Key Points
Question
Is preoperative skin antisepsis with povidone iodine in alcohol noninferior to chlorhexidine gluconate in alcohol to prevent surgical site infections after cardiac or abdominal surgery?
Findings
In this randomized clinical trial that included 3360 patients, surgical site infections after cardiac or abdominal surgery were identified in 80 patients (5.1%) in the povidone iodine group vs 97 (5.5%) in the chlorhexidine gluconate group, a difference of 0.4% that met the predefined noninferiority margin of an absolute difference of 2.5%.
Meaning
Povidone iodine was noninferior compared with chlorhexidine gluconate as preoperative skin antisepsis in preventing surgical site infections after cardiac or abdominal surgery.
Abstract
Importance
Preoperative skin antisepsis is an established procedure to prevent surgical site infections (SSIs). The choice of antiseptic agent, povidone iodine or chlorhexidine gluconate, remains debated.
Objective
To determine whether povidone iodine in alcohol is noninferior to chlorhexidine gluconate in alcohol to prevent SSIs after cardiac or abdominal surgery.
Design, Setting, and Participants
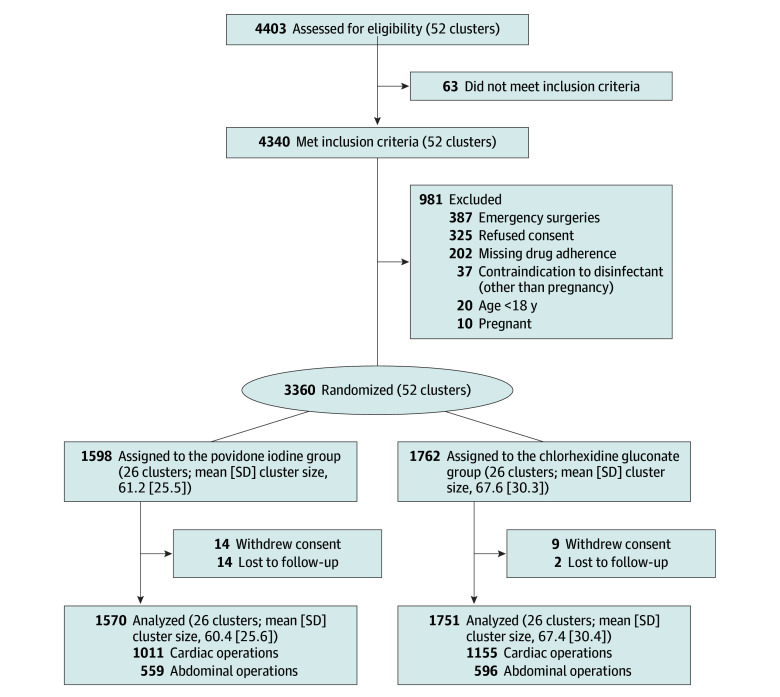
Multicenter, cluster-randomized, investigator-masked, crossover, noninferiority trial; 4403 patients undergoing cardiac or abdominal surgery in 3 tertiary care hospitals in Switzerland between September 2018 and March 2020 were assessed and 3360 patients were enrolled (cardiac, n = 2187 [65%]; abdominal, n = 1173 [35%]). The last follow-up was on July 1, 2020.
Interventions
Over 18 consecutive months, study sites were randomly assigned each month to either use povidone iodine or chlorhexidine gluconate, each formulated in alcohol. Disinfectants and skin application processes were standardized and followed published protocols.
Main Outcomes and Measures
Primary outcome was SSI within 30 days after abdominal surgery and within 1 year after cardiac surgery, using definitions from the US Centers for Disease Control and Prevention’s National Healthcare Safety Network. A noninferiority margin of 2.5% was used. Secondary outcomes included SSIs stratified by depth of infection and type of surgery.
Results
A total of 1598 patients (26 cluster periods) were randomly assigned to receive povidone iodine vs 1762 patients (26 cluster periods) to chlorhexidine gluconate. Mean (SD) age of patients was 65.0 years (39.0-79.0) in the povidone iodine group and 65.0 years (41.0-78.0) in the chlorhexidine gluconate group. Patients were 32.7% and 33.9% female in the povidone iodine and chlorhexidine gluconate groups, respectively. SSIs were identified in 80 patients (5.1%) in the povidone iodine group vs 97 (5.5%) in the chlorhexidine gluconate group, a difference of 0.4% (95% CI, −1.1% to 2.0%) with the lower limit of the CI not exceeding the predefined noninferiority margin of −2.5%; results were similar when corrected for clustering. The unadjusted relative risk for povidone iodine vs chlorhexidine gluconate was 0.92 (95% CI, 0.69-1.23). Nonsignificant differences were observed following stratification by type of surgical procedure. In cardiac surgery, SSIs were present in 4.2% of patients with povidone iodine vs 3.3% with chlorhexidine gluconate (relative risk, 1.26 [95% CI, 0.82-1.94]); in abdominal surgery, SSIs were present in 6.8% with povidone iodine vs 9.9% with chlorhexidine gluconate (relative risk, 0.69 [95% CI, 0.46-1.02]).
Conclusions and Relevance
Povidone iodine in alcohol as preoperative skin antisepsis was noninferior to chlorhexidine gluconate in alcohol in preventing SSIs after cardiac or abdominal surgery.
Trial Registration
ClinicalTrials.gov Identifier: NCT03685604
This multicenter, cluster-randomized, noninferiority trial investigates the effectiveness of povidone iodine vs chlorhexidine gluconate in alcohol as preoperative skin antisepsis in preventing surgical site infections in adults undergoing abdominal or cardiac surgery.
Introduction
Approximately 0.5% to 3% of patients undergoing surgery will experience infection at or adjacent to the surgical incision site.1 Microorganisms that colonize the patient’s skin are considered the major source of causative pathogens.2 Alcohol-based solutions have become the standard for preparing intact skin, either with chlorhexidine gluconate or povidone iodine compounds.3 The World Health Organization (WHO) favors alcohol-based solutions with chlorhexidine gluconate over povidone iodine, although the US Centers for Disease Control and Prevention (CDC) and the Surgical Infection Society recommend either of these compounds.3,4,5 Two meta-analyses concluded that chlorhexidine gluconate disinfectants significantly reduce the rate of surgical site infections (SSIs) compared with povidone iodine.6,7 More recent randomized clinical trials challenge these conclusions by observing that chlorhexidine gluconate is not superior to povidone iodine.8,9 Currently, the preferred preparation remains controversial because choice of drug, exposure time, and number of applications likely influence the results of skin preparation. There has been a lack of standardization of these factors in previous trials, which did not compare compounds with similar alcohol-based formulations but also had relatively small sample sizes, were single center, and/or were limited to specific surgical procedures.8,9,10,11
Alcohol provides no residual activity after application, whereas iodophors and chlorhexidine exhibit prolonged bacteriostatic activity on the skin. Some studies indicate that this effect may be less prominent in iodophors than in chlorhexidine.12 Chlorhexidine performed better compared with iodophors in other indications, such as the prevention of catheter-associated bloodstream infections or hospital-acquired infections in intensive care units,13 and therefore the UK National Institute for Health and Care Excellence cites chlorhexidine compounds as the first choice for surgical skin preparation.14 However, growing evidence of decreased susceptibility or even emergence of resistance to chlorhexidine is a matter of concern15 because this agent is widely used beyond operating rooms, for example, in intensive care units for bathing and for vascular access care.2,13 However, the evidence to promote a transition from povidone iodine to chlorhexidine gluconate for all surgical interventions due to putative inferiority of povidone iodine appears to be insufficient.8,9,16,17
Therefore a multicenter, cluster-randomized, crossover trial was initiated comparing povidone iodine with chlorhexidine gluconate (both formulated in alcohol), with products exclusively produced and labeled for the study by the manufacturer, including rigorous training and monitoring of the 3 sequential skin disinfectant applications18 and standardized active surveillance of SSI with regular on-site quality checks.19,20
Methods
Study Design
This multicenter, cluster-randomized, investigator-masked crossover study was designed as a noninferiority trial. The participating tertiary care centers were the University Hospital Basel, Inselspital Bern University Hospital, and University Hospital Zurich in Switzerland. Together, these hospitals serve a population of approximately 2.5 million people and have a total of 2647 beds.19 This study follows the CONSORT 2010 reporting guidelines for randomized trials and the respective extensions to noninferiority and cluster randomized trials.
Participants
All adult patients with scheduled elective abdominal or cardiac surgery (coronary artery bypass graft surgery, valve surgery, or other cardiac surgery with the exclusion of cardiac transplants) during the period from September 2018 until March 2020 were eligible for participation, as long as they were included in national SSI surveillance.21 Of note, 1 of the centers started recruitment 8 weeks later due to unresolved contract issues. Due to a relative contraindication for 1 of the compounds, povidone iodine, during pregnancy, pregnant patients were excluded. Patients with any contraindications to the study compounds or those undergoing emergent surgical procedures were excluded because adequate observation of the disinfection process could not be ensured and time limitations during emergencies do not always allow for meeting all steps for preparation of the surgical site, especially the recommended exposure time.
Regional human research ethics committees approved the study (Swiss Association of Research Ethics Committees approval EKNZ 2018-00962). Individual patient consent was waived because both study compounds were routinely used in the participating hospitals prior to the study, and all patients provided written general consent to allow their coded data to be used for clinical research. Patients who declined general consent were excluded from the study.
Randomization, Masking, and Data Monitoring
Patients were cluster-randomized with treatment assignment randomly allocated according to month within center (cluster-periods). Group assignments were centrally computer-generated using stratification per center and within centers using a 1:1 randomization ratio and blocks of 2. The head study nurse transmitted the cluster randomization to the site study nurse and the site study nurse checked the correct distribution of the disinfectants in the operating room tracts of the respective surgical specialties at the beginning of each month. Treatment assignment was masked toward the personnel assessing the outcomes, the site investigators, the primary investigator, and the study statistician; masking of patients and surgeons was not possible due to the intrinsic color of the study agents. Central monitoring was conducted by the University of Basel study board, and the Basel Clinical Trial Unit oversaw the trial and performed independent on-site validation and standardized monitoring visits. Routine on-site quality checks were conducted by the study center and by study-independent health care professionals.
Procedures
This trial investigated whether povidone iodine in alcohol as preparation of the surgical site is noninferior to chlorhexidine gluconate in alcohol in preventing SSI after cardiac or abdominal surgery. It compared the incidence of SSI after preparation of the surgical site prior to incision with 2 commercially available alcohol-based products containing povidone iodine or chlorhexidine gluconate, respectively. Before incision, the surgical site was disinfected with either povidone iodine (Braunoderm, B. Braun, containing 50.0 g propan-2-ol, 1 g povidone iodine in 100 mL, resulting in 10% free available iodine) or with chlorhexidine gluconate (Softasept CHX colored 2%, B. Braun Medical AG, composed of 20 mg chlorhexidine digluconate and 0.7 mL ethanol vol/vol per mL). Both compounds were produced, labeled, and distributed by a single manufacturer exclusively for the study to ensure maximum standardization of the products. Study products were delivered to the operating rooms by each hospital’s pharmacy according to the randomization of the unit and month. Only designated study flasks from the same production lot were accepted for use and were purchased using grant funding from the Swiss National Science Foundation. The application of the disinfectants followed a standardized procedure, as previously published. In brief, all centers were trained to apply the antiseptic agent with a gauze, allowing it to dry completely before applying a series of 3 applications for a total exposure time of at least 3 minutes.18
A single study coordinator trained local study personnel to ensure uniformity. At each center, 2 skin disinfection processes were observed each week and documented for agent used, number of disinfectant applications, and disinfectant exposure time. Follow-up of study patients consisted of telephone interviews and screening of medical records 30 days after the intervention in case of abdominal and cardiac operations and again after 1 year for cardiac operations. The extended follow-up time frame in cardiac surgery was chosen because the sternum is closed with wires that are considered foreign bodies, a situation in which a longer follow-up increases the sensitivity to detect SSIs.
SSI prevention measures were otherwise mostly uniform across the study centers because they all followed Swissnoso (Swiss National Center for Infection Prevention, a professional organization issuing national guidelines) recommendations. Hair removal was performed using clipping only in all centers. Graft harvesting (for coronary artery bypass graft surgery) was performed using a separate instrument tray. Preoperative chlorhexidine bathing was not implemented during the study period, nor was oral antibiotic bowel preparation for colon surgery. Moisturizing skin lotions in postoperative care were the same 2 formulations in all centers. One center differed from the others in that it did not implement preoperative Staphylococcus aureus decolonization using mupirocin nasal ointment for elective cardiac surgery during the study period. Antibiotics used for perioperative prophylaxis are listed in eTable 3 in Supplement 1.
Primary and Secondary Outcomes
The primary outcome was the occurrence of SSI within 30 days after abdominal surgery and 1 year after cardiac surgery, as specified by the CDC’s National Healthcare Safety Network definitions, focusing on the primary incision site.22 In spring 2020, the 1-year follow-up for patients who underwent cardiac surgery was shortened to 90 days due to the increased workload during the COVID-19 pandemic.20 A supplementary analysis was performed to investigate the effect of this shortening (eAppendix C, eTable 2 in Supplement 1).
Diagnosis of SSI was made by qualified infection control personnel. A board-certified infectious disease specialist confirmed each of these SSI diagnoses using the above-mentioned definitions.
Exploratory predefined subgroup analyses investigated the proportion of SSI and relative risk (RR) stratified by depth class (superficial incisional, deep incisional, organ space), type of surgery, timing of antimicrobial prophylaxis, duration of surgery (T-time23), wound contamination class according to the CDC’s National Healthcare Safety Network, sex, body weight (BMI [calculated as weight in kilograms divided by height in meters squared] of 25 as cutoff for overweight), American Society of Anesthesiologists score, presence of diabetes, arterial hypertension, active tobacco use, absence of anemia (hemoglobin >12 g/dL), absence of kidney failure by serum creatinine (>1.3 mg/dL as cutoff), C-reactive protein (dichotomous, 1 mg/dL as cutoff), along with in-hospital mortality and length of hospital stay.
Mortality was not considered the primary end point because the incidence was expected to be very low, and data on out-of-hospital mortality were not feasible because this would have required individual informed consent.
Statistical Analysis
The sample size was calculated for an intention-to-treat analysis. Based on the latest records of the national SSI surveillance data from 2011 to 2015 at the time of study planning, we estimated the crude SSI rate in cardiac surgery to be 5% and in abdominal surgery to be 10%, resulting in an overall crude mean SSI rate of 7.5%, assuming equal proportions of the respective surgery types. We designed the study to have 80% power for a 2-tailed significance level of 5%, with an absolute noninferiority margin of 2.5% (ie, 7.5% ± 2.5%). A narrow noninferiority margin was chosen given that small differences in preventive effectiveness translate to large absolute differences in infections because skin antisepsis is universally used in surgical procedures worldwide. We estimated that the study would need to enroll at least 1374 patients in each group to achieve the desired power (TrialSize package in R, function TwoSampleProportion.NIS [R Foundation]). Furthermore, we assumed up to 10% of patients would be lost to follow-up or drop out of the trial, leading to a total sample size of 1527 in each group, or 3054 patients overall. This original sample size calculation did not consider potential cluster correlations despite the cluster-randomized design. We performed a post hoc sensitivity analysis using an alternative sample size calculation accounting for cluster and cluster period effects in the context of a mixed-effects analysis (see eAppendix A in Supplement 1 for further details). Descriptive and univariable statistics were used to characterize the study patients and compare the baseline characteristics of the randomized patients.
The primary analysis compared the overall proportion of patients with SSI in the povidone iodine group with the chlorhexidine gluconate group to investigate the noninferiority of povidone iodine to the reference standard chlorhexidine gluconate. Noninferiority was defined as the lower Wald type 95% CI limit excluding the predefined noninferiority margin of −2.5%.
As secondary analyses, mixed-effects logistic models with SSI as end point, including center as fixed effect and period as random effect, and the estimates from a generalized estimating equation population mean model with jackknifed standard errors were also compared (eAppendix B, eTable 1 in Supplement 1). Univariable (mixed-effects) logistic regression models were fitted to confirm SSI risk factors and to determine whether SSI rates differed by procedure type. Period effects were investigated post hoc using box plots, histograms, and time series plots of the rate per period, stratified by intervention group (eFigures 3-6 in Supplement 1). A logistic interrupted time series model was fitted to analyze the extent of effects when transitioning from povidone iodine to chlorhexidine gluconate or vice versa. Handling of missing data is described in eAppendix D, eFigure 8, and eTables 4-5 in Supplement 1.
A level of 5% was deemed statistically significant, and all analyses were performed in R (R Foundation for Statistical Computing).
Results
A total of 4403 patients were assessed for the study and 3360 enrolled (52 cluster periods: 18 in center A, 18 in center B, and 16 in center C; eFigure 7 in Supplement 1 depicts the randomized cluster period allocations per center). Reasons for exclusion and details on patient flow are displayed in Figure 1. Data from 3321 patients were included in the analysis, a 0.5% loss to follow-up. Hospitals recruited similar percentages of eligible patients based on their size and general volume of surgical interventions (Table 1). Seven percent of patients declined general consent to use their coded data for scientific evaluation, and the Swiss data protection law did not allow access to determine the individual reasons for refusal. A shortened follow-up due to the COVID-19 pandemic was used in patients undergoing cardiac surgery in 16 of 52 cluster periods (see above). The cluster-randomized crossover design resulted in comparable rates from month to month and no significant center effect was observed (eFigure 2 in Supplement 1).
Figure 1. Patient Flow Diagram.
Table 1. Patient Characteristics.
| Patient levela | Cluster level, median (10th and 90th percentile)b | |||
|---|---|---|---|---|
| Povidone iodine (n = 1570) | Chlorhexidine gluconate (n = 1751) | Povidone iodine (n = 26) | Chlorhexidine gluconate (n = 26) | |
| Patient demographics | ||||
| Age, median (10th and 90th percentile), y | 65.0 (39.0 to 79.0) | 65.0 (41.0 to 78.0) | 65.0 (61.5 to 66.3) | 65.8 (62.5 to 68.5) |
| Sex, No. (%) | ||||
| Male | 1056 (67.3) | 1158 (66.1) | 69 (62 to 77) | 68 (58 to 77) |
| Female | 514 (32.7) | 593 (33.9) | ||
| BMI, median (10th and 90th percentile) [No.] | 26.6 (21.3 to 36.7) [1568] | 26.8 (21.0 to 36.5) [1746] | 26.5 (25.6 to 27.9) | 26.7 (25.4 to 27.5) |
| Smoked cigarettes, No./total No. (%) | 306/1241 (24.7) | 355/1387 (25.6) | 20 (13 to 28) | 19 (14 to 31) |
| Diabetes, No. (%) | 322 (20.5) | 348 (19.9) | 21 (11 to 25) | 20 (14 to 25) |
| Hypertension, No. (%) | 846 (53.9) | 944 (53.9) | 54 (45 to 66) | 53 (45 to 74) |
| ASA score, No./total No. (%)c | ||||
| I, healthiest | 26/1570 (1.7) | 21/1750 (1.2) | 0 (0 to 1) | 0 (0 to 0) |
| II | 216/1570 (13.8) | 229/1750 (13.1) | 14 (6 to 21) | 12 (6 to 18) |
| III | 469/1570 (29.9) | 532/1750 (30.4) | 26 (15 to 48) | 28 (16 to 55) |
| IV | 848/1570 (54.0) | 961/1750 (54.9) | 61 (28 to 76) | 62 (30 to 67) |
| V, sickest | 11/1570 (0.7) | 7/1750 (0.4) | 0 (0 to 0) | 0 (0 to 0) |
| Participating center, No. (%) | 58 (32 to 94) | 55 (36 to 110) | ||
| A | 734 (46.8) | 933 (53.3) | 85 (57 to 107) | 100 (89 to 123) |
| B | 560 (35.7) | 504 (28.8) | 58 (52 to 75) | 54 (46 to 67) |
| C | 276 (17.6) | 314 (17.9) | 36 (18 to 46) | 37 (30 to 50) |
| Length of hospital stay, median (10th and 90th percentile), d | 7.0 (2.0 to 15.0) | 7.0 (2.0 to 14.0) | 7.0 (7.0 to 9.0) | 7.0 (6.8 to 8.3) |
| Discharge destination, No. (%) | ||||
| Home | 721 (45.9) | 825 (47.1) | 48 (18 to 57) | 47 (32 to 55) |
| Rehabilitation clinic | 768 (48.9) | 824 (47.1) | 47 (39 to 74) | 50 (36 to 68) |
| Referral to acute care hospital | 43 (2.7) | 61 (3.5) | 2 (0 to 6) | 3 (0 to 12) |
| In-hospital mortality | 33 (2.1) | 35 (2.0) | 2 (0 to 4) | 2 (0 to 5) |
| Other | 5 (0.3) | 6 (0.3) | 0 (0 to 1) | 0 (0 to 1) |
| Laboratory findings | ||||
| Hemoglobin, median (10th and 90th percentile) [No.], g/dL | 13.5 (10.3 to 15.5) [1334] | 13.6 (10.3 to 15.5) [1541] | 13.6 (13.1 to 13.8) | 13.5 (13.1 to 13.9) |
| Leukocytes, median (10th and 90th percentile) [No.], /μL | 7100 (4989 to 10 970) [1334] | 7220 (4790 to 10 786) [1541] | 7138 (6580 to 7490) | 7110 (6765 to 7659) |
| Creatinine, median (10th and 90th percentile) [No.], mg/dL | 0.81 (0.58 to 1.21) [1317] | 0.82 (0.59 to 1.24) [1527] | 0.82 (0.76 to 0.88) | 0.82 (0.76 to 0.87) |
| C-reactive protein, median (10th and 90th percentile) [No.], mg/dL | 0.3 (0.1 to 5.7) [1252] | 0.3 (0.1 to 3.4) [1478] | 0.3 (0.2 to 0.4) | 0.3 (0.2 to 0.4) |
| Surgery characteristics | ||||
| Type of surgery, No. (%) | ||||
| Cardiac surgery | ||||
| Cardiac | 452 (28.8) | 506 (28.9) | 30 (22 to 40) | 29 (22 to 40) |
| Coronary bypass | 559 (35.6) | 649 (37.1) | 32 (22 to 44) | 33 (26 to 44) |
| Abdominal surgery | ||||
| Cholecystectomy | 174 (11.1) | 195 (11.1) | 9 (0 to 23) | 11 (0 to 21) |
| Colon | 162 (10.3) | 186 (10.6) | 11 (7 to 21) | 12 (6 to 22) |
| Hernia | 91 (5.8) | 50 (2.9) | 0 (0 to 21) | 0 (0 to 15) |
| Gastric bypass | 116 (7.4) | 138 (7.9) | 0 (0 to 18) | 0 (0 to 15) |
| Rectum, rectosigmoid, or rectal tissue | 16 (1.0) | 27 (1.5) | 0 (0 to 3) | 0 (0 to 3) |
| Overall surgery duration, median (10th and 90th percentile), min | 213 (59 to 327) | 212 (60 to 327) | 214 (180 to 252) | 211 (189 to 251) |
| Cardiac surgery | 240 (162 to 332) | 241 (159 to 337) | 245 (223 to 252) | 249 (228 to 264) |
| Abdominal surgery | 85 (41 to 289) | 88 (41 to 286) | 92 (72 to 236) | 97 (74 to 265) |
| Surgery duration T-time >75th percentile, No. (%)d | 370 (23.6) | 420 (24.0) | 23 (17 to 36) | 24 (18 to 37) |
| Cardiac surgery | 215 (21.3) | 255 (22.1) | 19 (13 to 30) | 22 (15 to 29) |
| Abdominal surgery | 155 (27.7) | 165 (27.7) | 29 (15 to 68) | 28 (16 to 75) |
| Timing of antibiotic prophylaxis, median (10th and 90th percentile), min relative to incisione | −39 (−73 to −17) | −41 (−72 to −18) | −39 (−72 to −17) | −41 (−72 to −18) |
| Cardiac surgery | −41 (−71 to −25) | −44 (−72 to −25) | −38 (−51 to −33) | −45 (−52 to −39) |
| Abdominal surgery | −30 (−81 to −12) | −33 (−68 to −11) | −40 (−53 to −24) | −36 (−52 to −21) |
| Endoscopic abdominal surgery, No. (%) | 408 (73.0) | 446 (74.8) | 69 (57 to 82) | 68 (61 to 79) |
| NHSN wound contamination class, No. (%)f | ||||
| I (clean) | 1034 (65.9) | 1146 (65.4) | 66 (58 to 79) | 69 (57 to 79) |
| II (clean-contaminated) | 366 (23.3) | 445 (25.4) | 20 (9 to 35) | 20 (10 to 35) |
| III (contaminated) | 86 (5.5) | 86 (4.9) | 5 (1 to 12) | 5 (2 to 10) |
| IV (infected) | 84 (5.4) | 74 (4.2) | 5 (0 to 9) | 4 (0 to 6) |
| Repeated operation, No. (%) | ||||
| No | 1467 (93.4) | 1632 (93.2) | 93 (82 to 100) | 93 (85 to 98) |
| Unplanned | 84 (5.4) | 103 (5.9) | 5 (0 to 14) | 6 (2 to 13) |
| Planned | 19 (1.2) | 16 (1.0) | 0 (0 to 3) | 0 (0 to 4) |
| Rehospitalization, No. (%) | 37 (2.4) | 41 (2.3) | 2 (0 to 4) | 2 (0 to 4) |
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); NHSN, National Healthcare Safety Network.
SI conversion factors: To convert hemoglobin to g/L, multiply by 10.0; leukocytes to ×109/L, multiply by 0.001; creatinine to μmol/L, multiply by 88.4; C-reactive protein to mg/L, multiply by 10.
No missing data unless otherwise indicated.
Median (10th and 90th percentile) of the respective median (continuous) or percentages (categorical) variable from each cluster.
Physical classification system of the ASA: I = healthy patient, V = moribund patient.
Measure for overlong surgical procedures used by the US National Nosocomial Infections Surveillance, comparing the effective duration of operation to a procedure category benchmark. Duration of operation greater than the 75th percentile per procedure category was considered overlong.
Surgical antimicrobial prophylaxis data for wound classes I and II stratified by cardiac vs abdominal (n = 2291).
Degree of contamination of the surgical wound at the time of surgery according to NHSN classification system: I = clean wound, IV = infected wound at time of surgery.
Baseline patient characteristics (Table 1), length of hospital stay, and time from intervention to infection did not significantly differ between the 2 groups. In particular, timing of administration, choice of agent for perioperative antimicrobial prophylaxis, and dosing met national and international recommendations.24,25,26
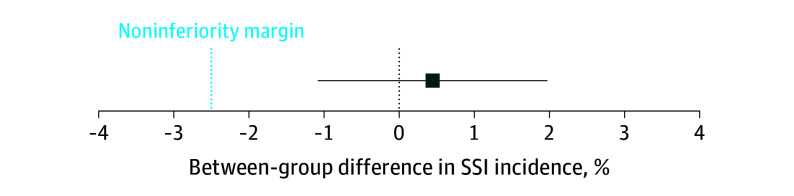
SSIs were detected in 80 of 1570 patients (5.1%) in the povidone iodine group and in 97 of 1751 patients (5.5%) in the chlorhexidine gluconate group, a difference of 0.4% (95% CI, −1.1% to 2.0%) with the lower limit of the CI not exceeding the predefined noninferiority margin of −2.5% (RR, 0.92 for povidone iodine compared with chlorhexidine gluconate [95% CI, 0.69-1.23]) (Table 2 and Figure 2). The results were similar when corrected for clustering (see eAppendix B, eTable 1 in Supplement 1). SSI rates were higher in the povidone iodine group for patients undergoing cardiac surgery (4.2% vs 3.3%; RR, 1.26 [95% CI, 0.82-1.94]), but lower in the povidone iodine group in patients undergoing abdominal surgery (6.8% vs 9.9%; RR, 0.69 [95% CI, 0.46-1.02]).
Table 2. Adjusted and Unadjusted Outcomes, Chlorhexidine Gluconate in Alcohol as Reference.
| Patient characteristics | Povidone iodine (n = 1570) | Chlorhexidine gluconate (n = 1751) | Absolute difference (95% CI), % | Relative risk (95% CI) | Adjusted relative risk (95% CI)a |
|---|---|---|---|---|---|
| Overall SSI, No. (%) | 80 (5.1) | 97 (5.5) | 0.4 (−1.1 to 2.0) | 0.92 (0.69 to 1.23) | 0.90 (0.66 to 1.22) |
| Type of SSI, No./total No. (%)b | |||||
| Superficial | 32/1526 (2.1) | 29/1691 (1.7) | −0.4 (−1.3 to 0.6) | 1.22 (0.74 to 2.01) | 1.20 (0.73 to 2.01) |
| Deep incisional | 15/1509 (1.0) | 20/1682 (1.2) | 0.2 (−0.6 to 1.0) | 0.84 (0.43 to 1.63) | 1.83 (0.43 to 1.63) |
| Organ space | 29/1523 (1.9) | 40/1702 (2.3) | 0.4 (−0.6 to 1.5) | 0.81 (0.51 to 1.30) | 0.77 (0.48 to 1.26) |
| Abdominal | 38/559 (6.8) | 59/596 (9.9) | 3.1 (0.3 to 6.5) | 0.69 (0.46 to 1.02) | |
| Superficial | 15/536 (2.8) | 14/551 (2.5) | −0.3 (−2.4 to 1.8) | 1.10 (0.54 to 2.60) | |
| Deep incisional | 3/524 (0.6) | 10/547 (1.9) | 1.4 (−0.2 to 2.9) | 0.31 (0.09 to 1.13) | |
| Organ space | 20/541 (3.7) | 35/572 (6.1) | 2.4 (0.3 to 5.1) | 0.60 (0.35 to 1.03) | |
| Cardiac | 42/1011 (4.2) | 38/1155 (3.3) | −0.9 (−2.6 to 0.8) | 1.26 (0.82 to 1.94) | |
| Superficial | 17/990 (1.7) | 15/1140 (1.3) | −0.4 (−1.5 to 0.7) | 1.31 (0.66 to 2.60) | |
| Deep incisional | 12/985 (1.2) | 10/1135 (0.9) | −0.3 (−1.3 to 0.6) | 1.38 (0.60 to 3.19) | |
| Organ space | 9/982 (0.9) | 5/1130 (0.4) | −0.5 (−1.2 to 0.3) | 2.07 (0.70 to 6.16) |
Abbreviation: SSI, surgical site infection.
Adjusted by surgery type in the Mantel-Haenszel test.
Classification as determined by infectious disease physician at last follow-up, applying US Centers for Disease Control and Prevention’s National Healthcare Safety Network definitions.
Figure 2. Difference of Overall Occurrence of Surgical Site Infection (SSI) Between Alcoholic Povidone Iodine and Chlorhexidine Gluconate With Noninferiority Margin and 95% CIs.
The difference is defined as chlorhexidine gluconate (reference) minus povidone iodine. CI whiskers do not cross the noninferiority line, meaning the hypothesis that povidone iodine is inferior to chlorhexidine gluconate is rejected.
Exploratory predefined subgroup analyses did not identify groups with significantly different SSI rates (eFigure 1 in Supplement 1), particularly when stratifying by superficial incisional, deep incisional, and organ space infections (Table 2). All analyses were performed on the complete records (no end point missingness).
Adherence to the skin disinfection procedure in the observed 396 operations was 98% for the used agent, 98% for the number of disinfectant applications, and 96% for the recommended exposure time to the disinfectant.
Discussion
Skin antisepsis with povidone iodine was shown to be noninferior to chlorhexidine gluconate, each formulated in alcohol, in SSI prevention. To the authors’ knowledge, this multicenter, cluster-randomized trial is the largest clinical trial to date comparing skin antisepsis with the 2 commonly used compounds in abdominal and cardiac surgery,6 with a high level of standardization of both the skin preparation process and the study compounds.18 Importantly, postdischarge patient follow-up was exceptionally good and ensured adequate identification of outcomes. The risk of confounding through contaminated operations, where proper skin preparation might be less relevant, was reduced by only including elective operations.25
The difference in SSI rates between the 2 compounds was remarkably small. In contrast, Tuuli et al reported a much larger difference in SSI rates when comparing povidone iodine (7.3%) with chlorhexidine gluconate (4%) in women undergoing cesarean delivery.27 Importantly, 3 of 4 similar studies were unable to demonstrate the superiority of chlorhexidine gluconate compared with aqueous povidone iodine per a meta-analysis of skin antisepsis and its effect on SSI after cesarean delivery.28 The study by Tuuli and colleagues, the most influential and only randomized clinical trial to support the superiority of chlorhexidine gluconate in that meta-analysis, had some distinctive aspects and issues that may have influenced the study results. The mean BMI was higher at 35, only cesarean births at a single center were considered, and 40% were unscheduled interventions, thus limiting the standardization of skin preparation.27 A systematic review by the Cochrane Library concluded that aqueous chlorhexidine gluconate might be moderately more effective than povidone iodine in reducing SSI after cesarean delivery.29 Surprisingly, chlorhexidine gluconate was associated with higher rates of SSI following bone and joint surgeries in another study.6 The authors speculated that the type of surgery may influence the SSI risk, and other factors may influence the study results, such as BMI.17 Two more randomized trials could not confirm the superiority of chlorhexidine over povidone iodine either,8,9 and a study published in 2024 compared chlorhexidine gluconate to iodine povacrylex with a similarly indifferent finding.30 The National Institute for Health and Care Research Unit of Global Surgery, a global research network including low- and middle-income countries, suggested reconsidering whether chlorhexidine gluconate should be the preferred disinfectant because it is “more expensive than other readily available alternatives,”8 adding another angle to the criticism of the possibly premature WHO SSI prevention guidelines that promoted chlorhexidine gluconate.
Several hypotheses may explain the difference in results from previous studies. For one, this difference may be related to the free iodine component in the different drugs: povidone iodine solutions contain a complex of iodine and polyvinylpyrrolidone and the microbiologically active compound (free iodine) is slowly liberated from the polyvinylpyrrolidone complex. Previous studies used a variety of concentrations and carriers of povidone iodine, with the microbiologically free iodine content rarely being reported. Commercial products can significantly differ in their free iodine, possibly explaining different results of clinical studies with povidone iodine.31,32 Similarly, the backbone of alcohol compounds is infrequently reported and rarely indicated as volume of alcohol per volume of fluid in a bottle, nor as grams per volume. Accordingly, previous trials are likely to have used different alcohol backbones in the comparators. Such a comparison of povidone iodine vs chlorhexidine gluconate would have been compromised by dissimilar alcohol concentrations or free iodine concentrations. Lastly, a 2021 meta-analysis hypothesized that povidone iodine may be more suitable for orthopedic and abdominal operations but less optimal for cesarean section,6 suggesting again that the type of surgical intervention may also influence the effect of the antiseptic agent.
Limitations
This study has limitations. First, individual patients were not randomized, but rather, a cluster-randomized crossover design was used. However, all elective patients undergoing a study procedure were eligible, which eliminated the risk of participation bias, and the monthly random crossover of the products resulted in comparable groups of patients, both between the intervention groups and also between study centers. Furthermore, the study team assessing outcomes, the site investigators, the primary investigator, and the study statistician were masked for the study allocation. Second, the original sample size calculation did not consider potential cluster correlations. However, additional sensitivity analysis was performed that confirmed the study to be adequately powered when considering cluster effects. Third, another possible limitation is the shortening of follow-up from 365 to 90 days in cardiac patients due to the constraints generated by the COVID-19 pandemic. As mentioned above, the effect of this shortening was examined in supplementary analyses, and it was concluded that an influence on study outcomes was unlikely.
Conclusions
Povidone iodine in alcohol as preoperative skin antisepsis was noninferior to chlorhexidine gluconate in alcohol in preventing SSIs after cardiac or abdominal surgery.
Appendix A: Note on sample size considerations.
Appendix B: Supplementary analyses on cluster, period and procedure effects.
Appendix C: Supplementary analysis on shortened follow-up during COVID-19.
Appendix D: Handling of missing data.
eFigure 1: Forest plot of predefined subgroup analyses.
eFigure 2: Infection rates for the periods, stratified by intervention and center.
eFigure 3: Interrupted time series analysis of the effect of switching in either direction, stratified by center.
eFigure 4: Infection rates for the periods, stratified by intervention.
eFigure 5: Infection rates for the periods, plotted at the start of the calendar month and year, stratified by intervention.
eFigure 6: Kaplan-Meier curve on probability of remaining free of surgical site infection.
eFigure 7: Randomized allocation of monthly clusters per center.
eFigure 8: Missingness patterns.
eTable 1: Fitted logistic regression models with SSI as endpoint, including center as fixed effect and period as random effect (Supplementary Analysis, Appendix B).
eTable 2: Comparison of the number of infections from cardiac surgeries for the Picasso trial and for the 18 months prior to the trial starting from Swissnoso Surveillance (Supplementary analysis Appendix C).
eTable 3: Preoperative antibiotic prophylaxis stratified by intervention.
eTable 4: Missingness in Table 1.
eTable 5: Missingness, comparison of complete case analysis an following multiple imputation.
Trial Protocol
Statistical Analysis Plan
Data Sharing Statement
References
- 1.Seidelman JL, Mantyh CR, Anderson DJ. Surgical site infection prevention: a review. JAMA. 2023;329(3):244-252. doi: 10.1001/jama.2022.24075 [DOI] [PubMed] [Google Scholar]
- 2.Wenzel RP. Surgical site infections and the microbiome: an updated perspective. Infect Control Hosp Epidemiol. 2019;40(5):590-596. doi: 10.1017/ice.2018.363 [DOI] [PubMed] [Google Scholar]
- 3.Berríos-Torres SI, Umscheid CA, Bratzler DW, et al. ; Healthcare Infection Control Practices Advisory Committee . Centers for Disease Control and Prevention guideline for the prevention of surgical site infection, 2017. JAMA Surg. 2017;152(8):784-791. doi: 10.1001/jamasurg.2017.0904 [DOI] [PubMed] [Google Scholar]
- 4.Fields AC, Pradarelli JC, Itani KMF. Preventing surgical site infections: looking beyond the current guidelines. JAMA. 2020;323(11):1087-1088. doi: 10.1001/jama.2019.20830 [DOI] [PubMed] [Google Scholar]
- 5.Ban KA, Minei JP, Laronga C, et al. American College of Surgeons and Surgical Infection Society: surgical site infection guidelines, 2016 update. J Am Coll Surg. 2017;224(1):59-74. doi: 10.1016/j.jamcollsurg.2016.10.029 [DOI] [PubMed] [Google Scholar]
- 6.Peel TN, Watson E, Lee SJ. Randomised controlled trials of alcohol-based surgical site skin preparation for the prevention of surgical site infections: systematic review and meta-analysis. J Clin Med. 2021;10(4):663. doi: 10.3390/jcm10040663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wade RG, Burr NE, McCauley G, Bourke G, Efthimiou O. The comparative efficacy of chlorhexidine gluconate and povidone-iodine antiseptics for the prevention of infection in clean surgery: a systematic review and network meta-analysis. Ann Surg. 2021;274(6):481-488.doi: 10.1097/SLA.0000000000004076 [DOI] [PubMed] [Google Scholar]
- 8.Ademuyiwa AO, Adisa AO, Bach S, et al. ; National Institute of Health Research Unit on Global Surgery . Alcoholic chlorhexidine skin preparation or triclosan-coated sutures to reduce surgical site infection: a systematic review and meta-analysis of high-quality randomised controlled trials. Lancet Infect Dis. 2022;22(8):1242-1251. doi: 10.1016/S1473-3099(22)00133-5 [DOI] [PubMed] [Google Scholar]
- 9.NIHR Global Research Health Unit on Global Surgery . Reducing surgical site infections in low-income and middle-income countries (FALCON): a pragmatic, multicentre, stratified, randomised controlled trial. Lancet. 2021;398(10312):1687-1699. doi: 10.1016/S0140-6736(21)01548-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maiwald M, Chan ES. The forgotten role of alcohol: a systematic review and meta-analysis of the clinical efficacy and perceived role of chlorhexidine in skin antisepsis. PLoS One. 2012;7(9):e44277. doi: 10.1371/journal.pone.0044277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maiwald M, Widmer AF. WHO’s recommendation for surgical skin antisepsis is premature. Lancet Infect Dis. 2017;17(10):1023-1024. doi: 10.1016/S1473-3099(17)30448-6 [DOI] [PubMed] [Google Scholar]
- 12.Wendt C, Frei R, Widmer AF. Decontamination, Disinfection, and Sterilization. Manual of Clinical Microbiology. 11th ed. American Society of Microbiology; 2015. [Google Scholar]
- 13.Guenezan J, Marjanovic N, Drugeon B, et al. ; CLEAN-3 trial investigators . Chlorhexidine plus alcohol versus povidone iodine plus alcohol, combined or not with innovative devices, for prevention of short-term peripheral venous catheter infection and failure (CLEAN 3 study): an investigator-initiated, open-label, single centre, randomised-controlled, two-by-two factorial trial. Lancet Infect Dis. 2021;21(7):1038-1048. doi: 10.1016/S1473-3099(20)30738-6 [DOI] [PubMed] [Google Scholar]
- 14.Team NGU. National Institute for Health and Care Excellence: Clinical Guidelines. Surgical Site Infections: Prevention and Treatment. National Institute for Health and Care Excellence; 2019. [Google Scholar]
- 15.Babiker A, Lutgring JD, Fridkin S, Hayden MK. Assessing the potential for unintended microbial consequences of routine chlorhexidine bathing for prevention of healthcare-associated infections. Clin Infect Dis. 2021;72(5):891-898. doi: 10.1093/cid/ciaa1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peel TN, Dowsey MM, Buising KL, Cheng AC, Choong PFM. Chlorhexidine-alcohol versus iodine-alcohol for surgical site skin preparation in an elective arthroplasty (ACAISA) study: a cluster randomized controlled trial. Clin Microbiol Infect. 2019;25(10):1239-1245. doi: 10.1016/j.cmi.2019.06.016 [DOI] [PubMed] [Google Scholar]
- 17.Tschudin-Sutter S, Frei R, Egli-Gany D, et al. No risk of surgical site infections from residual bacteria after disinfection with povidone-iodine-alcohol in 1014 cases: a prospective observational study. Ann Surg. 2012;255(3):565-569. doi: 10.1097/SLA.0b013e3182468b2d [DOI] [PubMed] [Google Scholar]
- 18.Roth JA, Schwab C, Atkinson A, et al. Are three antiseptic paints needed for safe preparation of the surgical field? a prospective cohort study with 239 patients. Antimicrob Resist Infect Control. 2020;9(1):120. doi: 10.1186/s13756-020-00780-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Troillet N, Aghayev E, Eisenring MC, Widmer AF; Swissnoso . First results of the Swiss National Surgical Site Infection Surveillance Program: who seeks shall find. Infect Control Hosp Epidemiol. 2017;38(6):697-704. doi: 10.1017/ice.2017.55 [DOI] [PubMed] [Google Scholar]
- 20.Kuster SP, Eisenring MC, Sax H, Troillet N; Swissnoso . Structure, process, and outcome quality of surgical site infection surveillance in Switzerland. Infect Control Hosp Epidemiol. 2017;38(10):1172-1181. doi: 10.1017/ice.2017.169 [DOI] [PubMed] [Google Scholar]
- 21.Atkinson A, Eisenring MC, Troillet N, et al. Surveillance quality correlates with surgical site infection rates in knee and hip arthroplasty and colorectal surgeries: a call to action to adjust reporting of SSI rates. Infect Control Hosp Epidemiol. 2021;42(12):1451-1457. doi: 10.1017/ice.2021.14 [DOI] [PubMed] [Google Scholar]
- 22.Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Am J Infect Control. 1999;27(2):97-132. [PubMed] [Google Scholar]
- 23.Culver DH, Horan TC, Gaynes RP, et al. ; National Nosocomial Infections Surveillance System . Surgical wound infection rates by wound class, operative procedure, and patient risk index. Am J Med. 1991;91(3B):152S-157S. doi: 10.1016/0002-9343(91)90361-Z [DOI] [PubMed] [Google Scholar]
- 24.Weber WP, Marti WR, Zwahlen M, et al. The timing of surgical antimicrobial prophylaxis. Ann Surg. 2008;247(6):918-926. doi: 10.1097/SLA.0b013e31816c3fec [DOI] [PubMed] [Google Scholar]
- 25.Weber WP, Mujagic E, Zwahlen M, et al. Timing of surgical antimicrobial prophylaxis: a phase 3 randomised controlled trial. Lancet Infect Dis. 2017;17(6):605-614. doi: 10.1016/S1473-3099(17)30176-7 [DOI] [PubMed] [Google Scholar]
- 26.Bratzler DW, Dellinger EP, Olsen KM, et al. ; American Society of Health-System Pharmacists (ASHP); Infectious Diseases Society of America (IDSA); Surgical Infection Society (SIS); Society for Healthcare Epidemiology of America (SHEA) . Clinical practice guidelines for antimicrobial prophylaxis in surgery. Surg Infect (Larchmt). 2013;14(1):73-156. doi: 10.1089/sur.2013.9999 [DOI] [PubMed] [Google Scholar]
- 27.Tuuli MG, Liu J, Stout MJ, et al. A randomized trial comparing skin antiseptic agents at cesarean delivery. N Engl J Med. 2016;374(7):647-655. doi: 10.1056/NEJMoa1511048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tolcher MC, Whitham MD, El-Nashar SA, Clark SL. Chlorhexidine-alcohol versus povidone-iodine for skin preparation before elective cesarean section: a prospective observational study. Am J Perinatol. 2019;36(2):118-123. doi: 10.1055/s-0038-1669907 [DOI] [PubMed] [Google Scholar]
- 29.Hadiati DR, Hakimi M, Nurdiati DS, Masuzawa Y, da Silva Lopes K, Ota E. Skin preparation for preventing infection following caesarean section. Cochrane Database Syst Rev. 2020;6(6):CD007462. doi: 10.1002/14651858.CD007462.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sprague S, Slobogean G, Wells JL, et al. ; The PREP-IT Investigators . Skin antisepsis before surgical fixation of extremity fractures. N Engl J Med. 2024;390(5):409-420. doi: 10.1056/NEJMoa2307679 [DOI] [PubMed] [Google Scholar]
- 31.Atemnkeng MA, Plaizier-Vercammen J, Schuermans A. Comparison of free and bound iodine and iodide species as a function of the dilution of three commercial povidone-iodine formulations and their microbicidal activity. Int J Pharm. 2006;317(2):161-166. doi: 10.1016/j.ijpharm.2006.03.013 [DOI] [PubMed] [Google Scholar]
- 32.Schubert R. Disinfectant properties of new povidone-iodine preparations. J Hosp Infect. 1985;6(Suppl 1):33-36. doi: 10.1016/S0195-6701(85)80043-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix A: Note on sample size considerations.
Appendix B: Supplementary analyses on cluster, period and procedure effects.
Appendix C: Supplementary analysis on shortened follow-up during COVID-19.
Appendix D: Handling of missing data.
eFigure 1: Forest plot of predefined subgroup analyses.
eFigure 2: Infection rates for the periods, stratified by intervention and center.
eFigure 3: Interrupted time series analysis of the effect of switching in either direction, stratified by center.
eFigure 4: Infection rates for the periods, stratified by intervention.
eFigure 5: Infection rates for the periods, plotted at the start of the calendar month and year, stratified by intervention.
eFigure 6: Kaplan-Meier curve on probability of remaining free of surgical site infection.
eFigure 7: Randomized allocation of monthly clusters per center.
eFigure 8: Missingness patterns.
eTable 1: Fitted logistic regression models with SSI as endpoint, including center as fixed effect and period as random effect (Supplementary Analysis, Appendix B).
eTable 2: Comparison of the number of infections from cardiac surgeries for the Picasso trial and for the 18 months prior to the trial starting from Swissnoso Surveillance (Supplementary analysis Appendix C).
eTable 3: Preoperative antibiotic prophylaxis stratified by intervention.
eTable 4: Missingness in Table 1.
eTable 5: Missingness, comparison of complete case analysis an following multiple imputation.
Trial Protocol
Statistical Analysis Plan
Data Sharing Statement