Version Changes
Revised. Amendments from Version 1
The mucus was diluted with CMC-Na to obtain gels with mucus concentrations of 24%, 48%, and 96% (w/w). This concentration produces a gel that is thick enough and does not flow out of the wound. The prepared mucus were stored in polypropylene conical tubes, covered with aluminum foil and placed in the refrigerator at 4 °C. This storage method preserved the angiogenic effect of the mucus. This was evidenced by the number of new blood vessels that were still significantly affected by the results of the study on day 7. Temperature and light could affect the stability of the mucus so that the used of opaque aluminum foil and cold temperatures could maintain product quality well. The minimum distance between wounds was 5 mm. The distance between the wounds in this study was made more than 2 cm so that it does not allow mucus to flow out or affect other groups due to animal movement. Positive control was not used in this study. The negative control showed a natural angiogenesis process for comparison with the mucus treatment group. The total rats in each groups are described in Figure 2. (Disclaimer: this image is only an illustration to explain the study grouping, not a photo of each mouse used in this study group).
Abstract
Background: Angiogenesis is the process through which new blood vessels are formed from existing ones. This process plays an important role in supplying the oxygen and nutrients needed for cellular metabolism and eliminating cell debris during wound healing. Snail mucus can bind to several factors that stimulate angiogenesis, including vascular endothelial growth factor, platelet-derived growth factor, and fibroblast growth factor. The aim of this study is to observe changes in angiogenesis during the healing of wounds topically applied with snail mucus.
Methods: Punch biopsy was performed on the back of male Wistar rats to obtain four wounds, and different concentrations of snail mucus were applied to each of these wounds. The animals were sacrificed on days 2, 4, and 7 to observe the extent of angiogenesis during wound healing by microscopy.
Results: Two-way ANOVA showed differences in number of blood vessels formed (p = 0.00) and day of observation (p = 0.00) between groups. Post hoc Tukey’s HSD test showed that 24% snail mucus treatment does not significantly affect wound healing (p = 0.488); by contrast, treatment with 48% and 96% snail mucus demonstrated significant effects on angiogenesis (p = 0.01). Spearman’s test showed interactive effects between snail mucus concentration and day of observation on the extent of angiogenesis (p = 0.001, R = 0.946).
Conclusion: Topical application of snail mucus gel can increase angiogenesis during wound healing in Wistar rat skin.
Keywords: new vessels, hematoxylin eosin, CMC-Na, glycosaminoglycans, heparan sulfate
Introduction
Oral surgery is an aspect of dentistry that is often associated with skin lesions 1, 2 . Cuts to the skin during oral and maxillofacial surgery may be achieved through incision or excision. Incision wounds are usually established during surgery, while excision wounds occur in trauma cases 3– 6 . Excision wound models generally have a diameter of 2–20 mm. Excision wounds provide complex and detailed views of the wound healing process and allow the examination of various wound healing parameters 7 . This fact underlies the selection of excision wound in the present study.
When a wound occurs in the body, physiological healing is performed by multiple biocellular and biochemical processes 8 . Wound healing refers to the process through which normal tissue is regenerated from damaged tissue 9 ; it involves cells, the extracellular matrix, and a number of mediators, such as growth factors and cytokines 10 . The wound healing process also involves hemostasis, regeneration of peripheral cells, and restoration of muscle tissue by collagen fibers 11 . Wound healing is a dynamic and complex process that involves multiple phases with overlaps from one phase to another 12 .
The wound healing process can be divided into three phases, namely, inflammation, proliferation, and tissue remodeling. This process can be observed using several parameters, such as re-epithelialization, number of polymorphonuclear leukocytes, number of fibroblasts, density of collagen fibers, and angiogenesis 13 . Angiogenesis is important in healing and refers to the process through which pre-existing blood vessels generate capillary buds to produce new blood vesselss 14 . Angiogenesis is triggered by tissue damage, which causes local hypoxia 15 . When local hypoxia occurs, cells respond by increasing their production of vascular endothelial growth factor (VEGF), one of the most important mediators for wound healing and a stimulant of capillary growth. Angiogenesis is then induced to fulfil requirements for nutrients, oxygen, and inflammatory cells 16 .
Wound healing can be enhanced by chemical and natural treatments 17 . Traditional medicines with natural health benefits and limited side effects have been developed by many researchers 18 . Snail mucus is widely used by cosmetics manufacturers as a skin care material 19 . The resulting products usually feature high contents of hyaluronic acid, proteoglycans, glycoprotein enzymes, and antibacterial peptides to protect the skin from damage 20 .
The African giant snail ( Achatina fulica) contains glycosaminoglycans 21 . Indeed, approximately 3%–5% of the dry weight of the snail is composed of glycosaminoglycans 22 . Glycosaminoglycans are a group of anionic polysaccharides that are typically isolated as proteoglycans connected to protein nuclei 23 . The biological activation of glycosaminoglycans stimulates the regulation of cell growth via the interaction of the glycosaminoglycan chains with growth factor proteins and their receptors 24 . The snail also contains acharan sulfate, which is stored as granules in the snail body and secreted by the animal under certain stimuli 25 .
Methods
Snail mucus gel preparation
The rat cages measured at least 40 cm long, 15 cm wide, and 10 cm high, and one cage housed one rat. The cages were covered with rice husks to achieve a stress-free environment for the rats, and the animals were provided food and water ad libitum. Snails are obtained from farms in Central Java. Identification and determination of Achatina fulica species were carried out in laboratory of animal biology. The snails were adapted to a cage with moist soil and banana leaves as food for three days. Then, the snails were fasted three days before the mucus extraction. Snail mucus was extracted by stimulating the surface of the snail body with an electric shock of 6 V for 60 s, one touches namely from repetition 2 ~ 4 times. This method does not cause pain or stress on the snail. A detailed description of all protocols can be found in the google patent numbered CN102846519B ( https://patents.google.com/patent/CN102846519B/en). The collected snail mucus was passed through batiste cloth to remove impurities, collected in a glass beaker, and homogenized. The snail mucus was sterilized by filtration through Whatman No. 4 filter paper. Finally, the mucus was diluted with CMC-Na to obtain gels with mucus concentrations of 24%, 48%, and 96% (w/w). This concentration produces a gel that is thick enough and does not flow out of the wound. The prepared mucus were stored in polypropylene conical tubes, covered with aluminum foil and placed in the refrigerator at 4 °C. This storage method preserved the angiogenic effect of the mucus. This was evidenced by the number of new blood vessels that were still significantly affected by the results of the study on day 7. Temperature and light could affect the stability of the mucus so that the used of opaque aluminum foil and cold temperatures could maintain product quality well.
Animals and group preparation
This research was approved by the Health and Medical Research Ethics Committee of the Faculty of Dentistry, Universitas Gadjah Mada (Approval No. 00272/KKEP/FKG-UGM/EC/2019). This study used nine rats based on calculations using the resource of equation method with the minimum sample calculation formula in research with ANOVA design. Sample calculation: Minimum n= 10/kr +1 =10/(4x3) +1 = 1.83. Maximum n= 20/kr +1 = 20/(4x3) +1 = 2.66 (k = number of treatments, r = number of repeated measurements). The conclusion is the use of 3 rats meets the sample size requirements.
The rats were obtained from breeding by laboratory of pharmaceutical. Healthy male Wistar rats were adapted to cages for 3 days. The rat cages measured at least 40 cm long, 15 cm wide, and 10 cm high, and one cage housed one rat. The cages were covered with rice husks to achieve a stress-free environment for the rats, and the animals were provided food and water ad libitum.
Animal treatment
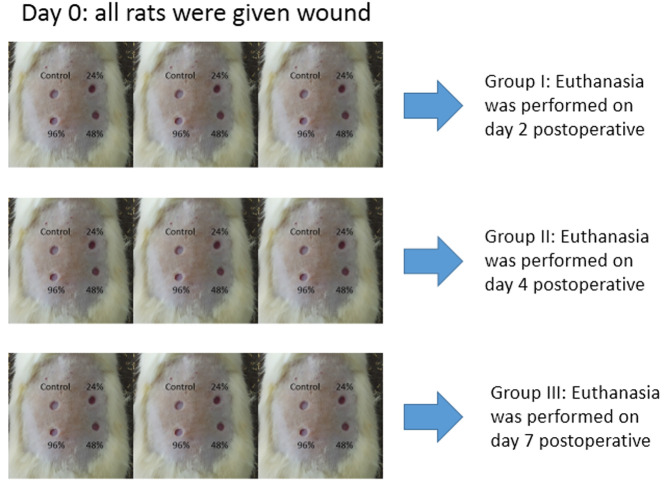
The rats were included in the study if they were 3–4 months old, weighed 250–300 mg, appeared healthy and physically active, and there were no visible anatomical defects. The rats were excluded in the study if they had postoperative infections or died before the euthanasia process. They were randomly divided into three groups of euthanasia day (three rats/group). Random numbers were generated using the standard = RAND() function in Microsoft Excel. The rats were anesthetized with 100 mg/kg BW ketamine and 4 mg/kg xylazine intramuscularly. The back of each rat was shaved, marked, and disinfected with 70% alcohol. A circular subcutaneous excision wound was made by punch biopsy of 5 mm. The skin on the back of a rat was folded and lifted by pinching the cranial and caudal skin between the thumb and forefinger. The rat was placed in the lateral position, and a biopsy punch was made through the folded skin (middle). Appearance of the resulting symmetrical and full-thickness wounds is shown in Figure 1. The minimum distance between wounds was 5 mm. The distance between the wounds in this study was made more than 2 cm so that it does not allow mucus to flow out or affect other groups due to animal movement. Each rat was given 4 wounds which were given different experiments (24%, 48%, 96% and control gels), so the total was nine rats with 36 wounds (each experiments were nine wounds which were observed on day two, four, and seven). Positive control was not used in this study. The negative control showed a natural angiogenesis process for comparison with the mucus treatment group. The total rats in each groups are described in Figure 2. (Disclaimer: this image is only an illustration to explain the study grouping, not a photo of each mouse used in this study group). The calculation of the number of samples and four wounds on the back of the rats and this fulfills the requirements of reduce, reuse, and recycle.
Figure 1. Installation of excision wounds.
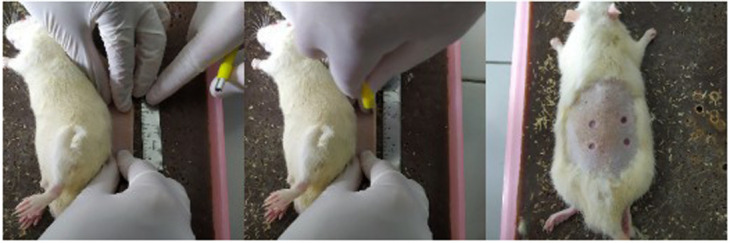
Figure 2. Experimental groups.
The rats were transferred to a warm cage until they regained complete consciousness and then returned to their original cages. The general condition and weight of the rats were recorded daily. The snail mucus (24%, 48%, 96%) and control gels (CMC-Na) were applied 1 ml once a day (in the morning) on each wound. Euthanasia was performed on postoperative days 2, 4, and 7 by overdoses of inhaled anesthetics ether. The skin of the treated wound area was removed for histological examination by hematoxylin–eosin staining. Observation and calculation of the number of new blood vessels formed were carried out using a binocular microscope equipped with an Optilab Advance V2 12,6MP camera in the five fields of view. Microscopic observations were performed at 40×, 100×, and 400× magnification. The number of new blood vessels formed was calculated by three observers. The number of new blood vessels were the only outcome measures used
Statistical analysis
Two-way analysis of variance (ANOVA) was used to determine significant differences in angiogenesis among the treatment groups. Post hoc Tukey’s test was conducted to determine which groups showed significant differences. Statistical calculations were performed using statistical package for the social sciences (IBM SPSS Statistics 23) software at a confidence level of 95% (α < 0.05).
Results
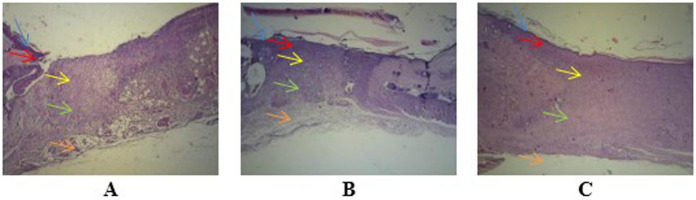
Observation at 40× magnification showed differences in the structure of wounds with healthy skin borders on days two, four, and seven at all perecentage of mucus ( Figure 3). Wounds observed on day two showed tissues filled with inflammatory cells without a surface epithelium. Wounds on day four revealed reductions in inflammatory tissue and a thin layer of connective tissue. The surface epithelium covering the wound was fairly thin. On day seven of healing, the wounds showed thick connective tissue formation and a surface epithelium layer clearly covering the wounded area. Inflammatory cells could not be clearly observed on day seven.
Figure 3.
Wound healing with gel 96% on day two ( A), day four ( B), and day seven ( C), as determined using histological preparations with hematoxylin–eosin staining at 40× magnification. Blue arrows indicate the epithelium, red arrows indicate the epidermis, yellow arrows indicate the papillary stratum, green arrows indicate the reticular stratum, and orange arrows indicate adipose layers. Observation of angiogenesis was carried out on the papillary and reticular dermal layers of the stratum.
Observation at 100× magnification was performed to determine relevant fields of view ( Figure 4). Angiogenesis was noted in the papillary and reticular strata. Visual fields for further observation were selected from five areas in these strata.
Figure 4.
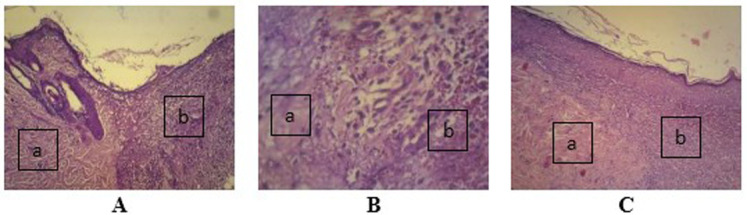
Wound healing with gel 96% on day two ( A), day four ( B), and day seven ( C), as determined using histological preparations with hematoxylin–eosin staining at 100× magnification. ( a) Healthy tissue and ( b) wound tissue.
The number of new blood vessels in the wounds was counted from five fields of view at 400× magnification. New blood vessels appeared in the lumen; the walls of these vessels were composed of endothelial cells and contained erythrocytes ( Figure 5). Endothelial cells at the edges of cell walls were purple in color, round, and flat. Erythrocytes appeared as red irregularly rounded cells without a nucleus.
Figure 5. Blood vessels in the histologic preparation.
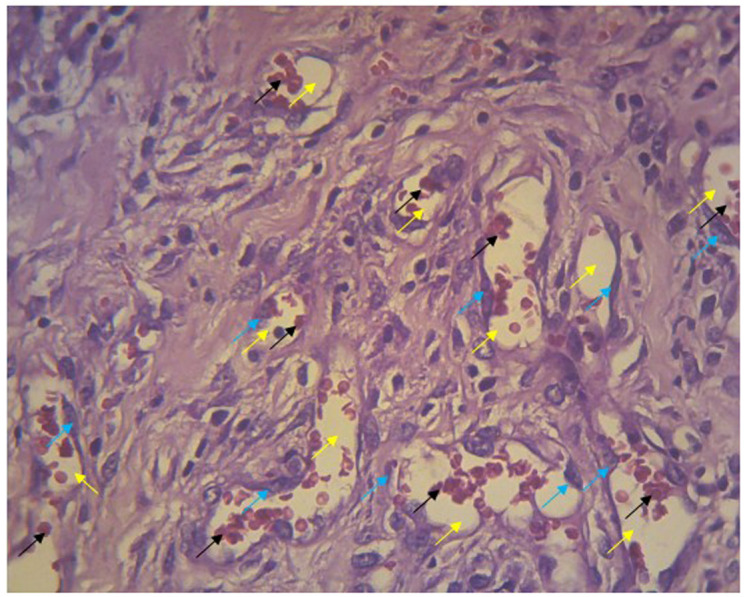
The walls of lumen vessels (yellow arrows) are formed by endothelial cells (blue arrows) and contain erythrocytes (black arrows). This picture was taken from experiments with gel 96%.
The number of new blood vessels formed are shown in Figure 6 and Table 1. The results of the calculations in Table 1 are depicted in Figure 7. The number of new blood vessels formed increased from day two to day four in all groups but was greatest in the 96% snail mucus treatment group (mean, 17.2). Whereas angiogenesis decreased from day four to day seven in the 96% and 48% snail mucus treatment groups, angiogenesis in the 24% snail mucus treatment and control groups increased over these days. The number of new blood vessels formed in the 96% snail mucus treatment group was consistently greater than those in the other treatment groups on each day assessed.
Figure 6. Angiogenesis in the wound area on days two, four, and seven, as observed from histological preparations with hematoxylin–eosin staining at 400× magnification.
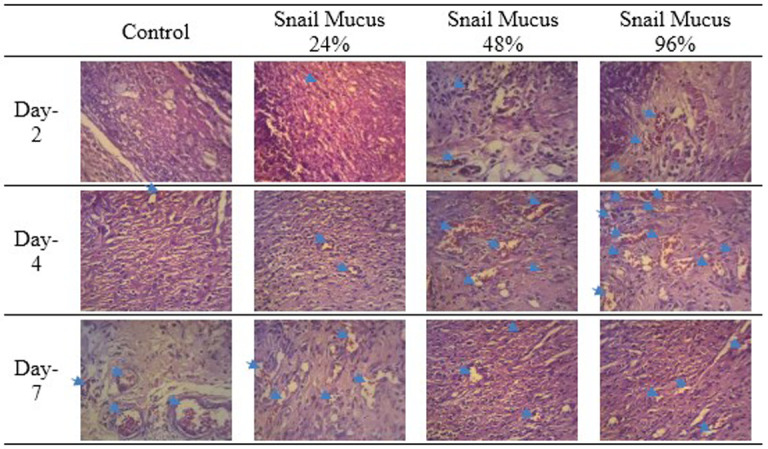
The greatest number of new blood vessels formed was observed on day four following the application of 96% snail mucus gel (p = 0.000).
Figure 7. Graphic of the numbers of new blood vessels formed among treatment groups and days.
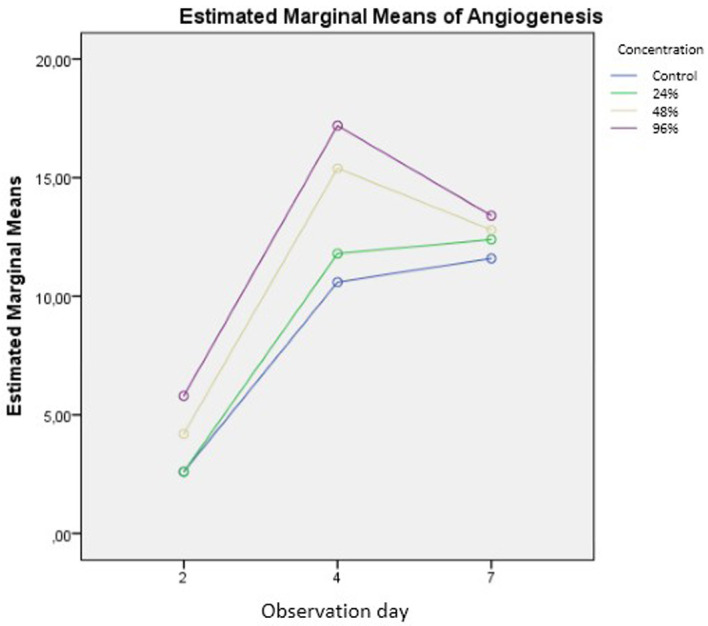
Table 1. Results of angiogenesis observations according to day and concentration.
| Obsevation
day |
Fields of
View |
Mucus Snail Gel Concentration | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 24% | 48% | 96% | ||||||||||
| Number/field | Total | Average | Number/
field |
Total | Average | Number/
field |
Total | Average | Number/
field |
Total | Average | ||
| Day 2 | 1 | 3 | 13 | 2.6 | 3 | 13 | 2.6 | 4 | 21 | 4.2 | 5 | 29 | 5.8 |
| 2 | 2 | 3 | 5 | 6 | |||||||||
| 3 | 2 | 2 | 5 | 6 | |||||||||
| 4 | 3 | 3 | 4 | 7 | |||||||||
| 5 | 3 | 2 | 3 | 5 | |||||||||
| Day 4 | 1 | 10 | 53 | 10.6 | 9 | 59 | 11.8 | 13 | 77 | 15.4 | 16 | 86 | 17.2 |
| 2 | 11 | 10 | 16 | 16 | |||||||||
| 3 | 9 | 15 | 17 | 18 | |||||||||
| 4 | 13 | 13 | 14 | 19 | |||||||||
| 5 | 10 | 12 | 17 | 17 | |||||||||
| Day 7 | 1 | 13 | 58 | 11.6 | 12 | 62 | 12.4 | 12 | 64 | 12.8 | 14 | 67 | 13.4 |
| 2 | 10 | 13 | 12 | 13 | |||||||||
| 3 | 10 | 12 | 14 | 12 | |||||||||
| 4 | 13 | 12 | 14 | 14 | |||||||||
| 5 | 12 | 13 | 12 | 14 | |||||||||
| Total
average |
41.33 | 44.67 | 54.00 | 60.67 | |||||||||
Data analysis
Two-way ANOVA was used to determine significant differences in the extent of angiogenesis and number of observation days among the treatment groups. Differences in number of observation days, concentration, and the interaction of number of observation days and concentration were significant with 0.000, 0.000, and 0.001 (p < 0.05), respectively. These results demonstrate that each of the independent variables analyzed has a significant effect on angiogenesis.
Post hoc testing of the two-way ANOVA results was conducted using Tukey’s HSD test. The difference in the number of new blood vessels formed between the control group and the 24% snail mucus treatment group was not significant. All other groups showed significant differences in mean number of new blood vessels formed. These results indicate that treatment with 48% and 96% snail mucus has significant effects on angiogenesis during skin wound healing.
Discussion
Wound healing is a biological process that involves complex interactions between cells, the extracellular matrix, and growth hormones. This process occurs in several phases, namely, hemostasis, inflammation, proliferation, and maturation 26 . Angiogenesis is an important process in wound healing 27 . The present study demonstrated that snail mucus can accelerate angiogenesis and wound healing. Prasojo et al. (2018) found that pure snail mucus without a carrier material can increase angiogenesis during wound healing compared with distilled water 28 . Harti et al. (2018) showed that heparan sulfate stimulates VEGF. The study found that 5% and 100% snail mucus creams could accelerate wound healing by stimulating lymphocyte proliferation 29 .
Xander and Toin (2013) 30 revealed that heparan sulfate is a proteoglycan that serves as a binder and storage unit for basic fibroblast growth factor (bFGF), which is secreted into the extracellular matrix. Heparan sulfate interacts with proangiogenic factors, such as fibroblast growth factor (FGF), VEGF, and platelet-derived growth factor (PDGF), on the surface of endothelial cells and causes these factors to bind to their corresponding receptors, thereby resulting in dimerization and various signaling processes. The extracellular matrix can release bFGF to stimulate inflammatory cell recruitment, fibroblast activation, and the formation of new blood vessels during injury 31, 32 . This hormonal mechanism may also occur in the increase of angiogenesis in this study. However, research on this subject is still our next research project. In addition, a snail mucus gel formulation is also in our next project, so that this gel will have a more effective absorption rate and can be stored for a long time. A previous study indicated that snail mucus with chitosan as a membrane can accelerate wound healing through anti-inflammatory activity. Apriyanti et al. (2017) 33 also showed that 5% snail mucus gel could increase angiogenesis in alveolar bone during the healing of periodontitis in Wistar rats.
Angiogenesis is a complex process involving various cells, hormones, and extracellular components 34 . Snail mucus contains proangiogenic glycosaminoglycans, heparan sulfate, heparin sulfate, and hyaluronic acid. These compounds can increase angiogenesis by triggering VEGF as the dominant angiogenetic growth factor against endothelial cells as blood vessel-forming cells 22, 35, 36 .
Endothelial cell proliferation marks the beginning of angiogenesis. Endothelial cells grow, migrate, and then attach to the extracellular matrix, where they differentiate into new blood vessels 37– 39 . Snail mucus can stimulate these processes remarkably. The results of this study demonstrated that the application of snail mucus could increase angiogenesis at all concentrations tested. The statistical analysis shows that the increase in angiogenesis is particularly significant at snail mucus concentrations of 48% and 96% (p = 0.000). Compared with the other groups, the 96% snail mucus treatment showed the greatest extent of angiogenesis ( Figure 7).
New blood vessels were formed on day two in all treatments. Wounds applied with snail mucus showed a greater number of new blood vessels formed compared with the control treatment (p = 0.000). This finding is consistent with the research of Bauer et al. (2005) 15 , who found that the initial factor triggering angiogenesis is the damage that occurs in endothelial tubules following tissue damage. Tissue damage causes local hypoxia. The hypoxic state of the tissue becomes an angiogenic stimulator as growth factors and cytokines are released from inflammatory cells accumulated in the wound area during the previous inflammatory process. These factors stimulate the proliferation and invasiveness of vascular cells to promote blood vessel growth 15, 16, 40 .
The most important mediators in the early phase of angiogenesis are VEGF, FGF, and PDGF 41 . Heparan sulfate in snail mucus can interact with these factors on the surface of endothelial cells and enhance their ability to bind to their corresponding receptors, resulting in dimerization and various signaling processes 30 . An adequate supply of nutrients, oxygen, and cells with essential functions in wound healing could hasten the wound healing process. Sufficient nutrition and oxygen are required for optimal wound healing. Angiogenesis provides a new vascular system that could deliver nutrients and oxygen to the wound area and enable wound healing. Cells necessary for wound healing, such as inflammatory cells, fibroblasts, and mesenchymal cells, which secrete various growth factors, may also be delivered to the wound site through this new vascular system 42, 43 . Mature endothelial cells can then form new blood vessel walls in the wound area 44 .
Day four of observation revealed the greatest number of new blood vessels formed in the 96% snail mucus treatment group. The proliferation phase of angiogenesis occurs on the fourth day after the initiation phase, which could explain the extent of angiogenesis observed on this day in the present study. Kalangi (2004) 45 stated that the proliferation phase begins with the degradation of old blood vessels by providing capillary shoot formation in hypoxic tissues to meet the nutritional and oxygen needs of parenchymal cells. These parenchymal cells secrete the most important proangiogenic growth factor, namely, VEGF-A. Then, there is a series of angiogenesis starting from (1) migration of endothelial cells distally from the original capillary vessels to stimulate angiogenesis; (2) proliferation of endothelial cells at the periphery of/distal to tubule formation; (3) stabilization of endothelial cells by interacting strongly with support cells, such as smooth muscle cells and pericytes; (4) maturation of endothelial cells via the formation of a lumen through intercellular and intracellular mechanisms, including the mobilization and proliferation of pericytes (from blood vessels) and smooth muscle cell (for large vessels) to support the endothelial wall and provide additional budding; (5) anastomosis with other endothelial buds and knot formation; and (6) development of circulation and adjustment of canals with arterial and venous segments 45– 47 .
Glycosaminoglycans and heparan sulfate in snail mucus significantly increased (p = 0.031) the number of new blood vessels formed in the 96% snail mucus treatment group on day four. Glycosaminoglycans stabilize cell membranes, increase the synthesis of hyaluronic acid, a known anti-inflammatory agent, and accelerate angiogenesis; as such, these compounds have positive effects on wound healing 48 . Angiogenesis can also be enhanced by the ability of snail mucus to bind divalent cations, such as copper(II) 29 . Heparan sulfate is a proteogenizer that can bind and store bFGF, which is secreted by the extracellular matrix to stimulate the recruitment of inflammatory cells, fibroblast activation, and angiogenesis 49 .
Compared with that on the day 4, the average number of new blood vessels formed in the 48% and 96% snail mucus treatment groups decreased on day 7. By contrast, the control and 24% snail mucus treatment groups revealed continuous increases in angiogenesis ( Table 1 and Figure 7) significantly (p = 0.000). This finding indicates that snail mucus not only increases the number of new blood vessels formed but also hastens the phases of angiogenesis. The decrease in number of new blood vessels formed on day seven in the 48% and 96% snail mucus treatment groups may be due to apoptosis. The number of new vessels formed is reduced until the density of blood vessels in the wound area returns to normal. This process is regulated by a selective apoptosis process that occurs simultaneously with the maturation of new blood vessels. Apoptosis refers to the automatic and programmed death of normal cells 50– 52 .
According to a study conducted by Ricard et al. (2014), the glycosaminoglycans and hyaluronic acid in snail mucus could increase the activity of pericyte cells. Pericyte cells are multifunctional cells capable of maintaining capillary stability and protecting capillaries from negative signals. Some selective apoptosis processes are regulated by pericyte cells 51, 53 . Pericytes are only present in newly formed blood vessels. Researchers believe that vessels without pericytes are susceptible to the influence of antiangiogenic agents 54 . The apoptosis of blood vessels in a wound area decreases following the reduction of antiangiogenic factors 55 . Mostafa (2014) 56 conducted a study on the effect of topical application of synthetic glycosaminoglycans on wound healing in mice and found an increase in wound closure speed; this study used synthetic glycosaminoglycans at a concentration of 2%. The glycosaminoglycans used in the present study are obtained naturally from snail mucus.
The most important mediators in angiogenesis are VEGF, FGF, and PDGF. This study did not examine hormonal levels of these factors to determine the effect of snail mucus on proangiogenic factors. Further research can be conducted to examine the effects of snail mucus on these factors and their corresponding receptors. In addition, a snail mucus gel formulation is also in our next project, so this gel will have a more effective absorption rate and can be stored for a long time.
Conclusion
Different concentrations of snail mucus gel revealed different effects on angiogenesis during the healing of punch biopsy wounds on the back skin of Wistar rats. Compared with the control and 24% and 48% snail mucus treatment groups, the 96% snail mucus treatment group showed the greatest improvements in angiogenesis on day 4 (p = 0.00). Snail mucus concentration and day of observation showed interactive effects on angiogenesis during skin wound healing in Wistar rats (R = 0.946). Specifically, the higher the snail mucus concentration and the greater the number of observation days, the faster the wound healing process.
Data availability
Underlying data
Figshare: Table 1. Results of angiogenesis observations according to day and concentration 56 . https://doi.org/10.6084/m9.figshare.13698871.v1
This project contains the following underlying data:
-
-
Table 1. The results of the calculations in Table 1 are depicted in Figure 7. The number of new blood vessels formed increased from day 2 to day 4 in all groups but was greatest in the 96% snail mucus treatment group (mean, 17.2). Whereas angiogenesis decreased from day 4 to day 7 in the 96% and 48% snail mucus treatment groups, angiogenesis in the 24% snail mucus treatment and control groups increased over these days. The number of new blood vessels formed in the 96% snail mucus treatment group was consistently greater than those in the other treatment groups on each day assessed.
Figshare: Figure 1. Installation of excision wounds 57 . https://doi.org/10.6084/m9.figshare.14045033.v1
This project contains the following data:
-
-
JPG file of the installation of excision wounds on the rats. The skin on the back of a rat was folded and lifted by pinching the cranial and caudal skin between the thumb and forefinger. The rat was placed in the lateral position, and a biopsy punch was made through the folded skin (middle). Appearance of the resulting symmetrical and full-thickness wounds. No modifications have been made to this image.
Figshare. Figure 3. Wound healing with gel 98% on day two (A), day four (B), and day seven (C) 58 . https://doi.org/10.6084/m9.figshare.14045075.v1
This project contains the following data:
-
-
JPG file showing the wound healing on day 2. Determined using histological preparations with hematoxylin–eosin staining at 100× magnification. (a) Healthy tissue and (b) wound tissue. Arrow on the image was made by Microsoft Word 2013. No others modifications have been made to this image.
Figshare. Figure 4. Wound healing with gel 98% on day two (A), day four (B), and day seven (C) 59 . https://doi.org/10.6084/m9.figshare.14045099
This project contains the following data:
-
-
JPG file of wound healing with gel 98% on day two (A), day four (B), and day seven (C). Determined using histological preparations with hematoxylin–eosin staining at 100× magnification. (a) Healthy tissue and (b) wound tissue. Alphabet on the image was made by Microsoft Word 2013. No others modifications have been made to this image.
Figshare. Figure 5. Blood vessels in the histologic preparation 60 . https://doi.org/10.6084/m9.figshare.14045309
This project contains the following data:
-
-
JPG file of blood vessels in the histologic preparation. The walls of lumen vessels (yellow arrows) are formed by endothelial cells (blue arrows) and contain erythrocytes (black arrows). This picture was taken from experiments with gel 96%. Arrow on the image was made by Microsoft Word 2013. No others modifications have been made to this image.
Figshare. Figure 6. New blood vessels formation in the wound area on days two, four, and seven 61 . https://doi.org/10.6084/m9.figshare.14045342
This project contains the following data:
-
-
JPG file of angiogenesis in the wound area on days two, four, and seven. Observed from histological preparations with hematoxylin–eosin staining at 400× magnification. The greatest number of new blood vessels formed was observed on day four following the application of 96% snail mucus gel (p = 0.000). Grouping, naming, and arrow on the image were made by Microsoft Word 2013. No other modifications have been made to this image.
Figshare. Figure 7. Graphic of the numbers of new blood vessels formed among treatment groups and days 62 . https://doi.org/10.6084/m9.figshare.14045351
This project contains the following data:
-
-
JPG graphic of the numbers of new blood vessels formed among treatment groups and days No other modifications have been made to this image.
Figshare. Raw data of new blood vessels observation 63 . https://doi.org/10.6084/m9.figshare.14045369
This project contains the following data:
-
-
.xlxs file of the results of angiogenesis observations according to day and concentration.
Data are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC BY 4.0 Public domain dedication).
Extended data
Figshare. Figure 3A. Wound healing with gel 96% on day two 64 . DOI: https://doi.org/10.6084/m9.figshare.14092499
Figshare. Figure 3B. Wound healing with gel 96% on day four as determined using histological preparations with hematoxylin–eosin staining at 40× magnification 65 . DOI: https://doi.org/10.6084/m9.figshare.14092898
Figshare. Figure 3C. Wound healing with gel 96% on day seven, as determined using histological preparations with hematoxylin–eosin staining at 40× magnification 66 . DOI: https://doi.org/10.6084/m9.figshare.14093035
Figshare. Figure 4A. Wound healing with gel 96% on day two as determined using histological preparations with hematoxylin–eosin staining at 100× magnification 67 . DOI: https://doi.org/10.6084/m9.figshare.14093131
Figshare. Figure 4B. Wound healing with gel 96% on day four as determined using histological preparations with hematoxylin–eosin staining at 100× magnification 68 . DOI: https://doi.org/10.6084/m9.figshare.14093227
Figshare. Figure 4C. Wound healing with gel 96% on day seven as determined using histological preparations with hematoxylin–eosin staining at 100× magnification 69 . DOI: https://doi.org/10.6084/m9.figshare.14093289
Figshare. Figure 5. Blood vessels in the histologic preparation. The walls of lumen vessels (yellow arrows) are formed by endothelial cells (blue arrows) and contain erythrocytes (black arrows). This picture was taken from experiments with gel 96% 70 . DOI: https://doi.org/10.6084/m9.figshare.14093357
Figshare. Figure 6 Day 2 24%. Angiogenesis in the wound area on day two with control as observed from histological preparations with hematoxylin–eosin staining at 400× magnification 71 . DOI: https://doi.org/10.6084/m9.figshare.14093419
Figshare. Figure 6 Day 2 48%. Angiogenesis in the wound area on days two with 48% of snail mucus as observed from histological preparations with hematoxylin–eosin staining at 400× magnification 72 . DOI: https://doi.org/10.6084/m9.figshare.14093731
Figshare. Figure 6 Day 2 96%. Angiogenesis in the wound area on days two with 96& of snail mucus as observed from histological preparations with hematoxylin–eosin staining at 400× magnification 73 . DOI: https://doi.org/10.6084/m9.figshare.14093829
Figshare. Figure 6 Day 2 Control. Angiogenesis in the wound area on days two, four, and seven, as observed from histological preparations with hematoxylin–eosin staining at 400× magnification 74 . DOI: https://doi.org/10.6084/m9.figshare.14093891
Figshare. Figure 6 Day 4 24%. Angiogenesis in the wound area on day four with 24% of snail mucus as observed from histological preparations with hematoxylin–eosin staining at 400× magnification 75 . DOI: https://doi.org/10.6084/m9.figshare.14093935
Figshare. Figure 6 Day 4 48%. Angiogenesis in the wound area on day four with 48% of snail mucus as observed from histological preparations with hematoxylin–eosin staining at 400× magnification 76 . DOI: https://doi.org/10.6084/m9.figshare.14094001
Figshare. Figure 6 Day 4 96%. Angiogenesis in the wound area on day four with 96% of snail mucus as observed from histological preparations with hematoxylin–eosin staining at 400× magnification 77 . DOI: https://doi.org/10.6084/m9.figshare.14094623
Figshare. Figure 6 Day 4 Control. Angiogenesis in the wound area on day four with control as observed from histological preparations with hematoxylin–eosin staining at 400× magnification 78 . DOI: https://doi.org/10.6084/m9.figshare.14094687
Figshare. Figure 6 Day 7 24%. Angiogenesis in the wound area on day seven with 24% of snail mucus as observed from histological preparations with hematoxylin–eosin staining at 400× magnification 79 . DOI: https://doi.org/10.6084/m9.figshare.14094751
Figshare. Figure 6 Day 7 48%. Angiogenesis in the wound area on day seven with 48% of snail mucus as observed from histological preparations with hematoxylin–eosin staining at 400× magnification 80 . DOI: https://doi.org/10.6084/m9.figshare.14094803
Figshare. Figure 6 Day 7 96%. Angiogenesis in the wound area on day seven with 96% of snail mucus as observed from histological preparations with hematoxylin–eosin staining at 400× magnification 81 . DOI: https://doi.org/10.6084/m9.figshare.14094849
Figshare. Figure 6 Day 7 Control. Angiogenesis in the wound area on day seven with control as observed from histological preparations with hematoxylin–eosin staining at 400× magnification 82 . DOI: https://doi.org/10.6084/m9.figshare.14094919
Acknowledgments
We thank our colleagues for providing their insights and expertise to this research.
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 2; peer review: 1 approved
References
- 1. Welsh JD: Medical History of Union Generals.OH: Kent State University Press. 2005. Reference Source [Google Scholar]
- 2. Politis C, Schoenaers J, Jacobs R, et al. : Wound healing problems in the mouth. Front Physiol. 2016;7:507. 10.3389/fphys.2016.00507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gál P, Toporcer T, Vidinský B, et al. : Simple interrupted percutaneous suture versus intradermal running suture for wound tensile strength measurement in rats: a technical note. Eur Surg Res. 2009;43(1):61–5. 10.1159/000219214 [DOI] [PubMed] [Google Scholar]
- 4. Harlaar JJ, van Ramshorst GH, Nieuwenhuizen J, et al. : Small stitches with small suture distances increase laparotomy closure strength. Am J Surg. 2009;198(3):392–5. 10.1016/j.amjsurg.2008.10.018 [DOI] [PubMed] [Google Scholar]
- 5. Lee YS, Yang HO, Shin KH, et al. : Suppression of tumor growth by a new glycosaminoglycan isolated from the African giant snail Achatina fulica. Eur J Pharmacol. 2003;465(1–2):191–8. 10.1016/s0014-2999(03)01458-4 [DOI] [PubMed] [Google Scholar]
- 6. Macpherson N, Lee S: Effect of different suture techniques on tension dispersion in cutaneous wounds: a pilot study. Australas J Dermatol. 2010;51(4):263–7. 10.1111/j.1440-0960.2010.00691.x [DOI] [PubMed] [Google Scholar]
- 7. Azevedo LH, de Sousa SC, Correa L, et al. : Mast cell concentration in the wound healing process of incisions made by different instruments. Lasers Med Sci. 2009;24(4):585–90. 10.1007/s10103-008-0616-5 [DOI] [PubMed] [Google Scholar]
- 8. de Oliveira Gonzalez AC, Costa TF, de Araújo Andrade Z, et al. : Wound healing - A literature review. An Bras Dermatol. 2016;91(5):614–20. 10.1590/abd1806-4841.20164741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Krafts KP: Tissue repair: The hidden drama. Organogenesis. 2010;6(4):225–33. 10.4161/org.6.4.12555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Velnar T, Bailey T, Smrkolj V: The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res. 2009;37(5):1528–42. 10.1177/147323000903700531 [DOI] [PubMed] [Google Scholar]
- 11. Cañedo-Dorantes L, Cañedo-Ayala M: Skin acute wound healing: A comprehensive review. Int J Inflam. 2019;2019:3706315. 10.1155/2019/3706315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guo S, Dipietro LA: Factors affecting wound healing. J Dent Res. 2010;89(3):219–29. 10.1177/0022034509359125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van der Laan AM, Piek JJ, van Royen N: Targeting angiogenesis to restore the microcirculation after reperfused MI. Nat Rev Cardiol. 2009;6(8):515–23. 10.1038/nrcardio.2009.103 [DOI] [PubMed] [Google Scholar]
- 14. Kumar V, Abbas AK, Fausto N, et al. : Robbins Basic Pathology. London: Elsevier. 2007. Reference Source [Google Scholar]
- 15. Bauer SM, Bauer RJ, Velazques OC: Angiogenesis, vasculogenesis, and induction of healing in chronic wounds. Vasc Endovascular Surg. 2005;39(4):293–305. 10.1177/153857440503900401 [DOI] [PubMed] [Google Scholar]
- 16. Szpaderska AM, Walsh CG, Steinberg MJ, et al. : Distinct patterns of angiogenesis in oral and skin wounds. J Dent Res. 2005;84(4):309–14. 10.1177/154405910508400403 [DOI] [PubMed] [Google Scholar]
- 17. Sivamani RK, Ma BR, Wehrli LN, et al. : Phytochemicals and naturally derived substances for wound healing. Adv Wound Care (New Rochelle). 2012;1(5):213–7. 10.1089/wound.2011.0330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yuan H, Ma Q, Ye L, et al. : The traditional medicine and modern medicine from natural products. Molecules. 2016;21(5):559. 10.3390/molecules21050559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dermatol Rev. Snail cream.2018. [Google Scholar]
- 20. Cilia G, Fratini F: Antimicrobial properties of terrestrial snail and slug mucus. J Complement Integr Med. 2018;15(3). 10.1515/jcim-2017-0168 [DOI] [PubMed] [Google Scholar]
- 21. Shim JY, Lee YS, Jung SH, et al. : Pharmacological activities of a new glycosaminoglycan, acharan sulfate isolated from the giant African snail Achatina fulica. Arch Pharm Res. 2002;25(6):889–94. 10.1007/BF02977010 [DOI] [PubMed] [Google Scholar]
- 22. Jeong J, Toida T, Muneta Y, et al. : Localization and characterization of acharan sulfate in the body of the giant African snail Achatina fulica. Comp Biochem Physiol B Biochem Mol Biol. 2001;130(4):513–9. 10.1016/s1096-4959(01)00468-7 [DOI] [PubMed] [Google Scholar]
- 23. Pomin VH, Mulloy B: Glycosaminoglycans and proteoglycans. Pharmaceuticals (Basel). 2018;11(1):27. 10.3390/ph11010027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim HS, Lee YH, Lee YR, et al. : Activation of professional antigen presenting cells by Acharan sulfate isolated from giant African snail, achatina fulica. Arch Pharm Res. 2007;30(7):866–70. 10.1007/BF02978838 [DOI] [PubMed] [Google Scholar]
- 25. Kim HJ, Ulagessan S: Antibacterial and antifungal activities of proteins extracted from seven different snails. Appl Sci. 2018;8(8):1362. 10.3390/app8081362 [DOI] [Google Scholar]
- 26. Gurtner GC, Werner S, Barrandon Y, et al. : Wound repair and regeneration. Nature. 2008;453(7193):314–21. 10.1038/nature07039 [DOI] [PubMed] [Google Scholar]
- 27. Zeng Z, Zhu BH: Arnebin-1 promotes the angiogenesis of human umbilical vein endothelial cells and accelerates the wound healing process in diabetic rats. J Ethnopharmacol. 2014;154(3):653–62. 10.1016/j.jep.2014.04.038 [DOI] [PubMed] [Google Scholar]
- 28. Prasojo S, Rahajoe PS, Hasan CY: Effect of Snail Mucus (Achatina fulica) on Increased Angiogenesis in Skin Incision Wounds of Mice (Mus musculus) (Immunohistochemical Study CD34). Thesis. Universitas Gadjah Mada. 2018. [Google Scholar]
- 29. Harti AS, Murharyati A, Sulisetyawati DS, et al. : The effectiveness of snail mucus (Achatina fulica) and chitosan toward limfosit proliferation in vitro. Asian J Pharm Clin Res. 2018;11(15):85–88. 10.22159/ajpcr.2018.v11s3.30041 [DOI] [Google Scholar]
- 30. van Wijk XMR, van Kuppevelt TH: Heparan sulfate in angiogenesis: a target for therapy. Angiogenesis. Springer 2014;17(3):443–62. 10.1007/s10456-013-9401-6 [DOI] [PubMed] [Google Scholar]
- 31. Ucuzian AA, Gassman AA, East AT, et al. : Molecular mediators of angiogenesis. J Burn Care Res. 2010;31(1):158–75. 10.1097/BCR.0b013e3181c7ed82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fuster MM, Wang L: Endothelial heparan sulfate in angiogenesis. Prog Mol Biol Transl Sci. 2010;93:179–212. 10.1016/S1877-1173(10)93009-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Apriyanti T, Herawati D, Lastiany SP: Effect of 5% Snail Mucus Gel ( Achatina fulica) Application on Alveolar Bone Angiogenesis in Periodontitis Healing Process (Study on Wistar). Yogyakarta: UGM ETD. 2017. [Google Scholar]
- 34. Lamalice L, Le Boeuf F, Huot J: Endothelial cell migration during angiogenesis. Circ Res. 2007;100(6):782–94. 10.1161/01.RES.0000259593.07661.1e [DOI] [PubMed] [Google Scholar]
- 35. Vieira TCRG, Costa-Filho A, Salgado NC, et al. : Acharan sulfate, the new glycosaminoglycan from Achatina fulica Bowdich 1822. Structural heterogeneity, metabolic labeling and localization in the body, mucus and the organic shell matrix. Eur J Biochem. 2004;271(4):845–54. 10.1111/j.1432-1033.2004.03989.x [DOI] [PubMed] [Google Scholar]
- 36. Dominic J: Formulation and evaluation of giant African snail (achatina fulica) mucus and chitosan composite films for cicatrization.University of San Agustin. Filipina. College of Pharmacy and Medical Technology. 2015. Reference Source [Google Scholar]
- 37. Ingber DE: Mechanical signaling and the cellular response to extracellular matrix in angiogenesis and cardiovascular physiology. Circ Res. 2002;91(10):877–87. 10.1161/01.res.0000039537.73816.e5 [DOI] [PubMed] [Google Scholar]
- 38. Norton KA, Popel AS: Effects of endothelial cell proliferation and migration rates in a computational model of sprouting angiogenesis. Sci Rep. 2016;6:36992. 10.1038/srep36992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mardiyantoro F, Prasetyaningrum N, Rahmastuti HT: Histopathological characteristics of dental socket healing on collagen density following use of pangas catfish (Pangasius djambal) gelatin. Majalah Kedokteran Gigi Indonesia. 2019;5(3):120–5. 10.22146/majkedgiind.39830 [DOI] [Google Scholar]
- 40. Fermillian A, Agustina D, Subagyo G: The effect of papaya leaf extract (Carica papaya L.) on healing process of buccal traumatic ulcer in Wistar rats. MKGI. 2019;5(1):15–22. 10.22146/majkedgiind.37026 [DOI] [Google Scholar]
- 41. Adair TH, Montani JP: Angiogenesis.San Rafael: Morgan & Claypool Life Sciences.2010. [PubMed] [Google Scholar]
- 42. Khakoo AY, Finkel T: Endothelial progenitor cells. Annu Rev Med. 2005;56:79–101. 10.1146/annurev.med.56.090203.104149 [DOI] [PubMed] [Google Scholar]
- 43. Milkiewicz M, Ispanovic E, Doyle JL, et al. : Regulators of angiogenesis and strategies for their therapeutic manipulation. Int J Biochem Cell Biol. 2006;38(3):333–57. 10.1016/j.biocel.2005.10.006 [DOI] [PubMed] [Google Scholar]
- 44. Fidler IJ, Ellis LM: Neoplastic angiogenesis--not all blood vessels are created equal. N Engl J Med. 2004;351(3):215–6. 10.1056/NEJMp048080 [DOI] [PubMed] [Google Scholar]
- 45. Kalangi SJR: The role of collagen in wound healing. Dexa Media. 2004;17(4):168–74. [Google Scholar]
- 46. Jimmy S, Elmarakby D, Gazia MA, et al. : The role of hepatic stellate cells and angiogenesis in liver regeneration following partial hepatectomy in adult male albino rats: histological and immunohistochemical study. Egyptian Journal of Histology. 2019;42(4):1070–1095. 10.21608/ejh.2019.9692.1087 [DOI] [Google Scholar]
- 47. Eldien HMS, Mostafa NA, ElTawab OA, et al. : Effect of hematopoietic stem cells and platelet-rich plasma on the healing of experimental skin burned tissues: A comparative study in adult male mice. Egyptian Journal of Histology. 2019;42(3):740–754. 10.21608/ejh.2019.8023.1083 [DOI] [Google Scholar]
- 48. Towheed TE, Maxwell L, Anastassiades TP, et al. : Glucosamine therapy for treating osteoarthritis. Cochrane Database Syst Rev. 2005;18(2):CD002946. 10.1002/14651858.CD002946.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ricard N, Tu L, Le Hiress M, et al. : Increased pericyte coverage mediated by endothelial-derived fibroblast growth Factor-2 and interleukin-6 is a source of smooth muscle-like cells in pulmonary hypertension. Circulation. 2014;129(15):1586–97. 10.1161/CIRCULATIONAHA.113.007469 [DOI] [PubMed] [Google Scholar]
- 50. Honnegowda TM, Kumar P, Udupa EGP, et al. : Role of angiogenesis and angiogenic factors in acute and chronic wound healing. Plast Aesthet Res. 2015;2:243–9. 10.4103/2347-9264.165438 [DOI] [Google Scholar]
- 51. DiPietro LA: Angiogenesis and wound repair: when enough is enough. J Leukoc Biol. 2016;100(5):979–984. 10.1189/jlb.4MR0316-102R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Attia G, Atef H, Elmansy R: Autologous platelet rich plasma enhances satellite cells expression of MyoD and exerts angiogenic and antifibrotic effects in experimental rat model of traumatic skeletal muscle injury. Egyptian Journal of Histology. 2017;40(4):443–458. 10.21608/ejh.2017.5686 [DOI] [Google Scholar]
- 53. Kelly-Goss MR, Sweat RS, Stapor PC, et al. : Targeting pericytes for angiogenic therapies. Microcirculation. 2014;21(4):345–57. 10.1111/micc.12107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dulmovits BM, Herman IM: Microvascular remodeling and wound healing: a role for pericytes. Int J Biochem Cell Biol. 2012;44(11):1800–12. 10.1016/j.biocel.2012.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wietecha MS, Król MJ, Michalczyk ER, et al. : Pigment epithelium-derived factor as a multifunctional regulator of wound healing. Am J Physiol Heart Circ Physiol. 2015;309(5):H812–26. 10.1152/ajpheart.00153.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rosanto YB: Table 1. Results of angiogenesis observations according to day and concentration. figshare. Dataset.2021. 10.6084/m9.figshare.13698871.v1 [DOI] [Google Scholar]
- 57. Rosanto YB: Installation of excision wounds. figshare. Figure.2021. 10.6084/m9.figshare.14045033.v1 [DOI] [Google Scholar]
- 58. Rosanto YB: Wound healing on day two. figshare. Figure.2021. 10.6084/m9.figshare.14045075.v1 [DOI] [Google Scholar]
- 59. Rosanto YB: Wound healing with gel 98% on day two (A), day four (B), and day seven (C). figshare. Figure.2021. 10.6084/m9.figshare.14045099.v1 [DOI] [Google Scholar]
- 60. Rosanto YB: Blood vessels in the histologic preparation. figshare. Figure.2021. 10.6084/m9.figshare.14045309.v1 [DOI] [Google Scholar]
- 61. Rosanto YB: Angiogenesis in the wound area on days two, four, and seven. figshare. Figure.2021. 10.6084/m9.figshare.14045342.v1 [DOI] [Google Scholar]
- 62. Rosanto YB: Graphic of the numbers of new blood vessels formed among treatment groups and days. figshare. Figure.2021. 10.6084/m9.figshare.14045351.v1 [DOI] [Google Scholar]
- 63. Rosanto YB: Raw data. figshare. Dataset.2021. 10.6084/m9.figshare.14045369.v1 [DOI] [Google Scholar]
- 64. Rosanto YB: Figure 3A. figshare. Figure.2021. 10.6084/m9.figshare.14092499.v1 [DOI] [Google Scholar]
- 65. Rosanto YB: Figure 3B. figshare. Figure.2021. 10.6084/m9.figshare.14092898.v1 [DOI] [Google Scholar]
- 66. Rosanto YB: Figure 3C. figshare. Figure.2021. 10.6084/m9.figshare.14093035.v1 [DOI] [Google Scholar]
- 67. Rosanto YB: Figure 4A. figshare. Figure.2021. 10.6084/m9.figshare.14093131.v1 [DOI] [Google Scholar]
- 68. Rosanto YB: Figure 4B. figshare. Figure.2021. 10.6084/m9.figshare.14093227.v1 [DOI] [Google Scholar]
- 69. Rosanto YB: Figure 4C. figshare. Figure.2021. 10.6084/m9.figshare.14093289.v1 [DOI] [Google Scholar]
- 70. Rosanto YB: Figure 4. figshare. Figure.2021. 10.6084/m9.figshare.14093357.v1 [DOI] [Google Scholar]
- 71. Rosanto YB: Figure 6 Day 2 24%. figshare. Figure.2021. 10.6084/m9.figshare.14093419.v1 [DOI] [Google Scholar]
- 72. Rosanto YB: Figure 6 Day 2 48%. figshare. Figure.2021. 10.6084/m9.figshare.14093731.v1 [DOI] [Google Scholar]
- 73. Rosanto YB: Figure 6 Day 2 96%. figshare. Figure.2021. 10.6084/m9.figshare.14093829.v1 [DOI] [Google Scholar]
- 74. Rosanto YB: Figure 6 Day 2 Control. figshare. Figure.2021. 10.6084/m9.figshare.14093891.v1 [DOI] [Google Scholar]
- 75. Rosanto YB: Figure 6 Day 4 24%. figshare. Figure.2021. 10.6084/m9.figshare.14093935.v1 [DOI] [Google Scholar]
- 76. Rosanto YB: Figure 6 Day 4 48%. figshare. Figure.2021. 10.6084/m9.figshare.14094001.v1 [DOI] [Google Scholar]
- 77. Rosanto YB: Figure 6 Day 4 96%. figshare. Figure.2021. 10.6084/m9.figshare.14094623.v1 [DOI] [Google Scholar]
- 78. Rosanto YB: Figure 6 Day 4 Control. figshare. Figure.2021. 10.6084/m9.figshare.14094687.v1 [DOI] [Google Scholar]
- 79. Rosanto YB: Figure 6 Day 7 24%. figshare. Figure.2021. 10.6084/m9.figshare.14094751.v1 [DOI] [Google Scholar]
- 80. Rosanto YB: Figure 6 Day 7 48%. figshare. Figure.2021. 10.6084/m9.figshare.14094803.v1 [DOI] [Google Scholar]
- 81. Rosanto YB: Figure 6 Day 7 96%. figshare. Figure.2021. 10.6084/m9.figshare.14094849.v1 [DOI] [Google Scholar]
- 82. Rosanto YB: Figure 6 Day 7 Control. figshare. Figure.2021. 10.6084/m9.figshare.14094919.v1 [DOI] [Google Scholar]