Abstract
The Setting International Standards in Analyzing Patient-Reported Outcomes and Quality of Life Endpoints Data (SISAQOL) initiative was established in 2016 to assess the quality and standardization of patient-reported outcomes (PRO) data analysis in randomized controlled trials (RCTs) on advanced breast cancer. The initiative identified deficiencies in PRO data reporting, including nonstandardized methods for handling missing data. This study evaluated the reporting of health-related quality of life (HRQOL) in Japanese cancer RCTs to provide insights into the state of PRO reporting in Japan. The study reviewed PubMed articles published from 2010 to 2018. Eligible studies included Japanese cancer RCTs with ≥50 adult patients (≥50% were Japanese) with solid tumors receiving anticancer treatments. The evaluation criteria included clarity of the HRQOL hypotheses, multiplicity testing, primary analysis methods, and reporting of clinically meaningful differences. Twenty-seven HRQOL trials were identified. Only 15% provided a clear HRQOL hypothesis, and 63% examined multiple HRQOL domains without adjusting for multiplicity. Model-based methods were the most common statistical methods for the primary HRQOL analysis. Only 22% of the trials explicitly reported clinically meaningful differences in HRQOL. Baseline assessments were reported in most trials, but only 26% reported comparisons between the treatment groups. HRQOL analysis was based on the intention-to-treat population in 19% of the trials, and 74% reported compliance at follow-up; however, 41% did not specify how missing values were handled. Although the rates of reporting clinical hypotheses and clinically meaningful differences were relatively low, the current state of HRQOL evaluation in the Japanese cancer RCT appears comparable to that of previous studies.
Keywords: health-related quality of life (hrqol), randomized trials, pro, hrqol, randomized controlled trial, statistical methods, health-related quality of life, patient reported outcomes
Introduction and background
In recent years, the importance of patient-reported outcomes (PRO) for health-related quality of life (HRQOL) has been increasing [1] in addition to conventional objective endpoints such as overall survival and response rate in the field of oncology. Although multiple guidelines have been proposed for reporting results [2-4], the use of HRQOL/PRO is not fully established owing to its ambiguity, various clinical hypotheses, and complex statistical methods. Between 2010 and 2020, only 8.3% and 30.2% of drugs approved by the FDA and the European Medicines Agency, respectively, in the oncology field were approved with PRO labeling, indicating that PRO has not yet been widely adopted in a form that can withstand evaluation by strict regulatory agencies such as the FDA [5].
In 2016, the Setting International Standards in Analyzing Patient-Reported Outcomes and Quality of Life Endpoints Data (SISAQOL) initiative was established to provide recommendations for standardizing the analysis of HRQOL and other PRO data in randomized controlled trials (RCT) regarding cancer [6]. In addition to the work of the FDA [7], the SISAQOL Consortium performed a systematic review to assess the variability, quality, and standards of PRO data analysis in RCTs on advanced breast cancer. The study findings showed a poor current situation in the reporting of PRO data and that the methods of analysis and handling of missing data have not been standardized [8].
Japanese researchers have also recognized the value of HRQOL/PRO as a critical endpoint in cancer-related clinical trials. In the 2021 revised guidelines for the clinical evaluation of anticancer drugs, the importance of PRO was explicitly stated for the first time [9], and the Japan Clinical Oncology Group (JCOG), one of the largest investigator-led cooperative groups in Japan, established a PRO/QOL research committee and published its policies [10]. However, no survey studies on HRQOL/PRO, such as those conducted by the SISAQOL, have been conducted for RCTs involving cancer in Japan, and it is unclear whether the current state of HRQOL/PRO in Japan is comparable to that in Europe and the United States.
This study aimed to evaluate reports of Japanese cancer RCTs that utilized PRO/QOL using the same criteria as the previous SISAQOL study to determine potential differences in the statistical analysis methods used between Japan and other countries and whether any specific issues are unique to Japanese trials.
Review
Methods
We used the methodology described in the Cochrane Handbook for Systematic Reviews of Interventions, and the results of this systematic review are reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [11]. We conducted a literature search of PubMed on April 9, 2019 using the following keywords: (quality of life[MeSH Terms] OR quality of life[Text Word]) AND cancer[Text Word] AND (Japan [All Fields]) AND (Randomized Controlled Trial) AND (neoplasm[MeSH Terms]) AND (Clinical Trial[ptyp] AND (“2010/01/01” [PDat]: “2018/03/30” [PDat]) AND Humans[Mesh]). Using this search strategy, we identified 125 potentially eligible articles written in English and reviewed the references of these publications for additional articles. We also performed a Web of Science search on April 25, 2019 and found one article.
The inclusion and exclusion criteria for the RCTs were similar to those reported by Pe et al. [8]. The inclusion criteria were the following: (1) RCT articles published between 2010 and 2018; (2) reporting of PRO findings; (3) a study population of adult patients (≥18 years of age) with solid tumor cancer receiving anticancer treatments (chemotherapy, targeted therapy, endocrine therapy, immunotherapy, surgery, radiotherapy, and endoscopy); (4) a sample size of at least 50 patients; and (5) Japanese persons comprising 50% of the enrolled patients (essentially, patients are enrolled for clinical trials from institutions within Japan.).
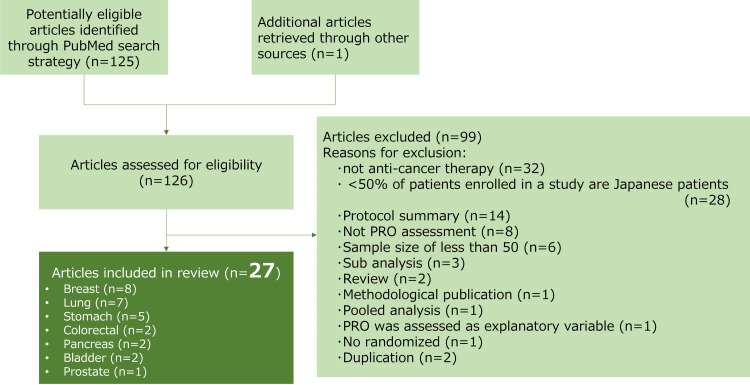
We excluded all RCTs that evaluated psychological, supportive, or supplementary interventions. Supplementary treatment was defined as an intervention other than anticancer therapies. We excluded purely methodological publications and review reports. We did not consider quality-adjusted life-year endpoints as PRO endpoints. Publications reporting interim analyses or analyses of patient subgroups were also excluded. Figure 1 shows a flowchart of the search strategy and the inclusion and exclusion criteria. JM and MT independently reviewed 126 eligible studies and assessed whether the reports met the inclusion and exclusion criteria. Any disagreements between them in the study assessments were discussed and resolved.
Figure 1. Study flowchart.
JM and GO independently extracted the information using predefined data abstraction forms. All data were checked for internal consistency, and disagreements were resolved by discussion. The following details were extracted: a general description of the article, research objectives, statistical analysis and clinical relevance, baseline assessment, and assessment of the amount and handling of missing data. The extracted information was summarized.
Results
Study Selection
Of the 126 eligible papers, 27 were selected for the systematic review [12-38]. General information and summary results of the 27 articles are shown in Table 1. Eight studies focused on breast cancer, seven on lung cancer, and five on stomach cancer; the other papers focused on colorectal, pancreatic, bladder, and prostate cancers. The details of the classification of the selected 27 trials are presented in Table 2.
Table 1. General information and summary of the 27 articles included in the study.
| Yes | No | Not reported or unclear | |
| Reporting of research objectives | |||
| Specific hypothesis | 4 | 10 | 13 |
| Statistical significance and clinical relevance | |||
| Multiple domains | 17 | 10 | 0 |
| If yes, was statistical correction used? | 0 | 3 | 14 |
| Repeated assessments | 24 | 3 | 0 |
| If yes, was a statistical technique used that allowed the inclusion of repeated assessment points, or was a statistical correction used? | 16 | 7 | 1 |
| Reporting of descriptive data | 17 | 10 | 0 |
| Primary statistical method | |||
| Linear mixed models | 7 | NA | NA |
| Wilcoxon rank-sum test or t-test | 6 | NA | NA |
| ANOVA or linear regression | 2 | NA | NA |
| Time to event | 2 | NA | NA |
| Repeated measures ANOVA | 2 | NA | NA |
| Proportion of patients or responder analysis | 1 | NA | NA |
| Others | 3 | NA | NA |
| Unreported or unclear | 4 | NA | NA |
| Reporting of clinical relevance | 6 | 21 | 0 |
| Change of X points from baseline | 4 | NA | NA |
| X points difference between arms | 1 | NA | NA |
| Change of X points from baseline and X points difference between arms | 1 | NA | NA |
| Baseline assessment | |||
| Assessed baseline | 24 | 2 | 1 |
| Compared baseline scores between treatments | 7 | 17 | 0 |
| Included baseline as a covariate | 12 | 11 | 1 |
| Assessing the prevalence and handling of missing data | |||
| Intention-to-treat population | 5 | 18 | 4 |
| Baseline compliance rates for each treatment arm | 16 | 11 | NA |
| Follow-up compliance rates for each treatment arm | 20 | 7 | NA |
| Strategy to handle missing data | 16 | 11 | NA |
Table 2. Details of the classification of the selected 27 trials.
| Author | Year | Cancer type | Disease status | Specific hypothesis | Multiple domains (more than one scale or domain included in the analysis) | QOL questionnaire | If yes, was statistical correction used (multiple domains were independently tested)? | Repeated assessments (more than one follow-up assessment included in the analysis) | If yes, was a statistical technique used that allowed the inclusion of repeated assessment points, or was a statistical correction used (if repeated assessments were independently tested)? | Reporting of descriptive data | Primary statistical technique | Not reported or unclear | (Generalized) linear mixed models, including pattern mixture models | Wilcoxon rank-sums test or between-subjects t-test | ANOVA or linear regression | Time to event | Repeated measures ANOVA | Proportion of patients or responder analysis | Others | Reporting of clinical relevance | Change of X points (from baseline) | X points difference (between arms) | Change of X points from baseline and X points differences (between arms) | Assessed baseline | Compared baseline scores between treatment arms | Included baseline as a covariate | Intention-to-treat population | Baseline compliance rates for each treatment arm | Follow-up compliance rates for each treatment arm | Strategy to handle missing data |
| Hagiwara, Y | 2018 | Pancreas | Curative | Yes | No | EQ-5D-3L | Yes | Yes | Yes | Yes | No | Yes | No | No | No | No | No | No | Yes | Yes | No | No | Yes | Yes | Yes | No | Yes | Yes | Yes | |
| Kawahara, T | 2018 | Breast | Unresectable | No | Yes | EORTC QLQ-C30, Patient Neurotoxicity Questionnaire | No | Yes | Yes | Yes | Yes | No | No | No | No | Yes | No | No | No | Yes | Yes | No | No | Yes | No | No | No | No | Yes | Yes |
| Ohashi et, Y | 2018 | Breast | Curative | No | Yes | QOL-ACD, QOL-ACD-B, FACT-ES | No | Yes | Yes | Yes | Yes | No | Yes | No | No | No | No | No | No | No | No | No | No | Yes | Yes | Yes | Yes | No | No | Yes |
| Yamamoto, D | 2017 | Breast | Unresectable | No | Yes | EORTC-QLQ-C30 | No | Yes | No | No | No | Yes | No | No | No | No | No | No | No | No | No | No | No | Yes | No | No | No | Yes | Yes | No |
| Shiroiwa, T | 2017 | Breast | Unresectable | No | No | EQ-5D | Yes | Yes | Yes | Yes | No | Yes | No | No | No | No | No | No | Yes | Yes | No | No | Yes | Yes | Yes | No | Yes | Yes | Yes | |
| Yoshino, S | 2016 | Gastric | Unresectable | No | No | FACT-Biological Response Modifier | Yes | No | No | No | Yes | No | No | No | No | No | No | No | No | No | No | No | Yes | No | No | No | Yes | No | No | |
| Yamazaki, K | 2016 | Colorectal | Unresectable | No | Yes | FACT-C, FACT/GOG-Ntx | Not reported or unclear | Yes | Yes | No | Yes | No | No | No | No | No | Yes | No | No | No | No | No | No | Yes | No | No | Not reported or unclear | No | No | Yes |
| Nakamura, M | 2016 | Gastric | Curative | Yes | Yes | FACT-Ga, FACT-G | Not reported or unclear | Yes | No | Yes | No | Yes | No | No | No | No | No | No | No | Yes | No | No | Yes | Yes | No | No | Yes | Yes | Yes | Yes |
| Yokomizo, A | 2016 | Bladder | Curative | Not reported or unclear | Yes | EORTC QLQ-C30 | Not reported or unclear | No | Yes | Yes | No | No | Yes | No | No | No | No | No | No | No | No | No | Yes | No | No | No | Yes | Yes | No | |
| Ito, Y | 2016 | Gastric | Curative | Yes | Yes | EORTC-QLQ-C30, STO22 | Not reported or unclear | Yes | No | No | Yes | No | No | Yes | No | No | No | No | No | Yes | No | Yes | No | Yes | No | No | No | Yes | Yes | Yes |
| Kubota, K | 2015 | NSCLC | Unresectable | Not reported or unclear | Yes | EORTC-QLQ-C30, QLQ-LC13 | Not reported or unclear | Yes | Yes | No | Yes | No | No | No | No | No | Yes | No | No | No | No | No | No | Yes | No | Yes | Not reported or unclear | No | Yes | No |
| Masuda, K | 2015 | Rectal | Curative | Not reported or unclear | No | Fecal Incontinence Quality of Life | Yes | No | Yes | Yes | No | No | Yes | No | No | No | No | No | No | No | No | No | Not reported or unclear | No | No | No | No | |||
| Abe, T | 2015 | NSCLC | Unresectable | Not reported or unclear | No | FACT-L | Yes | Yes | No | Yes | No | No | No | No | No | No | Yes | No | No | No | No | No | Yes | No | Yes | No | Yes | Yes | Yes | |
| Matsuyama, H | 2015 | Prostate | Curative | No | Yes | Expanded Prostate Cancer Index Composite | Not reported or unclear | Yes | Not reported or unclear | No | No | Yes | No | No | No | No | No | No | No | No | No | No | No | Yes | Yes | No | No | No | No | No |
| Tsukada, H | 2014 | NSCLC | Unresectable | Not reported or unclear | No | FACT-L | No | Yes | Yes | No | No | No | Yes | No | No | No | No | No | No | No | No | Yes | No | Yes | Yes | Yes | Yes | No | ||
| Sekine, I | 2013 | SCLC | Unresectable | Not reported or unclear | Yes | FACT-L, EQ-5D | Not reported or unclear | Yes | Yes | No | Yes | No | No | No | Yes | No | No | No | No | No | No | No | No | Yes | No | Yes | No | Yes | Yes | No |
| Ueno, H | 2013 | Pancreas | Unresectable | No | No | EQ-5D | Yes | Yes | No | Yes | No | No | No | No | No | No | No | Yes | No | No | No | No | Yes | No | No | Not reported or unclear | No | No | Yes | |
| Shimozuma, K | 2012 | Breast | Curative | Yes | Yes | Patient Neurotoxicity Questionnaire, FACT-G, FACT-Neuro-toxicity | Not reported or unclear | Yes | Yes | Yes | Yes | No | No | Yes | No | No | No | No | No | No | No | No | No | Yes | No | Yes | No | Yes | Yes | Yes |
| Oizumi, S | 2012 | NSCLC | Unresectable | Not reported or unclear | No | Care Notebook | Yes | Yes | Yes | Yes | No | No | No | No | Yes | No | No | No | Yes | Yes | No | No | Yes | Yes | No | No | Yes | Yes | No | |
| Takei, H | 2012 | Breast | Curative | Not reported or unclear | Yes | FACT-B, FACT-ES, CES-D | Not reported or unclear | Yes | Yes | No | Yes | No | Yes | No | No | No | No | No | No | No | No | No | No | Yes | No | Yes | No | Yes | Yes | Yes |
| Takiguchi, S | 2012 | Gastric | Curative | Not reported or unclear | Yes | EORTC-QLQ-C30, DAUGS 20 | Not reported or unclear | No | Yes | Yes | No | No | Yes | No | No | No | No | No | No | No | No | No | No | No | No | Yes | Yes | |||
| Ishigami, S | 2011 | Gastric | Curative | No | No | Original | Yes | No | Yes | Yes | No | No | Yes | No | No | No | No | No | No | No | No | No | No | No | No | Yes | No | |||
| Kawahara, M | 2011 | NSCLC | Unresectable | Not reported or unclear | Yes | FACT-L, FACT-Taxane, FACIT-Sp | Not reported or unclear | Yes | Yes | Yes | Yes | No | Yes | No | No | No | No | No | No | No | No | No | No | Yes | Yes | Yes | No | Yes | Yes | Yes |
| Shiroiwa, T | 2011 | Breast | Curative | No | Yes | EQ-5D, FACT-G, FACT-B, FACT-Taxane | Not reported or unclear | Yes | Yes | Yes | Yes | No | Yes | No | No | No | No | No | No | No | No | No | No | Yes | No | Yes | Yes | No | Yes | Yes |
| Ohsumi, S | 2011 | Breast | Curative | Not reported or unclear | Yes | FACT-B, FACT-ES, CES-D | Not reported or unclear | Yes | Yes | Yes | Yes | No | No | No | No | No | No | No | Yes | No | No | No | No | Yes | Yes | Yes | No | Yes | Yes | Yes |
| Koga, H | 2010 | Bladder | Curative | Not reported or unclear | Yes | EORTC-QLQ-C30 | Not reported or unclear | Yes | No | Yes | Yes | No | No | No | No | No | No | No | Yes | No | No | No | No | Yes | No | No | Not reported or unclear | No | No | No |
| Takeda, K | 2010 | NSCLC | Unresectable | Not reported or unclear | No | FACT-L | Yes | Yes | Yes | Yes | No | Yes | No | No | No | No | No | No | No | No | No | No | Yes | No | Not reported or unclear | Yes | Yes | Yes | Yes |
HRQOL Measurement
Several HRQOL questionnaires were used: the EORTC-QLQ-C30 [39] was most commonly used (seven studies, 26%), followed by the EQ-5D (five studies, 19%); EORTC-developed disease-specific questionnaires for gastric cancer, EORTC-QLQ-STO 22 [40,41], and the EORTC QLQ-LC13 [42] for lung cancer were used in one study each. The Functional Assessment of Cancer Therapy (FACT) questionnaire was widely used, including FACT-L for lung cancer [43], FACT-B for breast cancer [44], FACT-General for general cancer [45], and FACT-ES for endocrine therapy [46]. The Quality of Life Questionnaire for Patients treated with Anticancer Drugs (QOL-ACD) [47] and the QOL-ACD-B (breast) questionnaires developed in Japan were used in one study each. The Patient Neurotoxicity Questionnaire, a questionnaire for specific adverse events, was also used in two studies.
Reporting of Research Objectives
First, we examined whether a predefined hypothesis regarding HRQOL was stated. Only four studies (15%) were judged to have a predefined statement that noted a specific PRO domain and time point or time frame, 10 studies (37%) had statements that were considered unclear (for example, “to explore the relationships between the QOL”), and 13 studies (48%) had no statement.
Multiplicity Adjustment
To investigate the issue of multiplicity in testing, we assessed whether multiple domains of HRQOL were examined. Our findings revealed that of the 27 trials, 17 (63%) examined more than one HRQOL domain. However, none of these trials made clear adjustments for multiplicity in their analysis of multiple domains, and 14 provided an unclear description of such adjustments. In addition, HRQOL was assessed at multiple time points in 24 trials (89%), of which 16 (59%) described the multiplicity adjustment. Seven trials (26%) did not describe these adjustments.
Statistical Analysis and Clinical Relevance
Descriptive statistics for HRQOL were reported in 17 trials (63%). Various statistical methods were used for the primary HRQOL analysis. The most commonly used method was the linear mixed model, which was used in seven trials (26%). Model-based methods accounted for 11 trials (41%) in all analyses when ANOVA and repeated-measures ANOVA were included. Wilcoxon rank-sum tests and t-tests were used in six trials (22%) to compare simple means or medians. Two trials used time-to-event methods, whereas one trial used tests of proportions, such as the chi-square or Fisher’s exact test. Four trials did not report the primary analysis method.
Only six trials (22%) explicitly specified and reported clinically meaningful differences in HRQOL. Among these, four defined a clinically meaningful difference as a change from baseline, one trial defined it as a difference between treatment groups, and one trial used a combination of both definitions.
Of the 27 trials, baseline assessments were reported in 24 (89%), one (4%) was unclear, and two (7%) did not report baseline assessments. Among the 24 trials with baseline assessments, only seven reported comparisons between the treatment groups. However, 12 trials used baseline values as covariates in their statistical analyses.
The HRQOL analysis was based on the intention-to-treat population in five trials (19%) and the modified intention-to-treat population, which is defined as a population, not including all randomized patients. However, some patients were excluded from the entire population in 18 trials (67%), while four trials had unclear descriptions of the analysis population. Compliance rates were evaluated to determine the amount of missing data. Baseline compliance for the HRQOL assessment was reported in 16 trials (59%), and compliance at follow-up was reported in 20 trials (74%). Additionally, 11 trials (41%) did not report how the missing values were handled.
Discussion
This systematic review of 27 randomized clinical trials for anticancer treatment evaluated HRQOL in Japanese cancer patients, including the clarity of the HRQOL hypothesis, correction for multiplicity testing, primary analysis methods used, reporting of clinically meaningful differences, and reporting of missing data. Only 15% of the trials had a clear statement about the HRQOL-predefined hypothesis, and 63% examined more than one HRQOL domain without explicit adjustments for multiplicity. Various statistical methods were used for the primary HRQOL analysis, with model-based methods being the most common. Only 22% of the trials explicitly reported clinically meaningful differences in HRQOL, and baseline assessments were reported in most trials; however, only 26% reported comparisons between treatment groups. The HRQOL analysis was based on the intention-to-treat population in 19% of the trials, and compliance at follow-up was reported in 74% of the trials; however, 41% did not report how missing values were handled.
Our study aimed to compare the proportion of essential contents and the differences in statistical methods used in Japanese cancer RCT PRO/QOL papers with those in a study on unresectable/metastatic breast cancer evaluated by SISAQOL and to examine whether there are any unique issues specific to Japan. Regarding the presence of a specific hypothesis, only 12% of the articles in the SISAQOL study reported a specific hypothesis [8], with similarly low values in our study. This may be because HRQOL endpoints have many variations in hypotheses compared to the usual endpoints of cancer, such as overall survival or response rate, and HRQOL itself is often positioned as an exploratory secondary endpoint in clinical trials; therefore, many research plans may not have a clear hypothesis in advance [48]. Although guidelines such as ISOQOL [2] and CONSORT-PRO [3] require a clear description of hypotheses regarding HRQOL, it is impossible to describe them in a paper if they are not outlined in the research plan. Recently, an extended version of the SPIRIT guidelines, which specify the items to be included in research plans, was proposed for PRO research to provide evidence-based recommendations for the minimum content of a clinical trial protocol [49]. To improve the low rate of hypothesis description regarding HRQOL, these guidelines should be widely disseminated for clear hypotheses regarding HRQOL to be established from the research planning stage. In Japan, the JCOG has established and published policies regarding PRO and QOL research [10]. Such endeavors are crucial and are expected to remain significant in the future.
Various statistical methods were used for the primary statistical analysis, including model-based methods, the Wilcoxon rank-sum test, and the t-test, similar to those used in the previous SISAQOL study. In the SISAQOL study, model-based methods were used in 44% of studies, whereas the Wilcoxon rank-sum test and t-test were used in 17%. Appropriate statistical methods should be selected based on the clinical hypotheses and outcome types. If the method is chosen accordingly, it is considered sufficient. The SISAQOL-IMI consortium recommends statistical methods based on the outcome type and clinical hypotheses, which are useful for discussions between biostatisticians and clinicians [50]. However, no consensus has been reached for some clinical hypotheses, such as comparing QOL scores over time. A recent publication has reported details of recommendations regarding the views and opinions of PRO objectives and endpoints for RCTs from 41 stakeholders [51].
In the previous SISAQOL study, 42% of patients reported minimally important differences (MIDs), which is a measure of clinical relevance, whereas the reported rate was 22% in the present study. This may be related to the lower percentage of stated clinical hypotheses regarding HRQOL because, to clearly define a clinical hypothesis, its MIDs must be determined. Simultaneously, in cancer clinical trials, the sample size required to detect the primary endpoint, overall survival, or progression-free survival is usually larger than that required to detect an MID in HRQOL. Therefore, whether an MID was achieved is more important than whether a statistically significant difference was achieved [52]. MID has other challenges, as it can vary depending on the cancer type and domain. However, methods for defining MID are being established, and such efforts may contribute to the adoption and widespread use of MID, along with the prior establishment of its definition [53].
The reported rate of adjustment for multiplicity in statistical hypothesis testing was approximately 60%, similar to that of the SISAQOL study but not sufficiently high. At the very least, a prespecified adjustment for multiplicity is required for a drug to be accepted by regulatory agencies, such as the FDA, and listed on the drug label. In fact, in a review of FDA-approved drugs in clinical trials for breast cancer, the FDA reviewer’s comments suggested that, in addition to the lack of MIDs, inadequate analytical methods due to uncontrolled multiple comparisons may be the reason for the lack of drug product labeling [54].
The number of articles describing the handling of missing data and compliance rates tended to be higher than in the previous SISAQOL study. This finding may be partly because several of the Japanese clinical trials were sub-papers of RCTs limited to HRQOL/PRO endpoints, and the first author was a biostatistician. It is difficult to determine whether this is an appropriate procedure in cancer clinical trials, where there is much missing data and HRQOL often includes deaths. The ICH E9(R1) guidelines provide an estimand framework (treatment, population, variables, population-level summary, and handling of intercurrent events) for defining the treatment effect under investigation in a clinical trial [55]. The concept of estimands for HRQOL in cancer clinical trials has been proposed [56,57], and it is hoped that this and future studies will help to build a consensus.
Limitations
This study had some limitations. The findings were restricted to RCTs published between 2010 and 2018 in English and cannot be generalized to other published years and languages. Although the previous review by SISAQOL limited to advanced breast cancer [8], this study was not restricted to breast cancer to increase the number of publications. One limitation is the inability to compare with studies conducted under frameworks other than the SISAQOL project [58]. Although there were no notable differences between breast cancer and other cancer types, it is important to note that if there are differences based on the cancer type, descriptions regarding comparisons may not always be accurate. Furthermore, the description of HRQOL, a secondary endpoint, may have been omitted in papers with word count limits. In RCTs where HRQOL/PRO is evaluated as secondary endpoints, there is a tendency for HRQOL/PRO to be reported as separate papers, referred to as secondary papers. While such studies were limited in this review, it is expected that independent papers on HRQOL/PRO will contain a wealth of information.
Conclusions
Although the reporting rates of clinical hypotheses and MIDs in the previous reports tended to be similar to those in our study, the reporting rates for HRQOL/PRO compliance and the handling of missing values tended to be higher in previous reports. Overall, the statistical methods used for HRQOL/PRO evaluation in the Japanese cancer RCT were similar to those used in the previous SISAQOL study, indicating that the reporting methods of Japanese studies are not inferior to those of Western countries. The standardization of statistical and reporting methods is expected to progress domestically and internationally, following the guidelines presented by SISAQOL and regulatory agencies.
Acknowledgments
We would like to express our deep appreciation to Dr. Madeline Pe for her valuable comments on our research. Her expertise and insights greatly contributed to the improvement of this paper.
The authors have declared that no competing interests exist.
Funding Statement
The research was supported by the Japan Agency for Medical Research and Development (AMED) under Grant Number JP19ck0106498.
Author Contributions
Concept and design: Junki Mizusawa, Mitsumi Terada
Acquisition, analysis, or interpretation of data: Junki Mizusawa, Gakuto Ogawa, Mitsumi Terada, Hiroto Ishiki, Yuichiro Kikawa, Naomi Kiyota
Drafting of the manuscript: Junki Mizusawa, Gakuto Ogawa
Critical review of the manuscript for important intellectual content: Gakuto Ogawa, Mitsumi Terada, Hiroto Ishiki, Yuichiro Kikawa, Naomi Kiyota
References
- 1.Incorporating the patient experience into regulatory decision making in the USA, Europe, and Canada. Kluetz PG, O'Connor DJ, Soltys K. Lancet Oncol. 2018;19:267–274. doi: 10.1016/S1470-2045(18)30097-4. [DOI] [PubMed] [Google Scholar]
- 2.Patient-reported outcomes in randomized clinical trials: development of ISOQOL reporting standards. Brundage M, Blazeby J, Revicki D, et al. Qual Life Res. 2013;22:1161–1175. doi: 10.1007/s11136-012-0252-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reporting of patient-reported outcomes in randomized trials: the CONSORT PRO extension. Calvert M, Blazeby J, Altman DG, Revicki DA, Moher D, Brundage MD. JAMA. 2013;309:814–822. doi: 10.1001/jama.2013.879. [DOI] [PubMed] [Google Scholar]
- 4.Patient-reported outcomes labeling for oncology drugs: multidisciplinary perspectives on current status and future directions. Cella D, Chen CI, Quek RG, et al. Front Pharmacol. 2022;13:1031992. doi: 10.3389/fphar.2022.1031992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Has the quality of health-related quality of life reporting in cancer clinical trials improved over time? Towards bridging the gap with clinical decision making. Efficace F, Osoba D, Gotay C, Sprangers M, Coens C, Bottomley A. Ann Oncol. 2007;18:775–781. doi: 10.1093/annonc/mdl494. [DOI] [PubMed] [Google Scholar]
- 6.Analysing data from patient-reported outcome and quality of life endpoints for cancer clinical trials: a start in setting international standards. Bottomley A, Pe M, Sloan J, et al. Lancet Oncol. 2016;17:510–514. doi: 10.1016/S1470-2045(16)30510-1. [DOI] [PubMed] [Google Scholar]
- 7.US Food and Drug Administration review of statistical analysis of patient-reported outcomes in lung cancer clinical trials approved between January, 2008, and December, 2017. Fiero MH, Roydhouse JK, Vallejo J, King-Kallimanis BL, Kluetz PG, Sridhara R. Lancet Oncol. 2019;20:582. doi: 10.1016/S1470-2045(19)30335-3. [DOI] [PubMed] [Google Scholar]
- 8.Statistical analysis of patient-reported outcome data in randomised controlled trials of locally advanced and metastatic breast cancer: a systematic review. Pe M, Dorme L, Coens C, et al. Lancet Oncol. 2018;19:459–469. doi: 10.1016/S1470-2045(18)30418-2. [DOI] [PubMed] [Google Scholar]
- 9.Guidelines for clinical evaluation of anti-cancer drugs. Minami H, Kiyota N, Kimbara S, et al. Cancer Sci. 2021;112:2563–2577. doi: 10.1111/cas.14967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patient-reported outcome and quality of life research policy: Japan Clinical Oncology Group (JCOG) policy. Ishiki H, Kikawa Y, Terada M, et al. Jpn J Clin Oncol. 2023;53:195–202. doi: 10.1093/jjco/hyad007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Page MJ, McKenzie JE, Bossuyt PM, et al. BMJ. 2021;372:0. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Health-related quality of life of adjuvant chemotherapy with S-1 versus gemcitabine for resected pancreatic cancer: results from a randomised phase III trial (JASPAC 01) Hagiwara Y, Ohashi Y, Uesaka K, et al. Eur J Cancer. 2018;93:79–88. doi: 10.1016/j.ejca.2018.01.081. [DOI] [PubMed] [Google Scholar]
- 13.Patient-reported outcome results from the open-label randomized phase III SELECT BC trial evaluating first-line S-1 therapy for metastatic breast cancer. Kawahara T, Shimozuma K, Shiroiwa T, et al. Oncology. 2018;94:107–115. doi: 10.1159/000484142. [DOI] [PubMed] [Google Scholar]
- 14.Efficacy and safety of low-dose capecitabine plus docetaxel versus single-agent docetaxel in patients with anthracycline-pretreated HER2-negative metastatic breast cancer: results from the randomized phase III JO21095 trial. Yamamoto D, Sato N, Rai Y, et al. Breast Cancer Res Treat. 2017;161:473–482. doi: 10.1007/s10549-016-4075-6. [DOI] [PubMed] [Google Scholar]
- 15.Randomised phase III study of S-1 alone versus S-1 plus lentinan for unresectable or recurrent gastric cancer (JFMC36-0701) Yoshino S, Nishikawa K, Morita S, et al. Eur J Cancer. 2016;65:164–171. doi: 10.1016/j.ejca.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 16.Randomized clinical trial of defaecatory function after anterior resection for rectal cancer with high versus low ligation of the inferior mesenteric artery. Matsuda K, Hotta T, Takifuji K, et al. Br J Surg. 2015;102:501–508. doi: 10.1002/bjs.9739. [DOI] [PubMed] [Google Scholar]
- 17.Randomized phase III trial comparing weekly docetaxel plus cisplatin versus docetaxel monotherapy every 3 weeks in elderly patients with advanced non-small-cell lung cancer: the intergroup trial JCOG0803/WJOG4307L. Abe T, Takeda K, Ohe Y, et al. J Clin Oncol. 2015;33:575–581. doi: 10.1200/JCO.2014.55.8627. [DOI] [PubMed] [Google Scholar]
- 18.Running suture versus interrupted suture for vesicourethral anastomosis in retropubic radical prostatectomy: a randomized study. Matsuyama H, Matsumoto H, Nagao K, Harada N, Hara T, Sakano S. Int J Urol. 2015;22:271–277. doi: 10.1111/iju.12667. [DOI] [PubMed] [Google Scholar]
- 19.Randomized controlled trial comparing docetaxel-cisplatin combination with weekly docetaxel alone in elderly patients with advanced non-small-cell lung cancer: Japan Clinical Oncology Group (JCOG) 0207†. Tsukada H, Yokoyama A, Goto K, et al. Jpn J Clin Oncol. 2015;45:88–95. doi: 10.1093/jjco/hyu176. [DOI] [PubMed] [Google Scholar]
- 20.A randomized phase III study of single-agent amrubicin vs. carboplatin/etoposide in elderly patients with extensive-disease small-cell lung cancer. Sekine I, Okamoto H, Horai T, et al. Clin Lung Cancer. 2014;15:96–102. doi: 10.1016/j.cllc.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 21.Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. Ueno H, Ioka T, Ikeda M, et al. J Clin Oncol. 2013;31:1640–1648. doi: 10.1200/JCO.2012.43.3680. [DOI] [PubMed] [Google Scholar]
- 22.Taxane-induced peripheral neuropathy and health-related quality of life in postoperative breast cancer patients undergoing adjuvant chemotherapy: N-SAS BC 02, a randomized clinical trial. Shimozuma K, Ohashi Y, Takeuchi A, et al. Support Care Cancer. 2012;20:3355–3364. doi: 10.1007/s00520-012-1492-x. [DOI] [PubMed] [Google Scholar]
- 23.Quality of life with gefitinib in patients with EGFR-mutated non-small cell lung cancer: quality of life analysis of North East Japan Study Group 002 Trial. Oizumi S, Kobayashi K, Inoue A, et al. Oncologist. 2012;17:863–870. doi: 10.1634/theoncologist.2011-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Health-related quality of life, psychological distress, and adverse events in postmenopausal women with breast cancer who receive tamoxifen, exemestane, or anastrozole as adjuvant endocrine therapy: National Surgical Adjuvant Study of Breast Cancer 04 (N-SAS BC 04) Takei H, Ohsumi S, Shimozuma K, Takehara M, Suemasu K, Ohashi Y, Hozumi Y. Breast Cancer Res Treat. 2012;133:227–236. doi: 10.1007/s10549-011-1943-y. [DOI] [PubMed] [Google Scholar]
- 25.A comparison of postoperative quality of life and dysfunction after Billroth I and Roux-en-Y reconstruction following distal gastrectomy for gastric cancer: results from a multi-institutional RCT. Takiguchi S, Yamamoto K, Hirao M, et al. Gastric Cancer. 2012;15:198–205. doi: 10.1007/s10120-011-0098-1. [DOI] [PubMed] [Google Scholar]
- 26.Postoperative long-term evaluation of interposition reconstruction compared with Roux-en-Y after total gastrectomy in gastric cancer: prospective randomized controlled trial. Ishigami S, Natsugoe S, Hokita S, et al. Am J Surg. 2011;202:247–253. doi: 10.1016/j.amjsurg.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 27.Quality-of-life evaluation for advanced non-small-cell lung cancer: a comparison between vinorelbine plus gemcitabine followed by docetaxel versus paclitaxel plus carboplatin regimens in a randomized trial: Japan Multinational Trial Organization LC00-03 (BRI LC03-01) Kawahara M, Tada H, Tokoro A, et al. BMC Cancer. 2011;11:356. doi: 10.1186/1471-2407-11-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Comparison of EQ-5D scores among anthracycline-containing regimens followed by taxane and taxane-only regimens for node-positive breast cancer patients after surgery: the N-SAS BC 02 trial. Shiroiwa T, Fukuda T, Shimozuma K, Kuranami M, Suemasu K, Ohashi Y, Watanabe T. Value Health. 2011;14:746–751. doi: 10.1016/j.jval.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Health-related quality of life and psychological distress of breast cancer patients after surgery during a phase III randomized trial comparing continuation of tamoxifen with switching to anastrozole after adjuvant tamoxifen for 1-4 years: N-SAS BC 03. Ohsumi S, Shimozuma K, Ohashi Y, et al. Breast Cancer Res Treat. 2011;127:143–152. doi: 10.1007/s10549-011-1400-y. [DOI] [PubMed] [Google Scholar]
- 30.Maintenance intravesical bacillus Calmette-Guérin instillation for Ta, T1 cancer and carcinoma in situ of the bladder: randomized controlled trial by the BCG Tokyo Strain Study Group. Koga H, Ozono S, Tsushima T, et al. Int J Urol. 2010;17:759–766. doi: 10.1111/j.1442-2042.2010.02584.x. [DOI] [PubMed] [Google Scholar]
- 31.Randomized phase III trial of platinum-doublet chemotherapy followed by gefitinib compared with continued platinum-doublet chemotherapy in Japanese patients with advanced non-small-cell lung cancer: results of a west Japan thoracic oncology group trial (WJTOG0203) Takeda K, Hida T, Sato T, et al. J Clin Oncol. 2010;28:753–760. doi: 10.1200/JCO.2009.23.3445. [DOI] [PubMed] [Google Scholar]
- 32.Comparison of quality of life between 2-year and 3-or-more-year administration of leuprorelin acetate every-3-months depot in combination with tamoxifen as adjuvant endocrine treatment in premenopausal patients with endocrine-responsive breast cancer: a randomized controlled trial. Ohashi Y, Shiba E, Yamashita H, et al. Support Care Cancer. 2018;26:933–945. doi: 10.1007/s00520-017-3914-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Long-term health status as measured by EQ-5D among patients with metastatic breast cancer: comparison of first-line oral S-1 and taxane therapies in the randomized phase III SELECT BC trial. Shiroiwa T, Fukuda T, Shimozuma K, et al. Qual Life Res. 2017;26:445–453. doi: 10.1007/s11136-016-1388-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Randomized phase III study of bevacizumab plus FOLFIRI and bevacizumab plus mFOLFOX6 as first-line treatment for patients with metastatic colorectal cancer (WJOG4407G) Yamazaki K, Nagase M, Tamagawa H, et al. Ann Oncol. 2016;27:1539–1546. doi: 10.1093/annonc/mdw206. [DOI] [PubMed] [Google Scholar]
- 35.Randomized clinical trial comparing long-term quality of life for Billroth I versus Roux-en-Y reconstruction after distal gastrectomy for gastric cancer. Nakamura M, Nakamori M, Ojima T, et al. Br J Surg. 2016;103:337–347. doi: 10.1002/bjs.10060. [DOI] [PubMed] [Google Scholar]
- 36.Randomized controlled study of the efficacy, safety and quality of life with low dose bacillus Calmette-Guérin instillation therapy for nonmuscle invasive bladder cancer. Yokomizo A, Kanimoto Y, Okamura T, et al. J Urol. 2016;195:41–46. doi: 10.1016/j.juro.2015.08.075. [DOI] [PubMed] [Google Scholar]
- 37.Quality of life and nutritional consequences after aboral pouch reconstruction following total gastrectomy for gastric cancer: randomized controlled trial CCG1101. Ito Y, Yoshikawa T, Fujiwara M, et al. Gastric Cancer. 2016;19:977–985. doi: 10.1007/s10120-015-0529-5. [DOI] [PubMed] [Google Scholar]
- 38.A randomized phase III trial of oral S-1 plus cisplatin versus docetaxel plus cisplatin in Japanese patients with advanced non-small-cell lung cancer: TCOG0701 CATS trial. Kubota K, Sakai H, Katakami N, et al. Ann Oncol. 2015;26:1401–1408. doi: 10.1093/annonc/mdv190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. Aaronson NK, Ahmedzai S, Bergman B, et al. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 40.Clinical and psychometric validation of a questionnaire module, the EORTC QLQ-STO 22, to assess quality of life in patients with gastric cancer. Blazeby JM, Conroy T, Bottomley A, et al. Eur J Cancer. 2004;40:2260–2268. doi: 10.1016/j.ejca.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 41.Development of an EORTC disease-specific quality of life module for use in patients with gastric cancer. Vickery CW, Blazeby JM, Conroy T, et al. Eur J Cancer. 2001;37:966–971. doi: 10.1016/s0959-8049(00)00417-2. [DOI] [PubMed] [Google Scholar]
- 42.The EORTC QLQ-LC13: a modular supplement to the EORTC Core Quality of Life Questionnaire (QLQ-C30) for use in lung cancer clinical trials. Bergman B, Aaronson NK, Ahmedzai S, Kaasa S, Sullivan M. Eur J Cancer. 1994;30:635–642. doi: 10.1016/0959-8049(94)90535-5. [DOI] [PubMed] [Google Scholar]
- 43.Reliability and validity of the Functional Assessment of Cancer Therapy-Lung (FACT-L) quality of life instrument. Cella DF, Bonomi AE, Lloyd SR, Tulsky DS, Kaplan E, Bonomi P. Lung Cancer. 1995;12:199–220. doi: 10.1016/0169-5002(95)00450-f. [DOI] [PubMed] [Google Scholar]
- 44.Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument. Brady MJ, Cella DF, Mo F, et al. J Clin Oncol. 1997;15:974–986. doi: 10.1200/JCO.1997.15.3.974. [DOI] [PubMed] [Google Scholar]
- 45.The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. Cella DF, Tulsky DS, Gray G, et al. J Clin Oncol. 1993;11:570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 46.Assessment of quality of life in women undergoing hormonal therapy for breast cancer: validation of an endocrine symptom subscale for the FACT-B. Fallowfield LJ, Leaity SK, Howell A, Benson S, Cella D. Breast Cancer Res Treat. 1999;55:189–199. doi: 10.1023/a:1006263818115. [DOI] [PubMed] [Google Scholar]
- 47.Development of Quality of Life Questionnaire in Japan: quality of life assessment of cancer patients receiving chemotherapy. Kurihara M, Shimizu H, Tsuboi K, Kobayashi K, Murakami M, Eguchi K, Shimozuma K. Psychooncology. 1999;8:355–363. doi: 10.1002/(SICI)1099-1611(199907/08)8:4<355::AID-PON401>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 48.Systematic evaluation of the patient-reported outcome (PRO) content of clinical trial protocols. Kyte D, Duffy H, Fletcher B, et al. PLoS ONE. 2014;9:0. doi: 10.1371/journal.pone.0110229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guidelines for inclusion of patient-reported outcomes in clinical trial protocols: the SPIRIT-PRO Extension. Calvert M, Kyte D, Mercieca-Bebber R, et al. JAMA. 2018;319:483–494. doi: 10.1001/jama.2017.21903. [DOI] [PubMed] [Google Scholar]
- 50.International standards for the analysis of quality-of-life and patient-reported outcome endpoints in cancer randomised controlled trials: recommendations of the SISAQOL Consortium. Coens C, Pe M, Dueck AC, et al. Lancet Oncol. 2020;21:83–96. doi: 10.1016/S1470-2045(19)30790-9. [DOI] [PubMed] [Google Scholar]
- 51.Setting International Standards in Analyzing Patient-Reported Outcomes and Quality of Life Endpoints in Cancer Clinical Trials-Innovative Medicines Initiative (SISAQOL-IMI): stakeholder views, objectives, and procedures. Pe M, Alanya A, Falk RS, et al. Lancet Oncol. 2023;24:270–283. doi: 10.1016/S1470-2045(23)00157-2. [DOI] [PubMed] [Google Scholar]
- 52.Evidence-based guidelines for interpreting change scores for the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30. Cocks K, King MT, Velikova G, de Castro G Jr, Martyn St-James M, Fayers PM, Brown JM. Eur J Cancer. 2012;48:1713–1721. doi: 10.1016/j.ejca.2012.02.059. [DOI] [PubMed] [Google Scholar]
- 53.Minimally important differences for interpreting EORTC QLQ-C30 change scores over time: a synthesis across 21 clinical trials involving nine different cancer types. Musoro JZ, Coens C, Sprangers MA, et al. Eur J Cancer. 2023;188:171–182. doi: 10.1016/j.ejca.2023.04.027. [DOI] [PubMed] [Google Scholar]
- 54.Patient-reported outcomes in breast cancer FDA drug labels and review documents. Hong K, Majercak KR, Villalonga-Olives E, Perfetto EM. J Patient Rep Outcomes. 2021;5:36. doi: 10.1186/s41687-021-00308-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.ADDENDUM ON ESTIMANDS AND SENSITIVITY ANALYSIS IN CLINICAL. Addendum on estimands and sensitivity analysis in clinical trials to the guideline on statistical principles for clinical trials. [ Feb; 2024 ]. https://www.pmda.go.jp/files/000232860.pdf https://www.pmda.go.jp/files/000232860.pdf
- 56.Demystifying the estimand framework: a case study using patient-reported outcomes in oncology. Fiero MH, Pe M, Weinstock C, et al. Lancet Oncol. 2020;21:488–494. doi: 10.1016/S1470-2045(20)30319-3. [DOI] [PubMed] [Google Scholar]
- 57.Statistical methods and graphical displays of quality of life with survival outcomes in oncology clinical trials for supporting the estimand framework. Sakamaki K, Kawahara T. BMC Med Res Methodol. 2022;22:259. doi: 10.1186/s12874-022-01735-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.A systematic review of the quality of statistical methods employed for analysing quality of life data in cancer randomised controlled trials. Hamel JF, Saulnier P, Pe M, Zikos E, Musoro J, Coens C, Bottomley A. https://pubmed.ncbi.nlm.nih.gov/28738257/ Eur J Cancer. 2017;83:166–176. doi: 10.1016/j.ejca.2017.06.025. [DOI] [PubMed] [Google Scholar]