Abstract
Cultured cell lines infected with prions produce an abnormal isoform of the prion protein (PrPSc). In order to derive cell lines producing sufficient quantities of PrPSc for most studies, it has been necessary to subclone infected cultures and select the subclones producing the largest amounts of PrPSc. Since postinfection cloning can introduce differences between infected and uninfected cell lines, we sought an approach to generate prion-infected cell lines that would avoid clonal artifacts. Using an improved cell blot technique, which permits sensitive and rapid comparison of PrPSc levels in multiple independent cell cultures, we discovered marked heterogeneity with regard to prion susceptibility in tumor cell sublines. We exploited this heterogeneity to derive sublines which are highly susceptible to prion infection and used these cells to generate prion-infected lines without further subcloning. These infected sublines can be compared to the cognate uninfected cultures without interference from cloning artifacts. We also used susceptible cell lines and our modified cell blot procedure to develop a sensitive and reproducible quantitative cell culture bioassay for prions. We found that the sublines were at least 100-fold more susceptible to strain RML prions than to strain ME7 prions. Comparisons between scrapie-susceptible and -resistant cell lines may reveal factors that modulate prion propagation.
Prions are the transmissible pathogens that cause a class of neurodegenerative diseases in mammals including Creutzfeldt-Jakob disease in humans and scrapie in sheep. The only known component of the prion is an abnormal isoform of the prion protein (PrP) designated PrPSc. The precursor of PrPSc is PrPC, a glycophosphatidylinositol-anchored membrane protein of unknown function that is highly expressed in neurons of the central nervous system. Prion diseases can be transmitted to hamsters and mice, which are used extensively in laboratory studies of these conditions. However, the cost and complexity of prion studies in vivo have prompted many attempts to establish prion-infected cell lines. For unknown reasons, most cell lines are resistant to prion infection (4, 21). Stable propagation of prions has been established in only a few lines. The most successful of these have been derivatives of the C-1300 mouse neuroblastoma line, particularly the neuro-2A (N2a) subline (4, 20). Prion-infected N2a cells are traditionally referred to as ScN2a. Recently, a mouse hypothalamic cell line, GT1, has been infected and produces stable high titers of prions (ScGT1) (25). Although it is not widely used, the rat pheochromocytoma-derived line PC12 also has been reported to be infectible with mouse, but not rat, prions (21, 22).
Typical N2a cultures exposed to prions produce only low levels of infectious prions, apparently because only a small percentage of the cells become infected (19, 20). In order to obtain cultures that produce sufficient quantities of PrPSc for biochemical analysis, prion-exposed N2a cultures must be subcloned and the most highly infected sublines must be selected (4, 19). This method reliably produces ScN2a lines that generate large amounts of PrPSc and prion titers of about 105 50% infective dose (ID50) units/107 cells (4). About 80 to 90% of cells in these cloned, infected sublines are prion infected (reference 19 and data below).
To date, ScN2a has proved the most useful cell line for studying the cell biology of prion replication. This line has been used to determine the kinetics of PrPSc and the subcellular location of PrPSc formation (2, 6, 10, 12, 29, 30). These cells have also been used to seek inhibitors of scrapie formation, laying the groundwork for the development of antiprion therapeutic agents (5, 28). Recent studies using ScN2a cells transfected with mutant PrP sequences have mapped out regions of the PrP polypeptide responsible for dominant negative inhibition of prion propagation (13).
A number of studies have compared properties of uninfected and prion-infected cells to look for prion infection-specific alterations in cellular metabolism (7, 15, 32, 33). Such investigations may suffer from the fact that ScN2a cells are subcloned from a population of cells to which they are then compared. Tumor cells in culture are known to have heterogeneous properties due at least in part to genetic instability (16). Therefore, the observed differences between infected and uninfected cell lines do not necessarily arise as a consequence of prion infection. The observed differences might represent purely fortuitous artifacts of cloning or might be due to a selection artifact; i.e., properties specific to ScN2a cells may be those which render the cells susceptible to prion infection and might be found in only a minority of cells in the parent population.
We sought to generate prion-infected and uninfected cell lines in which artifactual differences between the lines would be eliminated. We first developed a method based on the cell blot technique for the rapid and sensitive detection of de novo prion infection in multiple independent cell cultures. Using this technique, we derived prion-susceptible cell lines from which we were able to generate prion-infected cultures without further subcloning. We also used these susceptible sublines and our modified cell blot method to develop a rapid and sensitive bioassay for one prion strain and to demonstrate the resistance of N2a to infection with other strains.
MATERIALS AND METHODS
Cell lines.
N2a cells were initially obtained from stocks from the American Type Cell Collection (ATCC). Lines designated N2a.AI or N2a.AF were taken from separate vials of cells and expanded for 5 to 10 passages before they were frozen. GT1-trk cells were procured as previously described (25).
Cell culture.
Cells were cloned by dilution into 96-well plates by standard techniques (9). Unless indicated otherwise, N2a cells were grown in Dulbecco's modified Eagle's medium containing 4.5 g of glucose per liter and supplemented with 10% fetal bovine serum and penicillin and streptomycin (high-glucose DMEM). In some cases, N2a cells were grown in minimal essential medium (MEM), supplemented as described above, or in DMEM containing 1 g of glucose per liter and supplemented as described above (low-glucose DMEM). GT1-trk cells were grown in high-glucose DMEM supplemented with G418 (300 mg/ml) and as described above. Plastic coverslips were obtained from Nunc.
Preparation of inocula.
For brain-derived homogenates, the whole brains of mice with signs of scrapie were suspended in 9 volumes of phosphate-buffered saline (PBS) and passed four times through successively smaller syringe needles from 16 to 22 gauge. Homogenates were stored at 20°C until use. For ScN2a-derived inocula, ScN2a cells were passaged at a 1:10 dilution from a confluent plate and grown for 4 days in DMEM. Cells were scraped off and suspended in PBS at a concentration of approximately 107 cells/ml on one 10-cm-diameter plate/ml. These cells were subjected to five rapid freeze-thaw cycles to kill all cells. The absence of viable cells was confirmed by plating 50 ml of the suspension in complete DMEM and observing for 2 weeks. After the freeze-thaw treatment, the cells were homogenized by passage through syringe needles as described above and stored at −20°C.
Inoculation of cells.
Cultures were split at a 1:10 dilution into 24-well tissue culture plates. Generally, either a 10% homogenate of brain or a homogenate with 107 cells/ml was added to the appropriate medium at a 1:30 ratio. In some cases, serial dilutions of this inoculum were made in the same medium. Approximately 600 μl of the inoculated medium was added to each well. Cells were grown in the presence of the inoculum for 4 days before splitting at a 1:10 ratio. Thereafter, cells were grown in uninoculated medium. N2a and GT1-trk cultures were generally split at a 1:10 ratio every 3 to 4 days. In cases where cell growth was slow, cultures were split at lower ratios.
Cell blotting.
The cell blot technique we used is a modified version of the previously described technique (27). Plastic coverslips were placed in the wells of a 24-well plate. Cells were plated at a 1:10 dilution into the wells. After 4 days in culture, the medium was removed and the wells were washed once in PBS. The coverslips were removed and placed cell side up on blotting paper. A suitably sized nitrocellulose membrane was wetted in distilled water and then soaked in lysis buffer (0.5% deoxycholate, 0.5% Triton X-100, 150 mM NaCl, and Tris HCl [pH 7.5]). This was backed with lysis buffer-soaked blotting paper, and using a glass plate, the nitrocellulose membrane was pressed firmly for 30 s onto the coverslips. The coverslips adhered to the membrane and were carefully removed with forceps or with an inverted micropipette tip attached to a vacuum. The membrane was air dried for 1 to 2 h. (At this point, it was sometimes stored in a plastic bag at −20°C for several days.) Before further processing, the blot was rewetted in lysis buffer. The blot was incubated in lysis buffer with proteinase K at 5 μg/ml for 1.5 h at 37°C with constant shaking. The blot was washed twice in distilled water and then incubated for 20 min with 5 mM phenylmethylsulfonyl fluoride in distilled water at room temperature. Next, the blot was immersed in denaturing buffer (3 M guanidine isothiocyanate, 10 mM Tris HCl [pH 8.0]) for 8 to 10 min. Until this step, all washes of the blot were treated as prion contaminated. The blot was washed three times in water and then blocked with 5% nonfat dry milk in TBST for 1 h before incubating with the polyclonal antibody R073 diluted 1:5,000 overnight in Tris-buffered saline with 0.1% Tween 20 (TBST) and 5% nonfat dry milk. Detection was performed with the ECL (Amersham) chemiluminescence technique per the manufacturer's instructions for Western blotting. Maximum sensitivity was achieved with exposure times of greater than 2 h. Densitometry was performed using NIH Image software on scanned images of photographic film.
Western blotting.
Confluent 6-cm-diameter plates (approximately 4 × 106 cells) were washed in PBS and then lysed by the addition of 300 μl of lysis buffer. The nuclear pellet was removed, and the protein concentration was determined by bicinchoninic acid assay as recommended by the manufacturer (Pierce). Proteinase K was added at a ratio of 1:50 to total protein, and the lysate was incubated at 37°C for 1 h. Insoluble material was precipitated by ultracentrifugation at 80,000 × g for 1 h. The pellet was solubilized in loading buffer, and Western blotting was performed according to standard procedures (24). Blots were probed with R073 antibody and detected with the ECL system.
RESULTS
Sensitivity of cell blotting.
We sought to develop a means to rapidly detect the earliest stages of de novo prion infection of cultured cells. We compared the sensitivity of the cell blot technique with that of Western blotting by mixing ScN2a cells with N2a cells at various ratios and assaying after coculture for 4 days. Cell blotting detected proteinase K-resistant PrP ScN2a/N2a ratios of 1:100. Western blotting performed on the entire lysate from plates with a 60-mm diameter could detect protease-resistant PrP at a 1:10 ratio (data not shown). Thus, cell blotting is sensitive to a smaller proportion of prion-infected cells and requires fewer cells be infected than Western blotting. This sensitivity facilitated rapid comparison of multiple independent cultures grown and processed in parallel.
Sublines vary in their susceptibility to prion infection.
Since uncloned cultures of N2a cells inoculated with prions produce only low levels of PrPSc but clonal sublines derived from these cultures produce high levels of PrPSc, we reasoned that uncloned populations of N2a cells might be composed of cells with different susceptibilities to prion infection. Therefore, we subcloned uninfected N2a into sublines and investigated the susceptibility of each subline to infection.
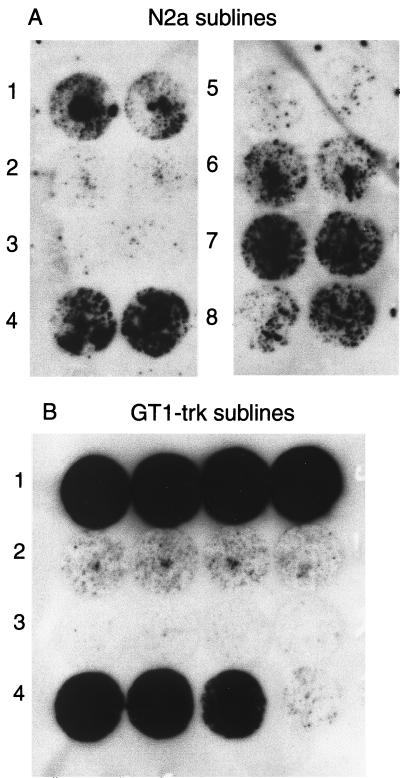
Typically, we isolated 10 to 20 sublines from a stock line. From each subline, a population of cells was inoculated with RML strain scrapie prions, while another population remained uninoculated. These subcultures were passaged in parallel to minimize differences due to culture conditions. After 18 to 30 days in culture, the sublines were assayed by cell blotting. Different sublines produced amounts of PrPSc varying from substantial to undetectable (Fig. 1). We refer to those sublines producing large amounts of PrPSc after exposure to prions as prion susceptible and to those sublines producing no detectable PrPSc after inoculation as prion resistant. In a typical experiment, we found about 20% of the sublines susceptible, about 20% resistant, and the remainder producing intermediate amounts of PrPSc. For each subline, the amount of PrPSc produced is consistent when inoculations are repeated in parallel or serially over a period of several months. After 4 to 6 months in continuous culture, there is some reduction in the amount of PrPSc produced by de novo inoculation of susceptible sublines with prions (data not shown). Susceptible and resistant sublines retained these properties after storage in liquid nitrogen for several months. Susceptible infected sublines have continued to produce large amounts of PrPSc for at least 6 months in continuous culture. Heterogeneity in susceptibility to prion infection is not solely a property of neuroblastoma cells, as we found similar variation in susceptibility to infection in GT1-trk cells (Fig. 1).
FIG. 1.
Sublines vary in susceptibility to scrapie infection. Sublines derived as described in Materials and Methods were inoculated with 30 μl of a homogenate (107 cells/ml) of ScN2a cells. (A) Eight N2a sublines inoculated in duplicate and then passaged for 25 days. Each circular blot in the figure is from a single independently inoculated and passaged population, with pairs of blots from the same subline shown side by side. Sublines 4 and 7 are susceptible, sublines 1, 6, and 8 are of intermediate susceptibility, and sublines 2, 3, and 5 are resistant. (B) GT1-trk sublines inoculated and passaged in quadruplicate for 24 days prior to blotting. Sublines 1 and 4 are susceptible, subline 2 is of intermediate susceptibility, and subline 3 is resistant. Note that the level of PrPSc is consistent in each inoculated culture derived from the same subline. (Cells were confluent on each coverslip prior to blotting except for one coverslip for GT1-trk subline 4, which had a low cell density.)
Infected susceptible sublines produce as much PrPSc as cloned ScN2a cells.
Thirty to 40 days after inoculation, cell blots of infected susceptible sublines seemed to show as much PrPSc as did blots of traditionally cloned ScN2a, which suggested to us that infected susceptible lines could be used in place of traditionally cloned ScN2a cells. Western blotting confirmed that equivalent amounts of PrPSc were produced in prion-infected susceptible sublines and cloned ScN2a cells (Fig. 2). We then compared the proportions of cells infected in susceptible sublines to the proportion infected in a traditionally cloned ScN2a line. Seven of nine lines cloned from an infected susceptible line produced PrPSc. All four sublines derived from an infected GT1-trk subline produced PrPSc (Fig. 3). In comparison, 18 of 23 sublines derived from the traditionally cloned ScN2a line produced PrPSc. We concluded that susceptible cells infected without further subcloning are infected to the same degree as cloned ScN2a cells.
FIG. 2.
Susceptible subclones produce as much PrPSc after infection as cloned ScN2a does. N2a cells are cells from ATCC stock, not subcloned. ScN2a are scrapie-infected cells derived by subcloning from an inoculated population of N2a. Lines N2a.3Sc and N2a.22Sc were inoculated with prions and then passaged without subcloning for 40 days prior to lysis. Previous cell blots (not shown) demonstrated N2a.3Sc to be prion susceptible, while N2a.22Sc was resistant. (A) Lysates not treated with protease. A total of 75 μg of protein is in each lane. The lower-molecular-weight forms of PrP typically seen in prion-infected N2a lines even without the addition of protease are present in ScN2a and N2a.3Sc lysates. (B) Proteinase K-treated lysates. The product of digestion of 500 μg of total protein is loaded in each lane. ScN2a and N2a.3Sc produce approximately equal amounts of protease-resistant PrP, while no protease-resistant PrP is detectable in N2a.22Sc.
FIG. 3.
Proportion of cells producing PrPSc is similar in inoculated susceptible sublines and ScN2a cells. N2a and GT1 sublines producing large amounts of PrPSc after inoculation with scrapie were further subcloned, and the subsublines were analyzed by cell blotting. (A) Each circular blot represents a separate subsubline derived from N2a.AI.15Sc; 7 of 9 sublines produced readily detectable amounts of proteinase K-resistant PrP. (B) Blots done in duplicate. All four GT1-trk.4Sc sublines were positive. (C) Subclones of clonally established ScN2a lines produced similar results, with 18 of 23 subclones producing detectable proteinase K-resistant PrP (each blot represents a separate subsubline).
Comparisons between infected and uninfected cultures.
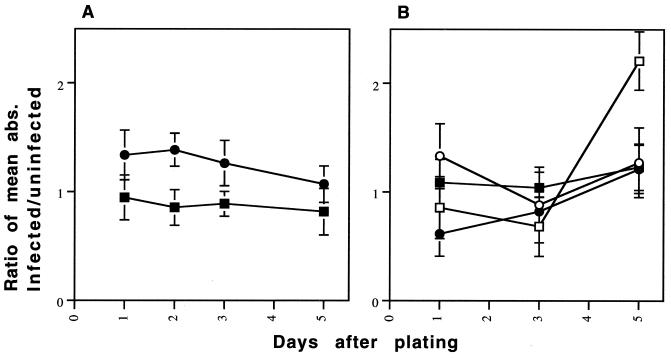
Having established prion-infected lines without cloning after infection, we used cognate pairs of infected and uninfected prion-susceptible sublines to determine whether scrapie infection affected properties of the cells other than the production of PrPSc. Because one of us (S.B.P.) has remarked on a tendency for ScN2a cell to assume a flatter, more epithelioid shape than N2a cells, we examined cell morphologies (30). Although every subline contained cells with a variety of shapes, different morphologies predominated in different sublines. Nevertheless, no consistent morphologic differences were seen between infected and uninfected cells of the same subline. Nor did we find that certain cell morphologies were associated with susceptibility to prion infection. We also compared the growth rates of infected and uninfected cells, looking for slowing of growth that might indicate a pathological effect of prion propagation in the infected cells. Although growth rate differed between sublines, it appeared to be unaffected by prion infection (Fig. 4).
FIG. 4.
Comparison of growth rates of scrapie-infected and uninfected cell lines. The graphs depict the results of two similar experiments. Cells were grown in 96-well plates. The number of viable cells were measured at each time point by the thiazolyl blue (MTT) assay. Each point represents the ratio of the mean absorbance (abs.) of 12 wells of scrapie-infected cells to the mean absorbance of 12 wells of the cognate uninfected cells. Measurements were taken at 1, 3, and 5 days. Each error bar represents the total relative standard deviation for the ratio. (A) A single prion-infected population from two different susceptible sublines is compared to its uninfected cognate line. (B) Two separately infected populations were derived from two different susceptible sublines (the two lines indicated by open symbols and solid symbols) are compared to the cognate uninfected population. A positively sloping line in either figure would indicate relatively faster growth in the scrapie-infected cells. The cell density increases each day in both scrapie-infected and uninfected populations (data not shown), but no consistent difference in the growth rates is seen between infected and uninfected sublines. The MTT assay was performed per the supplier's instructions (Sigma).
Kinetics of PrPSc production.
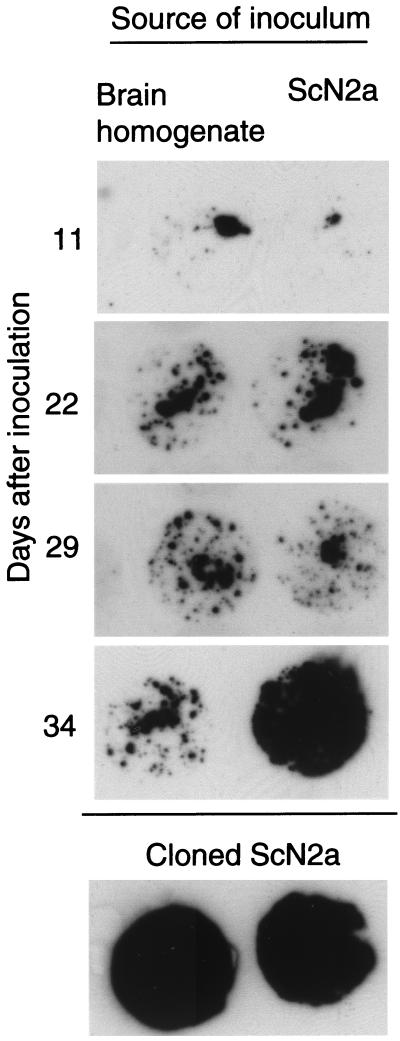
We sought to exploit the high sensitivity and reproducibility of our modified cell blot procedure and the increased susceptibility to prion infection of some of our sublines to develop a rapid bioassay for prions. First, we determined the time course of de novo PrPSc production in inoculated susceptible sublines (Fig. 5). Cells were cultured in the presence of 20 μl of a ScN2a homogenate, RML prion-infected mouse brain homogenate, or uninfected brain homogenate for 4 days. At 3- or 4-day intervals thereafter, cells were assayed and an aliquot was prepared for cell blotting. Cell blots performed 4 and 7 days after inoculation showed decreasing amounts of PrPSc, which we interpret to be residue of the inoculum. At 11 days, little or no proteinase K-resistant PrP is detected. Beginning 15 days postinoculation and increasing thereafter, some susceptible subclones produce detectable PrPSc. In repeated experiments using susceptible sublines, protease-resistant PrP could reliably be detected by 20 days after inoculation. Although the level of PrPSc increased with longer culture times, sensitivity did not increase; i.e., cultures negative at 20 days remained negative. Therefore, in subsequent experiments, cultures were assayed 20 or more days after inoculation.
FIG. 5.
Time course of accumulation of PrPSc in inoculated susceptible cells. A susceptible subline, N2a.AI.17, was inoculated with 20 μl of a 10% brain homogenate from an RML-infected CD-1 mouse (left column) or with 20 μl of ScN2a cells (107 cells/ml) (right column). At intervals after inoculation, cells were passaged and aliquots were plated for cell blotting. After 7 days (the first passage), a strong signal, thought to represent residual inoculum, was seen (not shown). The signal at day 11 may represent residual inoculum or de novo PrPSc formation. Increasing amounts of PrPSc after day 11 indicate de novo formation of PrPSc. By day 34, the amount of PrPSc in the culture inoculated with ScN2a homogenate approaches that of cloned ScN2a cells.
Effect of medium.
We next explored the effects of various culture media on PrPSc production in inoculated cells. Scrapie-susceptible N2a cells were grown in high-glucose DMEM, low-glucose DMEM, or MEM. The high- and low-glucose DMEM we used were standard formulations (Gibco-BRL) and are identical except for the glucose concentration and the presence of pyruvate in the low- but not high-glucose medium. MEM contains 1 g of glucose per liter and generally lower concentrations of amino acids and other nutrients than DMEM does. N2a cells grown in low-glucose media were morphologically indistinguishable from cells grown in high-glucose DMEM, although they grew more slowly, requiring 4 rather than 3 days to reach confluence from a 1:10 split. PrPSc was detected in all cultures exposed to high-titer inocula 20 days after inoculation, but the amount of PrPSc was consistently higher in the cells grown in high-glucose DMEM. Moreover, cultures grown in high-glucose DMEM were more sensitive to low titers of inoculum than those grown in MEM (Fig. 6). When cells grown and inoculated in MEM were switched to high-glucose DMEM for 4 days before blotting, they produced less PrPSc than cells grown in the high-glucose medium for the entire incubation period, which indicates that the lower PrPSc levels in these cells were not simply a reflection of their slower growth in low-glucose medium for the 4 days prior to blotting. Nor does the increased sensitivity seen with the high-glucose medium seem to be a conditioning effect of prolonged growth in the medium, as cultures switched from MEM to DMEM with 4.5 g of glucose per liter at the time of inoculation are as sensitive to low titers of inoculum as cells grown continuously in the high-glucose medium. Cells inoculated and grown continuously in DMEM with 1 g of glucose per liter behaved similarly to those inoculated and grown in MEM, suggesting that the concentration of glucose is a critical factor (data not shown). High-glucose DMEM consistently gave more than 1-log-unit greater sensitivity to inoculated prions than did the low-glucose media (Fig. 7). Consequently, we used high-glucose DMEM in cell culture bioassay experiments.
FIG. 6.
Effect of growth medium on susceptibility to scrapie infection. Cultures of a prion-susceptible N2a subline were inoculated with dilutions of a homogenate of ScN2a cells (107 cell/ml) and passaged in the indicated medium for 22 to 30 days before blotting. (A) Cultures inoculated and grown in high-glucose DMEM (DME) or MEM. PrPSc is detectable in cells grown in DMEM at a 2-log-unit dilution of the inoculum, but in cells grown in MEM, PrPSc is detected only in the undiluted inoculum. (B) Cultures inoculated and grown in high-glucose DMEM or MEM and then grown in high-glucose DMEM for 4 days prior to blotting. (C) Cultures grown for several weeks in either MEM or high-glucose DMEM and then switched to the other medium prior to inoculation. Uninoc., uninoculated.
FIG. 7.
Comparison of the cell blot densities of a susceptible N2a line grown in high-glucose DMEM or MEM. Cultures were inoculated with ScN2a cell homogenate. The amount inoculated is expressed as an estimate of the mouse ID50 titer, based on previous studies of ScN2a cells (4). Cell blotting was performed 25 days after inoculation. Density measurements were made using NIH Image software on scanned images of cell blots. Measurements were normalized by determining the ratio of the density of the blot to the mean density of blots of uninoculated cells on the same membrane. Each data point represents the average measurements of four separate cell blots and is expressed as a mean percent above background density [(normalized density − 1) × 100]. Error bars show the total relative standard deviation for the normalized density calculation. For cells grown in DMEM (solid squares), Student's t-test gives P values of 0.01 or lower for comparisons between any of the three inoculation titers and mean. However, for cells grown in MEM (open diamonds), P values are less than 0.05 only for the group receiving the highest inoculum titer. Thus, cell blot sensitivity is 1 to 2 log ID50 units greater for cells grown in DMEM than for cells grown in MEM.
Cell culture bioassay for prions.
In order to obtain a quantitative estimate of susceptibility of sublines to prions from different sources, we inoculated cultures with serial 10-fold dilutions of either brain homogenate from a CD-1 mouse infected with the RML strain of scrapie or homogenates of ScN2a cells infected with RML prions (4). Inoculation with dilutions to 10−2 of 10% RML mouse brain homogenate consistently produced detectable PrPSc on cell blots. ScN2a homogenate consistently produced PrPSc at 10−2 dilution and sometimes at 10−3 dilutions. A 10% RML brain homogenate contains about 106 ID50/ml, as determined by endpoint titration studies in intracerebrally inoculated CD-1 mice (8). Therefore, 10−2 dilution of this homogenate contains approximately 104 ID50 prions (Table 1). ScN2a homogenate, prepared as in this study, contains about 104 ID50 of prions per ml as determined by bioassay in mice, so a 10−2 dilution of a 30-ml inoculum contains on the order of 10 ID50 units of prions (4). Thus, this cell culture bioassay system is nearly as sensitive to ScN2a-derived prions as is bioassay by intracerebral inoculation of mice. However, when mouse brain homogenate is the inoculum, the cell culture assay is several orders of magnitude less sensitive than the mouse assay.
TABLE 1.
Effects of medium, source of inoculum, and prion strain on efficiency of de novo prion infection in susceptible N2a sublines
| Cell line (susceptible sublines) | Medium | Inoculuma | Maximum dilution of inoculum cell blot positive (log units) | Estimated minimum detectable titer (log units) |
|---|---|---|---|---|
| N2a | High-glucose DMEM | ScN2a homog. (RML) | −2 | 1 |
| N2a | Low-glucose MEM | ScN2a homog. (RML) | −1 | 2 |
| N2a | Low-glucose DMEM | ScN2a homog. (RML) | −1 | 2 |
| GT1-trk | High-glucose DMEM | ScN2a homog. (RML) | −2 | 1 |
| N2a | High-glucose DMEM | RML brain homog. | −2 | 4 |
| N2a | High-glucose DMEM | ME7 brain homog. | NAb | >6 |
homog., homogenate.
NA, not applicable. No infected cultures even when inoculum is concentrated 100-fold.
Attempts to infect sublines with various prion strains.
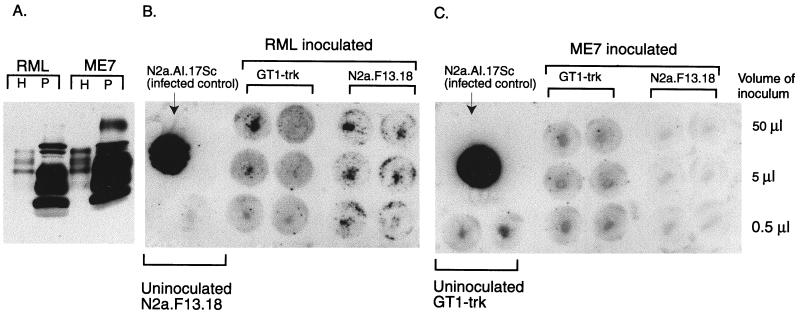
Encouraged by the high susceptibilities of some of our sublines to infection with RML prions, we attempted to establish N2a and GT-1 cells infected with other strains. However, RML-susceptible and -resistant sublines, derived from either GT1-trk or N2a cells, all failed to produce PrPSc after inoculation with brain homogenates from Prnpa/a mice infected with prion strain 139A, 301V, or ME7 (data not shown). The ME7 strain has a short incubation time in CD-1 mice, indistinguishable from that of the RML strain. For this reason, we made further attempts to establish cultures infected with this strain. We used phosphotungstic acid precipitation to concentrate PrPSc from mice infected with the ME7 and RML strains (23). We then inoculated susceptible N2a cells with the precipitated PrPSc from 0.25 g of brain, an approximately 100-fold increase over the usual undiluted inoculum. Again, only the RML inoculum resulted in detectable PrPSc in cells assayed 24 days after inoculation (Fig. 8). In order to exclude the possibility that ME7 inoculation resulted in a more protease-sensitive form of PrPSc, which might not be seen on our cell blots, we performed Western blotting on the insoluble fraction from lysates of inoculated cell cultures (31). Insoluble PrP was present only in RML-exposed cells (data not shown).
FIG. 8.
N2a and GT1-trk cells are resistant to infection with the ME7 strain of prions. (A) PrPSc was concentrated from RML- or ME7-infected mouse brains using phosphotungstic acid (PTA), as described in Materials and Methods. The Western blot compares 5 μl of 5% brain homogenate (H) to 5 μl of a 60-ml resuspension of the PTA-precipitated pellet (P) for both RML and ME7. Note that only PTA-precipitated samples were treated with proteinase K. The precipitation markedly increases the concentration of PrPSc. (B) Cell blots of GT1-trk and N2a.F13.18 cells exposed to PTA-concentrated RML 22 days before blotting. (C) GT1-trk and N2a.F13.18 cells exposed to PTA-concentrated ME7 22 days before blotting. No proteinase K-resistant PrP is seen in any ME7-inoculated culture, while all RML-inoculated N2a.F13.18 cultures and 50- and 5-ml RML-inoculated GT1-trk cultures are infected. Background staining is higher with GT1-trk cells than with N2a cells (compare the blots of uninoculated cultures).
DISCUSSION
Valid comparisons of infected and uninfected cell cultures.
Comparative studies between prion-infected and uninfected cells have the potential to demonstrate scrapie-specific alterations in cellular metabolism, alterations that may represent pathological consequences of prion propagation or compensatory mechanisms of the cell. However, the traditional method of deriving scrapie-infected cell lines results in selection or cloning artifacts that invalidate comparisons between the scrapie-infected line and its uninfected parent or sibling lines. We were able to derive highly susceptible sublines by subcloning uninfected N2a cells. We used these susceptible sublines to establish cultures in which virtually every cell was infected without further subcloning. Cognate pairs of uninfected and infected cell cultures from the same subline can be compared without danger of cloning or selection artifacts (Fig. 9).
FIG. 9.
Comparison of methods for deriving scrapie-infected cell cultures. In this schematic representation, different shapes depict the (presumably) genetic heterogeneity in the population of cultured cells. Triangles represent cells highly susceptible to prion infection, pentagons represent cells of intermediate susceptibility, and circles represent prion-resistant cells. The intensity of the red color represents the amount of PrPSc produced in a cell. In the traditional method (top), a population of cells (a) is inoculated with prions. Typically, the level of PrPSc in the inoculated culture (b) is low, necessitating a cloning step (c). The clonal population which is isolated may be representative of a minority of the cells in the heterogeneous parent culture, invalidating comparisons between the scrapie-infected clone (d) and the parent culture (a). In our improved approach (bottom), clonal populations (f) are derived from the parent culture before inoculation. These clonal populations vary in susceptibility to prion infection. Most or all cells in the most susceptible clonal populations will produce PrPSc upon inoculation with prions (g). These can be compared to the uninoculated cognate line (f) without artifacts due to cloning.
Our concerns about cloning artifacts are not merely hypothetical. Using the cell lines described here, we found that artifactual differences in cell metabolism are responsible for some previously reported differences between scrapie-infected and uninfected cells. First, based on a comparison of prion-infected and uninfected cell lines, one of us (S.B.P.) concluded that prion infection alters the expression of the 70- and 28-kDa heat shock proteins (32). Infected and uninfected pairs of sublines derived as in this study demonstrate no differences in heat shock protein expression (P. Bosque, S. Prusiner, et al., unpublished data). Thus, the previously reported differences appear to be due to a cloning artifact. Second, earlier studies had suggested that a redacted form of PrP, composed of only 106 amino acids, existed in a soluble, proteinase K-resistant form only in ScN2a cells (17). However, transfected cognate pairs of scrapie-infected and uninfected cells from the same subline express equal amounts of protease-resistant, redacted PrP (26). The previously perceived differences were due to artifactual differences in transfection efficiency between the ScN2a and N2a cells used in the original studies.
We used the cognate pairs of cell cultures to investigate two other properties we thought might be specifically associated with prion infection. Since prion propagation causes neuronal loss in animals, we reasoned that prion-infected cultures might grow more slowly than uninfected ones. However, we found no measurable difference in growth rates. In prion disease, neuronal cell death may be mediated by apoptosis (10a, 16a). Perhaps tumor cells in culture are more resistant to apoptotic cell death mediated by prion accumulation than are neurons in vivo. Alternatively, cell division may protect cultured cells from accumulating lethal quantities of PrPSc. We also compared the morphology of prion-infected and uninfected cells, because we had previously noticed a tendency for infected cells to assume a flatter, more epithelioid shape than uninfected cells. However, we saw no consistent differences in cell shape between infected and uninfected cognate pairs of cultures.
A rapid bioassay for prions.
The cell blot technique is more sensitive than the Western blot technique for the detection of PrPSc. We found that Western blotting could detect PrPSc when 10% of the cells confluent on a 6-cm-diameter dish were infected with prions, whereas cell blotting could detect PrPSc when 1% of the cells in a single well of a 24-well plate were infected. With respect to the number of infected cells, this represents an approximately 150-fold increase in sensitivity. The high sensitivity of this system made it possible for us to detect the early stages of prion propagation in de novo infected cells. We found that our modified version of cell blotting applied to susceptible sublines of N2a cells was a remarkably sensitive bioassay for infectious prions. Using ScN2a cell homogenate as an inoculum, on the order of ∼10 ID50 units could be detected in 20 days. This is more sensitive than any other in vitro assay for ScN2a-derived prions. Even the most rapid and sensitive in vivo assay, intracerebral inoculation of transgenic mice overexpressing PrP requires incubation times of about 75 days for such a low titer and would be significantly more expensive to perform (unpublished observations). The cell blot assay is less sensitive to brain-derived prions, with a detection limit of 104 ID50 units with RML prions. Why the cells are less susceptible to brain-derived prions is not clear. Possible explanations follow. (i) Prions have cell type-specific affinities so N2a cells would be more susceptible to homogeneous ScN2a prions than brain-derived prions, which are presumably derived from a mixture of cell types. (ii) ScN2a-derived prions are actually present at higher titers than are brain-derived prions but are cleared in vivo more efficiently than are brain-derived prions, effectively lowering the in vivo titer. (iii) Brain homogenate has some prion inhibitory factor, which is more effective with cultured cells than in vivo. (iv) Prions in the crude brain homogenates used in this study are more effectively mobilized by factors in the brain parenchymal milieu than by tumor cells in culture.
Strain specificity.
It is unclear why N2a and GT1-trk cells are susceptible to only the RML strain of prions. We considered the possibility that the insensitivity of these cells to some strains might be related to the longer incubation times of these strains in mice compared to that of the RML strain. In this case, the resistance of N2a and GT1-trk cells to longer-incubation strains might reflect a tendency of cells in culture to dilute out propagating prions by repeated cell division. For this reason, we focused our efforts on infecting N2a cells with the ME7 strain, which has an incubation time in Prnpa/a mice indistinguishable from that of the RML strain. (N2a cells are derived from a spontaneous tumor arising in A/J mice, a Prnpa/a mouse strain [14].) Nevertheless, ME7 failed to cause PrPSc production in RML-susceptible N2a cells, even with PrPSc concentrations in the ME7 inoculum at least 100 times higher than that necessary to cause infection with RML. Although we did not make a direct comparison with higher dilutions, this may reflect as much as a 104-fold or greater difference in susceptibility, since we successfully infected N2a with 100-fold dilutions of unconcentrated, RML-infected brain homogenate (Table 1). A caveat is that although the quantities of PrPSc in the concentrated RML and ME7 homogenates used in this study were approximately equal, the infectious titers were not directly compared by limiting dilution in intracerebrally inoculated mice. It is conceivable that despite the similar incubation times in CD-1 mice, the infectious titer is actually lower in the ME7 homogenate. Nevertheless, this demonstration that N2a cells are more sensitive to some prion strains than others is congruent with the observation that certain regions of the brain accumulate more PrPSc with some strains than others, presumably reflecting preferential replication of certain strains in certain subpopulations of brain neurons (3, 11). The apparent strain specificity of the present system restricts its usefulness as a rapid bioassay.
Scrapie-resistant sublines.
In addition to highly susceptible sublines, we also derived sublines that appear to be resistant to scrapie infection. Studies of resistant and susceptible sublines of tumor cells have proved useful in other fields, e.g., in analyzing the metabolism of anti-neoplastic agents (1, 18). An analogous approach using these scrapie-susceptible and -resistant subclones may detect cellular factors inhibiting or promoting prion propagation. In our initial analysis, we found that resistance of some sublines to scrapie infection may be attributable to a relatively low level of PrPC production (unpublished observations). However, we see a marked difference in susceptibility to scrapie infection even among sublines with moderate and high levels of PrP production. This suggests that factors other than PrPC levels, which vary from subline to subline, may be responsible for the different susceptibilities to scrapie. We are currently engaged in a search for these factors that confer susceptibility or resistance to prion infection.
In summary, we have developed a new approach to the study of prion-infected cells in culture. This approach systematically minimizes the possibility of clonal artifacts and permits the valid comparison of infected and uninfected cells. The techniques we apply here should be employed in future comparative studies of infected and uninfected cells. Using this approach, we were able to derive highly susceptible, uninfected cells, from which we developed a rapid and inexpensive quantitative bioassay for RML prions. Unfortunately, this cultured cell bioassay is highly dependent on the prion strain. Our observations may also lead to a new means for elucidating the cellular physiology of prion replication through the comparison of prion-susceptible and -resistant cell lines.
REFERENCES
- 1.Baguley B C, Marshall E S, Whittaker J R, Dotchin M C, Nixon J, McCrystal M R, Finlay G J, Matthews J H, Holdaway K M, van Zijl P. Resistance mechanisms determining the in vitro sensitivity to paclitaxel of tumour cells cultured from patients with ovarian cancer. Eur J Cancer. 1995;31A:230–237. doi: 10.1016/0959-8049(94)00472-h. [DOI] [PubMed] [Google Scholar]
- 2.Borchelt D R, Scott M, Taraboulos A, Stahl N, Prusiner S B. Scrapie and cellular prion proteins differ in their kinetics of synthesis and topology in cultured cells. J Cell Biol. 1990;110:743–752. doi: 10.1083/jcb.110.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruce M E, McBride P A, Farquhar C F. Precise targeting of the pathology of the sialoglycoprotein, PrP, and vacuolar degeneration in mouse scrapie. Neurosci Lett. 1989;102:1–6. doi: 10.1016/0304-3940(89)90298-x. [DOI] [PubMed] [Google Scholar]
- 4.Butler D A, Scott M R D, Bockman J M, Borchelt D R, Taraboulos A, Hsiao K K, Kingsbury D T, Prusiner S B. Scrapie-infected murine neuroblastoma cells produce protease-resistant prion proteins. J Virol. 1988;62:1558–1564. doi: 10.1128/jvi.62.5.1558-1564.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caughey B, Race R E. Potent inhibition of scrapie-associated PrP accumulation by Congo red. J Neurochem. 1992;59:768–771. doi: 10.1111/j.1471-4159.1992.tb09437.x. [DOI] [PubMed] [Google Scholar]
- 6.Caughey B, Raymond G J. The scrapie-associated form of PrP is made from a cell surface precursor that is both protease- and phospholipase-sensitive. J Biol Chem. 1991;266:18217–18223. [PubMed] [Google Scholar]
- 7.Doh-ura K, Perryman S, Race R, Chesebro B. Identification of differentially expressed genes in scrapie-infected mouse neuroblastoma cells. Microb Pathog. 1995;18:1–9. [PubMed] [Google Scholar]
- 8.Eklund C M, Kennedy R C, Hadlow W J. Pathogenesis of scrapie virus infection in the mouse. J Infect Dis. 1967;117:15–22. doi: 10.1093/infdis/117.1.15. [DOI] [PubMed] [Google Scholar]
- 9.Freshney R I. Animal cell culture: a practical approach. Oxford, England: IRL Press; 1992. [Google Scholar]
- 10.Gorodinsky A, Harris D A. Glycolipid-anchored proteins in neuroblastoma cells form detergent-resistant complexes without caveolin. J Cell Biol. 1995;129:619–627. doi: 10.1083/jcb.129.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10a.Gray F, Chrétien F, Adle-Biassette H, Dorandeu A, Ereau T, Delisle M B, Koop N, Ironside J W, Vital C. Neuronal apoptosis in Creutzfeldt-Jakob disease. J Neuropathol Exp Neurol. 1999;58:321–328. doi: 10.1097/00005072-199904000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Hecker R, Taraboulos A, Scott M, Pan K-M, Torchia M, Jendroska K, DeArmond S J, Prusiner S B. Replication of distinct prion isolates is region specific in brains of transgenic mice and hamsters. Genes Dev. 1992;6:1213–1228. doi: 10.1101/gad.6.7.1213. [DOI] [PubMed] [Google Scholar]
- 12.Kaneko K, Vey M, Scott M, Pilkuhn S, Cohen F E, Prusiner S B. COOH-terminal sequence of the cellular prion protein directs subcellular trafficking and controls conversion into the scrapie isoform. Proc Natl Acad Sci USA. 1997;94:2333–2338. doi: 10.1073/pnas.94.6.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaneko K, Zulianello L, Scott M, Cooper C M, Wallace A C, James T L, Cohen F E, Prusiner S B. Evidence for protein X binding to a discontinuous epitope on the cellular prion protein during scrapie prion propagation. Proc Natl Acad Sci USA. 1997;94:10069–10074. doi: 10.1073/pnas.94.19.10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klebe R J, Ruddle F H. Neuroblastoma: cell culture analysis of a differentiating stem cell system. J Cell Biol. 1969;43:69a. [Google Scholar]
- 15.Kristensson K, Feuerstein B, Taraboulos A, Hyun W C, Prusiner S B, DeArmond S J. Scrapie prions alter receptor-mediated calcium responses in cultured cells. Neurology. 1993;43:2335–2341. doi: 10.1212/wnl.43.11.2335. [DOI] [PubMed] [Google Scholar]
- 16.Leith J T. Mammalian tumor cell heterogeneity. Boca Raton, Fla: CRC Press; 1986. [Google Scholar]
- 16a.Lucassen P J, Williams A, Chung W C, Fraser H. Detection of apoptosis in murine scrapie. Neurosci Lett. 1999;198:185–188. doi: 10.1016/0304-3940(95)11995-9. [DOI] [PubMed] [Google Scholar]
- 17.Muramoto T, Scott M, Cohen F E, Prusiner S B. Recombinant scrapie-like prion protein of 106 amino acids is soluble. Proc Natl Acad Sci USA. 1996;93:15457–15462. doi: 10.1073/pnas.93.26.15457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perez M, Haschke B, Donato N J. Differential expression and translocation of protein tyrosine phosphatase 1B-related proteins in ME-180 tumor cells expressing apoptotic sensitivity and resistance to tumor necrosis factor: potential interaction with epidermal growth factor receptor. Oncogene. 1999;18:967–978. doi: 10.1038/sj.onc.1202368. [DOI] [PubMed] [Google Scholar]
- 19.Race R E, Caughey B, Graham K, Ernst D, Chesebro B. Analyses of frequency of infection, specific infectivity, and prion protein biosynthesis in scrapie-infected neuroblastoma cell clones. J Virol. 1988;62:2845–2849. doi: 10.1128/jvi.62.8.2845-2849.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Race R E, Fadness L H, Chesebro B. Characterization of scrapie infection in mouse neuroblastoma cells. J Gen Virol. 1987;68:1391–1399. doi: 10.1099/0022-1317-68-5-1391. [DOI] [PubMed] [Google Scholar]
- 21.Rubenstein R, Carp R I, Callahan S H. In vitro replication of scrapie agent in a neuronal model: infection of PC12 cells. J Gen Virol. 1984;65:2191–2198. doi: 10.1099/0022-1317-65-12-2191. [DOI] [PubMed] [Google Scholar]
- 22.Rubenstein R, Deng H, Race R E, Ju W, Scalici C L, Papini M C, Kascsak R, Carp R I. Demonstration of scrapie strain diversity in infected PC12 cells. J Gen Virol. 1992;73:3027–3031. doi: 10.1099/0022-1317-73-11-3027. [DOI] [PubMed] [Google Scholar]
- 23.Safar J, Wille H, Itri V, Groth D, Serban H, Torchia M, Cohen F E, Prusiner S B. Eight prion strains have PrPSc molecules with different conformations. Nat Med. 1998;4:1157–1165. doi: 10.1038/2654. [DOI] [PubMed] [Google Scholar]
- 24.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 25.Schätzl H M, Laszlo L, Holtzman D M, Tatzelt J, DeArmond S J, Weiner R I, Mobley W C, Prusiner S B. A hypothalamic neuronal cell line persistently infected with scrapie prions exhibits apoptosis. J Virol. 1997;71:8821–8831. doi: 10.1128/jvi.71.11.8821-8831.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Supattapone S, Bosque P, Muramoto T, Wille H, Aagaard C, Peretz D, Nguyen H-O B, Heinrich C, Torchia M, Safar J, Cohen F E, DeArmond S J, Prusiner S B, Scott M. Prion protein of 106 residues creates an artificial transmission barrier for prion replication in transgenic mice. Cell. 1999;96:869–878. doi: 10.1016/s0092-8674(00)80596-6. [DOI] [PubMed] [Google Scholar]
- 27.Taraboulos A, Jendroska K, Serban D, Yang S-L, DeArmond S J, Prusiner S B. Regional mapping of prion proteins in brains. Proc Natl Acad Sci USA. 1992;89:7620–7624. doi: 10.1073/pnas.89.16.7620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taraboulos A, Raeber A, Borchelt D, McKinley M P, Prusiner S B. Brefeldin A inhibits protease resistant prion protein synthesis in scrapie-infected cultured cells. FASEB J. 1991;5:A1177. [Google Scholar]
- 29.Taraboulos A, Scott M, Semenov A, Avrahami D, Laszlo L, Prusiner S B. Cholesterol depletion and modification of COOH-terminal targeting sequence of the prion protein inhibits formation of the scrapie isoform. J Cell Biol. 1995;129:121–132. doi: 10.1083/jcb.129.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taraboulos A, Serban D, Prusiner S B. Scrapie prion proteins accumulate in the cytoplasm of persistently infected cultured cells. J Cell Biol. 1990;110:2117–2132. doi: 10.1083/jcb.110.6.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tatzelt J, Prusiner S B, Welch W J. Chemical chaperones interfere with the formation of scrapie prion protein. EMBO J. 1996;15:6363–6373. [PMC free article] [PubMed] [Google Scholar]
- 32.Tatzelt J, Zuo J, Voellmy R, Scott M, Hartl U, Prusiner S B, Welch W J. Scrapie prions selectively modify the stress response in neuroblastoma cells. Proc Natl Acad Sci USA. 1995;92:2944–2948. doi: 10.1073/pnas.92.7.2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong K, Qiu Y, Hyun W, Nixon R, VanCleff J, Sanchez-Salazar J, Prusiner S B, DeArmond S J. Decreased receptor-mediated calcium response in prion-infected cells correlates with decreased membrane fluidity and IP3 release. Neurology. 1996;47:741–750. doi: 10.1212/wnl.47.3.741. [DOI] [PubMed] [Google Scholar]