Abstract
Background
Poor compliance with treatment often means that many people with schizophrenia or other severe mental illness relapse and may need frequent and repeated hospitalisation. Information and communication technology (ICT) is increasingly being used to deliver information, treatment or both for people with severe mental disorders.
Objectives
To evaluate the effects of psychoeducational interventions using ICT as a means of educating and supporting people with schizophrenia or related psychosis.
Search methods
We searched the Cochrane Schizophrenia Group Trials Register (2008, 2009 and September 2010), inspected references of identified studies for further trials and contacted authors of trials for additional information.
Selection criteria
All clinical randomised controlled trials (RCTs) comparing ICT as a psychoeducational and supportive tool with any other type of psychoeducation and supportive intervention or standard care.
Data collection and analysis
We selected trials and extracted data independently. For homogenous dichotomous data we calculated fixed‐effect risk ratios (RR) with 95% confidence intervals (CI). For continuous data, we calculated mean differences (MD). We assessed risk of bias using the criteria described in the Cochrane Handbook for Systematic Reviews of Interventions.
Main results
We included six trials with a total of 1063 participants. We found no significant differences in the primary outcomes (patient compliance and global state) between psychoeducational interventions using ICT and standard care.
Technology‐mediated psychoeducation improved mental state in the short term (n = 84, 1 RCT, RR 0.75, 95% CI 0.56 to 1.00; n = 30, 1 RCT, MD ‐0.51, 95% CI ‐0.90 to ‐0.12) but not global state (n = 84, 1 RCT, RR 1.07, 95% CI 0.82 to 1.42). Knowledge and insight were not effected (n = 84, 1 RCT, RR 0.89, 95% CI 0.68 to 1.15; n = 84, 1 RCT, RR 0.77, 95% CI 0.58 to 1.03). People allocated to technology‐mediated psychoeducation perceived that they received more social support than people allocated to the standard care group (n = 30, 1 RCT, MD 0.42, 95% CI 0.04 to 0.80).
When technology‐mediated psychoeducation was used as an adjunct to standard care it did not improve general compliance in the short term (n = 291, 3 RCTs, RR for leaving the study early 0.81, 95% CI 0.55 to 1.19) or in the long term (n = 434, 2 RCTs, RR for leaving the study early 0.70, 95% CI 0.39 to 1.25). However, it did improve compliance with medication in the long term (n = 71, 1 RCT, RR 0.45, 95% CI 0.27 to 0.77). Adding technology‐mediated psychoeducation on top of standard care did not clearly improve either general mental state, negative or positive symptoms, global state, level of knowledge or quality of life. However, the results were not consistent regarding level of knowledge and satisfaction with treatment.
When technology‐mediated psychoeducation plus standard care was compared with patient education not using technology the only outcome reported was satisfaction with treatment. There were no differences between groups.
Authors' conclusions
Using ICT to deliver psychoeducational interventions has no clear effects compared with standard care, other methods of delivering psychoeducation and support, or both. Researchers used a variety of methods of delivery and outcomes, and studies were few and underpowered. ICT remains a promising method of delivering psychoeducation; the equivocal findings of this review should not postpone high‐quality research in this area.
Keywords: Humans, Communication, Medical Informatics Applications, Patient Education as Topic, Patient Education as Topic/methods, Randomized Controlled Trials as Topic, Schizophrenia, Schizophrenia/therapy
Plain language summary
Patient education and support for people with schizophrenia by using information and communication technology
Information and Communication Technology (ICT) includes the use of computers, telephones, television and radio, video and audio recordings. It consists of all technical means used to handle information and communication. During the last twenty years there has been a growing trend towards the use of ICT for the delivery of education, treatment and social support for people with mental illness.
Education about illness and treatment has been found to be a good way to increase a person's awareness of their health. ICT has the potential to improve many aspects of overall care, including: better education and social support; improved information and management of illness; increased access to health services; improved quality of care; better contact and continuity with services and cut costs. Recent studies show that ICT and the web may also support people in their working lives and social relationships plus help cope with depression and anxiety. However, there is a lack of knowledge about the specific effectiveness of ICT for helping people with severe mental health problems such as schizophrenia.
This review includes six studies with a total of 1063 people. Although education and support using ICT shows great promise, there was no clear benefit of using ICT (when compared with standard or usual care and/or other methods of education and support) for people with severe mental illness. However, the authors of the review suggest that this should not put off or postpone future high quality research on ICT, which is a promising and growing area of much importance.
This Plain Language Summary has been written by Benjamin Gray, Service User and Service User Expert, Rethink Mental Illness. Email: ben.gray@rethink.org
Summary of findings
Summary of findings for the main comparison. Technology‐mediated psychoeducation compared to standard care intervention for people with schizophrenia.
| Technology‐mediated psychoeducation compared to standard care intervention for people with schizophrenia | ||||||
| Patient or population: patients with people with schizophrenia Settings: in and out‐patient psychiatric care unit and rehabilitation centres Intervention: technology‐mediated psychoeducation Comparison: standard care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard care intervention | Technology‐mediated psychoeducation | |||||
| Compliance/satisfaction: leaving the study early ‐ any reason Follow‐up: 52 weeks | Low1 | RR 1.24 (0.81 to 1.88) | 116 (2 studies) | ⊕⊝⊝⊝ very low2,3,4 | ||
| 200 per 1000 | 248 per 1000 (162 to 376) | |||||
| Moderate1 | ||||||
| 400 per 1000 | 496 per 1000 (324 to 752) | |||||
| High1 | ||||||
| 600 per 1000 | 744 per 1000 (486 to 1000) | |||||
| Mental state: not improved ‐ short‐term BPRS Follow‐up: 12 weeks | Low | RR 0.75 (0.56 to 1) | 84 (1 study) | ⊕⊕⊝⊝ low2,5,6 | ||
| 500 per 1000 | 375 per 1000 (280 to 500) | |||||
| Moderate | ||||||
| 700 per 1000 | 525 per 1000 (392 to 700) | |||||
| High | ||||||
| 900 per 1000 | 675 per 1000 (504 to 900) | |||||
| Global state: not improved ‐ short‐term GAF Follow‐up: 12 weeks | Low1 | RR 1.08 (0.82 to 1.42) | 84 (1 study) | ⊕⊕⊝⊝ low2,4,5 | ||
| 500 per 1000 | 540 per 1000 (410 to 710) | |||||
| Moderate1 | ||||||
| 700 per 1000 | 756 per 1000 (574 to 994) | |||||
| High1 | ||||||
| 900 per 1000 | 972 per 1000 (738 to 1000) | |||||
| Knowledge/insight | Moderate | Not estimable | 0 (0) | See comment | No trial reported this outcome. | |
| Quality of life | Moderate | Not estimable | 0 (0) | See comment | No trial reported this outcome. | |
| Needs | Moderate | Not estimable | 0 (0) | See comment | No trial reported this outcome. | |
| Social support Social Network Interview Follow‐up: 12 weeks | Low | RR 0.42 (0.04 to 0.80) | 60 (1 study) | |||
| 500 per 1000 | 210 per 1000 (20 to 400) | |||||
| Moderate | ||||||
| 700 per 1000 | 294 per 1000 (28 to 560) | |||||
| High | ||||||
| 900 per 1000 | 378 per 1000 (36 to 720) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BPRS: Brief Psychiatric Rating Scale; CI: Confidence interval; GAF: Global Assessment of Functioning; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Middle control group risk approximates that in trial control group. 2 Limitation in design: rated 'serious' ‐ randomisation not described. 3 Indirectness: rated as 'serious' ‐ implication of not being compliant. 4 Publication bias: rated 'likely' ‐ outcome data incomplete. 5 Assumed those who discontinued would not change if had remained in study. 6 Publication bias: rated 'likely' ‐ small single trial.
Summary of findings 2. Technology‐mediated psychoeducation + standard care intervention compared to standard care intervention for people with schizophrenia.
| Technology‐mediated psychoeducation + standard care intervention compared to standard care intervention for people with schizophrenia | ||||||
| Patient or population: patients with people with schizophrenia Settings: in and out‐patient psychiatric care unit and rehabilitation centres Intervention: technology‐mediated psychoeducation + standard care intervention Comparison: standard care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard care intervention | Technology‐mediated psychoeducation + standard care intervention | |||||
| Compliance/satisfaction: medication ‐ not compliant ‐ long‐term Follow‐up: 52 weeks | Low1 | RR 0.45 (0.27 to 0.77) | 71 (1 study) | ⊕⊝⊝⊝ very low2,3,4 | ||
| 500 per 1000 | 225 per 1000 (135 to 385) | |||||
| Moderate1 | ||||||
| 700 per 1000 | 315 per 1000 (189 to 539) | |||||
| High1 | ||||||
| 900 per 1000 | 405 per 1000 (243 to 693) | |||||
| Mental state: not improved ‐ short‐term BPRS Follow‐up: 12 weeks | Low1 | RR 0.86 (0.63 to 1.19) | 56 (1 study) | ⊕⊕⊝⊝ low5,6,7 | ||
| 500 per 1000 | 430 per 1000 (315 to 595) | |||||
| Moderate1 | ||||||
| 700 per 1000 | 602 per 1000 (441 to 833) | |||||
| High1 | ||||||
| 900 per 1000 | 774 per 1000 (567 to 1000) | |||||
| Global state: not improved ‐ short‐term GAF Follow‐up: 12 weeks | Low1 | RR 1.15 (0.86 to 1.54) | 56 (1 study) | ⊕⊕⊝⊝ low5,7 | ||
| 500 per 1000 | 575 per 1000 (430 to 770) | |||||
| Moderate1 | ||||||
| 700 per 1000 | 805 per 1000 (602 to 1000) | |||||
| High1 | ||||||
| 900 per 1000 | 1000 per 1000 (774 to 1000) | |||||
| Knowledge/insight: not improved ITAQ Follow‐up: 12 weeks | Low1 | RR 1 (0.76 to 1.31) | 56 (1 study) | ⊕⊕⊝⊝ low5,7 | ||
| 500 per 1000 | 500 per 1000 (380 to 655) | |||||
| Moderate1 | ||||||
| 700 per 1000 | 700 per 1000 (532 to 917) | |||||
| High1 | ||||||
| 900 per 1000 | 900 per 1000 (684 to 1000) | |||||
| Quality of life | Moderate | Not estimable | 0 (0) | See comment | No trial reported this outcome. | |
| Needs | Moderate | Not estimable | 0 (0) | See comment | No trial reported this outcome. | |
| Social support | Moderate | Not estimable | 0 (0) | See comment | No trial reported this outcome. | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BPRS: Brief Psychiatric Rating Scale; CI: Confidence interval; GAF: Global Assessment of Functioning; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Middle control group risk approximates that in trial control group. 2 Limitation in design: rated 'serious' ‐ randomisation not well described ‐ "Randomised participants matched (age, gender, length of illness, type of schizophrenia)" ‐ no further details. 3 Imprecision: rated as 'serious' ‐ small sample size. 4 Publication bias: rated as 'likely' ‐ small single trial. 5 Limitation in design: rated 'serious' ‐ randomisation not described. 6 Assumed those who discontinued would not change if had remained in study. 7 Publication bias: rated 'likely' ‐ outcome data incomplete.
Background
During the last two decades there has been a growing tendency towards the use of information and computer technology (ICT) in the delivery of treatments that support social relations of all citizens (European Commission 2001; Lewis 2003). ICT is increasingly used for range of purposes in business, government and health care (European Communities 2007). Globally, ICT has become an effective instrumement to communicate and increase awareness of mental health (Boyce 2011). The use of eHealth is particularly being supported by different EU agendas, most recently in the EU eGovernment action plan for the years 2011 to 2015 (European Commission 2010). There is, however, a lack of knowledge about effective ICT applications used in treatment delivery for people with schizophrenia (Podoll 2000).
Description of the condition
Schizophrenia is a severe mental disorder with a lifetime prevalence of about 1%. For many people afflicted with the illness, schizophrenia results in chronic disabilities (Kaplan 2007). Currently, around 24 million people worldwide suffer from schizophrenia (WHO 2011). The illness is characterised by profound disruption in thinking, affecting language, perception and sense of self. It often includes psychotic experiences such as hearing voices or delusions. It can impair functioning through the loss of capability to earn one's own livelihood or the disruption of studies (WHO 2006). Defects of cognitive functions such as problem‐solving ability, explicit memory, knowledge and general intellectual capacities have been reported (Kaplan 2007).
Other problems disturbing daily life are a lack of insight (Morrison 2006), relapse (Haro 2006), non‐compliance with treatment (Freudenreich 2004; Kikkert 2006) and feelings of isolation and loneliness (Chan 2004; Morrison 2006). Schizophrenia is associated with mortality rates that are two to three times higher than those observed in the general population (Auquier 2007) and the risk for suicide is higher than in the general population (Hor 2010; Palmer 2005). In addition, schizophrenia is associated with poor quality of life (Bobes 2006; Evans 2007).
Treatment of schizophrenia varies based on the different phases of patient illness. In the acute, stabilisation or maintenance phase, antipsychotic medication is the mainstay of treatment, with the newer atypical antipsychotic agents often thought to be superior (Kaplan 2007; Turner 2006). Most people with schizophrenia benefit from the combined use of antipsychotic drugs and psychosocial therapies (Tandon 2006). Lack of compliance, however, can be a problem. Stable patients who are maintained on an antipsychotic have a much lower relapse rate than patients who have their medication discontinued. It has been suggested that 16 to 23 per cent of patients receiving treatment will experience a relapse within a year and 53 to 72 per cent will relapse without medication (Kaplan 2007). Other treatment methods besides medication are psychosocial therapies, which include a variety of methods to increase social abilities, self sufficiency, practical skills and interpersonal communication in patients with schizophrenia (Kaplan 2007). A range of psychosocial treatments are also helpful, including family intervention, supported employment, cognitive‐behaviour therapy for psychosis, social skills training or teaching illness self management skills (Mueser 2005). For example, behavioural skills training focusing on patients’ symptoms may include videotapes or homework assignments for the specific skills. It has been shown that social skills training, along with pharmacological therapy, can be supportive and useful for patients (Kaplan 2007). However, willingness to follow through with a treatment plan is often related to a person's perception and understanding of their illness, and amongst other issues that may contribute to poor compliance with medication, people with schizophrenia may lack this insight (Kikkert 2006; Perkins 2000).
Description of the intervention
ICT consists of all technical means used to handle information and communication. It can include computers, network hardware and software, telephones, broadcast media, and audio and video processing and transmission (International Telecommunication Union 2009). ICT applications in health care can vary, and they can include telephones, mobile phones, computers, Internet, electronic databases and decision‐support systems. In recent years, a concept of eHealth has also increasingly been used to refer to ICT applications (Eysenbach 2001). eHealth, in turn, has been defined as the application of ICT across the whole range of functions that affect health care, from diagnosis to follow‐up (Silber 2003). It also refers to the use of modern information and communication technologies to meet the needs of citizens, patients, healthcare professionals and healthcare providers, as well as policymakers (Ministerial Declaration 2003).
eHealth can be defined as encompassing ICT‐enabled solutions providing benefits to health, be it at the individual or at the societal level (European Communities 2007). In a systematic review of published definitions of eHealth researchers found no clear consensus about the meaning of the term eHealth. They identified two universal themes (health and technology) and six less general themes (commerce, activities, stakeholders, outcomes, place and perspectives) (Oh 2005). E‐mental health can be understood as the use of digital technologies and new media for the delivery of screening, health promotion, prevention, early intervention, treatment or relapse prevention, as well as for improvement of health care delivery, e‐learning and online research in the field of mental health (Riper 2010).
How the intervention might work
Education about illness and treatment has been found to be an effective way to increase a person's awareness of their mental health problems (Gumley 2006; Perry 1999; Turkington 2002). Such increased awareness can lead to increased treatment compliance with consequent reduction of relapse, or readmission rates (Xia 2011). Education may also help people to evaluate their mood, self esteem and negative beliefs about themselves and others, which are to be considered when designing interventions (Smith 2006). In addition, clinical interventions that foster appraisals of recovery may improve emotional well‐being in people with psychosis (Watson 2006).
Use of ICT applications for patient education and support has the potential to improve information management, access to health services, quality of care, continuity of services and cost containment. In addition, they have the added benefit that many people turn to 'the web' to obtain information because of its anonymity and the fear of stigmatisation often associated with consulting mental heath professionals is reduced (Eysenbach 2001). Recent studies indicate that the Internet has shown promise in patient education, providing different methods to illustrate the educational material. Results show that computer‐delivered interventions could improve outcomes for a wide range of common mental health problems, such as depression, anxiety, work, social adjustment or general psychopathology in the general public (Christensen 2002; Proudfoot 2004).
Why it is important to do this review
Despite a considerable increase in the use of ICT‐based approaches in mental health services, its effect on people with schizophrenia has been less systematically evaluated (Podoll 2000; Walker 2006). Indeed, ICT methods used by patient groups who show poor compliance have received considerably less attention, despite being able to recruit well and maintain their participation (Jones 2001b). To avoid alienation of those with mental illness from the information society, a systematic evaluation of the effects of ICT in daily clinical practice for mental health services is needed.
Objectives
To evaluate the effects of using information and communication technology (ICT) as a means of educating and supporting people with schizophrenia or related psychoses compared to standard care, other methods of delivering psychoeducation and support, or both.
Methods
Criteria for considering studies for this review
Types of studies
We included all relevant randomised controlled trials. We excluded quasi‐randomised studies, for example, with alternation as the method of randomisation.
Types of participants
We included people with schizophrenia or schizophrenia‐like psychoses (schizophreniform and schizoaffective disorders). We included trials where it was implied that the majority of participants had a severe mental illness which was likely to be schizophrenia, including those with multiple diagnoses as well, and did not exclude trials due to age, nationality, gender, duration of illness or treatment setting.
Types of interventions
1. Technology‐mediated psychoeducation
Psychoeducation and support using ICT as sole method of delivery of treatment. ICT applications are varied and can include telehealth, telephones, mobile phones, computers and the Internet. These interventions can be provided by a single person with the main purpose of maintaining current functioning or assisting coping abilities in people who have a diagnosis of schizophrenia or schizophrenia‐like illness. The therapies can be aimed at individuals or groups of people. If the content of the therapy was not sufficiently clear after reading a clinical study, then we included any therapy that had supportive or support in its title. The intervention had to go beyond the provision of or access to an ICT application; there should be a planned strategy for ICT use.
We planned to classify ICT systems for the purposes of this review into broad categories, taking into consideration ease of access (mobile phones (high access), Internet, personal computer program (lower access)) and the complexity of the interface (simple, with minimal requirements expected from user, through to complex, with the need to enter complex data). However, we did not undertake this categorisation due to the low number of trials found.
2. ICT combination: technology‐mediated psychoeducation and standard care
Psychoeducation and support using ICT combined with standard care. Standard care is defined as the care a person would normally receive if they had not been included in the research trial. This would include interventions provided by health care professionals such as medication, hospitalisation, community psychiatric nursing input and/or day hospital but should explicitly state that formalised psychoeducation and support is not part of this package.
3. Psychoeducation with no technology
Psychoeducation intervention using only including supportive treatment (biological, psychological or social) such as problem‐solving therapy, psychoeducation, social skills training, cognitive‐behavioural therapy, family therapy or psychodynamic psychotherapy.
4. Standard care
Standard care or 'no' intervention is defined as the care a person would normally receive if they had not been included in the research trial. This would include interventions provided by health care professionals such as medication, hospitalisation, community psychiatric nursing input and/or day hospital but should explicitly state that formalised psychoeducation and support is not part of this package.
Types of outcome measures
We grouped outcomes into short‐term (up to 12 weeks), medium‐term (13 to 26 weeks) and long‐term (over 26 weeks).
Primary outcomes
1. Patient compliance
1.1 Compliance with treatment and medication
2. Global state
2.1 Relapse (both incidence of and time to relapse) 2.2 No clinically important change in global state (as defined by individual studies)
Secondary outcomes
1. Patient compliance
1.1 Compliance with follow‐up 1.2 Leaving the study early
2. Mental state
2.1 No clinically important change in general mental state 2.2 Average endpoint general mental state score 2.3 Average change in general mental state scores 2.4 No clinically important change in specific symptoms (positive symptoms of schizophrenia, negative symptoms of schizophrenia, depression, mania) 2.5 Average endpoint specific symptom score 2.6 Average change in specific symptom scores
3. Global state
3.1 Average endpoint global state score 3.2 Average change in global state scores
4. Level of knowledge/insight
4.1 No clinically important change in average of understanding of his/her illness and need for treatment 4.2 Average endpoint understanding/knowledge of illness scores 4.3 Average change in understanding/knowledge of illness scores 4.4 Average endpoint understanding/knowledge of treatment and medication effect scores 4.5 Average change in understanding/knowledge of treatment and medication effect scores
5. Behaviour
5.1 No clinically important change in general behaviour 5.2 Average endpoint general behaviour score 5.3 Average change in general behaviour scores 5.4 No clinically important change in specific aspects of behaviour 5.5 Average endpoint specific aspects of behaviour 5.6 Average change in specific aspects of behaviour
6. Quality of life
6.1 No clinically important change in quality of life 6.2 Average endpoint quality of life score 6.3 Average change in quality of life scores 6.4 No clinically important change in specific aspects of quality of life 6.5 Average endpoint specific aspects of quality of life 6.6 Average change in specific aspects of quality of life 6.7 Social support*
7. Satisfaction with treatment
7.1 Leaving the studies early 7.2 Recipient of care not satisfied with treatment 7.3 Recipient of care average satisfaction score 7.4 Recipient of care average change in satisfaction scores 7.5 Carer not satisfied with treatment 7.6 Carer average satisfaction score 7.7 Carer average change in satisfaction scores
8. Health and social needs*
9. Service utilisation
9.1 Hospitalisation 9.2 Time to hospitalisation 9.3 Duration of stay in hospital
10. Health economic outcomes
10.1 Direct treatment costs 10.2 Indirect treatment costs
11. Death
11.1 Suicide 11.2 Natural causes
*Additional outcome added for since publication of protocol. Please see Differences between protocol and review.
Search methods for identification of studies
Electronic searches
Electronic searches
We searched the Cochrane Schizophrenia Group Trials Register (June 2008, June 2009 and September 2010) using the phrase:
[((*communic* or *information* in title) or *computer* or *technolog* or * tele* or *phone* or *internet* or *digital* or *electronic* or *database* in title, abstract, index terms of REFERENCE) or (*computer* or tele* or *internet* or *technolog* or *electronic* in interventions of STUDY)].
This register is compiled by systematic searches of major databases, handsearches of journals and conference proceedings (see Cochrane Schizophrenia Group module).
Searching other resources
Reference searching
We inspected reference lists of identified studies for more relevant trials.
Personal contact
We contacted authors of relevant studies for inclusion to identify further relevant trials.
Data collection and analysis
The review authors read the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and acquired training by attending relevant courses and workshops.
Selection of studies
Review authors (MV, HH, LK) inspected all abstracts of studies identified as above and suggested potentially relevant reports. Where disagreement occurred we resolved it by discussion, or where there was still doubt, we acquired the full article for further inspection. Once the full articles were obtained, MV and HH inspected all full reports and independently decided whether they met inclusion criteria (i.e. Criteria for considering studies for this review). MV and HH were not blinded to the names of the authors, institutions or journal of publication. Where difficulties or disputes arose, we asked authors CA, LK and ML for help; if it was impossible to decide, we added these studies to those ‘Characteristics of studies awaiting classification’ table and we contacted the authors of the papers for clarification.
Data extraction and management
1. Extraction
MV, HH and LK extracted data from all included studies. Again, we discussed any disagreement, documented decisions and, if necessary, contacted authors of studies for clarification. We extracted data presented only in graphs and figures whenever possible, but we only included if two authors independently had the same result. When this was not possible and further information was necessary to resolve the dilemma, we did not enter these data but added the trial to the list of those awaiting assessment. We made attempts to contact authors through an open‐ended request in order to obtain missing information or for clarification whenever necessary. With remaining problems ML and CA helped clarify issues and we documented those final decisions.
2. Management
2.1 Forms
We extracted data onto standard, simple forms.
2.2 Scale‐derived data
We included continuous data from rating scales only if:
a) the psychometric properties of the measuring instrument had been described in a peer‐reviewed journal (Marshall 2000); and b) the measuring instrument was not written or modified by one of the trialists for that particular trial; and c) the measuring instrument is either i. a self report or ii. completed by an independent rater.
2.3 Endpoint versus change data
There are advantages of both endpoint and change data. Change data can remove a component of between‐person variability from the analysis. On the other hand calculation of change needs two assessments (baseline and endpoint); normally both sets of data would be available to trialists but if change scores are presented, the SD of the change is often not provided. We decided to primarily use endpoint data and only use change data if the former were not available. Endpoint and change data were combined in the analysis as we used mean differences rather than standardised mean differences throughout (Higgins 2011, chapter 9.4.5.2).
2.4 Skewed data
Continuous data on clinical and social outcomes are often not normally distributed. To avoid the pitfall of applying parametric tests to non‐parametric data, we aimed to apply the following standards to all data before inclusion:
a) standard deviations and means are reported in the paper or obtainable from the authors; b) when a scale starts from the finite number zero, the standard deviation, when multiplied by two, is less than the mean (as otherwise the mean is unlikely to be an appropriate measure of the centre of the distribution (Altman 1996); c) if a scale started from a positive value (such as PANSS, which can have values from 30 to 210) the calculation described above was modified to take the scale starting point into account. In these cases skew is present if 2SD > (S‐S min), where S is the mean score and S min is the minimum score. Endpoint scores on scales often have a finite start and endpoint and these rules can be applied. Skewed endpoint data from studies of less than 200 participants were entered in additional tables rather than into an analysis. Skewed data of means pose less of a problem if the sample size is large; if this condition was met, then skewed endpoint data were entered into syntheses.
When continuous data are presented on a scale that includes a possibility of negative values (such as change data), it is difficult to tell whether data are skewed or not and we entered such data into analysis.
2.5 Common measure
To facilitate comparison between trials, we intended to convert variables that can be reported in different metrics, such as length for course of illness (mean months per year).
2.6 Conversion of continuous to binary
We did not convert outcome measures to dichotomous data. Thus, we used the primary cut‐off presented by the original authors.
2.7 Direction of graphs
We entered data for unfavourable outcomes in such a way that the area to the left of the line of no effect indicates a favourable outcome for ICT. Where the outcomes were favourable, the area to the right of the line of no effect indicates a favourable outcome for ICT.
2.8 'Summary of findings' tables
We included the following short, medium or long‐term outcomes in a 'Summary of findings' table and one global and, preferably binary, measure of each.
1. Compliance 2. Mental state 3. Global state 4. Knowledge/insight 5. Quality of life 6. Needs 7. Social support
Assessment of risk of bias in included studies
Review authors (MV, HH, ML, LK, CEA) worked independently by using criteria described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) to assess trial quality. This new set of criteria is based on evidence of associations between overestimate of effect and high risk of bias of the article such as sequence generation, allocation concealment, blinding, incomplete outcome data and selective reporting.
Where inadequate details of randomisation and other characteristics of trials were provided, we contacted authors of the studies in order to obtain additional information.
We have noted the level of risk of bias in both the text of the review and in the Table 1.
Measures of treatment effect
1. Binary data ‘yes/no’ ‐ data
We calculated the risk ratio (RR) and its 95% confidence interval (CI) based on the random‐effects model, as this takes into account any differences between studies even if there is no statistically significant heterogeneity. It has been shown that RR is more intuitive (Boissel 1999) than odds ratios which tend to be interpreted as RR by clinicians (Deeks 2000). This misinterpretation then leads to an overestimate of the impression of the effect.
2. Continuous data
For continuous outcomes we estimated mean difference (MD) between groups. We preferred not to calculate effect size measures (standardised mean difference (SMD)).
Unit of analysis issues
1. Cluster trials
Studies increasingly employ 'cluster randomisation' (such as randomisation by clinician or practice) but analysis and pooling of clustered data poses problems. Firstly, authors often fail to account for intra class correlation in clustered studies, leading to a 'unit of analysis' error (Divine 1992) whereby P values are spuriously low, confidence intervals unduly narrow and statistical significance overestimated. This causes type I errors (Bland 1997; Gulliford 1999).
In subsequent versions of this review we will seek to contact first authors of studies to obtain intra‐class correlation co‐efficients of their clustered data and to adjust for this using accepted methods (Gulliford 1999). Where clustering had been incorporated into the analysis of primary studies, we also presented these data as if from a non‐cluster randomised study, but adjusted for the clustering effect.
We have sought statistical advice and have been advised that the binary data as presented in a report should be divided by a 'design effect'. This is calculated using the mean number of participants per cluster (m) and the intraclass correlation co‐efficient (ICC) [Design effect = 1+(m‐1)*ICC] (Donner 2002). If the ICC was not reported it was assumed to be 0.1 (Ukoumunne 1999).
2. Cross‐over trials
A major concern of cross‐over trials is the carry‐over effect. It occurs if an effect (e.g. pharmacological, physiological or psychological) of the treatment in the first phase is carried over to the second phase. As a consequence on entry to the second phase the participants can differ systematically from their initial state despite a wash‐out phase. For the same reason cross‐over trials are not appropriate if the condition of interest is unstable (Elbourne 2002). As both effects are very likely in severe mental illness, we used only data from the first phase of cross‐over studies.
3. Studies with multiple treatment groups
Where a study involved more than two treatment arms, if relevant, we presented the additional treatment arms in comparisons. Where the additional treatment arms were not relevant, we did not present these data.
Dealing with missing data
1. Overall loss of credibility
At some degree of loss of follow‐up data must lose credibility (Xia 2007). For any particular outcome if more than 40% of data was unaccounted for, we did not present these data or use them within analyses.
2. Last observation carried forward
We preferred not to make any assumptions about loss to follow‐up for continuous data and analysed results for those who completed the trial, since the use of methods such as the last observation carried forward (LOCF) introduce uncertainty about the reliability of the result (Leucht 2007). If LOCF data had been used in included trials, we reproduced these data and indicated that they were the result of LOCF assumptions.
Assessment of heterogeneity
1. Clinical heterogeneity
We considered all included studies initially, without seeing comparison data, to judge clinical heterogeneity. We simply inspected all studies for clearly outlying situations or people which we had not predicted would arise. When such situations or participant groups arose, these were fully discussed.
2. Methodological heterogeneity
We considered all included studies initially, without seeing comparison data, to judge methodological heterogeneity. We simply inspected all studies for clearly outlying methods which we had not predicted would arise. When such methodological outliers arose these were fully discussed.
3. Statistical heterogeneity
3. 1 Visual inspection
We visually inspected the forest plots to evaluate the possibility of heterogeneity of intervention effects across trials as evidenced by outlying trials with non‐overlapping confidence intervals. We also noted differences in the direction of effect estimates across trials.
3.2 Employing I2 statistics
We considered all the included studies within any comparison to judge clinical heterogeneity. Then visual inspection of graphs was used to investigate the possibility of statistical heterogeneity. This was supplemented using, primarily, the I2 statistic. This provides an estimate of the percentage of variability due to heterogeneity rather than chance alone. Where the I2 estimate was greater than or equal to 50%, we interpreted this as indicating the presence of high levels of heterogeneity (Higgins 2003). If inconsistency was high, we did not summate data, but presented the data separately and investigated the reasons for heterogeneity.
Assessment of reporting biases
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results (Egger 1997). These are described in Section 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We attempted to locate protocols of included trials. If the protocol was available, we compared outcomes in the protocol and in the published report. If the protocol was not available, we compared outcomes listed in the methods section of the trial report with actually reported results.
Data synthesis
We understand that there is no closed argument for preference for use of fixed or random‐effects models. The random‐effects method incorporates an assumption that the different studies are estimating different, yet related, intervention effects. This often seems to be true to us and the random‐effects model takes into account differences between studies even if there is no statistically significant heterogeneity. There is, however, a disadvantage to the random‐effects model. It puts added weight onto small studies which often are the most biased ones. Depending on the direction of effect these studies can either inflate or deflate the effect size. Therefore, we chose the fixed‐effect model for all analyses. The reader is, however, able to choose to inspect the data using the random‐effects model.
Subgroup analysis and investigation of heterogeneity
We did not perform any subgroup analyses.
Sensitivity analysis
1. Implication of randomisation
We planned sensitivity analyses when a trial was described as double‐blind, but it was implied that the study was randomised. The included trials were not described as double‐blind with implied randomisation and therefore we did not need to perform sensitivity analyses.
2. Assumption for lost binary data
Where assumptions had to be made regarding people lost to follow‐up (see Dealing with missing data) we compared the findings of the primary outcomes when we used our assumption compared with completer data only. If there was a substantial difference, we reported these results and discussed them but continued to employ our assumption.
3. Chinese studies
Concerns were raised about the quality of the Chinese trials (Hu 2011; Wu 2009). For this reason we performed sensitivity analysis for all primary outcomes in which Chinese trials were included.
4. Requirements of the interventions
Focusing solely on primary outcomes we also aimed to investigate whether interventions falling into pre‐defined categories (i) highly accessible and simple requirements; ii) less widely accessible and requirements that cannot be described as simple; iii) access by specific interface with complex requirements) were suggested to have different effects. However, due to the limited data we did not perform sensitivity analyses.
Results
Description of studies
For more detailed description of each study, please see Characteristics of included studies, Characteristics of excluded studies and Characteristics of ongoing studies.
Results of the search
We undertook the first search in June 2008 and re‐ran it in 2009 and 2010 (see Appendix 1). In 2008 we identified 505 studies. In 2009, the search offered us 574 new references (from 293 studies) and in September 2010, we found 231 new references (from 191 studies). Thus, altogether the search totalled 1310 studies to be inspected for possible selection. The review now contains six included studies, 18 studies are excluded and six are ongoing studies that may be relevant to this review (see Figure 1).
1.
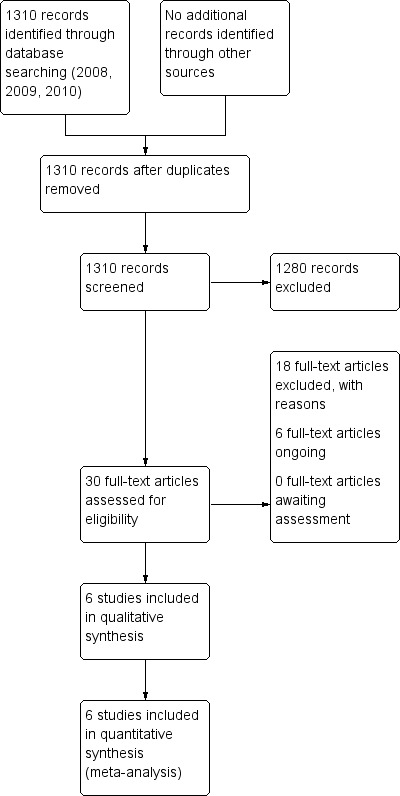
Study flow diagram
Included studies
This review includes six studies published between 2001 and 2010 (Chen 2007; Hansson 2008; Jones 2001b; Rotondi 2005; Salzer 2004; Valimaki 2010).
1. Methods
All studies were stated to be randomised, although description of the allocation varied. Randomisation was undertaken once people were matched (age, gender, length of illness and type of schizophrenia) in the Chen 2007 study. Hansson 2008 cluster‐randomised key workers. For further details please see the section below on Allocation (selection bias) and Blinding (performance bias and detection bias).
2. Study duration
Overall, the length of the trials varied although a majority used long‐term outcomes (over 26 weeks). Salzer 2004 followed people up to 13 months and three other studies up to 12 months (Chen 2007; Hansson 2008; Valimaki 2010). The length of the other two trials was six (Rotondi 2005) and three months (Jones 2001b).
3. Design
All of the six studies used a parallel study design. However, one employed a cluster technique (cluster by key worker, Hansson 2008). In this study over 500 participants took part but there were 134 clusters. The paper does not report intra class correlation coefficients (ICC) but we have had direct contact with the authors of the trial and they are aware of the importance of this statistic. They will forward these data when available. For this version of the review, however, we have had to assume the ICC to be 0.1 (Ukoumunne 1999). This results in a design effect of 1.28 for the intervention group and 1.20 for the control.
4. Participants
Participants totalled 1063 people with schizophrenia from the six trials between the years 2001 to 2010. Rotondi 2005 specified participants’ diagnosis as "schizophrenia and schizoaffective disorder" while Salzer 2004 used "schizophrenia‐spectrum". Diagnoses were based on different operationalised criteria such as ICD‐10 (Hansson 2008; Jones 2001b; Valimaki 2010), CCMD‐3 (Chen 2007) or DSM‐IV (Salzer 2004).
In three trials, participants were aged between 18 and 65 years (Hansson 2008; Jones 2001b; Valimaki 2010). Chen 2007 included some younger people (range 15 to 65 years). Rotondi 2005 reported age by mean (37.5 years) and Salzer 2004 did not report information about age.
Patients were in different states of illness. Chen 2007 reported that the mean length of patient illness was 10 years, while in Hansson 2008 study patients' duration of illness was about 16 years. The mean number of hospital admissions was about five in Hansson 2008. In the Valimaki 2010 study, patients were admitted into psychiatric hospitals and the mean length of their hospital stay was 60 days. In two studies patients were excluded if they had severe organic psychiatric illness (Hansson 2008) of if patients were acutely ill (Jones 2001b). On the other hand, Rotondi 2005 excluded patients if they had not had hospitalisation within the previous two years. In one study (Salzer 2004) patients' illness history was not described in detail.
5. Setting
Out of all six trials, 30 patients were treated in both in‐ and out‐patient settings (Rotondi 2005), 311 patients were recruited in an in‐patient setting (Valimaki 2010) and the rest (722 people) were treated in different types of out‐patient settings.
6. Study size
Overall, study size varied from 30 (Rotondi 2005) to 507 (Hansson 2008) patients, although in the largest trial participants were within 134 clusters.
7. Interventions
7.1 Intervention group
All psychoeducation or patient support interventions were included. Interventions used in the trials, however, varied considerably. We excluded staff support systems.
Chen 2007 used technology‐mediated psychoeducation and a standardised nursing intervention. ICT involved digital and spot network group and psychological online consultation.
In Hansson 2008, clinicians, in addition to continuing with standard treatment, implemented the manualised intervention 'DIALOG'. This is a computer‐mediated procedure to discuss quality of life domains with patients. Answers to all questions were entered directly onto a hand‐held computer or laptop using software specifically developed for the study. The intervention was repeated every two months so that participants' views on their situation and needs for care were explicit (Hansson 2008).
The Jones 2001a study involved five educational sessions using a computer intervention. In a ‘computer only’ group, patients were shown how to use the computer by a researcher. Three different types of screen display were used: general information, personal information from the viewing patient’s medical record with general information and questionnaires including medical record audit plus feedback displays. At the end of the session, participants received feedback information if requested (Jones 2001b).
Rotondi 2005 used the Schizophrenia Guide website as a telehealth intervention. The programme collected information on each participant’s usage of the website. The Schizophrenia Guide provided three online therapy groups; the ability to ask questions of the experts associated with the project and receive an answer; a library of previous asked and answered questions; activities in the community and news items that focus on mental health issues; and educational reading materials (Rotondi 2005).
Salzer 2004 employed a brief telephone‐based medication management programme (TMM) to enhance communication, insight, attitudes, knowledge and satisfaction among patients in weekly programme (altogether 52 weeks).
Lastly Valimaki 2010 used an IT‐based patient education programme to support well‐being. The programme offered need‐based computerised information and discussions with staff in five sessions, plus printed material based on patients' requests. This involved a total of five sessions for each participant.
7.2 Comparison group
There were also different comparison groups for each of the six studies. Chen 2007 used "general rehabilitation treatment" as standardised care. The Hansson 2008 control used key worker as "treatment as usual". Rotondi 2005 and Salzer 2004 both used "general treatment" as control. The Jones 2001a control involved a standardised nursing intervention as the comparison group. This was "community psychiatric nurse only" in which the hour‐long session with the community psychiatric nurse covered the same content as the computer system. Participants could also receive a printed summary. In addition, "combination of community psychiatric nurse and computer", which combined a first session offered by community nurse, sessions two to four by computer and the last session again with the nurse. In addition, patients were given relevant printed summaries from sessions. Finally Valimaki 2010 used a "standardised nursing intervention" as comparison group. In this case "traditional patient education" included five patient education sessions with printed leaflets for each participant.
8. Outcomes
8.1 General
The duration of follow‐up in the included trials varied between three and 12 months. Details of the scales used in this review to quantify different outcomes are provided below. Data from these scales were reported either in continuous form or as binary figures.
8.2 Binary data
8.2.1 Leaving the study early
All studies used a measure of whether a patient left the study early. In this review we assumed that if they were not included in analysis these people left early.
8.2.2 Drug compliance
In one study drug compliance was measured by counting payments for medication (Chen 2007).
8.3 Scales
8.3.1 Mental state scales
8.3.1.1 Positive and Negative Syndrome Scale ‐ PANSS (Kay 1987) This is a 30‐item scale, each of which can be defined on a seven‐point scoring system from absent to extreme. There are three sub‐scales for measuring the severity of general psychopathology, positive symptoms (PANSS‐P) and negative symptoms (PANSS‐N). A low score indicates lesser severity. Two studies reported data from this scale.
8.3.1.2 Brief Psychiatric Rating Scale ‐ BPRS (Overall 1962) The Brief Psychiatric Rating Scale (BPRS) is an 18‐item scale measuring positive symptoms, general psychopathology and affective symptoms. The original scale has 16 items, but a revised 18‐tem scale is commonly used. Scores can be ranged from 0 to 126. Each item is rated on a seven‐point scale varying from ‘not presented’ to ‘extremely severe’, with high scores indicating more severe symptoms. One study reported data from this scale.
8.3.1.3 Perceived stress ‐ Psychological distress (Zautra 1988) This is a scale including six sub‐scales: anxiety/dread (10 items), helplessness/hopelessness (four items) and confused thinking (two items), anxiety (seven items), suicidal ideation (three items) and depression (10 items). A high score indicates higher stress. One study reported data from this scale.
8.3.2 Global state scales
8.3.2.1 Global Assessment of Functioning ‐ GAF (APA 1994a) GAF is a global measure covering the range from positive mental health to severe psychopathology. It measures the degree of mental illness by rating psychological, social and occupational functioning. The 100‐point scales are divided into intervals each with 10 points. The 10‐point intervals have anchor points describing symptoms and functioning that are relevant for scoring. The anchor points for interval 1 to 10 describe the most severely ill and the anchor points for interval 91 to 100 describe the healthiest.
8.3.2.2 Social Disability Screening Schedule – SDSS (Cooper 1996; Shen 1985) The SDSS has been designed to yield continuous ratings of severity of substance dependence in compatibility with DSM‐IV and ICD‐10. High scores indicate poor outcome. One study reported data from this scale.
8.3.4 Knowledge/insight scales
8.3.4.1 Knowledge and Information about Schizophrenia Schedule ‐ KISS (Barrowclough 1987; Jones 2001a) KISS is a structured interview measuring the knowledge and information about schizophrenia schedule (KISS), which was developed for the pilot study partly on the basis of the KASI used for carers (Barrowclough 1987; Jones 2001a). One study reported data from this scale.
8.5.2 Insight and Treatment Attitude Questionnaire – ITAQ (McEvoy 1989) The ITAQ is an 11‐item semi‐structured interview that measures awareness of illness and attitude to medication and services, as well as follow‐up evaluation. Its scores range from 0 to 22, with a high score indicating better insight. One study reported data from the scale.
8.3.5 Quality of life scales
8.3.5.1 Manchester Short Assessment of Quality of Life ‐ MANSA (Slade 2005) This 16‐item measure comprises objective and subjective questions. Each item is rated on a seven‐point satisfaction scale, from 1 = ‘Couldn't be worse’ to 7 = ‘Couldn't be better’. The higher score indicates the better quality of life. One study reported data from the scale.
8.3.6 Satisfaction with treatment
8.3.6.1 The Client Satisfaction Questionnaire‐8 ‐ CSQ‐8 (Larsen 1979) CSQ‐8 is a self report statement of satisfaction with health and human services. The scale includes eight items with a four‐point Likert scale. The total score ranges from 8 to 32. A higher score indicates higher satisfaction (Larsen 1979). One study reported data from the scale.
8.3.6.2. Patient Satisfaction Scale ‐ PSS‐Fin PSS‐Fin is a Finnish adaptation of the Patient Satisfaction Scale (PSS, Kuosmanen 2009; Suhonen 2007). It includes 10 items in three sub‐scales (technical‐scientific, informational and interaction/support care needs). The answer format is a four‐point Likert‐type scale (1 = very dissatisfied, 2 = dissatisfied, 3 = satisfied, 4 = very satisfied). The PSS‐Fin has demonstrated good psychometric properties and conceptual rigour when used with surgical patients (Suhonen 2007). It has been used in a study with schizophrenia patients, but with no reliability testing (Kuosmanen 2009). A higher score indicates higher satisfaction. One study reported data from the scale.
8.3.7 Needs scales
8.3.7.1 Camberwell Assessment of Need ‐ Short Appraisal Schedule; patient‐rated version – CANSAS (Slade 2005) The Camberwell Assessment of Need ‐ Short Appraisal Schedule assesses health and social needs across 22 domains. The schedule summarises whether a person with mental health problems has difficulties in 22 different areas of life, and whether they are currently receiving any effective help with these difficulties. For each domain it distinguishes between ‘no need' (rating of 0), ‘met need’ (rating of 1) and ‘unmet need' (rating of 2). A higher score indicates higher unmet needs. One study reported data from the scale.
8.3.8 Social support scales
8.3.8.1 Social Network Interview (Fischer 1982; McCallister 1978; Zautra 1984) The Social Network Interview assess social network characteristics using an abridged 14‐item version of a scale. Ten items on this scale ask subjects to identify people who provide various types of instrumental social support (e.g. financial assistance or help with shopping or errands) and emotional social support (e.g. whom they confided in, discussed personal worries with, relied on for advice). The remaining four items ask subjects to identify individuals who are the sources of negative social experiences. Probes ask respondents to name individuals who cause problems by criticising the subject's behaviour, breaking promises to the subject, or consistently provoking feelings of anger.
8.4 Missing outcomes
One trial reported on our primary outcome compliance with medication (Chen 2007) and two trials reported on our primary outcome global state (Chen 2007; Jones 2001a). Data on compliance with medication from two trials could not be used (Salzer 2004; Valimaki 2010) and data on global state could not be used in two trials (Jones 2001a; Valimaki 2010). All six included studies reported compliance with intervention in terms of leaving the study early (Chen 2007; Hansson 2008; Jones 2001b, Rotondi 2005; Salzer 2004; Valimaki 2010). However, there were no data on behaviour, service utilisation, economic outcomes or mortality.
Excluded studies
We excluded 18 studies. Han 2008 and Shon 2002 had to be excluded because they were not randomised. We also excluded nine studies because they employed an intervention not appropriate for this review (Burgoyne 1983; Fan 2005; Frangou 2005; Kopelowicz 2006; Montes 2009; Park 2009; Pijnenborg 2010; Rossi 1994; Yi 2006). A further two studies were excluded because no data were usable due to the lack of presentation of means or standard deviations (Berns 2001; Borell 1995). Finally, we could not included five trials because the majority of participants were not people with schizophrenia (Cramer 1999; Crespo 2006; Janssen 2010; Kluger 1983; MacDonald 2000).
Studies awaiting assessment
No studies are awaiting assessment.
Ongoing studies
Six studies are ongoing (Hulsbosch 2008; Jahn 2008; Potts 2008; Prague 2008; Weisbrod 2007; Young 2009). These trials were started between 2006 and 2011 and should be completed between 2009 and 2013. We currently have no information on the results of these trials.
Risk of bias in included studies
Our overall impression of risk of bias in the included studies is represented in Figure 2. The suggestion is that there is a moderate risk of bias and therefore a risk of overestimating any positive effects of ICT use in psychoeducation or patient support.
2.
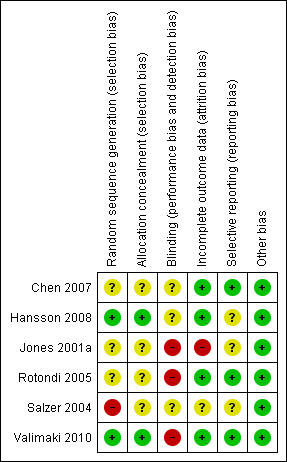
'Risk of bias' summary: review authors' judgements about each methodological quality item for each included study.
No study implied randomisation ‐ all clearly stated that randomisation took place. Therefore we have not undertaken any sensitivity analysis regarding this.
Allocation
All six studies were stated to be randomised but only two trials were clear about the means of allocation (Hansson 2008; Valimaki 2010). Three studies were not explicit about how allocation was achieved other than using the word "randomised" and therefore we categorised them as ‘unclear’ (Chen 2007; Jones 2001b; Rotondi 2005). One study randomly allocated participants matching age, length of illness and type of schizophrenia. However, sequence generation and allocation concealment was unclear (Salzer 2004).
Blinding
Three out of six studies reported that no form of blinding had been used (Jones 2001b; Rotondi 2005; Valimaki 2010). The remaining three studies did not report whether blinding had been used. Therefore we had to rate the risk of observed bias as (at best) ‘unclear’. This gathers further potential for overestimation of positive effects and underestimation of negative ones.
Incomplete outcome data
We considered four out of the six studies as reporting incomplete outcome data. Study attrition, however, was often not reported clearly and therefore trials may have used the technique of carrying forward the last observation of participants. This has introduced some more uncertainty into the results. It is unlikely that participants who left the studies early remained stable ‐ the assumption employed in the last observation carried forward technique.
When attrition rate was reported it varied between 11% (Hansson 2008) and 40% (Jones 2001a). In Jones 2001a high loss to follow‐up is a clear threat to the validity of findings. We took this into account when we interpreted the results of the review. Rotondi 2005 reported that there was no loss to follow‐up.
Selective reporting
The majority of data in this review originate from published reports. We have had no opportunity to see protocols for these trials to compare the outcomes reported in the full publications with what was measured during the conduct of the trial. We considered all included trials to be free of selective reporting.
Other potential sources of bias
We considered all six included studies to be free of other potential sources of bias. One Chinese trial (Chen 2007) was included. Strong and depressing evidence has emerged that many trials from the People's Republic of China which are stated to be randomised are not (Wu 2006). At this point we have not contacted the authors to verify the process by which they randomised. We will try to amend this situation for future versions of this review.
Effects of interventions
1. Comparison 1. Technology‐mediated psychoeducation versus standard care
1.1 Patient compliance: leaving the study early ‐ any reason
We found one study reporting short‐term and one reporting long‐term compliance with the intervention. Overall, there was no difference between groups (n = 116, two randomised controlled trials (RCTs), risk ratio (RR) 1.24, 95% confidence interval (CI) 0.81 to 1.88, Analysis 1.1).
1.1. Analysis.
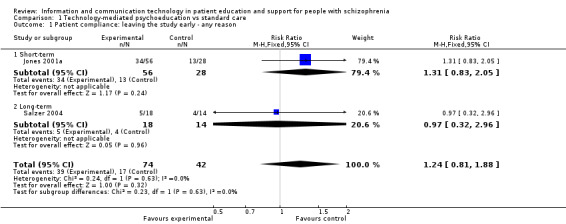
Comparison 1 Technology‐mediated psychoeducation vs standard care, Outcome 1 Patient compliance: leaving the study early ‐ any reason.
1.2 Mental state
1.2.1 Not improved (Brief Psychiatric Rating Scale (BPRS) measure) ‐ short‐term
One study reported mental state as a binary outcome (not improved). Fewer people allocated to technology‐mediated psychoeducation did not improve compared with those allocated to the standard care group (n = 84, RR 0.75, 95% CI 0.56 to 1.00, Analysis 1.2).
1.2. Analysis.
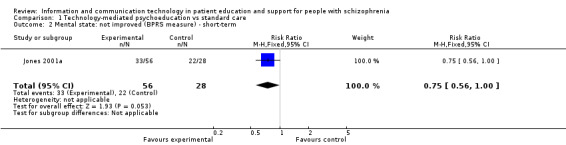
Comparison 1 Technology‐mediated psychoeducation vs standard care, Outcome 2 Mental state: not improved (BPRS measure) ‐ short‐term.
1.2.2 Average score (Perceived stress ‐ Psychological distress measure, high = good) ‐ short‐term
One small study reported mental state as a continuous outcome. Short‐term outcome favoured technology‐mediated psychoeducation (n = 30, mean difference (MD) ‐0.51, 95% CI ‐0.90 to ‐0.12, Analysis 1.3).
1.3. Analysis.
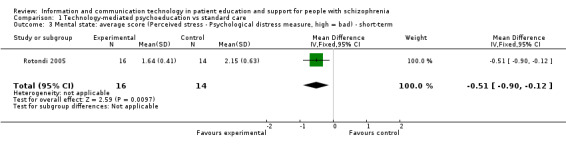
Comparison 1 Technology‐mediated psychoeducation vs standard care, Outcome 3 Mental state: average score (Perceived stress ‐ Psychological distress measure, high = bad) ‐ short‐term.
1.3 Global state: not improved (Global Assessment of Functioning (GAF) measure) ‐ short‐term
One study reported global state as a binary outcome. There was no difference between groups (n = 84, RR 1.07, 95% CI 0.82 to 1.42, Analysis 1.4).
1.4. Analysis.
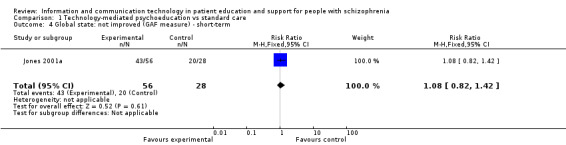
Comparison 1 Technology‐mediated psychoeducation vs standard care, Outcome 4 Global state: not improved (GAF measure) ‐ short‐term.
1.4 Knowledge and insight: not improved ‐ short‐term
Knowledge and insight were measured as two binary outcomes in one study. We found no difference between groups (n = 84, Insight and Treatment Attitude Questionnaire (ITAQ): RR 0.89, 95% CI 0.68 to 1.15, Knowledge and Information about Schizophrenia Schedule (KISS): RR 0.77, 95% CI 0.58 to 1.03, Analysis 1.5).
1.5. Analysis.
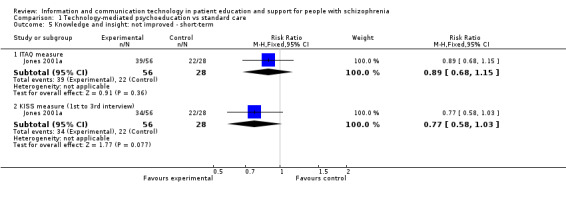
Comparison 1 Technology‐mediated psychoeducation vs standard care, Outcome 5 Knowledge and insight: not improved ‐ short‐term.
1.5 Social support: average score (Social Network Interview, high = good) ‐ short‐term
People allocated to technology‐mediated psychoeducation perceived that they received more social support than people allocated to the standard care group (n = 30, 1 RCT, MD 0.42, 95% CI 0.04 to 0.80, Analysis 1.6).
1.6. Analysis.
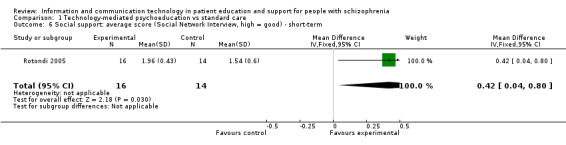
Comparison 1 Technology‐mediated psychoeducation vs standard care, Outcome 6 Social support: average score (Social Network Interview, high = good) ‐ short‐term.
2. Comparison 2. Technology‐mediated psychoeducation plus standard care versus standard care
2.1 Compliance
2.1.2 General ‐ leaving the study early ‐ any reason
We found five studies reporting compliance with the intervention. There was no difference between groups in the short term (n = 291, 3 RCTs, RR 0.81, 95% CI 0.55 to 1.19) or in the long term (n = 434, 2 RCTs, RR 0.70, 95% CI 0.39 to 1.25, Analysis 2.1).
2.1. Analysis.
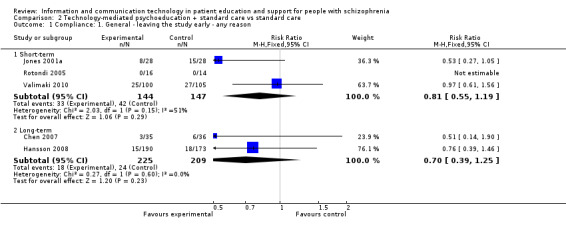
Comparison 2 Technology‐mediated psychoeducation + standard care vs standard care, Outcome 1 Compliance: 1. General ‐ leaving the study early ‐ any reason.
2.1.2 Medication ‐ not compliant ‐ long‐term
One study reported compliance with medication. People allocated to technology‐mediated psychoeducation plus standard care intervention were more compliant with medication than people allocated to the standard care group (n = 71, RR 0.45, 95% CI 0.27 to 0.77, Analysis 2.2).
2.2. Analysis.
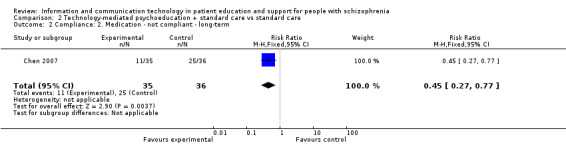
Comparison 2 Technology‐mediated psychoeducation + standard care vs standard care, Outcome 2 Compliance: 2. Medication ‐ not compliant ‐ long‐term.
2.2 Mental state
2.2.1 Not improved (BPRS measure) ‐ short‐term
We found one small study reporting mental state as a binary outcome (not improved). There was no difference between groups (n = 56, RR 0.86, 95% CI 0.63 to 1.19, Analysis 2.3).
2.3. Analysis.
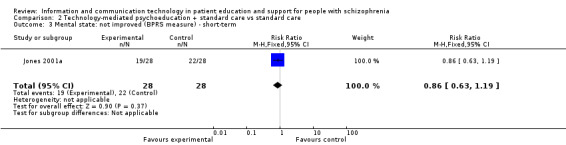
Comparison 2 Technology‐mediated psychoeducation + standard care vs standard care, Outcome 3 Mental state: not improved (BPRS measure) ‐ short‐term.
2.2.2 General ‐ overall total ‐ average endpoint score (Positive and Negative Syndrome Scale (PANSS) total, high = bad)
Mental state was measured with total score in one study with 71 participants. There were significant differences favouring the technology‐mediated psychoeducation plus standard care intervention in the short term (MD ‐6.07, 95% CI ‐9.77 to ‐2.37), in the medium term (MD ‐7.63, 95% CI ‐11.18 to ‐4.08) and in the long term (MD ‐11.90, 95% CI 14.93 to ‐8.87, Analysis 2.4)
2.4. Analysis.
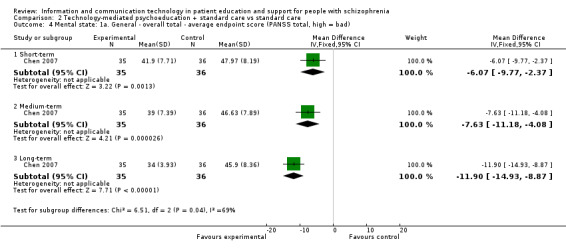
Comparison 2 Technology‐mediated psychoeducation + standard care vs standard care, Outcome 4 Mental state: 1a. General ‐ overall total ‐ average endpoint score (PANSS total, high = bad).
2.2.3 Mental state: 1b. General ‐ general symptoms sub‐score ‐ average endpoint (PANSS sub‐scale, high = bad) ‐ long‐term
One study measured mental state with general symptoms sub‐score in the long term. We found no difference between groups (n = 363, MD ‐0.46, 95% CI ‐2.44 to 1.52, Analysis 2.5).
2.5. Analysis.
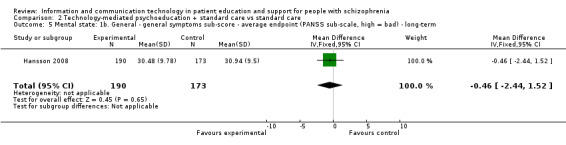
Comparison 2 Technology‐mediated psychoeducation + standard care vs standard care, Outcome 5 Mental state: 1b. General ‐ general symptoms sub‐score ‐ average endpoint (PANSS sub‐scale, high = bad) ‐ long‐term.
2.2.4 Mental state: 2. Specific ‐ negative symptoms ‐ average endpoint (PANSS sub‐scale, high = bad)
One study reported mental state measured with negative symptoms sub‐score in the long term. There was no difference between groups (n = 363, MD ‐0.45, 95% CI ‐1.77 to 0.87, Analysis 2.6).
2.6. Analysis.
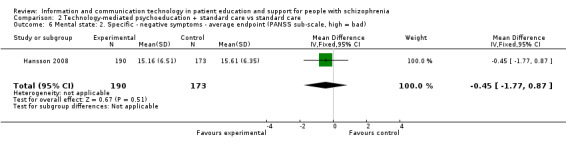
Comparison 2 Technology‐mediated psychoeducation + standard care vs standard care, Outcome 6 Mental state: 2. Specific ‐ negative symptoms ‐ average endpoint (PANSS sub‐scale, high = bad).
2.2.5 Mental state: 3. Specific ‐ positive symptoms ‐ average endpoint (PANSS sub‐scale, high = bad) ‐ long‐term
One study reported mental state measured as positive symptoms sub‐score in the long term. We found no difference between groups (n = 363, MD ‐0.75, 95% CI ‐1.96 to 0.46, Analysis 2.7).
2.7. Analysis.
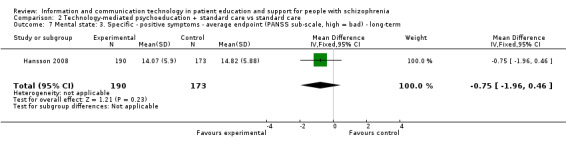
Comparison 2 Technology‐mediated psychoeducation + standard care vs standard care, Outcome 7 Mental state: 3. Specific ‐ positive symptoms ‐ average endpoint (PANSS sub‐scale, high = bad) ‐ long‐term.
2.3 Global state
2.3.1 Not improved (GAF measure) ‐ short‐term
There was no difference between groups (n = 56, 1 RCT, RR 1.15, 95% CI 0.86 to 1.54, Analysis 2.8).
2.8. Analysis.
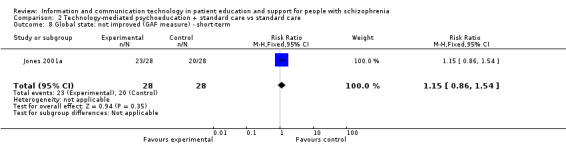
Comparison 2 Technology‐mediated psychoeducation + standard care vs standard care, Outcome 8 Global state: not improved (GAF measure) ‐ short‐term.
2.3.2 Average score (Social Disability Screening Schedule (SDSS), high = bad) ‐ long‐term
Global state among people allocated to the technology‐mediated psychoeducation plus standard care group improved more than among people allocated to the standard care group (n = 71, 1 RCT, MD ‐4.60, 95% CI ‐5.95 to ‐3.25, Analysis 2.9).
2.9. Analysis.
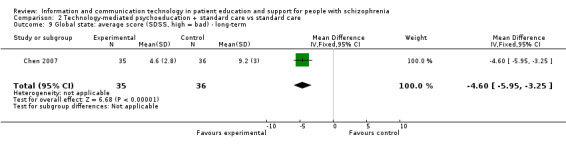
Comparison 2 Technology‐mediated psychoeducation + standard care vs standard care, Outcome 9 Global state: average score (SDSS, high = bad) ‐ long‐term.
2.4 Knowledge and insight: not improved
Knowledge and insight were measured with two binary outcomes in one study. We found no difference between groups measured with ITAQ (n = 56, RR 1.00, 95% CI 0.76 to 1.31). However, when knowledge by month three was measured with KISS, fewer people allocated to the technology‐mediated psychoeducation plus standard care group did not improve compared with those allocated to the standard care group (n = 56, RR 0.64, 95% CI 0.42 to 0.97, Analysis 2.10).
2.10. Analysis.
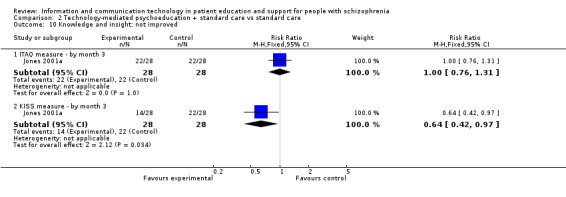
Comparison 2 Technology‐mediated psychoeducation + standard care vs standard care, Outcome 10 Knowledge and insight: not improved.
2.5 Quality of life: average score (Manchester Short Assessment of Quality of Life (MANSA), high = bad) ‐ long‐term
We found no difference between groups in quality of life (n = 363, 1 RCT, MD 0.12, 95% CI ‐0.00 to 0.24, Analysis 2.11).
2.11. Analysis.
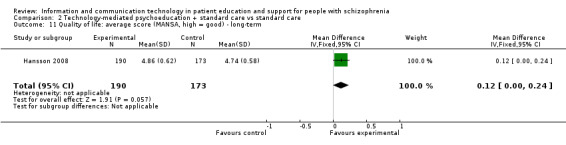
Comparison 2 Technology‐mediated psychoeducation + standard care vs standard care, Outcome 11 Quality of life: average score (MANSA, high = good) ‐ long‐term.
2.6 Satisfaction with treatment
2.6.1 Average score (Patient Satisfaction Scale (PSS‐Fin) measure, high = good) ‐ at discharge
We found no difference between groups in satisfaction with treatment at discharge (n = 145, 1 RCT, MD ‐0.02, 95% CI ‐0.19 to 0.15, Analysis 2.12).
2.12. Analysis.
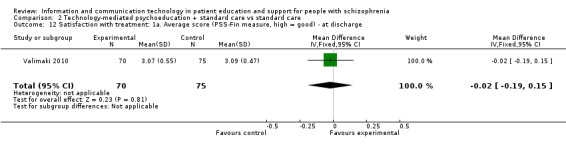
Comparison 2 Technology‐mediated psychoeducation + standard care vs standard care, Outcome 12 Satisfaction with treatment: 1a. Average score (PSS‐Fin measure, high = good) ‐ at discharge.
2.6.2 Average score (Patient Satisfaction Questionnaire (CSQ‐8), high = good) ‐ long‐term
One study reported patient satisfaction with treatment. Long‐term outcome favoured technology‐mediated psychoeducation (n = 363, MD 0.92, 95% CI 0.10 to 1.74, Analysis 2.13).
2.13. Analysis.
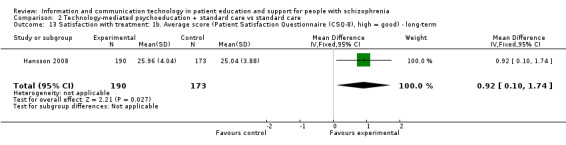
Comparison 2 Technology‐mediated psychoeducation + standard care vs standard care, Outcome 13 Satisfaction with treatment: 1b. Average score (Patient Satisfaction Questionnaire (CSQ‐8), high = good) ‐ long‐term.
2.7 Needs: average score (Camberwell Assessment of Need ‐ Short Appraisal Schedule (CANSAS), high = bad) ‐ long‐term
We found no difference between groups (n = 363, 1 RCT, MD ‐0.41, 95% CI ‐0.89 to 0.07, Analysis 2.14).
2.14. Analysis.
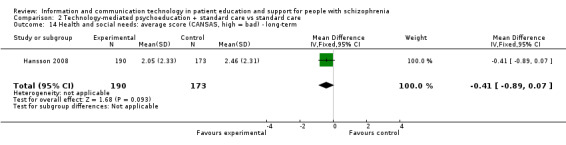
Comparison 2 Technology‐mediated psychoeducation + standard care vs standard care, Outcome 14 Health and social needs: average score (CANSAS, high = bad) ‐ long‐term.
2.8 Sensitivity analysis
Data from Chen 2007 were included in the primary outcome of 'compliance' (Analysis 2.1). This is by far the smallest of the two studies in this subgroup (long‐term) and removal of these data makes no substantive difference to the direction or precision of the result (n = 434, 2 RCTs, RR 0.70, 95% CI 0.39 to 1.25 versus 1 RCT, n = 363, RR 0.76, 95% CI 0.39 to 1.46).
3. Comparison 3. Technology‐mediated psychoeducation plus standar care intervention versus patient education not using technology
3.1 Satisfaction with treatment: average score (PSS‐Fin measure, high = good) ‐ at discharge
We found no difference between groups in satisfaction with treatment at discharge (n = 135, 1 RCT, MD ‐0.05, 95% CI ‐0.23 to 0.13, Analysis 3.1).
3.1. Analysis.
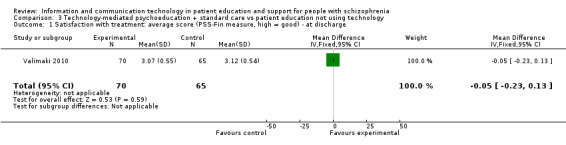
Comparison 3 Technology‐mediated psychoeducation + standard care vs patient education not using technology, Outcome 1 Satisfaction with treatment: average score (PSS‐Fin measure, high = good) ‐ at discharge.
Discussion
Summary of main results
1. Comparison 1. Technology‐mediated psychoeducation versus standard care intervention
Three studies with a total of 146 participants fell into this comparison.
1.1 Patient compliance
The overall rate of participants leaving the studies early was high (48%). There was no difference between groups. However, this finding was based on only two trials, limiting any interpretation but, nevertheless, such high attrition should be avoidable though empathetic study design ‐ or if it is not avoidable this then reflects badly on the acceptability of the trial and, perhaps, the applicability of the findings.
1.2 Mental state ‐ short‐term
Two different outcome measures revealed that technology‐mediated psychoeducation might improve mental state more than a standard care intervention. This is encouraging but the result must be interpreted with great caution as it was based on two small trials (total n = 114) reporting in different ways. More data are needed.
1.3 Global state
One trial reported no significant difference in effect on global state between interventions. This is not a difficult outcome to record and should have been feasible for all three trials.
1.4 Knowledge and insight
Jones 2001a used two different measures of knowledge and insight. The trial found no clear effect of technology‐mediated psychoeducation but the study was small (n = 84) and may have been underpowered. As with other outcomes, there is more work to be done.
1.5 Quality of life
No trial reported quality of life outcomes. As with other measures, recording of quality of life does not need to be complex and would seem to be of interest as an outcome in studies of this type.
1.6 Needs
No trial reported needs outcomes.
1.7 Social support
Perceived instrumental and emotional social support received from other people was higher for technology‐mediated patient education than for the standard care intervention. This result must be interpreted with great caution as it was based on one trial.
2. Comparison 2. Technology‐mediated psychoeducation plus standard care intervention versus standard care intervention
We included five studies with a total of 725 participants.
2.1 Patient compliance
Five studies reported compliance with the intervention and one study compliance with medication. In the short term, the rate of participants leaving studies early was high (26%). In the long term it was low (10%). There was no difference between groups in the short or long term suggesting a similar overall acceptability of interventions. On the other hand, the technology‐mediated psychoeducation plus standardised care intervention may improve compliance with medication more than the standard care. This result, however, is based on limited data from one small Chinese study (n = 77, Chen 2007) and the result must be interpreted with caution.
2.2 Mental state
Altogether three studies recorded outcome for mental state. There was no significant difference in mental state between interventions as measured in one study as a binary outcome in the short term. Two studies used the Positive and Negative Syndrome Scale (PANSS) scale. The results of these studies were not consistent. Very limited data from the Chen 2007 study suggest that the technology‐mediated psychoeducation plus standard care intervention might be slightly more effective than the standard care intervention for mental state in the short, medium and long term. On the other hand, no difference between interventions was found in general, negative or positive symptoms in a large study (n = 363) (Hansson 2008). Although findings tended to fall on the side of the line favouring the technology‐medicated approach, there were no convincing data that a real and important effect of the experimental intervention exists. The converse is that no data exist to refute effectiveness or to suggest that further investigation is not indicated.
2.3 Global state
Again we found inconsistent results. One study found no difference between groups on the measure of global state (Global Assessment of Functioning (GAF)); the other found in favour of the technology‐mediated psychoeducation plus standard care using the Social Disability Screening Schedule (SDSS). Both trials are small and this is also a finding that should be replicated.
2.4 Knowledge and insight
Knowledge and insight were measured as two binary outcomes in one study. We found no difference between interventions for insight. However, the technology‐mediated psychoeducation plus standard care intervention had more effect on knowledge than the standard care. This should not be taken as concrete evidence of a real effect and, as with other findings, should be replicated.
2.5 Quality of life ‐ long‐term
There was no significant difference in effect on quality of life between interventions. This was reported by only one trial, but it was the largest study with over 300 participating groups (Hansson 2008). That the technology‐mediated psychoeducation did not add discernibly to people's quality of life must be a cause for concern about its value overall.
2.6 Satisfaction with care
Here findings from the two better quality studies are not consistent. The Patient Satisfaction Scale (PSS‐Fin) measure used in Valimaki 2010 found no difference between groups, whereas the Client Satisfaction Questionnaire‐8 (CSQ‐8) in Hansson 2008 did. We are unclear if the difference in this latter finding (mean difference 0.92) really is of importance in everyday care ‐ but it remains an important research finding that would be good to replicate and build upon.
2.7 Health and social needs
There was no clear difference in effects on needs between interventions. Again, this important measure was used by only one study (Hansson 2008) and the finding was disappointing for those hoping to see a useful effect of the technology‐mediated treatment.
2.8 Social support
No trial within this comparison reported social support outcome.
3. Comparison 3. Technology‐mediated psychoeducation plus standard care versus patient education not using technology
We were able to include one study with 135 participants. No trial reported outcomes on compliance, mental state, global state, knowledge and insight, quality of life, needs or social support.
3.1 Satisfaction with treatment
There was no significant difference in satisfaction with interventions between groups. This small but important study left much unrecorded and unreported. It may well have been underpowered to show real differences and we, therefore, know very little about relative effects of delivering education in these two different ways.
Overall completeness and applicability of evidence
1. Completeness
No outcomes in this review involve large numbers of people or many trials that have been able to replicate findings in different settings. Moreover, general measures were used and more subtle findings are not recorded. For example, we identified few data on the outcome quality of life ‐ an important outcome of psychosocial interventions. There were no data on behaviour, service utilisation, economic outcomes or mortality. The evidence, therefore, is incomplete.
2. Applicability
Four of the six included trials were out‐patient based, one was out‐ and in‐patient based and one was undertaken only in hospital. The results are therefore most applicable to out‐patient settings. Moreover, a majority used long‐term outcomes (over 26 weeks) increasing the applicability of the evidence for people over the longer course of their illness.
Quality of the evidence
All studies were randomised, but details were frequently not presented. It is, therefore, unclear in almost all studies whether randomisation was really appropriately done. Interventions were heterogeneous and results have to be considered as indicative only. Furthermore, high numbers of participants leaving the studies early (overall 20%) and high variation in attrition rates between studies (from 0% to 40%) call the validity of many of the findings into question. There is a risk of overestimating the effects of the experimental interventions. All of these factors limit the quality of the evidence. Please refer to Figure 2 for a graphic representation of the methodological quality of included studies; these judgements are also reflected in Table 1 and Table 2.
Potential biases in the review process
We are interested in this area and had prior positive biases towards use of technology‐medicated approaches. We have tried not to allow these to influence us in the production of this review but it is possible that they did.
We have tried to be as comprehensive as possible in the identification of relevant trials but are aware that in this fast‐moving area we may have erroneously omitted studies or failed to identify relevant work. We would welcome any constructive comments or new data.
Agreements and disagreements with other studies or reviews
We know of no previous reviews comparing psychoeducational interventions using information and communication technology (ICT) as a means of educating and supporting people with schizophrenia or related psychosis with to standard care, other methods of delivering support and psychoeducation, or both. The only relevant review we know of is the Cochrane review on interactive health communication applications (IHCA) for people with chronic disease (Murray 2005). The results of this review showed more positive effects than our review. IHCA had a significant positive effect on knowledge, perceived social support, health behaviours and clinical outcomes. The results also suggested that IHCA might have a positive effect on self efficacy. The effects of IHCAs on emotional or economic outcomes were not possible to determine.
Authors' conclusions
Implications for practice.
1. For people with schizophrenia
It may be important to be aware that different psychoeducational interventions, including ICT‐based interventions, are available as means of support and education for people with schizophrenia. Although the effects of these interventions are not superior compared with standard care, other methods of delivering psychoeducation and support, or both, they can, however, offer an alternative method of support and education. Therefore, they should be taken seriously in psychiatric care.
2. For clinicians
Clinicians should be aware that psychoeducational interventions using ICT might have positive effect on patients’ compliance, mental state, global state, perceived social support and satisfaction with treatment. However, the evidence is limited because very high rates of participants left the studies early and the effect sizes were small. Certainly, more studies comparing psychoeducational interventions using ICT as a means of supporting people with schizophrenia or related psychosis with standard care, other methods of delivering support and psychoeducation, or both, are needed as is the input of clinicians into the design of these studies.
3. For managers/policymakers
This review has important implications for policymakers. As health policy tends to be nation‐specific, these implications are likely to vary from country to country. We have tried to outline the main implications for policy for countries with highly developed healthcare systems, as most of the work on psychoeducational interventions using ICT to date has been done in these countries. However, we have also outlined the implications for low‐income countries where ICT provides opportunities to facilitate access to health.
Our review suggests that psychoeducational interventions using ICT are a promising method to support and educate people with schizophrenia. These results support continued cautious investment in psychoeducational interventions using ICT, coupled with a rigorous programme of evaluation. Moreover, there is need to invest and develop less expensive technology to be used in underdeveloped countries. There is no evidence of the effects of psychoeducational interventions using ICT on health service utilisation. Thus, psychoeducational interventions using ICT should not be used as a method of cost containment, pending further research. Although these findings are not sufficiently robust to guide managers and policymakers, the development of an information society also requires the development of ICT‐based services for underserved populations such as people with schizophrenia.
Implications for research.
1. General
We would like to stress the importance of adhering to the CONSORT statement in future studies. Following these recommendations would clearly improve the conduct and reporting of clinical trials, ease critical appraisal, and help in formulating recommendations and making decisions in health care (Moher 2010).
2. Specific
2.1 Reviews
Several interesting trials had to be excluded as they did not quite fall into the scope of this review. We suggest that they could be the focus of future reviews (Table 3).
1. Suggested reviews.
| Title | Excluded study in this review |
| Mobile phone text message (SMS) prompting to increase attendance at a psychiatric outpatient clinic. |
Burgoyne 1983; Montes 2009; Pijnenborg 2010; Rossi 1994 |
| ICT monitoring to support compliance with medication. | Frangou 2005 |
| Motion control video games on clinical and extrapyramidal symptoms in patients with schizophrenia. | ‐ |
ICT: information and communication technology
2.2 Trials
Large, simple, well‐designed and well‐reported trials are still needed. Interventions should be clearly described in detail, especially regarding the use of ICT as a part of the intervention. We also recommend designing the interventions more comprehensively, based on existing knowledge on the effective factors. There should be research on existing and widely used interventions. On the other hand, innovative and future‐oriented interventions are also needed. Data collection should include at least quality of life, knowledge level, mental state, medication compliance, service use and economic evaluations. Moreover, outcome measures used and reported should be valid for evaluating the effects of interventions among people with schizophrenia, but should be simple and useful for research, clinical care and dissemination to carers and people with schizophrenia. All measures used should be understandable by all users of research. Binary as well as continuous data should be reported. We do realise that huge effort goes into the design and conduct of such studies and to propose a design at the end of a review could seem trite. However, we have learnt much from very detailed reading and use of the existing studies and have tried to summarise these in our suggested design (Table 4).
2. Suggested design of future trial.
| Methods | Allocation: randomised, fully explicit description of methods of randomisation and allocation concealment. Blindness: those collecting and analysing data unaware of the assigned treatment. Duration: 6 weeks treatment and follow‐up at least 52 weeks. Design: parallel. Location: simultaneously in multiple geographical locations (multicentre international study). |
| Participants | Diagnosis: schizophrenia. N = 600.* Age: range 18 to 65 years. Gender: mixed. Setting: community mental health services (out‐patients). Excluded: no access to technology, severe cognitive deficits, primary substance abuse. |
| Interventions | 1. Information technology intervention using one simple method (text message, virtual environment, Internet). N = 300. 2. Treatment as usual in community mental health services. N = 300. |
| Outcomes | Relapse: re‐hospitalisation. Quality of life: Quality of Life Questionnaire (Q‐Les‐Q) or simple binary question. Mental state: simple binary question/CGI. Satisfaction with treatment: Client Satisfaction Questionnaire (CSQ‐8) or simple binary question. Use of services: recorded use of health and social services. Economic outcomes. |
| Notes | * Powered to be able to identify a difference of about 20% between groups for primary outcome with adequate degree of certainty. |
CGI ‐ Clinical Global Impression
Acknowledgements
The authors would like to thank the Trial Search Co‐ordinator of the Cochrane Schizophrenia Group, Claire Irving and Samantha Roberts, for running the trial searches.
The methods section of this review this review uses text from the Cochrane Schizophrenia Group’s generic text which has been written to ensure consistency and clarity. We fully acknowledge use of this text which we have adapted for relevance to this particular review.
Appendices
Appendix 1. Searches 2008, 2009, 2010
In 2008 our search yielded 505 electronic records. Due to increasing number of research related to the topic, we repeated the search in June 2009 and September 2010. In 2009, the search yielded 574 new references (from 293 studies) and 231 references (from 191 studies) in 2010. Altogether searches yielded 1310 references, of which we rejected 1280 papers after the first inspection. We inspected the other 30 papers and rejected 18 as not relevant for the following mutually exclusive reasons: not a randomised controlled trial, participants were not people with schizophrenia, the intervention was not psychoeducational interventions using ICT as a means of educating and supporting patients. No new trials were identified for the comparison of interventions versus standard care. In this process six ongoing studies were recognised by the review authors to be relevant and were included in the section of ongoing studies. We considered the remaining six papers further. The total number of studies which met our inclusion criteria closely enough to be mentioned in either the included studies or excluded studies section was 24. The review cites 18 studies dating from 1983 to 2010 in the excluded studies section and six studies dating from 2001 to 2010 in the included studies section.
Data and analyses
Comparison 1. Technology‐mediated psychoeducation vs standard care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patient compliance: leaving the study early ‐ any reason | 2 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.81, 1.88] |
| 1.1 Short‐term | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.83, 2.05] |
| 1.2 Long‐term | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.32, 2.96] |
| 2 Mental state: not improved (BPRS measure) ‐ short‐term | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.56, 1.00] |
| 3 Mental state: average score (Perceived stress ‐ Psychological distress measure, high = bad) ‐ short‐term | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐0.51 [‐0.90, ‐0.12] |
| 4 Global state: not improved (GAF measure) ‐ short‐term | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.82, 1.42] |
| 5 Knowledge and insight: not improved ‐ short‐term | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 ITAQ measure | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.68, 1.15] |
| 5.2 KISS measure (1st to 3rd interview) | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.58, 1.03] |
| 6 Social support: average score (Social Network Interview, high = good) ‐ short‐term | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 0.42 [0.04, 0.80] |
Comparison 2. Technology‐mediated psychoeducation + standard care vs standard care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Compliance: 1. General ‐ leaving the study early ‐ any reason | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Short‐term | 3 | 291 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.55, 1.19] |
| 1.2 Long‐term | 2 | 434 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.39, 1.25] |
| 2 Compliance: 2. Medication ‐ not compliant ‐ long‐term | 1 | 71 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.27, 0.77] |
| 3 Mental state: not improved (BPRS measure) ‐ short‐term | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.63, 1.19] |
| 4 Mental state: 1a. General ‐ overall total ‐ average endpoint score (PANSS total, high = bad) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Short‐term | 1 | 71 | Mean Difference (IV, Fixed, 95% CI) | ‐6.07 [‐9.77, ‐2.37] |
| 4.2 Medium‐term | 1 | 71 | Mean Difference (IV, Fixed, 95% CI) | ‐7.63 [‐11.18, ‐4.08] |
| 4.3 Long‐term | 1 | 71 | Mean Difference (IV, Fixed, 95% CI) | ‐11.90 [‐14.93, ‐8.87] |
| 5 Mental state: 1b. General ‐ general symptoms sub‐score ‐ average endpoint (PANSS sub‐scale, high = bad) ‐ long‐term | 1 | 363 | Mean Difference (IV, Fixed, 95% CI) | ‐0.46 [‐2.44, 1.52] |
| 6 Mental state: 2. Specific ‐ negative symptoms ‐ average endpoint (PANSS sub‐scale, high = bad) | 1 | 363 | Mean Difference (IV, Fixed, 95% CI) | ‐0.45 [‐1.77, 0.87] |
| 7 Mental state: 3. Specific ‐ positive symptoms ‐ average endpoint (PANSS sub‐scale, high = bad) ‐ long‐term | 1 | 363 | Mean Difference (IV, Fixed, 95% CI) | ‐0.75 [‐1.96, 0.46] |
| 8 Global state: not improved (GAF measure) ‐ short‐term | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.86, 1.54] |
| 9 Global state: average score (SDSS, high = bad) ‐ long‐term | 1 | 71 | Mean Difference (IV, Fixed, 95% CI) | ‐4.6 [‐5.95, ‐3.25] |
| 10 Knowledge and insight: not improved | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 ITAQ measure ‐ by month 3 | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.76, 1.31] |
| 10.2 KISS measure ‐ by month 3 | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.42, 0.97] |
| 11 Quality of life: average score (MANSA, high = good) ‐ long‐term | 1 | 363 | Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.00, 0.24] |
| 12 Satisfaction with treatment: 1a. Average score (PSS‐Fin measure, high = good) ‐ at discharge | 1 | 145 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.19, 0.15] |
| 13 Satisfaction with treatment: 1b. Average score (Patient Satisfaction Questionnaire (CSQ‐8), high = good) ‐ long‐term | 1 | 363 | Mean Difference (IV, Fixed, 95% CI) | 0.92 [0.10, 1.74] |
| 14 Health and social needs: average score (CANSAS, high = bad) ‐ long‐term | 1 | 363 | Mean Difference (IV, Fixed, 95% CI) | ‐0.41 [‐0.89, 0.07] |
Comparison 3. Technology‐mediated psychoeducation + standard care vs patient education not using technology.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Satisfaction with treatment: average score (PSS‐Fin measure, high = good) ‐ at discharge | 1 | 135 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.23, 0.13] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Chen 2007.
| Methods | Allocation: randomly allocated, participants matched (age, gender, length of illness, type of schizophrenia). Blindness: no further details. Duration: 12 months. Design: parallel. Location: no further details. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3). N = 71. Age: range 15 to 65 years. Gender: mixed. History: length of illness 10 years (SD 4). Setting: community services (out‐patients). Excluded: no access to Internet, not living with family member or carers. | |
| Interventions | 1. Technology‐mediated psychoeducation + standardised nursing intervention: digital and spot network group (community prevention group) ‐ psychological online consultation. N = 35. 2. Standardised nursing intervention: community prevention group ‐ general rehabilitation. N = 36. |
|
| Outcomes | Compliance: drug taken compliance, leaving the study early. Patient compliance: leaving the study early, any reason. Mental State: relapse, PANSS. Global state: SDSS. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised participants matched (age, gender, length of illness, type of schizophrenia)". No further details. |
| Allocation concealment (selection bias) | Unclear risk | No further details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No further details. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rate was 13%. Analysis on intention‐to‐treat basis. |
| Selective reporting (reporting bias) | Low risk | We had no access to the protocol; there was no obvious selective reporting. |
| Other bias | Low risk | No clear conflicts of interest. |
Hansson 2008.
| Methods | Allocation: cluster (key worker), randomised. Blindness: researchers masked. Duration: 1 year. Design: parallel. Location: multicentre ‐ 6 countries. | |
| Participants | Diagnosis: schizophrenia (ICD‐10). N = 134 key workers, 507 people. Age (participants): range 18 to 65 years, mean ˜42 years (SD ˜12). Gender (participants): M 336, F 171. Setting: community mental health services. History (participants): duration of illness mean ˜16 years (SD ˜10), number of hospital admissions mean ˜ 5 (SD ˜7). Excluded (participants): no severe organic psychiatric illness, primary substance abuse, living in 24‐hour supported housing, expectation of discharge in next 24 months, insufficient contacts with co‐ordinator. | |
| Interventions | 1. Technology‐mediated psychoeducation (DIALOG; a computer‐mediated discussion of quality of life and needs for care) + key worker intervention. N = 64 key workers, 271 people. 2. Standardised intervention: key worker continuing with standard treatment. N = 70, 236 people. |
|
| Outcomes | Patient compliance: leaving the study early, any reason. Mental state: PANSS. Quality of life: MANSA. Satisfaction: CSQ‐8. Needs: Camberwell Assessment of Need Short Appraisal Schedule ‐ patient‐rated version. Unable to use ‐ Compliance: Helping Alliance Scale (no mean, SD). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated random block number generated sequence". Completed separately for each country and team. |
| Allocation concealment (selection bias) | Low risk | "Researcher not involved in the study generated the random allocation sequence". "Numbered sealed envelopes" then used to distribute allocation. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Attempts made to mask researchers ‐ but in 283/451 follow‐up interviews (63%) researchers stated they knew allocated treatment (correct in 275/283). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rate 11%, reasons reported, data analysed by intention‐to‐treat (including patients with at least one post randomisation observation). |
| Selective reporting (reporting bias) | Unclear risk | We had no access to the protocol; there was no obvious selective reporting. |
| Other bias | Low risk | No clear conflicts of interest. |
Jones 2001a.
| Methods | Allocation: randomly allocated. Blindness: no further details. Duration: 3 months after the last session. Design: parallel. Location: single. | |
| Participants | Diagnosis: schizophrenia (ICD‐10). N = 112. Age: range 18 to 65 years, no further details. Gender: M 75, F 37. History: mean BPRS ˜ 11 (SD 7), mean ITAQ ˜ 14 (SD 5), mean GAF ˜ 61 (SD 9), mean KISS ˜ 15 (SD 5). Setting: community services (out‐patients). Excluded: over 65 years, a uncertain diagnosis, acutely ill, chronic symptoms, physical problems restricting participation, were persistent defaulters, had recently been involved in education programme. | |
| Interventions | 1. Technology‐mediated psychoeducation: computer only ‐ combining information from medical records + general information about illness + questionnaires, 5 sessions + printed information material. N = 56. 2. Technology‐mediated psychoeducation + standardised nursing intervention: combination of computer and community psychiatric nurse ‐ combining information from medical records + general information about illness + questionnaires, 1st session with nurse, sessions 2 to 4 with computer only, 5th session with nurse + printed information material. N = 28. 3. Standardised nursing intervention: community psychiatric nurse only ‐ combining information from medical records + general information about illness + questionnaires, 5 sessions + printed information material. N = 28. |
|
| Outcomes | Patient compliance: leaving the study early, any reason.
Mental state: BPRS (binary).
Global state: GAF (binary).
Level of knowledge/insight: KISS, ITAQ (binary). Unable to use ‐ Mental state: average BPRS (no SD). Global state: average GAF (no SD). Level of knowledge/insight: average KISS, ITAQ (no SD). Health economic outcomes: direct treatment cost and use of community‐based service (modelling of cost of 3 interventions). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised" ‐ no further details. |
| Allocation concealment (selection bias) | Unclear risk | Unclear. |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding due to nature of interventions. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The overall attrition was 40%; assumed that those who discontinued would not have had change of condition if had remained in study. |
| Selective reporting (reporting bias) | Unclear risk | We had no access to the protocol; there was no obvious selective reporting. |
| Other bias | Low risk | No clear conflicts of interest. |
Rotondi 2005.
| Methods | Allocation: randomly allocated. Blindness: no further details. Duration: 6 months. Design: parallel. Location: multicentre. | |
| Participants | Diagnosis: schizophrenia, schizoaffective disorder. N = 30 (21 family members). Age: mean = 37.5 (SD = 10.7). Sex: F 21, M 9. History: Caucasian 14, African‐American 14, others 2, some post‐high school education 14, household income < USD 10,000, single 20. Excluded: under 14 years, non English speaking, no psychiatric hospitalisation within the previous 2 years. Setting: in and out‐patient psychiatric care unit and rehabilitation centres. | |
| Interventions | 1. Technology‐mediated psychoeducation: Telehealth psychoeducational intervention to decrease stress and social support ‐ 3 online therapy groups, question corner, question and answer library, news, education and reading material. N = 16. 2. Standardised nursing intervention: treatment as usual. N = 14. |
|
| Outcomes | Patient compliance: leaving the study early, any reason. Mental state: Perceived stress ‐ Psychological distress measure. Social support: Social Network Interview. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly assigned" ‐ no further details. |
| Allocation concealment (selection bias) | Unclear risk | No further details. |
| Blinding (performance bias and detection bias) All outcomes | High risk | No. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study follow‐up. |
| Selective reporting (reporting bias) | Low risk | We had no access to the protocol there was no obvious selective reporting. |
| Other bias | Low risk | No clear conflicts of interest. |
Salzer 2004.
| Methods | Allocation: randomly allocated demonstration study. Blindness: no further details. Duration: 12 months. Design: parallel. Location: single. | |
| Participants | Diagnosis: schizophrenia spectrum (DSM‐IV). N = 32. Age: no further details. Gender: no further details. History: also treated by psychiatrist who felt individual had problems with treatment compliance. Setting: out‐patient. Excluded: no further details. | |
| Interventions | 1. Technology‐mediated psychoeducation: brief telephone‐based medication management to enhance communication, insight, attitudes and knowledge, satisfaction, weekly, 52 weeks. N = 18. 2. Standardised care: treatment as usual. N = 14. |
|
| Outcomes | Patient compliance: leaving study early.* Unable to use ‐ Compliance: medication compliance, attitudes towards medication, subjective response to medication (data awaiting clarification). Mental state: symptoms and functioning (data awaiting clarification). Level of knowledge/insight: insight (data awaiting clarification). Satisfaction with treatment: treatment satisfaction (data awaiting clarification). Staff relationships (data awaiting clarification). Side effects: number of side effects, distress from side effects, number of extreme side effects (data awaiting clarification). |
|
| Notes | * We assumed if not included in analysis these people left early. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Randomly allocated", no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No further details. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The overall attrition rate was 28%. Only completer data were reported. |
| Selective reporting (reporting bias) | Unclear risk | We had no access to the protocol there was no obvious selective reporting. |
| Other bias | Low risk | No clear conflicts of interest. |
Valimaki 2010.
| Methods | Allocation: randomly allocated. Blindness: not for patients, professionals, due to nature of the intervention; blind to statistician. Duration: 12 months. Design: parallel. Location: multicentre. | |
| Participants | Diagnosis: schizophrenia (F20‐F29, ICD‐10). N = 311. Age: mean 38 (SD 13). Gender: M 184, F 127. History: PANSS total mean ˜ 72 (SD 20), no vocational education 150 persons, no academic education 30 persons, in‐hospital stay mean ˜ 60 days. Setting: in‐patient. Excluded: under 18 years, over 65 years, a uncertain diagnosis, not able to speak Finnish. | |
| Interventions | 1. Technology‐mediated psychoeducation: IT‐based patient education programme to support well‐being ‐ need‐based computerised information + discussions with staff + printed material, 5 sessions. N = 100. 2. Psychoeducation with no technology: patient education with standard leaflets ‐ patient education + discussion with staff + printed material, 5 sessions. N = 106. 3. Standard care: treatment as usual. N = 105. |
|
| Outcomes | Patient compliance: leaving the study early, any reason. Satisfaction with treatment: CSQ‐8. Unable to use ‐ Compliance: patients' compliance with treatment, DAI, follow‐up of medication compliance and treatment appointments ("will be reported later"). Mental state: PANSS ("will be reported later"). Global state: Sheehan Disability Scale, GAF ("will be reported later"). Level knowledge/insight: SAI, Knowledge test ("will be reported later"). Quality of life: EQ‐5D, MANSA ("will be reported later"). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patient randomisation was carried out centrally and manually". |
| Allocation concealment (selection bias) | Low risk | Randomisation "by a research director outside the study hospitals." |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding due to nature of interventions. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rate 30%, reasons reported, data analysed by intention‐to‐treat. |
| Selective reporting (reporting bias) | Low risk | "Other outcome data will be reported later". |
| Other bias | Low risk | No clear conflicts of interest. |
BPRS – Brief Psychiatric Rating Scale (Overall 1962) CANSAS – Camberwell Assessment of Need – Short Appraisal Schedule (Slade 2005) CCMD‐3 – Chinese Classification of Mental Disorders Version 3 (Chinese Society of Psychiatry 2001) CSQ‐8 – Client Satisfaction Questionnaire (Larsen 1979) DAI‐10 ‐ Drug Attitude Inventory (Hogan 1983) DIALOG – A computer‐mediated discussion of quality of life and needs for care DSM‐IV ‐ Diagnostic Statistical Manual, 4th Edition (APA 1994b) EQ‐5D – Health‐related quality of life questionnaire (EuroQol Group 1990) F – Female GAF – Global Assessment of Functioning (APA 1994a) ICD‐10 ‐ International Statistical Classification of Diseases and Related Health Problems (WHO 1992) ITAQ – Insight and Treatment Attitude Questionnaire (McEvoy 1989) KISS – Knowledge and Information about Schizophrenia Schedule (Barrowclough 1987; Jones 2001a) M – Male MANSA – Manchester Short Assessment of Quality of Life (Slade 2005) N – Number PANSS – Positive and Negative Syndrome Scale (Kay 1987) PSS‐Fin – Patient Satisfaction Scale (Suhonen 2007) SAI – The Schedule for the Assessment of Insight (David 1992) SD – Standard Deviation SDSS – Social Disability Screening Schedule (Shen 1985) WHO – World Health Organisation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Berns 2001 | Allocation: randomly allocated. Participants: people with schizophrenia. Interventions: electronic assistive device versus none. Outcomes: no data reported, abstract only and no reply from author. |
| Borell 1995 | Allocation: randomly allocated. Participants: people with schizophrenia. Interventions: technology‐mediated psychoeducation (including videos, discussion and individual plan) versus standard nursing intervention. Outcomes: not able to use data, no group specific data, only total group results available. |
| Burgoyne 1983 | Allocation: randomly allocated. Participants: out‐patients with mental disorder. No further details. Intervention: electronic prompting versus not prompting ‐ not psychoeducation delivered by ICT. Prompting interventions were excluded. |
| Cramer 1999 | Allocation: randomly allocated. Participants: depression/bipolar (24), schizoaffective/schizophrenia (26), post‐traumatic stress disorder (10). Under 50% participants with schizophrenia. We excluded studies where over 50% were other than patients with schizophrenia. |
| Crespo 2006 | Allocation: randomly allocated. Participants: no details available; setting was out‐patient clinic ‐ assumed that under 50% of participants are people with schizophrenia ‐ we excluded studies where over 50% were other than patients with schizophrenia. |
| Fan 2005 | Allocation: randomly allocated. Participants: people with schizophrenia. Interventions: community nursing package (including telephone contact) versus standard care (including telephone contact). Both interventions included telephone contact and not psychoeducation delivered by ICT. |
| Frangou 2005 | Allocation: randomly allocated. Participants: people with schizophrenia. Intervention: monitoring of medication compliance versus monitoring not using technology versus standard care ‐ not psychoeducation delivered by ICT. Interventions including only ICT for monitoring purpose were excluded. |
| Han 2008 | Allocation: no randomisation, participants "selected". |
| Janssen 2010 | Allocation: randomly allocated. Participants: psychiatrists, not people with schizophrenia. |
| Kluger 1983 | Allocation: randomly allocated. Participants: diagnosis unclear ‐ setting was out‐patient clinic ‐ assumed that under 50% of participants are people with schizophrenia. We excluded studies where over 50% were other than patients with schizophrenia. |
| Kopelowicz 2006 | Allocation: randomised, factorial design. Participants: people with schizophrenia. Interventions: olanzapine versus risperidone; occupational therapy versus work training ‐ latter included training in MS Word ‐ not psychoeducation delivered by ICT. |
| MacDonald 2000 | Allocation: a controlled prospective study, assignment by registration number. Participants: diagnosis unclear ‐ setting was out‐patient clinic ‐ assumed that under 50% of participants are people with schizophrenia. We excluded studies where over 50% were other than patients with schizophrenia. |
| Montes 2009 | Allocation: randomly allocated. Participants: people with schizophrenia. Interventions: telephone prompting versus no prompting ‐ not psychoeducation delivered by ICT. Prompting interventions were excluded. |
| Park 2009 | Allocation: randomly allocated. Participants: people with schizophrenia. Interventions: different antipsychotic medications ‐ special ICT glasses used to measure outcome ‐ not used as intervention. |
| Pijnenborg 2010 | Allocation: quasi‐randomised, order of attendance, cross‐over design, excluded because of problem with allocation. Participants: people with psychosis and limitations in goal‐directed behaviours. Interventions: SMS messages versus waiting longer for SMS messages. Both groups received ICT‐based intervention. |
| Rossi 1994 | Allocation: randomly allocated. Participants: people with schizophrenia. Intervention: prompting versus no prompt ‐ not psychoeducation delivered by ICT. Prompting interventions were excluded. |
| Shon 2002 | Allocation: "pre‐post test design", not randomised. |
| Yi 2006 | Allocation: randomly allocated. Participants: people with schizophrenia and depression. Interventions: education, guidance on use of medication, effort to control family expressed emotion, training on improving social function, and, when necessary, medical staff provide home‐visit or consultation over telephone versus standard care, telephoning not obligatory ‐ not psychoeducation delivered by ICT. |
ICT ‐ information and communication technology
Characteristics of ongoing studies [ordered by study ID]
Hulsbosch 2008.
| Trial name or title | Randomised controlled trial of home automation in mental health. |
| Methods | Allocation: randomly allocated. Blindness: no further details. Duration: no further details. Design: 2 or more arms, parallel. Location: no further details. |
| Participants | Diagnosis: people with severe mental illness (DSM‐IV). N = 100. Age: no further details. Gender: no further details. History: duration of illness at least 2 years. Setting: no further details. Excluded: no further details. |
| Interventions | 1. Technology‐mediated psychoeducation: digital communication device to assess the effects of home automation on client satisfaction with services, functioning, fulfilment of needs for care, quality of life and service contacts. The device opens the possibility for the services of regular/crisis consultation via webcam; computerised information about treatment plans, lab results, reminders and scheduled appointments, and support by significant others: clients can have webcam contact with family members or other significant contacts. 2. Standardised intervention: treatment as usual. |
| Outcomes | Global state: HoNOS. Quality of life: MANSA. Satisfaction with treatment: GGZ Thermometer. Needs: CANSAS. |
| Starting date | 1 July 2008 |
| Contact information | H. Kroon. Trimbos Institute ‐ Netherlands Institute of Mental Health and Addiction. P.O. Box 725, Da Costakade 45, 3500 AS, Utrecht, The Netherlands. +31 (0)30 2959286. hkroon@trimbos.nl. A.M. Hulsbosch. Trimbos Instituut, PO Box 725, 3500 AS, Utrecht, The Netherlands. 31 (0)30 2959315. lhulsbosch@trimbos.nl. |
| Notes |
Jahn 2008.
| Trial name or title | Cognitive Determinants of Psychoeducation and Information in Psychoses (COGPIP). |
| Methods | Interventional study with random allocation, parallel assignment. Allocation: randomly allocated. Blindness: no further details. Duration: no further details. Design: 2 or more arms, parallel. Location: no further details. |
| Participants | Diagnosis: schizophrenia, delusional disorders (ICD‐10). N = 120. |
| Interventions | 1. Standard treatment + daily computer‐based cognitive training (COGPACK; 10 1‐hour sessions over 2 weeks). 2. Standard treatment (including antipsychotic medications, art and occupational therapy, psychotherapy). |
| Outcomes | Compliance: compliance to treatment.
Global state: rehospitalisation. Mental state: psychopathological status. Level of knowledge/insight: illness knowledge, insight. Satisfaction with treatment. Service utilisation: days in hospital, psychosocial rehabilitation. |
| Starting date | February 2006. |
| Contact information | Thomas Jahn, Prof. Dr. phil. Dipl.‐Psych. +49 (0)89‐4140 4278. th.jahn@lrz.tum.de. |
| Notes |
Potts 2008.
| Trial name or title | A patient‐centered approach to improve screening for side effects of second generation antipsychotics (SGAs). |
| Methods | Allocation: randomly allocated. Blindness: no further details. Duration: no further details. Design: parallel, open label, active control. Location: no further details. |
| Participants | Diagnosis: of a psychotic disorder (schizophrenia, affective psychosis, major depression with psychotic features). Currently prescribed any SGA medication (aripiprazole, clozapine, olanzapine, quetiapine, risperidone, ziprasidone) by a clinician in a mental health clinic. Had at least 2 outpatient visits with the prescribing clinician in the past year. N = 240. Age: 18 to 70 years. Gender: no further details. History: no further details. Setting: no further details. Excluded: no further details. |
| Interventions | 1. Patient‐centred computerised tool: (1) rates of screening for and identification of health problems associated with the metabolic side effects of SGAs; (2) patterns of patient‐centred communication around screening for metabolic side effects and patients' self efficacy in communicating with their psychiatrists about screening; (3) patients' preferences for obtaining health information and participating in decision‐making about screening; and (4) patients' perceptions of their psychiatrists' participatory decision‐making styles around screening. 2. Compared to enhanced treatment as usual (e‐TAU). |
| Outcomes | Compliance: number of days in the study period that a patient's screening for metabolic side effects of second‐generation antipsychotic medications adheres to guidelines. |
| Starting date | October 2008. |
| Contact information | Wendy Potts, MS. (410) 706‐ 6638. wpotts@psych.umaryland.edu. |
| Notes |
Prague 2008.
| Trial name or title | Study with information technology (IT) ‐ aided preventive program in schizophrenia. |
| Methods | Allocation: prospective, randomised. Blindness: double‐blinded. Duration: no further details. Design: parallel. Location: no further details. |
| Participants | Diagnosis: schizophrenia and schizoaffective disorder (ICD‐10). N = 150. Age: 16 to 60 years. Gender: no further details. History: no further details. Setting: no further details. Excluded: no further details. |
| Interventions | 1. Information Technology‐aided Program of Relapse Prevention in Schizophrenia (ITAREPS) will decrease the number of hospitalisations in patients with schizophrenia or schizoaffective disorder. 2. TAU: placebo comparator ‐ in the treatment as usual study arm (control, non‐active ITAREPS), the e‐mail ALERT message feedback will not be activated. In this group, even in the presence of early warning sings, the investigators will be kept blinded to the EWSQ scores, will receive no ALERT message and thus no early pharmacologic intervention based on the ITAREPS program will be prompted. Treatment in the control group will consist of routine clinical and medication management with the frequency of visits common in the outpatient clinical settings. |
| Outcomes | Mental state: EWSQ. Assessment of the natural course of the psychotic illness. Global state: the reduction of the number of hospitalisations for psychotic relapses. Correlation between baseline CGI and number of hospitalisations at endpoint. Service utilisation: number of hospitalisation days. |
| Starting date | November 2008. |
| Contact information | Filip Spaniel, M.D., PhD. Prague Psychiatrc Center. |
| Notes |
Weisbrod 2007.
| Trial name or title | Complex problem solving training in schizophrenic patients |
| Methods | Allocation: randomised. Blindness: single‐blind. Duration: no further details. Design: parallel, active control. Location: no further details. |
| Participants | Diagnosis: schizophrenia, schizoaffective disorder. N = 100. Age: no further details. Gender: no further details. History: no further details. Setting: no further details. Excluded: no further details. |
| Interventions | 1. Behavioural complex problem‐solving training (10 sessions of 45 minutes complex problem‐solving training over 3 weeks (including 30 minutes of computerised planning and problem‐solving training with Plan‐A‐Day and 15 minutes group session for transfer to everyday situations). 2. Basic cognitive training (basic cognitive training 10 sessions over 3 weeks of 45 min basic cognitive training (including 45 min computerised training of attention, processing speed, memory). |
| Outcomes | Global State: Osnabrücker Arbeitsfähigkeitsprofil "learning ability" sub‐scale. Behaviour: Behavioural Assessment of the Dysexecutive Syndrome (BADS, Zoo Map Score), Tower of London, Plan‐A‐Day S Solution Time. |
| Starting date | August 2007. |
| Contact information | Matthias Weisbrod, MD+49‐7202‐613342 matthias.weisbrod@kkl.srh.de. Stefan Kaiser, MD +49‐6221‐5637804 stefan_kaiser@med.uni‐heidelberg.de. |
| Notes |
Young 2009.
| Trial name or title | Web‐based delivery of MOVE! to veterans with serious mental illness (SMI). |
| Methods | Allocation: randomised, prospective. Blindness: single‐blind. Duration: no further details. Design: parallel with 3 arms, active control. Location: no further details. |
| Participants | Diagnosis: schizophrenia, schizoaffective disorder, bipolar disorder or recurrent major depressive disorder with psychosis (DSM‐IV). N = 300. Age: 18 and over. Gender: no further details. History: no psychiatric hospitalisation during the month prior to enrolment, receipt of an antipsychotic medication for at least 3 months prior to enrolment, a BMI of 28 or higher. Setting: no further details. Excluded: no further details. |
| Interventions | 1. In‐person MOVE! SMI for weight management ‐ psychoeducation. 2. Web‐based MOVE! SMI for weight management ‐ psychoeducation. 3. Usual care + educational handouts regarding weight loss. |
| Outcomes | Mental state: psychopathology.
Global state: health‐related functioning.
Behaviour: motivation ‐ readiness to change, dietary habits, physical activity, self efficacy. Quality of life. Satisfaction with treatment: satisfaction and usability of intervention. Physical measure: BMI. |
| Starting date | January ‐ 2011. |
| Contact information | Amy.Cohen@va.gov. kathleen.oksas@va.gov. |
| Notes |
BMI – Body Mass Index CANSAS – Camberwell Assessment of Need – Short Appraisal Schedule (Slade 2005) CGI – Clinical Global Impression (Guy 1976) DSM‐IV ‐ Diagnostic Statistical Manual, 4th Edition (APA 1994b) EWSQ – Early Warning Signs Questionnaire (Spaniel 2007) HoNOS – Health of the Nation Outcome Scales (Wing 1998) ICD‐10 ‐ International Statistical Classification of Diseases and Related Health Problems (WHO 1992) MANSA – Manchester Short Assessment of Quality of Life (Slade 2005) SGA ‐ second generation antipsychotic SMI ‐ serious mental illness TAU ‐ treatment as usual
Differences between protocol and review
We have substantively reworded the protocol for this review. The rewording improves its clarity but it did not change the procedures by which we undertook the review.
Additional outcome social support and health and social needs were added for since publication of protocol.
We did not use sensitivity analysis as described in the original protocol. The included trials were not described as double‐blind with implied randomisation and therefore we did not need to perform sensitivity analyses. Moreover, due to the limited data we did not perform sensitivity analyses according to the requirements of the interventions. However, concerns were raised about the quality of the Chinese trials (Hu 2011; Wu 2009) and we performed sensitivity analysis for all primary outcomes in which Chinese trials were included.
We have added search terms to identify relevant literature related to the topic of the review. The search was re‐run three times.
We did not use the Jadad 1996 criteria for assessment of methodological quality. Instead we assessed the risk of bias using the criteria described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Contributions of authors
Maritta Välimäki ‐ initiation of the review, protocol production, data extraction, analysis, data interpretation, writing the final report.
Heli Hätönen ‐ protocol production, data extraction, analysis, data interpretation, writing the final report.
Mari Lahti ‐ searching, data extraction, analysis.
Lauri Kuosmanen ‐ protocol production, data extraction, data interpretation.
Clive E Adams ‐ data extraction, analysis, data interpretation, writing the final report.
Sources of support
Internal sources
University of Turku, Finland.
University of Nottingham, UK.
External sources
No sources of support supplied
Declarations of interest
All authors are undertaking a trial of short message texting to encourage compliance with medication for people with schizophrenia. In addition MV, HH and LK have been undertaking a trial included in this review (Valimaki 2010).
New
References
References to studies included in this review
Chen 2007 {published data only}
- Chen L‐S, Li Z‐G, Chen Y‐F, Xiao C‐L, Huang J‐J, Bao G‐L, et al. Comparative study of relapse prevention in schizophrenia by network [利用网络防治精神分裂症复发对照研究]. Linchuang Jingshen Yixue Zazhi [临床精神医学杂志] 2007;17(4):234‐6. [Google Scholar]
Hansson 2008 {published data only}
- Bylund C. Structured patient‐clinician communication using DIALOG improves patient quality of life. Evidence‐Based Mental Health 2008;11(3):89. [DOI] [PubMed] [Google Scholar]
- Hansson L, Svensson B, Bjorkman T, Bullenkamp J, Lauber C, Martinez‐Leal R, et al. What works for whom in a computer‐mediated communication intervention in community psychiatry? Moderators of outcome in a cluster randomized trial. Acta Psychiatrica Scandinavica 2008;118(5):404‐9. [DOI] [PubMed] [Google Scholar]
- Priebe S, McCabe R, Bullenkamp J, Hansson L, Lauber C, Martinez‐Leal R, et al. Structured patient‐clinician communication and 1‐year outcome in community mental healthcare: cluster randomised controlled trial. British Journal of Psychiatry 2007;191:420‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jones 2001a {published data only}
- Jones R. Development and evaluation of personalised patient information for patients with schizophrenia living in the community. Current Controlled Trials 2004.
- Jones R. Development and evaluation of personalised patient information for patients with schizophrenia living in the community. National Research Register 2000. [National Research Register N0466011493]
- Jones R. Development and evaluation of personalised patient information for patients with schizophrenia living in the community. National Research Register 2001; Vol. 1. [N0466011493]
- Jones RB, Atkinson JM, Coia DA, Paterson L, Morton AR, McKenna K, et al. Randomised trial of personalised computer based information for patients with schizophrenia. BMJ 2001;322(7290):835‐40. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rotondi 2005 {published data only}
- Rotondi AJ. Schizophrenia patient and family continuity of care. http://www.clinicaltrials.gov 2003. [NCT00051233]
- Rotondi AJ. Support to patients and families. http://www.clinicaltrials.gov 2005. [NCT00177983]
- Rotondi AJ, Haas G, Anderson C, Ganguli R, Keshavan M, Newhill C, et al. A randomized trial of a telehealth intervention to provide in‐home psychoeducation to persons with schizophrenia and their families: intervention design and preliminary findings. Schizophrenia Bulletin 2005;31(2):533. [Google Scholar]
- Rotondi AJ, Haas GL, Anderson CM, Newhill CE, Spring MB, Ganguli R, et al. A clinical trial to test the feasibility of a telehealth psychoeducational intervention for persons with schizophrenia and their families: intervention and 3‐month findings. Rehabilitation Psychology 2005;50(4):325‐36. [AMED: 0081778; CINAHL: 2009074611] [DOI] [PMC free article] [PubMed] [Google Scholar]
Salzer 2004 {published data only}
- Salzer MS, Tunner T, Charney NJ. A low‐cost, telephone intervention to enhance schizophrenia treatment: a demonstration study. Schizophrenia Research 2004;66(1):75‐6. [EMBASE: 2004010745] [DOI] [PubMed] [Google Scholar]
Valimaki 2010 {published data only}
- Kuosmanen L, Valimaki M, Joffe G, Pitkanen A, Hatonen H, Patel A, et al. The effectiveness of technology‐based patient education on self‐reported deprivation of liberty among people with severe mental illness: a randomized controlled trial. Nordic Journal of Psychiatry 2009;63(5):383‐9. [DOI] [PubMed] [Google Scholar]
- Valimaki M. Information technology in mental health. http://www.controlled‐trials.com 2010.
References to studies excluded from this review
Berns 2001 {published data only}
- Berns S. Electronic assistive device to help patients with schizophrenia. National Alliance for Research on Schizophrenia and Depression (http://www.mhsource.com/narsad/bd/studyops.html) (accessed February 2001).
Borell 1995 {published data only}
- Borell P, Orhagen T, D'Elia G. Feasibility and effects of a patient information program in schizophrenia [Sjukdomsrelaterad information vid schizofreni: klinisk tillämpning och effekter]. Scandinavian Journal of Behaviour Therapy 1995;24(3‐4):75‐86. [PSYCINFO: 1996‐18099‐002] [Google Scholar]
Burgoyne 1983 {published data only}
- Burgoyne RW, Acosta FX, Yamamoto J. Telephone prompting to increase attendance at a psychiatric outpatient clinic. American Journal of Psychiatry 1983;140:345‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cramer 1999 {published data only}
- Cramer JA, Rosenheck R. Enhancing medication compliance for people with serious mental illness. Journal of Nervous and Mental Disease 1999;187(1):53‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Crespo 2006 {published data only}
- Crespo Iglesias JM, Gonzalez Garcia A, Santos Miguelez B, Naya Fernandez S. Improvement of the attendance to the first psychiatric appointments after telephone reminder [Mejoria de la asistencia a las primeras citas en psiquiatria tras aviso telefonico]. Anales de Psiquiatria 2006;22(3):95‐101. [MEDLINE: ] [Google Scholar]
Fan 2005 {published data only}
- Fan X‐H. The effects of community nursing intervention on the recovery of patients with primary schizophrenia. Journal of the Linyi Medical College 2005;27(6):419‐21. [Google Scholar]
Frangou 2005 {published data only}
- Frangou S. Remote home monitoring of patients. National Research Register 2001; Vol. 3. [N0042099581]
- Frangou S. Remote home monitoring of patients. National Research Register 2002; Vol. 1. [N0042099581]
- Frangou S, Sachpazidis I, Stassinakis A, Sakas G. Telemonitoring of medication adherence in patients with schizophrenia. Telemedicine Journal and E‐Health 2005;11(6):675‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Han 2008 {published data only}
- Han DH, Renshaw PF, Sim ME, Kim JI, Arenella LS, Lyoo IK . The effect of internet video game play on clinical and extrapyramidal symptoms in patients with schizophrenia. Schizophrenia Research 2008;103(1‐3):338‐40. [DOI] [PubMed] [Google Scholar]
Janssen 2010 {published data only}
- Janssen B, Ludwig S, Eustermann H, Menke R, Haerter M, Berger M, et al. Improving outpatient treatment in schizophrenia: effects of computerised guideline implementation‐‐results of a multicenter‐study within the German research network on schizophrenia. European Archives of Psychiatry and Clinical Neuroscience 2010;260(1):51‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kluger 1983 {published data only}
- Kluger MP, Karras A. Strategies for reducing missed initial appointments in a community mental health center. Community Mental Health Journal 1983;19(2):137‐43. [MEDLINE: ; 6653081] [DOI] [PubMed] [Google Scholar]
Kopelowicz 2006 {published data only}
- Kopelowicz A, Liberman RP, Wallace CJ, Aguirre F, Mintz J. Differential performance of job skills in schizophrenia: an experimental analysis. Journal of Rehabilitation 2006;72(4):31‐9. [Google Scholar]
MacDonald 2000 {published data only}
- MacDonald J. Telephone prompting improves attendance at community mental health centres ‐ provided the phone is answered. Unpublished report 1994. [MEDLINE: ]
- MacDonald J, Brown N, Ellis P. Using telephone prompts to improve initial attendance at a community mental health centre. Psychiatric Services 2000;51(6):812‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Montes 2009 {published data only}
- Montes J, Saiz Ruiz J, Claret J, Maurino J, Diez T. Effect of a nurse telephone follow‐up on therapeutic adherence of patients with schizophrenia. World Psychiatry 2009;8(Suppl 1):274‐81. [MEDLINE: ] [Google Scholar]
Park 2009 {published data only}
- Park KM, Ku J, Park IH, Park JY, Kim SI, Kim JJ. Improvement in social competence in patients with schizophrenia: a pilot study using a performance‐based measure using virtual reality. Human Psychopharmacology ‐ Clinical and Experimental 2009;24(8):619‐27. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pijnenborg 2010 {published data only}
- Pijnenborg GHM, Withaar FK, Brouwer WH, Timmerman ME, Bosch RJ, Evans JJ. The efficacy of SMS text messages to compensate for the effects of cognitive impairments in schizophrenia. British Journal of Clinical Psychology 2010;49:259‐74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rossi 1994 {published data only}
- Rossi, E. Effects of a simple strategy on attendance and satisfaction with medication clinic services in the chronically mentally ill [PhD thesis]. United States International University, USA, 1994. [Google Scholar]
Shon 2002 {published data only}
- Shon KH, Park SS. Medication and symptom management education program for the rehabilitation of psychiatric patients in Korea: the effects of promoting schedule on self‐efficacy theory. Yonsei Medical Journal 2002;43(5):579‐89. [DOI] [PubMed] [Google Scholar]
Yi 2006 {published data only}
- Yi L‐F, Zhu S‐Y. Interventional effect of regular psychological therapy on the improvement of schizophrenia accompanied by depressive mood in the community: one‐year follow‐up. Chinese Journal of Clinical Rehabilitation [Zhongguo lin chuang kang fu] 2006;10(22):18‐20. [Google Scholar]
References to ongoing studies
Hulsbosch 2008 {published data only}
- Hulsbosch AM, Kroon H. Randomised controlled trial of home automation in mental health. http://www.trialregister.nl/trialreg/index.asp 2008.
Jahn 2008 {published data only}
- Jahn T. Cognitive determinants of psychoeducation and information in psychoses. http://www.clinicaltrials.gov 2008.
Potts 2008 {published data only}
- Potts W, Kreyenbuhl JA. A patient‐centered approach to improve screening for side effects of second generation antipsychotics (SGAs). http://www.clinicaltrials.gov 2008.
Prague 2008 {published data only}
- Eli Lilly Prague Psychiatric Center. Study with information technology (IT) ‐ aided preventive program in schizophrenia. http://www.clinicaltrials.gov 2008.
Weisbrod 2007 {published data only}
- Weisbrod M, Kaiser S. Complex problem solving training in schizophrenic patients. http://www.clinicaltrials.gov 2007. [NCT00507988]
Young 2009 {published data only}
- Young AS, Cohen AN, Oksas K. Web‐based delivery of move! to veterans with serious mental illness (SMI). http://www.clinicaltrials.gov 2009.
Additional references
Altman 1996
- Altman DG, Bland JM. Detecting skewness from summary information. BMJ 1996;313(7066):1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
APA 1994a
- American Psychiatric Association. Global assessment of functioning GAF. DSM IV ‐ Diagnostic Statistical Manual. 4th Edition. American Psychiatric Association, 1994. [Google Scholar]
APA 1994b
- APA. DSM IV ‐ Diagnostic Statistical Manual. 4th Edition. American Psychiatric Association, 1994. [Google Scholar]
Auquier 2007
- Auquier P, Lancon C, Rouillon F, Lader M. Mortality in schizophrenia. Pharmacoepidemiology & Drug Safety 2007;16(12):1308‐12. [DOI] [PubMed] [Google Scholar]
Barrowclough 1987
- Barrowclough C, Tarrier N, Watts S, Vaughn C, Bamrah JS, Freeman HL. Assessing the functional value of relatives' knowledge about schizophrenia: a preliminary report. British Journal of Psychiatry 1987;151:1‐8. [DOI] [PubMed] [Google Scholar]
Bland 1997
- Bland JM, Kerry SM. Statistics notes. Trials randomised in clusters. BMJ 1997;315:600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bobes 2006
- Bobes J, Carcía‐Portilla MP, Bascarán MT, Saíz PA, Bousoño M. Quality of life in schizophrenia patients. Dialogues in Clinical Neurosciences 2007;9:215‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boissel 1999
- Boissel JP, Cucherat M, Li W, Chatellier G, Gueyffier F, Buyse M, et al. The problem of therapeutic efficacy indices. 3. Comparison of the indices and their use. Therapie 1999;54(4):405‐11. [PubMed] [Google Scholar]
Boyce 2011
- Boyce N. The Lancet Technology. The Lancet 2011;378(9840):1691. [Google Scholar]
Chan 2004
- Chan S, Yu IW. Quality of life of clients with schizophrenia. Journal of Advanced Nursing 2004;45:72‐83. [DOI] [PubMed] [Google Scholar]
Chinese Society of Psychiatry 2001
- Chinese Society of Psychiatry. CCMD‐3 – Chinese Classification of Mental Disorders. 3rd Edition. Chinese Society of Psychiatry, 2001. [Google Scholar]
Christensen 2002
- Christensen H, Griffiths KM, Korten A. Web‐based cognitive behavior therapy: analysis of site usage and changes in depression and anxiety scores. Journal of Medical Internet Research 2002;4(1):e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cooper 1996
- Cooper JE, Sartorius N. Mental Health Disorders in China: Results of the National Epidemiological Survey in 12 Areas. London: Gaskell, 1996. [Google Scholar]
David 1992
- David A, Buchanan A, Reed A, Almeida O. The assessment of insight in psychosis. British Journal of Psychiatry 1992;161(5):599‐602. [DOI] [PubMed] [Google Scholar]
Deeks 2000
- Deeks J. Issues in the selection for meta‐analyses of binary data. Proceedings of the 8th International Cochrane Colloquium; 2000 Oct 25‐28th; Cape Town, South Africa. 2000.
Divine 1992
- Divine GW, Brown JT, Frazer LM. The unit of analysis error in studies about physicians' patient care behavior. Journal of General Internal Medicine 1992;7:623‐9. [DOI] [PubMed] [Google Scholar]
Donner 2002
- Donner A, Klar N. Issues in the meta‐analysis of cluster randomized trials. Statistics in Medicine 2002;21:2971‐80. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey‐Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple graphical test. BMJ 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne D, Altman DG, Higgins JPT, Curtina F, Worthingtond HV, Vaile A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
European Commission 2001
- European Commission 2001. Communication from the Commission to the Council and the European Parliament. Information and communication technologies in development. The role of ICTs in EU development policy. http://ec.europa.eu/development/icenter/repository/com2001_0770en01_en.pdf (assessed 7 July 2009).
European Commission 2010
- European Commission. Action plan 2011 ‐ 2015. http://ec.europa.eu/information_society/activities/egovernment/action_plan_2011_2015/index_en.htm (accessed 6 September 2011).
European Communities 2007
- European Communities. eHealth priorities and strategies in European countries. http://ec.europa.eu/information_society/activities/health/docs/policy/ehealth‐era‐full‐report.pdf (accessed 6 September 2011).
EuroQol Group 1990
- EuroQol Group. EQ‐5D – Health Related Quality of Life Questionnaire. EuroQol, 1990. [Google Scholar]
Evans 2007
- Evans S, Banerjee S, Leese M, Huxley P. The impact of mental illness on quality of life: a comparison of severe mental illness, common mental disorder and healthy population samples. Quality of Life Research 2007;16:17‐29. [DOI] [PubMed] [Google Scholar]
Eysenbach 2001
- Eysenbach G. What is eHealth?. Journal of Medical Internet Research 2001;3(2):e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fischer 1982
- Fischer CS. What do we mean by “friend”? An inductive study. Social Networks 1982;3:287–306. [Google Scholar]
Freudenreich 2004
- Freudenreich O, Cather C, Evins AE, Henderson DC, Goff DC. Attitudes of schizophrenia outpatients toward psychiatric medications: relationship to clinical variables and insight. Journal of Clinical Psychiatry 2004;65(10):1372‐6. [DOI] [PubMed] [Google Scholar]
Gulliford 1999
- Gulliford MC, Ukoumunne OC, Chinn S. Components of variance and intraclass correlations for the design of community‐based surveys and intervention studies: data from the Health Survey for England 1994. American Journal of Epidemiology 1999;149:876‐83. [DOI] [PubMed] [Google Scholar]
Gumley 2006
- Gumley A, Karatzias A, Power K, Reilly J, McNay L, O'Grady M. Early intervention for relapse in schizophrenia: impact of cognitive behavioural therapy on negative beliefs about psychosis and self‐esteem. British Journal of Clinical Psychology 2006;45(2):247‐60. [DOI] [PubMed] [Google Scholar]
Guy 1976
- Guy W. Clinical Global Impressions. ECDEU Assessment Manual for Psychopharmacology. Revised. Rockville, MD: National Institute of Mental Health, 1976:218‐22. [Google Scholar]
Haro 2006
- Haro JM, Novick D, Suarez D, Alonso J, Lépine JP, Ratcliffe M. SOHO study group. Remission and relapse in the outpatient care of schizophrenia: three‐year results from the schizophrenia outpatient health outcomes study. Journal of Clinical Psychopharmacology 2006;26(6):571‐8. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hogan 1983
- Hogan TP, Awad AG, Eastwood R. A self‐report scale predictive of drug compliance in schizophrenics: reliability and discriminative validity. Psychological Medicine 1983;13:177‐83. [DOI] [PubMed] [Google Scholar]
Hor 2010
- Hor K, Taylor M. Suicide and schizophrenia: a systematic review of rates and risk factors. Journal of Psychopharmacology 2010;24(Suppl 4):81‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hu 2011
- Hu Y, Huang Y, Ding J, Liu Y, Fan D, Li T, et al. Status of clinical research in China. Lancet 2011;377:124‐5. [DOI] [PubMed] [Google Scholar]
International Telecommunication Union 2009
- International Telecommunication Union. Measuring the information society. The ICT development index. http://www.itu.int/net/pressoffice/backgrounders/general/pdf/5.pdf (accessed 6 September 2011).
Jadad 1996
- Jadad A, Moore A, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
Jones 2001b
- Jones RB, Atkinson JM, Coia DA, Paterson L, Morton AR, McKenna K, et al. Randomised trial of personalised computer based information for patients with schizophrenia. BMJ 2001;322(7290):835‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaplan 2007
- Kaplan HI, Sadock BJ. Comprehensive Textbook of Psychiatry. Williams & Wilkins: Baltimore, MD, 1995. [Google Scholar]
Kay 1987
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin 1987;13(2):261‐76. [DOI] [PubMed] [Google Scholar]
Kikkert 2006
- Kikkert MJ, Schene AH, Koeter MWJ, Robson D, Born A, Helm H, et al. Medication adherence in schizophrenia: exploring patients', carers' and professionals' views. Schizophrenia Bulletin 2006;32(4):786‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kuosmanen 2009
- Kuosmanen L, Välimäki M, Joffe G, Pitkänen A, Hätönen H, Patel A, et al. The effectiveness of technology‐based patient education on self‐reported deprivation of liberty among people with severe mental illness: a randomised controlled trial. Nordic Journal of Psychiatry 2009;63:383‐9. [DOI] [PubMed] [Google Scholar]
Larsen 1979
- Larsen DL, Attkisson CC, Hargreaves WA, Nguyen TD. Assessment of client/patient satisfaction: development of a general scale. Evaluation and Program Planning 1979;2:197‐207. [DOI] [PubMed] [Google Scholar]
Leucht 2007
- Leucht S, Engel RR, Bauml J, Davis JM. Is the superior efficacy of new generation antipsychotics an artifact of LOCF?. Schizophrenia Bulletin 2007;33(1):183‐91. [PUBMED: 16905632] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lewis 2003
- Lewis D. Computers in patient education. CIN: Computers, Informatics, Nursing 2003;21(2):88‐96. [DOI] [PubMed] [Google Scholar]
Marshall 2000
- Marshall M, Lockwood A, Adams C, Bradley C, Joy C, Fenton M. Unpublished rating scales ‐ a major source of bias in randomised controlled trials of treatments for schizophrenia?. British Journal of Psychiatry 2000;176:249‐52. [DOI] [PubMed] [Google Scholar]
McCallister 1978
- McAllister L, Fischer CS. A procedure for surveying personal networks. Sociological Methods & Research 1978;7:131‐48. [Google Scholar]
McEvoy 1989
- McEvoy JP, Apperson LJ, Appelbaum PS, Ortlip P, Brecosky J, Hammill K, et al. Insight in schizophrenia. Its relationship to acute psychopathology. Journal of Nervous and Mental Disease 1989;177(1):43‐7. [DOI] [PubMed] [Google Scholar]
Ministerial Declaration 2003
- Ministerial declaration. http://europa.eu.int/information_society/eeurope/ehealth/conference/2003/doc/min_dec_22_may_03 (accessed 22 October 2010).
Moher 2010
- Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trial. BMJ 2010;340:c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morrison 2006
- Morrison AP, French P, Lewis SW, Roberts M, Raja S, Neil ST, et al. Psychological factors in people at ultra‐high risk of psychosis: comparisons with non‐patients and associations with symptoms. Psychological Medicine 2006;36(10):1395‐404. [DOI] [PubMed] [Google Scholar]
Mueser 2005
- Mueser K, McGurk S. Schizophrenia. Lancet 2005;363(9426):2063‐72. [DOI] [PubMed] [Google Scholar]
Murray 2005
- Murray E, Burns J, See TS, Lai R, Nazareth I. Interactive health communication applications for people with chronic disease. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD004274.pub4] [DOI] [PubMed] [Google Scholar]
Oh 2005
- Oh H, Rizo C, Enkin M, Jadad A. What Is eHealth: a systematic review of published definitions. Journal of Medical Internet Research 2005;7(1):e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Overall 1962
- Overall JE, Gorham DR. The brief psychiatric rating scale. Psychological Reports 1962;10:799‐812. [Google Scholar]
Palmer 2005
- Palmer B, Pankratz S, Bostwick JM. Lifetime risk of suicide in schizophrenia. Archives of General Psychiatry 2005;62:247‐53. [DOI] [PubMed] [Google Scholar]
Perkins 2000
- Perkins DO, Stroup TS, Lieberman JA. Psychotic disorder measures. Handbook of Psychiatric Measures. Washington, DC: American Psychiatric Association, 2000:485‐90. [Google Scholar]
Perry 1999
- Perry A, Tarrier N, Morris R, McCarthy E, Limb K. Randomised controlled trial of efficacy of teaching patients with bipolar disorder to identify early symptoms of relapse and obtain treatment. BMJ 1999;318:149‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Podoll 2000
- Podoll K, Habermeyer E, Nöller B, Ebel H, Sas H. Internet as a delusional topic in paranoid schizophrenia [Internet als wahnthema bei paranoider schizophrenie]. Nervenarzt 2000;71:912‐4. [DOI] [PubMed] [Google Scholar]
Proudfoot 2004
- Proudfoot J, Ryden C, Everitt B, Shapiro DA, Goldberg D, Mann A, et al. Clinical efficacy of computerised cognitive‐behavioural therapy for anxiety and depression in primary care: randomised controlled trial. British Journal of Psychiatry 2004;185:46‐54. [DOI] [PubMed] [Google Scholar]
Riper 2010
- Riper H, Andersson G, Christensen H, Cuijpers P, Lange A, Eysenbach G. Theme issue on e‐mental health: a growing field in internet research. Journal of Medical Internet Research 2010;12(5):e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shen 1985
- Shen Y, Wang C. A Handbook of Epidemiological Investigation of Mental Illness. Beijing: Renming Health Press, 1985. [Google Scholar]
Silber 2003
- Silber D. The case for eHealth. Proceedings of the European commission's conference on eHealth; 2003 May 22‐23; Brussels. 2003.
Slade 2005
- Slade M, Leese M, Cahill S, Thornicroft G. Patient‐rated mental health needs and quality of life improvement. British Journal of Psychiatry 2005;187:256‐61. [DOI] [PubMed] [Google Scholar]
Smith 2006
- Smith B, Fowler DG, Freeman D, Bebbington P, Bashforth H, Garety P, et al. Emotion and psychosis: links between depression, self‐esteem, negative schematic beliefs and delusions and hallucinations. Schizophrenia Research 2006;86(1‐3):181‐8. [DOI] [PubMed] [Google Scholar]
Spaniel 2007
- Španiel F, Novák T, Motlová L, Hrdlička J, Höschl C. Information technology aided relapse prevention program in schizophrenia (ITAREPS): reliability and validity of the early warning signs questionnaire. Psychiatry 2007;11:157–9. [Google Scholar]
Suhonen 2007
- Suhonen R, Leino‐Kilpi H, Välimäki M, Kim HS. The patient satisfaction scale ‐ an empirical investigation into the Finnish adaptation. Journal of Evaluation in Clinical Practice 2007;13:31‐8. [DOI] [PubMed] [Google Scholar]
Tandon 2006
- Tandon R, Targum SD, Nasrallah HA, Ross R. The treatment effectiveness in schizophrenia consortium institution. Strategies for maximizing clinical effectiveness in the treatment of schizophrenia. Journal of Psychiatric Practice 2006;12(6):348‐63. [DOI] [PubMed] [Google Scholar]
Turkington 2002
- Turkington D, Kingdon DG, Turner T. Effectiveness of a brief cognitive‐behavioural therapy intervention in the treatment of schizophrenia. British Journal of Psychiatry 2002;180:523‐7. [DOI] [PubMed] [Google Scholar]
Turner 2006
- Turner MS, Stewart DW. Review of the evidence for the long‐term efficacy of atypical antipsychotic agents in the treatment of patients with schizophrenia and related psychoses. Journal of Psychopharmacology 2006;20(6):20‐37. [DOI] [PubMed] [Google Scholar]
Ukoumunne 1999
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PGJ. Methods for evaluating area‐wide and organisation‐based interventions in health and health care: a systematic review. Health Technology Assessment 1999;3(5):iii‐92. [PubMed] [Google Scholar]
Walker 2006
- Walker H. Computer‐based education for patients with psychosis. Nursing Standard 2006;20(30):49‐56. [DOI] [PubMed] [Google Scholar]
Watson 2006
- Watson PW, Garety PA, Weinman J, Dunn G, Bebbington PE, Fowler D, et al. Emotional dysfunction in schizophrenia spectrum psychosis: the role of illness perceptions. Psychological Medicine 2006;36(6):761‐70. [DOI] [PubMed] [Google Scholar]
WHO 1992
- World Health Organization. ICD 10 ‐ International Statistical Classification of Diseases and Related Health Problems. WHO, 1992. [Google Scholar]
WHO 2006
- World Health Organization. Schizophrenia. http://www.who.int (assessed 24 August 2009).
WHO 2011
- World Health Organization. Schizophrenia. http://www.who.int/mental_health/management/schizophrenia/en/ (accessed August 2011).
Wing 1998
- Wing JK, Beevor AS, Curtis RH. Health of the nation outcome scales (Ho NOS). Research and development. British Journal of Psychiatry 1998;172:11‐8. [DOI] [PubMed] [Google Scholar]
Wu 2009
- Wu T, Li Y, Bian Z, Liu G, Moher D. Randomized trials published in some Chinese journals: how many are randomized?. Trials 2009;10:46. [PUBMED: 19573242] [DOI] [PMC free article] [PubMed] [Google Scholar]
Xia 2007
- Xia J, Adams CE, Bhagat N, Bhagat V, Bhoopathi P, El‐Sayeh H, et al. The Leeds outcomes stakeholders survey (LOSS) study. Proceedings of the 15th Cochrane Colloquium; 2007 Oct 23‐27; Sao Paulo. The Cochrane Collaboration, 2007.
Xia 2011
- Xia J, Merinder LB, Belgamwar MR. Psychoeducation for schizophrenia. Cochrane Database of Systematic Reviews 2011, Issue 6. [DOI: 10.1002/14651858.CD002831.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zautra 1984
- Zautra AJ. Life events and demoralization in the elderly. Grant proposal ‐ Department of Psychology, Arizona State University; Tempe, AZ 1984.
Zautra 1988
- Zautra AJ, Guarnaccia CA, Reich JW. The factor structure of mental health measures for older adults. Journal of Consulting and Clinical Psychology 1988;56:514‐9. [DOI] [PubMed] [Google Scholar]


