Abstract
Rationale & Objective:
The toxins contributing to uremic symptoms in patients with CKD are unknown. We sought to apply complementary statistical modeling approaches to data from untargeted plasma metabolomic profiling to identify solutes associated with uremic symptoms in patients with CKD.
Study Design:
Cross-sectional.
Setting & Participants:
1,761 Chronic Renal Insufficiency Cohort (CRIC) participants with CKD not on dialysis.
Predictors:
Measurement of 448 known plasma metabolites.
Outcomes:
The uremic symptoms fatigue, anorexia, pruritus, nausea, paresthesia, and pain were assessed by single items on the Kidney Disease Quality of Life-36 (KDQOL) instrument.
Analytical Approach:
Multivariable adjusted linear regression, Lasso linear regression, and random forest models were used to identify metabolites associated with symptom severity. After adjustment for multiple comparisons, metabolites selected in at least two of the three modeling approaches were deemed “overall” significant.
Results:
Participant mean eGFR was 43 mL/min/1.73 m2, with 44% self-identifying as female and 41% Non-Hispanic Black. The prevalence of uremic symptoms ranged from 22 – 55%. We identified 17 metabolites for which a higher level was associated with greater severity of at least one uremic symptom, and 9 metabolites inversely associated with uremic symptom severity. Many of these metabolites demonstrated at least a moderate correlation with eGFR (Pearson’s r ≥ 0.5), and some were also associated with risk of developing kidney failure or death in multivariable adjusted Cox regression models.
Limitations:
Lack of a second independent cohort for external validation of our findings.
Conclusions:
Metabolomic profiling was used to identify multiple solutes associated with uremic symptoms in adults with CKD, but future validation and mechanistic studies are needed.
Keywords: chronic kidney disease, metabolomics, uremic symptoms, Chronic Renal Insufficiency Cohort (CRIC), multivariable model, machine learning
Plain Language Summary
Individuals living with chronic kidney disease (CKD) often experience symptoms related to CKD, traditionally called “uremic” symptoms. It is likely that CKD results in alterations in the level of numerous circulating substances that, in turn, cause uremic symptoms; however, the identity of these solutes is not known. In this study, we used metabolomic profiling in patients with CKD to gain insights into the pathophysiology of uremic symptoms. We identified 26 metabolites whose levels were significantly associated with at least one of the symptoms of fatigue, anorexia, itchiness, nausea, paresthesia, and pain. The results of this study lay the groundwork for future research into the biological causes of symptoms in patients with CKD.
Introduction
Uremic symptoms, traditionally conceived of including fatigue, anorexia, pruritus, nausea, paresthesia, and pain,1 contribute significantly to the higher morbidity experienced by patients with chronic kidney disease (CKD).2-4 In a recent study of non-dialysis CKD patients, nearly every patient reported at least one symptom related to kidney disease over a 5-year span.5 Importantly, patients with kidney disease have repeatedly identified “symptoms” as a top research priority.6,7
Uremic symptoms, at least in part, are attributed to the accumulation of circulating toxins as a result of declining kidney function. The observations that such symptoms often improve after dialysis initiation, and largely resolve following kidney transplantation, support this hypothesis.8,9 However, the identity of specific metabolites causing symptoms is unknown, and this remains a key barrier to the development of novel and targeted treatment strategies.
Through rapid and unbiased assessment of hundreds of solutes, metabolomics studies have the potential to accelerate discovery of candidate uremic toxins. In this study, we applied untargeted metabolomics to identify solutes associated with uremic symptoms in patients with non-dialysis CKD. We hypothesized that plasma levels of select metabolites would be independently associated with uremic symptoms among participants of the Chronic Renal Insufficiency Cohort (CRIC) Study.
Methods
Study population
The CRIC Study is a prospective, multi-center observational study of adults with CKD in the United States. Between 2003 and 2008, 3,939 participants not on dialysis, ages 21-74 years, and with an estimated glomerular filtration rate (eGFR) between 20 to 70 mL/min/1.73 m2 were enrolled across seven clinical centers. Inclusion and exclusion criteria for the CRIC Study have been published previously.10
Out of 3,520 CRIC participants who completed the Year 1 visit, 1,800 were randomly selected for blood metabolomic profiling. After excluding participants with dialysis-dependent CKD prior to the Year 1 visit (n=27) or with missing data on all six uremic symptoms at the Year 1 visit (n=12), a total of 1,761 participants were included in the current analysis. For the present study, “baseline” is defined as the Year 1 CRIC Study visit.
All participants provided written informed consent for the CRIC Study. The Institutional Review Boards of the participating centers approved the study, and the research was conducted in accordance with the Declaration of Helsinki.
Metabolomics
The Broad Institute Metabolomics Platform11 was used to profile fasting blood samples stored at −80°C using a combination of three liquid chromatography-tandem mass spectrometry (LC-MS) injections.12 Detailed methods of metabolite measurement for the present study are described in Item S1. Metabolite profiling identified 489 known metabolites in 1,800 samples. Metabolites below the level of detection in >80% of samples (n=12) were excluded, leaving 477 metabolites for evaluation. Missing metabolite values were imputed with 50% of the lowest measured value (median number of missing metabolites: 0, IQR 0-1). Metabolite data were then transformed as the natural log of the metabolite abundance and standardized to a mean of 0 and standard deviation of 1.
Uremic Symptoms
Symptoms were captured annually in CRIC by the Kidney Disease Quality of Life-36 (KDQOL-36) instrument.13 We a priori selected fatigue, anorexia, pruritus, nausea, paresthesia, and pain as co-primary outcomes. Of the symptoms assessed by the KDQOL, these six best meet the definition of uremia set forth by Hostetter and Meyer1 as symptoms accompanying kidney failure and not primarily explained by alterations in extracellular volume, inorganic ion concentration, or lack of known renal synthetic products. Consistent with prior studies, uremic symptom severity was determined by the response to single items on the symptom subscale of the KDQOL-36.3,8,14 Participants reported to what extent they were bothered by each symptom during the past 4 weeks on a Likert scale with 5 response options ranging from “Not at all bothered” to “Extremely bothered.” For this analysis, higher symptom severity scores correspond to worse symptom severity. Of the 1,761 participants included in this analysis, a small number were missing data on individual symptoms (N; fatigue 1, pruritus 3, nausea 2, paresthesia 2, pain 2) and were excluded from the analysis for that symptom.
Covariates
Covariates included in adjusted models were determined a priori, including age, sex, race and ethnicity, body mass index (BMI), history of diabetes, hypertension, and cardiovascular disease, current smoking status, eGFR and urine protein-creatinine ratio (PCR). Race and ethnicity were self-reported. eGFR was calculated using the CRIC GFR estimating equation, which includes serum creatinine and cystatin C levels in addition to age, sex, and race.15 The CRIC equation was selected due to its internal validity with reference to measured GFR. Urine PCR was measured by 24-hour urine collection or spot urine protein-to-creatinine ratio. Missing values for BMI (n=12), eGFR (n=8), and urine PCR (n=123) were imputed using estimated values from linear regression of the variable on time when two or more observations were available. When only one observation was available, missing values were filled in by carrying the value from the Year 0 visit forward. A small number of missing values for covariates remained (BMI=1, urine PCR=4), and these observations were not included in models that were adjusted for covariates.
Statistical analysis
Participant characteristics at baseline were summarized using means ± standard deviations or medians and interquartile ranges and counts (frequencies) for continuous and categorical variables, respectively. The linear-by-linear trend test was used to determine the association between categories of eGFR and category of uremic symptom severity (none, mild, or ≥moderate).
We applied three methods to identify metabolites associated with uremic symptom severity (Figure 1): ordinary least squares (OLS) linear regression, Lasso linear regression, and random forest. As there is not a single-best statistical method to analyze high-dimensional data,16-18 we utilized different statistical approaches under the assumption that metabolites identified across multiple models are more likely to represent true patterns in the data.16 Lasso and random forest models were selected as complementary approaches to linear regression models given their ability to account for collinearity in high-dimensional data.19-21 We designated “overall significant” features as metabolites found to be significantly associated with uremic symptoms in at least 2 of the 3 modeling approaches.
Figure 1.
Analysis plan for identifying metabolites associated with uremic symptom severity. Lasso and random forest models were chosen as complementary approaches to account for multicollinearity among metabolites. Metabolites selected by 2 or more of the 3 modeling approaches were considered overall significant. The statistical significance for repeated linear regression models was determined using Benjamini-Hochberg method to control for a false discovery rate (FDR) threshold of 0.05. Stability selection with Lasso regression was applied to 100 bootstrapped samples with replacement and the importance factor (IF) was then calculated as the proportion of bootstrapped samples in which the variable (i.e., metabolite) was selected by the Lasso. Variable importance in random forest models was determined by quantifying the decrease in model accuracy as a result of excluding a particular variable. Significant metabolites were defined as those included in the top 5% most important features in ≥5/10 of the training iterations.
OLS linear regression models with metabolite level as the independent variable and uremic symptom score, modeled as a continuous outcome, as the dependent variable were adjusted for all covariates listed above. To account for multiple comparisons, we used the Benjamini-Hochberg correction for controlling the false discovery rate (FDR) at or below 5%, based on the total number of metabolites.22,23
Lasso linear regression models were adjusted for the same covariates as OLS linear regression, using the Stata plugin estimator for selecting the regularization parameter (λ), which was developed to achieve an optimal sparsity rate.24 As a method of internal cross-validation, we used stability selection to identify the metabolites with the strongest association to each uremic symptom.25 100 bootstrapped samples were drawn from the data set, with replacement, and Lasso regression was computed on each sample. Then, the importance factor (IF) was calculated as the number of times a metabolite was selected by Lasso in a bootstrapped sample, divided by the total number of samples (i.e., 100). We set a significance threshold of IF ≥ 0.50.
We used the R caret (Kuhn, 2022) package to build random forest models with symptom severity modeled as a binary outcome (no symptom reported versus other) to avoid classification problems that can arise from class imbalance. Random forests were trained with 10-fold cross-validation, repeated twice, and each training iteration was repeated 10 times per symptom. We did not include covariates other than metabolites as possible features in random forest models because inclusion of variables with different scales of measurement or number of categories can result in biases when calculating variable importance.26 Variable importance was determined by quantifying the decrease in model accuracy as a result of excluding a particular variable. Significant metabolites were defined as those included in the top 5% most important features in ≥5/10 of the training iterations, as has been done in other recent studies utilizing random forests to analyze metabolomics data in CKD.27
In secondary analyses, we examined the association of the “overall significant” metabolites associated with uremic symptoms with progression to kidney failure requiring dialysis or kidney transplant and death, using multivariable adjusted Cox proportional hazards models. Ascertainment of clinical outcomes in CRIC has been previously described.10 We also repeated the primary analysis with the addition of 29 known medication-related analytes to explore potential associations between medications and symptoms.
We carried out multiple sensitivity analyses to test the robustness of our findings. We repeated the primary analysis using responses from the Modification of Diet in Renal Disease (MDRD) symptom list, a different questionnaire used in CRIC to collect symptom data, to define uremic symptom severity. The MDRD patient symptom form asks participants to rate symptom severity on a scale from 0 to 3, corresponding to none, mild, moderate, or severe, and the number of days in the past month that the symptom was experienced.28-30 A uremic symptom score was calculated as the product of the symptom severity score and the number of days the symptom was experienced. Second, as our outcome variable was measured using a 5-point Likert scale, we repeated the primary analysis using ordinal logistic regression rather than linear regression to model the association between metabolite level and uremic symptom severity. Third, considering the possibility that metabolites are an intermediary step in the causal pathway between decreasing kidney function and uremic symptom severity, the inclusion of eGFR and urine PCR in models may result in the attenuation of biologically meaningful associations. Therefore, we repeated the analyses using linear regression models that did not adjust for eGFR and urine PCR. Finally, we used multivariable adjusted linear regression models to estimate the association of eGFR and uremic symptom score and then observed the change, if any, to the magnitude of this association when each overall significant metabolite was added to the model.
Analyses were done using Stata SE 17.0 (StataCorp, College Station, TX, USA), except instances where R (R Core Team, 2022) and RStudio (Rstudio Team, 2022) were used, as mentioned.
Results
Descriptive characteristics
Baseline characteristics of the 1,761 CRIC Study participants included in the current study are shown in Table 1. Mean age (± SD) of participants was 59 ± 11 years, with 44% self-identifying as female and 41% as Non-Hispanic Black. The mean eGFR was 43 ± 17 mL/min/1.73 m2, and the median urine PCR was 133 mg/g (interquartile range 53 – 674 mg/g). There were no significant differences in characteristics between CRIC participants included in the metabolomics analysis and participants not included (Table S1).
Table 1.
Characteristics of 1,761 CRIC Study participants included in present study
| Characteristics | Overall |
|---|---|
| Mean age (SD), yr | 59 (±11) |
| Female, N (%) | 778 (44) |
| Race/ethnicity, N (%) | |
| Non-Hispanic Black | 729 (41) |
| Non-Hispanic White | 769 (44) |
| Other* | 263 (15) |
| Mean eGFR (SD), mL/min/1.73 m2 | 43 (±17) |
| CKD G-Stage, N (%) | |
| eGFR ≥ 60 mL/min/1.73 m2 | 285 (16) |
| eGFR 45-59 mL/min/1.73 m2 | 435 (25) |
| eGFR 30-44 mL/min/1.73 m2 | 587 (33) |
| eGFR < 30 mL/min/1.73 m2 | 454 (26) |
| Median protein-creatinine ratio (25th - 75th percentiles), mg/g | 133 (53 – 674) |
| Mean body mass index (SD), kg/m2 | 32 (±8) |
| Diabetes, N (%) | 862 (49) |
| Hypertension, N (%) | 1565 (89) |
| Cardiovascular disease, N (%) | 637 (36) |
| Current smoking, N (%) | 206 (12) |
| Uremic symptom prevalence, N (%) | |
| Fatigue | 937 (53) |
| Anorexia | 387 (22) |
| Pruritus | 787 (45) |
| Nausea | 497 (28) |
| Paresthesia | 800 (45) |
| Pain | 970 (55) |
Of the 1,800 CRIC participants with measured metabolites at the Year 1 visit, 27 participants were excluded from the current study for developing dialysis-dependent CKD at or prior to the Year 1 visit and 12 participants were excluded for missing data on all 6 uremic symptoms. A uremic symptom was considered present if the patient reported mild or higher severity over the preceding four weeks on the KDQOL survey.
Other group includes Hispanic, Asian/Pacific Islander, and American Indian individuals. Missingness: BMI (n=1), urine protein-creatinine ratio (n=4), fatigue (n=1), pruritus (n=3), nausea (n=2), paresthesia (n=2), pain (n=2)
Abbreviations: SD, standard deviation; IQR, interquartile range
The most prevalent uremic symptoms among CRIC participants were pain (55%), fatigue (53%), pruritus (45%) and paresthesia (45%), with nausea (28%) and anorexia (22%) less commonly reported (Table 1). Only 16% (n=276) of participants did not report any uremic symptoms at the baseline visit, while 55% (n=964) of participants reported moderate or higher symptom severity for at least one of the six uremic symptoms. Participants with lower eGFR tended to report higher symptom severity for all six uremic symptoms (Figure 2; unadjusted test for trend p<0.001 for all).
Figure 2.
Prevalence of uremic symptom severity stratified by eGFR category among 1,761 CRIC Study participants. Uremic symptom severity was assessed by single items on the symptom subscale of the KDQOL-36 instrument using a Likert scale from 1 (none) to 5 (extremely bothered). Mild symptom severity was defined by a response of “somewhat bothered” and ≥moderate symptom severity was defined as a response of “moderately bothered,” “very much bothered,” or “extremely bothered.” All symptoms demonstrated a statistically significant association with eGFR category (p<0.001). A small number of participants were missing data on uremic symptom severity and not included in the histogram for that symptom (N; fatigue 1, pruritus 3, nausea 2, paresthesia 2, pain 2).
Metabolites associated with uremic symptoms
Metabolites associated with specific uremic symptoms are reported in Table 2, with descriptions of their biological function, if known, presented in Table S2. In total, 26 metabolites were significantly associated with at least one uremic symptom, meaning the metabolite was significant in at least 2 of the 3 modeling approaches. Certain metabolites were associated with multiple uremic symptoms, with anorexia and nausea sharing the largest number of common metabolites. The unadjusted correlation between metabolite level and eGFR for the overall significant metabolites is shown in Figure 3. Complete results for all 3 modeling approaches are shown in Tables S3-S5. When the analyses were repeated including medication-derived metabolites, 3 metabolites of acetaminophen were associated with pain and 2 with fatigue, gabapentin was associated with pain and paresthesia, and piperine was significantly associated with nausea (Table S6).
Table 2.
Overall significant metabolites associated with uremic symptom severity in the CRIC Study
| Symptom | Metabolite | Analytic model | |||
|---|---|---|---|---|---|
| Lasso | Random forest |
Linear regression |
β * | ||
| Fatigue | Threitol | ü | ü | -- | 3.5 |
| Anorexia | Pseudouridine | -- | ü | ü | 4.9 |
| C-glycosyltryptophan | -- | ü | ü | 3.8 | |
| N-acetylaspartic acid | ü | ü | ü | 3.0 | |
| Imidazole propionate | ü | -- | ü | 2.3 | |
| Diacetylspermine | ü | ü | ü | 2.0 | |
| Sucrose/lactose/trehalose | -- | ü | ü | 2.0 | |
| Erythronate/threonate | -- | ü | ü | 1.7 | |
| C18:0 sphingomyelin | -- | ü | ü | 1.6 | |
| Phenylalanine | -- | ü | ü | −1.6 | |
| L-NMMA | -- | ü | ü | −1.9 | |
| 2-aminobutyrate | -- | ü | ü | −1.9 | |
| Valine | ü | ü | ü | −2.0 | |
| Tryptophan | -- | ü | ü | −2.0 | |
| Uridine | ü | ü | ü | −2.6 | |
| Pruritus | DMGV | ü | ü | ü | 3.2 |
| Salicylurate | -- | ü | ü | −2.9 | |
| Nausea | C-glycosyltryptophan | -- | ü | ü | 3.4 |
| N-acetylaspartic acid | -- | ü | ü | 2.7 | |
| DMGV | ü | -- | ü | 2.6 | |
| Sucrose/lactose/trehalose | ü | ü | ü | 2.3 | |
| N4-acetylcytidine | -- | ü | ü | 2.3 | |
| Diacetylspermine | -- | ü | ü | 1.8 | |
| C24:1 ceramide (d18:1) | -- | ü | ü | 1.4 | |
| Uridine | -- | ü | ü | −1.6 | |
| Tryptophan | -- | ü | ü | −2.1 | |
| 2-aminobutyrate | -- | ü | ü | −2.1 | |
| Paresthesia | Glucuronate | ü | ü | -- | 2.8 |
| Sucrose/lactose/Trehalose | ü | ü | -- | 2.3 | |
| Pain | N4-acetylcytidine | ü | ü | ü | 4.5 |
| C34:2 phosphatidylethanolamine | -- | ü | ü | 2.8 | |
| C16:1 sphingomyelin | -- | ü | ü | 2.4 | |
| C18:1 sphingomyelin | -- | ü | ü | 2.3 | |
| C36:4 phosphatidylethanolamine | -- | ü | ü | 2.1 | |
| Asparagine | -- | ü | ü | −2.1 | |
| 2-aminobutyrate | -- | ü | ü | −2.1 | |
| Indole-3-propionate | ü | ü | ü | −3.0 | |
Coefficients are from multivariable adjusted linear regression models and represent estimated change in symptom score associated with a 1-SD change in metabolite level (on the natural log scale)
The metabolites shown in this table were statistically significantly associated with uremic symptoms in ≥2/3 modeling approaches used, and thus, have been designated as “overall significant.” Statistical significance for each modeling approach was defined as shown in Figure 1. Coefficients from fully adjusted linear regression models are reported to indicate the direction of association. For example, higher levels of DMGV and lower levels of salicylurate were associated with greater pruritus severity. A small number of participants were missing data on uremic symptom severity (N; fatigue 1, pruritus 3, nausea 2, paresthesia 2, pain 2).
Abbreviations: L-NMMA, also known as L-targinine; DMGV, dimethylguanidino valeric acid
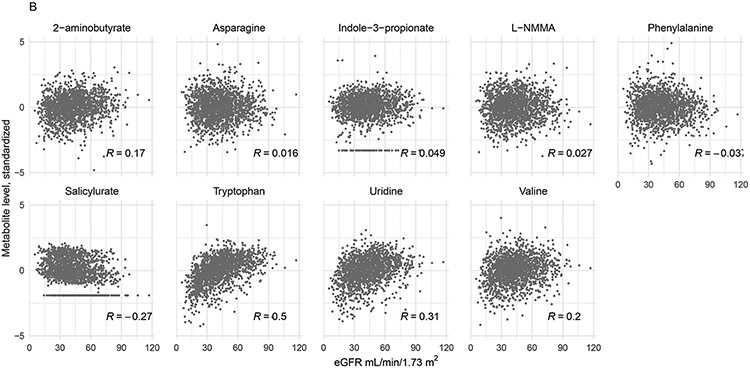
Figure 3.
Scatter plots of unadjusted relationship between eGFR and metabolite level (log-transformed and standardized). Panel A depicts metabolites positively associated with uremic symptom severity, e.g., a higher metabolite level was associated with worse symptom severity. Panel B depicts metabolites for which a lower level was associated with higher uremic symptom severity. Pearson correlation coefficients are shown. For this plot, metabolite levels less than or greater than 5 times the standard deviation of the metabolite distribution were omitted (n=24).
Association with outcomes
We then examined associations between the final set of 26 overall significant metabolites and progression to kidney failure and death, with a mean (± SD) length of follow-up of 11 ± 4 years (Table 3). Three of the 26 overall significant metabolites were positively associated with progression to kidney failure, while 3 metabolites had a statistically significant negative association with this outcome. Fourteen of the 26 metabolites were significantly associated with mortality, with a positive association for 8 metabolites and a negative association for 6 metabolites. In all cases, apart from the association between salicylurate and higher risk of kidney failure, the direction of the associations between metabolite levels and these outcomes was consistent with the direction of the association between the metabolite and uremic symptom severity.
Table 3.
Association of metabolites associated with uremic symptom severity with long-term outcomes
| Metabolite | Symptoms associated with metabolite level |
Kidney failure | Death |
|---|---|---|---|
| HR (95% CI) | HR (95% CI) | ||
| Metabolites positively associated with uremic symptom severity | |||
| C16:1 sphingomyelin | Pain | 1.08 (0.98 - 1.18) | 1.01 (0.93 - 1.10) |
| C18:0 sphingomyelin | Anorexia | 1.03 (0.94 - 1.13) | 1.03 (0.95 - 1.12) |
| C18:1 sphingomyelin | Pain | 0.99 (0.91 - 1.09) | 0.98 (0.90 - 1.07) |
| C24:1 ceramide | Nausea | 0.96 (0.88 - 1.05) | 1.08 (1.00 - 1.17) |
| C34:2 PE | Pain | 0.95 (0.86 - 1.04) | 1.21 (1.10 - 1.32) |
| C36:4 PE | Pain | 0.94 (0.86 - 1.04) | 1.14 (1.04 - 1.24) |
| C-glycosyltryptophan | Anorexia, nausea | 1.27 (1.03 - 1.58) | 1.36 (1.13 - 1.63) |
| Diacetylspermine | Anorexia, nausea | 0.93 (0.84 - 1.04) | 1.41 (1.27 - 1.55) |
| DMGV | Pruritus, nausea | 1.05 (0.93 - 1.18) | 1.06 (0.94 - 1.18) |
| Erythronate/threonate | Anorexia | 1.11 (0.95 - 1.29) | 1.07 (0.95 - 1.20) |
| Glucuronate | Paresthesia | 1.05 (0.94 - 1.19) | 1.09 (0.98 - 1.20) |
| Imidazole propionate | Anorexia | 1.03 (0.93 - 1.14) | 1.18 (1.08 - 1.29) |
| N4-acetylcytidine | Nausea, pain | 1.07 (0.94 - 1.22) | 1.34 (1.20 - 1.49) |
| N-acetylaspartic acid | Anorexia, nausea | 1.04 (0.94 - 1.15) | 1.03 (0.94 - 1.14) |
| Pseudouridine | Anorexia | 1.32 (1.08 - 1.60) | 1.16 (0.98 - 1.37) |
| Sucrose/lactose/trehalose | Anorexia, nausea, paresthesia | 1.01 (0.90 - 1.13) | 1.19 (1.08 - 1.31) |
| Threitol | Fatigue | 1.06 (0.91 - 1.23) | 1.13 (1.00 - 1.27) |
| Metabolites negatively associated with uremic symptom severity | |||
| 2-aminobutyrate | Anorexia, nausea, pain | 0.93 (0.86 - 1.01) | 0.91 (0.85 - 0.97) |
| Asparagine | Pain | 1.03 (0.94 - 1.12) | 0.90 (0.83 - 0.97) |
| Indole-3-propionate | Pain | 0.99 (0.90 - 1.08) | 0.86 (0.80 - 0.93) |
| L-NMMA | Anorexia | 1.01 (0.93 - 1.10) | 0.85 (0.78 - 0.92) |
| Phenylalanine | Anorexia | 0.89 (0.82 - 0.98) | 0.95 (0.88 - 1.03) |
| Salicylurate | Pruritus | 1.11 (1.00 - 1.22) | 1.07 (0.99 - 1.17) |
| Tryptophan | Anorexia, nausea | 0.83 (0.76 - 0.92) | 0.87 (0.80 - 0.96) |
| Uridine | Anorexia, nausea | 0.93 (0.85 - 1.02) | 0.84 (0.77 - 0.91) |
| Valine | Anorexia | 0.87 (0.79 - 0.95) | 0.93 (0.86 - 1.01) |
Metabolites positively associated with uremic symptom severity indicate that higher metabolite level was associated with worse uremic symptom score, whereas a negative association implies that lower metabolite level was associated with worse symptom severity. Models adjusted for age, sex, race/ethnicity, body mass index, diabetes, hypertension, cardiovascular disease, current smoking, eGFR, urine PCR, and clinical site. Bold font denotes statistical significance (p<0.05). Abbreviations: PE, phosphatidylethanolamine; L-NMMA, also known as L-targinine; DMGV, dimethylguanidino valeric acid
Sensitivity analyses
Using the MDRD symptom list to define uremic symptom severity, rather than the KDQOL-36 questionnaire, resulted in fewer metabolites meeting the threshold of overall significance, but the results were largely consistent with the primary analysis (Table S7). Use of ordinal logistic regression rather than linear regression to model the association between metabolite level and uremic symptom severity resulted in the selection of 5, 4, and 2 additional metabolites as overall significant for anorexia, nausea, and pain, respectively (Table S8). Omitting eGFR and urine PCR from multivariable linear regression models resulted in the selection of between 1 and 11 additional metabolites as overall significant for all six symptoms (Table S9). Finally, the estimated association between eGFR and symptom severity score was statistically significant, though small in magnitude, for all uremic symptoms except pain (Table S10). For some, but not all, of the overall significant metabolites, the addition of metabolite level to the linear regression models resulted in marked attenuation of the estimated association of eGFR and symptom score.
Discussion
An unbiased metabolomic approach presents a unique opportunity to identify novel circulating solutes associated with uremic symptoms. With this study, we aimed to contribute to the growing recognition in the field of uremic toxins that patient symptoms are an important outcome for which causative toxins must be identified.31 We used multiple complementary statistical approaches to identify metabolites associated with 6 pre-specified uremic symptoms among adult patients with non-dialysis CKD in the CRIC Study. Nevertheless, the observational nature of our study design precludes any interpretation of causality, and replication in an external cohort is required to bolster any conclusions. Instead, as a hypothesis-generating study, the metabolites identified here offer a starting point for future studies of uremic symptom pathophysiology, with the eventual goal of developing novel therapeutic targets.
We replicate some findings from a study that utilized metabolomics data from the MDRD Study to investigate uremic symptoms in patients with non-dialysis CKD.32 The MDRD Study identified an association between C-glycosyltryptophan and “lack of pep and energy”, pseudouridine and “tiring easily”, and glucuronate and “loss of appetite.” We also found a significant association between these metabolites and uremic symptoms, though the specific symptoms were different between the two studies (see Table S11 for a comparison of results). The MDRD Study used a different platform to measure metabolites and a different instrument to assess symptoms, which likely partially accounts for discrepancies between the results. We believe our study significantly adds to this prior work given our larger sample size and use of multiple statistical approaches to strengthen our findings. Metabolomics has also been used to study pruritus among patients on hemodialysis.33,34 There are no common metabolites between our study and the two studies of pruritus in hemodialysis patients, but it is likely that the metabolomic signatures of patients on hemodialysis differ significantly from those not on dialysis, particularly given the effects of the hemodialysis treatment itself on circulating metabolites.35
The metabolites significantly associated with uremic symptoms in the present study include solutes from classes of compounds that have long been identified as candidate uremic toxins, solutes not previously associated with the uremic syndrome but with interesting biological activity, and other solutes with unknown physiologic roles.36 We briefly discuss what is known regarding some of the metabolites in the following paragraphs and direct readers to Table S2 for additional sources of information. Altered lipid metabolism is a hallmark of CKD and the uremic syndrome, and we find associations between several lipid metabolites and anorexia, nausea, and pain in the current study. Phosphatidylethanolamine and ceramide levels are associated with CKD stage and progression,37 and sphingomyelin metabolites have been linked to neurotoxicity via free radicals in certain neurodegenerative disorders.38,39 Relatedly, CKD is characterized by chronic inflammation and oxidative stress.40 N4-acetylcytidine, associated with nausea and pain, was historically found to be elevated in the serum of patients with CKD on dialysis,41 and has since been associated with other pro-inflammatory conditions, such as multiple sclerosis and malignancy.42 Imidazole proprionate, associated with anorexia in the present study, is a gut microbiome-derived histidine metabolite associated with systemic inflammation and altered microbiome in patients with prediabetes and type 2 diabetes.43
Polyamines have long been recognized as a class of uremic retention solutes with possible toxic effects on erythropoiesis, macrophage activation, and neuronal function.44-46 Diacetylspermine, a polyamine metabolite, was significantly associated with anorexia and nausea in the present study and is elevated, along with spermine, in several types of malignancies.47 While not a polyamine, we also found as association between N-acetylaspartic acid and anorexia and nausea. This metabolite is an amino acid derivative formed by acetylation of L-aspartic acid and is abundant in the mammalian brain and localizes to neurons.48 Though its physiologic roles are not entirely understood, it is hypothesized to act as a neuronal osmolyte49 and shown to induce inflammation and smooth muscle contractility in the gastrointestinal tract.50 Lastly, dimethylguanidino valerate (DMGV) is interesting as one of only 2 metabolites to be significantly associated with pruritus and as a product of asymmetric dimethylarginine (ADMA) transamination, which has long been recognized as a putative uremic toxin given its inhibition of the endothelial protective compound nitric oxygen synthase and its association with cardiovascular outcomes in hemodialysis patients.51,52 Levels of circulating DMGV are a marker for fatty liver disease and positively associated with incident type 2 diabetes, incident coronary artery disease, and cardiovascular mortality.53,54
A recently advanced hypothesis posits that metabolic pathways generating uremic toxins may also give rise to beneficial molecules.55 As an example, the various metabolites of tryptophan, such as indoxyl sulfate and kynurenine, have well described toxic effects,56 whereas the tryptophan metabolites melatonin, indole, and indole-3-propionic acid can be beneficial.55,57 Interestingly, we find an association between higher tryptophan levels and less anorexia and nausea and higher levels of indole-3-propionic acid and lower pain severity. The latter molecule is a powerful anti-oxidant58 and suppresses indoxyl sulfate-induced expression of pro-fibrotic and inflammatory genes in rat kidneys.59
Implicit in the aim of the present study is the hypothesis that one or more metabolites identified herein may lie on a causal pathway between alterations in kidney function common to CKD and the development of uremic symptoms. We are not, however, able to directly test this hypothesis given the limitations of our observational data, and there are several caveats of our study design which must be acknowledged. First, the underlying hypothesis must be reconciled with findings from a recent observational study using CRIC Study data that reported only a modest association between decreasing eGFR and uremic symptom severity.14 Indeed, Figure 2 illustrates a modest apparent correlation between eGFR and symptom severity in the present study and demonstrates high prevalence of symptoms, particularly fatigue and pain, among patients with high eGFR. However, eGFR is an estimate of only one facet of kidney function, and prior studies have shown that eGFR values are insufficient to evaluate the accumulation of uremic retention solutes;60,61 thus, we have included patients with a wide range of eGFR in the present study. Symptoms experienced by patients with CKD are multifactorial, and the specific level below which symptoms are more likely to be caused by alterations in kidney function is not known. Determining the specificity of a candidate uremic toxin for a symptom directly caused by alterations in kidney function would require further mechanistic studies.
A significant limitation of this study is the lack of an external validation cohort employing the same metabolomic platform. We emphasize that ours is a hypothesis-generating study, and our findings require further validation. Additionally, it is not possible to discern the direction of influence between any metabolite and uremic symptoms from an observational study such as this (i.e., does an abnormal level of the metabolite cause the symptom or vice versa). Steps toward establishing a causal role for a metabolite in any disease state include development and use of an orthogonal assay for absolute solute quantification, demonstrating biological plausibility in animal models, performing intraindividual validation, and finally, demonstrating clinical change with removal or reduction of the candidate uremic toxin. Nevertheless, as discussed above, we cross referenced our findings with those from the MDRD Study and found similarities. Our finding of an association between metabolites of acetaminophen with fatigue and pain and metabolites of gabapentin with paresthesia and pain lends a degree of further validation to our findings. Despite this limitation, we devised an analytic approach that attempted to increase the robustness of our findings by utilizing multiple complementary statistical methods and internal cross validation to avoid overfitting of our data. Our use of Lasso and random forest models allowed for more flexible modeling, considering metrics of kidney function, e.g., eGFR and urine protein, are strongly collinear with some metabolite levels.
In conclusion, metabolomic profiling in the CRIC Study presents several novel associations between metabolites and specific uremic symptoms in adults with CKD. We present these findings as a possible springboard for further investigation into the biological mechanisms that may underlie these associations.
Supplementary Material
Item S1. Supplementary Methods: Metabolomic profiling
Table S1. Characteristics of 3,481 CRIC Study participants at the Year 1 visit, stratified by inclusion in the current analysis.
Table S2. Description of overall significant metabolites with accompanying references.
Table S3. Metabolites significantly associated with uremic symptoms in the CRIC study using multivariable adjusted linear regression models.
Table S4. Metabolites significantly associated with uremic symptoms in the CRIC study using Lasso linear regression with stability selection to determine variable importance.
Table S5. Metabolites significantly associated with uremic symptoms in the CRIC study using random forest with cross-validation and multiple iterations.
Table S6. Medication metabolites associated with uremic symptom severity as determined by multiple modeling approaches.
Table S7. Metabolites associated with uremic symptom severity as determined by multiple modeling approaches, using the MDRD symptom list to assess symptoms.
Table S8. Additional metabolites significantly associated with uremic symptom severity in the CRIC Study when utilizing ordinal logistic regression rather than linear regression models.
Table S9. Metabolites associated with uremic symptom severity in the CRIC Study, without adjusting for eGFR in linear regression analysis.
Table S10. Coefficients from multivariable adjusted linear regression models of symptom severity on eGFR, followed by changes to the coefficient for eGFR after adding overall significant metabolites for each symptom to the models.
Table S11. Comparison of overall significant metabolites identified in the present study (CRIC Study) and in a prior study by Hu et al. (Kidney Int., 2022) identifying metabolites significantly associated with uremic symptoms among 695 participants of the Modification of Diet in Renal Disease (MDRD) Study.
Support:
Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number F32DK131793 (principal investigator [PI]: KEW). This study was supported by the CKD Biomarkers Consortium, including U01 DK106981 (PI: EPR) and U01 DK106982 (PI: MRD). Funding for the CRIC Study was obtained under a cooperative agreement from National Institute of Diabetes and Digestive and Kidney Diseases (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, U01DK060902 and U24DK060990). In addition, this work was supported in part by: the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award NIH/NCATS UL1TR000003, Johns Hopkins University UL1 TR-000424, University of Maryland GCRC M01 RR-16500, Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research, Michigan Institute for Clinical and Health Research (MICHR) UL1TR000433, University of Illinois at Chicago CTSA UL1RR029879, Tulane COBRE for Clinical and Translational Research in Cardiometabolic Diseases P20 GM109036, Kaiser Permanente NIH/NCRR UCSF-CTSI UL1 RR-024131, Department of Internal Medicine, University of New Mexico School of Medicine Albuquerque, NM R01DK119199. The funders did not have any role in study design, data collection, analysis, reporting, or the decision to submit for publication.
CRIC Study Investigators:
Laura M. Dember, MD, J. Richard Landis, PhD, Raymond R. Townsend, MD, Lawrence Appel, MD, MPH, Jeffrey Fink, MD, MS, Mahboob Rahman, MD, MS, Edward J. Horwitz, MD, Jonathan J. Taliercio, DO, Panduranga Rao, MD, James H. Sondheimer, MD, James P. Lash, MD, Jing Chen, MD, MMSc, MSc, Alan S. Go, MD, Afshin Parsa, MD, MPH, and Tracy Rankin, PhD, MPH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Additional Information: Author CYH is a CRIC Study Investigator.
Financial Disclosure: The authors declare that they have no relevant financial interests.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the National Institute of Diabetes Digestive and Kidney Disease, the Department of Health and Human Services or the Government of the Unites States of America.
Prior Presentation: Presented in part at the American Society of Nephrology Kidney Week; November 4, 2022; Orlando, Florida.
References
- 1.Meyer TW, Hostetter TH. Uremia. N Engl J Med. 2007;357(13):1316–1325. doi: 10.1056/NEJMra071313 [DOI] [PubMed] [Google Scholar]
- 2.Porter AC, Lash JP, Xie D, et al. Predictors and Outcomes of Health-Related Quality of Life in Adults with CKD. Clin J Am Soc Nephrol. 2016;11(7):1154–1162. doi: 10.2215/CJN.09990915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sukul N, Speyer E, Tu C, et al. Pruritus and Patient Reported Outcomes in Non-Dialysis CKD. Clin J Am Soc Nephrol. 2019;14(5):673–681. doi: 10.2215/CJN.09600818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grams ME, Surapaneni A, Appel LJ, et al. Clinical events and patient-reported outcome measures during CKD progression: findings from the CRIC study. Nephrol Dial Transplant. 2021;36(9):1685–1693. doi: 10.1093/ndt/gfaa364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faye M, Legrand K, Gall LL, et al. Five-Year Symptom Trajectories in Nondialysis-Dependent CKD Patients. Clin J Am Soc Nephrol. 2022;17(11):1588–1597. doi: 10.2215/CJN.06140522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hemmelgarn BR, Pannu N, Ahmed SB, et al. Determining the research priorities for patients with chronic kidney disease not on dialysis. Nephrol Dial Transplant. 2017;32(5):847–854. doi: 10.1093/ndt/gfw065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manns B, Hemmelgarn B, Lillie E, et al. Setting research priorities for patients on or nearing dialysis. Clin J Am Soc Nephrol. 2014;9(10):1813–1821. doi: 10.2215/CJN.01610214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor K, Chu N, Chen X, et al. Kidney Disease Symptoms Before and After Kidney Transplantation. Clin J Am Soc Nephrol. 2021;16(7):1083–1093. doi: 10.2215/CJN.19031220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Rooij ENM, Meuleman Y, de Fijter JW, et al. Symptom Burden before and after Dialysis Initiation in Older Patients. Clin J Am Soc Nephrol. 2022;17(12):1719. doi: 10.2215/CJN.09190822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feldman HI, Appel LJ, Chertow GM, et al. The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and Methods. J Am Soc Nephrol. 2003;14(7 Suppl 2):S148–153. doi: 10.1097/01.asn.0000070149.78399.ce [DOI] [PubMed] [Google Scholar]
- 11.Rhee EP, Waikar SS, Rebholz CM, et al. Variability of Two Metabolomic Platforms in CKD. Clin J Am Soc Nephrol. 2019;14(1):40–48. doi: 10.2215/CJN.07070618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wen D, Zheng Z, Surapaneni A, et al. Metabolite profiling of CKD progression in the chronic renal insufficiency cohort study. JCI Insight. 2022;7(20):e161696. doi: 10.1172/jci.insight.161696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hays RD, Kallich JD, Mapes DL, Coons SJ, Carter WB. Development of the Kidney Disease Quality of Life (KDQOL™) Instrument. Qual Life Res. 1994;3(5):329–338. [DOI] [PubMed] [Google Scholar]
- 14.Wulczyn KE, Zhao SH, Rhee EP, Kalim S, Shafi T. Trajectories of Uremic Symptom Severity and Kidney Function in Patients with Chronic Kidney Disease. Clin J Am Soc Nephrol. 2022;17(4):496–506. doi: 10.2215/CJN.13010921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson AH, Yang W, Hsu C yuan, et al. Estimating GFR Among Participants in the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2012;60(2):250–261. doi: 10.1053/j.ajkd.2012.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Antonelli J, Claggett BL, Henglin M, et al. Statistical Workflow for Feature Selection in Human Metabolomics Data. Metabolites. 2019;9(7):143. doi: 10.3390/metabo9070143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bartel J, Krumsiek J, Theis FJ. Statistical Methods for the Analysis of High-Throughput Metabolomics Data. Comput Struct Biotechnol J. 2013;4(5):e201301009. doi: 10.5936/csbj.201301009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ogutu JO, Schulz-Streeck T, Piepho HP. Genomic selection using regularized linear regression models: ridge regression, lasso, elastic net and their extensions. BMC Proc. 2012;6(Suppl 2):S10. doi: 10.1186/1753-6561-6-S2-S10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tibshirani R. Regression Shrinkage and Selection via the Lasso. J R Stat Soc Ser B Methodol. 1996;58(1):267–288. [Google Scholar]
- 20.Breiman L. Random Forests. Mach Learn. 2001;45(1):5–32. doi: 10.1023/A:1010933404324 [DOI] [Google Scholar]
- 21.Chen T, Cao Y, Zhang Y, et al. Random Forest in Clinical Metabolomics for Phenotypic Discrimination and Biomarker Selection. Evid Based Complement Alternat Med. 2013;2013:e298183. doi: 10.1155/2013/298183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J R Stat Soc Ser B Methodol. 1995;57(1):289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- 23.Newson RB. Frequentist Q-values for Multiple-test Procedures. Stata J. 2010;10(4):568–584. doi: 10.1177/1536867X1101000403 [DOI] [Google Scholar]
- 24.Belloni A, Chen D, Chernozhukov V, Hansen C. Sparse Models and Methods for Optimal Instruments with an Application to Eminent Domain. Econometrica. 2012;80(6):2369–2429. [Google Scholar]
- 25.Meinshausen N, Bühlmann P. Stability selection. J R Stat Soc Ser B Stat Methodol. 2010;72(4):417–473. doi: 10.1111/j.1467-9868.2010.00740.x [DOI] [Google Scholar]
- 26.Strobl C, Boulesteix AL, Zeileis A, Hothorn T. Bias in random forest variable importance measures: Illustrations, sources and a solution. BMC Bioinformatics. 2007;8(1):25. doi: 10.1186/1471-2105-8-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee AM, Hu J, Xu Y, et al. Using Machine Learning to Identify Metabolomic Signatures of Pediatric Chronic Kidney Disease Etiology. J Am Soc Nephrol. 2022;33(2):375–386. doi: 10.1681/ASN.2021040538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klahr S, Levey AS, Beck GJ, et al. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group. N Engl J Med. 1994;330(13):877–884. doi: 10.1056/NEJM199403313301301 [DOI] [PubMed] [Google Scholar]
- 29.Rocco MV, Gassman JJ, Wang SR, Kaplan RM. Cross-sectional study of quality of life and symptoms in chronic renal disease patients: The modification of diet in renal disease study. Am J Kidney Dis. 1997;29(6):888–896. doi: 10.1016/S0272-6386(97)90463-7 [DOI] [PubMed] [Google Scholar]
- 30.Zhang X, Bansal N, Go AS, Hsu CY. Gastrointestinal symptoms, inflammation and hypoalbuminemia in chronic kidney disease patients: a cross-sectional study. BMC Nephrol. 2015;16:211. doi: 10.1186/s12882-015-0209-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosner MH, Reis T, Husain-Syed F, et al. Classification of Uremic Toxins and Their Role in Kidney Failure. Clin J Am Soc Nephrol. 2021;16(12):1918–1928. doi: 10.2215/CJN.02660221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu JR, Myint L, Levey AS, et al. A metabolomics approach identified toxins associated with uremic symptoms in advanced chronic kidney disease. Kidney Int. 2022;101(2):369–378. doi: 10.1016/j.kint.2021.10.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bolanos CG, Pham NM, Mair RD, Meyer TW, Sirich TL. Metabolomic analysis of uremic pruritus in patients on hemodialysis. PloS One. 2021;16(2):e0246765. doi: 10.1371/journal.pone.0246765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu Q, Zhang H, Ding J rong, et al. UPLC-QTOF MS-Based Serum Metabolomic Profiling Analysis Reveals the Molecular Perturbations Underlying Uremic Pruritus. BioMed Res Int. 2018;2018:4351674. doi: 10.1155/2018/4351674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rhee EP, Souza A, Farrell L, et al. Metabolite profiling identifies markers of uremia. J Am Soc Nephrol. 2010;21(6):1041–1051. doi: 10.1681/ASN.2009111132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vanholder R, Glorieux G, De Smet R, Lameire N, European Uremic Toxin Work Group. New insights in uremic toxins. Kidney Int Suppl. 2003;(84):S6–10. doi: 10.1046/j.1523-1755.63.s84.43.x [DOI] [PubMed] [Google Scholar]
- 37.Baek J, He C, Afshinnia F, Michailidis G, Pennathur S. Lipidomic Approaches To Dissect Dysregulated Lipid Metabolism In Kidney Disease. Nat Rev Nephrol. 2022;18(1):38–55. doi: 10.1038/s41581-021-00488-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen SD, Yin JH, Hwang CS, Tang CM, Yang DI. Anti-apoptotic and anti-oxidative mechanisms of minocycline against sphingomyelinase/ceramide neurotoxicity: implication in Alzheimer’s disease and cerebral ischemia. Free Radio Res. 2012;46(8):940–950. doi: 10.3109/10715762.2012.674640 [DOI] [PubMed] [Google Scholar]
- 39.Vr V, Am O, S V, et al. Brain and blood metabolite signatures of pathology and progression in Alzheimer disease: A targeted metabolomics study. PLoS Med. 2018;15(1):e1002482. doi: 10.1371/journal.pmed.1002482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vanholder R, Fouque D, Glorieux G, et al. Clinical management of the uraemic syndrome in chronic kidney disease. Lancet Diabetes Endocrinol. 2016;4(4):360–373. doi: 10.1016/S2213-8587(16)00033-4 [DOI] [PubMed] [Google Scholar]
- 41.Niwa T, Takeda N, Yoshizumi H. RNA metabolism in uremic patients: accumulation of modified ribonucleosides in uremic serum. Technical note. Kidney Int. 1998;53(6):1801–1806. doi: 10.1046/j.1523-1755.1998.00944.x [DOI] [PubMed] [Google Scholar]
- 42.Jin G, Xu M, Zou M, Duan S. The Processing, Gene Regulation, Biological Functions, and Clinical Relevance of N4-Acetylcytidine on RNA: A Systematic Review. Mol Ther Nucleic Acids. 2020;20:13–24. doi: 10.1016/j.omtn.2020.01.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Molinaro A, Bel Lassen P, Henricsson M, et al. Imidazole propionate is increased in diabetes and associated with dietary patterns and altered microbial ecology. Nat Commun. 2020;11(1):5881. doi: 10.1038/s41467-020-19589-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kushner D, Beckman B, Nguyen L, et al. Polyamines in the anemia of end-stage renal disease. Kidney Int. 1991;39(4):725–732. doi: 10.1038/ki.1991.88 [DOI] [PubMed] [Google Scholar]
- 45.Szabó C, Southan GJ, Wood E, Thiemermann C, Vane JR. Inhibition by spermine of the induction of nitric oxide synthase in J774.2 macrophages: requirement of a serum factor. Br J Pharmacol. 1994; 112(2):355–356. doi: 10.1111/j.1476-5381.1994.tb13078.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Segal JA, Skolnick P. Spermine-induced toxicity in cerebellar granule neurons is independent of its actions at NMDA receptors. J Neurochem. 2000;74(1):60–69. doi: 10.1046/j.1471-4159.2000.0740060.x [DOI] [PubMed] [Google Scholar]
- 47.Tse RTH, Wong CYP, Chiu PKF, Ng CF. The Potential Role of Spermine and Its Acetylated Derivative in Human Malignancies. Int J Mol Sci. 2022;23(3):1258. doi: 10.3390/ijms23031258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu H, Bai M, Xie X, et al. Impaired Amino Acid Metabolism and Its Correlation with Diabetic Kidney Disease Progression in Type 2 Diabetes Mellitus. Nutrients. 2022;14(16):3345. doi: 10.3390/nu14163345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moffett JR, Ross B, Arun P, Madhavarao CN, Namboodiri MAA. N-Acetylaspartate in the CNS: From Neurodiagnostics to Neurobiology. Prog Neurobiol. 2007;81(2):89–131. doi: 10.1016/j.pneurobio.2006.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Surendran S. Upregulation of N-acetylaspartic acid alters inflammation, transcription and contractile associated protein levels in the stomach and smooth muscle contractility. Mol Biol Rep. 2009;36(1):201–206. doi: 10.1007/s11033-007-9167-2 [DOI] [PubMed] [Google Scholar]
- 51.Shafi T, Hostetter TH, Meyer TW, et al. Serum Asymmetric and Symmetric Dimethylarginine and Morbidity and Mortality in Hemodialysis Patients. Am J Kidney Dis. 2017;70(1):48–58. doi: 10.1053/j.ajkd.2016.10.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vallance P, Leone A, Calver A, Collier J, Moncada S. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet Lond Engl. 1992;339(8793):572–575. doi: 10.1016/0140-6736(92)90865-z [DOI] [PubMed] [Google Scholar]
- 53.Ottosson F, Ericson U, Almgren P, et al. Dimethylguanidino Valerate: A Lifestyle-Related Metabolite Associated With Future Coronary Artery Disease and Cardiovascular Mortality. J Am Heart Assoc. 2019;8(19):e012846. doi: 10.1161/JAHA.119.012846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O’Sullivan JF, Morningstar JE, Yang Q, et al. Dimethylguanidino valeric acid is a marker of liver fat and predicts diabetes. J Clin Invest. 2017;127(12):4394–4402. doi: 10.1172/JCI95995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vanholder R, Nigam SK, Burtey S, Glorieux G. What If Not All Metabolites from the Uremic Toxin Generating Pathways Are Toxic? A Hypothesis. Toxins. 2022;14(3):221. doi: 10.3390/toxins14030221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vanholder R, Pletinck A, Schepers E, Glorieux G. Biochemical and Clinical Impact of Organic Uremic Retention Solutes: A Comprehensive Update. Toxins. 2018;10(1). doi: 10.3390/toxins10010033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bansal T, Alaniz RC, Wood TK, Jayaraman A. The bacterial signal indole increases epithelial-cell tight-junction resistance and attenuates indicators of inflammation. Proc Natl Acad Sci U S A. 2010;107(1):228–233. doi: 10.1073/pnas.0906112107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chyan YJ, Poeggeler B, Omar RA, et al. Potent neuroprotective properties against the Alzheimer beta-amyloid by an endogenous melatonin-related indole structure, indole-3-propionic acid. J Biol Chem. 1999;274(31):21937–21942. doi: 10.1074/jbc.274.31.21937 [DOI] [PubMed] [Google Scholar]
- 59.Yisireyili M, Takeshita K, Saito S, Murohara T, Niwa T. Indole-3-propionic acid suppresses indoxyl sulfate-induced expression of fibrotic and inflammatory genes in proximal tubular cells. Nagoya J Med Sci. 2017;79(4):477–486. doi: 10.18999/nagjms.79.4.477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eloot S, Schepers E, Barreto DV, et al. Estimated Glomerular Filtration Rate Is a Poor Predictor of Concentration for a Broad Range of Uremic Toxins. Clin J Am Soc Nephrol. 2011;6(6):1266–1273. doi: 10.2215/CJN.09981110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mair RD, Sirich TL, Meyer TW. Uremic Toxin Clearance and Cardiovascular Toxicities. Toxins. 2018;10(6):226. doi: 10.3390/toxins10060226 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Item S1. Supplementary Methods: Metabolomic profiling
Table S1. Characteristics of 3,481 CRIC Study participants at the Year 1 visit, stratified by inclusion in the current analysis.
Table S2. Description of overall significant metabolites with accompanying references.
Table S3. Metabolites significantly associated with uremic symptoms in the CRIC study using multivariable adjusted linear regression models.
Table S4. Metabolites significantly associated with uremic symptoms in the CRIC study using Lasso linear regression with stability selection to determine variable importance.
Table S5. Metabolites significantly associated with uremic symptoms in the CRIC study using random forest with cross-validation and multiple iterations.
Table S6. Medication metabolites associated with uremic symptom severity as determined by multiple modeling approaches.
Table S7. Metabolites associated with uremic symptom severity as determined by multiple modeling approaches, using the MDRD symptom list to assess symptoms.
Table S8. Additional metabolites significantly associated with uremic symptom severity in the CRIC Study when utilizing ordinal logistic regression rather than linear regression models.
Table S9. Metabolites associated with uremic symptom severity in the CRIC Study, without adjusting for eGFR in linear regression analysis.
Table S10. Coefficients from multivariable adjusted linear regression models of symptom severity on eGFR, followed by changes to the coefficient for eGFR after adding overall significant metabolites for each symptom to the models.
Table S11. Comparison of overall significant metabolites identified in the present study (CRIC Study) and in a prior study by Hu et al. (Kidney Int., 2022) identifying metabolites significantly associated with uremic symptoms among 695 participants of the Modification of Diet in Renal Disease (MDRD) Study.