Abstract
Recombinant adeno-associated virus vectors (rAAV) show promise in preclinical trials for the treatment of genetic diseases including hemophilia. Liver-directed gene transfer results in a slow rise in transgene expression, reaching steady-state levels over a period of 5 weeks concomitant with the conversion of the single-stranded rAAV molecules into high-molecular-weight concatemers in about 5% of hepatocytes. Immunohistochemistry and RNA in situ hybridization show that the transgene product is made in about ∼5% of hepatocytes, suggesting that most rAAV-mediated gene expression occurs in hepatocytes containing the double-stranded concatemers. In this study, the mechanism(s) involved in stable transduction in vivo was evaluated. While only ∼5% of hepatocytes are stably transduced, in situ hybridization experiments demonstrated that the vast majority of the hepatocytes take up AAV-DNA genomes after portal vein infusion of the vector. Two different vectors were infused together or staggered by 1, 3, or 5 weeks, and two-color fluorescent in situ hybridization and molecular analyses were performed 5 weeks after the infusion of the second vector. These experiments revealed that a small but changing subpopulation of hepatocytes were permissive to stable transduction. Furthermore, in animals that received a single infusion of two vectors, about one-third of the transduced cells contained heteroconcatemers, suggesting that dimer formation was a critical event in the process of concatemer formation. To determine if the progression through the cell cycle was important for rAAV transduction, animals were continuously infused with 5′-bromo-2′-deoxyuridine (BrdU), starting at the time of administration of a rAAV vector that expressed cytoplasmic β-galactosidase. Colabeling for β-galactosidase and BrdU revealed that there was no preference for transduction of cycling cells. This was further confirmed by demonstrating no increase in rAAV transduction efficiencies in animals whose livers were induced to cycle at the time of or after vector administration. Taken together, our studies suggest that while virtually all hepatocytes take up vector, unknown cellular factors are required for stable transduction, and that dimer formation is a critical event in the transduction pathway. These studies have important implications for understanding the mechanism of integration and may be useful for improving liver gene transfer in vivo.
Recombinant adeno-associated virus vectors (rAAV) have been used to deliver therapeutic and in some cases curative amounts of the factor IX gene into mice and dogs with hemophilia B (8, 16, 23, 24). After intraportal delivery, factor IX levels slowly rise during the first 5 weeks to reach a steady-state concentration in plasma. During this period, the number of single-stranded rAAV vector genomes slowly decreases and there is a concomitant increase in the number of high-molecular-weight concatemers (15). With a dose of 6.4 × 1010 viral particles, pulsed-field gel electrophoresis and fluorescent in situ hybridization (FISH) analysis of isolated hepatic nuclei showed that there are concatemers and integrated rAAV proviral genomes in about 5% of hepatocytes (15, 24). More recently, integration of rAAV into the mouse genome has been confirmed by the cloning of mouse chromosomal AAV vector junction fragments from liver (17). The number of hepatocytes that express the rAAV-mediated transgene product, as determined by RNA in situ hybridization and protein immunohistochemistry, is similar to the number of hepatocytes that contain the concatemers (24). This suggests that most if not all of the gene expression from the liver comes from the hepatocytes that contain the integrated concatemers.
In cell culture, most but not all integrants contain a single-copy proviral genome (14, 22, 29). Some of the in vitro studies were done using rAAV vectors that express neomycin phosphotransferase in G418-treated cells where all nonintegrated transduction events would be quickly lost due to cell division. Whether the rapid cell division in cultured cells, selective pressure, metabolic state of the cells, or cell type is responsible for the differences observed between in vivo and in vitro studies is not known.
The mechanism(s) by which rAAV genomes transduce liver is not known, and it is not clear why only a small proportion of hepatocytes are stably transduced, as opposed to other tissues like brain, muscle, and retina, where high rates of transduction can be obtained around the injection site (7, 13, 25, 28). One possible explanation is that unlike other tissues, with intravascular infusion into the liver, rAAV vector virions are not able to bind or enter the majority of hepatocytes. A previous study showed that 10 to 25% of hepatocytes were transduced with rAAV genomes when the vector was coadministered with an adenovirus (6). This suggested that many hepatocytes take up rAAV; however, it was not conclusive, because of the possibility that rAAV entry into the cells was influenced by the adenovirus particles.
The studies described here were designed to better understand the process of liver transduction in vivo. To do this, we studied (i) whether all hepatocytes are equally capable of taking up the vector shortly after its administration and/or whether there is a defined population of hepatocytes that are permissive to stable transduction, (ii) the relative frequency of independent transduction events, (iii) heteromultimer formation within individual hepatocytes, and (iv) the importance of the cell cycle for transduction.
These factors are important not only because they may lead to insights into the mechanism(s) of vector integration, but also because with the vector genome size limitations, they may allow a means of using two vectors to make a single functional gene. In early studies, dual vector transduction has been suggested in the brain (3, 13, 26) and muscle (19, 31) but the molecular state of the genomes (heterogenomes versus two homogenomes) was not determined. However, very recent results clearly establish the formation of heteroconcatemers in muscle (30). If transduction is a random event, then in the liver, where the frequency of transduced hepatocytes is low, the probability that two vectors will transduce a single cell may be much lower than that expected for the muscle and brain, where a high proportion of transduced cells are found concentrated at the injection site. Thus, it is possible that the mechanism(s) involved in stable transduction will ultimately turn out to be different for different tissues.
MATERIALS AND METHODS
rAAV vector preparation and characterization.
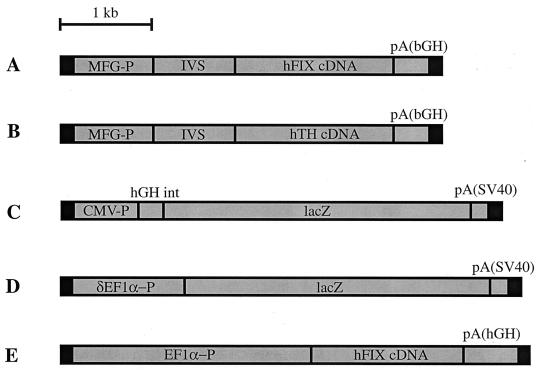
Preparation and characterization of rAAV-FIX from pSSV9-MFG-hFIX, rAAV-TH from pSSV-MFG-TH, rAAV-EF1α-FIX, and rAAV-CMV-LacZ were performed as described previously (10, 17, 24). rAAV-EF1α-LacZ contains a truncated EF1α promoter (17) driving the Escherichia coli lacZ gene. The total particle titer of the rAAV vectors was determined by a dot-blot assay. The structures of the vectors used are given in Fig. 1.
FIG. 1.
Molecular structures of the vectors. (A) rAAV-FIX; (B) rAAV-TH; (C) rAAV-CMV-LacZ; (D) rAAV-EF1α-LacZ; (E) rAAV-EF1α-FIX. The black boxes at both vector ends represent the inverted terminal repeats. MFG-P, Moloney murine leukemia virus long terminal repeat promoter; IVS, mRNA splice donor/splice acceptor; hFIX cDNA, human coagulation factor IX cDNA; pA(bGH), bovine growth hormone polyadenylation signal; hTH cDNA, human tyrosine hydroxylase cDNA; CMV-P; human cytomegalovirus immediate-early gene enhancer/promoter; hGH int, human growth hormone intron; lacZ, the bacterial lacZ gene; pA(SV40), simian virus 40 mRNA polyadenylation signal; EF1α-P, human polypeptide elongation factor 1α gene enhancer/promoter; δEF1α-P, truncated EF1α-P; pA(hGH), human growth hormone polyadenylation signal.
Animal studies.
All methods of vector infusion, partial hepatectomy, and DNA isolation have been described previously (9, 15, 24). Animals were treated according to the National Institutes of Health guidelines for animal care and the guidelines of the University of Washington and Stanford University. Adult female C57BL/6, C57BL/6 scid, and C57BL/6 Rag-1 mice were purchased from Jackson Laboratory and infused with rAAV vectors via the portal vein as described below. Blood samples were taken from the retro-orbital plexus.
For 5′-bromo-2′-deoxyuridine (BrdU)-labeling studies, immediately after portal vein infusion of the vector and 200 mg of BrdU per kg, a miniosmotic pump that can continuously administer BrdU solution in 0.5 N sodium bicarbonate at a rate of 1 mg/day for 14 days (model 2002; Alzet, Palo Alto, Calif.) was implanted into the subcutaneous space on the dorsal surface. To allow for immediate release of BrdU from the pump, all pumps were incubated in saline at 37°C for at least 4 h prior to implantation as recommended by the manufacturer. These pumps were replaced twice during the experiment, every 12 days for up to 36 days, to allow for continuous labeling of hepatocytes. Additional subcutaneous injection of 200 mg of BrdU per kg was administered during the surgical procedure of pump replacement to ensure continuous labeling. Continuous infusion of BrdU at this rate caused no liver damage as determined by serum alanine aminotransferase measurements (data not shown).
FISH in hepatic nuclei.
From each group of mice infused with both rAAV-FIX and rAAV-TH, primary mouse hepatocytes were isolated by the collagenase perfusion method 5 weeks after the infusion of the second vector, rAAV-TH (15). The hepatocytes were subsequently suspended in Williams's medium supplemented with 10% fetal calf serum, filtered, and washed three times with the same medium. Mouse interphase nuclei were then prepared from freshly isolated primary hepatocytes by a standard methanol-acetic acid method. The rAAV-FIX probe made from the rAAV plasmid, pSSV9-MFG-hFIX, was labeled by nick translation, incorporating biotin-14-dATP (GIBCO BRL), and the rAAV-TH probe made from the pSSV-MFG-TH plasmid was labeled by nick translation, incorporating digoxigenin-11-dUTP (Boehringer Mannheim). Labeled DNA (100 ng) was ethanol precipitated along with 10 μg of salmon sperm DNA for use on each slide. The slides were hybridized with both probes in 50% formamide–2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–10% dextran sulfate at 37°C overnight. The next day, the slides were washed in 50% formamide–2× SSC at 42°C and 2× SSC at 42°C. Texas red-conjugated avidin was used in combination with biotinylated goat anti-avidin antibody for detection of hybridization signals generated from the rAAV-FIX probe. Mouse anti-digoxigenin antibody was used in combination with fluorescein isothiocyanate-conjugated rabbit anti-mouse immunoglobulin G and fluorescein isothiocyanate-conjugated goat anti-rabbit immunoglobulin G to detect hybridization signals generated from the rAAV-TH probe. The nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). Photomicroscopy was performed with a standard epifluorescence microscope (Zeiss Co.).
Southern analyses.
Genomic DNA was prepared from primary mouse hepatocytes from the mice injected with rAAV-FIX and rAAV-TH. A 15-μg sample of each DNA was digested with BamHI and XbaI at 37°C overnight and then electrophoresed through a 0.8% agarose gel and transferred to a nylon membrane (Hybond N+; Amersham). The membrane was prehybridized and then hybridized with an hFIX cDNA probe at 65°C using a Rapid-Hyb buffer (Amersham). The final stringency of washing was 0.1× SSC–0.1% sodium dodecyl sulfate at 65°C. After autoradiography, the blot was stripped and then hybridized with a TH cDNA probe under the same conditions. The hFIX cDNA probe is 1.4 kb in size, and the TH cDNA probe is 1.2 kb. Both probes were labeled to a specific activity of 108 cpm/μg with [α-32P]dCTP, using a random-primer labeling kit (Stratagene).
To determine the vector copy number per cell (the number of double-stranded vector genomes per diploid genomic equivalent) in the transduced livers of rAAV-EF1α-FIX-injected mice, 20 μg of genomic DNA extracted from livers was digested with XhoI that cuts three times within the vector, electrophoresed together with copy number standards, and subjected to Southern blot analysis as described previously (17), with a 1.9-kb vector sequence-specific probe. The intensity of the bands was quantitated by densitometry.
PCR detection methods.
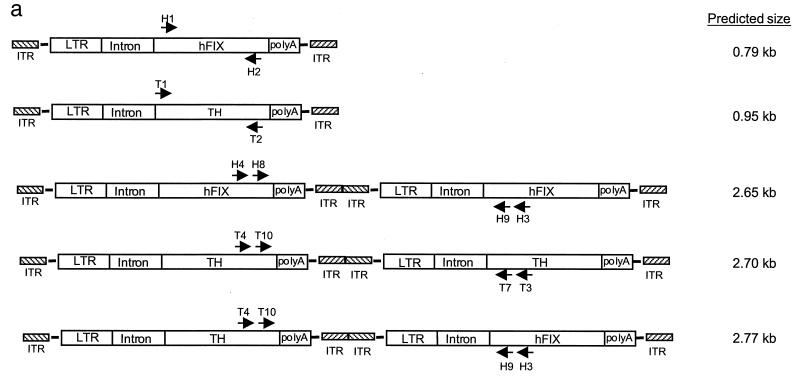
rAAV-FIX and rAAV-TH vector genomes were detected in the transduced mouse livers by one-round PCR experiments using internal primer sets within the cDNA sequence of hFIX and TH, respectively. Two sets of primers were used in nested-PCR experiments to amplify concatemers composed of the same vector genomes and mixed vector genomes, respectively. After electrophoresis of the PCR products, the gel was blotted and hybridized with a hFIX cDNA probe. Subsequently, the blots were stripped and hybridized with a TH cDNA probe. The hFIX and TH probes and the hybridization procedures used were the same as the ones in the Southern analysis. The primer sequences are as follows: H1, 5′ GATGGAGATCAGTGTGAGTCCAATCCATGT; H2, 5′ AGTTTAAACCTAGACCGATACATTCACCGA; H3, 5′ CTCTCAAGGTTCCCTTGAACAAACTCTTCC; H4, 5′ ACTGAAGTGGAAGGGACCAGTTTCTTAAC; H8, 5′ GCTGGGGTGAAGAGTGTGCAATGAAAGGCA; H9, 5′ CCTTGAACAAACTCTTCCAATTTACCTG; T1, 5′ GGCCATCATGGTAAGAGG; T2, 5′ ACTGGGTGCACTGGAACAC; T3, 5′ AAACGTCTCAAACACCTTCACAGCTC; T4, 5′ TATCCGCCACGCGTCCTCGCCCATGCACTC; T7, 5′ CTCTGCCTGCTTGGCGTCCAGCTCAGACAC; and T10, 5′ ACCAAGACCAGACGTACCAGTCAGTCTAC.
DNA in situ hybridization in liver tissue.
Livers from mice were fixed in 10% neutral buffered formalin, dehydrated, and paraffin embedded. Sections (5 μm) were cut onto Superfrost Plus charged slides and baked at 60°C for 1 h. Plasmid pAAV-EF1α-LacZ DNA was labeled with digoxigenin-11-dUTP using the DIG High Prime (Roche Molecular) random-primer labeling kit.
Slides were deparaffinized in xylene and rehydrated through a series of graded ethanols (100, 95, and 70%) into water. They were then treated in 0.2 N HCl for 20 min at room temperature, (RT), briefly rinsed in water, digested with 100 μg of proteinase K per ml in phosphate-buffered saline for 20 min at 37°C, and washed for 5 min in water. The sections were postfixed for 1 min in 10% neutral buffered formalin and rinsed in water, treated with 0.1 M triethanolamine–0.25% acetic anhydride for 10 min at RT, and washed twice in phosphate-buffered saline for 3 min each. The slides were then dehydrated through a series of graded ethanols (70, 95, and 100%) and allowed to air dry. The tissues were denatured in 70% formamide–2× SSC for 5 min at 75°C, immediately dipped into a series of cold 70, 95, and 100% ethanols, and once again allowed to air dry. Then 0.75 μg of labeled probe was added to a hybridization solution consisting of 60 μl of 2× hybridization buffer (4× SSC, 2× Denhardt's solution, 0.2 M sodium phosphate buffer [pH 6.5]), 60 μl of 20% dextran sulfate, and 0.06 μg of salmon sperm DNA per μl. The final probe concentration was 6 μg/ml. The probe was denatured at 75°C for 5 min and quickly placed in an ice bath. Approximately 20 μl of diluted probe was applied under a coverslip and hybridized in a moist chamber at 42°C overnight. The next day, the coverslips were removed and the slides were subjected to a series of posthybridization washes including a 2× SSC–0.1 mM dithiothreitol wash for 30 min at RT followed by a 0.1× SSC–0.1 mM dithiothreitol wash for 30 min at 37°C and a final 3-min rinse in 0.1× SSC.
A blocking buffer (2× SSC–0.05% Triton X-100) supplemented with 30% normal sheep serum was applied to the sections, and the slides were incubated in a moist chamber for 2 h at RT. They were then washed twice in Tris-buffered saline (TBS) for 10 min at RT. Anti-DIG-AP (Roche Molecular) was applied at a dilution of 1:500 in a buffer (0.3% Triton X-100, TBS) supplemented with 5% normal sheep serum, and the slides were incubated in a moist chamber overnight at RT. Tissue sections were washed twice the next day in TBS for 15 min at RT and placed in an alkaline phosphatase buffer (a mixture of 0.3 M Tris-HCl [pH 9.5], 0.15 M MgCl2, and 0.3 M NaCl in equal parts, made immediately prior to use) for 5 min at RT. Nitroblue tetrazolium chloride–5-bromo-4-chloro-3-indolylphosphate (NBT-BCIP stock solution from Roche Molecular) color substrate was used at a dilution of 40 μl of stock solution to 10 ml of alkaline phosphatase buffer for 1 h 45 min to detect the signals. Slides were washed well in water, allowed to air dry, and coverslipped with a nonxylene medium.
X-Gal and BrdU double staining of transduced hepatocytes.
The mice injected with rAAV-EF1α-LacZ and labeled with BrdU were sacrificed 36 days after vector administration, and the liver and duodenum were harvested, embedded in OCT compound (Tissue-Tek; Sakura Finetek, U.S.A. Inc., Torrance, Calif.), and frozen in 2-methylbutane chilled with dry ice. Sections (10 μm) were subjected to X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) staining for analysis of cytoplasmic β-galactosidase expression (9). For the X-Gal and BrdU double staining, 7-μm-thick sections were prepared, stained with X-Gal, and immunostained against incorporated BrdU with sheep anti-BrdU antibody (Maine Biotechnology Services, Inc., Portland, Maine) as described elsewhere (18).
Statistical methods.
The data obtained from FISH were assessed statistically. The number of transduction events by viral vectors in any cell was assumed to follow a Poisson distribution. We conditioned on the number of cells having no viral vectors integrated to estimate the parameter of the Poisson distribution, i.e., P(X = 0) = exp(−λ). Then the expected number of cells with 1, 2, 3, etc., transduction or integration events by viral vectors was calculated by assuming a Poisson distribution with parameter lambda. The fit of the data to this random model was evaluated using a chi-square goodness-of-fit statistic, with cells classified as having 1, 2, or more transduction or integration events. Since it was not possible to determine if cells containing two red or green signals had undergone individual transduction events (see Table 1), they were counted as indicating a single transduction event. This would only underestimate the number of double transduction events and decrease the probability of statistical significance.
TABLE 1.
Summary of coinfusion of two vectors
| Time points | No. of nuclei examined | No. (%) of nuclei that werea:
|
Total no. (%) of transduced cellsa | |||
|---|---|---|---|---|---|---|
| Red | Green | Red and green | Yellow | |||
| Day 0, rAAV-FIX; day 0, rAAV-TH (T1) | 2,010 | 22 (1.1 ± 0.13) | 15 (0.8 ± 0.11) | 8 (0.4 ± 0.07) | 19 (0.9 ± 0.22) | 64 (3.2 ± 0.53) |
| Day 0, rAAV-FIX; day 7, rAAV-TH (T2) | 2,210 | 28 (1.3 ± 0.13) | 48 (2.2 ± 0.11) | 3 (0.1 ± 0.0) | 13 (0.6 ± 0.15) | 92 (4.2 ± 0.1) |
| Day 0, rAAV-FIX; day 21, rAAV-TH (T3) | 1,820 | 30 (1.7 ± 0.04) | 56 (3.1 ± 0.04) | 5 (0.3 ± 0.12) | 0 | 91 (5.0 ± 0.04) |
| Day 0, rAAV-FIX; day 35, rAAV-TH (T4) | 1,940 | 42 (2.2 ± 0.03) | 89 (4.6 ± 0.01) | 0 | 0 | 131 (6.8 ± 0.02) |
There were three animals per group. The nuclei counted per group are roughly equally divided among the three animals. The percentage of positive hepatocytes is given with the standard deviations in parentheses. The standard deviations were calculated using the individual transduction efficiencies from three mice per experimental group. On occasion, individual nuclei were found that contained two separate red or green signals. Because it was not possible to differentiate between two independent transduction events in the same cell and a single transduction event followed by nuclear DNA synthesis, these nuclei were scored as single transduction events. This would slightly underestimate the number of hepatocytes that had undergone double transduction events.
RESULTS
Functional rAAV genomes are taken up by most hepatocyte nuclei in vivo.
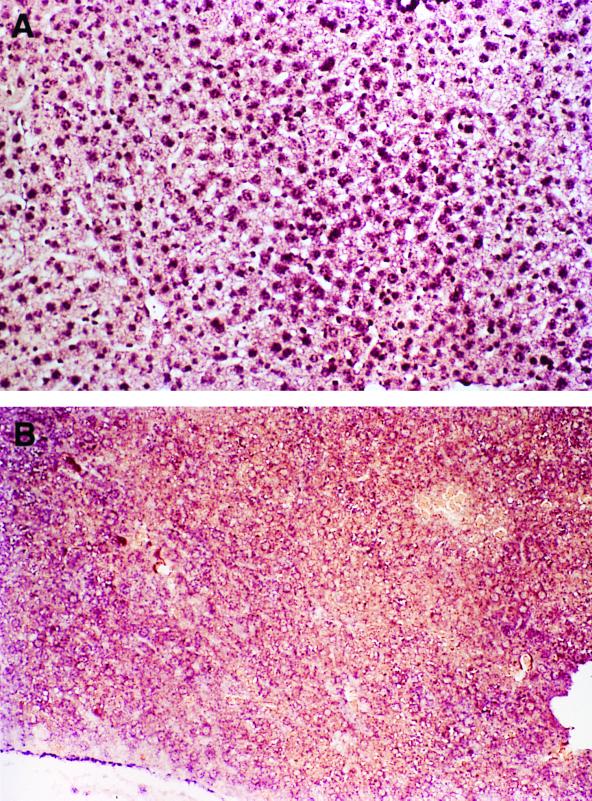
To attempt to understand why only a small percentage of the hepatocytes become transduced in vivo, an experiment was performed to establish whether all or most of the hepatocytes take up the vector genomes. The liver sections from C57BL/6 mice injected with rAAV-EF1α-LacZ (Fig. 1D) at an estimated multiplicity of infection of 1,000 (1.2 × 1011 particles per mouse, with 108 hepatocytes/liver) were prepared 24 h postinjection and examined for rAAV single-stranded vector genomes by DNA in situ hybridization. As shown in Fig. 2a and b, most if not all of the hepatocyte nuclei had a positive signal for rAAV, establishing that the low rate-limiting step for transduction was not selective nuclear entry of the single-stranded vector genomes.
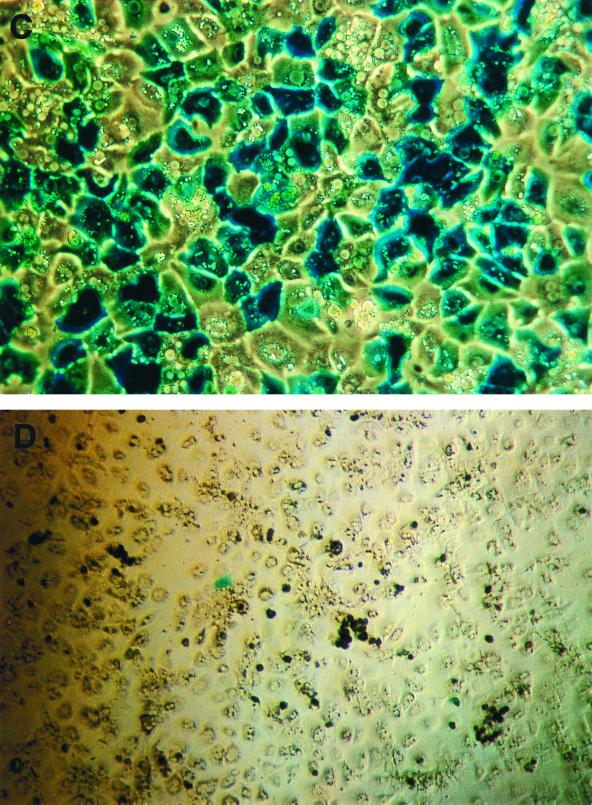
FIG. 2.
DNA in situ hybridization and biological assay for functional rAAV genomes. (A and B) C57BL/6 mice (n = 2) were injected with 1.2 × 1011 particles of rAAV-EF1α-LacZ by intraportal infusion. One day later, the animals were sacrificed and the livers were examined for the presence of rAAV genomes. (A) Representative liver sample from one of two rAAV-treated animals. Original magnification, ×200. (B) Liver sample from a non-rAAV control. Original magnification, ×100. Note the presence of staining in most of the hepatocyte nuclei in panel A. (C and D) C57BL/6 mice (n = 2) were injected with 1.0 × 1011 particles of AAV-CMV-LacZ by intraportal infusion. At 18 h after rAAV was administered, mouse hepatocytes were prepared and plated in six-well dishes. At 6 h later, the cells were infected with wild-type adenovirus type 2 at a multiplicity of infection of 100 (C [original magnification, ×100]) or not infected (D [original magnification, ×50]). At 24 h later, the cells were stained with X-Gal.
To establish that these cells contained a substantial number of nuclear rAAV genomes that were actually capable of transduction, two mice infused with 1.0 × 1011 particles of rAAV-CMV-LacZ were sacrificed 18 h later and primary mouse hepatocytes were cultured from these animals. At 6 h after isolation, the hepatocytes were infected for 24 h with a wild-type adenovirus type 2 (controls were cultured without adenovirus) at a dose known to infect about 90% of hepatocytes (11). The wild-type adenovirus was used to supply helper gene function to allow second-strand rAAV synthesis to proceed into a potential transcriptionally active molecule (5, 6, 19, 31). From 65 to 71% (n = 2 animals) of the adenovirus-infected and less than 0.1% of the non-adenovirus-infected hepatocytes stained positive with X-Gal, demonstrating that the majority of hepatocytes have “transduction-competent” rAAV genomes (Fig. 2c and d) shortly after vector administration in vivo.
These single-stranded competent genomes were lost over time, because when a similar study was performed 5 weeks after infusion of 1.8 × 10" rAAV-EF1α-LacZ particles into C57BL/6 Rag-1 mice (n = 3) (to avoid immune system responses to the E. coli β-galactosidase), there was only a slight (threefold) enhancement from 0.7 ± 0.1% (representing stable rAAV transduction) to 2.1 ± 0.3% X-Gal-positive cells with the addition of wild-type adenovirus. This is consistent with our previous findings that single-stranded rAAV vector genomes decrease over a 5- to 13-week period (15).
A changing subpopulation of hepatocytes is permissive to stable rAAV transduction, and dimer formation is an important event in achieving stable transduction.
To further evaluate the mechanism(s) of transduction in vivo, a mixed-vector experiment was performed. A total of 1.0 × 1011 particles of rAAV encoding human factor IX (rAAV-FIX) were infused per C57BL/6-scid mouse (scid mice were used to eliminate the humoral immune response to the vector and avoid interference with repeat dosing) into four groups of animals (n = 3/group) through their portal vein. Each group of mice was then infused with 1.0 × 1011 particles of rAAV encoding tyrosine hydroxylase (rAAV-TH) at the same time (T1) or 1 week (T2), 3 weeks (T3), or 5 weeks (T4) after rAAV-FIX infusion. Interphase nuclei were prepared from primary hepatocytes isolated from each group of mice 5 weeks after rAAV-TH infusion to determine the distribution of the two genomes in the nucleus by two-color FISH (Fig. 3) (data are summarized in Table 1). Consistent with our previous studies (15, 24), we found that a small percentage of the nuclei (3.2% to 6.8%) contained rAAV signals (a quantitative measure of transduction efficiency) (Table 1).
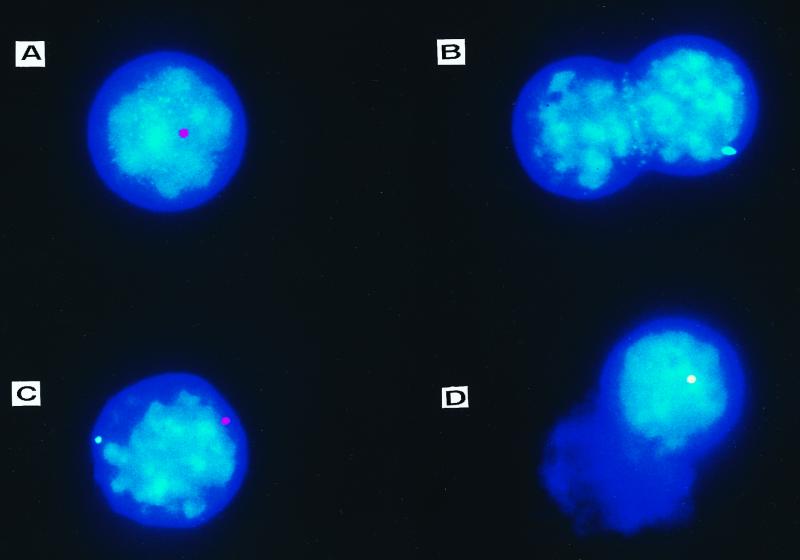
FIG. 3.
FISH from mice transduced in vivo with rAAV-FIX and rAAV-TH vectors. C57BL/6-scid mice were infused with 1.0 × 1011 particles of each rAAV-FIX and rAAV-TH vectors. At 5 weeks later, mouse interphase nuclei from freshly isolated primary hepatocytes were subjected to FISH with fluorescence-labeled rAAV-FIX and/or rAAV-TH probes. Nuclei contained only rAAV-FIX signal (A), only rAAV-TH signal (B), separate rAAV-FIX and rAAV-TH signals (C), or overlapping rAAV-FIX and rAAV-TH signals (D). The discrete red hybridization signals were produced by hybridizing with the rAAV-FIX probe, the green signals were produced by hybridizing with the rAAV-TH probe, and the yellow signal was generated from overlapping red and green signals. Rare cells contained two red or two green signals (not shown). Because some hepatocytes undergo DNA synthesis without nuclear or cell division, it is not possible to determine whether these doubly labeled single-color signals represent two independent events or DNA synthesis after transduction; these were counted as single red or green positive cells.
Most of the rAAV stably transduced positive nuclei contained either rAAV-FIX genomes (red) (Fig. 3A) or rAAV-TH genomes (green) (Fig. 3B) (Table 1). However, when rAAV-FIX and rAAV-TH were infused at the same time, 42% of the transduced cells contained both genomes (Fig. 3C and D). While 0.9% of the hepatocytes (28% of transduced hepatocytes) contained a hybrid genome signal (yellow), indicative of a single transduction event using a mixture of the two vector genomes (Fig. 3C), 0.4% of the hepatocytes (12.5% of transduced hepatocytes) contained separate red and green signals in the same nucleus (Fig. 3D), indicating two separate transduction events at different loci within the same cell. These results have intriguing implications. If many of the input vector genomes were involved in a transduction event, we would expect that most of the FISH signals would contain a red-green mix (yellow), whereas if a single genome were used, a yellow signal would never be observed. The approximate 1:1:1 red/green/yellow ratio of rAAV-positive nuclei was most consistent with dimer formation as a critical event in the transduction pathway.
Next, we examined whether rAAV transduction in hepatocytes was a random event. For this purpose, we assumed that a cell containing both a red and green signal was the subject of two independent transduction events. Yellow signals were counted as a single transduction event because the probability of two independent events occurring in close enough proximity to yield a yellow signal would be small. Furthermore, even if side-by-side transduction events were to occur on rare occasions, their exclusion as double transduction events would only decrease the statistical significance. By assuming that the transduction of a viral vector in any cell follows a Poisson distribution, the fit of the data to a random model was evaluated using a chi-square goodness-of-fit statistic, with cells classified as having one or multiple transduction events. From this random model, the number of cells with dual transduction events at time point T1 was predicted to occur at much lower frequency (0.05%) than the actual observed frequency (at least 0.4% [Table 1]) (P < 0.00001). These analyses indicate that rAAV transduction was not a random event and that there is a finite number of hepatocytes permissive to rAAV transduction. As the time between the injection of vector 1 and vector 2 increases, the number of dual-transduction events decreases but the total number of transduced hepatocytes increases, suggesting that cells permissive to transduction change over time. Taken together, our data suggest that there exists a relatively small subpopulation of hepatocytes that are permissive to transduction but that these cells are not static and change over time.
Molecular analysis of mixed rAAV concatemers in vivo.
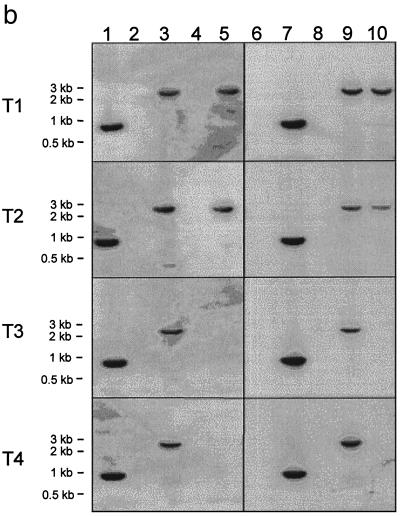
To begin to understand the molecular nature of the transduced rAAV genome in cells, Southern and PCR analyses were performed. Southern analysis of the genomic DNA digested with enzymes that cleave twice within either vector demonstrated the presence of both rAAV-FIX and rAAV-TH in the transduced mouse liver in all four groups of mice (data not shown). Furthermore, we confirmed the presence of both vector genomes in mouse liver by PCR amplification using internal primer sets (Fig. 4a) within the cDNA sequence of hFIX and TH (Fig. 4b, lanes 1 and 2 and lanes 6 and 7). Since it was more difficult to amplify longer sequences from genomic DNA, we used a nested-PCR amplification method with two sets of vector-specific primers, as shown in Fig. 4a, to detect the presence of head-to-tail concatemers, circular monomers or dimers composed of the same vector genomes, or mixed-vector genomes. After electrophoresis of the PCR products, the gel was blotted and hybridized with the hFIX cDNA probe. Subsequently, the same blots were stripped and hybridized with the TH cDNA probe. The control monomer vector band and concatemers composed of rAAV-FIX genomes hybridized with the hFIX probe only but not with the TH probe (Fig. 4b, lanes 1, 3, 6, and 8), whereas control monomer and concatemers of rAAV-TH hybridized with the TH probe only but not with the hFIX probe (lanes 2, 4, 7, and 9). The majority of the molecules are in a head-to-tail orientation; however, with longer exposure, minor bands of ∼0.9 kb corresponding to the tail-to-tail orientation could sometimes be detected (data not shown).
FIG. 4.
PCR detection of the vector genomes from transduced mouse hepatocytes. (a) Proposed structure of the amplified fragments. Horizontal arrows indicate the location and direction of PCR primers, and the sizes of the expected fragments are given. (b) The vector genomes were amplified from liver DNA of animals in each of the four groups (T1, both rAAV vectors infused at the same time; T2, vectors administered 1 week apart; T3, vectors administered 3 weeks apart; and T4, vectors administered 5 weeks apart) as summarized in Table 1. The presence of vector genomes in the mouse liver was confirmed by PCR experiments using sequence-specific primer sets within the cDNA sequence of hFIX (H1 and H2) (lanes 1 and 6) and TH (T1 and T2) (lanes 2 and 7). Two sets of primers as shown in panel a were used in nested PCR experiments to amplify vector-vector junctions composed of the same vector genomes and mixed-vector genomes, respectively. After electrophoresis of the PCR products, the gel was blotted and hybridized with an hFIX cDNA probe (lanes 1 to 5). Subsequently, the blots were stripped and hybridized with a TH cDNA probe (lanes 6 to 10). Primer sets consisting of H3-H4 and H8-H9 amplified head-to-tail rAAV-FIX molecules that hybridized with the hFIX probe but not the TH probe (lanes 3 and 8), primer sets consisting of T3-T4 and T7-T10 amplified head-to-tail rAAV-TH molecules which hybridized with the TH probe but not the hFIX probe (lanes 4 and 9), and primer sets consisting of H3-T4 and H9-T10 amplified head-to-tail rAAV-TH-rAAV-FIX mixed dimers which hybridized with both hFIX and TH probes (lanes 5 and 10). While a 2.7-kb band could arise from a PCR artifact, the livers from mice infused at the later time points (T3 and T4), which do contain both vector genomes in the same liver samples, do not give the 2.7-kb band.
Most interestingly, a single band of ∼2.7 kb was amplified by PCR and hybridized with both hFIX and TH probes; it was found to represent a mixed-genome species (Fig. 4b, lanes 5 and 10 in samples T1 and T2 only). The mixed concatemers containing rAAV-FIX and rAAV-TH genomes in a head-to-tail orientation were detected only in hepatocytes of mice given the two constructs simultaneously or 1 week apart, not in those of mice given the constructs 3 and 5 weeks apart; this was consistent with the presence or absence of yellow FISH signals (Fig. 3) in transduced hepatocytes. Larger molecules consisting of (rAAV-TH)n-(rAAV-FIX)n in either head-to-tail, head-to-head, or tail-to-tail forms may also exist but may not be detectable because of heterogeneity, primer selection, or length of the PCR product. Thus, further studies are required to establish the most prevalent molecular structure (if one exists) of the heterogenomes.
The permissive subpopulation of hepatocytes comprises both non-S-phase and S-phase cells.
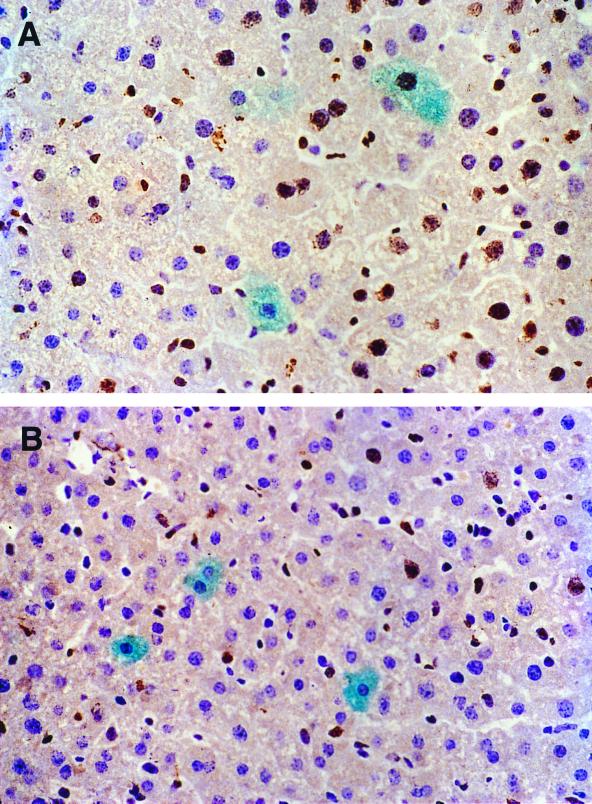
Although most hepatocytes at a single time point are quiescent, a small percentage are in the cell cycle, and some of these do not undergo cytokinesis; this results in polyploid nuclei and/or doubly nucleated hepatocytes (4). Thus, a substantial fraction of hepatocytes could cycle over the 5-week period required for rAAV transduction of hepatocytes. In addition, it has been demonstrated that rAAV vectors preferentially transduce cells in S phase in vitro (21). Therefore, we designed studies to determine if the cell cycle status could account for the permissive subpopulation of hepatocytes that were transduced by rAAV vectors in vivo. We first compared the rAAV-EF1α-LacZ transduction efficiency in the two groups of mice that were not hepatectomized or were partially hepatectomized 48 h (time to maximal hepatocellular division) prior to vector administration. Continuous labeling with BrdU for 36 days revealed that 50% of the hepatocytes passed through S phase after vector administration in the hepatectomized group while 15% of the hepatocytes were labeled in the nonhepatectomized group (Fig. 5; Table 2); however, there was no significant difference in the number of β-galactosidase-positive hepatocytes between these two groups. This suggested that cell division at or around the time of vector injection was not a requirement for transduction of mouse hepatocytes. More importantly, among the β-galactosidase-positive hepatocytes, the BrdU-positive cells appeared to be of the same or lower prevalence compared with the BrdU-negative cells in both the partially hepatectomized and nonhepatectomized animals (Fig. 5; Table 2). This substantiates the notion that there was no preference for transduction in hepatocytes that had passed through the S phase in vivo.
FIG. 5.
Determination of rAAV transgene expression in BrdU-labeled hepatocytes. C57BL/6 Rag-1 mice were injected with 1.8 × 1011 particles of rAAV-EF1α-LacZ with (n = 3) (A) or without (n = 4) (B) a prior hepatectomy. The animals were continuously labeled with BrdU for 36 days, as described in Materials and Methods. At 36 days, liver sections were costained with X-Gal and BrdU. The cytoplasmic blue and nuclear brown staining represents β-galactosidase and BrdU, respectively. (A) Magnification, ×400. One doubly labeled hepatocyte is present in the lower left corner. (B) Magnification, ×200. The β-galactosidase-positive cells are negative for BrdU.
TABLE 2.
BrdU and β-galactosidase labeling of hepatocytes
| Mouse | Labeling index (%)a | X-Gal-positive cells (%)a | Labeling index in X-Gal-positive cells (%)a |
|---|---|---|---|
| Nonhepatectomized group | |||
| 1 | 13.3 (314/2,367) | 0.9 (27/3,117) | 4.3 (12/278) |
| 2 | 14.2 (325/2,286) | 0.6 (13/2,139) | 8.6 (8/93) |
| 3 | 14.3 (346/2,417) | 0.7 (16/2,329) | 4.7 (6/112) |
| 4 | 16.0 (388/2,420) | 0.3 (7/2,367) | 4.9 (9/182) |
| Mean | 14.5 ± 1.0 | 0.6 ± 0.2 | 5.6 ± 1.7 |
| Partially hepatectomized group | |||
| 5 | 57.9 (1,715/3,014) | 0.5 (12/2,217) | 46.7 (71/152) |
| 6 | 43.2 (905/2,097) | 1.1 (22/2,049) | 38.4 (117/305) |
| 7 | 48.7 (1,032/2,121) | 0.6 (12/2,133) | 38.2 (79/207) |
| Mean | 49.9 ± 6.1 | 0.7 ± 0.3 | 41.1 ± 4.0 |
The numbers in parentheses are the number of BrdU-positive cells or X-Gal-positive cells/total number of cells counted.
We next wanted to know if stimulating cell division by partial hepatectomy after vector injection would enhance transduction and/or change the molecular forms of the vector. To address this question, C57BL/6 mice (13 to 16 weeks old) injected with rAAV-EF1α-FIX vector intraportally at a dose of 1.7 × 1011 particles were partially hepatectomized on days 3, 10, 17, and 24 postinjection. All the mice that underwent hepatectomy were sacrificed 7 days after the procedure (days 10, 17, 24, and 31 after vector administration) for rAAV DNA analysis. Plasma hFIX levels, rAAV genome number per cell, and rAAV vector forms were compared in rAAV-treated, nonhepatectomized and partially hepatectomized animals. Consistent with our previous studies, the hFIX levels increased over time (15) but were accompanied by a reduction in the number of vector genomes in the nonhepatectomized mice (Fig. 6). The hFIX levels in the partially hepatectomized group were consistently lower than those in the nonhepatectomized group, presumably representing loss of the vector genomes that had the potential to participate in the protein expression. The loss of vector genomes was most prevalent between the 3- and 10-day partial hepatectomy groups, a period when a large proportion of vector genomes have not integrated (15). The loss of DNA and the lower hFIX gene expression may have simply been due to the dilution of the nonintegrated vector genomes per hepatocyte during cell division. The smaller decrease in the number of vector genomes observed between the 17- and 24-day partially hepatectomized animals (Fig. 6) probably occurred because some of the stable transduced hepatocytes contained integrated rAAV genomes by this time that would not be diluted by cellular division. Finally, cell cycle activation after vector injection did not promote the conversion of the low-molecular-weight episomal forms to the high-molecular-weight species (data not shown). Taken together, the results show that cell cycling did not facilitate the rAAV transduction in hepatocytes.
FIG. 6.
Comparison of transgene expression and vector copy number per cell in the liver between mice partially hepatectomized after rAAV administration and control mice without hepatectomy. Sixteen adult C57BL/6 mice were injected with rAAV-EF1α-FIX. Three mice were partially hepatectomized on days 3, 10, 17, and 24 and sacrificed 7 days after the hepatectomy. hFIX levels in mouse plasma were determined by an enzyme-linked immunosorbent assay, and the vector copy number per diploid amount of DNA in livers was determined by Southern blot analysis. DNA signals represent all double-stranded vector sequences (see Materials and Methods). Error bars represent standard deviation. The number above each bar indicates the number of mouse samples analyzed. PHx, partial hepatectomy.
DISCUSSION
Possible mechanisms of concatemerization leading to stable transduction.
The mechanisms involved in rAAV integration have been studied in tissue culture and are now starting to be addressed in vivo (reviewed in reference 20). It is becoming apparent that the process of integration may not be the same under different experimental conditions, and it is likely that the metabolic state of the cell and/or the cell type is a crucial factor.
Our results are consistent with the mechanism that the random linkage of two genomes is a critical event or that only a few input rAAV genomes are directly involved in the process of transduction in the absence of helper viruses (12). The presence of the concatemeric mixed genomes argues against the models where AAV proviral concatemers are the exclusive result of the amplification of a single input genome in a rolling-circle model, or as a monomer-length input genome that integrates and is then amplified in place. The formation of vector concatemers may involve joining of existing single-stranded genomes and/or rolling-circle replicative mechanisms. The presence of mixed concatemeric structures indicates that recombination between two vector monomers may occur. In view of the great stability of single-stranded rAAV genomes in the liver for at least 5 weeks (15) and the recent evidence of circular intermediates of monomer and dimer viral genomes in a head-to-tail array in muscle tissue (2, 27, 30), our data are consistent with our current hypothesis that two genomes somehow become linked and then become larger concatermers before, during, or after chromosomal integration. Further experiments are required to clarify the molecular mechanism.
Permissive state of cells required for transduction.
The reason why only a small, changing population of hepatocytes is permissible to rAAV transduction is not known but may be associated with a special metabolic state of the cell. Alternatively, the permissive state could be nothing more than a stochastic event where a large threshold number of genomes is needed for transduction even if only a few genomes are used and required in the initial steps of concatemer formation. If this were true, we would expect most, if not all, of the transduced hepatocytes to be periportal, because they would have received the highest concentration of vector after an intraportal infusion. However, there is only a slight preference for transduction of periportal hepatocytes (24). Dose-response studies with careful quantitation of transgene product and the number of transduced hepatocytes may help to distinguish between these different possibilities.
Clearly, our studies demonstrate that the cell cycle is not an important factor for rAAV transduction. In a previous study, regenerating muscle was less permissive to rAAV transduction than was mature muscle (25). However, the situation for muscle is different from that for liver, because regenerating hepatocytes are fully differentiated cells whereas the cells involved in muscle replacement are not terminally differentiated. In our studies, we demonstrate that rAAV uptake is not the limiting step to transduction in regenerating livers. Moreover, in livers that had taken up rAAV genomes and then underwent a regenerative stimulus by partial hepatectomy, there was no enhancement in transduction.
There is still no way to easily reconcile these studies with the studies of Russell et al., who showed enhanced rAAV transduction in cultured cells that had passed through the S phase (21). There are multiple issues, including the possibility of low levels of wild-type helper virus that may have been present in earlier viral preparations, the difference in timing when the cultured cells were examined for rAAV-mediated gene expression compared to the in vivo studies (48 h and 36 days, respectively), and/or the presence of [3H]thymidine (used for S-phase labeling), which itself can increase rAAV transduction (1). Cultured cells may not behave in the same way as cells in vivo, or the differences may be related to the different cell types studied.
It is important to resolve the process involved in the permissive nature of hepatocytes that allows stable transduction, because it may allow the presently limited transduction efficiency in vivo to be expanded. However, the inability to analyze single cells or their clones at the molecular level makes this a challenging task for future studies.
ACKNOWLEDGMENTS
C.H.M. and H.N. contributed equally to this work.
We thank Doug Bolgiano for assistance with the statistical analysis.
This work was supported by NIH grant HL53682. C.H.M. was supported by individual NIH-NRSA grant HL09754.
Footnotes
Present address: Division of Molecular Medicine, Department of Pediatrics, Harvard Medical School, Boston, MA 02115.
REFERENCES
- 1.Alexander I E, Russell D W, Miller A D. DNA-damaging agents greatly increase the transduction of non-dividing cells by adeno-associated virus vectors. J Virol. 1994;68:8282–8287. doi: 10.1128/jvi.68.12.8282-8287.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duan D, Sharma P, Yang J, Yue Y, Dudus L, Zhang Y, Fisher K, Engelhardt J. Circular intermediates of recombinant adeno-associated virus have defined structural characteristics responsible for long-term episomal persistence in muscle tissue. J Virol. 1998;72:8568–8577. doi: 10.1128/jvi.72.11.8568-8577.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fan D S, Ogawa M, Fujimoto K I, Ikeguchi K, Ogasawara Y, Urabe M, Nishizawa M, Nakano I, Yoshida M, Nagatsu I, Ichinose H, Nagatsu T, Kurtzman G J, Ozawa K. Behavioral recovery in 6-hydroxydopamine-lesioned rats by cotransduction of striatum with tyrosine hydroxylase and aromatic l-amino acid decarboxylase genes using two separate adeno-associated virus vectors. Hum Gene Ther. 1998;9:2527–2535. doi: 10.1089/hum.1998.9.17-2527. [DOI] [PubMed] [Google Scholar]
- 4.Fausto N. Hepatic regeneration. In: Zakim D, Boyer T D, editors. Hepatology. 3rd ed. Vol. 1. Philadelphia, Pa: The W. B. Saunders Co.; 1996. pp. 35–58. [Google Scholar]
- 5.Ferrari F K, Samulski T, Shenk T, Samulski R J. Second-strand synthesis is a rate-limiting step for efficient transduction by recombinant adeno-associated virus vectors. J Virol. 1996;70:3227–3234. doi: 10.1128/jvi.70.5.3227-3234.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fisher K J, Gao G-P, Weitzman M D, DeMatteo R, Burda J F, Wilson J M. Transduction with recombinant adeno-associated virus for gene therapy is limited by leading-strand synthesis. J Virol. 1996;70:520–532. doi: 10.1128/jvi.70.1.520-532.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flannery J G, Zolotukhin S, Vaquero M I, LaVail N M, Muzyczka N, Hauswirth W W. Efficient photoreceptor-targeted gene expression in vivo by recombinant adeno-associated virus. Proc Natl Acad Sci USA. 1997;94:6916–6921. doi: 10.1073/pnas.94.13.6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herzog R, Yang E, Couto L, Hagstrom J, Elwell D, Fields P, Burton M, Bellinger D, Read M, Brinkhous K, Podsakoff G, Nichols T, Hurtzman G, High K. Long-term correction of canine hemophilia B by gene transfer of blood coagulation factor IX mediated by adeno-associated viral vector. Nat Med. 1999;5:56–63. doi: 10.1038/4743. [DOI] [PubMed] [Google Scholar]
- 9.Kay M A, Li Q, Liu T J, Leland F, Toman C, Finegold M, Woo S L C. Hepatic gene therapy: persistent expression of human alpha 1-antitrypsin in mice after direct gene delivery in vivo. Hum Gene Ther. 1992;3:641–647. doi: 10.1089/hum.1992.3.6-641. [DOI] [PubMed] [Google Scholar]
- 10.Kessler P D, Podsakoff G M, Chen X, McQuiston S A, Colosi P C, Matelis L A, Kurtzman G J, Byrne B J. Gene delivery to skeletal muscle results in sustained expression and systemic delivery of a therapeutic protein. Proc Natl Acad Sci USA. 1996;93:14082–14087. doi: 10.1073/pnas.93.24.14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Q, Kay M A, Finegold M, Stratford-Perricaudet L D, Woo S L C. Assessment of recombinant adenoviral vectors for hepatic gene therapy. Hum Gene Ther. 1993;4:403–409. doi: 10.1089/hum.1993.4.4-403. [DOI] [PubMed] [Google Scholar]
- 12.Linden R M, Winocour E, Berns K I. The recombination signals for adeno-associated virus site-specific integration. Proc Natl Acad Sci USA. 1996;93:7966–7972. doi: 10.1073/pnas.93.15.7966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mandel R J, Rendahl K G, Spratt S K, Snyder R O, Cohen L K, Leff S E. Characterization of intrastriatal recombinant adeno-associated virus-mediated gene transfer of human tyrosine hydroxylase and human GTP-cyclohydrolase I in a rat model of Parkinson's disease. J Neurosci. 1998;18:4271–4284. doi: 10.1523/JNEUROSCI.18-11-04271.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McLaughlin S K, Collis P, Hermonat P L, Muzyczka N. Adeno-associated virus general transduction vectors: analysis of proviral structures. J Virol. 1988;62:1963–1973. doi: 10.1128/jvi.62.6.1963-1973.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miao C, Snyder R, Schowalter D, Patijn G, Donahue B, Winther B, Kay M A. The kinetics of rAAV integration in the liver. Nat Genet. 1998;19:13–15. doi: 10.1038/ng0598-13. [DOI] [PubMed] [Google Scholar]
- 16.Nakai H, Herzog R, Hagstrom J, Walter J, Kung S-H, Yang E, Tai S, Iwaki Y, Kurtzman G, Fisher K, Colosi P, Couto L, High K. Adeno-associated viral vector-mediated gene transfer of human blood coagulation factor IX into mouse liver. Blood. 1998;91:4600–4607. [PubMed] [Google Scholar]
- 17.Nakai H, Iwaki Y, Kay M A, Couto L B. Isolation of recombinant adeno-associated virus (rAAV) vector-cellular DNA junctions from mouse liver. J Virol. 1999;77:5438–5447. doi: 10.1128/jvi.73.7.5438-5447.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park F, Ohashi K, Chiu W, Kay M A. Efficient lentiviral transduction of liver requires cell cycling in vivo. Nat Genet. 2000;24:49–52. doi: 10.1038/71673. [DOI] [PubMed] [Google Scholar]
- 19.Rendahl K G, Leff S E, Otten G R, Spratt S K, Bohl D, Van Roey M, Donahue B A, Cohen L K, Mandel R J, Danos O, Snyder R O. Regulation of gene expression in vivo following transduction by two separate rAAV vectors. Nat Biotechnol. 1998;16:757–761. doi: 10.1038/nbt0898-757. [DOI] [PubMed] [Google Scholar]
- 20.Russell D, Kay M A. rAAV and hematology. Blood. 1999;94:864–874. [PMC free article] [PubMed] [Google Scholar]
- 21.Russell D W, Miller A D, Alexander I E. Adeno-associated virus vectors preferentially transduce cells in S phase. Proc Natl Acad Sci USA. 1994;91:8915–8919. doi: 10.1073/pnas.91.19.8915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rutledge E A, Russell D W. Adeno-associated virus vector integration junctions. J Virol. 1997;71:8429–8436. doi: 10.1128/jvi.71.11.8429-8436.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Snyder R, Miao C, Meuse L, Tubb J, Donahue B, Lin H, Stafford D, Patel S, Thompson A, Nichols T, Read M, Bellinger D, Brinkhous K, Kay M. Correction of hemophilia B in canine and murine models using recombinant adeno-associated viral vectors. Nat Med. 1999;5:64–70. doi: 10.1038/4751. [DOI] [PubMed] [Google Scholar]
- 24.Snyder R, Miao C, Patijn G, Spratt S, Danos O, Nagy D, Gown A, Winther B, Meuse L, Cohen L, Thompson A, Kay M. Persistent and therapeutic concentrations of human factor IX in mice after hepatic gene transfer of recombinant AAV vectors. Nat Genet. 1997;16:270–276. doi: 10.1038/ng0797-270. [DOI] [PubMed] [Google Scholar]
- 25.Snyder R O, Spratt S K, Lagarde C, Bohl D, Kaspar B, Sloan B, Cohen L K, Danos O. Efficient and stable adeno-associated virus-mediated transduction in the skeletal muscle of adult immunocompetent mice. Hum Gene Ther. 1997;8:1891–1900. doi: 10.1089/hum.1997.8.16-1891. [DOI] [PubMed] [Google Scholar]
- 26.Szczypka M S, Mandel R J, Donahue B A, Snyder R O, Palmiter R D. Viral gene delivery selectively restores feeding and prevents lethality of dopamine-deficient mice. Neuron. 1999;22:167–178. doi: 10.1016/s0896-6273(00)80688-1. [DOI] [PubMed] [Google Scholar]
- 27.Vincent-Lacaze N, Snyder R O, Gluzman R, Bohl D, Lagarde C, Danos O. Structure of adeno-associated virus vector DNA following transduction of the skeletal muscle. J Virol. 1999;73:1949–1955. doi: 10.1128/jvi.73.3.1949-1955.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiao X, Li J, Samulski R J. Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector. J Virol. 1996;70:8098–8108. doi: 10.1128/jvi.70.11.8098-8108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang C C, Xiao X, Zhu X, Ansardi D C, Epstrein N D, Frey M R, Matera A G, Samulski R J. Cellular recombination pathways and viral terminal repeat hairpin structures are sufficient for adeno-associated virus integration in vivo and in vitro. J Virol. 1997;71:9231–9246. doi: 10.1128/jvi.71.12.9231-9247.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang J, Zhou W, Zhang Y, Zidon T, Ritchie T, Engelhardt J F. Concatamerization of adeno-associated virus circular genomes occurs through intermolecular recombination. J Virol. 1999;73:9468–9477. doi: 10.1128/jvi.73.11.9468-9477.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ye X, Rivera V M, Zoltick P, Cerasoli F, Schnell M A, Gao G P, Hughes J V, Gilman M, Wilson J M. Regulated delivery of therapeutic proteins after in vivo somatic gene transfer. Science. 1999;283:88–91. doi: 10.1126/science.283.5398.88. [DOI] [PubMed] [Google Scholar]