Abstract
We used a sensitive assay to test whether an adeno-associated virus (AAV) productive replication cycle can occur in immortalized human keratinocytes carrying episomal human papillomavirus type 16 (HPV-16) DNA. Following transfection with cloned AAV DNA, infectious AAV was produced, and the infectivity was blocked by anti-AAV antiserum. The HPV-16 E2 protein substantially increased the yield of AAV. Other HPV early proteins did not, in our experiments, show this ability. E2 has been shown to be able to affect p53 levels and to block cell cycle progression at mitosis. We tested the effect of changes in p53 expression on AAV replication and found that large differences in the level of p53 did not alter AAV DNA replication. In extension of this, we found that cellular help for AAV in response to stress was also independent of p53. To test if a mitotic block could trigger AAV DNA replication, we treated the cells with the mitotic inhibitor nocodazole. AAV DNA replication was stimulated by the presence of nocodazole in these and a number of other cell types tested. Yields of infectious virus, however, were not increased by this treatment. We conclude that the HPV-16 E2 protein stimulates AAV multiplication in these cells and propose that this occurs independently of the effects of E2 on p53 and cell cycle progression. Since the effect of E2 was not seen in keratinocytes lacking the HPV-16 episome, we suggest that E2 can help AAV by working in concert with other HPV-16 proteins.
Adeno-associated viruses (AAVs) are small, single-stranded DNA viruses with a genome of approximately 4,700 nucleotides (6, 54). They belong to the family Parvoviridae, of which they constitute the genus Dependovirus, and are widespread in the human population. The Dependovirus genus is so named because its members are believed to rely, for full replication, on the presence of a helper virus (reviewed in reference 7) or on the induction of a cellular genotoxic stress response. The best characterized of AAV's helper viruses are adenoviruses and herpesviruses, while many cell types can be rendered permissive for AAV replication by treatment with carcinogens, UV irradiation, or hydroxyurea (50, 66–68). Under conditions that are nonpermissive for replication, AAV establishes a latent infection (reviewed in reference 11). These viruses are not, as yet, linked with any pathology.
It has been reported that human papillomaviruses (HPVs) are among those viruses able to provide help for the replication of AAV (62). An HPV-AAV interaction would be of interest with respect to seroepidemiological studies carried out some time ago that highlighted a negative correlation between AAV infection and the development of cervical cancer (20, 37, 53). This type of cancer is now known to be strongly associated with infection by certain anogenital HPV types (14, 42; reviewed in reference 69). Since AAV shows oncosuppressive effects in many systems (3, 12, 25–28, 58; reviewed in references 44 and 49), help for its replication by HPV might be expected to selectively kill, or hinder the growth of, HPV-infected cells. This could perhaps explain the negative correlation between seropositivity for AAV and cervical cancer. AAV or its DNA has been detected in human genital tissue by a number of investigators (19, 24, 36, 61, 63).
HPVs are small, double-stranded DNA viruses which now constitute a group of over 80 types. They replicate in squamous epithelium as multicopy extrachromosomal plasmids and normally cause benign lesions. In some lesions, a progression occurs to high-grade dysplasia and invasive carcinoma, particularly in the anogenital region. Of the HPV types able to infect the anogenital epithelia, some, such as types HPV-6 and -11, are classified as low risk with respect to progression toward cervical carcinoma. Others, such as HPV-16 and -18, are classified as high risk for such progression.
The replication of HPV is very much linked to the differentiation state of the host epithelial cell, since the productive stage of the viral life cycle occurs only in the terminally differentiating epithelial layers (2, 57; reviewed in reference 30). In these differentiating cells, the copy number of the viral episome is amplified and late genes encoding capsid proteins are expressed, allowing the production of viral progeny. In cervical lesions that have progressed to carcinoma, the viral DNA is found with high frequency to have integrated into the cellular DNA, resulting in the loss of the integrity of the E1 or E2 early gene. The loss of E2 function in particular is believed to be a critical event in the development of high-grade lesions (23, 43, 51). This could be because its loss leads to deregulation of cell division, due to the role of E2 in regulating transcription of HPV transforming proteins E6 and E7 (5, 23).
In the work described here, we set out to determine whether introduction of AAV DNA into an HPV-16-immortalized keratinocyte line containing approximately 1,000 copies of episomal HPV DNA (15) per cell would, as a result of HPV-mediated help, lead to the production of AAV. This system has the advantage of using the natural host cell for HPV already containing HPV DNA in the episomal state. A scenario where AAV would infect cervical epithelium harboring such an HPV infection is perhaps the most relevant with respect to the potential of AAV to inhibit oncogenicity. It was also of interest to test the effect of increased expression of HPV early genes in these cells, as it is known that the transcription of these genes is increased in differentiating cells (56, 57). We found that a productive replication cycle of AAV occurred in these immortalized keratinocytes, providing evidence that HPV can contribute help for AAV replication and production of infectious particles. Our additional finding that introduction of HPV-16 E2 increases infectious AAV production led to the investigation of possible mechanisms for HPV-generated help. E2 has been reported to increase p53 levels (31) and transcriptional activity (13) apparently by two separate mechanisms, one involving repression of E6 transcription and the other occurring independently of E2 binding to the E6/E7 promoter. AAV's multifunctional replication protein Rep78 has recently been reported to interact with p53 (4). We therefore looked at the possibility of changes in p53 expression being involved in permissiveness for AAV replication. We also investigated the possibility that E2 may mediate help for AAV via increasing the HPV copy number or as a result of its recently reported ability to cause cell cycle arrest (13, 17).
MATERIALS AND METHODS
Cells.
S12 cells are derived from an HPV-16-immortalized line, W12, obtained from a low-grade cervical intraepithelial lesion (15), and contain the HPV-16 episome at approximately 1,000 copies/cell. The S12 cells were grown in a 3:1 mixture of Ham F-12 medium (Gibco) and Dulbecco's minimal essential medium (DMEM) (Gibco) containing 5% fetal bovine serum (FBS) and penicillin-streptomycin plus supplements (all from Sigma) as follows: adenine (24 μg/ml), insulin (5 μg/ml), cholera toxin (8.4 ng/ml), hydrocortisone (0.4 μg/ml), and epidermal growth factor (10 ng/ml). H1299 is a p53-null human small cell lung carcinoma line. H1299 cells expressing wild-type human p53 from the tetracycline-regulated promoter (9, 22) are designated here H1299p53. H24 cells are a clone of H1299 which constitutively express the tetracycline repressor protein but are devoid of the p53 construct (9). These were used as the parental clone for H1299p53 construction. H24 and H1299p53 cells were obtained as a kind gift from R. Iggo and E. Saller (46). The H1299p53-tetracycline system used here allows induction of p53 expression by removal of tetracycline from the medium. The H1299p53 cells were grown in DMEM supplemented with 10% FBS and antibiotics and, where repression of p53 was desired, the continuous presence of tetracycline at 1 μg/ml. Wild-type p53 generated in these cells has been shown to have normal capacity for p21 (9, 45) and mdm-2 (45) induction. Other cell lines used in this work were Cos-1, simian virus 40 (SV40)-transformed monkey cells; HeLa, HPV-18-transformed human keratinocytes; HaCat, spontaneously immortalized human keratinocytes; 293 cells, human kidney epithelial cells transformed with adenovirus E1A and E1B genes; and 293-T cells, a derivative of 293 cells additionally containing the SV40 large T antigen. These cells were cultured in DMEM supplemented with 5% FBS and antibiotics. All cells used were grown as monolayer cultures at 37°C with 5% CO2.
Viruses.
AAV infections were done with wild-type AAV-2 prepared in HeLa cells using adenovirus as helper. The stock underwent three rounds of CsCl purification and before use was heated at 60°C for 30 min in order to inactivate any contaminating adenovirus. Virus titration was done by in situ hybridization assay as described previously (67). Infections were carried out at a multiplicity of infection (MOI) of between 2 × 103 and 5 × 103 unless indicated otherwise. Adenovirus-2 was the adenovirus strain used throughout this work; this stock had been twice plaque purified, and its titer had been determined on HeLa cells. It was used at an MOI of approximately 5 PFU/cell, and its continued AAV-free status was checked at each use.
DNA and transfections.
The wild-type AAV genome was obtained by excision from the pAV2 plasmid (34) and cloned into the pSP72 vector (Promega). For all transfections of AAV DNA, the AAV genome was released from the vector by digestion with BglII (Boehringer Mannheim). For cotransfections of AAV DNA with HPV-16 early genes, the HPV-16 DNAs used were cloned plasmids of the E1 to E5 genes expressed under the control of the Moloney murine leukemia virus promoter in the vector pBabepuro. The expression of HPV proteins from these vectors has been verified, either in S12 cells or in other systems. Full-length HPV-16 DNA used was either cloned into pSP64 (Promega) and released using BamHI or cloned into Bluescribe (the Bluescribe HPV-16 was a kind gift from M. Dürst). All transfections were done using DOTAP (Boehringer Mannheim) according to the manufacturer's recommended conditions. Routine transfection efficiency checks using β-galactosidase expression showed that S12 was consistently transfected at an efficiency of between 25 and 40%.
Isolation of infectious AAV from transfected cells.
It was observed that DOTAP-AAV DNA complexes withstood ultracentrifugation, retaining the ability to transfect cells. Due to the concern that they could still be present in cells at the time of harvesting, procedures were developed to break up any such complexes and, following this, to digest AAV DNA released. In order to do this, extensive testing was carried out using fresh DOTAP-AAV DNA transfection complexes to establish a protocol where neither they nor free AAV DNA could contribute to the results, while aiming to preserve maximally the integrity of AAV particles. The final general protocol chosen for the work was as follows (details applying to specific experiments are as noted in the figure legends). Transfected cells were cultured for 6 days with one passage. Cells were then freeze-thawed three times into the harvested culture medium. A step to further disrupt membranes and help release any membrane-associated AAV aggregates was carried out at this stage by vortexing briefly with an approximately 1/4 volume of diethyl ether. After centrifugation at 80 × g for 5 min, the ether phase was removed by aspiration. The freeze-thawed lysate was then centrifuged at 120,000 × g for 30 min in a Sorvall SS34 rotor to remove cellular debris. The supernatants were then centrifuged at 130,000 × g for 6.5 h in a TST28.38 rotor (Kontron) to pellet AAV produced. The pellet obtained after ultracentrifugation was resuspended in 0.5 to 1 ml of sterile phosphate-buffered saline (PBS) or serum-free DMEM. Ether extraction was carried out on this suspension, using 1 to 2 volumes of diethyl ether. Ether was added and mixed by vortexing for approximately 20 s, and the phases were separated by centrifugation at maximum speed for 2 min in a microcentrifuge (Sigma). The ether phase was withdrawn using a sterile glass Pasteur pipette, and the extraction was repeated three more times. MgCl2 was added, where necessary, to a final concentration of 10 mM, DNase I (Boehringer Mannheim) was added at 15 to 80 U/sample, and the suspension was incubated for at least 1 h at 37°C. Previous testing with DMEM had shown that DNase functioned efficiently in this medium without further addition of MgCl2. Routine controls were carried out within these AAV isolation experiments to check efficient functioning of the DNase. Traces of ether were allowed to evaporate during a 1-h incubation at 40°C, and then the resuspended pellet was allowed to adsorb for 1 h at 37°C onto plates of HeLa cells seeded with approximately 4 × 106 cells/dish on the previous day. Following the adsorption period, the 1-ml suspension was removed. The cells were then fed with fresh medium and infected with adenovirus. At approximately 48 h post-infection with adenovirus, cells were harvested and DNA was extracted. As indicated in the figure legends, either total DNA or low-molecular-weight DNA was prepared. Low-molecular-weight DNA was extracted as described previously (29). Agarose gel electrophoresis and Southern blotting were used to analyze the DNA, and hybridization was performed with a 32P-labeled AAV DNA probe.
Total DNA extraction.
The cell pellet, resuspended in sterile PBS, was lysed in an equal volume of 1% sodium dodecyl sulfate (SDS)–200 mM NaCl–20 mM Tris Cl (pH 8.0)–50 mM EDTA–0.2 mg of proteinase K per ml. After digestion for at least 6 h at 50°C, the DNA was sheared with a homogenizer (Polytron) for 1 min/sample. Two rounds of phenol-chloroform extraction were followed by precipitation at −20°C with 2.5 volumes of 100% ethanol. After resuspension in TE (10 mM Tris Cl [pH 7.4], 1 mM EDTA), a digestion was carried out at 37°C for 1 h with RNase A (Boehringer Mannheim) at a concentration of 20 μg/ml. DNA concentrations were measured by use of a DU-64 spectrophotometer (Beckman Instruments). For certain experiments, DNA was isolated by use of a QIAamp DNA purification kit (Qiagen) according to the manufacturer's recommended conditions.
Agarose gel electrophoresis and Southern blotting.
Agarose (0.65 to 1%)–Tris-acetate gels were run for a total of approximately 16 h at 25 V at a temperature of 4°C. DNA was vacuum transferred onto positively charged nylon membranes (Hybond N+), and UV transillumination was used for DNA fixation (Stratagene UV Stratalinker 2400; 254 nm; 190 mJ/cm2). Prehybridization and hybridization were carried out at 65°C in a hybridization oven (Hybaid). A solution of 0.5 M NaH2PO4–7% SDS, containing 1 mM EDTA and with a pH of 7.2, was used for both these steps. The AAV DNA used as a probe was the same as that used for the AAV DNA transfections described above. All probes were labeled by the random-primer method according to the manufacturer's protocol (Boehringer Mannheim) with either [α-32P]dATP or [α-32P]dCTP (NEN DuPont). Posthybridization washing was carried out for approximately 30 min at room temperature in 0.4% SDS–2× SSC (1× SSC is 150 mM NaCl with 15 mM sodium citrate) and then at 65°C for approximately 25 min in 0.1% SDS–0.1× SSC before exposure to X-ray film at −70°C.
Anti-AAV-2 antiserum.
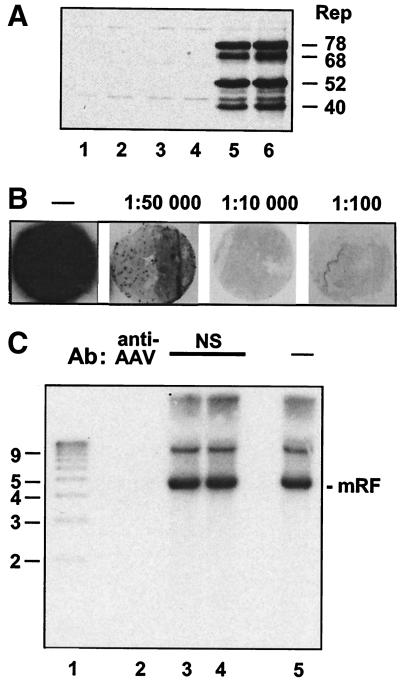
We obtained guinea pig anti-AAV-2 antiserum in lyophilized form from the National Institute for Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Md. (catalogue no. V-257-502-558). Before use, it was titrated against infectious wild-type AAV as follows: approximately 3 × 105 infectious units (IU) of AAV was preincubated on a rotating wheel for 1.5 h at room temperature either with serial dilutions of the antiserum or with no antiserum. Following this, the antiserum-treated (and control) virus was tested in the same in situ hybridization assay as that used for AAV titration (67). Dilutions of antiserum between 1/100 and 1/1,000 effectively blocked the AAV infection (Fig. 1B), and dilutions of approximately 1/100 were subsequently used in our experiments. Control antiserum was unable to block AAV infection at the same concentration as or double the concentrations used in these experiments (Fig. 1C).
FIG. 1.
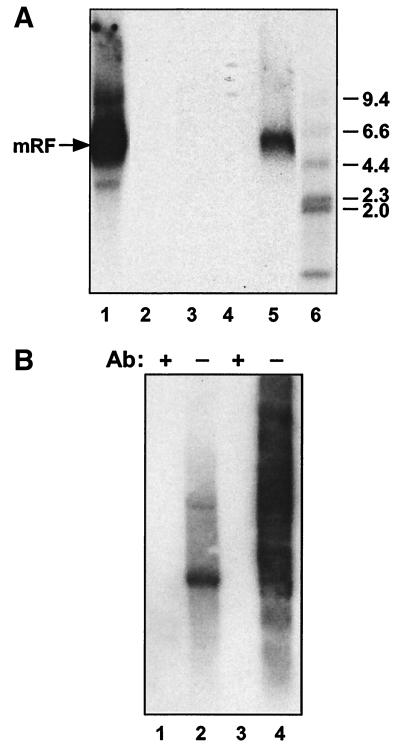
(A) Elimination of transfection complexes with chloroform and ether. Transfection complexes were treated with chloroform or ether following ultracentrifugation to test the ability of these treatments to eliminate infectivity. Treated or untreated complexes were adsorbed onto plates of HeLa cells for 1 h before washing of the cells and infection with adenovirus. At 48 h postinfection, an equal fraction of each plate was analyzed for the presence of Rep proteins by SDS-polyacrylamide gel electrophoresis and Western blotting. Lane 1, HeLa cells infected with adenovirus alone. Lane 2, 8 μg of free AAV DNA processed as described below for transfection complex in lane 5. Lanes 3 and 4, chloroform (lane 3)- or ether (lane 4)-treated transfection complex. Lane 5, untreated transfection complex. Lane 6, AAV DNA transfection plus adenovirus infection of HeLa cells. (B) Titration of the anti-AAV-2 antiserum. Infectious AAV was incubated with serial dilutions of the anti-AAV-2 antiserum before it was used to infect HeLa cells, subsequently superinfected with adenovirus. An in situ hybridization assay for infectious AAV titration was carried out as described previously (67). Briefly, after incubation for 30 h under a layer of 0.9% agarose mixed with DMEM containing 2% FBS, the cell layer was blotted onto circular nitrocellulose filters. DNA was denatured and neutralized before UV fixation, prehybridization, and hybridization to a 32P-labeled AAV DNA probe. Antibody dilutions used are noted above the corresponding filters. The filter designated with a minus sign (−) corresponds to the equivalent AAV infection in the absence of antibody preincubation. (C) Blocking of infectivity by anti-AAV-2 antiserum is specific. Approximately 2 × 106 IU of AAV was preincubated with the anti-AAV-2 antiserum at a dilution of approximately 1/100 or with a control antiserum at a protein concentration the same as or double that of the anti-AAV-2 antiserum. AAV-2 preincubated with the antisera and the same amount of untreated AAV were then used to infect plates of HeLa cells containing approximately 6 × 106 cells/plate. These plates were then superinfected with adenovirus. At 48 h postinfection, they were harvested for total DNA extraction, and 10 μg/sample was analyzed by agarose gel electrophoresis and Southern blotting. Lane 1, 1-kb marker (sizes in kilobases are indicated). Lane 2, AAV preincubated with anti-AAV-2 antiserum. Lanes 3 and 4, AAV preincubated with control antiserum (NS) at the same protein concentration as (lane 3) or double the protein concentration of (lane 4) the specific antiserum. Lane 5, AAV with no antiserum preincubation (−). Ab, antibody; mRF, monomer replicative form.
Protein analysis by Western blotting.
Cell pellets were washed and resuspended in PBS and then lysed either in an equal volume of 2× SDS loading buffer, prepared according to standard procedures (47), or in reporter lysis buffer (Promega) containing a protease inhibitor cocktail (Calbiochem). After being boiled for denaturation, samples were analyzed on SDS–8% polyacrylamide gels, and total cellular proteins were transferred to nitrocellulose by electrophoresis (in transfer buffer containing 20% methanol, 25 mM Tris, and 186 mM glycine). For detection of human p53, the mouse monoclonal antibody DO1 (a kind gift from R. Iggo and E. Saller) was used. The secondary antibody was peroxidase-linked anti-mouse immunoglobulin detected using an ECL chemiluminescence kit (Amersham). For detection of the AAV Rep protein, a rabbit antiserum raised against glutathione S-transferase–Rep (a kind gift from P. Saudan) was used while the secondary antibody was peroxidase-linked anti-rabbit immunoglobulin detected as described above.
UV irradiation of H1299p53 and H24 cells.
UV irradiation of H1299p53 and H24 cells was done using a wavelength of 254 nm in a Stratalinker 2400 (Stratagene) at 17 J/m2. Prior to irradiation, culture medium was removed, and 2 ml of sterile PBS was added to the cells. The culture dish was then irradiated with the lid removed. Immediately following this, PBS was removed and the cells were fed with fresh medium.
RESULTS
Assay for AAV production in S12 cells.
A sensitive assay, based on that reported previously (62), was developed to detect AAV particles synthesized in cells transfected by lipofection with AAV DNA. Modifications added were designed to increase the specificity of the assay to detect AAV replication resulting from viral particles synthesized in transfected cells. The principle of looking for AAV production in a particular cell type relied on transfection of AAV DNA into the cells so that any virus isolated had been synthesized within the cells. Since quantities of virus were expected to be small, the assay incorporated an amplification step using a secondary infection with the isolated AAV coinfecting detector plates with adenovirus. Since DOTAP-AAV DNA complexes remaining in transfected cells at the time of harvesting could potentially lead to artifacts, a method had to be found to eradicate these without destroying AAV virions. Figure 1A shows that transfection complexes containing AAV DNA were inactivated by treatment with diethyl ether or chloroform. Parvoviruses are naked icosahedral particles and are resistant to lipid solvents. We confirmed that AAV is not destroyed by ether and used this treatment in subsequent experiments (see Materials and Methods).
To further ensure that infection was due to virions, for use in this work an anti-AAV-2 antiserum was titrated against infectious AAV and tested for specificity in blocking AAV infection. At a concentration of anti-AAV-2 antiserum which blocked infection very efficiently (Fig. 1B), control antiserum was completely ineffective (Fig. 1C).
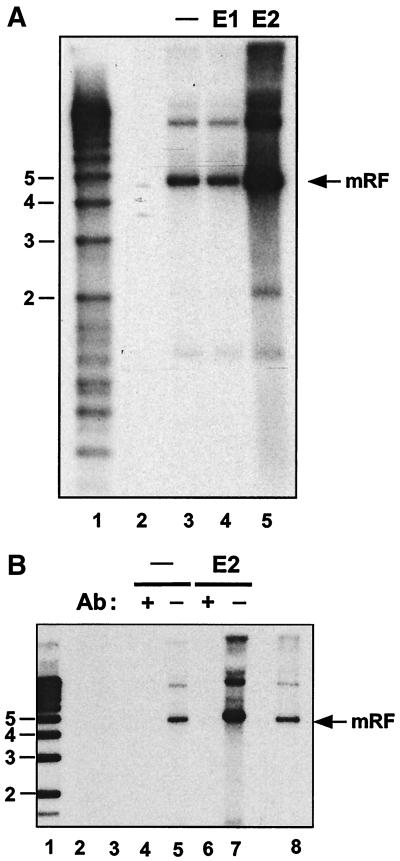
By using this assay, we found that transfection of full-length AAV DNA into S12 cells resulted in the production of readily detectable amounts of infectious AAV (see Fig. 2A, lane 3, and B, lane 5). AAV was not detected when HeLa indicator cells were infected with adenovirus alone or when AAV DNA was subjected to the viral purification procedure (Fig. 2B, lanes 2 and 3, respectively). Use of the anti-AAV-2 antiserum to block infectivity showed that the AAV replication that we detected had resulted from infectious AAV isolated from the transfected cells (Fig. 2B, compare lanes 4 and 5).
FIG. 2.
(A) HPV-16 E2 increases infectious AAV production in S12 human keratinocytes. S12 cells were cotransfected with AAV DNA and either the empty vector alone, HPV-16 E1 DNA, or HPV-16 E2 DNA. Six days posttransfection, virus was extracted and treated as described in Materials and Methods. This extract was used to infect HeLa cells, which were then superinfected with adenovirus. At approximately 48 h postinfection, total DNA was extracted and 10 μg/sample was analyzed for AAV DNA by agarose gel electrophoresis and Southern blotting. Lane 1, 1-kb marker (sizes in kilobases are indicated). Lane 2, HeLa cells infected with adenovirus alone. Lanes 3, 4, and 5, HeLa cells infected with AAV-containing extract isolated from S12 cells transfected with the following DNA: AAV plus empty vector (lane 3), AAV plus HPV-16 E1 (lane 4), and AAV plus HPV-16 E2 (lane 5). (B) Replication of AAV isolated from S12 cells is blocked by anti-AAV-2 antiserum. A transfection experiment was carried out as described for panel A, with the following modifications. Incubation of the virus-containing extract from the transfected S12 cells with the anti-AAV-2 antibody (Ab) was carried out prior to infection of HeLa cell plates. Lane 1, 1-kb marker (sizes in kilobases are indicated). Lane 2, HeLa cells infected with adenovirus alone. Lane 3, 5 μg of AAV DNA was treated with DNase in parallel with the samples in lanes 4 to 7 before adsorption onto HeLa cells. Lanes 4 and 5, HeLa cells infected with the AAV-containing extract of S12 cells transfected with AAV DNA plus empty vector either with (lane 4) or without (lane 5) preincubation of the extract with anti-AAV-2 antiserum. Lanes 6 and 7, HeLa cells infected with the AAV-containing extract of S12 transfected with AAV DNA plus HPV-16 E2 DNA either with (lane 6) or without (lane 7) preincubation of the extract with anti-AAV-2 antiserum. Lane 8, marker DNA for AAV replication in the presence of adenovirus. mRF, monomer replicative form.
It has been reported previously that transfection of AAV and HPV-16 DNAs into another keratinocyte line, HaCat, led to a cytopathic effect (62). Transfection of AAV DNA into S12 cells, which already contain the HPV episome, would be expected to give rise to a similar situation such as a cotransfection. In S12, however, there was no detectable cytopathic effect, and when the HPV-16 copy number was increased by transfecting additional HPV-16 DNA into the cells together with the AAV DNA, still no cytopathic effect was seen. These contrasting findings may indicate that the content of the two viral genomes in the cell, or the relative molecular ratio of the two genomes, is critical in determining whether or not cells will survive such a coinfection. There is evidence that the relative ratio of the two genomes is important with respect to the ability of AAV to suppress the tumorigenic phenotype in cervical carcinoma cell lines (58). The difference in result could also be due to a variation in the levels of HPV gene expression between the two systems.
AAV production in S12 cells is stimulated by HPV-16 E2.
Because S12 cells already contain the HPV-16 immortalizing proteins E6 and E7, we tested whether any of the other early genes, E1, E2, E4, or E5, would, when introduced into this background, be able to increase AAV particle synthesis. Cotransfection of AAV DNA together with the E2 gene gave rise to a marked increase in viral production (Fig. 2A, compare lanes 5 and 3, and Fig. 2B, compare lanes 7 and 5). Although the amounts of AAV produced in these experiments were easily detectable, they were clearly lower than those obtained from an adenovirus-AAV coinfection. The latter situation yielded approximately 104 infectious particles per cell, whereas the S12 cells yielded 100-fold less. In order to demonstrate that the signal seen here was indeed due to infectious virus, the inoculum was incubated, prior to adsorption onto HeLa cells, with anti-AAV-2 antiserum. This treatment resulted in the complete loss of the signal (Fig. 2B, compare lanes 6 and 7). Using this system of analysis, cotransfection of E1 (Fig. 2A) and E4 and E5 (data not shown) with AAV was not able to show help for AAV.
HPV-16 episomes in S12 cells.
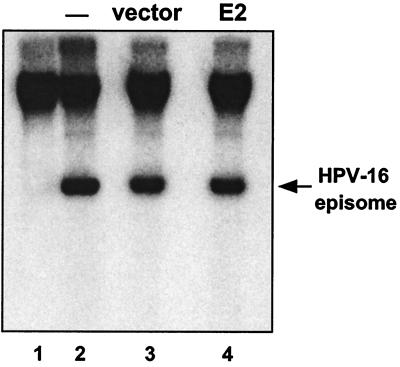
Since E2 is a protein with a variety of known functions and effects, several possible mechanisms of action could be envisaged regarding help for AAV. Firstly, we wanted to determine whether or not E2 was increasing the copy number of the HPV episome in S12 cells. Since E2 is important for viral DNA replication, it was hypothesized that an overexpression of E2 may cause a rapid increase in viral copy number (17, 18). This could be involved in the development of permissiveness for AAV's growth cycle, for example, by increasing the number of templates for HPV gene products. We found, however, that in our system the viral copy number was not detectably affected by the presence of the E2-expressing vector (Fig. 3).
FIG. 3.
HPV episomal copy number in S12 cells transfected with HPV-16 E2. S12 cells were transfected with HPV-16 E2-expressing plasmid or vector alone and cultured for 3 days posttransfection. Total DNA was extracted, and 10 μg/sample was analyzed by agarose gel electrophoresis and Southern blotting. Hybridization was carried out using 32P-labeled HPV-16 L1 (capsid protein gene) DNA as the probe. Upon extensive passage, S12 cells lose the episomal copies of the HPV genome, though integrated copies remain. Lane 1, high-passage (≈50) S12 cells. Lane 2, low-passage (≈15) S12 cells as used in our experiments. Lanes 3 and 4, S12 cells transfected with empty vector DNA (lane 3) or with HPV-16 E2 DNA (lane 4).
AAV DNA amplification is independent of p53.
E2 is a transcription factor and has a well-known role in controlling the HPV upstream regulatory region promoter from where the mRNAs encoding E6 and E7, two of the HPV transforming proteins, are transcribed. Although it has been reported that low amounts of E2 can stimulate the activity of the E6/E7 promoter, while increasing amounts repress its activity (55), the predominant effect of E2 on this promoter is thought to be a repression of E6/E7 transcription. Since in our experiments we overexpressed E2 in S12 cells, we considered it more likely that E2 would be repressing E6 and E7 transcription. When levels of E6 protein in HPV-immortalized or -transformed cells are down-regulated, one consequence is thought to be an increased level of the p53 protein, due to the ability of E6 from high-risk HPV types to target p53 for ubiquitin-mediated degradation (48). E2-mediated help for AAV may be linked, via repression of E6 expression, with a rise in cellular p53 levels. To investigate the effect of p53 on AAV replication, we used cells in which the level of p53 can be regulated. H1299 is a human, p53-null lung carcinoma cell line. In the H1299 derivative, H1299p53, p53 expression is under the control of a tetracyline-responsive promoter whereby, in the absence of tetracycline, wild-type human p53 is expressed. By comparing the results of infecting H1299p53 with AAV in the presence and in the absence of tetracycline, we could ask what effect variation in p53 level has on the ability of AAV to replicate its DNA. Assays for helper activity were done at the level of AAV DNA replication: this allowed a more rapid assay for help and the use of infectious virus as input, which is more efficient than DNA transfections. Situations that allow AAV replication to the extent of viral particle synthesis are believed to also show a corresponding degree of viral DNA replication (65).
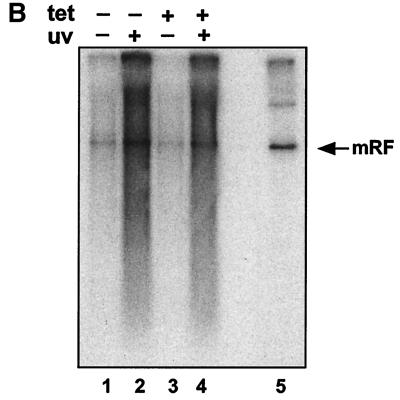
AAV replication can be induced by subjecting infected cells to genotoxic stress or DNA-damaging agents such as UV irradiation (50, 66, 67). p53 is known to play an important role in the cellular response to stressful treatments. We therefore extended this experiment to include treatment of AAV-infected H1299p53 cells with UV irradiation in the presence or absence of p53 expression. The results showed that large changes in the level of p53 (Fig. 4A) did not affect the amount of monomer replicative-form DNA produced in these cells (Fig. 4B, lanes 1 and 3). Furthermore, the stimulation of viral DNA replication in response to UV irradiation occurred independently of the level of p53 expression (Fig. 4B, lanes 2 and 4).
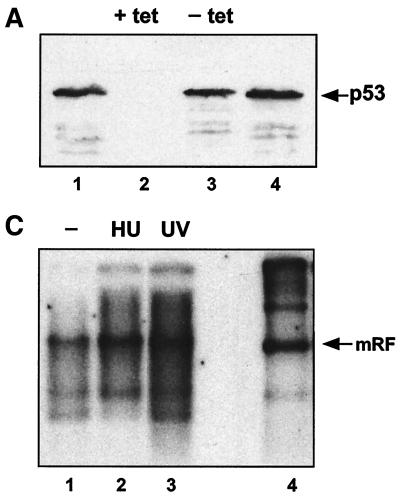
FIG. 4.
(A) Tetracycline-repressible p53 expression in H1299. H1299p53 cells were seeded at approximately 3 × 106 cells/dish and grown for 48 h in the presence (+ tet) or absence (− tet) of tetracycline. At this time point, two plates (one with and one without tetracycline) were harvested. (The remaining plates were used to test the ability of these cells to amplify AAV DNA in response to UV [see panel B].) The two harvested plates were analyzed for the presence of p53 using SDS-polyacrylamide gels and Western blotting using 1/12 of the extract from each dish. Lanes 1 and 4, HaCat cell extract as a positive control for p53 detection. Lanes 2 and 3, H1299p53 in the presence (lane 2) or absence (lane 3) of tetracycline. (B) The AAV DNA amplification response to UV irradiation is independent of expression of p53 in H1299 cells. H1299p53 cells were used to test for dependence of UV-induced AAV DNA amplification on p53 expression level. Plates cultured in parallel with those used for panel A, in the presence (tet +) or absence (tet −) of tetracycline, were either treated with UV or left untreated. All plates were then infected with AAV. On the third day postinfection, total DNA was extracted and 5 μg/sample was analyzed by agarose gel electrophoresis and Southern blotting. Lanes 1 and 2, AAV-infected H1299p53 cultured in the absence of tetracycline; lane 1, no UV irradiation; lane 2, cells were UV irradiated. Lanes 3 and 4, AAV-infected H1299p53 cultured in the presence of tetracycline; lane 3, no UV irradiation; lane 4, cells were UV irradiated. Lane 5, marker DNA for AAV replication in the presence of adenovirus. (C) p53-null cells can amplify AAV DNA in response to UV irradiation. Approximately 2 × 106 cells of the H24 clone of H1299 cells (which lack the p53 expression construct) per dish were either treated with 1 mM hydroxyurea (Sigma) for approximately 24 h prior to infection or, immediately prior to infection, treated with UV. These plates and a control untreated plate were then infected with AAV, and at 24 h postinfection, total DNA was extracted and 5 μg/sample was analyzed by agarose gel electrophoresis and Southern blotting. Lane 1, AAV-infected H24 cells. Lanes 2 and 3, H24 cells infected as described for lane 1 but with either hydroxyurea (HU) (lane 2) or UV irradiation (lane 3) pretreatment of the cells. Lane 4, marker DNA for AAV replication in the presence of adenovirus. mRF, monomer replicative form.
In order to verify whether AAV DNA amplification in response to UV and DNA-damaging agents in general can occur in the complete absence of p53 (tetracyline-mediated repression cannot be assumed to be complete), the ability of UV irradiation and hydroxyurea pretreatment to induce AAV DNA replication was tested in H24 cells, a clone of H1299 lacking the p53 expression construct and therefore p53 null. A clear increase in viral DNA replication was seen as a result of either treatment (Fig. 4C), showing that increased cellular permissiveness for AAV can be induced as a response to DNA-damaging agents in the absence of functional p53. Since we found that AAV replication did not depend on p53, we concluded that E2-mediated help for AAV was not likely to be linked with its reported ability to affect p53 levels. We therefore went on to investigate the possibility that E2 may help AAV via another of its effects on cells, namely, the interference with cell cycle control.
Treatment with the mitotic inhibitor nocodazole leads to AAV DNA amplification.
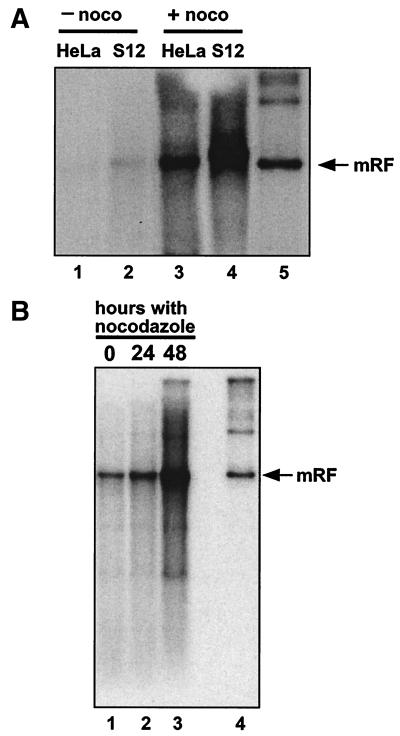
Recent work carried out by us and others (16, 17) implicates E2 in the abrogation of a mitotic checkpoint. In the presence of HPV E6 and E7, E2 can cause a G2/M block while allowing continued DNA rereplication, even in the absence of cell cycle progression (17; K. Raj, unpublished data). If interference in the regulation of mitosis is involved in E2-mediated help for AAV in S12 cells, one could expect agents such as the mitotic spindle inhibitor nocodazole, which blocks cells in G2/M, to have a similar effect. AAV-infected S12 or HeLa cells treated with nocodazole showed a clear amplification of viral DNA replication (Fig. 5A). Other cell lines, Cos-1, CV1, HaCat, Saos-2, OD4, NB-E, and the p53-null H24 clone, were tested, and all of them showed increased AAV DNA replication in the presence of nocodazole (the result for Cos-1 is shown in Fig. 5B). These results indicate that blocking cells in G2/M is sufficient in a number of cell lines infected with AAV to allow an amplification of the viral DNA. On the basis of this, it is possible that cellular stress caused by agents that block mitosis, including HPV E2, may contribute help for AAV replication. However, we found no evidence that a mitotic block caused by nocodazole is sufficient to increase viral particle production. The induction of a mitotic block may, in part, explain help for AAV by E2; it is likely, however, that additional factors are involved in the effect of this protein.
FIG. 5.
(A) Nocodazole treatment leads to increased AAV DNA replication in S12 and HeLa cells. Approximately 2 × 106 S12 or HeLa cells/dish were infected with AAV and subsequently cultured in the presence (+ noco) or absence (− noco) of nocodazole (obtained from Sigma and used in all experiments at a concentration of 0.04 μg/ml of culture medium) for 72 h postinfection. At this point, total DNA was extracted and 10 μg/sample was analyzed by agarose gel electrophoresis and Southern blotting. Lanes 1 and 3, AAV-infected HeLa cells cultured in the absence (lane 1) or the presence (lane 3) of nocodazole. Lanes 2 and 4, AAV-infected S12 cells cultured in the absence (lane 2) or the presence (lane 4) of nocodazole. Lane 5, marker DNA for AAV replication in the presence of adenovirus. (B) Nocodazole treatment leads to increased AAV DNA replication in Cos-1 cells. Approximately 3 × 106 Cos-1 cells/dish were infected with AAV and subsequently cultured for 48 h during which time they were exposed to nocodazole for 0, 24, or 48 h. At 48 h postinfection, total DNA was extracted and 5 μg/sample was analyzed by agarose gel electrophoresis and Southern blotting. Lanes 1 to 3, AAV-infected Cos-1 cells exposed to nocodazole postinfection for 0 (lane 1), 24 (lane 2), or 48 (lane 3) h. Lane 4, marker DNA for AAV replication in the presence of adenovirus. mRF, monomer replicative form.
SV40 large T antigen also provides helper functions for AAV.
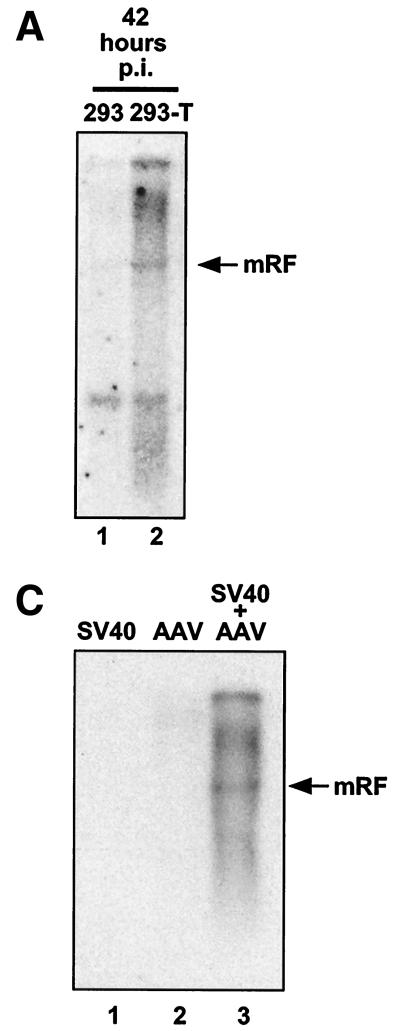
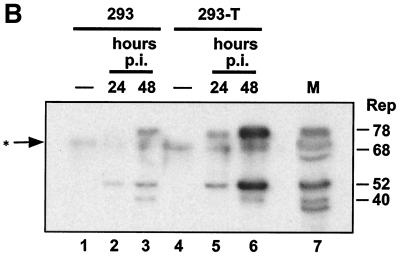
The large T antigen of SV40 encodes functions analogous to the immortalization and replication functions of HPV early proteins E6, E7, E1, and E2. We therefore used our assay to determine whether infectious AAV was produced after transfection of AAV DNA into Cos-1 cells, which constitutively express the SV40 large T protein (21). Infectious AAV production was detected in these cells (Fig. 6). It was not detected in the indicator cells when these were infected with adenovirus alone or when either free AAV DNA or AAV DNA transfection complexes were subjected to the viral purification procedures (Fig. 6A). Its infectivity was blocked by anti-AAV antiserum (Fig. 6B). The amounts of AAV produced in Cos-1 cells were comparable to the amounts obtained from S12 cells transfected with E2. Comparative AAV infections of 293 and 293-T cells were also carried out. 293-T cells are derived from 293 cells, both lines containing and expressing adenovirus E1A and E1B genes (1). 293-T cells additionally constitutively express the SV40 large T protein. These lines were infected in parallel, and both AAV DNA and Rep protein production were examined. The amounts of AAV replicative-form DNAs were enhanced in 293-T cells compared to those in 293 cells at 42 h postinfection (Fig. 7A), while Rep production was considerably increased in 293-T cells at 24 and 48 h postinfection (Fig. 7B). These results suggest that the presence of the large T antigen stimulates Rep expression and AAV DNA replication. Additionally, a coinfection of SV40 and AAV in Cos-1 cells (a simian cell line had to be used since SV40 does not efficiently infect human cells) clearly led to help for AAV DNA replication (Fig. 7C). Although other SV40-induced effects cannot be excluded, the results in Fig. 7A and B suggest that this is at least partly due to the high amounts of large T antigen in the coinfected cells. SV40 large T antigen, which shares a number of functions with the HPV early proteins that we are studying here, thus also can provide, directly or indirectly, helper functions for AAV.
FIG. 6.
(A) Cos-1 cells transfected with AAV DNA can produce infectious AAV. Cos-1 cells were transfected with AAV DNA. Six days posttransfection, procedures were carried out to extract and treat virus as described in Materials and Methods. This extract was used to infect HeLa cells which were then superinfected with adenovirus. At approximately 48 h postinfection, low-molecular-weight DNA was extracted and half the total extract per sample was analyzed by agarose gel electrophoresis and Southern blotting. Lane 1, HeLa cells infected with the AAV-containing extract of Cos-1 cells transfected with AAV DNA. Lane 2, HeLa cells infected with adenovirus alone. Lane 3, 10 μg of AAV DNA was treated with DNase I in parallel with the sample in lane 1 and then adsorbed onto HeLa cells. Lane 4, control transfection complex containing AAV DNA treated with ether and DNase as described above for samples was adsorbed onto HeLa cells. Lane 5, low-molecular-weight DNA from an AAV-adenovirus coinfection used here as a marker for the monomer replicative form (mRF) of AAV DNA. Lane 6, λ DNA cut with HindIII (sizes in kilobases are indicated). (B) Replication of AAV isolated from Cos-1 cells is blocked by anti-AAV-2 antiserum. A transfection experiment was carried out, and the results were analyzed as for the experiment shown in panel A, except that the virus-containing extract was incubated with anti-AAV-2 antiserum prior to infection of HeLa cell plates. Lanes 1 and 2, HeLa cells infected with the AAV-containing extract of Cos-1 cells transfected with AAV DNA; lane 1, with anti-AAV-2 antiserum preincubation of the extract; lane 2, no antiserum. Lanes 3 and 4, HeLa cells infected with the AAV-containing extract of Cos-1 cells that had been transfected with twice as much AAV DNA as used for lanes 1 and 2; lane 3, with anti-AAV-2 antiserum; lane 4, without antiserum. Ab, antibody.
FIG. 7.
(A) Amplification of AAV DNA in 293-T cells. Approximately 6 × 106 293 and 293-T cells were infected with AAV at an MOI of ≅20 IU/cell. At 42 h postinfection (p.i.), total DNA was extracted and 5 μg/sample was analyzed by agarose gel electrophoresis and Southern blotting. Lane 1, AAV-infected 293 cells. Lane 2, AAV-infected 293-T cells. (B) Rep synthesis in 293 and 293-T cells. Approximately 6 × 106 293 and 293-T cells were infected with AAV. At 24 and 48 h postinfection (p.i.), the presence of Rep proteins was analyzed by SDS-polyacrylamide gel electrophoresis and Western blotting. Lane 1, uninfected 293 cells. Lanes 2 and 3, AAV-infected 293 cells, 24 (lane 2) and 48 (lane 3) h postinfection. Lane 4, uninfected 293-T cells. Lanes 5 and 6, AAV-infected 293-T cells, 24 (lane 5) and 48 (lane 6) h postinfection. Lane 7, Rep-positive marker extract. The band between Rep78 and Rep68 marked with an asterisk is a background band. The band below Rep40 is a degradation product. (C) SV40 helps AAV DNA replication. Approximately 2 × 106 Cos-1 cells were simultaneously infected with SV40 at an MOI of ≅20 IU/cell and with AAV. Six days postinfection, total DNA was extracted and 10 μg/sample was analyzed for AAV DNA by agarose gel electrophoresis and Southern blotting. Lane 1, SV40 infection alone. Lane 2, AAV infection alone. Lane 3, SV40 and AAV coinfection. mRF, monomer replicative form.
DISCUSSION
In this work, we asked whether infectious AAV particles were produced after transfection of cloned AAV DNA into HPV-16 episome-containing S12 cells. AAV was detected, and the yield was increased by cotransfection of the HPV-16 E2 gene. S12 cells were derived from the W12 line (15), which originated from a low-grade cervical carcinoma containing HPV-16 DNA. In addition to E6 and E7, other HPV-16 early genes may be transcribed, at least at low levels, from the episomal templates in these cells. E2 is therefore not acting alone in this assay but rather in the presence of other HPV proteins. The effect of E2 was not seen in cells derived from S12 which had lost the HPV-16 episomal DNA nor in other keratinocytes lacking the viral episome. These results substantiate the suggestion made elsewhere (62) that HPV can contribute certain helper functions to a permissive environment for AAV replication.
Infection by AAV is common in the population, but the identity of the natural host cell is not clear. AAV DNA or virions have been detected in low amounts in several tissues, including genital and placental tissue (19, 24, 36, 61, 63), and antibodies against AAV are prevalent in sera. AAV may, therefore, be able to establish low-level persistent infections. The amounts of AAV that we detected from E2-containing S12 cells were also low, about 100-fold lower than those for a laboratory coinfection of adenovirus and AAV. They may well be sufficient, however, to maintain the presence of AAV in genital or other tissues.
Others have shown that transfection with AAV DNA also leads to detectable AAV production in 293 cells, which express the adenovirus E1A and E1B proteins (64). It cannot be excluded that the cellular stress of the transfection process, or the high number of AAV genomes entering the cell, may contribute to the AAV production following such transfections. We believe, however, that these results and ours indicate a low level of permissiveness for AAV replication in some transformed cell lines which, by the use of less sensitive assays, is undetected.
As mentioned above, the introduction of the HPV E2 gene into S12 cells was able to increase AAV production. The effect of increased early gene expression is of interest because this is believed to occur concomitantly with keratinocyte differentiation (56, 57). E2 is a sequence-specific DNA-binding protein that plays several roles in the HPV life cycle. By binding simultaneously to sites near the origin of replication and to the DNA replication protein E1, E2 takes part in the initiation of HPV DNA replication. As a transcription factor, E2 has the ability either to repress or, in certain circumstances, to activate transcription. By repressing the HPV E6/E7 promoter, E2 increases p53 levels because E6 targets p53 for degradation. E2 may also activate p53 in a manner independent of E2 binding to the E6/E7 promoter (13, 31). Finally, E2 interferes with the cell cycle. This is, in part, a result of the increase in p53 activity just mentioned (13, 31) and partly p53 independent (38). E2 has recently been shown also to impose a mitotic block, by a mechanism that is not yet clear.
We found no evidence that E2 provides help for AAV via effects on HPV-16 DNA replication, for example by increasing the number of templates for the transcription of other HPV genes. Since the activity of p53 is stimulated not only by E2 but also by DNA-damaging agents (for example, UV irradiation) that are able to induce helper functions for AAV replication, we were prompted to examine in some detail the response of AAV DNA amplification to changes in p53 levels. For these experiments, we used a p53-null human lung carcinoma line, H1299, modified to express human p53 from the tetracycline-repressible promoter (H1299p53). The fact that AAV DNA replication occurred to the same extent in these cells whether or not p53 was expressed suggests that changes in p53 level alone do not affect the replication of AAV. Furthermore, H1299p53, under p53-expressing or -repressed conditions, showed the same level of AAV DNA amplification in response to UV treatment, implying that AAV DNA amplification in response to this form of cellular stress is not affected by changes in p53 levels, despite p53's role in the response to stress. Since, although known to be efficient, tetracycline-mediated repression is unlikely to completely abolish all p53 expression, an additional and similar experiment was carried out using H24, a clone of H1299 lacking the p53 construction. This experiment confirmed that AAV DNA amplification in response to DNA-damaging agents can occur completely independently of p53. An interaction between p53 and Rep78 has recently been reported (4). Although the biological significance of this interaction is not yet clear, our results suggest that it is not involved in AAV DNA replication. We concluded from these experiments that the ability of E2 to lead to increased AAV replication was unlikely to be linked to any p53 up-regulation pathway. Our results also suggest that changes in p53 expression alone do not affect the permissiveness of a cell for AAV DNA replication under either steady-state or genotoxic stress conditions.
Evidence is accumulating that HPV E6 and E7 can abrogate mitotic checkpoints, thus allowing serial rounds of viral and cellular DNA replication to occur in the absence of intervening mitosis when spindle inhibitors such as nocodazole are used to block completion of mitosis (59, 60). This ability of E6 and E7 is believed to be due to the requirement for functional p53 and pRb in maintaining the integrity of mitotic control checkpoints and the inactivation of these key cell cycle regulatory proteins by high-risk types of HPV E6 and E7, respectively. Cells with intact checkpoint controls will normally be arrested upon treatment with nocodazole, with 4N DNA content. However, work with cells deficient in either p53 or pRb has shown that lack of these proteins allows some cells to progress toward hyperploidy upon treatment with nocodazole (10, 32, 40). Both nocodazole and E2 can, in the presence of high-risk E6 and E7, cause a mitotic block and rereplication of cellular DNA (K. Raj, unpublished observations). It has been proposed elsewhere (17) that an E2-induced cell cycle block allowing cells to undergo continuous DNA rereplication may be a method used by HPV to expand its own genome in differentiated keratinocytes. By blocking cells at this stage of the cell cycle, a form of continual S phase may be established. E2 may operate in part by a similar mechanism in facilitating AAV replication. If E2's helper effect for AAV depends on the ability of E2 to block the G2/M transition in the cell cycle, other agents that block mitosis, for example, nocodazole, should show helper activity, too. We show that nocodazole treatment of S12 cells as well as several other cell lines allows increased AAV DNA replication, in line with the idea that a mitotic block may be sufficient to provide some help for AAV. These cells are immortalized or transformed—many by viral proteins—implicating cell cycle control defects. However, although nocodazole was efficient at inducing AAV DNA replication, we found that it was insufficient to increase the production of virus. We conclude therefore that E2's ability to stimulate the yield of AAV particles goes further than the effect of a mitotic block alone, though this may be a factor.
There appear to be at least two levels of help for AAV replication. The first level, DNA amplification, can occur without concomitant virus production. On the other hand, appearance of virus presumably requires previous DNA synthesis plus additional factors. The requirement of AAV replication for DNA replicative or S-phase enzymes is well established: part of the help provided for AAV by adenovirus is via the induction of host cell S phase, thus stimulating the production of cellular DNA replication enzymes used by AAV. This property of S-phase induction is shared by HPV and SV40. Although AAV's known helper herpes simplex virus does not have this property, it is believed to help AAV via the direct provision of its own DNA replication enzymes (65). A group of proteins associated with S phase has been shown to be sufficient upon addition of Rep protein to support AAV DNA replication (39). Cell synchronization experiments also highlighted a link between AAV replication and the S phase (68).
It is clear, however, that in order for AAV to undergo a full cycle of replication with synthesis of new viral particles, permissiveness for AAV DNA replication is not sufficient. The SV40 large T antigen provides an example of a factor that can be decisive in determining the extent of AAV replication. By genotoxic stress treatments, AAV DNA replication could be readily induced in many cell lines (50, 66, 68), but infectious virus synthesis was detectable only in lines additionally containing the large T antigen (66, 68). A second level of help has to be provided beyond that allowing AAV DNA amplification, and the large T antigen seems to be able to provide this. Here we suggest that the HPV E2 protein also has such a property.
Recently discovered properties of E2 may shed light on how it functions. In the absence of a helper virus, AAV transcription is normally inhibited. This involves an autoinhibition by the Rep protein of its own production via binding to the viral p5 promoter (33, 41). Rep transcription is inhibited, in addition, by binding to this same promoter of the cellular transcription factor YY1 (8). One adenovirus helper function for AAV is to relieve this YY1-mediated repression of Rep transcription via direct interaction of its E1A protein with YY1 (52). This interferes with YY1-mediated repression and allows the p5 promoter to be transactivated. A clue to what E2 may be doing in the cell to augment AAV production may be the recent report that HPV-18 E2 also directly interacts with the YY1 transcription factor (35). Perhaps, through this interaction, E2 has an ability to interfere with p5 repression by YY1. This could be tested should a mutant E2 protein that is defective for YY1 interaction become available. Another clue may be found in binding experiments carried out in our laboratory. Though this work is at a preliminary stage, an interaction between the HPV-16 E2 protein and Rep has been found, both in vitro by glutathione S-transferase–Rep pull-down assays and in vivo by immunoprecipitation (P. Saudan and K. Raj, unpublished data). This interaction between E2 and Rep could influence Rep functions at several stages of the AAV growth cycle. Further investigation will show whether an E2-YY1 interaction may be able to alleviate YY1-mediated repression of Rep production and whether an E2-Rep interaction could prove to provide other keys toward establishing permissive conditions for AAV.
ACKNOWLEDGMENTS
We thank Beatrice Bentele for cell culture and Nicole Paduwat for technical help. We are very grateful to Richard Iggo and Elisabeth Saller for DO1 antibody and H1299p53 and H24 cells, Philippe Saudan for the anti-Rep antibody and the AAV clone, and Matthias Dürst for the HPV-16 clone used in this work. We thank Bernhard Hirt and Philippe Saudan for helpful discussions concerning the work and Lew Pizer for critical reading of the manuscript.
This work was supported by the Fonds National Suisse de la Recherche Scientifique and Recherche Suisse Contre le Cancer.
REFERENCES
- 1.Aiello L, Guilfoyle R, Huebner K, Weinmann R. Adenovirus 5 DNA sequences present and RNA sequences transcribed in transformed human embryo kidney cells (HEK-Ad-5 or 293) Virology. 1979;94:460–469. doi: 10.1016/0042-6822(79)90476-8. [DOI] [PubMed] [Google Scholar]
- 2.Amtmann E, Sauer G. Bovine papilloma virus transcription: polyadenylated RNA species and assessment of the direction of transcription. J Virol. 1982;43:59–66. doi: 10.1128/jvi.43.1.59-66.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bantel-Schaal U, Stohr M. Influence of adeno-associated virus on adherence and growth properties of normal cells: adeno-associated parvoviruses inhibit growth of cells derived from malignant human tumors. J Virol. 1992;66:773–779. doi: 10.1128/jvi.66.2.773-779.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Batchu R B, Shammas M A, Wang J Y, Munshi N C. Interaction of adeno-associated virus Rep78 with p53: implications in growth inhibition. Cancer Res. 1999;59:3592–3595. [PubMed] [Google Scholar]
- 5.Bernard B A, Bailly C, Lenoir M C, Darmon M, Thierry F, Yaniv M. The human papillomavirus type 18 (HPV18) E2 gene product is a repressor of the HPV18 regulatory region in human keratinocytes. J Virol. 1989;63:4317–4324. doi: 10.1128/jvi.63.10.4317-4324.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berns K I, Bohenzky R A. Adeno-associated viruses: an update. Adv Virus Res. 1987;32:243–306. doi: 10.1016/s0065-3527(08)60479-0. [DOI] [PubMed] [Google Scholar]
- 7.Berns K I, Linden R M. The cryptic life style of adeno-associated virus. Bioessays. 1995;17:237–245. doi: 10.1002/bies.950170310. [DOI] [PubMed] [Google Scholar]
- 8.Chang L S, Shi Y, Shenk T. Adeno-associated virus P5 promoter contains an adenovirus E1A-inducible element and a binding site for the major late transcription factor. J Virol. 1989;63:3479–3488. doi: 10.1128/jvi.63.8.3479-3488.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen X, Ko L J, Jayaraman L, Prives C. p53 levels, functional domains, and DNA damage determine the extent of the apoptotic response of tumor cells. Genes Dev. 1996;10:2438–2451. doi: 10.1101/gad.10.19.2438. [DOI] [PubMed] [Google Scholar]
- 10.Cross S M, Sanchez C A, Morgan C A, Schimke M K, Ramel S, Idzerda R L, Raskind W H, Reid B J. A p53-dependent mouse spindle checkpoint. Science. 1995;267:1353–1356. doi: 10.1126/science.7871434. [DOI] [PubMed] [Google Scholar]
- 11.Cukor G, Blocklow N R, Hoggan M D, Berns K I. Biology of adeno-associated virus. In: Berns K I, editor. The parvoviruses. New York, N.Y: Plenum Press; 1984. pp. 33–60. [Google Scholar]
- 12.de la Maza L M, Carter B J. Inhibition of adenovirus oncogenicity in hamsters by adeno-associated virus DNA. J Natl Cancer Inst. 1981;67:1323–1326. [PubMed] [Google Scholar]
- 13.Desaintes C, Demeret C, Goyat S, Yaniv M, Thierry F. Expression of the papillomavirus E2 protein in HeLa cells leads to apoptosis. EMBO J. 1997;16:504–514. doi: 10.1093/emboj/16.3.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Durst M, Gissmann L, Ikenberg H, zur Hausen H. A papillomavirus DNA from a cervical carcinoma and its prevalence in cancer biopsy samples from different geographic regions. Proc Natl Acad Sci USA. 1983;80:3812–3815. doi: 10.1073/pnas.80.12.3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flores E R, Lambert P F. Evidence for a switch in the mode of human papillomavirus type 16 DNA replication during the viral life cycle. J Virol. 1997;71:7167–7179. doi: 10.1128/jvi.71.10.7167-7179.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fournier N, Raj K, Saudan P, Utzig S, Sahli R, Simanis V, Beard P. Expression of human papillomavirus 16 E2 protein in Schizosaccharomyces pombe delays the initiation of mitosis. Oncogene. 1999;18:4015–4021. doi: 10.1038/sj.onc.1202775. [DOI] [PubMed] [Google Scholar]
- 17.Frattini M G, Hurst S D, Lim H B, Swaminathan S, Laimins L A. Abrogation of a mitotic checkpoint by E2 proteins from oncogenic human papillomaviruses correlates with increased turnover of the p53 tumor suppressor protein. EMBO J. 1997;16:318–331. doi: 10.1093/emboj/16.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frattini M G, Laimins L A. Binding of the human papillomavirus E1 origin-recognition protein is regulated through complex formation with the E2 enhancer-binding protein. Proc Natl Acad Sci USA. 1994;91:12398–12402. doi: 10.1073/pnas.91.26.12398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friedman-Einat M, Grossman Z, Mileguir F, Smetana Z, Ashkenazi M, Barkai G, Varsano N, Glick E, Mendelson E. Detection of adeno-associated virus type 2 sequences in the human genital tract. J Clin Microbiol. 1997;35:71–78. doi: 10.1128/jcm.35.1.71-78.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Georg-Fries B, Biederlack S, Wolf J, zur Hausen H. Analysis of proteins, helper dependence, and seroepidemiology of a new human parvovirus. Virology. 1984;134:64–71. doi: 10.1016/0042-6822(84)90272-1. [DOI] [PubMed] [Google Scholar]
- 21.Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981;23:175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- 22.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ham J, Dostatni N, Gauthier J M, Yaniv M. The papillomavirus E2 protein: a factor with many talents. Trends Biochem Sci. 1991;16:440–444. doi: 10.1016/0968-0004(91)90172-r. [DOI] [PubMed] [Google Scholar]
- 24.Han L, Parmley T H, Keith S, Kozlowski K J, Smith L J, Hermonat P L. High prevalence of adeno-associated virus (AAV) type 2 rep DNA in cervical materials: AAV may be sexually transmitted. Virus Genes. 1996;12:47–52. doi: 10.1007/BF00370000. [DOI] [PubMed] [Google Scholar]
- 25.Hermonat P L. Adeno-associated virus inhibits human papillomavirus type 16: a viral interaction implicated in cervical cancer. Cancer Res. 1994;54:2278–2281. [PubMed] [Google Scholar]
- 26.Hermonat P L. The adeno-associated virus Rep78 gene inhibits cellular transformation induced by bovine papillomavirus. Virology. 1989;172:253–261. doi: 10.1016/0042-6822(89)90127-x. [DOI] [PubMed] [Google Scholar]
- 27.Hermonat P L. Inhibition of bovine papillomavirus plasmid DNA replication by adeno-associated virus. Virology. 1992;189:329–333. doi: 10.1016/0042-6822(92)90710-7. [DOI] [PubMed] [Google Scholar]
- 28.Hermonat P L, Plott R T, Santin A D, Parham G P, Flick J T. Adeno-associated virus Rep78 inhibits oncogenic transformation of primary human keratinocytes by a human papillomavirus type 16-ras chimeric. Gynecol Oncol. 1997;66:487–494. doi: 10.1006/gyno.1997.4789. [DOI] [PubMed] [Google Scholar]
- 29.Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967;26:365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- 30.Howley P M. Papillomaviruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2045–2076. [Google Scholar]
- 31.Hwang E S, Naeger L K, DiMaio D. Activation of the endogenous p53 growth inhibitory pathway in HeLa cervical carcinoma cells by expression of the bovine papillomavirus E2 gene. Oncogene. 1996;12:795–803. [PubMed] [Google Scholar]
- 32.Khan S H, Wahl G M. p53 and pRb prevent rereplication in response to microtubule inhibitors by mediating a reversible G1 arrest. Cancer Res. 1998;58:396–401. [PubMed] [Google Scholar]
- 33.Kyostio S R, Owens R A, Weitzman M D, Antoni B A, Chejanovsky N, Carter B J. Analysis of adeno-associated virus (AAV) wild-type and mutant Rep proteins for their abilities to negatively regulate AAV p5 and p19 mRNA levels. J Virol. 1994;68:2947–2957. doi: 10.1128/jvi.68.5.2947-2957.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laughlin C A, Tratschin J D, Coon H, Carter B J. Cloning of infectious adeno-associated virus genomes in bacterial plasmids. Gene. 1983;23:65–73. doi: 10.1016/0378-1119(83)90217-2. [DOI] [PubMed] [Google Scholar]
- 35.Lee K Y, Broker T R, Chow L T. Transcription factor YY1 represses cell-free replication from human papillomavirus origins. J Virol. 1998;72:4911–4917. doi: 10.1128/jvi.72.6.4911-4917.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malhomme O, Dutheil N, Rabreau M, Armbruster-Moraes E, Schlehofer J R, Dupressoir T. Human genital tissues containing DNA of adeno-associated virus lack DNA sequences of the helper viruses adenovirus, herpes simplex virus or cytomegalovirus but frequently contain human papillomavirus DNA. J Gen Virol. 1997;78:1957–1962. doi: 10.1099/0022-1317-78-8-1957. [DOI] [PubMed] [Google Scholar]
- 37.Mayor H D, Drake S, Stahmann J, Mumford D M. Antibodies to adeno-associated satellite virus and herpes simplex in sera from cancer patients and normal adults. Am J Obstet Gynecol. 1976;126:100–104. doi: 10.1016/0002-9378(76)90472-5. [DOI] [PubMed] [Google Scholar]
- 38.Naeger L K, Goodwin E C, Hwang E S, DeFilippis R A, Zhang H, DiMaio D. Bovine papillomavirus E2 protein activates a complex growth-inhibitory program in p53-negative HT-3 cervical carcinoma cells that includes repression of cyclin A and cdc25A phosphatase genes and accumulation of hypophosphorylated retinoblastoma protein. Cell Growth Differ. 1999;10:413–422. [PubMed] [Google Scholar]
- 39.Ni T H, McDonald W F, Zolotukhin I, Melendy T, Waga S, Stillman B, Muzyczka N. Cellular proteins required for adeno-associated virus DNA replication in the absence of adenovirus coinfection. J Virol. 1998;72:2777–2787. doi: 10.1128/jvi.72.4.2777-2787.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Notterman D, Young S, Wainger B, Levine A J. Prevention of mammalian DNA reduplication, following the release from the mitotic spindle checkpoint, requires p53 protein, but not p53-mediated transcriptional activity. Oncogene. 1998;17:2743–2751. doi: 10.1038/sj.onc.1202210. [DOI] [PubMed] [Google Scholar]
- 41.Pereira D J, McCarty D M, Muzyczka N. The adeno-associated virus (AAV) Rep protein acts as both a repressor and an activator to regulate AAV transcription during a productive infection. J Virol. 1997;71:1079–1088. doi: 10.1128/jvi.71.2.1079-1088.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reeves W C, Caussy D, Brinton L A, Brenes M M, Montalvan P, Gomez B, de Britton R C, Morice E, Gaitan E, de Lao S L, et al. Case-control study of human papillomaviruses and cervical cancer in Latin America. Int J Cancer. 1987;40:450–454. doi: 10.1002/ijc.2910400403. [DOI] [PubMed] [Google Scholar]
- 43.Romanczuk H, Howley P M. Disruption of either the E1 or the E2 regulatory gene of human papillomavirus type 16 increases viral immortalization capacity. Proc Natl Acad Sci USA. 1992;89:3159–3163. doi: 10.1073/pnas.89.7.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rommelaere J, Cornelis J J. Antineoplastic activity of parvoviruses. J Virol Methods. 1991;33:233–251. doi: 10.1016/0166-0934(91)90024-t. [DOI] [PubMed] [Google Scholar]
- 45.Saller E. Ph.D. thesis. Lausanne, Switzerland: University of Lausanne; 1999. [Google Scholar]
- 46.Saller E, Tom E, Brunori M, Otter M, Estreicher A, Mack D H, Iggo R. Increased apoptosis induction by 121F mutant p53. EMBO J. 1999;18:4424–4437. doi: 10.1093/emboj/18.16.4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 48.Scheffner M, Werness B A, Huibregtse J M, Levine A J, Howley P M. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- 49.Schlehofer J R. The tumor suppressive properties of adeno-associated viruses. Mutat Res. 1994;305:303–313. doi: 10.1016/0027-5107(94)90250-x. [DOI] [PubMed] [Google Scholar]
- 50.Schlehofer J R, Ehrbar M, zur Hausen H. Vaccinia virus, herpes simplex virus, and carcinogens induce DNA amplification in a human cell line and support replication of a helper virus-dependent parvovirus. Virology. 1986;152:110–117. doi: 10.1016/0042-6822(86)90376-4. [DOI] [PubMed] [Google Scholar]
- 51.Schneider-Maunoury S, Croissant O, Orth G. Integration of human papillomavirus type 16 DNA sequences: a possible early event in the progression of genital tumors. J Virol. 1987;61:3295–3298. doi: 10.1128/jvi.61.10.3295-3298.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shi Y, Seto E, Chang L S, Shenk T. Transcriptional repression by YY1, a human GLI-Kruppel-related protein, and relief of repression by adenovirus E1A protein. Cell. 1991;67:377–388. doi: 10.1016/0092-8674(91)90189-6. [DOI] [PubMed] [Google Scholar]
- 53.Sprecher-Goldberger S, Thiry L, Lefebvre N, Dekegel D, de Halleux F. Complement-fixation antibodies to adenovirus-associated viruses, cytomegaloviruses and herpes simplex viruses in patients with tumors and in control individuals. Am J Epidemiol. 1971;94:351–358. doi: 10.1093/oxfordjournals.aje.a121330. [DOI] [PubMed] [Google Scholar]
- 54.Srivastava A, Lusby E W, Berns K I. Nucleotide sequence and organization of the adeno-associated virus 2 genome. J Virol. 1983;45:555–564. doi: 10.1128/jvi.45.2.555-564.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Steger G, Corbach S. Dose-dependent regulation of the early promoter of human papillomavirus type 18 by the viral E2 protein. J Virol. 1997;71:50–58. doi: 10.1128/jvi.71.1.50-58.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stoler M H, Whitbeck A, Wolinsky S M, Broker T R, Chow L T, Howett M K, Kreider J W. Infectious cycle of human papillomavirus type 11 in human foreskin xenografts in nude mice. J Virol. 1990;64:3310–3318. doi: 10.1128/jvi.64.7.3310-3318.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stoler M H, Wolinsky S M, Whitbeck A, Broker T R, Chow L T. Differentiation-linked human papillomavirus types 6 and 11 transcription in genital condylomata revealed by in situ hybridization with message-specific RNA probes. Virology. 1989;172:331–340. doi: 10.1016/0042-6822(89)90135-9. [DOI] [PubMed] [Google Scholar]
- 58.Su P F, Wu F Y. Differential suppression of the tumorigenicity of HeLa and SiHa cells by adeno-associated virus. Br J Cancer. 1996;73:1533–1537. doi: 10.1038/bjc.1996.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thomas J T, Laimins L A. Human papillomavirus oncoproteins E6 and E7 independently abrogate the mitotic spindle checkpoint. J Virol. 1998;72:1131–1137. doi: 10.1128/jvi.72.2.1131-1137.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thompson D A, Belinsky G, Chang T H, Jones D L, Schlegel R, Munger K. The human papillomavirus-16 E6 oncoprotein decreases the vigilance of mitotic checkpoints. Oncogene. 1997;15:3025–3035. doi: 10.1038/sj.onc.1201495. [DOI] [PubMed] [Google Scholar]
- 61.Tobiasch E, Rabreau M, Geletneky K, Larue-Charlus S, Severin F, Becker N, Schlehofer J R. Detection of adeno-associated virus DNA in human genital tissue and in material from spontaneous abortion. J Med Virol. 1994;44:215–222. doi: 10.1002/jmv.1890440218. [DOI] [PubMed] [Google Scholar]
- 62.Walz C, Deprez A, Dupressoir T, Durst M, Rabreau M, Schlehofer J R. Interaction of human papillomavirus type 16 and adeno-associated virus type 2 co-infecting human cervical epithelium. J Gen Virol. 1997;78:1441–1452. doi: 10.1099/0022-1317-78-6-1441. [DOI] [PubMed] [Google Scholar]
- 63.Walz C M, Anisi T R, Schlehofer J R, Gissmann L, Schneider A, Muller M. Detection of infectious adeno-associated virus particles in human cervical biopsies. Virology. 1998;247:97–105. doi: 10.1006/viro.1998.9226. [DOI] [PubMed] [Google Scholar]
- 64.Wang X S, Srivastava A. Rescue and autonomous replication of adeno-associated virus type 2 genomes containing Rep-binding site mutations in the viral p5 promoter. J Virol. 1998;72:4811–4818. doi: 10.1128/jvi.72.6.4811-4818.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weindler F W, Heilbronn R. A subset of herpes simplex virus replication genes provides helper functions for productive adeno-associated virus replication. J Virol. 1991;65:2476–2483. doi: 10.1128/jvi.65.5.2476-2483.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yakinoglu A O, Heilbronn R, Burkle A, Schlehofer J R, zur Hausen H. DNA amplification of adeno-associated virus as a response to cellular genotoxic stress. Cancer Res. 1988;48:3123–3129. [PubMed] [Google Scholar]
- 67.Yakobson B, Hrynko T A, Peak M J, Winocour E. Replication of adeno-associated virus in cells irradiated with UV light at 254 nm. J Virol. 1989;63:1023–1030. doi: 10.1128/jvi.63.3.1023-1030.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yakobson B, Koch T, Winocour E. Replication of adeno-associated virus in synchronized cells without the addition of a helper virus. J Virol. 1987;61:972–981. doi: 10.1128/jvi.61.4.972-981.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.zur Hausen H. Papillomaviruses in anogenital cancer as a model to understand the role of viruses in human cancers. Cancer Res. 1989;49:4677–4681. [PubMed] [Google Scholar]