Abstract
Background and Aims
Cognitive-affective processes, including hypervigilance and symptom-specific anxiety, may contribute to chronic laryngeal symptoms and are potentially modifiable; however, a validated instrument to assess these constructs is lacking. The aims of this study were to develop and validate the Laryngeal Cognitive-Affective Tool (LCAT) instrument.
Methods
This two-phase single-center prospective study enrolled participants from 11/2021 to 6/2023. In the initial phase 1:1 patient cognitive interviews and multidisciplinary team consensus were conducted to develop the LCAT. In the second phase asymptomatic and symptomatic participants completed a series of questionnaires to examine psychometric properties of the LCAT.
Results
268 participants were included: 8 in the initial phase and 260 in the validation phase (56 asymptomatic; 204 symptomatic). A 15-item LCAT was developed. In the validation phase, mean total LCAT and hypervigilance/anxiety sub-scores were significantly higher in symptomatic vs asymptomatic participants (p<0.01). The LCAT had excellent internal consistency (α=0.942) and split-half reliability (Guttman=0.853). Using a median split, a score of 33 or greater was defined as elevated.
Conclusions
The 15-item LCAT evaluates laryngeal hypervigilance and symptom-specific anxiety among patients with laryngeal symptoms. It has excellent reliability and construct validity. The LCAT highlights burdensome cognitive-affective processes which can accordingly help tailor treatments.
Keywords: Gastroesophageal reflux, Patient reported outcome measures, Psychosocial functioning, Laryngopharyngeal Reflux
Introduction
Laryngeal symptoms, as measured by the reflux symptom index (RSI), are common, with an estimated prevalence of 5–30% in the general population.1–5 Laryngeal symptoms are often presumed to be a consequence of gastroesophageal reflux disease (GERD).1,6,7 As such, patients frequently receive a diagnosis of laryngopharyngeal reflux (LPR) based on clinical presentation alone, empirically trial proton pump inhibitor therapy, and in the setting of non-response, undergo a slew of diagnostic tests8. Albeit an arduous journey, most patients remain symptomatic8,9 and report reduced health-related quality of life (QOL) as well as higher rates of anxiety and depression.10 Further, this paradigm imparts a significant economic burden, estimated at 50 billion annual health care dollars.11 Recognizing and addressing mechanisms that contribute to laryngeal symptoms is critically needed.
Emerging literature supports an interaction between cognitive-affective processes and laryngeal symptoms.8–10,12–15 For instance, hypervigilance (heightened awareness and discomfort from physiologic sensations) can manifest as an increased focus on throat sensations in social situations. Symptom-specific anxiety (persistent worrying over laryngeal symptoms and their consequences), can present as troublesome anxiety over developing throat cancer in an individual with hoarseness. Hypervigilance and symptom-specific anxiety have been shown to be elevated in patients with chronic laryngeal symptoms, regardless of whether pathologic GERD is present.10,12–14 These processes, while designed by the brain to be protective, likely contribute to symptom burden and are modifiable with directed behavioral interventions.8,15
Instruments exist to measure cognitive-affective processes. For instance the esophageal hypervigilance and anxiety scale [EHAS])16 is increasingly utilized across patients with esophageal symptoms to identify populations that may benefit from esophageal directed behavioral therapies. However, an instrument to assess hypervigilance and symptom-specific anxiety in patients with chronic laryngeal symptoms does not exist. Therefore, this study aimed to develop and validate the Laryngeal Cognitive-Affective Tools (LCAT).
Methods
Study Design
This prospective two-phase single-center study enrolled participants over 2.5 years (11/2021–6/2023). The initial phase aimed to develop the Laryngeal Cognitive-Affective Tool (LCAT) and the second phase aimed to examine the validity of the LCAT. This study was funded by NIH DK135513 and the protocol was approved the by Institutional Review Board (IRB).
Development Phase
In the initial development phase, a multidisciplinary team of a GI clinical psychologist, esophageal specialist, laryngologist, and speech-language pathologist proposed the initial LCAT. The LCAT was developed by adapting the 15-items of the EHAS to specifically target laryngeal symptoms and included two sub-scales: symptom-specific anxiety and hypervigilance. The original 15-item EHAS16 utilized components from four validated questionnaires: the Sullivan Pain Catastrophizing Scale17, the Visceral Sensitivity Index18, the McCracken Pain Vigilance and Awareness Scale19,20 and the Rosenstiel & Keefe Coping Strategies Questionnaire.21 After obtaining consent, 8 participants underwent a 1:1 cognitive interview with a trained research assistant, utilizing the Cognitive Aspects of Survey Methodology (CASM)22 to assess: 1) Question comprehension, 2) Information retrieval, 3) Judgement and estimation, 4) Documenting responses. During the interviews a script was utilized and each LCAT question was asked to the patient who provided an answer based on a Likert scale. Then a series of questions were asked to determine how the participant arrived at their answer and to assess their understanding (Supplemental Figure 1). Next, the multidisciplinary team reviewed participant responses. If two or more participants exhibited difficulty responding to or confusion in understanding the content on any of the four CASM levels, the team proposed and agreed upon modifications.
Participants were English speaking adults (≥18 years) who were experiencing ≥6 months of laryngeal symptoms (mucus in throat, throat clearing, cough, dysphonia, globus sensation, or sore throat). Individuals were excluded if unable to provide consent, imprisoned, cognitively impaired, or required legal adult representation. Participants provided informed consent and received compensation.
Validation Phase
The validation phase (9/2022–6/2023) enrolled English speaking adults (18–99 years) into two cohorts: Symptomatic - who self-identified as having chronic laryngeal symptoms for ≥6 months, and Asymptomatic - who denied chronic throat symptoms. Symptomatic patients were recruited from routine outpatient GI visits. Asymptomatic patients were recruited from routine outpatient GI visits and through campus survey invitation. Participants were excluded if unable to consent, self-complete patient reported questionnaires without being read to them or use a computer or smart device to complete the questionnaires, as well as if they were imprisoned. Participants were provided a survey link through UCSD REDCap. After providing informed consent, participants completed a series of online questionnaires, and demographic data were collected. Participants received compensation for their participation.
Questionnaires
The following validated series of questionnaires were included to assess esophageal and laryngeal symptom burden, as well as the impact on QOL, anxiety, depression, and a patient’s ability to adjust to their symptoms.
Gastroesophageal Reflux Questionnaire (GerdQ):
A validated 6-item questionnaire that evaluates GERD symptoms. Scores of ≥ 8 are indicative of a high likelihood of GERD.23
Reflux Symptom Index (RSI):
A validated 9-item questionnaire aimed at evaluating laryngeal symptoms. Scores of > 13 were considered abnormal.24
Voice Handicap Index (VHI):
A validated 30-item questionnaire aimed at evaluating voice symptoms. Scores of 0–30 indicate mild symptom severity, 31–60 moderate severity, and 61–120 severe symptoms.25
Illness Cognition Questionnaire (ICQ):
A validated 18-item questionnaire that evaluates illness beliefs in patients with chronic conditions with three subscales: helplessness, acceptance, and perceived benefits. Higher scores denote elevations for each subscale. No total score is calculated for the ICQ; individual scale scores range from 6–24.26
Northwestern Esophageal QOL Questionnaire (NEQOL):
A validated 14-item questionnaire aimed at measuring the impact of esophageal symptoms on patient QOL. Higher scores indicate a better QOL, with scores ranging from 0–56.27
NIH PRO Measurement Information System (PROMIS) for Depression and Anxiety:
Two validated questionnaires, the depression questionnaire containing eight items and the anxiety scale containing seven items, that evaluate psychological distress. Raw scores are converted into T-scores. For PROMIS Anxiety, a raw score of 16 equates to a T-score of 55.1, a raw score of 20 to a T-score of 60.0, and a raw score of 28 to a T-score of 70.2. For PROMIS Depression a raw score of 17 equates to a T-score of 55.3, a raw score of 23 to a T-score of 60.7 and a raw score of 33 to a T-score of 70.4, For both scores, T-scores of <55 are considered none-slight, 55.0–59.9 mild, 60.0–69.9 moderate and 70.0 and over as severe anxiety or depression. 28
Sample Size:
Psychometric standards suggest a ratio of 10 participants per item of a scale (10 × 15 items) undergoing principal components factor analysis (PCFA), which equals 150 participants for the LCAT.29 Other guidelines suggest that a minimum of 200 is considered “fair” for PCFA.30 Thus, the pre-specified goal in this study was 240 participants: 200 symptomatic and 40 asymptomatic.
Statistical Analyses
The primary objective was to assess the psychometric properties of the LCAT. To assess the reliability of the score, the following methods were used: 1) Measurement of internal consistency using Cronbach α, which evaluates how each item in a questionnaire are related and 2) Split-half reliability (Guttman statistic), which evaluates if two halves of the test result in similar scores and was conducted with the entire sample. A reliability score of 0.70 or higher is considered acceptable.31,32 Construct validity, which measures how well a questionnaire is accurately evaluating what it is supposed to, was assessed through Pearson’s correlations with previously validated questionnaires mentioned above. The LCAT subscale structure was evaluated through PCFA with varimax rotation, which is a method that allows for identification of specific factors (i.e., subscales) that questionnaires are measuring. Eigenvalues were computed and plotted, with those greater than 1.0 indicating a potential subscale. The rotated component matrix was assessed for item correlations and subscale fit. Inter-item correlations to assess for multicollinearity to identify redundant items were assessed via Pearson’s correlations; items with a correlation >0.75 were classified as redundant and evaluated for removal. In addition, a median split, a method to turn continuous variables into categorical values, was computed to determine a threshold for normal vs abnormal LCAT score.
In the validation phase it was noted that a sub-group who self-identified as not having chronic laryngeal symptoms (asymptomatic) indicated an elevated RSI (>13) and a sub-group of self-identified symptomatic participants indicated a normal RSI (≤13). To ensure that potentially symptomatic patients were not being missed, a post-hoc sensitivity analysis was conducted to compare LCAT scores between participants with an RSI >13 and RSI ≤13.
Two-sample t-tests (Welch for unequal standard deviations) for continuous variables, and Pearson’s Chi-Squared tests for binary variables, were used to compare symptomatic and asymptomatic patients as well as patients with and without an elevated RSI (>13). Multivariable linear regression analyses comparing PROs in the symptomatic and asymptomatic group, controlling for age, were also run.
Analyses were conducted via SPSS v23 for Macintosh (Chicago, IL) and R v4.2.0 (Vienna, Austria).
Results
Overall, 268 participants enrolled in and completed this study: 8 in the development phase and 260 in the validation phase.
Development Phase
Of the 8 participants ages ranged from 25–74 years, 4 (50%) were male, and the mean length of symptoms was 46.5 (SD 49.1) months. Based on participant responses and review with the multidisciplinary team, 5 questions from the original EHAS were modified (Supplemental Table 1) resulting in the final 15-item LCAT. The LCAT includes a five-point Likert scale (0=”Strongly Disagree” to 4=”Strongly Agree”) that assess symptoms over the past month with scores ranging from 0 to 60.
Validation Phase
Of 260 participants in the validation phase, 56 were asymptomatic and 204 were symptomatic. Mean age was 52.9 (SD 16.8) years, 71% (184/258) were female, and mean body mass index was 27.6 (6.9) kg/m2 (Table 1). The population included the following racial groups: 61% White, 3% Black, 8% Asian, 11% Hispanic, 1% Native American and 16% other/unknown. Symptomatic patients were significantly older than asymptomatic volunteers (54.7 (16.1) vs. 46.1 (17.7), p<0.01). Two sample t-tests are presented in Table 1.
Table 1:
Baseline Characteristics
| Overall n=260 | Asymptomatic Group n=56 | Symptomatic Group n=204 | P-value | |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) (n 258) | 52.9 (16.8) | 46.1 (17.7) | 54.7 (16.1) | <0.01 |
| Sex (female) (n 258) | 184 (71%) | 41 (75%) | 143 (70%) | 0.55 |
| Body Mass Index (kg/m2) (n 239) | 27.6 (6.9) | 27.5 (8.2) | 27.6 (6.7) | 0.97 |
| Questionnaires | ||||
| Total LCAT Score | 31.0 (15.1) | 17.8 (16.1) | 34.6 (12.7) | <0.01 |
| LCAT Symptom-specific Anxiety Subscale (Q1–9) | 16.7 (9.9) | 9.1 (9.8) | 18.8 (8.9) | <0.01 |
| LCAT Hypervigilance Subscale (Q10–15) | 14.3 (6.2) | 8.6 (7.4) | 15.8 (4.8) | <0.01 |
| GerdQ | 8.9 (3.0) | 7.7 (2.9) | 9.3 (3.0) | <0.01 |
| ICQ Helplessness Subscale | 10.7 (4.4) | 8.3 (3.6) | 11.4 (4.3) | <0.01 |
| ICQ Acceptance Subscale | 15.0 (4.4) | 15.0 (6.0) | 14.9 (3.9) | 0.84 |
| ICQ Perceived Benefits Subscale | 12.1 (4.8) | 10.9 (5.1) | 12.5 (4.6) | 0.03 |
| NEQOL (n 258) | 33.7 (15.3) | 45.5 (12.9) | 30.5 (14.3) | <0.01 |
| PROMIS Anxiety (n 257) | 16.8 (7.2) | 13.2 (6.0) | 17.8 (7.2) | <0.01 |
| PROMIS Depression (n 256) | 14.8 (7.8) | 11.2 (5.1) | 15.8 (8.1) | <0.01 |
| VHI (n 252) | 21.8 (26.7) | 6.7 (13.2) | 26.0 (28.0) | <0.01 |
| RSI (n 256) | 19.3 (11.7) | 7.3 (8.2) | 22.6 (10.3) | <0.01 |
Subscale Identification
The PCFA yielded 2 components with an Eigenvalue > 1.0. Component 1 (Eigenvalue = 8.363) representing 55.76% of the variance in LCAT score while Component 2 (Eigenvalue = 1.282) represented 8.54% of the variance. In the rotated component matrix, LCAT items 1–8 (Symptom-Specific Anxiety questions) loaded onto Component 1, while items 9–15 (Hypervigilance questions) loaded onto Component 2. This structure mirrored that of the original EHAS. The rotated PCFA matrix is presented in Table 2.
Table 2:
Rotated Component Matrix with Factor Loading for LCAT Consistent with Two Subscales
| Laryngeal Cognitive-Affective Tool | Factor 1 (Symptom-specific Anxiety) | Factor 2 (Symptom-specific Hypervigilance) |
|---|---|---|
| Symptom Specific Anxiety Questions (1–9) | ||
| 1) Can’t keep my throat symptoms out of my mind. | 0.735 | |
| 2) Hard time enjoying myself because I cannot get my mind off throat symptoms. | 0.786 | |
| 3) Throat symptoms are overwhelming. | 0.789 | |
| 4) As soon as I awake, I worry that I will have throat discomfort | 0.732 | |
| 5) I often worry about my throat. | 0.692 | |
| 6) My throat symptoms are terrible and never going to get better. | 0.808 | |
| 7) Nothing I can do to reduce my throat symptoms. | 0.721 | |
| 8) Discomfort in my throat scares me. | 0.623 | |
| 9) I want my throat symptoms to go away. | 0.559 | 0.567 |
| Symptom-Specific Hypervigilance Questions (10–15) | ||
| 10) Quick to notice changes in my throat symptoms. | 0.840 | |
| 11) Focus on my throat symptoms in social situations. | 0.752 | |
| 12) Notice throat symptoms even if I am busy | 0.732 | |
| 13) I focus on my throat. | 0.633 | |
| 14) Very sensitive to my throat | 0.749 | |
| 15) Keep track of my throat symptoms. | 0.607 | |
Notes: Items are paraphrased for Copyright Restrictions. Questionnaire prompt indicates for patients to answer if they agree with the following statements based on their experience with throat symptoms over the past month.
Questionnaire Scores
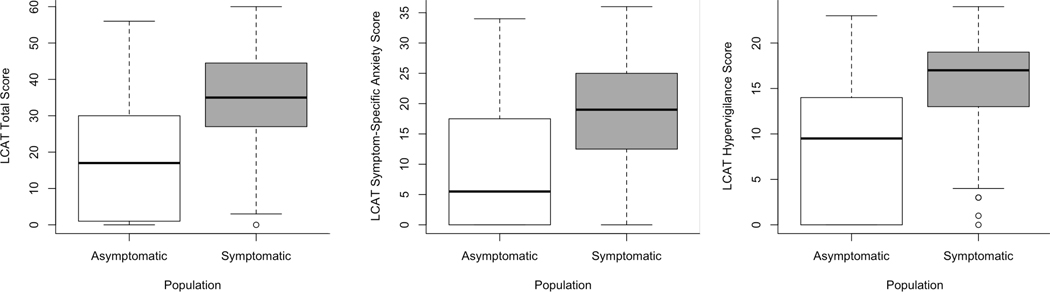
Mean total LCAT score was 31.0 (15.1) and significantly higher in the symptomatic group (34.6 (12.7)) compared to the asymptomatic group (17.8 (16.1); p<0.01). Further, the LCAT hypervigilance sub-score and LCAT symptom specific anxiety sub-score were significantly higher among the symptomatic group compared to the asymptomatic group (Table 1, Figure 1). In addition, symptomatic participants reported significantly higher GerdQ, ICQ: Helplessness, ICQ: Perceived Benefits, PROMIS Anxiety and Depression, and VHI scores as well as significantly lower NEQOL scores when compared to asymptomatic participants (Table 1). Multivariable linear regression models comparing questionnaire scores between the asymptomatic and symptomatic group yielded similar results when controlling for age.
Figure 1:
Boxplots comparing LCAT total, hypervigilance, and symptom-specific anxiety sub-scores between asymptomatic and symptomatic patients.
LCAT Score Validation
The LCAT had excellent internal consistency (α=0.942) and split-half reliability (Guttman=0.853). Construct validity of the LCAT is supported by moderate relationships with RSI (r=0.69), NEQOL (r= −0.78), ICQ Helplessness Subscale (r=0.61), PROMIS-anxiety (r=0.61), PROMIS-depression (r=0.44), and VHI (r=0.46) and weak relationship with GerdQ (r=0.34) and ICQ Acceptance Subscale (r= −0.24). all p-values <0.01. There was no relationship with ICQ Perceived Benefits Subscale (r=0.03, p=0.62) (Table 3).
Table 3:
Pearson’s Correlations with LCAT and LCAT Subscales
| LCAT Total | LCAT Symptom-Specific Anxiety | LCAT Hypervigilance | |
|---|---|---|---|
| LCAT Total | -- | -- | -- |
| LCAT Symptom-Specific Anxiety | 0.96* | -- | -- |
| LCAT Hypervigilance | 0.90* | 0.75* | -- |
| GerdQ | 0.34* | 0.31* | 0.34* |
| ICQ Helplessness | 0.61* | 0.64* | 0.46* |
| ICQ Acceptance | −0.24* | −0.30* | −0.11 |
| ICQ Perceived Benefits | 0.03 | −0.02 | 0.11 |
| NEQOL | −0.78* | −0.80* | −0.63* |
| PROMIS Anxiety | 0.61* | 0.62* | 0.48* |
| PROMIS Depression | 0.44* | 0.46* | 0.35* |
| VHI | 0.46* | 0.45* | 0.39* |
| RSI | 0.69* | 0.67* | 0.60* |
P<0.01
Inter-item Correlations
Pearson’s correlations between LCAT items 1–15 ranged from 0.26 (Q7 and Q15) - 0.73 (Q11 and Q12), all p-values <0.01, indicating each question was unique and did not achieve multicollinearity, thus no items were removed.
Defining an LCAT Threshold
Using a median split, a total score of ≥33 defined an elevated LCAT. In total 137/260 (53%) had an LCAT score ≥33. A significantly greater proportion of the symptomatic group had an elevated LCAT compared to the asymptomatic group (125/204 (61%) vs 12/56 (21%); (p<0.01)).
Sensitivity Analysis
Among the entire group, 88 (34%) had a normal RSI (≤ 13) and 168 (66%) had an elevated RSI (>13). Individuals with elevated RSI were more likely to have elevated total LCAT scores and sub-scores, GerdQ, ICQ: Helplessness, PROMIS Anxiety and Depression, and VHI scores and lower NEQOL scores (all p-values <0.05) (Supplemental Table 2). A significantly greater proportion of those with an elevated RSI had an LCAT score ≥33 compared to those with a normal RSI (16/88 (18%) vs 121/168 (72%); (p<0.01)).
Discussion
Laryngeal hypervigilance and symptom-specific anxiety are common among patients experiencing laryngeal symptoms, whether in overlap with other pathologic conditions such as GERD, or in silo, and represent an important therapeutic target to alleviate symptoms and improve QOL. Given the lack of instruments to measure laryngeal hypervigilance and symptom-specific anxiety, we developed and validated the 15-item LCAT, which demonstrates excellent internal consistency, reliability, and construct validity. Further, LCAT sub-scales differentiated symptom-specific anxiety and hypervigilance as related but unique cognitive-affective processes.12,16,33–35 Therefore, the LCAT is a valid instrument that clinicians can utilize to measure the burden of laryngeal hypervigilance and symptom anxiety in patients and elucidate the mechanisms of symptom experiences, provide education to patients, and target therapies towards such cognitive-affective processes.
The relationship between cognitive-affective processes and esophageal symptom burden is well-established in GERD33,36, eosinophilic esophagitis34, and major motor disorders;16,35 however, our understanding of how to empirically and reliably measure these processes in laryngeal symptoms is nascent. Our group previously examined 77 patients with laryngeal symptoms and found similar rates of heightened hypervigilance and symptom-specific anxiety among those with and without pathologic GERD, as measured by the EHAS.12 Similarly, Wong et al. evaluated 269 patients with laryngeal and GERD symptoms and found a higher burden of esophageal hypervigilance and symptom-specific anxiety (measured by EHAS) in patients with both GERD and LPR symptoms, LPR predominant symptoms, and GERD predominant symptoms when compared to controls. Further, there were no difference in reflux burden in these groups.13 Though these studies suggest that patients with laryngeal symptoms experience high rates of hypervigilance and symptom anxiety, the EHAS was developed for esophageal symptoms and is not specific to laryngeal symptoms. Only a few PROs focus on QOL and depression in patients with laryngeal symptoms, 37,38 and no PROs are available to assess cognitive-affective processes such as laryngeal specific hypervigilance or symptom-specific anxiety.
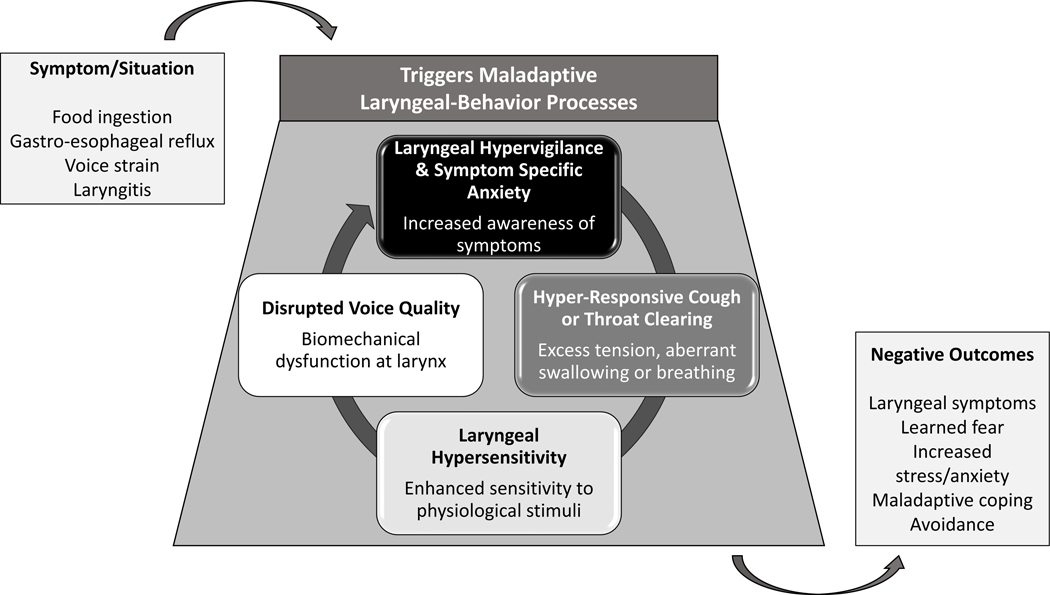
Our multidisciplinary experience, as well as the research highlighted above, suggests a complex biopsychosocial interplay between laryngeal behaviors, cognitive-affective processes, and in some patients, pathologic reflux disease (Figure 2). In some individuals, laryngeal irritation from a reflux episode, a food trigger, or pharyngitis results in hyper-responsive laryngeal behaviors such as cough/throat clearing or biomechanical changes in the vocal cords presenting as dysphonia. These behaviors can be associated with, and exacerbated by, heightened levels of hypervigilance and symptom-specific anxiety. Collectively, compensatory behaviors to try to remedy discomfort (e.g., throat clearing), heightened awareness to throat sensations and the effectiveness (or lack thereof) of attempts to improve symptoms are normal responses to physical discomfort. With time, the brain may become habituated to assessing throat sensations and the associated worry can lead to impairments across QOL domains. What is intended to be protective then backfires on the patient. Fortunately, these responses are modifiable with brain-gut behavioral interventions, such as cognitive-behavioral therapy for GI illness or gut-directed hypnotherapy,15,39 which can be administered by a clinical psychologist, or other interventions targeting laryngeal hyper-responsive behaviors (such as cough/throat clearing), which can be administered by a specialized speech-language pathologist. With the development of the LCAT, there is now a method to measure and quantify these cognitive-affective processes in clinical practice, which can help guide clinicians as to when adding behavioral approaches may be of utility. In addition, this score allows practitioners to assess the effectiveness of these therapies over time and monitor patient outcomes, to ensure treatment optimization.
Figure 2:
A biopsychosocial model outlining the complex interplay between laryngeal symptoms, hyper-responsive laryngeal behaviors/biomechanical dysfunction, cognitive-affective processes, and in some patients, pathologic reflux disease, which can all lead to negative patient outcomes.
This is the first study to develop and validate an instrument to measure laryngeal hypervigilance and symptom-specific anxiety. This prospective study was conducted across a large well-characterized population of individuals in accordance with pre-specified sample estimates and instrument validation methods. A group of patients without chronic laryngeal symptoms were also evaluated to determine the performance of these questionnaires in this population. Median split identified an LCAT score of ≥33 as elevated; however, this should not be considered a diagnostic indicator but rather suggests that a patient may need additional evaluation and support.
The single center study design, lack of participant diversity, as well as the study being conducted at a tertiary care center, may limit generalizability across populations. Information on treatments or underlying disease pathology was not collected and it is possible that some patients who self-identified as asymptomatic were so in the setting of treatment. Interestingly, we found that there was some overlap in total and sub-scale LCAT scores between the asymptomatic and symptomatic cohorts (Figure 1). To account for this potentially low symptom burden in some patients, sensitivity analyses utilizing RSI score cutoffs of 13 were evaluated and yielded similar results. Assessment of change in LCAT scores across treatment outcome measures are needed to understand how this score changes after therapy.
In conclusion, the LCAT is a newly validated 15-item instrument with excellent reliability and construct validity to measure laryngeal hypervigilance and symptom specific anxiety. The use of this questionnaire in practice could help clinicians to better understand symptom perception and burden and thereby tailor therapies targeting these cognitive-affective processes. Further, the LCAT can be used in research to better characterize populations, as well as evaluate the longitudinal utility of interventions over time.
Supplementary Material
Grant Support:
NIH 5T32DK007202-46 (Ghosh, PI); NIH DK125266 (Yadlapati, PI); NIH DK135513 (Yadlapati, PI); University of California San Diego Academic Senate Grant P025945.
Abbreviations:
- CASM
Cognitive Aspects of Survey Methodology
- EHAS
Esophageal Hypervigilance and Anxiety Scale
- GERD
Gastroesophageal reflux disease
- GerdQ
Gastroesophageal Reflux Disease Questionnaire
- ICQ
Illness Cognition Questionnaire
- LCAT
Laryngeal Cognitive-Affective Tool
- LPR
Laryngopharyngeal reflux
- NEQOL
Northwestern Esophageal Quality of Life Questionnaire
- PCFA
Principal Components Factor Analysis
- PPI
Proton pump inhibitor
- PRO
Patient Reported Outcomes
- PROMIS
Patient Reported Outcomes Measurement Information System
- QOL
Quality of life
- RSI
Reflux Symptom Index
- UCSD
University of California San Diego
- VHI
Voice Handicap Index
Footnotes
Conflicts of Interest
AK, MG, ZCB, EW: None
TT: Abyle Health (Consultant, Advisory Board), Takeda (Consultant), Healthline (Consultant)
PW: FemtoVox (Consultant)
JEP: AlfaSigma (Consultant); Endogastric solutions (Consultant, Speakers Bureau); Ethicon/J&J (Advisory Committee/Board Member, Consultant, Speakers Bureau); Medtronic (Advisor or Review Panel Member, Consultant, Intellectual Property/Patents, Royalties, Speakers Bureau); Phathom (Consultant); Takeda (Consultant, Speakers Bureau)
RY: Consultant for Medtronic, Phathom Pharmaceuticals, StatLinkMD, Reckitt Benckiser Healthcare Ltd, Medscape; Research Support: Ironwood Pharmaceuticals; Advisory Board with Stock Options: RJS Mediagnostix
Data Sharing Statement:
Data, analytic methods, and study materials will be made available to other researchers by request whose used of the proposed data has been approved. Data is available on request to mgreytak@health.ucsd.edu.
References
- 1.Lechien JR, Akst LM, Hamdan AL, et al. Evaluation and Management of Laryngopharyngeal Reflux Disease: State of the Art Review. Otolaryngol Head Neck Surg. May 2019;160(5):762–782. doi: 10.1177/0194599819827488 [DOI] [PubMed] [Google Scholar]
- 2.Chen XM, Li Y, Guo WL, Wang WT, Lu M. [Prevalence of laryngopharyngeal reflux disease in Fuzhou region of China]. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. Dec 07 2016;51(12):909–913. doi: 10.3760/cma.j.issn.1673-0860.2016.12.006 [DOI] [PubMed] [Google Scholar]
- 3.Spantideas N, Drosou E, Bougea A, Assimakopoulos D. Laryngopharyngeal reflux disease in the Greek general population, prevalence and risk factors. BMC Ear Nose Throat Disord. 2015;15:7. doi: 10.1186/s12901-015-0020-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kamani T, Penney S, Mitra I, Pothula V. The prevalence of laryngopharyngeal reflux in the English population. Eur Arch Otorhinolaryngol. Oct 2012;269(10):2219–25. doi: 10.1007/s00405-012-2028-1 [DOI] [PubMed] [Google Scholar]
- 5.Lowden M, McGlashan JA, Steel A, Strugala V, Dettmar PW. Prevalence of symptoms suggestive of extra-oesophageal reflux in a general practice population in the UK. Logoped Phoniatr Vocol. 2009;34(1):32–5. doi: 10.1080/14015430902735847 [DOI] [PubMed] [Google Scholar]
- 6.Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R, Group GC. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. Aug 2006;101(8):1900–20; quiz 1943. doi: 10.1111/j.1572-0241.2006.00630.x [DOI] [PubMed] [Google Scholar]
- 7.Olson NR. Laryngopharyngeal manifestations of gastroesophageal reflux disease. Otolaryngol Clin North Am. Oct 1991;24(5):1201–13. [PubMed] [Google Scholar]
- 8.Yadlapati R, Chan WW. Modern Day Approach to Extra-Esophageal Reflux: Clearing the Murky Lens. Clin Gastroenterol Hepatol. Feb 28 2023;doi: 10.1016/j.cgh.2022.12.038 [DOI] [PMC free article] [PubMed]
- 9.Chen JW, Vela MF, Peterson KA, Carlson DA. AGA Clinical Practice Update on the Diagnosis and Management of Extraesophageal Gastroesophageal Reflux Disease: Expert Review. Clin Gastroenterol Hepatol. Jun 2023;21(6):1414–1421.e3. doi: 10.1016/j.cgh.2023.01.040 [DOI] [PubMed] [Google Scholar]
- 10.Liu K, Krause A, Yadlapati R. Quality of Life and Laryngopharyngeal Reflux. Dig Dis Sci. Jul 06 2023;doi: 10.1007/s10620-023-08027-8 [DOI] [PMC free article] [PubMed]
- 11.Francis DO, Rymer JA, Slaughter JC, et al. High economic burden of caring for patients with suspected extraesophageal reflux. Am J Gastroenterol. Jun 2013;108(6):905–11. doi: 10.1038/ajg.2013.69 [DOI] [PubMed] [Google Scholar]
- 12.Krause AJ, Greytak M, Burger ZC, Taft T, Yadlapati R. Hypervigilance and Anxiety are Elevated Among Patients With Laryngeal Symptoms With and Without Laryngopharyngeal Reflux. Clin Gastroenterol Hepatol. Oct 26 2022;doi: 10.1016/j.cgh.2022.10.017 [DOI] [PMC free article] [PubMed]
- 13.Wong MW, Hsiao SH, Wang JH, et al. Esophageal Hypervigilance and Visceral Anxiety Contribute to Symptom Severity of Laryngopharyngeal Reflux. Am J Gastroenterol. May 01 2023;118(5):786–793. doi: 10.14309/ajg.0000000000002151 [DOI] [PubMed] [Google Scholar]
- 14.Wong MW, Bair MJ, Chang WC, et al. Clinical and psychological characteristics in gastroesophageal reflux disease patients overlapping with laryngopharyngeal reflux symptoms. J Gastroenterol Hepatol. Oct 2019;34(10):1720–1726. doi: 10.1111/jgh.14651 [DOI] [PubMed] [Google Scholar]
- 15.Krause AJ, Walsh EH, Weissbrod PA, Taft TH, Yadlapati R. An update on current treatment strategies for laryngopharyngeal reflux symptoms. Ann N Y Acad Sci. 04 2022;1510(1):5–17. doi: 10.1111/nyas.14728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taft TH, Triggs JR, Carlson DA, et al. Validation of the oesophageal hypervigilance and anxiety scale for chronic oesophageal disease. Aliment Pharmacol Ther. May 2018;47(9):1270–1277. doi: 10.1111/apt.14605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sullivan MJ, Bishop SR, Pivaik J. The Pain Catastrophizing Scale: Development and validation. Psychological Assessment. 1995;7(4):524–532. doi: 10.1037/1040-3590.7.4.524 [DOI] [Google Scholar]
- 18.Labus JS, Bolus R, Chang L, et al. The Visceral Sensitivity Index: development and validation of a gastrointestinal symptom-specific anxiety scale. Aliment Pharmacol Ther. Jul 01 2004;20(1):89–97. doi: 10.1111/j.1365-2036.2004.02007.x [DOI] [PubMed] [Google Scholar]
- 19.McCracken LM. “Attention” to pain in persons with chronic pain: A behavioral approach. 1997;28(2):271–284. doi: 10.1016/S0005-7894(97)80047-0 [DOI] [Google Scholar]
- 20.Roelofs J, Peters ML, McCracken L, Vlaeyen JWS. The pain vigilance and awareness questionnaire (PVAQ): further psychometric evaluation in fibromyalgia and other chronic pain syndromes. Pain. Feb 2003;101(3):299–306. doi: 10.1016/S0304-3959(02)00338-X [DOI] [PubMed] [Google Scholar]
- 21.Rosenstiel AK, Keefe FJ. The use of coping strategies in chronic low back pain patients: relationship to patient characteristics and current adjustment. Pain. Sep 1983;17(1):33–44. doi: 10.1016/0304-3959(83)90125-2 [DOI] [PubMed] [Google Scholar]
- 22.Schwarz N Cognitive Aspects of Survey Methodology. Appl Cognit Psychol. 2007;21:277–287. doi: 10.1002/acp.1340 [DOI] [Google Scholar]
- 23.Jones R, Junghard O, Dent J, et al. Development of the GerdQ, a tool for the diagnosis and management of gastro-oesophageal reflux disease in primary care. Aliment Pharmacol Ther. Nov 15 2009;30(10):1030–8. doi: 10.1111/j.1365-2036.2009.04142.x [DOI] [PubMed] [Google Scholar]
- 24.Belafsky PC, Postma GN, Koufman JA. Validity and reliability of the reflux symptom index (RSI). J Voice. Jun 2002;16(2):274–7. doi: 10.1016/s0892-1997(02)00097-8 [DOI] [PubMed] [Google Scholar]
- 25.Jacobson BH, Johnson A, Grywalski C, et al. The Voice Handicap Index (VHI). American Journal of Speech-Language Pathology. 1997;6(3):66–70. [Google Scholar]
- 26.Evers AW, Kraaimaat FW, van Lankveld W, Jongen PJ, Jacobs JW, Bijlsma JW. Beyond unfavorable thinking: the illness cognition questionnaire for chronic diseases. J Consult Clin Psychol. Dec 2001;69(6):1026–36. [PubMed] [Google Scholar]
- 27.Bedell A, Taft TH, Keefer L, Pandolfino J. Development of the Northwestern Esophageal Quality of Life Scale: A Hybrid Measure for Use Across Esophageal Conditions. Am J Gastroenterol. Apr 2016;111(4):493–9. doi: 10.1038/ajg.2016.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pilkonis PA, Choi SW, Reise SP, et al. Item banks for measuring emotional distress from the Patient-Reported Outcomes Measurement Information System (PROMIS®): depression, anxiety, and anger. Assessment. Sep 2011;18(3):263–83. doi: 10.1177/1073191111411667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mundfrom DJ, Shaw DG, Lu Ke T. Minimum Sample Size Recommendations for Conducting Factor Analyses. International Journal of Testing. 2005;5(2):159–168. doi: 10.1207/s15327574ijt0502_4 [DOI] [Google Scholar]
- 30.Comrey AL, Lee HB. A first course in factor analysis, 2nd ed. A first course in factor analysis, 2nd ed. Lawrence Erlbaum Associates, Inc; 1992:xii, 430-xii, 430. [Google Scholar]
- 31.Taber KS. The Use of Cronbach’s Alpha When Developing and Reporting Research Instruments in Science Education. Research in Science Education. 2018;48:1273–1296. [Google Scholar]
- 32.TenHouten WD. Scale Gradient Analysis: A Statistical Method for Constructing and Evaluating Guttman Scales. Sociometry. 1969;32(1):80–98. doi: 10.2307/2786636 [DOI] [Google Scholar]
- 33.Guadagnoli L, Yadlapati R, Taft T, Pandolfino JE, Tye M, Keefer L. Esophageal hypervigilance is prevalent across gastroesophageal reflux disease presentations. Neurogastroenterol Motil. Aug 2021;33(8):e14081. doi: 10.1111/nmo.14081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taft TH, Carlson DA, Simons M, et al. Esophageal Hypervigilance and Symptom-Specific Anxiety in Patients with Eosinophilic Esophagitis. Gastroenterology. Oct 2021;161(4):1133–1144. doi: 10.1053/j.gastro.2021.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carlson DA, Gyawali CP, Roman S, et al. Esophageal Hypervigilance and Visceral Anxiety Are Contributors to Symptom Severity Among Patients Evaluated With High-Resolution Esophageal Manometry. Am J Gastroenterol. Mar 2020;115(3):367–375. doi: 10.14309/ajg.0000000000000536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kahrilas PJ, Keefer L, Pandolfino JE. Patients with refractory reflux symptoms: What do they have and how should they be managed? Neurogastroenterol Motil. Sep 2015;27(9):1195–201. doi: 10.1111/nmo.12644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Francis DO, Patel DA, Sharda R, et al. Patient-Reported Outcome Measures Related to Laryngopharyngeal Reflux: A Systematic Review of Instrument Development and Validation. Otolaryngol Head Neck Surg. Dec 2016;155(6):923–935. doi: 10.1177/0194599816664330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carrau RL, Khidr A, Gold KF, et al. Validation of a quality-of-life instrument for laryngopharyngeal reflux. Arch Otolaryngol Head Neck Surg. Apr 2005;131(4):315–20. doi: 10.1001/archotol.131.4.315 [DOI] [PubMed] [Google Scholar]
- 39.Schneider SL, Clary MS, Fink DS, et al. Voice therapy associated with a decrease in the reflux symptoms index in patients with voice complaints. Laryngoscope. May 2019;129(5):1169–1173. doi: 10.1002/lary.27583 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data, analytic methods, and study materials will be made available to other researchers by request whose used of the proposed data has been approved. Data is available on request to mgreytak@health.ucsd.edu.