Abstract
Recombinant adeno-associated virus (rAAV) vectors have been shown to be useful for efficient gene delivery to a variety of dividing and nondividing cells. Mechanisms responsible for the long-term, persistent expression of the rAAV transgene are not well understood. In this study we investigated the kinetics of rAAV-mediated human factor IX (hFIX) gene transfer into human primary myoblasts and myotubes. Transduction of both myoblasts and myotubes occured with a similar and high efficiency. After 3 to 4 weeks of transduction, rAAV with a cytomegalovirus (CMV) promoter showed 10- to 15-fold higher expression than that with a muscle-specific creatine kinase enhancer linked to β-actin promoter. Factor IX expression from transduced myoblasts as well as myotubes reached levels as high as approximately 2 μg of hFIX/106 cells/day. Southern blot analyses of high-molecular-weight (HMW) cellular genomic and Hirt DNAs isolated from rAAV/CMVhFIXm1-transduced cells showed that the conversion of single-stranded vector genomes to double-stranded DNA forms, but not the level of the integrated forms in HMW DNA, correlated with increasing expression of the transgene. Together, these results indicate that rAAV can transduce both proliferating and terminally differentiated muscle cells at about the same efficiency, that expression of transgenes increases linearly over their lifetime with no initial lag phase, and that increasing expression correlates with the appearance of double-stranded episomal rAAV genomes. Evidence showing that the rAAV virions can copackage hFIX, presumably nonspecifically, was also obtained.
Adeno-associated virus type 2 (AAV-2) is a nonpathogenic (10, 11), 4.7-kb single-stranded DNA virus that requires coinfection with helper adenovirus or herpes simplex virus for efficient replication (5, 12, 30). The viral genome contains two open reading frames that express four Rep and three Cap proteins and is flanked by the 145-bp terminal repeats, which are the only cis-acting elements that are essential for replication, packaging, or integration (reviewed in reference 47). Thus, the entire AAV genome, except for these repeats, can be replaced by a transgene to form a recombinant AAV (rAAV) (43, 57). The natural tissue tropism of wild-type AAV is for lung epithelial cells; however, the recombinant vector has been used to also efficiently target skeletal muscle, liver, retina, brain, and gut epithelium (15, 20, 23, 24, 32, 34, 35, 60, 71, 77). This nearly ubiquitous tropism can be partly explained by the fact that AAV-2 apparently uses widely expressed molecules as coreceptors, including heparan sulfate proteoglycan (primary attachment receptor), fibroblast growth factor receptor 1, and αVβ5-integrin (52, 62, 63; J. Qui, H. Mizukami, and K. E. Brown, Letter, Nat. Med. 5:467–468, 1999).
Either strand of the virus can be packaged in virions as a single-stranded DNA (47). In an infected cell, this single-stranded virion DNA is converted to a double-stranded form by poorly defined mechanisms. In the absence of helper virus coinfection, wild-type AAV integrates as concatamer and preferentially but not exclusively at the AAVS1 locus on human chromosome 19 by a process that requires viral Rep protein(s) (33, 36, 40, 58). Upon subsequent helper virus infection, the wild-type AAV genome is rescued and the virus enters the lytic cycle, forming progeny virions and thus completing the viral infection cycle. On the other hand, the genome maturation kinetics is not well understood for the rAAV vector genome. rAAV, delivered in the absence of helper virus and Rep protein, persists as a single-stranded genome for a certain period in transduced tissues. Using host cell enzymes, the single-stranded rAAV genome is converted to double-stranded forms that may persist as linear or circular episomes and may also appear in the high-molecular-weight (HMW) DNA. Integration of rAAV occurs at a low frequency, as monomers or low-copy-numbers concatamers, and with a loss of chromosome 19 specificity (13, 33, 43, 48, 51, 53, 55). Poor understanding of rAAV genome maturation has added to the confusion regarding what form(s) of rAAV, integrated or episomal, is truly responsible for long-term transgene expression and persistence, both of which are the hallmarks of rAAV-mediated gene transfer in muscle, liver, and other target organs.
Skeletal muscle has turned out to be a promising tissue for rAAV-mediated systemic delivery of transgene products. Efficient systemic delivery of transgene products in skeletal muscle has been clearly demonstrated (6, 16, 18, 73). High rAAV transducibility, easy accessibility, low turnover of cells, and high vascularity are salient features that make skeletal muscle a preferred rAAV delivery tissue. The ability of skeletal muscle to exhibit persistent expression of the rAAV transgene has been well described (15, 23, 34, 71). The lifelong transgene expression obtained in mice after rAAV-mediated gene transfer is in part due to the inability to elicit a cell-mediated immune response (31). These features of rAAV in muscle have led to preclinical testing with hemophilia B mouse and dog models and now to initial translational clinical testing (for a review, see reference 54). Hemophilia B is caused by deficiency of blood coagulation factor IX (FIX), a key protein that occupies a pivotal position in the coagulation cascade (17, 37, 41).
A single intramuscular injection of rAAV containing the human, mouse, or canine FIX (hFIX, mFIX, or cFIX) expression unit into immunocompetent normal or hemophiliac mice or dogs can result in the production of biologically active FIX (27, 28, 76). In mice intramuscularly injected with rAAV vector containing the hFIX expression unit (rAAV/hFIX), the level of hFIX increases gradually over 5 to 10 weeks after a 1- to 2-week initial lag phase. Using rAAV/β-galactosidase vector, such gradual increase in expression of transgene has also been correlated with a concomitant increase in the integrated form of the rAAV vector (15). Similarly, the integrated form of rAAV correlates with transgene expression in liver-directed rAAV in mice (44). Other groups have suggested a role for episomal rAAV forms in the long-term persistence and expression in muscle (19). To clarify these issues, we analyzed the kinetics of rAAV transduction using a human skeletal muscle cell assay system. Primary human skeletal muscle cells can be maintained for almost a month in culture, much longer than mouse primary muscle cells, allowing us to analyze the detailed initial kinetics of rAAV transduction.
MATERIALS AND METHODS
rAAV vectors.
Plasmid pTR/Me4βAhFIXm1 containing the 2.8-kb human FIX minigene (hFIXm1) (38) under the transcriptional control of four tandem copies of muscle creatine kinase enhancer (Me4) and β-actin promoter (βA) (69) was constructed by inserting the Me4-βA-hFIXm1 cassette between the AAV terminal repeats in pTR-UF2 (described in reference 77). The Me4-βA-hFIXm1 fragment (4.4-kb) was obtained from pBS/Me4βAhFIXm1 by KpnI-BamHI digestion. This fragment was inserted at the KpnI-BamHI sites of pTR-UF2 containing AAV terminal repeats and bovine growth hormone (bGH) poly(A) signal, thus generating pTR/Me4βAhFIXm1 (Fig. 1). pTR/CMVhFIXm1 was constructed by inserting hFIXm1 (2.8-kb) into pTR-UF2 at the BamHI site between the human cytomegalovirus (CMV) immediate-early gene enhancer/promoter and bGH poly(A) signal (Fig. 1). The rAAV genomes produced from these vectors, rAAV/CMVhFIXm1 and rAAV/Me4βAhFIXm1, were 4.0 and 4.9 kb in size, respectively.
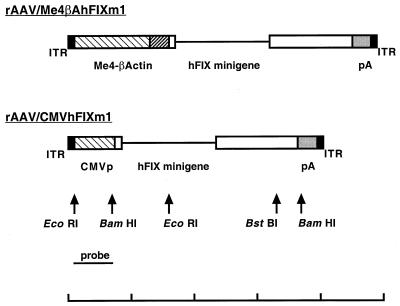
FIG. 1.
Structure of rAAV/Me4βAhFIXm1 and rAAV/CMVhFIXm1 constructs. rAAV/Me4βAhFIXm1 contains the hFIX minigene under the transcriptional control of four tandem copies of the minimal MCK enhancer (Me4; total size 1.2 kb) and 280-bp β-actin promoter. rAAV/CMVhFIXm1 contains the 620-bp CMV immediate-early gene enhancer-promoter, hFIX minigene, and bGH poly(A) signal, flanked by AAV inverted terminal repeats. The CMV promoter probe (0.62-kb EcoRI-BamHI fragment) used for Southern analysis is shown by a dark line. The internal EcoRI fragment detected by the CMV promoter probe is 1.9 kb (arrow a in Fig. 4 and 5). The line at the bottom represents a 5-kb scale, with each mark representing 1 kb.
Helper-free rAAV vectors were prepared by the triple plasmid cotransfection method as previously described (39, 72). Briefly, 293 cells in a 100-mm-diameter culture dish were cotransfected with 5 μg of rAAV plasmid, 5 μg of AAV helper plasmid (pACG2), and 15 μg of adenovirus helper plasmid (pXX6) (1:1:1 molar ratio). At 48 h posttransfection, the rAAV virions were released from the cells by three freeze-thaw cycles and purified by (NH4)2SO4 fractionation and two sequential CsCl2 gradient centrifugations. The titer of rAAV was then determined by the dot-blot hybridization assay. Following DNase digestion and proteinase K treatment, 5-μl samples from each fraction were denatured and probed with a radiolabelled BamHI fragment of hFIX cDNA. Serial dilutions of linearized rAAV plasmid solution were used as DNA concentration standards to determine the viral particle number titer (virus particles per milliliter). In our experience, the number of infectious units is in the order of 500 to 1,000 times smaller than the rAAV particle number. The rAAV preparation was then dialyzed against 10 mM Tris (pH 7.5)–140 mM NaCl–1 mM MgCl2–3% glycerol with three or four changes of dialysis solution over 4 to 12 h. Virus aliquots were stored at −70°C until use. No visible cytopathic effect was seen on myoblast cultures after infection with these rAAV preparations. Viral titers obtained were between 4.5 × 1011 and 2 × 1012 particles per ml for the several preparations used in this paper.
Isolation and maintenance of human myoblasts.
Primary human myoblasts were isolated from biopsy samples of healthy abdominal muscle tissue (rectus abdominus) which were obtained from patients undergoing surgery (at sites unrelated to abdominal muscle) at the Department of Surgery. Written consent was obtained in accordance with institutional guidelines. Muscle tissue biopsy specimens freshly obtained from 44- or 2-year-old individuals were immediately placed in ice-cold human myoblast growth medium (HMB-GM) (see below) and left overnight at 4°C. Myoblasts were isolated by using anti-CD56 antibody linked to magnetic beads (B.-G. Chen and K. Kurachi, unpublished data) or by a cloning method as previously described (74). The primary myoblasts obtained were more than 99% free of fibroblasts, as judged by desmin immunofluorescence staining (data not shown), and were capable of complete differentiation into myotubes. The primary myoblasts can be maintained in culture for at least 25 to 30 doublings (about 8 to 10 passages) (data not shown). The cells used in this report were between passages 3 and 5. Myoblasts were routinely maintained on 0.25% gelatin (Sigma, St. Louis, Mo.)-coated tissue culture dishes. The tissue culture dishes were coated with gelatin by pipetting 0.25% (wt/vol) solution of gelatin made in water, swirling the dishes to wet the surface, and removing the solution before plating the cells. The growth-factor rich media, HMB-GM, used for myoblasts in this paper were (i) Ham's F10 medium (Sigma) supplemented with 20% heat-inactivated fetal bovine serum (FBS; Gibco-BRL, Gaithersburg, Md.), 5 ng of bovine fibroblast growth factor basic (R & D Systems, Minneapolis, Minn.) per ml, and penicillin-streptomycin (pen-strep; Gibco-BRL); and (ii) skeletal muscle growth medium (SKGM; Clonetics/Whittakar, San Diego, Calif.) supplemented with 20% Dulbecco's modified Eagle's medium (DMEM; Gibco-BRL), 5% FBS, and pen-strep. The differentiation medium used was DMEM supplemented with 2.5% FBS and pen-strep.
Recombinant AAV transduction.
For rAAV transduction, 3 × 105 human myoblasts were routinely plated on 60-mm-diameter gelatin-coated culture dishes and grown for 3 days to 90% confluency (∼106 cells). The myoblasts were then differentiated by being grown for 3 days in DMEM containing 2.5% FBS. The medium was replaced every day. Fully differentiated myotubes or 90% confluency myoblasts were infected with rAAV at a multiplicity of infection (MOI) of 1 × 104, 1 × 105, or 5 × 105 particles/cell (ca. 1 × 1010, 1 × 1011, or 5 × 1011 particles/dish) for 2 h at 37°C with continuous rocking in 0.5 ml made up with Opti-MEM-I medium (Gibco-BRL). The volume was then adjusted to 2 ml with medium containing BaSO4-treated FBS (75) and supplemented with 10 μg of vitamin K1 per ml (AquaMEPHYTON; Merck, West Point, Pa.). The medium was collected daily, and fresh medium was added. In some experiments, the infections were done in six-well plates. The medium collected was centrifuged for 30 s in a microcentrifuge to remove cell debris and stored at −70°C until use. Levels of hFIX produced in the medium were estimated by enzyme-linked immunosorbent assay (ELISA), using pooled normal human plasma (George King Bio-Medical Inc., St. Overland Park, Kans.) as the standard, as previously described (38). All samples were assayed in duplicate, and the sensitivity of this ELISA was of the subnanogram order.
Isolation and analysis of HMW and Hirt DNAs.
HMW (cellular genomic and possibly some very large concatameric forms of rAAV) DNA was isolated from infected cells at various times postinfection (p.i.). Cells were gently rinsed with ice-cold phosphate-buffered saline and were harvested by scraping into 2 ml of ice-cold phosphate-buffered saline. Cells from three 60-mm-diameter dishes were pooled for each time point, and the genomic DNA fraction was obtained by proteinase digestion of cells as described previously (45, 56). Stringy DNA (HMW DNA), precipitated upon addition of 70% ethanol (final volume) to cell lysates, was spooled out using a plastic pipette tip, transferred to a tube containing 70% ethanol, and pelleted by brief centrifugation at room temperature. The DNA pellet was air dried and allowed to resuspend overnight in 20 to 100 μl of TE (10 mM Tris-HCl [pH 8.0], 1 mM EDTA) at 4°C. After removal of HMW DNA, the remaining original 70% ethanol solution was centrifuged at room temperature for 15 min in a microcentrifuge to obtain Hirt DNA (unintegrated low-molecular-weight DNA). The Hirt DNA pellet was washed with 70% ethanol and resuspended in 25 μl of TE containing RNase A (at 0.1 mg/ml [final concentration]). This spooling method, which is routinely used for obtaining high-quality genomic DNA from culture cells and various tissues, was used rather than conventional Hirt precipitation (29) because this enabled us to prepare both high-quality HMW and Hirt DNA preparations from the same cells. Southern blot analysis of restricted genomic DNA (4 to 10 μg) was carried out on a 0.75% agarose gel using 32P-radiolabelled probes (56). Specifically hybridized bands were quantitated on a PhosphorImager (STORM system; Molecular Dynamics, Sunnyvale, Calif.).
Transfection of human myoblasts.
Primary myoblasts were transfected using FuGENE 6 (Roche, Indianapolis, Ind.) as recommended by the manufacturer, with modifications. Briefly, 2 × 105 myoblasts were plated per well of a six-well plate coated with 0.25% gelatin and incubated overnight at 37°C. After 1 day, cells at 70 to 80% confluency were transfected by centrifugation (30 min at 1,180 − g and 32°C) after addition of a mixture of 3 μg of plasmid DNA (precondensed by incubating with 3 μg of histone H1 protein [Roche] for 5 min at room temperature) and 9 μl of FuGENE 6 on cells in a final volume of 1 ml of medium. Centrifugation was performed using microtiter plate carrier. After 4 h, the transfection medium was replaced with 1 ml of growth medium (SKGM) supplemented with BaSO4-treated FBS and 10 μg of vitamin K1 per ml. The medium was collected every 24 h, and the hFIX produced was assayed by ELISA. Expression obtained in the 24- to 48-h posttransfection period in repeated experiments was reported.
Western blot analysis of rAAV/CMVhFIXm1 virions.
rAAV preparations were diluted to 1011 particles/ml in 50 mM Tris (pH 6.7). Sodium dodecyl sulfate (SDS)-polyacrylamide gel-loading buffer (5×) was added to the samples and boiled for 5 min, and 2 or 4 μl was resolved on an SDS–10% polyacrylamide gel and electroblotted onto polyvinylidene difluoride membranes as previously described (42). The hFIX proteins on blots were visualized by sequential treatment with goat anti-hFIX polyclonal antibody (obtained from Enzyme Research Laboratories Inc., South Bend, Ind.) (used at 1:1,000 dilution in Tris-buffered saline [TBS] [pH 7.5]), horseradish peroxidase-conjugated swine antigoat immunoglobulin G (Roche) (1:5,000 dilution in TBS) and chemiluminescent reagents (ECL-PLUS [Amersham]). The titers of rAAV/CMV-β-gal and wild-type AAV-2 used as controls in these experiments were 5.5 × 1011 and 1.3 × 1011 particles/ml, respectively. These vectors were produced from rAAV plasmids, pAB-11 and pSub201, respectively, using the helper-free system as described above.
RESULTS AND DISCUSSION
Transduction of human myotubes with rAAV/CMVhFIXm1 results in much higher expression of transgene than does that of rAAV/Me4βAhFIXm1.
Previously we showed that Me4βA muscle-specific promoter can drive high hFIX expression in primary mouse myoblasts (69). To evaluate the expression of hFIX transgene from the Me4βA promoter in rAAV-mediated gene transfer, we constructed rAAV/Me4βAhFIXm1 vector and compared it with the constitutive vector rAAV/CMVhFIXm1 (Fig. 1). Figure 2 shows the kinetics of hFIX production from human myotubes after transduction with rAAV/Me4βAhFIXm1 or rAAV/CMVhFIXm1. It is important to note that longitudinal and detailed analysis of kinetics of rAAV transduction and transgene expression was possible only by using human muscle cells, which could be maintained viable in culture for as long as 1 month. Mouse myotubes, on the other hand, could not be maintained for more than 7 to 9 days in culture (data not shown).
FIG. 2.
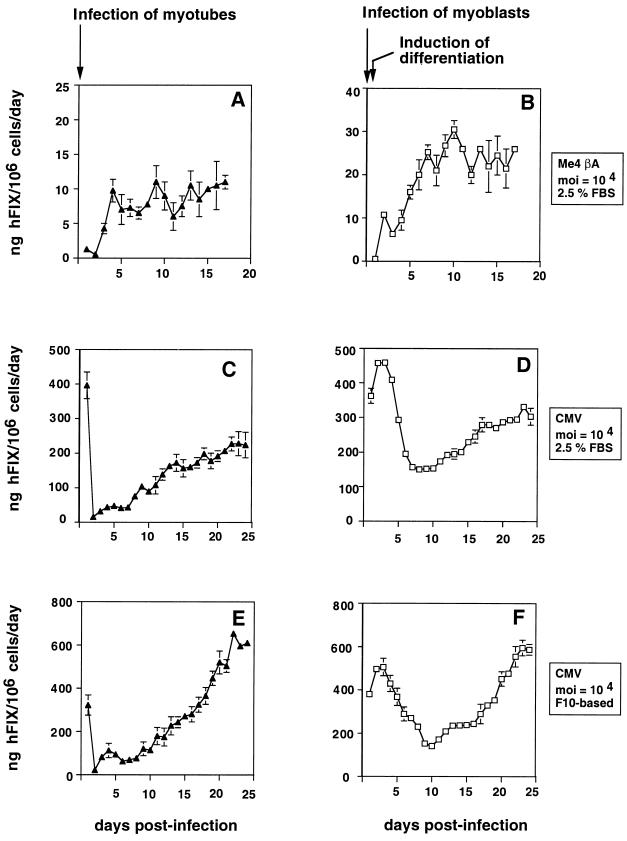
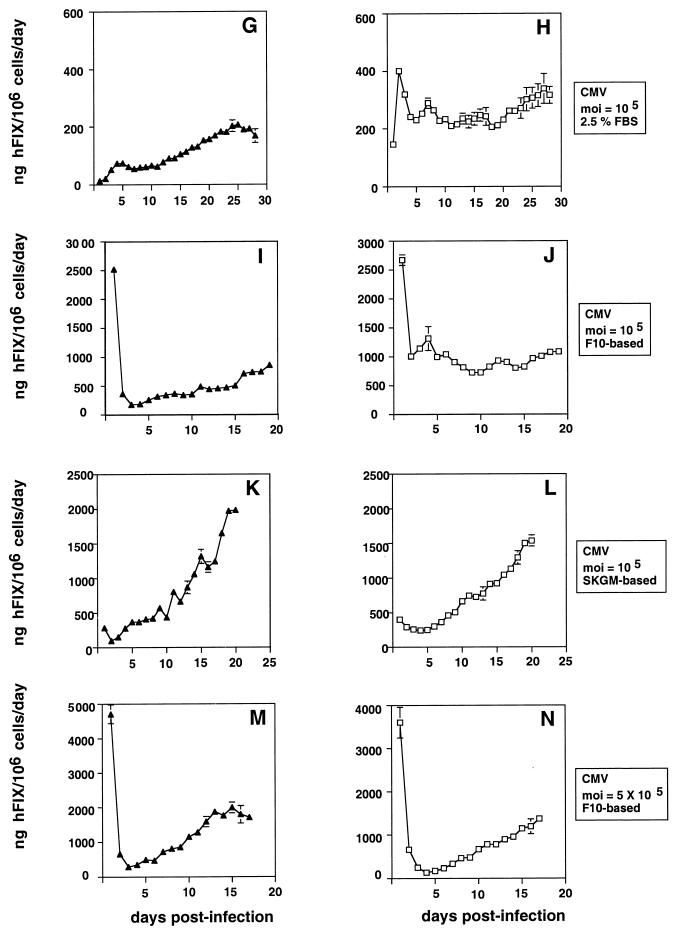
Production of hFIX from human muscle cells transduced with rAAV/Me4βAhFIXm1 or rAAV/CMVhFIXm1 vector. (A, C, E, G, I, K, and M) hFIX production after direct transduction of myotubes (solid triangles); (B, D, F, H, J, L, and N) hFIX production from myotubes generated from transduced myoblasts (open squares). Cells were transduced with rAAV/Me4βAhFIXm1 at a MOI of 1 × 104 (A and B) or rAAV/CMVhFIXm1 at a MOI of 1 × 104 (C to F), 1 × 105 (G to L), and 5 × 105 (M and N). Cells were maintained in differentiation medium (A to D, G and H), F10-based medium (E, F, I, J, M, and N), or SKGM-based medium (K and L). hFIX secreted per day was assayed by ELISA and is shown as mean and standard error of the mean (n = 2 for panels A, B, E, and F; n = 3 for panels C, D, K, L, M, and N). For panels G, H, I, and J, the experiment was started with nine 60-mm dishes, and three dishes were used at each time point as described in the text. For panels F, J, L, and N, myoblasts were differentiated by being transferred to differentiation medium for 3 days p.i., and then maintaining the cells in F10- or SKGM-based rich medium as described in the text. No high or transient expression of hFIX was seen in panels K and L, presumably because a different rAAV batch was used for them, which is different from those used for other experiments.
The human muscle cells were infected at a MOI of 104 rAAV particles per cell and maintained in differentiation medium, and the hFIX secreted was assayed by ELISA (Fig. 2A to D). Myotubes directly transduced with rAAV/Me4βAhFIXm1 secreted ∼14 ng of hFIX/106 cells/day, while myotubes transduced with rAAV/CMVhFIXm1 produced almost 15-fold more hFIX than did those transduced with rAAV/Me4βAhFIXm1, almost 200 ng of hFIX/106 cells/day toward the end of the 4 weeks of the experimental period (Fig. 2A and C). In these experiments, the cell numbers refer to myoblasts prior to differentiation. Similar results were also obtained from myotubes derived from myoblasts transduced prior to differentiation (Fig. 2B and D). In the latter case, rAAV/Me4βAhFIXm1- or rAAV/CMVhFIXm1-transduced cells secreted ∼30 or ∼300 ng of hFIX/106 cells/day, respectively, toward the end of experimental period. Production of hFIX from human myoblasts transfected with rAAV vectors also showed a relative difference in hFIX expression levels; i.e., pTR/CMVhFIXm1-transfected myoblasts produced fourfold more hFIX (15 ± 5 ng/ml; n = 4) than did the pTR/Me4βAhFIXm1-transfected myoblasts (4 ± 1 ng/ml; n = 4). These experiments suggested that the CMV promoter is substantially stronger than the Me4βA transcriptional unit in human muscle cells. This is consistent with observations made by others (1, 27, 28). The overall hFIX production level from rAAV/Me4βAhFIXm1-transduced human muscle cells was much lower than that from pdLMe4βAhFIXm1-transfected mouse myoblasts (∼750 ng of hFIX/106 cells/day) (68). This difference may be due in part to the source of enhancer-promoter used in Me4βA (the enhancer and promoter were of mouse and chicken origin, respectively) (69). Therefore, we used rAAV/CMVhFIXm1 for the following studies of the mechanisms responsible for rAAV transduction of human skeletal muscle cells and the transgene expression kinetics involved.
rAAV transduction of human myotubes and myoblasts results in similar and high hFIX production.
Human skeletal myotubes were transduced with rAAV/CMVhFIXm1 at a MOI of 1 × 104, 1 × 105, or 5 × 105 and maintained in differentiation medium (supplemented with 2.5% FBS) or in rich medium (F10- or SKGM-based medium) (Fig. 2C to N). Both F10- and SKGM-based media are routinely used as muscle cell growth media. We tested both to see if these different media have an effect on transgene expression. In addition, we chose to maintain cells throughout in differentiation medium because cells survive longer under these culture conditions, allowing us to extend the duration of transgene expression. As shown in Table 1 and Fig. 2C to N, a viral dose-response relationship was obtained from transduced myotubes maintained in the F10-based medium. These cells showed maximal hFIX production of ∼600, ∼800, or ∼1,700 ng of hFIX/106 cells/day when transduced at a MOI of 1 × 104, 1 × 105, or 5 × 105, respectively (Fig. 2E, I, and M; Table 1). Myotubes in differentiation medium produced lower levels of hFIX (∼200 ng of hFIX/106 cells/day) (Fig. 2C and G). Similar to the results with myotubes maintained in F10-based medium, the myotubes in SKGM-based medium also produced high levels of hFIX (Fig. 2K; Table 1). A similar dose-response relationship was also obtained from myotubes derived from myoblasts, which were transduced at 90% confluency prior to differentiation (Fig. 2D, F, H, J, L, and N; and Table 1). However, unlike direct transduction of myotubes, the expression of hFIX did not necessarily increase linearly. This may be in part due to the rAAV transduction process and subtle unknown effects of induction of differentiation, although further studies are required to find the reason. The overall maximal hFIX expression in these experiments was 1,500 to 1,900 ng of hFIX/106 cells/day (Table 1). This supports the high capability of skeletal muscle to express hFIX transgene, agreeing with previous observations in mouse muscle cells (68, 73).
TABLE 1.
Average maximal expression of hFIX from human myotubes
| MOI | Infection of myotubes (after differentiation)a:
|
Infection of cells prior to differentiationa:
|
||||
|---|---|---|---|---|---|---|
| 2.5% FBSb | F10-based mediumb | SKGM-based mediumb | 2.5% FBS | F10-based medium | SKGM-based medium | |
| 1 × 104 | 200 | 600 | NDc | 300 | 600 | ND |
| 1 × 105 | 200 | 800 | 1900 | 310 | 1,000 | 1,500 |
| 5 × 105 | ND | 1,700 | ND | ND | 1,300 | ND |
Total expression of hFIX in nanograms per 106 cells per day.
The cells were maintained in either of the three culture media as described in the text and Fig. 2.
ND, not done.
Expression of hFIX from rAAV-transduced human skeletal muscle cells occurs with no lag phase.
As shown in Fig. 2, the rAAV/CMVhFIXm1-transduced human myotubes showed no appreciable lag period but showed a linear increase in hFIX production (Fig. 2C, E, G, I, K, and M). In both mice and dogs intramuscularly injected with rAAV/FIX, a lag phase of about 2 weeks followed by a slow rise over 5 to 8 weeks before reaching stable constant levels is observed (27, 28). A similar lag phase was also reported in β-galactosidase production in mice intramuscularly injected with rAAV/β-gal (15). However, in some experiments, wherein rAAV/erythropoitin was intramuscularly delivered to mice, no such lag phase was observed and transgene expression was observed from as early as 4 days p.i. (61, 67). One possible explanation for the observed lag in detectable hFIX production in mice is a need to saturate hFIX binding sites on endothelial cells and vascular tissue prior to the appearance of detectable hFIX in the circulation (14, 27) whereas the in vitro culture assay system is not affected by such hFIX binding. Our observations with human muscle cells strongly support the notion that there is no appreciable initial lag phase in hFIX production and are consistent with the conceivable mechanism of slow but steady conversion of single- to double-stranded rAAV genome.
rAAV virions can copackage hFIX protein.
In some experiments, as shown in Fig. 2, rAAV/CMVhFIXm1-transduced muscle cells exhibited a high and transient hFIX production during the first 2 days of transduction. Some previous reports suggested that the CMV promoter has a differential activity in different tissues and at various stages of organogenesis and differentiation (7, 8, 59). The initial spike in hFIX levels is probably not due to myoblast differentiation-specific effects, because this transient expression was also observed in transduction of fully differentiated myotubes (Fig. 2C, E, I, and M). Since rAAV vectors can package either plus or minus strands (9), the single-stranded DNA of both polarities must be present in infected cells. Therefore, it is possible that some of these strands anneal and form transcriptionally active double-stranded forms. This scenario, however, provides only a partial explanation because of the transient nature of hFIX expression. The notion of carryover of the rAAV plasmid vector used for transfection of the 293 packaging cells to target cell transduction medium is inconceivable, because of the several specific steps involved in rAAV purification.
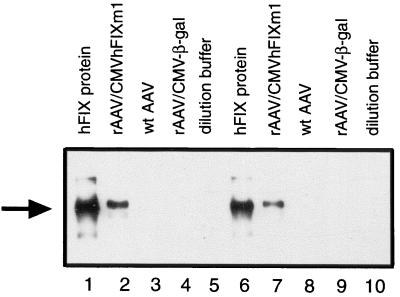
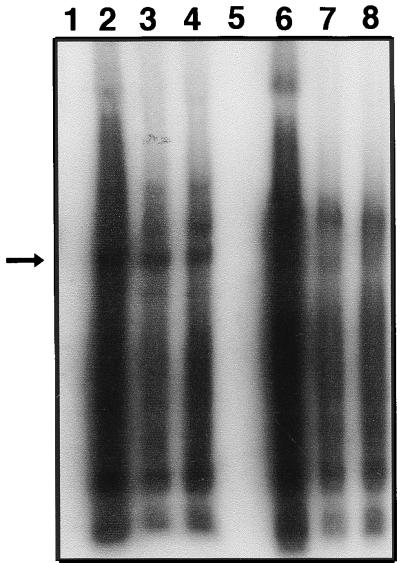
rAAV may nonspecifically trap transgene products, as previously suggested (4, 65). As shown in Fig. 2, the purified rAAV virions were not removed after 2 h of infection and the medium was made up to a total of 2 ml. Therefore, 1 day p.i., the medium still contained the rAAV virions that failed to infect. Using a quantitative dot blot assay and a CMV promoter probe, we showed that a substantial number of particles failed to infect cells and remained in the medium after 1 day (Table 2). These virions, if containing hFIX, might have contributed to the hFIX levels measured by ELISA. We directly tested rAAV/CMVhFIXm1 virions in an hFIX-specific ELISA. Dilutions of rAAV/CMVhFIXm1 virions at 1011, 1010, or 109 particles/ml gave an average of 2,282, 272, or 8 ng of hFIX/ml, respectively. Results of Western blot analysis of rAAV/CMVhFIXm1 virion proteins is shown in Fig. 3. Wild-type AAV-2 or rAAV/CMV-β-gal virions prepared by the same helper-free system as for rAAV/CMVhFIXm1 were used as controls along with the purified hFIX protein. rAAV/CMVhFIXm1 virion protein-containing lanes showed the anti-FIX reactive band of 56 kDa (lanes 2 and 7) at the same position as the plasma-derived hFIX controls (lanes 1 and 6). Protein preparations from rAAV/CMV-β-gal or wild-type AAV-2 virions or dilution buffer alone did not show any corresponding bands (lanes 3 to 5 and 8 to 10). These results strongly supported the notion that rAAV virions copackaged hFIX, and thus the transient hFIX expression at 1 day p.i. in Fig. 2 can be almost fully accounted for by hFIX protein contained in the rAAV virions and is not due to transgene expression from infected cells. We previously observed a transient partial correction of whole-blood clotting time in dogs after 1 day of intramuscular injection of rAAV/CMVhFIXm1 (46). This whole-blood clotting time correction could not be expected on the basis of hFIX transgene expression. We propose that such partial WBCT correction may be due to the delivery of hFIX protein copackaged in the rAAV virions.
TABLE 2.
Uninfected rAAV particles present in culture medium
| MOI | Cell type infected | Total no. of cellsa | Total no. of rAAV particles added | No. of particles remaining after 1 day | Total no. of infective particlesb | Effective MOIc |
|---|---|---|---|---|---|---|
| 104 | Myotubes | 4.4 × 105 | 4.4 × 109 | 0.8 × 109 | 3.6 × 109 | 8.1 × 103 |
| 104 | Myoblasts | 4.4 × 105 | 4.4 × 109 | 4 × 109 | 0.4 × 109 | 0.9 × 103 |
| 105 | Myotubes | 0.78 × 106 | 0.78 × 1011 | 0.9 × 1010 | 0.69 × 1011 | 8.8 × 104 |
| 105 | Myoblasts | 0.8 × 106 | 0.8 × 1011 | 0.3 × 1011 | 0.5 × 1011 | 6.2 × 104 |
For a MOI of 104, the cells were infected in six-well plates; the other experiments were done in 6-cm dishes.
Total infective particles is defined as a total rAAV added minus particles remaining after 1 day.
Effective MOI is defined as total infective particles divided by total number of cells.
FIG. 3.
Western blot analysis of rAAV virion-packaged proteins. rAAV preparations diluted to 1011 particles/ml were resolved on an SDS–10% polyacrylamide gel and electroblotted onto polyvinylidene difluoride membranes. The hFIX proteins on blots were visualized by the chemiluminescent detection system. Lanes 2 to 4 and 7 to 9 were loaded with 4 or 2 μl of rAAV vectors, respectively. Lanes 1 and 6 were loaded with 8 and 4 ng of purified plasma-derived hFIX protein, respectively. Lanes 2 and 7 were loaded with rAAV/CMVhFIXm1. Lanes 3 and 8 and lanes 4 and 9 were loaded with wild-type AAV or rAAV/CMV-β-gal, respectively. Lanes 5 and 10 contain the dilution buffer alone. The arrow indicates the position of the hFIX protein (56 kDa).
Integration of the rAAV transgene in cellular genomes does not correlate with transgene expression.
One of the major unresolved issues regarding the rAAV transduction mechanism is whether rAAV genome integration into the host cell genome is needed for transgene expression. The human muscle cell model described above provides a powerful system to study the mechanisms governing rAAV transduction, because the cell number (nucleus number) is effectively maintained unchanged upon differentiation and thus any alterations observed in the rAAV genome in these cells can be directly correlated to the changes in transgene expression. Furthermore, it permits us to analyze actively proliferating cells and terminally differentiated cells derived from them.
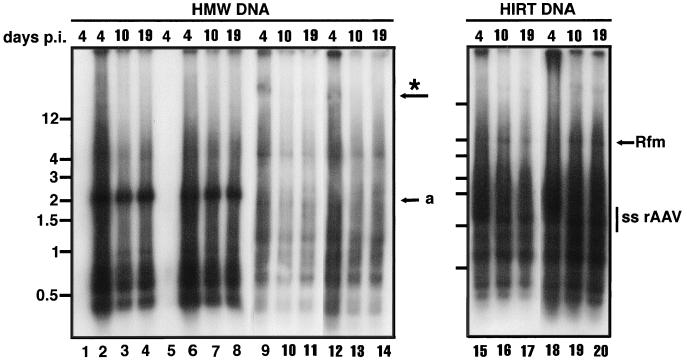
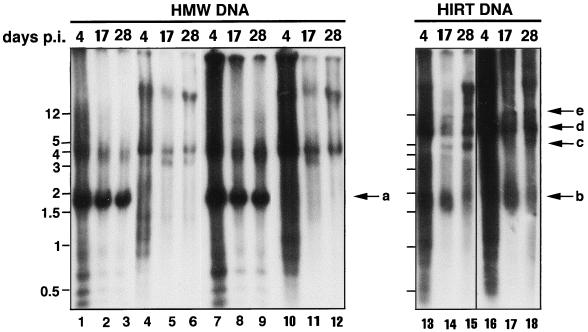
We isolated cellular genomic (HMW) DNA from rAAV/CMVhFIXm1-transduced myotubes that were infected at a MOI of 105 and maintained in F10-based rich medium (Fig. 2I). Presumably, this fraction may also contain some very large concatameric forms of the rAAV vector. The HMW DNA was subjected to Southern blot analysis using a CMV promoter-specific probe (Fig. 1). As expected, this probe showed strict transgene specificity, and no hybridization was seen with the DNA isolated from uninfected myotubes (Fig. 4, lane 5). To compare the amounts of rAAV genomes in HMW DNA fractions at various times after transduction, the HMW DNA were isolated from myotubes at 4, 10, and 19 days p.i. and digested with EcoRI, which cleaves at the 5′ end of the promoter and within the hFIX intron to release a 1.9-kb band (Fig. 1 and 4). As shown in Fig. 4 (lane 6), transgenes were clearly present in HMW DNA on day 4 p.i., the earliest time point analyzed in this study. The amount of transgene released from HMW DNA on day 10 p.i. was not significantly different from that obtained from the HMW DNA on day 19 p.i. (lanes 7 and 8). To rule out a culture medium-specific effect, HMW DNA from transduced myotubes, which were maintained in differentiation medium, was also analyzed (Fig. 2G). As expected, the undigested HMW DNA from transduced myotubes hybridized with the CMV promoter probe showed the presence of transgene in DNA larger than 12 kb (Fig. 5, lanes 10 to 12). Similar to the above results, the amount of transgenes released from HMW DNA on day 17 p.i. was not significantly different from the amount released on day 28 p.i. in the transduced myotubes (lanes 8 and 9).
FIG. 4.
Southern blot analysis of DNA isolated from rAAV/CMVhFIXm1-transduced cells. HMW and Hirt DNA were isolated from muscle cells transduced at a MOI of 105 and maintained in F10-based medium (Fig. 2I and J). HMW or Hirt DNA was isolated 4 days p.i. (lanes 2, 6, 9, 12, 15, and 18), 10 days p.i. (lanes 3, 7, 10, 13, 16, and 19), or 19 days p.i. (lanes 4, 8, 11, 14, 17, and 20). Lanes: 2 to 4 and 9 to 11, HMW DNA from cells transduced at the myoblast stage; 6 to 8 and 12 to 14, HMW DNA from cells transduced at the myotube stage; 1 and 5 respective mock-infected control DNA isolated on day 4. HMW DNA (4 μg) was digested with EcoRI and subjected to Southern blot analysis with the CMV promoter probe. Lanes 1 to 8 contain EcoRI-digested HMW DNAs, and lanes 9 to 14 contain undigested HMW DNAs. Arrow a indicates the position of the 1.9-kb internal EcoRI fragment. The possible position for the >12-kb genomic DNA signal, more prominant in Fig. 5, is shown by an asterisk. Lanes 15 to 17 contain Hirt DNA from cells transduced at the myoblast stage; lanes 18 to 20 contain Hirt DNA from cells transduced at the myotube stage. Hirt DNA isolated from cells cultured in the rich medium tends to give less discernible bands, and undigested or digested genomic DNA show bands of >4-kb (lanes 9 to 14) and <1.9 kb (lanes 2 to 4 and 6 to 8), respectively. These bands, however, are not seen when genomic DNA prepared from rAAV-transduced cells was grown in differentiation medium (see Fig. 5). At present, little is known about why genomic DNA from cells maintained in rich medium with much active metabolism tends to have these bands.
FIG. 5.
Southern blot analysis of DNA isolated from rAAV/CMVhFIXm1-transduced cells. HMW and Hirt DNA was isolated from muscle cells transduced at a MOI of 105 and maintained in differentiation medium (Fig. 2G and H). HMW or Hirt DNA was isolated 4 days p.i. (lanes 1, 4, 7, 10, 13, and 16), 17 days p.i. (lanes 2, 5, 8, 11, 14, and 17), or 28 days p.i. (lanes 3, 6, 9, 12, 15, and 18). Lanes: 1 to 6, HMW DNA from cells transduced at the myoblast stage; 7 to 12, HMW DNA from cells transduced at the myotube stage. HMW DNA samples (10 μg) were digested with EcoRI and subjected to Southern blot analysis with the CMV promoter probe. The undigested samples are shown in lanes 4 to 6 and 10 to 12. The Hirt DNA profile of rAAV/CMVhFIXm1-transduced cells is shown in lanes 13 to 18. Lanes: 13 to 15, Hirt DNA from cells transduced at the myoblast stage; 16 to 18, Hirt DNA from cells transduced at the myotube stage. Arrow a indicates the position of the 1.9-kb internal EcoRI fragment. Arrow b indicates the single-stranded rAAV genomes, and arrows c, d, and e indicate possible double-stranded forms of rAAV. Determination of which band refers to monomer, dimer, or circular forms has yet to be done. The source of faint bands migrating at <1.9 kb in some digested genomic DNA samples (lanes 1 to 3 and 7 to 9) is not known. Since these <1.9-kb and ∼4-kb bands appear resistant, at least partially, to restriction digestion, they may be some trapped single-stranded DNAs.
HMW DNA isolated from cells transduced prior to differentiation was also analyzed (Fig. 2H and J). HMW DNA isolated from cells which were transduced prior to differentiation and the resulting myotubes maintained in differentiation medium showed transgene bands larger than 12 kb (Fig. 5, lanes 4 to 6), similar to those obtained with HMW DNA isolated from myotubes that were directly transduced (lanes 10 to 12). Importantly, the transgene amount on day 28 p.i. was not significantly different from that on day 17 p.i. (lanes 2 and 3). On the other hand, the results obtained from the analysis of HMW DNA isolated from cells that were transduced prior to differentiation and maintained in F10-based rich medium showed a somewhat nonlinear increase in hFIX expression (Fig. 2J and Fig. 4, lanes 1 to 4). Nevertheless, the observations from directly transduced myotubes and those from cells transduced prior to differentiation and maintained in differentiation medium suggested that the amount of vector genomes in HMW DNA (presumably containing integrated rAAV genomes) does not correlate with the transgene expression level in human muscle cells. Thus, the amount of HMW transgene, often used as an important indicator of increasing and persistent transgene expression, appears not to be a valid parameter for increasing transgene expression in human muscle cells.
The HMW DNA fraction contains head-to-tail junctions indicative of integrated and/or concatameric forms of rAAV.
The HMW DNA fraction may contain integrated as well as some large concatameric forms of rAAV and/or small amounts of trapped episomal forms. To show if a junction fragment could be released from the HMW DNA, we digested HMW DNA with an enzyme with a single recognition site, BstBI, and analyzed it by Southern blotting using the CMV promoter probe as described above. The head-to-tail, head-to-head, or tail-to-tail junctions should release a 4-, 6.3-, and 1.8-kb fragment, respectively, and the end fragment should be detected as a 3.1-kb band upon BstBI digestion (Fig. 1). As shown in Fig. 6, we detected only the 4-kb fragment, which is indicative of a head-to-tail junction (lanes 2 to 4). Such junctions are most probably released from the integrated and/or cellular genome-associated large concatameric forms of rAAV. Although it may not be significant, the possible presence of some circular episomes trapped in the HMW DNA fraction cannot be ruled out at this stage of study.
FIG. 6.
Detection of head-to-tail junctions in HMW DNA. HMW DNA (4 μg) isolated from transduced myotubes (Fig. 2I) were digested with BstBI (lanes 1 to 4) or NotI (lanes 5 to 8) and analyzed by Southern blot analysis with the CMV promoter-specific probe. HMW DNA was isolated 4 days p.i. (lanes 2 and 6), 10 days p.i. (lanes 3 and 7), or 19 days p.i. (lanes 4 and 8). Lanes 1 and 5 contain DNA from mock-infected controls. BstBI recognizes a single site in the vector. The arrow indicates the expected head-to-tail junction. No NotI site exists in the vector. NotI digestion did not release any distinct band and showed a profile similar to that of undigested samples (Fig. 4, lanes 9 to 14).
Upon reprobing of the blots (Fig. 5, HMW DNA) containing EcoRI-digested fragments of transduced myoblasts or myotubes with the rAAV ITR probe, a strong band between 1.5 and 2 kb was observed, which may arise from the free right end of a linear DNA and/or head-to-tail junction of integrated, concatameric, or episomal DNA (data not shown). Since free linear forms are unlikely to be isolated in the HMW DNA fraction, we conclude that both rAAV concatamers (at least those of four units, since the hybridization with undigested genomic DNA was seen in DNA larger than 12 kb [Fig. 4, lanes 9 to 14, and Fig. 5, lanes 4 to 6 and 10 to 12]) and circular episomal forms may be present in the HMW fraction. However, as shown above, these forms do not increase concurrently with transgene expression (Fig. 4 and 5). Thus, these results argue against the proposed mechanism wherein ongoing amplification of the transgene sequences in integrated or episomal concatamers is the primary mechanism leading to a steady and long-term increase in expression.
Some groups have shown integration of rAAV by the isolation of cellular-rAAV DNA junctions (references 48, 55, and 70 and references therein). These analyses have shown that integration of rAAV is a low frequency event. Pulsed-field gel electrophoresis and fluorescent in situ hybridization analyses have also been performed to demonstrate chromosome-associated rAAV (23, 44). Such analysis remains to be done on HMW DNA in our human muscle cell assay system.
The conversion of single-stranded vector genomes to double-stranded DNA forms in Hirt DNA, but not the stably integrated form, correlates with the expression of transgene.
The slow but linear rise in hFIX level (in transduced myotubes in this study) suggests that the pathway for conversion of the single-stranded input viral genome to the transcriptionally active form may be limited by several steps. The conversion of single-stranded input vector to a double-stranded form is suggested to be one of the primary rate-limiting steps (21, 22). To determine if conversion of the single-stranded input viral genome to episomal forms is responsible for the slow linear rise in transgene expression in myotubes, we analyzed the Hirt DNA fraction. The HMW DNA was isolated by spooling, and the remaining DNA was considered the Hirt fraction (see Materials and Methods). The Hirt DNA was fractionated on an agarose gel and subjected to Southern blot analysis using a CMV promoter-specific probe.
The Southern blot analysis of Hirt DNA isolated from cells maintained in F10-based medium (Fig. 2I and J) is shown in Fig. 4 (lanes 15 to 20). A smear of hybridization, which is typically observed at the early stage in mice intramuscularly injected with rAAV (15, 67), was seen at 4 days p.i. both in transduced myotubes and when cells were transduced prior to differentiation (Fig. 4, lanes 15 and 18). This smear, representing the single-stranded input rAAV vector (marked as ss rAAV), resolved into more distinct bands on later days and the double-stranded monomer Rf form appeared (lanes 16, 17, 19 and 20). These results obtained with human muscle cells are generally consistent with previously published observations with other cell types, which show slow conversion of single-stranded input rAAV vector to the double-stranded monomer Rf form (3, 21, 22).
A correlation between the slow conversion of single-stranded input rAAV vector to double-stranded DNA forms was clearly visible when the Hirt DNA isolated from cells maintained in differentiation medium was analyzed (Fig. 2G and H). As shown in Fig. 5, a smear of hybridization was seen in transduced myotubes after 4 days, which resolved into distinct bands by day 17 (lanes 16 and 17). When the Hirt DNA profiles in day 17 (lane 17) and day 28 (lane 18) samples were compared, the smear representing single-stranded vector DNA slowly disappeared and double-stranded DNA forms (linear monomers, dimers, higher forms, and possibly circular episomal forms) concomitantly increased in amount. Similar results were obtained when Hirt DNA from cells that were transduced prior to differentiation was analyzed (lanes 13 to 15). PhosphorImager analysis suggested a decrease of 20 to 30% in single-stranded vector genomes and an increase in double-stranded monomer and dimer forms of 100 to 200% from days 17 to 28 (Fig. 5, compared lane 14 to 15 or lane 17 to 18). In transduced myotubes, the levels of hFIX increased from 128 to 168 ng of hFIX/106 cells/day, and in cells transduced prior to differentiation, the increase in expression was from 242 to 316 ng of hFIX/106 cells/day for the period from days 17 to 28 p.i. (Fig. 2G and H). In the former case, the levels did rise to ∼200 ng of hFIX/106 cells/day between days 24 and 25, and the apparent fall at the end is in part due to loss of cells by detachment. Together, these results indicated that the increase in the amount double-stranded forms of rAAV but not host cell genome-integrated forms correlates with the increase in expression of the hFIX transgene.
Possible significance of nonintegrated rAAV in transgene expression in muscle.
Several reports have concluded that the integrated from of rAAV is responsible for the transgene expression and persistence in muscle (15, 23, 27, 71). In these studies, the head-to-tail concatamers of rAAV often detected by PCR or Southern blotting were taken as indicators of integrated rAAV (15, 23, 27, 71). Miao et al. (44) reached a similar conclusion in studies of transduced mouse liver. Up to 70-copy concatamers of rAAV were found by pulsed-field gel electrophoresis and fluorescent in situ hybridization of metaphase or interphase chromosome in transduced mouse liver, suggesting the presence of an integrated form of rAAV (44). Although no data exist so far, others have raised the possibility that rAAV concatamers are not integrated but are in a tight association with cellular DNA and/or nuclear matrix (2, 26, 67). An expected feature of rAAV genome maturation, which is generally observed, is the gradual loss of single-stranded DNA in transduced mouse muscle (15, 67). Clark et al. (15) observed increasing transgene expression correlating with increasing levels of transgenes in genomic DNA fraction (presumably integrated), whereas Vincent-Lacaze et al. (67) found a somewhat inverse relationship. Such conflicting observations demonstrate the difficulty in performing a reliable analysis of DNA isolated from tissues of animals. The site of rAAV injection and isolation of vector after the animal has aged for a period will introduce numerous variables, including organ growth and the possibility that cells processed for DNA do not truly represent the site of injection.
We developed a human muscle cell-based assay system to study the kinetics and molecular events involved in rAAV transduction and transgene expression. Our observations support the notion that the rAAV transgene level in the genomic DNA (HMW) fraction does not correlate with transgene expression level during the period of linear increase of transgene expression. At this stage, however, we cannot completely rule out the possibility that HMW fraction transgenes are responsible, at least in part, for long-term persistent hFIX expression in animals (27, 28). We further showed that the recruitment of single-stranded rAAV to double-stranded forms (linear or circular episomal) correlates with expression.
The importance of episomal forms is increasingly recognized as the dominant factor in rAAV-mediated expression and persistence. In one of the earliest studies, rAAV genomes were present as approximately 8 kb (double-stranded dimer size) in Hirt DNA and none were detected in the genomic DNA fraction isolated 14 days p.i. from a rAAV/β-gal or rAAV/neo-transduced IB3-1 cell line, a tetraploid human cystic fibrosis bronchial epithelial cell line (25). Similar results were obtained in vivo from rAAV-transduced primary bronchial epithelial cells isolated from monkeys (2). These experiments did not rule out the possibility that the 8-kb DNA represents only double-stranded dimer forms and/or episomal forms (2, 25). The episomal forms of rAAV may contain linear or circular double-stranded monomers, dimers, or higher forms. Not surprisingly, circular episomal forms have been selectively rescued from transduced mouse muscle (19). Our experiments with the human muscle cell assay system suggest that the unintegrated low-molecular-weight episomal rAAV forms may be primarily responsible for transgene expression and possibly play a critical role in long-term persistence of expression. Wild-type AAV or rAAV vectors require Rep68 or Rep78 protein for site-specific integration (50, 64). The plasmids containing ITR elements exhibit long-term persistence in transfected cells (19, 49, 66). Thus, the rAAV episomal forms, unable to efficiently integrate in the absence of Rep68 and Rep78, may stably persist in transduced human myotubes and function as the predominant source of transcriptionally active DNA. However, our results do not rule out the possibility that some of these rAAV episomes are further converted into higher concatmeric forms and/or integrate into genomic DNA, which may also contribute to the lifelong persistence of transgene expression observed in mice. Various rAAV episomal forms in the Hirt DNA fraction remain to be analyzed for a specific fraction(s) responsible for hFIX expression. In summary, we have successfully developed a human muscle cell assay system for further dissecting the transduction mechanisms of rAAV.
ACKNOWLEDGEMENTS
This work was supported by grants to K.K. from NIH (HL53713) and TKT Inc., Boston, Mass., and to R.J.S. from NIH (DK54419-01), the Multipurpose Arthritis Center of the University of Michigan (P60-AR20557), and the Comprehensive Cancer Center of the University of Michigan (P30-CA46592). A.K.M. is a Fellow of the American Heart Association. P.E.M. is supported by a Judith Graham Pool Fellowship from National Hemophilia Foundation and by NIH (HL03960-01).
We thank Jeff Bartlett (University of Florida, Gainesville, Fla.) and Julie Chappen (Roche, Indianapolis, Ind.) for sharing the information regarding the use of histone H1 for human myoblast transfection. We also thank J. S. Huo for critical reading of the manuscript.
REFERENCES
- 1.Addison C L, Hitt M, Kunsken D, Graham F L. Comparison of the human versus murine cytomegalovirus immediate early gene promoters for transgene expression by adenoviral vectors. J Gen Virol. 1997;78:1653–1661. doi: 10.1099/0022-1317-78-7-1653. [DOI] [PubMed] [Google Scholar]
- 2.Afione S A, Conrad C K, Kearns W G, Chunduru S, Adams R, Reynolds T C, Guggino W B, Cutting G R, Carter B J, Flotte T R. In vivo model of adeno-associated virus vector persistence and rescue. J Virol. 1996;70:3235–3241. doi: 10.1128/jvi.70.5.3235-3241.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Afione S A, Wang J, Walsh S, Guggino W B, Flotte T R. Delayed expression of adeno-associated virus vector DNA. Intervirology. 1999;42:213–220. doi: 10.1159/000024980. [DOI] [PubMed] [Google Scholar]
- 4.Alexander I E, Russell D W, Miller A D. Transfer of contaminants in adeno-associated virus vector stocks can mimic transduction and lead to artifactual results. Hum Gene Ther. 1997;8:1911–1920. doi: 10.1089/hum.1997.8.16-1911. [DOI] [PubMed] [Google Scholar]
- 5.Atchinson R W, Casto B C, Hammon W M. Adenovirus-associated defective virus particles. Science. 1965;149:754–756. doi: 10.1126/science.149.3685.754. [DOI] [PubMed] [Google Scholar]
- 6.Barr E, Leiden J M. Systemic delivery of recombinant proteins by genetically modified myoblasts. Science. 1991;254:1507–1509. doi: 10.1126/science.1962212. [DOI] [PubMed] [Google Scholar]
- 7.Baskar J F, Smith P P, Ciment G S, Hoffmann S, Tucker C, Tenney D J, Colberg-Poley A M, Nelson J A, Ghazal P. Developmental analysis of the cytomegalovirus enhancer in transgenic animals. J Virol. 1996;70:3215–3226. doi: 10.1128/jvi.70.5.3215-3226.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baskar J F, Smith P P, Nilaver G, Jupp R A, Hoffmann S, Peffer N J, Tenney D J, Colberg-Poley A M, Ghazal P, Nelson J A. The enhancer domain of the human cytomegalovirus major immediate-early promoter determines cell type-specific expression in transgenic mice. J Virol. 1996;70:3207–3214. doi: 10.1128/jvi.70.5.3207-3214.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berns K I, Rose J A. Evidence for a single-stranded adenovirus-associated virus genome: isolation and separation of complementary single strands. J Virol. 1970;5:693–699. doi: 10.1128/jvi.5.6.693-699.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blacklow N R, Hoggan M D, Kapikian A Z, Austin J B, Rowe W P. Epidemiology of adenovirus-associated virus infection in a nursery population. Am J Epidemiol. 1968;88:368–378. doi: 10.1093/oxfordjournals.aje.a120897. [DOI] [PubMed] [Google Scholar]
- 11.Blacklow N R, Hoggan M D, Rowe W P. Serologic evidence for human infection with adenovirus-associated viruses. J Natl Cancer Inst. 1968;40:19–27. [PubMed] [Google Scholar]
- 12.Buller R M, Janik J E, Sebring E D, Rose J A. Herpes simplex virus types 1 and 2 completely help adenovirus-associated virus replication. J Virol. 1981;40:241–247. doi: 10.1128/jvi.40.1.241-247.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheung A K M, Hoggan M D, Hauswirth W W, Berns K I. Integration of the adeno-associated virus genome into cellular DNA in latently infected human Detroit 6 cells. J Virol. 1980;33:739–748. doi: 10.1128/jvi.33.2.739-748.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheung W-F, van den Born J, Kühn K, Kjellén L, Hudson B G, Stafford D W. Identification of the endothelial cell binding site for factor IX. Proc Natl Acad Sci USA. 1996;93:11068–11073. doi: 10.1073/pnas.93.20.11068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clark K R, Sferra T J, Johnson P R. Recombinant adeno-associated viral vectors mediate long-term transgene expression in muscle. Hum Gene Ther. 1997;8:659–669. doi: 10.1089/hum.1997.8.6-659. [DOI] [PubMed] [Google Scholar]
- 16.Dai Y, Roman M, Naviaux R K, Verma I M. Gene therapy via primary myoblasts: long-term expression of factor IX protein following transplantation in vivo. Proc Natl Acad Sci USA. 1992;89:10892–10895. doi: 10.1073/pnas.89.22.10892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davie E W, Ratnoff O D. Waterfall sequence for intrinsic blood clotting. Science. 1964;145:1310–1312. doi: 10.1126/science.145.3638.1310. [DOI] [PubMed] [Google Scholar]
- 18.Dhawan J, Pan L C, Pavlath G K, Travis M A, Lanctot A M, Blau H M. Systemic delivery of human growth hormone by injection of genetically engineered myoblasts. Science. 1991;254:1509–1512. doi: 10.1126/science.1962213. [DOI] [PubMed] [Google Scholar]
- 19.Duan D, Sharma P, Yang J, Yue Y, Dudus L, Zhang Y, Fisher K J, Engelhardt J F. Circular intermediates of recombinant adeno-associated virus have defined structural characteristics responsible for long-term episomal persistence in muscle tissue. J Virol. 1998;72:8568–8577. doi: 10.1128/jvi.72.11.8568-8577.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.During M J, Xu R, Young D, Kaplitt M G, Sherwin R S, Leone P. Peroral gene therapy of lactose intolerance using an adeno-associated virus vector. Nat Med. 1998;4:1131–1135. doi: 10.1038/2625. [DOI] [PubMed] [Google Scholar]
- 21.Ferrari F K, Samulski T, Shenk T, Samulski R J. Second-strand synthesis is a rate-limiting step for efficient transduction by recombinant adeno-associated virus vectors. J Virol. 1996;70:3227–3234. doi: 10.1128/jvi.70.5.3227-3234.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fisher K J, Gao G-P, Weitzman M D, DeMatteo R, Burda J F, Wilson J M. Transduction with recombinant adeno-associated virus for gene therapy is limited by leading-strand synthesis. J Virol. 1996;70:520–532. doi: 10.1128/jvi.70.1.520-532.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fisher K J, Jooss K, Alston J, Yang Y, Haecker S E, High K, Pathak R, Raper S E, Wilson J M. Recombinant adeno-associated virus for muscle directed gene therapy. Nat Med. 1997;3:306–312. doi: 10.1038/nm0397-306. [DOI] [PubMed] [Google Scholar]
- 24.Flotte T R, Afione S A, Solow R, Drumm M L, Markakis D, Guggino W B, Zeitlin P L, Carter B J. Expression of the cystic fibrosis transmembrane conductance regulator from a novel adeno-associated virus promoter. J Biol Chem. 1993;268:3781–3790. [PubMed] [Google Scholar]
- 25.Flotte T R, Afione S A, Zeitlin P L. Adeno-associated virus vector gene expression occurs in nondividing cells in the absence of vector DNA integration. Am J Respir Cell Mol Biol. 1994;11:517–521. doi: 10.1165/ajrcmb.11.5.7946381. [DOI] [PubMed] [Google Scholar]
- 26.Hargrove P W, Vanin E F, Kurtzman G J, Nienhuis A W. High-level globin gene expression mediated by a recombinant adeno-associated virus genome that contains the 3′ gamma globin gene regulatory element and integrates as tandem copies in erythroid cells. Blood. 1997;89:2167–2175. [PubMed] [Google Scholar]
- 27.Herzog R W, Hagstrom J N, Kung S-H, Tai S J, Wilson J M, Fisher K J, High K A. Stable gene transfer and expression of human blood coagulation factor IX after intramuscular injection of recombinant adeno-associated virus. Proc Natl Acad Sci USA. 1997;94:5804–5809. doi: 10.1073/pnas.94.11.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herzog R W, Yang E Y, Couto L B, Hagstrom J N, Elwell D, Fields P A, Burton M, Bellinger D A, Read M S, Brinkhous K M, Podsakoff G M, Nichols T C, Kurtzman G J, High K A. Long-term correction of canine hemophilia B by gene transfer of blood coagulation factor IX mediated by adeno-associated viral vector. Nat Med. 1999;5:56–63. doi: 10.1038/4743. [DOI] [PubMed] [Google Scholar]
- 29.Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967;26:365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- 30.Hoggan M D, Blacklow N R, Rowe W P. Studies of small DNA viruses found in various adenovirus preparations: physical, biological and immunological characteristics. Proc Natl Acad Sci USA. 1966;55:1467–1474. doi: 10.1073/pnas.55.6.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jooss K, Yang Y, Fisher K J, Wilson J M. Transduction of dendritic cells by DNA viral vectors directs the immune response to transgene products in muscle fibers. J Virol. 1998;72:4212–4223. doi: 10.1128/jvi.72.5.4212-4223.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaplitt M G, Leone P, Samulski R J, Xiao X, Pfaff D W, O'Malley K L, During M J. Long-term gene expression and phenotypic correction using adeno-associated virus vectors in the mammalian brain. Nat Genet. 1994;8:148–154. doi: 10.1038/ng1094-148. [DOI] [PubMed] [Google Scholar]
- 33.Kearns W G, Afione S A, Fulmer S B, Pang M G, Erikson D, Egan M, Landrum M J, Flotte T R, Cutting G R. Recombinant adeno-associated virus (AAV-CFTR) vectors do not integrate in a site-specific fashion in an immortalized epithelial cell line. Gene Ther. 1996;3:748–755. [PubMed] [Google Scholar]
- 34.Kessler P D, Podsakoff G M, Chen X, McQuiston S A, Colosi P C, Matelis L A, Kurtzman G J, Byrne B J. Gene delivery to skeletal muscle results in sustained expression and systemic delivery of a therapeutic protein. Proc Natl Acad Sci USA. 1996;93:14082–14087. doi: 10.1073/pnas.93.24.14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koeberl D D, Alexander I E, Halbert C L, Russell D W, Miller A D. Persistent expression of human clotting factor IX from mouse liver after intravenous injection of adeno-associated virus vectors. Proc Natl Acad Sci USA. 1997;94:1426–1431. doi: 10.1073/pnas.94.4.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kotin R M, Siniscalco M, Samulski R J, Zhu X, Hunter L, Laughlin C A, McLaughlin S, Muzyczka N, Rocchi M, Berns K I. Site-specific integration by adeno-associated virus. Proc Natl Acad Sci USA. 1990;87:2211–2215. doi: 10.1073/pnas.87.6.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kurachi K, Kurachi S, Furukawa M, Yao S-N. Biology of factor IX. Blood Coagul Fibrinolysis. 1993;4:953–974. [PubMed] [Google Scholar]
- 38.Kurachi S, Hitomi Y, Furukawa M, Kurachi K. Role of intron I in expression of the human factor IX gene. J Biol Chem. 1995;270:5276–5281. doi: 10.1074/jbc.270.10.5276. [DOI] [PubMed] [Google Scholar]
- 39.Li J, Samulski R J, Xiao X. Role for highly regulated rep gene expression in adeno-associated virus vector production. J Virol. 1997;71:5236–5243. doi: 10.1128/jvi.71.7.5236-5243.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Linden R M, Wincour E, Berns K I. The recombination signals for adeno-associated virus site-specific integration. Proc Natl Acad Sci USA. 1996;93:7966–7972. doi: 10.1073/pnas.93.15.7966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Macfarlane R G. An enzyme cascade in the blood clotting mechanism, and its function as a biochemical amplifier. Nature. 1964;202:498–499. doi: 10.1038/202498a0. [DOI] [PubMed] [Google Scholar]
- 42.Malik A K, Weller S K. Use of transdominant mutants of the origin binding protein (UL9) of herpes simplex virus type-1 to define functional domains. J Virol. 1996;70:7859–7866. doi: 10.1128/jvi.70.11.7859-7866.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McLaughlin S K, Collis P, Hermonat P L, Muzyczka N. Adeno-associated virus general transduction vectors: analysis of proviral structures. J Virol. 1988;62:1963–1973. doi: 10.1128/jvi.62.6.1963-1973.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miao C H, Snyder R O, Schowalter D B, Patijn G A, Donahue B, Winther B, Kay M A. The kinetics of rAAV integration in the liver. Nat Genet. 1998;19:13–14. doi: 10.1038/ng0598-13. [DOI] [PubMed] [Google Scholar]
- 45.Miller S A, Dykes D D, Polesky H F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Monahan P E, Tazelaar J, Xiao X, Nichols T C, Bellinger D A, Read M S, Walsh C E, Samulski R J. Direct intramuscular injection with recombinant AAV vectors results in sustained expression in a dog model of hemophilia. Gene Ther. 1998;5:40–49. doi: 10.1038/sj.gt.3300548. [DOI] [PubMed] [Google Scholar]
- 47.Muzyczka N. Use of adeno-associated virus as a general transduction vector for mammalian cells. Curr Top Microbiol Immunol. 1992;158:97–129. doi: 10.1007/978-3-642-75608-5_5. [DOI] [PubMed] [Google Scholar]
- 48.Nakai H, Iwaki Y, Kay M A, Couto L B. Isolation of recombinant adeno-associated virus vector-cellular DNA junctions from mouse liver. J Virol. 1999;73:5438–5447. doi: 10.1128/jvi.73.7.5438-5447.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Philip R, Brunette E, Kilinski L, Murugesh D, McNally M A, Ucar K, Rosenblatt J, Okarma T B, Lebkowski J S. Efficient and sustained gene expression in primary T lymphocytes and primary and cultured tumor cells mediated by adeno-associated virus plasmid DNA complexed to cationic liposomes. Mol Cell Biol. 1994;14:2411–2418. doi: 10.1128/mcb.14.4.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pieroni L, Fipaldini C, Monciotti A, Cimini D, Sgura A, Fattori E, Epifano O, Cortese R, Palombo F, La Monica N. Targeted integration of adeno-associated virus-derived plasmids in transfected human cells. Virology. 1998;249:249–259. doi: 10.1006/viro.1998.9332. [DOI] [PubMed] [Google Scholar]
- 51.Ponnazhagan S, Erikson D, Kearns W G, Zhou S Z, Nahreini P, Wang X-S, Srivastava A. Lack of site-specific integration of the recombinant adeno-associated virus 2 genomes in human cells. Hum Gene Ther. 1997;8:275–284. doi: 10.1089/hum.1997.8.3-275. [DOI] [PubMed] [Google Scholar]
- 52.Qing K, Mah C, Hansen J, Zhou S, Dwarki V, Srivastava A. Human fibroblast growth factor receptor 1 is a co-receptor for infection by adeno-associated virus 2. Nat Med. 1999;5:71–77. doi: 10.1038/4758. [DOI] [PubMed] [Google Scholar]
- 53.Rivadeneira E D, Popescu N C, Zimonjic D B, Cheng G S, Nelson P J, Ross M D, DiPaolo J A, Klotman M E. Sites of recombinant adeno-associated virus integration. Int J Oncol. 1998;12:805–810. doi: 10.3892/ijo.12.4.805. [DOI] [PubMed] [Google Scholar]
- 54.Russell D W, Kay M A. Adeno-associated virus vectors and hematology. Blood. 1999;94:864–874. [PMC free article] [PubMed] [Google Scholar]
- 55.Rutledge E A, Russell D W. Adeno-associated virus vector integration junctions. J Virol. 1997;71:8429–8436. doi: 10.1128/jvi.71.11.8429-8436.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 57.Samulski R J, Chang L S, Shenk T. Helper-free stocks of recombinant adeno-associated viruses: normal integration does not require viral gene expression. J Virol. 1989;63:3822–3828. doi: 10.1128/jvi.63.9.3822-3828.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Samulski R J, Zhu X, Xiao X, Brook J D, Housman D E, Epstein N, Hunter L A. Targeted integration of adeno-associated virus (AAV) into human chromosome 19. EMBO J. 1991;10:3941–3950. doi: 10.1002/j.1460-2075.1991.tb04964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shering A F, Bain D, Stewart K, Epstein A L, Castro M G, Wilkinson G W G, Lowenstein P R. Cell type-specific expression in brain cell cultures from a short human cytomegalovirus major immediate early promoter depends on whether it is inserted into herpesvirus or adenovirus vectors. J Gen Virol. 1997;78:445–459. doi: 10.1099/0022-1317-78-2-445. [DOI] [PubMed] [Google Scholar]
- 60.Snyder R O, Miao C H, Patijn G A, Spratt S K, Danos O, Nagy D, Gown A M, Winther B, Meuse L, Cohen L K, Thompson A R, Kay M A. Persistent and therapeutic concentrations of human factor IX in mice after hepatic gene transfer of recombinant AAV vectors. Nat Genet. 1997;16:270–276. doi: 10.1038/ng0797-270. [DOI] [PubMed] [Google Scholar]
- 61.Snyder R O, Spratt S K, Lagarde C, Bohl D, Kasper B, Sloan B, Cohen L K, Danos O. Efficient and stable adeno-associated virus-mediated transduction in the skeletal muscle of adult immunocompetent mice. Hum Gene Ther. 1997;8:1891–1900. doi: 10.1089/hum.1997.8.16-1891. [DOI] [PubMed] [Google Scholar]
- 62.Summerford C, Bartlett J S, Samulski R J. αVβ5 integrin: a co-receptor for adeno-associated virus type 2 infection. Nat Med. 1999;5:78–82. doi: 10.1038/4768. [DOI] [PubMed] [Google Scholar]
- 63.Summerford C, Samulski R J. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J Virol. 1998;72:1438–1445. doi: 10.1128/jvi.72.2.1438-1445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Surosky R T, Urabe M, Godwin S G, McQuiston S A, Kurtzman G J, Ozawa K, Natsoulis G. Adeno-associated virus Rep proteins target DNA sequences to a unique locus in the human genome. J Virol. 1997;71:7951–7959. doi: 10.1128/jvi.71.10.7951-7959.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tenenbaum L, Hamdane M, Pouzet M, Avalosse B, Stathopoulos A, Jurysta F, Rosenbaum C, Hanemann C O, Levivier M, Velu T. Cellular contaminants of adeno-associated virus vector stocks can enhance transduction. Gene Ther. 1999;6:1045–1053. doi: 10.1038/sj.gt.3300904. [DOI] [PubMed] [Google Scholar]
- 66.Vieweg J, Boczkowski D, Roberson K M, Edwards D W, Philip M, Philip R, Rudoll T, Smith C, Robertson C, Gilboa E. Efficient gene transfer with adeno-associated virus-based plasmids complexed to cationic liposomes for gene therapy of human prostate cancer. Cancer Res. 1995;55:2366–2372. [PubMed] [Google Scholar]
- 67.Vincent-Lacaze N, Snyder R O, Gluzman R, Bohl D, Lagarde C, Danos O. Structure of adeno-associated virus vector DNA following transduction of the skeletal muscle. J Virol. 1999;73:1949–1955. doi: 10.1128/jvi.73.3.1949-1955.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang J-M, Zheng H, Blaivas M, Kurachi K. Persistent systemic production of human factor IX in mice by skeletal myoblast-mediated gene transfer: feasibility of repeat application to obtain therapeutic levels. Blood. 1997;90:1075–1082. [PubMed] [Google Scholar]
- 69.Wang J-M, Zheng H, Sugahara Y, Tan J, Yao S-N, Olson E, Kurachi K. Construction of human factor IX expression vectors in retroviral vector frames optimized for muscle cells. Hum Gene Ther. 1996;7:1743–1756. doi: 10.1089/hum.1996.7.14-1743. [DOI] [PubMed] [Google Scholar]
- 70.Wu P, Phillips M I, Bui J, Terwilliger E F. Adeno-associated virus vector-mediated transgene integration into neurons and other nondividing cell targets. J Virol. 1998;72:5919–5926. doi: 10.1128/jvi.72.7.5919-5926.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xiao X, Li J, Samulski R J. Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector. J Virol. 1996;70:8098–8108. doi: 10.1128/jvi.70.11.8098-8108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xiao X, Li J, Samulski R J. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J Virol. 1998;72:2224–2232. doi: 10.1128/jvi.72.3.2224-2232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yao S-N, Kurachi K. Expression of human factor IX in mice after injection of genetically modified myoblasts. Proc Natl Acad Sci USA. 1992;89:3357–3361. doi: 10.1073/pnas.89.8.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yao S-N, Kurachi K. Implanted myoblasts not only fuse with myofibers but also survive as muscle precursor cells. J Cell Sci. 1993;105:957–963. doi: 10.1242/jcs.105.4.957. [DOI] [PubMed] [Google Scholar]
- 75.Yao S-N, Kurachi K. A simple treatment of serum for precise determination of recombinant factor IX in the culture media. BioTechniques. 1992;12:525–526. [PubMed] [Google Scholar]
- 76.Yao S-N, Smith K J, Kurachi K. Primary myoblast-mediated gene transfer: persistent expression of human factor IX in mice. Gene Ther. 1994;1:99–107. [PubMed] [Google Scholar]
- 77.Zolotukhin S, Potter M, Hauswirth W W, Guy J, Muzyczka N. A “humanized” green fluorescent protein cDNA adapted for high-level expression in mammalian cells. J Virol. 1996;70:4646–4654. doi: 10.1128/jvi.70.7.4646-4654.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]