Abstract
Introduction
Chronic kidney disease of uncertain etiology (CKDu) is an incompletely defined phenotype of chronic kidney disease (CKD) affecting young individuals mostly in agricultural communities in Central America and South Asia. CKDu is a diagnosis of exclusion made in individuals from endemic regions.
Methods
We conducted a systematic review of the primary literature on urinary and plasma kidney injury biomarkers measured in the setting of CKDu (through February 2023). The literature was identified via a Web of Science search and hand search of the references of previously identified literature. Search terms included “CKDu,” “Mesoamerican Nephropathy,” “CKD of unknown etiology,” “Chronic Interstitial Nephritis in Agricultural Communities,” “biomarker,” “urin∗,” and/or “plasma.”
Results
A total of 25 papers were included. The 2 most frequently measured biomarkers were urinary kidney injury molecule-1 (KIM-1) and urinary neutrophil gelatinase-associated lipocalin (NGAL). There was substantial variability in study design, laboratory assay methods, and statistical methodology, which prohibited meta-analysis.
Conclusion
Biomarkers that identify tubulointerstitial disease early and accurately may substantially accelerate progress in the study of CKDu and facilitate public health approaches that eventually lead to its prevention and elimination. To date, the literature is limited by relatively small sample sizes and methodological limitations which should be addressed in future studies.
Keywords: biomarkers, chronic kidney disease of uncertain etiology, CKDu, kidney injury, Mesoamerican nephropathy
Graphical abstract
CKDu is an incompletely defined phenotype of CKD affecting predominantly young workers in rural communities of Central America and South Asia.1, 2, 3, 4, 5 CKDu is a progressive tubulointerstitial nephropathy, and its cause or causes are unknown. Potential causes include environmental or occupational exposure to nephrotoxins, heat stress, infection, and genetic predisposition.6, 7, 8 The disease typically affects individuals younger than 50 years, men, and those who engage in strenuous manual labor; several regions, particularly in India and Sri Lanka, report similar disease rates in women. CKDu—or a similar disease process—can also occur in nonagricultural communities.9 Rapid decline in kidney function and early mortality in CKDu have devastating effects on families and communities.5,7
The current working definition of CKDu is an estimated glomerular filtration rate (eGFR) <60 ml/min per 1.73 m2 in the absence of type 2 diabetes, longstanding hypertension, heavy proteinuria, or a known underlying syndrome (e.g., polycystic kidney disease, glomerulonephritis, and congenital disorder).1,2 Kidney biopsies in individuals from endemic regions suggest a tubulointerstitial disease process.10 Considering that eGFR <60 ml/min per 1.73 m2 lacks specificity for pathophysiology, there is no precise definition of CKDu, and it remains essentially a diagnosis of exclusion.10,11 Novel plasma or urinary biomarkers of kidney disease could potentially improve the evaluation and diagnosis of CKDu. Ideally, a biomarker would distinguish CKDu from other CKD phenotypes and identify the disease early in its development, allowing for more effective screening and the introduction of therapies to prevent disease progression. Despite a growing interest in using biomarkers to phenotype disease, there are no validated CKDu-specific biomarkers.
The evidence to date for biomarkers in CKDu is predominantly from cross-sectional analyses of relatively small samples in endemic regions.12 Biomarker studies in other forms of CKD are heavily skewed toward cohorts in the United States comprised of individuals with traditional CKD risk factors.13 Although several investigators have identified biomarkers that hold promise in predicting incident CKD or CKD prognosis, results from these studies are not necessarily applicable to CKDu due to the differences in etiology and pathophysiology. Given the young age of onset of CKDu and the fact that the normal GFR of healthy younger individuals exceeds 100 ml/min per 1.73 m2,14 the current definition of CKDu as an eGFR < 60 ml/min per 1.73 m2 identifies the disease very late into its course. Novel biomarkers of injury, if sensitive and accurate for the identification of tubulointerstitial injury that is thought to lead to CKDu, may ultimately transform our understanding of its epidemiology and pathogenesis.
Methods
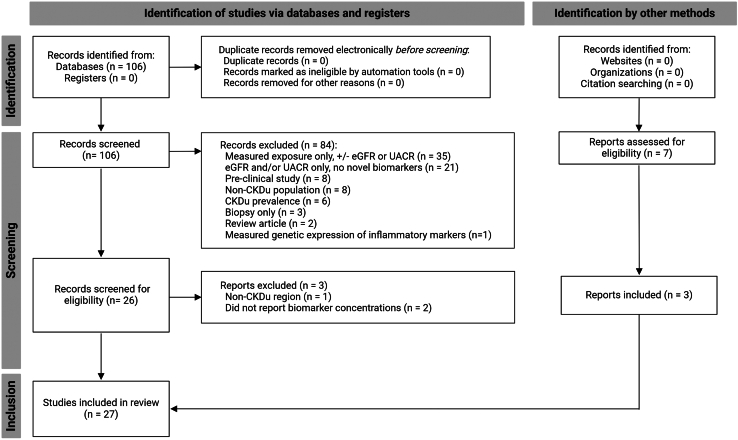
We identified the primary literature on urinary and plasma kidney injury biomarkers measured in CKDu via Web of Science through January 2024 (Supplementary Materials). The search was not limited by date or language. We excluded review papers, articles that only measured serum creatinine or urinary albumin without additional biomarkers, and animal model studies (Figure 1). Supplementary searches in PubMed yielded only duplicates of the Web of Science results, including searches using the terminology “Chronic Interstitial Nephritis in Agricultural Communities.” Additional manuscripts were selected via hand search of the references of previously identified literature. We collected data on the biomarker concentration, type of assay used for each biomarker, sample collection procedures, country, number of participants, percentage women, details regarding adjustment for urinary creatinine (for urine biomarkers), and main study findings. These criteria were not published as a protocol, nor was a protocol registered. For pooled presentation of data, biomarker concentrations were transformed to common units for comparison across studies. In this manuscript, the term ‘sex’ is used to describe sex assigned at birth to discuss potential physiologic differences. Determination of sex was not specified by any of the manuscripts reviewed.
Figure 1.
PRISMA diagram. See Supplemental Materials for details of the Web of Science search used to identify the literature for this systematic review. Adapted from: Page MJ, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi:10.1136/bmj.n71. For more information, visit: http://www.prisma-statement.org/. CKDu, chronic kidney disease of uncertain etiology; eGFR, estimated glomerular filtration rate; UACR, urinary albumin-to-creatinine ratio. Figure created with BioRender.com.
We identified 27 papers in total, only 2 of which measured kidney injury biomarkers in serum or plasma. All studies meeting criteria were included regardless of quality, to highlight the methodological issues affecting CKDu biomarker research and due to the low overall number of studies available for review. Methodological variability, including differences in assay, study population, exposure, and outcome, precluded pooled or statistical comparison of the findings across studies. Therefore, studies are grouped based on types of biomarkers relevant for outcome assessment and each study’s findings are presented after a brief introduction of the pathobiology of the biomarker. Figures were made with BioRender.com, Microsoft Excel (version 2311 for Windows; Microscoft Corporation, Redmond, WA), and GraphPad PRISM (version 10.2.1 for Windows; GraphPad Software, Boston, MA).
Kidney Injury Biomarkers in CKDu
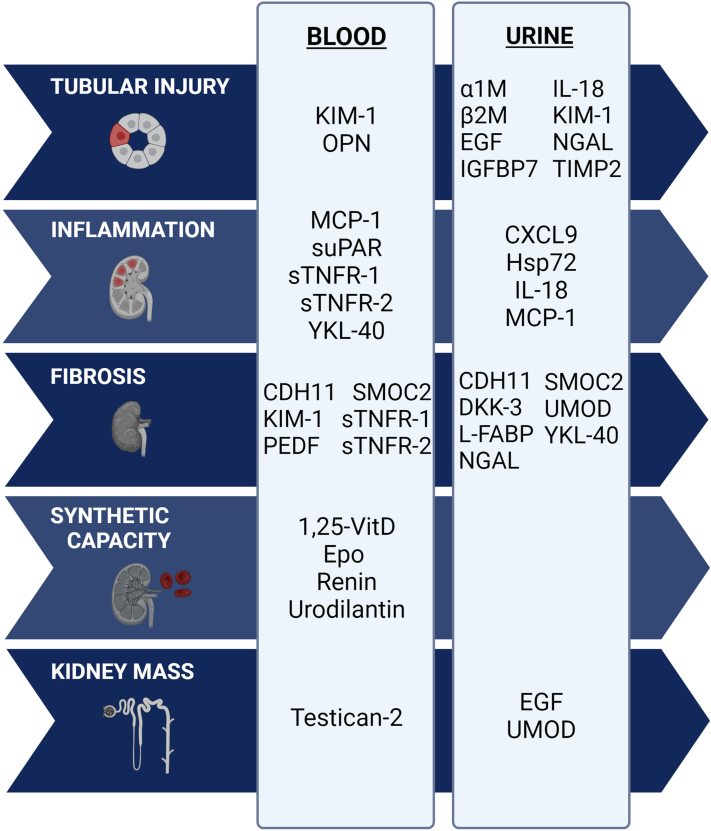
Kidney injury biomarkers can be classified according to the pathophysiologic process being captured (e.g., tubular injury, inflammation, fibrosis, synthetic capacity, or kidney mass) or the site of filtration, secretion, or metabolism along the nephron (Figure 2).13 Relatively few biomarker studies in CKD have compared biomarker levels against histopathology or used biomarkers as a diagnostic tool for differential diagnosis (notable exceptions include biomarkers of glomerular processes such as antineutrophilic cytoplasmic antibody vasculitis and membranous nephropathy).15, 16, 17, 18 This review summarizes the literature on kidney biomarkers that have been studied in CKDu (Table 1, Table 2, Figure 3), including those initially studied in association with acute kidney injury (AKI); low-molecular weight proteins that are typically catabolized by the tubules; and finally, biomarkers associated with injury, inflammation, and repair. The review concludes with a brief discussion of opportunities to advance the field.
Figure 2.
Selected biomarkers of kidney disease as represented by hypothesized mechanism of injury. Although these biomarkers are grouped according to the available evidence for their involvement in kidney injury pathways, there is likely overlap in the pathophysiology of each biomarker. Figure created with BioRender.com. 1,25-Vit D, 1,25-dihydroxyvitamin D; α1M, alpha-1-microglobulin; β2M, beta-2-microglobulin; CDH11, cadherin 11; CXCL9, CXC motif chemokine ligand 9; DKK-3, dickopf 3; EGF, epidermal growth factor; eGFR, estimated glomerular filtration rate; Epo, erythropoietin; FGF-23, fibroblast growth factor 23; GST-π, glutathione-S-transferase pi; Hsp72, heat shock protein 72; IGFBP7, insulin-like growth factor binding protein 7; IL-18, interleukin 18; KIM-1, kidney injury molecule-1; L-FABP, liver-fatty acid binding protein; MCP-1, monocyte chemoattractant protein-1; NGAL, neutrophil gelatinase-associated lipocalin; OPN, osteopontin; PEDF, pigment epithelium-derived factor; SMOC2, SPARK-related modular calcium binding 2; sTNFR-1, soluble tumor necrosis factor-1; suPAR, soluble urokinase plasminogen activator receptor; TIMP-2, tissue inhibitor of metalloproteinase 2; UACR, urinary albumin-to-creatinine ratio; UMOD, uromodulin.
Table 1.
CKDu studies that include measurements of kidney injury biomarkers
| Study | Country | Total Na No. controls % women |
Biomarker(s) | Normalized to urinary creatinine? | Assay / instrument | Other measures of kidney function | Specimen collection procedures |
|---|---|---|---|---|---|---|---|
| Adult populations | |||||||
| Gunawickrama et al. Diseases, 202271 | Sri Lanka | 56 total | Plasma IL-1α | N/A | ThermoFisher Scientific assays (ELISA): BMS243-2 (IL-1 α), KAC1261 (IL-6), BMS228 (IFNγ), BMS2034 (TNFα), BMS281 (MCP-1) | SCr, eGFR (CKD-EPICr 2009 and MDRD) | No details on the timing of sample collection |
| No controls | Plasma IL-6 | ||||||
| No participant demographics | Plasma MCP-1 | ||||||
| Plasma TNFα | |||||||
| Plasma INFγ | |||||||
| Swa et al. Kidney Dialysis, 202265 | Sri Lanka | 134 total | Serum RBP-4 | N/A | Luminex xMAP biomarker assay, select samples confirmed with Abcam ELISA (RBP-4) | SCr, eGFR (CKD-EPICr 2009) | No details on the timing of sample collection |
| 90 controls | |||||||
| 39.6% women | |||||||
| Abdul et al. Int J Environ Res Public Health, 202131 | Sri Lanka | 210 total | Urinary KIM-1 | Yes | Cusabio ELISA (KIM-1 and B2M), Ray Biotech ELIZA (NGAL) | SCr, eGFR (CKD-EPICr 2009) | First morning void (6–8 am) |
| No controls | Urinary NGAL | ||||||
| 40.9% women | Urinary B2M | ||||||
| De Silva et al. PLOS Negl Trop Dis, 201629 | Sri Lanka | 223 total | Urinary KIM-1 | Yes | Cusabio ELISA (KIM-1), Ray Biotech ELISA (NGAL) | SCr, eGFR (CKD-EPICr 2009) | First morning void |
| 106 controls | Urinary NGAL | ||||||
| Excluded women | |||||||
| Ekanayake et al. Int J Environ Res Public Health, 202232 | Sri Lanka | 228 total | Urinary KIM-1 | Yes | Cusabio ELISA (both KIM-1 and NGAL) | SCr, eGFR (CKD-EPICr 2021) | First morning void |
| 168 controls | Urinary NGAL | ||||||
| 40.7% women | |||||||
| Fernando et al. KIR, 201930 | Sri Lanka | 406 total | Urinary KIM-1 | Only in some analyses | Luminex xMAP custom biomarker bead assay kits (CERTKD-05 and HKI6/MAG-99-K-02) from Merck Millipore; using Luminex MAGPIX analyzer. | SCr, eGFR (unspecified equation) | Random spot urine |
| 164 controls) | Urinary NGAL | ||||||
| 40% women | Urinary Cystatin c | ||||||
| Urinary B2M | |||||||
| Urinary OPN | |||||||
| Urinary A1M | |||||||
| Urinary TIMP-1 | |||||||
| Urinary RBP4 | |||||||
| Nanayakkara et al. Environ Health Prev Med, 201263 | Sri Lanka | 237 total | Urinary A1M | Yes | Latex agglutination immunoassay (A1M), calorimetric assay (NAG). | SCr, eGFR (MDRD) | Random spot urine |
| 131 controls | Urinary NAG | ||||||
| 54.5% women | |||||||
| Wijkstrom et al. PLOS One, 201894 | Sri Lanka | 11 total | Urinary A1M | Only for A1M | R&D ELISA (KIM-1). Others not specified. | SCr, eGFR (CKD-EPICr 2009) | Random spot urine at time of kidney biopsy (or 5–6 mo later if missing) |
| No controls | Urinary NGAL | ||||||
| No women | Urinary KIM-1 | ||||||
| Wanigasuriya et al. Ceylon Med J, 201748 | Sri Lanka | 116 total | Urinary KIM-1 | Yes | Millipore multiplexed Luminex kit (all biomarkers). | Random spot urine; time of collection not indicated | |
| 79 controls | Urinary Clusterin | ||||||
| 14.5% women | Urinary B2M | ||||||
| Urinary Fibrinogen | |||||||
| Kulasooriya et al. Int J Envi Res Public Health, 202135 | Sri Lanka | 218 total | Urinary NGAL | No | Particle-enhanced turbidimetric immunoassay method (Roche cobas C 501) | SCr | Preshift first morning void (5–8 am), postshift spot urine (4–6 pm), evening spot urine (7–10 pm) |
| 67 controls | |||||||
| 40.3% women | |||||||
| Siriwardhana et al. BMC Nephrol, 201460 | Sri Lanka | 60 total | Urinary B2M | No | Bioquant B-2MG BQO10T (B2M) | Random spot urine | |
| 30 controls | |||||||
| 33.3% women | |||||||
| Herath et al. Journal of Water and Health, 201873 | Sri Lanka | 84 total | Urinary L-FABP | Yes | Not described | No details on timing of sample collection. | |
| No controls | |||||||
| No participant demographics | |||||||
| Hansson et al. Occup Environ Med, 202247 | Nicaragua | 68 total | Urinary KIM-1 | Yes | Bio-Plex Pro RBM Kidney Toxicity Panel 1 (includes all studied biomarkers in a single assay) | SCr, eGFR (CKD-EPICr) | Random spot urine; early morning hour preshift; before and after harvest season |
| 46 controls | Urinary MCP-1 | ||||||
| Excluded women | Urinary Calbindin | ||||||
| Urinary GST-π | |||||||
| Urinary Clusterin | |||||||
| Urinary IL-18 | |||||||
| Wesseling et al. Envi Res, 201634 | Nicaragua | 54 total | Urinary KIM-1 | Yes | Bioporto ELISA (NGAL), BioAssay Works ELISA (KIM-1), Western Blot using ENZO LIfe Science monoclonoal antibodies (Hsp72) | eGFR (CKD-EPICr 2009) | Random spot urine; preshift (3–5 am) or postshift (4–7 pm) at workplace; before and after harvest season |
| 25 controls | Urinary NGAL | ||||||
| Excluded women | Urinary Hsp72 | ||||||
| Laws et al. AJKD, 201633 | Nicaragua | 284 total | Urinary NGAL | Yes | Bioporto ELISA (NGAL), MBL ELISA (IL-18), colorimetric assay by Roche (NAG) | eGFR (CKD-EPICr 2009) | Random spot urine; before and after harvest season. |
| No controls | Urinary IL-18 | ||||||
| 22% women | Urinary NAG | ||||||
| Petropoulos et al. Kidney360, 202041 | Nicaragua | 251 total | Urinary NAG | Adjusted for urine Cr in regression models | Bioporto ELISA (NGAL and IL-18), Roche colorimetric assay (NAG) | SCr, eGFR (CKD-EPICr 2009) | Preharvest random spot urine |
| No controls | Urinary NGAL | ||||||
| 22% women | Urinary IL-18 | ||||||
| Gonzalez-Quiroz et al. J Am Soc Nephrol, 201828 | Nicaragua | 104 total | Urinary NGAL | Yes | ELH-Lipocalin2 RayBiotech (NGAL) | SCr, eGFR (CKD-EPICr 2009) | Random spot urine |
| No controls | |||||||
| Excluded women | |||||||
| Gonzalez-Quiroz et al. BMC Nephrol, 201927 | Nicaragua | 105 total | Urinary NGAL | Yes | ELH-Lipocalin2 RayBiotech (NGAL) | SCr, eGFR (CKD-EPICr 2009 and CKD-EPICr-Cys 2012) | Random spot urine |
| No controls | |||||||
| Excluded women | |||||||
| Butler-Dawson et al. J Expo Sci Environ Epidemiol, 202237 | Guatemala | 222 total | Urinary NGAL | Yes | Quantikine ELISA by R&D Systems (NGAL) | SCr, eGFR (CKD-EPICr 2009) | Random spot urine, variable times (morning and afternoon) while workers were in the field |
| No controls | |||||||
| Excluded women | |||||||
| Diaz de Leon-Martinez et al. Envi Sci Pollut Res Int, 201936 | Mexico | 34 total | Urinary KIM-1 | No | Magnetic Luminex Performance Assay | SCr, eGFR (CKD-EPICr) | First morning void |
| No controls | Urinary NGAL | ||||||
| 100% women (excluded men) | Urinary Cystatin c | ||||||
| Pediatric Populations | |||||||
| De Silva et al. Children, 202125 | Sri Lanka | 909 total adolescents | Urinary KIM-1 | Yes | Cusabio Technology ELISA (both KIM-1 and NGAL) | First morning void (6–8 am) | |
| No controls | Urinary NGAL | ||||||
| 53.2% female | |||||||
| Gunasekara et al. Sci Rep, 202295 | Sri Lanka | 804 total adolescents | Urinary KIM-1 | Yes | Cusabio Technology ELISA (KIM-1 and NGAL) | First morning void | |
| 164 controls | Urinary NGAL | ||||||
| 53.2% female | |||||||
| Gunasekara et al. Ped Neph39 | Sri Lanka | 922 total adolescents | Urinary KIM-1 | Yes | Cusabio Technology ELISA (KIM-1 and NGAL) | Early morning void | |
| 677 controls | Urinary NGAL | ||||||
| 54.7% female | |||||||
| Sandamini et al. World J Ped, 202267 | Sri Lanka | 892 total adolescents | Urinary Cystatin c | Yes | Cusabio CSB-E08384h (Cystatin c) | UACR | First morning void |
| 471 controls | |||||||
| 52.9% female | |||||||
| Ramirez-Rubio et al. NDT, 201642 | Nicaragua | 200 total adolescents | Urinary NGAL | Yes | Bioporto ELISA (NGAL), MBL ELISA (IL-18), Roche colorimetric assay (NAG) | Random spot urine. | |
| No controls | Urinary NAG | ||||||
| 50% female | Urinary IL-18 | ||||||
| Leibler et al. Ped Neph26 | Nicaragua | 210 total adolescents | Urinary KIM-1 | Yes | MesoScale Discovery Platform (all biomarkers) | Random spot urine. | |
| No controls | Urinary NGAL | ||||||
| 49.5% female | Urinary IL-18 | ||||||
| Urinary MCP-1 | |||||||
| Urinary YKL-40 | |||||||
| Cardenas-Gonzalez et al. Environ Res38 | Mexico | 83 total children (5-12y) | Urinary KIM-1 | Adjusted for urine Cr in selected regression models | Microbead-based assay developed by collaborators (KIM-1 and NGAL) | SCrUACR | Random spot urine |
| No controls | Urinary NGAL | ||||||
| 43.4% female |
A1M, alpha-1-microglobulin; B2M, beta-2-microglobulin; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; CKDu, CKD of unknown etiology; ELISA, enzyme linked immunosorbent assay; GST-π, glutathione-S-transferase pi; INFy, interferon gamma; IL-1a, interleukin 1 alpha; IL-6, interleukin 6; IL-18, interleukin 18; KIM-1, kidney injury molecule 1; L-FABP, liver-fatty acid binding protein; MCP-1, monocyte chemoattractant factor 1; NAG, N-acetyl-beta-D-glucosaminidase; NGAL, neutrophil gelatinase-associated lipocalin; No., number of; RBP-4, retinol binding protein 4; SCr, serum creatinine; TIMP1, tissue inhibitor matrix metalloproteinase 1; TNFa, tumor necrosis factor alpha; UACR, urinary albumin-to-creatinine ratio.
Total N represents the number of participants in which kidney injury biomarkers were measured. Some studies may have included additional participants in the design and measured kidney injury biomarkers in a subsample.
Table 2.
Biomarker values and estimated glomerular filtration rate (eGFR) in studies that included both CKDu cases and controls
| Study | CKDu definition | Control group definition | Biomarker(s) studied | Biomarker concentration |
eGFR (ml/min per 1.73 m2) |
||
|---|---|---|---|---|---|---|---|
| CKDu | Control | CKDu | Control | ||||
| De Silva. PLOS Negl Trop Dis, 201629 | Two values of UACR ≥30 mg/g in the absence of prior glomerular disease, pyelonephritis, nephrolithiasis, snake bite, diabetes, HbA1c >6.5%, or HTN (N = 14)a | Residence in a nonendemic area (N = 54)a | KIM-1 (μg/g) | 64 ± 11 | 1.3 ± 0.2 | 96.5 ± 4.5 | 110.0 ± 2.6 |
| NGAL (ug/g) | 2 ± 0.6 | 0.3 ± 0.03 | |||||
| Ekanayake. Int J Environ Res Publ Health, 202232 | Clinically diagnosed with CKDu (N = 40) | Factory workers from CKDu-endemic regions (N = 33)b | KIM-1 (ng/mg) | 5 [4–9] | 2 [0.5–2] | 20.0 [9.9–34.8] | 115.1 [108.4–131.3] |
| NGAL (ng/mg) | 22 [4–154] | 2 [0.7-5] | |||||
| Fernando. KIR, 201930 | Biopsy features consistent with CKDu from individuals living in a CKDu-endemic area for ≥ 5 years. Other causes of CKD were excluded by clinical judgment (N = 75) | Age- and sex-matched residents of nonendemic regions with no evidence of kidney disease (N = 85) | A1M (ng/g) | 870 [6473] | 54 [58.4] | Not reported | Not reported |
| B2M (ng/g) | 383 [1519] | 48 [70] | 42.7% with eGFR > 60 | 98.8% with eGFR > 60 | |||
| Cystatin c (ng/g) | 4 [17] | 1 [1] | |||||
| NGAL (ng/g) | 20 [35] | 3 [6] | |||||
| OPN (ng/g) | 68 [99] | 50 [40] | |||||
| RBP4 (ng/g) | 478 [551] | 188 [200] | |||||
| KIM-1 (ng/g) | 0.05 [0.08] | 0.05 [0.06] | |||||
| TIMP-1 (ng/g) | 0.6 [1] | 0.4 [0.8] | |||||
| Nanayakkara. Environ Health Prev Med, 201263 | CKDu previously diagnosed through a screening program (N = 106) | Apparently healthy adults from the general population of Kyoto, Japan (N = 50)c | A1M (mg/g) | Overall sample mean not reportedd | GM 0.6 ± 6 | Not reported | Not reported |
| NAG (U/g) | Overall sample mean not reported | GM 0.9 ± 4 | 28% with eGFR >60 | ||||
| Wanigasuriya. Ceylon Med J, 201748 | Fulfilled the diagnostic criteria for suspected CKDu and had a SCr ≥2 mg/dl (N = 37) | Nonfarmer residents of a nonendemic area (N = 40)e | Fibrinogen (ng/mg) | 20 [9–134] | 6 [4–8] | 23.5 ± 1.2 | 76.1 ± 8.3 |
| B2M (ng/mg) | 4643 [249–15375] | 118 [44–181] | |||||
| Clusterin (ng/mg) | 372 [222–1587] | 836 [366–1525] | |||||
| KIM-1 (pg/mg) | 505 [346–1008] | 403 [311–672] | |||||
| Cystatin c (ng/mg) | 681 [43–3650] | 243 [96–362] | |||||
| Siriwardhana. BMC Nephrol, 201460 | Diagnosed as having CKDu by a nephrologist. Known causes of CKD (e.g., glomerular disease, diabetes, longstanding HTN, snake bite, leptospirosis, congenital kidney disease, nephrolithiasis) were excluded by clinical judgment. (N = 30) | Age-matched and sex-matched residents of the same area; SCr <1.0 mg/dl, random glucose <140 mg/dl, blood pressure <120/80, and negative proteinuria (N = 30) | B2M (μg/ml) | 1.24 ± 0.71 | 0.16 ± 0.05 | Not reported | Not reported |
A1M, alpha-1-microglobulin; B2M, beta-2-microglobulin; CKDu, chronic kidney disease of uncertain etiology; eGFR, estimated glomerular filtration rate; GM, geometric mean; HbA1c, hemoglobin A1c; HTN, hypertension; KIM-1, kidney injury molecule 1; NAG, N-acetyl-beta-D-glucosaminidase; NGAL, neutrophil gelatinase-associated lipocalin; RBP-4, retinol binding protein 4; SCr, serum creatinine; TIMP1, tissue inhibitor matrix metalloproteinase 1; UACR, urinary albumin-to-creatinine ratio.
Not all studies in the table employed a traditional case-control design. In some instances, case-control status was determined after receiving the test results of population-wide screening programs.
Values are reported as mean ± SD or median [interquartile range].
This study evaluated participants from 2 endemic and 2 nonendemic regions separately. Data are presented for 1 of the 2 groups for simplicity.
This study included multiple occupational groups. Factory workers were selected as the representative control as they were the only nonagricultural occupation.
This study also included healthy, nonaffected relatives as a control group, which is not presented in the table for simplicity.
This study provided mean biomarker concentration stratified by eGFR category only for those participants identified as having CKDu.
This study also included a sample of farmer controls, which are not presented in the table for simplicity.
Figure 3.
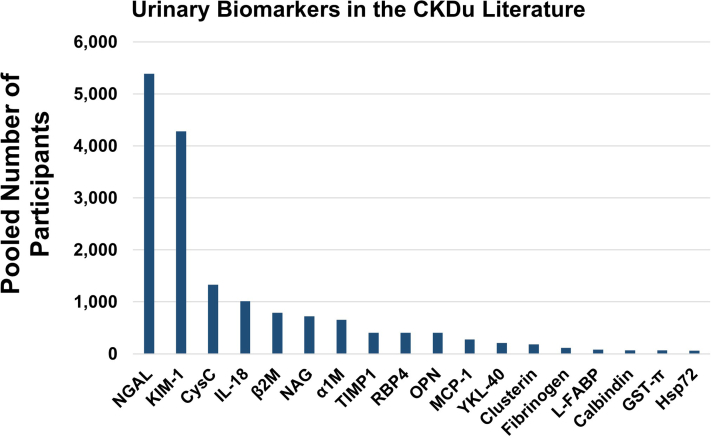
The total number of study participants in which novel urinary biomarkers have been measured in the setting of CKDu. These counts include both healthy controls and suspected patients with CKDu who have undergone urinary biomarker testing. These data represent studies published through January 2024. α1M, alpha-1-microglobulin; β2M, beta-2-microglobulin; CKDu, chronic kidney disease of uncertain etiology; CysC, cystatin c; GST-π, Glutathione-S-transferase pi; Hsp72, heat shock protein 72s; IL-18, interleukin 18; KIM-1, kidney injury molecule-1; L-FABP, liver-fatty acid binding protein; MCP-1, monocyte chemoattractant protein-1; NAG, N-acetyl-beta-D-glucosaminidase; NGAL, neutrophil gelatinase-associated lipocalin; OPN, osteopontin; RBP-4, retinal binding protein-4; TIMP-1, tissue inhibitor of metalloproteinase 1.
Results
Proteins Studied Initially as Biomarkers of AKI
Early work in the field of kidney biomarkers focused on rodent models of ischemic AKI and subsequently on predicting AKI in patients undergoing cardiac surgery.19,20 More recent work has investigated these acute tubular injury markers in CKD, given the bidirectional relationship between AKI and CKD21 and, in the case of CKDu, the tubulointerstitial predominance of its histopathology. As traditional markers of acute injury to the tubules, these “AKI” biomarkers have been primarily studied in the context of cross-shift AKI in field workers. It should be noted that many biomarkers have been studied in both AKI and CKD, and that the concept of “AKI” versus “CKD” biomarkers may be a false dichotomy.
NGAL
NGAL is a protein expressed by proximal and distal tubular epithelial cells. NGAL promotes cellular apoptosis, inhibits bacterial growth, and regulates iron metabolism. Urinary levels of NGAL increase after ischemic kidney injury and cisplatin-induced nephrotoxicity.22,23 Cells in the liver, colon, lungs, stomach, and genitourinary organs express NGAL in response to epithelial damage, inflammation, and neoplasia.24
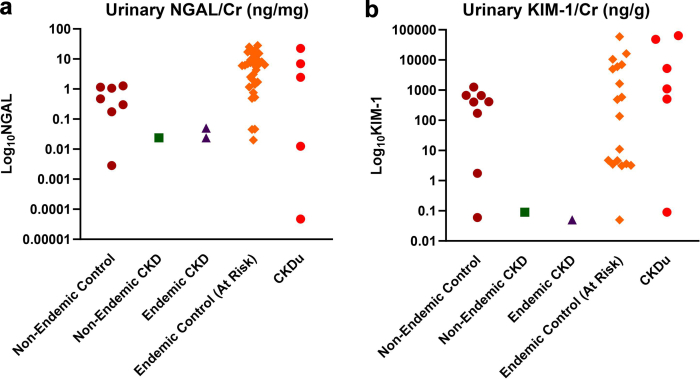
To date, urinary NGAL is the most studied urinary biomarker in CKDu and has been measured in over 5000 patients and controls (Figures 3 and 4, Supplementary Table S2). Reference ranges have been proposed for adolescents in CKDu-endemic regions of Sri Lanka but cannot be directly compared to studies using different ELISA kits.25,26
Figure 4.
Urinary concentration of (a) NGAL and (b) KIM-1 by case-control status reported in each study. Each marker represents the mean or median biomarker concentration for the representative subgroup(s) in each study. To facilitate comparisons across normalized and nonnormalized values, values are shown in units of ng/g creatinine or either ng/L for KIM-1, and ng/mg or ng/ml for NGAL. Because approximate urine output is 1L/d and approximate urine creatinine excretion is 1g/d, the units ng/g and ng/l are roughly comparable within an order of magnitude. Data represented in this figure are compiled from the studies of adult populations listed in Supplementary Table S2. Figure created in GraphPad Prism (v10.0.2). CKD, chronic kidney disease; CKDu, CKD of uncertain etiology; Cr, creatinine; KIM-1, kidney injury molecule 1; NGAL, neutrophil gelatinase-associated lipocalin.
Although the literature is limited, urinary NGAL has not been shown to predict eGFR decline in CKDu, to date. In a longitudinal study of 105 young Nicaraguan adults, adding urinary NGAL to traditional predictors of eGFR decline (e.g., age, albuminuria, and occupation) did not improve the prediction of rapid decline in eGFR.27 The authors reported consistent, negative findings across regression models, including a model adjusting for baseline eGFR and urinary albumin-to-creatinine ratio only and a separate model adjusting for repeated eGFR and urinary albumin-to-creatinine ratio measures over a 1-year period. The study only measured urinary NGAL at baseline and may have been underpowered, because only approximately 10% experienced a rapid decline.27 In this population, urinary NGAL was mostly higher at baseline in those who subsequently experienced a rapid decline despite similar baseline eGFR.28
Urinary NGAL has also been tested to differentiate CKDu cases from nonendemic controls and other forms of CKD. In emerging CKDu regions of Sri Lanka, urinary NGAL inversely correlated to eGFR (n = 223).29 In a separate cohort of Sri Lankan adults, urinary NGAL performed moderately well with area under the receiver operating characteristics curve (AUC-ROC) ranging between 0.767 and 0.827 for comparisons across participants with CKDu, nonendemic CKD, and controls from both endemic and nonendemic regions (n = 406).30 (The AUC-ROC is a summary measure of classification models, ranging from 0.5 [random classifier] to 1.0 [perfect classifier]).
Urinary NGAL has been compared across agricultural occupational groups in healthy adults living in CKDu-endemic regions. For example, urinary NGAL was higher in apparently healthy sugarcane famers in arid regions than vegetable or paddy farmers in the wetlands of Sri Lanka (n = 210),31 whereas another study found that urinary NGAL was significantly higher in paddy farmers than either fisherfolk or plantation workers (n = 228).32 These findings are potentially consistent with the hypothesis of a work-related environmental or toxic exposure as an underlying cause of CKDu; though all participants were healthy at the time of the study, they were not followed-up with longitudinally to assess the development of incident CKDu. Furthermore, the authors reported significant regional differences with respect to participants’ age, smoking status, alcohol and tobacco use, exposure to herbicides, and other factors that may impact the observed associations and were not accounted for in the analyses.
Urinary NGAL has also been compared between agricultural and nonagricultural workers. Sugarcane workers in Nicaragua experienced a greater increase in urinary NGAL concentration than factory workers over the course of a 9-week harvest season (n = 284).33 In a subsequent study, sugarcane workers demonstrated a rise in preshift urinary NGAL and a greater increase in serum creatinine compared to a control group of primarily office workers (n = 54).34 In Sri Lanka, agricultural workers in CKDu-endemic regions reported higher heat stress symptoms than agricultural workers in nonendemic regions and nonagricultural controls (n = 218); urinary NGAL was positively associated with self-reported heat stress symptoms.35 However, the authors note that the relationship was primarily driven by a few participants with extreme NGAL values and extreme heat stress symptoms.
Urinary NGAL has also been studied in relation to specific agrochemical and environmental exposures. In a cross-sectional study from Sri Lanka, investigators found no significant association between urinary NGAL and either urinary paraquat or glycophosphate after adjusting for age, sex, and location (n = 210).31 In another study from Mexico of aflatoxin exposure, there was no significant association between urinary NGAL and serum aflatoxin adducts (AFB1-Lysine) (n = 34).36 In 222 apparently healthy Guatemalan sugarcane workers, urinary NGAL was positively associated with urinary cadmium concentration but not arsenic, lead, nickel, or uranium.37 In children living in San Luis Potosi, Mexico (a region with mining and industrial activities), urinary NGAL was not associated with urinary heavy metals (arsenic, chromium, or cadmium) in multivariable models adjusting for clinical characteristics and urine creatinine concentration (n = 83).38 Among adolescents in Sri Lanka, urinary fluoride concentration was weakly associated with urinary NGAL and not suggestive of a significant risk factor for kidney injury (n = 922).39 Important limitations of these studies include lack of biomarker normalization to urinary creatinine concentration36 and lack of adjustment for eGFR,31,36, 37, 38 because urine heavy metal and agrochemicals can accumulate in the setting of advanced kidney disease due to reduced clearance. Normalization to 24-hour urinary creatinine concentration is preferred in populations with advanced kidney disease, though impractical. Additional limitations of assessing agrochemical exposure in cross-sectional studies are discussed in a recent review.40
Among Nicaraguan workers, urinary NGAL was higher in participants with positive leukocyte esterase on urine dipstick, suggesting that NGAL may be neutrophil-derived after urinary tract infections (n = 200).41,42 In one study, higher urinary NGAL levels were associated with greater self-reported urinary symptoms, but only in the late harvest season, which the authors hypothesized may be related to increased crystalluria (n = 251).41 None of the participants who reported urinary symptoms and provided a sample for culture had positive urine culture results.41
KIM-1
KIM-1 was identified as the gene most highly expressed in the kidney after 24 to 48 hours of ischemia in rat models.43 KIM-1 is a transmembrane glycoprotein expressed primarily on the apical membrane of dedifferentiated proximal tubular cells after ischemic or toxic injury; KIM-1 is also expressed in renal cell carcinoma and at lower levels in the liver, spleen, and lymphocytes; and in cochlear cells following cisplatin-induced cochlear damage.44 After acute injury, KIM-1 functions as a phosphatidylserine receptor that confers on tubular epithelial cells the ability to recognize and phagocytose dead cells in the tubule.45 When expressed chronically, KIM-1 appears to lead to progressive kidney inflammation and fibrosis through activation of the innate immune system and leukocyte recruitment.46
To date, urinary KIM-1 is the second most widely studied kidney injury biomarker in CKDu and has been measured in nearly 4300 participants (Figures 3 and 4). One study established reference ranges for urinary KIM-1 in apparently healthy adolescent populations in CKDu-endemic regions of Nicaragua (n = 210).26 In several, but not all, studies of healthy adolescents, women had higher urinary KIM-1 concentration than men.12,25,26
To date, 2 studies have measured urinary KIM-1 longitudinally. In a study of healthy Nicaraguan sugarcane workers, preshift urinary KIM-1 increased significantly within the first week of the harvest season, and this group subsequently demonstrated persistent decrease in eGFR over the study despite return of urinary KIM-1 to baseline levels (n = 54).34 Another study of Nicaraguan sugarcane cutters stratified the population between those who did and did not experience a ≥0.3 mg/dl (26.5 μmol/dl) increase in serum creatinine over the harvest season (n = 68).47 Urinary KIM-1 increased in those who experienced a decline in kidney function, and remained unchanged in others with stable kidney function.47
In studies from Sri Lanka, urinary KIM-1 was higher in patients with CKDu than both endemic and nonendemic controls (n = 233),29 and also higher in apparently healthy individuals across 5 different occupational groups (n = 228).32 A third study found similar urinary KIM-1 levels across sugarcane, vegetable, and paddy farmers (n = 210).31 Not all studies have found a difference in urinary KIM-1 between cases and controls48 and, in 1 study, KIM-1 could not differentiate CKDu cases from nonendemic CKD (n = 406).30 The divergent findings may be due to the population sampled and study design, differences in definition CKDu, or differences in the assays used to measure KIM-1.
Urinary KIM-1 has also been studied in relation to specific agrochemical and other environmental exposures. In one study from Sri Lanka, investigators found a correlation between urinary KIM-1 and levels of urinary arsenic and mercury; differences in urinary KIM-1 between CKDu cases and controls were not statistically significant (n = 116).48 Similarly, in a small pilot study of indigenous women from San Luis Potosi, Mexico, urinary KIM-1 correlated with serum aflatoxin adducts (AFB1-Lysine).36 Urinary KIM-1 was not associated with urinary concentrations of paraquat or glyphosate in healthy agricultural workers in Sri Lanka (n = 210)31 and was minimally associated with urinary fluoride concentration in Sri Lankan adolescents (n = 922).39
Interleukin-18 (IL-18)
IL-18 is a systemic proinflammatory cytokine that activates NFkB and interferon-γ inflammatory cascades. In the kidney, proximal tubular cells release IL-18 in response to hypoxic injury49 and after tubular necrosis.50,51 In rodent models, mice that lack the ability to activate IL-18 sustained less severe tubular injury following local hypoxia,52 and neutralizing IL-18 reduced tubular fibrosis following injury.53
Urinary IL-18 has been studied across occupation groups in CKDu-endemic regions. In a study of Nicaraguan workers, change in urinary IL-18 over the course of the harvest season was approximately 61% higher in field workers than in nonfield workers; however, increases in urinary IL-18 were not associated with eGFR decline.33 Urinary IL-18 was consistently higher in field workers than in nonfield workers, though the difference between groups varied by specific job category (n = 284).33 For example, more physically demanding jobs were associated with higher urinary IL-18 than those requiring less strenuous manual labor. In another study of Nicaraguan workers, urinary IL-18 was not consistently higher in agricultural workers compared to nonagricultural workers (n = 251).41 Workers reporting urinary symptoms and with leukocyte esterase on urinalysis had higher urinary IL-18 after adjusting for demographic and occupational characteristics (n = 251).41 Highlighting the importance of assay platforms in biomarker studies, 1 study among Nicaraguan sugarcane cutters using a commercially available multiplex immunoassay system was limited in examining IL-18 because 95% of values measured in urine were below the lower limit of detection (n = 68).47 Several studies have suggested sex differences in urinary IL-18 in healthy adolescents (women with higher IL-18 than men); however, the clinical implications are not well understood.26,42
Low-Molecular Weight Proteins Normally Catabolized by the Tubules
The tubules catabolize many low-molecular weight proteins that are filtered from the circulation across the glomerulus.54 Proximal tubular injury can lead to impaired uptake and catabolism, resulting in tubular proteinuria.55, 56, 57 Given the tubulointerstitial pattern of injury in CKDu, elevations of these proteins in urine may indicate early CKDu before detectable changes in eGFR. It should be noted however that the CKDu literature lacks longitudinal data to evaluate the role of biomarkers in assessing disease progression.
Beta-2-microglobulin (B2M)
B2M is a component of major histocompatibility complex class I molecules and circulates as an unbound monomer. It is freely filtered at the glomerulus and catabolized in the proximal tubule; both serum and urine B2M have been measured in various clinical settings, including kidney disease. Urinary B2M was measured as an indicator of tubular dysfunction in epidemiologic investigations of Itai-itai disease, the name given to chronic cadmium poisoning of individuals residing in the Jinzu river basin region of Japan in the 1930s from nearby mining activities.58,59
In 2 small cohorts of patients in Sri Lanka, urinary B2M was significantly higher in patients with CKDu than in healthy controls (n = 116 and n = 90).48,60 In a cross-sectional study from Sri Lanka in patients with CKDu, nonendemic CKD, and controls from both endemic and nonendemic regions (n = 406), B2M had an AUC-ROC ranging between 0.696 and 0.852 in comparisons across the groups.30
N-Acetyl-Beta-D-Glucosaminidase (NAG)
NAG is a lysosomal tubular enzyme that is elevated in the urine after proximal tubular injury.61 Urinary concentrations of NAG were measured in early studies of Itai-itai disease.62 Among sugarcane workers in Nicaragua, those with the greatest increases in urinary NAG over the harvest period also experienced a decline in eGFR (n = 284).33 In a follow-up study of 251 of these participants who responded to a symptom questionnaire, urinary NAG was associated with hematuria and self-reported urinary symptoms.41
Alpha-1-Microglobulin
Alpha-1-microglobulin is a low-molecular weight protein synthesized in the liver and catabolized by the tubules after glomerular filtration; it was previously measured in studies of Itai-itai disease.58 In a cross-sectional study from Sri Lanka in patients with CKDu, nonendemic CKD, and controls from both endemic and nonendemic regions (n = 406), alpha-1-microglobulin had the highest discriminatory capacity in a panel of 8 biomarkers, with AUC-ROC values of 0.891 to 0.914.30 In a study of Sri Lankan adults (n = 237), healthy controls had the lowest levels of urinary alpha-1-microglobulin and those with stage 3 clinical CKDu had the highest concentrations.63 The interpretation of these findings is limited by the small sample size at each stage of CKD.
Retinol Binding Protein-4 (RBP-4)
RBP-4 is a lipocalin that is predominantly synthesized by the liver and adipocytes and freely filtered and catabolized by the tubules.64 A pilot study in Sri Lanka assessed the diagnostic utility of RBP-4 in CKDu (n = 406). A panel of RBP-4, osteopontin (OPN), and KIM-1 distinguished patients with CKDu from nonendemic CKD (AUC-ROC, 0.976) but not endemic CKD from nonendemic CKD.30 These results have not been replicated in other CKDu populations. RBP-4 can also be measured in the serum. In a study of 134 Sri Lankan adults, serum RBP-4 was higher in those with CKDu than in healthy controls but not different in other forms of CKD.65
Cystatin C (CysC)
CysC is a low-molecular weight protein in the cystatin protease inhibitor family that is produced at a constant rate by all nucleated cells.66 CysC is freely filtered and typically catabolized by the proximal tubule. Serum CysC is a marker of eGFR, whereas urinary CysC is a biomarker of kidney injury. In healthy states, little to no CysC is detectable in the urine. In the setting of kidney disease, impaired proximal tubular catabolism of CysC can lead to elevated urinary concentrations. Several studies have measured urinary CysC in CKDu,30,36,67 including 1 pediatric study which sought to establish reference intervals in healthy Sri Lankan children.67 In a study of Sri Lankan adults, urinary CysC was unable to distinguish between cases of CKDu and nonendemic CKD (ROC-AUC, 0.585) (n = 160).30 In women living in San Luis Potosí, Mexico, urinary CysC was weakly correlated to serum aflatoxin concentration (AFB1-Lysine) (n = 34).36
Other Biomarkers of Injury, Inflammation, and Repair
OPN
OPN is a matricellular protein that is primarily expressed in the bone, kidney, and intestines, but can be expressed in nearly every organ in response to inflammation. In a mouse model, tubular OPN expression was upregulated in early CKD, before histologic evidence of direct tubular injury.68 Kidney-released OPN after acute ischemic injury has also been shown to mediate cross-organ lung damage.69 OPN has only been measured in 1 CKDu study to date (n = 406). Urinary OPN concentrations were higher in patients with CKDu compared to nonendemic CKD and endemic CKD in Sri Lanka.30 In isolation, OPN demonstrated relatively low sensitivity and specificity for distinguishing CKDu from other forms of kidney disease, with AUC-ROC values ranging from 0.586 to 0.756.30
Monocyte Chemoattractant Protein-1 (MCP-1)
MCP-1 is a protein produced by epithelial cells, macrophages, neutrophils, and endothelial cells in response to proinflammatory cytokines. In the kidney, MCP-1 is released by tubular epithelial cells and attracts monocytes, T lymphocytes, natural killer cells, and tissue macrophages to damaged tissue.70
Both serum and urinary MCP-1 have been studied in CKDu-endemic regions.47,71 Among healthy Nicaraguan agricultural workers at risk for CKDu (n = 68), those who experienced a >0.30 mg/dl (26.5 μmol/dl) increase in serum creatinine also exhibited rising urinary MCP-1 across the harvest period.47 Those whose kidney function was unchanged had stable urinary MCP-1 concentrations.47
Clusterin
Clusterin is a glycoprotein that is involved in inhibiting the terminal complement cascade and may have an antiapoptotic role in the kidney.72 In Sri Lanka, CKDu cases had higher urinary clusterin concentration than nonendemic controls; however, these patients were not compared to nonendemic CKD (n = 117).48 In another study, elevated urinary clusterin was not associated with incident kidney injury in Nicaraguan sugarcane workers (n = 68); however, 65% of the samples were below the limit of detection.47
Other Biomarkers Studied in CKDu
Urinary biomarkers with just 1 publication to date include glutathione-S-transferase π (a distal tubular marker),47 tissue inhibitor of metalloproteinase-1 (a glycoprotein),30 YKL-40 (a glycoprotein that modulates the extracellular matrix),26 calbindin (a calcium binding protein),47 heat shock protein 72 (a stress-inducible protein chaperone),34 fibrinogen (an acute phase protein involved in hemostasis),48 and liver-fatty acid binding protein (a cytosolic lipid binding protein).73 Longitudinal data are lacking to support the associations between these biomarkers and CKDu progression or incidence. A brief review of biomarkers not yet studied in CKDu which may be considered for future studies is included in the Supplementary Materials.
Methodological Limitations of the Existing Evidence and Recommendations for Future Biomarker Studies in CKDu
Important methodological considerations and recommendations for future biomarker studies in CKDu are outlined in Table 3 and discussed in further detail below. In Table 2, we summarize differences in biomarker concentrations between CKDu cases and controls in the context of different study populations and methodologies. In Supplementary Table S1, we summarize the few longitudinal studies of kidney injury biomarkers identified in the CKDu literature.
Table 3.
Recommendations for future studies
| Important considerations | Recommendations |
|---|---|
| Methodology | |
| Choice of comparator group for CKDu biomarker studies using a case-control design | Comparisons of kidney biomarker values in CKDu versus other groups should consider potential differences in kidney function between groups, because GFR may influence blood or urine levels of injury markers independent of CKDu status. Similarly, investigations of environmental exposures and biomarkers should include a comparator group with similar kidney function for the same reason. |
| Exclusion of individuals in the CKDu affected group who have alternative possible explanations for kidney disease | CKDu is typically a diagnosis of exclusion. Factors that may lead to errors in disease classification include diabetes mellitus, severe hypertension preceding CKDu diagnosis, heavy proteinuria, and hematuria. To reduce confounding, strategies such as exclusion of individuals with comorbidities, statistical adjustment for these factors, and stratified analyses can increase confidence that results are representative of CKDu. |
| Outcomes data | Longitudinal follow-up to ascertain kidney disease progression, regression, or stability will add insight into the utility of biomarker measurements in CKDu. |
| Assessment of kidney function | The optimal test(s) to estimate GFR in CKDu studies is still unclear. Most studies have utilized serum creatinine and others may begin to incorporate cystatin C. Measurements performed in reference laboratories would be ideal to allow interstudy comparisons. Assessing albuminuria quantitatively may also aid future studies of CKDu. |
| Environmental exposure quantification | Use of quantifiable exposure data, such as wet-bulb globe measured temperature and laboratories with validated methods for measuring chemical levels in environmental samples or chemical metabolites in biologic samples can improve interstudy comparability of environmental exposures. |
| Quality control metrics | Quality control metrics include coefficients of variation, ideally from blind split replicates. In studies comparing biomarker values across groups, measurement of samples in batches by disease status or site can introduce “batch effects” that lead to spurious differences; inclusion of bridging samples or randomly distributing samples across plates can help minimize batch effects. |
| Statistical methods | |
| Adjustment or stratification | Directed acyclic graphs can be used to aid investigators in determining the appropriate method and variables to include to minimize bias. Plausible confounders could include age, sex, eGFR, duration of exposure to suspected causal agent(s) but will vary based on study design. Stratification can be used to assess heterogeneity of effect by prespecified, hypothesis-driven subgroups. |
| Normalization of biomarker concentrations | Urine concentration can range over 2 orders of magnitude, resulting in large differences in biomarker concentration due to water intake or dehydration. Adjustment for urinary creatinine can account for differences in urine volume. Additional adjustments have also been proposed to account for other variables that may influence urine creatinine concentration.92 |
| Power calculations | Null findings in biomarker studies may be due to insufficient statistical power. Power should be considered in early phases of study design and power calculations should be transparently reported in publications to guide other researchers. |
| Common divisor for urine biomarkers | Urine biomarkers are often reported as a ratio to urine creatinine to account for urinary volume and concentration. Correlating the levels of 2 biomarkers in the urine (e.g., UKIM-1/UCr and UACR; or urinary metal concentration (normalized to UCr) and UKIM-1/UCr) introduces a spurious correlation because of the common divisor, UCr. The likely extent of the spurious correlation can be estimated using variances of the 2 numerators.85,86 |
| Comparisons of biomarker levels in CKDu vs. healthy controls | Because of the large potential effect of impaired glomerular filtration rate on kidney biomarker concentrations in the urine or blood, levels may differ between CKDu and healthy controls due to kidney function rather than CKDu itself. Selecting appropriate comparison groups with overlapping levels of kidney function can allow for statistical adjustment of kidney function. |
| Reporting | |
| CKDu definition | The optimal definition of CKDu for epidemiologic research is not known and locally accepted criteria can vary within geographic regions. Describing the specific clinical criteria used to determine participants’ CKDu status allows for interstudy comparisons. |
| Sampling technique for biospecimens | Describe details of biologic sample collection and processing, which include factors such as timing of urine or blood collection, time and temperature before centrifugation, and time to refrigeration or freezing. Quality control information should also be reported for both biologic specimens and environmental samples. |
| Reporting of descriptive data | Comprehensive reporting of key descriptive data (e.g., minimum, maximum, measures of central tendency, variance) can improve the ability of future investigators to perform high quality systematic reviews.93 |
CKDu, chronic kidney disease of uncertain etiology; eGFR, estimated glomerular filtration rate; UACR, urinary albumin to creatinine ratio; UCr, urinary creatinine; UKIM-1, urinary kidney injury molecule-1.
Biomarker studies in CKDu are heterogeneous across all aspects of study design, participant selection, laboratory methodology, field collection protocols, and statistical analysis. In case-control studies, the definition of CKDu can vary by including (or not) individuals with hypertension or heavy proteinuria. Several studies of CKDu include patients with traditional CKD risk factors, including diabetes or moderate-to-heavy proteinuria (urinary albumin-to-creatinine ratio > 1000 mg/g). Methods for specimen collection are also variable (e.g., random spot urine or morning void), which can limit comparison of biomarker levels across studies. Identifying appropriate control groups that arise from the same source population as cases and are sampled independently of exposure is also challenging pragmatically, and several studies included suboptimal control groups for this reason.
Standardization of collection procedures and laboratory methodology is essential in kidney biomarker research because it increases the reliability, comparability, and reproducibility of study findings. Interassay differences and batch effects are a major source variability.74,75 It is not possible to directly compare absolute biomarker values across studies using different assays. Preanalytical factors are also critical and not always reported or standardized across studies.76 The complex logistics of transporting samples from remote field sites to central laboratories, first in-country and then to analytic laboratories in other countries such as the United States, also introduces multiple opportunities for temperature excursions that may compromise sample integrity and lead to measurement error.77,78 Previous studies have shown that hemolysis impacts NGAL assay accuracy, for example.79,80 This is particularly relevant to CKDu studies conducted at field sites, where mechanical agitation of blood specimens during transportation to the laboratory may introduce hemolysis. As an illustration of the variability across studies in biomarker levels, in Figure 4, we show published urine NGAL and KIM-1 concentrations across multiple studies and highlight the wide range of concentrations reported. Postanalytical methodologic variability is also common in the CKDu literature, particularly surrounding standardization of biomarkers to urinary creatinine81, 82, 83, 84 and the potential for spurious associations with environmental exposures due to a common divisor (urinary creatinine).85,86
Future studies will benefit from consistently reporting information that would allow for quantitative synthesis of the associations between biomarkers and kidney function in CKDu. These factors include preanalytic details (e.g., urine sample collection procedures, power analysis, as well as comprehensive clinical and demographic information of study participants), analytic details (e.g., assay selection and quality control), and postanalytical practices (e.g., selection of eGFR estimating equation and inclusion of confounders in regression modeling), along with a clearly defined case definition for CKDu. Understanding more fully the study population and procedures may facilitate greater quantitative comparisons than were possible in this manuscript (e.g., pooled effect estimates and formal tests of heterogeneity).
Applicability of Biomarkers to CKDu in the Absence of a Gold Standard Diagnosis and Future Directions
Biomarkers can be used for multiple applications and contexts, as described in the FDA-NIH Biomarker Working Group’s “BEST (Biomarkers, EndpointS, and other Tools) Resource.”87 Categories for biomarkers include diagnostic, monitoring, pharmacodynamic, predictive, prognostic, safety, and susceptibility. Because longitudinal studies clarify the relationship between biomarkers and trajectories of kidney function in CKDu, biomarkers could be used for diseased activity monitoring and prognostication. In Table 4, we present examples of how CKDu biomarkers could be used in the future, adopting the framework of the BEST Glossary.
Table 4.
Application of the NIH-FDA BEST (Biomarker, EndpointS, and other Tools) framework to CKDu biomarkers
| Biomarker type | Definition | Example of use in CKDu |
|---|---|---|
| Diagnostic biomarker | A biomarker used to detect or confirm presence of a disease or condition of interest or to identify individuals with a subtype of the disease. | Community screening in conjunction with standard measures of kidney function to identify subclinical disease |
| Monitoring/response biomarker | A biomarker measured repeatedly for assessing status of a disease or medical condition or for evidence of exposure to (or effect of) a medical product or an environmental agent. | Serial monitoring in high-risk occupations or geographic regions to identify subclinical injury |
| Predictive biomarker | A biomarker used to identify individuals who are more likely than similar individuals without the biomarker to experience a favorable or unfavorable effect from exposure to a medical product or an environmental agent. | Preoccupational screening |
| Prognostic biomarker | A biomarker used to identify likelihood of a clinical event, disease recurrence or progression in patients who have the disease or medical condition of interest. | Predicting risk of progression to kidney failure; selection of individuals for closer clinical monitoring |
| Safety biomarker | A biomarker measured before or after an exposure to a medical product or an environmental agent to indicate the likelihood, presence, or extent of toxicity as an adverse effect. | Preoccupational screening |
| Susceptibility/risk biomarker | A biomarker that indicates the potential for developing a disease or medical condition in an individual who does not currently have clinically apparent disease or the medical condition. | Preoccupational screening; community screening to identify subclinical disease |
CKDu, chronic kidney disease of uncertain etiology; eGFR, estimated glomerular filtration rate; UCr, urinary creatinine; UKIM-1, urinary kidney injury molecule-1.
The lack of a readily available gold standard diagnosis makes biomarker validation studies challenging in CKDu because misclassification of disease status can distort the apparent performance characteristics of a biomarker under investigation; for example, a young individual with eGFR of 90 ml/min per 1.73 m2 may have suffered significant kidney injury and have been exposed to the cause(s) of CKDu but not manifest the disease by the current standard definition, because of renal functional reserve and the high baseline eGFR of young healthy individuals. If a biomarker is measured in such an individual and found to be high, classifying the measurement as a false positive would be a misinterpretation. Investigators evaluating biomarker concentrations in the setting of case-control or cross-sectional study designs should be aware of this potential pitfall and use multiple complementary analytic approaches to overcome the limitations of such study designs.
The NIH-funded Chronic Kidney Diseases of UnceRtain Etiology (CKDu) in Agricultural Communities (CURE) Research Consortium (CURE)88 was initiated to enable discovery science to understand CKDu etiology.89, 90, 91 CURE has planned a prospective cohort study involving individuals across the spectrum of kidney function at risk for CKDu.88, 89, 90, 91 This international research consortium offers an important opportunity to evaluate the role of kidney injury biomarkers in defining CKDu, as well as accumulate evidence on how CKDu may be diagnosed in earlier stages.
Conclusion
The evidence base, to date, on biomarkers of CKDu is still premature but growing. No biomarker(s) have yet been shown to serve as a sufficiently sensitive and specific marker to diagnose CKDu or its antecedent exposures. Larger studies with comprehensive exposure assessment, longitudinal follow-up, and biopsies for diagnosis will substantially accelerate progress in the study of CKDu biomarkers and facilitate public health approaches that eventually lead to its prevention and elimination.
Appendix
List of Chronic Kidney Disease of UnceRtain Etiology (CKDu) in Agricultural Communities (CURE) Research Consortium
Juan José Amador, MD, MPH; Vivek Bhalla, MD; Daniel Brooks, DSc; Sophie E. Claudel, MD; Jennifer Crowe, PhD, MPH; Mariela Arias-Hidalgo, PhD; Lawrence S. Engel, PhD; Nora Franceschini, MD, MPH; David Friedman, MD, PhD, MPH; Ramón García-Trabanino, MD, MS; Marvin González-Quiroz, MD, PhD; Emmanuel Jarquín, MD; Vivekanand Jha, MBBS, MD, DM; Bonnie Joubert, PhD; Karen Kesler, PhD; Jill Lebov, PhD; Adeera Levin, MD; Susan Mendley, MD; Sumit Mohan, MD; Ana Navas-Acien, MD, PhD; Afshin Parsa, MD, MPH; Madeleine K. Scammell, DSc; and Sushrut S. Waikar, MD MPH.
Disclosure
SSW reports personal fees from Motric Bio, Vertex, PepGen, GSK, and Ikena, outside the submitted work. All the other authors declared no competing interests.
Acknowledgments
Funding was from R38HL143584 (SEC), U01DK130060 (SSW). Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the National Institute of Environmental Health Sciences (NIEHS), and Fogarty International Center (FIC) under Award Numbers U24DK130043, U01DK130060, U01DK130057, U01DK130044, U01DK130058, and U01DK130046. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funding sources had no role in the study design, analysis, interpretation of results, or decision to publish.
Footnotes
PRISMA Checklist.
Web of Science Search (2/13/2023).
CKD Biomarkers and Multi-Omics Platforms Not Yet Studied in CKDu.
Supplementary References.
Table S1. Longitudinal studies of kidney injury biomarkers in CKDu.
Table S2. Urinary Biomarker Concentrations.
Contributor Information
Sushrut S. Waikar, Email: swaikar@bu.edu.
Chronic Kidney Disease of UnceRtain Etiology (CKDu) in Agricultural Communities (CURE) Research Consortium:
Juan José Amador, Vivek Bhalla, Daniel Brooks, Sophie E. Claudel, Jennifer Crowe, Mariela Arias-Hidalgo, Lawrence S. Engel, Nora Franceschini, David Friedman, Ramón García-Trabanino, Marvin González-Quiroz, Emmanuel Jarquín, Vivekanand Jha, Bonnie Joubert, Karen Kesler, Jill Lebov, Adeera Levin, Susan Mendley, Sumit Mohan, Ana Navas-Acien, Afshin Parsa, Madeleine K. Scammell, and Sushrut S. Waikar
Supplementary Material
PRISMA Checklist.
Web of Science Search (2/13/2023).
CKD Biomarkers and Multi-Omics Platforms Not Yet Studied in CKDu.
Supplementary References.
Table S1. Longitudinal studies of kidney injury biomarkers in CKDu.
Table S2. Urinary Biomarker Concentrations.
References
- 1.Anupama Y.J., Sankarasubbaiyan S., Taduri G. Chronic kidney disease of unknown etiology: case definition for India - a perspective. Indian J Nephrol. 2020;30:236–240. doi: 10.4103/ijn.IJN. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wijewickrama E.S., Gunawardena N., Jayasinghe S., Herath C. CKD of unknown etiology (CKDu) in Sri Lanka: a multilevel clinical case definition for surveillance and epidemiological studies. Kidney Int Rep. 2019;4:781–785. doi: 10.1016/j.ekir.2019.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mendley S.R., Levin A., Correa-Rotter R., et al. Chronic kidney diseases in agricultural communities: report from a workshop. Kidney Int. 2019;96:1071–1076. doi: 10.1016/j.kint.2019.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anand S., Caplin B., Gonzalez-Quiroz M., et al. Epidemiology, molecular, and genetic methodologies to evaluate causes of CKDu around the world: report of the working group from the ISN International Consortium of Collaborators on CKDu. Kidney Int. 2019;96:1254–1260. doi: 10.1016/j.kint.2019.09.019. [DOI] [PubMed] [Google Scholar]
- 5.Smyth B., Glaser J., Butler-Dawson J., et al. Challenges and opportunities in interventions for chronic kidney disease of unknown origin (CKDu): report from the International Society of Nephrology Consortium of Collaborators on CKDu. Kidney Int. 2023;103:6–12. doi: 10.1016/j.kint.2022.10.013. [DOI] [PubMed] [Google Scholar]
- 6.Nayak S., Rehman T., Patel K., et al. Factors associated with chronic kidney disease of unknown etiology (CKDu): a systematic review. Healthcare (Switzerland) 2023;11 doi: 10.3390/healthcare11040551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan American Health Organization . Pan American Health Organization; Washington, D.C.: 2017. Epidemic of Chronic Kidney Disease in Agricultural Communities in Central America Case Definitions, Methodological Basis, and Approaches for Public Health Surveillance. [Google Scholar]
- 8.Lunyera J., Mohottige D., von Isenburg M., Jeuland M., Patel U.D., Stanifer J.W. CKD of uncertain etiology: A systematic review. Clin J Am Soc Nephrol. 2016;11:379–385. doi: 10.2215/CJN.07500715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gifford F.J., Gifford R.M., Eddleston M., Dhaun N. Endemic nephropathy around the world. Kidney Int Rep. 2017;2:282–292. doi: 10.1016/j.ekir.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gunawardena S., Dayaratne M., Wijesinghe H., Wijewickrama E. A systematic review of renal pathology in chronic kidney disease of uncertain etiology. Kidney Int Rep. 2021;6:1711–1728. doi: 10.1016/j.ekir.2021.03.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lusco M.A., Fogo A.B., Wernerson A.Ö., Najafian B., Alpers C.E. AJKD atlas of renal pathology: CKD of unknown cause (CKDu); Mesoamerican nephropathy. Am J Kidney Dis. 2017;70:e17–e18. doi: 10.1053/j.ajkd.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Gunasekara T.D.K.S.C., De Silva P.M.C.S., Herath C., et al. The utility of novel renal biomarkers in assessment of chronic kidney disease of unknown etiology (CKDu): a review. Int J Environ Res Public Health. 2020;17:1–21. doi: 10.3390/ijerph17249522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu C., Debnath N., Mosoyan G., et al. Systematic review and meta-analysis of plasma and urine biomarkers for CKD outcomes. J Am Soc Nephrol. 2022;33:1657–1672. doi: 10.1681/ASN.2022010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delanaye P., Glassock R.J., Pottel H., Rule A.D. An age-calibrated definition of chronic kidney disease: rationale and benefits. Clin Biochem Rev. 2016;37:17–26. [PMC free article] [PubMed] [Google Scholar]
- 15.Francis J.M., Beck L.H., Salant D.J. Membranous nephropathy: a journey from bench to bedside jean. Am J Kidney Dis. 2016;68:138–147. doi: 10.1053/j.gastro.2016.08.014.CagY. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lerner G.B., Virmani S., Henderson J.M., Francis J.M., Beck L.H. A conceptual framework linking immunology, pathology, and clinical features in primary membranous nephropathy. Kidney Int. 2021;100:289–300. doi: 10.1016/j.kint.2021.03.028. [DOI] [PubMed] [Google Scholar]
- 17.Guchelaar N.A.D., Waling M.M., Adhin A.A., van Daele P.L.A., Schreurs M.W.J., Rombach S.M. The value of anti-neutrophil cytoplasmic antibodies (ANCA) testing for the diagnosis of ANCA-associated vasculitis, a systematic review and meta-analysis. Autoimmun Rev. 2021;20 doi: 10.1016/j.autrev.2020.102716. [DOI] [PubMed] [Google Scholar]
- 18.Mehta P., Balakrishnan A., Phatak S., Pathak M., Ahmed S. Diagnostic accuracy of antineutrophil cytoplasmic antibodies (ANCA) in predicting relapses of ANCA-associated vasculitis: systematic review and meta-analysis. Rheumatol Int. 2023;43:437–448. doi: 10.1007/s00296-022-05192-3. [DOI] [PubMed] [Google Scholar]
- 19.Parikh C.R., Coca S.G., Thiessen-Philbrook H., et al. Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. J Am Soc Nephrol. 2011;22:1748–1757. doi: 10.1681/ASN.2010121302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koyner J.L., Garg A.X., Coca S.G., et al. Biomarkers predict progression of acute kidney injury after cardiac surgery. J Am Soc Nephrol. 2012;23:905–914. doi: 10.1681/ASN.2011090907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chawla L.S., Eggers P.W., Star R.A. Kimmel PL. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med. 2014;371:58–66. doi: 10.1016/B978-1-4377-0134-0.10085-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mishra J., Qing M.A., Prada A., et al. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14:2534–2543. doi: 10.1097/01.ASN.0000088027.54400.C6. [DOI] [PubMed] [Google Scholar]
- 23.Mishra J., Mori K., Ma Q., Kelly C., Barasch J., Devarajan P. Neutrophil gelatinase-associated lipocalin: a novel early urinary biomarker for cisplatin nephrotoxicity. Am J Nephrol. 2004;24:307–315. doi: 10.1159/000078452. [DOI] [PubMed] [Google Scholar]
- 24.Soni S.S., Cruz D., Bobek I., et al. NGAL: A biomarker of acute kidney injury and other systemic conditions. Int Urol Nephrol. 2010;42:141–150. doi: 10.1007/s11255-009-9608-z. [DOI] [PubMed] [Google Scholar]
- 25.De Silva P.M.C.S., Gunasekara T.D.K.S.C., Gunarathna S.D., et al. Urinary biomarkers of renal injury KIM-1 and NGAL: reference intervals for healthy pediatric population in Sri Lanka. Children (Basel) 2021;8:684. doi: 10.3390/children8080684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leibler J.H., Ramirez-rubio O., José J., et al. Biomarkers of kidney injury among children in a high-risk region for chronic kidney disease of uncertain etiology. Pediatr Nephrol. 2021;36:387–396. doi: 10.1007/s00467-020-04595-3. [DOI] [PubMed] [Google Scholar]
- 27.Gonzalez-Quiroz M., Smpokou E.T., Pearce N., Caplin B., Nitsch D. Identification of young adults at risk of an accelerated loss of kidney function in an area affected by Mesoamerican nephropathy. BMC Nephrol. 2019;20:1–8. doi: 10.1186/s12882-018-1193-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gonzalez-Quiroz M., Smpokou E.T., Silverwood R.J., et al. Decline in kidney function among apparently healthy young adults at risk of Mesoamerican nephropathy. J Am Soc Nephrol. 2018;29:2200–2212. doi: 10.1681/ASN.2018020151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Silva P.M.C.S., Mohammed Abdul K.S., Eakanayake E.M.D.V., et al. Urinary biomarkers KIM-1 and NGAL for detection of chronic kidney disease of uncertain etiology (CKDu) among agricultural communities in Sri Lanka. PLOS Negl Trop Dis. 2016;10:1–17. doi: 10.1371/journal.pntd.0004979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fernando B.N.T.W., Alli-Shaik A., Hemage R.K.D., et al. Pilot study of renal urinary biomarkers for diagnosis of CKD of uncertain etiology. Kidney Int Rep. 2019;4:1401–1411. doi: 10.1016/j.ekir.2019.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abdul K.S.M., De S.P.M.C.S., Ekanayake E.M.D.V., et al. Ekanayake EMD V, Thakshila WAKG. Occupational paraquat and glyphosate exposure may decline renal functions among rural farming communities in Sri Lanka. Int J Environ Res Public Health. 2021;18:3278. doi: 10.3390/ijerph18063278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ekanayake E.M.D.V., Gunasekara T.D.K.S.C., De Silva P.M.C.S., Jayasinghe S., Chandana E.P.S., Jayasundara N. Applicability of novel urinary biomarkers for the assessment of renal injury in selected occupational groups in Sri Lanka: a comparative study with conventional markers. Int J Environ Res Public Health. 2022;19:5264. doi: 10.3390/ijerph19095264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laws R.L., Brooks D.R., Amador J.J., et al. Biomarkers of kidney injury among Nicaraguan sugarcane workers. Am J Kidney Dis. 2016;67(Biomarkers):209–217. doi: 10.1053/j.ajkd.2015.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wesseling C., Aragón A., González M., et al. Kidney function in sugarcane cutters in Nicaragua – a longitudinal study of workers at risk of Mesoamerican nephropathy. Environ Res. 2016;147:125–132. doi: 10.1016/j.envres.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 35.Kulasooriya P.N., Jayasekara K.B., Nisansala T., et al. Utility of self-reported heat stress symptoms and NGAL biomarker to screen for chronic kidney disease of unknown origin (CKDu) in Sri Lanka. Int J Environ Res Public Health. 2021;18:1–11. doi: 10.3390/ijerph181910498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diaz de León-Martínez L., Díaz-Barriga F., Barbier O., et al. Evaluation of emerging biomarkers of renal damage and exposure to aflatoxin-B 1 in Mexican indigenous women: a pilot study. Environ Sci Pollut Res Int. 2019;26:12205–12216. doi: 10.1007/s11356-019-04634-z. [DOI] [PubMed] [Google Scholar]
- 37.Butler-dawson J., James K.A., Krisher L., et al. Environmental metal exposures and kidney function of Guatemalan sugarcane workers. J Expo Sci Environ Epidemiol. 2022;32:461–471. doi: 10.1038/s41370-021-00292-x.Environmental. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cardenas-Gonzalez M., Osorio-Yanez C., Gaspar-Ramirez O., et al. Environmental exposure to arsenic and chromium in children is associated with kidney injury Molecule-1. Environ Res. 2016;150:653–662. doi: 10.1016/j.envres.2016.06.032.Environmental. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gunasekara T.D.K.S.C., De Silva P.M.C.S., Chandana E.P.S., et al. Environmental fluoride exposure and implications on potential pediatric kidney health risks: an approach with urinary biomarkers. Pediatr Nephrol. 2024;39:1469–1480. doi: 10.1007/s00467-023-06218-z. [DOI] [PubMed] [Google Scholar]
- 40.Zúñiga-Venegas L.A., Hyland C., Muñoz-Quezada M.T., et al. Health effects of pesticide exposure in Latin American and the Caribbean populations: A scoping review. Environ Health Perspect. 2022;130 doi: 10.1289/EHP9934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petropoulos Z.E., Laws R.L., Jose J., et al. Kidney function, self-reported symptoms, and urine findings in Nicaraguan sugarcane workers. Kidney360. 2020;1:1042–1051. doi: 10.34067/KID.0003392020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramírez-Rubio O., Amador J.J., Kaufman J.S., et al. Urine biomarkers of kidney injury among adolescents in Nicaragua, a region affected by an epidemic of chronic kidney disease of unknown aetiology. Nephrol Dial Transplant. 2016;31:424–432. doi: 10.1093/ndt/gfv292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ichimura T., Bonventre J.V., Bailly V., et al. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem. 1998;273:4135–4142. doi: 10.1074/jbc.273.7.4135. [DOI] [PubMed] [Google Scholar]
- 44.Bonventre J.V. Kidney injury molecule-1 (KIM-1): a urinary biomarker and much more. Nephrol Dial Transplant. 2009;24:3265–3268. doi: 10.1093/ndt/gfp010. [DOI] [PubMed] [Google Scholar]
- 45.Ichimura T., Asseldonk E.J.P.V., Humphreys B.D., Gunaratnam L., Duffield J.S., Bonventre J.V. Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J Clin Invest. 2008;118:1657–1668. doi: 10.1172/JCI34487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Humphreys B.D., Xu F., Sabbisetti V., et al. Chronic epithelial kidney injury molecule-1 expression causes murine kidney fibrosis. J Clin Invest. 2013;123:4023–4035. doi: 10.1172/JCI45361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hansson E., Wegman D.H., Wesseling C., et al. Markers of kidney tubular and interstitial injury and function among sugarcane workers with cross- harvest serum creatinine elevation. Occup Environ Med. 2022;79:396–402. doi: 10.1136/oemed-2021-107989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wanigasuriya K., Jayawardene I., Amarasiriwardena C., Wickremasinghe R. Novel urinary biomarkers and their association with urinary heavy metals in chronic kidney disease of unknown aetiology in Sri Lank: a pilot study. Ceylon Med J. 2017;62:210–217. doi: 10.4038/cmj.v62i4.8568. [DOI] [PubMed] [Google Scholar]
- 49.Parikh C.R., Jani A., Melnikov V., Faubel S., Edelstein C.L. Urinary interleukin-18 as a marker of human acute tubular necrosis. Am J Kidney Dis. 2004;43:405–414. doi: 10.1053/j.ajkd.2003.10.040. [DOI] [PubMed] [Google Scholar]
- 50.VanderBrink B., Asanuma H., Hile Ka Z.H., Rink R.C., Meldrum K.K. IL-18 stimulates a positive feedback loop during renal obstruction via the IL-18 receptor. J Urol. 2011;186:1502–1508. doi: 10.1016/j.juro.2011.05.046.IL-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Franke E.I., Vanderbrink B.A., Hile K.L., et al. Renal IL-18 production is macrophage independent during obstructive injury. PLoS One. 2012;7 doi: 10.1371/journal.pone.0047417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Edelstein C.L., Hoke T.S., Somerset H., et al. Proximal tubules from caspase-1-deficient mice are protected against hypoxia-induced membrane injury. Nephrol Dial Transplant. 2007;22:1052–1061. doi: 10.1093/ndt/gfl775. [DOI] [PubMed] [Google Scholar]
- 53.Bani-Hani A.H., Leslie J.A., Asanuma H., et al. IL-18 neutralization ameliorates obstruction-induced epithelial-mesenchymal transition and renal fibrosis. Kidney Int. 2009;76:500–511. doi: 10.1038/ki.2009.216. [DOI] [PubMed] [Google Scholar]
- 54.Waldmann T.A., Strober W., Mogielnicki R.P. The renal handling of low molecular weight proteins. II. Disorders of serum protein catabolism in patients with tubular proteinuria, the nephrotic syndrome, or uremia. J Clin Invest. 1972;51:2162–2174. doi: 10.1172/jci107023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chan W.Y., Rennert O.M. Cadmium nephropathy. Ann Clin Lab Sci. 1981;11:229–238. doi: 10.1007/978-1-4684-4214-4_26. [DOI] [PubMed] [Google Scholar]
- 56.Manuel Y., Colle A., Leclercq M., Tonnelle C. Low molecular weight proteinuria. Contrib Nephrol. 1975;1:156–162. doi: 10.1146/annurev.me.30.020179.001215. [DOI] [PubMed] [Google Scholar]
- 57.Beetham R., Newman D. Urinary albumin and low molecular weight protein excretion in the nephrotic syndrome - sequential studies during corticosteroid treatment. Ann Clin Biochem. 1992;29:450–453. doi: 10.1177/000456329202900414. [DOI] [PubMed] [Google Scholar]
- 58.Aoshima K. Epidemiology of renal tubular dysfunction in the inhabitants of a cadmium-polluted area in the Jinzu River Basin in Toyama Prefecture. Tohoku J Exp Med. 1987;152:151–172. doi: 10.1620/tjem.152.151. [DOI] [PubMed] [Google Scholar]
- 59.Ikeda M., Watanabe T., Nakatsuka H., et al. Cadmium exposure in general populations in Japan: a review. J Food Saf. 2015;3:118–135. doi: 10.14252/foodsafetyfscj.2015020. [DOI] [Google Scholar]
- 60.Siriwardhana E.R.I., Perera P.A., Sivakanesan R., Abeysekara T., Nugegoda D.B., Weerakoon K.G. Is the staple diet eaten in Medawachchiya, Sri Lanka, a predisposing factor in the development of chronic kidney disease of unknown etiology? - A comparison based on urinary β2-microglobulin measurements. BMC Nephrol. 2014;15:1–9. doi: 10.1186/1471-2369-15-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vaidya V.S., Niewczas M.A., Ficociello L.H., et al. Regression of microalbuminuria in type 1 diabetes is associated with lower levels of urinary tubular injury biomarkers, kidney injury molecule-1, and N-acetyl-Β-D-glucosaminidase. Kidney Int. 2011;79:464–470. doi: 10.1038/ki.2010.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nogawa K., Yamada Y., Honda R., Tsuritani I., Ishizaki M., Sakamoto M. Urinary N-acetyl-B-D-Glucosaminidase and β2-microglobulin in “Itai-Itai” disease. Toxicol Lett. 1983;16:317–322. doi: 10.1016/0378-4274(83)90193-5. [DOI] [PubMed] [Google Scholar]
- 63.Nanayakkara S., Senevirathna S.T.M.L.D., Karunaratne U., et al. Evidence of tubular damage in the very early stage of chronic kidney disease of uncertain etiology in the North Central Province of Sri Lanka: A cross-sectional study. Environ Health Prev Med. 2012;17:109–117. doi: 10.1007/s12199-011-0224-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ratajczyk K., Konieczny A., Czekaj A., et al. The clinical significance of urinary retinol-binding Protein 4: a review. Int J Environ Res Public Health. 2022;19 doi: 10.3390/ijerph19169878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Swa H.L.F., Fernando B.N.T., Premarathna S., et al. Evaluating serum RBP4 as an auxiliary biomarker for CKDu diagnosis. Kidney Dial. 2022;2:576–587. doi: 10.3390/kidneydial2040052. [DOI] [Google Scholar]
- 66.Mcmurray M.D., Trivax J.E., Mccullough P.A. Editorial]. Serum cystatin C, renal filtration function, and left ventricular remodeling. Circ Heart Fail. 2009;2:86–89. doi: 10.1161/CIRCHEARTFAILURE.109.856393. [DOI] [PubMed] [Google Scholar]
- 67.Sandamini P.M.M.A., De Silva P.M.C.S., Gunasekara T.D.K.S.C., et al. Urinary cystatin C: pediatric reference intervals and comparative assessment as a biomarker of renal injury among children in the regions with high burden of CKDu in Sri Lanka. World J Pediatr. 2022;18:196–205. doi: 10.1007/s12519-022-00513-9. [DOI] [PubMed] [Google Scholar]
- 68.Stubbs J.R., Zhang S., Jansson K.P., et al. Critical role of osteopontin in maintaining urinary phosphate solubility in CKD. Kidney360. 2022;3:1578–1589. doi: 10.34067/kid.0007352021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Khamissi F.Z., Ning L., Kefaloyianni E., et al. Identification of kidney injury–released circulating osteopontin as causal agent of respiratory failure. Sci Adv. 2022;8:1–19. doi: 10.1126/sciadv.abm5900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zoja C., Liu X.H., Donadelli R., et al. Renal expression of monocyte chemoattractant protein-1 in lupus autoimmune mice. J Am Soc Nephrol. 1997;8:720–729. doi: 10.1681/asn.v85720. [DOI] [PubMed] [Google Scholar]
- 71.Gunawickrama S.H.N.P., Hewavitharana I.G., Nanayakkara P.G.C.L., Gunawickrama K.B.S. Chronic Kidney Disease of Unknown Etiology (CKDu) in Sri Lanka: hematological changes and pro-inflammation suggest likely predictors of advance disease, as renal outcomes show prevalent normoalbuminuria. Diseases. 2022;10:2. doi: 10.3390/diseases10010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dieterle F., Perentes E., Cordier A., et al. Urinary clusterin, cystatin C, Β2-microglobulin and total protein as markers to detect drug-induced kidney injury. Nat Biotechnol. 2010;28:463–469. doi: 10.1038/nbt.1622. [DOI] [PubMed] [Google Scholar]
- 73.Herath H.M.S., Kawakami T., Nagasawa S., et al. Arsenic, cadmium, lead, and chromium in well water, rice, and human urine in Sri Lanka in relation to chronic kidney disease of unknown etiology. J Water Health. 2018;16:212–222. doi: 10.2166/wh.2018.070. [DOI] [PubMed] [Google Scholar]
- 74.Hsu C., Ballard S., Batlle D., et al. Cross-disciplinary biomarkers research: lessons learned by the CKD biomarkers consortium. Clin J Am Soc Nephrol. 2015;10:894–902. doi: 10.2215/CJN.11541114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Whitcomb B., Perkins N., Albert P., Schisterman E. Treatment of batch in the detection, calibration, and quantification of immunoassays in large-scale epidemiologic studies. Epidemiology. 2010;21(suppl 4):S44–S50. doi: 10.1097/EDE.0b013e3181dceac2.Treatment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Andersson L., Haraldsson B., Johansson C., Barregard L. Methodological issues on the use of urinary alpha-1-microglobuline in epidemiological studies. Nephrol Dial Transplant. 2008;23:1252–1256. doi: 10.1093/ndt/gfm729. [DOI] [PubMed] [Google Scholar]
- 77.Schuh M.P., Nehus E., Ma Q., et al. Long-term stability of urinary biomarkers of acute kidney injury in children. Am J Kidney Dis. 2016;67:56–61. doi: 10.1053/j.ajkd.2015.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Giesen C., Lieske J. The influence of processing and storage conditions on renal protein biomarkers. Clin J Am Soc Nephrol. 2016;11:1726–1728. doi: 10.2215/CJN.08800816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pedersen K.R., Ravn H.B., Hjortdal V.E., Nørregaard R., Povlsen J.V. Neutrophil gelatinase-associated lipocalin (NGAL): validation of commercially available ELISA. Scand J Clin Lab Investig. 2010;70:374–382. doi: 10.3109/00365513.2010.486868. [DOI] [PubMed] [Google Scholar]
- 80.Revuelta-López E., Barallat J., Cserkoóvá A., et al. Pre-analytical considerations in biomarker research: focus on cardiovascular disease. Clin Chem Lab Med. 2021;59:1747–1760. doi: 10.1515/cclm-2021-0377. [DOI] [PubMed] [Google Scholar]
- 81.Waikar S.S., Sabbisetti V.S., Bonventre J.V. Normalization of urinary biomarkers to creatinine during changes in glomerular filtration rate. Kidney Int. 2010;78:486–494. doi: 10.1038/ki.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Adedeji A.O., Pourmohamad T., Chen Y., et al. Investigating the value of urine volume, creatinine, and cystatin C for urinary biomarkers normalization for drug development studies. Int J Toxicol. 2019;38:12–22. doi: 10.1177/1091581818819791. [DOI] [PubMed] [Google Scholar]
- 83.Wettersten N., Katz R., Shlipak M.G., et al. Urinary biomarkers and kidney outcomes: impact of indexing versus adjusting for urinary creatinine. Kidney Med. 2021;3:546–554.e1. doi: 10.1016/j.xkme.2021.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tang K.W.A., Toh Q.C., Teo B.W. Normalisation of urinary biomarkers to creatinine for clinical practice and research – when and why. Singapore Med J. 2015;56:7–10. doi: 10.11622/smedj.2015003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pearson K. Mathematical contributions to the theory of evolution—on a form of spurious correlation which may arise when indices are used in the measurement of organs. Proceedings of the Royal Society of London. The Royal Society. 1897;60:489–498. [Google Scholar]
- 86.Kim J.H. Spurious correlation between ratios with a common divisor. Statistics & Probability Letters. 1999;44:383–386. doi: 10.1016/S0167-7152(99)00030-9. www.elsevier.nl/locate/stapro [DOI] [Google Scholar]
- 87.FDA-NIH Biomarker Working Group . US Food and Drug Administration; Silver Spring (MD): 2021. BEST (Biomarkers, EndpointS, and other Tools) [PubMed] [Google Scholar]
- 88.CKDu Cure Consortium 2022. https://ckducureconsortium.org/
- 89.National Institutes of Health Chronic kidney diseases of UnceRtain etiology (CKDu) in agricultural communities (CURE) research consortium - field epidemiology sites (U01 - clinical trial not allowed) Request for application. 2020 https://grants.nih.gov/grants/guide/rfa-files/RFA-DK-20-017.html [Google Scholar]
- 90.National Institutes of Health Chronic kidney diseases of UnceRtain etiology (CKDu) in agricultural communities (CURE) research consortium: Scientific Data Coordinating Center (U24 - clinical trial not allowed) Request for Application. 2020 https://grants.nih.gov/grants/guide/rfa-files/RFA-DK-20-019.html [Google Scholar]
- 91.National Institutes of Health Chronic kidney diseases of UnceRtain etiology (CKDu) in agricultural communities (CURE) research consortium – renal science core (U01 - clinical trial not allowed) Request for application. 2020 https://grants.nih.gov/grants/guide/rfa-files/RFA-DK-20-018.html [Google Scholar]
- 92.O’Brien K.M., Upson K., Cook N.R., Weinberg C.R. Environmental chemicals in urine and blood: improving methods for creatinine and lipid adjustment. Environ Health Perspect. 2016;124:220–227. doi: 10.1289/ehp.1509693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.U.S. Department of Health and Human Services National Toxicology Program . 2015. Handbook for Conducting a Literature-Based Health Assessment Using OHAT Approach for Systematic Review and Evidence Integration.https://ntp.niehs.nih.gov/sites/default/files/ntp/ohat/pubs/handbookmarch2019_508.pdf [Google Scholar]
- 94.Wijkström J., Jayasumana C., Dassanayake R., Priyawardane N., Godakanda N., Siribaddana S., Ring A., Hultenby K., Söderberg M., Elinder C.G., Wernerson A. Morphological and clinical findings in Sri Lankan patients with chronic kidney disease of unknown cause (CKDu): Similarities and differences with Mesoamerican Nephropathy. PLoS One. 2018 Mar 7;13(3):e0193056. doi: 10.1371/journal.pone.0193056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gunasekara T.D.K.S.C., De Silva P.M.C.S., Ekanayake E.M.D.V., Thakshila W.A.K.G., Pinipa R.A.I., Sandamini P.M.M.A., Gunarathna S.D., Chandana E.P.S., Jayasinghe S.S., Herath C., Siribaddana S., Jayasundara N. Urinary biomarkers indicate pediatric renal injury among rural farming communities in Sri Lanka. Sci Rep. 2022 May 16;12(1):8040. doi: 10.1038/s41598-022-10874-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.