Summary
Patients with cirrhosis are prone to developing acute kidney injury (AKI), a complication associated with a markedly increased in-hospital morbidity and mortality, along with a risk of progression to chronic kidney disease. Whereas patients with cirrhosis are at increased risk of developing any phenotype of AKI, hepatorenal syndrome (HRS), a specific form of AKI (HRS-AKI) in patients with advanced cirrhosis and ascites, carries an especially high mortality risk. Early recognition of HRS-AKI is crucial since administration of splanchnic vasoconstrictors may reverse the AKI and serve as a bridge to liver transplantation, the only curative option. In 2023, a joint meeting of the International Club of Ascites (ICA) and the Acute Disease Quality Initiative (ADQI) was convened to develop new diagnostic criteria for HRS-AKI, to provide graded recommendations for the work-up, management and post-discharge follow-up of patients with cirrhosis and AKI, and to highlight priorities for further research.
Keywords: hepatorenal syndrome, acute kidney injury, liver transplantation, acute disease quality initiative, international club of ascites, cirrhosis, biomarker, renal replacement therapy, ascites, albumin, terlipressin
Introduction
Acute kidney injury (AKI) occurs in up to 60% of hospitalized patients with cirrhosis and is associated with increased morbidity and mortality.1–9 In 2012, the Acute Disease Quality Initiative (ADQI) VIII and the International Club of Ascites (ICA) proposed diagnostic criteria for AKI10 which were further revised in 2015 by the ICA.11 Over the last decade, there have been significant advances in the field.12 In 2023, a joint meeting of ADQI (ADQI XXIX) and the ICA was reconvened to refine the diagnostic criteria for AKI and hepatorenal syndrome (HRS), review their epidemiology and pathophysiology, explore the role of biomarkers in the diagnosis and prognostication of AKI, examine current and novel therapies for the prevention and treatment of AKI, and create a potential paradigm for the post-discharge care of patients who experience AKI or acute kidney disease (AKD), especially as they progress to chronic kidney disease (CKD). The goals of the meeting were to provide recommendations for clinical practice and identify knowledge gaps to inform a research framework for this clinically important area.
Methods
The ADQI-ICA consensus conference chairs (MKN, FD and RLM) convened a diverse international panel of clinicians representing hepatology, nephrology, intensive care, surgery, and pharmacology. The conference was held over 2 days and followed the established ADQI process (http://www.ADQI.org) using a modified Delphi method to achieve consensus.13 Conference participants were divided into five working groups. In the pre-conference phase, each group identified a list of key questions and conducted a systematic literature search. During the conference, a series of plenary and breakout sessions were held where work groups developed consensus positions and recommendations that were refined through iterative discussions in plenary sessions. Statements were then proposed and supported by evidence, and by consensus where evidence was limited. The quality of the overall evidence and the strength of recommendations were graded using the GRADE (Grading of Recommendations Assessment, Development and Evaluation) criteria (Table S1).14–16 Following the meeting, the contributions of all groups were merged and reconciled by the steering group to generate this conference report following revision and approval by each of the participants.
Epidemiology and definition of kidney dysfunction in patients with cirrhosis
How should definitions for AKI, AKD, CKD and renal recovery in patients with cirrhosis be harmonized between kidney disease: Improving global outcomes (KDIGO) and ICA?
Consensus statements
• In patients with cirrhosis, we recommend defining AKI using KDIGO criteria: increase in serum creatinine (SCr) ≥0.3 mg/dl (26.5 μmol/L) within 48 h or ≥50% from baseline value known or presumed to have occurred within the prior 7 days and/or urine output (UO) ≤0.5 ml/kg for ≥6 h (strong recommendation, grade A).
In patients with cirrhosis, we recommend defining AKD and CKD using KDIGO criteria (strong recommendation, grade A).
In patients with cirrhosis, we recommend defining complete recovery from AKI as a return of SCr to within 0.3 mg/dl (26.5 μmol/L) of baseline (strong recommendation, grade B).
Rationale:
AKI, AKD and CKD are classified by KDIGO according to duration and severity of structural and functional abnormalities (Fig. 1).17 The ICA currently defines and stages AKI by KDIGO SCr criteria only11; however, oliguria is a sensitive and early marker of AKI that is associated with worse outcomes in critically ill patients with cirrhosis.9 Most cases of AKI will fulfil both SCr and UO criteria but clinical judgement should be utilised, taking into consideration that UO at baseline may be low in patients with cirrhosis and ascites. Measurement of UO, especially, outside the intensive care unit (ICU) is often inaccurate, and the frequent use of diuretics may affect inter-pretation; however, when possible, close monitoring of UO should be performed in order to detect moderate to severe AKI earlier, and reduce fluid overload.18 Recently, a combination of damage and functional biomarkers was proposed by ADQI to be used, along with clinical information, to define AKI and improve diagnostic and staging accuracy,19 but their role in patients with cirrhosis remains to be determined. CKD is defined as persistent glomerular filtration rate (GFR) <60 ml/min/1.73 m2 and/or markers of kidney damage for >3 months.17 Some individuals may have significant abnormalities of structure and/or function (GFR <60 ml/min/1.73 m2 or increase in SCr by >50%) within a duration of ≤3 months that do not fulfil the definitions of AKI or CKD; this period is described as AKD (Fig. 1).20 AKI is a subset of AKD, therefore, any patient with AKI, by definition, has AKD.
Fig. 1. Clinical course and outcomes of AKI in patients with cirrhosis.
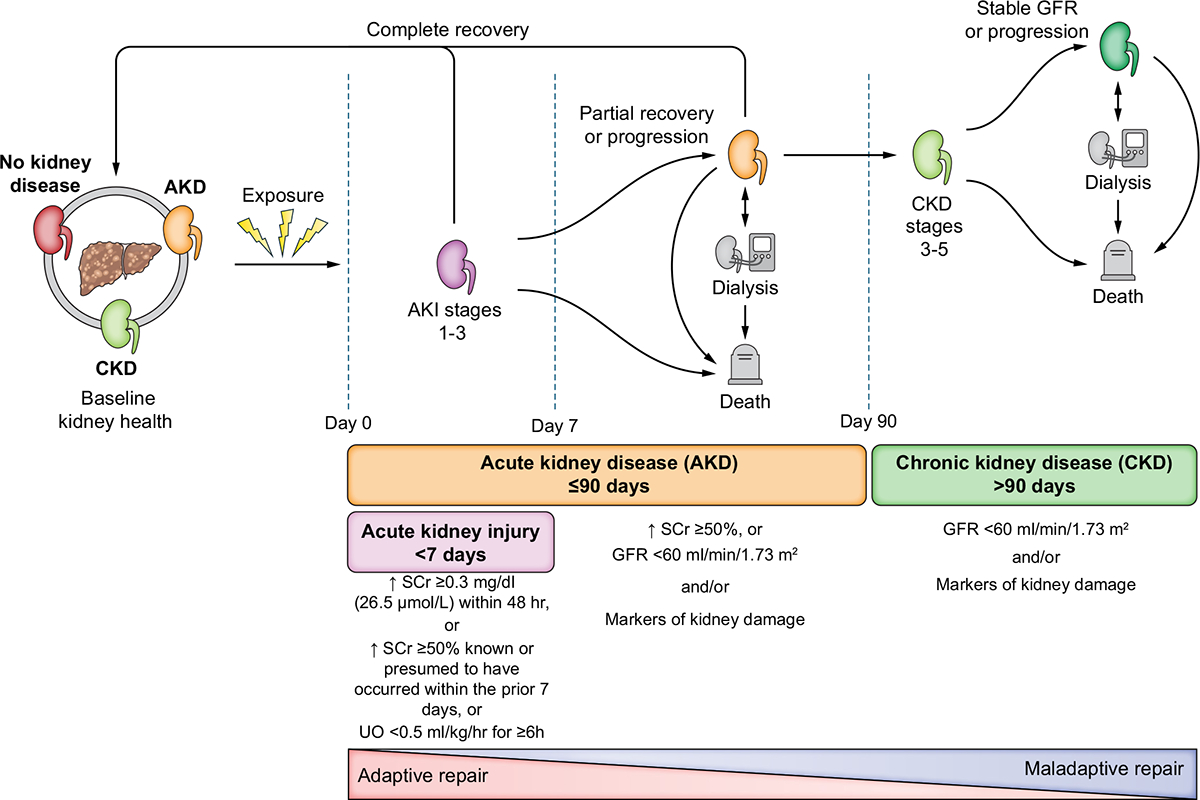
AKI, AKD and CKD form a continuum whereby initial kidney injury can lead to recovery (adaptive repair), persistent renal injury, and/or eventually CKD (maladaptive repair). Multiple episodes of AKI may occur over the course of an illness within one individual. After AKI resolves, patients may still have abnormalities in kidney function and/or structure that fulfil the criteria for AKD. AKI is a subset of AKD, therefore, all patients with AKI are considered to have AKD. The absence of criteria for AKI, AKD or CKD represents no kidney disease (NKD). Liver or liver-kidney transplantation in select patients may occur at any time. Patients who meet HRS criteria are considered to have HRS-AKI, HRS-AKD or HRS-CKD based on the timing and duration of kidney dysfunction. Patients with HRS-AKD meeting AKI criteria are classified as having HRS-AKI. HRS for less than 90 days would be classified as HRS-AKD, while HRS persisting for more than 90 days would be classified as HRS-CKD. In contrast, a patient with pre-existing CKD (e.g., diabetic nephropathy) who develops HRS-AKI would be classified as having HRS-AKI on CKD. AKD, acute kidney disease; AKI, acute kidney injury; CKD, chronic kidney disease; HRS, hepatorenal syndrome. Adapted from Acute Disease Quality Initiative 29, www.ADQI.org, CC BY 2.0 (https://creativecommons.org/licenses/by/2.0/)
To date, a universal definition of post-AKI renal recovery is not available and remains controversial. Distinct phenotypes based on clinical course have been described in critical illness, defining full recovery as a return of SCr to within 0.3 mg/dl (26.5 μmol/L) of baseline, which also aligns with ADQI21 and ICA recommendations.11,21–23 However, it is important to appreciate that use of the creatinine criteria may result in overestimation of recovery by ignoring the loss of muscle mass that occurs during critical illness.24
What reference SCr value should be used to define AKI in patients with cirrhosis?
Consensus statements
We recommend using the lowest stable SCr value obtained in the previous 3 months for the diagnosis and staging of AKI. If no values are available in the previous 3 months, the most recent value up to 12 months prior may be used (strong recommendation, grade D).
In the absence of a known baseline SCr, we suggest using the lower of either SCr on admission or SCr back calculated from an estimated GFR (eGFR) of 75 ml/min/1.73 m2 as the reference value (weak recommendation, grade B).
Rationale:
A SCr value is required to diagnose and stage AKI, to evaluate the extent of renal recovery, and to establish a reference point in studies examining the long-term consequences of AKI.25 What is considered a baseline SCr remains controversial and is inconsistently defined in the general population, especially in patients without any previous values.25,26 The use of known SCr values is superior to imputation,27 and therefore, all efforts should be made to identify a prior SCr level, preferably within the previous 3 months.28 When more than one SCr value is available, utilising median SCr reduces the biases from outliers and normal physiologic variation and is reliable for estimating baseline kidney function in the general population.25,27–30 However, if significant fluctuations exist across multiple SCr values, clinical judgement is crucial to determine the SCr that best reflects the most appropriate baseline value. If no SCr values are available from the prior 3 months, the most recent SCr value up to 12 months prior may be used as a reference SCr, with attention paid to the clinical trajectory to ensure that decrements in kidney function on presentation are truly acute and not due to the presence of progressive CKD.27 Thus, it is imperative that all patients with presumed AKI be evaluated for the presence of pre-existing CKD using all available data (i.e. clinical history, physical exam, laboratory data, and renal ultrasound).
In the rare instance when no previous baseline SCr values are available, the ICA has suggested the first documented SCr value on hospital admission be used as the reference SCr.11 However, this may underestimate the incidence and severity of AKI, and potentially miss the diagnosis of community-acquired AKI, as the SCr may have already increased prior to hospitalization.9 In patients with no prior SCr value available, KDIGO recommends the lower value of the admission SCr or SCr derived from eGFR (assuming a baseline GFR of 75 ml/min/1.73 m2) be used to decide the reference SCr.9,17,30,31 This method was studied in a retrospective study of 3,458 patients with cirrhosis.9 The average SCr on the day of admission in patients who developed stage 3 AKI was 1.6 mg/dl, however, an imputed SCr derived from back-calculation was 1.0 mg/dl, a value closer to the known baseline SCr (1.1 mg/dl). There is currently no superior alternative, thus we propose that KDIGO recommendations be followed until a better methodology is verified. The above recommendations for baseline SCr are to facilitate the clinical diagnosis of AKI and should not replace clinical judgement, as AKI remains a clinical diagnosis.
What are the diagnostic criteria for AKI due to HRS (HRS-AKI)?
Consensus statements
HRS-AKI is a phenotype of AKI that is specific to patients with advanced cirrhosis and ascites; it may also occur in the presence of tubular injury, proteinuria, and/or pre-existing CKD (not graded).
We recommend the following diagnostic criteria for HRS-AKI: a) cirrhosis with ascites; b) increase in SCr ≥0.3 mg/dl (26.5 μmol/L) within 48 h or ≥50% from baseline value, known or presumed, to have occurred within the prior 7 days and/or UO ≤0.5 ml/kg for ≥6 h; c) absence of improvement in SCr and/or UO within 24 h following adequate volume resuscitation (when clinically indicated); and d) absence of strong evidence for an alternative explanation as the primary cause of AKI (not graded).
We recommend against systematic administration of albumin for 48 h as a requisite for the diagnosis of HRS-AKI (strong recommendation, grade D).
We recommend replacing the historical terms HRS type 1 and 2 with the terms HRS-AKI, HRS-AKD and HRS-CKD, depending on the timing and duration of kidney dysfunction (strong recommendation, grade D).
Rationale:
HRS phenotype describes renal dysfunction in patients with cirrhosis and ascites (a sine qua non in the diagnosis of HRS), caused by reduced renal perfusion through haemodynamic alterations in the arterial circulation and overactivity of the endogenous vasoactive systems (Fig. 2).12,32,33 Systemic inflammation contributes to neurohumoral and vasodilatory derangements resulting in functional AKI (HRS-AKI) that persists despite adequate fluid resuscitation and may be reversible with vasoconstrictive therapy. In patients with cirrhosis and ascites who present with AKI, HRS-AKI (Box 1) is an essential part of the differential diagnosis and may not always occur in isolation. Even where other aetiologies of AKI coexist, HRS-AKI may be the primary cause of AKI. Therefore, appropriate, and rapid work-up and diagnosis of the cause of AKI are crucial in ensuring timely recognition and treatment of HRS-AKI. Intravascular volume should be assessed34–36 in all patients who present with AKI. In those with clinical and haemodynamic evidence of intravascular volume depletion, assessment of response to fluid resuscitation34–36 should be completed within 24 h, to ensure early diagnosis and initiation of treatment for HRS-AKI. In patients who are euvolemic or have evidence of intravascular fluid overload, 48 h of albumin infusion for the diagnosis of HRS-AKI is not appropriate and will lead to fluid accumulation. In addition, 48 h of systematic administration of albumin may also delay the initiation of terlipressin in patients who are euvolemic at baseline. Where volume status is equivocal and/or difficult to assess, to exclude any reduction in intravascular volume as the cause of AKI, a fluid challenge (250–500 ml of crystalloid or 1–1.5 g/kg of 20–25% albumin) may be prescribed and, if there is no improvement in SCr and/or UO within 24 h, a diagnosis of HRS-AKI should be considered.
Fig. 2. Contemporary concepts in the pathophysiology of AKI.
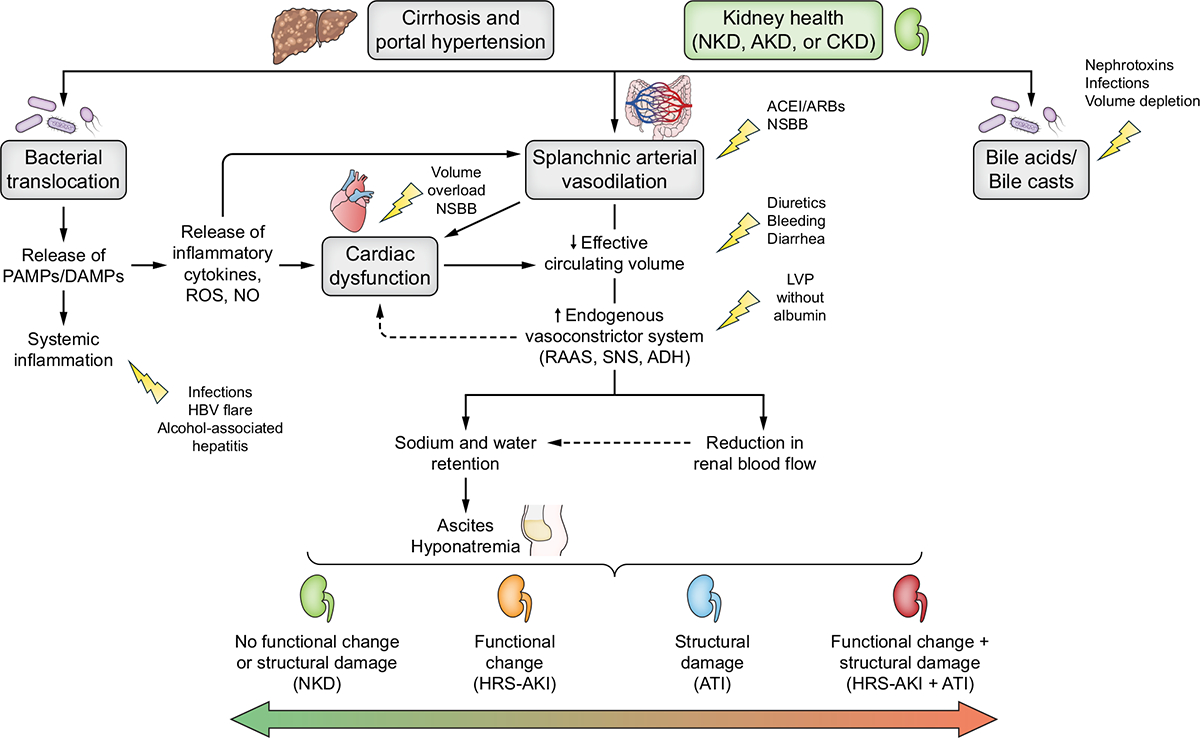
Multiple simultaneous mechanisms can contribute to the development of different phenotypes of AKI in patients with cirrhosis. Background susceptibility to renal injury varies across individuals, according to non-modifiable (e.g., comorbidity burden) and modifiable factors (e.g., sepsis) and includes liver-related (e.g., severity of liver disease, decompensating events), kidney-related (e.g., CKD, eGFR), cardiovascular (e.g., cirrhotic cardiomyopathy), comorbidities (e.g., hypertension, diabetes), and external factors (e.g., nephrotoxic drugs, sepsis, excessive diuretics or laxatives). The clinical condition of the liver, kidney, and heart, in addition to concomitant precipitating events and exposures (yellow arrows) may lead to a variety of clinical AKI phenotypes. The different phenotypes of AKI include presence of functional changes (i.e. increase serum creatinine and/or cystatin C, and decrease urine output), structural damage (i.e. albuminuria, urinary casts, urinary biomarkers) or both. The arrows show progression (red), regression or recovery (green) between the different phenotypes. ACEi, angiotensin converting enzyme inhibitor; ADH, anti-diuretic hormone; AKD, acute kidney disease; AKI, acute kidney injury; ATI, acute tubular injury; ARB, angiotensin receptor blocker; CKD, chronic kidney disease; DAMPs, damage-associated molecular patterns; HBV, hepatitis B virus; LVP, large volume paracentesis; NKD, no kidney disease; NO, nitric oxide; NSBBs, non-selective beta-blockers; PAMPs, pathogen-associated molecular patterns; RAAS, renin-angiotensin-aldosterone system; ROS, reactive oxygen species; SNS, sympathetic nervous system. Adapted from Acute Disease Quality Initiative 29, www.ADQI.org, CC BY 2.0 (https://creativecommons.org/licenses/bi/2.0/)
Box 1. ICA-ADQI new diagnostic criteria for HRS-AKI.
Cirrhosis with ascites
Increase in serum creatinine ≥0.3 mg/dl (26.5 μmol/L) within 48 h or ≥50% from baseline value known or presumed to have occurred within the prior 7 days and/or urinary output ≤0.5 ml/kg for ≥6 h
Absence of improvement in serum creatinine and/or urine output within 24 h following adequate volume resuscitation (when clinically indicated)
Absence of strong evidence for an alternative explanation as the primary cause of AKI
Presence of underlying kidney disease does not exclude a diagnosis of superimposed HRS-AKI and HRS-AKI may coexist with other causes of AKI. Examples of alternative causes of AKI include septic shock requiring vasopressors, drug-induced AKI, obstruction, or acute glomerular injury. Patients who meet HRS criteria are considered to have HRS-AKI, HRS-AKD or HRS-CKD based on timing and duration of kidney dysfunction. ADQI, Acute Disease Quality Initiative; AKD, acute kidney disease; AKI, acute kidney injury; CKD, chronic kidney disease; HRS, hepatorenal syndrome; ICA, International Club of Ascites.
Strong evidence for an alternative explanation such as septic shock requiring vasopressors, acute glomerular injury, obstruction, or nephrotoxin-induced AKI (where an improvement in renal function is expected after withdrawal of drugs) as the primary cause of AKI should be sought. Analysis of urinary sediment and damage markers may be useful to detect acute glomerular and/or severe tubular damage, although the thresholds for biomarkers remain to be determined (Table S2). Given the increasing prevalence of metabolic syndrome and diabetes-related kidney disease, isolated proteinuria might be related to comorbidities in the patient and pre-existing CKD and/or proteinuria does not rule out HRS-AKI.
We acknowledge that clinical uncertainty will persist in some cases and whilst enrolment in clinical trials requires that many uncertain cases are excluded (best interest of advancing science), clinical practice mandates that uncertainty is managed in the best interest of the patient. There will be cases where a provisional diagnosis of HRS-AKI is made on the best available evidence and is excluded later as more information becomes available. Since HRS-AKI can coexist with other causes of AKI, patients with co-existing structural damage may still respond to treatment with vasoconstrictors given the presence of altered haemodynamics. Thus, although a lack of HRS-AKI-targeted vasoconstrictor response should trigger re-evaluation for other causes of AKI, non-response does not exclude a coexisting diagnosis of HRS-AKI.
A rapid reduction in kidney function, previously referred to as HRS type-1, is most often precipitated by infections, in particular spontaneous bacterial peritonitis (SBP), however variceal bleed and large volume paracentesis (LVP) without sufficient albumin administration have also been implicated.37 Conversely, HRS type-2 was characterised by a slower and more chronic decline in renal function in the setting of refractory ascites. We recommend using the terminology HRS-AKI, HRS-AKD or HRS-CKD based on timing and duration of kidney dysfunction, instead of the historical HRS type-1 and type-2. HRS for less than 90 days would be classified as HRS-AKD, while HRS persisting for more than 90 days would be classified as HRS-CKD. Patients with HRS-AKD meeting AKI criteria are classified as having HRS-AKI. In contrast, a patient with pre-existing CKD (e.g., diabetic nephropathy) who develops HRS-AKI would be classified as having HRS-AKI on CKD.
What is the epidemiology and what are the outcomes of kidney dysfunction in patients with cirrhosis?
Consensus statement
AKI and AKD are common in patients with cirrhosis; prognosis depends on the severity of kidney and liver disease (not graded).
Risk of de novo CKD is high following AKI and is associated with worse clinical outcomes (not graded).
Rationale:
Incidence and outcomes of AKI in patients with cirrhosis vary according to the heterogeneity in severity of illness (both kidney and liver health), the aetiology of AKI, variations in AKI definitions, the diversity of clinical settings and, importantly, inconsistent reporting of outcomes. A diagnosis of AKI (even stage 1 AKI) has been shown to be associated with an increased risk of mortality at 30 days, 90 days and 1 year, compared to no AKI, even following recovery from AKI.5,9 Risk factors with the strongest association for developing AKI include CKD, sepsis, SBP, and presence of ascites.9,38,39 In-hospital renal replacement therapy (RRT) is required in between 5–47% of patients, with mortality rates between 60–80%.1,6,40,41 Independence from RRT is unlikely if not achieved by 3 months post-discharge and occurs in only 26% within 1 year post-discharge.6,9,42
The incidence of AKD, defined by KDIGO43,44 or as AKI persisting beyond 7 days3, is approximately 30% in patients with cirrhosis, with risk factors including older age, stage 2/3 AKI, CKD, diabetes, ascites, infection and community-acquired AKI.3,43 The prevalence of CKD in patients with cirrhosis has increased over the years, probably owing to increased recognition coupled with the increased prevalence of metabolic risk factors.3,45 The transition from AKI or AKD to CKD is poorly described in patients with cirrhosis, but emerging data suggest that the risk of developing de novo CKD is high in AKI survivors, occurring in 14–25% of patients, and is associated with worse clinical outcomes including increased risk of hospital readmission, further episodes of AKI, refractory ascites, and bacterial infections during follow-up.33,43,45
Pathophysiology of AKI in patients with cirrhosis
The degree of liver, kidney, and cardiac derangement, together with concomitant precipitating events and exposures may lead to a variety of clinical phenotypes of AKI (Fig. 2).12 Susceptibility to AKI follows development of portal hypertension through increased intrahepatic resistance from liver fibrosis and vasodilation of splanchnic vascular beds secondary to bacterial translocation and systemic inflammation. Vasodilatation leads to a decrease in effective central blood volume that, in turn, leads to activation of sodium/water conservation and vasoconstrictive neurohumoral pathways. Progression of cirrhosis and portal hypertension leads to further vasodilatation and consequently increased activation of these neurohumoral systems, leading to ascites, extreme renal vasoconstriction and HRS-AKI.
Cardiac dysfunction may contribute to AKI development although the mechanisms are controversial. In the early phase of decompensated cirrhosis, the cardiac output (CO) increases but release of cardio-depressive substances leads to subclinical changes in the myocardium46 and impairment of cardiovascular reflexes which, coupled with cardio-depression and diastolic dysfunction, is termed ”cirrhotic cardiomyopathy”.47 Small cohort studies have suggested that a relative reduction of CO results in renal hypoperfusion and might predict the development of HRS-AKI.48,49 Use of non-selective beta-blockers to prevent variceal bleeding has been associated with a greater risk of developing HRS-AKI and to increased mortality in selected patients with refractory ascites and documented inappropriate CO.50–53 However, two recent studies demonstrated significantly higher CO in patients with HRS-AKI compared to those without.54,55 Consequently, the predominant pathophysiological mechanism behind HRS-AKI may not be directly related to reduced CO but rather driven by an inability to increase CO in response to stress, a hallmark of cirrhotic cardiomyopathy.55 Collectively these seemingly disparate findings suggest that perhaps there is a “window” during the development of HRS-AKI in which impaired cardiac response to stress leads to a low CO. Interventions which worsen this trajectory (e.g., non-selective beta-blockers, un-guided volume expansion) may in fact impede renal recovery. However, whether interventions that protect or improve CO result in improved renal function is currently unknown.56
Systemic inflammation is common in patients with decompensated cirrhosis (Fig. 2).57,58 Bacterial/bacterial product translocation and/or overt infection, which is associated with release of PAMPs (pathogen-associated molecular patterns), are fundamental in the development of HRS-AKI, particularly in patients with acute-on-chronic liver failure (ACLF). PAMPs activate innate host immunity, and release of proinflammatory cytokines, vasodilators and reactive oxygen species which may all impair renal function.59,60 Renal tubular Toll-like receptor 4 is also upregulated in patients with AKI, likely through bacterial translocation.61
The toxic effect of bile acids on tubular cells has been documented and the mechanisms leading to toxicity have been demonstrated recently in animal models.62 However, in the absence of diagnostic tests, the thresholds of bile acids and serum bilirubin associated with AKI in patients with severe cholestasis remain largely unknown.63
What are the determinants of susceptibility and trajectory for AKI and its recovery in patients with cirrhosis?
Consensus statement
Modifiable and non-modifiable factors affect susceptibility to AKI and determine the severity as well as the trajectory of recovery (not graded).
Rationale:
Background susceptibility to AKI varies across individuals according to liver- (e.g. severity of liver disease, ACLF, decompensating events) and kidney-related factors (CKD, baseline kidney function), cardiovascular status (e.g. cirrhotic cardiomyopathy), concurrent comorbidities (e.g. hypertension, diabetes), and external elements which may be either modifiable (e.g., presence of infection, liver disease aetiology, nephrotoxins, volume depletion) or non-modifiable (e.g., comorbidity burden). The trajectory of post-AKI recovery is influenced by resolution of the precipitating events, the aetiology and severity of AKI, presence of underlying CKD, renal reserve, the severity of liver disease, degree of adaptative and maladaptive repair, and regenerative mechanisms.64 Adaptive repair is characterised by tubular proliferation, repair and regeneration of endothelial cells, which leads to resolution and return to normal kidney structure.65 Maladaptive repair is characterised by fibrosis, tubular loss and delayed resolution of inflammation with subsequent loss of functional renal reserve and has been shown to play a central role in the transition from AKI to CKD.65 Factors associated with a switch from adaptive to maladaptive repair are thought to include advanced age, AKI phenotype, severity, duration and frequency of injury, and baseline kidney health.66
Patients with decompensated cirrhosis are prone to develop repeated episodes of AKI following sepsis, hypovolemia and circulatory changes associated with LVP and may develop irreversible chronic kidney changes. While no data exist on maladaptive repair in HRS-AKI, there is recognition that HRS-AKI may not be an entirely functional entity due purely to haemodynamic derangements. Patients with intense renal vasoconstriction and systemic inflammation (as seen in HRS-AKI) may have sustained kidney hypoxia, resulting in concomitant acute tubular injury (ATI), as demonstrated on kidney biopsy findings of patients with HRS67,68 and by the overlap in biomarkers in patients with HRS-AKI and ATI.69–73
Prevention and work-up of AKI in patients with cirrhosis
What are the approaches for prevention of AKI in patients with cirrhosis?
Consensus statements
We recommend strategies to mitigate the risk of AKI that include a personalised kidney-liver health (KLH) assessment to inform susceptibility to AKI, nephrotoxin stewardship, and liver-specific recommendations for anticipated and unanticipated exposures (best practice statement).
We recommend 20–25% albumin for the prevention of AKI following LVP and in patients with SBP (strong recommendation, grade B). The dose and duration of albumin administration should be guided by patients’ haemodynamic and volume status (best practice statement).
We recommend against the systematic use of albumin in patients with decompensated cirrhosis for a) the prevention of AKI in patients with non-SBP infections, and b) solely to maintain a serum albumin concentration >3.0 g/dl (strong recommendation, grade A).
Rationale:
A comprehensive KLH assessment offers opportunities for surveillance measures and targeted prevention strategies, both before an anticipated exposure and following an AKI-inducing event (Fig. 3).74 Prevention of AKI in patients with cirrhosis includes general measures that apply to all patients at risk of AKI,17 as well as those unique to patients with cirrhosis (Table 1).75–77 Nephrotoxin stewardship entails assessment of potential exposure, surveillance for drug-related events and ensuring safe medication use.78–80 Approximately 30% of patients with cirrhosis experience a potentially avoidable adverse drug event.81 Drug dosing can be particularly challenging in patients with cirrhosis as relatively lower SCr concentrations may lead to overestimation of GFR.82
Fig 3. Kidney-liver health assessment.
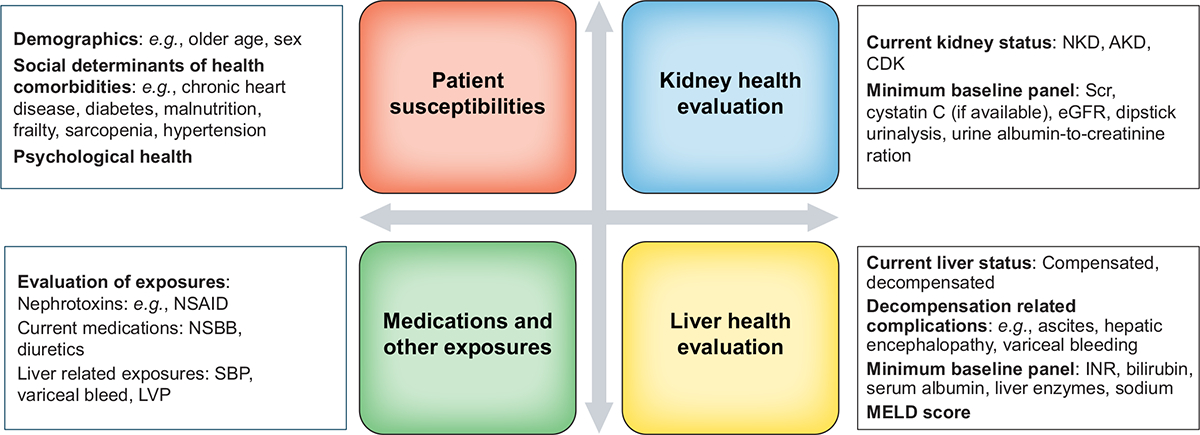
Kidney-liver health assessment is a ‘living’ process that should be repeated if the patient’s condition changes and following planned or unplanned exposure, both during hospitalization and post-AKI care in the outpatient setting. AKD, acute kidney disease; AKI, acute kidney injury; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; INR, international normalised ratio; MELD, model for end-stage liver disease; NKD, no kidney disease; NSAID, non-steroidal anti-inflammatory drug; NSBBs, non-selective beta-blockers. Adapted from Acute Disease Quality Initiative 29, www.ADQI.org, CC BY 2.9 (https://creativecommons.org/licenses/by/2.0/)
Table 1.
Strategies to prevent AKI in patients with cirrhosis.
| Exposure | Preventive interventions |
|---|---|
| Iodinated contrast media exposure |
|
| Volume depletion (e.g., diarrhoea, over diuresis) |
|
| LVP (>5 L of ascites removed in a single session) |
|
| Variceal bleeding | |
| Spontaneous bacterial peritonitis |
|
| Bacterial infections other than spontaneous bacterial peritonitis |
|
| Nephrotoxic medications |
|
| Major abdominal surgery |
|
| alfapump® (abdominal cavity to bladder pump for the treatment of ascites) |
|
AKI, acute kidney injury; CIN, contrast-induced nephrotoxicity; LVP, large volume paracentesis; MAP, mean arterial pressure; NSAID, non-steroidal anti-inflammatory drug; PRBCs, packed red blood cells; SCr, serum creatinine.
The role of intravenous albumin in the prevention of AKI has been studied in several randomised-controlled trials (RCTs) (Table S3). In patients with SBP, treatment with antibiotics in addition to 20% albumin administration (at an arbitrary dose of 1.5 g/kg on day 1 and 1.0 g/kg on day 3) has been associated with lower rates of AKI and mortality compared to antibiotics alone.83 However, this benefit has only been demonstrated in patients with serum bilirubin >4 mg/dl or SCr >1.0 mg/dl.83–85 Administration of albumin should consider the patient’s haemodynamic and volume status. Whether all patients with SBP should receive routine albumin administration, or the optimal dose86 and duration of albumin treatment for the prevention of AKI in patients with SBP, remain to be determined. Systematic administration of albumin in hospitalized patients with non-SBP infection87–90 or the use of daily albumin to target an albumin level >3.0 g/dl91 have been associated with higher risk of pulmonary oedema with no effect on AKI incidence or survival. RCTs on the long-term administration of 20–25% albumin in the outpatient setting in patients with uncomplicated ascites have led to conflicting results.92,93 The lack of survival benefit in MACHT may be because few patients completed the 12-month follow-up (10% in those receiving albumin and 20% in the placebo group), as many underwent liver transplantation (LT).93 Meanwhile, in the ANSWER trial, patients receiving albumin were seen more frequently compared to those in the control group, thus the observed survival benefit could have resulted from earlier detection and treatment of complications.92 Therefore, there is insufficient evidence to recommend long-term outpatient administration of albumin for the prevention of AKI in patients with uncomplicated ascites.
Compared to alternative treatments, administration of 20–25% albumin (6–8 g for every litre over 5 L of ascites removed) during LVP is associated with lower incidence of post-paracentesis circulatory dysfunction, a known trigger for AKI, specifically HRS-AKI.94,95 In patients with refractory ascites, transjugular intrahepatic portosystemic shunt (TIPS) has been shown to be effective at controlling ascites and may thereby prevent the development of HRS-AKI.96 An implantable medical device, alfapump® (Sequana Medical NV, Ghent, Belgium) enables mobilization of ascitic fluid to the bladder for urinary excretion and has been shown to reduce the frequency of LVPs in patients with refractory ascites. However, it is not widely available and has been associated with AKI if the volume of ascites removed early after insertion is high, thus regular administration of albumin may be required to prevent AKI.97–100
What diagnostic tools should be included in the work-up of patients with cirrhosis and AKI?
Consensus statements
We recommend using similar tools for the diagnostic work-up for AKI in patients with cirrhosis as used in those without cirrhosis (best practice statement).
We suggest using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) eGFR equation without the race variable, and preferably with cystatin C (CysC), for assessment of kidney function, though the performance at low GFR and in those with ascites may be suboptimal (weak recommendation, grade B).
In addition to SCr, we suggest complementary use of functional and damage-related markers to aid in timely detection of AKI, characterisation of different AKI phenotypes and to guide treatment strategies (weak recommendation, grade B).
Rationale:
The diagnostic evaluation of patients with cirrhosis and AKI includes clinical history, assessment of intravascular volume status, and detection of potential precipitants. Assessment of intravascular volume remains challenging as most currently available haemodynamic monitoring tools have not been studied in patients with cirrhosis.34,35 Point-of-care ultrasonography has been suggested as a tool to assess volume status at the bedside; however, it is prone to interobserver variability and is challenging to use in patients with significant ascites.101–103 Examination of urinary sediment is difficult in patients with elevated bilirubin levels due to staining of cells and casts. Additionally, significant interobserver variability and discordance with kidney biopsy have been reported.68,104,105 Complications from percutaneous renal biopsy are documented in up to 30% of cases compared to 0.9% in the general population;106,107 however, low complication rates have been reported using the transvenous route, even in patients with coagulation disorders.68,105,108
Assessment of kidney function
Diagnosis of AKI may be missed or delayed in patients with cirrhosis given SCr is influenced by reduced muscle mass, increased volume of distribution in the setting of fluid overload,109 and interference with bilirubin.110 SCr may also be falsely lowered by large volume blood transfusions. CysC allows for earlier diagnosis of AKI in patients with cirrhosis with rising levels often preceding changes in SCr by 48 h, and is a useful prognostic marker for renal outcomes and mortality.111–115 In a large prospective study in patients with cirrhosis, the addition of CysC to the components of the model for end-stage liver disease (MELD) score was superior to MELD for prediction of overall mortality.111 In addition, CysC provides a better estimation of renal function, especially in patients with prolonged critical illness, and may help in drug dosing and management of nephrotoxic drugs.24,116
eGFR equations, such as MDRD or CKD-EPI equations, were developed and validated in patients with CKD and are inaccurate for the assessment of renal function in patients with AKI, as they require SCr to be in a ‘steady state’. eGFR is one of the factors used to determine candidacy for simultaneous liver and kidney transplantation (SLKT), yet current equations tend to overestimate the true GFR by 10 to 20 ml/min/1.73 m2, especially in those with a GFR <40 ml/min/1.73 m2, ascites, or both.117,118 In patients with cirrhosis with a GFR <60 ml/min/1.73 m2, use of the CKD-EPI-CysC eGFR equation demonstrated the least bias (overestimated GFR by 10.3 ml/min/1.73 m2) with acceptable precision and accuracy.117 Thus, efforts to enable increased, routine and timely use of CysC, especially to confirm eGFR in patients who are at risk of or have CKD, should be undertaken as this may also allow clinicians to better identify candidates for SLKT.26,117,119 Recently, several eGFR equations were developed specifically in patients with cirrhosis to allow for more accurate GFR estimation in this patient population.120–122 In 2021, a new CKD-EPI equation, which included the removal of race as a variable, was introduced and widely implemented in the US as an important step in efforts to eliminate disparities in the care of patients with kidney disease; however, this equation has not been widely adopted outside the US.119,123 Preliminary data suggest acceptable performance in patients with cirrhosis, though their role in patients with low GFR and ascites remains to be studied.124 A meta-analysis of studies on timed urine collection for GFR estimation by creatinine clearance in patients with cirrhosis demonstrated overestimation of true GFR, especially in those with low GFR (<60 ml/min/1.73 m2).125 In patients without cirrhosis, the composite of timed urinary urea clearance and creatinine clearance (former tends to underestimate, and the latter overestimate, the true GFR) showed superior performance over CKD-EPI equations and creatinine clearance alone when compared to measured GFR, especially in patients with GFR <60 ml/min/1.73 m2126; however, this has not been studied in patients with cirrhosis.
AKI phenotyping: Role of biomarkers
The combined use of functional (e.g., Scr, CysC) and damage (e.g., albuminuria, urinary neutrophil gelatinase-associated lipocalin [uNGAL]) biomarkers enables more accurate differential diagnosis of the aetiology and mechanisms of AKI in patients with cirrhosis and potentially enables the identification of AKI sub-phenotypes suitable for specific therapeutic interventions (Fig. 4).127 Biomarkers may also help to detect those at risk of AKI in whom interventions may limit renal damage.113,128,129 However, in the absence of a detectable SCr rise (i.e., subclinical AKI), more data are required to define context-specific thresholds for damage-related markers that could act as precise diagnostic criteria for AKI. As further damage and functional biomarkers are discovered and qualified, we believe incorporating them into the proposed conceptual framework (Fig. 4) is an important step towards improving our understanding of the mechanisms and pathophysiology of AKI in patients with cirrhosis (Fig. 2), refining the determination of prognosis and selecting time points and targets for interventions.127,130–132
Fig. 4. Proposed framework for evaluating AKI phenotypes based on combination of functional and damage markers.
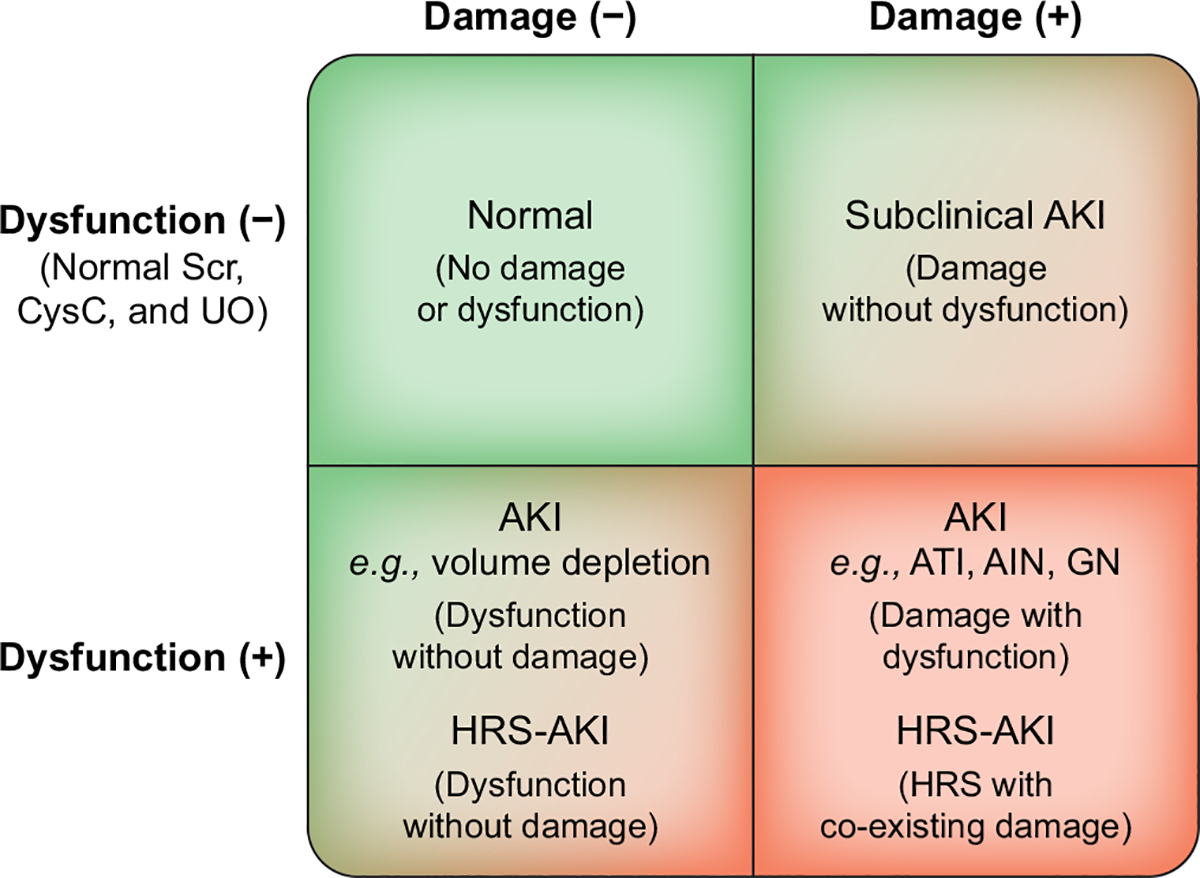
At any given point in time, patients would fall into one of the four quadrants, based on the results of the representative functional and damage marker tests and could be assessed over time to see their transitions across the categories. The ability to detect a state of damage alone (right upper quadrant) represents a “subclinical” state from which loss of function might develop after several days or not at all. Markers of kidney damage may include albuminuria/proteinuria, hematuria, urinary casts, and biomarkers. Bottom left quadrant indicates an acute change in kidney filtration but without detectable kidney damage such as seen in patients with volume depletion. Patients who meet criteria for HRS may be either without evidence of damage (left lower quadrant) or have co-existing damage (right lower quadrant). Sequential assessments could provide information on which of the factors is prevalent for ongoing injury or resolution and offer opportunities for targeted intervention. It is expected that the process is dynamic, and patients may move from one phenotype to another during the course of their illness. Modified, with permission, from Acute Disease Quality Initiative 10, www.ADQI.org. AIN, acute interstitial nephritis; AKI, acute kidney injury; ATI, acute tubular injury; GN, glomerulonephritis; HRS, hepatorenal syndrome. Adapted from Acute Disease Quality Initiative 29, www.ADQI.org, CC BY 2.0 (https://creativecommons.org/licenses/by/2.0/)
Various markers have been assessed in patients with cirrhosis (Table S2).71,133,134 Measurement of the fractional excretion of sodium (FENa) to differentiate ATI from HRS-AKI has been thought to be unhelpful since FENa <1% is common in patients with cirrhosis, even in the absence of AKI.70,135 However, if using a lower threshold of FENa of <0.1–0.2% (which may not be possible as many laboratories do not report urine sodium values <20 mEq/L) in combination with other urinary biomarkers and clinical judgement, the test may have improved specificity in identifying HRS-AKI.70,72 uNGAL is one of the most promising and widely studied injury biomarkers, with levels significantly increasing in a stepwise manner from HRS-AKI to ATI.70–73,133,136,137 A uNGAL value of ~220–250 μg/g creatinine (Bioporto Diagnostics, Hellerup, Denmark) has been demonstrated to distinguish patients with ATI from other phenotypes,69,72 with response rates to terlipressin seen in 70% of patients with uNGAL <220 μg/g of creatinine compared to only 33% in those with uNGAL >220 μg/g of creatinine.136 Of note, these studies have shown overlap between different phenotypes, which may be due to a combination of patient population heterogeneity, presence of underlying CKD, differences in assays used, results based on adjudicated gold standards rather than histopathological diagnosis, or reflecting possible progression along a continuum from functional to structural causes of AKI. Combining markers such as urinary kidney injury molecule-1, uNGAL, and CysC was better than using one marker alone in identifying HRS-AKI, especially after adding clinical parameters.138 Whether the target level of uNGAL that would differentiate between the AKI phenotypes, and/or response to terlipressin would be different with the new diagnostic criteria for HRS-AKI set forth by the authors remains to be determined.
Management of AKI in patients with cirrhosis
What strategies are applicable to the management of AKI in patients with cirrhosis?
Consensus statements
We recommend personalised strategies for the management of AKI based on the individual patient’s kidney-liver health profile and AKI phenotype (best practice statement).
We recommend a combination of physical examination, imaging studies, and static and dynamic measurements to guide fluid management, with frequent reassessment throughout all phases of treatment to avoid volume overload (best practice statement).
We recommend crystalloids, preferentially balanced solutions, as first-line therapy for patients with AKI requiring fluid resuscitation, unless a specific indication exists for the use of other fluids (strong recommendation, grade B).
We recommend discontinuation of all fluids and initiation of diuretic therapy or RRT in patients with AKI who demonstrate signs or symptoms of volume overload (best practice statement).
There is insufficient evidence to support routine measurement of intra-abdominal pressure (IAP) in patients with tense ascites and AKI (not graded).
We recommend initiation of RRT be individualised, with consideration of clinical context and anticipated or observed life-threatening AKI-related complications (best practice statement).
We recommend expedited evaluation for LT in patients with decompensated cirrhosis following an episode of AKI (best practice statement).
There is insufficient evidence to recommend TIPS or extracorporeal liver support for the treatment of AKI (not graded).
Rationale:
Initial management of patients with AKI should follow KDIGO consensus recommendations for AKI, as well as specific guidelines for patients with cirrhosis, which include discontinuation and/or avoidance of nephrotoxins, and optimisation of haemodynamic and volume status.75–77 Fluid administration requires careful titration based on severity of kidney disease, degree of oliguria, and phase of resuscitation.34–36 Assessment of fluid responsiveness should include careful history and physical examination, vital signs, and a combination of available variables including imaging studies, as well as static and dynamic measurements.34–36 No study has demonstrated superiority of a particular method, and therefore the choice of tool depends on the patient’s location (ICU vs. general ward) and clinical discretion. Repeated assessment of volume status and close monitoring of UO should be undertaken so that complications of iatrogenic volume overload can be prevented.
Fluid choice should be individualised and guided by specific patient condition: blood products in cases of gastrointestinal bleeding, crystalloids (preferentially balanced solutions such as lactated ringers or PlasmaLyte) in cases of volume depletion, and 20–25% albumin in those with SBP or HRS-AKI, with close attention to patient haemodynamics and volume status.11,34,35,75,77 Albumin is often used with the notion that it is more likely to maintain oncotic pressure than crystalloids; however, numerous RCTs in critically ill patients have failed to demonstrate any difference in 30-day or 90-day mortality or need for RRT between groups.139,140 In patients with advanced cirrhosis, not only does serum albumin concentration decrease, but its structure and anti-oxidant functions are also altered, reducing its capacity to bind to bacterial products and reactive oxygen species, potentially exacerbating systemic inflammation.141 Experimental studies suggest that infusion of normal “exogenous” albumin has beneficial effects on controlling systemic inflammation and improving circulatory status, which could also contribute to the prevention or reversal of AKI; however, this effect has not been observed in clinical practice.142 Results from two RCTs comparing albumin to crystalloids in patients with cirrhosis and sepsis-induced hypotension have been conflicting, which may be explained by differences in type of albumin solution (5% vs. 20%), type of crystalloid (0.9% saline vs. plasmalyte), and the short duration of the studies (7 vs. 28 days) (Table S3).143,144 Although the use of albumin was associated with a significantly greater improvement in haemodynamics in the short term, the response was not sustained and did not improve renal outcomes or need for RRT compared to crystalloids.143,144
Interactions between ascites, IAP, and AKI are complex. In theory, IAP and intra-abdominal compartment syndrome related to large volume ascites may induce AKI by increasing central venous pressure and reducing right ventricular output, and thus CO.95,145 In critically ill patients with HRS and tense ascites, paracentesis plus albumin infusion resulted in an increase in creatinine clearance, which correlated with the decrease in IAP.145,146 However, LVP which reduces IAP is also known to trigger circulatory changes that may contribute to impaired kidney function without the use of albumin.147 Currently, there is insufficient evidence to support routine measurement of IAP in patients with tense ascites and no evidence to support systematic LVP in patients with increased IAP.148,149
Renal replacement therapy
Recent RCTs have not shown a benefit of accelerated initiation of RRT in critically ill patients; however, patients with cirrhosis were either excluded or largely underrepresented.150–152 The timing of RRT in patients with cirrhosis should be individualised, taking into account the trajectory of both kidney and liver health and be considered before overt complications have developed.17,35,153–155 Early initiation of RRT should be considered in patients with signs or symptoms of intravascular volume overload without adequate response to diuretics (even in the absence of AKI) or in those in whom volume overload cannot be corrected without serious adverse effects.35,155 Patients with cirrhosis and AKI-related metabolic changes are prone to develop encephalopathy and, uremic symptoms can often overlap with hepatic encephalopathy. As such, initiation of RRT should be considered earlier, especially if encephalopathy persists despite treatment. Choice of RRT modality depends on availability, resources, and inherent risks with intervention.154 Among patients listed or undergoing evaluation for LT, initiation of RRT should be viewed as a tool to optimise a patient’s condition and as a bridge to LT. For those who are not candidates for LT, we recommend discussion with the patient and/or caregivers regarding goals of therapy, and the poor long-term prognosis, as transplant-free survival is extremely low, especially in those with very high MELD scores.40,156,157
Transplantation
Episodes of AKI are associated with a high risk of short-term mortality, especially in patients with high MELD scores, and therefore patients may benefit from an expedited inpatient transplant evaluation.156,158 Predicting the severity and duration of kidney dysfunction that results in non-recovery of renal function following LT remains a challenge.159–161 Current US policies for SLKT incorporate duration of AKI (eGFR ≤25 ml/min for ≥6 weeks, with or without dialysis) and CKD at the time of transplant and introduced a safety net approach which guaranteed prioritisation of kidney transplantation in patients with an eGFR ≤20 ml/min within 1 year following LT.162 However, factors such as aetiology of AKI,163 older age, and comorbidities (such as diabetes) known to impact post-transplant renal recovery are not included. Biomarkers predictive of AKI recovery after LT could enhance decision-making algorithms regarding the need for SLK.164,165 Kidney transplant alone in patients on chronic dialysis may be a feasible option among selected patients with compensated cirrhosis without clinically significant portal hypertension, especially in the setting of a treatable aetiology of liver disease.166
Transjugular intrahepatic portosystemic shunt
While TIPS placement has been shown to improve GFR over time in patients with refractory ascites, a complication of portal hypertension that shares its pathophysiology with HRS-AKI, it has been studied only sparingly as a treatment for HRS-AKI.167–171 A RCT examining TIPS for the treatment of HRS-AKI is currently underway.172
Extracorporeal liver support
Extracorporeal liver support such as adsorbent columns, albumin dialysis, and plasma exchange have been investigated for use in ACLF and treatment of HE, but not as AKI-specific therapies.173,174 Treatment of AKI in patients with ACLF may require targeting not only removal of known substances, such as creatinine and urea, but also removal of a wide spectrum of pathogenic factors and mediators of inflammatory response that are implicated in the pathophysiology of ACLF.175–177
What strategies are specific to the management of HRS-AKI?
Consensus statements
We recommend initiating vasoconstrictor therapy (terlipressin as first-line agent), in combination with 20–25% albumin, immediately upon establishing a diagnosis of HRS-AKI (strong recommendation, grade A).
We recommend close monitoring of volume status during treatment for HRS-AKI. The dose of albumin should be adjusted daily based on patients’ volume status, with immediate discontinuation of albumin if there is evidence of volume overload (best practice statement).
We recommend increasing the dose of terlipressin every 24 h if SCr has not decreased by 25% from baseline (strong recommendation, grade D) and increasing the dose of norepinephrine every 4 h if MAP has not increased by ≥10 mmHg from baseline (strong recommendation, grade B).
We recommend discontinuation of vasoconstrictors for HRS-AKI if (a) SCr returns to within 0.3 mg/dl of baseline; (b) a severe adverse reaction develops; (c) kidney function does not improve after 48 h on maximum tolerated doses; (d) RRT is indicated; or (e) maximum of 14 days of therapy (strong recommendation, grade B).
We recommend LT, in select patients, as the definitive treatment for HRS-AKI regardless of response to vasoconstrictor-directed therapy (strong recommendation, grade A).
Rationale:
Terlipressin is the most studied and consistently effective vasoconstrictor for the treatment of HRS-AKI and its use (preferably as a continuous infusion)178 is recommended as a first-line agent (Table 2).11,75–77 Meta-analysis and systematic reviews have shown norepinephrine to have comparative effects to terlipressin for reversal of HRS-AKI, with the exception of one study in patients with ACLF wherein terlipressin was demonstrated to be superior.179–182 If terlipressin is not available or contraindicated, treatment with norepinephrine may be more appropriate than an initial trial with midodrine and octreotide.183–185 However, norepinephrine requires ICU admission and placement of a central venous catheter for continuous infusion.
Table 2.
Vasoconstrictors used for the treatment of HRS-AKI.
| Vasoconstrictor | Route/dose | Comments |
|---|---|---|
| Terlipressin | Continuous infusion*: 2–12 mg/day or i.v. bolus**: 1–2 mg every 6 h |
|
| Norepinephrine | Continuous infusion: 0.5–3 mg/h |
|
| Midodrine + octreotide | Oral: 7.5–15 mg every 8 h Subcutaneous: 100–200 μg every 8 h |
|
Criteria for discontinuation
| ||
Dose titration
| ||
All vasoconstrictors are given in combination with albumin.
ICU, intensive care unit; MAP, mean arterial pressure; RRT, renal replacement therapy; SCr, serum creatinine; UO, urine output.
Continuous infusion of terlipressin may be associated with a lower incidence of side effects compared to i.v. bolus, most likely due to lower cumulative daily dose.178.
1 vial = 0.85 mg terlipressin (North American FDA label) = 1 mg terlipressin acetate.
Current guidelines recommend daily use of 20–25% albumin (20–40 g/day) during the treatment of HRS-AKI; however, the optimal dosing of albumin and length of administration are not well defined. Cautious use of albumin is recommended, with discontinuation if there is evidence of pulmonary oedema.
Clinical trials examining the efficacy of vasoconstrictors for HRS-AKI have used historical definitions of HRS (i.e., type-1 HRS) and demonstrated that vasoconstrictors are more effective at improving renal function when initiated at SCr <2.25 mg/dl and when achieving an increase of MAP ≥15 mmHg.185–188 Re-evaluation for alternative causes of AKI should be considered if there is an increase in MAP ≥15 mmHg from baseline during vasoconstrictor treatment for HRS-AKI without improvement in SCr. What MAP goal (absolute value or an increase in MAP from baseline) should be targeted during treatment with vasoconstrictors requires further investigation. Whether the current definitions set forth here by the authors will improve the rates of HRS-AKI reversal remains to be determined.
Patients receiving vasoconstrictors should be monitored for adverse events (mainly ischaemic) which are usually mitigated by drug discontinuation, lowering the dose, or in the case of terlipressin, changing from bolus dosing to continuous infusion.178 Higher incidence of pulmonary oedema has been reported in patients receiving terlipressin compared to placebo,189–192 which may be related to a combination of several pathways in an already critically ill patient population (Fig. 5).193,194 Cautious use of terlipressin is recommended in patients with evidence of volume overload, and temporary suspension of albumin together with administration of diuretics may prevent complete discontinuation of vasoconstrictor treatment.190
Fig. 5. Differential effects of various HRS-AKI treatments on vascular beds, cardiac function, and renal perfusion, as well as pulmonary effects.
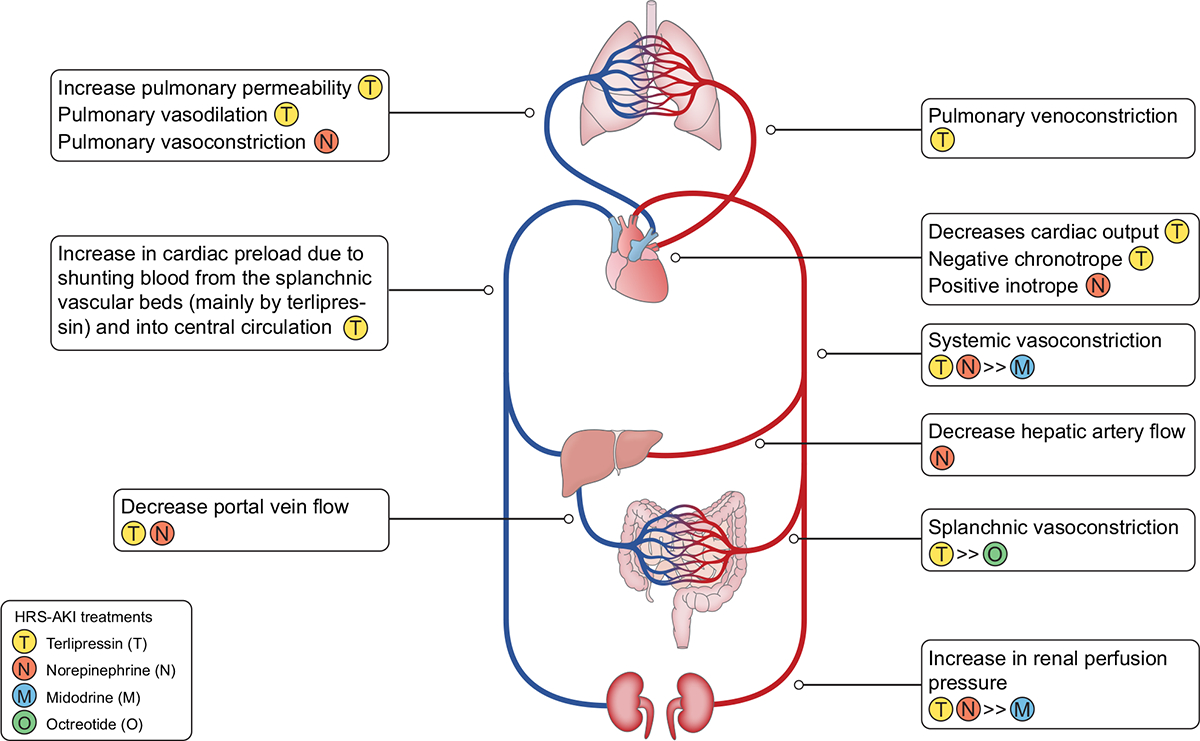
Terlipressin (T) increases renal perfusion pressure but also decreases cardiac output. By increasing cardiac preload (through shunting of splanchnic blood to central blood), increasing cardiac afterload (due to increase in systemic vascular resistance), and effecting pulmonary vasculature231–234 (pulmonary artery dilation, pulmonary vein constriction, as well as possibly an increase in pulmonary capillary permeability), when combined with large doses of albumin, may be associated with an increased incidence of pulmonary oedema. Norepinephrine (N) has a positive inotropic effect and causes systemic vasoconstriction, which then also increases renal perfusion pressure. In contrast to terlipressin, norepinephrine constricts pulmonary arteries without any effect on the pulmonary vein. Midodrine (M) causes weak systemic vasoconstriction and octreotide (O) causes temporary splanchnic vasoconstriction, effects that lead to an only modest increase in renal perfusion. Adapted from Acute Disease Quality Initiative 29, www.ADQI.org/ CC BY 2.0 (https://creativecommons.org/licenses/by/2.0/)
Reversal of HRS-AKI reduces the risk of CKD and need for RRT after LT195; however, there is no improvement in transplant-free survival, thus the use of vasoconstrictors should be seen as a bridge to transplantation or renal recovery, rather than a definitive cure. Pharmacological treatment of HRS-AKI lowers the MELD score by lowering SCr, affecting waiting list priority. This can be detrimental for patients awaiting LT in regions with long waiting times and higher average MELD scores at time of transplant, especially when MELD score is updated at very close intervals.156,192 Some countries partially mitigate this issue by “holding” the SCr at its apex after the initiation of a vasoconstrictor or assigning extra points for those treated for HRS-AKI, regardless of treatment response, to ensure patients who are treated are not disadvanged.196 As new treatments and prognostic scores become available, revisions to transplant allocation policies will be needed to best serve this high-risk patient population.
Post-AKI/AKD outpatient follow-up in patients with cirrhosis
What are the key elements of an appropriate post-AKI/AKD care bundle following hospital discharge in patients with cirrhosis?
Consensus statements
We recommend tailoring the care bundle for post-AKI/AKD outpatient follow-up according to the severity of both kidney and liver disease, with the delivery of care requiring close collaboration between hepatologists and nephrologists (best practice statement).
We recommend personalised palliative care evaluation with goals including reduction in symptom burden, patient/caregiver wellbeing and goals of care discussions (best practice statement).
Rationale:
Patients discharged following an episode of AKI are at an increased risk of recurrent episodes of AKI, progression to CKD, dialysis dependency and mortality.197–202 The post-hospital discharge period is a critical time in which dynamic liver and kidney function changes impact outcomes including mortality, transplant candidacy, and quality of life.203 Almost half of patients with cirrhosis discharged after an episode of AKI are re-admitted within 3 months, with 22% of readmissions due to renal and metabolic issues such as AKI, anasarca, or hyponatremia.204 Patients with cirrhosis who are discharged after an episode of AKI should have, at a minimum, a KLH assessment within 1 month of discharge, the timing of which can be individualised according to the risk-phenotype of the patient at the time of discharge (Fig. 6).205 From the clinical point of view, the stage of cirrhosis should be assessed during the first outpatient visit, with a focus on evaluating the presence and severity of specific complications. In assessing renal recovery, it is important to emphasise that using SCr may result in overestimation of kidney function and measurement of serum CysC levels should be considered when available.206–208 Screening for albuminuria should be routine following an episode of AKI as it has been shown to identify those patients who are at a higher risk of CKD progression.209
Fig. 6. Recommended structure of post-discharge follow-up according to the evaluation of the kidney axis (severity, duration, and recovery of AKI) and the liver axis (compensated vs. decompensated cirrhosis) at the time of hospital discharge.
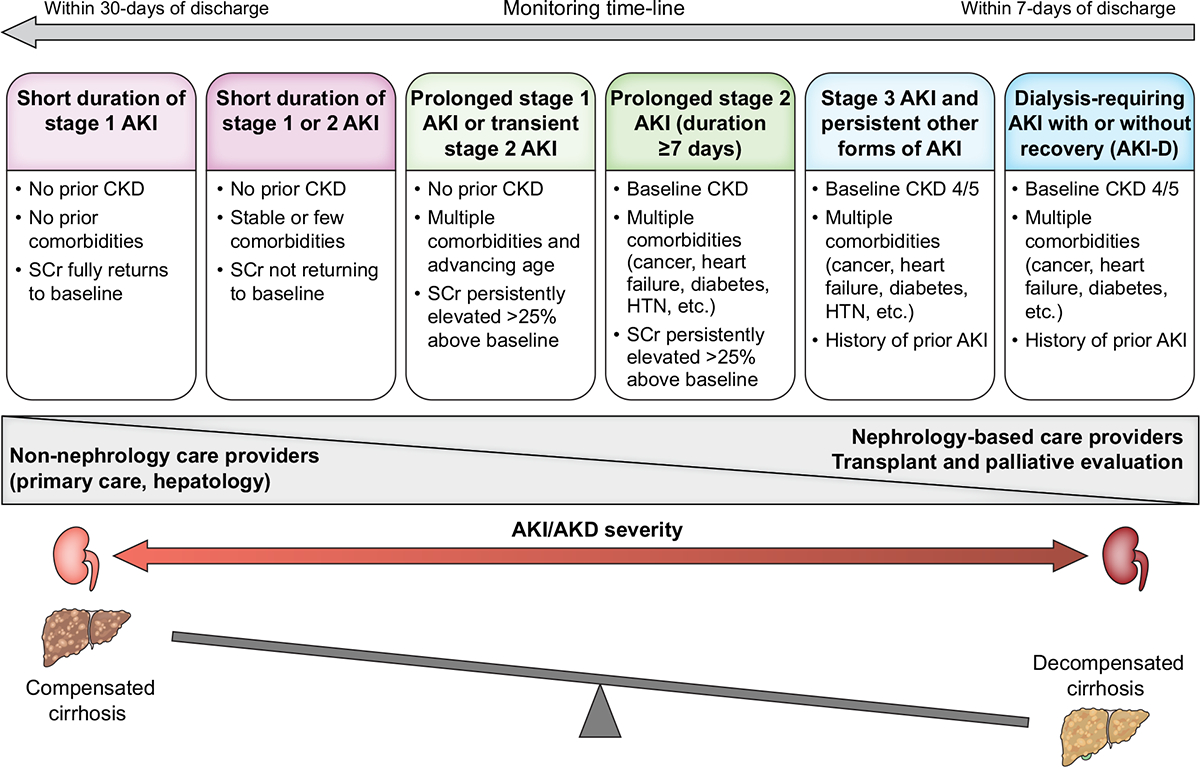
Limited data are available to inform the timing and nature of monitoring for patients with cirrhosis who experience AKI or AKD in hospital. The post-discharge follow-up will depend on the state of kidney and liver health at the time of discharge. We suggest that these patients should have their kidney function checked within 1 month of hospital discharge, at a minimum, to confirm the extent of recovery or progression of kidney disease. Patients with persistent kidney dysfunction at 90 days should be formally assessed for the development or progression of CKD. Patients with less severe AKI or AKD can be monitored in primary care or by the base specialist with the degree of nephrology involvement in follow-up monitoring increasing with the duration and severity of AKI or AKD during hospitalization. Adapted, with permission, from Acute Disease Quality Initiative 24, www.ADQI.org. AKD, acute kidney disease; AKI, acute kidney injury; AKI-D, acute kidney injury treated with dialysis; CKD, chronic kidney disease; HTN, hypertension; NKD, no kidney disease; SCr, serum creatinine. Adapted from Acute Disease Quality Initiative 29, www.ADQI.org, CC BY 2.0 (https://creativecommons.org/licenses/by/2.0/)
KLH management focuses on five key domains that include education, medication management, disease-modifying interventions, and dynamic transplant and palliative care evaluations. Communication of future risk of CKD and recurrent AKI episodes helps the patient adhere to preventative measures and avoid further kidney insults.210 Medication reconciliation is crucial during the first outpatient visit since dose modifications and initiation of medications such as diuretics are frequently necessary.211 The routine post-discharge evaluation of the global domains of frailty in addition to assessment of symptom burden and health-related quality of life, can allow for potentially modifiable gaps to be identified and addressed.212,213 More than one-third of caregivers of patients with cirrhosis have been shown to be affected by one or more major adverse impacts on their own lives and a substantial portion of family members are forced to stop working to provide care.214
Palliative care is not synonymous with end-of-life care and is associated with improvements in advance care planning, patient and caregiver satisfaction, and lower healthcare utilisation without detriment to patient survival, yet it remains underutilised in patients with cirrhosis (<5%), especially in patients never placed on the waiting list.215–218 Recognising AKI as a marker of worse prognosis in cirrhosis, persistent kidney dysfunction following discharge can be a trigger for reassessing goals of care by a multidisciplinary team, especially for those patients who are not candidates for LT and with a life expectancy of less than a few months.215 In such instances, a palliative approach to care is important to ensure that patient’s goals are elicited and translated into care that best meets their needs before an acute medical crisis occurs.
Paediatric perspective
AKI is common in children with cirrhosis and carries significant morbidity and mortality.219,220 Cholestatic diseases such as biliary atresia are the leading aetiology of paediatric cirrhosis, precluding direct extrapolation of evidence from adult patients. The reported prevalence of paediatric HRS-AKI (<10%) is likely a gross underrepresentation as none of the proposed consensus definitions of HRS-AKI have been validated in children. CysC is a superior test for AKI detection and should be explored in combination with damage-related biomarkers to improve diagnostic accuracy in paediatric cirrhosis.221 Despite the different case-mix, the pathophysiology of HRS-AKI seems to be similar in children, thus paediatric HRS-AKI should be responsive to splanchnic vasoconstrictors like terlipressin.222 Paediatric cirrhotic cardiomyopathy, in part related to bile acids, contributes to the pathogenesis of AKI in cirrhosis and is associated with need for continuous RRT and post-transplant outcomes.223,224
Conclusion and perspectives
AKI in patients with cirrhosis, especially HRS-AKI, is strongly associated with both short- and long-term adverse events. Over the last decade, there have been significant advances in our understanding of the pathophysiology and epidemiology of AKI in patients with cirrhosis. Our consensus recommendations are based not only on existing data but also on expert opinion, as much of the strength of evidence is poor and much evidence comes from studies in patients without cirrhosis. We acknowledge that some of the current literature contains limitations as many of the studies were performed prior to changes in the definition of HRS-AKI and further research is needed. However, utilising a multidisciplinary approach, we endeavoured to apply, as precisely as possible, lessons learned from AKI in the general population to the specific population of patients with cirrhosis. With the new diagnostic criteria for HRS-AKI, the integration (into routine practice) of appropriately selected biomarkers that can identify different sub-phenotypes of AKI should be increasingly explored, as this holds the key to further improvements in the care of patients with HRS-AKI. Consequently, it is imperative to develop research questions to address these knowledge gaps (Table S4). Overall, we believe that an integrated approach involving various specialties is imperative in the management of AKI in patients with cirrhosis, both in the inpatient and outpatient settings.
Supplementary Material
Key points:
According to the Acute Disease Quality Initiative (ADQI) and International Club of Ascites (ICA) joint multidisciplinary consensus meeting, acute kidney injury (AKI) in patients with cirrhosis is defined using KDIGO criteria: increase in serum creatinine ≥0.3 mg/dl (26.5 μmol/L) within 48 h or ≥50% from baseline value known or presumed to have occurred within the prior 7 days and/or urine output ≤0.5 ml/kg for ≥6 h.
The lowest, stable serum creatinine value obtained in the previous 3 months may be used for the diagnosis and staging of AKI. If no values are available in the previous 3 months, the most recent value up to 12 months prior may be used.
Hepatorenal syndrome-AKI (HRS-AKI) is a phenotype of AKI that is specific to patients with advanced cirrhosis and ascites, which may also occur in the presence of tubular injury, proteinuria, and/or pre-existing chronic kidney disease.
The following diagnostic criteria for HRS-AKI should be: a) cirrhosis with ascites; b) increase in serum creatinine ≥0.3 mg/dl (26.5 μmol/L) within 48 h or ≥50% from baseline value, known or presumed, to have occurred within the prior 7 days and/or urine output ≤0.5 ml/kg for ≥6 h; c) absence of improvement in serum creatinine and/or urine output within 24 h following adequate volume resuscitation (when clinically indicated); and d) absence of strong evidence for an alternative explanation as the primary cause of AKI.
The ADQI and ICA joint multidisciplinary consensus meeting recommends against systematic administration of albumin for 48 h as a requisite for the diagnosis of HRS-AKI.
Vasoconstrictor therapy (terlipressin as first-line agent), in combination with 20–25% albumin, should be initiated immediately upon establishing a diagnosis of HRS-AKI.
Financial support
This conference was kindly supported by unrestricted educational grants from Mallinckrodt Pharmaceuticals, Grifols, Sphingotec GmbH, Baxter, Fresenius, BioPorto, Ocelot, Biotest and Sequana Medical.
Conflict of interest
MKN has received consulting/advisory board fees from Mallinckrodt Pharmaceuticals, Ocelot and Baxter; JAK has received grant support and consulting fees from Astute Medical/BioMerieux, Novaratis; Fulltime employee, Spectral Medical; LF has received research support from Baxter and Ortho Clinical Diagnostics; MO has received research funding from Baxter, Biomerieux and La Jolla Pharma; ASA has received consulting/advisory board fees from Mallinckrodt Pharmaceuticals and Ocelot; JAN has received consulting fees from Baxter, Outset Medical, and Vifor Pharma; JCO has received consulting fees from Mallinckrodt Pharmaceuticals; SP has received consulting fees from Plasma Protein Therapeutics association, advisory board fees from Mallinckrodt Pharmaceuticals and speaking fees from Grifols and Medscape; LBV has received research support from W.L. Gore & Associates; ECV has received research support to institution from Salix; AAA’s institution has received research funding from Baxter, Bioporto, and Medtronic; PA has received advisory board fees from Biovie, Biomarin, and speaking fees from Grifols, CSL Bhering and Kedrion; JMB has received consulting/advisory board fees from Mallinckrodt; PG has received research funding from Gilead & Grifols, has consulted or attended advisory boards for Gilead, RallyBio, SeaBeLife, Merck, Sharp and Dohme (MSD), Ocelot Bio and Behring, and received speaking fees from Pfizer; MK has received speaker fees from Baxter, Fresenius Medical Care and B.Braun; CR has been consultant or member of advisory board and/or Speaker Bureau for Asahi medical, Aferetica, Baxter, B.Braun, Biomerieux, Cytosorbents, Fresenoius mecial care, Medtronic, ESTOR, Medica, Jafron, OCD, GE, Toray, Nipro, Sphingotec; JCV has received honoraria from Mallinckrodt Pharmaceuticals (consulting, speaking bureau), Travere Therapeutics (advisory) and Calliditas (advisory); RLM has received consulting/advisory board fees from Baxter, Fresenius, AM Pharma, Alexion, Unicycive, Guard, Novartis, Renibus, Mallinckrodt and Sphingotec; FD has received consulting/advisory board fees from Chiesi and Biotest; CF, SA, SWB, AD, YSG, PSK, SKG, NL, EM, RM, SM, RHP, PT have nothing to declare.
Please refer to the accompanying ICMJE disclosure forms for further details.
Abbreviations
- ACLF
acute-on-chronic liver failure
- ADQI
Acute Disease Quality Initiative
- AKI
acute kidney injury
- AKD
acute kidney disease
- ATI
acute tubular injury
- ATN
acute tubular necrosis
- CKD
chronic kidney disease
- CKD-EPI
chronic kidney disease epidemiology collaboration
- CO
cardiac output
- CysC
cystatin C
- FENa
fractional excretion of sodium
- GFR
glomerular filtration rate
- HRS
hepatorenal syndrome
- ICA
International Club of Ascites
- ICU
intensive care unit
- KDIGO
Kidney Disease: Improving Global Outcomes
- KLH
kidney-liver health
- LT
liver transplantation
- LVP
large volume paracentesis
- MAP
mean arterial pressure
- MELD
model for end-stage liver disease
- PAMP
pathogen-associated molecular pattern
- RCT
randomised-controlled trial
- RRT
renal replacement therapy
- SBP
spontaneous bacterial peritonitis
- SCr
serum creatinine
- SLK
simultaneous liver and kidney
- TIPS
transjugular intrahepatic portosystemic shunts
- uNGAL
urinary neutrophil gelatinase-associated lipocalin
- UO
urine output
Footnotes
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhep.2024.03.031.
References
Author names in bold designate shared co-first authorship
- [1].Patidar KR, Belcher JM, Regner KR, et al. Incidence and outcomes of acute kidney injury including hepatorenal syndrome in hospitalized patients with cirrhosis in the US. J Hepatol 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Tariq R, Hadi Y, Chahal K, et al. Incidence, mortality and predictors of acute kidney injury in patients with cirrhosis: a systematic review and meta-analysis. J Clin Transl Hepatol 2020;8(2):135–142. 10.14218/JCTH.2019.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Patidar KR, Naved MA, Grama A, et al. Acute kidney disease is common and associated with poor outcomes in patients with cirrhosis and acute kidney injury. J Hepatol 2022;77(1):108–115. 10.1016/j.jhep.2022.02.009. [DOI] [PubMed] [Google Scholar]
- [4].Patidar KR, Shamseddeen H, Xu C, et al. Hospital-acquired versus community-acquired acute kidney injury in patients with cirrhosis: a prospective study. Am J Gastroenterol 2020;115(9):1505–1512. 10.14309/ajg.0000000000000670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Tandon P, James MT, Abraldes JG, et al. Relevance of new definitions to incidence and prognosis of acute kidney injury in hospitalized patients with cirrhosis: a retrospective population-based cohort study. PLoS One 2016;11(8):e0160394. 10.1371/journal.pone.0160394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wang PL, Silver SA, Djerboua M, et al. Recovery from dialysis-treated acute kidney injury in patients with cirrhosis: a population-based study. Am J Kidney Dis 2022;80(1):55–64 e1. 10.1053/j.ajkd.2021.09.025. [DOI] [PubMed] [Google Scholar]
- [7].Worden A, Pike F, Allegretti AS, et al. The prognostic impact of acute kidney injury recovery patterns in critically ill patients with cirrhosis. Liver Transpl 2023;29(3):246–258. 10.1097/LVT.0000000000000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cullaro G, Rubin JB, Fortune BE, et al. Association between kidney dysfunction types and mortality among hospitalized patients with cirrhosis. Dig Dis Sci 2022;67(7):3426–3435. 10.1007/s10620-021-07159-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Amathieu R, Al-Khafaji A, Sileanu FE, et al. Significance of oliguria in critically ill patients with chronic liver disease. Hepatology 2017;66(5):1592–1600. 10.1002/hep.29303. [DOI] [PubMed] [Google Scholar]
- [10].Nadim MK, Kellum JA, Davenport A, et al. Hepatorenal syndrome: the 8th international consensus conference of the acute dialysis quality initiative (ADQI) group. Crit Care 2012;16(1):R23. 10.1186/cc11188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Angeli P, Gines P, Wong F, et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. J Hepatol 2015;62(4):968–974. 10.1016/j.jhep.2014.12.029. [DOI] [PubMed] [Google Scholar]
- [12].Nadim MK, Garcia-Tsao G. Acute kidney injury in patients with cirrhosis. N Engl J Med 2023;388(8):733–745. 10.1056/NEJMra2215289. [DOI] [PubMed] [Google Scholar]
- [13].Kellum JA, Bellomo R, Ronco C. Acute dialysis quality initiative (ADQI): methodology. Int J Artif Organs 2008;31(2):90–93. 10.1177/039139880803100202. [DOI] [PubMed] [Google Scholar]
- [14].Guyatt GH, Oxman AD, Kunz R, et al. Going from evidence to recommendations. BMJ 2008;336(7652):1049–1051. 10.1136/bmj.39493.646875.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Dewidar O, Lotfi T, Langendam MW, et al. Good or best practice statements: proposal for the operationalisation and implementation of GRADE guidance. BMJ Evid Based Med 2023;28(3):189–196. 10.1136/bmjebm-2022-111962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924–926. 10.1136/bmj.39489.470347[AD]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney inter., Suppl 2012;2:1–138. [Google Scholar]
- [18].Jin K, Murugan R, Sileanu FE, et al. Intensive monitoring of urine output is associated with increased detection of acute kidney injury and improved outcomes. Chest 2017;152(5):972–979. 10.1016/j.chest.2017.05.011. [DOI] [PubMed] [Google Scholar]
- [19].Ostermann M, Zarbock A, Goldstein S, et al. Recommendations on acute kidney injury biomarkers from the acute disease quality initiative consensus conference: a consensus statement. JAMA Netw Open 2020;3(10): e2019209. 10.1001/jamanetworkopen.2020.19209. [DOI] [PubMed] [Google Scholar]
- [20].Lameire NH, Levin A, Kellum JA, et al. Harmonizing acute and chronic kidney disease definition and classification: report of a kidney disease: improving global outcomes (KDIGO) consensus conference. Kidney Int 2021;100(3):516–526. [DOI] [PubMed] [Google Scholar]
- [21].Chawla LS, Bellomo R, Bihorac A, et al. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol 2017;13(4):241–257. 10.1038/nrneph.2017.2. [DOI] [PubMed] [Google Scholar]
- [22].Forni LG, Darmon M, Ostermann M, et al. Renal recovery after acute kidney injury. Intensive Care Med 2017;43(6):855–866. 10.1007/s00134-017-4809-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kellum JA, Sileanu FE, Bihorac A, et al. Recovery after acute kidney injury. Am J Respir Crit Care Med 2017;195(6):784–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Haines RW, Fowler AJ, Liang K, et al. Comparison of cystatin C and creatinine in the assessment of measured kidney function during critical illness. Clin J Am Soc Nephrol 2023;18(8):997–1005. 10.2215/CJN.0000000000000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Siew ED, Matheny ME. Choice of reference serum creatinine in defining acute kidney injury. Nephron 2015;131(2):107–112. 10.1159/000439144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ostermann M, Bellomo R, Burdmann EA, et al. Controversies in acute kidney injury: conclusions from a kidney disease: improving global outcomes (KDIGO) conference. Kidney Int 2020;98(2):294–309. 10.1016/j.kint.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Siew ED, Ikizler TA, Matheny ME, et al. Estimating baseline kidney function in hospitalized patients with impaired kidney function. Clin J Am Soc Nephrol 2012;7(5):712–719. 10.2215/CJN.10821011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sawhney S, Bell S, Black C, et al. Harmonization of epidemiology of acute kidney injury and acute kidney disease produces comparable findings across four geographic populations. Kidney Int 2022;101(6):1271–1281. 10.1016/j.kint.2022.02.033. [DOI] [PubMed] [Google Scholar]
- [29].Siew ED, Matheny ME, Ikizler TA, et al. Commonly used surrogates for baseline renal function affect the classification and prognosis of acute kidney injury. Kidney Int 2010;77(6):536–542. 10.1038/ki.2009.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Al-Jaghbeer M, Dealmeida D, Bilderback A, et al. Clinical decision support for in-hospital AKI. J Am Soc Nephrol 2018;29(2):654–660. 10.1681/ASN.2017070765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bataineh A, Dealmeida D, Bilderback A, et al. Sustained effects of a clinical decision support system for acute kidney injury. Nephrol Dial Transpl 2020;35(10):1819–1821. 10.1093/ndt/gfaa099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Arroyo V, Gines P, Gerbes AL, et al. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. International Ascites Club. Hepatology 1996;23(1):164–176. 10.1002/hep.510230122. [DOI] [PubMed] [Google Scholar]
- [33].Angeli P, Garcia-Tsao G, Nadim MK, et al. News in pathophysiology, definition and classification of hepatorenal syndrome: a step beyond the International Club of Ascites (ICA) consensus document. J Hepatol 2019;71(4):811–822. 10.1016/j.jhep.2019.07.002. [DOI] [PubMed] [Google Scholar]
- [34].Durand F, Kellum JA, Nadim MK. Fluid resuscitation in patients with cirrhosis and sepsis: a multidisciplinary perspective. J Hepatol 2023. 10.1016/j.jhep.2023.02.024. [DOI] [PubMed] [Google Scholar]
- [35].Nadim MK, Durand F, Kellum JA, et al. Management of the critically ill patient with cirrhosis: a multidisciplinary perspective. J Hepatol 2016;64(3):717–735. 10.1016/j.jhep.2015.10.019 (In eng). [DOI] [PubMed] [Google Scholar]
- [36].Hoste EA, Maitland K, Brudney CS, et al. Four phases of intravenous fluid therapy: a conceptual model. Br J Anaesth 2014;113(5):740–747. 10.1093/bja/aeu300 (In eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Salerno F, Gerbes A, Gines P, et al. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut 2007;56(9):1310–1318. 10.1136/gut.2006.107789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Desai AP, Knapp SM, Orman ES, et al. Changing epidemiology and outcomes of acute kidney injury in hospitalized patients with cirrhosis - a US population-based study. J Hepatol 2020;73(5):1092–1099. 10.1016/j.jhep.2020.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Leão GS, de Mattos AA, Picon RV, et al. The prognostic impact of different stages of acute kidney injury in patients with decompensated cirrhosis: a prospective cohort study. Eur J Gastroenterol Hepatol 2021;33(1S):e407–e412. 10.1097/meg.0000000000002120. [DOI] [PubMed] [Google Scholar]
- [40].Allegretti AS, Parada XV, Eneanya ND, et al. Prognosis of patients with cirrhosis and AKI who initiate RRT. Clin J Am Soc Nephrol 2018;13(1):16–25. 10.2215/CJN.03610417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Saraiva IE, Ortiz-Soriano VM, Mei X, et al. Continuous renal replacement therapy in critically ill patients with acute on chronic liver failure and acute kidney injury: a retrospective cohort study. Clin Nephrol 2020;93(4):187–194. 10.5414/CN109983. [DOI] [PubMed] [Google Scholar]
- [42].Velez JCQ, Wong F, Reddy KR, et al. The effect of terlipressin on renal replacement therapy in patients with hepatorenal syndrome. Kidney360 2023. 10.34067/kid.0000000000000132 (In eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Tonon M, Rosi S, Gambino CG, et al. Natural history of acute kidney disease in patients with cirrhosis. J Hepatol 2021;74(3):578–583. 10.1016/j.jhep.2020.08.037. [DOI] [PubMed] [Google Scholar]
- [44].Wong F, Garcia-Tsao G, Reddy KR, et al. Prognosis of hospitalized patients with cirrhosis and acute kidney disease. Liver Int 2022;42(4):896–904. 10.1111/liv.15154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Bassegoda O, Huelin P, Ariza X, et al. Development of chronic kidney disease after acute kidney injury in patients with cirrhosis is common and impairs clinical outcomes. J Hepatol 2020;72(6):1132–1139. 10.1016/j.jhep.2019.12.020. [DOI] [PubMed] [Google Scholar]
- [46].Zardi EM, Zardi DM, Chin D, et al. Cirrhotic cardiomyopathy in the pre- and post-liver transplantation phase. J Cardiol 2016;67(2):125–130. 10.1016/j.jjcc.2015.04.016 (In eng). [DOI] [PubMed] [Google Scholar]
- [47].Izzy M, VanWagner LB, Lin G, et al. Redefining cirrhotic cardiomyopathy for the modern era. Hepatology 2020;71(1):334–345. 10.1002/hep.30875 (In eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ruiz-del-Arbol L, Monescillo A, Arocena C, et al. Circulatory function and hepatorenal syndrome in cirrhosis. Hepatology 2005;42(2):439–447. 10.1002/hep.20766 (In eng). [DOI] [PubMed] [Google Scholar]
- [49].Krag A, Bendtsen F, Henriksen JH, et al. Low cardiac output predicts development of hepatorenal syndrome and survival in patients with cirrhosis and ascites. Gut 2010;59(1):105–110. 10.1136/gut.2009.180570 (In eng). [DOI] [PubMed] [Google Scholar]
- [50].Krag A, Wiest R, Albillos A, et al. The window hypothesis: haemodynamic and non-haemodynamic effects of b-blockers improve survival of patients with cirrhosis during a window in the disease. Gut 2012;61(7):967–969. 10.1136/gutjnl-2011-301348 (In eng). [DOI] [PubMed] [Google Scholar]
- [51].Giannelli V, Roux O, Laouénan C, et al. Impact of cardiac function, refractory ascites and beta blockers on the outcome of patients with cirrhosis listed for liver transplantation. J Hepatol 2020;72(3):463–471. 10.1016/j.jhep.2019.10.002 (In eng). [DOI] [PubMed] [Google Scholar]
- [52].Sersté T, Melot C, Francoz C, et al. Deleterious effects of beta-blockers on survival in patients with cirrhosis and refractory ascites. Hepatology 2010;52(3):1017–1022. 10.1002/hep.23775 (In eng). [DOI] [PubMed] [Google Scholar]
- [53].Mandorfer M, Bota S, Schwabl P, et al. Nonselective β blockers increase risk for hepatorenal syndrome and death in patients with cirrhosis and spontaneous bacterial peritonitis. Gastroenterology 2014;146(7):1680–16890.e1. 10.1053/j.gastro.2014.03.005 (In eng). [DOI] [PubMed] [Google Scholar]
- [54].Danielsen KV, Wiese S, Busk T, et al. Cardiovascular mapping in cirrhosis from the compensated stage to hepatorenal syndrome: a magnetic resonance study. Am J Gastroenterol 2022;117(8):1269–1278. 10.14309/ajg.0000000000001847 (In eng). [DOI] [PubMed] [Google Scholar]
- [55].Koshy AN, Farouque O, Cailes B, et al. Impaired cardiac reserve on dobutamine stress echocardiography predicts the development of hepatorenal syndrome. Official J Am Coll Gastroenterol ACG 2020;115(3):388–397. 10.14309/ajg.0000000000000462. [DOI] [PubMed] [Google Scholar]
- [56].Israelsen M, Dahl EK, Madsen BS, et al. Dobutamine reverses the cardio-suppressive effects of terlipressin without improving renal function in cirrhosis and ascites: a randomized controlled trial. Am J Physiol Gastrointest Liver Physiol 2020;318(2):G313–g321. 10.1152/ajpgi.00328.2019 (In eng). [DOI] [PubMed] [Google Scholar]
- [57].Arroyo V, Angeli P, Moreau R, et al. The systemic inflammation hypothesis: towards a new paradigm of acute decompensation and multiorgan failure in cirrhosis. J Hepatol 2021;74(3):670–685. 10.1016/j.jhep.2020.11.048 (In eng). [DOI] [PubMed] [Google Scholar]
- [58].Trebicka J, Amoros A, Pitarch C, et al. Addressing profiles of systemic inflammation across the different clinical phenotypes of acutely decompensated cirrhosis. Front Immunol 2019;10(Original Research). 10.3389/fimmu.2019.00476 (In English). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Zhang IW, Curto A, López-Vicario C, et al. Mitochondrial dysfunction governs immunometabolism in leukocytes of patients with acute-on-chronic liver failure. J Hepatol 2022;76(1):93–106. 10.1016/j.jhep.2021.08.009 (In eng). [DOI] [PubMed] [Google Scholar]
- [60].Moreau R, Clària J, Aguilar F, et al. Blood metabolomics uncovers inflammation-associated mitochondrial dysfunction as a potential mechanism underlying ACLF. J Hepatol 2020;72(4):688–701. 10.1016/j.jhep.2019.11.009 (In eng). [DOI] [PubMed] [Google Scholar]
- [61].Shah N, Mohamed FE, Jover-Cobos M, et al. Increased renal expression and urinary excretion of TLR4 in acute kidney injury associated with cirrhosis. Liver Int 2013;33(3):398–409. 10.1111/liv.12047 (In eng). [DOI] [PubMed] [Google Scholar]
- [62].Ghallab A, Gonzalez D, Strangberg E, et al. Inhibition of the renal apical sodium dependent bile acid transporter prevents cholemic nephropathy in mice with obstructive cholestasis. J Hepatol 2023. 10.1016/j.jhep.2023.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Fickert P Detour of bile acid routes as therapeutic roadmap for cholemic nephropathy. J Hepatol 2023. 10.1016/j.jhep.2023.11.008. [DOI] [PubMed] [Google Scholar]
- [64].Basile DP, Bonventre JV, Mehta R, et al. Progression after AKI: understanding maladaptive repair processes to predict and identify therapeutic treatments. J Am Soc Nephrol 2016;27(3):687–697. 10.1681/ASN.2015030309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Ferenbach DA, Bonventre JV. Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat Rev Nephrol 2015;11(5):264–276. 10.1038/nrneph.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Chawla LS, Eggers PW, Star RA, et al. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med 2014;371(1):58–66. 10.1056/NEJMra1214243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Mandal AK, Lansing M, Fahmy A. Acute tubular necrosis in hepatorenal syndrome: an electron microscopy study. Am J Kidney Dis 1982;2(3):363–374. 10.1016/s0272-6386(82)80096-6. [DOI] [PubMed] [Google Scholar]
- [68].Trawale JM, Paradis V, Rautou PE, et al. The spectrum of renal lesions in patients with cirrhosis: a clinicopathological study. Liver Int 2010;30(5):725–732. 10.1111/j.1478-3231.2009.02182.x. [DOI] [PubMed] [Google Scholar]
- [69].Allegretti AS, Parada XV, Endres P, et al. Urinary NGAL as a diagnostic and prognostic marker for acute kidney injury in cirrhosis: a prospective study. Clin Transl Gastroenterol 2021;12(5):e00359. 10.14309/ctg.0000000000000359 (In eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Belcher JM, Sanyal AJ, Peixoto AJ, et al. Kidney biomarkers and differential diagnosis of patients with cirrhosis and acute kidney injury. Hepatology 2014;60(2):622–632. 10.1002/hep.26980 (In eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Francoz C, Nadim MK, Durand F. Kidney biomarkers in cirrhosis. J Hepatol 2016;65(4):809–824. 10.1016/j.jhep.2016.05.025 (In eng). [DOI] [PubMed] [Google Scholar]
- [72].Huelin P, Sola E, Elia C, et al. Neutrophil gelatinase-associated lipocalin for assessment of acute kidney injury in cirrhosis: a prospective study. Hepatology 2019;70(1):319–333. 10.1002/hep.30592. [DOI] [PubMed] [Google Scholar]
- [73].Verna EC, Brown RS, Farrand E, et al. Urinary neutrophil gelatinase-associated lipocalin predicts mortality and identifies acute kidney injury in cirrhosis. Dig Dis Sci 2012;57(9):2362–2370. 10.1007/s10620-012-2180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Kashani K, Rosner MH, Haase M, et al. Quality improvement goals for acute kidney injury. Clin J Am Soc Nephrol 2019;14(6):941–953. 10.2215/CJN.01250119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol 2018;69(2):406–460. 10.1016/j.jhep.2018.03.024. [DOI] [PubMed] [Google Scholar]
- [76].Biggins SW, Angeli P, Garcia-Tsao G, et al. Diagnosis, evaluation, and management of ascites, spontaneous bacterial peritonitis and hepatorenal syndrome: 2021 practice guidance by the American association for the study of liver diseases. Hepatology 2021;74(2):1014–1048. 10.1002/hep.31884. [DOI] [PubMed] [Google Scholar]
- [77].Singh V, De A, Mehtani R, et al. Asia-Pacific association for study of liver guidelines on management of ascites in liver disease. Hepatol Int 2023;17(4):792–826. 10.1007/s12072-023-10536-7. [DOI] [PubMed] [Google Scholar]
- [78].Stottlemyer BA, Abebe KZ, Palevsky PM, et al. Expert consensus on the nephrotoxic potential of 195 medications in the non-intensive care setting: a modified Delphi method. Drug Saf 2023:1–11. 10.1007/s40264-023-01312-5 (In eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Kane-Gill SL. Nephrotoxin stewardship. Crit Care Clin 2021;37(2):303–320. 10.1016/j.ccc.2020.11.002 (In eng). [DOI] [PubMed] [Google Scholar]
- [80].Ehrmann S, Helms J, Joret A, et al. Nephrotoxic drug burden among 1001 critically ill patients: impact on acute kidney injury. Ann Intensive Care 2019;9(1):106. 10.1186/s13613-019-0580-1 (In eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Franz CC, Hildbrand C, Born C, et al. Dose adjustment in patients with liver cirrhosis: impact on adverse drug reactions and hospitalizations. Eur J Clin Pharmacol 2013;69(8):1565–1573. 10.1007/s00228-013-1502-z (In eng). [DOI] [PubMed] [Google Scholar]
- [82].Delcò F, Tchambaz L, Schlienger R, et al. Dose adjustment in patients with liver disease. Drug Saf 2005;28(6):529–545. 10.2165/00002018-200528060-00005 (In eng). [DOI] [PubMed] [Google Scholar]
- [83].Sort P, Navasa M, Arroyo V, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med 1999;341(6):403–409. 10.1056/NEJM199908053410603. [DOI] [PubMed] [Google Scholar]
- [84].Poca M, Concepcion M, Casas M, et al. Role of albumin treatment in patients with spontaneous bacterial peritonitis. Clin Gastroenterol Hepatol 2012;10(3):309–315. 10.1016/j.cgh.2011.11.012. [DOI] [PubMed] [Google Scholar]
- [85].Terg R, Gadano A, Cartier M, et al. Serum creatinine and bilirubin predict renal failure and mortality in patients with spontaneous bacterial peritonitis: a retrospective study. Liver Int 2009;29(3):415–419. 10.1111/j.1478-3231.2008.01877.x. [DOI] [PubMed] [Google Scholar]
- [86].Chen TA, Tsao YC, Chen A, et al. Effect of intravenous albumin on endotoxin removal, cytokines, and nitric oxide production in patients with cirrhosis and spontaneous bacterial peritonitis. Scand J Gastroenterol 2009;44(5):619–625. 10.1080/00365520902719273 (In eng). [DOI] [PubMed] [Google Scholar]
- [87].Thevenot T, Bureau C, Oberti F, et al. Effect of albumin in cirrhotic patients with infection other than spontaneous bacterial peritonitis. A randomized trial. J Hepatol 2015;62(4):822–830. 10.1016/j.jhep.2014.11.017. [DOI] [PubMed] [Google Scholar]
- [88].Guevara M, Terra C, Nazar A, et al. Albumin for bacterial infections other than spontaneous bacterial peritonitis in cirrhosis. A randomized, controlled study. J Hepatol 2012;57(4):759–765. 10.1016/j.jhep.2012.06.013. [DOI] [PubMed] [Google Scholar]
- [89].Fernandez J, Angeli P, Trebicka J, et al. Efficacy of albumin treatment for patients with cirrhosis and infections unrelated to spontaneous bacterial peritonitis. Clin Gastroenterol Hepatol 2020;18(4):963–973 e14. 10.1016/j.cgh.2019.07.055. [DOI] [PubMed] [Google Scholar]
- [90].Leao GS, John Neto G, Jotz RF, et al. Albumin for cirrhotic patients with extraperitoneal infections: a meta-analysis. J Gastroenterol Hepatol 2019;34(12):2071–2076. 10.1111/jgh.14791. [DOI] [PubMed] [Google Scholar]
- [91].China L, Freemantle N, Forrest E, et al. A randomized trial of albumin infusions in hospitalized patients with cirrhosis. N Engl J Med 2021;384(9):808–817. 10.1056/NEJMoa2022166. [DOI] [PubMed] [Google Scholar]
- [92].Caraceni P, Riggio O, Angeli P, et al. Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial. Lancet 2018;391(10138):2417–2429. 10.1016/s0140-6736(18)30840-7 (In eng). [DOI] [PubMed] [Google Scholar]
- [93].Solà E, Solé C, Simón-Talero M, et al. Midodrine and albumin for prevention of complications in patients with cirrhosis awaiting liver transplantation. A randomized placebo-controlled trial. J Hepatol 2018;69(6):1250–1259. 10.1016/j.jhep.2018.08.006 (In eng). [DOI] [PubMed] [Google Scholar]
- [94].Bernardi M, Caraceni P, Navickis RJ, et al. Albumin infusion in patients undergoing large-volume paracentesis: a meta-analysis of randomized trials. Hepatology 2012;55(4):1172–1181. 10.1002/hep.24786. [DOI] [PubMed] [Google Scholar]
- [95].Ginès P, Titó L, Arroyo V, et al. Randomized comparative study of therapeutic paracentesis with and without intravenous albumin in cirrhosis. Gastroenterology 1988;94(6):1493–1502. 10.1016/0016-5085(88)90691-9 (In eng). [DOI] [PubMed] [Google Scholar]
- [96].Bureau C, Thabut D, Oberti F, et al. Transjugular intrahepatic portosystemic shunts with covered stents increase transplant-free survival of patients with cirrhosis and recurrent ascites. Gastroenterology 2017;152(1):157–163. 10.1053/j.gastro.2016.09.016. [DOI] [PubMed] [Google Scholar]
- [97].Stirnimann G, Berg T, Spahr L, et al. Final safety and efficacy results from a 106 real-world patients registry with an ascites-mobilizing pump. Liver Int 2022;42(10):2247–2259. 10.1111/liv.15337 (In eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Bureau C, Adebayo D, Chalret de Rieu M, et al. Alfapump® system vs. large volume paracentesis for refractory ascites: a multicenter randomized controlled study. J Hepatol 2017;67(5):940–949. 10.1016/j.jhep.2017.06.010 (In eng). [DOI] [PubMed] [Google Scholar]
- [99].Lepida A, Marot A, Trepo E, et al. Systematic review with meta-analysis: automated low-flow ascites pump therapy for refractory ascites. Aliment Pharmacol Ther 2019;50(9):978–987. 10.1111/apt.15502. [DOI] [PubMed] [Google Scholar]
- [100].Sola E, Sanchez-Cabus S, Rodriguez E, et al. Effects of alfapump system on kidney and circulatory function in patients with cirrhosis and refractory ascites. Liver Transpl 2017;23(5):583–593. 10.1002/lt.24763. [DOI] [PubMed] [Google Scholar]
- [101].Díaz-Gómez JL, Mayo PH, Koenig SJ. Point-of-Care ultrasonography. N Engl J Med 2021;385(17):1593–1602. 10.1056/NEJMra1916062 (In eng). [DOI] [PubMed] [Google Scholar]
- [102].Kaptein EM, Oo Z, Kaptein MJ. Hepatorenal syndrome misdiagnosis may be reduced using inferior vena cava ultrasound to assess intravascular volume and guide management. Ren Fail 2023;45(1):2185468. 10.1080/0886022X.2023.2185468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Velez JCQ, Petkovich B, Karakala N, et al. Point-of-Care echocardiography unveils misclassification of acute kidney injury as hepatorenal syndrome. Am J Nephrol 2019;50(3):204–211. 10.1159/000501299 (In eng). [DOI] [PubMed] [Google Scholar]
- [104].Palsson R, Colona MR, Hoenig MP, et al. Assessment of interobserver reliability of nephrologist examination of urine sediment. JAMA Netw Open 2020;3(8):e2013959. 10.1001/jamanetworkopen.2020.13959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Jouet P, Meyrier A, Mal F, et al. Transjugular renal biopsy in the treatment of patients with cirrhosis and renal abnormalities. Hepatology 1996;24(5):1143–1147. 10.1002/hep.510240527. [DOI] [PubMed] [Google Scholar]
- [106].Wadei HM, Heckman MG, Rawal B, et al. Renal outcomes of liver transplant recipients who had pretransplant kidney biopsy. Transplantation 2014;98(12):1323–1330. 10.1097/tp.0000000000000215 (In eng). [DOI] [PubMed] [Google Scholar]
- [107].Corapi KM, Chen JL, Balk EM, Gordon CE. Bleeding complications of native kidney biopsy: a systematic review and meta-analysis. Am J Kidney Dis 2012;60(1):62–73. 10.1053/j.ajkd.2012.02.330 (In eng). [DOI] [PubMed] [Google Scholar]
- [108].Calmus Y, Conti F, Cluzel P, et al. Prospective assessment of renal histopathological lesions in patients with end-stage liver disease: effects on long-term renal function after liver transplantation. J Hepatol 2012;57(3):572–576. 10.1016/j.jhep.2012.04.028. [DOI] [PubMed] [Google Scholar]
- [109].Macedo E, Bouchard J, Soroko SH, et al. Fluid accumulation, recognition and staging of acute kidney injury in critically-ill patients. Crit Care 2010;14(3):R82. 10.1186/cc9004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Verna EC, Connelly C, Dove LM, et al. Center-related bias in MELD scores within a liver transplant UNOS region: a call for standardization. Transplantation 2020;104(7):1396–1402. 10.1097/TP.0000000000003031. [DOI] [PubMed] [Google Scholar]
- [111].Maiwall R, Kumar A, Bhardwaj A, et al. Cystatin C predicts acute kidney injury and mortality in cirrhotics: a prospective cohort study. Liver Int 2018;38(4):654–664. 10.1111/liv.13600. [DOI] [PubMed] [Google Scholar]
- [112].Markwardt D, Holdt L, Steib C, et al. Plasma cystatin C is a predictor of renal dysfunction, acute-on-chronic liver failure, and mortality in patients with acutely decompensated liver cirrhosis. Hepatology 2017;66(4):1232–1241. 10.1002/hep.29290. [DOI] [PubMed] [Google Scholar]
- [113].Slack AJ, McPhail MJ, Ostermann M, et al. Predicting the development of acute kidney injury in liver cirrhosis–an analysis of glomerular filtration rate, proteinuria and kidney injury biomarkers. Aliment Pharmacol Ther 2013;37(10):989–997. 10.1111/apt.12299 (In eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Wan ZH, Wang JJ, You SL, et al. Cystatin C is a biomarker for predicting acute kidney injury in patients with acute-on-chronic liver failure. World J Gastroenterol 2013;19(48):9432–9438. 10.3748/wjg.v19.i48.9432 (In eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Jaques DA, Spahr L, Berra G, et al. Biomarkers for acute kidney injury in decompensated cirrhosis: a prospective study. Nephrology (Carlton) 2019;24(2):170–180. 10.1111/nep.13226. [DOI] [PubMed] [Google Scholar]
- [116].Barreto EF, Rule AD, Murad MH, et al. Prediction of the renal elimination of drugs with cystatin C vs creatinine: a systematic review. Mayo Clin Proc 2019;94(3):500–514. 10.1016/j.mayocp.2018.08.002. [DOI] [PubMed] [Google Scholar]
- [117].Singapura P, Ma TW, Sarmast N, et al. Estimating glomerular filtration rate in cirrhosis using creatinine-based and cystatin C-based equations: systematic review and meta-analysis. Liver Transpl 2021;27(11):1538–1552. 10.1002/lt.26216. [DOI] [PubMed] [Google Scholar]
- [118].Francoz C, Nadim MK, Baron A, et al. Glomerular filtration rate equations for liver-kidney transplantation in patients with cirrhosis: validation of current recommendations. Hepatology 2014;59(4):1514–1521. 10.1002/hep.26704. [DOI] [PubMed] [Google Scholar]
- [119].Delgado C, Baweja M, Crews DC, et al. A unifying approach for GFR estimation: recommendations of the NKF-ASN task force on reassessing the inclusion of race in diagnosing kidney disease. J Am Soc Nephrol 2021;32(12):2994–3015. 10.1681/ASN.2021070988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Kalafateli M, Wickham F, Burniston M, et al. Development and validation of a mathematical equation to estimate glomerular filtration rate in cirrhosis: the royal free hospital cirrhosis glomerular filtration rate. Hepatology 2017;65(2):582–591. 10.1002/hep.28891 (In eng). [DOI] [PubMed] [Google Scholar]
- [121].Mindikoglu AL, Dowling TC, Magder LS, et al. Estimation of glomerular filtration rate in patients with cirrhosis by using new and conventional filtration markers and dimethylarginines. Clin Gastroenterol Hepatol 2016;14(4):624–632. 10.1016/j.cgh.2015.06.021.e2. (In eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Asrani SK, Jennings LW, Trotter JF, et al. A model for glomerular filtration rate assessment in liver disease (GRAIL) in the presence of renal dysfunction. Hepatology 2019;69(3):1219–1230. 10.1002/hep.30321. [DOI] [PubMed] [Google Scholar]
- [123].Inker LA, Eneanya ND, Coresh J, et al. New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med 2021;385(19):1737–1749. 10.1056/NEJMoa2102953 (In eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Panchal S, Serper M, Bittermann T, et al. Impact of race-adjusted glomerular filtration rate estimation on eligibility for simultaneous liver-kidney transplantation. Liver Transpl 2022;28(6):959–968. 10.1002/lt.26310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Proulx NL, Akbari A, Garg AX, et al. Measured creatinine clearance from timed urine collections substantially overestimates glomerular filtration rate in patients with liver cirrhosis: a systematic review and individual patient meta-analysis. Nephrol Dial Transpl 2005;20(8):1617–1622. 10.1093/ndt/gfh839. [DOI] [PubMed] [Google Scholar]
- [126].Selistre L, de Souza V, Nicola C, et al. Average creatinine-urea clearance: revival of an old analytical technique? Clin Kidney J 2023;16(8):1298–1306. 10.1093/ckj/sfad050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Murray PT, Mehta RL, Shaw A, et al. Potential use of biomarkers in acute kidney injury: report and summary of recommendations from the 10th Acute Dialysis Quality Initiative consensus conference. Kidney Int 2014;85(3):513–521. 10.1038/ki.2013.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Treeprasertsuk S, Wongkarnjana A, Jaruvongvanich V, et al. Urine neutrophil gelatinase-associated lipocalin: a diagnostic and prognostic marker for acute kidney injury (AKI) in hospitalized cirrhotic patients with AKI-prone conditions. BMC Gastroenterol 2015;15:140. 10.1186/s12876-015-0372-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Lima C, Gorab DL, Fernandes CR, et al. Role of proenkephalin in the diagnosis of severe and subclinical acute kidney injury during the perioperative period of liver transplantation. Pract Lab Med 2022;31:e00278. 10.1016/j.plabm.2022.e00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Jo SK, Yang J, Hwang SM, et al. Role of biomarkers as predictors of acute kidney injury and mortality in decompensated cirrhosis. Sci Rep 2019;9(1): 14508. 10.1038/s41598-019-51053-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Cruz DN, Bagshaw SM, Maisel A, et al. Use of biomarkers to assess prognosis and guide management of patients with acute kidney injury. Contrib Nephrol 2013;182:45–64. 10.1159/000349965. [DOI] [PubMed] [Google Scholar]
- [132].McCullough PA, Shaw AD, Haase M, et al. Diagnosis of acute kidney injury using functional and injury biomarkers: workgroup statements from the tenth Acute Dialysis Quality Initiative Consensus Conference. Contrib Nephrol 2013;182:13–29. 10.1159/000349963. [DOI] [PubMed] [Google Scholar]
- [133].Asrani SK, Shankar N, da Graca B, et al. Role of novel kidney biomarkers in patients with cirrhosis and after liver transplantation. Liver Transpl 2022;28(3):466–482. 10.1002/lt.26344. [DOI] [PubMed] [Google Scholar]
- [134].Juanola A, Ma AT, Pose E, Gines P. Novel biomarkers of AKI in cirrhosis. Semin Liver Dis 2022;42(4):489–500. 10.1055/a-1954-4136. [DOI] [PubMed] [Google Scholar]
- [135].Belcher JM, Garcia-Tsao G, Sanyal AJ, et al. Urinary biomarkers and progression of AKI in patients with cirrhosis. Clin J Am Soc Nephrol 2014;9(11):1857–1867. 10.2215/CJN.09430913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Gambino C, Piano S, Stenico M, et al. Diagnostic and prognostic performance of urinary neutrophil gelatinase-associated lipocalin in patients with cirrhosis and acute kidney injury. Hepatology 2023;77(5):1630–1638. 10.1002/hep.32799 (In eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Fagundes C, Pepin MN, Guevara M, et al. Urinary neutrophil gelatinase-associated lipocalin as biomarker in the differential diagnosis of impairment of kidney function in cirrhosis. J Hepatol 2012;57(2):267–273. 10.1016/j.jhep.2012.03.015. [DOI] [PubMed] [Google Scholar]
- [138].Lei L, Li LP, Zeng Z, et al. Value of urinary KIM-1 and NGAL combined with serum Cys C for predicting acute kidney injury secondary to decompensated cirrhosis. Sci Rep 2018;8(1):7962. 10.1038/s41598-018-26226-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Finfer S, Bellomo R, Boyce N, et al. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med 2004;350(22):2247–2256. 10.1056/NEJMoa040232. [DOI] [PubMed] [Google Scholar]
- [140].Caironi P, Tognoni G, Masson S, et al. Albumin replacement in patients with severe sepsis or septic shock. N Engl J Med 2014;370(15):1412–1421. 10.1056/NEJMoa1305727. [DOI] [PubMed] [Google Scholar]
- [141].Alcaraz-Quiles J, Casulleras M, Oettl K, et al. Oxidized albumin triggers a cytokine storm in leukocytes through P38 mitogen-activated Protein kinase: role in systemic inflammation in decompensated cirrhosis. Hepatology 2018;68(5):1937–1952. 10.1002/hep.30135. [DOI] [PubMed] [Google Scholar]
- [142].Arroyo V, Garcia-Martinez R, Salvatella X. Human serum albumin, systemic inflammation, and cirrhosis. J Hepatol 2014;61(2):396–407. 10.1016/j.jhep.2014.04.012. [DOI] [PubMed] [Google Scholar]
- [143].Maiwall R, Kumar A, Pasupuleti SSR, et al. A randomized-controlled trial comparing 20% albumin to plasmalyte in patients with cirrhosis and sepsis-induced hypotension [ALPS trial]. J Hepatol 2022;77(3):670–682. 10.1016/j.jhep.2022.03.043 (In eng). [DOI] [PubMed] [Google Scholar]
- [144].Philips CA, Maiwall R, Sharma MK, et al. Comparison of 5% human albumin and normal saline for fluid resuscitation in sepsis induced hypotension among patients with cirrhosis (FRISC study): a randomized controlled trial. Hepatol Int 2021;15(4):983–994. 10.1007/s12072-021-10164-z (In eng). [DOI] [PubMed] [Google Scholar]
- [145].Umgelter A, Reindl W, Wagner KS, et al. Effects of plasma expansion with albumin and paracentesis on haemodynamics and kidney function in critically ill cirrhotic patients with tense ascites and hepatorenal syndrome: a prospective uncontrolled trial. Crit Care 2008;12(1):R4. 10.1186/cc6765 (In eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Umgelter A, Reindl W, Franzen M, et al. Renal resistive index and renal function before and after paracentesis in patients with hepatorenal syndrome and tense ascites. Intensive Care Med 2009;35(1):152–156. 10.1007/s00134-008-1253-y. [DOI] [PubMed] [Google Scholar]
- [147].Pozzi M, Osculati G, Boari G, et al. Time course of circulatory and humoral effects of rapid total paracentesis in cirrhotic patients with tense, refractory ascites. Gastroenterology 1994;106(3):709–719. 10.1016/0016-5085(94)90706-4. [DOI] [PubMed] [Google Scholar]
- [148].Seethapathy H, Sharma S, Zhao S, et al. Acute kidney injury following paracentesis among inpatients with cirrhosis. Kidney Int Rep 2020;5(8):1305–1308. 10.1016/j.ekir.2020.05.014 (In eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Ginés P, Arroyo V, Quintero E, et al. Comparison of paracentesis and diuretics in the treatment of cirrhotics with tense ascites. Results of a randomized study. Gastroenterology 1987;93(2):234–241. 10.1016/0016-5085(87)91007-9 (In eng). [DOI] [PubMed] [Google Scholar]
- [150].Gaudry S, Hajage D, Schortgen F, et al. Initiation strategies for renal-replacement therapy in the intensive care unit. N Engl J Med 2016;375(2):122–133. 10.1056/NEJMoa1603017 (In eng). [DOI] [PubMed] [Google Scholar]
- [151].Barbar SD, Clere-Jehl R, Bourredjem A, et al. Timing of renal-replacement therapy in patients with acute kidney injury and sepsis. N Engl J Med 2018;379(15):1431–1442. 10.1056/NEJMoa1803213 (In eng). [DOI] [PubMed] [Google Scholar]
- [152].Wald R, Bagshaw SM. Timing of initiation of renal-replacement therapy in acute kidney injury. Reply N Engl J Med 2020;383(18):1797–1798. 10.1056/NEJMc2027489 (In eng). [DOI] [PubMed] [Google Scholar]
- [153].Bouchard J, Mehta RL. Timing of kidney support therapy in acute kidney injury: what are we waiting for? Am J Kidney Dis 2022;79(3):417–426. 10.1053/j.ajkd.2021.07.014. [DOI] [PubMed] [Google Scholar]
- [154].Ostermann M, Joannidis M, Pani A, et al. Patient selection and timing of continuous renal replacement therapy. Blood Purif 2016;42(3):224–237. 10.1159/000448506. [DOI] [PubMed] [Google Scholar]
- [155].Rosner MH, Ostermann M, Murugan R, et al. Indications and management of mechanical fluid removal in critical illness. Br J Anaesth 2014;113(5):764–771. 10.1093/bja/aeu297. [DOI] [PubMed] [Google Scholar]
- [156].Nadim MK, DiNorcia J, Ji L, et al. Inequity in organ allocation for patients awaiting liver transplantation: rationale for uncapping the model for end-stage liver disease. J Hepatol 2017;67(3):517–525. 10.1016/j.jhep.2017.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Scherer JS, Holley JL. The role of time-limited trials in dialysis decision making in critically ill patients. Clin J Am Soc Nephrol 2016;11(2):344–353. 10.2215/cjn.03550315 (In eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Braun HJ, Mello A, Kothari R, et al. Expedited evaluation for liver transplantation: a critical look at processes and outcomes. Clin Transpl 2022;36(3):e14539. 10.1111/ctr.14539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Dong V, Nadim MK, Karvellas CJ. Post-liver transplant acute kidney injury. Liver Transpl 2021;27(11):1653–1664. 10.1002/lt.26094. [DOI] [PubMed] [Google Scholar]
- [160].Nadim MK, Sung RS, Davis CL, et al. Simultaneous liver-kidney transplantation summit: current state and future directions. Am J Transpl 2012;12(11):2901–2908. 10.1111/j.1600-6143.2012.04190.x. [DOI] [PubMed] [Google Scholar]
- [161].Levitsky J, Baker T, Ahya SN, et al. Outcomes and native renal recovery following simultaneous liver-kidney transplantation. Am J Transpl 2012;12(11):2949–2957. 10.1111/j.1600-6143.2012.04182.x. [DOI] [PubMed] [Google Scholar]
- [162].U.S. Department of Health & Human Services: organ procurement and transplantation network data. Available at: https://optn.transplant.hrsa.gov/data.
- [163].Nadim MK, Genyk YS, Tokin C, et al. Impact of the etiology of acute kidney injury on outcomes following liver transplantation: acute tubular necrosis versus hepatorenal syndrome. Liver Transpl 2012;18(5):539–548. 10.1002/lt.23384. [DOI] [PubMed] [Google Scholar]
- [164].Levitsky J, Asrani SK, Klintmalm G, et al. Discovery and validation of a biomarker model (PRESERVE) predictive of renal outcomes after liver transplantation. Hepatology 2020;71(5):1775–1786. 10.1002/hep.30939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Levitsky J, Asrani SK, Abecassis M, et al. External validation of a pretransplant biomarker model (REVERSE) predictive of renal recovery after liver transplantation. Hepatology 2019;70(4):1349–1359. 10.1002/hep.30667. [DOI] [PubMed] [Google Scholar]
- [166].Dodge JL, Lee BT, Kassem ACZ, et al. The conundrum of patients with compensated cirrhosis requiring kidney transplantation; kidney alone or simultaneous liver kidney transplantation. Transplantation 2023;107(2):429–437. 10.1097/TP.0000000000004311. [DOI] [PubMed] [Google Scholar]
- [167].Allegretti AS, Ortiz G, Cui J, et al. Changes in kidney function after transjugular intrahepatic portosystemic shunts versus large-volume paracentesis in cirrhosis: a matched cohort analysis.. The Official Journal of the National Kidney Foundation Am J Kidney Dis 2016. S0272-S6386(16)00211–0 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Charilaou P, Devani K, Petrosyan R, et al. Inpatient mortality benefit with transjugular intrahepatic portosystemic shunt for hospitalized hepatorenal syndrome patients. Dig Dis Sci 2020;65(11):3378–3388. 10.1007/s10620-020-06136-2. [DOI] [PubMed] [Google Scholar]
- [169].Ponzo P, Campion D, Rizzo M, et al. Transjugular intrahepatic portosystemic shunt in cirrhotic patients with hepatorenal syndrome - chronic kidney disease: impact on renal function. Dig Liver Dis 2022;54(8):1101–1108. 10.1016/j.dld.2021.09.008. [DOI] [PubMed] [Google Scholar]
- [170].Song T, Rossle M, He F, et al. Transjugular intrahepatic portosystemic shunt for hepatorenal syndrome: a systematic review and meta-analysis. Dig Liver Dis 2018;50(4):323–330. 10.1016/j.dld.2018.01.123. [DOI] [PubMed] [Google Scholar]
- [171].Guevara M, Gines P, Bandi JC, et al. Transjugular intrahepatic portosystemic shunt in hepatorenal syndrome: effects on renal function and vasoactive systems. Hepatology 1998;28(2):416–422. 10.1002/hep.510280219. [DOI] [PubMed] [Google Scholar]
- [172].Ripoll C, Platzer S, Franken P, et al. Liver-HERO: hepatorenal syndrome-acute kidney injury (HRS-AKI) treatment with transjugular intrahepatic portosystemic shunt in patients with cirrhosis-a randomized controlled trial. Trials 2023;24(1):258. 10.1186/s13063-023-07261-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Banares R, Ibanez-Samaniego L, Torner JM, et al. Meta-analysis of individual patient data of albumin dialysis in acute-on-chronic liver failure: focus on treatment intensity. Therap Adv Gastroenterol 2019;12:1756284819879565. https://www.ncbi.nlm.nih.gov/pubmed/31632458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Kribben A, Gerken G, Haag S, et al. Effects of fractionated plasma separation and adsorption on survival in patients with acute-on-chronic liver failure. Gastroenterology 2012;142(4):782–789 e3. 10.1053/j.gastro.2011.12.056. [DOI] [PubMed] [Google Scholar]
- [175].Niewinski G, Raszeja-Wyszomirska J, Hrenczuk M, et al. Intermittent high-flux albumin dialysis with continuous venovenous hemodialysis for acute-on-chronic liver failure and acute kidney injury. Artif Organs 2020;44(1):91–99. 10.1111/aor.13532. [DOI] [PubMed] [Google Scholar]
- [176].Agarwal B, Canizares RB, Saliba F, et al. Randomized, controlled clinical trial of the DIALIVE liver dialysis device versus standard of care in patients with acute-on-chronic liver failure. J Hepatol 2023;79(1):79–92. 10.1016/j.jhep.2023.03.013. [DOI] [PubMed] [Google Scholar]
- [177].Popescu M, David C, Marcu A, et al. Artificial liver support with CytoSorb and MARS in liver failure: a retrospective propensity matched analysis. J Clin Med 2023;12(6). 10.3390/jcm12062258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Cavallin M, Piano S, Romano A, et al. Terlipressin given by continuous intravenous infusion versus intravenous boluses in the treatment of hepatorenal syndrome: a randomized controlled study. Hepatology 2016;63(3):983–992. 10.1002/hep.28396 (In eng). [DOI] [PubMed] [Google Scholar]
- [179].Facciorusso A, Chandar AK, Murad MH, et al. Comparative efficacy of pharmacological strategies for management of type 1 hepatorenal syndrome: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol 2017;2(2):94–102. 10.1016/S2468-1253(16)30157-1. [DOI] [PubMed] [Google Scholar]
- [180].Pitre T, Kiflen M, Helmeczi W, et al. The comparative effectiveness of vasoactive treatments for hepatorenal syndrome: a systematic review and network meta-analysis. Crit Care Med 2022;50(10):1419–1429. 10.1097/CCM.0000000000005595. [DOI] [PubMed] [Google Scholar]
- [181].Best LM, Freeman SC, Sutton AJ, et al. Treatment for hepatorenal syndrome in people with decompensated liver cirrhosis: a network meta-analysis. Cochrane Database Syst Rev 2019;9(9):CD013103. 10.1002/14651858.CD013103.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Arora V, Maiwall R, Rajan V, et al. Terlipressin is superior to noradrenaline in the management of acute kidney injury in acute on chronic liver failure. Hepatology 2020;71(2):600–610. 10.1002/hep.30208. [DOI] [PubMed] [Google Scholar]
- [183].Cavallin M, Kamath PS, Merli M, et al. Terlipressin plus albumin versus midodrine and octreotide plus albumin in the treatment of hepatorenal syndrome: a randomized trial. Hepatology (Baltimore, Md) 2015;62(2):567–574. 10.1002/hep.27709. [DOI] [PubMed] [Google Scholar]
- [184].El-Desoki Mahmoud EI, Abdelaziz DH, Abd-Elsalam S, et al. Norepinephrine is more effective than midodrine/octreotide in patients with hepatorenal syndrome-acute kidney injury: a randomized controlled trial. Front Pharmacol 2021;12:675948. 10.3389/fphar.2021.675948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Velez JCQ, Karakala N, Tayebi K, et al. Responsiveness to vasoconstrictor therapy in hepatorenal syndrome type 1. Kidney360 2023;4(4):e448–e456. 10.34067/KID.0000000000000068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Velez JC, Kadian M, Taburyanskaya M, et al. Hepatorenal acute kidney injury and the importance of raising mean arterial pressure. Nephron 2015;131(3):191–201. 10.1159/000441151 (In eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Moore K, Jamil K, Verleger K, et al. Real-world treatment patterns and outcomes using terlipressin in 203 patients with the hepatorenal syndrome. Aliment Pharmacol Ther 2020. 10.1111/apt.15836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Maddukuri G, Cai CX, Munigala S, et al. Targeting an early and substantial increase in mean arterial pressure is critical in the management of type 1 hepatorenal syndrome: a combined retrospective and pilot study. Dig Dis Sci 2014;59(2):471–481. 10.1007/s10620-013-2899-z. [DOI] [PubMed] [Google Scholar]
- [189].Sanyal AJ, Boyer T, Garcia-Tsao G, et al. A randomized, prospective, double-blind, placebo-controlled trial of terlipressin for type 1 hepatorenal syndrome. Gastroenterology 2008;134(5):1360–1368. 10.1053/j.gastro.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Martín-Llahí M, Pépin MN, Guevara M, et al. Terlipressin and albumin vs albumin in patients with cirrhosis and hepatorenal syndrome: a randomized study. Gastroenterology 2008;134(5):1352–1359. 10.1053/j.gastro.2008.02.024 (In eng). [DOI] [PubMed] [Google Scholar]
- [191].Boyer TD, Sanyal AJ, Wong F, et al. Terlipressin plus albumin is more effective than albumin alone in improving renal function in patients with cirrhosis and hepatorenal syndrome type 1. Gastroenterology 2016;150(7):1579–1589 e2. 10.1053/j.gastro.2016.02.026. [DOI] [PubMed] [Google Scholar]
- [192].Wong F, Pappas SC, Curry MP, et al. Terlipressin plus albumin for the treatment of type 1 hepatorenal syndrome. N Engl J Med 2021;384(9):818–828. 10.1056/NEJMoa2008290 (In eng). [DOI] [PubMed] [Google Scholar]
- [193].Pichler RH, Swenson ER, Leary PJ, et al. Terlipressin: hopes fulfilled or dashed? Clin J Am Soc Nephrol 2022;17(1):140–142. 10.2215/CJN.06710521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Allegretti AS, Subramanian RM, Francoz C, et al. Respiratory events with terlipressin and albumin in hepatorenal syndrome: a review and clinical guidance. Liver Int 2022;42(10):2124–2130. 10.1111/liv.15367 (In eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Piano S, Gambino C, Vettore E, et al. Response to terlipressin and albumin is associated with improved liver transplant outcomes in patients with hepatorenal syndrome. Hepatology 2021;73(5):1909–1919. 10.1002/hep.31529 (In eng). [DOI] [PubMed] [Google Scholar]
- [196].Angeli P, Gines P. Hepatorenal syndrome, MELD score and liver transplantation: an evolving issue with relevant implications for clinical practice. J Hepatol 2012;57(5):1135–1140. 10.1016/j.jhep.2012.06.024 (In eng). [DOI] [PubMed] [Google Scholar]
- [197].Kellum JA, Sileanu FE, Bihorac A, et al. Recovery after acute kidney injury. Am J Respir Crit Care Med 2017;195(6):784–791. 10.1164/rccm.201604-0799OC (In eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Siew ED, Parr SK, Abdel-Kader K, et al. Predictors of recurrent AKI. J Am Soc Nephrol 2016;27(4):1190–1200. 10.1681/ASN.2014121218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Harel Z, Wald R, Bargman JM, et al. Nephrologist follow-up improves all-cause mortality of severe acute kidney injury survivors. Kidney Int 2013;83(5):901–908. 10.1038/ki.2012.451 (In eng). [DOI] [PubMed] [Google Scholar]
- [200].Ly H, Ortiz-Soriano V, Liu LJ, et al. Characteristics and outcomes of survivors of critical illness and acute kidney injury followed in a pilot acute kidney injury clinic. Kidney Int Rep 2021;6(12):3070–3073. 10.1016/j.ekir.2021.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Singh G, Hu Y, Jacobs S, et al. Post-discharge mortality and rehospitalization among participants in a comprehensive acute kidney injury rehabilitation program. Kidney360 2021;2(9):1424–1433. 10.34067/kid.0003672021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].See EJ, Jayasinghe K, Glassford N, et al. Long-term risk of adverse outcomes after acute kidney injury: a systematic review and meta-analysis of cohort studies using consensus definitions of exposure. Kidney Int 2019;95(1):160–172. 10.1016/j.kint.2018.08.036. [DOI] [PubMed] [Google Scholar]
- [203].Mindikoglu AL, Hernaez R, Liu Y, et al. Renal trajectory patterns are associated with postdischarge mortality in patients with cirrhosis and acute kidney injury. Clin Gastroenterol Hepatol 2020;18(8):1858–1866 e6. 10.1016/j.cgh.2019.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Bajaj JS, Reddy KR, Tandon P, et al. The 3-month readmission rate remains unacceptably high in a large North American cohort of patients with cirrhosis. Hepatology 2016;64(1):200–208. 10.1002/hep.28414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].de Franchis R, Bosch J, Garcia-Tsao G, et al. Baveno VII - renewing consensus in portal hypertension. J Hepatol 2022;76(4):959–974. 10.1016/j.jhep.2021.12.022 (In eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Gharaibeh KA, Hamadah AM, El-Zoghby ZM, et al. Cystatin C predicts renal recovery earlier than creatinine among patients with acute kidney injury. Kidney Int Rep 2018;3(2):337–342. 10.1016/j.ekir.2017.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Torre A, Aguirre-Valadez JM, Arreola-Guerra JM, et al. Creatinine versus cystatin C for estimating GFR in patients with liver cirrhosis. Am J Kidney Dis 2016;67(2):342–344. 10.1053/j.ajkd.2015.09.022. [DOI] [PubMed] [Google Scholar]
- [208].Ahn HS, Kim YS, Kim SG, et al. Cystatin C is a good predictor of hepatorenal syndrome and survival in patients with cirrhosis who have normal serum creatinine levels. Hepatogastroenterology 2012;59(116):1168–1173. 10.5754/hge11616. [DOI] [PubMed] [Google Scholar]
- [209].Hsu CY, Hsu RK, Liu KD, et al. Impact of AKI on urinary Protein excretion: analysis of two prospective cohorts. J Am Soc Nephrol 2019;30(7):1271–1281. 10.1681/ASN.2018101036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Watson KE, Dhaliwal K, Robertshaw S, et al. Consensus recommendations for sick day medication guidance for people with diabetes, kidney, or cardiovascular disease: a modified Delphi process. Am J Kidney Dis 2023;81(5):564–574. 10.1053/j.ajkd.2022.10.012. [DOI] [PubMed] [Google Scholar]
- [211].Volk ML, Fisher N, Fontana RJ. Patient knowledge about disease self-management in cirrhosis. Am J Gastroenterol 2013;108(3):302–305. 10.1038/ajg.2012.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Tandon P, Montano-Loza AJ, Lai JC, et al. Sarcopenia and frailty in decompensated cirrhosis.. Suppl 1 J Hepatol 2021;75(Suppl 1):S147–S162. 10.1016/j.jhep.2021.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Tandon P, Zanetto A, Piano S, et al. Liver transplantation in the patient with physical frailty. J Hepatol 2023;78(6):1105–1117. 10.1016/j.jhep.2023.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Roth K, Lynn J, Zhong Z, et al. Dying with end stage liver disease with cirrhosis: insights from SUPPORT. Study to understand prognoses and preferences for outcomes and risks of treatment. J Am Geriatr Soc 2000;48(S1):S122–S130. https://www.ncbi.nlm.nih.gov/pubmed/10809465. [PubMed] [Google Scholar]
- [215].Tandon P, Walling A, Patton H, et al. AGA clinical practice update on palliative care management in cirrhosis: expert review. Clin Gastroenterol Hepatol 2021;19(4):646–656 e3. 10.1016/j.cgh.2020.11.027. [DOI] [PubMed] [Google Scholar]
- [216].Rogal SS, Hansen L, Patel A, et al. AASLD Practice Guidance: palliative care and symptom-based management in decompensated cirrhosis. Hepatology 2022;76(3):819–853. 10.1002/hep.32378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Esteban JPG, Rein L, Szabo A, et al. Attitudes of liver and palliative care clinicians toward specialist palliative care consultation for patients with end-stage liver disease. J Palliat Med 2019;22(7):804–813. 10.1089/jpm.2018.0553. [DOI] [PubMed] [Google Scholar]
- [218].Kavalieratos D, Corbelli J, Zhang D, et al. Association between palliative care and patient and caregiver outcomes: a systematic review and meta-analysis. JAMA 2016;316(20):2104–2114. 10.1001/jama.2016.16840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Claude C, Deep A, Kneyber M, et al. pCLIF-SOFA is a reliable outcome prognostication score of critically ill children with cirrhosis: an ESPNIC multicentre study. Ann Intensive Care 2020;10(1):137. 10.1186/s13613-020-00753-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Deep A, Saxena R, Jose B. Acute kidney injury in children with chronic liver disease. Pediatr Nephrol 2019;34(1):45–59. 10.1007/s00467-018-3893-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Vijay P, Lal BB, Sood V, et al. Cystatin C: best biomarker for acute kidney injury and estimation of glomerular filtration rate in childhood cirrhosis. Eur J Pediatr 2021;180(11):3287–3295. 10.1007/s00431-021-04076-1. [DOI] [PubMed] [Google Scholar]
- [222].Saxena R, Anand A, Deep A. Use of terlipressin in critically ill children with liver disease. BMC Nephrol 2020;21(1):360. 10.1186/s12882-020-01914-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Gorgis NM, Kennedy C, Lam F, et al. Clinical consequences of cardiomyopathy in children with biliary atresia requiring liver transplantation. Hepatology 2019;69(3):1206–1218. 10.1002/hep.30204. [DOI] [PubMed] [Google Scholar]
- [224].Virk MK, Mian MUM, Bashir DA, et al. Elevated bile acids are associated with left ventricular structural changes in biliary atresia. Hepatol Commun 2023;7(5). 10.1097/HC9.0000000000000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Ul Abideen Z, Mahmud SN, Salih M, et al. Contrast-induced acute kidney injury in patients with liver cirrhosis: a retrospective analysis. Cureus 2018;10(5):e2707. 10.7759/cureus.2707 (In eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Filomia R, Maimone S, Caccamo G, et al. Acute kidney injury in cirrhotic patients undergoing contrast-enhanced computed tomography. Medicine (Baltimore) 2016;95(38):e4836. 10.1097/MD.0000000000004836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Campion D, Ponzo P, Risso A, et al. A prospective, multicenter, three-cohort study evaluating contrast-induced acute kidney injury (CI-AKI) in patients with cirrhosis. J Hepatol 2023. 10.1016/j.jhep.2023.10.010. [DOI] [PubMed] [Google Scholar]
- [228].Guevara M, Fernandez-Esparrach G, Alessandria C, et al. Effects of contrast media on renal function in patients with cirrhosis: a prospective study. Hepatology 2004;40(3):646–651. 10.1002/hep.20373. [DOI] [PubMed] [Google Scholar]
- [229].Lodhia N, Kader M, Mayes T, et al. Risk of contrast-induced nephropathy in hospitalized patients with cirrhosis. World J Gastroenterol 2009;15(12):1459–1464. 10.3748/wjg.15.1459 (In eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Tergast TL, Schulte B, Griemsmann M, et al. Application of CT contrast medium is not associated with an increased risk for acute kidney injury in patients with decompensated cirrhosis. Aliment Pharmacol Ther 2023;57(1):136–145. 10.1111/apt.17289. [DOI] [PubMed] [Google Scholar]
- [231].Schultz J, Andersen A, Lyhne MD, et al. Terlipressin increases systemic and lowers pulmonary arterial pressure in experimental acute pulmonary embolism. Crit Care Med 2020;48(4):e308–e315. 10.1097/CCM.0000000000004243. [DOI] [PubMed] [Google Scholar]
- [232].Altintas E, Akkus N, Gen R, et al. Effects of terlipressin on systolic pulmonary artery pressure of patients with liver cirrhosis: an echocardio-graphic assessment. World J Gastroenterol 2004;10(15):2278–2280. 10.3748/wjg.v10.i15.2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Kalambokis GN, Pappas K, Tsianos EV. Differential effects of terlipressin on pulmonary and systemic hemodynamics in patients with cirrhosis and pulmonary hypertension: an echo study. Angiology 2012;63(3):199–205. 10.1177/0003319711411704. [DOI] [PubMed] [Google Scholar]
- [234].Abdelazziz MM, Abdelhamid HM. Terlipressin versus norepinephrine to prevent milrinone-induced systemic vascular hypotension in cardiac surgery patient with pulmonary hypertension. Ann Card Anaesth 2019;22(2):136–142. 10.4103/aca.ACA_83_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


