiatric Institute (NYSPI) between November 2018 and March 2020 through clinical and research study referrals. The data were managed using the RedCap system (RedCap, Nashville, TN, USA) at the NYSPI.
The inclusion criteria were patients aged 55 to 90 years without a dementia diagnosis and with a Folstein Mini‐Mental State Examination (MMSE) score of ≥23 (range: 0 to 30; a higher score indicates better cognition). The study physician evaluated the participants for whether they met the exclusion criteria, which included dementia, clinical depression (Geriatric Depression Scale score of >5), a history of primary psychiatric disease, neurological disorders (eg, Parkinson's disease, stroke, or traumatic brain injury), infectious or inflammatory disease (eg, rheumatic arthritis), medical conditions associated with clinical fatigue (eg, cancer, immunological disease, anemia, or moderate–severe cardiovascular diseases), and taking medications with sedative properties that affect daily functioning (eg, high‐dose opioids, anticholinergics, or benzodiazepines, greater than 1‐mg lorazepam equivalents per day).
During patient screening, a research physician performed a full medical evaluation using the Cumulative Illness Rating Scale‐Geriatric (CIRS‐G). 20 Study candidates with a history of chronic diseases associated with pathological fatigue or physical restriction of age‐appropriate activities were excluded from the present study; these conditions included pulmonary diseases, neurological deficits, skeletomuscular abnormalities, depression, cancer, or other conditions, apart from obstructive sleep apnea (OSA).
2.2. Measures
2.2.1. Cognitive diagnosis
We used the Alzheimer's Disease Neuroimaging Initiative diagnostic criteria. All participants had an MMSE score ≥23 out of 30. CN healthy controls had a Wechsler Memory Scale (WMS)‐III Logical Memory delayed recall score of ≥12 and a Clinical Dementia Rating (CDR) score of 0. In contrast, patients with aMCI had a WMS‐III Logical Memory delayed recall score of 11 or less and a CDR score of 0.5, as well as cognitive concerns of their own or from the physician, family members, or caregiver.
2.2.2. Measures and variables
We assessed perceived fatigability using the National Institutes of Health (NIH) Neuro–Quality of Life (QOL) fatigue short‐form subscale 21 and also used the NIH Patient‐Reported Outcomes Measurement Information System (PROMIS) scales, 22 including Sleep Disturbance, Sleep‐related Impairment (SRI), and Cognitive Function–Abilities (CogAb). All short‐form scales used semistructured interview questions, focusing on the changes from an individual's “usual state” or previously obtained levels. Each question asked about the frequency of functional failures in performing day‐to‐day activities in specific categories (eg, sleep, fatigue, and social roles and participation). These instruments focus on assessing the frequency of symptoms that interfere with or interrupt day‐to‐day functioning from the patient's perspective. Each item was scored from 1 to 5 (1 = never, 2 = rarely; 3 = sometimes; 4 = often; and 5 = always). It should be noted that, among the eight items in each scale (short form), some items used positive words, while others used negative words. For example, to obtain a higher score regarding CogAb, a participant must reject a negative statement that does not apply to them or agree with a positive statement and then choose the frequency for each state. These relatively new instruments are similar to the clinical evaluation of disease or condition severity using the International Statistical Classification of Diseases and Related Health Problems codes and the Diagnostic and Statistical Manual of Mental Disorders (5th edition) criteria.
In this study, we instructed the participants to answer each question based on their experiences over the prior 6 months or longer. NIH health measures provide conversion tables to transform the raw score into a T‐score for each scale, with a mean of 50 and a standard deviation (SD) of 10 in a study population or subpopulation. We evaluated CR using the standardized and validated CR Index Questionnaire (CRIq), which includes lifelong education, work/occupation experiences, and leisure time proxies.
2.2.3. Sleep disturbances and sleep‐related impairment
The NIH PROMIS Sleep Disturbance 8a (version 1.0) is used to assess subjective sleep quality at night. There are eight items on the Sleep Disturbance Scale, with five assessing insomnia‐related symptoms (eg, “I had difficulty falling asleep”) and the remaining three questions for sleep quality (eg, “My sleep was refreshing”). The SRI 8a (version 1.0) is used to evaluate daytime cognitive function directly related to sleep quality (eg, “I had a hard time concentrating because of my poor sleep”). The two short forms are scored from 1 to 5 (1 = not at all; 2 = a little bit; 3 = somewhat; 4 = quite a bit; and 5 = very much). These two short forms have greater measurement precision than the Pittsburgh Sleep Quality Index and Epworth Sleepiness Scale. 23 Higher Sleep Disturbance scores indicate poorer sleep quality, and higher SRI scores indicate worse daytime cognitive functioning and alertness.
2.2.4. Fatigability
The clinical level of perceived fatigability is defined by a set of parameters for assessing the frequency of failure to perform everyday activities associated with fatigue, referenced by an individual's normal state in multiple aspects of QOL. The NIH Neuro‐QOL fatigability subscale 8a (version 1.0) includes physical (eg, “I was too tired to do household chores”), functional (eg, “I was too tired to leave the house to do things”), psychological (eg, “I was frustrated by being too tired to do the things I wanted to do”), and social (eg, “I had to limit my social activity because I was too tired”) domains. Each question was scored from 1 to 5 (1 = never, 2 = rarely; 3 = sometimes; 4 = often; and 5 = always). A higher score suggests greater difficulty in initiating daily activities owing to fatigability.
2.2.5. Cognitive abilities
The PROMIS CogAb short form 8a (version 2.0) includes five cognition‐related question items (eg, “My memory is as good as usual”) and three effort‐related question items (eg, “I have been able to think clearly without extra effort”). Each item is scored from 1 to 5 (1 = never, 2 = rarely; 3 = sometimes; 4 = often; and 5 = always). A higher computed score suggests greater abilities to maintain cognition, function, and behavior, including motivational behavior. 24
2.2.6. Cognitive reserve
The CRIq 25 is a standardized and validated measure of CR with three proxy measures: lifelong years of education, work complexity, and leisure time. Each proxy questionnaire set has a formula for computing a standard score. The CRIq leisure activity score is the relative time spent performing life activities given in a list assessed weekly and for a number of years, including reading, performing domestic chores and leisure hobbies, using modern technologies, participating in social gatherings and voluntary work, caring for children and pets, and managing one's accounts. The CRIq score is a standardized score that includes three proxies. There are five categories: high (scores 130 or higher), medium‐high (scores 115 to 130), medium (scores 85 to 114), medium‐low (scores 70 to 84), and low (scores less than 70).
2.2.7. STOP‐Bang Sleep Apnea Screening Questionnaire
The STOP‐Bang Sleep Apnea Screening Questionnaire consists of eight yes/no questions, with scores ranging from 0 to 8 (addressing the topics of Loud Snore, Tired, Observed apnea, high blood Pressure, Body Mass Index [BMI], age, neck size, and gender). 26 The STOP‐BANG OSA screening questionnaire has been evaluated in epidemiological and sleep clinical studies and in various racial and ethnic populations. Polysomnography is the gold standard for diagnosing OSA, but it is expensive and cumbersome. Screening for OSA with this questionnaire is an effective and practical approach to identifying people with moderate to severe OSA. The STOP‐Bang questionnaire has a high sensitivity in identifying moderate to severe OSA (Apnea/Hypopnea Index > 15); a score of 4 or higher suggests a high pretest probability of moderate to severe OSA. 27
2.3. Statistical analyses
Analyses were performed using SPSS 28.0 (IBM Corp., Armonk, NY, USA). Demographic and clinical measures of interest were described using means and SD for continuous measures and proportions for categorical measures. The Pearson correlation was computed to assess bivariate associations between continuous measures. For the remaining analyses, all measures were standardized to mean = 0 and standard deviation = 1 so that parameter estimates were interpreted in units of SD.
Differences in demographic and clinical variables between the aMCI and CN groups were examined using independent samples t‐test‐ for continuous variables after testing for normality and the chi‐squared test for categorical variables (Table 1). We used G*Power software to examine the number of participants required in each group to meet a minimum of 80% power to conduct parametric testing. 28 We also computed z‐scores for SRI, fatigability, and CogAb because the Neuro‐QOL and PROMIS t‐scores were generated from adults aged 18 to 90 years. We examined the relationships between two variables using two‐tailed Pearson correlations (Table 2).
TABLE 1.
Demographics and characteristics of participants.
| Cognitive status | aMCI versus CN | |||||
|---|---|---|---|---|---|---|
| Variables | Total (n = 54) | aMCI (n = 41) | CN (n = 13) | P (two‐tailed) | Effect size (Cohen's d) | |
| Age (year), mean (± SD) | 70.2 (± 8.8) | 72.7 (± 8.5) | 69.4 (± 8.8) | 0.25 | 0.37 | |
| Sex (female), n (%) | 37 (68.5%) | 27 (65.9%) | 10 (76.9%) | 0.46 | 0.24 | |
| Education (year), mean (± SD) | 16.0 (± 2.0) | 16.3 (± 3.0) | 160 (± 2.0) | 0.71 | 0.12 | |
| Caucasians n (%) | 48 (88.5%) | 36 (81.0%) | 12 (92.3%) | 0.81 | 0.08 | |
| BMI, mean (± SD) | 24.6 (± 3.8) | 27.0 (± 4.3) | 24.6 (± 3.8) | 0.07 | 0.56 | |
| MMSE, mean (± SD) | 27.4 (± 4.2) | 27.2 (± 4.7) | 28.2 (± 1.9) | 0.46 | 0.23 | |
| Immediate recall, mean (± SD) | 11.6 (± 3.6) | 10.2 (± 2.9) | 15.8 (± 2.3) | <0.001*** | 2.00 | |
| Delayed recall, mean (± SD) | 9.5 (± 5.0) | 7.5 (± 2.6) | 15.9 (± 5.4) | <0.001*** | 2.41 | |
|
NIH PROMIS Neuro‐QOL T‐score mean (± SD) |
Cognitive abilities | 50.0 (± 8.6) | 42.6 (± 6.9) | 50.5 (± 8.0) | 0.002** | 1.02 |
| Fatigue | 44.2 (± 7.3) | 46.7 (± 7.0) | 39.2 (± 5.8) | 0.003** | 0.98 | |
| Sleep disturbances | 50.6 (± 9.5) | 53.1 (± 8.4) | 42.4 (± 8.1) | <0.001*** | 1.28 | |
| Sleep‐related impairment | 48.3 (± 10.0) | 51.1 (± 9.1) | 39.4 (± 7.5) | <0.001*** | 1.34 | |
| Cognitive reserve index questionnaire (Computed scores) | Education | 116.1 (± 26.3) | 113.9 (± 29.2) | 123.1 (± 11.3) | 0.28 | 0.35 |
| Working activities | 114.6 (± 28.0) | 114.6 (± 31.3) | 114.5 (± 14.2) | 1.00 | 0.003 | |
| Leisure time | 121.1 (± 23.6) | 115.9 (± 22.7) | 137.5 (± 18.9) | 0.003** | 0.93 | |
| Total score | 127.6 (± 19.1) | 125.9 (± 20.0) | 133.2 (± 15.3) | 0.24 | 0.38 | |
Note: Values are mean ± standard deviation (SD) or %. Cognitive status was defined according to the Alzheimer's Disease Neuroimaging Initiative (ADNI) criteria as cognitively normal (CN; healthy control group) and amnestic mild cognitive impairment (aMCI). NIH Person‐centered measures for adults were used for PROMIS nondisease specific scales (Cognitive Abilities, Sleep Disturbance, and Sleep‐Related Impairment) and Neuro‐QOL (quality of life) for neurological diseases (Fatigue). BMI, body mass index; MMSE, Mini‐Mental State Examination. Thresholds for assessing effect size were as follows: small (Cohen's d = 0.2), medium (Cohen's d = 0.5), and large (Cohen's d = 0.8).
*p < 0.05, **p < 0.01, ***p < 0.001.
TABLE 2.
Pearson correlation coefficient (r) between variables of interest.
| Cognitively normal group | 1 | 2 | 3 | 4a | 4b | 4c | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Age | 1 | −0.07 | 0.04 | 0.77 | 0.15 | 0.36 | 0.51 | −0.37 | −0.21 | 0.80 | 0.20 |
| 2 | Sex | 1 | −0.21 | 0.17 | 0.01 | 0.47 | 0.32 | −0.04 | −0.10 | 0.06 | 0.32 | |
| 3 | Body mass index | 1 | 0.09 | −0.48 | −0.56 | −0.47 | −0.39 | −0.18 | −0.10 | 0.30 | ||
| 4a | CR‐education | 1 | 0.16 | 0.44 | 0.64 | −0.34 | −0.11 | 0.55 | 0.21 | |||
| 4b | CR‐working | 1 | 0.50 | 0.73 | 0.21 | −0.22 | −0.17 | −0.37 | ||||
| 4c | CR‐leisure time | 1 | 0.90 | 0.16 | 0.07 | 0.39 | −0.07 | |||||
| 4 | CR‐total | 1 | 0.06 | −0.08 | 0.33 | −0.12 | ||||||
| 5 | Cognitive abilities | 1 | −0.14 | −0.50 | −0.30 | |||||||
| 6 | Sleep disturbances | 1 | 0.23 | 0.02 | ||||||||
| 7 | Sleep‐related impairment | 1 | 0.15 | |||||||||
| 8 | Fatigability | 1 | ||||||||||
| Amnestic mild cognitive impairment group | 1 | 2 | 3 | 4a | 4b | 4c | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | age | 1 | −0.10 | −0.17 | 0.31 | 0.11 | 0.05 | 0.17 | 0.07 | −0.21 | 0.09 | 0.02 |
| 2 | sex | 1 | −0.10 | −0.05 | −0.22 | −0.22 | −0.28 | −0.20 | −0.05 | 0.11 | 0.35 | |
| 3 | BMI | 1 | −0.21 | −0.22 | −0.12 | −0.13 | −0.11 | 0.15 | 0.00 | −0.09 | ||
| 4a | CR‐education | 1 | 0.63 | −0.01 | 0.02 | 0.05 | 0.15 | 0.22 | 0.12 | |||
| 4b | CR‐working | 1 | 0.31 | 0.52 | 0.30 | 0.00 | −0.01 | −0.13 | ||||
| 4c | CR‐leisure time | 1 | 0.84 | 0.24 | −0.06 | −0.29 | −0.41 | |||||
| 4 | CR‐total | 1 | 0.36 | −0.17 | −0.27 | −0.41 | ||||||
| 5 | Cognitive abilities | 1 | −0.21 | −0.37 | ‐0.69 | |||||||
| 6 | Sleep disturbances | 1 | 0.62 | 0.16 | ||||||||
| 7 | Sleep‐related impairment | 1 | 0.51 | |||||||||
| 8 | Fatigability | 1 | ||||||||||
Note: Cognitive reserve (CR) was assessed using the validated Cognitive Reserve Index Questionnaire, including three proxies (education, working, and leisure time). CR‐total is a total score of computed the proxies. Cognitive abilities, sleep disturbances, sleep‐related impairment were assessed using the NIH PROMIS scales and fatigue was assessed using the NIH Neuro‐QOL fatigability subscale. Thresholds for assessing the Pearson correlation coefficient were r = 0.8, r = 0.6, and r = 0.3 for strong, moderate, and weak relationships between two variables, respectively.
A moderated mediation model (model 7) was fit to assess whether fatigability mediated the effect of SRI on CogAb and to explore whether the effect of SRI on fatigability differed across different levels of CR (Figure 1). The full model comprises two subregression models. The first‐stage model entails regressing fatigability (mediator) onto SRI, CR, and their interaction (SRI × CR). The second‐stage model involved simultaneously regressing CogAb onto SRI and fatigability. 29
FIGURE 1.
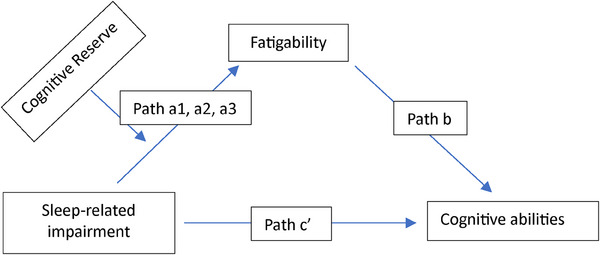
The proposed moderated mediation model. This moderated mediation model tested the indirect effect of sleep‐related impairment (SRI; independent variable) on cognitive abilities (dependent variable) through the mediator fatigability, with the indirect effect being moderated by cognitive reserve (CR). The full model is comprised of two subregression models. The first‐stage model entails regressing fatigability (mediator) onto SRI, CR (at low [<−1 SD, path a1], mean [SD = 0, path a2], and high [>+1 SD, path a3] values of CR), and their interactions (SRI × CR). The second‐stage model entails regressing cognitive abilities onto SRI and fatigability simultaneously.
The path of a linear model included the effects of SRI (continuous), CR (continuous), and their two‐way interaction on fatigability. If the interaction CR × SRI was significant, then the effect of SRI on fatigability was assessed at low (<−1 SD), mean, and high (>+1 SD) levels of CR. The path b linear model included the effects of SRI and fatigability on CogAb. Each model was adjusted for age and sex. If moderated mediation was significant (the effect of path a interaction by path b), then the indirect effect of SRI on CogAb mediated by fatigability was computed at low CR (path a1), mean CR (path a2), and high CR (path a3) levels to test our hypothesis (Figure 2, Table 3). Bootstrapped samples (n = 1000) were computed to obtain valid standard errors and corresponding 95% confidence intervals (CIs) for indirect effects instead of p‐values.
FIGURE 2.
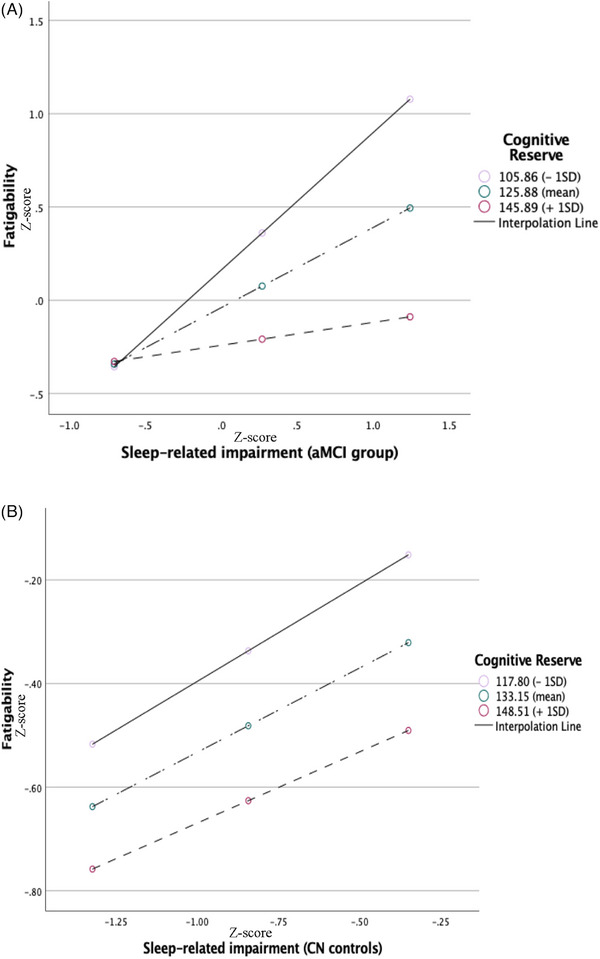
Effect of sleep‐related impairment on fatigability moderated by cognitive reserve. The illustrations represent the moderation effect of cognitive reserve on the relationship between sleep‐related impairment and fatigability at low (−1 SD), mean (0 SD), and high (+1 SD) levels of cognitive reserve in (A) patients with amnestic mild cognitive impairment and (B) cognitively normal healthy controls.
TABLE 3.
Results of the moderated mediation model with fatigability in older adults with amnestic mild cognitive impairment.
| Mediator: Fatigability | Outcome: Cognitive abilities | |||||
|---|---|---|---|---|---|---|
| Predictor | B (SE) | p | B (SE) | p | ||
| Sleep‐related impairment (SRI) | 2.36 (0.86) | <0.001*** | −0.035 (0.14) | 0.81 | ||
| Cognitive reserve (CR) | −0.010 (0.007) | 0.16 | ||||
| CR x SRI | −0.015 (0.007) | 0.03* | ||||
| Fatigability | −0.699 (0.144) | <0.001*** | ||||
| Moderator: CR | Levels | Indirect effect | p | B (SE) | 95% LLCI | 95% ULCI |
|---|---|---|---|---|---|---|
| Path a1: Lower level (<−1 SD) | CRIq = 105.7 | 0.737 (0.19) | <0.001*** | −0.49 (0.25) | −1.15 | −0.18 |
| Path a2: Mean level (0 SD) | CRIq = 125.9 | 0.430 (0.14) | 0.003** | −0.29 (0.13) | −0.62 | −0.11 |
| Path a3: Higher level (>+1 SD) | CRIq = 145.9 | 0.123 (0.20) | 0.54 | −0.08 (0.12) | −0.35 | 0.18 |
| Index of moderated mediation | 0.01 (0.007) | 0.007 | 0.04 |
Note: The moderated mediation model was controlled by age and sex. CR moderated SRI on fatigability at low‐CR (Path a1), mean‐CR (Path a2), and high‐CR (Path a3) levels.
Abbreviations: CRIq, Cognitive Reserve Index Questionnaire; LLCL, lower limit confidence interval; ULCL, upper limit confidence interval.
*p < 0.05; **p < 0.01; ***p < 0.001.
3. RESULTS
As shown in Table 1, 54 individuals without depression participated in this study, comprising 13 CN controls and 41 patients diagnosed with aMCI. The majority of the participants had at least college education or higher, and more than two thirds were women. A total of 16.7% and 31.6% of participants in the CN and aMCI groups, respectively, were apolipoprotein E (APOE) ε4 carriers, and there were no significant differences in age, sex, education, BMI, MMSE scores, and % APOE ε4 positivity between the groups. There were no significant differences in CR education and work proxies between the groups, whereas CR leisure (time spent on leisure activities) was significantly greater in the CN than in the aMCI group. We compared the t‐scores for CogAb, fatigue, Sleep Disturbance, and SRI, with significant differences in these variables between the groups with large Cohen's d effect sizes (0.98 to 1.34). The differences remained significant when using z‐scores.
As shown in Table 2, CogAb correlated with SRI (r = −0.374; p < 0.05), fatigability (r = −0.689; p < 0.001), and CR (r = 0.355; p < 0.05); fatigue correlated with SRI (r = 0.513; p < 0.01) and CR (r = −0.413; p < 0.01); and SRI correlated with Sleep Disturbance (r = 0.617; p < 0.01) in the aMCI group. In the CN group, CogAb did not Johnson–Neyman significant regions correlate with Sleep Disturbance, SRI, CR, or fatigability. In the aMCI group, fatigability had a significant negative effect on CR leisure after controlling for covariates and APOE ε4 positivity (adjusted R2 = 0.21, F(6, 34) = 3.18, p = 0.003).
Finally, moderated mediation analysis was performed using Hayes’ Process Macro (Model 7) in SPSS (Figure 1). Figure 2 illustrates the path moderation effect at low (<−1 SD), mean, and high (>+1 SD) CR values. In the aMCI group (Figure 2A), high SRI was associated with high fatigability at lower CR (CRIq score of <105.8, Beta [B] [standard error (SE)] = 0.74 [0.20], p = 0.0004) and mean CR (CRIq score = 126.9, B [SE] = 0.43 [0.14], p = 0.003); however, it was not significantly associated with higher CR (CRIq score of >145.9, B [SE] = 0.12 [0.12], p = 0.63). Furthermore, the moderator CR values defining the Johnson–Neyman significant regions were CRIq scores of <135 (p < 0.05), 135.34 (p = 0.05), and >136 (p > 0.05), indicating that the effect of SRI on CogAb mediated by fatigability was not significant when CRIq scores were greater than 136. Age and sex were not significantly associated with fatigue (B [SE] = 0.01 [0.02], p = 0.55 and B [SE] = 0.52 [0.27], p = 0.06, respectively). In contrast, the effect of SRI on fatigability did not differ across CR levels in the CN group (Figure 2B).
As shown in Table 3 (aMCI only), CR moderated the effect of SRI on fatigability (CR × SRI interaction: B [SE] = −0.15 [0.007], p = 0.03) after controlling for age and sex. There was a significantly moderated mediation, where the indirect effect of SRI on CogAb mediated through fatigability varied by the level of CR in the aMCI group. The conditional indirect effect of SRI on fatigability on CogAb was significant for low CR (−1 SD, CRIq score of <105.7) and mean CR (SD = 0, CRIq score = 125.9), but not for high CR (+1 SD, CRIq score of >136). The moderated mediation index was statistically significant.
In the aMCI group, one quarter of the participants had a high pretest probability of moderate to severe OSA (STOP‐Bang scores of ≥4) or were diagnosed with OSA by a sleep physician. None of the OSA+ participants had received active OSA treatment for 1 year or longer at the time of screening. Among the patients with aMCI and OSA, 40% were women.
4. DISCUSSION
The objective of the present study was to examine patient adaptability or susceptibility to sleep–wake disturbances of perceived fatigability at different levels of CR. We found that SRI, perceived fatigability, and CogAb were significantly worse in patients with aMCI than in CN controls. Further, patients with aMCI who had low and mean levels of CR showed that the effects of sleep–wake disturbances contributed to CogAb decline mediated through the exacerbation of fatigability. In contrast, those with high levels of CR were able to tolerate SRI and fatigability, which in part protected against CogAb decline. In contrast, CN controls could fully compensate for sleep–wake disturbances and fatigability, regardless of their level of CR.
4.1. Independent and interrelated relationships among SRI, fatigability, and cognitive abilities
As shown in Table 1, the differences between patients with aMCI and CN controls in SRI, fatigability, and CogAb were significant with large effect sizes (0.98 to 1.34). The results indicated that patients with aMCI experienced poor sleep‐associated impaired cognitive function in performing day‐to‐day activities, owing to an increase in fatigability. Furthermore, the results from the moderated mediation analysis showed that indirect effects of SRI significantly affected CogAb mediated through perceived fatigability in patients with aMCI (Figure 1). Therefore, fatigability negatively influences perceived CogAb in patients with aMCI. In contrast, sleep disturbances did not cause pathological fatigue in CN controls; therefore, their energy and functionality could be restored by rest (Table 2). Our findings agreed with the literature showing that individuals with sleep–wake dysregulation have worse cognitive function and a higher prevalence of AD. 30 , 31 Further, greater fatigability in patients with aMCI can be explained by the neuroinflammation hypothesis of AD. 9 , 32 Fatigability could be a clinical expression of increased proinflammatory cytokines in response to Aβ and tau pathology, which are associated with poorer cognitive functioning. 11 , 33
4.2. Connectome between the sleep/wake‐fatigability paradigm and CR framework
The concept of CR can explain the fact that some individuals can tolerate more brain structural and functional abnormalities than others, although there is no immunity to the accumulation of AD. 34 We examined the effects of SRI on perceived fatigability at three levels of CR (low [−1 SD], mean, and high [+1 SD]) (Figure 1). We found that greater SRI was significantly associated with higher fatigability (Table 2) in patients with aMCI at low and mean CR levels (Table 3), but not at high CR levels (Table 3, path a3). The indirect effect of SRI on cognitive abilities was mediated through the exacerbation of fatigability at the low and mean CR levels (Figure 2A), whereas high levels of CR could attenuate the effect of sleep‐wake disturbances on fatigability, in part preserving cognitive abilities despite having aMCI. In contrast, the relationship between SRI and fatigability was not altered by the level of CR in CN controls (Figure 2b), thus maintaining normal cognition.
Furthermore, the average leisure proxy score of CR was significantly lower in patients with aMCI than in CN controls, with a large effect size (Cohen's d = 0.93). Perceived fatigability affected the engagement in leisure activities by 16.6% in patients with aMCI and by 0.5% in CN controls, suggesting that perceived fatigability in patients with aMCI played a crucial role in participation in leisure activities. This result is consistent with a systematic review and meta‐analysis of aMCI including 58 studies in the literature (up to March 2020; n = 7871; 53% female) reporting that real‐world functioning was strongly associated with activities of daily living with leisure activities (r = 0.27) compared with cognitively stimulating leisure activities only (r = 0.16). 35
There are a series of changes associated with sleep–wake patterns 36 in cognition, function, and behavior from resilience (compensation) to impairment (decompensation) to the loss of circadian rhythmicity during transitions from preclinical cognitive impairment to dementia. 37 In patients with aMCI, SRI explained 26.3% of fatigability and 14% of declined CogAb, suggesting that nearly 75% of perceived fatigability was caused by other contributing and facilitating factors. In addition, fatigability had a strong negative effect on CogAb, explaining 47.6% of the decline in patients with aMCI. The result remained significant after controlling for covariates (age, sex, education, and BMI) as well as APOE ε4 positivity and SRI, the two known determinants of CogAb in AD, indicating that fatigability was not exclusively the consequence of sleep–wake disturbances, APOE ε4 positivity, older age, and obesity. Therefore, other potential factors contributing to clinical fatigability should be investigated.
The findings of our study are consistent with those of studies showing that (1) untreated chronic and persistent sleep–wake dysregulation could progress to SRI when there is a transition from cognitive normality to aMCI 2 , 38 ; (2) sleep–wake disturbances with evidence of altered rest/activity rhythms are linked to AD pathology in the brain 30 , 31 ; and (3) persistent fatigability that cannot be relieved with rest is a manifestation of neuroinflammation associated with Aβ and tau pathology in aMCI 39 , 40 and abnormal structures in the brain (eg, white matter hyperintensities in AD). Moreover, findings from our study and other studies highlight the importance of cognitive training and behavioral rehabilitation programs for cognitively stimulating leisure and recreational activities to preserve CR in later life and maintain independent living and QOL. 41
4.3. OSA and AD pathology, a double insult to the brain
OSA is a systemic disease that causes major structural and functional damage to organs including the brain (eg, cerebral small vessel disease). 42 Estimates suggest that up to 60% of community‐dwelling people aged 65 years or older have OSA. 43 In a large cohort study of older adults, people self‐reporting having OSA but not noncompliance with continuous positive airway pressure therapy had a cognitive transition from aMCI to dementia 8 years earlier than expected, based on the findings from the Alzheimer's Disease Neuroimaging Initiative study. 44 OSA is associated with decreased non‐REM stage 3 sleep owing to sleep fragmentation. The lack of deep sleep in OSA is associated with decreased brain clearance of waste products, such as Aβ, compared with that in people without OSA. 45
In this study, SRI (daytime cognitive and functional deficits directly related to sleep disturbances) was significantly greater in patients with aMCI with OSA (Figure SA), suggesting that sleep–wake behavior causes additional damage to the brain during the preclinical stages of AD. Furthermore, all variables of interest remained significant between patients with aMCI without OSA and CN controls (Figure SB), indicating that cognitive status was determined by AD pathology rather than OSA.
The characteristics of sleep–wake behavior and fatigability associated with OSA are clinically different from the abnormal sleep–wake rhythms and fatigability linked to AD pathology. A brief nap or dozing off can quickly eliminate sleepiness and help patients with OSA recover from mental fatigue. However, fatigability associated with AD pathology cannot be alleviated by rest, and there is no effective treatment for the irregularity of sleep–wake rhythms once Aβ plaques damage the biological clock. 30
4.4. Future research direction
Fatigability has been the focus of research on neurodegenerative disorders to develop therapeutic interventions to improve functionality and QOL. 46 In 2000, the Multiple Sclerosis (MS) Council released the brochure “Fatigue: What You Should Know—A Guide for People with MS,” which provided multiple sclerosis‐related fatigue education for patients and their families. In 2013, experts from the World Parkinson's Congress workgroup identified Parkinson's disease‐related fatigue as a high priority for research after patients voted fatigue as the leading symptom. In contrast, there is a paucity of data in the literature that addresses the impact of fatigability on CogAb when performing day‐to‐day activities in AD. As illustrated in Figure 3, future studies are needed to investigate the central mechanisms and pathways linking clinical manifestations of sleep–wake behavior, fatigability, and CogAb to altered brain structures (eg, white matter hyperintensities and function, impaired neural connectivity) 47 through AD biomarkers, including increased proinflammatory cytokines, 32 which are beyond the scope of the present study. Further studies on the concept of AD‐related fatigue are warranted.
FIGURE 3.
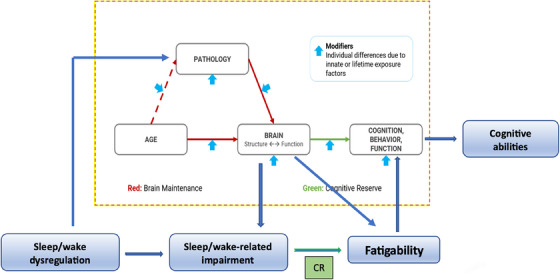
Connectome between the Sleep–Fatigability paradigm and the Cognitive Reserve (CR) framework. The results of this study supported our Sleep–Fatigability paradigm which is also consistent with the literature, in which (1) untreated chronic and persistent sleep–wake dysregulation could progress to sleep‐related impairment with cognitive transition from cognitive normal to amnestic mild cognitive impairment; (2) sleep‐related impairment with altered rest/activity rhythms is associated with Alzheimer's pathology in the brain; and (3) persistent fatigue that cannot be relieved with rest is a manifestation of neuroinflammation associated with beta‐amyloid and tau pathology in amnestic mild cognitive impairment and abnormal structures in the brain (eg, white matter hyperintensities in Alzheimer's disease).
4.5. Limitations
Our participants had an average of 16 years of education and the majority were of Caucasian ethnicities. Therefore, our results may not be applicable to other ethnic groups or individuals with lower educational levels. Additionally, we excluded participants with active depression. Thus, our results may not be applicable to individuals with fatigue due to depression. Additionally, this was a cross‐sectional study with 13 participants in the CN control group and 41 participants in the aMCI group. Since the variables had large effect sizes (0.98 to 1.3) between the two groups, only 12 participants were required in the control group and 36 were required in the aMCI group to reach greater than 80% power in this pilot study. We considered this to be a limitation because a larger sample size with a balanced number of participants in each comparison group would generate more reliable results. In addition, we did not objectively assess sleep disturbances and SRI using actigraphy.
5. CONCLUSIONS
This study highlights a novel area of research by linking the sleep–wake–fatigability paradigm to the CR framework, emphasizing inter‐ and intra‐individual variability in fatigability associated with sleep–wake behavior during the preclinical stage of AD. Based on the study results and emerging evidence in the literature, we conclude that patients diagnosed with aMCI with high CR levels are resilient to sleep–wake disturbances and fatigability, and are thus less susceptible to AD pathology in the brain. In contrast, those with below‐mean CR levels have less adaptability to SRI and fatigability, resulting in a more rapid decline in CogAb. In addition, improving cognitively stimulating leisure activities is critical for older adults to maintain CR to protect against cognitive decline. Nonetheless, further studies on the mechanisms and pathways behind sleep–wake behavior and fatigability and how these are associated with brain function and neural compensation, through the application of the CR concept, are warranted.
CONFLICT OF INTEREST STATEMENT
Dr. Kerner receives support from Columbia University's Clinical and Translational Research (CTSA) and NIH/NIA. Drs. Goldberg, Qin, Andrews, Pelton, and Devanand receive support from the NIH/NIA. All authors have no conflict of interest to declare. Author disclosures are available in the supporting information.
CONSENT STATEMENT
The study protocol was approved by the Institutional Review Board of the New York State Psychiatric Institute (# 7779) and performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments. All participants provided written informed consent before data collection.
Supporting information
Supplementary Figure A. Comparison variables of interest between amnestic mild cognitive impairment and cognitively normal control groups.
Supplementary Figure B. Comparison variables of interest between patients with and without obstructive sleep apnea in the mild cognitive impairment group.
Supporting Information
ACKNOWLEDGMENTS
This study was supported by NIH UL1TR001873, K23AG066945; R01AG052440; and R01AG051346.
Kerner N, Goldberg TE, Cohen HR, et al. Sleep–wake behavior, perceived fatigability, and cognitive reserve in older adults. Alzheimer's Dement. 2024;20:4020–4031. 10.1002/alz.13802
REFERENCES
- 1. Logan RW, McClung CA. Rhythms of life: circadian disruption and brain disorders across the lifespan. Nat Rev Neurosci. 2019;20(1):49‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lim AS, Kowgier M, Yu L, Buchman AS, Bennett DA. Sleep fragmentation and the risk of incident Alzheimer's disease and cognitive decline in older persons. Sleep. 2013;36(7):1027‐1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lee YF, Gerashchenko D, Timofeev I, Bacskai BJ, Kastanenka KV. Slow wave sleep is a promising intervention target for Alzheimer's disease. Front Neurosci. 2020;14:705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Spira AP, Gamaldo AA, An Y, et al. Self‐reported sleep and beta‐amyloid deposition in community‐dwelling older adults. JAMA Neurol. 2013;70(12):1537‐1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Naismith SL, Hickie IB, Terpening Z, et al. Circadian misalignment and sleep disruption in mild cognitive impairment. J Alzheimers Dis. 2014;38(4):857‐866. [DOI] [PubMed] [Google Scholar]
- 6. Aaronson LS, Teel CS, Cassmeyer V, et al. Defining and measuring fatigue. Image J Nurs Sch. 1999;31(1):45‐50. [DOI] [PubMed] [Google Scholar]
- 7. Eldadah BA. Fatigue and fatigability in older adults. PM R. 2010;2(5):406‐413. [DOI] [PubMed] [Google Scholar]
- 8. Heneka MT, Carson MJ, El Khoury J, et al. Neuroinflammation in Alzheimer's disease. Lancet Neurol. 2015;14(4):388‐405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kelley KW, Bluthe RM, Dantzer R, et al. Cytokine‐induced sickness behavior. Brain Behav Immun. 2003;17:S112‐S118. Suppl 1. [DOI] [PubMed] [Google Scholar]
- 10. Ju YE, Lucey BP, Holtzman DM. Sleep and Alzheimer disease pathology–a bidirectional relationship. Nat Rev Neurol. 2014;10(2):115‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kress GJ, Liao F, Dimitry J, et al. Regulation of amyloid‐beta dynamics and pathology by the circadian clock. J Exp Med. 2018;215(4):1059‐1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shih YH, Pai MC, Huang YC, Wang JJ. Sundown syndrome, sleep quality, and walking among community‐dwelling people with Alzheimer disease. J Am Med Dir Assoc. 2017;18(5):396‐401. [DOI] [PubMed] [Google Scholar]
- 13. Zhu L, Zee PC. Circadian rhythm sleep disorders. Neurol Clin. 2012;30(4):1167‐1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. AlzheimerAssociation . Alzheimer's disease facts and figures. Alzheimers Demen. 2023. [Google Scholar]
- 15. Jack CR Jr, Bennett DA, Blennow K, et al. NIA‐AA Research Framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14(4):535‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stern Y. Cognitive reserve in ageing and Alzheimer's disease. Lancet Neurol. 2012;11(11):1006‐1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stern Y, Arenaza‐Urquijo EM, Bartres‐Faz D, et al. Whitepaper: defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimers Dement. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Song S, Stern Y, Gu Y. Modifiable lifestyle factors and cognitive reserve: a systematic review of current evidence. Ageing Res Rev. 2022;74:101551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Scarmeas N, Levy G, Tang MX, Manly J, Stern Y. Influence of leisure activity on the incidence of Alzheimer's disease. Neurology. 2001;57(12):2236‐2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Miller MD, Paradis CF, Houck PR, et al. Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Res. 1992;41(3):237‐248. [DOI] [PubMed] [Google Scholar]
- 21. Cella D, Lai JS, Nowinski CJ, et al. Neuro‐QOL: brief measures of health‐related quality of life for clinical research in neurology. Neurology. 2012;78(23):1860‐1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.HealthMeasures. Neuro‐QOL, PROMIS. 2022.
- 23. Yu L, Buysse DJ, Germain A, et al. Development of short forms from the PROMIS sleep disturbance and sleep‐related impairment item banks. Behav Sleep Med. 2011;10(1):6‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Forstmeier S, Maercker A. Motivational reserve: lifetime motivational abilities contribute to cognitive and emotional health in old age. Psychol Aging. 2008;23(4):886‐899. [DOI] [PubMed] [Google Scholar]
- 25. Nucci M, Mapelli D, Mondini S. Cognitive Reserve Index questionnaire (CRIq): a new instrument for measuring cognitive reserve. Aging Clin Exp Res. 2012;24(3):218‐226. [DOI] [PubMed] [Google Scholar]
- 26. Chung F, Abdullah HR, Liao P. STOP‐Bang questionnaire: a practical approach to screen for obstructive sleep apnea. Chest. 2016;149(3):631‐638. [DOI] [PubMed] [Google Scholar]
- 27. Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. deliberations of the sleep apnea definitions task force of the American academy of sleep medicine. J Clin Sleep Med. 2012;8(5):597‐619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kang H. Sample size determination and power analysis using the G*Power software. J Educ Eval Health Prof. 2021;18:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Preacher KJ, Rucker DD, Hayes AF. Addressing moderated mediation hypotheses: theory, methods, and prescriptions. Multivariate Behav Res. 2007;42(1):185‐227. [DOI] [PubMed] [Google Scholar]
- 30. Musiek ES, Bhimasani M, Zangrilli MA, Morris JC, Holtzman DM, Ju YS. Circadian rest‐activity pattern changes in aging and preclinical Alzheimer disease. JAMA Neurol. 2018;75(5):582‐590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Duncan MJ, Veasey SC, Zee P. Editorial: roles of sleep disruption and circadian rhythm alterations on neurodegeneration and Alzheimer's disease. Front Neurosci. 2021;15:737895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lacourt TE, Vichaya EG, Chiu GS, Dantzer R, Heijnen CJ. The high costs of low‐grade inflammation: persistent fatigue as a consequence of reduced cellular‐energy availability and non‐adaptive energy expenditure. Front Behav Neurosci. 2018;12:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kinney JW, Bemiller SM, Murtishaw AS, Leisgang AM, Salazar AM, Lamb BT. Inflammation as a central mechanism in Alzheimer's disease. Alzheimers Dement (N Y). 2018;4:575‐590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stern Y, Alexander GE, Prohovnik I, Mayeux R. Inverse relationship between education and parietotemporal perfusion deficit in Alzheimer's disease. Ann Neurol. 1992;32(3):371‐375. [DOI] [PubMed] [Google Scholar]
- 35. Berezuk C, Scott SC, Black SE, Zakzanis KK. Cognitive reserve, cognition, and real‐world functioning in MCI: a systematic review and meta‐analysis. J Clin Exp Neuropsychol. 2021;43(10):991‐1005. [DOI] [PubMed] [Google Scholar]
- 36. Peter‐Derex L, Yammine P, Bastuji H, Croisile B. Sleep and Alzheimer's disease. Sleep Med Rev. 2015;19:29‐38. [DOI] [PubMed] [Google Scholar]
- 37. Smevik H, Habli S, Saksvik SB, et al. Poorer sleep health is associated with altered brain activation during cognitive control processing in healthy adults. Cereb Cortex. 2023;33(11):7100‐7119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Musiek ES, Xiong DD, Holtzman DM. Sleep, circadian rhythms, and the pathogenesis of Alzheimer disease. Exp Mol Med. 2015;47:e148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Parbo P, Ismail R, Hansen KV, et al. Brain inflammation accompanies amyloid in the majority of mild cognitive impairment cases due to Alzheimer's disease. Brain. 2017;140(7):2002‐2011. [DOI] [PubMed] [Google Scholar]
- 40. Popp J, Oikonomidi A, Tautvydaite D, et al. Markers of neuroinflammation associated with Alzheimer's disease pathology in older adults. Brain Behav Immun. 2017;62:203‐211. [DOI] [PubMed] [Google Scholar]
- 41. Manly JJ, Touradji P, Tang MX, Stern Y. Literacy and memory decline among ethnically diverse elders. J Clin Exp Neuropsychol. 2003;25(5):680‐690. [DOI] [PubMed] [Google Scholar]
- 42. Kerner NA, Roose SP. Obstructive sleep apnea is linked to depression and cognitive impairment: evidence and potential mechanisms. Am J Geriatr Psychiatry. 2016;24(6):496‐508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ancoli‐Israel S, Kripke DF, Klauber MR, Mason WJ, Fell R, Kaplan O. Sleep‐disordered breathing in community‐dwelling elderly. Sleep. 1991;14(6):486‐495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Osorio RS, Gumb T, Pirraglia E, et al. Sleep‐disordered breathing advances cognitive decline in the elderly. Neurology. 2015;84(19):1964‐1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ju YS, Zangrilli MA, Finn MB, Fagan AM, Holtzman DM. Obstructive sleep apnea treatment, slow wave activity, and amyloid‐beta. Ann Neurol. 2019;85(2):291‐295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kluger BM, Krupp LB, Enoka RM. Fatigue and fatigability in neurologic illnesses: proposal for a unified taxonomy. Neurology. 2013;80(4):409‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hua L, Gao F, Xia X, et al. Individual‐specific functional connectivity improves prediction of Alzheimer's disease's symptoms in elderly people regardless of APOE epsilon4 genotype. Commun Biol. 2023;6(1):581. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure A. Comparison variables of interest between amnestic mild cognitive impairment and cognitively normal control groups.
Supplementary Figure B. Comparison variables of interest between patients with and without obstructive sleep apnea in the mild cognitive impairment group.
Supporting Information


