Abstract
Pulmonary hypertension (PH) adds a substantial disease burden, including higher mortality, when associated with interstitial lung disease (ILD), a severe, chronic, progressive condition. Yet little is known of the lived experiences, perspectives, priorities, and viewpoints of patients and carers living with PH‐ILD. The Voice of the Patient meeting at the center of this qualitative research study aims to provide these difficult‐to‐obtain insights from a European perspective for the first time. The multistakeholder approach brought together four PH‐ILD patients, three primary caregivers, two patient associations, clinical experts, sponsor representatives, and a facilitator. Of the six major themes identified in the thematic analysis, symptoms, and physical limitations were the most impactful. Shortness of breath was the most bothersome symptom affecting patients daily. Further symptoms included fatigue, cough, dizziness, syncope, edema, and palpitations. Physical limitations focused on reduced mobility, impacting patients’ ability to perform daily tasks, hobbies, sports, and to enjoy travel. Existing antifibrotic and pulmonary arterial hypertension‐targeted treatments were perceived as beneficial. However, despite advances in treatment, severe disease burdens and high unmet medical needs persist from the perspectives of patients. Most meaningful to patients’ daily wellbeing was supplemental oxygen, enabling greater mobility. Patients and carers reported difficulties and barriers in navigating the healthcare system and obtaining adequate information to reduce their considerable uncertainties, documenting the substantial challenges that rare and complex conditions such as PH‐ILD pose for routine clinical practice beyond PH expert centers and indicating an urgent need for high‐quality patient‐ and clinician‐directed information to support patient‐centered care.
Keywords: health‐related quality of life, interstitial lung disease, patient experience data, patient‐involvement, pulmonary hypertension
INTRODUCTION
Pulmonary hypertension (PH) is a condition characterized by elevated pressure in the pulmonary vasculature, which may be associated with interstitial lung diseases (ILDs), including idiopathic pulmonary fibrosis (IPF), and classified as Group 3 PH. 1 , 2 Nonsevere PH (pulmonary vascular resistance, PVR, of ≤5 wood units, WU) is common and reported in up to 86% of patients in lung transplant candidates, 3 while severe PH (PVR > 5 WU) occurs in approximately 9% of patients with advanced ILD. 4 Notably, even nonsevere PH negatively impacts symptoms, exacerbations, and survival in ILD. 5 , 6 , 7 A recent real‐world study found mortality correlated significantly with mPAP, and survival in ILD patients was worse for any PH level than in patients without PH (3‐year survival: No PH 58%, Mild PH 32%, Moderate PH 28%, Severe PH 33%, p = 0.002). 6
As often in rare conditions, there is a lack of standardized approaches to the diagnosis of PH‐ILD in routine clinical practice, leading to delays between the manifestation of symptoms and correct diagnosis. 8 , 9 A recent Delphi expert panel provided several indicators for suspicion of PH in patients with ILD, according to symptoms and signs, findings on chest CT scan or other imaging, abnormalities in pulse oximetry, elevations in brain natriuretic peptide (BNP) or N‐terminal pro‐brain natriuretic peptide (NT‐proBNP), and unexplained worsening in gas exchange or 6‐min walk distance. 8 Transthoracic echocardiography is the most widely used noninvasive tool to assess PH, and right heart catheterization (RHC) is essential for confirming diagnosis, but the 2022 European Society of Cardiology/European Respiratory Society (ESC/ERS) guidelines on PH only indicate it when better phenotyping or an actionable treatment option is available. 1 For this reason, outside of PH expert centers, RHC remains infrequently indicated. 6 , 8 , 9
While inhaled Treprostinil is approved in the United States for the treatment of PH‐ILD based on the INCREASE study, there are currently no approved treatments available in Europe. 9 , 10 The 2022 ESC/ERS guideline recommends an individualized approach to treatment: PDE‐5 inhibitors may be considered in patients with severe PH‐ILD, and inhaled Treprostinil on a case‐by‐case basis regardless of severity. 1 The lack of satisfactory treatments, poorer prognosis, and decreased survival has led to the recognition of a high unmet medical need in PH‐ILD, and to a great interest in innovative treatments. 9
Directly involving patients in drug development to generate meaningful, high‐quality patient experience data (PED) is key in ensuring that innovative therapies effectively address patients’ unmet needs. In its Patient‐Focused Drug Development (PFDD) initiative, the US Food and Drug Administration (FDA) defines PED as quantitative data generated with clinical outcome assessments (COAs), including patient‐reported outcomes (PRO), as well as qualitative and patient preference research. 11 The European Medicines Agency (EMA) supports the collection and use of evidence‐based PED for benefit‐risk decision‐making, 12 and the interest of payers and health technology assessment (HTA) bodies is equally increasing. 13
In Idiopathic Pulmonary Fibrosis (IPF), patients report that the aspects most important to them are not routinely captured by clinical studies, despite trial protocols including PRO measures such as the St George's Respiratory Questionnaire, Living with Pulmonary Fibrosis, Short Form 36, and the EQ‐5D. 14 , 15 , 16 A lack of specificity and validity results in studies often not detecting a clinically meaningful PRO‐score difference in response to therapy, supporting the need for further research on the health‐related quality of life (HRQoL) impacts in PH‐ILD. 14 , 15 Qualitative research, such as we employ here, provides an alternative where quantitative analyses have limitations. 11 , 12 , 14 , 15
FDA's PFDD gathered patients’ perspectives in Voice of the Patient (VOP) meetings, which included IPF and in pulmonary arterial hypertension. 16 , 17 DuBrock et al. undertook qualitative interviews with three clinical experts and 14 individuals with PH‐ILD in the United States. 18 Yet little is known of the real‐life experience of Group‐3 PH patients from a European perspective. We aimed to address this gap in the PED literature with a European PH‐ILD VOP meeting, which systematically collected and analyzed qualitative insights on:
the experiences, perspectives, priorities, and what matters most of European patients and carers living with PH‐ILD, providing context and understanding of the burden of disease and unmet medical needs
the diagnostic and therapeutic journey of PH‐ILD patients in routine practice, and similarities and differences between the US and European experience
patient preferences and priorities for a potential future treatment in PH‐ILD.
METHODS
The European PH‐ILD VOP meeting was designed and implemented in full adherence of the principles developed by the European Patient Focused Medicines Development initiative (PFMD) and published in the Patient Engagement Management (PEM) Suite, which includes best practices, qualitative guidance, patient remuneration and fair market value, legal and contractual tools, and practical how‐to guides. 19 Processes were adapted to national patient engagement guidelines of the countries of residence of the participants and Spanish guidance as the country in which the meeting's sponsor is based. 20
The meeting emulated the approach of the FDA's PFDD VOP meetings to systematically gather patients’ perspectives. 16 , 17 This format provides the opportunity to hear directly from patients, patient caregivers, and patient representatives about their lived experiences with PH‐ILD. Discussion focussed on two main groups of topics: first, disease symptoms and daily impacts that matter most to patients, and second, perspectives on current and potential future approaches to diagnosis and treatment.
The importance of carers is increasingly recognized. This particularly includes the impacts experienced by these individuals as a result of supporting patients which are affected by severe and chronic diseases, and that often require complex, time‐consuming care. 16 , 21 Therefore, we included individuals with experience functioning as the primary caregiver for patients living with PH‐ILD (in practice patient and carer pairs).
For each topic, the participating patients and caregivers first shared their perspectives to begin the dialog, followed by a facilitated discussion inviting comments and follow‐up questions from the other VOP participants, which brought together representatives of the Pulmonary Hypertension Association Europe (PHA Europe) 22 and the European Pulmonary Fibrosis Federation (EU‐PFF), 23 clinical experts, pharmaceutical industry experts nominated by the sponsor, and the meeting facilitator. 16 , 17
To address the often‐limited mobility and difficulties with travel of participants, for example due to the need for ambulatory oxygen, the VOP meeting was held virtually through a web‐enabled video application. Recent research supports the validity and methodologic rigor of using virtual patient meetings, yielding similar insights as compared to traditional in‐person formats. 24
Participating patients and carers executed a “Participant Consultancy Agreement,” which outlined their role and responsibilities, their rights to privacy and confidentiality, their fair market compensation, as well as a signed consent form. The agreement and participants’ remuneration were based on the WECAN and PFMD “Guiding Principles on Reasonable Agreements between Patient Advocates and Pharmaceutical Companies.” 25
Participants
Patient and caregiver candidates for the PH‐ILD VOP meeting were identified and approached by the participating patient associations, PHA Europe and EU‐PFF. A participant information leaflet outlined the aims and topics of the VOP meeting, role, time commitment, rights, and an invitation to contact the meeting coordinator (no personal identifying data was transmitted directly). Twenty‐minute screening calls were scheduled via email to ascertain candidates’ eligibility to participate in the meeting based on the following inclusion criteria:
Male and female participants, 18 years of age and older.
Confirmed diagnosis of PH‐ILD 6 months before the meeting (the cutoff of 6 months enables participants to share an in‐depth lived experience).
Comorbidities were included, with the sole exception of PH‐COPD.
Spouses, relatives, or close friends of patients functioning as primary carer.
In an overall state of health so the participant can responsibly take part in a 3‐h virtual meeting from their home and communicate effectively.
Execution of a Participant Agreement, including signed informed consent.
Screening calls were conducted by a single researcher (D. S.) with a formal questionnaire, which was developed with the support of clinical experts, and relied on self‐reporting without further review of patient medical records. Any apparent clinical discrepancies reported by the candidates were probed and clarified during the calls. Patients with a diagnosis of COPD were excluded. A total of 10 patients and five carers were screened, of which five patients and two related caregivers had to be excluded. Further details of the exclusion criteria are provided in Table 1.
Table 1.
Characteristics of the VOP meeting participants.
| Patient and caregiver characteristics | Totals |
|---|---|
| Participant eligibility, total eligible/total screened | |
| Patients | 4/10 |
| Caregivers | 3/5 |
| Exclusion criteria (multiple criteria provided) | |
| Diagnosis of PH‐ILD not confirmed (patients and caregivers) | 7 |
| Patient's state of health too severe | 2 |
| Informed consent not executed | 1 |
| Sex, n | |
| Patients, male | 2 |
| Patients, female | 2 |
| Caregivers, male | 2 |
| Caregivers, female | 1 |
| Age, years mean (range) | |
| Patients | 58.8 (33–80) |
| Caregivers | 39.7 (24–62) |
| Country of residence, n | |
| United Kingdom | 5 |
| Slovakia | 2 |
| Diagnosis of PH‐ILD, years mean (range) | |
| Time since diagnosis | 2.0 (0.5–6.0) |
| ILD subtype, n of 4 patients | |
| Idiopathic pulmonary fibrosis | 3/4 |
| Systemic sclerosis | 1/4 |
| Antifibrotic treatment, n of 4 patients | |
| Prifenidone | 1/4 |
| Prifenidone discontinued (drug–drug interactions) | 1/4 |
| Prifendione prescribed, waiting for insurance approval | 1/4 |
| Nintedanib | 0/4 |
| PH therapy, n of 4 patients | |
| Oral PDE5i, single therapy | 1/4 |
| Oral PDE5i/ERA dual therapy | 1/4 |
| PDE5i/ERA/prostacyclin analog (oral/parenteral) triple therapy | 1/4 |
| No PH therapy | 1/4 |
| Supplemental oxygen, n of 4 patients | |
| Oxygen at exertion (ambulatory) | 4/4 |
| Oxygen at rest | 2/4 |
| Comorbidities, n of 4 patients | |
| Depression | 1/4 |
| Mixed connective tissue disease (MCTD) | 1/4 |
| Lupus and scleroderma | 1/4 |
| Basal cell carcinoma | 1/4 |
| Pulmonary embolism | 1/4 |
| Concomitant medications, n of 4 patients | |
| Immunosuppressant | 1/4 |
| Analgesics/morphine patches | 1/4 |
| Omeprazole | 1/4 |
| Corticosteroid | 1/4 |
| Warfarin | 1/4 |
Note: All characteristics are provided exactly as reported by the participants, the clinical accuracy and completeness of patient‐reported medical details may vary.
Abbreviations: ERA, endothelin receptor antagonist; ILD, interstitial lung disease; PDE5i, phosphodiesterase 5 inhibitor; PH, pulmonary hypertension; VOP, Voice of the Patient.
Four patients and three primary caregivers attended the virtual meeting and comprised the European PH‐ILD VOP panel. Patients reported prescriptions of antifibrotic medication as well as off‐label treatment with pulmonary arterial hypertension (PAH) drugs. Patients routinely relied on supplemental oxygen, two of four also requiring oxygen at rest. Two of the three primary caregivers were spouses of patients, and in one case the son, resulting in a large age range (Table 1).
Qualitative analysis
Phenomenology is the qualitative framework we employed to gain an in‐depth understanding of the lived experiences of people living with PH‐ILD and the meanings that these individuals perceive of those experiences. 21 , 26 , 27
Open coding thematic analysis was adopted to analyze the unstructured data from the original video recording and the verbatim meeting transcript and to identify those themes most important to patients and caregivers. In an open coding technique, the data is broken down into first‐level concepts, or major themes, and second‐level categories, or sub‐themes. 21 , 26 , 27 , 28 , 29 For example, participants discussed their most bothersome symptoms in detail. “Most frequently reported symptoms of PH‐ILD” therefore became a concept or major theme, and the related symptoms became categories or sub‐themes. The mapping process from coded statements, which are verbatim quotes by the participants and reported without any interpretation, to sub‐themes and then to major theme(s) is shown in Figure 1 (see the supplemental materials for the mapping of the further major‐ and sub‐themes).
Figure 1.
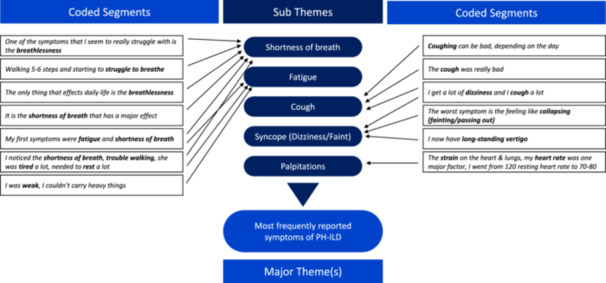
Thematic analysis—mapping of coded statements into major‐ and sub‐themes. ILD, interstitial lung disease; PH, pulmonary hypertension.
With a larger number of transcripts, qualitative data analysis software such as NVivo 14 is used to systematically code unstructured data. 21 , 27 , 29 Given the highly condensed data from the video recording and transcript, we elected to manually code participant statements, emphasizing the “human touch” of the interactions during the 3‐h VOP meeting. Analysis of the qualitative data was conducted in a systematic manner by three members of the research team. The initial thematic analysis was performed by D. S., who as the facilitator of the VOP meeting was familiar with the data and therefore able to gain an immediate impression of the thematic framework, as a first analytic step. 21 , 27 , 29 The initial analysis was independently reviewed by researchers L. P. and G. K., who re‐assessed the proposed major‐ and sub‐themes. Identified discrepancies were reviewed across researchers and resolved through examination of the source material and discussion. Once this resolution was completed, a theme map of all identified major‐themes and sub‐themes and their relationships was created (Figure 2). Comparison between the European lived experience and the US‐based PH‐ILD, IPF, and PAH populations was performed based on the major theme reported in all qualitative publications: most frequently reported symptoms. 16 , 17 , 18
Figure 2.
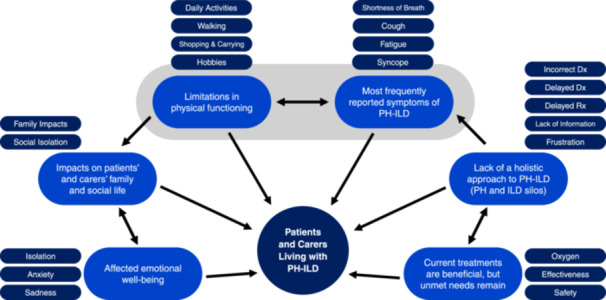
Identified major‐ and sub‐themes most meaningful to PH‐ILD patients and carers. Dx, diagnosis; ILD, interstitial lung disease; PH, pulmonary hypertension; Rx, treatment.
RESULTS
Qualitative thematic analysis of the European PH‐ILD VOP meeting identified six major themes most important to the participants: (1) most frequently reported symptoms of PH‐ILD, (2) limitations in physical functioning, (3) impacts on patients’ and carers’ family and social life, (4) affected emotional well‐being, (5) current treatments are beneficial, but unmet needs remain, and (6) the lack of a holistic approach to PH‐ILD (PH and ILD silos). The resulting thematic map of the major‐ and sub‐themes and their relationships is shown in Figure 2.
The symptoms of PH‐ILD, which patients and—by extension of their familiarity with the patients—the carers unanimously reported experiencing, were the first and primary topic of discussion. This included shortness of breath, fatigue, cough, dizziness, syncope (reported by patients as feeling faint), edema (reported by patients as swelling), and palpitations. Of the symptoms affecting patients daily, shortness of breath was identified as the most burdensome:
One of the symptoms that I seem to really struggle with is the breathlessness, but also the chest tightness caused through PH, it's not just being out of breath, it is a stifle that I feel. People that are unaware of the disease assume that oxygen is something that loosens it, but it doesn't.
It is the shortness of breath that has a major effect on my ability to do tasks. I've always been very active; it is just a struggle now to do a lot of things.
It seems that the breathlessness is very much a common factor, pretty much any activity will make me breathless.
My first symptoms were fatigue, and shortness of breath, I was weak, I couldn't carry heavy things, but the worst symptom is the feeling like collapsing.
Carer: Simple things in regard to the PH, like walking 5‐6 steps and starting to struggle to breathe, and it's gotten worse over time, as you would expect.
I get a lot of dizziness and I cough a lot, which is part of the IPF. I've had pitting oedema in my legs, which my GP was concerned about, he prescribed me compression stockings to stop the swelling.
The next major topic prompting discussion were limitations in physical function, resulting in patients having to rest or even being unable to perform daily activities and those things most important to them, such as home do it yourself improvements, traveling, hobbies, and sports. This was perceived as closely related to the severity of symptoms, particularly shortness of breath. There was an animated back and forth between participants on coping mechanisms and how to live with day‐to‐day limitations:
I love travelling, I'm a foodie by heart, I've gone from walking slowly to wheelchair in a year, and obviously due to accessibility with the wheelchair and the oxygen, it's been rather difficult, and unfortunately I'm a 33‐year‐old woman who spends now most of my time at home.
Even stuff like putting up a blind at the windows, having to drill holes and go up a ladder, it takes it out of me. It takes me a lot longer to do things and I have to rest.
I couldn't walk in a supermarket, even with oxygen, I think that'd be really difficult for me.
Before I got sick, I danced Oriental and Egyptian dance, going to the competitions with other women, dance is a very nice sport. I liked going to the mountains, hiking, going to the gym, it is now very hard for me.
The next major themes pertained to impacts on patients’ and carers’ family and social life and their emotional well‐being. The identified sub‐topics included personal isolation as well as social isolation impacting the entire family, anxiety and fear, sadness, particularly also expressed by the carers, and financial burdens. This was a very frank and open discussion given the virtual format and the fact that patients and carers did not know each other or most of the other participants beforehand:
It takes a long time now to plan something, especially if you have these needs now with the oxygen and the wheelchair. I don't have many friends anymore, my social circle kind of drifted off, and that has been a drastic change in my life.
People don't understand the limitations and no matter how many times you explain, it usually falls on deaf ears. Others cannot understand your disease, and what the disease is doing with your body.
My life is changed, I had to change my activities, I like a smaller group of people. I like to go to the theatre, but I plan this very well, where I go, whom I go with, where I park my car and so on.” The carer: “Her social circle got a little smaller and it is sad that these things had to change.
We used to travel a lot. We'd fly to European cities. And now I'm just really scared about doing that. I saw the complications of taking oxygen with me, and what would happen if I was abroad and I had an exacerbation. If we're going to stay in a hotel, it must have a lift, there's no way I can do stairs.
And I haven't been intimate with my wife for a long time, because we're both so worried that I have lack of breath.
Thankfully we have got the money, and we can afford to pay people to do things. We got a little mobility scooter so I can at least go out with my wife.
For the second major topic of the VOP meeting, discussion turned to patients’ and carers’ perspectives on current and potential future approaches to diagnosis and treatment. Current therapies were regarded as helpful, particularly oxygen, which patients report enables greater physical activity and increases mobility. Patients and carers stated being grateful for any support and improvement available, although there were no illusions regarding a reversal or cure of the condition. Despite treatment benefits, it was clear from the perspective of all participants that the burden of disease remains high overall and that unmet medical needs persist. Expectations for future PH‐ILD treatments—a cure aside—therefore concentrated on an improvement of patients’ quality of life, particularly related to disease symptoms and physical functioning. Adverse events were experienced as manageable, even though therapy switches and discontinuations were reported due to side effects and drug‐drug interactions:
For the PH I was started off with sildenafil and macitentan, but due to the side effects I was having I was put on tadalafil instead, which has been a bit better.
In terms of the antifibrotic medication side effects, there is a huge, long list, but I don't suffer from the skin rash in the sun and it hasn't affected my bowels either, The biggest thing is a loss of appetite – I don't know if that is the condition or the drugs. Initially it made me feel nauseated, but that's not been so bad recently.
I had to stop taking pirfenidone because it was interfering with the warfarin.
I was put on full‐time oxygen, so I'm now on 3 litres at rest, and 6 to 8 litres on exertion, which has helped a lot with the heartbeat and in taking some of that strain from the lungs and the heart.
I would say the oxygen really helped, the coughing was way less and maybe even the shortness of breath.
So, if I'm doing something, I'll use that liquid oxygen which is a continuous flow at 6 L/min and that does help but it is not a cure.” The carer: “He does use oxygen to move around, but he really couldn't walk around without it, the answer is it is very beneficial.
I don't mind taking the drugs, or whatever kind of therapy it is, it's a small price to pay if something can improve my quality of life and the length of my life.
The sixth and final major theme, which we summarized as “Lack of a holistic approach to PH‐ILD (PH and ILD silos),” was an unforeseen outcome. Originally we aimed to discuss patients’ experiences with their diagnostic journeys. This, however, turned into a documentation of the struggles and barriers patients and carers experience when navigating the healthcare system and seeking adequate information. This was perceived as resulting from a lack of knowledge and understanding of PH‐ILD as a rare form of PH, particularly among general and emergency room practitioners, as well as a lack of coordination between the different medical specialties involved. All this led to diagnostic odysseys which resulted in delayed or incorrect diagnoses, and delays in treatment. Most meaningfully, given the severity and progressive nature of PH‐ILD, these experiences left patients and carers feeling frustrated, insecure, ill‐informed, and confused about their condition and symptoms, planned diagnostic measures, and follow‐ups and regarding their treatments. In contrast, one patient reported a very positive healthcare experience:
I saw a consultant who looked at the CT‐scan and said I had IPF, and I've got 3 to 5 years to live. There was never any mention of PH at that point. I was shocked by the brutality of it all, to be told so bluntly.
It was near 18 months from first presenting at the GP with shortness of breath to getting the antifibrotic drug, which I'm very angry about, that it took that amount of time. Has that shortened my life by 18 months?
The IPF was not diagnosed at the two visits to the local hospital but when I saw the other (respiratory) consultant and that was the original diagnosis in 2004. The original diagnosis was based purely on the x‐rays but then confirmed by various CT scans. PH was never mentioned, that only came up when I went to a local centre of excellence in April of this year (2022) and was finally diagnosed following RHC.
I always thought I just had IPF, and it is difficult to know which one is contributing to the other and making it worse. I'm confused by the relationship between the PH and the IPF.
I need to understand the relationship between the IPF and the PH, and I'd like to have more follow‐up from the hospitals on the condition, I haven't got another follow‐up appointment, I don't know when that will be. I think they should be looking after me better, whether it is 6‐months reviews or whatever.
The GPs don't really know anything about this, he said you need to go to the emergency room, and at the local hospital, they didn't know anything, they said I have a chest infection and sent me home with steroids and antibiotics.
My GP turned around and said, you are one of my complex patients, you need to know more about your body than I do. That puts the responsibility on me.
My therapy was very well coordinated with my cardiologist, rheumatologist and pneumologist. I think my doctors treat me very well and I'm very thankful to them, they are very professional. I think I take good drugs.
Figure 3 compares the lived experience of PH‐ILD, PAH and IPF patients in terms of most frequently reported symptoms between our qualitative analysis, the research by DuBrock et al., 18 and the two FDA VOP meetings. 16 , 17 It should be noted that many of the currently available PAH‐targeted therapies prescribed off‐label in PH‐ILD, as well as antifibrotic therapy, which constitutes the current standard of care in ILD/IPF, were not yet approved at the time of the FDA meetings. 1 , 9 , 10 These novel drug approvals, which collectively represent significant advances in the treatment of these conditions, 9 , 10 did, however, not appear have a substantial impact on the type and ranking of the symptoms most frequently reported by patients. These remain remarkably similar across the three studied populations of PH‐ILD, PAH, and IPF, and across nearly a decade of time, apart from cough in PAH, which is ranked lower as compared with the lung conditions. This mirrors the discussion of currently prescribed treatments in Europe in our VOP meeting, where treatment benefits are appreciated, but high unmet medical needs continue to persist from the perspectives of patients and carers.
Figure 3.

Comparison of the most frequently reported symptoms—PH‐ILD, PAH and IPF. ILD, interstitial lung disease; IPF, idiopathic pulmonary fibrosis; PAH, pulmonary arterial hypertension; PH, pulmonary hypertension. *DuBrock et al. 18 **FDA 17 ***FDA. 16
DISCUSSION
For the first time in Europe, we provide an in‐depth understanding of patients’ and carers’ lived experiences of PH associated with ILD and describe what matters most to these individuals, by systematically gathering and thematically analyzing their perspectives, needs, and priorities, with an approach emulated from the FDA's PFDD VOP meetings. 16 , 17 The multistakeholder approach, including patients and carers, representatives of patient associations, clinical experts, and pharmaceutical industry experts facilitated a comprehensive and broad discussion. Prioritizing patients and caregivers to first share their perspectives and to highlight those aspects most important to them focussed the scope of discussion. The virtual format allowed patients with advanced disease and oxygen therapy to attend the meeting from the comfort of their own home. The relative anonymity of participants seemed to result in a richer discussion of sensitive topics as compared with a traditional in‐person format. 24
The life‐altering effect of PH‐ILD impacts every aspect of patients’ lives. We identified six major themes (Figure 2); most meaningful among these were symptoms and physical functioning. Patients reported shortness of breath as the most bothersome symptom which affects them daily. Further symptoms included fatigue, cough, dizziness, syncope, edema, and palpitations. The most prominent physical limitation was reduced mobility, which affects patients’ ability to perform daily tasks, such as walking, climbing stairs and shopping, as well as restricting those activities most important to them, including travel, hobbies, and sports. In patients’ and carers’ perception, these physical limitations are closely related to the severity of breathlessness, explaining why shortness of breath is the most bothersome symptom, and why participants unanimously reported oxygen as being essential to their well‐being, enabling greater activity and mobility. At the same time, oxygen was not considered a disease modifying or curative treatment, and one patient found no relief from breathlessness with it. PAH‐targeted and antifibrotic medications were reported as beneficial and appreciated, and adverse events as manageable. Despite these treatments, there was a strong consensus that high unmet medical needs remain, with a preference for an improvement of symptoms and functionality when discussing future treatments of PH‐ILD.
Understanding the crucial importance of the complex shortness of breath “physical limitation” oxygen can help inform ongoing research into those therapeutic approaches best suited to significantly improve the symptoms and the health‐related quality of life (HRQoL) of patients living with PH‐ILD, and how to collect, analyze and report these patient‐relevant data meaningfully. 14 , 15 , 16 , 17 , 18 , 21 Our qualitative insights suggest a rethink regarding the PRO measures typically selected for clinical trials. Rather than broad measures of HRQoL, it may be preferable to focus on more proximal concepts such as validated symptom scores, including dyspnea, cough, and fatigue, in combination with patient‐ and observer‐reported assessments of functionality and activities of daily living. Ultimately this may warrant the development and validation of a PH‐ILD‐specific symptom/functionality measure. 14 , 16 , 21 These measures could function as key secondary endpoints in randomized trials, including a pre‐specification of clinically meaningful changes in scores in response to treatment. These recommendations are aligned with the most recent FDA PFDD guidance on “incorporating clinical outcome assessments into endpoints for regulatory decision‐making.” 30
Our VOP findings correspond with those of the US‐based PH‐ILD population analyzed by DuBrock et al. (n = 14). 18 Shortness of breath, fatigue, cough, and swelling were the most frequently reported symptoms of PH‐ILD, and shortness of breath was also identified as the single most bothersome symptom for most US patients (71.4%). Interview participants described experiencing impacts related to PH symptoms, including limitations in activities of daily living and impacts on physical functioning, family life, social life, as well as emotional impacts. This supports the conclusion that persons living with PH‐ILD in the United States and Europe have comparable lived experiences and unmet medical needs. Figure 3 documents the similarities between the most frequently reported symptoms in PH‐ILD, PAH, and IPF populations.
An unforeseen topic of the VOP meeting was the difficulties participants described in navigating the healthcare system and obtaining adequate information on the condition, symptoms, diagnosis, and treatment of PH‐ILD. This is experienced as delayed or incorrect diagnoses and treatments, suboptimal coordination between specialists involved in PH and ILD care, as well as GPs and emergency rooms often lacking knowledge of the disease. These reports echo a growing body of literature documenting barriers to receiving expert care for individuals living with complex rare diseases. 31 , 32 , 33 The diagnostic odysseys rare disease patients go through, and the negative impact on outcomes are equally well documented. 32 , 33
The absence of timely and adequate information resulted in patients reporting marked confusion in understanding the relationship between their PH and the underlying lung disease, how their symptoms are related to which diagnosis, and to prescribed therapy, very similar to the US PH‐ILD patients interviewed by DuBrock et al. 18
These findings indicate an urgent need for patient‐ and clinician‐directed education: the participating patient associations (PHA Europe and EU‐PFF) envisage joint educational initiatives to disseminate best practices for diagnosis and treatment, including the 2022 ESC/ERS guidelines, highlighting patient needs in clinical practice, a faster referral to PH expert centers and a multidisciplinary approach. 1 , 6 , 31 , 32 , 33 The European Reference Networks for Rare Diseases (ERN‐Lung), 34 established PH and PH‐ILD registries such as the Spanish Registry of Pulmonary Hypertension Associated with Respiratory Disease (REHAR) 6 and the Comparative, Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension (COMPERA), 7 , 35 as well as the pharmaceutical industry could partner with patient associations to advance these goals.
There were remarkably few differences in perspectives between patients and their carers during our VOP meeting. Overgaard et al., who interviewed 24 couples together for their qualitative research in IPF also reported few disagreements. 21 Qualitative research in other conditions, however, has reported differing viewpoints between carers and patients, 26 and PHA Europe has found that carers speak more freely about their challenges when patients are not present in surveys. Given the life‐altering impact of PH‐ILD, mutual support and agreement between patients and carers is likely to be of substantial importance and may represent an interesting topic for future research. 16 , 21 , 31 , 32 , 33
This qualitative thematic analysis has several limitations, notably the limited number of patient and caregiver participants, owing to challenges in identifying screening candidates and in reliably confirming a PH‐ILD diagnosis based on patients’ statements. Candidates with more advanced disease caused concerns that they may not be able to participate to the 3‐h VOP meeting, despite the virtual format. Collaborating with established registries may provide access to patients with a confirmed diagnosis, representing a potential approach for future qualitative research involving complex, small, heterogeneous populations, which are notoriously difficult to recruit. Because of the limited cohort, IPF was overrepresented; while IPF represents the largest PH‐ILD subtype, 36 future works should include a greater proportion of other aetiologies. Similarly, the number of European countries represented is limited, the United Kingdom being the main country of residence. Access to healthcare and patients’ experiences may differ across Europe, therefore the barriers to access and lack of information on the disease reported here are not generalizable and should be evaluated in the specific country or region.
In conclusion, this study reports on the experiences of people living with PH‐ILD in Europe, which are similar to those of US PH‐ILD, 18 PAH, and IPF populations. Additional qualitative research to expand on these findings is warranted to support the patient‐centered design of future clinical trials, observational studies, and registries in PH‐ILD, so that research can effectively incorporate what matters most to patients and carers. 6 , 11 , 12 , 14 , 15 , 16 , 17 , 21 , 30 , 31 , 32 , 33
AUTHOR CONTRIBUTIONS
Claudia Roca Herms, Gabriela Silvina Bacchini Jeanneret, Nuria Gonzalez‐Rojas Guix, Miriam Fernandez Delgado, and David Schwicker conceptualized and designed the VOP meeting. Héctor Gálvez García and Melquiades Calzado Vinardell led the meeting organization. Hall Skaara and Steve Jones facilitated the identification and recruitment of the meeting participants. David Schwicker performed the screening calls and recruited the participants, developed the qualitative methodology, and performed the initial thematic analysis. Lucilla Piccari and Gabor Kovacs reviewed and finalized the thematic analysis; all authors except Gabor Kovacs participated in the VOP meeting; all authors contributed to the preparation of the manuscript.
CONFLICTS OF INTEREST STATEMENT
H. S. and S. J. declare no conflicts of interest; C. R. H., G. S. B. J., N. G. G., M. F. D., H. G. G. and M. C. V. are employees of Ferrer; L. P. reports grants from Janssen and Ferrer, lecture honoraria from Janssen, Ferrer, MSD and United Therapeutics, participation on advisory boards with Janssen, Ferrer and United Therapeutics, and travel support from Janssen, Ferrer and MSD, outside the submitted work; G. K. reports personal fees and nonfinancial support from Johnson & Johnson, Bayer, GSK, MSD, Boehringer Ingelheim, Chiesi, Vitalaire, Ferrer, AOP and Astra Zeneca within the last 3 years; D. S. is a consultant for Ferrer, Pierre Fabre, Galecto, Bridge Biotherapeutics, and is a shareholder of Moderna and argenx.
ETHICS STATEMENT
The VOP meeting was deemed exempt from ethical review board approval based on interpretation of the principles developed by the European Patient Focused Medicines Development initiative (PFMD).
Supporting information
Supporting information.
ACKNOWLEDGMENTS
The authors would like to gratefully acknowledge the valuable contributions of the participating patients and caregivers who openly shared their lived experiences, without whom this VOP meeting and qualitative research would have been impossible to carry out. We thank Kim Adams for reviewing the text of the manuscript. Funding for this research and article was provided by Ferrer Internacional S.A., Barcelona, Spain.
Piccari L, Kovacs G, Jones S, Skaara H, Herms CR, Jeanneret GSB, Vinardell MC, Guix NG‐R, Delgado MF, García HG, Schwicker D. The European Voice of the Patient living with pulmonary hypertension associated with interstitial lung disease: Diagnosis, symptoms, impacts, and treatments. Pulm Circ. 2024;14:e12405. 10.1002/pul2.12405
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, Carlsen J, Coats AJS, Escribano‐Subias P, Ferrari P, Ferreira DS, Ghofrani HA, Giannakoulas G, Kiely DG, Mayer E, Meszaros G, Nagavci B, Olsson KM, Pepke‐Zaba J, Quint JK, Rådegran G, Simonneau G, Sitbon O, Tonia T, Toshner M, Vachiery JL, Vonk Noordegraaf A, Delcroix M, Rosenkranz S. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2023;61:Article 2200879. 10.1183/13993003.00879-2022 [DOI] [PubMed] [Google Scholar]
- 2. Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, Williams PG, Souza R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53:1801913. 10.1183/13993003.01913-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nathan SD, Shlobin OA, Ahmad S, Koch J, Barnett SD, Ad N, Burton N, Leslie K. Serial development of pulmonary hypertension in patients with idiopathic pulmonary fibrosis. Respiration. 2008;76(3):288–294. 10.1159/000114246 [DOI] [PubMed] [Google Scholar]
- 4. Shorr AF, Wainright JL, Cors CS, Lettieri CJ, Nathan SD. Pulmonary hypertension in patients with pulmonary fibrosis awaiting lung transplant. Eur Respir J. 2007;30:715–721. 10.1183/09031936.00107206 [DOI] [PubMed] [Google Scholar]
- 5. Nemoto K, Oh‐ishi S, Akiyama T, Yabuuchi Y, Goto H, Nonaka M, Sasatani Y, Tachi H, Arai N, Ishikawa H, Hyodo K, Hase I, Miura Y, Takaku T, Hayashihara K, Saito T. Borderline pulmonary hypertension is associated with exercise intolerance and increased risk for acute exacerbation in patients with interstitial lung disease. BMC Pulm Med. 2019;19:167. 10.1186/s12890-019-0932-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Piccari L, Wort SJ, Meloni F, Rizzo M, Price LC, Martino L, Salvaterra E, Scelsi L, López Meseguer M, Blanco I, Callari A, Pérez González V, Tuzzolino F, McCabe C, Rodríguez Chiaradía DA, Vitulo P. The effect of borderline pulmonary hypertension on survival in chronic lung disease. Respiration. 2022;101:717–727. 10.1159/000524263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Olsson KM, Hoeper MM, Pausch C, Grünig E, Huscher D, Pittrow D, Rosenkranz S, Gall H. Pulmonary vascular resistance predicts mortality in patients with pulmonary hypertension associated with interstitial lung disease: results from the COMPERA registry. Eur Respir J. 2021;58:2101483. 10.1183/13993003.01483-2021 [DOI] [PubMed] [Google Scholar]
- 8. Rahaghi FF, Kolaitis NA, Adegunsoye A, de Andrade JA, Flaherty KR, Lancaster LH, Lee JS, Levine DJ, Preston IR, Safdar Z, Saggar R, Sahay S, Scholand MB, Shlobin OA, Zisman DA, Nathan SD. Screening strategies for pulmonary hypertension in patients with interstitial lung disease: a multidisciplinary delphi study. Chest. 2022;162(1):145–155. 10.1016/j.chest.2022.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Waxman AB, Elia D, Adir Y, Humbert M, Harari S. Recent advances in the management of pulmonary hypertension with interstitial lung disease. Eur Respir Rev. 2022;31:210220. 10.1183/16000617.0220-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Waxman A, Restrepo‐Jaramillo R, Thenappan T, Ravichandran A, Engel P, Bajwa A, Allen R, Feldman J, Argula R, Smith P, Rollins K, Deng C, Peterson L, Bell H, Tapson V, Nathan SD. Inhaled treprostinil in pulmonary hypertension due to interstitial lung disease. N Engl J Med. 2021;384:325–334. 10.1056/NEJMoa2008470 [DOI] [PubMed] [Google Scholar]
- 11.U.S. Department of Health and Human Services Food and Drug Administration (FDA). Patient‐focused drug development: methods to identify what is important to patients. Guidance for industry, food and drug administration staff, and other stakeholders [Internet]. 2022. Feb 25 [cited 2024 Jan 25]. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/patient-focused-drug-development-methods-identify-what-important-patients
- 12. European Medicines Agency (EMA) . Engagement Framework: EMA and patients, consumers and their organizations [Internet]. 2022. Jan 20 [cited 2024 Jan 25]. Available from: https://www.ema.europa.eu/en/documents/other/engagement-framework-european-medicines-agency-patients-consumers-their-organisations_en.pdf
- 13. Nabarette H, Chastenay MH, Dupont JCK, Ganache I, Single ANV. Patient and citizen participation at the organizational level in health technology assessment: an exploratory study in five jurisdictions. Int J Technol Assess Health Care. 2023;39(1):e51. 10.1017/S0266462323000417 [DOI] [PubMed] [Google Scholar]
- 14. Cox IA, Borchers Arriagada N, de Graaff B, Corte TJ, Glaspole I, Lartey S, Walters EH, Palmer AJ. Health‐related quality of life of patients with idiopathic pulmonary fibrosis: a systematic review and meta‐analysis. Eur Respir Rev. 2020;29:200154. 10.1183/16000617.0154-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Morel T, Cano SJ. Measuring what matters to rare disease patients—reflections on the work by the IRDiRC taskforce on patient‐centered outcome measures. Orphanet J Rare Dis. 2017;12:171. 10.1186/s13023-017-0718-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The Voice of the Patient, A series of reports from the U.S. Food and Drug Administration's (FDA's) Patient‐Focused Drug Development Initiative. Idiopathic Pulmonary Fibrosis, Public Meeting: September 26, 2014, Report Date: March, 2015, Center for Drug Evaluation and Research (CDER) U.S. Food and Drug Administration (FDA) [Internet]. 2015. Mar [cited 2024 Jan 25]. Available from: https://www.fda.gov/media/91396/download
- 17.The Voice of the Patient, A series of reports from the U.S. Food and Drug Administration's (FDA's) Patient‐Focused Drug Development Initiative. Pulmonary Arterial Hypertension, Public Meeting: May 13, 2014, Report Date: December, 2014, Center for Drug Evaluation and Research (CDER) U.S. Food and Drug Administration (FDA) [Internet]. 2014. Dec [cited 2024 Jan 25]. Available from: https://www.fda.gov/media/90479/download
- 18. DuBrock HM, Nathan SD, Reeve BB, Kolaitis NA, Mathai SC, Classi PM, Nelsen AC, Olayinka‐Amao B, Norcross LN, Martin SA. Pulmonary hypertension due to interstitial lung disease or chronic obstructive pulmonary disease: a patient experience study of symptoms and their impact on quality of life. Pulm Circ. 2021;11(2):1–9. 10.1177/20458940211005641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patient Focused Medicines Development initiative (PFMD), Patient Engagement Management (PEM) Suite, global hub for practical tools to plan, assess and execute any patient engagement initiative [Internet]. 2021. [cited 2024 Jan 25]. Available from: https://pemsuite.org
- 20.Patient Focused Medicines Development initiative (PFMD), Spain Patient Engagement Guidelines (FARMAINDUSTRIA Code) [Internet]. 2021. Sep [cited 2024 Jan 25]. Available from: https://patientengagement.synapseconnect.org/resources/spain-patient-engagement-guidelines
- 21. Overgaard D, Kaldan G, Marsaa K, Nielsen TL, Shaker SB, Egerod I. The lived experience with idiopathic pulmonary fibrosis: a qualitative study. Eur Respir J. 2016;47:1472–1480. 10.1183/13993003.01566-2015 [DOI] [PubMed] [Google Scholar]
- 22.The Pulmonary Hypertension Association Europe (PHA Europe) [Internet]. 2021. Jan [cited 2024 Jan 25]. Available from: https://www.phaeurope.org
- 23.The European Pulmonary Fibrosis Federation (EU‐PFF) [Internet]. 2022. Jul [cited 2024 Jan 25]. Available from: https://www.eu-pff.org
- 24. Dwyer AA, Uveges M, Dockray S, Smith N. Advancing qualitative rare disease research methodology: a comparison of virtual and in‐person focus group formats. Orphanet J Rare Dis. 2022;17:354. 10.1186/s13023-022-02522-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patient Focused Medicines Development initiative (PFMD), Guiding Principles on Reasonable Agreements between Patient Advocates and Pharmaceutical Companies. Consultancy Agreement between a patient advocate and a pharmaceutical company. WECAN—Final Consensus Document, 2018, V6.2 [Internet]. 2020. Mar [cited 2024 Jan 25]. Available from: https://patientengagement.synapseconnect.org/resources/consultancy-agreement-between-a-patient-advocate-and-a-pharmaceutical-company?uuid=a94fb598-3885-4f40-bfab-34f5b6a7dde1
- 26. Benner P, editor. Interpretive phenomenology: embodiment, caring, and ethics in health and illness [Internet]. Thousand Oaks, California, USA: Sage Publications; 1994. 2012 Jun [cited 2024 Jan 25]. 10.4135/9781452204727. Available from: [DOI] [Google Scholar]; https://sk.sagepub.com/books/interpretive-phenomenology
- 27. Prem Senthil M, Khadka J, Gilhotra JS, Simon S, Pesudovs K. Exploring the quality‐of‐life issues in people with retinal diseases: a qualitative study. J Patient Rep Outcomes. 2017;1:15. 10.1186/s41687-017-0023-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McCormack C, Cullivan S, Kehoe B, McCaffrey N, Gaine S, McCullagh B, Moyna NM, Hardcastle SJ. “It is the fear of exercise that stops me”—attitudes and dimensions influencing physical activity in pulmonary hypertension patients. Pulm Circ. 2021;11(4):1–9. 10.1177/20458940211056509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Using NVivo 14 for qualitative research [Internet]. Lumivero, Denver, CO. 2023. Mar [cited 2024 Jan 25]. Available from: https://lumivero.com/products/nvivo/nvivo-product-tour/ [Google Scholar]
- 30.Patient‐focused drug development: incorporating clinical outcome assessments into endpoints for regulatory decision‐making. Guidance for industry, food and drug administration staff, and other stakeholders. U.S. Department of Health and Human Services Food and Drug Administration. Procedural Draft Guidance [Internet]. 2023. Apr [cited 2024 Jan 25]. Available from: https://www.fda.gov/media/166830/download
- 31. Sisk B, Lin S, Kerr AM. Factors affecting the ability of patients with complex vascular anomalies to navigate the healthcare system. Orphanet J Rare Dis. 2024;19:18. 10.1186/s13023-024-03018-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Llubes‐Arrià L, Sanromà‐Ortíz M, Torné‐Ruiz A, Carillo‐Álvarez E, García‐Expósito J, Roca J. Emotional experience of the diagnostic process of a rare disease and the perception of support systems: a scoping review. J Clin Nurs. 2022;31:20–31. 10.1111/jocn.15922 [DOI] [PubMed] [Google Scholar]
- 33. Willmen T, Willmen L, Pankow A, Ronicke S, Gabriel H, Wagner AD. Rare diseases: why is a rapid referral to an expert center so important. BMC Health Serv Res. 2023;23:904. 10.1186/s12913-023-09886-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. RN‐LUNG: patient‐centric network of European healthcare providers and patient organisations, committed Europe‐wide and globally to reducing morbidity and mortality from rare lung diseases [Internet]. 2017. Mar [cited 2024 Jan 25]. Available from: https://ern-lung.eu
- 35. Hoeper MM, Pausch C, Olsson KM, Huscher D, Pittrow D, Grünig E, Staehler G, Vizza CD, Gall H, Distler O, Opitz C, Gibbs JSR, Delcroix M, Ghofrani HA, Ewert R, Kaemmerer H, Kabitz HJ, Skowasch D, Behr J, Milger K, Halank M, Wilkens H, Seyfarth HJ, Held M, Dumitrescu D, Tsangaris I, Vonk‐Noordegraaf A, Ulrich S, Klose H, Claussen M, Eisenmann S, Schmidt KH, Rosenkranz S, Lange TJ. Prognostic value of improvement endpoints in pulmonary arterial hypertension trials: a COMPERA analysis. J Heart Lung Transplant. 2022;41(No 7):971–981. 10.1016/j.healun.2022.03.011 [DOI] [PubMed] [Google Scholar]
- 36. Nikkho SM, Richter MJ, Shen E, Abman SH, Antoniou K, Chung J, Fernandes P, Hassoun P, Lazarus HM, Olschewski H, Piccari L, Psotka M, Saggar R, Shlobin OA, Stockbridge N, Vitulo P, Vizza CD, Wort SJ, Nathan SD. Clinical significance of pulmonary hypertension in interstitial lung disease: a consensus statement from the Pulmonary Vascular Research Institute's innovative drug development initiative—Group 3 pulmonary hypertension. Pulm Circ. 2022;12:e12127. 10.1002/pul2.12127 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


