Abstract
Volumetric muscle loss (VML) overwhelms the native regenerative capabilities of skeletal muscle and has few effective treatments to regain lost muscle mass and function. Tissue engineered muscle constructs designed to promote neuromuscular regeneration are a promising therapeutic avenue. To date, there has been no engineered muscle construct for VML treatment that has incorporated a pharmacologic agent to promote neuromuscular regeneration. Here, we have modified electrospun fibrin microfiber bundles, which have demonstrated muscle regenerative potential, with the heparan sulfate proteoglycan, agrin, to stimulate innervation post-VML. Myoblasts cultured on microfiber bundles with either soluble or chemically tethered agrin demonstrated statistically significant increased clustering of acetylcholine receptors (AChRs) with soluble agrin displaying AChR clusters throughout the myofiber bundles, and tethered agrin displaying AChR clusters only at 10 μm from the substrate surface. Following implantation into murine VML defects for 4 weeks, constructs pre-treated with soluble or tethered agrin resulted in statistically significant increased neuromuscular junctions, regenerating myofibers, vascular infiltration, neural infiltration, and nuclear yes-associated protein (YAP) expression within the defect site compared to the control without agrin. The agrintethered microfiber bundles provided sustained agrin signaling within the regenerating site during the 4-week post-implantation periods and further augmented the density of regenerating myofibers in regenerated tissue with statistical significance compared to constructs with soluble agrin. These data demonstrate the neuromuscular regenerative potential of engineered muscle constructs pre-treated to induce AChR clustering with locally delivered agrin at the site of VML regeneration.
Keywords: Volumetric muscle loss, Neuromuscular junction, Electrospun hydrogels, Agrin, Tissue engineered skeletal muscle
1. Introduction
The repair mechanism for skeletal muscle is severely compromised following volumetric muscle loss (VML), thus leading to chronic functional deficits [1, 2]. Current treatments are often unsuccessful in restoring muscle function and are limited by donor site morbidity, lack of donor tissue, and the need for highly skilled surgical teams [3]. It was recently demonstrated that VML is accompanied by significant motoneuron axotomy and lost interaction between neurons and the injured skeletal muscle, a probable cause for the heightened levels of lost muscle function seen post-VML [4]. Tissue engineered grafts have great potential to create clinical treatment options for regeneration of muscle with VML, but previous approaches to regenerate the injured muscle remain limited in their ability to encourage neuromuscular junction (NMJ) regeneration within the healing tissue. Engineered muscle constructs that incorporate methods to encourage neural infiltration and the formation of functional NMJs post-VML provide promising avenues to restore interaction between the muscle and nerve.
Prior strategies to promote NMJ regeneration post-VML have included neurotization [5, 6], the use of rehabilitative exercise [7–10], and implantation of a pre-innervated construct [11]. These approaches have moderately increased neural infiltration, force recovery, and NMJ formation within and around the defect site. Despite incremental neuromuscular regeneration, the nerve and NMJ densities within the defects remain low and the newly formed NMJs exhibit abnormal morphologies. In addition, there has been little effort to quantify neuromuscular regeneration across the entire defect area, instead relying on subsets of the defect region at high magnifications that likely do not accurately represent variability in expression across the defect region. In native tissues, the mature NMJ contains densely clustered postsynaptic acetylcholine receptors (AChR) optimized for efficient signal transfer across the neuromuscular synapse and effective muscle contraction [12]. Agrin, a large heparan sulfate proteoglycan secreted by the nerve terminal, is vital for AChR cluster stabilization during embryonic development [13] and has been utilized extensively to induce AChR clustering in cultured myotubes [14–22]. Muscle constructs containing agrin physically mixed into a fibrin hydrogel and seeded with mouse C2C12 myoblasts were implanted subcutaneously in a non-VML defect near the peroneal nerve for 8 weeks and resulted in increased nerve infiltration, NMJ formation, and vascular infiltration [14], demonstrating the potential neuromuscular therapeutic benefits of scaffold-mediated agrin delivery in vivo. In this study, we resolved to test whether the delivery of soluble or chemically-tethered agrin could promote improved NMJ regeneration in the treatment of VML defects.
Although release of bioactive molecules from engineered constructs has been utilized in VML treatment to promote vascular infiltration [23–26] and myogenesis [25–27], there have been no previously published constructs used for VML treatment that have incorporated a bioactive factor to induce nerve infiltration and the formation of neuromuscular junctions. In addition, prior VML treatments with scaffold-mediated delivery of bioactive molecules have utilized physical entrapment or passive adsorption to the scaffold prior to implantation, which rely on diffusion of the bioactive agent to the surrounding tissues [28]. In contrast, scaffolds incorporating immobilized bioactive agents offer the ability to control spatiotemporal presentation and local signaling to the regenerating tissue, avoid poor targeting efficiency, and extend factor bioactivity over time following implantation [29–31]. Our group has previously utilized electrospun fibrin microfiber bundles that mimic the native properties of skeletal muscle combined with C2C12 myoblasts to promote functional and histological regeneration post-VML [32]. In the current study, we have enhanced our in vitro muscle constructs to promote neuromuscular regeneration in VML defects through the delivery of agrin. We tested the hypothesis that immobilized agrin tethered to the surface of C2C12 myoblast-seeded microfiber bundles would maintain its in vitro bioactivity and induce AChR clustering in myotubes cultured on the 3D constructs. In addition, we tested the ability of tethered agrin constructs implanted in a murine VML defect model to improve neuromuscular regeneration via recruitment of acetylcholine receptors to overlap with nerves and form neuromuscular junctions at 4 weeks compared to constructs pre-treated with soluble or zero agrin.
2. Materials and Methods
2.1. Electrospinning Fibrin Scaffolds
Fibrin scaffolds were electrospun in a sterile environment with sterile solutions as previously described [32, 33]. Parallel syringes containing solutions of fibrinogen (Sigma) or sodium alginate (Sigma) were connected via a Y-connector and extruded by syringe pumps with an applied voltage of 3 to 5 kV applied to a 27-G needle tip utilizing 1% fibrinogen with an extrusion rate of 4 ml/h and 0.75% alginate with an extrusion rate of 1 ml/h. Poly(ethylene oxide) (PEO, average Mv ~ 4,000 kDa, Sigma) was added to each solution at 0.2 wt% to increase viscosity during electrospinning. The electrospun hydrogel solutions were collected for 5.75 min on a rotating dish (~35 rpm) containing 50 mM CaCl2 and 20 U/ml thrombin (Sigma) as crosslinking agents. Samples were allowed to crosslink for an additional 3–5 min after electrospinning and were then wrapped around a 1.5 × 1.5 cm poly(acrylonitrile-butadiene-styrene) frame 4 times to yield a hydrogel microfiber bundle ~700 μm in diameter. Scaffolds were incubated overnight in 250 mM sodium citrate (Sigma) at room temperature to dissolve the alginate and then transferred to DI water for storage up to 2 weeks.
2.2. Protein Tethering to Scaffolds
Scaffolds with tethered proteins underwent the following tethering protocol one day prior to cell seeding. All incubation steps were performed at room temperature. Activation buffer was prepared using 0.1 M 4-morpholine ethane sulfonic acid (MES; Sigma) and 0.5 M sodium chloride (Sigma) and adjusted to pH 5–6. N-Ethyl-N′-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC; Sigma) and N-hydroxysuccinimide (NHS; ThermoFisher Scientific) were dissolved at 10 mg/ml in activation buffer immediately prior to use. Agrin (R&D Systems) or Cy3 was diluted to 2, 10, 50, or 100 μg/ml (0.08, 0.4, 2, 4 μg/fiber) in sterile PBS (pH 7.2–7.5). Scaffolds were incubated with activation buffer on a rocker at 100 rpm for two 30-min washes then transferred to EDC/NHS buffer on a rocker at 120 rpm for 15 min. Scaffolds were then transferred to customized small-volume molds designed to suspend the scaffolds within 40 μl of agrin or Cy3 on a rocker at 80 rpm for 3 h. Scaffolds were then washed with DI water for two 20-min washes and stored in fresh DI water overnight.
2.3. Cell Culture and Seeding on Scaffolds
C2C12 myoblasts were expanded in growth medium: high-glucose DMEM (Invitrogen), 10% fetal bovine serum (FBS; Atlanta Biologicals), and 1% penicillin/streptomycin (P/S; Invitrogen) and used for experiments at passage 8. In preparation for cell seeding, scaffolds were incubated in high glucose DMEM for 20 min at 37 °C. The scaffolds were then transferred to 6-well cell culture plates coated with 2% agarose type VII (Sigma) to minimize cell adhesion to the plate surface, which increases the seeding efficiency. For all experiments, a total seeding volume of 40 μl at 7,500 cells/μl was pipetted onto the scaffold surface in a series of 5 μl droplets (for a total seeding density of 300,000 cells/scaffold). Scaffolds were incubated for 1 h at 37°C with hydration levels maintained by addition of 15 μl of C2C12 growth medium at 30 min. After a 1-h incubation, 3 ml of C2C12 growth medium containing 30 μg/ml aprotinin (Affymetrix) was added to each well and care was taken to ensure the scaffolds had been submerged in the media. On day 3 of culture myoblasts were transferred to C2C12 induction medium: high-glucose DMEM, 2% horse serum (ThermoFisher Scientific), 1% P/S, 1% insulin-transferrin-selenium (ITS; Corning Cellgro), and 30 μg/ml aprotinin and media was changed every other day. For soluble agrin constructs, recombinant rat agrin protein (agrin; R&D Systems) was added to C212 induction medium at 5 or 50 ng/ml at day 6 of culture and replenished at day 7 of culture. Constructs with no agrin or tethered agrin remained in C2C12 induction medium for the extent of in vitro culture. These constructs were used for experiments on day 7 of culture, while soluble agrin constructs were used for experiments at day 8 of culture.
2.4. Agrin Release Kinetics
Scaffolds were electrospun and underwent agrin tethering with 50 μg/ml agrin as described above. Control scaffolds with untethered agrin were fabricated by incubating scaffolds in small-volume molds containing 50 μg/ml agrin for 3 hours without the prior Activation Buffer and EDC/sulfoNHS incubation steps. Immediately following the agrin incubation, all scaffolds were rinsed once in 1 ml of 1% BSA in PBS (Reagent Diluent Buffer; R&D Systems) which was collected and stored at −20 °C. Scaffolds were then incubated in fresh buffer at 37 °C. Excess residual agrin remaining within the small-volume molds following scaffold removal from the molds was also collected in buffer and stored at −20 °C. At 1, 4, and 7 days post-tethering the buffer was removed, replaced with fresh buffer, and stored at −20 °C. Control wells that served as a 100% release control contained 50 μg/ml agrin in 1 ml of buffer and were incubated at 37 °C with 100 μl samples collected at days 1, 4, and 7. Agrin concentration for all samples was analyzed via ELISA (DuoSet ELISA for Rat Agrin, DY550; R&D Systems) with optical density determined using a microplate reader set to 450 nm.
2.5. In Vitro Mechanical Testing
Scaffolds that were either untreated or pre-treated with EDC/SNHS buffer as described above were loaded into a custom bioreactor and stiffness analyzed as described previously [34, 35]. Briefly, scaffolds were adjusted to zero strain, then stretched beyond 15% strain at ~1% strain/s as force sensors generated a stress-strain curve. Elastic modulus was calculated from a linear fit of the first 15% strain and corresponding stress. Diameter of the fibers was used to estimate the cross-sectional area for stress calculation and was measured as an average of the diameter of two different regions of each fiber due to variability along the fiber length.
2.6. Whole Mount Immunostaining
Scaffolds were fixed in 4% formaldehyde at 4 °C on a rocker at 80 rpm for 3 h. Following 3 washes with PBS, scaffolds were blocked and permeabilized (Block/Perm Solution) for 3 h with 0.2% Triton X-100, 10% normal goat serum (Sigma), and 2% bovine serum albumin (BSA; Sigma) in PBS. Scaffolds were then incubated with mouse anti-myosin heavy chain, fast (1:400; Sigma) diluted in Block/Perm Solution overnight at 4 °C on a rocker at 135 rpm, followed by three 1-hour washes with PBS. Scaffolds were then incubated with DyLight 488-conjugated goat anti-mouse (1:400; Jackson ImmunoResearch) and tetramethylrhodamine α-bungarotoxin (1:50; Thermo Fisher) diluted in 10% normal goat serum, 2% BSA, and 0.1% Tween in PBS overnight at 4 °C on a rocker at 135 rpm, followed by three 1 hour washes with PBS. DAPI was incorporated in the second wash at a dilution of 1:2000. Samples were then imaged with a Zeiss LSM 510 confocal microscope. All in vitro staining was performed with triplicate samples per group and imaged with at least two 20× z-stack images per sample. To quantify AChR clusters, bungarotoxin stains were analyzed using Fiji software [36] with a size range of 3 to 225 μm2. Cluster density was determined as the total area of positive bungarotoxin staining divided by the total area of MHC. Cluster spatial location was determined by quantifying the percent area coverage of each slice within a z-stack image using a size threshold of 3–225 μm2 and determining the distance above the scaffold with the Fiji Plot Z-axis Profile plugin.
2.7. VML Defect Model
Animal and surgical procedures were approved by the Institutional Animal Care and Use Committee at Johns Hopkins University School of Medicine. Unilateral VML defects in 21 two-month-old female NOD-scid IL2Rgnull (NSG) immunodeficient mice (Jackson Lab) were utilized for in vivo testing of no (zero) agrin (n = 7 mice), soluble agrin (n = 7 mice), and tethered agrin (n = 7 mice) constructs. Immunodeficient mice were utilized to minimize rejection of the implanted allogeneic C2C12 cells. Mice were randomly assigned across all groups. The average body weight ± error per group was as follows: no agrin: 21.9 ± 0.7 g; soluble agrin: 20.4 ± 0.7 g; tethered agrin: 21.0 ± 0.7 g. The body weights between groups were analyzed via one-way ANOVA and were not statistically different (with p values of 0.48, 0.75, and 0.82). In vitro constructs were prepared and cultured with C2C12 cells as described above. VML defects were created as previously described [32]. Briefly, mice were anesthetized with isoflurane, the tibialis anterior (TA) muscle was exposed, and a partial-thickness defect was created along the entire length of the TA (including motor endplate region) with approximately 30–50% of the muscle removed. After bleeding ceased, four scaffolds were placed in the defect site and ligated on both ends to the remaining TA muscle with non-absorbable sutures (6–0 Nylon, Express Medical Supplies). Surgical glue (Histoacryl, B. Braun) and sutures were used to close the skin. Rimadyl was injected subcutaneously post-surgery for pain management (5 mg/kg). The average weight of removed muscle was as follows: no agrin: 8.2 ± 0.8 mg; soluble agrin: 11.2 ± 0.7 mg; tethered agrin: 9.5 ± 1.0 mg. The removed muscle weights between groups were analyzed via one-way ANOVA and were not statistically different (with p values of 0.16, 0.69, and 0.34). Mice were sacrificed at 4 weeks post-implantation by isoflurane overdose and cervical dislocation. Upon harvest, implanted scaffolds and surrounding TA muscle tissue were removed and cryopreserved.
2.8. In Vivo Functional Testing
In vivo assessment of mouse TA muscle contractility was performed at 4 weeks as previously described [32, 37]. Briefly, mice were anesthetized with isoflurane and the leg was stabilized in a custom-made apparatus. The foot was taped to a foot plate connected to a torque sensor and stepper motor and the ankle was positioned at the optimum angle to achieve maximum ankle dorsiflexor torque (in which the TA muscle accounts for the majority of ankle dorsiflexion). Electrodes were placed subcutaneously near the fibular head to stimulate the fibular nerve and induce dorsiflexion. Voltage and frequency were optimized for each leg during tetanic contractions (300 ms duration) and the maximal isometric torque was quantified per leg at each time point and normalized to the body weight of that mouse (n=7). Functional data for zero agrin (n=7), uninjured (n=20) and untreated (n=6) groups is from previously published results [32].
2.9. Histology
TA muscles were sectioned on a cryostat (Leica) at a thickness of 10 μm for cross-sections and 70 μm for longitudinal sections. For cross-sections, serial sections were collected on glass slides with approximately five to six sections along the length of the TA collected per slide. Masson’s Trichrome stains were imaged on a Zeiss Axio Imager upright microscope. For immunohistochemistry, slides were fixed in cold 4% formaldehyde for 10 min and rinsed with PBS three times for 15 min each, then blocked in 10% normal donkey or goat serum (Sigma) in PBS for 1 h at room temperature (RT). For β tubulin III staining, slides were blocked in 10% normal donkey serum, 2% BSA, and 0.2% Triton X-100 in PBS for 3 h at RT. For agrin labeling, slides were fixed in a 1:1 mixture of ice-cold acetone and methanol for 10 min, rinsed with PBS, then blocked in 5% normal donkey serum and 5% BSA in PBS for 1 h at RT. For YAP labeling, slides were quenched in 50 mM ammonium chloride for 5 min at RT prior to blocking in 10% donkey serum and 1% BSA in PBS for 2 h at RT. Slides were incubated with antigen-specific primary antibodies in blocking solution overnight at 4°C. Primary antibodies included mouse anti-embryonic myosin (5 μg/ml; DSHB), mouse anti-myosin heavy chain (fast-twitch) (1:400; Sigma Aldrich), goat anti-mouse CD31 (1:100; R&D Systems), rabbit anti-β tubulin III (1:200; Sigma), mouse anti-Pax7 (5 μg/ml; DSHB), rabbit anti-Ki67 (1:1000; Abcam), and rabbit anti-YAP (1:100; Cell Signaling Technology). After three 5 min washes with PBS, slides were incubated with Cy3-conjugated donkey anti-mouse, Alexa Fluor 488-conjugated donkey anti-goat, DyLight 488-conjugated goat anti-mouse, or Alexa Fluor 647-conjugated donkey anti-rabbit (1:400; Jackson ImmunoResearch) and DAPI (1:2000) diluted in blocking solution for 1 hour at RT. Slides staining for α-bungarotoxin (1:50; Thermo Fisher) or phalloidin-TRITC (1:40; Sigma) incorporated the stains into the secondary antibody stain. Slides were washed three times for 5 min then mounted with 50% glycerol and imaged on a Zeiss Axio Observer inverted fluorescence microscope or Zeiss Axio Observer 7. Two sections were quantified from each slide per sample, with one section at a proximal location and one at a middle location longitudinally within the TA defect region. In cross-section, the defect region was identified using DAPI staining to manually demarcate an area within the muscle section with a marked increased nuclear density and was verified by at least two researchers. To quantify staining within the defect regions, tiled images covering the entire defect region were taken on a Zeiss Axio Observer 7 with a 20× objective. An AChR cluster was determined to be within an NMJ when the area of positive bungarotoxin staining was within 2 μm of an area with positive β-tubulin III staining.
2.10. Statistics
Statistical analysis was performed using GraphPad Prism 5 software. Statistical significance was determined by t-test or one-way ANOVA with Dunnett or Bonferroni post-test. In the force-frequency curve, the torque frequency data was analyzed with a separate t test for each frequency pairing. Error bars represent standard error of the mean (SE). *: p < 0.05; **: p < 0.01; ***: p < 0.001; ǂ: not significant.
3. Results
3.1. AChR Cluster 3D Spatial Distribution is Determined by Agrin Delivery Method
Electrospun fibrin hydrogels designed to mimic the hierarchical structure and alignment of skeletal muscle were fabricated as previously described. A protocol to chemically tether proteins to the electrospun fibrin scaffolds using the EDC zero-length crosslinker was initially developed and validated using a fluorescent Cy3 antibody which has a similar size to agrin (Fig. S1A). Agrin was chemically tethered to acellular scaffolds at 0.08–2 μg/scaffold prior to seeding with C2C12 myoblasts, after which C2C12-seeded constructs were cultured for 7 days (Fig. 1A). Tethered agrin constructs exhibited significantly higher mechanical stiffness than controls without EDC crosslinking (Fig. S1B), due to increased crosslinks in fibrin scaffold, which is visible in scaffold cross-sections (Fig. 1B). Soluble agrin was tested in parallel and was delivered to muscle constructs at 10 or 100 ng/scaffold for 24 or 48 h at days 6–8 of culture. Both soluble and tethered agrin induced some amount of AChR clustering in myotubes on 3D constructs at all concentrations, with differences in cluster density dependent on duration and concentration (Fig. S2A–C). To control for nonspecific adsorption of agrin to the scaffold, untethered control scaffolds were fabricated by incubation with agrin at 2 μg/scaffold without the addition of the EDC crosslinker. Despite evidence of some agrin binding, the bioactivity of scaffolds with untethered agrin was 2.8 times lower than those with agrin tethered at the same concentration (Fig. S2C–F). Tethered agrin at 2 μg/scaffold and soluble agrin at 10 ng/scaffold for 48 h had the highest cluster densities while retaining normal myotube morphology. This construct was selected for subsequent experiments. Few AChR clusters were visible in day 7 muscle constructs with no agrin, and both soluble and tethered agrin resulted in myotubes with significantly higher AChR cluster density, area, and length than no agrin controls (Fig. 1C–F). Interestingly, tethered and soluble agrin resulted in significant differences in the spatial location of AChR clusters when analyzed in 3D using confocal imaging. Localization of clusters to the scaffold side of myotubes in the tethered agrin group was visible in immunostained cross-sections as well (Fig. 1G). Clusters induced by tethered agrin were primarily located about 10 μm from the scaffold surface while clusters induced by soluble agrin were visible throughout the myotube layer with two similar peaks in cluster density at about 10 and 30 μm from the scaffold surface (Fig. 1H).
Figure 1. Soluble and tethered agrin increased acetylcholine receptor clustering with spatial effects in 3D muscle constructs.
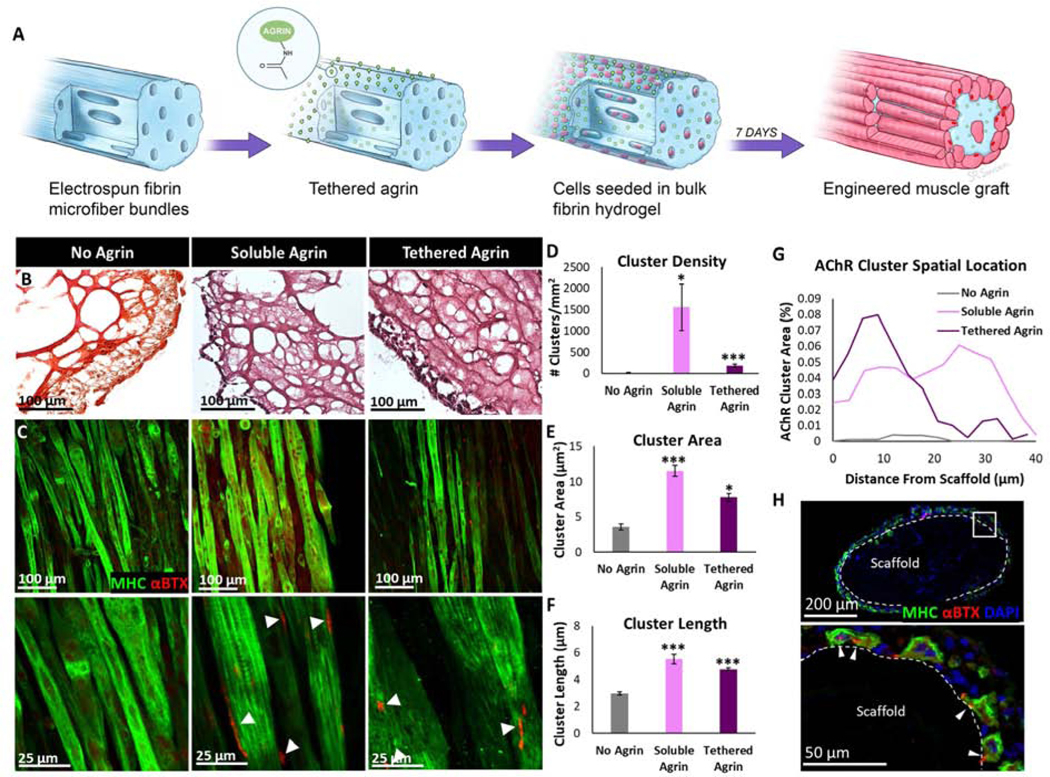
A) Schematic illustrating agrin tethering and cell seeding on electrospun fibrin microfiber bundles. B) Masson’s Trichrome stain of cell-seeded scaffold cross-sections demonstrating differences in crosslinking density in tethered agrin scaffolds. C) Aligned myotubes positive for myosin heavy chain (MHC; green) in all groups exhibit AChR clusters positive for α-bungarotoxin (αBTX; red) in agrin treatment groups. D) Density of AChR clusters is significantly increased in agrin treatment groups. E,F) The area and length of individual AChR clusters in agrin treatments groups was significantly higher than clusters in constructs with no agrin. G) Cross-section of cell-seeded tethered agrin scaffold immunostained for MHC and αBTX demonstrating the spatial location of AChR clusters at the scaffold surface. H) The spatial location of AChR clusters differed significantly between groups: tethered agrin induced clusters close to the scaffold surface while soluble agrin induced AChR clusters independent of distance from the scaffold surface. *: p < 0.05; ***: p < 0.001
3.2. Agrin Pre-Treatment did not Improve Muscle Function in VML Defects
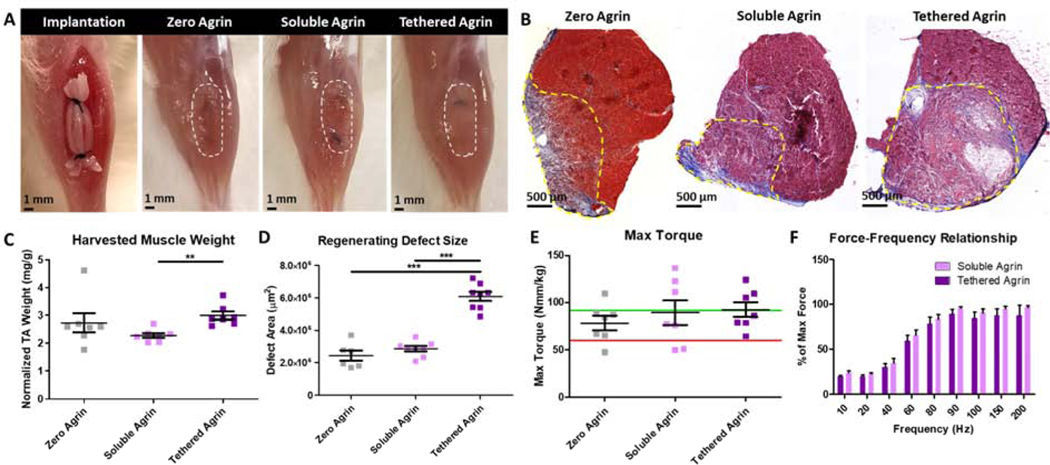
At 4 weeks post-implantation in VML defects, all treatment groups exhibited a healthy gross muscle morphology with no signs of infection or graft rejection. Gross images revealed that scaffolds in defects treated with tethered agrin remained visible while those in other groups had resorbed (Fig. 2A). Masson’s Trichrome staining of histologic sections demonstrated low levels of fibrosis and defect volume retention in all groups. Defects treated with soluble agrin contained dense muscle tissue similar to no agrin controls visible via red Masson’s Trichrome staining, while defects treated with tethered agrin also resulted in dense muscle tissue but contained regions with undegraded scaffold lacking the red stain (Fig. 2B). Immunostaining for agrin demonstrated visibly higher levels of agrin on all four implanted scaffolds in the tethered agrin treatment group when compared to soluble or no agrin, demonstrating that tethering agrin to the scaffold surface causes persistent agrin presentation to the surrounding tissues up to four weeks post-implantation (Fig. S3). Explanted muscles treated with tethered agrin had a higher wet weight and significantly higher defect cross-sectional area compared to soluble and no agrin (Fig. 2C,D). Despite these differences in histology and size for the tethered agrin group, there was no difference in either maximum torque or the force-frequency relationship between groups (Fig. 2E,F).
Figure 2. Muscle function following VML defects improved equally in no-agrin and agrin-treated groups.
A) Gross morphology at scaffold implantation and 4 weeks post-implantation for no agrin, soluble, and tethered agrin constructs. B) Cross-sections of the explanted tibialis anterior muscle stained for Masson’s Trichrome demonstrate differences in defect area (yellow dashed line) and morphology between groups. C,D) Differences were present between groups in the harvested TA weight and defect size in cross-section. E,F) No difference in muscle function was seen between treatment groups. Green line: average max torque of age-matched uninjured TA muscle; Red line: average max torque of untreated VML defects at 4 weeks post-injury. **: p < 0.01; ***: p < 0.001
3.3. Agrin Pre-Treatment Increased NMJ Formation with Differences in NMJ Morphology
Immunostaining for myosin heavy chain (MHC) demonstrated densely packed myofibers in the defect regions for zero and soluble agrin, while tethered agrin had dense MHC+ regions in addition to distinct areas lacking MHC+ muscle (Fig. 3A). Tethered agrin treatment groups contained higher total MHC area compared to no agrin (Fig. 3B), although this is likely due to the increased defect size for tethered agrin as the normalized myofiber area did not differ between tethered agrin and no agrin controls, and was higher for soluble agrin compared to tethered agrin (Fig. S4). Neurofilament and acetylcholine receptor clusters were visible within the defects of all groups, with tethered agrin treatment groups containing more total nerve area than no agrin (Fig. 3C). NMJs defined by positive αBTX staining within 2 μm of a nerve were quantified and soluble agrin treatment groups had statistically more AChR cluster area within a NMJ than no agrin (Fig. 3D). Tethered agrin samples also had a higher average percent of AChR cluster area within a NMJ compared to no agrin but with increased variability between samples.
Figure 3. VML treatment with soluble and tethered agrin constructs increases NMJ formation in vivo.
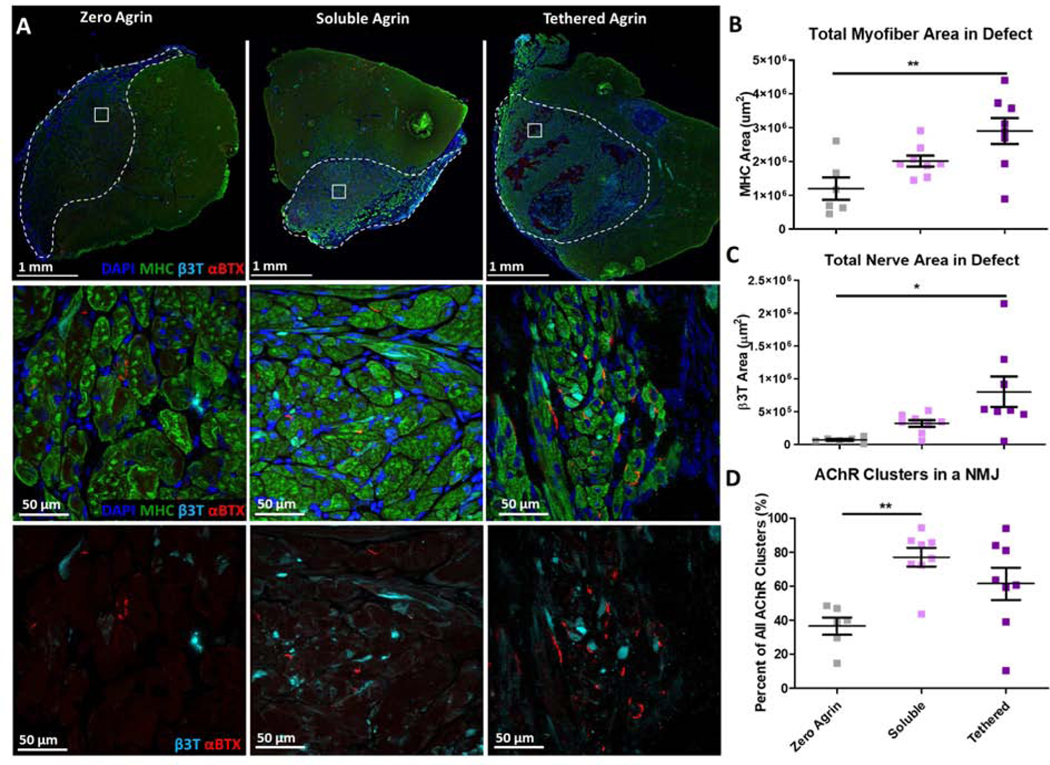
A) Cross-sections of explanted muscle stained for myosin heavy chain (MHC; green), neurofilament (β3T; cyan), and acetylcholine receptors (αBTX; red) with dense myotube-positive area and NMJs within the defect area (white dashed line). B) Tethered agrin constructs resulted in more total myofiber area within the defect region than other treatment groups. C) Tethered agrin constructs resulted in more total nerve area in the defect region than other treatment groups. D) Both soluble and tethered agrin constructs resulted in higher percentages of AChR clusters within an NMJ than no agrin controls. *: p < 0.05; **: p < 0.01
Due to the presence of NMJs within the defect regions of all treatment groups, longitudinal sections were utilized to assess for differences in NMJ morphology within the defect that would otherwise not be visible in cross-section (Fig. S4A). NMJs for all VML treatment groups were smaller than those in native skeletal muscle and had a less mature morphology (Fig. S4B). Despite differences in size and shape, visible overlap between the AChR clusters and neurofilament was visible in the soluble and tethered agrin groups, but not zero agrin (Fig. S4C). NMJs visible within defects treated with tethered agrin constructs had an immature plaque morphology, while the soluble agrin group contained some NMJs with more complex architectures.
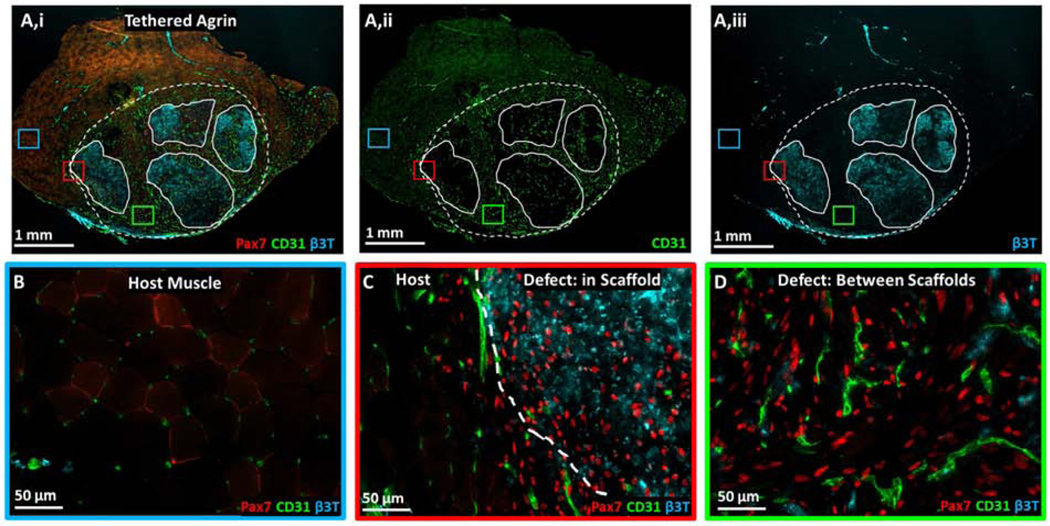
3.4. Spatial Differences in Protein Expression were Present within Implanted Grafts
Interesting patterns of protein expression were visible within the defect regions of the tethered agrin samples. Overall, the defect regions were highly positive for Pax7, CD31, and β3-tubulin compared to host muscle (Fig. 4A,B). Four regions of persistent, undegraded scaffold were visible within the defect regions that were primarily negative for mature muscle, as demonstrated above with Masson’s Trichrome and MY32 staining. Spatial differences in protein expression were visible within undegraded scaffold regions compared to between the scaffolds. Expression of Pax7 was fairly consistent across the defect region both within the four implanted scaffolds and between scaffolds (Fig. 4C,D) while blood vessel infiltration into the defect region was primarily between the implanted scaffolds (Fig. 4Aii,D). Overall, defect regions in tethered agrin groups contained higher total neurofilament as well as higher neurofilament density compared to other treatment groups (Fig. 3C, Fig. S5). Interestingly, undegraded scaffolds within the tethered agrin defect region were highly positive for the neurofilament stain β3-tubulin but not the areas between the scaffolds (Fig. 4Aiii,C,D).
Figure 4. Muscle stem cell, vascular, and neural proteins were upregulated within and surrounding implanted tethered agrin constructs compared to host muscle.
A) Low magnification image of TA cross-section treated with tethered agrin constructs and immunostained for (i) Pax7 (red), CD31 (green), and β3-tubulin (cyan), (ii) CD31 alone, or (iii) β3-tubulin alone. Dashed line: defect region; Solid lines: undegraded scaffold regions. Insets: three regions with corresponding high magnification images demonstrating variations in protein expression in host muscle (blue inset), at the host-defect border (red inset), and within the defect region but between implanted scaffolds (green inset). B) High magnification image of host muscle with low levels of Pax7, CD31, and β3T staining. C) High magnification image of the border between host muscle and an implanted tethered-agrin scaffold within the defessct region. Increases in Pax7, CD31, and β3T expression are visible going into the defect region. D) High magnification image within the defect region but between implanted scaffolds. Pax7 and CD31 remain upregulated between scaffolds but β3T expression is decreased.
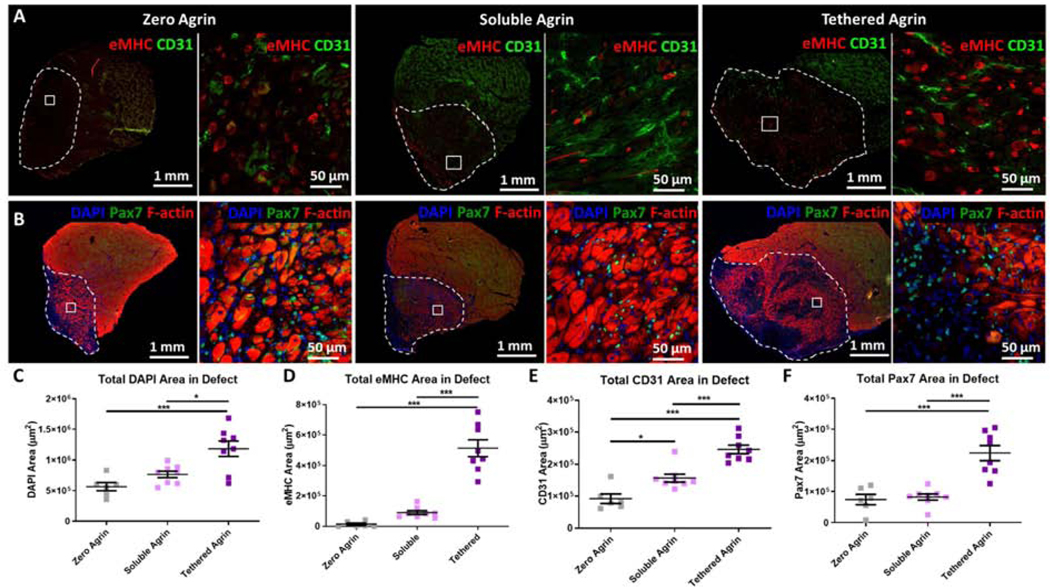
3.5. Tethered Agrin Resulted in Increased Blood Vessels and Regenerating Myofibers
VML treatment with soluble and tethered agrin resulted in significant differences in a range of regenerative metrics. Total expression of embryonic myosin (eMHC), a marker of regenerating myofibers, was significantly higher in defects treated with tethered agrin compared to zero or soluble agrin (Fig. 5A,D) and the density of eMHC expression differed significantly between both agrin treatment groups and the zero agrin control as well as between soluble and tethered agrin, with tethered agrin resulting in the highest overall eMHC density (Fig. S5). Soluble and tethered agrin constructs also resulted in increases in total blood vessels within the defect region compared to zero agrin (Fig. 5A,E). Tethered agrin resulted in higher total blood vessel content compared to soluble agrin but when normalized to the defect area, soluble agrin groups contained a higher blood vessel density than tethered agrin groups (Fig. S5). Total expression of the muscle stem cell marker Pax7 also increased due to tethered agrin constructs (Fig. 5B,F) and dense Pax7+ cells were visible even within undegraded scaffold regions of tethered agrin groups that did not contain MHC+ myofibers (Fig. 5B). Total number of nuclei within the defect region was also significantly higher due to tethered agrin compared to both other treatment groups (Fig 5C). These results indicate that persistent agrin presentation in tethered agrin treatment groups within regenerating VML defects causes significantly higher levels of eMHC+ regenerating myofibers compared to soluble and zero agrin treatment groups. Tethered agrin also increased total nuclear area, blood vessel infiltration, and satellite cell presence within VML defects.
Figure 5. Tethered agrin increases the presence of regenerating myofibers, blood vessels, and satellite cells within VML defects.
A) Differences in expression within the defect site (white dashed line) between groups are visible via immunostaining for embryonic myosin (eMHC; red) and blood vessels (CD31; green). Inset: high magnification image. B) Immunostaining for satellite cells (Pax7; green) and filamentous actin (F-actin; red) demonstrated differences in satellite cell recruitment between treatment groups. C) Defects treated with tethered agrin contained more nuclei than zero or soluble agrin groups. D) Tethered agrin resulted in significantly higher embryonic myosin positive area within the defect region than zero or soluble agrin. E) Significant differences in total blood vessel coverage were seen between all treatment groups with tethered agrin inducing the highest amount of CD31 area within the defect region. F) Tethered agrin constructs resulted in higher Pax7 area within the defect region than zero or soluble agrin. *: p < 0.05; ***: p < 0.001
Due to the striking increase in both total and normalized eMHC+ myofiber area within defects treated with tethered agrin compared to other treatment groups, we next evaluated co-expression of eMHC and nuclear YAP, a transcriptional coactivator with nuclear expression in its active form (Fig. S6). Defects treated with tethered agrin constructs resulted in significantly higher total nuclear YAP expression compared to those treated with zero and soluble agrin (Fig. S6A,B) while the density of nuclear YAP within defects was equal between groups (Fig. S7A). Both soluble and tethered agrin groups also had significantly higher total levels of cells coexpressing eMHC and nuclear YAP compared to zero agrin (Fig. S6C). Due to the high levels of Pax7+ cells within defect regions of all groups, we also evaluated co-expression of Pax7 and the proliferation marker Ki67 (Fig. S7B). Although tethered agrin constructs resulted in significantly more Ki67 compared to soluble agrin, there was no difference in the level of Pax7 and Ki67 co-expression between groups. These data demonstrate that persistent agrin presentation within regenerating VML defects results in both higher total nuclear YAP expression and co-expression of eMHC+ regenerating myofibers with nuclear YAP, as well as increased overall cell proliferation.
4. Discussion
Successful regeneration of VML defects includes the formation of mature NMJs that allow for efficient neural signal propagation and subsequent muscle contraction. Significant advances in the field of skeletal muscle tissue engineering have demonstrated varied levels of muscle tissue regeneration and functional recovery post-VML [9, 23, 46–48, 38–45], but remain limited in their ability to promote neuromuscular regeneration. To date, there have been no previously published engineered skeletal muscle constructs used for VML treatment that have incorporated pharmacologic factors specific to neuromuscular regeneration. Agrin has been utilized to induce AChR clustering in cultured myotubes [14–22] and agrin-loaded muscle constructs have resulted in increased nerve infiltration and NMJ formation upon subcutaneous implantation in a non-VML model [14]. We thus identified agrin as a putative pharmacologic factor to induce neuromuscular regeneration post-VML and modified our previously developed electrospun muscle constructs [32, 33] to incorporate agrin delivery within in vitro cultured muscle constructs as well as to the regenerating environment post-implantation.
Two methods of agrin delivery to 3D cultured myoblasts were examined for their neuromuscular potential in vitro and in vivo. Agrin provided in solution within cell culture media most closely mimics previous studies utilizing soluble agrin to induce AChR clustering in myotube monolayers via transient delivery to mature myotubes. Preliminary studies demonstrated that soluble agrin applied to C2C12 myoblast monolayers (day 2) did not cause AChR clustering, while agrin applied to fused myotubes late in culture (day 8) was successful in inducing AChR clustering (data not shown). We thus applied agrin at a late time point (days 6–8) in the soluble agrin group. Chemically tethering agrin to electrospun fibrin scaffolds changes the cell microenvironment by enabling agrin signaling to cells throughout the duration of culture. In addition to inducing AChR clustering, in vitro agrin application to myotubes has been shown to increase contractile properties and dystrophin expression [18], myotube motility [49], and maturation of the excitation-contraction coupling machinery [50] further motivating prolonged agrin signaling via tethering to the scaffold surface. In addition, implanted constructs incorporating tethered agrin enable sustained local agrin signaling over time to the regenerating VML environment post-implantation with potential extended therapeutic benefit. Treatment of VML with an agrin-loaded muscle construct and investigation into the effect of sustained agrin signaling within the regenerating muscle environment post-VML has not previously been studied.
Scaffold-mediated delivery of immobilized bioactive factors may be limited by the potential for decreased factor bioactivity by denaturing or deactivating the protein during the tethering process [51]. The EDC crosslinking paradigm used in this study has been used previously to tether agrin to polymeric microparticles for application to in vitro cultured myotubes with preserved agrin bioactivity [16]. In the current study, the bioactivity of tethered agrin was also maintained and resulted in significantly more AChR clustering than passively adsorbed agrin. The difference in the in vitro 3D spatial location of AChR clusters seen between soluble and tethered agrin delivery methods is similar to previous reports where spatial control of AChR clusters is controlled by localized agrin signaling [16] and further motivates the potential benefit of tethering agrin to the implanted scaffold to prolong agrin signaling in vivo such that AChR clusters are induced to form within the defect site.
The EDC tethering protocol resulted in increased crosslinking of microfiber bundles and a significantly higher mechanical stiffness than other treatment groups. This has potential implications for both in vitro myogenesis and regeneration upon implantation. In the current study, in vitro myotubes formed within tethered agrin constructs had no visible difference in morphology compared to myotubes in the other experimental groups. Following implantation in VML defects for four weeks, tethered agrin constructs resulted in regions with distinct undegraded scaffold clearly visible via histological staining and likely due to the increased crosslinking and mechanical stiffness. In addition, despite resulting in a larger defect containing a higher area of eMHC+ regenerating fibers, tethered agrin constructs did not have a measurably higher maximum isometric torque compared to defects treated with zero or soluble agrin constructs. This is similar to previous studies demonstrating that VML treatment with acellular scaffolds provides functional improvements at similar levels to cell-seeded scaffolds without corresponding histological muscle regeneration [7, 9, 32, 41, 43, 52, 53]. In fact, a recent meta-analysis of various VML treatments found that while treatment may have resulted in improved muscle regeneration compared to untreated VML, there was little functional difference between the various treatment options [54]. Our current findings are consistent with those reported in literature in that VML treatment with myoblast-seeded constructs containing soluble, tethered, or no agrin resulted in similar levels of functional improvement after one month. It is possible that a longer recovery time post-injury would enable measurable differences in function between groups as the regenerating myofibers and nascent neuromuscular junctions increase in maturity.
Improved neuromuscular regeneration was seen for VML treatment with both soluble and tethered agrin constructs compared to no agrin constructs. The total and normalized neurofilament areas within defects treated with tethered agrin were higher than no agrin, and soluble agrin constructs resulted in a higher percentage of AChR clusters within a NMJ compared to no agrin. This is in keeping with a previous study where agrin-loaded muscle constructs were implanted subcutaneously and resulted in increased numbers of NMJs at 2, 4, and 8 weeks post-implantation [14]. Quantification of NMJ density and morphology post-VML has been primarily limited to thin histological cross-sections, which do not enable morphological analysis of the complete NMJ [55]. In the current study, when NMJs were viewed via thick longitudinal sections, differences in morphology were apparent between agrin treatment groups, with soluble agrin constructs inducing a more mature NMJ morphology than tethered agrin constructs. While smaller than native NMJs, the NMJs in the soluble agrin group were assessed at a single 4-week time-point. It has been previously demonstrated in monolayer cultures that agrin preferentially induces AChR clusters with an immature plaque shape [21], possibly explaining the higher incidence of an immature, plaque-shaped NMJ morphology in VML defects exposed to sustained agrin presentation over time via tethered agrin constructs.
The sustained presentation of agrin, combined with increased scaffold stiffness of tethered agrin constructs, appears to have caused spatial differences in protein expression within the regenerating defect region. Soluble and tethered agrin constructs resulted in increased overall blood vessel infiltration, but blood vessels in zero and soluble agrin samples were uniform across the defect region, while tethered agrin samples resulted in blood vessels primarily infiltrating between implanted scaffolds. This was likely due to increased stiffness of tethered agrin constructs and subsequent decreased ability for blood vessel penetration to the scaffold interior. In future studies, we will assess the role of increased scaffold stiffness by utilizing EDC-treated scaffolds lacking agrin. Implanted tethered agrin constructs also contained high densities of neurofilament within the undegraded scaffold regions compared to regions between the implanted scaffolds and within host muscle tissue. Agrin treatment has been previously linked to increased nerve outgrowth [14] and neural proteolytic cleavage of agrin has been shown to induce formation of dendritic filopodia synaptic precursors [56]. The persistent agrin presence at four weeks post-implantation within tethered agrin scaffolds may have induced the high levels of neurofilament seen here. Although the presence of agrin within the defect region four weeks post-implantation was highest for tethered agrin constructs, agrin was visible at low levels in the other two treatment groups as well. Agrin is endogenously expressed at neuromuscular junctions and some neural synapses [57, 58] and immunostaining for agrin within the defect region may be identifying endogenous protein expression in addition to the implanted agrin constructs.
A high incidence of eMHC+ regenerating myofibers within the defect is perhaps the most striking result of implanted tethered agrin constructs. Embryonic myosin is a transient marker of regenerating skeletal muscle and we have demonstrated previously that C2C12-seeded electrospun fibrin scaffolds implanted in VML defects resulted in significant eMHC expression at 2 weeks, but not 4 weeks, post-implantation [32]. Here, both agrin groups resulted in a significant increase in eMHC expression within the defect region compared to no agrin controls, with tethered agrin resulting in the highest amount of eMHC. It appears that agrin treatment induces a prolonged state of eMHC expression within VML defects, with the sustained signaling of tethered agrin causing the highest level of eMHC. Agrin administration has recently been shown to suppress cardiomyocyte maturation and enable cardiac regeneration in adult mice with a link to increased nuclear YAP expression [59]. It is possible that the skeletal muscle constructs pre-treated with soluble and tethered agrin are causing a similar suppressed maturation state of the myofibers present within VML defect regions, resulting in sustained eMHC expression. Additionally, tethered agrin constructs resulted in increased total nuclear YAP expression and both agrin treatment groups resulted in increased myofibers with co-expression of eMHC and nuclear YAP. Agrin has recently been correlated with YAP activation and increased nuclear YAP expression in various tissues [58, 60], and nuclear YAP expression has been linked to increased cell proliferation and decreased myoblast maturity [61, 62]. In addition, muscle YAP expression impacts the size and location of AChR clusters, NMJ formation, and NMJ regeneration in response to injury [63]. The sustained agrin delivery provided by implanted tethered agrin constructs may thus promote the increased nuclear YAP, increased cell proliferation, and increased expression of eMHC found within regenerating VML defect regions here. While prolonged agrin signaling within the post-VML regenerating environment provides therapeutic benefit, optimization of the construct degradation timeline to match the rate of muscle regeneration would likely further improve the construct regenerative potential by enabling increased blood vessel and myofiber infiltration to the scaffold interior. Due to the increased fibrin crosslinking caused by EDC, utilization of a lower EDC concentration or alternative tethering chemistry could increase the construct degradation rate. As soluble agrin constructs provided improved blood vessel and myofiber density as well as increased NMJ maturity, pretreating muscle constructs with both soluble and tethered agrin could combine the benefits of both delivery approaches. In addition, defect regions in tethered agrin treatment groups were significantly larger than the other groups, indicating possible overgrowth within the regenerating muscle in response to sustained agrin signaling and a potential need for regulating the temporal release of agrin in future studies.
5. Conclusions
In conclusion, engineered muscle constructs pre-treated with agrin form dense AChR clusters in vitro and promote neuromuscular regeneration upon implantation in VML defects for four weeks. While all treatment groups resulted in improved muscle function, constructs pre-treated with both soluble and tethered agrin caused increased neurofilament and blood vessel infiltration, NMJ formation, and the presence of eMHC+ regenerating myofibers compared to no agrin controls. In addition, sustained in vivo agrin signaling in tethered agrin treatment groups caused heightened neurofilament and eMHC density compared to soluble agrin. Future studies should further investigate the role of agrin in the regenerating muscle environment post-VML over longer time periods, as well as incorporate the delivery of other factors to promote neuromuscular regeneration post-VML.
Supplementary Material
Acknowledgements
Funding was provided by the Maryland Stem Cell Research Fund (2016-MSCRFI-2692), the Wilmer Core Grant for Vision Research, Microscopy and Imaging Core Module (EY001765), the NIH (NIAMS NRSA F31 AR071759 and K01 AR074048), and the MDA DG 577897.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Conflict of Interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Availability
The raw/processed data required to reproduce these findings cannot be shared at this time due to technical or time limitations.
References
- 1.Garg K, Ward CL, Hurtgen BJ, et al. (2015) Volumetric Muscle Loss : Persistent Functional Deficits Beyond Frank Loss of Tissue. J Orthop Res 33:40–46. 10.1002/jor.22730 [DOI] [PubMed] [Google Scholar]
- 2.Gilbert-Honick J, Grayson W (2019) Vascularized and Innervated Skeletal Muscle Tissue Engineering. Adv Healthc Mater 1900626:1–27. 10.1002/adhm.201900626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grogan BF, Hsu JR (2011) Volumetric muscle loss. J Am Acad Orthop Surg 19:35–37 [DOI] [PubMed] [Google Scholar]
- 4.Corona BT, Flanagan KE, Brininger CM, et al. (2018) Impact of volumetric muscle loss injury on persistent motoneuron axotomy. Muscle and Nerve 57:799–807. 10.1002/mus.26016 [DOI] [PubMed] [Google Scholar]
- 5.VanDusen KW, Syverud BC, Williams ML, et al. (2014) Engineered skeletal muscle units for repair of volumetric muscle loss in the tibialis anterior muscle of a rat. Tissue Eng Part A 20:2920–30. 10.1089/ten.TEA.2014.0060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaufman T, Kaplan B, Perry L, et al. (2019) Innervation of an engineered muscle graft for reconstruction of muscle defects. Am J Transplant 19:37–47. 10.1111/ajt.14957 [DOI] [PubMed] [Google Scholar]
- 7.Aurora A, Roe JL, Corona BT, Walters TJ (2015) An acellular biologic scaffold does not regenerate appreciable de novo muscle tissue in rat models of volumetric muscle loss injury. Biomaterials 67:393–407. 10.1016/j.biomaterials.2015.07.040 [DOI] [PubMed] [Google Scholar]
- 8.Corona BT, Garg K, Ward CL, et al. (2013) Autologous minced muscle grafts: a tissue engineering therapy for the volumetric loss of skeletal muscle. Am J Physiol Physiol 305:C761–C775. 10.1152/ajpcell.00189.2013 [DOI] [PubMed] [Google Scholar]
- 9.Quarta M, Cromie M, Chacon R, et al. (2017) Bioengineered constructs combined with exercise enhance stem cell-mediated treatment of volumetric muscle loss. Nat Commun 8:15613. 10.1038/ncomms15613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakayama KH, Alcazar C, Yang G, et al. (2018) Rehabilitative exercise and spatially patterned nanofibrillar scaffolds enhance vascularization and innervation following volumetric muscle loss. npj Regen Med 3:16. 10.1038/s41536-018-0054-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Das S, Browne KD, Laimo FA, et al. (2019) Pre-Innervated Tissue Engineered Muscle Promotes a Pro-Regenerative Microenvironment Following Volumetric Muscle Loss. bioRxiv 840124. 10.1101/840124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singhal N, Martin PT (2011) Role of extracellular matrix proteins and their receptors in the development of the vertebrate neuromuscular junction. Dev Neurobiol 71:982–1005. 10.1002/dneu.20953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Witzemann V (2006) Development of the neuromuscular junction. Cell Tissue Res 326:263–271. 10.1007/s00441-006-0237-x [DOI] [PubMed] [Google Scholar]
- 14.Ko IK, Lee BK, Lee SJ, et al. (2013) The effect of in vitro formation of acetylcholine receptor (AChR) clusters in engineered muscle fibers on subsequent innervation of constructs in vivo. Biomaterials 34:3246–3255. 10.1016/j.biomaterials.2013.01.029 [DOI] [PubMed] [Google Scholar]
- 15.Zhang BGX, Quigley AF, Bourke JL, et al. (2016) Combination of agrin and laminin increase acetylcholine receptor clustering and enhance functional neuromuscular junction formation In vitro. Dev Neurobiol 76:551–565. 10.1002/dneu.22331 [DOI] [PubMed] [Google Scholar]
- 16.Scott JB, Ward CL, Corona BT, et al. (2017) Achieving Acetylcholine Receptor Clustering in Tissue-Engineered Skeletal Muscle Constructs In vitro through a Materials-Directed Agrin Delivery Approach. Front Pharmacol 7:1–16. 10.3389/fphar.2016.00508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferns MJ, Campanelli JT, Hoch W, et al. (1993) The ability of agrin to cluster AChRs depends on alternative splicing and on cell surface proteoglycans. Neuron 11:491–502. 10.1016/0896-6273(93)90153-I [DOI] [PubMed] [Google Scholar]
- 18.Bian W, Bursac N (2012) Soluble miniagrin enhances contractile function of engineered skeletal muscle. FASEB J 26:955–965. 10.1096/fj.11-187575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tourovskaia A, Li N, Folch A (2008) Localized acetylcholine receptor clustering dynamics in response to microfluidic focal stimulation with agrin. Biophys J 95:3009–3016. 10.1529/biophysj.107.128173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee LK, Kunkel DD, Stollberg J (2002) Mechanistic distinctions between agrin and laminin-1 induced aggregation of acetylcholine receptors. BMC Neurosci 3:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bruneau EG, Macpherson PC, Goldman D, et al. (2005) The effect of agrin and laminin on acetylcholine receptor dynamics in vitro. Dev Biol 288:248–258. 10.1016/j.ydbio.2005.09.041 [DOI] [PubMed] [Google Scholar]
- 22.Burkin DJ, Kim JE, Gu M, Kaufman SJ (2000) Laminin and alpha7beta1 integrin regulate agrin-induced clustering of acetylcholine receptors. J Cell Sci 113:2877–86 [DOI] [PubMed] [Google Scholar]
- 23.Perry L, Landau S, Flugelman MY, Levenberg S (2018) Genetically engineered human muscle transplant enhances murine host neovascularization and myogenesis. Commun Biol 1:. 10.1038/s42003-018-0161-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou W, He D-Q, Liu J-Y, et al. (2015) Angiogenic gene-modified myoblasts promote vascularization during repair of skeletal muscle defects. J Tissue Eng Regen Med 9:1404–1416. 10.1002/term [DOI] [PubMed] [Google Scholar]
- 25.Grasman JM, Do DM, Page RL, Pins GD (2015) Rapid release of growth factors regenerates force output in volumetric muscle loss injuries. Biomaterials 72:49–60. 10.1016/j.biomaterials.2015.08.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hagiwara K, Chen G, Kawazoe N, et al. (2016) Promotion of muscle regeneration by myoblast transplantation combined with the controlled and sustained release of bFGFcpr. J Tissue Eng Regen Med 10:325–333. 10.1002/term [DOI] [PubMed] [Google Scholar]
- 27.Ju YM, Atala A, Yoo JJ, Lee SJ (2014) In situ regeneration of skeletal muscle tissue through host cell recruitment. Acta Biomater 10:4332–4339. 10.1016/j.actbio.2014.06.022 [DOI] [PubMed] [Google Scholar]
- 28.Nakayama KH, Shayan M, Huang NF (2019) Engineering Biomimetic Materials for Skeletal Muscle Repair and Regeneration. Adv Healthc Mater 8:e1801168. 10.1002/adhm.201801168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kearney CJ, Mooney DJ (2013) Macroscale delivery systems for molecular and cellular payloads. Nat Mater 12:1004–1017. 10.1038/nmatXXXX [DOI] [PubMed] [Google Scholar]
- 30.Tayalia P, Mooney DJ (2009) Controlled growth factor delivery for tissue engineering. Adv Mater 21:3269–3285. 10.1002/adma.200900241 [DOI] [PubMed] [Google Scholar]
- 31.Kuhl PR, Griffith-Cima LG (1996) Tethered epidermal growth factor as a paradigm for growth factor–induced stimulation. Nat Med 2:1022–1027. 10.1038/nm1196-1211 [DOI] [PubMed] [Google Scholar]
- 32.Gilbert-Honick J, Iyer SR, Somers SM, et al. (2018) Engineering functional and histological regeneration of vascularized skeletal muscle. Biomaterials 164:70–79. 10.1016/j.biomaterials.2018.02.006 [DOI] [PubMed] [Google Scholar]
- 33.Gilbert-Honick J, Ginn B, Zhang Y, et al. (2018) Adipose-derived Stem/Stromal Cells on Electrospun Fibrin Microfiber Bundles Enable Moderate Muscle Reconstruction in a Volumetric Muscle Loss Model. Cell Transplant 27:1644–1656. 10.1177/0963689718805370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cook CA, Huri PY, Ginn BP, et al. (2016) Characterization of a Novel Bioreactor System for 3D Cellular Mechanobiology Studies. Biotechnol Bioeng 113:1825–1837. 10.1002/bit.25946 [DOI] [PubMed] [Google Scholar]
- 35.Somers SM, Zhang NY, Morrissette-McAlmon JBF, et al. (2019) Myoblast maturity on aligned microfiber bundles at the onset of strain application impacts myogenic outcomes. Acta Biomater 94:232–242. 10.1016/j.actbio.2019.06.024 [DOI] [PubMed] [Google Scholar]
- 36.Schindelin J, Arganda-Carreras I, Frise E, et al. (2012) Fiji: An open-source platform for biological-image analysis. Nat Methods 9:676–682. 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iyer SR, Valencia AP, Hernandez-Ochoa EO, Lovering RM (2016) In vivo assessment of muscle contractility in animal studies. Methods Mol Biol 1460:293–307. 10.1007/978-1-4939-3810-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ward CL, Ji L, Corona BT (2015) An Autologous Muscle Tissue Expansion Approach for the Treatment of Volumetric Muscle Loss. Biores Open Access 4:198–208. 10.1089/biores.2015.0009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aguilar CA, Greising SM, Watts A, et al. (2018) Multiscale analysis of a regenerative therapy for treatment of volumetric muscle loss injury. Cell Death Discov 4:. 10.1038/s41420-018-0027-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goldman SM, Henderson BEP, Walters TJ, Corona BT (2018) Co-delivery of a laminin-111 supplemented hyaluronic acid based hydrogel with minced muscle graft in the treatment of volumetric muscle loss injury. PLoS One 1–15. 10.1371/journal.pone.0191245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kasukonis B, Kim J, Brown L, et al. (2016) Co-delivery of Infusion Decellularized Skeletal Muscle with Minced Muscle Autografts Improved Recovery from Volumetric Muscle Loss Injury in a Rat Model. Tissue Eng Part A 22:1151–1163. 10.1089/ten.TEA.2016.0134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li MTA, Willett NJ, Uhrig BA, et al. (2014) Functional analysis of limb recovery following autograft treatment of volumetric muscle loss in the quadriceps femoris. J Biomech 47:2013–2021. 10.1016/j.jbiomech.2013.10.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Corona BT, Ward CL, Baker HB, et al. (2014) Implantation of In Vitro Tissue Engineered Muscle Repair Constructs and Bladder Acellular Matrices Partially Restore In Vivo Skeletal Muscle Function in a Rat Model of Volumetric Muscle Loss Injury. Tissue Eng Part A 20:705–715. 10.1089/ten.tea.2012.0761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Madden L, Juhas M, Kraus WE, et al. (2015) Bioengineered human myobundles mimic clinical responses of skeletal muscle to drugs. Elife 4:e04885. 10.7554/eLife.04885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Page RL, Malcuit C, Vilner L, et al. (2011) Restoration of skeletal muscle defects with adult human cells delivered on fibrin microthreads. Tissue Eng Part A 17:2629–2640. 10.1089/ten.TEA.2011.0024 [DOI] [PubMed] [Google Scholar]
- 46.Passipieri JA, Baker HB, Siriwardane M, et al. (2017) Keratin hydrogel enhances in vivo skeletal muscle function in a rat model of volumetric muscle loss. Tissue Eng Part A 23:556–571. 10.1089/ten.TEA.2016.0458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baker HB, Passipieri JA, Siriwardane M, et al. (2017) Cell and Growth Factor Loaded Keratin Hydrogels for Treatment of Volumetric Muscle Loss (VML) in a Mouse Model. Tissue Eng Part A 23:572–584. 10.1089/ten.TEA.2016.0457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Juhas M, Engelmayr GC, Fontanella AN, et al. (2014) Biomimetic engineered muscle with capacity for vascular integration and functional maturation in vivo. Proc Natl Acad Sci 111:5508–5513. 10.1073/pnas.1402723111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Uhm CS, Neuhuber B, Lowe B, et al. (2001) Synapse-forming axons and recombinant agrin induce microprocess formation on myotubes. J Neurosci 21:9678–9689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bandi E, Jevšek M, Mars T, et al. (2008) Neural agrin controls maturation of the excitation-contraction coupling mechanism in human myotubes developing in vitro. Am J Physiol - Cell Physiol 294:66–73. 10.1152/ajpcell.00248.2007 [DOI] [PubMed] [Google Scholar]
- 51.Chen RR, Mooney DJ (2003) Polymeric growth factor delivery strategies for tissue engineering. Pharm Res 20:1103–1112. 10.1023/A:1025034925152 [DOI] [PubMed] [Google Scholar]
- 52.Sicari BM, Rubin JP, Dearth CL, et al. (2014) An Acellular Biologic Scaffold Promotes Skeletal Muscle Formation in Mice and Humans with Volumetric Muscle Loss. Sci Transl Med 6:1–10. 10.1126/scitranslmed.3008085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Corona BT, Wu X, Ward CL, et al. (2013) The promotion of a functional fibrosis in skeletal muscle with volumetric muscle loss injury following the transplantation of muscle-ECM. Biomaterials 34:3324–3335. 10.1016/j.biomaterials.2013.01.061 [DOI] [PubMed] [Google Scholar]
- 54.Greising SM, Corona BT, McGann C, et al. (2019) Therapeutic Approaches for Volumetric Muscle Loss Injury: A Systematic Review and Meta-Analysis. Tissue Eng Part B Rev 25:. 10.1089/ten.teb.2019.0207 [DOI] [PubMed] [Google Scholar]
- 55.Pratt SJP, Iyer SR, Shah SB, Lovering RM (2018) Imaging analysis of the neuromuscular junction in dystrophic muscle. In: Methods in Molecular Biology. pp 57–72 [DOI] [PubMed] [Google Scholar]
- 56.Matsumoto-Miyai K, Sokolowska E, Zurlinden A, et al. (2009) Coincident Pre- and Postsynaptic Activation Induces Dendritic Filopodia via Neurotrypsin-Dependent Agrin Cleavage. Cell 136:1161–1171. 10.1016/j.cell.2009.02.034 [DOI] [PubMed] [Google Scholar]
- 57.Koulen P, Honig LS, Fletcher EL, Kröger S (1999) Expression, distribution and ultrastructural localization of the synapse-organizing molecule agrin in the mature avian retina. Eur J Neurosci 11:4188–4196. 10.1046/j.1460-9568.1999.00848.x [DOI] [PubMed] [Google Scholar]
- 58.Chakraborty S, Hong W (2018) Linking extracellular matrix agrin to the hippo pathway in liver cancer and beyond. Cancers (Basel) 10:15–18. 10.3390/cancers10020045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bassat E, Mutlak YE, Genzelinakh A, et al. (2017) The extracellular matrix protein agrin promotes heart regeneration in mice. Nature 547:179–184. 10.1038/nature22978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chakraborty S, Njah K, Pobbati AV., et al. (2017) Agrin as a Mechanotransduction Signal Regulating YAP through the Hippo Pathway. Cell Rep. 10.1016/j.celrep.2017.02.041 [DOI] [PubMed] [Google Scholar]
- 61.Watt KI, Judson R, Medlow P, et al. (2010) Yap is a novel regulator of C2C12 myogenesis. Biochem Biophys Res Commun 393:619–624. 10.1016/j.bbrc.2010.02.034 [DOI] [PubMed] [Google Scholar]
- 62.Tremblay AM, Missiaglia E, Galli GG, et al. (2014) The Hippo transducer YAP1 transforms activated satellite cells and is a potent effector of embryonal rhabdomyosarcoma formation. Cancer Cell. 10.1016/j.ccr.2014.05.029 [DOI] [PubMed] [Google Scholar]
- 63.Zhao K, Shen C, Lu Y, et al. (2017) Muscle yap is a regulator of neuromuscular junction formation and regeneration. J Neurosci 37:3465–3477. 10.1523/JNEUROSCI.2934-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw/processed data required to reproduce these findings cannot be shared at this time due to technical or time limitations.