Abstract
Mounting evidence indicates complex interaction between the immune system and the nervous system, challenging the traditional view about the immune privilege of the brain. Innate lymphoid cells (ILCs) and innate-like T cells are unique families of immune cells that functionally mirror traditional T cells but may function via antigen- and T cell antigen receptor (TCR)-independent mechanisms. Recent work indicates that various ILCs and innate-like T cell subsets are present in the brain barrier tissue, where they play important roles in regulating brain barrier integrity, brain homeostasis and cognitive function. In this review, we discuss recent advances in understanding the intricate roles for innate and innate-like lymphocytes in regulating brain and cognitive function.
1. Introduction
The interaction between the immune system and the brain remains an intriguing enigma. Of note, the brain is a relatively immune-privileged organ largely devoid of immune cells save for microglia in the parenchyma. Nevertheless, mounting evidence indicates that the activities of non-microglial immune cells may have profound effects on brain and cognitive function(Rua and McGavern 2018, Alves de Lima, Rustenhoven et al. 2020). Recent work indicates that brain barrier regions, including blood-brain and blood-cerebrospinal fluid (CSF) barriers, appear to be hubs of brain-immune cell interaction (Rua and McGavern 2018, Alves de Lima, Rustenhoven et al. 2020). The precise mechanisms by which these brain barrier-resident immune cells may influence brain and cognitive function are beginning to be revealed.
Recent work on innate lymphoid cells (ILCs) and innate-like T cells has greatly expanded our current knowledge of immune cell diversity and function (Artis and Spits 2015, Yang and Bhandoola 2016, Das, Harly et al. 2018). The ILC family includes cytotoxic natural killer (NK) cells and three known cytokine-producing helper ILC subsets, including group-1 innate lymphoid cells (ILC1), group-2 innate lymphoid cells (ILC2), and group-3 innate lymphoid cells (ILC3) (Artis and Spits 2015, Yang and Bhandoola 2016, Das, Harly et al. 2018). Unlike traditional T cells that respond to specific antigens, ILCs lack clonally restricted T-cell antigen receptors (TCRs) and respond to antigen-independent (innate) microenvironmental signals (Artis and Spits 2015, Yang and Bhandoola 2016, Das, Harly et al. 2018). However, despite the lack of TCRs, ILCs transcriptionally and functionally mirror traditional T cells. NK cells resemble CD8+ cytotoxic T cells at the molecular and functional levels, whereas helper ILCs functionally and transcriptionally mirror CD4+ Th1, Th2, and Th17 cells (Artis and Spits 2015, Yang and Bhandoola 2016, Das, Harly et al. 2018). scRNA-seq revealed that ILCs are close to T cells at the transcriptome level (Rustenhoven, Drieu et al. 2021, Yamawaki, Lu et al. 2021, Alkon, Bauer et al. 2022). As such, ILCs are generally considered analogues to T cells at the molecular and functional levels, despite the lack of TCRs.
Interestingly, certain bona-fide T cells with functional TCRs possess innate-like properties and may function via TCR- and antigen-independent mechanisms, similar to ILCs (Bendelac, Savage et al. 2007, Godfrey, Koay et al. 2019, Hinks and Zhang 2020, Ribot, Lopes et al. 2021). These T cells are generally referred to as innate-like T cells. Known innate-like T cells include γδ T cells, NKT cells and mucosal-associated invariant T cells (MAITs) (Bendelac, Savage et al. 2007, Godfrey, Koay et al. 2019, Hinks and Zhang 2020, Ribot, Lopes et al. 2021). The function and regulation of innate-like T cells in human health and disease remain to be fully characterized.
While the roles of NK cells in brain tumors and other brain disorders have been extensively explored (Sedgwick, Ghazanfari et al. 2020, Balatsoukas, Rossignoli et al. 2022), recent studies have also revealed the presence of other ILCs and innate-like T cells in brain barrier tissues, including the meninges and the choroid plexus (Ribeiro, Brigas et al. 2019, Alves de Lima, Rustenhoven et al. 2020, Fung, Sankar et al. 2020, Zhang, Bailey et al. 2022). In this review, we will summarize recent advances in understanding the characteristics of brain-barrier resident ILCs and innate-like T cells and their potential roles in regulating brain homeostasis and cognitive function. This review focuses on brain barrier resident group-2 innate lymphoid cells (ILC2s), γδ T cells, and MAIT cells.
NKT cells are another type of innate-like T cells implicated in demyelination diseases (Podbielska, O’Keeffe et al. 2018). Due to the lack of reports describing potential roles for NKT cells in regulating cognitive function, we do not focus on NKT cells in this review.
2. Characterization of innate lymphocytes in the brain barriers
Recent work has revealed the presence of several innate lymphocytes subsets in the brain barrier tissue, including ILC2s, γδ T cells, and MAIT cells. We listed the surface markers used to identify these innate lymphocytes in mouse brain barrier tissue in Table 1. Innate lymphocytes in human brain barrier tissue remain to be characterized.
Table 1:
Surface markers used to identify main subsets of murine innate lymphocytes in the brain barriers.
| Cell name | Markers |
|---|---|
| ILC2 | CD45+, CD3−, CD19−, IL-2Rα+, IL-7Rα+, CD90/Thy1+, Gata3+, ST2/IL33R+ |
| γδ T cells | CD45+, CD3+, TCRδ+ |
| MAIT cells | CD45+, CD3+, Thy1.2+, IL-18R+, IL-7Rα+, CD90/Thy1+, MR1-tetramer binding+ |
2.1. Characterization of ILCs in the meninges and choroid plexus.
The ILC family comprises NK cells and 3 subsets of helper ILCs, including ILC1s, ILC2s and ILC3s (Artis and Spits 2015). Despite the lack of clonally distributed antigen receptors, ILCs resemble T cells at the molecular and functional levels (Artis and Spits 2015, Yang and Bhandoola 2016, Das, Harly et al. 2018). ILC1s express high levels of Th1-characteristic transcription factor T-bet and are important producers of IFN-γ (Artis and Spits 2015, Yang and Bhandoola 2016, Das, Harly et al. 2018). ILC2s express the Th2-signature transcription regulator Gata3 and produce high levels of the Th2 characteristic cytokines IL-5 and IL-13 (Artis and Spits 2015, Yang and Bhandoola 2016, Das, Harly et al. 2018). ILC3 express the Th17-characteristic transcription factor Rorγτ and produce IL-22 and IL-17 (Artis and Spits 2015, Yang and Bhandoola 2016, Das, Harly et al. 2018). Nevertheless, unlike traditional T cells that undergo antigen-stimulated clonal expansion and activation, ILCs are activated by innate microenvironmental signals such as cytokines, alarmin proteins and cellular metabolites (Artis and Spits 2015, Yang and Bhandoola 2016, Das, Harly et al. 2018). In addition, helper ILCs are mostly non-circulating tissue-resident cells that are enriched in non-lymphoid organs (Gasteiger, Fan et al. 2015). Inside many organs, such as the lung and gut, ILCs might constitutively produce basal levels of effector molecules even at homeostasis, and thus, they might play important roles in orchestrating tissue homeostasis and organ function (Ricardo-Gonzalez, Van Dyken et al. 2018, Bielecki, Riesenfeld et al. 2021).
ILC2s are the major helper ILC subset residing in the brain barriers (Gadani, Smirnov et al. 2017, Fung, Zhang et al. 2021, Rustenhoven, Drieu et al. 2021). In the meninges, ILC2s are enriched along the dura venous sinus (Gadani, Smirnov et al. 2017). IHC staining indicates that ILC2s might colocalize with adventitial stromal cells (ASCs) (Dahlgren, Jones et al. 2019). ASCs produce IL-33 that promotes ILC2s survival and activity. ILC2s are a major population of tissue-resident lymphocytes in the meninges (Dahlgren, Jones et al. 2019). ILC2s are present in the meninges in both young and aged mice, and the number of meningeal ILC2s gradually increases with aging (Fung, Sankar et al. 2020). ILC2s are absent in the brain parenchyma under physiological states in both young and aged mice (Fung, Sankar et al. 2020). The choroid plexus (CP), a tissue specialized in generating CSF, also harbors a variety of immune cells. ILCs are nearly absent in the CP of young mice (Fung, Sankar et al. 2020). However, a subset of ILC2s accumulate in the choroid plexus of aged mice and humans (Fung, Sankar et al. 2020). CP ILC2s and meningeal ILC2s differ greatly in functional activities and molecular properties (Fung, Sankar et al. 2020). Compared to meningeal ILC2s, CP ILC2s have enhanced long-term self-renewal capability, increased proliferative activity and notable resistance to cellular senescence (Fung, Sankar et al. 2020). While meningeal ILC2s can only proliferate for up to 2 weeks in vitro, CP ILC2s can proliferate for more than 4 weeks without signs of exhaustion or senescence. The expression of genes encoding senescence markers such as p16 is significantly lower in cultured CP ILC2s than in cultured meningeal ILC2s. Following IL-33 stimulation, CP ILC2s exhibit increased proliferative and cytokine-producing activity compared to meningeal ILC2s. The expression of lipid metabolism genes, such as Arginase 1, is much higher in CP ILC2s than in meningeal ILC2s. The mechanisms that underlie these functional and molecular differences between meningeal and CP ILC2s are yet to be deciphered.
Studies with other organs indicate that ILC2s in different tissues have distinct developmental origins, which might endow them with differential functional capabilities (Schneider, Lee et al. 2019). For example, ILC2s in the lungs of adult mice predominantly derive from prenatal/perinatal stages, whereas ILC2s in the skin mainly derive from bone marrow precursors in adult mice (Schneider, Lee et al. 2019). ILC2s of an adult origin appear to respond more strongly to thymic stromal lymphopoietin (TSLP) and IL-25, whereas ILC2s of perinatal and postnatal origins might respond more strongly to IL-33 (Schneider, Lee et al. 2019). In addition to bone marrow or fetal liver precursors, the thymus might also be a significant source of a unique subset of ILC2s that express high levels of intracellular CD3 (Bajana, Pankow et al. 2022, Sun and Bajana 2022). In addition, calvaria bone marrow might provide a shortcut for early immune cell development, generating meningeal immune cells with an immature phenotype (Brioschi, Wang et al. 2021, Cugurra, Mamuladze et al. 2021). The ontogeny of meningeal and CP ILC2s remains to be deciphered. The surprising resistance to cellular senescence points to an interesting hypothesis that CP ILC2s in aged mice might be newly generated “young” cells that derive from adult precursors, whereas meningeal ILC2s might be “old” cells with a perinatal or prenatal origin. While this and other hypotheses remain to be tested by future investigation, it is possible that the functional difference between meningeal ILC2s and CP ILC2s is associated with distinct developmental origins.
2.2. Characterization of innate-like T cells in the meninges and choroid plexus.
Innate-like T cells are bona fide T cells that express TCRs. Similar to traditional T cells, innate-like T cells are derived from the thymus and undergo antigen-mediated positive and negative selection for their development and maturation. Nevertheless, innate-like T cells already acquire effector programs during early development and may function via both TCR-dependent and TCR-independent mechanisms. The three known subsets of innate-like T cells are γδT cells, MAIT cells, and NKT cells (Bendelac, Savage et al. 2007, Godfrey, Koay et al. 2019, Hinks and Zhang 2020, Ribot, Lopes et al. 2021).
2.2.1. MAIT cells in the meninges
MAIT cells are the predominant type of innate-like T cells in humans (Hinks and Zhang 2020, Nel, Bertrand et al. 2021). The majority of human MAIT cells express CD8. MAIT cells constitute around 10% to 50% of CD8+ T cells in non-lymphoid organs in humans (Godfrey, Koay et al. 2019, Hinks, Marchi et al. 2019, Hinks and Zhang 2020). MAIT cells are much less abundant in laboratory mice (Rahimpour, Koay et al. 2015). Research with MAIT cells in mouse models are still considered to be of high translational potential, because MAIT cells are the major innate-like T cells in humans.
MAIT cells are an interesting subset of lymphocytes whose development depends on microbiota (Constantinides, Link et al. 2019, Legoux, Bellet et al. 2019). MAIT cells possess an invariant αβTCR receptor that recognizes microbe-derived riboflavin biogenesis intermediates (Treiner, Duban et al. 2003, Huang, Martin et al. 2009, Kjer-Nielsen, Patel et al. 2012). MAIT cells are generally divided into interferon-γ-producing MAIT1 cells and IL-17-producing MAIT17 cells (Rudak, Choi et al. 2018, Constantinides, Link et al. 2019, Legoux, Bellet et al. 2019). In humans, MAIT cells also secrete cytotoxic molecules and possess potent cytotoxic activity (Rudak, Choi et al. 2018, Constantinides, Link et al. 2019, Legoux, Bellet et al. 2019). MAIT cells may function via both TCR-dependent and TCR-independent mechanisms (van Wilgenburg, Scherwitzl et al. 2016, van Wilgenburg, Loh et al. 2018, Lamichhane, Schneider et al. 2019). The TCRs of MAIT cells can be activated by riboflavin metabolites presented by MR1-expressing cells. MAIT cells may also survive and proliferate in the absence of TCR stimulators with cytokines alone (Ussher, Bilton et al. 2014, Loh, Wang et al. 2016, van Wilgenburg, Scherwitzl et al. 2016, van Wilgenburg, Loh et al. 2018). These unique features of MAIT cells indicate that they may play important physiological roles that straddle the boundary between innate and adaptive immunity.
Our recent work indicates that MAIT cells are present in the meninges and accumulate with age (Zhang, Bailey et al. 2022). MAIT cells have been identified in both the leptomeningeal tissue isolated from the outer surface of the cerebrum and the dura/arachnoid meningeal tissue isolated from the inner cavalry (Zhang, Bailey et al. 2022). Because it is difficult to separate arachnoid mater and the pia mater, the precise anatomic location in which MAIT cells reside remains unclear (Zhang, Bailey et al. 2022). Mouse meningeal MAIT cells lack the expression of cytotoxic molecules but express both IL-17 and IFNγ (Zhang, Bailey et al. 2022). The separation between IFNγ-expressing MAIT1 cells and IL-17-expressing MAIT17 cells is unclear by scRNA-seq (Zhang, Bailey et al. 2022). Interestingly, meningeal MAIT cells express high levels of a variety of anti-oxidant molecules including Selenop, Selenof, and Fth1 (Zhang, Bailey et al. 2022). These anti-oxidant molecules are also highly expressed by MAIT cells in other non-lymphoid organs, such as the liver, skin and lung (Constantinides, Link et al. 2019). Consistent with their innate-like properties, meningeal MAIT cells can survive and produce effector molecules, both in the presence and in the absence of TCR-signaling (Zhang, Bailey et al. 2022). Interestingly, TCR activation enhances the expression of cytokines by meningeal MAIT cells, but decreases the expression of anti-oxidant molecules by MAIT cells (Zhang, Bailey et al. 2022). It is thus possible that homeostatic MAIT cells (without TCR activation) and TCR-activated MAIT cells might play distinct physiological roles. Homeostatic MAIT cells might be generally anti-inflammatory; whereas TCR-activated MAIT cells might be more prone to produce pro-inflammatory cytokines. This hypothesis remains to be tested by future investigation. Together, these results indicate that MAIT cells might play an important physiological role in restricting tissue oxidative stress.
2.2.2. γδT cells in the meninges
γδT cells are T cells that express the γδTCR. γδT cells are another important subset of innate-like T cells. Similar to MAIT cells, γδ T cells already acquire effector programs during early development in the thymus (Parker and Ciofani 2020). Following egression from the thymus, γδ T cells express high levels of T-bet and Rorγτ that endow them with Th1 and Th17 effector function (Parker and Ciofani 2020). γδ T cells may have functional redundancy with MAIT cells (Constantinides, Link et al. 2019). γδ T deficient mice exhibit striking compensatory expansion of MAIT cells in the skin (Constantinides, Link et al. 2019). The functional similarity and difference between MAIT cells and γδ T cells warrant future investigation.
Recent reports revealed that γδ T cells are present with relative abundance in the meninges of young mice (Ribeiro, Brigas et al. 2019, Alves de Lima, Rustenhoven et al. 2020). γδ T cells appear to be the major source of IL-17 in the meninges at homeostasis (Ribeiro, Brigas et al. 2019, Alves de Lima, Rustenhoven et al. 2020). The expression of IL-17 by meningeal γδ T cells is independent of inflammatory signals (Ribeiro, Brigas et al. 2019). Thus, meningeal γδ T cells possess a basal level of activity at homeostasis, indicating that they might be implicated in regulating tissue homeostasis.
3. The role of brain barrier-resident innate and innate-like lymphocytes in regulating brain and cognitive function
Recent work indicates that brain barrier-resident ILCs and innate-like T cells play an important role in maintaining normal brain function. Here, we will summarize recent studies that indicate important roles for brain barrier ILC2s, MAIT cells and γδT cells in maintaining normal brain and cognitive function.
3.1. ILC2s in aging and CNS injury
A variety of immune cells reside in the CP, a tissue specialized in generating CSF. Recent work indicates that ILC2s accumulate in the CP with aging and constitute a major subset of innate lymphocytes in the CP of aged mice and humans (Fung, Sankar et al. 2020). Activation of ILC2s by administration of IL-33 or i.c.v. transfer of pre-activated ILC2s reduced aging-associated neuroinflammation and improved spatial memory in the object location task and Morris water maze in aged mice (Fung, Sankar et al. 2020). The precise mechanisms by which ILC2 activation may alleviate aging-associated cognitive deficits remain to be fully explored. An intuitive hypothesis is that cytokines and growth factors secreted by CP-resident immune cells may be released into the CSF, thus affecting the activity of neurons and glial cells in the brain parenchyma. Indeed, CP ILC2s can produce a range of cytokines and growth factors, including very high amounts of IL-5 and amphiregulin (Fung, Sankar et al. 2020). IL-5 may repress the activity of aging-associated proinflammatory T cells, enhance Treg activity, and promote plasma cell differentiation and immunoglobin secretion (Horikawa and Takatsu 2006, Emslie, D’Costa et al. 2008, Tran, Hodgkinson et al. 2012, Fung, Sankar et al. 2020). Amphiregulin may repress astrogliosis and enhance neuron survival (Falk and Frisen 2002, Zhan, Zheng et al. 2015, Ito, Komai et al. 2019). In addition, activated ILC2s also produce IL-13 that may regulate learning and memory by controlling astrocyte function (Brombacher, Nono et al. 2017). These activities may together lead to reduced neuroinflammation and improved cognitive function. Interestingly, ILC2s fail to accumulate in the brain barriers of aged 3x Tg-AD mice with amyloid-β and tau pathologies (Fung, Zhang et al. 2021). Potential roles for ILC2s in human neurodegenerative diseases remain to be deciphered. Together, these studies suggest a role for CP-resident innate lymphocytes in influencing cognitive function in ageing and neurodegeneration.
ILC2s are also abundant in the meninges of both young and aged adult mice. Meningeal ILC2s might play a protective role in CNS injury. Meningeal ILC2s were reported to exhibit neuroprotective properties by upregulating the expression of CGRP and other neuroprotective molecules in a mouse model of spinal cord contusion (Gadani, Smirnov et al. 2017). In patients with traumatic brain injury (TBI), ILC2s were present in the dura and increased in the CSF (Baban, Braun et al. 2021). IL-10-producing regulatory ILC2s induced by AMP-activated protein kinase (AMPK) might help improve neurological outcomes in a controlled cortical impact mouse model of experimental TBI (Baban, Braun et al. 2021). ILC2s also accumulated in brain-associated tissue without altering the outcome of brain injury in neonatal mouse models of hypoxic ischemia (Zelco, Rocha-Ferreira et al. 2020). Thus, ILC2s might also play an important role in mitigating CNS injury.
3.2. The role of γδT cells in regulating brain and cognitive function
γδT cells are known to be a major source of IL-17 at homeostasis (Ribot, Lopes et al. 2021). Recent work indicates that γδT cells are present in the meninges and that meningeal γδT cells may produce IL-17 independent of inflammatory signals (Ribeiro, Brigas et al. 2019, Alves de Lima, Rustenhoven et al. 2020). A recent report indicated that γδT cell-derived IL-17 may affect the activity of prefrontal cortex neurons, restricting anxiety-like behavior (Alves de Lima, Rustenhoven et al. 2020). Young (8- to 10-week-old) TCRδ−/− mice exhibited decreased anxiety behavior in the elevated plus maze test and the open field test (Alves de Lima, Rustenhoven et al. 2020). Administration of anti-TCR γδ depleting antibody or anti-IL-17a neutralizing antibody into the cisterna magna of wild-type mice also decreased anxiety (Alves de Lima, Rustenhoven et al. 2020). Attenuation of neuronal IL-17a signaling in the medial prefrontal cortex sufficed to decrease anxiety-like behavior in mice (Alves de Lima, Rustenhoven et al. 2020). Conversely, recombinant IL-17a injection into the cerebrospinal fluid of TCRδ−/− mice increased anxiety levels (Alves de Lima, Rustenhoven et al. 2020). These findings suggested that meningeal γδT cell-derived IL-17a, through neuronal IL-17Ra signaling, controls anxiety-like behavior in mice (Alves de Lima, Rustenhoven et al. 2020).
Another study indicated that γδT cells and IL-17 may affect cognition. Ten- to twenty-four-week-old mice lacking γδT cells (TCRδ−/−) or IL-17 cells (IL-17−/−) displayed deficient short-term spatial working memory in the Y-maze test while retaining long-term spatial reference memory in the Morris water maze (MWM) test (Ribeiro, Brigas et al. 2019). Bone marrow chimera mice devoid of most meningeal γδ17 T cells also suffered from short-term, but not long-term, memory deficits (Ribeiro, Brigas et al. 2019). TCRδ−/− and IL-17−/− mice displayed long-term potentiation (LTP) and basal transmission deficits in the CA1 region of hippocampal slices after short-term Y-maze (Ribeiro, Brigas et al. 2019). Moreover, IL-17 increased glial brain-derived neurotrophic factor production and modulated hippocampal neuronal plasticity (Ribeiro, Brigas et al. 2019). These results indicated that meningeal γδT cell-derived IL-17 promoted short-term memory by increasing the glutamatergic synaptic plasticity of hippocampal neurons (Ribeiro, Brigas et al. 2019).
In some other studies, a pathogenic role for γδT cells in neuroinflammation has been highlighted. γδ17 T cells were found to promote leukocyte infiltration and exacerbate ischemic brain injury in mouse models (Shichita, Sugiyama et al. 2009, Gelderblom, Weymar et al. 2012, Benakis, Brea et al. 2016). In addition, IFN-γ-producing γδT cells were shown to mediate demyelination upon coronavirus infection (Dandekar, O’Malley et al. 2005). Thus, normal brain function might require an appropriate concentration of IL-17 and other γδT cell-derived proteins.
3.3. The role of MAIT cells in regulating neuroinflammation and cognitive function.
Our recent work with MAIT cells suggests another possible mechanism by which meningeal lymphocytes might influence cognitive function. MAIT cells are the most abundant innate-like T cells in humans (Legoux, Salou et al. 2020). MAIT cells are present in mouse leptomeninges, and their abundance increases with age (Zhang, Bailey et al. 2022). Interestingly, scRNA-seq identified that meningeal MAIT cells expressed high levels of a variety of anti-oxidant molecules (Zhang, Bailey et al. 2022). The absence of MAIT cells led to reactive oxidative species (ROS) accumulation in the leptomeninges in 7-month-old mice (Zhang, Bailey et al. 2022). The accumulated ROS impaired the expression of tight junction and adherens junction proteins on the arachnoid meningeal barrier, leading to leakage of the meningeal barrier (Zhang, Bailey et al. 2022). In MAIT cell-deficient (Mr1−/−) mice, the meningeal barrier was broken down, which inflamed the brain parenchyma and disrupted cognitive function (Zhang, Bailey et al. 2022). MAIT cell transfer to Mr1−/− mice prevented microgliosis and defects in spatial memory in the Y-maze and Morris water maze tests (Zhang, Bailey et al. 2022). Antioxidant glutathione treatment partially alleviated microgliosis and cognitive impairment in 7-month-old Mr1−/− mice (Zhang, Bailey et al. 2022). Together, these data highlight an important role for meningeal lymphocytes in maintaining meningeal barrier integrity, and indicate that the maintenance of meningeal barrier integrity is essential for protecting daily brain function.
Another study indicated that MAIT cells might also restrict neuroinflammation in experimental autoimmune encephalomyelitis (EAE) using V(alpha)19i TCR transgene mice with an increased abundance of MAIT cells (Croxford, Miyake et al. 2006). Consistent with these findings, MAIT cell abundance and activity might be altered in multiple sclerosis (MS) patients (Treiner and Liblau 2015). The mechanisms underlying the potential regulatory function of MAIT cells in EAE or MS warrant future investigation.
4. Summary
Together, recent evidence with innate lymphocytes supports the hypothesis that the activity of brain barrier resident lymphocytes impacts brain homeostasis and cognitive function. Multiple-coexisting mechanisms may underlie the interaction between brain barrier-resident innate lymphocytes and the cells in brain parenchyma. CP-resident lymphocytes may directly release cytokines and growth factors into the CSF. They may also stimulate or repress the production and release of effector molecules by other CP-resident immune cells. As cytokines and growth factors circulate in the CSF, these CP cell-derived proteins may regulate the activity of neurons and glial cells in the brain parenchyma. For meningeal lymphocytes, multiple mechanisms might underlie the capability of meningeal lymphocytes to influence brain and cognitive function. Meningeal lymphocytes might be the most proximal source of cytokines that affect the function of neurons and glial cells in the cortex. Meningeal lymphocyte-secreted proteins may also circulate into the CSF, controlling the activities of neurons and glial cells in other brain parenchyma regions. Finally, meningeal lymphocytes may play an important role in maintaining meningeal barrier integrity, thus restricting neuroinflammation and protecting cognitive function. Similar processes may occur for lymphocytes residing in blood‒brain barriers.
In Figure 1, we propose a model to summarize the co-existing mechanisms underlying the interaction between brain barrier-resident innate lymphocytes and the cells in brain parenchyma. Of note, the detailed cellular and molecular pathways, such as the specific effector molecules and the direct target cells, remain to be fully discovered. We thus provide a general model, instead of providing a list of specific pathways or cells.
Figure 1. Potential mechanisms underlying the interaction between brain barrier-resident innate lymphocytes and the cells in the brain parenchyma.
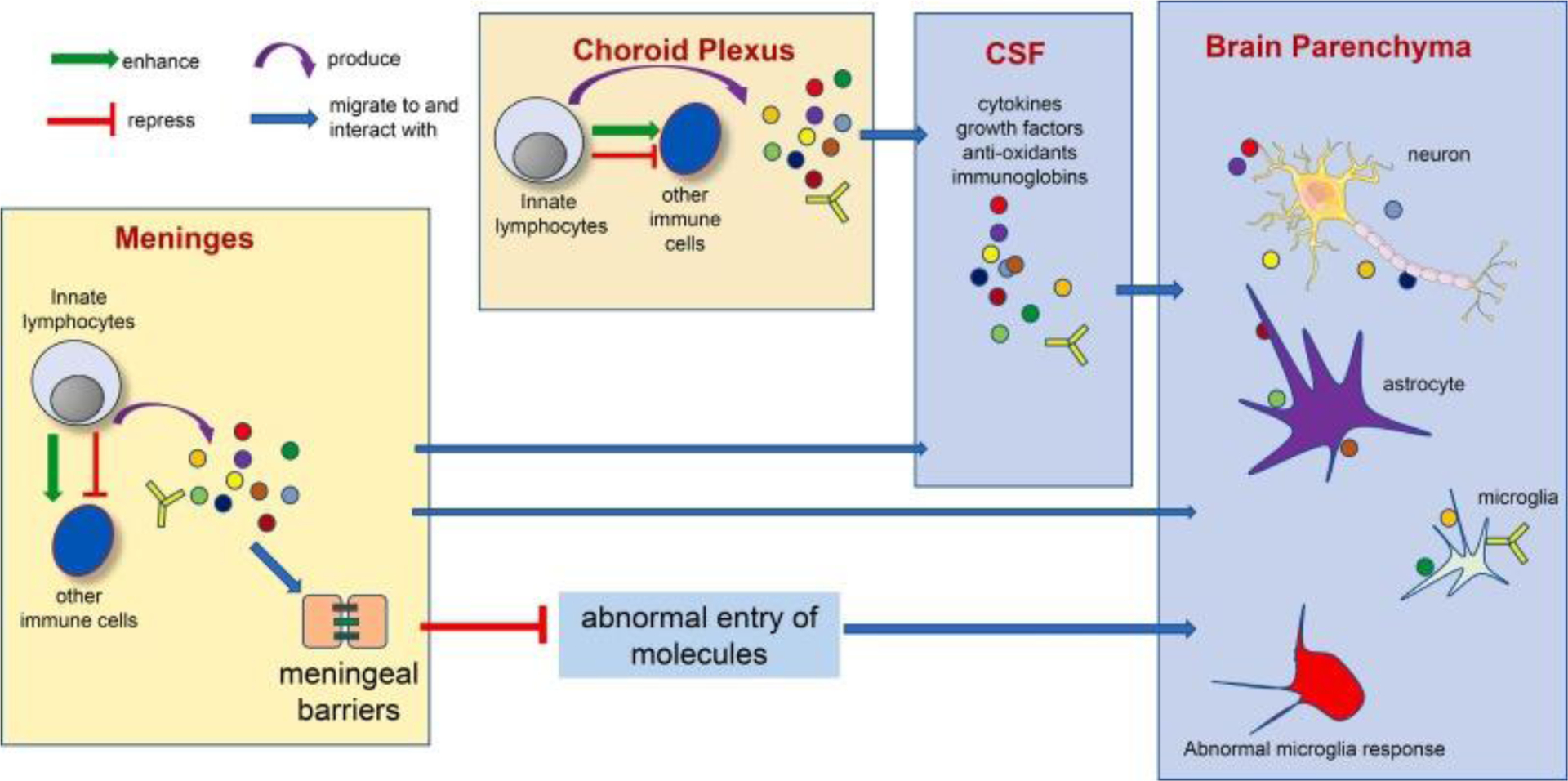
Multiple co-existing mechanisms might under the interaction between brain barrier-resident innate lymphocytes and the cells in the brain parenchyma. Innate lymphocytes at the brain barrier tissue may produce effector molecules that access cells in the brain parenchyma via CSF circulation. Innate lymphocytes may also stimulate or repress other immune cells to produce such effector molecules. Innate lymphocytes may also play an important role in regulating brain barrier integrity, thus preventing abnormal entry of undesirable molecules into the brain parenchyma and restricting neuroinflammation. Of note, the interaction between innate lymphocytes and the nervous system is bilateral. The nervous system may in turn control innate lymphocyte activity via various mechanisms (Yano and Artis 2022).
Several major questions remain unresolved. What are the similarities and differences in the development and function of lymphocytes at different brain barriers? What are the precise trafficking paths and kinetics of effector molecules secreted by brain barrier resident lymphocytes? What are the specific molecular pathways linking neuroinflammation and cognitive impairment? Future efforts to uncover these mysteries may provide exciting insights into understanding the immune cell pathways that control brain homeostasis and how such pathways go awry in neurological disorders.
Funding
This work was supported by the National Institutes of Health (NIH) grants R01HL137813 (to Q.Y.), R01AG057782 (to Q.Y.), R01HL155021 (to Q.Y.), R01NS110749 (to K.L.Z.), U01AG072464 (to K.L.Z.), and support to the Child Health Institute of New Jersey from the Robert Wood Johnson Foundation (Grant #74260).
Footnotes
Declaration of Competing Interest
None.
Data availability
No data was used for the research described in the article.
References
- Alkon N, Bauer WM, Krausgruber T, Goh I, Griss J, Nguyen V, Reininger B, Bangert C, Staud C, Brunner PM, Bock C, Haniffa M and Stingl G (2022). “Single-cell analysis reveals innate lymphoid cell lineage infidelity in atopic dermatitis.” J Allergy Clin Immunol 149(2): 624–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves de Lima K, Rustenhoven J, Da Mesquita S, Wall M, Salvador AF, Smirnov I, Martelossi Cebinelli G, Mamuladze T, Baker W, Papadopoulos Z, Lopes MB, Cao WS, Xie XS, Herz J and Kipnis J (2020). “Meningeal gammadelta T cells regulate anxiety-like behavior via IL-17a signaling in neurons.” Nat Immunol 21(11): 1421–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves de Lima K, Rustenhoven J and Kipnis J (2020). “Meningeal Immunity and Its Function in Maintenance of the Central Nervous System in Health and Disease.” Annu Rev Immunol 38: 597–620. [DOI] [PubMed] [Google Scholar]
- Artis D and Spits H (2015). “The biology of innate lymphoid cells.” Nature 517(7534): 293–301. [DOI] [PubMed] [Google Scholar]
- Baban B, Braun M, Khodadadi H, Ward A, Alverson K, Malik A, Nguyen K, Nazarian S, Hess DC, Forseen S, Post AF, Vale FL, Vender JR, Hoda MN, Akbari O, Vaibhav K and Dhandapani KM (2021). “AMPK induces regulatory innate lymphoid cells after traumatic brain injury.” JCI Insight 6(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajana S, Pankow A, Liu K, Michniowska M, Urban JF Jr., Chen WR and Sun XH (2022). “Correlation between circulating innate lymphoid cell precursors and thymic function.” iScience 25(2): 103732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balatsoukas A, Rossignoli F and Shah K (2022). “NK cells in the brain: implications for brain tumor development and therapy.” Trends Mol Med 28(3): 194–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benakis C, Brea D, Caballero S, Faraco G, Moore J, Murphy M, Sita G, Racchumi G, Ling L, Pamer EG, Iadecola C and Anrather J (2016). “Commensal microbiota affects ischemic stroke outcome by regulating intestinal gammadelta T cells.” Nat Med 22(5): 516–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendelac A, Savage PB and Teyton L (2007). “The biology of NKT cells.” Annu Rev Immunol 25: 297–336. [DOI] [PubMed] [Google Scholar]
- Bielecki P, Riesenfeld SJ, Hutter JC, Torlai Triglia E, Kowalczyk MS, Ricardo-Gonzalez RR, Lian M, Amezcua Vesely MC, Kroehling L, Xu H, Slyper M, Muus C, Ludwig LS, Christian E, Tao L, Kedaigle AJ, Steach HR, York AG, Skadow MH, Yaghoubi P, Dionne D, Jarret A, McGee HM, Porter CBM, Licona-Limon P, Bailis W, Jackson R, Gagliani N, Gasteiger G, Locksley RM, Regev A and Flavell RA (2021). “Skin-resident innate lymphoid cells converge on a pathogenic effector state.” Nature 592(7852): 128–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brioschi S, Wang WL, Peng V, Wang M, Shchukina I, Greenberg ZJ, Bando JK, Jaeger N, Czepielewski RS, Swain A, Mogilenko DA, Beatty WL, Bayguinov P, Fitzpatrick JAJ, Schuettpelz LG, Fronick CC, Smirnov I, Kipnis J, Shapiro VS, Wu GF, Gilfillan S, Cella M, Artyomov MN, Kleinstein SH and Colonna M (2021). “Heterogeneity of meningeal B cells reveals a lymphopoietic niche at the CNS borders.” Science 373(6553). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brombacher TM, Nono JK, De Gouveia KS, Makena N, Darby M, Womersley J, Tamgue O and Brombacher F (2017). “IL-13-Mediated Regulation of Learning and Memory.” J Immunol 198(7): 2681–2688. [DOI] [PubMed] [Google Scholar]
- Constantinides MG, Link VM, Tamoutounour S, Wong AC, Perez-Chaparro PJ, Han SJ, Chen YE, Li K, Farhat S, Weckel A, Krishnamurthy SR, Vujkovic-Cvijin I, Linehan JL, Bouladoux N, Merrill ED, Roy S, Cua DJ, Adams EJ, Bhandoola A, Scharschmidt TC, Aube J, Fischbach MA and Belkaid Y (2019). “MAIT cells are imprinted by the microbiota in early life and promote tissue repair.” Science 366(6464). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croxford JL, Miyake S, Huang YY, Shimamura M and Yamamura T (2006). “Invariant V(alpha)19i T cells regulate autoimmune inflammation.” Nat Immunol 7(9): 987–994. [DOI] [PubMed] [Google Scholar]
- Cugurra A, Mamuladze T, Rustenhoven J, Dykstra T, Beroshvili G, Greenberg ZJ, Baker W, Papadopoulos Z, Drieu A, Blackburn S, Kanamori M, Brioschi S, Herz J, Schuettpelz LG, Colonna M, Smirnov I and Kipnis J (2021). “Skull and vertebral bone marrow are myeloid cell reservoirs for the meninges and CNS parenchyma.” Science 373(6553). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlgren MW, Jones SW, Cautivo KM, Dubinin A, Ortiz-Carpena JF, Farhat S, Yu KS, Lee K, Wang C, Molofsky AV, Tward AD, Krummel MF, Peng T and Molofsky AB (2019). “Adventitial Stromal Cells Define Group 2 Innate Lymphoid Cell Tissue Niches.” Immunity 50(3): 707–722.e706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandekar AA, O’Malley K and Perlman S (2005). “Important roles for gamma interferon and NKG2D in gammadelta T-cell-induced demyelination in T-cell receptor beta-deficient mice infected with a coronavirus.” J Virol 79(15): 9388–9396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Harly C, Yang Q and Bhandoola A (2018). “Lineage specification in innate lymphocytes.” Cytokine Growth Factor Rev 42: 20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emslie D, D’Costa K, Hasbold J, Metcalf D, Takatsu K, Hodgkin PO and Corcoran LM (2008). “Oct2 enhances antibody-secreting cell differentiation through regulation of IL-5 receptor alpha chain expression on activated B cells.” J Exp Med 205(2): 409–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk A and Frisen J (2002). “Amphiregulin is a mitogen for adult neural stem cells.” J Neurosci Res 69(6): 757–762. [DOI] [PubMed] [Google Scholar]
- Fung ITH, Sankar P, Zhang Y, Robison LS, Zhao X, D’Souza SS, Salinero AE, Wang Y, Qian J, Kuentzel ML, Chittur SV, Temple S, Zuloaga KL and Yang Q (2020). “Activation of group 2 innate lymphoid cells alleviates aging-associated cognitive decline.” Journal of Experimental Medicine 217(4): e20190915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung ITH, Zhang Y, Shin DS, Sankar P, Sun X, D’Souza SS, Song R, Kuentzel ML, Chittur SV, Zuloaga KL and Yang Q (2021). “Group 2 innate lymphoid cells are numerically and functionally deficient in the triple transgenic mouse model of Alzheimer’s disease.” J Neuroinflammation 18(1): 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadani SP, Smirnov I, Wiltbank AT, Overall CC and Kipnis J (2017). “Characterization of meningeal type 2 innate lymphocytes and their response to CNS injury.” J Exp Med 214(2): 285–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasteiger G, Fan X, Dikiy S, Lee SY and Rudensky AY (2015). “Tissue residency of innate lymphoid cells in lymphoid and nonlymphoid organs.” Science 350(6263): 981–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelderblom M, Weymar A, Bernreuther C, Velden J, Arunachalam P, Steinbach K, Orthey E, Arumugam TV, Leypoldt F, Simova O, Thom V, Friese MA, Prinz I, Holscher C, Glatzel M, Korn T, Gerloff C, Tolosa E and Magnus T (2012). “Neutralization of the IL-17 axis diminishes neutrophil invasion and protects from ischemic stroke.” Blood 120(18): 3793–3802. [DOI] [PubMed] [Google Scholar]
- Godfrey DI, Koay HF, McCluskey J and Gherardin NA (2019). “The biology and functional importance of MAIT cells.” Nat Immunol 20(9): 1110–1128. [DOI] [PubMed] [Google Scholar]
- Hinks TSC, Marchi E, Jabeen M, Olshansky M, Kurioka A, Pediongco TJ, Meehan BS, Kostenko L, Turner SJ, Corbett AJ, Chen Z, Klenerman P and McCluskey J (2019). “Activation and In Vivo Evolution of the MAIT Cell Transcriptome in Mice and Humans Reveals Tissue Repair Functionality.” Cell Rep 28(12): 3249–3262 e3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinks TSC and Zhang XW (2020). “MAIT Cell Activation and Functions.” Front Immunol 11: 1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikawa K and Takatsu K (2006). “Interleukin-5 regulates genes involved in B-cell terminal maturation.” Immunology 118(4): 497–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Martin E, Kim S, Yu L, Soudais C, Fremont DH, Lantz O and Hansen TH (2009). “MR1 antigen presentation to mucosal-associated invariant T cells was highly conserved in evolution.” Proc Natl Acad Sci U S A 106(20): 8290–8295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Komai K, Mise-Omata S, Iizuka-Koga M, Noguchi Y, Kondo T, Sakai R, Matsuo K, Nakayama T, Yoshie O, Nakatsukasa H, Chikuma S, Shichita T and Yoshimura A (2019). “Brain regulatory T cells suppress astrogliosis and potentiate neurological recovery.” Nature 565(7738): 246–250. [DOI] [PubMed] [Google Scholar]
- Kjer-Nielsen L, Patel O, Corbett AJ, Le Nours J, Meehan B, Liu L, Bhati M, Chen Z, Kostenko L, Reantragoon R, Williamson NA, Purcell AW, Dudek NL, McConville MJ, O’Hair RA, Khairallah GN, Godfrey DI, Fairlie DP, Rossjohn J and McCluskey J (2012). “MR1 presents microbial vitamin B metabolites to MAIT cells.” Nature 491(7426): 717–723. [DOI] [PubMed] [Google Scholar]
- Lamichhane R, Schneider M, de la Harpe SM, Harrop TWR, Hannaway RF, Dearden PK, Kirman JR, Tyndall JDA, Vernall AJ and Ussher JE (2019). “TCR- or Cytokine-Activated CD8(+) Mucosal-Associated Invariant T Cells Are Rapid Polyfunctional Effectors That Can Coordinate Immune Responses.” Cell Rep 28(12): 3061–3076.e3065. [DOI] [PubMed] [Google Scholar]
- Legoux F, Bellet D, Daviaud C, El Morr Y, Darbois A, Niort K, Procopio E, Salou M, Gilet J, Ryffel B, Balvay A, Foussier A, Sarkis M, El Marjou A, Schmidt F, Rabot S and Lantz O (2019). “Microbial metabolites control the thymic development of mucosal-associated invariant T cells.” Science 366(6464): 494–499. [DOI] [PubMed] [Google Scholar]
- Legoux F, Salou M and Lantz O (2020). “MAIT Cell Development and Functions: the Microbial Connection.” Immunity 53(4): 710–723. [DOI] [PubMed] [Google Scholar]
- Loh L, Wang Z, Sant S, Koutsakos M, Jegaskanda S, Corbett AJ, Liu L, Fairlie DP, Crowe J, Rossjohn J, Xu J, Doherty PC, McCluskey J and Kedzierska K (2016). “Human mucosal-associated invariant T cells contribute to antiviral influenza immunity via IL-18-dependent activation.” Proc Natl Acad Sci U S A 113(36): 10133–10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nel I, Bertrand L, Toubal A and Lehuen A (2021). “MAIT cells, guardians of skin and mucosa?” Mucosal Immunol 14(4): 803–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker ME and Ciofani M (2020). “Regulation of γδ T Cell Effector Diversification in the Thymus.” Front Immunol 11: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podbielska M, O’Keeffe J and Hogan EL (2018). “Autoimmunity in multiple sclerosis: role of sphingolipids, invariant NKT cells and other immune elements in control of inflammation and neurodegeneration.” J Neurol Sci 385: 198–214. [DOI] [PubMed] [Google Scholar]
- Rahimpour A, Koay HF, Enders A, Clanchy R, Eckle SB, Meehan B, Chen Z, Whittle B, Liu L, Fairlie DP, Goodnow CC, McCluskey J, Rossjohn J, Uldrich AP, Pellicci DG and Godfrey DI (2015). “Identification of phenotypically and functionally heterogeneous mouse mucosal-associated invariant T cells using MR1 tetramers.” J Exp Med 212(7): 1095–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro M, Brigas HC, Temido-Ferreira M, Pousinha PA, Regen T, Santa C, Coelho JE, Marques-Morgado I, Valente CA, Omenetti S, Stockinger B, Waisman A, Manadas B, Lopes LV, Silva-Santos B and Ribot JC (2019). “Meningeal gammadelta T cell-derived IL-17 controls synaptic plasticity and short-term memory.” Sci Immunol 4(40). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribot JC, Lopes N and Silva-Santos B (2021). “gammadelta T cells in tissue physiology and surveillance.” Nat Rev Immunol 21(4): 221–232. [DOI] [PubMed] [Google Scholar]
- Ricardo-Gonzalez RR, Van Dyken SJ, Schneider C, Lee J, Nussbaum JC, Liang HE, Vaka D, Eckalbar WL, Molofsky AB, Erle DJ and Locksley RM (2018). “Tissue signals imprint ILC2 identity with anticipatory function.” Nat Immunol 19(10): 1093–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rua R and McGavern DB (2018). “Advances in Meningeal Immunity.” Trends Mol Med 24(6): 542–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudak PT, Choi J and Haeryfar SMM (2018). “MAIT cell-mediated cytotoxicity: Roles in host defense and therapeutic potentials in infectious diseases and cancer.” J Leukoc Biol 104(3): 473–486. [DOI] [PubMed] [Google Scholar]
- Rustenhoven J, Drieu A, Mamuladze T, de Lima KA, Dykstra T, Wall M, Papadopoulos Z, Kanamori M, Salvador AF, Baker W, Lemieux M, Da Mesquita S, Cugurra A, Fitzpatrick J, Sviben S, Kossina R, Bayguinov P, Townsend RR, Zhang Q, Erdmann-Gilmore P, Smirnov I, Lopes MB, Herz J and Kipnis J (2021). “Functional characterization of the dural sinuses as a neuroimmune interface.” Cell 184(4): 1000–1016 e1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C, Lee J, Koga S, Ricardo-Gonzalez RR, Nussbaum JC, Smith LK, Villeda SA, Liang HE and Locksley RM (2019). “Tissue-Resident Group 2 Innate Lymphoid Cells Differentiate by Layered Ontogeny and In Situ Perinatal Priming.” Immunity 50(6): 1425–1438.e1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedgwick AJ, Ghazanfari N, Constantinescu P, Mantamadiotis T and Barrow AD (2020). “The Role of NK Cells and Innate Lymphoid Cells in Brain Cancer.” Front Immunol 11: 1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shichita T, Sugiyama Y, Ooboshi H, Sugimori H, Nakagawa R, Takada I, Iwaki T, Okada Y, Iida M, Cua DJ, Iwakura Y and Yoshimura A (2009). “Pivotal role of cerebral interleukin-17-producing gammadeltaT cells in the delayed phase of ischemic brain injury.” Nat Med 15(8): 946–950. [DOI] [PubMed] [Google Scholar]
- Sun XH and Bajana S (2022). “ILC Differentiation in the Thymus.” Adv Exp Med Biol 1365: 25–39. [DOI] [PubMed] [Google Scholar]
- Tran GT, Hodgkinson SJ, Carter NM, Verma ND, Plain KM, Boyd R, Robinson CM, Nomura M, Killingsworth M and Hall BM (2012). “IL-5 promotes induction of antigen-specific CD4+CD25+ T regulatory cells that suppress autoimmunity.” Blood 119(19): 4441–4450. [DOI] [PubMed] [Google Scholar]
- Treiner E, Duban L, Bahram S, Radosavljevic M, Wanner V, Tilloy F, Affaticati P, Gilfillan S and Lantz O (2003). “Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1.” Nature 422(6928): 164–169. [DOI] [PubMed] [Google Scholar]
- Treiner E and Liblau RS (2015). “Mucosal-Associated Invariant T Cells in Multiple Sclerosis: The Jury is Still Out.” Front Immunol 6: 503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ussher JE, Bilton M, Attwod E, Shadwell J, Richardson R, de Lara C, Mettke E, Kurioka A, Hansen TH, Klenerman P and Willberg CB (2014). “CD161++ CD8+ T cells, including the MAIT cell subset, are specifically activated by IL-12+IL-18 in a TCR-independent manner.” Eur J Immunol 44(1): 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wilgenburg B, Loh L, Chen Z, Pediongco TJ, Wang H, Shi M, Zhao Z, Koutsakos M, Nussing S, Sant S, Wang Z, D’Souza C, Jia X, Almeida CF, Kostenko L, Eckle SBG, Meehan BS, Kallies A, Godfrey DI, Reading PC, Corbett AJ, McCluskey J, Klenerman P, Kedzierska K and Hinks TSC (2018). “MAIT cells contribute to protection against lethal influenza infection in vivo.” Nat Commun 9(1): 4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wilgenburg B, Scherwitzl I, Hutchinson EC, Leng T, Kurioka A, Kulicke C, de Lara C, Cole S, Vasanawathana S, Limpitikul W, Malasit P, Young D, Denney L, Moore MD, Fabris P, Giordani MT, Oo YH, Laidlaw SM, Dustin LB, Ho LP, Thompson FM, Ramamurthy N, Mongkolsapaya J, Willberg CB, Screaton GR and Klenerman P (2016). “MAIT cells are activated during human viral infections.” Nat Commun 7: 11653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamawaki TM, Lu DR, Ellwanger DC, Bhatt D, Manzanillo P, Arias V, Zhou H, Yoon OK, Homann O, Wang S and Li CM (2021). “Systematic comparison of high-throughput single-cell RNA-seq methods for immune cell profiling.” BMC Genomics 22(1): 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q and Bhandoola A (2016). “The development of adult innate lymphoid cells.” Curr Opin Immunol 39: 114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano H and Artis D (2022). “Neuronal regulation of innate lymphoid cell responses.” Curr Opin Immunol 76: 102205. [DOI] [PubMed] [Google Scholar]
- Zelco A, Rocha-Ferreira E, Nazmi A, Ardalan M, Chumak T, Nilsson G, Hagberg H, Mallard C and Wang X (2020). “Type 2 Innate Lymphoid Cells Accumulate in the Brain After Hypoxia-Ischemia but Do Not Contribute to the Development of Preterm Brain Injury.” Front Cell Neurosci 14: 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan L, Zheng L, Hosoi T, Okuma Y and Nomura Y (2015). “Stress-induced neuroprotective effects of epiregulin and amphiregulin.” PLoS One 10(2): e0118280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Bailey JT, Xu E, Singh K, Lavaert M, Link VM, D’Souza S, Hafiz A, Cao J, Cao G, Sant’Angelo DB, Sun W, Belkaid Y, Bhandoola A, McGavern DB and Yang Q (2022). “Mucosal-associated invariant T cells restrict reactive oxidative damage and preserve meningeal barrier integrity and cognitive function.” Nat Immunol 23(12): 1714–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.


