Summary
Background
Since 2014, Brazil has gradually implemented the Xpert MTB/RIF (Xpert) test to enhance early tuberculosis (TB) and drug-resistant (DR-TB) detection and control, yet its nationwide impact remains underexplored. Our study conducts an intervention time-series analysis (ITSA) to evaluate how the Xpert's implementation has improved TB and DR-TB detection nationwide.
Methods
1,061,776 cases from Brazil's National TB Registry (2011–2022) were reviewed and ITSA (2011–2019) was used to gauge the impact of the Xpert's adoption on TB and DR-TB notification. Granger Causality and dynamic regression modelling determined if incorporating Xpert testing as an external regressor enhanced forecasting accuracy for Brazil's future TB trends.
Findings
Xpert implementation resulted in a 9.7% increase in TB notification and substantial improvements in DR-TB (63.6%) and drug-susceptible TB (92.1%) detection compared to expected notifications if it had not been implemented. Xpert testing counts also presented a time-dependent relationship with DR-TB detection post-implementation, and improved predictions in forecasting models, which depicted a potential increase in TB and DR-TB detection in the next six years.
Interpretation
This study underscores the critical role of Xpert's adoption in boosting TB and DR-TB detection in Brazil, reinforcing the case for its widespread use in disease control. Improvements in prediction accuracy resulting from integrating Xpert data are crucial for allocating resources and reducing the incidence of TB. By acknowledging Xpert's role in both disease control and improving predictions, we advocate for its expanded use and further research into advanced molecular diagnostics for effective TB and DR-TB control.
Funding
FIOCRUZ.
Keywords: Xpert MTB/RIF, Intervention time-series, Drug-resistant tuberculosis, Laboratory diagnostics, Public health measures, Brazil
Research in context.
Evidence before this study
Previous studies demonstrated the impact of implementing genotypic testing methods, such as the Xpert MTB/RIF (Xpert), on tuberculosis (TB) and drug-resistance (DR) screening across different countries. We performed a literature review on November 18th 2023, using PubMed for papers published examining the effect of the Xpert implementation on case notification of TB and drug-resistant TB (DR-TB) infected individuals. Using the following search (“Xpert” OR “GeneXpert” OR “GeneXpert MTB/RIF” OR “Rapid Molecular Test”) AND “tuberculosis” AND (((“Brazil”) AND (“association” OR “impact” OR “intervention”)) with no language, geographical or temporal restrictions, the search resulted in 22 papers retrieved ranging from 2014 to 2023. The evidence indicates that the Xpert possess an association with increased TB and DR-TB notification, however results were limited to specific cities and/or states and did not encompass the entire nation. Additionally, the studies did not investigate the long-term impacts of Xpert adoption on TB and DR-TB control, considering its gradual implementation into the public health system, in addition to focusing only on post-implementation data.
Added value of this study
Our study adds valuable insights to the existing literature by assessing both the immediate and sustained impacts of the Xpert's implementation in improving TB notification case counts in Brazil, a high-burden TB country. This includes a specific look at DR-TB and drug-susceptible (DS-TB) case detection, alongside the reach of other TB diagnostic laboratory methods. We present a comprehensive nationwide analysis of 1,061,776 TB cases reported to the Brazilian disease registry data between 2011 and 2022, while previous research only considered data strictly from individual municipalities and regions of Brazil or solely assessed the data post Xpert implementation. Our approach integrating Intervention Time Series analysis (2011–2019) with Cross-Correlation Function showcased the sustained improvements in TB case detection and resistance screening post-Xpert implementation, especially when compared to expected notifications if Xpert had not been implemented. Our findings also established the Xpert's role in improving predictions of future TB and DR-TB trends, allowing us to enhance the accuracy of forecasting models of TB and DR-TB case detection for the next 6 years. Thereby, this pioneering approach extends beyond standard effect assessment, by providing a dual perspective on both immediate and long-term effects of health interventions exemplified by the adoption of Xpert technology to improve TB and DR-TB control in Brazil's healthcare system.
Implications of all the available evidence
Our comprehensive findings illuminate the vital role of the adoption of Xpert technology in improving the detection of TB and DR-TB in Brazil, a country significantly affected by TB. The study highlights the need for continuous support of the widespread adoption of the Xpert system, which has been intricately linked to enhanced TB and especially DR-TB case detection. Furthermore, we propose direct, actionable public health interventions aimed at bridging the gap between academic research and practical application. Our study not only provides evidence for the role of Xpert's implementation in enhancing case detection and testing coverage for other diagnostic methods but also suggests comprehensive strategies including targeted regional measures to mitigate geographical disparities in testing coverage. Moreover, we demonstrate the benefits of incorporating Xpert testing counts into TB and DR-TB case notification forecasting models, aiming to enhance their accuracy measures, which is crucial for planning and resource allocation in TB and drug-resistance control programs. Additionally, our novel methodology can serve as a model for evaluation of the long-term effectiveness of other public health interventions, such as the Xpert implementation, enriching our understanding of their sustained effect on targeted strategies. This convergent perspective of evidence and application serves as a call for policymakers to continue adopting innovative, evidence-based interventions to reinforce the Brazilian TB healthcare system and contribute to the global fight against TB.
Introduction
Tuberculosis (TB) remains a major public health concern worldwide, particularly in low- and middle-income countries.1 Of note, Brazil ranks among the 30 highest TB burden countries, a situation exacerbated by inequality and poverty which contribute to the nation's elevated social and epidemiological TB risk factors.1 To address this challenge, the Brazilian government has created strategies to mitigate this extensive disease burden. A notable step was the gradual adoption of the Xpert MTB/RIF (Xpert) technology, starting in 2014.2 This rapid molecular test (RMT) enables faster detection of Mycobacterium tuberculosis (MTB) and rifampicin resistance (RR), aiding in the timely management of suspected cases.2 Notably, substantial advancements in case detection and drug-resistance screening have been observed in other high-burden TB countries following Xpert's implementation.3,4
Furthermore, Brazil has recently established a highly praised interministerial committee, created for its multi-sectoral approach towards eradicating the TB epidemic, extending beyond just the healthcare sector.5 These goals are facilitated by the Brazilian Tuberculosis Database System (SINAN-TB), a health monitoring system that aggregates epidemiological data from all TB cases nationwide.6 Supported by a policy of mandatory TB notification and regular updates endorsed by the Ministry of Health (MoH), the SINAN-TB, has been pivotal in exploring the intricacies of TB epidemiology in Brazil, including its interplay with complex comorbidities like HIV and diabetes.6
Nevertheless, a significant gap persists in understanding and managing drug resistance (DR) to anti-TB drugs in Brazil. Therefore, given the escalating global prevalence of TB and drug-resistant TB (DR-TB),1 our study conducts an intervention time-series analysis (ITSA) together with cross-correlation function (CCF) to evaluate the association of the Xpert's gradual implementation with continued improvements in TB and DR-TB detection nationwide, as well as how it affected the testing coverage of other laboratory diagnosis methods. Furthermore, we investigated the potential of using Xpert testing data to improve prediction models of future disease burden.
Methods
Ethics statement
This study solely utilized data obtained from a public government platform (https://portalsinan.saude.gov.br/). These data were pre-processed by the Brazilian MoH to check for duplicates, completeness, and consistency, following the guidelines of Resolution Number 466/12 on Research Ethics by the National Health Council, Brazil.7
Overall study design
We performed a nationwide epidemiological study, using data from the SINAN-TB database, to determine the impact of Xpert implementation on TB and DR-TB case notification in Brazil, as well as its influence on testing coverage of other TB diagnosis and DR screening methods. Finally, this study also aimed to explore the potential of leveraging Xpert testing rates to enhance predictive models for TB burden.
Data collection
Data from all notified TB cases, diagnosed by microbiologic confirmation (bacteriology in sputum smears/positive culture/molecular testing), chest radiography, histopathology, or clinical manifestations, were collected across the 26 Brazilian states and the Federal District (Brasília), between 2011 and 2022. The laboratory diagnosis protocol followed criteria established by the Brazilian MoH,8 further detailed in the Supplementary Methods. In this study, we defined our exclusion criteria to omit cases under 10 years old and post-mortem notifications, due to distinct diagnostic protocols,8 as well as cases with Xpert testing data before its official implementation in 2014, which likely represents either early-adopters of the prior to its official rollout or data entry errors.
Our dataset was structured in a case-based format, meaning each TB case was represented by a unique row. This approach allowed for detailed, individual-level analysis of each case. Additionally, our data was further aggregated into national-level yearly measures of case notification and testing coverage for TB and DR-TB. While the SINAN-TB system boasts an extensive national coverage with data from all notified TB cases being consistently maintained and verified by the Brazilian MoH,9 the data's completeness varies significantly across regions.
Notification of TB cases
In Brazil, the notification of TB cases is mandatory and composed of four primary stages: diagnosis, notification, treatment accessibility and update notification. Diagnosis: the diagnosis of TB typically occurs across various healthcare facilities, including healthcare centres, family healthcare units, hospitals, specialty clinics, and private medical offices, employing a mix of epidemiological, imaging, clinical, or laboratory tests. A detailed description of the other three stages can be found in Supplementary Methods.
Study definitions
Variables were defined as follows: (i) phenotypic drug-sensibility (DS) test: culture-based DS test; (ii) Xpert: RMT for MTB detection and DR screening; (iii) MTB Culture: result of the smear culture test for MTB; (iv) Smear microscopy: result of the first smear test for acid-fast bacilli.
Additionally, both the Xpert and phenotypic DS tests were used to calculate confirmed DR-TB and DS-TB cases. In such estimations, results from the phenotypic test were considered in cases of discordance between the DS test results, due to its greater accuracy.10 DR-TB categorization incorporated any form of resistance found in the phenotypic test, including isoniazid or rifampicin monoresistance, multidrug-resistance (MDR), or resistance to other first-line drugs, including pyrazinamide and ethambutol. Resistance indicated by both methods or solely by the Xpert, including when phenotypic test results were unavailable, were also considered. DS-TB cases were classified as those with susceptibility to all drugs assessed by the culture-based method, or as rifampicin susceptible by the Xpert, including scenarios in which only Xpert testing was performed.
Statistical analysis
Exploratory data analysis
Exploratory data analysis, using descriptive statistics to present the data as frequency (#) and proportions, evaluated the notification, testing coverage, and results of the following TB laboratory diagnosis methods: Xpert, smear microscopy, MTB culture, and phenotypic DS. All statistical analyses included in this paper were performed using R (version 4.3.1), with the package descriptions shown in Supplementary Table S1.
Intervention time-series analysis
In assessing the impact of Xpert adoption on TB and DR-TB control in Brazil, we conducted a comprehensive ITSA using national-level data from the SINAN-TB database, which was stratified into yearly intervals covering the period between 2011 and 2019. To avoid confounding effects on TB detection and reporting caused by the COVID-19 pandemic, we removed the data from 2020 to 2022 exclusively from ITSA analysis. ITSA is a statistical approach designed to evaluate the effect of interventions over time, being particularly suited to scenarios where intervention is introduced gradually, such as the implementation of Xpert in Brazil. ITSA compares observed outcomes against what might have occurred without the intervention, incorporating models that account for both level changes (immediate effects) and slope changes (changes in trend over time), in addition to handling more complex temporal patterns such as autocorrelation and non-stationarity.
Initially, an automated algorithm was employed to detect significant changes that cause large fluctuations in our time-series data, classified as anomalies. Specifically, we aimed to identify Level Shift anomalies which indicate a sudden and permanent structural change in the series level, suggesting an intervention in the data.11 Through this, we aimed to pinpoint the exact year in which effects triggered by Xpert's implementation began to statistically affect yearly measures of case notification and testing coverage for TB and DR-TB in Brazil, which was classified as the intervention year. Details on anomaly detection/evaluation are depicted in the Supplementary Methods.
Next, to perform an ITSA, we use the intervention year detected as a level shift in the model to segment our data into pre- and post-intervention phases. Following previously published studies for accurately forecasting small-sized time-series,12,13 we then employed ARIMA and ForecastHybrid models12,13 to predict the scenario without Xpert adoption (counterfactual scenario) from data collected prior to its implementation and compared their accuracy measures with Mean Absolute Percentage Error (MAPE). Afterwards, the most accurate predictions of the hypothetical (counterfactual) scenarios were then compared to the real (observed) values post-intervention to assess the impact of Xpert implementation. Notably, data from pandemic-affected years were excluded from this analysis to accurately isolate the effect of Xpert's implementation.
Of note, through the usage of differencing techniques included in both the anomaly detection and ITSA analysis, we aimed to effectively filter out possible underlying trends and seasonal variations of TB infection that existed in our data and could be attributed to a series of other epidemiological factors. This methodology aimed to meticulously isolate Xpert's impact on TB and DR-TB case detection.
Correlation and granger causality analysis
Next, we aimed to determine possible associations between annual Xpert testing counts and TB case notifications, post Xpert's initial rollout. Initially, Spearman's rank correlation tests were performed, aiming to determine the strength and direction of a monotonic relationship in the overall trend of Xpert testing with TB and DR-TB case detection and testing notifications. To adjust for the small sample sizes and ensure precision in our analysis, p-values were calculated using a precomputed exact null distribution algorithm.14 Additionally, we aimed to further explore this relationship by employing the CCF, which is considered a superior method in assessing correlations between time-series data due to its ability to determine the time-lagged correlations between data.15 This was used to indicate if changes in Xpert testing counts, post the test's initial implementation, possessed an immediate or lagged impact on TB and DR-TB notifications. Finally, we also explored if including the annual coverage of Xpert testing into the model training could improve the accuracy of prediction models for future TB and DR-TB case notification using the Toda-Yamamoto procedure, a method for testing Granger Causality. Granger Causality is a statistical technique that allows us to analyse the causal relationships between time series variables. In our study, this technique evaluates whether past values of one time series can provide information that aids in predicting another time series.16 Further explanations of how the Toda-Yamamoto procedure was performed can be found in the Supplementary Methods.
Dynamic regression forecasting models
Dynamic regression forecasting models were developed using the ARIMA and ForecastHybrid techniques, to further access the relationship and Xpert testing counts with case notification and testing coverage of TB and DR-TB in Brazil. These models are particularly useful because they are designed to predict future trends by combining historical data with the influence of external factors to improve forecasting accuracy.17
In our study, we leveraged dynamic regression forecasting models by incorporating both annual Xpert testing counts as an external regressor and historical data from each time series in ARIMA and ForecastHybrid models. We then compared these dynamic models to univariate versions, based solely on historical data, aiming to explore how Xpert testing counts may impact and assist in predicting the future trends of TB and DR-TB notifications. To assess model accuracy, we segmented the time-series into training data (in-sample: 2011–2020) and test data (out-of-sample: from 2021 to 2022). Then, the models with the lowest MAPE were selected to predict a possible scenario for TB and DR-TB notifications during 2023–2028, with the results further investigated through the exploration of significant trends using the Mann–Kendall test and Sen's slope estimates.
Finally, a specific analysis was performed to assess the impacts of Xpert's implementation on DR-TB and DS-TB case subgroups, according to their different DS testing methods, including phenotypic, Xpert, and both tests (Supplementary Figures S2 and S3). A more detailed explanation of each analysis is provided in the Supplementary Methods.
Geographical analysis
Additionally, we investigated regional variations regarding the coverage of diagnostic tests and case notification of DR-TB cases. To address the inherent geographical disparities in TB infection per region, we calculated notification rates from 2011 to 2022. The calculations were made by first, assessing the number of notified resistant and susceptible TB cases and the total number of cases that performed each TB diagnostic method (testing coverage), and then comparing this data to all TB cases reported per region in the SINAN-TB database. The results were then expressed per 100 cases. For calculating Xpert testing coverage per region, we used data exclusively from the post-implementation period of 2014–2022. We also analysed state-wise distribution of TB notifications and testing coverage, using the total number of reported cases. To capture the most recent trends and assess the pandemic's impact on TB diagnostics and reporting, we also included the data from the era affected by the COVID-19 pandemic in our geographical analysis.
Role of the funding source
The funding source had no role in study design, data collection, analysis and interpretation, or the decision to submit the work for publication.
Results
TB diagnostic tests reported to the SINAN-TB database
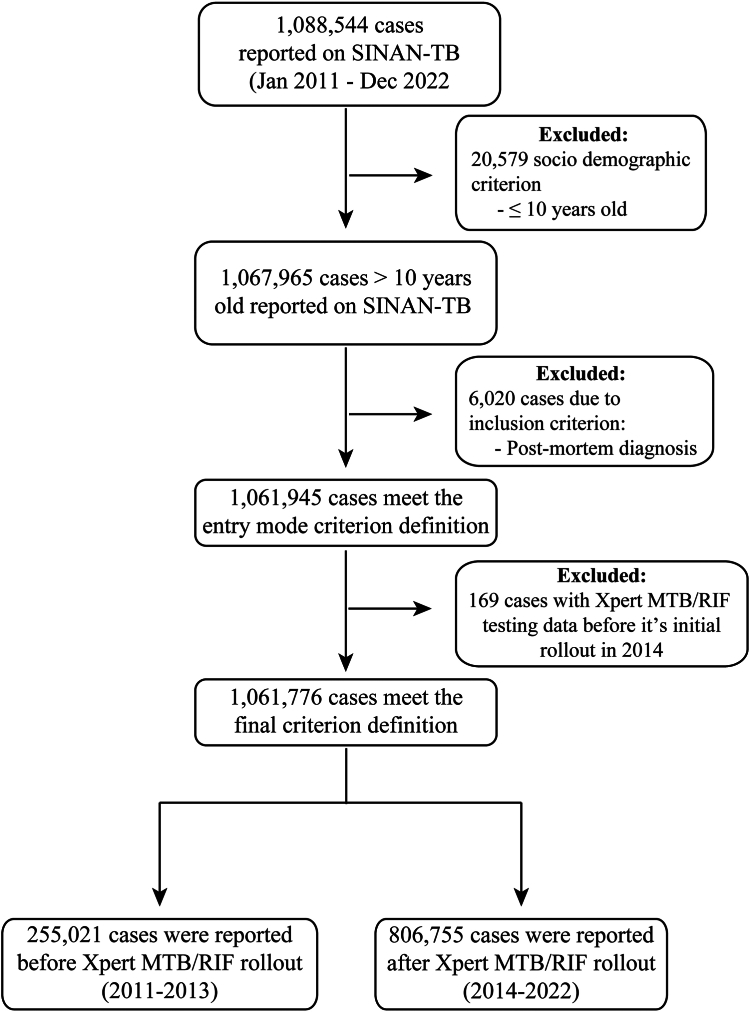
Our study encompassed 1,088,544 cases reported between 2011 and 2022, of which 26,768 cases were removed due to the exclusion criteria, resulting in 1,061,776 TB cases. The findings of this research were derived from two sub-datasets: one spanning from 2014 to 2022, to assess the characteristics associated with the results of Xpert; and another from 2011 to 2019, employed for evaluating the implementation's impact over time while excluding the influence of the COVID-19 pandemic. For descriptive analyses focused on exhibiting Xpert testing coverage, results, and geographical distributions, 255,021 pre-implementation cases were omitted (2011–2013), resulting in 806,755 cases from 2014 to 2022 (Fig. 1).
Fig. 1.
Flow chart of TB cases notified in Brazil between 2011 and 2022. Abbreviations: TB, Tuberculosis; SINAN-TB, Brazilian Tuberculosis Database System.
Initially, we found substantial data gaps for phenotypic DS testing, with 58% data missing (619,623/1,061,776 cases). Similarly, regarding testing coverage, only smear microscopy was broadly utilized, with less than half of TB cases performing MTB culture (33%; 354,154/1,057,569), Xpert (37%; 256,495/695,143), or phenotypic DS testing (27%; 321,784/442,153). DR screening with phenotypic tests revealed a predominance of isoniazid monoresistance (36%; 3711/10,345), followed by resistance to other first-line drugs (27%; 2770/10,345), MDR-TB (23%; 2323/10,345), and rifampicin monoresistance (14%; 1541/10,345) (Table 1).
Table 1.
Status and results of laboratory diagnostic testing data in SINAN-TB.
| Xpert MTB/RIF | Smear microscopy | Culture | Phenotypic DST | |
|---|---|---|---|---|
| Notification status, n (%) | ||||
| Reported | 695,143 (86.1%) | 1,061,254 (99.05%) | 1,057,569 (99.6%) | 442,153 (58.4%) |
| Not reported | 111,612 (13.9%) | 522 (0.05%) | 4207 (0.4%) | 619,623 (41.6%) |
| Total | 806,755 | 1,061,776 | 1,061,776 | 1,061,776 |
| Testing coverage, n (%) | ||||
| Performed | 256,495 (36.9%) | 761,936 (71.8%) | 354,154 (35.5%) | 321,784 (27.2%) |
| Not performed | 438,648 (63.1%) | 264,692 (24.9%) | 703,415 (66.5%) | 120,369 (72.8%) |
| Not applicable | N/A | 34,626 (3.3%) | N/A | N/A |
| Total | 695,143 | 1,061,254 | 1,057,569 | 442,153 |
| MTB detection, n (%) | ||||
| MTB positive | 205,981 (80.3%) | 545,730 (71.6%) | 210,364 (27.7%) | N/A |
| MTB negative | 38,218 (14.9%) | 216,206 (28.4%) | 98,122 (12.9%) | N/A |
| Inconclusive | 12,296 (4.8%) | N/A | N/A | N/A |
| Waiting results | N/A | N/A | 45,668 (59.4%) | N/A |
| Total | 256,495 | 761,936 | 354,154 | 321,784 |
| Resistance screening, n (%) | ||||
| Drug-Susceptible | 198,447 (96.3%) | N/A | N/A | 95,858 (79.6%) |
| Drug-Resistance | 7534 (3.7%) | N/A | N/A | 10,345 (8.6%) |
| Waiting results | N/A | N/A | N/A | 14,166 (11.8%) |
| Resistance subtypes, n (%) | ||||
| Rifampicin monoresistance | 7534 (100%) | N/A | N/A | 3711 (35.8%) |
| Isoniazid monoresistance | N/A | N/A | N/A | 2323 (22.4%) |
| Multidrug-Resistance | N/A | N/A | N/A | 2770 (26.7%) |
| Resistant to other first-line TB drugs | N/A | N/A | N/A | 1541 (14.8%) |
| Total | 7534 | 10,345 |
This table presents a consolidated view of TB testing data, including notification status, testing coverage, MTB detection rates, and resistance screening outcomes. Reported numbers reflect cases within the Brazilian national TB notification system (SINAN), and percentages indicate the proportion of each category relative to the total number of cases tested with each respective diagnostic method. ‘na’ denotes data not applicable or not available. All testing and reporting are performed in accordance with the protocols of the Unified Health System (SUS) and SINAN set by the Ministry of Health.9 Abbreviations: MTB, Mycobacterium tuberculosis; DST, Drug–Sensitivity Test.
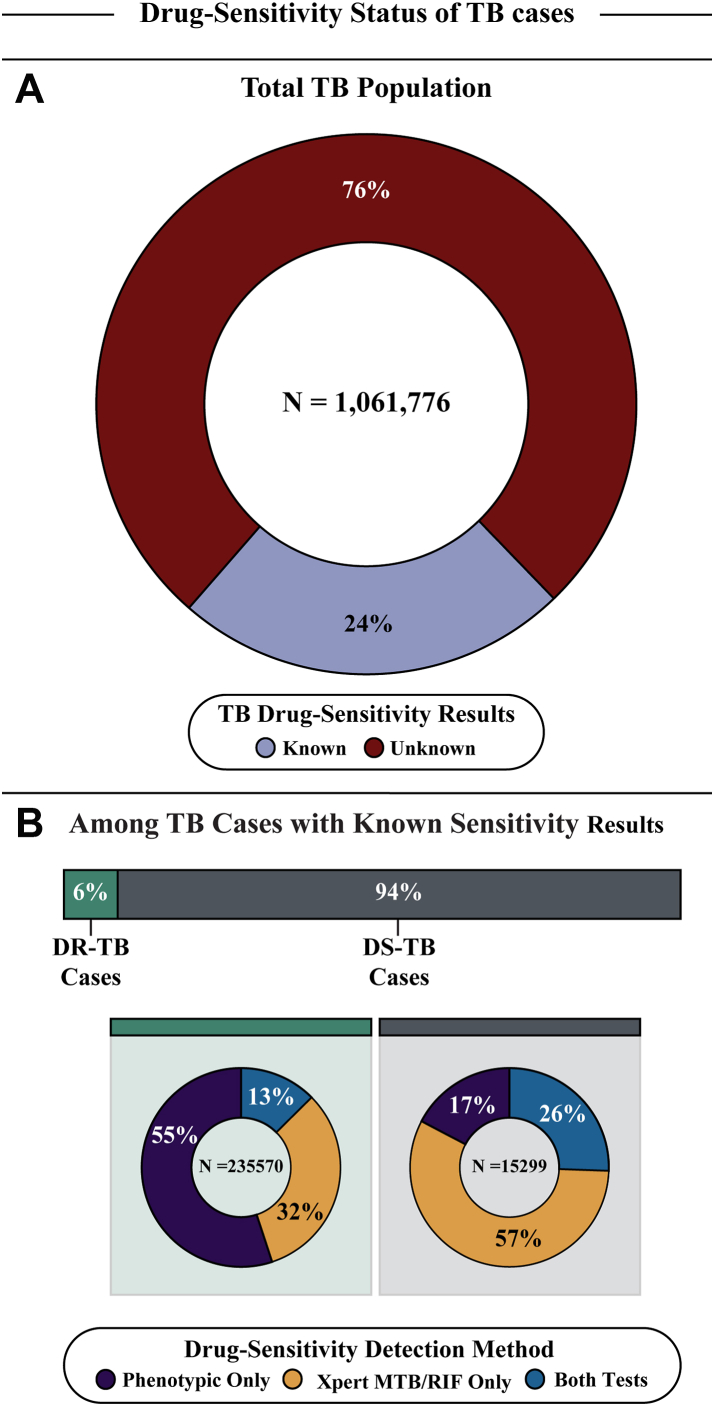
Furthermore, only 24% of TB cases had confirmed DS results (250,869/1,061,776); of these, 94% were DS-TB (235,570/250,869), which were primarily detected by Xpert (83%; 194,829/235,570), either alone (57%; 134,621/235,570) or with phenotypic tests (26%; 60,208/235,570). For DR-TB, most cases were detected solely by phenotypic testing (55%; 8422/15,299), with Xpert diagnosing 32% (4954/15,299), and both methods were used in 13% (1923/15,299) of cases (Fig. 2).
Fig. 2.
Distribution of TB cases by drug–sensitivity status and detection methods. This figure illustrates the distribution of TB cases based on drug-sensitivity results and the methods used for detection. Panel A depicts the proportion of the total TB population with known and unknown drug-sensitivity results, with 24% of cases having known drug-sensitivity results. While, Panel B provides a detailed breakdown of TB cases with known sensitivity results, further categorized into Drug-Resistant TB (DR-TB) and Drug-Susceptible TB (DS-TB), with the respective detection methods used, including solely Phenotypic DS test or Xpert MTB/RIF, and cases detected by both tests. Abbreviations: TB, Tuberculosis; DR-TB, Drug-resistant Tuberculosis; DS-TB, Drug-susceptible Tuberculosis.
Impact of Xpert implementation on the TB control
Next, we investigated the impact of Xpert implementation on TB and DR-TB notifications and testing coverage for other TB detection methods in Brazil. An ITSA, using data from 2011 to 2019, was conducted on the yearly notification counts of total TB cases, including DR-TB and DS-TB, as well as the testing coverage of phenotypic DS, MTB culture, and smear microscopy methods.
Significant anomalies, identified as level shifts, were found in all variables. However, the year in which the effects of Xpert adoption began to statistically impact the mean level of each time-series model differed between variables (Supplementary Table S2). After identifying the intervention year, we then created forecasting models using pre-intervention data with both ARIMA and ForecastHybrid, comparing their out-sample MAPE values on the post-intervention data to select the most accurate models for predicting counterfactuals. As seen in the Supplementary Tables S3 and S4, the forecast models with highest accuracy, as well as the potential range of scenarios from the intervention's impact also differed according to time-series data.
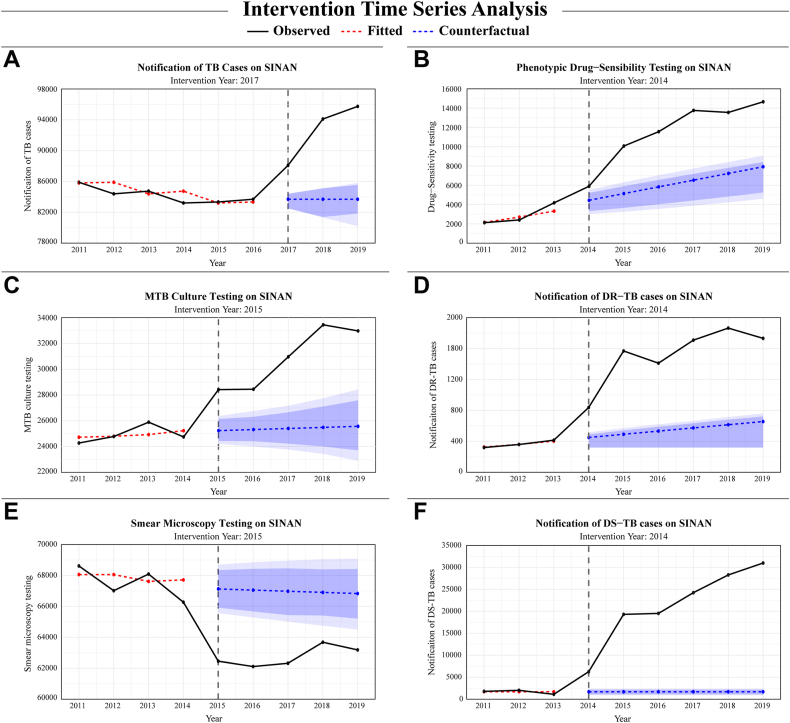
Next, counterfactual measures were compared to observed values, demonstrating the intervention's significant impact on all models. Specifically, DS-TB case notifications displayed the most substantial improvements with an average increase of 92.1% [95% PI: 95.8%–88.9%]. Similarly, DR-TB notifications rose by 63.6% [79.0%–57.9%] with observed cases numbering 1521 and counterfactual projections at 553 [320–641].
Additionally, phenotypic DS testing showed a significant increase of 46.9% [95% PI: 67.6%–36.6%], based on an observed count of 11,580 and a counterfactual of 6151 [3749–7340]. While, MTB culture coverage was also enhanced, rising by 17.7% [23.3%–11.5%] from an observed figure of 30,849 to a counterfactual of 25,402 [23,662–27,293] testing coverages exhibited significant increases. Conversely, total TB case notifications exhibited a lower difference between observed (92,643) and counterfactual cases (83,690 [81,347–85,104]), with a positive effect of 9.7% [12.2%–8.1%] (Table 2 and Fig. 3).
Table 2.
Comparison between the post-intervention and the counterfactual average and last year values.
| Time-series data: |
Average |
Last year (2019) |
|||||
|---|---|---|---|---|---|---|---|
| Models | Intervention year | Observed | Counterfactual (95% P.I.) | Difference (%) | Observed | Counterfactual (95% P.I.) | Difference (%) |
| Total TB cases | 2017 | 92,643 | 83,690 [81,347–85104] | 9.7% | 95,744 | 83,690 [80,207–85806] | 12.6% |
| Phenotypic DS testing | 2014 | 11,580 | 6151 [3749–7340] | 46.9% | 14,657 | 7849 [4594–9060] | 46.4% |
| DR-TB cases | 2014 | 1521 | 553 [320–641] | 63.6% | 1732 | 656 [320–761] | 62.1% |
| DS-TB cases | 2014 | 21,431 | 1694 [893–2389] | 92.1% | 30,990 | 1694 [893–2389] | 94.5% |
| MTB culture testing | 2015 | 30,849 | 25,402 [23,662–27293] | 17.7% | 32,970 | 25,571 [22,906–28431] | 22.4% |
| Smear microscopy testing | 2015 | 62,752 | 66,981 [65,026–68929] | −6.7% | 63,185 | 66,832 [64,503–69085] | −5.8% |
Data represents average values from the last year (2019) for the variables analysed. The “Observed” column represents the actual values during the intervention period, while the “Counterfactual” column presents estimated values without the intervention, with a 95% prediction interval (P.I.). The “Difference (%)” column indicates the percentage difference between observed and counterfactual values, reflecting the impact of the intervention, calculated with the following formula: Percentage Difference = ((Counterfactual - Observed)/Observed) ∗ 100. Abbreviations: TB, Tuberculosis; DR-TB, Drug-resistant tuberculosis; DS-TB, Drug-susceptible tuberculosis; MTB, Mycobacterium tuberculosis.
Fig. 3.
Intervention time-series analysis to measure the impact of Xpert implementation. This analysis provides a comparative view of the real-world outcomes observed after Xpert implementation (illustrated by the black lines) versus the predicted observations in a scenario without its implementation (represented by the blue lines). The intervention year was chosen through a comprehensive anomaly detection analysis, which identified significant level-shifts in each time-series. Prediction intervals are presented at two confidence levels: 80% and 95%. These intervals are visually differentiated by two distinct shades of blue, with the lighter shade representing the broader 95% interval. Using this approach, we assessed the number of TB cases reported (A), DS tests (B), Mtb culture tests performed (C), DR-TB cases reported (D), smear microscopy (E), and cases of DS-TB notified (F). Abbreviations: TB, Tuberculosis; DR-TB, Drug-resistant tuberculosis; DS-TB, Drug-susceptible tuberculosis; MTB, Mycobacterium tuberculosis.
In contrast, smear microscopy testing exhibited smaller observed values (62,752) when compared to the counterfactuals (66,981 [65,026–68929]), highlighted by an average decrease of 6.7% [−3.6% to −9.8%] (Table 2 and Fig. 3).
Predictive influence of the Xpert testing on TB case detection
Afterwards, we evaluated associations between the annual Xpert testing counts with each of our time-series data. Spearman rho's results displayed robust positive significant correlations with total TB case notification, including DR-TB and DS-TB, as well as phenotypic DS and MTB culture testing coverage. Conversely, a strong negative correlation was found with annual microscopy testing (Table 3, Supplementary Table S5).
Table 3.
Correlation and causality analysis between Xpert MTB-RIF testing and anomaly time-series data.
| Time-series data |
Cross-Correlation |
Toda-Yamamoto procedure for granger causality |
||
|---|---|---|---|---|
| Target | Lag of highest correlation | Correlation coefficient | F-statistic | p-value |
| Total TB cases | 0 | 0.76 | 4.82 | 0.030 |
| DR-TB cases | 0 | 0.92 | 4.14 | 0.020 |
| DS-TB cases | 0 | 0.96 | 4.61 | 0.047 |
| Phenotypic DS testing | 0 | 0.95 | 0.24 | 0.260 |
| MTB culture testing | 0 | 0.91 | 5.24 | 0.028 |
| Smear microscopy testing | 1 | −0.75 | 4.76 | 0.035 |
This table represents results from three analyses: Spearman's rank correlation test, Cross-Correlation Function, and Toda-Yamamoto Procedure for Granger Causality. For Spearman's rank correlation, we reported both Spearman's rho and the associated p-value calculated using exact null distribution. In the Cross-Correlation analysis, we showed the correlation coefficients and the respective lag of highest correlation. All displayed cross-correlation coefficients were deemed significant based on ACF plots with a p-value less than 0.05. The Granger Causality analysis employed the Toda-Yamamoto procedure, presenting both the F-statistic and p-values. p-values lower than 0.05 are considered statistically significant and are highlighted in bold.
Furthermore, CCF highlighted the time–sensitive relationships between annual increases in Xpert testing and the TB and DR-TB detection rates. This analysis increases in the number of tests were associated with immediate corresponding changes in TB and DR-TB detection rates (lag = 0). Notably, an increase in the Xpert testing was also correlated with a decrease in smear microscopy testing. However, the decrease in smear microscopy testing only occurred one year after the increase in Xpert testing (lag = 1). Our findings indicate that the effect of an increase in Xpert testing on TB and DR-TB detection rates, as well as on reducing smear microscopy testing is not merely temporary. Instead, the results suggest a prolonged and sustained impact caused by Xpert testing, rather than a one-off change (Table 3).
Pivotal to our findings, the Granger Causality test expanded our understanding about the abovementioned relationships by highlighting how including Xpert testing coverage as an external predictor can improve TB forecasting models on distinct settings. Our results indicate that Xpert testing can be used to predict detection of total TB cases (p = 0.030), including DS-TB, and DR-TB (p = 0.020, 0.047, Table 3), in addition to changes in MTB culture or smear microscopy testing coverage (p = 0.028, 0.035, Table 3).
Dynamic regression forecasts of TB case notification in Brazil
We employed dynamic regression forecasting models for all time-series variables, using annual Xpert testing coverage as an external regressor to further test its predictive power. These results revealed that Xpert testing was associated with improved prediction accuracy, as indicated by lower MAPE values compared to univariate versions for the following models: DR-TB (6.10%; 110.02/1810.50), DS-TB (2.08%; 746.60/35,881) and overall TB cases (4.71%; 4531.75/96,342) as well as smear microscopy (6.80%; 4214.70/61,957.50) and MTB culture (9.02%; 3195.33/35,440.5) testing. Conversely, for phenotypic DS testing, the univariate models displayed more accurate predictions (3.51%; 506.21/14,432.50), coherent with Granger Causality results (Table 4).
Table 4.
Observed and forecasted values for TB and DR-TB testing and notification case counts.
| Year | Total TB cases | DR-TB cases | DS-TB cases | Phenotypic DS testing | Smear microscopy testing | MTB culture testing | Xpert MTB/RIF testing |
|---|---|---|---|---|---|---|---|
| 2011 | 85,892 | 320 | 1788 | 2124 | 68,618 | 24,273 | 0 |
| 2012 | 84,389 | 359 | 2015 | 2400 | 67,016 | 24,778 | 0 |
| 2013 | 84,740 | 415 | 1112 | 4177 | 68,093 | 25,891 | 0 |
| 2014 | 83,205 | 839 | 6269 | 5890 | 66,272 | 24,746 | 2673 |
| 2015 | 83,334 | 1569 | 19,298 | 10,064 | 62,457 | 28,419 | 19,967 |
| 2016 | 83,690 | 1411 | 19,518 | 11,556 | 62,113 | 28,449 | 19,198 |
| 2017 | 88,084 | 1709 | 24,238 | 13,760 | 62,324 | 30,962 | 25,425 |
| 2018 | 94,102 | 1865 | 28,275 | 13,552 | 63,681 | 33,444 | 32,971 |
| 2019 | 95,744 | 1732 | 30,990 | 14,657 | 63,185 | 32,970 | 35,895 |
| 2020∗ | 85,912 | 1459 | 30,305 | 13,324 | 54,262 | 29,341 | 35,288 |
| 2021∗ | 90,867 | 1777 | 33,033 | 14,039 | 58,314 | 31,334 | 38,965 |
| 2022∗ | 101,817 | 1844 | 38,729 | 14,826 | 65,601 | 39,547 | 46,113 |
| 2023 | 92,308 [86,072–99124] | 1752 [1351–2332] | 35,559 [33,140–45933] | 15,943 [12,603–19553] | 61,205 [54,166–67212] | 32,972 [29,486–37113] | 50,091 [40,654–61527] |
| 2024 | 92,148 [85,912–98964] | 1800 [1173–2583] | 36,125 [32,412–48772] | 17,075 [12,384–21897] | 61,301 [54,262–67308] | 32,827 [29,341–36968] | 53,556 [39,171–67442] |
| 2025 | 93,117 [86,881–99933] | 1909 [1131–2836] | 38,682 [32,958–52236] | 18,206 [12,401–24192] | 60,721 [53,682–66728] | 33,703 [30,217–37844] | NA |
| 2026 | 95,001 [88,765–101817] | 2069 [1149–3098] | 42,851 [34,609–56317] | 19,337 [12,696–26294] | 59,595 [52,555–65601] | 35,406 [31,920–39547] | NA |
| 2027 | 96,049 [89,813–102865] | 2183 [1144–3309] | 45,548 [35,825–59486] | 20,469 [13,072–28179] | 58,967 [51,928–64974] | 36,353 [32,867–40495] | NA |
| 2028 | 96,962 [90,726–103778] | 2289 [1144–3515] | 48,006 [36,897–62611] | 21,600 [13,445–30067] | 58,421 [51,382–64428] | 37,178 [33,692–41320] | NA |
The table presents observed values from 2011 to 2022 and forecasted values for the years 2023–2028. The values in bold font type represent the forecasted values, while the non-bold values correspond to the observed data. The forecasted values are accompanied by the 95% prediction intervals with lower and upper bounds. These projections stem from models integrating historical data and Xpert testing counts as an external regressor in the case of Total TB, DR-TB, and DS-TB Cases, as well as Smear Microscopy and MTB Culture testing. Phenotypic DS Testing and Xpert MTB/RIF testing models relied exclusively on historical data. Note that these projections represent one possible scenario based on historical trends and Xpert testing, not accounting for other influencing factors. For Xpert testing, predictions were made only until 2024. Data from 2020 to 2022 were labelled with an asterisk (∗) to denote the years impacted by the COVID-19 pandemic. Abbreviations: TB, Tuberculosis; DR-TB, Drug-resistant TB; DS-TB, Drug-susceptible; DS, Drug-sensitivity; MTB, Mycobacterium tuberculosis.
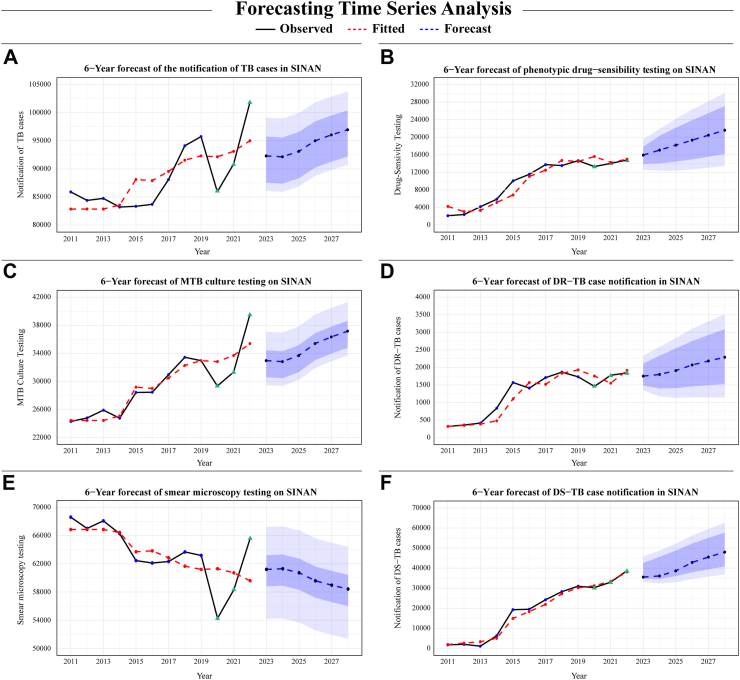
Subsequently, 6-year forecasts using the best-performing models projected a possible scenario for future trends of TB and DR-TB in Brazil. To enhance the precision of dynamic regression models, we also performed a 2-year forecast of yearly Xpert testing using only historical data, which possessed an out-of-sample MAPE accuracy value of 5.04% (726.91/14432.5) and an upward trend (Table 5 and Supplementary Figure S3). The 6-year predictions then incorporated both these forecasted values alongside the historical last four years of Xpert testing data as external regressors.
Table 5.
Trend analysis of TB and DR-TB cases and diagnostic methods over time.
| Subgroup | Mann–Kendall test for trend tau value | Sen's slope estimates | p-value |
|---|---|---|---|
| Total TB cases | |||
| Historical | 0.515 | 965 [167–1929] | 0.024 |
| Historical + Forecasted | 0.608 | 807 [535–1061] | 0.0004 |
| DR-TB cases | |||
| Historical | 0.758 | 147 [48–227] | 0.0007 |
| Historical + Forecasted | 0.830 | 103 [55–125] | <0.0001 |
| DS-TB cases | |||
| Historical | 0.909 | 3598 [2703–4376] | <0.0001 |
| Historical + Forecasted | 0.922 | 2697 [2139–3126] | <0.0001 |
| Phenotypic DS testing | |||
| Historical | 0.848 | 1236 [715–1753] | 0.0001 |
| Historical + Forecasted | 0.935 | 1127 [873–1155] | <0.0001 |
| MTB culture testing | |||
| Historical | 0.758 | 1126 [577–1517] | 0.0008 |
| Historical + Forecasted | 0.765 | 759 [584–918] | <0.0001 |
| Smear microscopy testing | |||
| Historical | −0.515 | −771 [(−1520) to (−142)] | 0.024 |
| Historical + Forecasted | −0.621 | −537 [(−654) to (−308)] | 0.0003 |
This table presents the results of the Mann–Kendall test for trend analysis across different categories of Tuberculosis (TB) cases and associated diagnostic methods. Sen's Slope Estimates provide the estimated yearly change, with the range in square brackets indicating the 95% confidence interval. Statistical significance was indicated by a p-value lower than 0.05 and depicted in bold. Abbreviations: TB, Tuberculosis; DR-TB, Drug-resistant TB; DS-TB, Drug-susceptible; DS, Drug-sensitivity; MTB, Mycobacterium tuberculosis.
Using models that included historical data from each variable and Xpert testing data, our forecast showed one potential scenario indicating an increase in TB detection, including confirmed DR-TB and DS-TB cases. Our models also demonstrated that, after a spike in 2022, case counts were anticipated to revert closer to the 2021 levels, but eventually most models approach the peak reported in 2022 with time (Fig. 4). These findings were further elucidated by the MK test for trend and Sen's slope estimates, denoting significant positive trends across most time-series data. Smear microscopy, notably, was once again an exception, highlighting a decreasing trend in forecasted values (Table 5).
Fig. 4.
6-Year dynamic regression model forecast for TB and DR-TB testing and notification case counts. This illustrative analysis presents a 6-year forecast of observations in each outcome time-series present in this study. For (A) Total TB case notification, (C) MTB culture testing, (D) DR-TB case notification, (E) Smear Microscopy testing and (F) DS-TB case notification, external regressors in the form of the Xpert testing coverage were integrated into the forecast analysis. Conversely, the forecasting for (B) phenotypic drug-sensitivity testing was constructed using univariate models. Of note, these projections represent one possible scenario based on historical trends and Xpert testing, not accounting for other influencing factors. Prediction intervals are presented at two confidence levels: 80% and 95%. These intervals are visually differentiated by two distinct shades of blue, with the lighter shade representing the broader 95% interval. Additionally, the green triangle represents data from 2020 to 2022 which was affected by the COVID-19 pandemic. Abbreviations: TB, Tuberculosis; DR-TB, Drug-resistant tuberculosis; DS-TB, Drug-susceptible tuberculosis; MTB, Mycobacterium tuberculosis.
Geographical evaluation of DR-TB notification among Brazilian regions
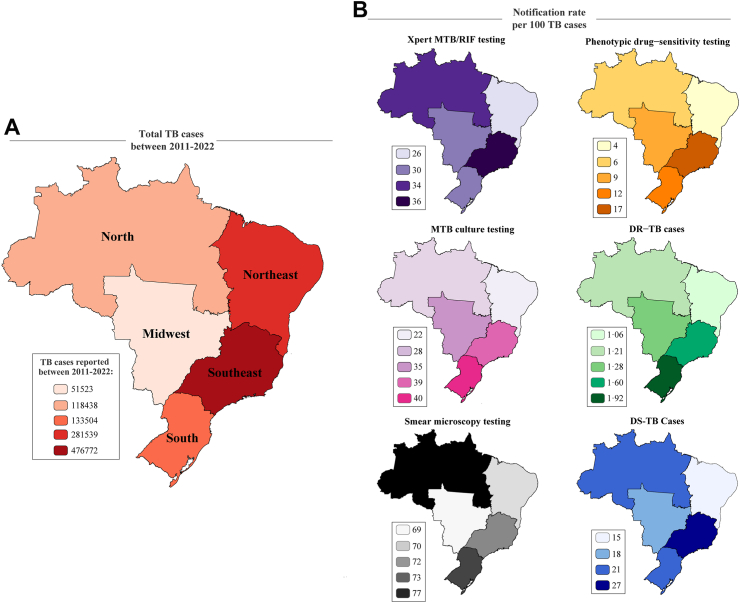
Finally, significant disparities were found regarding the incidence of DR-TB and laboratory diagnostic test coverage among different regions of Brazil. The Southeast and South regions exhibited higher rates for DR-TB and DS-TB incidence, as well as MTB culture, and phenotypic DS testing coverage, whereas the Northeast and North regions consistently reported lower testing and incidence rates (Supplementary Table S6 and Fig. 5). Notably, Amazonas state in the North region had an exceptionally high testing rate for the Xpert, demonstrating disparities inside the same region (Supplementary Figure S4). In contrast, the smear microscopy test demonstrated high rates uniformly across all regions, displaying less geographical variation.
Fig. 5.
TB and DR-TB case notifications and testing according to Brazilian macro-region. (A) Total TB cases reported between 2011 and 2022. (B) Notification and testing of TB and DR-TB. Xpert in Purple; Phenotypic drug-sensitivity test in Yellow; DR-TB cases in Green; DS-TB cases in Blue; Smear Microscopy in Black and MTB culture in Pink. Abbreviations: TB, Tuberculosis; DR-TB, Drug-resistant tuberculosis; DS-TB, Drug-susceptible tuberculosis; MTB, Mycobacterium tuberculosis.
Integration of the study's main findings with public health interventions
In Table 6, we directly translate our study's critical findings into practical applications within the public health domain, presenting intricate implications between each result with potential interventions and policy shifts crucial in advancing public health strategies for TB control in Brazil. Thus, this table aims to bridge the gap between our intricate assessment of effects of implementing the Xpert test on the Brazilian TB healthcare system and real-world disease control measures. It emphasizes the importance of evidence-based decision-making in creating or improving public health measures based on scientific findings.
Table 6.
Public health impact and policy implications derived from Study's main findings.
| Main findings | Public health impact and policy implications |
|---|---|
| Substantial data gaps on laboratory diagnostic testing and notification | Strengthen data collection and analysis to fill existing information gaps |
| Xpert's Influence in Enhancing TB and DR-TB Detection and Coverage | Invest in broader implementation of Xpert and expand the number of laboratories and healthcare professionals qualified for its usage |
| Using Xpert testing as a potential tool to increase accuracy in prediction models of TB and DR-TB Burden | Harness Xpert's capabilities to improve prediction models for customized screening strategies forecasting future burden, while also advancing research and usage of more complex multivariate models to further enhance the accuracy of TB and DR-TB trend predictions. |
| High Prevalence of Isoniazid-Monoresistance and MDR TB | Advocate for the adoption of comprehensive molecular tests like Xpert MTB/XDR to detect a wider range of drug resistances. |
| Geographical Discrepancies in TB Testing Accessibility | Implement tailored regional interventions to mitigate testing coverage disparities, especially in underserved regions with high socio-epidemiological risks |
| Novel Methodology for Assessment of Public Health Interventions | This methodology provides a robust framework for policymakers and health researchers to systematically assess the sustained impact of future health interventions, beyond their immediate effects, both for TB and other infectious diseases. |
The table presents connections between the key findings from our study and their projected implications for public health interventions and policies in Brazil. The left column details the main outcomes derived from the comprehensive analysis of the SINAN-TB database regarding Xpert's impact on the Brazilian TB healthcare system, whereas the right column proposes actionable steps to harness these findings for enhanced TB control. Abbreviations: Xpert, Xpert MTB/RIF test; TB, Tuberculosis; DR-TB, Drug-resistant Tuberculosis; MDR, Multidrug-resistance.
Discussion
The global spread of antimicrobial resistance remains a major impediment to worldwide eradication of TB.1,18 Notably, the introduction of molecular tests such as the Xpert, has revolutionized TB diagnosis, as well as resistance screening by providing quick and accurate results for both MTB and RR detection.10 Our evaluation of the SINAN-TB database highlighted the significant advancements in TB and DR-TB case detection achieved with the Xpert's gradual implementation into Brazil's healthcare system. Our findings underscore the tangible benefits of the integration of Xpert technology, alongside showcasing how Xpert testing counts can be used to improve forecasting models of TB and DR-TB case detection.
By combining ITSA with CCF, our results reveal that continued increases in Xpert testing counts were associated with prolonged improvements in case detection and changes in the diagnostic approach for suspected cases.1 Such associations were even more pronounced for DR-TB and DS-TB detection, exceeding its effects in other models and aligning with national TB control objectives for early detection and management of bacterial resistance.19 We hypothesize that this increase in DR screening can be attributed to both rapid detection of rifampicin-resistance by the Xpert test, as well as its positive impact on the slower phenotypic DS testing.
Interestingly, smear microscopy testing models exhibited inferior post-intervention values and negative correlations with Xpert testing. This shift mirrors the evolution in infectious diseases diagnostic practices with the advent of advanced faster tools.10 Notably, smear microscopy's sensitivity fluctuates between 40 and 80%, and its specificity lies within 94–98%.20 In contrast, Xpert achieves a higher sensitivity range of 78.6–94.7% and a similar specificity of 88.9–99.3%.21 This superior performance aligns with the growing preference for molecular tests over smear microscopy, attributed to their rapidity and precision. Additionally, Xpert's capacity to also detect RR, exhibiting a sensitivity of 88.9–98.1% and specificity of 97.2–98.9%, further solidifies its advantage and endorses the shift towards more advanced diagnostic methods.21
However, differing results were found regarding the effects of Xpert's integration into the Brazilian healthcare system during its initial phase. Most commonly, the onset of impact ranged from 2014 to 2015, coinciding with the test's gradual adoption in 92 key Brazilian cities that composed a Rapid Molecular Testing Network for TB.22 Nonetheless, a notable 2–3-year delay was observed until the test's adoption began to significantly affect the trend of total TB case notifications when compared to the other models. Interestingly, this overlapped with the broader dissemination of Xpert machine availability throughout Brazil between 2017 and 2018, when the network expanded to 128 cities, which accounted for 65% of new TB cases.23 Additionally, CCF findings indicated that following the start of Xpert's impact in 2017, the continuous rise in Xpert testing began to significantly contribute to the increase in case detection without delays.
Of note, similar improvements in TB case detection following Xpert's rollout were reported in specific regions of Brazil and other high TB burden countries.3,24,25 Our study builds upon the previous works by extending the evaluation of Xpert's impact on TB and DR-TB detection rates at a nationwide level, while also incorporating a comprehensive assessment of temporal trends and the effectiveness of Xpert's adoption in various phases of its implementation. Additionally, our novel assessment captures both immediate impacts post Xpert's implementation and sustained benefits following the initial rollout. These aspects include the test's significant role in improving case detection and resistance screening, as well as its impact in reducing diagnosis time and simplifying logistics.3
Furthermore, our dynamic regression modelling and Granger Causality suggests that Xpert testing counts could help refine epidemiological predictions for TB and DR-TB management in Brazil.16 This finding indicates possible use of case testing data in strategy development for increases in TB case detection. Although our forecasts suggest a potential rise in TB and DR-TB case notifications, we hypothesise these predictions may be linked with reduced underreporting due to Xpert's usage, rather than an actual increase in disease incidence.1 Nonetheless, our research indicates that incorporating external factors like Xpert testing data can be beneficial to epidemiological predictions and may contribute to future research into multivariate forecasting of TB burden in Brazil.
While Xpert implementation has improved DR-TB detection by primarily screening for RR, it falls short in identifying isoniazid monoresistance, MDR and other DR forms.26 Notably, these latter resistance subtypes are only detected by slower culture-based methods, which are infrequently used in regions like North and Northeast. Thus, the adoption of more advanced tests, such as the Xpert MTB/XDR, might provide an even greater enhancement to DR-TB detection by screening for a broader range of resistance traits.26
Although this study represents significant advancements, our methodology still faces limitations, including the reliance on secondary data with gaps, high levels of missing data and limited data periods, which, along with socio-regional developments and other confounding factors in TB and DR-TB burden unincorporated in our models, can affect forecast accuracy. Moreover, the lack of consistent monthly data restricted us to annual time-series, hindering the detection of more complex temporal patterns. Nevertheless, efforts were made to mitigate some limitations by adopting previously recommended forecasting methods for short-term time-series,12 coupled with using the Toda-Yamamoto procedure for Granger Causality testing, to strengthen the validity of our analysis.16 Furthermore, the spearman's rank correlation was calculated using a precomputed exact null distribution, aiming to minimize the risk of identifying spurious correlations.
Despite limitations, our study generated compelling evidence that implementing the Xpert technology into TB healthcare has improved case detection in Brazil, particularly in screening for DR-TB. Thus, we advocate for its broader implementation, including bolstering molecular diagnosis infrastructure and improving the training of healthcare professionals in its use. Moreover, the Xpert's successful adoption in Brazil, a country severely affected by TB, provide important insights for other nations with high disease burden by emphasizing the positive impact that implementing advanced diagnostic technologies can have on TB detection and DR screening. These efforts are crucial for improving diagnostic capabilities and developing effective strategies aimed at rapid and precise detection of TB and DR-TB.
In conclusion, our research uses a pioneering methodology to highlight the pivotal role of innovative diagnostic tools like the Xpert system in reshaping the landscape of TB and DR-TB case detection, as well as in improving prediction modelling for the future disease detection in Brazil. Therefore, by adopting and further developing such technologies, significant strides can be taken towards improving TB and DR control and, ultimately, towards the goal of TB eradication.
Contributors
Conceptualization, K.V-S., B.B-D., M.A-P. and B.B.A.; Data verification and curation, K.VS-., B.B-D., A.T.Q.L., M.M.R, M.A-P., and B.B.A.; Investigation, K.V-S., B.B-D, J.P.M-P., G.A., P.F.R., T.R.S., M.A-P., B.B.A., and B.B.A.; Formal analysis, KV-S., M.A-P., and B.B.A.; Funding acquisition, T.R.S., M.C.S., M.M.R., and B.B.A.; Methodology, KV-S, B.B-D., M.A-P., and B.B.A.; Project administration, T.R.S., M.A-P. and B.B.A.; Resources, K.V-S., B.B.-D., T.R.S., M.A-P. and B.B.A.; Software, KV-S, B.B-D., M.A-P., and B.B.A.; Supervision, M.A-P. and B.B.A.; Writing—original draft, K.V-S., B.B-D., J.P.M-P., M.A-P., and B.B.A.; Writing—review and editing, all authors.
Data sharing statement
The data that support the findings of this study will be available upon reasonable request to the corresponding author of the study.
Editor's note
The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Declaration of interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgements
The study was supported by the Intramural Research Program of the Fundação Oswaldo Cruz (B.B.A.), Intramural Research Program of the Fundação José Silveira (B.B.A), the National Institute of Allergy and Infectious Diseases [U01-AI069923 to TRS, BBA, and MCS and U01-AI115940 to B.B.A.]. KV-S received a fellowship from the Fundação de Amparo à Pesquisa da Bahia (FAPESB), B.B.D received a fellowship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Finance code: 001). B.B.A, and M.C.S. are senior investigators and fellows from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil. The authors thank Mrs. Elze Leite (FIOCRUZ, Brazil) for logistics and administrative support.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lana.2024.100804.
Contributor Information
Klauss Villalva-Serra, Email: klaussvs1@gmail.com.
Bruno B. Andrade, Email: bruno.andrade@fiocruz.br.
Appendix A. Supplementary data
References
- 1.World Health Organization . 2023. Global tuberculosis report 2023.https://iris.who.int/ [Google Scholar]
- 2.Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde . 2020. Teste rápido molecular para Tuberculose (TRM-TB) [Google Scholar]
- 3.Wu Z., Rueda Z.V., Li T., et al. Effect of the Xpert MTB/RIF on the detection of pulmonary tuberculosis cases and rifampicin resistance in Shanghai, China. BMC Infect Dis. 2020;20 doi: 10.1186/S12879-020-4871-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sachdeva K.S., Raizada N., Sreenivas A., et al. Use of Xpert MTB/RIF in decentralized public health settings and its effect on pulmonary TB and DR-TB case finding in India. PLoS One. 2015;10 doi: 10.1371/JOURNAL.PONE.0126065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bagcchi S. Brazil tackles its tuberculosis burden. Lancet Infect Dis. 2023;23:790. doi: 10.1016/S1473-3099(23)00383-3. [DOI] [PubMed] [Google Scholar]
- 6.Rocha M.S., Bartholomay P., Cavalcante M.V., et al. Notifiable diseases information system (SINAN): main features of tuberculosis-relatid notification and data analysis. Epidemiologia e Servicos de Saude. 2020;29 doi: 10.5123/S1679-49742020000100009. [DOI] [PubMed] [Google Scholar]
- 7.Ministério da Saúde B. 2012. Resolução No 466, conselho nacional de saúde resolução No 466, de 12 de dezembro de 2012; pp. 1–19. [Google Scholar]
- 8.Ministério da Saúde . 2022. Manual e recomendações para o diagnóstico laboratorial de tuberculose e micobactérias não tuberculosas de interesse em saúde pública no Brasil. [Google Scholar]
- 9.Ministério da Saúde . 2018. Manual sinan – normas e rotinas 2a edição – portal da vigilância em Saúde.http://vigilancia.saude.mg.gov.br/index.php/download/manual-sinan-normas-e-rotinas-2a-edicao/ [Google Scholar]
- 10.Procop G.W. Laboratory diagnosis and susceptibility testing for Mycobacterium tuberculosis. Microbiol Spectr. 2016;4 doi: 10.1128/MICROBIOLSPEC.TNMI7-0022-2016. [DOI] [PubMed] [Google Scholar]
- 11.López-de-Lacalle J. 2019. Package ‘tsoutliers’; pp. 1–29. [Google Scholar]
- 12.Cruz-Nájera M.A., Treviño-Berrones M.G., Ponce-Flores M.P., et al. Short time series forecasting: recommended methods and techniques. Symmetry. 2022;14:1231. [Google Scholar]
- 13.Schaffer A.L., Dobbins T.A., Pearson S.A. Interrupted time series analysis using autoregressive integrated moving average (ARIMA) models: a guide for evaluating large-scale health interventions. BMC Med Res Methodol. 2021;21:1–12. doi: 10.1186/s12874-021-01235-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van De Wiel M.A., Di Bucchianico A. Fast computation of the exact null distribution of Spearman's ρ and Page's L statistic for samples with and without ties. J Stat Plan Inference. 2001;92:133–145. [Google Scholar]
- 15.Derrick T., Thomas J. Time series analysis: the cross-correlation function. 2004. https://dr.lib.iastate.edu/handle/20.500.12876/52528
- 16.Lima V., Dellajustina F.J., Shimoura R.O., et al. Granger causality in the frequency domain: derivation and applications. Rev Bras Ensino Física. 2020;42 [Google Scholar]
- 17.Hyndman R.J., Athanasopoulos G. 2nd ed. OTexts - OTexts.com/fpp2.; Melbourne, Australia: 2018. Forecasting: principles and practice. [Google Scholar]
- 18.World Health Organization Global research agenda for antimicrobial resistance in human health. 2023. https://www.who.int/publications/m/item/global-research-agenda-for-antimicrobial-resistance-in-human-health
- 19.Vigilância S. De, Nacional P., Controle D. Manual de recomendações para o controle da tuberculose no Brasil 2014. 2014. www.saude.gov.br/
- 20.Silva D.R., Rabahi M.F., Sant'Anna C.C., et al. Diagnosis of tuberculosis: a consensus statement from the Brazilian thoracic association. J Bras Pneumol. 2021;47 doi: 10.36416/1806-3756/e20210054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zifodya J.S., Kreniske J.S., Schiller I., et al. Xpert Ultra versus Xpert MTB/RIF for pulmonary tuberculosis and rifampicin resistance in adults with presumptive pulmonary tuberculosis. Cochrane Database Syst Rev. 2021;2021 doi: 10.1002/14651858.CD009593.pub5. [DOI] [PubMed] [Google Scholar]
- 22.Secretaria de Vigilância a Saúde . Brasília; 2015. Rede de teste rápido para tuberculose no Brasil. [Google Scholar]
- 23.Teste rápido molecular para tuberculose amplia rede de diagnóstico — Ministério da Saúde. https://www.gov.br/saude/pt-br/assuntos/noticias/2018/marco/teste-rapido-molecular-para-tuberculose-amplia-rede-de-diagnostico
- 24.Iúdice T.N.D.S., da Conceição M.L., de Brito A.C., et al. The role of GeneXpert® for tuberculosis diagnostics in Brazil: an examination from a historical and epidemiological perspective. Trop Med Infect Dis. 2023;8:483. doi: 10.3390/tropicalmed8110483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aguilar-Jiménez J.R., Pelissari D.M., Diaz-Quijano F.A. How has the municipal availability of the GeneXpert®MTB/RIF system affected the detection of drug-resistant tuberculosis in Brazil? Trop Med Int Health. 2024;29 doi: 10.1111/TMI.13945. [DOI] [PubMed] [Google Scholar]
- 26.Mvelase N.R., Mlisana K.P. Xpert MTB/XDR for rapid detection of drug-resistant tuberculosis beyond rifampicin. Lancet Infect Dis. 2022;22:156–157. doi: 10.1016/S1473-3099(21)00481-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.