Significance
The great majority of studies estimating the impact of climate change on biodiversity rely on spatial occurrence data and climate envelope modeling. A range-wide network of tree-ring time series data for an aridland pine shows that individual-scale responses to time-varying climate are opposite in sign to species-scale responses to spatial climate variation for half or more of the species’ distribution. Instead of half the distribution benefiting from warming, the entire distribution suffers with warming, making the trailing edge everywhere. Time series data reveal a transient risk of extinction, which requires evolutionary change of individual-scale climate tolerances for a species to persist (evolutionary rescue). Scale-dependent climate responses are reported for hundreds of species in the literature, questioning the climate envelope approach.
Keywords: biodiversity, climate change, scale, species distribution modeling, time-series data
Abstract
Given the importance of climate in shaping species’ geographic distributions, climate change poses an existential threat to biodiversity. Climate envelope modeling, the predominant approach used to quantify this threat, presumes that individuals in populations respond to climate variability and change according to species-level responses inferred from spatial occurrence data—such that individuals at the cool edge of a species’ distribution should benefit from warming (the “leading edge”), whereas individuals at the warm edge should suffer (the “trailing edge”). Using 1,558 tree-ring time series of an aridland pine (Pinus edulis) collected at 977 locations across the species’ distribution, we found that trees everywhere grow less in warmer-than-average and drier-than-average years. Ubiquitous negative temperature sensitivity indicates that individuals across the entire distribution should suffer with warming—the entire distribution is a trailing edge. Species-level responses to spatial climate variation are opposite in sign to individual-scale responses to time-varying climate for approximately half the species’ distribution with respect to temperature and the majority of the species’ distribution with respect to precipitation. These findings, added to evidence from the literature for scale-dependent climate responses in hundreds of species, suggest that correlative, equilibrium-based range forecasts may fail to accurately represent how individuals in populations will be impacted by changing climate. A scale-dependent view of the impact of climate change on biodiversity highlights the transient risk of extinction hidden inside climate envelope forecasts and the importance of evolution in rescuing species from extinction whenever local climate variability and change exceeds individual-scale climate tolerances.
Climate is understood to be fundamental in determining species’ geographic distributions (1–3); thus, it is expected that climate change will exacerbate the loss of biodiversity (4, 5). The most prevalent approach used to predict how species will respond to climate change is species distribution modeling, which infers a “climate envelope”—the range of climatic conditions expected to allow a species to persist—based on the climatic conditions where the species is present (or present vs. absent). Climate envelope models are then used to project a species’ geographic distribution under future climate scenarios (4, 6; SI Appendix, Fig. S1 and Biodiversity Forecasting with Occurrence Data). Based on this approach, it is estimated that 18 to 37% of species are “committed to extinction” by climate change (4, 6).
However, ecologists know that in addition to climate, species’ distributions are influenced by biotic interactions, dispersal, ecological disturbances, and evolution, among other processes (7, 8). Climate envelope forecasting relies on correlations between climate and any other range-limiting processes, and the assumption that species’ distributions are at equilibrium with climate (or other range-limiting processes), both with respect to model calibration and forward projection (3, 9–11). In reality, the processes influencing species’ abundances and geographic distributions operate on time scales ranging from short to long and spatial scales ranging from small to large (4, 8, 12–14). Near-term (transient) range dynamics are influenced by fast ecological processes (phenotypic plasticity and its demographic consequences), whereas long-term (equilibrium) range dynamics are also influenced by slow processes (evolution, dispersal, community sorting; 12–14). This is recognized in the biodiversity forecasting literature in that many studies include alternative scenarios of “no-dispersal” vs. “full dispersal,” i.e., to account for the fact that a species’ rate of dispersal may be slow relative to the rate of changing climate (12, 15).
Perhaps less well appreciated is that the correlative, equilibrium nature of climate envelope forecasting presumes that individuals will respond to temporal variation in climate according to species-level responses to spatial variation in climate (inferred from occurrence data). For example, with respect to changing temperature, a species’ distribution will track the movement of its thermal envelope poleward or upward in elevation with warming if individuals in populations at the cool edge of the species’ distribution respond positively to warming whereas those at the warm edge respond negatively (Fig. 1I). This is the leading edge-trailing edge paradigm for range dynamics (12, 15). In other words, climate envelope forecasting presumes that climate responses are invariant, both across biological scales (species vs. individual) and across space vs. time (Fig. 1, hypothesis 1; 16–19). This assumption is justified, with respect to biological scale, by the abundant center hypothesis (20) and center-periphery hypothesis (21; SI Appendix, Fig. S2 and “Climate Responses across Scales” ), and with respect to space vs. time as an example of space-for-time substitution, a widespread practice in ecology. These assumptions underpinning climate envelope forecasting have been criticized both on conceptual and empirical grounds (9–11, 16, 18, 19, 21–23, and citations in SI Appendix, Table S1), yet the practice remains dominant.
Fig. 1.
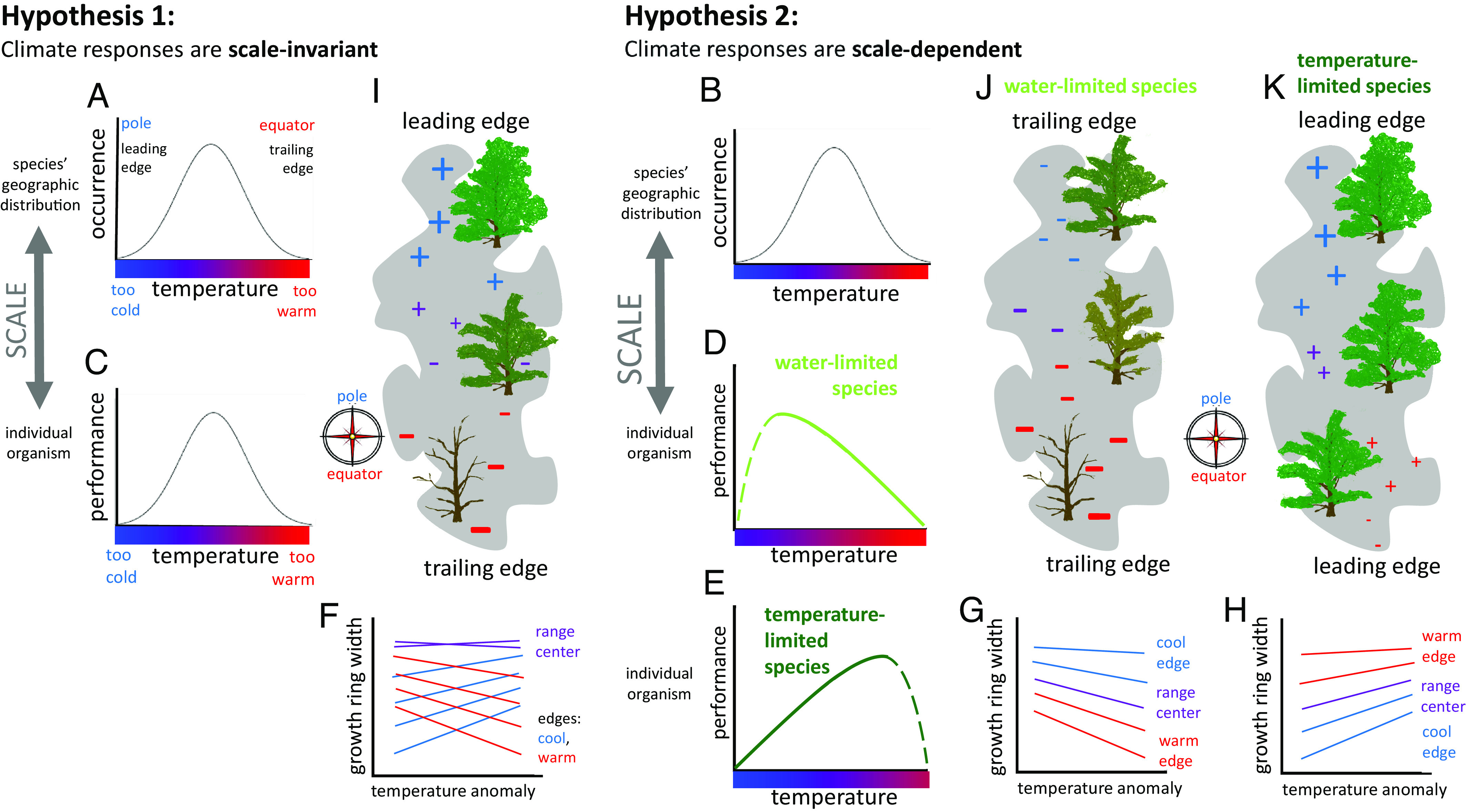
Hypotheses about climate responses at the species vs. individual scale (A–E) and corresponding predictions about climate-growth relationships (F–H) and range dynamics with warming (I–K). Under hypothesis 1, species-scale climate responses, inferred from occurrence data (A), match individual-scale climate responses, inferred from performance data (C); under hypothesis 2, species- and individual-scale climate responses do not match (B vs. D and E), individual-scale performance is either negatively sensitive to temperature variation across time and space (water-limited performance, D) or positively temperature sensitive (temperature-limited performance, E), with solid lines indicating the response to interannual temperature variability within its historical range, to which the organism is adapted, and dashed lines indicating the response to more extreme temperature variability. Panels (F–H) show the predicted sensitivity of tree growth-ring width to time-varying temperature (rescaled as local temperature anomalies) reflecting the local slope in panels (C–E) at warm (red) to average (purple) to cool (blue) locations across the species’ distribution. Panels (I–K) show three contrasting predicted responses (+/−) of individual-level performance to warming across species’ geographic distributions, also derived from panels (C–E), with the size of the symbol indicating the magnitude (slope) of individual-level sensitivity to temperature variation. A temperature-limited species may experience declining performance (−) at its low-latitude edge (panel K), if warming exceeds individual-scale thermal tolerances.
An alternative possibility is that individual- or population-level climate responses differ from species-level climate responses (Fig. 1, hypothesis 2; 24–28), i.e., that transient responses reflecting fast processes like individual-scale plasticity differ from equilibrium responses reflecting slow processes like evolution and dispersal. Instead of changing in sign from positive to negative across a species’ distribution (Fig. 1F), individual-level responses to time-varying climate may be similar throughout a species’ geographic distribution if the same limiting factor constrains physiology and hence performance everywhere (Fig. 1 G and H). Consider the climatic factors that limit performance in plants: they include inadequate temperature and inadequate soil moisture (29–31). Plants found in cold places might be expected to respond positively to warmer-than-average conditions, within the range of temperature variation they have historically been exposed to and are therefore adapted to, whereas plants in a soil moisture-limited context would be expected to respond negatively to warmer-than-average temperatures (32, 33; see SI Appendix, Climate Responses across Scales—Individual Scale). Hence, all individuals in all populations of a moisture-limited species should suffer with warming (Fig. 1J) and all individuals in all populations of a temperature-limited species should benefit from warming (Fig. 1K). We describe predictions with respect to temperature because it is changing relatively predictably compared to precipitation (34), with consequences that include large-scale mortality events (35, 36). Parallel predictions apply to any other nonstationary climate variables, and how they might interact with one another, affecting individual performance. If transient vs. equilibrium responses to climate differ, species-level climate response curves, which result from the net effect of fast and slow processes influencing the geographic distribution, may not be predictive of how individuals within populations across the distribution will respond to time-varying climate. The space-for-time substitution that underlies climate envelope forecasting may not be reliable if spatial patterns are driven by additional processes, operating on longer time scales, than those generating patterns through time.
It is important to understand which of these hypotheses better reflects real-world patterns of variation in individual performance, particularly because they lead to contrasting predictions about how species’ geographic distributions will be affected at near-term (transient) time scales by changing climate (Fig. 1 I–K). Assessing climate responses at the individual vs. species scale, and in response to spatial vs. temporal climate variation, has thus far been difficult because the data needs are high: long-term, annually resolved records of individual performance in response to climate variability throughout a species’ geographic distribution, in addition to the occurrence data that are much more available and commonly used. Spatial networks of biogenic time series from naturally occurring individuals are a special kind of data that make it possible to infer the plastic capacity of genotypes to tolerate different conditions in different years, as those genotypes are currently distributed across a species’ range (37). Past empirical studies that have compared individual- or population-level responses to climate against species-level responses instead have tended to focus on spatial variation in average performance with climate (17, 38–40) or performance measured at single point in time (41), not individual-scale plastic responses to time-varying climate, or they have been relatively limited in spatial and temporal scope (21, 42, 43).
Here, we evaluate climate responses across scales, where scale refers both to the species-level geographic distribution vs. individual organism, and to space vs. time. We compare a species’ occurrence against its individual-level performance with respect to climate and we investigate how individual-level performance varies with climate across space vs. time. We focus on a tree species because trees are positioned at the nexus of the biodiversity and climate crises—forests harbor a great deal of Earth’s biodiversity at the same time that they play an important role in the feedback between Earth’s terrestrial biosphere and its climate via carbon cycling (44, 45). In addition, many tree species form annual growth rings that can be sampled to generate time series data encompassing individuals’ lifespans (46). Specifically, we studied Pinus edulis, a tree at the dry edge of the temperate coniferous forest biome that is found under a wide range of temperature conditions [mean annual temperature (MAT) of 4 to 17 °C; SI Appendix, Fig. S3]. We used a tree-ring collection that is more unbiased and representative of this species than any other available to quantify individual-level responses to temporal variation in climate, spatial variation in average climate conditions, and their interactions. These climate responses based on tree-ring time series data were compared to separately inferred species-level climate responses based on occurrence data, i.e., the presence vs. absence of P. edulis in forest inventory plots across its distribution. If climate responses are the same at the individual and species scale, the slope of the response to time-varying temperature should change in sign across P. edulis’ distribution: from positive at cold locations to negative at warm locations (Fig. 1F). If instead climate responses differ between the individual and species scale, the sign of the slope of the response to time-varying climate could be the same throughout the species’ distribution. A priori, we expect P. edulis’ performance (growth) to be soil moisture limited; hence, we expect to see lower-than-average growth in warmer-than-average years—a negative response to time-varying temperature (Fig. 1 D and G). We also considered spatial variation in the response to precipitation variability, and how precipitation modifies the response to variation in temperature, since soil moisture limitation is influenced by their combination.
Results
Tree-ring data confirm the a priori expectation that P. edulis’ performance is soil moisture-limited. Growth rings of P. edulis are wider in a wetter-than-average year and at wetter locations: We found positive effects of interannual variation in winter precipitation [βwinter precip = 0.2045, 95% CI = (0.1942 − 0.2149)] and monsoon precipitation [βmonsoon precip = 0.0451, 95% CI = (0.0348 − 0.0533)], as well as mean annual precipitation (MAP) on growth-ring width [βMAP = 0.2355, 95% CI = (0.194 − 0.2771); SI Appendix, Fig. S4]. In addition, year-to-year variation in fall and spring temperatures negatively affects growth-ring width [Fig. 2; βfall temp = −0.0723, 95% CI = (−0.0826 − −0.0618), βspring temp = −0.0965, 95% CI = (−0.1076 − −0.0857)].
Fig. 2.
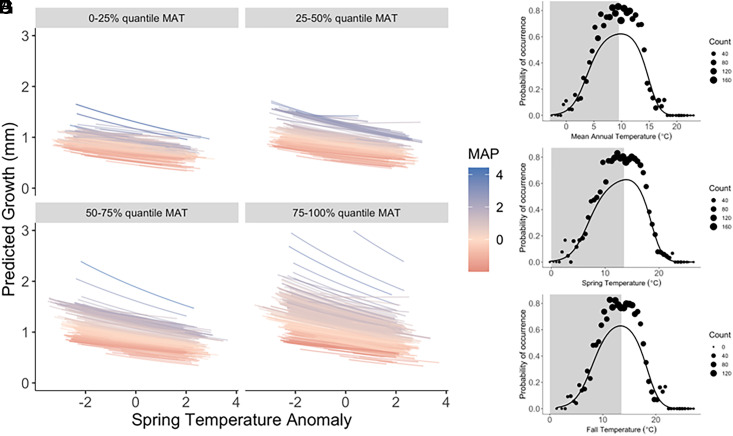
Responses to temperature variation. (A–D) The model-predicted responses to spring (April–June) temperature anomalies of all 1,558 P. edulis trees in the dataset, at locations that vary from cool to warm [panels (A–D) show individual tree responses grouped by quantiles of mean annual temperature (MAT), averaged over the period 1895 to 2018], with each response colored by the mean annual precipitation (MAP) at that location, from dry (red) to wet (blue). Responses are plotted for a constant tree size of 20 cm. (E–G) Species-scale climate responses to spatial variation in temperature—the probability of occurrence of P. edulis as a function of average climate conditions at FIA plot locations. Shading in (D–F) indicates that part of the species’ climate envelope where the individual-level response in (A–D) is opposite in sign.
Of particular interest, with respect to the hypotheses and predictions in Fig. 1, is how P. edulis’ responses to time-varying climate shift across gradients of mean annual temperature (MAT) and MAP. Five of the eight interactions between spatially varying MAT and MAP and time-varying climate predictors are significantly different from zero (SI Appendix, Fig. S4), but these interactions do not change the sign of the effect across the geographic distribution. The sensitivity of P. edulis’ growth to spring and fall temperature variability is negative essentially everywhere, from cool to warm sites, and dry to wet sites (Fig. 2 A–D and SI Appendix, Fig. S5; see histograms of tree-level climate sensitivities in SI Appendix, Fig. S6 A and B). P. edulis’ model-predicted sensitivity to year-to-year precipitation variability is also (nearly) uniformly positive (Fig. 3 A–D and SI Appendix, Fig. S7; see histograms in SI Appendix, Fig. S6 C and D). The statistically significant interaction effects do however indicate there is variation across P. edulis’ distribution in how sensitive its performance is to time-varying climate: Lower growth in response to a warmer-than-average spring is especially pronounced at the wet and warm edge of the species’ distribution (blue lines, Fig. 2D), and lower growth in response to a drier-than-average winter is especially pronounced at the dry and warm edge of the species’ distribution (red lines, Fig. 3D).
Fig. 3.
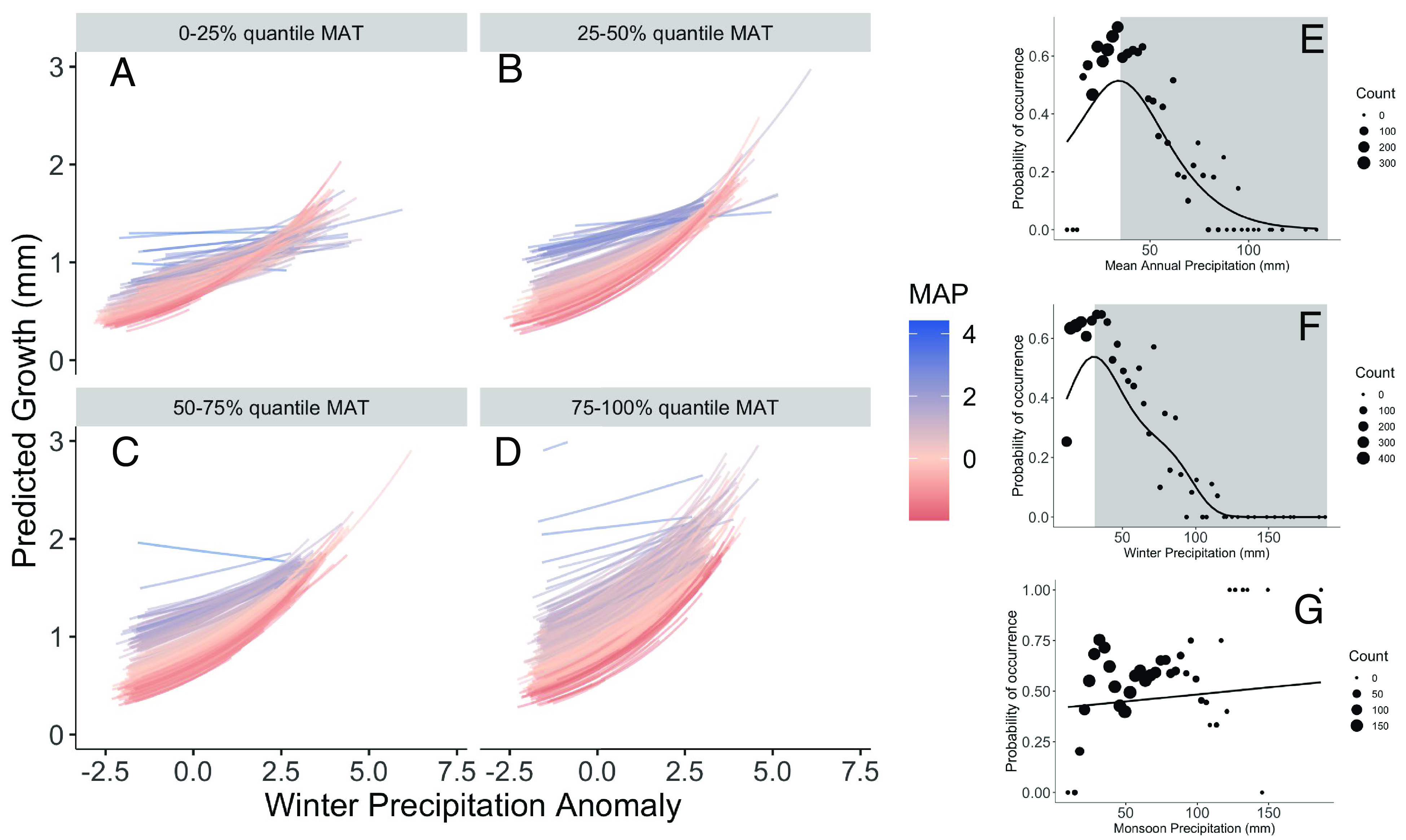
Responses to precipitation variation. (A–D) The model-predicted responses to winter precipitation anomalies of all 1,558 common pinon trees in the dataset, at locations that vary from cool to warm [panels (A–D) show individual tree responses grouped by quantiles of mean annual temperature (MAT), averaged over the period 1895 to 2018], with each response colored by the mean annual precipitation (MAP) at that location, from dry (red) to wet (blue). Winter precipitation is the cumulative total from November of the previous year through March of the current year. Responses are plotted for a constant tree size of 20 cm. (E–G) Species-scale climate responses to spatial variation in precipitation—the probability of occurrence of P. edulis as a function of average climate conditions at FIA plot locations. Shading in (E and F) indicates that part of the species’ climate envelope where the individual-level response in (A-D) is opposite in sign.
For five of the six climate variables, species-level climate response curves based on presence–absence data were very different from the individual tree-scale climate responses detected using tree-ring data. Probability of occurrence of P. edulis increases then decreases with each of the temperature variables (mean annual temperature, mean spring temperature, and mean fall temperature), i.e., in a unimodal, symmetric pattern (Fig. 2 E–G). P. edulis’ occurrence in response to spatial variation in mean annual precipitation and average winter precipitation peaks at relatively low values (dry locations) and declines across most of the range of each of these precipitation variables in the study domain, leading to right-skewed responses (Fig. 3 E and F). Only one climate response was qualitatively similar at the species vs. individual scale: Both probability of occurrence (Fig. 3G) and tree-scale growth (SI Appendix, Fig. S7) increase with monsoon precipitation. Counter to the a priori expectation that growth should be lower at the warm edge of the distribution of a soil moisture-limited species, growth increased with mean annual temperature across P. edulis’ distribution [βMAT = 0.2083, 95% CI = (0.1661 – 0.2506), SI Appendix, Fig. S4], a result that we interpret in terms of Liebig’s Law of the Minimum (SI Appendix, Climate Responses and Fig. S8). That is, the sign of the response of individual tree-scale growth to spatial variation in temperature (positive) is the opposite of its response to in situ time-varying temperature (negative).
Discussion
This analysis of tree-ring and occurrence data for P. edulis demonstrates that its climate responses are scale dependent—different patterns are observed at the species vs. individual scale, and in response to spatial vs. temporal climate variability. Across the entire geographic distribution of P. edulis, trees respond negatively to warmer-than-average spring and fall temperatures (Fig. 2 A–D and SI Appendix, Fig. S5, respectively), whereas species-scale temperature tolerances, inferred across space from occurrence data, are unimodal and symmetric (Fig. 2 E–G). Further, instead of observing peak performance at climatically average locations (i.e., average MAT), average growth-ring width increases with MAT. Trees grow faster at warmer-than-average locations, even though they grow less in warmer-than-average years across the entire distribution. The contrast between species-level and individual-level responses to variation in precipitation is also striking: Individuals respond positively to more precipitation, across both time and space (Fig. 3 A–D and SI Appendix, Fig. S7), as would be expected for a soil moisture-limited species, whereas the species-level probability of occurrence is mostly a declining function of spatial variation in precipitation (Fig. 3 E and F).
Individual growth rate is but one part of the life cycle; hence, the mismatch between climate responses inferred from tree-ring vs. occurrence data might be caused by vital rates other than growth (survival, recruitment) responding to climate variability in a compensatory manner (“demographic compensation”; 47). However, demographic analyses of forest inventory data have shown that P. edulis’ population growth rate is lowest at warm and dry sites, particularly because of a negative effect of spatial variation in temperature on survival, which is by far the strongest driver of variation in population growth rate across this species’ distribution (3). In addition, there is a large literature showing that warm drought events cause tree mortality (36, 48, 49), with well-documented physiological causes (cavitation, hydraulic failure; 50–52). Because the response of P. edulis’ survival to both spatial and temporal variation in temperature is negative, and because the sensitivity of population growth rate to recruitment is so weak in a long-lived organism, demographic compensation is not a plausible explanation for the observed mismatch between the responses of individual-scale growth vs. species-scale occurrence to climate variability.
An alternative explanation for contrasting responses of individual- and population-level demographic rates vs. species-level occurrence to climate variation is that climate is not the only factor influencing P. edulis’ geographic distribution. Demographic analyses have shown that the wet and cool limits of P. edulis’ distribution, where climatic conditions are good both for individual-level performance and population-level fitness, result from the influence of climate on vegetation and fire regime (3), combined with the fact that P. edulis is fire-intolerant. With increasing mean annual precipitation and decreasing mean annual temperature, hence increased productivity of herbaceous fine fuels, P. edulis is replaced by its congener Pinus ponderosa, a species that is fire-tolerant (53, 54). That is, climate does not limit the wet and cool edge of P. edulis’ distribution directly, it does so indirectly through its influence on an ecological disturbance process (fire). Fire suppression in the 20th century and concomitant expansion of fire-intolerant P. edulis further demonstrates that climate is not the only factor shaping this species’ abundance and geographic distribution (3). The case of P. edulis highlights the much more general (and well-known) problem of causality embedded in space-for-time substitution and climate envelope forecasting (22, 23)—just because climate can be correlated with occurrence does not mean that it (directly) is the causal factor that determines range limits.
We have shown that in P. edulis, individual-scale climate responses (based on time-series data) are the opposite of species-scale climate responses (based on occurrence data) for approximately half the species’ distribution with respect to MAT (shading, Fig. 2E) and the majority of the distribution with respect to MAP (shading, Fig. 3E). The broader implications of this are troubling: The organism–environment relationships that are central to forecasting the impact of climate change on biodiversity are scale dependent, compromising the assumption that climate responses at the species scale are predictive of climate responses at the individual scale. This begs the question, how widespread are the patterns observed in P. edulis?
Previous studies of tree-ring data offer some insight, even if they were not designed to address this question. Canham et al. (55) found ubiquitous negative temperature sensitivity using >23,000 tree-ring time series sampled from 14 dominant tree species of temperate deciduous forests across the northeastern United States. Ubiquitous negative temperature sensitivity was also found by Klesse et al. (56), based on >30,000 tree-ring time series for Douglas-fir (Pseudotsuga menziesii), a species with a geographic distribution spanning 36 degrees of latitude in western North America. Climate sensitivities consistent with soil moisture-limitation of tree growth (negative temperature sensitivity, positive precipitation sensitivity) were also found across the geographic distribution of P. ponderosa, another widely distributed tree of western North America (57). Northern hemisphere and global analyses of tree-ring data show that tree growth is negatively sensitive to summer temperature variability across the midlatitudes and positively sensitive to summer temperature variability at high-latitude and high-elevation sites (30, 31). In other words, there is a striking contrast between what is well-established in dendrochronology (and ecosystem ecology, earth system sciences)—that climate limitation of tree growth (and terrestrial ecosystem productivity) is consistent, or largely so, across broad geographic extents—vs. the expectation embedded in occurrence-based forecasting that climate limitations should switch in sign across every species’ distribution.
Other tree-ring studies also found contrasting responses to spatial vs. temporal variation in temperature as we did. Canham et al. (55) found that 14 species of temperate deciduous trees grow at a higher rate at more southerly (warmer) locations, in contrast to their ubiquitous negative sensitivity to interannual temperature variability. Klesse et al. (56) also found this pattern, across the very large distribution of Douglas-fir. Because of this switch in sign, forecasts made based on the relationship between time-averaged tree growth and spatial variation in temperature would suggest these forests should benefit from warming, whereas forecasts based on the relationship between time-varying performance and temperature would suggest the opposite. A unique study of P. ponderosa demonstrated just this: Forecasts based on the species’ response to spatial variation in temperature predict increased tree growth, whereas forecasts made based on population-level responses to time-varying temperature predict reduced growth (58; and for an example from grasslands, see ref. 59). Further, model validation via hindcasting showed that ponderosa pine’s observed responses to climate variability and change in the recent past were much better predicted by its statistically inferred response to time-varying temperature (58). This suggests forecasts of increased forest productivity based on spatial patterns of climate variability should be viewed with caution (e.g., refs. 60 and 61). The larger point is to be aware, when making ecological forecasts of any kind, that organism-environment relationships are likely to be scale-dependent (13, 62, 63).
Beyond tree-ring time series data, there is a growing body of evidence of contrasting climate responses at different biological scales or across space vs. time, including mismatches between a species’ peak occurrence compared to its peak abundance, population growth rate, or individual-level performance, detailed in SI Appendix, Table S1. This evidence comes from multispecies surveys of the literature as well as original studies contradicting the abundant center and center-periphery hypotheses (17, 21, 38–40), along with more detailed studies of demographic variation across space and time showing that individual- and population-scale responses to climate variation do not match occurrence-based, species-scale climate responses (64, 65). This emerging body of work suggests that our findings are not a pattern unique to a single aridland pine species.
At the same time, it should be expected that some species’ distributions do span conditions under which performance is negatively vs. positively sensitive to time-varying temperature (or other performance-limiting and nonstationary aspects of climate). While we dichotomized the possibilities in Fig. 1 for the sake of clarity, in fact, a continuum of patterns between these extremes is possible. Spatial variation in the response to interannual variation in temperature was found in Artemisia tridentata (a dominant shrub in a shrub-steppe ecosystem) based on time series data at 131 monitoring sites across its distribution—negative responses at warm locations and positive responses at cold locations (66). A similar pattern was found with respect to some climate variables but not others, based on repeat censuses of 746 populations of wood frogs (Lithobates sylvaticus) at 27 locations across its distribution—a switch from positive to negative sensitivity of demographic performance to interannual variation in climate (18). We suggest that a key knowledge gap to be filled is the characterization of individual- and population-scale responses to time-varying climate as opposed to species-scale “climate envelopes,” with a focus on the biogeography of limiting factors, to better anticipate the near-term impact of climate change on biodiversity.
A Critical Transition: From Near-Term to Long-Term Dynamics.
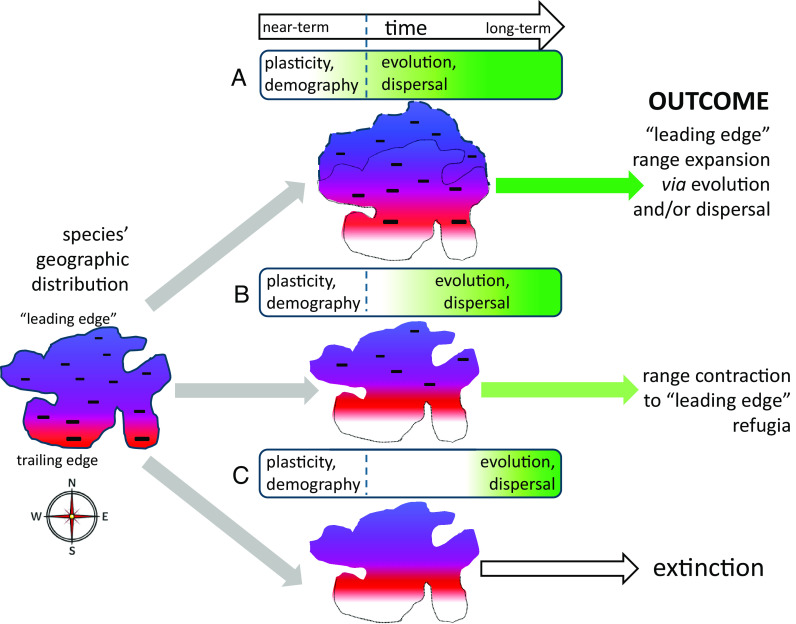
For P. edulis and other species in which performance is consistently lower in warmer-than-average years, the entire distribution is a “trailing edge” when faced with warming: All populations should experience decreased performance. The risk, hidden by the correlative, equilibrium nature of climate envelope forecasting, is that this short-term (negative) response is not replaced by the long-term (positive) response at what the occurrence-based approach identifies as the “leading edge,” as illustrated in Fig. 4. Whether negative transient vs. positive equilibrium dynamics prevail at the “leading edge” should be expected to depend on the breadth of plasticity (i.e., reaction norms, thermal performance curves) relative to the pace of climate change, the pace of in situ evolution of reaction norms, and the pace of migration of genotypes with better-adapted reaction norms from elsewhere (Fig. 4; 11, 13, 14, 24, 67–70). If rates of evolution and/or dispersal keep pace with climate change, a smooth transition from negative transient to positive equilibrium dynamics is possible (Fig. 4A). If evolution and/or dispersal are slower than climate change, but not too much slower, there may be range contraction to leading-edge refugia, but potentially recovery (Fig. 4B). In P. edulis, for example, population growth rate is least sensitive to change in temperature and precipitation at the climatically benign, cool, wet (high-elevation) edge of its distribution (3)—i.e., the “leading edge.” There, climate change-driven decline should be less rapid, and there is the potential for evolutionary rescue. If in situ evolution and/or dispersal of better-adapted genotypes are too slow compared to climate change, extinction may be the outcome, rather than the equilibrium expectation of persistence and expansion at the leading edge (Fig. 4C).
Fig. 4.
Three possible outcomes, in response to warming, for a northern hemisphere species in which individual-level performance is negatively sensitive to time-varying temperature (indicated with negative symbols throughout the geographic distribution). The species is more strongly negatively impacted by warming toward its warm, southern (trailing) edge, indicated by the size of the symbols. The (A) best-case, (B) middle-case, and (C) worst-case scenarios differ with respect to how fast evolution and/or dispersal (green shading) are relative to the rate of changing climate, leading to outcomes that reflect little to strong influence of transient dynamics, respectively. Evolution and dispersal do not necessarily operate on the same time scale but are grouped here as “slow” processes compared to “fast” processes (plasticity and demography).
We conclude that occurrence-based models can underestimate the threat to biodiversity posed by changing climate, in that species-scale climate tolerances can be a poor proxy for individual- and population-scale climate responses, and fail to capture the transient risk of extinction arising from the actual responses of individuals and populations to climate variability and change. A strong reliance in the biodiversity forecasting community on occurrence-based approaches may create a blind spot regarding the possibility that performance and suitability may actually decrease with changing climate at the so-called “leading edge” of a species’ distribution. The expectation of expansion at the “leading edge” can be a false one, at least in the near term, and failure to expand there may not be caused by dispersal limitation, rather, it can be limited by the evolution of individual-scale climate tolerances. At the same time, there may be other ways in which occurrence-based approaches overestimate extinction risk—for example, by overfitting to occurrence data using many climate predictors and very flexible models.
For biodiversity forecasting to improve, i.e., address potential under- and overestimation of extinction risk, there is a need to characterize and better understand responses of individuals and populations to time-varying climate. This includes, for example, expanding studies of thermal performance curves to encompass genotypes sampled across species’ distributions (26, 37, 71), quantifying how climate-performance relationships are modified by the intensity and duration of acute exposures such as heat waves (72–74), and leveraging transplant experiments to parse the genetic vs. plastic basis of responses to climate variability and extremes (28, 75–77). Studies of demography in the wild across time and space also are a powerful source of information (64, 65, 78, 79), because they can reveal trade-offs between fitness in different parts of the life cycle (e.g., competitiveness and hence individual growth rate vs. stress-tolerance and hence survival rate) and the impact of shifting biotic interactions, community composition, and ecosystem processes. These data will provide the grounding in physiology and demography needed to more reliably scale the impact of climate change from individuals to species (33, 63, 80–84). With the accumulation of such data across species and environments, patterns may emerge that further facilitate prediction. For example, it’s been suggested that individual- vs. species-scale thermal tolerances are more closely equivalent in marine ectotherms than terrestrial ectotherms (85), and that individual-scale thermal tolerances are narrow in tropical terrestrial ectotherms, heightening their vulnerability to climate change (86–88). In addition, there is a need to better characterize rates of evolution and dispersal compared to changing climate, and how these interact with other global change drivers (e.g., land transformation and fragmentation), to gauge the timescales at which range dynamics may shift from transient, individual-scale responses to equilibrium, species-scale responses. Thirty years ago, Levin (89) remarked that “the problem of pattern and scale is the central problem in ecology.” Addressing this central problem in ecology is key to better assessing the risk of biodiversity loss posed by climate change, the disruption of ecosystem services associated with that loss, and acting to prevent it.
Materials and Methods
P. edulis Engelm. is a stress-tolerant pine endemic to the Colorado Plateau of the southwestern United States, where the climate is semiarid and continental (90; SI Appendix, Fig. S3). Our study is based on tree-ring samples collected from 1,558 trees located in 977 plots in the U.S. Forest Service’s probabilistically designed Forest Inventory and Analysis (FIA) plot network, which fully encompasses P. edulis’ geographic distribution (91). We processed these samples to generate annually resolved time series of growth-ring widths following standard protocols of dendrochronology (SI Appendix, Tree-Ring Data). We then used Bayesian hierarchical regression to model variation in growth-ring widths as a function of climate variability across space and time. Climate variables were derived from 4-km resolution PRISM monthly data (92), i.e., aggregated to climate normals (MAT, MAP; years 1895 to 2018), which vary strictly across space, and time-varying seasonal climate variables (monsoon and winter precipitation, fall and spring temperature). The seasonal climate time series were locally scaled and centered; hence, they represent site-specific anomalies of precipitation and temperature. Tree size (stem diameter at root collar) was also included as a predictor because growth-ring widths are known to change with tree size. We included all 2-way interactions between fixed effects, including interactions between spatially varying climate normals (cold vs. warm locations) and time-varying climate (e.g., spring temperature), which we use to distinguish between hypotheses 1 vs. 2 of Fig. 1. Additional details about the regression modeling are available in SI Appendix, including Bayesian model implementation, evaluation of model convergence, and comparison of the fit to data of nine alternative regression models (SI Appendix, Table S2 and Fig. S9).
Using generalized additive models (GAMs), we quantified the probability of occurrence of P. edulis as a function of the same climate variables used to predict individual tree growth variability. We used data on the presence vs. absence of P. edulis derived from the FIA plot network in Arizona, Colorado, New Mexico, and Utah. Hence, the occurrence of P. edulis was assessed relative to the forested portion of the study domain (defined by FIA as 10% tree cover). We tested GAMs with 3, 4, and 5 knots to evaluate the influence of model flexibility on climate response curves (SI Appendix, Table S3). Scripts for all data analysis are found in a public GitHub repository (93).
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
We benefited from the critical feedback of the following: Daniel Perret, Courtney Giebink, Jaime Yazzie, Peter Adler, Michael Stemkovski, Annie Schiffer, Michael Donoghue, Deborah Goldberg, and Pieter Zuidema. We thank Tyson Swetnam for assistance with CyVerse computing resources, which are supported by US NSF awards DBI-0735191, DBI-1265383, and DBI-1743442. Our thanks also go to Nicole Antebi for assistance in producing Fig. 1. M.E.K.E., K.A.H., and S. M. N. D. were supported by US NSF award DBI-1802893. In addition, M.E.K.E. benefitted from participation in a working group on ecosystem acclimation supported by US NSF DBI-2225103. J.R.T. was supported by the U.S. Department of Energy through the Los Alamos National Laboratory. Los Alamos National Laboratory is operated by Triad National Security, LLC, for the National Nuclear Security Administration of U.S. Department of Energy (Contract No. 89233218CNA000001). S.K. was supported by SNF Sinergia project CALDERA, no. 183571. R.J.D. was supported by the Utah Agricultural Experiment Station, Utah State University, Logan, Utah, and this contribution is approved as journal paper no. 9650. This article was prepared in part by employees of the USDA Forest Service as part of official duties and is therefore in the public domain. The findings and conclusions in this publication are those of the authors and should not be construed to represent any official USDA or US government determination or policy.
Author contributions
M.E.K.E., S.M.N.D., K.A.H., and J.R.T. designed research; S.M.N.D., K.A.H., and J.R.T. performed research; M.E.K.E., S.M.N.D., K.A.H., J.R.T., and E.L.S. analyzed data; M.E.K.E., K.A.H., and E.L.S. supervision; S.M.N.D., R.J.D., E.L.S., and J.D.S. generated data; and M.E.K.E., S.M.N.D., K.A.H., J.R.T., R.J.D., S.K., E.L.S., and J.D.S. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
Tree-ring time series data have been deposited in CyVerse (DOI: 10.25739/7c3a-z340) (91). Previously published PRISM climate data were used in this work (92). Scripts for conducting all aspects of the data analysis are available in the GitHub repository (https://github.com/dey3434/PIED-Project) (93).
Supporting Information
References
- 1.Merriam C. H., The Geographic Distribution of Animals and Plants in North America, Merriam C. H., Ed. (US Dept of Agriculture, 1895), pp. 203–214. [Google Scholar]
- 2.von Humboldt A., Bonpland A., Jackson S. T., Romanowski S., Essay on the Geography of Plants (University of Chicago Press, 2008). [Google Scholar]
- 3.Schultz E. L., et al. , Climate-driven, but dynamic and complex? A reconciliation of competing hypotheses for species’ distributions. Ecol. Lett. 25, 38–51 (2022). [DOI] [PubMed] [Google Scholar]
- 4.Thomas C. D., et al. , Extinction risk from climate change. Nature 427, 5 (2004). [DOI] [PubMed] [Google Scholar]
- 5.Pörtner H.-O., et al. , IPBES-IPCC co-sponsored workshop report on biodiversity and climate change. Zenodo. 10.5281/ZENODO.4782538. Accessed 7 June 2022. [DOI] [Google Scholar]
- 6.Urban M. C., Accelerating extinction risk from climate change. Science 348, 571–573 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Sexton J. P., McIntyre P. J., Angert A. L., Rice K. J., Evolution and ecology of species range limits. Annu. Rev. Ecol. Evol. Syst. 40, 415–436 (2009). [Google Scholar]
- 8.Franklin J., Mapping Species Distributions: Spatial Inference and Prediction Cambridge University Press, Cambridge, UK, 2010). [Google Scholar]
- 9.Guisan A., et al. , Making better biogeographical predictions of species’ distributions. J. Appl. Ecol. 43, 386–392 (2006). [Google Scholar]
- 10.Zurell D., Jeltsch F., Dormann C., Schröder B., Static species distribution models in dynamically changing systems: How good can predictions really be? Ecography 32, 733–744 (2009). [Google Scholar]
- 11.Chevin L.-M., Lande R., Mace G. M., Adaptation, plasticity, and extinction in a changing environment: Towards a predictive theory. PLoS Biol. 8, e1000357 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Svenning J.-C., Sandel B., Disequilibrium vegetation dynamics under future climate change. Am. J. Bot. 100, 1266–1286 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Adler P. B., White E. P., Cortez M. H., Matching the forecast horizon with the relevant spatial and temporal processes and data sources. Ecography 43, 1729–1739 (2020). [Google Scholar]
- 14.Williams J. W., Ordonez A., Svenning J.-C., A unifying framework for studying and managing climate-driven rates of ecological change. Nat. Ecol. Evol. 5, 17–26 (2021). [DOI] [PubMed] [Google Scholar]
- 15.Lenoir J., Svenning J.-C., Climate-related range shifts—a global multidimensional synthesis and new research directions. Ecography 38, 15–28 (2015). [Google Scholar]
- 16.Pelini S. L., et al. , Translocation experiments with butterflies reveal limits to enhancement of poleward populations under climate change. Proc. Natl. Acad. Sci. U.S.A. 106, 11160–11165 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGill B. J., Trees are rarely most abundant where they grow best. J. Plant Ecol. 5, 46–51 (2012). [Google Scholar]
- 18.Amburgey S. M., et al. , Range position and climate sensitivity: The structure of among-population demographic responses to climatic variation. Glob. Change Biol. 24, 439–454 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Gaüzère P., Devictor V., Mismatches between birds’ spatial and temporal dynamics reflect their delayed response to global changes. Oikos 130, 1284–1296 (2021). [Google Scholar]
- 20.Brown J. H., On the relationship between abundance and distribution of species. Am. Nat. 124, 255–279 (1984). [Google Scholar]
- 21.Pironon S., et al. , Geographic variation in genetic and demographic performance: New insights from an old biogeographical paradigm. Biol. Rev. 92, 1877–1909 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Damgaard C., A critique of the space-for-time substitution practice in community ecology. Trends Ecol. Evol. 34, 416–421 (2019). [DOI] [PubMed] [Google Scholar]
- 23.Lovell R. S. L., Collins S., Martin S. H., Pigot A. L., Phillimore A. B., Space-for-time substitutions in climate change ecology and evolution. Biol. Rev. 98, 2243–2270 (2023). [DOI] [PubMed] [Google Scholar]
- 24.Stillman J. H., Acclimation capacity underlies susceptibility to climate change. Science 301, 65–65 (2003). [DOI] [PubMed] [Google Scholar]
- 25.Pörtner H. O., Farrell A. P., Physiology and climate change. Science 322, 690–692 (2008). [DOI] [PubMed] [Google Scholar]
- 26.Angert A. L., Sheth S. N., Paul J. R., Incorporating population-level variation in thermal performance into predictions of geographic range shifts. Integr. Comp. Biol. 51, 733–750 (2011). [DOI] [PubMed] [Google Scholar]
- 27.Martínez B., Arenas F., Trilla A., Viejo R. M., Carreño F., Combining physiological threshold knowledge to species distribution models is key to improving forecasts of the future niche for macroalgae. Glob. Change Biol. 21, 1422–1433 (2015). [DOI] [PubMed] [Google Scholar]
- 28.Bennett S., Wernberg T., Arackal Joy B., de Bettignies T., Campbell A. H., Central and rear-edge populations can be equally vulnerable to warming. Nat. Commun. 6, 10280 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Churkina G., Running S. W., Contrasting climatic controls on the estimated productivity of global terrestrial biomes. Ecosystems 1, 206–215 (1998). [Google Scholar]
- 30.George S. St., Ault T. R., The imprint of climate within Northern Hemisphere trees. Q. Sci. Rev. 89, 1–4 (2014). [Google Scholar]
- 31.Wilmking M., et al. , Global assessment of relationships between climate and tree growth. Glob. Change Biol. 26, 3212–3220 (2020). [DOI] [PubMed] [Google Scholar]
- 32.Breshears D., et al. , The critical amplifying role of increasing atmospheric moisture demand on tree mortality and associated regional die-off. Front. Plant Sci. 4, 266 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palmquist K. A., et al. , Divergent climate change effects on widespread dryland plant communities driven by climatic and ecohydrological gradients. Glob. Change Biol. 27, 5169–5185 (2021). [DOI] [PubMed] [Google Scholar]
- 34.Intergovernmental Panel on Climate Change (IPCC), Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change (Cambridge University Press, 2021), 10.1017/9781009157896. [DOI] [Google Scholar]
- 35.Ruthrof K. X., et al. , Subcontinental heat wave triggers terrestrial and marine, multi-taxa responses. Sci. Rep. 8, 13094 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hammond W. M., et al. , Global field observations of tree die-off reveal hotter-drought fingerprint for Earth’s forests. Nat. Commun. 13, 1761 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morrongiello J. R., Thresher R. E., Smith D. C., Aquatic biochronologies and climate change. Nat. Clim. Change 2, 849–857 (2012). [Google Scholar]
- 38.Thuiller W., et al. , Does probability of occurrence relate to population dynamics? Ecography 37, 1155–1166 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bohner T., Diez J., Extensive mismatches between species distributions and performance and their relationship to functional traits. Ecol. Lett. 23, 33–44 (2019). [DOI] [PubMed] [Google Scholar]
- 40.Midolo G., Wellstein C., Faurby S., Individual fitness is decoupled from coarse-scale probability of occurrence in North American trees. Ecography 44, 789–801 (2021). [Google Scholar]
- 41.Chakraborty D., Schueler S., Lexer M. J., Wang T., Genetic trials improve the transfer of Douglas-fir distribution models across continents. Ecography 42, 88–101 (2019). [Google Scholar]
- 42.Baer K. C., Maron J. L., Ecological niche models display nonlinear relationships with abundance and demographic performance across the latitudinal distribution of Astragalus utahensis (Fabaceae). Ecol. Evol. 10, 8251–8264 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diez J. M., Giladi I., Warren R., Pulliam H. R., Probabilistic and spatially variable niches inferred from demography. J. Ecol. 102, 544–554 (2014). [Google Scholar]
- 44.Arora V. K., et al. , Carbon–concentration and carbon–climate feedbacks in CMIP6 models and their comparison to CMIP5 models. Biogeosciences 17, 4173–4222 (2020). [Google Scholar]
- 45.Friedlingstein P., et al. , Global Carbon Budget 2020. Earth Syst. Sci. Data 12, 3269–3340 (2020). [Google Scholar]
- 46.Evans M. E. K., Black B. A., Falk D. A., Giebink C. L., Schultz E. L., "Growth rings across the tree of life: Demographic insights from biogenic time series data" in Demographic Methods across the Tree of Life, Salguero-Gomez R., Gamelon M., Eds. (Oxford University Press, 2021). [Google Scholar]
- 47.Doak D. F., Morris W. F., Demographic compensation and tipping points in climate-induced range shifts. Nature 467, 959–962 (2010). [DOI] [PubMed] [Google Scholar]
- 48.Anderegg W. R. L., Kane J. M., Anderegg L. D. L., Consequences of widespread tree mortality triggered by drought and temperature stress. Nat. Clim. Change 3, 30–36 (2013). [Google Scholar]
- 49.Allen C. D., Breshears D. D., McDowell N. G., On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere 6, art129 (2015). [Google Scholar]
- 50.McDowell N. G., Allen C. D., Darcy’s law predicts widespread forest mortality under climate warming. Nat. Clim. Change 5, 669–672 (2015). [Google Scholar]
- 51.Choat B., et al. , Triggers of tree mortality under drought. Nature 558, 531–539 (2018). [DOI] [PubMed] [Google Scholar]
- 52.Breshears D. D., et al. , Underappreciated plant vulnerabilities to heat waves. New Phytol. 231, 32–39 (2021). [DOI] [PubMed] [Google Scholar]
- 53.Keeley J. E., Evolution of life histories in pines. Ecol. Biogeogr. Pinus 69, 445–453 (1998). [Google Scholar]
- 54.He T., Pausas J. G., Belcher C. M., Schwilk D. W., Lamont B. B., Fire-adapted traits of Pinus arose in the fiery Cretaceous. New Phytol. 194, 751–759 (2012). [DOI] [PubMed] [Google Scholar]
- 55.Canham C. D., Murphy L., Riemann R., McCullough R., Burrill E., Local differentiation in tree growth responses to climate. Ecosphere 9, e02368 (2018). [Google Scholar]
- 56.Klesse S., et al. , Continental-scale tree-ring-based projection of Douglas-fir growth: Testing the limits of space-for-time substitution. Glob. Change Biol. 26, 5146–5163 (2020). [DOI] [PubMed] [Google Scholar]
- 57.McCullough I. M., Davis F. W., Williams A. P., A range of possibilities: Assessing geographic variation in climate sensitivity of ponderosa pine using tree rings. For. Ecol. Manag. 402, 223–233 (2017). [Google Scholar]
- 58.Perret D. L., Evans M. E. K., Sax D. F., A species’ response to spatial climatic variation does not predict its response to climate change. Proc. Natl. Acad. Sci. U.S.A. 121, e2304404120 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Felton A. J., et al. , Climate disequilibrium dominates uncertainty in long-term projections of primary productivity. Ecol. Lett. 25, 2688–2698 (2022). [DOI] [PubMed] [Google Scholar]
- 60.Weiskittel A. R., Crookston N. L., Rehfeldt G. E., Projected future suitable habitat and productivity of Douglas-fir in western North America. Schweiz. Z. Forstwes. 163, 70–78 (2012). [Google Scholar]
- 61.Wang J., Taylor A. R., D’Orangeville L., Warming-induced tree growth may help offset increasing disturbance across the Canadian boreal forest. Proc. Natl. Acad. Sci. U.S.A. 120, e2212780120 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Scholes R. J., Taking the Mumbo out of the jumbo: Progress towards a robust basis for ecological scaling. Ecosystems 20, 4–13 (2017). [Google Scholar]
- 63.Denny M., Benedetti-Cecchi L., Scaling up in ecology: Mechanistic approaches. Annu. Rev. Ecol. Evol. Syst. 43, 1–22 (2012). [Google Scholar]
- 64.DeMarche M. L., et al. , Latitudinal gradients in population growth do not reflect demographic responses to climate. Ecol. Appl. 31, e2242 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oldfather M. F., Koontz M. J., Doak D. F., Ackerly D. D., Range dynamics mediated by compensatory life stage responses to experimental climate manipulations. Ecol. Lett. 24, 772–780 (2021). [DOI] [PubMed] [Google Scholar]
- 66.Kleinhesselink A. R., Adler P. B., The response of big sagebrush (Artemisia tridentata) to interannual climate variation changes across its range. Ecology 99, 1139–1149 (2018). [DOI] [PubMed] [Google Scholar]
- 67.Etterson J. R., Shaw R. G., Constraint to adaptive evolution in response to global warming. Science 294, 151–154 (2001). [DOI] [PubMed] [Google Scholar]
- 68.Hoffmann A. A., Sgrò C. M., Climate change and evolutionary adaptation. Nature 470, 479–485 (2011). [DOI] [PubMed] [Google Scholar]
- 69.Bontrager M., et al. , Adaptation across geographic ranges is consistent with strong selection in marginal climates and legacies of range expansion. Evolution 75, 1316–1333 (2021). [DOI] [PubMed] [Google Scholar]
- 70.Buckley L. B., Kingsolver J. G., Evolution of thermal sensitivity in changing and variable climates. Annu. Rev. Ecol. Evol. Syst. 52, 563–586 (2021). [Google Scholar]
- 71.Kuo E. S. L., Sanford E., Geographic variation in the upper thermal limits of an intertidal snail: Implications for climate envelope models. Mar. Ecol. Prog. Ser. 388, 137–146 (2009). [Google Scholar]
- 72.Schulte P. M., Healy T. M., Fangue N. A., Thermal performance curves, phenotypic plasticity, and the time scales of temperature exposure. Integr. Comp. Biol. 51, 691–702 (2011). [DOI] [PubMed] [Google Scholar]
- 73.Kingsolver J. G., Woods H. A., Beyond thermal performance curves: Modeling time-dependent effects of thermal stress on ectotherm growth rates. Am. Nat. 187, 283–294 (2016). [DOI] [PubMed] [Google Scholar]
- 74.Williams C. M., et al. , Biological impacts of thermal extremes: Mechanisms and costs of functional responses matter. Integr. Comp. Biol. 56, 73–84 (2016). [DOI] [PubMed] [Google Scholar]
- 75.Housset J. M., et al. , Tree rings provide a new class of phenotypes for genetic associations that foster insights into adaptation of conifers to climate change. New Phytol. 218, 630–645 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Montwé D., Isaac-Renton M., Hamann A., Spiecker H., Cold adaptation recorded in tree rings highlights risks associated with climate change and assisted migration. Nat. Commun. 9, 1574 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.King N. G., Evidence for different thermal ecotypes in range centre and trailing edge kelp populations. J. Exp. Mar. Biol. Ecol. 514–515, 10–17 (2019). [Google Scholar]
- 78.DeMarche M. L., Doak D. F., Morris W. F., Both life-history plasticity and local adaptation will shape range-wide responses to climate warming in the tundra plant Silene acaulis. Glob. Change Biol. 24, 1614–1625 (2018). [DOI] [PubMed] [Google Scholar]
- 79.DeMarche M. L., Doak D. F., Morris W. F., Incorporating local adaptation into forecasts of species’ distribution and abundance under climate change. Glob. Change Biol. 25, 775–793 (2019). [DOI] [PubMed] [Google Scholar]
- 80.Tredennick A. T., et al. , Forecasting climate change impacts on plant populations over large spatial extents. Ecosphere 7, e01525 (2016). [Google Scholar]
- 81.Buckley L. B., Cannistra A. F., John A., Leveraging organismal biology to forecast the effects of climate change. Integr. Comp. Biol. 58, 38–51 (2018). [DOI] [PubMed] [Google Scholar]
- 82.Buckley L. B., Kingsolver J. G., Environmental variability shapes evolution, plasticity and biogeographic responses to climate change. Glob. Ecol. Biogeogr. 28, 1456–1468 (2019). [Google Scholar]
- 83.Lasky J. R., Hooten M. B., Adler P. B., What processes must we understand to forecast regional-scale population dynamics? Proc. R. Soc. B Biol. Sci. 287, 20202219 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Louthan A. M., DeMarche M. L., Shoemaker L. G., Climate sensitivity across latitude: Scaling physiology to communities. Trends Ecol. Evol. 36, 931–942 (2021). [DOI] [PubMed] [Google Scholar]
- 85.Sunday J. M., Bates A. E., Dulvy N. K., Thermal tolerance and the global redistribution of animals. Nat. Clim. Change 2, 686–690 (2012). [Google Scholar]
- 86.Deutsch C. A., et al. , Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl. Acad. Sci. U.S.A. 105, 6668–6672 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tewksbury J. J., Huey R. B., Deutsch C. A., Putting the heat on tropical animals. Science 320, 1296–1297 (2008). [DOI] [PubMed] [Google Scholar]
- 88.Huey R. B., et al. , Predicting organismal vulnerability to climate warming: Roles of behaviour, physiology and adaptation. Philos. Trans. R. Soc. B Biol. Sci. 367, 1665–1679 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Levin S. A., The problem of pattern and scale in ecology: The Robert H. Macarthur award lecture. Ecology 73, 1943–1967 (1992). [Google Scholar]
- 90.Miller R. F., et al. The ecology, history, ecohydrology, and management of pinyon and juniper woodlands in the Great Basin and Northern Colorado Plateau of the western United States. Gen Tech Rep RMRS-GTR-403 Fort Collins CO US Dep. Agric. For. Serv. Rocky Mt. Res. Stn. 403, 284 (2019). [Google Scholar]
- 91.Evans M. E. K., et al. , Pinus edulis ring-width data. CyVerse Data Commons. 10.25739/7c3a-z340. Deposited 29 April 2024. [DOI]
- 92.PRISM Climate Group, PRISM Climate Data. https://prism.oregonstate.edu. Accessed 16 December 2020.
- 93.Dey S. M. N., Heilman K. A., Tipton J. R., Evans M. E. K., project Pinus edulis: Bayesian regression analysis of tree-ring data. GitHub. https://github.com/dey3434/PIED-Project. Created 1 September 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
Tree-ring time series data have been deposited in CyVerse (DOI: 10.25739/7c3a-z340) (91). Previously published PRISM climate data were used in this work (92). Scripts for conducting all aspects of the data analysis are available in the GitHub repository (https://github.com/dey3434/PIED-Project) (93).