Abstract
Lactate is a byproduct of glycolysis, and before the Warburg effect was revealed (in which glucose can be fermented in the presence of oxygen to produce lactate) it was considered a metabolic waste product. At present, lactate is not only recognized as a metabolic substrate that provides energy, but also as a signaling molecule that regulates cellular functions under pathophysiological conditions. Lactylation, a post-translational modification, is involved in the development of various diseases, including inflammation and tumors. Liver disease is a major health challenge worldwide. In normal liver, there is a net lactate uptake caused by gluconeogenesis, exhibiting a higher net lactate clearance rate compared with any other organ. Therefore, abnormalities of lactate and lactate metabolism lead to the development of liver disease, and lactate and lactate metabolism-related genes can be used for predicting the prognosis of liver disease. Targeting lactate production, regulating lactate transport and modulating lactylation may be potential treatment approaches for liver disease. However, currently there is not a systematic review that summarizes the role of lactate and lactate metabolism in liver diseases. In the present review, the role of lactate and lactate metabolism in liver diseases including liver fibrosis, non-alcoholic fatty liver disease, acute liver failure and hepatocellular carcinoma was summarized with the aim to provide insights for future research.
Key words: lactate, lactate metabolism, acute liver failure, non-alcoholic fatty liver disease, hepatocellular carcinoma
1. Introduction
Lactate, with the chemical structure CH3CH(OH)COOH, was first reported in 1780. Initially considered a waste product under hypoxia conditions, it was hypothesized to have multiple harmful effects (1). In the 1920s, Warburg first observed that tumor tissues have an increased uptake of glucose compared with normal tissues. This led to the proposal of aerobic glycolysis, which also revealed that glucose can be fermented in the presence of oxygen to produce lactate, a process that increases the intra and extracellular concentrations of lactate, known as the Warburg effect (2). Accumulation of lactate in the tissue microenvironment is a prominent feature of inflammatory diseases (such as asthma and arthritis) and tumors (3-5), highlighting the important role of lactate in these conditions. In 1985, Brooks proposed the 'lactate shuttle hypothesis', suggesting that lactate serves as a fuel to coordinate systemic metabolism and as a signaling molecule in intercellular, inter-tissue and inter-organ signal transduction (6,7). With increasing research, it is now considered that lactate not only serves as an energy source, but also acts as a signaling molecule and through protein lactylation (1,8-10).
The liver serves a vital role in various physiological processes, including glucose metabolism, fatty acid metabolism, lipid metabolism, immune response and the secretion of various cytokines (such as TGF-β, IL-6 and IL-10) (11-13). Liver diseases encompass non-alcoholic fatty liver (NAFL) disease (NAFLD), liver fibrosis, liver cirrhosis, acute liver failure (ALF) and hepatocellular carcinoma (HCC) (11). Liver diseases pose a notable global health burden, with an estimated 150 million cases of liver diseases resulting in ~2 million mortalities annually (14,15). In the normal liver, there is a net lactate uptake due to gluconeogenesis, and the liver has the highest net lactate clearance rate compared with the other organs of the body, which is expected to be ≤70% of the systemic clearance (9,16). Thus, lactate and lactate metabolism serve an important role in liver diseases. However, there is not currently a systematic review that summarizes the role of lactate and lactate metabolism in liver diseases. The aim of the present review was to summarize the roles of lactate and lactate metabolism in the liver and in the development of liver diseases. Additionally, the present review may provide new directions and guidance for future research and the treatment of liver diseases.
2. Methodology
The studies cited in the present review were published between 1994 and 2024, with the majority published between 2011 and 2024. All of the studies cited in the present review were found on the PubMed database using the following keywords: Lactate, lactate metabolism, lactate metabolism-related genes (LMRGs), glucose metabolism, glycolysis, lactate shuttle, monocarboxylate transporter (MCT), G protein-coupled receptor 81 (GPR81), histone lactylation, non-histone lactylation, NAFLD, liver fibrosis, ALF and HCC.
3. Lactate and lactate metabolism
Production and clearance of lactate
As the final product of glycolysis, lactate is primarily produced under hypoxia conditions (7). In the cytoplasm, glucose undergoes a series of catalytic reactions to form pyruvate, which is subsequently converted to lactate in the presence of lactate dehydrogenase (LDH)A (Fig. 1). Another metabolic pathway that produces lactate includes the conversion of alanine to glutamate via alanine aminotransferase, which is also a minor source of lactate production in tumor cells (17). Lactate exists in the body as D-lactate, L-lactate and racemic DL-lactate. Among these, L-lactate is the primary form in the human body, participating in various biological processes (such as energy regulation and the regulation of fatty acid metabolism) (9). D-lactate serves as the main metabolite in bacteria (such as Lactobacillus and colibacillus) found in the gut, and may be involved in the transport of metabolic substrates (such as H+, pyruvic acid and malate) within the body (9,18).
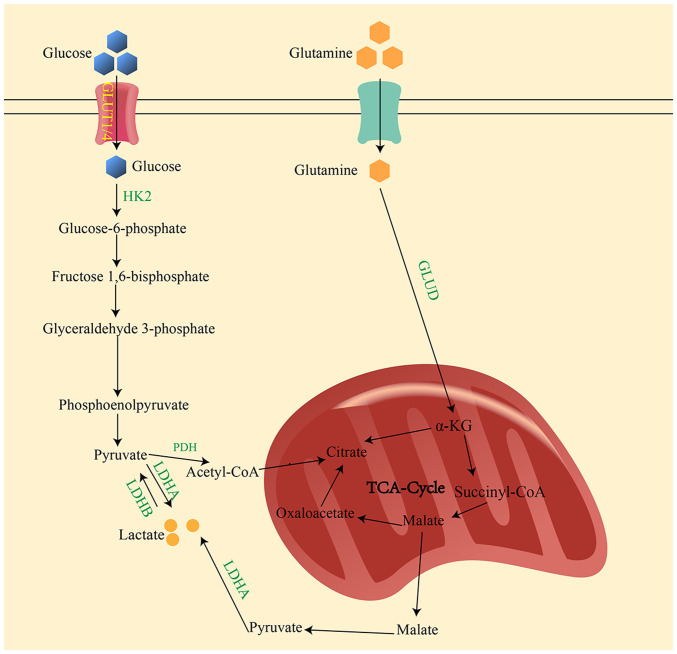
Figure 1.
Main production pathway of lactate. In the cytoplasm, glucose is converted to pyruvate through a series of catalytic reactions. Under normal oxygenation, pyruvate is transported to the mitochondria for the TCA cycle. However, under hypoxic conditions, pyruvate is catalyzed by LDHA to lactate. Glutamate is converted to α-ketoglutarate by GLUD in the mitochondria. Subsequently, α-KG is converted to malate, which is then transported out of the mitochondria and oxidized to pyruvate in the cellular matrix. Finally, lactic acid is produced by the action of LDHA. GLUT1/4, glucose transporter 1/4; HK2, hexokinase 2; PDH, pyruvate dehydrogenase; LDHA/B, lactate dehydrogenase A/B; TCA, tricarboxylic acid; GLUD, glutamate dehydrogenase; α-KG, α-ketoglutarate.
Accumulation of lactate in the body can be hazardous and causes lactic acidosis (19). Consequently, the body should efficiently and rapidly remove lactate from tissues and circulation through metabolism. The primary method of lactate clearance is through the oxidative formation of pyruvate. This is followed by the formation of acetyl-CoA, catalyzed by pyruvate dehydrogenase, which is then used in the tricarboxylic acid cycle for the formation of CO2, water and for providing energy (20,21). Another clearance pathway involves activating gluconeogenesis in the liver and skeletal muscle cells in response to hormones such as glucagon and cortisol, in which lactate is converted into glucose, which is then released into the bloodstream and further metabolized to provide energy to the body (9,22). Under normal circumstances, the liver exhibits the highest lactate clearance rate in the body (9,16). When liver function is impaired, it leads to dysregulation of lactate metabolism, and in patients with chronic liver disease, lactate clearance is markedly reduced leading to lactate accumulation (23).
Lactate transport
Lactate, and its function as a signaling molecule, has been investigated. It primarily exerts its effects by being transported into cells via MCTs or by signaling through its specific receptor, GPR81 (24,25).
MCTs belong to the solute carrier l6A subfamily (25). MCT1 and 4 are primarily associated with lactate transport and are expressed in various tissues including the muscle, heart, nerve and liver (26). The main function of MCT1 is to import lactate into cells, and that of MCT4 is to export lactate from cells; they work synergistically to promote lactate shuttling between cells, which serves an essential role in maintaining lactate homeostasis (27-29). Abnormal expression of MCTs can lead to the onset of various diseases including cancer (9,30,31). In the liver, MCT1 transports L-lactate into hepatocytes for gluconeogenesis (32). The expression of MCT1 and the concentration of lactate are positively associated (33). Therefore, MCTs serve a vital role in liver diseases (Table I) (34-43).
Table I.
Function of MCTs in liver diseases.
| Liver diseases | MCT1 function | MCT4 function | (Refs.) |
|---|---|---|---|
| Liver fibrosis | Promote liver fibrosis | Not reported | (34) |
| NAFLD | Promote food anticipatory activity and liver steatosis. Knockdown of MCT1 in mice attenuates NAFLD | Not reported | (35-37) |
| HCC | Upregulation of MCT1 in regulatory T cells promotes resistance to anti-PD-1 therapy in patients with HCC | Promotes the proliferation, invasion and metastasis of tumor cells. It is associated with the poor prognosis of patients with HCC. Inhibition of MCT4 can increase immunotherapy in HCC | (38-43) |
NAFLD, non-alcoholic fatty liver disease; HCC, hepatocellular carcinoma; MCT, monocarboxylate transporter; PD-1, programmed death-1.
GPR81 is widely distributed in tissues and organs such as fat, kidney and liver (44). Lactate may inhibit IL1β expression in macrophages by acting on GPR81 and arrestin β 2 to inhibit Toll-like receptor (TLR) 4-triggered NLR family pyrin domain containing 3 activation. Furthermore, low concentrations of lactate can attenuate acute liver injury (45). Additionally, lactate can inhibit lipolysis by activating GPR81 on the surface of adipocytes, which downregulates cAMP levels to balance the energy metabolism between glucose and lipids (46). Metformin can increase GPR81 expression, improving mouse NAFLD symptoms in a GPR81-dependent manner (47). However, knowledge of the role of lactate/GRP81 in liver disease is limited and additional studies are required.
4. Functions of lactate and lactate metabolism
As a metabolic substrate, the main function of lactate is to generate pyruvate, which is catalyzed by LDHB. Lactate also serves as a precursor of gluconeogenesis in the synthesis of glucose, which is then used an energy source (Table II) (7,48-61). Additionally, lactate regulates fatty acid metabolism and acts as a signaling molecule to modulate cellular functions including the modulation of inflammatory responses and cell proliferation (9). Besides these functions, Zhang et al (62) showed that lactate can regulate transcription through an epigenetic modification known as lactylation. Lactylation is a post-translational modification that occurs after the translation of proteins, directly promoting gene transcription (62,63). When lactate levels are increased, lactate is converted into lactyl CoA due to the action of a currently unknown enzyme, and histone lysine residues are lactylated by an effector protein (P300). Lactylation can be modulated by effector proteins such as P300/cyclic AMP response element-binding protein (CBP) (9,62). Due to lactylation being considered a common post-translational modification, investigating the role of lactylated proteins in the occurrence and development of liver diseases in future research will broaden the understanding of the mechanisms underlying disrupted lactate metabolism in liver diseases.
Table II.
Functions of lactate and lactate metabolism.
| Function | Description | (Refs.) |
|---|---|---|
| Energy regulation | Primary fuel in the TCA cycle. Supplementary sources of glucose | (48-52) |
| Regulation of fatty acid metabolism | Lactate promotes fatty acid synthesis by increasing the intracellular pool of acetyl- CoA as well as by increasing the activity of acetyl coenzyme A carboxylase (a key enzyme that regulates fatty acid synthesis). Furthermore, lactate induces CD4+ T cells to upregulate the expression of the lactate transporter SLC5A12, which mediates the uptake of lactate by CD4+ T cells, forming a positive feedback loop to increase the synthesis of fatty acids | (9,53) |
| Histone lactylation | Associated with lactate concentrations. Promotes the transition of macrophages from a pro-inflammatory phenotype to a reparative phenotype. HK2 promotes lactylation by acting on the H3K181a lactylation site, then promoting liver fibrosis. AK2 and lactylation of H3 histone contribute to the progression of HCC | (9,54-57) |
| Non-histone lactylation | By analyzing tumors and adjacent tissues from patients with HCC, 9,275 lactylation sites were identified, of which 9,256 were located on non-histone proteins. The role of non-histone lactylation has not been revealed in studies on liver diseases | (9,56) |
| LMRGs | Lactate metabolism contributes to tumor-induced immune suppression, a major obstacle to effective immune therapy. LMRGs can be used as predictors of tumor clinical prognosis. There are 66 LMRGs differentially expressed in HCC, mainly associated with metabolic processes and oxidative reactions. FKTN, PDSS1, PET117, PUS1, RARS1 and RNASEH1 were associated with the prognosis of HCC and were used to calculate the LMRG score; patients with a high LMRGS score had a poor prognosis, and the LMRGS score was positively associated with the expression of immune checkpoints such as PD-1. Further research is required to determine the predictive role of LMRGs | (58-61) |
HK2, hexokinase 2; AK2, adenosine kinase 2; HCC, hepatocellular carcinoma; PD-1, programmed death-1; LMRGs, lactate metabolism- related genes; TCA, tricarboxylic acid.
5. Lactate and lactate metabolism in liver diseases
Lactate serves an important role in inflammation, immune energy metabolism and signaling pathway activation, affecting inflammation processes and tumor immune tolerance (9). The liver is an important metabolic organ that coordinates various metabolic activities (such as lipid fatty acid metabolism and immune responses) and serves an essential role in several glucose metabolic pathways, including gluconeogenesis, glycogenolysis and glycolysis (11,64,65). Normal lactate levels are 0.5-1.7 mmol/l (66), but lactate clearance rates do not have a defined normal range. Elevated lactate levels are observed in liver diseases, especially in patients with chronic liver disease (67). Arterial serum lactate levels >2 mmol/l are associated with higher organ failure scores and higher mortality (68). Comparison of the 28-day survival in patients with liver cirrhosis admitted to the intensive care unit (ICU) reveals that admission lactate (1.2-3.4 mmol/l) are notably lower in surviving patients compared with in those who died (2-9.7 mmol/l), whereas lactate clearance (-9 to 50%) was markedly higher in surviving patients compared with in those who died (-33 to 43%). Therefore, lactate is associated with short-term mortality in critically ill patients with cirrhosis and can be used as a prognostic indicator (68). Lactate and lactate metabolism are involved in liver fibrosis, NAFLD and HCC development (Fig. 2) (55-57). Lactate, LDH and LMRGs may be used as predictors of liver failure and HCC prognosis (Table III) (59,68,69). Lactate can not only be used as a clinical prognostic marker for liver disease, but can also be a target for studying the pathogenesis and potential therapeutic approaches for liver disease.
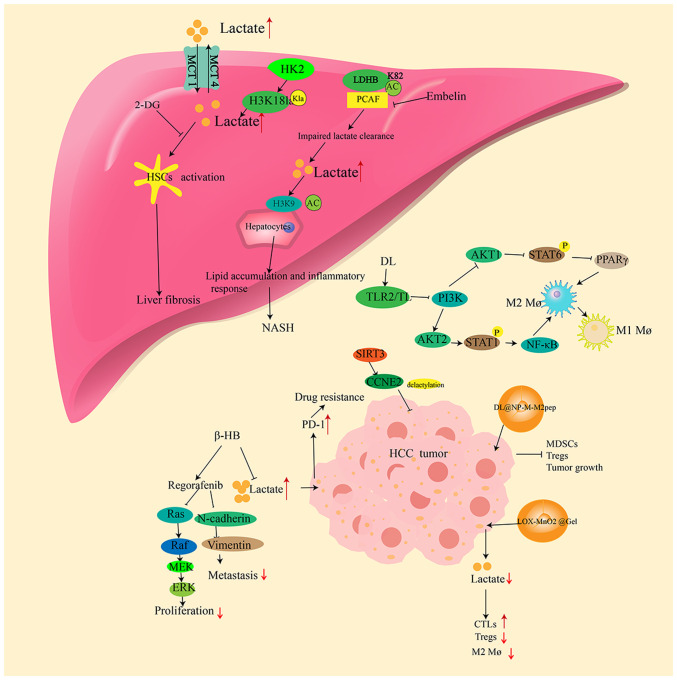
Figure 2.
Role of lactate in liver diseases. In the liver, lactate is transported into and out of cells via MCT1 and MCT4, respectively. The increase of lactate in the liver can promote liver fibrosis through the activation of HSCs. HK2 induces histone lactylation at H3K181a to promote lactate production, which induces liver fibrosis. PCAF inhibits the activity of LDHB by acting at the K82 site of LDHB to promote acetylation, thereby inhibiting lactate clearance and thus contributing to the development of NAFLD. D-lactate inhibits the PI3K/AKT1/STAT6/PPARγ pathway by interacting with TLR2/TLR9, which activates AKT2/STAT1/NF-κB to promote the transformation of M2 Mø to M1 Mø. In HCC, lactate promotes PD-1 expression thereby increasing drug resistance. However, β-HB inhibits lactate production, and suppresses HCC proliferation and migration by inhibiting the B-Raf/MAPK pathway and EMT, as well as increasing the drug sensitivity of HCC. SIRT3 induces the delactylation of CCNE2 to inhibit the development of HCC. The nano particle formulation, DL@ NP-M-M2pep, acts on macrophages to inhibit MDSCs, Tregs and tumor growth. Another nanoformulation, LOX-MnO2 @Gel, reduces the lactate levels to restore the intratumoral function of CTLs, inhibit Tregs and reduce M2 Mø. MCT1/4, monocarboxylate transporter 1/4; HSCs, hepatic stellate cells; HK2, hexokinase 2; PCAF, P300/cyclic AMP response element-binding protein-associated factor; LDHB, lactate dehydrogenase B; NAFLD, non-alcoholic fatty liver disease; TLR2/9, Toll-like receptor; M2 Mø, M2 macrophages; M1 Mø, M1 macrophages; HCC, hepatocellular carcinoma; PD-1, programmed death-1; β-HB, β-hydroxybutyrate; EMT, epithelial-mesenchymal transition; SIRT3, sirtuin 3; CCNE2, cyclin E2; MDSCs, myeloid-derived suppressor cells; Tregs, regulatory T cells; CTLs, cytotoxic T lymphocytes; 2-DG, 2-deoxy-D-glucose; AC, acetylated; NASH, non-alcoholic steatohepatitis; DL, D-lactate; PPARγ, peroxisome proliferator-activated receptor γ; P, phosphorylated.
Table III.
Function of lactate in liver diseases.
| Liver diseases | Function | (Refs.) |
|---|---|---|
| Liver fibrosis | Increases in lactate levels could promote HSC activation and liver fibrosis. Lactate could promote HSC activation through lactylation. HK2 deficiency could lead to a reduction of H3K181a inhibiting lactylation and HSC activation. HK2/H3K18la axis is a potential target for the treatment of liver fibrosis | (55,74,75) |
| NAFLD | Lactate levels in the blood and liver gradually increase with lesion aggravation. PCAF-dependent K82 acetylation reduces LDHB activity and inhibits lactate clearance, and upregulation of LDHB-K82Q increases histone acetylation and promotes NAFLD | (23) |
| ALF | Increased lactate levels, which could be used as a predictor even though they have low specificity, could inform decision process of the transplant team that may benefit the prognosis of the patient. Markedly elevated serum LDH levels, but of low diagnostic value | (84-103) |
| HCC | Increased lactate levels. Accumulation of lactate in the TME could lead to acidification of the extracellular environment, which can inhibit the function of T cells and NK cells, and enhance the immunosuppressive function of TAMs, MDSCs and regulatory T cells thereby promoting tumor progression. Increased lactate levels along with increased drug resistance in HCC. Knockdown of LDHA in mice markedly inhibited the growth of HCC; however, it is ineffective in vivo when LDHA is used as a target for drug development. Targeted delivery of D-lactate to macrophages could inhibit the growth of HCC. LDH levels could be used as a prognostic indicator for HCC. Lactylation of adenylate kinase 2 promotes the progression of HCC. SIRT3 induces delactylation of CCNE2 to inhibit the development of HCC. H3 histones lactylation could promote the progression of HCC. Knockdown of HK2 suppressed the incidence of HCC in mice | (9,56,57,76,108,117-123,128,129,133-142) |
HK2, hexokinase 2; HSCs, hepatic stellate cells; NAFLD, non-alcoholic fatty liver disease; PCAF, P300/cyclic AMP response element-binding protein-associated factor; LDH, lactate dehydrogenase; ALF, acute liver failure; HCC, hepatocellular carcinoma; TME, tumor microenvironment; TAMs, tumor-associated macrophages; MDSCs, myeloid-derived suppressor cells; SIRT3, sirtuin 3; CCNE2, cyclin E2; NK, natural killer.
Liver fibrosis
Liver fibrosis is a wound healing response to various injuries to the liver and has a high morbidity rate affecting >100 million individuals worldwide (11). Liver fibrosis is caused by a variety of factors, including viral hepatitis, alcoholic liver disease and NAFLD (11). Additionally, as liver fibrosis progresses, liver function becomes impaired, and further progression to liver cirrhosis will cause ascites and esophagogastric fundus venous hypertension, which decreases the quality of life of the patient, and it may progress to HCC, affecting the prognosis of the patient (70,71). At present, besides liver transplantation, there are no effective methods to cure liver fibrosis (72). Liver fibrosis mainly occurs due to the activation and transformation of quiescent hepatic stellate cells (HSCs) into myofibroblasts, leading to excessive extracellular matrix deposition (11,73). Research indicates that metabolic reprogramming, including aerobic glycolysis, can activate HSCs (74,75), and inhibition of aerobic glycolysis can suppress HSC activation. After HSC activation, lactate is involved in subsequent processes, including gene expression (74,75).
In highly glycolytic proliferating cells, such as cancer cells, hexokinase (HK)2 expression accelerates glucose metabolism (76). In HSCs, HK2 deletion or pharmacological inhibition of lactate production can reduce histone lactylation at H3K181a in HSCs, thereby inhibiting HSC activation. This inhibition is reversed by supplementing exogenous lactate (55). Furthermore, HSC activation is inhibited, and hepatic fibrosis is attenuated in mice with an HSC-specific knockout of HK2 (55). Thus, intervention of the HK2/H3K18la axis is a potential therapeutic strategy for liver fibrosis. Increased expression of MCT1 also promotes liver fibrosis formation in non-alcoholic steatohepatitis (NASH) mouse models (34). Increases in lactate levels promotes HSC activation as well as lactate transport and lactylation, which are associated with the development of liver fibrosis. Therefore, the role of lactate metabolism in the development of liver fibrosis should be investigated further in the future.
NAFLD
The prevalence of NAFLD has made it a major global health issue and the overall prevalence of NAFLD worldwide is estimated to be 32.4% (77). NAFLD comprises a spectrum of liver conditions, in which hepatic steatosis (fatty liver) alone is referred to as NAFL and NASH is defined as a more serious condition with inflammation and hepatocyte damage (steatohepatitis) (77,78). As the disease progresses, a number of patients may develop liver cirrhosis or HCC (79). Studies show that with an increasing severity of liver disease, especially when the disease progresses from steatosis to NASH, lactate levels gradually increase in both the blood and liver (80,81). Protein acetylation is a major mechanism in the development of chronic liver diseases (82). LDHB activity is markedly reduced in the liver of patients with NAFLD or NASH (23). Similarly, in high fat diet (HFD)-induced NAFLD mouse models, the LDHB activity is decreased (23). Mass spectrometry reveals that during the construction of the mouse model, the HFD exacerbates lactate accumulation, decreases the liver lactate clearance rate and alters the expression of the acetyltransferases P300/CBP-associated factor (PCAF) (23). PCAF is the major regulatory factor for LDHB acetylation at the K82 site (23). PCAF-dependent K82 acetylation reduces LDHB activity and inhibits lactate clearance. Another study suggests that H3K9 acetylation may aggravate lipid accumulation, and overexpression of LDHB-K82Q (which inhibits the activation of LDHB) in mice increases H3K9 histone acetylation, promotes lipid accumulation and inflammatory reactions, and results in an exacerbation of NAFLD (23). Inhibition of PCAF reduces LDHB acetylation and alleviates hepatic steatosis in NASH mice. This provides a potential therapeutic target for NAFLD. MCT1 can promote liver steatosis, and the knockdown of MCT1 in a mouse model attenuates the symptoms of NAFLD (35-37). However, further studies are required to investigate the role of lactate metabolic processes in NAFLD.
ALF
ALF is a syndrome characterized by brain dysfunction, coagulation disorders and multi-organ dysfunction resulting from acute liver injury (83). A number of studies have investigated the role of lactate in ALF. Bernal et al (84) demonstrate that arterial blood lactate levels can predict a poor prognosis of acetaminophen-induced ALF. There is a notable association between lactate levels and survival in response to ALF, including acetaminophen-induced, non-acetaminophen-induced and edible mushroom-induced AFL, and despite the low specificity of lactate as a predictor of prognosis in patients with ALF, patients with high lactate levels have lower survival rates (68,85-93). Furthermore, a study suggests that lactate levels lack specificity as a criterion for urgent liver transplantation in patients with ALF (94). Bernal (95) argues that the decision-making process for liver transplantation is a dynamic one, and the level of lactate remains an important component of the overall assessment of patients with ALF, aiding transplant teams in making decisions that contribute to the prognosis of the patients. Early postoperative lactate levels are effective markers for clinically relevant post-hepatectomy liver failure (PHLF) (96). Furthermore, elevated perioperative lactate levels and decreased lactate clearance are associated with the incidence of PHLF (97). Therefore, lactate levels may assist in clinical decision-making for patients with a liver transplant, such as timely administration of preventive treatment and enhanced observation. The lactate clearance rate serves as an independent predictor of mortality in critically ill patients with liver cirrhosis and acute-on-chronic liver failure (ACLF) (98). Additionally, in patients with ACLF requiring ICU admission, a lactate and organ failures predictive model, constructed from lactate levels and a number of organ failures, demonstrates that the lactate level and the number of organ failures at the time of admission to the ICU predicts patient prognosis. Therefore, this may allow for an improved risk stratification in order to optimize strategies for organ support (99).
In patients with ALF, serum LDH levels are markedly higher compared with those in patients with acute or chronic hepatitis, or liver cirrhosis. Immunohistochemistry also shows a relative increase in LDH expression levels (100). While LDH was previously considered to have low diagnostic value in liver diseases due to its production by various cells throughout the body (101), a recent study reveals that a high lactate/albumin ratio is associated with an increased mortality during hospitalization in patients with liver cirrhosis (102). This ratio serves as an independent predictive indicator of in-hospital mortality in patients with cirrhosis (102). Therefore, the present study suggests that lactate can be used as a prognostic predictor for cirrhosis (103) as well as ALF. During the development of ALF, the proteins involved in lactate metabolism must be altered, therefore, it may be possible to identify more specific LMRGs to predict the prognosis of ALF in the future.
HCC
Liver cancer is the 6th most common cancer and the 4th leading cause of cancer-related mortality in the world (104). The most common type of primary liver cancer is HCC, which accounts for 80-90% of cases (104). Current effective treatments for HCC include surgery, liver transplantation, chemotherapy and targeted therapy, but overall survival (OS) remains unsatisfactory (105). Further research is required for the treatment of HCC.
The tumor microenvironment (TME) serves an important role in cancer progression, consisting of tumor cells, immune cells, stromal cells, blood vessels and the extracellular matrix (106). Lactate accumulation exacerbates hypoxia thereby activating hypoxia-inducible factor-1α to further promote lactate production (107). Lactate accumulation in the TME can lead to extracellular acidification, inhibiting the function of T cells and natural killer (NK) cells, while enhancing the immunosuppressive functions of tumor-associated macrophages (TAMs), myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Tregs), thereby promoting tumor progression (9). Lactate also promotes hypoxia and angiogenesis, further contributing to the immunosuppressive functions of the TME (9).
Lactate induces the expression of programmed death-1 (PD-1) in monocytes and neutrophils (108). In addition, lactate can induce PD-1 expression by activating signaling pathways mediated by TGF-β, IFN-γ and TNF-α (105-108). Increased lactate activates the TGF-β/Smad signaling pathway and subsequently activates epithelial-mesenchymal transition (EMT)-related genes to promote tumor progression (109,110). Lactate can lead to an increase in the levels of hepatocyte growth factor (HGF) in cancer-associated fibroblasts and subsequently activate the mesenchymal epithelial transition-dependent signaling pathway in cancer cells, maintaining resistance to tyrosine kinase inhibitors (TKIs) (111). In HCC, lactate promoted HCC progression by modulating HGF (112). Lactate upregulates IFN-γ expression in M2 TAMs and promotes T-cell apoptosis through the PD-1/PD-ligand (L)1 pathway (113). In HCC, lactate promotes PD-L1 upregulation by increasing TNF-α expression; blocking TNF-α inhibits PD-L1 expression in TAMs (114). Both lactate and lactylation promotes IL-6 secretion to promote tumor progression (115,116). Hence, increases in lactate levels increases HCC resistance and decreases HCC treatment efficacy (117).
Elevated lactate levels are observed in the tumor and surrounding tissues, and in the serum of patients with HCC (108,118). Lenvatinib administered in combination with celecoxib reduces lactate-induced PD-L1 neutrophil survival and thus, reduces the levels of PD-L1 neutrophils increasing the antitumor effect of lenvatinib in subcutaneous and orthotopic HCC mouse models (108). Overexpression of LDHA in HCC cells increases the invasive capacity of HCC cells, and knockdown of LDHA inhibits the metastatic potential in xenograft mice (119). Ketogenesis primarily occurs in the liver, and β-hydroxybutyrate (β-HB) is a ketone produced during this process (120). Exogenous administration of β-HB decreases LDHA expression and lactate production in sorafenib-resistant HCC cells, enhancing the drug sensitivity of HCC sorafenib-resistant cells by inhibiting the B-Raf/MAPK pathway and EMT (117). However, in xenograft models, β-HB inhibits the expression of proliferating cell nuclear antigen and LDHA without markedly improving tumor size and weight (117). While β-HB treatment may reverse sorafenib resistance by downregulating lactate production, further research is needed to confirm its effectiveness in vivo (117). microRNA (miR)-34a serves an anticancer role in HCC cells. By establishing a radioresistant HCC cell line, miR-34a levels are markedly downregulated in HepG2 radioresistant cells, and overexpression of miR-34a re-sensitizes cells to radiation treatment by inhibiting LDHA (121). The potassium inwardly-rectifying channel, subfamily J, member 11 potassium channel can interact with LDHA and enhance its enzymatic activity to promote HCC progression (122). Knockdown of LDHA in mice notably inhibits the growth of HCC, while the selective loss of CD8+ and increases of CD4+ T lymphocytes in the TME are observed (123). However, further studies are needed to investigate the mechanism of HCC development inhibition by knockdown of LDHA. A number of drugs that target LDHA are effective in in vitro experiments but are found to be ineffective when experimenting in vivo (123,124). Additionally, a number of effective in vivo LDHA inhibitors often exhibit off-target effects, suggesting that their antitumor activity may not be solely due to LDHA inhibition (125,126). Thus, further investigation is required to determine whether LDHA is a suitable target for HCC treatment.
Research shows that D-lactate can reach the liver through the portal vein and enhance the ability of Kupffer cells to clear pathogens from the bloodstream (127). In addition, D-lactate also interacts with TLR2 and/or TLR9 on macrophages to inhibit the PI3K/Akt pathway while activating the NF-κB pathway to promote the transition of M2 TAMs to M1 TAMs (128). Targeted delivery of D-lactate to macrophages through the nanoformulation DL@NP-M-M2 macrophage-binding peptide (DL@NP-M-M2pep) markedly inhibits tumor growth in mouse models, improving survival rates (128). It reverses the immunosuppression in TME by inhibiting MDSCs and Tregs while activating NK cells and DCs (128). Another study used nanotechnology to construct a nanoparticle-hydrogel composite system, LOX-MnO2@Gel; this system depletes lactate from the TME through a cascade catalytic reaction, then restores intratumoral cytotoxic T lymphocyte function, reduces the ratio of Tregs/M2-like macrophages, enhances antitumor immune responses and transforms the immunosuppressive TME into an immunocompetent one (129). This approach markedly inhibits residual tumor growth, suppresses lung metastasis and prolongs mouse survival in subcutaneous and in situ HCC mouse models (129). Combining metabolic therapy with immunotherapy provides new insights for the treatment of HCC recurrence post-ablation, but long-term research is required. Targeting lactate has also been shown to be a possible treatment for HCC.
LDH can serve a predictive role in the treatment of sorafenib in patients with renal cell, rectal and lung cancer (130-132). Earlier studies have also shown the same predictive value in HCC (133). However, another study suggests that baseline LDH levels are not associated with the prognosis of patients with HCC undergoing sorafenib treatment (134). In a previous study, baseline LDH levels in patients with HCC were found to be influenced by the degree of liver fibrosis, independent of HCC staging (135). Lower baseline LDH levels were identified as an independent prognostic factor for an improved response to sorafenib. The study also found that a marked increase in serum LDH levels during sorafenib administration may indicate the potential development of ALF (135). In patients with HCC undergoing liver resection, low LDH levels are associated with improved OS and recurrence-free survival (136). Preoperative serum LDH levels can assess the long-term prognosis of patients with HCC undergoing transarterial chemoembolization (TACE) (137). In addition, an increase in LDH after undergoing TACE also implies poorer OS (138). Another study involving 2,327 patients with HCC indicated that high LDH levels and a high ratio of alkaline phosphatase/LDH are associated with poor OS (139). The positive rates of LDHC mRNA expression in serum and in serum exosomes of patients with HCC were 68 and 60%, respectively. The LDHC expression levels were negatively associated with HCC prognosis, serving as a predictor for HCC prognosis (140). Therefore, current research supports LDH as a prognostic indicator for HCC treatment.
Lactate transport also serves a role in the development of HCC. MCT1 is highly expressed in HCC tissues compared with adjacent tissues (38). Additionally, MCT4 is highly expressed in HCC cells and tissues compared with normal hepatocytes and adjacent tissues. MCT4 can promote tumor cell proliferation, invasion and metastasis, and is strongly associated with the poor prognosis of patients with HCC (39,40). It is also involved in HCC progression by promoting the expression of trafficking protein particle complex subunit 5 (41). When MCT4 is inhibited, it leads to the disruption of pH homeostasis in HCC cells, which induces apoptosis and inhibits migration and invasion (42).
LMRGs can be used as predictors of tumor clinical prognosis (59-61). There are 66 LMRGs differentially expressed in HCC, mainly associated with metabolic processes and oxidative reactions (59). FKTN, PDSS1, PET117, PUS1, RARS1 and RNASEH1 are associated with the prognosis of HCC and may be used to calculate the LMRG score; patients with a high LMRGs score have a poor prognosis, and the LMRGs score is positively associated with the expression of immune checkpoints such as PD-1 (59). However, further research is needed to determine the predictive role of LMRGs.
Lactylation primarily affects enzymes involved in metabolism, and is associated with cellular energy metabolism. Adenylate kinase 2 (AK2) is a key enzyme in the transfer of phosphate groups between adenosine monophosphate and ATP to produce adenosine diphosphate. AK2 lactylation markedly reduces AK2 enzyme activity, leading to energetic disturbances in HCC cells, which promotes HCC progression (56). The NAD-dependent deacetylase sirtuin 3 can inhibit the development of HCC by promoting cell cycle protein E2 delactylation (141). Pan et al (57) also reveal that histone lactate levels in tumor tissues of patients with HCC are notably higher compared with those in adjacent tissues, and that lactylation of H3 histones can promote the progression of HCC. Silencing HK2 in HCC cells inhibits tumorigenesis and promotes cell death, and knockdown of HK2 in mice suppresses the incidence of HCC by inhibiting lactate production (55). By analyzing the relevant lactonization genes of patients with HCC in The Cancer Genome Atlas and the International Cancer Genome Consortium databases, Cheng et al (142) found that 16 lactylation-related genes were associated with the prognosis of HCC, and eight differential genes, which were further filtered to be included in the lactylation score, were found to be negatively associated with prognosis; therefore, lactylation-related genes have the potential to serve as a prognostic biomarker for HCC in the future. Lactate production, lactate shuttling and lactylation are involved in the development of HCC, therefore, targeting lactate metabolism is a potential approach to treating HCC; however, additional research is required.
6. Lactate and lactate metabolism in the treatment of liver diseases
Efforts have been made to identify effective treatment methods and diagnostic markers for liver diseases. When liver diseases progress to liver cirrhosis and HCC, the prognosis for patients is poor. Lactate levels can be useful for assessing the prognosis of ALF and liver cirrhosis (84-103), and LDH can also be used as a predictor of therapeutic sensitivity in HCC (133-140). LDHA, HK2, MCTs and HK2 are also potential targets for the treatment of liver diseases (23,35-37,42,43,55,123). Lactate-targeted amelioration of liver disease mainly occurs through the regulation of lactate production, lactate transport and lactylation (Table IV).
Table IV.
Drug of target lactate in liver diseases.
| Drug | Target | Function | (Refs.) |
|---|---|---|---|
| Curcumol | KLF5/LDHA | Attenuates liver fibrosis | (145) |
| 2-DG | HK2 | Improves liver fibrosis, and increases the antitumor effects of anti-PD-1 and sorafenib, while showing antitumor effects in anti-PD-1-resistant tumors | (147-152) |
| 3-BrPA | HK2 | Inhibits HCC cell proliferation and motility, and improves the efficacy of sorafenib in HCC models | (153-155) |
| Quercetin | HK2 | Inhibits the proliferation HCC cells | (156) |
| ORPH | Glycolysis | Inhibits the growth and metastasis of HCC | (157) |
| Galloflavin | LDHA | Attenuates liver injury in the mouse model of ALF, and inhibits the proliferation of HCC cells | (158,159) |
| Quinoline-3-sulfonamides | LDHA | Inhibits the proliferation of HCC cells | (159) |
| Oxamate | LDHA | Enhances the antitumor activity of sorafenib, imatinib and sunitinib against HCC cells | (160) |
| VB124 | MCT4 | Enhances T cell infiltration and the efficacy of anti-PD-1 immunotherapy in a HCC mouse model | (164) |
| Demethylzeylasteral | Lactylation | Inhibits the development of HCC | (57) |
| RJA | Lactylation | Inhibits the proliferation and migration of HCC cells | (165) |
2-DG, 2-deoxy-D-glucose; KLF5, Kruppel like factor 5; LDH, lactate dehydrogenase; HK2, hexokinase 2; PD-1, programmed death-1; 3-BrPA, 3-bromopyruvate; HCC, hepatocellular carcinoma; ORPH, oviductus ranae protein hydrolysate; ALF, acute liver failure; MCT, monocarboxylate transporter; RJA, royal jelly acid.
Reducing lactate production
Glycolysis is an important source of lactate production, and glucose transport is also a regulator of lactate production. Lactate production can be regulated by targeting glycolytic pathway-related proteins including LDH, glucose transporter, MCT, HK2 and pyruvate kinase M2 (PKM2) (143,144). Reducing lactate can inhibit the activation of HSCs, thereby suppressing the occurrence and development of liver fibrosis (55). Kruppel like factor 5 (KLF5) promotes glycolysis, leading to an increased LDHA expression. Curcumol inhibits liver fibrosis by blocking the KLF5/LDHA feedback loop (145). The Wnt/β-catenin signaling pathway enhances LDHA stability, promoting glycolysis and liver fibrosis. In mice, the specific deletion of LDHA in HSCs alleviates liver fibrosis (146). Therefore, the inhibition of LDHA may be an effective treatment for liver fibrosis. By inhibiting HK, 2-deoxy-D-glucose (2-DG) inhibits glycolysis, which improves liver fibrosis (147). The combination of 2-DG with sorafenib inhibits HCC cell proliferation and improves sorafenib resistance (148,149). However, a number of studies suggest that 2-DG has no marked impact on tumor growth at doses that do not cause severe adverse reactions (150,151). A recent study, in which 2-DG is delivered to the liver via nanoparticles, demonstrates that it increases the antitumor effects of sorafenib while producing antitumor effects in anti-PD-1-resistant tumors (152). Additionally, 3-bromopyruvate is a HK2 inhibitor that suppresses HCC cell proliferation and movement, enhances sorafenib efficacy, and is considered a potential sensitizer for clinical chemotherapy (153-155). Quercetin is a bioactive flavonoid that can inhibit HK2-dependent glycolysis and thus, inhibit HCC progression (156). Oviductus ranae protein hydrolysate (ORPH) has immunomodulatory and anti-glioma activities. ORPH can inhibit HCC progression by targeting the miR-491-5p/PKM2 axis to inhibit glycolysis (157). Galloflavin, an LDHA inhibitor, alleviates liver damage in ALF mouse models (158). Quinoline-3-sulfonamides are also inhibitors of LDHA, and quinoline-3-sulfonamides and galloflavin also inhibit the proliferation of HCC cells (159). As an inhibitor of LDHA, oxamate enhances the antitumor activity of sorafenib, imatinib and sunitinib in HCC (160). Liver fibrosis, liver injury and HCC can be ameliorated by targeting lactate production; however, long-term studies are required to investigate the application of this in clinical treatment.
Inhibition of lactate transport
MCT1 and 4 serve important roles in the occurrence and development of liver diseases. Inhibiting the transport of lactate is a potential target for cancer therapy (161). Therefore, inhibiting lactate transport may be beneficial for improving liver diseases. In mice, knocking out MCT1 alleviates symptoms of NAFLD (162). Upregulation of MCT1 in Tregs promotes resistance to PD-1 therapy in patients with HCC (43). A previous study demonstrates that ARC155858, an inhibitor of MCT1, can inhibit proliferation and lipid synthesis in HCC cells, but further in vivo studies are required to confirm this finding (163). Thus, in-depth studies are required to determine whether an MCT1 inhibitor can improve liver diseases, and further research is warranted in the future. Inhibition of MCT4 disrupts the intracellular pH homeostasis and initiates apoptosis in HCC cells (42). The MCT4 inhibitor VB124 enhances T cell infiltration and the efficacy of anti-PD-1 immunotherapy in a HCC mouse model (164).
Inhibition of lactylation
Inhibiting lactylation may also be a therapeutic strategy for HCC. Demethylzeylasteral, a triterpenoid anti-tumor compound, can inhibit the development of HCC by suppressing the lactylation of H3 histones, thereby inhibiting the tumorigenicity induced by liver cancer stem cells (57). Royal jelly acid (RJA), a major unsaturated fatty acid in natural compound royal jelly, inhibits the proliferation and migration of HCC cells and promotes apoptosis. In a subcutaneous HCC model, RJA inhibits tumor growth by inhibiting the lactylation of H3K9la and H3K14la sites on H3 histone (165). Glypican-3 (GPC3), a member of the glypican family, is expressed at high levels in HCC and has diagnostic value (166). Recent research indicates that GPC3 promotes lactate production, contributing to HCC development by enhancing the overall lactate levels and c-myc lactylation (167). In future research, a new direction may be to target lactylation to study the treatment of liver diseases. In addition, LMRGs are also potential markers and therapeutic targets for predicting the prognosis of liver diseases.
7. Conclusion
Lactate and lactate metabolism serve an essential role in the development and progression of liver diseases. Abnormalities in lactate production and transport, and lactylation contribute to the development of liver disease, while lactate levels can predict the prognosis of ALF and liver cirrhosis. LDH can be used as a predictor of the therapeutic sensitivity of HCC. Targeting lactate production and transport, regulating circulating lactate levels and inhibiting lactylation may serve as potential future strategies for the treatment of liver disease. Several studies have already been conducted (57,145,147-160,164,165), but the role of lactate transport and lactylation in liver disease should be further investigated in the future. Lactate metabolism involves a number of genes; LMRGs may exist as biomarkers and therapeutic targets for liver diseases such as liver fibrosis, ALF and HCC. In-depth basic and clinical studies are required to confirm the role of lactate metabolism in liver diseases. Summarizing the currently available studies may help guide future research.
Acknowledgements
Not applicable.
Funding Statement
The present study was supported by grants from the National Natural Science Foundation of China (grant nos. 81960507, 82073087 and 82160112), the collaborative Innovation Center of Chinese Ministry of Education (grant no. 2020-39), the Science and Technology Bureau fund of Zunyi city [grant no. ZUN SHI KE HE HZ ZI (2019)93-Hao], and the Science and Technology Plan Project of Guizhou Province [grant nos. QIAN KE HE JI cHU-ZK (2021) YI BAN451 and QIAN KE HE LH ZI (2017)7095 HAO].
Availability of data and materials
Not applicable.
Authors' contributions
SY drafted the manuscript. HC, TT, LZ, XY and ZY participated in the literature search and analysis of the data to be included in the review. XL, YW, JA and GW were involved in the design of the study and assisted in the preparation of the figures and tables. HJ and BT edited and revised the manuscript. All authors read and approved the final version of the manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Ferguson BS, Rogatzki MJ, Goodwin ML, Kane DA, Rightmire Z, Gladden LB. Lactate metabolism: Historical context, prior misinterpretations, and current understanding. Eur J Appl Physiol. 2018;118:691–728. doi: 10.1007/s00421-017-3795-6. [DOI] [PubMed] [Google Scholar]
- 2.Liberti MV, Locasale JW. The warburg effect: How does it benefit cancer cells? Trends Biochem Sci. 2016;41:211–218. doi: 10.1016/j.tibs.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Certo M, Tsai CH, Pucino V, Ho PC, Mauro C. Lactate modulation of immune responses in inflammatory versus tumour microenvironments. Nat Rev Immunol. 2021;21:151–161. doi: 10.1038/s41577-020-0406-2. [DOI] [PubMed] [Google Scholar]
- 4.Syed M, Kammala AK, Callahan B, Oskeritzian CA, Subramanian H. Lactic acid suppresses MRGPRX2 mediated mast cell responses. Cell Immunol. 2021;368:104422. doi: 10.1016/j.cellimm.2021.104422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Souto-Carneiro MM, Klika KD, Abreu MT, Meyer AP, Saffrich R, Sandhoff R, Jennemann R, Kraus FV, Tykocinski L, Eckstein V, et al. Effect of increased lactate dehydrogenase a activity and aerobic glycolysis on the proinflammatory profile of autoimmune CD8+ T cells in rheumatoid arthritis. Arthritis Rheumatol. 2020;72:2050–2064. doi: 10.1002/art.41420. [DOI] [PubMed] [Google Scholar]
- 6.Brooks GA. Lactate shuttles in nature. Biochem Soc Trans. 2002;30:258–264. doi: 10.1042/bst0300258. [DOI] [PubMed] [Google Scholar]
- 7.Brooks GA. Cell-cell and intracellular lactate shuttles. J Physiol. 2009;587(Pt 23):5591–5600. doi: 10.1113/jphysiol.2009.178350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang C, Pan RY, Guan F, Yuan Z. Lactate metabolism in neurodegenerative diseases. Neural Regen Res. 2024;19:69–74. doi: 10.4103/1673-5374.374142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li X, Yang Y, Zhang B, Lin X, Fu X, An Y, Zou Y, Wang JX, Wang Z, Yu T. Lactate metabolism in human health and disease. Signal Transduct Target Ther. 2022;7:305. doi: 10.1038/s41392-022-01151-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaffney DO, Jennings EQ, Anderson CC, Marentette JO, Shi T, Schou Oxvig AM, Streeter MD, Johannsen M, Spiegel DA, Chapman E, et al. Non-enzymatic lysine lactoylation of glycolytic enzymes. Cell Chem Biol. 2020;27:206–213.e6. doi: 10.1016/j.chembiol.2019.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yao S, Yang X, An J, Jin H, Wen G, Wang H, Tuo B. Role of the S100 protein family in liver disease (Review) Int J Mol Med. 2021;48:166. doi: 10.3892/ijmm.2021.4999. [DOI] [PubMed] [Google Scholar]
- 12.Kang JH, Toita R, Murata M. Liver cell-targeted delivery of therapeutic molecules. Crit Rev Biotechnol. 2016;36:132–143. doi: 10.3109/07388551.2014.930017. [DOI] [PubMed] [Google Scholar]
- 13.Gao B. Hepatoprotective and anti-inflammatory cytokines in alcoholic liver disease. J Gastroenterol Hepatol. 2012;27(Suppl 2):S89–S93. doi: 10.1111/j.1440-1746.2011.07003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019;70:151–171. doi: 10.1016/j.jhep.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 15.Paik JM, Golabi P, Younossi Y, Mishra A, Younossi ZM. Changes in the global burden of chronic liver diseases from 2012 to 2017: The growing impact of NAFLD. Hepatology. 2020;72:1605–1616. doi: 10.1002/hep.31173. [DOI] [PubMed] [Google Scholar]
- 16.van Hall G. Lactate kinetics in human tissues at rest and during exercise. Acta Physiol (Oxf) 2010;199:499–508. doi: 10.1111/j.1748-1716.2010.02122.x. [DOI] [PubMed] [Google Scholar]
- 17.Feron O. Pyruvate into lactate and back: From the Warburg effect to symbiotic energy fuel exchange in cancer cells. Radiother Oncol. 2009;92:329–333. doi: 10.1016/j.radonc.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 18.de Bari L, Atlante A, Guaragnella N, Principato G, Passarella S. D-Lactate transport and metabolism in rat liver mitochondria. Biochem J. 2002;365(Pt 2):391–403. doi: 10.1042/bj20020139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bennis Y, Bodeau S, Batteux B, Gras-Champel V, Masmoudi K, Maizel J, De Broe ME, Lalau JD, Lemaire-Hurtel AS. A study of associations between plasma metformin concentration, lactic acidosis, and mortality in an emergency hospitalization context. Crit Care Med. 2020;48:e1194–e1202. doi: 10.1097/CCM.0000000000004589. [DOI] [PubMed] [Google Scholar]
- 20.Jha MK, Lee IK, Suk K. Metabolic reprogramming by the pyruvate dehydrogenase kinase-lactic acid axis: Linking metabolism and diverse neuropathophysiologies. Neurosci Biobehav Rev. 2016;68:1–19. doi: 10.1016/j.neubiorev.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Soreze Y, Boutron A, Habarou F, Barnerias C, Nonnenmacher L, Delpech H, Mamoune A, Chrétien D, Hubert L, Bole-Feysot C, et al. Mutations in human lipoyltransferase gene LIPT1 cause a Leigh disease with secondary deficiency for pyruvate and alpha-ketoglutarate dehydrogenase. Orphanet J Rare Dis. 2013;8:192. doi: 10.1186/1750-1172-8-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Emhoff CA, Messonnier LA, Horning MA, Fattor JA, Carlson TJ, Brooks GA. Gluconeogenesis and hepatic glycogenolysis during exercise at the lactate threshold. J Appl Physiol (1985) 2013;114:297–306. doi: 10.1152/japplphysiol.01202.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang T, Chen K, Yao W, Zheng R, He Q, Xia J, Li J, Shao Y, Zhang L, Huang L, et al. Acetylation of lactate dehydrogenase B drives NAFLD progression by impairing lactate clearance. J Hepatol. 2021;74:1038–1052. doi: 10.1016/j.jhep.2020.11.028. [DOI] [PubMed] [Google Scholar]
- 24.Brown TP, Ganapathy V. Lactate/GPR81 signaling and proton motive force in cancer: Role in angiogenesis, immune escape, nutrition, and Warburg phenomenon. Pharmacol Ther. 2020;206:107451. doi: 10.1016/j.pharmthera.2019.107451. [DOI] [PubMed] [Google Scholar]
- 25.Felmlee MA, Jones RS, Rodriguez-Cruz V, Follman KE, Morris ME. Monocarboxylate transporters (SLC16): Function, regulation, and role in health and disease. Pharmacol Rev. 2020;72:466–485. doi: 10.1124/pr.119.018762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halestrap AP. The SLC16 gene family-structure, role and regulation in health and disease. Mol Aspects Med. 2013;34:337–349. doi: 10.1016/j.mam.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Sun S, Li H, Chen J, Qian Q. Lactic Acid: No longer an inert and end-product of glycolysis. Physiology (Bethesda) 2017;32:453–463. doi: 10.1152/physiol.00016.2017. [DOI] [PubMed] [Google Scholar]
- 28.Contreras-Baeza Y, Sandoval PY, Alarcón R, Galaz A, Cortés-Molina F, Alegría K, Baeza-Lehnert F, Arce-Molina R, Guequén A, Flores CA, et al. Monocarboxylate transporter 4 (MCT4) is a high affinity transporter capable of exporting lactate in high-lactate microenvironments. J Biol Chem. 2019;294:20135–20147. doi: 10.1074/jbc.RA119.009093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Halestrap AP. Monocarboxylic acid transport. Compr Physiol. 2013;3:1611–1643. doi: 10.1002/cphy.c130008. [DOI] [PubMed] [Google Scholar]
- 30.Valença I, Ferreira AR, Correia M, Kühl S, van Roermund C, Waterham HR, Máximo V, Islinger M, Ribeiro D. Prostate cancer proliferation is affected by the subcellular localization of MCT2 and accompanied by significant peroxisomal alterations. Cancers (Basel) 2020;12:3152. doi: 10.3390/cancers12113152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang G, Zhang Y, Dong D, Wang F, Ma X, Guan F, Sun L. MCT1 regulates aggressive and metabolic phenotypes in bladder cancer. J Cancer. 2018;9:2492–2501. doi: 10.7150/jca.25257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Halestrap AP. The monocarboxylate transporter family-Structure and functional characterization. IUBMB Life. 2012;64:1–9. doi: 10.1002/iub.573. [DOI] [PubMed] [Google Scholar]
- 33.Droździk M, Szeląg-Pieniek S, Grzegółkowska J, Łapczuk-Romańska J, Post M, Domagała P, Miętkiewski J, Oswald S, Kurzawski M. Monocarboxylate transporter 1 (MCT1) in liver pathology. Int J Mol Sci. 2020;21:1606. doi: 10.3390/ijms21051606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Min K, Yenilmez B, Kelly M, Echeverria D, Elleby M, Lifshitz LM, Raymond N, Tsagkaraki E, Harney SM, DiMarzio C, et al. Lactate transporter MCT1 in hepatic stellate cells promotes fibrotic collagen expression in nonalcoholic steatohepatitis. bioRxiv [Preprint] 2023.05.03.539244. 2023 doi: 10.7554/eLife.89136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martini T, Ripperger JA, Chavan R, Stumpe M, Netzahualcoyotzi C, Pellerin L, Albrecht U. The hepatic monocarboxylate transporter 1 (MCT1) contributes to the regulation of food anticipation in mice. Front Physiol. 2021;12:665476. doi: 10.3389/fphys.2021.665476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carneiro L, Asrih M, Repond C, Sempoux C, Stehle JC, Leloup C, Jornayvaz FR, Pellerin L. AMPK activation caused by reduced liver lactate metabolism protects against hepatic steatosis in MCT1 haploinsufficient mice. Mol Metab. 2017;6:1625–1633. doi: 10.1016/j.molmet.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lengacher S, Nehiri-Sitayeb T, Steiner N, Carneiro L, Favrod C, Preitner F, Thorens B, Stehle JC, Dix L, Pralong F, et al. Resistance to diet-induced obesity and associated metabolic perturbations in haploinsufficient monocarboxylate transporter 1 mice. PLoS One. 2013;8:e82505. doi: 10.1371/journal.pone.0082505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fan Q, Yang L, Zhang X, Ma Y, Li Y, Dong L, Zong Z, Hua X, Su D, Li H, Liu J. Autophagy promotes metastasis and glycolysis by upregulating MCT1 expression and Wnt/β-catenin signaling pathway activation in hepatocellular carcinoma cells. J Exp Clin Cancer Res. 2018;37:9. doi: 10.1186/s13046-018-0673-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao HJ, Zhao MC, Zhang YJ, Zhou DS, Xu L, Li GB, Chen MS, Liu J. Monocarboxylate transporter 4 predicts poor prognosis in hepatocellular carcinoma and is associated with cell proliferation and migration. J Cancer Res Clin Oncol. 2015;141:1151–1162. doi: 10.1007/s00432-014-1888-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen HL, OuYang HY, Le Y, Jiang P, Tang H, Yu ZS, He MK, Tang YQ, Shi M. Aberrant MCT4 and GLUT1 expression is correlated with early recurrence and poor prognosis of hepatocellular carcinoma after hepatectomy. Cancer Med. 2018;7:5339–5350. doi: 10.1002/cam4.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Niu Z, Yang F, Li H, Wang J, Ni Q, Ma C, Zhu H, Chang H, Zhou X, Lu J, Gao H. MCT4 promotes hepatocellular carcinoma progression by upregulating TRAPPC5 gene. J Hepatocell Carcinoma. 2022;9:289–300. doi: 10.2147/JHC.S352948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao Y, Li W, Li M, Hu Y, Zhang H, Song G, Yang L, Cai K, Luo Z. Targeted inhibition of MCT4 disrupts intracellular pH homeostasis and confers self-regulated apoptosis on hepatocellular carcinoma. Exp Cell Res. 2019;384:111591. doi: 10.1016/j.yexcr.2019.111591. [DOI] [PubMed] [Google Scholar]
- 43.Zhou J, Shao Q, Lu Y, Li Y, Xu Z, Zhou B, Chen Q, Li X, Xu X, Pan Y, et al. Monocarboxylate transporter upregulation in induced regulatory T cells promotes resistance to anti-PD-1 therapy in hepatocellular carcinoma patients. Front Oncol. 2022;12:960066. doi: 10.3389/fonc.2022.960066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu J, Cai M, Liu Y, Liu B, Xue X, Ji R, Bian X, Lou S. The roles of GRP81 as a metabolic sensor and inflammatory mediator. J Cell Physiol. 2020;235:8938–8950. doi: 10.1002/jcp.29739. [DOI] [PubMed] [Google Scholar]
- 45.Hoque R, Farooq A, Ghani A, Gorelick F, Mehal WZ. Lactate reduces liver and pancreatic injury in Toll-like receptorand inflammasome-mediated inflammation via GPR81-mediated suppression of innate immunity. Gastroenterology. 2014;146:1763–1774. doi: 10.1053/j.gastro.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ahmed K, Tunaru S, Tang C, Müller M, Gille A, Sassmann A, Hanson J, Offermanns S. An autocrine lactate loop mediates insulin-dependent inhibition of lipolysis through GPR81. Cell Metab. 2010;11:311–319. doi: 10.1016/j.cmet.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 47.Wu G, Dai Y, Yan Y, Zheng X, Zhang H, Li H, Chen W. The lactate receptor GPR81 mediates hepatic lipid metabolism and the therapeutic effect of metformin on experimental NAFLDs. Eur J Pharmacol. 2022;924:174959. doi: 10.1016/j.ejphar.2022.174959. [DOI] [PubMed] [Google Scholar]
- 48.Hui S, Ghergurovich JM, Morscher RJ, Jang C, Teng X, Lu W, Esparza LA, Reya T, Le Zhan, Yanxiang Guo J, et al. Glucose feeds the TCA cycle via circulating lactate. Nature. 2017;551:115–118. doi: 10.1038/nature24057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dienel GA. Brain glucose metabolism: Integration of energetics with function. Physiol Rev. 2019;99:949–1045. doi: 10.1152/physrev.00062.2017. [DOI] [PubMed] [Google Scholar]
- 50.Lhomme T, Clasadonte J, Imbernon M, Fernandois D, Sauve F, Caron E, da Silva Lima N, Heras V, Martinez-Corral I, Mueller-Fielitz H, et al. Tanycytic networks mediate energy balance by feeding lactate to glucose-insensitive POMC neurons. J Clin Invest. 2021;131:e140521. doi: 10.1172/JCI140521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gómez-Valadés AG, Pozo M, Varela L, Boudjadja MB, Ramírez S, Chivite I, Eyre E, Haddad-Tóvolli R, Obri A, Milà-Guasch M, et al. Mitochondrial cristae-remodeling protein OPA1 in POMC neurons couples Ca(2+) homeostasis with adipose tissue lipolysis. Cell Metab. 2021;33:1820–1835.e9. doi: 10.1016/j.cmet.2021.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Faubert B, Li KY, Cai L, Hensley CT, Kim J, Zacharias LG, Yang C, Do QN, Doucette S, Burguete D, et al. Lactate metabolism in human lung tumors. Cell. 2017;171:358–371.e9. doi: 10.1016/j.cell.2017.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pucino V, Certo M, Bulusu V, Cucchi D, Goldmann K, Pontarini E, Haas R, Smith J, Headland SE, Blighe K, et al. Lactate buildup at the site of chronic inflammation promotes disease by inducing CD4(+) T cell metabolic rewiring. Cell Metab. 2019;30:1055–1074.e8. doi: 10.1016/j.cmet.2019.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Irizarry-Caro RA, McDaniel MM, Overcast GR, Jain VG, Troutman TD, Pasare C. TLR signaling adapter BCAP regulates inflammatory to reparatory macrophage transition by promoting histone lactylation. Proc Natl Acad Sci USA. 2020;117:30628–30638. doi: 10.1073/pnas.2009778117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rho H, Terry AR, Chronis C, Hay N. Hexokinase 2-mediated gene expression via histone lactylation is required for hepatic stellate cell activation and liver fibrosis. Cell Metab. 2023;35:1406–1423.e8. doi: 10.1016/j.cmet.2023.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang Z, Yan C, Ma J, Peng P, Ren X, Cai S, Shen X, Wu Y, Zhang S, Wang X, et al. Lactylome analysis suggests lactylation-dependent mechanisms of metabolic adaptation in hepatocellular carcinoma. Nat Metab. 2023;5:61–79. doi: 10.1038/s42255-022-00710-w. [DOI] [PubMed] [Google Scholar]
- 57.Pan L, Feng F, Wu J, Fan S, Han J, Wang S, Yang L, Liu W, Wang C, Xu K. Demethylzeylasteral targets lactate by inhibiting histone lactylation to suppress the tumorigenicity of liver cancer stem cells. Pharmacol Res. 2022;181:106270. doi: 10.1016/j.phrs.2022.106270. [DOI] [PubMed] [Google Scholar]
- 58.Hayes C, Donohoe CL, Davern M, Donlon NE. The oncogenic and clinical implications of lactate induced immunosuppression in the tumour microenvironment. Cancer Lett. 2021;500:75–86. doi: 10.1016/j.canlet.2020.12.021. [DOI] [PubMed] [Google Scholar]
- 59.Li Y, Mo H, Wu S, Liu X, Tu K. A novel lactate metabolism-related gene signature for predicting clinical outcome and tumor microenvironment in hepatocellular carcinoma. Front Cell Dev Biol. 2022;9:801959. doi: 10.3389/fcell.2021.801959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang L, Tan P, Sun H, Zeng Z, Pan Y. Integrative dissection of novel lactate metabolism-related signature in the tumor immune microenvironment and prognostic prediction in breast cancer. Front Oncol. 2022;12:874731. doi: 10.3389/fonc.2022.874731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang Z, Zhang S, Li J, Yuan Y, Chen S, Zuo M, Li W, Feng W, Chen M, Liu Y. Prognostic value of lactate metabolism-related gene expression signature in adult primary gliomas and its impact on the tumor immune microenvironment. Front Oncol. 2022;12:1008219. doi: 10.3389/fonc.2022.1008219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang D, Tang Z, Huang H, Zhou G, Cui C, Weng Y, Liu W, Kim S, Lee S, Perez-Neut M, et al. Metabolic regulation of gene expression by histone lactylation. Nature. 2019;574:575–580. doi: 10.1038/s41586-019-1678-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Izzo LT, Wellen KE. Histone lactylation links metabolism and gene regulation. Nature. 2019;574:492–493. doi: 10.1038/d41586-019-03122-1. [DOI] [PubMed] [Google Scholar]
- 64.Oosterveer MH, Schoonjans K. Hepatic glucose sensing and integrative pathways in the liver. Cell Mol Life Sci. 2014;71:1453–1467. doi: 10.1007/s00018-013-1505-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lei Y, Han P, Chen Y, Wang H, Wang S, Wang M, Liu J, Yan W, Tian D, Liu M. Protein arginine methyltransferase 3 promotes glycolysis and hepatocellular carcinoma growth by enhancing arginine methylation of lactate dehydrogenase A. Clin Transl Med. 2022;12:e686. doi: 10.1002/ctm2.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lazzeri C, Gensini GF, Sori A, Bernardo P, Chiostri M, Tommasi E, Grossi F, Valente S. Dynamic behaviour of lactate values during mild hypothermia in patients with cardiac arrest. Eur Heart J Acute Cardiovasc Care. 2014;3:176–182. doi: 10.1177/2048872613514014. [DOI] [PubMed] [Google Scholar]
- 67.Scheiner B, Lindner G, Reiberger T, Schneeweiss B, Trauner M, Zauner C, Funk GC. Acid-base disorders in liver disease. J Hepatol. 2017;67:1062–1073. doi: 10.1016/j.jhep.2017.06.023. [DOI] [PubMed] [Google Scholar]
- 68.Drolz A, Horvatits T, Rutter K, Landahl F, Roedl K, Meersseman P, Wilmer A, Kluwe J, Lohse AW, Kluge S, et al. Lactate improves prediction of short-term mortality in Critically Ill patients with cirrhosis: A multinational study. Hepatology. 2019;69:258–269. doi: 10.1002/hep.30151. [DOI] [PubMed] [Google Scholar]
- 69.Gao Y, Zhang H, Zhong H, Yang S, Wang Q. Lactate and blood ammonia on admission as biomarkers to predict the prognosis of patients with acute mushroom poisoning and liver failure: A retrospective study. Toxicol Res (Camb) 2021;10:850–855. doi: 10.1093/toxres/tfab068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Roehlen N, Crouchet E, Baumert TF. Liver fibrosis: Mechanistic concepts and therapeutic perspectives. Cells. 2020;9:875. doi: 10.3390/cells9040875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dulai PS, Singh S, Patel J, Soni M, Prokop LJ, Younossi Z, Sebastiani G, Ekstedt M, Hagstrom H, Nasr P, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology. 2017;65:1557–1565. doi: 10.1002/hep.29085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tan Z, Sun H, Xue T, Gan C, Liu H, Xie Y, Yao Y, Ye T. Liver fibrosis: Therapeutic targets and advances in drug therapy. Front Cell Dev Biol. 2021;9:730176. doi: 10.3389/fcell.2021.730176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sherman MH. Stellate cells in tissue repair, inflammation, and cancer. Annu Rev Cell Dev Biol. 2018;34:333–355. doi: 10.1146/annurev-cellbio-100617-062855. [DOI] [PubMed] [Google Scholar]
- 74.Mejias M, Gallego J, Naranjo-Suarez S, Ramirez M, Pell N, Manzano A, Suñer C, Bartrons R, Mendez R, Fernandez M. CPEB4 increases expression of PFKFB3 to induce glycolysis and activate mouse and human hepatic stellate cells, promoting liver fibrosis. Gastroenterology. 2020;159:273–288. doi: 10.1053/j.gastro.2020.03.008. [DOI] [PubMed] [Google Scholar]
- 75.Trivedi P, Wang S, Friedman SL. The power of plasticity-metabolic regulation of hepatic stellate cells. Cell Metab. 2021;33:242–257. doi: 10.1016/j.cmet.2020.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shangguan X, He J, Ma Z, Zhang W, Ji Y, Shen K, Yue Z, Li W, Xin Z, Zheng Q, et al. SUMOylation controls the binding of hexokinase 2 to mitochondria and protects against prostate cancer tumorigenesis. Nat Commun. 2021;12:1812. doi: 10.1038/s41467-021-22163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Riazi K, Azhari H, Charette JH, Underwood FE, King JA, Afshar EE, Swain MG, Congly SE, Kaplan GG, Shaheen AA. The prevalence and incidence of NAFLD worldwide: A systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2022;7:851–861. doi: 10.1016/S2468-1253(22)00165-0. [DOI] [PubMed] [Google Scholar]
- 78.Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67:123–133. doi: 10.1002/hep.29466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Afonso MB, Rodrigues PM, Simão AL, Castro RE. Circulating microRNAs as potential biomarkers in non-alcoholic fatty liver disease and hepatocellular carcinoma. J Clin Med. 2016;5:30. doi: 10.3390/jcm5030030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jeppesen JB, Mortensen C, Bendtsen F, Møller S. Lactate metabolism in chronic liver disease. Scand J Clin Lab Invest. 2013;73:293–299. doi: 10.3109/00365513.2013.773591. [DOI] [PubMed] [Google Scholar]
- 81.Ha TS, Shin TG, Jo IJ, Hwang SY, Chung CR, Suh GY, Jeon K. Lactate clearance and mortality in septic patients with hepatic dysfunction. Am J Emerg Med. 2016;34:1011–1015. doi: 10.1016/j.ajem.2016.02.053. [DOI] [PubMed] [Google Scholar]
- 82.Li J, Wang T, Xia J, Yao W, Huang F. Enzymatic and nonenzymatic protein acetylations control glycolysis process in liver diseases. FASEB J. 2019;33:11640–11654. doi: 10.1096/fj.201901175R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vazquez JH, Kennon-McGill S, Byrum SD, Mackintosh SG, Jaeschke H, Williams DK, Lee WM, Dranoff JA, McGill MR, Acute Liver Failure Study Group Proteomics indicates lactate dehydrogenase is prognostic in acetaminophen-induced acute liver failure patients and reveals altered signaling pathways. Toxicol Sci. 2022;187:25–34. doi: 10.1093/toxsci/kfac015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bernal W, Donaldson N, Wyncoll D, Wendon J. Blood lactate as an early predictor of outcome in paracetamol-induced acute liver failure: A cohort study. Lancet. 2002;359:558–563. doi: 10.1016/S0140-6736(02)07743-7. [DOI] [PubMed] [Google Scholar]
- 85.Macquillan GC, Seyam MS, Nightingale P, Neuberger JM, Murphy N. Blood lactate but not serum phosphate levels can predict patient outcome in fulminant hepatic failure. Liver Transpl. 2005;11:1073–1079. doi: 10.1002/lt.20427. [DOI] [PubMed] [Google Scholar]
- 86.Dabos KJ, Newsome PN, Parkinson JA, Davidson JS, Sadler IH, Plevris JN, Hayes PC. A biochemical prognostic model of outcome in paracetamol-induced acute liver injury. Transplantation. 2005;80:1712–1717. doi: 10.1097/01.tp.0000187879.51616.e0. [DOI] [PubMed] [Google Scholar]
- 87.Schmidt LE, Larsen FS. Prognostic implications of hyperlactatemia, multiple organ failure, and systemic inflammatory response syndrome in patients with acetaminophen-induced acute liver failure. Crit Care Med. 2006;34:337–343. doi: 10.1097/01.CCM.0000194724.70031.B6. [DOI] [PubMed] [Google Scholar]
- 88.Cholongitas EB, Betrossian A, Leandro G, Shaw S, Patch D, Burroughs AK. King's criteria, APACHE II, and SOFA scores in acute liver failure. Hepatology. 2006;43:881. doi: 10.1002/hep.21121. author reply 882. [DOI] [PubMed] [Google Scholar]
- 89.Gow PJ, Warrilow S, Lontos S, Lubel J, Wongseelashote S, MacQuillan GC, Jones RM, Bellomo R, Angus PW. Time to review the selection criteria for transplantation in paracetamol-induced fulminant hepatic failure? Liver Transpl. 2007;13:1762–1763. doi: 10.1002/lt.21301. [DOI] [PubMed] [Google Scholar]
- 90.Agrawal T, Maiwall R, Rajan V, Bajpai M, Jagdish RK, Sarin SK, Trehanpati N. Higher circulating natural killer cells and lower lactate levels at admission predict spontaneous survival in non-acetaminophen induced acute liver failure. Clin Immunol. 2021;231:108829. doi: 10.1016/j.clim.2021.108829. [DOI] [PubMed] [Google Scholar]
- 91.Karvellas CJ, Tillman H, Leung AA, Lee WM, Schilsky ML, Hameed B, Stravitz RT, McGuire BM, Fix OK, United States Acute Liver Failure Study Group Acute liver injury and acute liver failure from mushroom poisoning in North America. Liver Int. 2016;36:1043–1050. doi: 10.1111/liv.13080. [DOI] [PubMed] [Google Scholar]
- 92.Feldman AG, Sokol RJ, Hardison RM, Alonso EM, Squires RH, Narkewicz MR, Pediatric Acute Liver Failure Study Group Lactate and Lactate: Pyruvate ratio in the diagnosis and outcomes of pediatric acute liver failure. J Pediatr. 2017;182:217–222.e3. doi: 10.1016/j.jpeds.2016.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Haidar MK, Morton N, Roederer T, Mayronne S, Bawo L, Kerkula J, Porten K, Baud FJ. Evaluating lactate prognostic value in children suspected of acetaminophen-induced liver failure in Liberia. Pediatr Res. 2020;88:605–611. doi: 10.1038/s41390-020-0783-z. [DOI] [PubMed] [Google Scholar]
- 94.Schmidt LE, Larsen FS. Is lactate concentration of major value in determining the prognosis in patients with acute liver failure? Hardly. J Hepatol. 2010;53:211–212. doi: 10.1016/j.jhep.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 95.Bernal W. Lactate is important in determining prognosis in acute liver failure. J Hepatol. 2010;53:209–210. doi: 10.1016/j.jhep.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 96.Niederwieser T, Braunwarth E, Dasari BVM, Pufal K, Szatmary P, Hackl H, Haselmann C, Connolly CE, Cardini B, Öfner D, et al. Early postoperative arterial lactate concentrations to stratify risk of post-hepatectomy liver failure. Br J Surg. 2021;108:1360–1370. doi: 10.1093/bjs/znab338. [DOI] [PubMed] [Google Scholar]
- 97.Popescu M, Dima S, Brasoveanu V, Tudor A, Simionescu M, Tomescu D. High perioperative lactate levels and decreased lactate clearance are associated with increased incidence of posthepatectomy liver failure. Hepatobiliary Pancreat Dis Int. 2021;20:592–594. doi: 10.1016/j.hbpd.2021.03.005. [DOI] [PubMed] [Google Scholar]
- 98.Gao F, Huang XL, Cai MX, Lin MT, Wang BF, Wu W, Huang ZM. Prognostic value of serum lactate kinetics in critically ill patients with cirrhosis and acute-on-chronic liver failure: A multicenter study. Aging (Albany NY) 2019;11:4446–4462. doi: 10.18632/aging.102062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cardoso FS, Abraldes JG, Sy E, Ronco JJ, Bagulho L, Mcphail MJ, Karvellas CJ. Lactate and number of organ failures predict intensive care unit mortality in patients with acute-on-chronic liver failure. Liver Int. 2019;39:1271–1280. doi: 10.1111/liv.14083. [DOI] [PubMed] [Google Scholar]
- 100.Kotoh K, Kato M, Kohjima M, Tanaka M, Miyazaki M, Nakamura K, Enjoji M, Nakamuta M, Takayanagi R. Lactate dehydrogenase production in hepatocytes is increased at an early stage of acute liver failure. Exp Ther Med. 2011;2:195–199. doi: 10.3892/etm.2011.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cassidy WM, Reynolds TB. Serum lactic dehydrogenase in the differential diagnosis of acute hepatocellular injury. J Clin Gastroenterol. 1994;19:118–121. doi: 10.1097/00004836-199409000-00008. [DOI] [PubMed] [Google Scholar]
- 102.Krispin I, Mahamid M, Goldin E, Fteiha B. Elevated lactate/albumin ratio as a novel predictor of in-hospital mortality in hospitalized cirrhotics. Ann Hepatol. 2023;28:100897. doi: 10.1016/j.aohep.2023.100897. [DOI] [PubMed] [Google Scholar]
- 103.Nie Y, Liu LX, Chen T, Zhang Y, Zhu X. Serum lactate level predicts 6-months mortality in patients with hepatitis B virus-related decompensated cirrhosis: A retrospective study. Epidemiol Infect. 2021;149:e26. doi: 10.1017/S0950268820003143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301–1314. doi: 10.1016/S0140-6736(18)30010-2. [DOI] [PubMed] [Google Scholar]
- 105.Chen Z, Xie H, Hu M, Huang T, Hu Y, Sang N, Zhao Y. Recent progress in treatment of hepatocellular carcinoma. Am J Cancer Res. 2020;10:2993–3036. [PMC free article] [PubMed] [Google Scholar]
- 106.Hanahan D, Coussens LM. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 107.Lee DC, Sohn HA, Park ZY, Oh S, Kang YK, Lee KM, Kang M, Jang YJ, Yang SJ, Hong YK, et al. A lactate-induced response to hypoxia. Cell. 2015;161:595–609. doi: 10.1016/j.cell.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 108.Deng H, Kan A, Lyu N, He M, Huang X, Qiao S, Li S, Lu W, Xie Q, Chen H, et al. Tumor-derived lactate inhibit the efficacy of lenvatinib through regulating PD-L1 expression on neutrophil in hepatocellular carcinoma. J Immunother Cancer. 2021;9:e002305. doi: 10.1136/jitc-2020-002305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Xu Y, Hao X, Ren Y, Xu Q, Liu X, Song S, Wang Y. Research progress of abnormal lactate metabolism and lactate modification in immunotherapy of hepatocellular carcinoma. Front Oncol. 2022;12:1063423. doi: 10.3389/fonc.2022.1063423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tu CE, Hu Y, Zhou P, Guo X, Gu C, Zhang Y, Li A, Liu S. Lactate and TGF-β antagonistically regulate inflammasome activation in the tumor microenvironment. J Cell Physiol. 2021;236:4528–4537. doi: 10.1002/jcp.30169. [DOI] [PubMed] [Google Scholar]
- 111.Apicella M, Giannoni E, Fiore S, Ferrari KJ, Fernández-Pérez D, Isella C, Granchi C, Minutolo F, Sottile A, Comoglio PM, et al. Increased lactate secretion by cancer cells sustains non-cell-autonomous adaptive resistance to MET and EGFR targeted therapies. Cell Metab. 2018;28:848–65 e6. doi: 10.1016/j.cmet.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 112.Huang X, Gan G, Wang X, Xu T, Xie W. The HGF-MET axis coordinates liver cancer metabolism and autophagy for chemotherapeutic resistance. Autophagy. 2019;15:1258–1279. doi: 10.1080/15548627.2019.1580105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shan T, Chen S, Chen X, Wu T, Yang Y, Li S, Ma J, Zhao J, Lin W, Li W, et al. M2-TAM subsets altered by lactic acid promote T-cell apoptosis through the PD-L1/PD-1 pathway. Oncol Rep. 2020;44:1885–1894. doi: 10.3892/or.2020.7767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lu LG, Zhou ZL, Wang XY, Liu BY, Lu JY, Liu S, Zhang GB, Zhan MX, Chen Y. PD-L1 blockade liberates intrinsic antitumourigenic properties of glycolytic macrophages in hepatocellular carcinoma. Gut. 2022;71:2551–2560. doi: 10.1136/gutjnl-2021-326350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Stone SC, Rossetti RAM, Alvarez KLF, Carvalho JP, Margarido PFR, Baracat EC, Tacla M, Boccardo E, Yokochi K, Lorenzi NP, Lepique AP. Lactate secreted by cervical cancer cells modulates macrophage phenotype. J Leukoc Biol. 2019;105:1041–1054. doi: 10.1002/JLB.3A0718-274RR. [DOI] [PubMed] [Google Scholar]
- 116.Chu X, Di C, Chang P, Li L, Feng Z, Xiao S, Yan X, Xu X, Li H, Qi R, et al. Lactylated histone H3K18 as a potential biomarker for the diagnosis and predicting the severity of septic shock. Front Immunol. 2022;12:786666. doi: 10.3389/fimmu.2021.786666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Suk FM, Wu CY, Fang CC, Chen TL, Liao YJ. β-HB treatment reverses sorafenib resistance by shifting glycolysis-lactate metabolism in HCC. Biomed Pharmacother. 2023;166:115293. doi: 10.1016/j.biopha.2023.115293. [DOI] [PubMed] [Google Scholar]
- 118.Baltazar F, Afonso J, Costa M, Granja S. Lactate beyond a waste metabolite: Metabolic affairs and signaling in malignancy. Front Oncol. 2020;10:231. doi: 10.3389/fonc.2020.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sheng SL, Liu JJ, Dai YH, Sun XG, Xiong XP, Huang G. Knockdown of lactate dehydrogenase A suppresses tumor growth and metastasis of human hepatocellular carcinoma. FEBS J. 2012;279:3898–3910. doi: 10.1111/j.1742-4658.2012.08748.x. [DOI] [PubMed] [Google Scholar]
- 120.Fukao T, Lopaschuk GD, Mitchell GA. Pathways and control of ketone body metabolism: On the fringe of lipid biochemistry. Prostaglandins Leukot Essent Fatty Acids. 2004;70:243–251. doi: 10.1016/j.plefa.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 121.Li X, Lu P, Li B, Yang R, Chu Y, Zhang Z, Wan H, Niu C, Wang C, Luo K. Sensitization of hepatocellular carcinoma cells to irradiation by miR-34a through targeting lactate dehydrogenase-A. Mol Med Rep. 2016;13:3661–3667. doi: 10.3892/mmr.2016.4974. [DOI] [PubMed] [Google Scholar]
- 122.Zhang K, Mu L, Ding MC, Xu R, Ding ZJ, Liang J. NFκB mediated elevation of KCNJ11 promotes tumor progression of hepatocellular carcinoma through interaction of lactate dehydrogenase A. Biochem Biophys Res Commun. 2018;495:246–253. doi: 10.1016/j.bbrc.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 123.Serra M, Di Matteo M, Serneels J, Pal R, Cafarello ST, Lanza M, Sanchez-Martin C, Evert M, Castegna A, Calvisi DF, et al. Deletion of lactate dehydrogenase-a impairs oncogene-induced mouse hepatocellular carcinoma development. Cell Mol Gastroenterol Hepatol. 2022;14:609–624. doi: 10.1016/j.jcmgh.2022.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cui W, Lv W, Qu Y, Ma R, Wang YW, Xu YJ, Wu D, Chen X. Discovery of 2-((3-cyanopyridin-2-yl)thio)acetamides as human lactate dehydrogenase A inhibitors to reduce the growth of MG-63 osteosarcoma cells: Virtual screening and biological validation. Bioorg Med Chem Lett. 2016;26:3984–3987. doi: 10.1016/j.bmcl.2016.06.083. [DOI] [PubMed] [Google Scholar]
- 125.Le A, Cooper CR, Gouw AM, Dinavahi R, Maitra A, Deck LM, Royer RE, Vander Jagt DL, Semenza GL, Dang CV. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci USA. 2010;107:2037–2042. doi: 10.1073/pnas.0914433107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chen CY, Feng Y, Chen JY, Deng H. Identification of a potent inhibitor targeting human lactate dehydrogenase A and its metabolic modulation for cancer cell line. Bioorg Med Chem Lett. 2016;26:72–75. doi: 10.1016/j.bmcl.2015.11.025. [DOI] [PubMed] [Google Scholar]
- 127.McDonald B, Zucoloto AZ, Yu IL, Burkhard R, Brown K, Geuking MB, McCoy KD. Programing of an intravascular immune firewall by the gut microbiota protects against pathogen dissemination during infection. Cell Host Microbe. 2020;28:660–668.e4. doi: 10.1016/j.chom.2020.07.014. [DOI] [PubMed] [Google Scholar]
- 128.Han S, Bao X, Zou Y, Wang L, Li Y, Yang L, Liao A, Zhang X, Jiang X, Liang D, et al. d-lactate modulates M2 tumor-associated macrophages and remodels immunosuppressive tumor microenvironment for hepatocellular carcinoma. Sci Adv. 2023;9:eadg2697. doi: 10.1126/sciadv.adg2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chen Y, Bei J, Chen M, Cai W, Zhou Z, Cai M, Huang W, Lin L, Guo Y, Liu M, et al. Intratumoral lactate depletion based on injectable nanoparticles-hydrogel composite system synergizes with immunotherapy against postablative hepatocellular carcinoma recurrence. Adv Healthc Mater. 2024;13:e2303031. doi: 10.1002/adhm.202303031. [DOI] [PubMed] [Google Scholar]
- 130.Kubackova K, Bortlicek Z, Pavlik T, Melichar B, Linke Z, Pokorna P, Vyzula R, Prausova J, Buchler T, Czech Renal Cancer Cooperative Group Prognostic factors in renal cell carcinoma patients treated with sorafenib: Results from the Czech registry. Target Oncol. 2015;10:385–392. doi: 10.1007/s11523-014-0343-8. [DOI] [PubMed] [Google Scholar]
- 131.Scartozzi M, Giampieri R, Maccaroni E, Del Prete M, Faloppi L, Bianconi M, Galizia E, Loretelli C, Belvederesi L, Bittoni A, Cascinu S. Pre-treatment lactate dehydrogenase levels as predictor of efficacy of first-line bevacizumab-based therapy in metastatic colorectal cancer patients. Br J Cancer. 2012;106:799–804. doi: 10.1038/bjc.2012.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hermes A, Gatzemeier U, Waschki B, Reck M. Lactate dehydrogenase as prognostic factor in limited and extensive disease stage small cell lung cancer-a retrospective single institution analysis. Respir Med. 2010;104:1937–1942. doi: 10.1016/j.rmed.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 133.Faloppi L, Scartozzi M, Bianconi M, Svegliati Baroni G, Toniutto P, Giampieri R, Del Prete M, De Minicis S, Bitetto D, Loretelli C, et al. The role of LDH serum levels in predicting global outcome in HCC patients treated with sorafenib: implications for clinical management. BMC Cancer. 2014;14:110. doi: 10.1186/1471-2407-14-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sacco R, Mismas V, Granito A, Musettini G, Masi G, Caparello C, Vivaldi C, Felder M, Bresci G, Fornaro L, Italian Liver Cancer (IT.LI.CA) group Correlation between LDH levels and response to sorafenib in HCC patients: An analysis of the ITA. LI.CA database. Int J Biol Markers. 2015;30:e65–e72. doi: 10.5301/jbm.5000117. [DOI] [PubMed] [Google Scholar]
- 135.Yada M, Miyazaki M, Motomura K, Masumoto A, Nakamuta M, Kohjima M, Sugimoto R, Aratake Y, Higashi N, Morizono S, et al. The prognostic role of lactate dehydrogenase serum levels in patients with hepatocellular carcinoma who are treated with sorafenib: the influence of liver fibrosis. J Gastrointest Oncol. 2016;7:615–623. doi: 10.21037/jgo.2016.03.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wu SJ, Lin YX, Ye H, Xiong XZ, Li FY, Cheng NS. Prognostic value of alkaline phosphatase, gamma-glutamyl transpeptidase and lactate dehydrogenase in hepatocellular carcinoma patients treated with liver resection. Int J Surg. 2016;36(Pt A):143–151. doi: 10.1016/j.ijsu.2016.10.033. [DOI] [PubMed] [Google Scholar]
- 137.Gan Y, Gao F, Du B, Liu Y, Xue Q, Fu J. Effects of preoperative serum lactate dehydrogenase levels on long-term prognosis in elderly patients with hepatocellular carcinoma undergoing transcatheter arterial chemoembolization. Front Surg. 2022;9:982114. doi: 10.3389/fsurg.2022.982114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhuang G, Xie Y, Hong J, Lin S, Chen T, Fang W. Arterial chemoembolization for patients with hepatocellular carcinoma and elevated lactate dehydrogenase is associated with low survival: A cohort study. Infect Agent Cancer. 2022;17:31. doi: 10.1186/s13027-022-00443-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Su K, Huang W, Li X, Xu K, Gu T, Liu Y, Song J, Qian K, Xu Y, Zeng H, et al. Evaluation of lactate dehydrogenase and alkaline phosphatase as predictive biomarkers in the prognosis of hepatocellular carcinoma and development of a new nomogram. J Hepatocell Carcinoma. 2023;10:69–79. doi: 10.2147/JHC.S398632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cui Z, Li Y, Gao Y, Kong L, Lin Y, Chen Y. Cancer-testis antigen lactate dehydrogenase C4 in hepatocellular carcinoma: A promising biomarker for early diagnosis, efficacy evaluation and prognosis prediction. Aging (Albany NY) 2020;12:19455–19467. doi: 10.18632/aging.103879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jin J, Bai L, Wang D, Ding W, Cao Z, Yan P, Li Y, Xi L, Wang Y, Zheng X, et al. SIRT3-dependent delactylation of cyclin E2 prevents hepatocellular carcinoma growth. EMBO Rep. 2023;24:e56052. doi: 10.15252/embr.202256052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cheng Z, Huang H, Li M, Liang X, Tan Y, Chen Y. Lactylation-Related gene signature effectively predicts prognosis and treatment responsiveness in hepatocellular carcinoma. Pharmaceuticals (Basel) 2023;16:644. doi: 10.3390/ph16050644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Doherty JR, Cleveland JL. Targeting lactate metabolism for cancer therapeutics. J Clin Invest. 2013;123:3685–3692. doi: 10.1172/JCI69741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kooshki L, Mahdavi P, Fakhri S, Akkol EK, Khan H. Targeting lactate metabolism and glycolytic pathways in the tumor microenvironment by natural products: A promising strategy in combating cancer. BioFactors. 2022;48:359–383. doi: 10.1002/biof.1799. [DOI] [PubMed] [Google Scholar]
- 145.Li Y, Zhou Y, Xia S, Chen L, Yang T, Zhao D, Zhang Z, Shao J, Xu X, Zhang F, Zheng S. Blockade of KLF5/LDH-A feedback loop contributes to Curcumol inhibition of sinusoidal endothelial cell glycolysis and mitigation of liver fibrosis. Phytomedicine. 2023;114:154759. doi: 10.1016/j.phymed.2023.154759. [DOI] [PubMed] [Google Scholar]
- 146.Wang F, Chen L, Kong D, Zhang X, Xia S, Liang B, Li Y, Zhou Y, Zhang Z, Shao J, et al. Canonical Wnt signaling promotes HSC glycolysis and liver fibrosis through an LDH-A/HIF-1α transcriptional complex. Hepatolog. 2024;79:606–623. doi: 10.1097/HEP.0000000000000569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ban D, Hua S, Zhang W, Shen C, Miao X, Liu W. Costunolide reduces glycolysis-associated activation of hepatic stellate cells via inhibition of hexokinase-2. Cell Mol Biol Lett. 2019;24:52. doi: 10.1186/s11658-019-0179-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Reyes R, Wani NA, Ghoshal K, Jacob ST, Motiwala T. Sorafenib and 2-deoxyglucose synergistically inhibit proliferation of both sorafenib-sensitive and -resistant HCC cells by inhibiting ATP production. Gene Expr. 2017;17:129–140. doi: 10.3727/105221616X693855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tomizawa M, Shinozaki F, Motoyoshi Y, Sugiyama T, Yamamoto S, Ishige N. 2-Deoxyglucose and sorafenib synergistically suppress the proliferation and motility of hepatocellular carcinoma cells. Oncol Lett. 2017;13:800–804. doi: 10.3892/ol.2016.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Raez LE, Papadopoulos K, Ricart AD, Chiorean EG, Dipaola RS, Stein MN, Rocha Lima CM, Schlesselman JJ, Tolba K, Langmuir VK, et al. A phase I dose-escalation trial of 2-deoxy-D-glucose alone or combined with docetaxel in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2013;71:523–530. doi: 10.1007/s00280-012-2045-1. [DOI] [PubMed] [Google Scholar]
- 151.Stein M, Lin H, Jeyamohan C, Dvorzhinski D, Gounder M, Bray K, Eddy S, Goodin S, White E, Dipaola RS. Targeting tumor metabolism with 2-deoxyglucose in patients with castrate-resistant prostate cancer and advanced malignancies. Prostate. 2010;70:1388–1394. doi: 10.1002/pros.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sasaki K, Nishina S, Yamauchi A, Fukuda K, Hara Y, Yamamura M, Egashira K, Hino K. Nanoparticle-Mediated Delivery of 2-Deoxy-D-Glucose induces antitumor immunity and cytotoxicity in liver tumors in mice. Cell Mol Gastroenterol Hepatol. 2021;11:739–762. doi: 10.1016/j.jcmgh.2020.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tomizawa M, Shinozaki F, Motoyoshi Y, Sugiyama T, Yamamoto S, Ishige N. Suppressive effects of 3-bromopyruvate on the proliferation and the motility of hepatocellular carcinoma cells. Oncol Rep. 2016;35:59–63. doi: 10.3892/or.2015.4370. [DOI] [PubMed] [Google Scholar]
- 154.Yoo JJ, Yu SJ, Na J, Kim K, Cho YY, Lee YB, Cho EJ, Lee JH, Kim YJ, Youn H, Yoon JH. Hexokinase-II inhibition synergistically augments the anti-tumor efficacy of sorafenib in hepatocellular carcinoma. Int J Mol Sci. 2019;20:1292. doi: 10.3390/ijms20061292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sun X, Sun G, Huang Y, Hao Y, Tang X, Zhang N, Zhao L, Zhong R, Peng Y. 3-Bromopyruvate regulates the status of glycolysis and BCNU sensitivity in human hepatocellular carcinoma cells. Biochem Pharmacol. 2020;177:113988. doi: 10.1016/j.bcp.2020.113988. [DOI] [PubMed] [Google Scholar]
- 156.Wu H, Pan L, Gao C, Xu H, Li Y, Zhang L, Ma L, Meng L, Sun X, Qin H. Quercetin inhibits the proliferation of glycolysis-addicted HCC cells by reducing hexokinase 2 and Akt-mTOR pathway. Molecules. 2019;24:1993. doi: 10.3390/molecules24101993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Xu Q, Dou C, Liu X, Yang L, Ni C, Wang J, Guo Y, Yang W, Tong X, Huang D. Oviductus ranae protein hydrolysate (ORPH) inhibits the growth, metastasis and glycolysis of HCC by targeting miR-491-5p/PKM2 axis. Biomed Pharmacother. 2018;107:1692–1704. doi: 10.1016/j.biopha.2018.07.071. [DOI] [PubMed] [Google Scholar]
- 158.Ferriero R, Nusco E, De Cegli R, Carissimo A, Manco G, Brunetti-Pierri N. Pyruvate dehydrogenase complex and lactate dehydrogenase are targets for therapy of acute liver failure. J Hepatol. 2018;69:325–335. doi: 10.1016/j.jhep.2018.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Billiard J, Dennison JB, Briand J, Annan RS, Chai D, Colón M, Dodson CS, Gilbert SA, Greshock J, Jing J, et al. Quinoline 3-sulfonamides inhibit lactate dehydrogenase A and reverse aerobic glycolysis in cancer cells. Cancer Metab. 2013;1:19. doi: 10.1186/2049-3002-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Manerba M, Di Ianni L, Govoni M, Roberti M, Recanatini M, Di Stefano G. LDH inhibition impacts on heat shock response and induces senescence of hepatocellular carcinoma cells. Eur J Pharm Sci. 2017;105:91–98. doi: 10.1016/j.ejps.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 161.Payen VL, Mina E, Van Hée VF, Porporato PE, Sonveaux P. Monocarboxylate transporters in cancer. Mol Metab. 2020;33:48–66. doi: 10.1016/j.molmet.2019.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hadjihambi A, Konstantinou C, Klohs J, Monsorno K, Le Guennec A, Donnelly C, Cox IJ, Kusumbe A, Hosford PS, Soffientini U, et al. Partial MCT1 invalidation protects against diet-induced non-alcoholic fatty liver disease and the associated brain dysfunction. J Hepatol. 2023;78:180–190. doi: 10.1016/j.jhep.2022.08.008. [DOI] [PubMed] [Google Scholar]
- 163.Jeon JY, Lee M, Whang SH, Kim JW, Cho A, Yun M. Regulation of acetate utilization by monocarboxylate transporter 1 (MCT1) in hepatocellular carcinoma (HCC) Oncol Res. 2018;26:71–81. doi: 10.3727/096504017X14902648894463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Fang Y, Liu W, Tang Z, Ji X, Zhou Y, Song S, Tian M, Tao C, Huang R, Zhu G, et al. Monocarboxylate transporter 4 inhibition potentiates hepatocellular carcinoma immunotherapy through enhancing T cell infiltration and immune attack. Hepatology. 2023;77:109–123. doi: 10.1002/hep.32348. [DOI] [PubMed] [Google Scholar]
- 165.Xu H, Li L, Wang S, Wang Z, Qu L, Wang C, Xu K. Royal jelly acid suppresses hepatocellular carcinoma tumorigenicity by inhibiting H3 histone lactylation at H3K9la and H3K14la sites. Phytomedicine. 2023;118:154940. doi: 10.1016/j.phymed.2023.154940. [DOI] [PubMed] [Google Scholar]
- 166.Zhou F, Shang W, Yu X, Tian J. Glypican-3: A promising biomarker for hepatocellular carcinoma diagnosis and treatment. Med Res Rev. 2018;38:741–767. doi: 10.1002/med.21455. [DOI] [PubMed] [Google Scholar]
- 167.Yao G, Yang Z. Glypican-3 knockdown inhibits the cell growth, stemness, and glycolysis development of hepatocellular carcinoma cells under hypoxic microenvironment through lactylation. Arch Physiol Biochem. 2023 May 2; doi: 10.1080/13813455.2023.2206982. Online ahead of print. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.