Abstract
Adverse pregnancy outcomes are common among pregnant individuals and are associated with long-term risk of cardiovascular disease. Individuals with adverse pregnancy outcomes also have an increased incidence of cardiovascular disease risk factors after delivery. Despite this, evidence-based approaches to managing these patients after pregnancy to reduce cardiovascular disease risk are lacking. In this scientific statement, we review the current evidence on interpregnancy and postpartum preventive strategies, blood pressure management, and lifestyle interventions for optimizing cardiovascular disease using the American Heart Association Life’s Essential 8 framework. Clinical, health system, and community-level interventions can be used to engage postpartum individuals and to reach populations who experience the highest burden of adverse pregnancy outcomes and cardiovascular disease. Future trials are needed to improve screening of subclinical cardiovascular disease in individuals with a history of adverse pregnancy outcomes, before the onset of symptomatic disease. Interventions in the fourth trimester, defined as the 12 weeks after delivery, have great potential to improve cardiovascular health across the life course.
Keywords: AHA Scientific Statements; cardiovascular diseases; diabetes, gestational; postpartum period; pregnancy; pregnancy complications; primary prevention
Adverse pregnancy outcomes (APOs) can arise from stress of metabolic and vascular changes during pregnancy.1–3 APOs include myriad maternal or fetal complications, including hypertensive disorders of pregnancy (HDP; gestational hypertension, preeclampsia, eclampsia, and HELLP [hemolysis, elevated liver enzymes, low platelets] syndrome), gestational diabetes, placental abruption, spontaneous preterm birth, fetal growth restriction, and small-for-gestational-age infant.1 The prevalence of APOs is estimated to be 10% to 20% in the literature and varies by race and ethnicity.2
These APOs portend higher risk of future long-term complications, including increased lifetime risk of atherosclerotic cardiovascular disease (ASCVD), heart failure, stroke, chronic kidney disease (CKD), and vascular dementia.1,3 This risk appears to be largely mediated by the increased incidence of cardiovascular disease (CVD) risk factors, including diabetes and hypertension.4,5 Individuals with gestational diabetes, for instance, are 8 times more likely to develop subsequent type 2 diabetes compared with those without gestational diabetes.6 Women with HDP have a 2- to 4-fold higher risk of developing chronic hypertension compared with women with normotensive pregnancies at 10 to 20 years or later after delivery.7,8 These CVD risk factors, in turn, place postpartum individuals at a higher risk of developing subsequent CVD and other end-organ effects and thus are important targets for postpartum interventions.
Furthermore, disparities by race and ethnicity, socioeconomic status, and geography (rural versus urban-dwelling individuals) affect the prevalence of APOs and subsequent cardiovascular sequelae. Compared with non-Hispanic White women, non-Hispanic Black women have an increased risk of developing HDP and severe maternal morbidity in the peripartum period and higher CVD risk across their life span.9,10 CVD is a leading cause of pregnancy-related deaths and causes the most deaths in Black birthing individuals.10,11 Given that race and ethnicity are social constructs, social determinants of health, economic status, and structural racism are key contributors to racial disparities in prenatal CVD risk factors, maternal access to care, and maternal and fetal outcomes.11,12 An in-depth review of social determinants of health, their role in the development of CVD risk factors, morbidity and mortality in women and men, and the need for policy-level changes to mitigate disparities has been published previously.13
Despite these well-established associations, evidence-based guidelines are lacking to provide clinicians recommendations on how to reduce CVD risk and optimize cardiovascular health (CVH) after APOs. Given the robust data from epidemiological and clinical studies linking APOs and CVD, APOs have been highlighted as a CVD risk factor in multisociety scientific statements from the American Heart Association (AHA) and American College of Obstetricians and Gynecologists (ACOG).14 No randomized clinical trials have evaluated the effects of postpartum interventions on long-term maternal CVD outcomes. Yet, the need for interventional strategies supported by rigorous evidence remains. In particular, the fourth trimester, defined as the 12 weeks after delivery, is an optimal time to engage postpartum individuals in care to reduce maternal morbidity and improve care transitions. The concept of the fourth trimester can be further extended to the first year after delivery as a critical time to assess long-term CVD risk and to implement lifestyle changes in order to improve maternal CVH across the life course with potential downstream impact on the CVH health of offspring.
This scientific statement is organized into 3 parts. First, current evidence on interpregnancy CVH, postpartum blood pressure (BP) trends, and lifestyle interventions is reviewed. Next, a pragmatic approach to postpartum management and preventive strategies for optimizing CVH using the AHA Life’s Essential 8 framework15 is presented (Figure 1). Last, this scientific statement highlights the need for postpartum CVD risk factor screening after an APO and future directions to define the role for screening for subclinical CVD in individuals with a history of APOs. In this scientific statement, we use gender-neutral language to refer to all individuals capable of pregnancy. We refer to women and men on the basis of presumed gender identity when used in published studies and guidelines.
Figure 1. Opportunities to improve CVH in the postpartum period after adverse pregnancy outcomes.15.
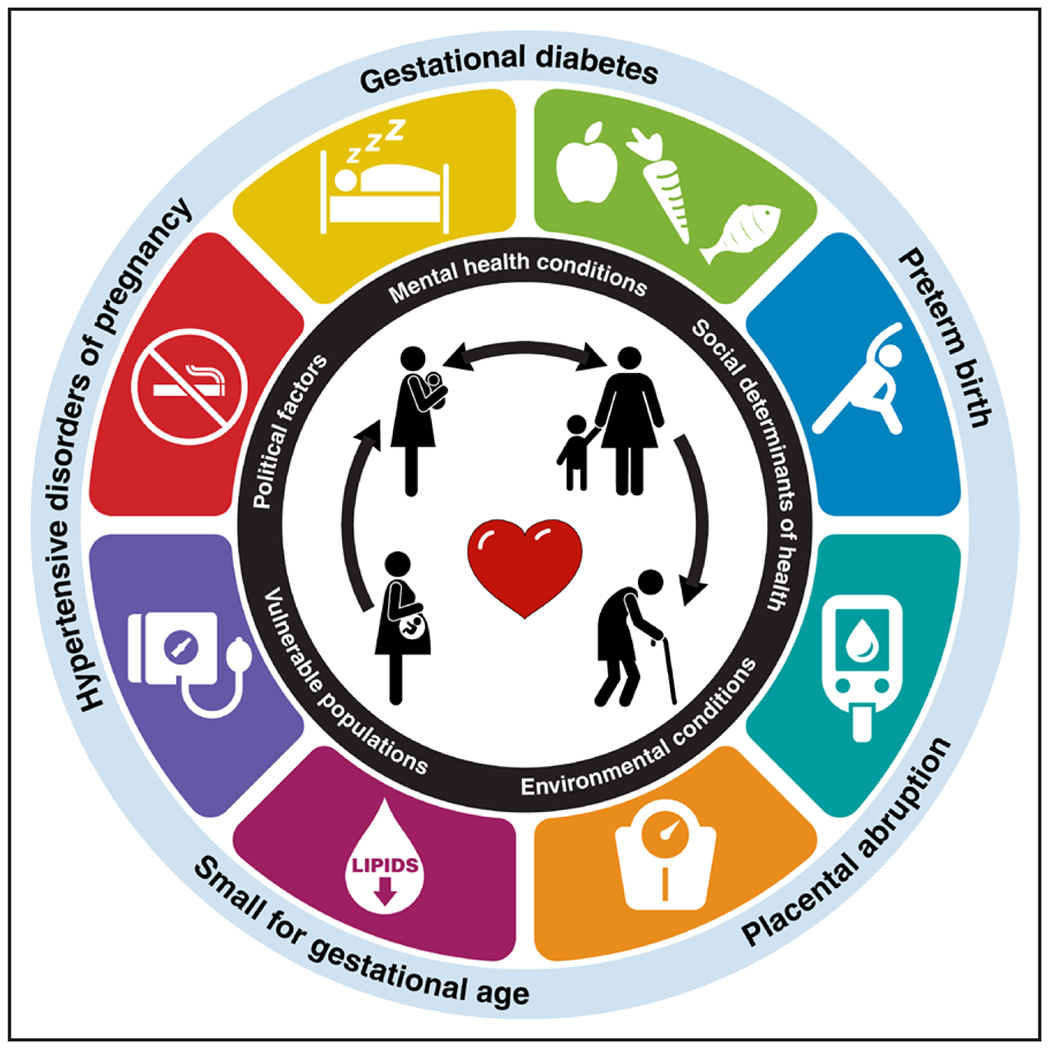
Improvements in postpartum maternal cardiovascular health (CVH) may help reduce risk of future adverse pregnancy outcomes (bidirectional arrow), which may additionally attenuate maternal and offspring cardiovascular risk. Modified with permission from Lloyd-Jones et al.15 © 2022 American Heart Association, Inc.
POSTPARTUM AND INTERPREGNANCY HEALTH
Specific components of postpartum care have long-term implications for maternal CVH, especially for those who have experienced APOs. The interpregnancy period is an ideal opportunity to further reduce risk of future pregnancy complications for individuals who plan on future pregnancy. Postpartum and interpregnancy counseling for specific APOs is summarized in Table 1.
Table 1.
Postpartum and Interpregnancy Counseling for Patients With Specific APOs
| Counseling | Management | Lactation considerations | Subsequent pregnancy | Contraception | |
|---|---|---|---|---|---|
| HDP | BP returns to baseline levels by 12 wk postpartum, but hypertension can persist in some patients 2- to 4-fold increased risk of developing chronic hypertension 2-fold increased risk of developing subsequent CVD; risk is higher in patients with preeclampsia with early onset, severe features, or recurrence |
Wean BP medication as appropriate Ideal BP <120/80 mm Hg with BP goal <130/80 mm Hg for patients with treated chronic hypertension Lifestyle changes for patients with stage I or stage II hypertension Glucose and lipid screening if not up to date Serum creatinine and proteinuria assessment if history of elevated creatinine, AKI, proteinuria, or prepregnancy kidney disease* |
Breastfeeding may reduce future risk of chronic hypertension Avoid angiotensin receptor blockers and ACE inhibitors, except for enalapril and captopril, which are considered safe while breastfeeding Diuretics may affect milk supply if used in high doses |
Patients with preeclampsia have risk of recurrence in future pregnancy. Discuss aspirin in future pregnancy to reduce recurrence risk. Good BP control before and during pregnancy can reduce preeclampsia risk |
Avoid estrogen-containing contraception (eg, combined oral contraceptive pills or patch) if hypertensive or treated hypertension |
| Gestational diabetes | 8-fold increased risk of developing T2D Increased risk of subsequent CVD |
2-h oral GTT at 4–12 wk postpartum Lipid screening if not up to date Weight loss and exercise for diabetes prevention; consider metformin if prediabetic; refer to DPP |
Breastfeeding may reduce future risk of T2D | Risk of recurrent gestational diabetes in future pregnancy High prepregnancy weight associated with increased recurrence risk |
|
| Other APOs: placental abruption, SGA infant, preterm birth | Increased risk of subsequent CVD | Lipid and glucose screening if not up to date |
ACE indicates angiotensin-converting enzyme; AKI, acute kidney injury; APO, adverse pregnancy outcome; BP blood pressure; CVD, cardiovascular disease; DPP, Diabetes Prevention Program; GTT, glucose tolerance test; HDP, hypertensive disorder of pregnancy; SGA, small-for-gestational-age; and T2D, type 2 diabetes.
Creatinine is usually checked within 1 week after delivery Proteinuria (as assessed by 24-hour urine collection, albumin-to-creatinine ratio, or protein-to-creatinine ratio) can persist for months after delivery, and repeat assessment at 6 to 12 months may be reasonable.
Breastfeeding has a multitude of benefits for maternal and offspring CVH. Longer duration of breastfeeding is associated with lower risk of type 2 diabetes, especially for those with a history of gestational diabetes, hypertension, and myocardial infarction.16 Breastfed infants self-regulate intake and volume, develop early programming for self-regulation, and have improved CVH profiles in adulthood.17 Breastfeeding rates may be suboptimal for populations that experience structural barriers to breastfeeding such as inadequate parental leave and lactation support, which require policy-level interventions.18
Most contraceptive options are safe in postpartum individuals with a history of APOs.9,19 Combined estrogen-progestin hormonal contraception increases BP and generally is avoided in patients with stage 2 hypertension (even if treated), those with migraine with aura, and those with multiple CVD risk factors.9 Combined hormonal contraception is likely safe in normotensive individuals with a history of HDP. Postpartum individuals are advised to avoid short-interval pregnancies (<6 months between birth and conception), especially with a history of preterm birth.16
The year after delivery is a critical time to optimize CVH in order to lower future risk of APOs, especially preeclampsia, for those who may have a subsequent pregnancy. Prior preeclampsia significantly increases the risk of preeclampsia in a subsequent delivery, with the greatest risk of recurrence associated with onset before 37 weeks’ gestation.20 Up to one-third of individuals with gestational diabetes will be diagnosed with diabetes or impaired glucose metabolism within 12 weeks after delivery.21,22 Prior gestational diabetes is also associated with recurrent gestational diabetes in future pregnancy. Prepregnancy hypertension and diabetes are associated with preeclampsia, preterm birth, and intrauterine fetal demise.21 Optimal control of hypertension and diabetes before and during pregnancy is associated with lower risk of adverse maternal and neonatal outcomes, emphasizing the importance of CVD risk factor diagnosis and treatment during the interpregnancy period.23–25 During subsequent pregnancy, low-dose aspirin (81 mg/d) reduces the risk and severity of preeclampsia for individuals with chronic hypertension, type 2 diabetes, history of preeclampsia, or other risk factors.26
POSTPARTUM BP MANAGEMENT
Postpartum hypertension often results from preeclampsia or a gestational hypertension diagnosis during pregnancy or chronic hypertension. A small proportion of patients with previously normotensive pregnancies will develop preeclampsia de novo in the postpartum period.27 Risk factors for de novo postpartum preeclampsia are similar to those for preeclampsia before delivery. Patients most commonly present with headache or other neurological symptoms.28 Postpartum hypertension and preeclampsia contribute to serious short-term maternal complications such as stroke, seizures, and cardiomyopathy27,29 and early warning signs and symptoms are often missed.30 Careful management of postpartum BP may reduce maternal morbidity and mortality.31,32
BP Trajectory
BP in individuals with HDP diagnosed during pregnancy typically peaks between postpartum days 3 and 6 as a result of the delayed mobilization of extravascular fluid into the intravascular space and physiological decline of vasodilatory hormones.33 BP then rapidly decreases over the first 3 weeks after delivery, with slower declines thereafter. Several factors during the immediate postpartum period may exacerbate the risk of hypertension, including the generous use of intravenous fluids and the preferential use of nonsteroidal anti-inflammatory drugs (NSAIDs) for postpartum analgesia. Although postpartum NSAID use was not found to be associated with higher BP in a meta-analysis, studies were limited by small sample sizes and durations of follow-up.34 NSAID therapy in the setting of renal disease and chronic hypertension may cause worsening hypertension, similar to nonpregnant individuals. We suggest additional investigations to address the impact of longer duration of postpartum NSAID use for those at risk and careful consideration of analgesic options until then.
As for alternative analgesics, a recent study showed that 4 g acetaminophen taken daily over a 2-week period increases systolic BP in older hypertensive individuals.35 The results of this study should be interpreted with caution in the context of the lower acetaminophen dosing commonly used for postpartum analgesia. Given limited analgesic options in the postpartum period, future studies should address the safety of acetaminophen use in the postpartum period.
Diuretics should also be considered for postpartum hypertension, which may be exacerbated by an increase in intravascular fluid volume after delivery. A recent randomized controlled clinical trial showed that a 5-day course of furosemide in postpartum women with HDP was associated with a 60% reduction in hypertension at day 7 after delivery.36
Patient education and postpartum follow-up are critical to the prevention and early treatment of severe hypertension and its complications. Home BP monitoring, including text-based communication, and telemedicine programs have improved BP monitoring rates, have increased postpartum obstetric visits, and are associated with fewer emergency department visits and hospital readmissions for hypertension.31,37–39 It is important to note that remote BP monitoring has been found to be feasible in racially diverse populations and reduces disparities in postpartum BP monitoring.40 Home BP monitoring programs increasingly are becoming the standard of care and may offer opportunities to better understand BP trajectories and the risk of developing chronic hypertension. Future studies should investigate how home BP monitoring may reduce inequities in access to care in other populations, including rural-dwelling individuals.
In single-center cohort studies, an estimated 18% to 57% of women will continue to need antihypertensive medication or have BP values meeting criteria for stage 2 hypertension when evaluated between 6 weeks and 4 months postpartum.29,41–43 Ambulatory BP monitoring may additionally detect hypertension missed during office visits.29,44 Factors associated with persistent hypertension include older maternal age, obesity, higher BP in early pregnancy, preeclampsia with severe features, and discharge from the delivery admission on BP medications.41–43 Nationwide cohort studies in Denmark and France suggest lower rates of chronic hypertension at 1 year after delivery, although a diagnosis of HDP was still associated with up to a 12-fold to 25-fold higher risk of hypertension within the first year of delivery compared with women with normotensive pregnancies.7,45 Differences in the estimate of chronic hypertension in the year after delivery may be related to patient or geographic factors, study design, or the definition of chronic hypertension outcome (eg, measured BP during office or study visit versus prescription fill for BP medication). The diagnosis of secondary hypertension requiring further evaluation should be considered in the presence of classic clues, signs, and symptoms, including maternal age <35 years; severe or resistant hypertension; the presence of laboratory abnormalities such as hypokalemia, albuminuria, or elevated creatinine; and obesity-related obstructive sleep apnea. Renin and aldosterone levels decrease within the 6 weeks after delivery, after which time case detection of hyperaldosteronism may improve.
Individuals with CKD have an elevated risk of developing preeclampsia because of comorbid conditions such as hypertension and vascular and metabolic abnormalities. In turn, preeclampsia can cause acute kidney injury or proteinuria, or both, regardless of prepregnancy kidney disease, and increase future risk of CKD.46,47 Both acute kidney injury and CKD further contribute to elevated CVD risk. Repeat assessment of postpartum renal function and proteinuria may help identify those at risk for both CVD and CKD and facilitate early diagnosis and treatment. The optimal timing of proteinuria testing is not established and may vary with clinical context.
Hypertension After an APO: Longer-Term Implications
Individuals who experience HDP have increased risk of developing chronic hypertension in the early postpartum years. A study of 4484 nulliparous women followed up prospectively since early in pregnancy found that those who experienced HDP had more than double the risk of developing new chronic hypertension within 2 to 7 years after delivery (adjusted relative risk, 2.7 [95% CI, 2.0–3.6]).48 Women who had medically indicated preterm birth (before 37 weeks’ gestation) and HDP had the highest risk of developing subsequent new hypertension (relative risk, 4.3 [95% CI, 2.7–6.7]).48 Hypertension, particularly in women, confers an increased risk of cardiovascular and cerebrovascular disease; the INTERSTROKE study found that the population attributable risk of hypertension for stroke was 52.3% in women compared with 45.2% in men,49 and the INTERHEART study similarly found that hypertension was a stronger risk factor for myocardial infarction in women compared with men.50 Of note, women experience increased stroke risk at lower BP compared with men.51 Women with a history of 1 or more APOs experience myocardial infarction and stroke at younger ages compared with those without APOs.52,53 Early detection and treatment of hypertension after pregnancy thus may yield downstream benefits for prevention of myocardial infarction, heart failure, and stroke, especially among those who have experienced 1 or more APOs. A history of HDP and other APOs furthermore should be considered a strong risk factor in the evaluation of a young adult with cardiac or stroke-like symptoms; these symptoms are more likely to be misdiagnosed as mimics (eg, anxiety attack, complicated migraine) in young women.54–57
Capturing Hypertension Outside the Primary Care Setting
Significant barriers exist in accessing regular primary care postpartum in birthing individuals. Therefore, post-pregnancy hypertension may go undiagnosed and untreated until the next pregnancy or years later when end-organ manifestations develop prematurely.52 Every medical encounter, however, presents an opportunity for early hypertension detection (Figure 2). Urgent care or emergency department visits for injuries, minor illnesses, or headache may identify elevations in BP in young adults, which should not be attributed to exclusively anxiety or pain because elevated BP in these settings is associated with long-term ASCVD.58 These patients should receive prompt counseling and referral to primary care to ensure that a follow-up BP check is scheduled. Many other specialists besides those in primary care may interact with pregnancy-capable adults of reproductive age. Neurologists often see patients with headache disorders, which affect more than half of women 20 to 64 years of age59; these encounters also offer an opportunity to screen for and detect elevated BP, which in turn may reflect untreated sleep apnea in a patient with frequent headaches.60 Dermatologists frequently treat acne or other skin conditions in individuals capable of pregnancy, which may be a consequence of underlying conditions (eg, polycystic ovarian syndrome) associated with hypertension.61 Educational outreach to emergency department and specialist health care professionals on CVD complications of pregnancy is necessary to improve patient screening, referral, and treatment.
Figure 2. Opportunities to identify and treat chronic hypertension in postpartum individuals.
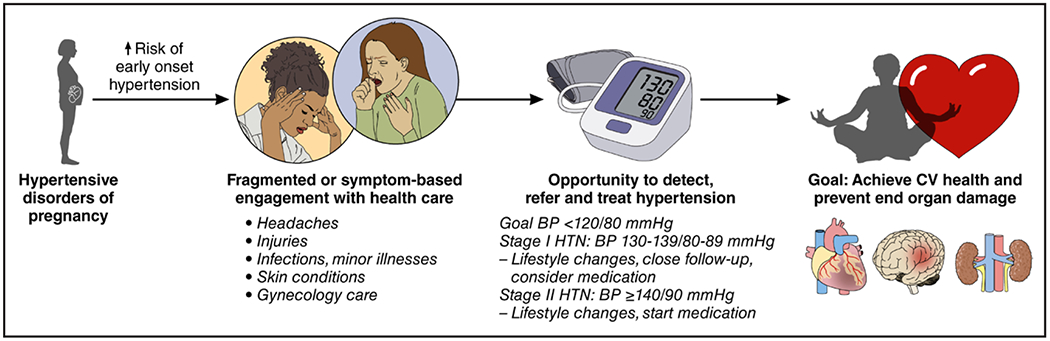
BP indicates blood pressure; CV, cardiovascular; and HTN, hypertension.
EVIDENCE FOR POSTPARTUM LIFESTYLE MODIFICATION INTERVENTIONS
Lifestyle interventions that target achieving a healthy weight, healthy diet, and regular physical activity reflect Life’s Essential 8, the AHA’s approach to optimal CVH across the life course.15 A cross-sectional study of 26 543 women of childbearing age reported that only 4.8% had ideal CVH across 7 metrics, with non-Hispanic Black women least likely to have ideal CVH, especially for BP, blood glucose, and body mass index.62
Postpartum Weight Management Interventions
Obesity increases risk for APOs, including HDP, gestational diabetes, and pregnancy loss. Obesity also increases risk for future CVD after APOs. Risk for obesity itself is increased by postpartum weight retention; indeed, 60% of individuals retain weight a year after delivery, with many gaining additional weight.63 The postpartum period is thus a critical window for healthy weight management and cardiovascular risk reduction through lifestyle modifications.
In post hoc analyses, the landmark Diabetes Prevention Program (DPP) demonstrated that an intensive lifestyle intervention targeting weight loss and physical activity successfully reduced diabetes incidence by 50% compared with placebo among individuals with a history of gestational diabetes.64 Of note, participants with prior gestational diabetes were not the primary focus in this trial and were enrolled 12 years postpartum on average.64 The primary goals of the intensive lifestyle intervention were to reach and maintain a weight loss of at least 7% of initial body weight, achieved through behavioral strategies such as goal setting, self-monitoring, and dietary changes in fat and calorie intake, and engaging in at least 150 minutes of moderate-intensity physical activity per week. This approach has been disseminated at scale through the National Diabetes Prevention Program, a public-private partnership to deliver a 12-month lifestyle intervention aiming for 5% weight loss and 150 min/wk of physical activity for individuals at high risk for type 2 diabetes (including those with a history of gestational diabetes).65 Subsequent trials have built on the success of the DPP lifestyle intervention specifically to reduce postpartum cardiometabolic risk.
Among randomized clinical trials evaluating lifestyle interventions focused on postpartum weight within the past 5 years, 6 randomized clinical trials tested interventions delivered exclusively in the postpartum period (Supplemental Table).66–71 Of these, 3 trials included individuals with gestational diabetes,66,69,70 and 2 trials focused primarily or exclusively on Hispanic participants.67,70 Four of the 5 trials reported significant intervention effects on primary outcomes of body weight.66–69 For trials with follow-up periods of ≥6 months, between-group differences in weight change ranged from 4.5 kg at 6 months69 to 2.3 to 3.3 kg at 12 months.66,67 Intervention components in these successful trials included lifestyle coaching; goal setting for calorie intake and physical activity; self-monitoring and feedback of weight, diet, and physical activity health behaviors; text messages; and multimedia educational material. Many leveraged digital technologies. Only 1 trial reporting significant intervention effects on postpartum weight focused on participants of underrepresented races and ethnicities or low-income; this was also the largest trial, with >370 participants in the Special Supplemental Nutrition Program for Women, Infants, and Children.67 This trial demonstrated that an internet-based intervention, derived in part from the DPP and integrated into the Special Supplemental Nutrition Program for Women, Infants, and Children care delivery setting, effectively promoted postpartum weight management.
An additional 5 randomized clinical trials tested interventions extending from pregnancy to postpartum.72–76 Three of the 5 trials reported significant intervention effects on primary outcomes of body weight. One of the largest trials tested the comparative effectiveness of a health system–based, DPP-derived intervention on 12-month postpartum weight loss in 2280 individuals with gestational diabetes receiving care through Kaiser Permanente Northern California.72 The primarily telehealth intervention resulted in 28% greater odds of meeting weight goals compared with usual care in this pragmatic trial. Parents as Teachers (PAT) is a national evidence-based home visiting program designed to improve parenting knowledge and skills. The PAT program plus lifestyle intervention during pregnancy and 12 months postpartum was compared with the PAT standard protocol among low-income Black individuals. The combined intervention, which incorporated an adaptation of the DPP within the standard PAT curriculum and home visits, resulted in significantly greater likelihood of returning to baseline weight at 12 months postpartum among trial completers (n=185/267).74 In a different patient cohort, the combination of the PAT program plus lifestyle intervention resulted in no differences in weight compared with the PAT program alone at 12 months postpartum and may be related to study design, lifestyle curriculum, and smaller sample size.73,77,78
These studies found no between-group differences in self-reported physical activity. A study that measured step count with a pedometer found improvement from baseline to 12 weeks in the intervention group compared with the control group, but this was not maintained at 1 year.71 Last, a study measuring physical activity with a wrist-worn accelerometer found no between-group differences in physical activity at 6 or 12 months.67 Of concern is that in most of these studies, participants demonstrated a low volume of exercise at follow-up. Considering that the AHA’s Life’s Essential 8 recommends at least 150 minutes of moderate to vigorous physical activity each week, future studies might strengthen the physical activity component of their interventions to yield the many physical and psychosocial benefits, which was the focus of another recent AHA scientific statement.79
Limitations in prior trials include small sample sizes, self-reported rather than objectively measured weights and physical activity, and high attrition, with the lowest retention in trials attempting to retain Black women from rural geographic regions. Furthermore, because most intervention components were delivered as a package, it is unclear which components were most successful. A multiphase optimization strategy framework could determine the active ingredient of these lifestyle interventions.80
Weight loss interventions may also include pharmacological options such as glucagon-like peptide 1 receptor agonists or bariatric surgery for eligible patients.
Postpartum Interventions Targeted to Populations With HDP
Few studies have focused on improving CVH after pregnancy complicated by HDP.81–83 Among postpartum individuals with recent HDP, a gamified digital health intervention (involving text messages providing feedback, social incentives, and points for meeting goals) with wearable activity trackers was effective in increasing daily step counts over a 12-week period.82 A key strength of this study was the diverse recruitment, with 55% of individuals self-reporting Black race. A more longitudinal intervention study, Heart Health 4 Moms, randomized participants with a history of preeclampsia within the previous 5 years to an online intervention program that included educational modules, a community forum, and a lifestyle coach. The intervention group reported a statistically significant increase in knowledge of CVD risk, increased healthy eating, and less physical inactivity.83
Although existing evidence supports the effectiveness of lifestyle interventions to reduce risk of postpartum weight retention, additional studies are needed to understand the impact of such interventions on other CVH metrics such as BP and glucose control. Opportunities for impact include delivering digital and telehealth interventions and reaching postpartum patients within health systems and community-based settings such as the Special Supplemental Nutrition Program for Women, Infants, and Children and home visitation programs (Figure 3).67,74 Indeed, of National Diabetes Prevention Program participants with a remote history of gestational diabetes, 77.1% (29 037) joined an online program (ie, asynchronous delivery) rather than an in-person, distance-learning (synchronous), or combined-modality program.84 Programs that are delivered online, programs delivered in participants’ own homes or community settings, or a hybrid of both formats may offer the flexibility necessary to meet the needs of younger birthing individuals. Additional trials are needed that are focused on individuals with APOs, address clinical outcomes, and address methodological limitations such as small sample sizes, trial attrition, and self-reported rather than measured outcomes. With some exceptions, the trials discussed previously target individual-level behavior change. Upstream interventions spanning multiple levels–from communities and built environments to policy and systems change–offer opportunities to address the social determinants of health that contribute to maternal CVH disparities and to increase intervention reach, effectiveness, and sustainability.85,86 Additional trials are needed that focus on groups burdened by structural racism, who contribute to disparities in APOs and subsequent CVD outcomes.
Figure 3. Interventions to reduce CVD risk according to an ecological framework.
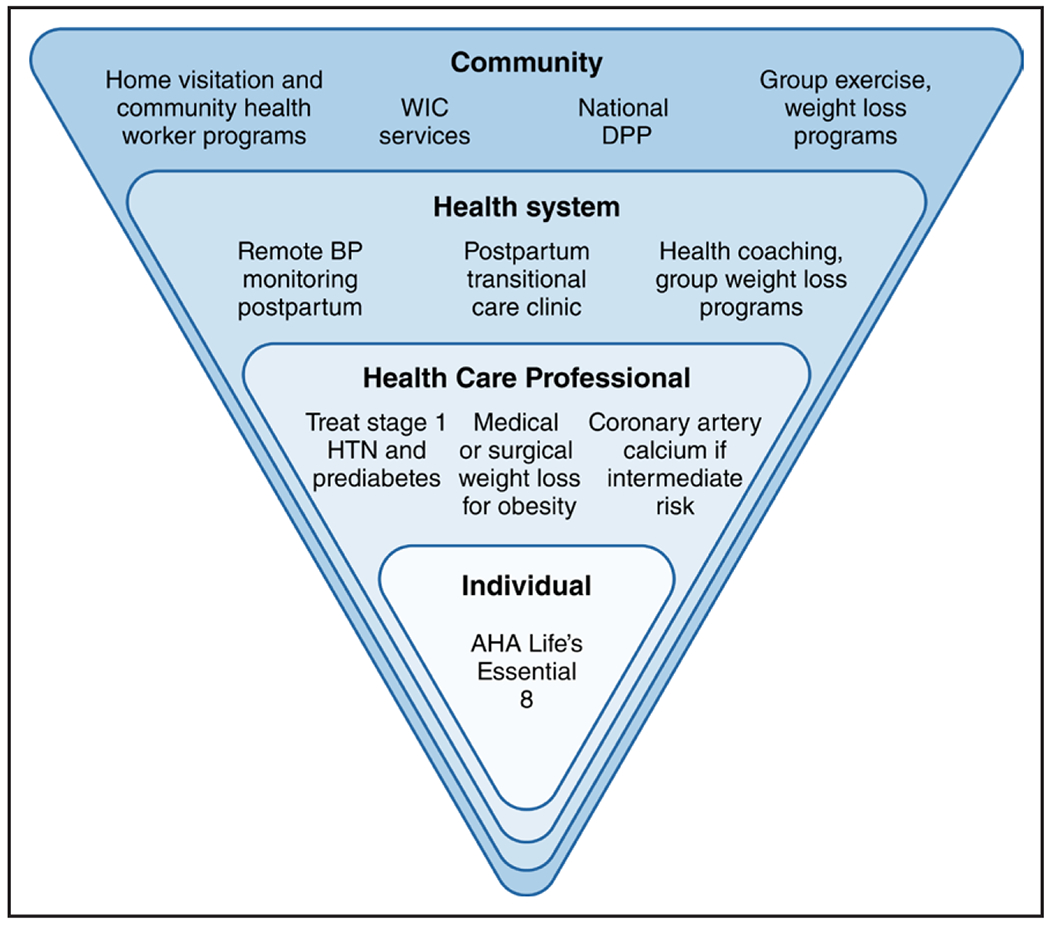
National policies to reduce maternal cardiovascular disease (CVD) risk include Medicaid expansion to ensure access to preventive care and contraception. AHA indicates American Heart Association; BP, blood pressure; DPP, Diabetes Prevention Program; HTN, hypertension; and WIC, Special Supplemental Nutrition Program for Women, Infants, and Children.
POSTPARTUM OPTIMIZATION OF CVH: A PRAGMATIC APPROACH FOR CLINICIANS
The postpartum and interpregnancy time frames are critical time windows in which implementation of a comprehensive multidisciplinary plan and careful consideration of CVD risk factors are important to reduce adverse maternal outcomes and for the implementation of innovative care delivery models for those affected by APOs.15,87,88 We provide in this section a timeline and framework for risk factor screening and specific interventions to improve CVH based on the AHA’s Life’s Essential 8 construct (Figure 4).15
Figure 4. Strategies and timeline to reduce cardiovascular risk after APOs.
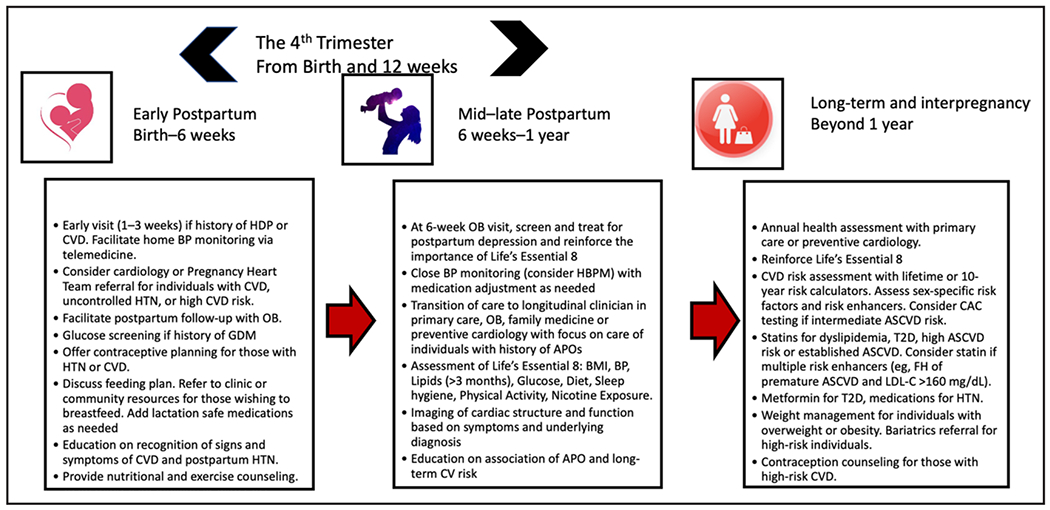
APO indicates adverse pregnancy outcome; ASCVD, atherosclerotic cardiovascular disease; BMI, body mass index; BP, blood pressure; CAC, coronary artery calcium; CV, cardiovascular; CVD, cardiovascular disease; FH, family history; GDM, gestational diabetes; HBPM, home blood pressure monitoring; HDP, hypertensive disorders of pregnancy; HTN, hypertension; LDL-C, low-density lipoprotein cholesterol; OB, obstetrician; and T2D, type 2 diabetes.
A recent AHA scientific statement recommended frequent cardiac risk factor screening assessments in the first year postpartum at 6 weeks, 12 weeks, 6 months, and 12 months, with appropriate transition from postpartum to longitudinal primary care around the 8- to 12-week mark.1 Screening and promoting CVH across the life course could be implemented with the Life’s Essential 8 framework as outlined in a recent AHA presidential advisory.15 Current literature promotes a framework that intervenes early and provides sustainable solutions within 1 year postpartum. Many interventions can be achieved by use of telemedicine, digital health, and self-monitoring at the individual, health system, and community levels (Figure 3). Reinforcement of lifestyle counseling and ongoing sex-specific risk assessment over time will be needed to potentially modify the long-term risk trajectory of CVD.
Identification of barriers and implementation of solutions that incorporate different team members to improve retention of individuals are essential given that up to 40% of women do not participate in postpartum care and only an estimated 18% to 25% of postpartum patients with APOs or chronic health conditions are seen by a primary care clinician within 6 months of delivery.88–90 Obstetrician-gynecologists are the primary care clinician for many individuals capable of pregnancy. The ACOG and AHA have released a joint presidential advisory calling for enhanced collaboration and coordination of care between obstetrician-gynecologists and cardiologists, particularly for CVD risk factor screening and integrated models of care.14 Table 2 lists strategies to improve postpartum care engagement.
Table 2.
Strategies to Improve Engagement With Postpartum Visits With Multidisciplinary Care Coordination
| Types | Specific Challenges | Solutions |
|---|---|---|
| Patient level | Competing priorities Lack of self-prioritization Dependent care |
Booking the postpartum follow-up prenatally Enlisting family and friends to assist with practical needs such as infant care, household chores, transportation Patient counseling Postpartum doula services |
| Practice level | Staffing shortages Clinician bias Maternity care deserts |
Telemedicine, digital tools, texts, phone calls to encourage participation Screening for social determinants of health and community referrals |
| Ecological level | Adverse social determinants such as health literacy, lack of language support, transportation, unsafe neighborhoods, access to clinics, underresourced and rural-dwelling communities | Patient navigators speaking native languages Home visiting nurses to check postpartum BP Community health workers embedded in the neighborhood to deliver health education and safe postpartum practices Medicaid expansion to 1 y postpartum Parental leave policies Enhanced services to meet social determinants of health such as housing and transportation |
BP indicates blood pressure.
BP Monitoring and Pharmacotherapy
Early BP treatment for postpartum severe hypertension is critical to prevent adverse maternal events. The choice of medications should no longer be limited by concerns of fetal exposure in utero and can be extended to those that are safe during breastfeeding. Labetalol, nifedipine, and amlodipine are commonly used postpartum.91 Calcium channel blockers offer the convenience of once-daily dosing but may cause headaches, which can affect medication adherence. Enalapril can be used safely during lactation.” Timely volume management in the postpartum period coupled with the judicious use of NSAIDs for pain control may reduce maternal hypertension morbidity and circumvent the need for hypertension-related hospitalizations. Home BP monitoring programs should be used, when available, to improve BP monitoring and treatment in the weeks after delivery.
Current BP guidelines from the American College of Cardiology/AHA93 and ACOG22 do not specify BP goals in the postpartum period. The threshold for BP treatment postpartum, as a result, is extrapolated from ACOG guidelines to treat BP ≥160/110 mm Hg in pregnant patients with preeclampsia.39 Treatment thresholds for chronic hypertension in pregnancy recently have changed on the basis of the CHAP trial (Chronic Hypertension and Pregnancy) to <140/90 mm Hg. However, CHAP was a trial of individuals with chronic hypertension, and current BP targets remain higher for management of preeclampsia or postpartum hypertension because lower BP goals in these subsets of the populations have not been well studied.94 In contrast, UK-based National Institute for Health and Care Excellence guidelines for hypertension in pregnancy92 support postpartum BP goals that are consistent with hypertension goals in nonpregnant adults.95 Although US-based studies are needed to confirm the optimal BP threshold in postpartum individuals, it may be reasonable to consider postpartum BP goals based on those established for nonpregnant, age-matched individuals. For patients initiated on BP treatment during pregnancy or the postpartum period, medications should be continued until normal BP readings are achieved. Self-management of BP medication using established algorithms in the context of close clinical follow-up has been shown to be feasible in select populations.96
Individuals with persistent hypertension after delivery should be treated according to current American College of Cardiology/AHA BP guidelines.93 Stage 1 hypertension is especially common after HDP, and affected individuals should receive intensive lifestyle counseling and close follow-up to assess CVD risk and need for medication initiation. Future studies are needed to determine the effectiveness of pharmacological treatment of stage 1 hypertension to prevent progression to subclinical CVD among individuals with prior HDP.97
Blood Glucose Monitoring
Women with gestational diabetes should be screened for dysglycemia at 4 to 12 weeks postpartum with a 2-hour 75-g oral glucose tolerance test, as recommended by the American Diabetes Association.25 Given the low rates of oral glucose tolerance test uptake,98,99 glucose screening with hemoglobin A1c or fasting glucose should be considered within the first year postpartum for those who do not complete an oral glucose tolerance test. Early postpartum oral glucose tolerance test testing (ie, before hospital discharge) is another promising strategy to improve diabetes screening. Lifelong screening for diabetes or prediabetes is recommended every 1 to 3 years for individuals with a history of gestational diabetes and may be considered for those with a history of any APO.
Blood Lipids
Given low rates of lipid screening among women of reproductive age,100 checking a lipid panel within the first year postpartum to establish a baseline is reasonable to screen for familial hypercholesterolemia and for assessment of ASCVD risk. Lipid levels may take up to 3 months to return to prepregnancy levels and are minimally affected by lactation or use of oral contraceptive pills; these effects are unlikely to be clinically significant.101 Early lipid testing at the 6-week postpartum visit may be indicated in patients with suspected familial hypercholesterolemia or those who experience barriers to accessing primary care. Intensive lifestyle changes are recommended for all individuals with dyslipidemia. Statins can be used in individuals who are not lactating with established indications according to the 2018 cholesterol guidelines.102
Nicotine Exposure
Avoidance of nicotine exposure is recommended in all individuals to improve CVH. Thus, the ACOG recommends that all types of tobacco and nicotine use, including cigarette smoking or vaping products and hookahs, be avoided in the postpartum period and that clinicians offer psychosocial, behavioral, and pharmacotherapy interventions.103
Postpartum Weight Management and Health Behaviors
Current evidence supports the effectiveness of lifestyle interventions to reduce the risk of postpartum weight retention and to improve postpartum weight in those with overweight or obesity. Referral to lifestyle interventions such as the National Diabetes Prevention Program or programs incorporating DPP elements is recommended for individuals with a history of gestational diabetes and can be considered for individuals with other APOs. Behavioral health coaches, digital technology, and integrated community-based programs hold particular promise for postpartum individuals. Many of these multicomponent interventions also have the potential to improve diet and physical activity. The US Department of Health and Human Services recommends 150 minutes of moderate-intensity exercise per week in the postpartum period. Exercise routines may be resumed or started postpartum gradually and after discussion with the obstetrician-gynecologist. Brisk walking with a baby in a carrier or stroller can help postpartum individuals achieve exercise goals and improve CVH, especially if time and childcare help are limited.
Sleep
The peripartum period is a vulnerable time for maternal sleep disturbances. After delivery, individuals experience increased nocturnal awakenings, more fragmented sleep, and impaired sleep efficiency. Poor sleep quality is also associated with postpartum depression. Nonpharmacological interventions such as massage and exercise have been found to improve subjective reports of maternal sleep.104 Pregnancy is also a risk factor for obstructive sleep apnea, which is associated with APOs and adverse neonatal outcomes.105 Obstructive sleep apnea can take up to 6 months to resolve, and some patients may go on to develop persistent obstructive sleep apnea.106 All patients with obstructive sleep apnea during pregnancy should undergo evaluation by a sleep medicine specialist after delivery.105
Additional Postpartum Factors Influencing CVH
Lactation
The ACOG recommends exclusive breast milk for an infant’s first 6 months of life, with continued breastfeeding as foods are introduced during the first year of life and up to 2 years. The effects of lactation on reduction of cardiometabolic risk factors should be stressed.107–109 Policy-level interventions to promote optimal support for lactating individuals, including paid maternity leave, access to lactation consultants, and affordable supplies for pumping, are needed simultaneously.
Postpartum Depression and Mood
Up to 10% of birthing individuals experience postpartum depression during the first year after delivery, and regular screening at obstetrician-gynecologists visits is recommended by several organizations.110,111 Maternal screening at pediatric clinics and colocating maternal and pediatric care are proposed solutions to improving maternal health.112 There are several validated screening scales for early recognition of postpartum depression, and early recognition in the postpartum period can lead to appropriate therapeutic interventions to improve maternal outcomes.
FUTURE DIRECTIONS TO ASSESS RISK OF SUBCLINICAL AND CLINICAL CVD
The 2019 American College of Cardiology/AHA primary prevention guidelines identified APOs as a risk-enhancing factor for ASCVD to guide clinician-patient discussions of lipid-lowering therapy among individuals 40 to 79 years of age after quantitative assessment of 10-year ASCVD risk with the Pooled Cohort Equations.113 Most postpartum individuals, however, are <40 years of age and are likely to be at low absolute 10-year risk. For adults 20 to 59 years of age, the guidelines recommend that 30-year risk assessment can be helpful. However, implementation, communication, and evidence-based interventions based on 30-year CVD risk assessment remain poorly defined, with no specific guidance for those who have experienced an APO. Therefore, as highlighted in expert consensus documents and by national funding agencies, critical barriers to actualize strategies for CVD prevention in young adults remain, particularly among postpartum individuals who experience an APO.114,115
Detection of subclinical CVD (eg, atherosclerosis on computed tomography, silent cerebrovascular disease, or left ventricular remodeling on echocardiogram) before the development of overt symptomatic disease among risk-enhanced populations can inform personalization of preventive interventions (eg, intensive BP lowering, lipid-lowering therapy).116 Current guidelines recommend consideration of coronary artery calcium (CAC) in middle-aged to older adults who are at intermediate 10-year risk of ASCVD but do not specifically recommend the use of CAC among those with a history of APOs.113 Available observational data suggest that women with a history of APOs have a higher prevalence of CAC in midlife, but it remains unclear whether APOs are a marker or mediator of future ASCVD. Among women in CREw-IMAGO (Cardiovascular Risk Profile: Imaging and Gender-Specific Disorders), any CAC was significantly more prevalent in women who self-reported a history of preeclampsia (20%) compared with those with normotensive pregnancies (13%).117 However, presence of CAC was not significantly different by preeclampsia status in women <45 years of age, which suggests limited utility of this tool in younger women. This is not surprising because CAC represents a later stage of the disease course that is often not present in women until later in life. In the Swedish Cardiopulmonary Bioimage Study, which enrolled 10 528 women 50 to 65 years of age with at least 1 live birth, a history of APO was associated with a significantly higher prevalence of any atherosclerosis (including noncalcified plaque).118 Therefore, identifying clinical populations in whom screening for atherosclerosis may better identify risk, personalizing clinical management, and improving hard outcomes are important targets of future clinical trials.
Individuals with a history of APO, in addition to increased risk for ASCVD, are at higher risk for heart failure, and distinguishing risks for CVD subtypes may inform differential strategies for screening, detection, and prevention. In a study at a single tertiary care center of 132 women with mean age of 38 years at the time of echocardiogram, having a history of HDP ≈10 years earlier compared with women with normotensive pregnancies was associated with a higher prevalence of left ventricular remodeling.119 Although physiological remodeling of the left ventricle occurs during pregnancy, it remains unclear whether the presence of pathological left ventricular remodeling postpartum is the result of persistence of abnormal changes due to an APO or if it preceded pregnancy according to preexisting CVD risk factor profiles among those likely to develop an APO. In addition, limited data are available on laboratory-based biomarker levels (eg, BNP [B-type natriuretic peptide], high-sensitivity troponin) in the postpartum period.
Although great attention has been paid in recent years to the association between APOs and heightened short- and long-term risks of CVD, the pathways and mechanisms by which APOs are related to increased risk of CVD remain unclear, which limits the optimization of prevention strategies. Some potential future directions to prioritize include the following: (1) mechanistic studies to understand early vascular dysfunction after APO that may occur in the absence of CVD risk factors; (2) elucidation of the target population for, timing of, and methods for detection of subclinical CVD and cerebrovascular disease with laboratory and imaging-based biomarkers before the onset of clinically symptomatic disease (eg, ASCVD, heart failure, and stroke) in individuals who experience an APO; (3) incorporation of a life course approach in pregnancy and postpartum studies that integrates upstream or prepregnancy risk factors with a focus on prevention of APOs themselves; (4) multilevel strategies that prioritize primordial prevention (ie, prevention of risk factor development such as hypertension and diabetes); and (5) implementation studies to identify best strategies to modify long-term trajectory of CVD risk and to address significant and persistent gaps in evidence-based treatment once risk is identified. This is currently being investigated in ENRICH (Early Intervention to Promote Cardiovascular Health of Mothers and Children), an ongoing National Heart, Lung, and Blood Institute–funded multicenter trial testing the effectiveness of an implementation-ready intervention to promote CVH and to reduce CVH disparities.120
CONCLUSIONS
Interventions to mitigate APO-associated short-term and long-term CVD risks such as promotion of CVH based on the AHA Life’s Essential 8 have great potential, particularly in the first 12 months postpartum, but require future study. Health system–based interventions that focus on transitions of care in the postpartum period, prioritize interrelated health and social needs of individuals at risk of readmission and disease progression, improve patient awareness, and offer postpartum screening for cardiovascular risk factors are the crux of postpartum care. Further studies on long-term reduction of CVD risk and implementation of policy-level changes that mitigate the adverse impact of social determinants of health with innovative health care delivery models and value-based care are essential, especially in resource-limited areas.
Supplementary Material
Footnotes
The American Heart Association makes every effort to avoid any actual or potential conflicts of interest that may arise as a result of an outside relationship or a personal, professional, or business interest of a member of the writing panel. Specifically, all members of the writing group are required to complete and submit a Disclosure Questionnaire showing all such relationships that might be perceived as real or potential conflicts of interest.
This statement was approved by the American Heart Association Science Advisory and Coordinating Committee on December 1, 2023, and the American Heart Association Executive Committee on January 12, 2024. A copy of the document is available at https://professional.heart.org/statements by using either “Search for Guidelines & Statements” or the “Browse by Topic” area. To purchase additional reprints, call 215-356-2721 or Meredith.Edelman@wolterskluwer.com
Disclosures
Writing Group Disclosures
| Writing group member | Employment | Research grant | Other research support | Speakers’ bureau/honoraria | Expert witness | Ownership interest | Consultant/advisory board | Other |
|---|---|---|---|---|---|---|---|---|
| Jennifer Lewey | University of Pennsylvania, Perelman School of Medicine | NHLBI (grant funding)† | None | None | None | None | None | None |
| Laxima S. Mehta | The Ohio State University | None | None | None | None | None | None | None |
| Theresa M. Beckie | University of South Florida College of Nursing/College of Medicine | None | None | None | None | None | None | None |
| Haywood L. Brown | Morsani College of Medicine, University of South Florida | None | None | None | None | None | Merck for Mothers†; HRSA Healthy Start†; Myriad Genetics*; Hologic* | None |
| Susan D. Brown | UC Davis | NHLBI (PI of R01 HL142996)†; UC Davis, Wyeth Court Settlement cy-pres award (coinvestigator of the UC Davis Center for Women’s Cardiovascular and Brain Health)†; NIDDK (PI of R01 DK122087)†; NIH/NIDDK (coinvestigator of R01 DK118455)†; NIH/NIDDK (coinvestigator of P30 DK092924)†; NIH/NIDDK (PI of K26DK138246)†; NIH/OD (coinvestigator of UG3OD035540)† | None | NIH (Summer Institute on Randomized Behavioral Trials)* | None | None | None | None |
| Vesna D. Garovic | Mayo Clinic | None | None | None | None | None | None | None |
| Sadiya S. Khan | Northwestern University Feinberg School of Medicine | None | None | None | None | None | None | None |
| Eliza C. Miller | Columbia University | NIH/NINDS (K23NS107645)†; NIH/NIA (R21AG069111)†; NIH NINDS (R01NS122815)†; NIH NICHD (R21HD110992)† | None | None | Expert for defense (stroke) 2022–2023†; expert for plaintiff (stroke) 2022–2023† | None | None | None |
| Garima Sharma | Inova Heart and Vascular Institute, Inova Fairfax Hospital | None | None | None | None | None | None | None |
Reviewer Disclosures
| Reviewer | Employment | Research grant | Other research support | Speakers’ bureau/honoraria | Expert witness | Ownership interest | Consultant/advisory board | Other |
|---|---|---|---|---|---|---|---|---|
| Karen | Saint Luke’s Hospital | None | None | None | None | None | None | None |
| Anna Grodzinsky | Saint Luke’s Mid America Heart Institute | None | None | None | None | None | None | None |
| Michael Honigberg | Massachusetts General Hospital | AHA (“Health-related social needs and the risk of hypertension in young adult and early midlife women: the impact of pregnancy”)† | None | None | None | None | Miga Health*; CRISPR Therapeutics*;Comanche Biopharma* | None |
| Rina Mauricio | University of Texas Southwestern | None | None | None | None | None | None | None |
| Anum Minhas | Johns Hopkins University | NIH KL2TR003099†; AMAG Pharmaceuticals Preeclampsia and Prematurity Grant†; Preeclampsia Foundation Vision Grant†; Doris Duke Early Clinician Investigator Award† | None | None | None | None | None | None |
| Karol Watson | David Geffen School of Medicine at UCLA | None | None | None | None | None | None | None |
REFERENCES
- 1.Parikh NI, Gonzalez JM, Anderson CAM, Judd SE, Rexrode KM, Hlatky MA, Gunderson EP, Stuart JJ, Vaidya D; on behalf of the American Heart Association Council on Epidemiology and Prevention; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular and Stroke Nursing; and the Stroke Council. Adverse pregnancy outcomes and cardiovascular disease risk: unique opportunities for cardiovascular disease prevention in women: a scientific statement from the American Heart Association. Circulation. 2021;143:e902–e916. doi: 10.1161/CIR.0000000000000961 [DOI] [PubMed] [Google Scholar]
- 2.Lane-Cordova AD, Khan SS, Grobman WA, Greenland P, Shah SJ. Long-term cardiovascular risks associated with adverse pregnancy outcomes: JACC review topic of the week. J Am Coll Cardiol. 2019;73:2106–2116. doi: 10.1016/j.jacc.2018.12.092 [DOI] [PubMed] [Google Scholar]
- 3.Garovic VD, Dechend R, Easterling T, Karumanchi SA, McMurtry Baird S, Magee LA, Rana S, Vermunt JV, August P; on behalf of the American Heart Association Council on Hypertension; Council on the Kidney in Cardiovascular Disease, Kidney in Heart Disease Science Committee; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Lifestyle and Cardiometabolic Health; Council on Peripheral Vascular Disease; and Stroke Council. Hypertension in pregnancy: diagnosis, blood pressure goals, and pharmacotherapy: a scientific statement from the American Heart Association [published correction appears in Hypertension. 2022;79:e70]. Hypertension. 2022;79:e21–e41. doi: 10.1161/HYP.0000000000000208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Honigberg MC, Zekavat SM, Aragam K, Klarin D, Bhatt DL, Scott NS, Peloso GM, Natarajan P Long-term cardiovascular risk in women with hypertension during pregnancy. J Am Coll Cardiol. 2019;74:2743–2754. doi: 10.1016/j.jacc.2019.09.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haug EB, Horn J, Markovitz AR, Fraser A, Klykken B, Dalen H, Vatten LJ, Romundstad PR, Rich-Edwards JW, Åsvold BO. Association of conventional cardiovascular risk factors with cardiovascular disease after hypertensive disorders of pregnancy: analysis of the Nord-Trøndelag Health Study. JAMA Cardiol. 2019;4:628–635. doi: 10.1001/jamacardio.2019.1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dennison RA, Chen ES, Green ME, Legard C, Kotecha D, Farmer G, Sharp SJ, Ward RJ, Usher-Smith JA, Griffin SJ. The absolute and relative risk of type 2 diabetes after gestational diabetes: a systematic review and meta-analysis of 129 studies. Diabetes Res Clin Pract. 2021;171:108625. doi: 10.1016/j.diabres.2020.108625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Behrens I, Basit S, Melbye M, Lykke JA, Wohlfahrt J, Bundgaard H, Thilaganathan B, Boyd HA. Risk of post-pregnancy hypertension in women with a history of hypertensive disorders of pregnancy: nationwide cohort study. BMJ. 2017;358:j3078. doi: 10.1136/bmj.j3078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bellamy L, Casas J-P, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335:974. doi: 10.1136/bmj.39335.385301.BE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehta LS, Warnes CA, Bradley E, Burton T, Economy K, Mehran R, Safdar B, Sharma G, Wood M, Valente AM, et al. ; on behalf of the American Heart Association Council on Clinical Cardiology; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular and Stroke Nursing; and Stroke Council. Cardiovascular considerations in caring for pregnant patients: a scientific statement from the American Heart Association [published corrections appear in Circulation. 2020;141:e904 and Circulation. 2021;143:e792–e793]. Circulation. 2020;141:e884–e903. doi: 10.1161/CIR.0000000000000772 [DOI] [PubMed] [Google Scholar]
- 10.Bond RM, Gaither K, Nasser SA, Albert MA, Ferdinand KC, Njoroge JN, Parapid B, Hayes SN, Pegus C, Sogade B, et al. ; Association of Black Cardiologists. Working agenda for black mothers: a position paper from the Association of Black Cardiologists on solutions to improving Black maternal health. Circ Cardiovasc Qual Outcomes. 2021;14:e007643. doi: 10.1161/CIRCOUTCOMES.120.007643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehta LS, Sharma G, Creanga AA, Hameed AB, Hollier LM, Johnson JC, Leffert L, McCullough LD, Mujahid MS, Watson K, et al. ; on behalf of the American Heart Association Advocacy Coordinating Committee. Call to action: maternal health and saving mothers: a policy statement from the American Heart Association. Circulation. 2021;144:e251–e269. doi: 10.1161/CIR.0000000000001000 [DOI] [PubMed] [Google Scholar]
- 12.Crear-Perry J, Correa-de-Araujo R, Lewis Johnson T, McLemore MR, Neilson E, Wallace M. Social and structural determinants of health inequities in maternal health. J Womens Health (Larchmt). 2021;30:230–235. doi: 10.1089/jwh.2020.8882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Powell-Wiley TM, Baumer Y, Baah FO, Baez AS, Farmer N, Mahlobo CT, Pita MA, Potharaju KA, Tamura K, Wallen GR. Social determinants of cardiovascular disease. Circ Res. 2022;130:782–799. doi: 10.1161/CIRCRESAHA.121.319811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown HL, Warner JJ, Gianos E, Gulati M, Hill AJ, Hollier LM, Rosen SE, Rosser ML, Wenger NK; on behalf of the American Heart Association and the American College of Obstetricians and Gynecologists. Promoting risk identification and reduction of cardiovascular disease in women through collaboration with obstetricians and gynecologists: a presidential advisory from the American Heart Association and the American College of Obstetricians and Gynecologists. Circulation. 2018;137:e843–e852. doi: 10.1161/CIR.0000000000000582 [DOI] [PubMed] [Google Scholar]
- 15.Lloyd-Jones DM, Allen NB, Anderson CAM, Black T, Brewer LC, Foraker RE, Grandner MA, Lavretsky H, Perak AM, Sharma G, et al. ; on behalf of the American Heart Association. Life’s Essential 8: updating and enhancing the American Heart Association’s construct of cardiovascular health: a presidential advisory from the American Heart Association. Ciroulation. 2022;146:e18–e43. doi: 10.1161/CIR.0000000000001078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American College of Obstetricians and Gynecologists, Society for Maternal-Fetal Medicine. Obstetric care consensus No 8: interpregnancy care. Obstet Gynecol. 2019;133:e51–e72. doi: 10.1097/AOG.0000000000003025 [DOI] [PubMed] [Google Scholar]
- 17.Parikh NI, Hwang S-J, Ingelsson E, Benjamin EJ, Fox CS, Vasan RS, Murabito JM. Breastfeeding in infancy and adult cardiovascular disease risk factors. Am J Med. 2009;122:656–63.e1. doi: 10.1016/j.amjmed.2008.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barriers to breastfeeding: supporting initiation and continuation of breastfeeding: ACOG committee opinion, number 821. Obstet Gynecol. 2021;137:e54–e62. doi: 10.1097/AOG.0000000000004249 [DOI] [PubMed] [Google Scholar]
- 19.Curtis KM, Tepper NK, Jatlaoui TC, Berry-Bibee E, Horton LG, Zapata LB, Simmons KB, Pagano HP, Jamieson DJ, Whiteman MKUS. Medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1–130. doi: 10.15585/mmwr.rr6503a1 [DOI] [PubMed] [Google Scholar]
- 20.Wainstock T, Sheiner E. Clinical factors associated with preeclampsia recurrence. Pregnancy Hypertens. 2022;30:31–35. doi: 10.1016/j.preghy.2022.08.004 [DOI] [PubMed] [Google Scholar]
- 21.ACOG practice bulletin No. 190: gestational diabetes mellitus. Obstet Gynecol. 2018;131:e49–e64. doi: 10.1097/AOG.0000000000002501 [DOI] [PubMed] [Google Scholar]
- 22.American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin No. 203: chronic hypertension in pregnancy. Obstet Gynecol. 2019;133:e26–e50. doi: 10.1097/AOG.0000000000003020 [DOI] [PubMed] [Google Scholar]
- 23.Wu D, Gao L, Huang O, Ullah K, Guo M, Liu Y, Zhang J, Chen L, Fan J, Sheng J, et al. Increased adverse pregnancy outcomes associated with stage 1 hypertension in a low-risk cohort. Hypertension. 2020;75:772–780. doi: 10.1161/HYPERTENSIONAHA.119.14252 [DOI] [PubMed] [Google Scholar]
- 24.Tita AT, Szychowski JM, Boggess K, Dugoff L, Sibai B, Lawrence K, Hughes BL, Bell J, Aagaard K, Edwards RK, et al. ; Chronic Hypertension and Pregnancy (CHAP) Trial Consortium. Treatment for mild chronic hypertension during pregnancy. N Engl J Med. 2022;386:1781–1792. doi: 10.1056/NEJMoa2201295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, Hilliard ME, Isaacs D, Johnson EL, et al. ; on behalf of the American Diabetes Association. 15 Management of diabetes in pregnancy: Standards of Care in Diabetes–2023. Diabetes Care. 2023;46:S254–S266. doi: 10.2337/dc23-S015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.ACOG committee opinion No. 743: low-dose aspirin use during pregnancy. Obstet Gynecol. 2018;132:e44–e52. doi: 10.1097/AOG.0000000000002708 [DOI] [PubMed] [Google Scholar]
- 27.Goel A, Maski MR, Bajracharya S, Wenger JB, Zhang D, Salahuddin S, Shahul SS, Thadhani R, Seely EW, Karumanchi SA, et al. Epidemiology and mechanisms of de novo and persistent hypertension in the postpartum period. Circulation. 2015;132:1726–1733. doi: 10.1161/CIRCULATIONAHA.115.015721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hauspurg A, Jeyabalan A. Postpartum preeclampsia or eclampsia: defining its place and management among the hypertensive disorders of pregnancy. Am J Obstet Gynecol. 2022;226:S1211–S1221. doi: 10.1016/j.ajog.2020.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ditisheim A, Wuerzner G, Ponte B, Vial Y, Irion O, Burnier M, Boulvain M, Pechère-Bertschi A. Prevalence of hypertensive phenotypes after preeclampsia: a prospective cohort study. Hypertension. 2018;71:103–109. doi: 10.1161/HYPERTENSIONAHA.117.09799 [DOI] [PubMed] [Google Scholar]
- 30.Gestational hypertension and preeclampsia: ACOG practice bulletin, number 222. Obstet Gynecol. 2020;135:e237–e260. doi: 10.1097/AOG.0000000000003891 [DOI] [PubMed] [Google Scholar]
- 31.Hirshberg A, Zhu Y, Smith-McLallen A, Srinivas SK. Association of a remote blood pressure monitoring program with postpartum adverse outcomes. Obstet Gynecol. 2023;141:1163–1170. doi: 10.1097/AOG.0000000000005197 [DOI] [PubMed] [Google Scholar]
- 32.Elgendy IY, Bukhari S, Barakat AF, Pepine CJ, Lindley KJ, Miller EC; American College of Cardiology Cardiovascular Disease in Women Committee. Maternal stroke. Circulation. 2021;143:727–738. doi: 10.1161/CIRCULATIONAHA.120.051460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walters BN, Walters T. Hypertension in the puerperium. Lancet. 1987;2:330. doi: 10.1016/s0140-6736(87)90912-3 [DOI] [PubMed] [Google Scholar]
- 34.Bellos I, Pergialiotis V, Antsaklis A, Loutradis D, Daskalakis G. Safety of non-steroidal anti-inflammatory drugs in postpartum period in women with hypertensive disorders of pregnancy: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2020;56:329–339. doi: 10.1002/uog.21997 [DOI] [PubMed] [Google Scholar]
- 35.MacIntyre IM, Turtle EJ, Farrah TE, Graham C, Dear JW, Webb DJ; PATH-BP (Paracetamol in Hypertension–Blood Pressure) Investigators. Regular acetaminophen use and blood pressure in people with hypertension: the PATH-BP trial. Circulation. 2022;145:416–423. doi: 10.1161/CIRCULATIONAHA.121.056015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lopes Perdigao J, Lewey J, Hirshberg A, Koelper N, Srinivas SK, Elovitz MA, Levine LD. Furosemide for accelerated recovery of blood pressure postpartum in women with a hypertensive disorder of pregnancy: a randomized controlled trial. Hypertension. 2021;77:1517–1524. doi: 10.1161/HYPERTENSIONAHA.120.16133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hirshberg A, Downes K, Srinivas S. Comparing standard office-based follow-up with text-based remote monitoring in the management of postpartum hypertension: a randomised clinical trial. BMJ Qual Saf. 2018;27:871–877 doi: 10.1136/bmjqs-2018-007837 [DOI] [PubMed] [Google Scholar]
- 38.Hauspurg A, Lemon LS, Quinn BA, Binstock A, Larkin J, Beigi RH, Watson AR, Simhan HN. A postpartum remote hypertension monitoring protocol implemented at the hospital level. Obstet Gynecol. 2019;134:685–691. doi: 10.1097/AOG.0000000000003479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niu B, Mukhtarova N, Alagoz O, Hoppe K. Cost-effectiveness of telehealth with remote patient monitoring for postpartum hypertension. J Matern Fetal Neonatal Med. 2022;35:7555–7561. doi: 10.1080/14767058.2021.1956456 [DOI] [PubMed] [Google Scholar]
- 40.Hirshberg A, Sammel MD, Srinivas SK. Text message remote monitoring reduced racial disparities in postpartum blood pressure ascertainment. Am J Obstet Gynecol. 2019;221:283–285. doi: 10.1016/j.ajog.2019.05.011 [DOI] [PubMed] [Google Scholar]
- 41.Hauspurg A, Lemon L, Cabrera C, Javaid A, Binstock A, Quinn B, Larkin J, Watson AR, Beigi RH, Simhan H. Racial differences in postpartum blood pressure trajectories among women after a hypertensive disorder of pregnancy. JAMA Netw Open. 2020;3:e2030815. doi: 10.1001/jamanetworkopen.2020.30815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levine LD, Nkonde-Price C, Limaye M, Srinivas SK. Factors associated with postpartum follow-up and persistent hypertension among women with severe preeclampsia. J Perinatol. 2016;36:1079–1082. doi: 10.1038/jp.2016.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giorgione V, Khalil A, O’Driscoll J, Thilaganathan B. Peripartum screening for postpartum hypertension in women with hypertensive disorders of pregnancy. J Am Coll Cardiol. 2022;80:1465–1476. doi: 10.1016/j.jacc.2022.07.028 [DOI] [PubMed] [Google Scholar]
- 44.Benschop L, Duvekot JJ, Versmissen J, van Broekhoven V, Steegers EAP, Roeters van Lennep JE. Blood pressure profile 1 year after severe preeclampsia. Hypertension. 2018;71:491–498. doi: 10.1161/HYPERTENSIONAHA.117.10338 [DOI] [PubMed] [Google Scholar]
- 45.Boucheron P, Lailler G, Moutengou E, Regnault N, Gabet A, Deneux-Tharaux C, Kretz S, Grave C, Mounier-Vehier C, Tsatsaris V, et al. Hypertensive disorders of pregnancy and onset of chronic hypertension in France: the nationwide CONCEPTION study. Eur Heart J. 2021;43:ehab686. [DOI] [PubMed] [Google Scholar]
- 46.Barrett PM, McCarthy FP, Evans M, Kublickas M, Perry IJ, Stenvinkel P, Khashan AS, Kublickiene K. Hypertensive disorders of pregnancy and the risk of chronic kidney disease: a Swedish registry-based cohort study. PLoS Med. 2020;17:e1003255. doi: 10.1371/journal.pmed.1003255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kattah AG, Asad R, Scantlebury DC, Bailey KR, Wiste HJ, Hunt SC, Mosley TH, Kardia SLR, Turner ST, Garovic VD. Hypertension in pregnancy is a risk factor for microalbuminuria later in life. J Clin Hypertens (Greenwich). 2013;15:617–623. doi: 10.1111/jch.12116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haas DM, Parker CB, Marsh DJ, Grobman WA, Ehrenthal DB, Greenland P, Bairey Merz CN, Pemberton VL, Silver RM, Barnes S, et al. ; NHLBI nuMoM2b Heart Health Study. Association of adverse pregnancy outcomes with hypertension 2 to 7 years postpartum. J Am Heart Assoc. 2019;8:e013092. doi: 10.1161/JAHA.119.013092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O’Donnell MJ, Chin SL, Rangarajan S, Xavier D, Liu L, Zhang H, Rao-Melacini P, Zhang X, Pais P, Agapay S, et al. ; INTERSTROKE Investigators. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet. 2016;388:761–775. doi: 10.1016/S0140-6736(16)30506-2 [DOI] [PubMed] [Google Scholar]
- 50.Anand SS, Islam S, Rosengren A, Franzosi MG, Steyn K, Yusufali AH, Keltai M, Diaz R, Rangarajan S, Yusuf S; INTERHEART Investigators. Risk factors for myocardial infarction in women and men: insights from the INTERHEART study. Eur Heart J. 2008;29:932–940. doi: 10.1093/eurheartj/ehn018 [DOI] [PubMed] [Google Scholar]
- 51.Ji H, Niiranen TJ, Rader F, Henglin M, Kim A, Ebinger JE, Claggett B, Merz CNB, Cheng S. Sex differences in blood pressure associations with cardiovascular outcomes. Circulation. 2021;143:761–763. doi: 10.1161/CIRCULATIONAHA.120.049360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leon LJ, McCarthy FP, Direk K, Gonzalez-Izquierdo A, Prieto-Merino D, Casas JP, Chappell L. Preeclampsia and cardiovascular disease in a large UK pregnancy cohort of linked electronic health records: a CALIBER study. Circulation. 2019;140:1050–1060. doi: 10.1161/CIRCULATIONAHA.118.038080 [DOI] [PubMed] [Google Scholar]
- 53.Miller EC, Kauko A, Tom SE, Laivuori H, Niiranen T, Bello NA; FinnGen Investigators. Risk of midlife stroke after adverse pregnancy outcomes: the FinnGen study. Stroke. 2023;54:1798–1805. doi: 10.1161/STROKEAHA.123.043052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bushnell C, Howard VJ, Lisabeth L, Caso V, Gall S, Kleindorfer D, Chaturvedi S, Madsen TE, Demel SL, Lee S-J, et al. Sex differences in the evaluation and treatment of acute ischaemic stroke. Lancet Neurol. 2018;17:641–650. doi: 10.1016/S1474-4422(18)30201-1 [DOI] [PubMed] [Google Scholar]
- 55.Maserejian NN, Link CL, Lutfey KL, Marceau LD, McKinlay JB. Disparities in physicians’ interpretations of heart disease symptoms by patient gender: results of a video vignette factorial experiment. J Womens Health (Larchmt). 2009;18:1661–1667. doi: 10.1089/jwh.2008.1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stevens CJ, Shaffer JA, Edwards KS, Masters KS, Leon KK, Wood MJ, Pittman Wagers T. Younger age impacts perceptions of care received in the emergency department among women with spontaneous coronary artery dissection. J Womens Health (Larchmt). 2022;31:1165–1172. doi: 10.1089/jwh.2021.0162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liberman AL, Zhang C, Lipton RB, Kamel H, Parikh NS, Navi BB, Segal AZ, Razzak J, Newman-Toker DE, Merkler AE. Short-term stroke risk after emergency department treat-and-release headache visit. Headache. 2022;62:1198–1206. doi: 10.1111/head.14387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oras P, Häbel H, Skoglund PH, Svensson P. Elevated blood pressure in the emergency department. Hypertension. 2020;75:229–236. doi: 10.1161/HYPERTENSIONAHA.119.14002 [DOI] [PubMed] [Google Scholar]
- 59.Stovner LJ, Hagen K, Linde M, Steiner TJ. The global prevalence of headache: an update, with analysis of the influences of methodological factors on prevalence estimates. J Headache Pain. 2022;23:34. doi: 10.1186/s10194-022-01402-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ohayon MM. Prevalence and risk factors of morning headaches in the general population. Arch Intern Med. 2004;164:97–102. doi: 10.1001/archinte.164.1.97 [DOI] [PubMed] [Google Scholar]
- 61.Reckelhoff JF. Androgens and blood pressure control: sex differences and mechanisms. Mayo Clin Proc. 2019;94:536–543. doi: 10.1016/j.mayocp.2018.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zheng Y, Wen X, Bian J, Zhao J, Lipkind HS, Hu H. Racial, ethnic, and geographic disparities in cardiovascular health among women of childbearing age in the United States. J Am Heart Assoc. 2021;10:e020138. doi: 10.1161/JAHA.120.020138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Olson CM, Strawderman MS, Hinton PS, Pearson TA. Gestational weight gain and postpartum behaviors associated with weight change from early pregnancy to 1 y postpartum. Int J Obes Relat Metab Disord. 2003;27:117–127. doi: 10.1038/sj.ijo.0802156 [DOI] [PubMed] [Google Scholar]
- 64.Ratner RE, Christophi CA, Metzger BE, Dabelea D, Bennett PH, Pi-Sunyer X, Fowler S, Kahn SE; Diabetes Prevention Program Research Group. Prevention of diabetes in women with a history of gestational diabetes: effects of metformin and lifestyle interventions. J Clin Endocrinol Metab. 2008;93:4774–4779. doi: 10.1210/jc.2008-0772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gruss SM, Nhim K, Gregg E, Bell M, Luman E, Albright A. Public health approaches to type 2 diabetes prevention: the US National Diabetes Prevention Program and beyond. Curr Diab Rep. 2019;19:78. doi: 10.1007/s11892-019-1200-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nicklas JM, Zera CA, England LJ, Rosner BA, Horton E, Levkoff SE, Seely EW. A web-based lifestyle intervention for women with recent gestational diabetes mellitus: a randomized controlled trial. Obstet Gynecol. 2014;124:563–570. doi: 10.1097/AOG.0000000000000420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Phelan S, Hagobian T, Brannen A, Hatley KE, Schaffner A, Muñoz-Christian K, Tate DF. Effect of an internet-based program on weight loss for low-income postpartum women: a randomized clinical trial. JAMA. 2017;317:2381–2391. doi: 10.1001/jama.2017.7119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Basharat S, Gilani SA, Burq AI, Bashir S. Low glycaemic index diet is effective in managing weight among obese postpartum women. J Pak Med Assoc. 2018;68:548–553. [PubMed] [Google Scholar]
- 69.Holmes VA, Draffin CR, Patterson CC, Francis L, Irwin J, McConnell M, Farrell B, Brennan SF, McSorley O, Wotherspoon AC, et al. ; PAIGE Study Group. Postnatal lifestyle intervention for overweight women with previous gestational diabetes: a randomized controlled trial. J Clin Endocrinol Metab. 2018;103:2478–2487. [DOI] [PubMed] [Google Scholar]
- 70.Palnati M, Marcus BH, Pekow P, Rosal MC, Manson JE, Chasan-Taber L. The Impact of a lifestyle intervention on postpartum weight retention among at-risk Hispanic women. Am J Prev Med. 2021;61:44–54. doi: 10.1016/j.amepre.2021.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huseinovic E, Bertz F, Leu Agelii M, Hellebö Johansson E, Winkvist A, Brekke HK. Effectiveness of a weight loss intervention in postpartum women: results from a randomized controlled trial in primary health care. Am J Clin Nutr. 2016;104:362–370. doi: 10.3945/ajcn.116.135673 [DOI] [PubMed] [Google Scholar]
- 72.Ferrara A, Hedderson MM, Brown SD, Albright CL, Ehrlich SF, Tsai A-L, Caan BJ, Sternfeld B, Gordon NP, Schmittdiel JA, et al. The comparative effectiveness of diabetes prevention strategies to reduce postpartum weight retention in women with gestational diabetes mellitus: the Gestational Diabetes’ Effects on Moms (GEM) cluster randomized controlled trial. Diabetes Care. 2016;39:65–74. doi: 10.2337/dc15-1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tussing-Humphreys LM, Thomson JL, Hemphill NO, Goodman MH, Landry AS. Maternal weight in the postpartum: results from the Delta Healthy Sprouts Trial. Matern Health Neonatol Perinatol. 2017;3:20. doi: 10.1186/s40748-017-0058-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Haire-Joshu D, Cahill AG, Stein RI, Cade WT, Woolfolk CL, Moley K, Mathur A, Schwarz CD, Schechtman KB, Klein S. Randomized controlled trial of home-based lifestyle therapy on postpartum weight in underserved women with overweight/obesity. Obesity (Silver Spring). 2019;27:535–541. doi: 10.1002/oby.22413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu J, Wilcox S, Hutto B, Turner-McGrievy G, Wingard E. Effects of a lifestyle intervention on postpartum weight retention among women with elevated weight. Obesity (Silver Spring). 2022;30:1370–1379. doi: 10.1002/oby.23449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Simpson SA, Coulman E, Gallagher D, Jewell K, Cohen D, Newcombe RG, Huang C, Robles-Zurita JA, Busse M, Owen-Jones E, et al. Healthy Eating and Lifestyle in Pregnancy (HELP): a cluster randomised trial to evaluate the effectiveness of a weight management intervention for pregnant women with obesity on weight at 12 months postpartum. Int J Obes (Lond). 2021;45:1728–1739. doi: 10.1038/s41366-021-00835-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thomson JL, Tussing-Humphreys LM, Goodman MH, Landry AS. Enhanced curriculum intervention did not result in increased postnatal physical activity in rural, southern, primarily African American women. Am J Health Promot. 2018;32:464–472. doi: 10.1177/0890117117736090 [DOI] [PubMed] [Google Scholar]
- 78.Thomson JL, Tussing-Humphreys LM, Landry AS, Goodman MH. No improvements in postnatal dietary outcomes were observed in a two-arm, randomized, controlled, comparative impact trial among rural, southern, African-American women. J Acad Nutr Diet. 2018;118:1196–1207. doi: 10.1016/j.jand.2017.11.010 [DOI] [PubMed] [Google Scholar]
- 79.Lane-Cordova AD, Jerome GJ, Paluch AE, Bustamante EE, LaMonte MJ, Pate RR, Weaver RG, Webber-Ritchey KJ, Gibbs BB; on behalf of the Committee on Physical Activity of the American Heart Association Council on Lifestyle and Cardiometabolic Health. Supporting physical activity in patients and populations during life events and transitions: a scientific statement from the American Heart Association. Circulation. 2022;145:e117–e128. doi: 10.1161/CIR.0000000000001035 [DOI] [PubMed] [Google Scholar]
- 80.Collins LM, Strayhorn JC, Vanness DJ. One view of the next decade of research on behavioral and biobehavioral approaches to cancer prevention and control: intervention optimization. Transl Behav Med. 2021;11:1998–2008. doi: 10.1093/tbm/ibab087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Scholten RR, Thijssen DJH, Lotgering FK, Hopman MTE, Spaanderman MEA. Cardiovascular effects of aerobic exercise training in formerly preeclamptic women and healthy parous control subjects. Am J Obstet Gynecol. 2014;211:516.e1–516.e11. doi: 10.1016/j.ajog.2014.04.025 [DOI] [PubMed] [Google Scholar]
- 82.Lewey J, Murphy S, Zhang D, Putt ME, Elovitz MA, Riis V, Patel MS, Levine LD. Effectiveness of a text-based gamification intervention to improve physical activity among postpartum individuals with hypertensive disorders of pregnancy: a randomized clinical trial. JAMA Cardiol. 2022;7:591–599. doi: 10.1001/jamacardio.2022.0553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rich-Edwards JW, Stuart JJ, Skurnik G, Roche AT, Tsigas E, Fitzmaurice GM, Wilkins-Haug LE, Levkoff SE, Seely EW. Randomized trial to reduce cardiovascular risk in women with recent preeclampsia. J Womens Health (Larchmt). 2019;28:1493–1504. doi: 10.1089/jwh.2018.7523 [DOI] [PubMed] [Google Scholar]
- 84.Cannon MJ, Ng BP, Lloyd K, Reynolds J, Ely EK. Delivering the National Diabetes Prevention Program: assessment of enrollment in in-person and virtual organizations. J Diabetes Res. 2022;2022:2942918. doi: 10.1155/2022/2942918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yuhas M, Moore CF, Garay J, Brown SD. Improving maternal cardiovascular health in underserved populations: a narrative review of behavioral intervention trials targeting postpartum weight retention. Curr Atheroscler Rep. 2022;24:689–699. doi: 10.1007/s11883-022-01045-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hill-Briggs F, Adler NE, Berkowitz SA, Chin MH, Gary-Webb TL, Navas-Acien A, Thornton PL, Haire-Joshu D. Social determinants of health and diabetes: a scientific review. Diabetes Care. 2020;44:258–279. doi: 10.2337/dci20-0053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McCloskey L, Bernstein J, Bridging the Chasm Collaborative, Amutah-Onukagha N, Anthony J, Barger M, Belanoff C, Bennett T, Bird CE, Bolds D, Brenna BW. Bridging the chasm between pregnancy and health over the life course: a national agenda for research and action. Womens Health Issues. 2021;31:204–218. doi: 10.1016/j.whi.2021.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McKinney J, Keyser L, Clinton S, Pagliano C. ACOG committee opinion No 736: optimizing postpartum care. Obstet Gynecol. 2018;132:784–785. doi: 10.1097/AOG.0000000000002849 [DOI] [PubMed] [Google Scholar]
- 89.Lewey J, Levine LD, Yang L, Triebwasser JE, Groeneveld PW. Patterns of postpartum ambulatory care follow-up care among women with hypertensive disorders of pregnancy. J Am Heart Assoc. 2020;9:e016357 doi: 10.1161/JAHA.120.016357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gemkow JW, Liss DT, Yang T-Y Padilla R, King PL, Pereyra S, Cox-Batson S, Tenfelde S, Masinter L. Predicting postpartum transition to primary care in community health centers. Am J Prev Med. 2022;63:689–699. doi: 10.1016/j.amepre.2022.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sharma KJ, Greene N, Kilpatrick SJ. Oral labetalol compared to oral nifedipine for postpartum hypertension: a randomized controlled trial. Hypertens Pregnancy. 2017;36:44–47. doi: 10.1080/10641955.2016.1231317 [DOI] [PubMed] [Google Scholar]
- 92.Webster K, Fishburn S, Maresh M, Findlay SC, Chappell LC, Guideline C Diagnosis and management of hypertension in pregnancy: summary of updated NICE guidance. BMJ. 2019;366:l5119. doi: 10.1136/bmj.l5119 [DOI] [PubMed] [Google Scholar]
- 93.Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Himmelfarb CD, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13–e115. doi: 10.1161/HYP.0000000000000065 [DOI] [PubMed] [Google Scholar]
- 94.American College of Obstetricians and Gynecologists. Clinical guidance for the integration of the findings of the Chronic Hypertension and Pregnancy (CHAP) Study. Accessed December 8, 2022. https://acog.org/en/clinical/clinical-guidance/practice-advisory/articles/2022/04/clinical-guidance-for-the-integration-of-the-findings-of-the-chronic-hypertension-and-pregnancy-chap-study
- 95.Nice Institute for Health Care and Excellence. Hypertension in adults: diagnosis and management. 2019. Accessed June 19, 2023. https://www.nice.org.uk/guidance/ng136/chapter/recommendations#treating-and-monitoring-hypertension [PubMed]
- 96.Cairns AE, Tucker KL, Leeson P, Mackillop LH, Santos M, Velardo C, Salvi D, Mort S, Mollison J, Tarassenko L, et al. ; SNAP-HT Investigators. Self-management of postnatal hypertension: the SNAP-HT trial. Hypertension. 2018;72:425–432. doi: 10.1161/HYPERTENSIONAHA.118.10911 [DOI] [PubMed] [Google Scholar]
- 97.Jones DW, Whelton PK, Allen N, Clark D, Gidding SS, Muntner P, Nesbitt S, Mitchell NS, Townsend R, Falkner B; on behalf of the American Heart Association Council on Hypertension; Council on the Kidney in Cardiovascular Disease; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Lifelong Congenital Heart Disease and Heart Health in the Young; and Stroke Council. Management of stage 1 hypertension in adults with a low 10-year risk for cardiovascular disease: filling a guidance gap: a scientific statement from the American Heart Association. Hypertension. 2021;77:e58–e67 doi: 10.1161/HYP.0000000000000195 [DOI] [PubMed] [Google Scholar]
- 98.D’Amico R, Dalmacy D, Akinduro JA, Hyer M, Thung S, Mao S, Fareed N, Bose-Brill S. Patterns of postpartum primary care follow-up and diabetes-related care after diagnosis of gestational diabetes. JAMA Netw Open. 2023;6:e2254765. doi: 10.1001/jamanetworkopen.2022.54765 [DOI] [PubMed] [Google Scholar]
- 99.Brown SD, Hedderson MM, Zhu Y Tsai A-L, Feng J, Quesenberry CP Ferrara A. Uptake of guideline-recommended postpartum diabetes screening among diverse women with gestational diabetes: associations with patient factors in an integrated health system in the USA. BMJ Open Diabetes Res Care. 2022;10:e002726. doi: 10.1136/bmjdrc-2021-002726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kuklina EV, Yoon PW, Keenan NL. Prevalence of coronary heart disease risk factors and screening for high cholesterol levels among young adults, United States, 1999–2006. Ann Fam Med. 2010;8:327–333. doi: 10.1370/afm.1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Montes A, Walden CE, Knopp RH, Cheung M, Chapman MB, Albers JJ. Physiologic and supraphysiologic increases in lipoprotein lipids and apoproteins in late pregnancy and postpartum: possible markers for the diagnosis of “prelipemia.” Arteriosclerosis. 1984;4:407–417 doi: 10.1161/01.atv.4.4.407 [DOI] [PubMed] [Google Scholar]
- 102.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines [published corrections appear in Circulation. 2019;139:e1182–e1186 and Circulation. 2023;148:e5]. Circulation. 2019;139:e1082–e1143. doi: 10.1161/CIR.0000000000000625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tobacco and nicotine cessation during pregnancy: ACOG committee opinion, number 807. Obstet Gynecol. 2020;135:e221–e229. doi: 10.1097/AOG.0000000000003822 [DOI] [PubMed] [Google Scholar]
- 104.Owais S, Chow CHT, Furtado M, Frey BN, Van Lieshout RJ. Nonpharmacological interventions for improving postpartum maternal sleep: a systematic review and meta-analysis. Sleep Med Rev. 2018;41:87–100. doi: 10.1016/j.smrv.2018.01.005 [DOI] [PubMed] [Google Scholar]
- 105.Dominguez JE, Street L, Louis J. Management of obstructive sleep apnea in pregnancy. Obstet Gynecol Clin North Am. 2018;45:233–247. doi: 10.1016/j.ogc.2018.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Street LM, Aschenbrenner CA, Houle TT, Pinyan CW, Eisenach JC. Gestational obstructive sleep apnea: biomarker screening models and lack of postpartum resolution. J Clin Sleep Med. 2018;14:549–555. doi: 10.5664/jcsm.7042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schwarz EB, Ray RM, Stuebe AM, Allison MA, Ness RB, Freiberg MS, Cauley JA. Duration of lactation and risk factors for maternal cardiovascular disease. Obstet Gynecol. 2009;113:974–982. doi: 10.1097/01.AOG.0000346884.67796.ca [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Aune D, Norat T, Romundstad P, Vatten LJ. Breastfeeding and the maternal risk of type 2 diabetes: a systematic review and dose-response meta-analysis of cohort studies. Nutr Metab Cardiovasc Dis. 2014;24:107–115. doi: 10.1016/j.numecd.2013.10.028 [DOI] [PubMed] [Google Scholar]
- 109.Yu J, Pudwell J, Dayan N, Smith GN. Postpartum breastfeeding and cardiovascular risk assessment in women following pregnancy complications. J Womens Health (Larchmt). 2020;29:627–635. doi: 10.1089/jwh.2019.7894 [DOI] [PubMed] [Google Scholar]
- 110.ACOG committee opinion No. 757: screening for perinatal depression. Obstet Gynecol. 2018;132:e208–e212. doi: 10.1097/AOG.0000000000002927 [DOI] [PubMed] [Google Scholar]
- 111.Siu AL, Bibbins-Domingo K, Grossman DC, Baumann LC, Davidson KW, Ebell M, García FAR, Gillman M, Herzstein J, Kemper AR, et al. ; US Preventive Services Task Force (USPSTF). Screening for depression in adults: US Preventive Services Task Force recommendation statement. JAMA. 2016;315:380–387. doi: 10.1001/jama.2015.18392 [DOI] [PubMed] [Google Scholar]
- 112.Earls MF, Yogman MW, Mattson G, Rafferty J; Committee on Psychosocial Aspects of Child and Family Health. Incorporating recognition and management of perinatal depression into pediatric practice. Pediatrics. 2019;143:e20183259. doi: 10.1542/peds.2018-3259 [DOI] [PubMed] [Google Scholar]
- 113.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines [published corrections appear in Circulation. 2019;140:e649–e650, Circulation. 2020;141:e60, and Circulation. 2020;141:e774]. Circulation. 2019;140:e596–e646. doi: 10.1161/CIR.0000000000000678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Stone NJ, Smith SC, Orringer CE, Rigotti NA, Navar AM, Khan SS, Jones DW, Goldberg R, Mora S, Blaha M, et al. Managing atherosclerotic cardiovascular risk in young adults: JACC state-of-the-art review. J Am Coll Cardiol. 2022;79:819–836. doi: 10.1016/j.jacc.2021.12.016 [DOI] [PubMed] [Google Scholar]
- 115.Navar AM, Fine LJ, Ambrosius WT, Brown A, Douglas PS, Johnson K, Khera AV, Lloyd-Jones D, Michos ED, Mujahid M, et al. Earlier treatment in adults with high lifetime risk of cardiovascular diseases: what prevention trials are feasible and could change clinical practice? Report of a National Heart, Lung, and Blood Institute (NHLBI) workshop. Am J Prev Cardiol. 2022;12:100430. doi: 10.1016/j.ajpc.2022.100430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Smith EE, Saposnik G, Biessels GJ, Doubal FN, Fornage M, Gorelick PB, Greenberg SM, Higashida RT, Kasner SE, Seshadri S; on behalf of the American Heart Association Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Functional Genomics and Translational Biology; and Council on Hypertension. Prevention of stroke in patients with silent cerebrovascular disease: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2017;48:e44–e71. doi: 10.1161/STR.0000000000000116 [DOI] [PubMed] [Google Scholar]
- 117.Benschop L, Brouwers L, Zoet GA, Meun C, Boersma E, Budde RPJ, Fauser BCJM, de Groot CMJ, van der Schouw YT, Maas AHEM, et al. ; CREW Consortium. Early onset of coronary artery calcification in women with previous preeclampsia. Circ Cardiovasc Imaging. 2020;13:e010340. doi: 10.1161/CIRCIMAGING.119.010340 [DOI] [PubMed] [Google Scholar]
- 118.Sederholm Lawesson S, Swahn E, Pihlsgård M, Andersson T, Angerås O, Bacsovics Brolin E, Bergdahl E, Blomberg M, Christersson C, Gonçalves I, et al. Association between history of adverse pregnancy outcomes and coronary artery disease assessed by coronary computed tomography angiography. JAMA. 2023;329:393–404. doi: 10.1001/jama.2022.24093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Countouris ME, Villanueva FS, Berlacher KL, Cavalcante JL, Parks WT, Catov JM. Association of hypertensive disorders of pregnancy with left ventricular remodeling later in life. J Am Coll Cardiol. 2021;77:1057–1068. doi: 10.1016/j.jacc.2020.12.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.ENRICH. Early intervention to promote cardiovascular health of mothers and children. Accessed December 1, 2023. https://hvenrich.org/ENRICH_Main.asp
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


