By 2010 the number of people with diabetes is expected to exceed 350 million. Late diabetic complications will cause considerable morbidity in 5-10% of these patients and place an enormous burden on society. Transplantation of insulin producing islet cells isolated in vitro from a donor pancreas could be a cure for type 1 and some cases of type 2 diabetes. Currently, however, lack of sufficient donor organs and the side effects of immunosuppressive therapy limit its potential. Ways to overcome these problems include deriving islet cells from other sources such as pigs, human pancreatic duct cells, fetal pancreatic stem cells, embryonic stem cells, and by therapeutic cloning. This article outlines these developments and discusses how islet cell transplantation is likely to become the treatment of choice for most insulin dependent diabetics within the next five to 10 years.
Methods
Our article is based on information from the following core references: the international islet transplant registry; recently published articles describing improvements in islet cell transplantation, reporting treatment of diabetes in animal models with islet cells grown in vitro, and describing novel molecular mechanisms in pancreatic endocrine development (including our own recent work); papers in embryonic and adult stem cell research that have had a major influence on our thinking; and the seminal work from the Roslin Institute and other groups on nuclear transfer.
The limitations of conventional treatment
In many cases current diabetes drug therapies do not provide sufficiently tight control of blood glucose to avoid diabetic late complications.1,2 Transplantation of whole donor pancreas is an effective form of treatment but is of limited application since it entails major surgery and long term immunosuppression. This failure to prevent the morbidity associated with diabetes places an enormous burden not only on patients and their relatives but also on society. The costs of treating late diabetic complications are set to escalate because of the predicted sharp rise in the number of people with diabetes. Thus, both patients and society have much to gain from development of improved treatment for diabetes.
Predicted developments
Increased use of islet cell transplantation to treat diabetes
Introduction of new methods for in vitro generation of β cells, either from pancreatic duct cells or from stem cell cultures
Treatment of type 2 diabetes with individually tailored β cells produced by cloning or from haemopoietic stem cells
Development of immunosuppressive therapy specific to the autoimmune response seen in type 1 diabetes, allowing transplantation of individually tailored β cells with minimal side effects
Islet cell transplantation
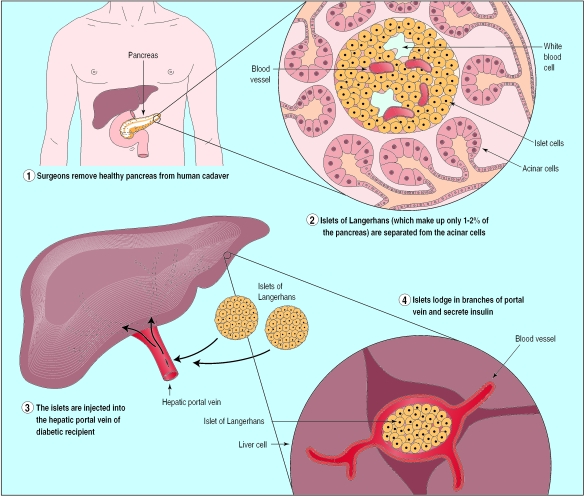
This is an effective treatment for diabetes, but its use is limited by shortage of donor material. Allogeneic islet transplantation has been explored as a treatment for type 1 diabetes. Islet cells are extracted from a donor pancreas and injected into the portal vein of the liver (fig 1). The procedure has to be carried out two or three times and requires as many short term hospitalisations over a period of two to three months. When successful, this treatment has improved patients' diabetes.3 However, the need for intense immunosuppression to prevent graft rejection has, until recently, limited this approach to patients who are already immunosuppressed either for a previous organ graft or because of simultaneous kidney transplantation.4 It is also possible that the immunosuppressive regimen itself may have prevented success in some cases, because most protocols use agents that inhibit islet cell function or induce peripheral insulin resistance.5 As a result, only 10% of the patients on the International Islet Cell Transplantation Registry are recorded as being insulin independent a year after receiving a transplant.4
Figure 1.
Islet cell transplantation by injection into hepatic portal vein
Promising results have recently been reported from transplantation of large amounts of islet cells from cadaveric pancreases that were not HLA matched into seven patients with type 1 diabetes who had multiple hypoglycaemic episodes or uncontrolled diabetes despite compliance with the prescribed insulin treatment.6 All the patients showed normalisation of glycated haemoglobin concentration and lasting independence from insulin injections at an average of 11 months' follow up. The islet cells were purified in medium free from foreign protein, and this, combined with a glucocorticoid-free immunosuppressive regimen, successfully prevented rejection. Notably, both host versus graft and autoimmune reactions were apparently avoided. This was a small uncontrolled study, however, and its encouraging results need to be confirmed in larger randomised controlled trials.
Even if further studies confirm the effectiveness of this approach, the need to obtain two to four donor pancreases for each patient and the uncertainty regarding long term side effects from immunosuppression are likely to limit its application to patients with very poorly controlled diabetes.
Alternative sources of islet cells
The shortage of human donor pancreases for islet cell transplantation has led to a search for alternative sources of islet cells. Several sources have been suggested—from pigs, induction from human pancreatic duct cells, fetal pancreatic stem cells, and induction of insulin producing B cells by therapeutic cloning—and each has its own advantages and disadvantages.
Xenogeneic islet cells
Porcine islet cells have been suggested as a virtually unlimited supply of insulin producing cells for transplantation. However, the immunological barrier to xenogeneic grafts is substantially greater than the barrier to human grafts. Hence, the development of transgenic pigs that express human genes and the extension of cloning technology to pigs have been welcomed because these technologies promise the production of “humanised” pigs. Such pigs would lack xenoantigens that are immunologically important but not essential for survival, and the technology might even allow production of pigs individually matched for recipients' HLA type.
The disadvantage of xenografts from transgenic pigs is the possible risk to public health from cross species infection with porcine endogenous retroviruses that then adapt to human hosts. Retroviruses result in permanent infection, and there are reports that porcine endogenous retroviruses from porcine cell lines and lymphocytes can infect human cells in vitro.7,8 These concerns have led the US Food and Drug Administration to halt trials of porcine xenografts until those patients who have already received grafts are assessed for infection. Although 10 Swedish patients have received porcine islet xenografts and have no sign of infection in their lymphocytes,9 a recent study in genetically modified mice (non-obese diabetic plus severe combined immunodeficiency) that had received porcine islet cells found infection with porcine endogenous retroviruses in several tissues.10
Further information on pig cloning and the use of pigs for xenografts can be found at www.sciencemag.org/cgi/content/full/289/5486/1886.
Expansion and transdifferentiation of pancreatic duct cells
While the nature of pancreatic stem cells is still uncertain, recent advances in this area prompted a high level meeting sponsored by the National Institutes of Health on stem cells and pancreatic development.11 Peck and colleagues have reported that pancreatic ductal epithelial cells isolated from adult non-obese diabetic mice that are still prediabetic can be grown in long term cultures and induced to produce functioning islets of Langerhans containing α, β, and δ cells.12 These in vitro generated islets were capable of lowering blood glucose concentrations to near normal when implanted in diabetic non-obese mice. The mice remained normoglyacemic for the three months' duration of the study. Human pancreatic duct cells have also been grown successfully in vitro and induced to differentiate, but the ability of these cells to restore blood glucose in vivo is still unproved.13 This promising line of research is being pursued by several laboratories. Not only does the use of adult donor ductal cells avoid the controversy of using fetal cells but there are fewer biological problems associated with making β cells from duct cells than from, for example, embryonic stem cells.
The use of fetal pancreatic stem cells and β cell precursor
In the past few years huge advances have been made in the understanding of fetal endocrine development. These provide an important guide to further attempts to produce islet cells in vitro.
The identification of endocrine precursor cells in the developing pancreas and the regulation of their differentiation by a specific cellular pathway (the Delta Notch pathway—see appendix on BMJ 's website for details) raises the exciting possibility that modulation of cellular signalling can be used in vitro to grow and differentiate endocrine precursor cells, taken either from embryonic pancreas from aborted fetuses or by using pancreatic duct cells. Once the molecular details are solved suitable culture conditions can be developed to supply an unlimited number of allogeneic β cells for transplantation.
Embryonic stem cells
Stem cells are potent biological units that have been used for decades in many aspects of biology. The mammalian body consists of some 200 distinct cell types, which all derive from the fertilised egg cell. The fertilised human egg divides and gives rise to the early embryo, which, at the blastula stage, contains a cluster of apparently totipotent cells—termed the inner cell mass—from which clonal embryonic stem cells can be derived. Such embryonic stem cells can be propagated indefinitely in vitro and can be induced to differentiate into several distinct lineages in vitro, including cardiomyocytes and neural cells, but differentiation into endodermal cell types has not yet been reported. As figure 2 shows, the stem cells have to follow the appropriate developmental pathway in order to become insulin producing β cells.
Figure 2.
Schematic representation of developmental pathway an embryonic stem cell must follow in order to become an insulin producing β cell. Little is known about the signals that govern the choices at the various branch points
Soria and coworkers derived insulin producing cells from mouse embryonic stem cells by using embryonic stem cells transfected with an insulin promoter (driving expression of the neo gene, a marker for antibiotic resistance), which allowed them to selectively induce insulin producing cells.14 However, this procedure gives rise to proliferating cells, and thereby potentially malignant cells, rather than mature, post-mitotic cells. Nevertheless, this experiment shows that embryonic stem cells can differentiate along a pancreatic endocrine path. This technique might be developed to provide a source of β cells by using more elaborate selection schemes that are compatible with normal β cell development.
For more information on the use of embryonic stem cells, see www.sciencemag.org/cgi/content/full/283/5407/1468#F1
Therapeutic cloning
The transfer of the nucleus of a somatic cell (such as from breast tissue ) into a donor oocyte from which the nucleus has been removed can be used to clone a mammalian species. The oocyte with the replaced nucleus carries the genetic information of the donor. This technique was used to clone Dolly the sheep.15 Blastocysts can be developed in vitro from such manipulated oocytes, and embryonic stem cells that are genetically matched to the donor can be derived from the inner cell mass of the blastocysts (fig 3), a procedure that takes several months.
Figure 3.
Therapeutic cloning (use of nuclear replacement to generate patient specific β cells). An enucleated host oocyte is injected with a nucleus derived from a somatic cell from a particular patient. The oocyte is then allowed to develop to the blastocyst stage in vitro. Embryonic stem cells can then be isolated from the blastocyst and used as the starting point for in vitro β cell development
Such a procedure for generating β cells from embryonic stem cells could be developed (a ready supply of oocytes could be provided from registered fertility clinics) to produce effective therapy for diabetics. The great advantage of this cloning technology is, of course, that the embryonic stem cells would generate β cells with a patient's own genetic information, thus avoiding allogeneic host versus graft reactions.
For more information on therapeutic cloning see www.sciencemag.org/cgi/content/full/288/5472/1775
When the nature of pancreatic β cell ontogeny is fully understood we may be able to mimic this process in vitro to propagate β cells—either starting with duct cells derived from pancreatic donor specimens or by the use of other appropriate human stem cells (such as from bone marrow or even blood samples). This development would clearly be welcome because it would avoid the need for therapeutic cloning, with all the attendant controversy of creating human embryos solely for medical use.
The future
Research in islet cell and stem cell transplantation is set to develop rapidly. Within the next five years it should be possible to generate sufficient β cells in vitro to solve the current shortage of insulin producing cells from donor islets. This would allow doctors to treat not only those patients with poorly controlled diabetes but also those with less severe disease but who are identified (by monitoring glycated haemoglobin levels) as being at risk of developing long term complications. This could considerably reduce the personal and societal burden of morbidity from late diabetic complications
Of the techniques described above, the most promising is generation of β cells from pancreatic duct cells. It is inherently a shorter biological step to make a β cell from a duct cell than it is from other possible cells, such as embryonic stem cells and haemopoietic stem cells, because these are not closely related in lineage. However, safe suppression of autoimmunity for patients with type 1 diabetes must be achieved before this promising new technology can lead to a dramatic shift in clinical practice. In type 1 diabetes this reaction is as important as the standard graft versus host reaction. For patients with type 2 diabetes (where autoimmunity is no problem), fully histocompatible and patient specific β cells could well be developed within the next 10 years, either by manipulation of adult stem cells or by therapeutic cloning. Hopefully, safe and efficient interventions to curtail the autoimmune reaction will have evolved by then, allowing patients with type 1 diabetes to benefit from this development also.
Supplementary Material
Footnotes
Competing interests: The authors are employed by and have financial interests in Novo Nordisk A/S, which manufactures insulin.
Further details about the origin of pancreatic endocrine cells appear on the BMJ's website
References
- 1.UK Prospective Diabetes Study Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 2.Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 3.Ricordi C. Human islet cell transplantation: new perspectives for an old challenge. Diabetes Rev. 1996;4:356–369. [Google Scholar]
- 4.Brendel M, Hering B, Schulz A, Bretzel R. International islet transplant registry. Giessen: Justus-Liebig University of Giessen; 1999. pp. 1–20. [Google Scholar]
- 5.Zeng Y, Ricordi C, Lendoire J, Carroll PB, Alejandro R, Bereiter DR, et al. The effect of prednisone on pancreatic islet autografts in dogs. Surgery. 1993;113:98–102. [PMC free article] [PubMed] [Google Scholar]
- 6.Shapiro A, Lakey J, Ryan E, Korbutt G, Toth E, Warnock G, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 7.Patience C, Takeuchi Y, Weiss RA. Infection of human cells by an endogenous retrovirus of pigs. Nat Med. 1997;3:282–286. doi: 10.1038/nm0397-282. [DOI] [PubMed] [Google Scholar]
- 8.Wilson CA, Wong S, Muller J, Davidson CE, Rose TM, Burd P. Type C retrovirus released from porcine primary peripheral blood mononuclear cells infects human cells. J Virol. 1998;72:3082–3087. doi: 10.1128/jvi.72.4.3082-3087.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heneine W, Tibell A, Switzer WM, Sandstrom P, Rosales GV, Mathews A, et al. No evidence of infection with porcine endogenous retrovirus in recipients of porcine islet-cell xenografts. Lancet. 1998;352:695–699. doi: 10.1016/S0140-6736(98)07145-1. [DOI] [PubMed] [Google Scholar]
- 10.Van der Laan LJ, Lockey C, Griffeth BC, Frasier FS, Wilson CA, Onions DE, et al. Infection by porcine endogenous retrovirus after islet xenotransplantation in SCID mice. Nature. 2000;407:90–94. doi: 10.1038/35024089. [DOI] [PubMed] [Google Scholar]
- 11.Serup P. Panning for pancreatic stem cells. Nat Gen. 2000;25:134–135. doi: 10.1038/75960. [DOI] [PubMed] [Google Scholar]
- 12.Ramiya VK, Maraist M, Arfors KE, Schatz DA, Peck AB, Cornelius JG. Reversal of insulin-dependent diabetes using islets generated in vitro from pancreatic stem cells. Nat Med. 2000;6:278–282. doi: 10.1038/73128. [DOI] [PubMed] [Google Scholar]
- 13.Bonner-Weir S, Taneja M, Weir GC, Tatarkiewicz K, Song KH, Sharma A, et al. In vitro cultivation of human islets from expanded ductal tissue. Proc Natl Acad Sci U S A. 2000;97:7999–8004. doi: 10.1073/pnas.97.14.7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soria B, Roche E, Berná G, León-Quinto T, Reig J, Martín F. Insulin-secreting cells derived from embryonic stem cells normalize glycemia in streptozotocin-induced diabetic mice. Diabetes. 2000;49:157–162. doi: 10.2337/diabetes.49.2.157. [DOI] [PubMed] [Google Scholar]
- 15.Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KH. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997;385:810–813. doi: 10.1038/385810a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.