Abstract
OBJECTIVES:
Although clinicians may use methylene blue (MB) in refractory septic shock, the effect of MB on patient-important outcomes remains uncertain. We conducted a systematic review and meta-analysis to investigate the benefits and harms of MB administration in patients with septic shock.
DATA SOURCES:
We searched six databases (including PubMed, Embase, and Medline) from inception to January 10, 2024.
STUDY SELECTION:
We included randomized clinical trials (RCTs) of critically ill adults comparing MB with placebo or usual care without MB administration.
DATA EXTRACTION:
Two reviewers performed screening, full-text review, and data extraction. We pooled data using a random-effects model, assessed the risk of bias using the modified Cochrane tool, and used Grading of Recommendations Assessment, Development, and Evaluation to rate certainty of effect estimates.
DATA SYNTHESIS:
We included six RCTs (302 patients). Compared with placebo or no MB administration, MB may reduce short-term mortality (RR [risk ratio] 0.66 [95% CI, 0.47–0.94], low certainty) and hospital length of stay (mean difference [MD] –2.1 d [95% CI, –1.4 to –2.8], low certainty). MB may also reduce duration of vasopressors (MD –31.1 hr [95% CI, –16.5 to –45.6], low certainty), and increase mean arterial pressure at 6 hours (MD 10.2 mm Hg [95% CI, 6.1–14.2], low certainty) compared with no MB administration. The effect of MB on serum methemoglobin concentration was uncertain (MD 0.9% [95% CI, –0.2% to 2.0%], very low certainty). We did not find any differences in adverse events.
CONCLUSIONS:
Among critically ill adults with septic shock, based on low-certainty evidence, MB may reduce short-term mortality, duration of vasopressors, and hospital length of stay, with no evidence of increased adverse events. Rigorous randomized trials evaluating the efficacy of MB in septic shock are needed.
REGISTRATION:
Center for Open Science (https://osf.io/hpy4j).
Keywords: critical care medicine, methylene blue, septic shock
KEY POINTS.
Question: What is the efficacy and safety of methylene blue (MB) administration, as compared with placebo or usual care without MB, in patients with septic shock?
Findings: Six randomized controlled trials were included. Compared with placebo or no MB administration, MB may reduce short-term mortality, duration of vasopressors, and hospital length of stay. Few adverse events were reported across the included trials.
Meaning: In patients with septic shock, administration of MB may improve patient outcomes, but more rigorous randomized data surrounding the efficacy and safety of MB are needed.
Sepsis and septic shock are caused by a dysregulated host response to infection, resulting in organ dysfunction, and potentially leading to death (1). Worldwide, septic shock remains one of the leading causes of death, particularly in low- and middle-income countries (2). Along with early initiation of antimicrobials and source control, treatment is focused on fluid resuscitation and vasopressor administration to maintain organ perfusion (3). Prolonged and high doses of vasoactive medications may be associated with adverse effects, such as tachyarrhythmia, myocardial dysfunction, and peripheral ischemia (4, 5). Therefore, there is an urgent need for alternative therapeutic agents to assist the hemodynamic support of patients with profound shock secondary to sepsis.
Methylene blue (MB) can restore vascular tone through specific inhibition of endothelial and inducible nitric oxide synthase, and its downstream enzyme soluble guanylate cyclase (sGC) (6). MB has been shown to restore vasoregulation in vasoplegic conditions of nitrous oxide up-regulation, such as septic shock (7), improving hemodynamics in patients with profound vasoplegia. MB has demonstrated effects in patients with hypotension following cardiac surgery (postcardiopulmonary bypass) and in acute poisoning from calcium channel blockers (8, 9). Despite this, evidence examining the use of MB in septic shock remains sparse, including only small randomized clinical trials (RCTs). Furthermore, the risk of MB-associated adverse effects is unclear (10). To address this critical knowledge gap, we conducted a systematic review and meta-analysis summarizing the efficacy and safety of MB in adult patients with septic shock.
MATERIALS AND METHODS
We followed the Preferred Reporting Items for Systematic Review and Meta-Analysis statement guidelines (11). We registered the protocol with the Center for Open Science (https://osf.io/hpy4j). Institutional review board approval was not required, as all study data had been published previously, and we did not include individual patient data.
Data Sources and Search Strategy
We searched six databases (Medline, PubMed, Embase, Scopus, Web of Science, and the Cochrane Database of Systematic Reviews) from inception to January 10, 2024. An experienced health sciences librarian assisted in the development of the search strategy. The search strategy is shown in Supplemental Figure 1 (http://links.lww.com/CCX/B354). We conducted further surveillance searches using the “related articles” feature (12).
Study Selection
Two reviewers (S.M.F. and A.T.) independently screened titles and abstracts identified through the searches using Covidence (Melbourne, Australia). The same two reviewers independently assessed full texts of the selected articles from phase one. Disagreements were resolved by discussion. We sought to include all studies, published in any language, describing retrospective and prospective observational studies, or RCTs. We included studies meeting all of the following criteria: 1) enrolled or presented a subgroup analysis of adult patients (16 yr old or older), with a diagnosis of septic shock, 2) Evaluated the use of IV MB at any dose, duration of therapy, and with any timing, and 3) Compared patients receiving MB to those not receiving MB, or those administered placebo. “Sepsis” and “septic shock” were defined using any criteria used by the primary study authors, including the systemic inflammatory response syndrome criteria (13), or the more recent Sepsis-3 criteria using organ dysfunction (Sequential Organ Failure Assessment score ≥ 2) (1). We initially sought to include observational studies, regardless of whether the results were adjusted for known confounders. However, once the review was completed, we found no observational studies meeting our criteria and focused entirely on RCTs.
Data Extraction
Two investigators (S.M.F. and A.T.) abstracted the following variables from the included articles: authors; year of publication; trial design and dates; eligibility criteria; number of patients; dose, timing, and method of MB administration; and efficacy and safety outcomes, using a predesigned data extraction sheet (Supplemental Table 1, http://links.lww.com/CCX/B354). We focused on the following efficacy outcomes, as defined by the trial authors: short-term mortality (longest reported up to 60 d, or in-hospital [14]), long-term mortality (longest reported 61 d or later, and not including in-hospital), shock reversal (as defined by trial authors, e.g., time to vasopressor discontinuation, time to lactate clearance, etc), vasopressor-free days, duration of mechanical ventilation, and length of stay in the ICU and hospital. Adverse events included any of the following: extravasation of MB, tissue necrosis, methemoglobinemia (within 72 hr of drug administration), hemolytic anemia, serotonin syndrome, and any other serious events (as defined by individual study authors). Two investigators (S.M.F. and A.T.) independently collected outcome information. For studies reporting the median and interquartile range, we estimated the mean and sd, using previously published methods (15). Disagreements were resolved through discussion.
Risk-of-Bias Assessment
Two reviewers (S.M.F. and A.T.) independently assessed the risk of bias in the included studies, using the modified Cochrane Risk of Bias Tool for Randomized Controlled Trials (CLARITY Group). We judged each criterion for each trial as low or probably low risk of bias, or high or probably high risk of bias. Studies with high or probably high risk of bias were considered as “high risk of bias.” Reviewers resolved disagreements through discussion.
Data Synthesis and Analysis
We performed meta-analyses using the random-effects method with inverse variable weighting (16), and the Review Manager software (Version 5.3, Copenhagen, Denmark). In keeping with published guidance, and due to methodological heterogeneity, we planned to pool RCTs separately from observational studies (17). For RCTs, we present pooled risk ratios (for dichotomous outcomes), or mean difference (MD; for continuous outcomes), both with 95% CIs. For observational studies, we planned to present pooled unadjusted and adjusted odds ratios, along with 95% CI. We assessed for statistical heterogeneity (or “inconsistency,” in Grading of Recommendations, Assessment, Development, and Evaluation [GRADE] terminology) using the I2 statistic, the chi-square test for homogeneity, and visual inspection of the forest plots.
Subgroup and Sensitivity Analyses
We performed preplanned sensitivity analyses, excluding trials judged to be “high” risk of bias. We also performed a subgroup analysis comparing trials administering MB as a bolus only versus as a continuous infusion (with or without a bolus dose). For any statistically significant subgroup effects, we assessed credibility using the Instrument to assess the Credibility of Effect Modification Analyses (18).
Assessment of Certainty of Evidence
We used the GRADE approach to assess the certainty of evidence for each outcome (19). Input was received from all coinvestigators to ensure agreement with the certainty assessments. We categorized the overall certainty in estimates for each outcome into 1 of 4 levels: high, moderate, low, or very low. The certainty assessments are based not only on the magnitude of the effect estimate alone, but additionally encompass the risk of bias, consistency, directness, and precision. We describe results using the appropriate GRADE narrative statements, based on certainty of evidence (“probably,” “may,” etc) (20).
RESULTS
Search Results and Study Characteristics
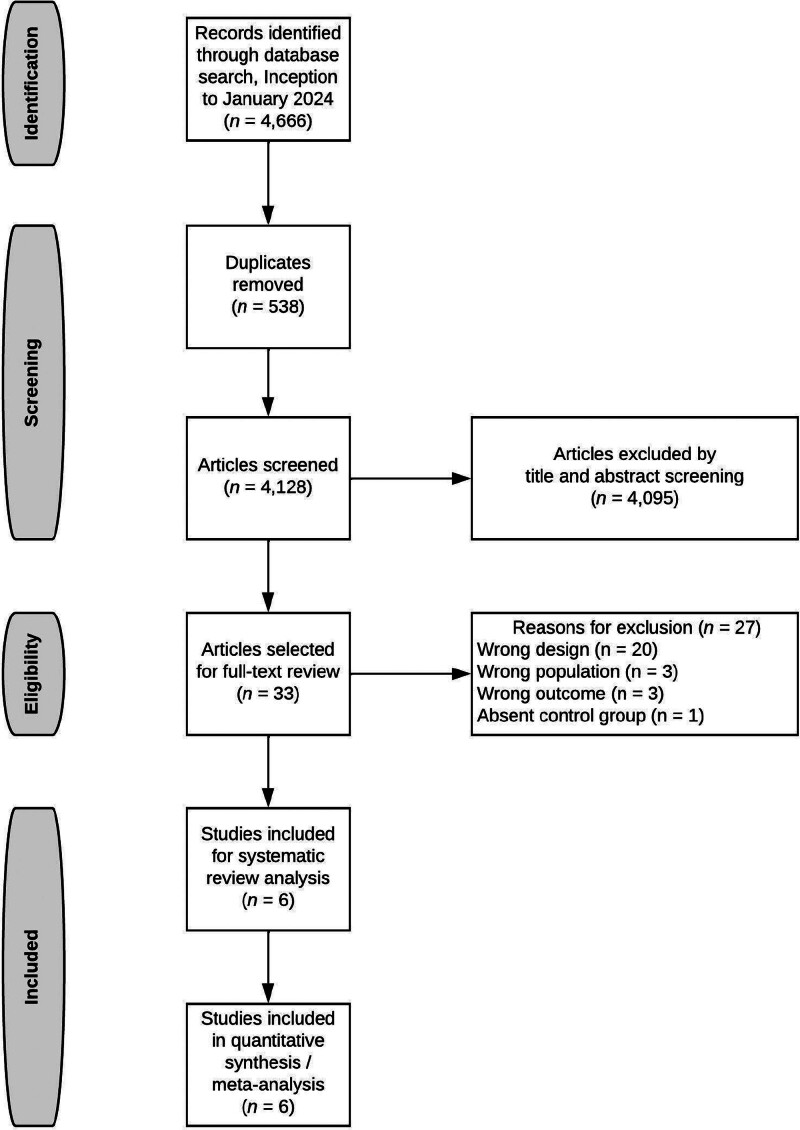
Of 4,128 citations (Fig. 1), we reviewed 33 full texts and included Six eligible studies (all RCTs) (21–26), examining 302 total patients. There were no disagreements among reviewers. Three of these RCTs were published in China (23, 24, 26), and were translated by one of the study authors (L.W.). We did not identify any observational studies meeting the eligibility criteria. Study characteristics are shown in Table 1, with further details depicted in Supplemental Table 2 (http://links.lww.com/CCX/B354). All trials were conducted in critically ill patients. One trial administered MB as a single bolus (23), and five trials used a continuous infusion (with or without bolus) (21, 22, 24–26). Only two trials (21, 22) detailed the timing of MB administration relative to the initiation of vasopressors. Risk-of-bias assessment of the included trials is shown in Supplemental Table 3 (http://links.lww.com/CCX/B354). Only two trials were judged to have “low” risk of bias (21, 25), and detailed methods of blinding MB administration. The other four trials were judged to have “high” risk of bias.
Figure 1.
Flowchart summarizing evidence search and study selection.
TABLE 1.
Characteristics of the Six Randomized Trials
| References | Country (Sites) | Population | Inclusion Criteria | Exclusion Criteria | Dose of Methylene Blue | Total Sample Size | Mean Age (yr), % Male |
|---|---|---|---|---|---|---|---|
| Ibarra-Estrada et al (21) | Mexico (1) | ICU | 18 yr old or older, with septic shock (Sepsis-3 criteria) | > 24 hr of norepinephrine infusion; pregnancy; high probability of death; concurrent hemorrhage; obstructive or hypovolemic shock; pending surgery; major burn injury; personal or family history of G6PD deficiency; allergy to MB, phenothiazines, or food dyes; recent selective serotonin reuptake inhibitor intake | IV infusion of 100 mg MB in 500 mL of 0.9% normal saline over 6 hr daily, given within 24 hr of initiation of norepinephrine; 3 d total | 92 | 46.5, 60.9% |
| Kirov et al (22) | Norway (1) | ICU | 18 yr old or older, with septic shock (≥ 2 SIRS criteria and a mean arterial pressure of < 70 mm Hg at 30 min despite fluid resuscitation or receiving vasopressors) | Pregnancy, receiving corticosteroids, immunosuppressants, chemotherapy, known irreversible underlying disease (such as end-stage neoplasms) | IV bolus of 2 mg/kg for 15 min, within 2 hr of randomization; followed by infusion of escalating doses of 0.25–2 mg/kg/hr for 1 each | 20 | 57.4, 55% |
| Li (23) | China (1) | ICU | Septic shock (unclear definition) | Abnormal liver and kidney function, patients with incomplete data | IV bolus of 2 mg/kg for 15 min | 66 | 52.7, 59.1% |
| Lu et al (24) | China (1) | ICU | 18 yr old or older, with septic shock (Sepsis-3 criteria) | History of previous myocardial infarction or stroke in the preceding 3 mo, pregnancy, known anaphylaxis to MB. nitrate use in previous 3 d | IV bolus of 2 mg/kg with or without 2mg/kg infusion over 24 hr | 54 | 64, 53.7% |
| Memis et al (25) | Turkey (1) | Operating room and ICU | 18 yr old or older, with septic shock (≥ 2 SIRS criteria and a mean arterial pressure of < 70 mm Hg at 30 min despite fluid resuscitation or receiving vasopressors) | Pregnancy, receiving corticosteroids, immunosuppressants; chemotherapy, known irreversible underlying disease (such as end-stage neoplasms) | IV infusion of 0.5 mg/kg/h over 6 hr | 30 | 52.4, 60% |
| Xiong et al (26) | China (1) | Operating room and ICU | Septic shock (unclear definition) undergoing emergency surgery | Abnormal liver or kidney function, previous methemoglobinemia, previous carbon monoxide or cyanide poisoning | IV infusion of 0.5–1.0 mg/kg/hr during surgery | 40 | 47.5, 52.5% |
G6PD = glucose-6-phosphate dehydrogenase, MB = methylene blue, SIRS = systemic inflammatory response syndrome.
Efficacy Outcomes
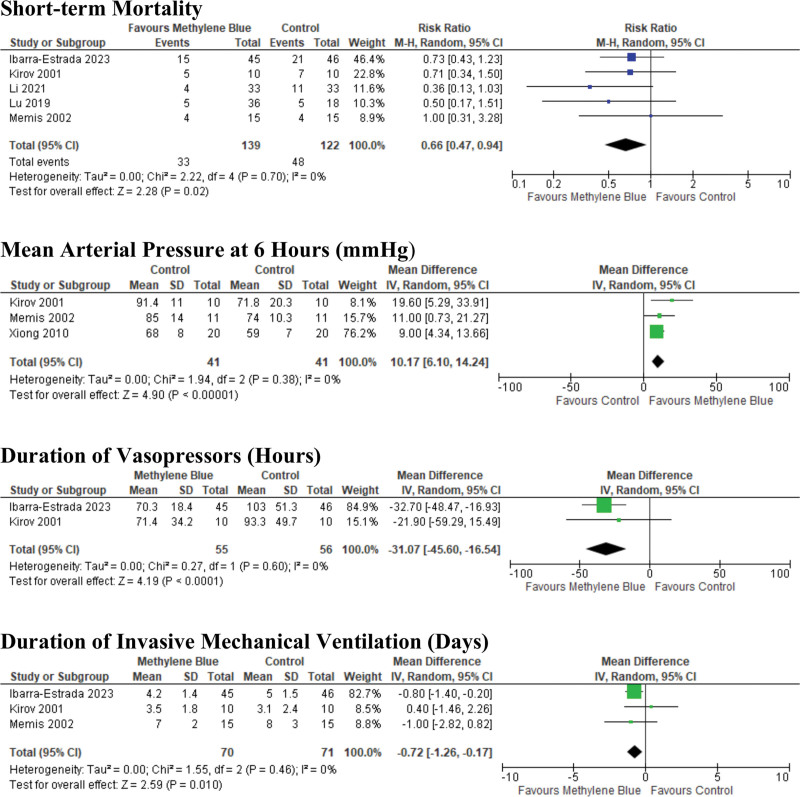
The primary efficacy outcomes are shown in Table 2, and forest plots are displayed in Figure 2 and Supplemental Figure 2 (http://links.lww.com/CCX/B354). GRADE Evidence Profile is included in Supplemental Table 4 (http://links.lww.com/CCX/B354). We found that MB may reduce short-term mortality in patients with septic shock (RR 0.66 [95% CI, 0.47–0.94]), based on low-certainty evidence, downgraded for imprecision and risk of bias among the included trials. With regard to reversal of shock, MB may increase mean arterial pressure (MAP) at 6 hours after administration (MD 10.2 mm Hg [95% CI, 6.1–14.3], low certainty) and decrease duration of vasopressors (MD –31.1 hr [95% CI, –16.5 to –45.6], low certainty). MB may decrease hospital length of stay (MD –2.1 d [95% CI, –1.4 to –2.8], low certainty), ICU length of stay (MD –1.05 d [95% CI, –2.6 to +0.5], low certainty), and may have no effect on the duration of invasive mechanical ventilation (MD –0.7 d [95% CI, –1.3 to –0.2], low certainty).
TABLE 2.
Pooled Efficacy and Safety Outcomes Comparing Effect of Methylene Blue With Placebo or Usual Care
| Outcome | Studies (Patients) | Pooled Risk Ratio or Mean Difference (95% CI) | p | I2 (%) | Grading of Recommendations Assessment, Development, and Evaluation Certainty |
|---|---|---|---|---|---|
| Short-term mortality | 5 (260) | 0.66 (0.47 to 0.95) | 0.02 | 0 | Low |
| Mean arterial pressure at 6 hr (mm Hg) | 3 (82) | 10.2 (6.1 to 14.2) | < 0.00001 | 0 | Low |
| Duration of vasopressors (hr) | 2 (111) | –31.1 (–45.6 to –16.5) | < 0.00001 | 0 | Low |
| ICU length of stay (d) | 3 (133) | –1.1 (–2.6 to 0.5) | 0.19 | 37 | Very low |
| Hospital length of stay (d) | 3 (177) | –2.1 (–2.8 to –1.4) | < 0.0001 | 0 | Low |
| Duration of invasive mechanical ventilation (d) | 3 (141) | –0.7 (–1.3 to –0.2) | 0.01 | 0 | Low |
| Methemoglobin concentration (%) | 3 (133) | 0.9 (–0.2 to 2.0) | 0.11 | 99 | Very low |
Figure 2.
Forest plots depicting the efficacy of methylene blue versus placebo or usual care.
Adverse Events
Adverse events were not uniformly reported across trials, but are summarized in Table 3. The only adverse event that was amenable to meta-analysis was methemoglobin concentration, as reported in Three trials (21, 22, 25) (Table 2); the effect of MB on this outcome was uncertain (MD 0.9% [95% CI, –0.2 to 2.0], very low certainty). Otherwise, few adverse events were reported across trials.
TABLE 3.
Adverse Events Described Across Trials
| References | Adverse Events |
|---|---|
| Ibarra-Estrada et al (21) | Green-blue discoloration of urine in 42/45 (93%) of patients receiving methylene blue. No difference in ejection fraction, arterial oxygen concentration, serum creatinine, bilirubin, or liver enzymes |
| Kirov et al (22) | The majority of patients (unclear proportion) developed a noticeable blue-gray skin color for 1–3 d. Blue discoloration of urine noted for 2–4 d. No difference in ejection fraction, arterial oxygen concentration, or laboratory values |
| Li (23) | Not recorded |
| Lu et al (24) | Not recorded |
| Memis et al (25) | None identified |
| Xiong et al (26) | Not recorded |
Subgroup and Sensitivity Analyses
Forest plots for sensitivity analyses are shown in Supplemental Figure 3 (http://links.lww.com/CCX/B354). Following the exclusion of trials at high risk of bias, the effect of MB on mortality was attenuated (RR 0.77 [95% CI, 0.48–1.24]), but MB was still associated with reduced duration of vasopressors (based only on the trial from Ibarra-Estrada et al [21]), and reduced duration of invasive mechanical ventilation (MD –0.8 d [95% CI, –1.4 d to –0.3 d]). The subgroup analysis excluding trials only using bolus of MB (i.e., without infusion) yielded similar results to the primary analysis.
DISCUSSION
We conducted a systematic review and meta-analysis to evaluate the efficacy and safety of MB administration in patients with septic shock. Although existing systematic reviews have been performed evaluating the use of MB, none have specifically evaluated patients with septic shock, and few have attempted to capture adverse events, therefore leading to variability in included trials, and conflicting conclusions, particularly with regard to mortality (27–29). Furthermore, these existing reviews have not incorporated GRADE certainty estimates, which are especially important given the paucity of available evidence. Although this review includes a small number of trials and patients, we found low-certainty evidence suggesting that MB may reduce short-term mortality in patients with septic shock. We also found that administration of MB may increase MAP, decrease the duration of exposure to vasopressors, and reduce hospital length of stay. There were minimal adverse events reported across trials. Taken together, this review highlights the potential role of MB administration as a possible therapeutic strategy for septic shock and demonstrates that further high-quality randomized studies are necessary.
Sepsis and septic shock are often characterized by a systemic inflammatory response, resulting in profound vasodilation, which can lead to dysregulation of endothelial homeostasis and endothelial dysfunction (30). The nitric oxide pathway plays a particular role in this pathophysiology, as nitrosylation of the heme iron within soluble guanylate cyclase leads to increased synthesis of cyclic guanosine monophosphate, which subsequently acts on several targets (such as protein kinases), resulting in vasodilation. Clinically, this manifests in hypotension and impaired perfusion and oxygenation of tissues, leading to progressive organ dysfunction and death. The ability of MB to inhibit nitric oxide synthesis may attenuate the resultant vasodilation and maintain or improve tissue perfusion. This phenomenon has been consistently demonstrated in animal models of septic shock (31), but limited data exist in humans.
The results of this review suggest that MB administration may reduce short-term mortality, providing emerging evidence of potential benefits in patients with septic shock. This review builds upon the existing evidence supporting the use of MB in other states of vasoplegia, such as postcardiopulmonary bypass and toxicity from beta-blockers and calcium channel blockers (8, 9). If large, geographically representative trials show that MB, a relatively inexpensive treatment (32), reduces mortality in septic shock, the findings may be generalizable to resource-constrained settings globally, where the burdens of sepsis incidence and mortality are high (2, 33). In addition, we documented low-certainty evidence that MB may facilitate reversal of shock and decrease the duration of vasopressors, which may indirectly support its potential to reduce mortality. As vasopressors may be associated with important adverse effects such as arrhythmia and myocardial dysfunction (4, 5), the benefits of MB may partly be explained by a reduction in exposure to these agents. Existing evidence also suggests that the harms of vasopressors may be more pronounced in older patients (65 yr old or older), where higher exposure has been associated with increased mortality (34). This is particularly important given the aging population, as older patients have higher incidence of septic shock and worse outcomes (35). Therefore, MB may have an even more important role in these high-risk patients, pending additional evidence about adverse effects in this vulnerable population.
We also sought to evaluate potential harms or adverse events associated with MB administration—an important consideration in the evaluation of any potential therapy. Although reporting of adverse events across the included trials was inconsistent, we found little reported evidence of harm associated with MB administration. Although often used as a treatment for methemoglobinemia, MB can paradoxically also increase methemoglobin levels (6). However, we found no difference across trials in methemoglobin concentrations following MB administration in the three trials that measured methemoglobin concentrations. Two trials described discoloration of the urine and skin (21, 22), but this was not reported to be associated with patient-important outcomes. Furthermore, no differences in arterial oxygen concentration, renal function, or liver function were reported. Therefore, although based on low-certainty evidence, with an important degree of ongoing uncertainty, the existing randomized trial evidence does not demonstrate an increased risk of adverse events associated with MB administration.
Although this review highlights the potential of MB as a therapeutic strategy in septic shock, the inherent uncertainty in the literature suggests that clinical equipoise regarding its utility may exist (10). Given the limited data describing the current use of MB in septic shock, a practice audit and clinician survey would be particularly useful components of a future research program on this topic. The use of MB has frequently been cited for “refractory” shock (6), but given the high mortality associated with such conditions, any potential benefit of MB is likely to be when administered earlier when organ dysfunction is still potentially reversible. Administration of MB in included trials has focused upon early, adjunctive use (21); a better understanding of current and optimal drug timing and dosing is crucial. Finally, while this review summarizes the existing trial data evaluating the use of MB in septic shock, the evidence base is still fairly small, including only 302 patients across all trials; highlighting the need for further randomized evidence.
This review has important strengths, including the investigation of a novel intervention and an important research question. We screened all available articles from numerous databases and translated articles as required. We performed sensitivity and subgroup analyses and used GRADE to rate certainty in the effect estimates. This review also has important limitations. First, there were few randomized trials, with variable inclusion criteria (reflecting changing definitions for “sepsis”), and the overall number of included patients was small, contributing to imprecision. Second, the majority of the included trials were judged to be at high risk of bias, largely due to lack of blinding and lack of concealment. That said, administration of MB is difficult to blind, due to the dark color of the drug itself, and the changes to urine and skin color. We accounted for these issues of imprecision and individual trial risk of bias in GRADE assessments. Nonetheless, it should be noted that the primary pooled point estimates largely included trials with a high risk of bias. Third, there were limited data available describing adverse events, which were not systematically reported across trials. Thus, conclusions regarding the safety of MB should be tempered. There were also limited data available describing cointerventions, such as the type and volume of fluid resuscitation, corticosteroids, and adjuncts such as midodrine or thiamine. Finally, we did not have sufficient available data to examine subgroups of patients with septic shock based on clinical characteristics (e.g., age, sex, source of infection) or biologic profile (e.g., inflammatory markers, etc). Heterogeneity of treatment effect with MB is plausible, and certain subgroups of patients may benefit, while others may experience harm, which would require exploration in very large randomized trials.
CONCLUSIONS
In this systematic review and meta-analysis, based on low certainty of evidence, we found that the administration of MB may reduce short-term mortality, duration of vasopressors, and hospital length of stay, with minimal evidence of adverse events. This review highlights the need for large, rigorous randomized trials evaluating the effect of MB in patients with septic shock on clinically important and patient-important outcomes.
Supplementary Material
Footnotes
The study was supported by the Lakeridge Health Foundation, Lakeridge Health Corporation (Oshawa, ON, Canada).
Dr. Seely holds patents related to multiorgan variability analysis and has shares in Therapeutic Monitoring Systems. Dr. Cook is supported by a Canada Research Chair in Critical Care Knowledge Translation. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Drs. Fernando, Soliman, and Rochwerg conceived the study idea. Drs. Fernando, Tran, and Rochwerg coordinated the systematic review. Drs. Fernando and Tran designed the search strategy, screened abstracts and full texts, acquired the data, and judged the risk of bias in the studies. Dr. Tran verified the data and performed the analyses. Dr. Rochwerg created the Grading of Recommendations Assessment, Development, and Evaluation evidence profiles. All authors interpreted the data analyses, co-wrote and revised the article for intellectual content, provided their final approval for article submission, and agreed to be accountable for all aspects of the work.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Contributor Information
Alexandre Tran, Email: alexandre.tran@medportal.ca.
Karim Soliman, Email: ksoliman@lh.ca.
Barbara Flynn, Email: bflynn@lh.ca.
Thomas Oommen, Email: toommen@lh.ca.
Li Wenzhe, Email: 13062801628@163.com.
Neill K.J. Adhikari, Email: Neill.Adhikari@sunnybrook.ca.
Salmaan Kanji, Email: skanji@toh.ca.
Andrew J.E. Seely, Email: aseely@toh.ca.
Alison E. Fox-Robichaud, Email: afoxrob@mcmaster.ca.
Randy S. Wax, Email: rwax@lh.ca.
Deborah J. Cook, Email: debcook@mcmaster.ca.
François Lamontagne, Email: francois.lamontagne@usherbrooke.ca.
Bram Rochwerg, Email: rochwerg@mcmaster.ca.
REFERENCES
- 1.Singer M, Deutschman CS, Seymour CW, et al. : The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016; 315:801–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rudd KE, Johnson SC, Agesa KM, et al. : Global, regional, and national sepsis incidence and mortality, 1990-2017: Analysis for the Global Burden of Disease Study. Lancet 2020; 395:200–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evans L, Rhodes A, Alhazzani W, et al. : Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med 2021; 47:1181–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McIntyre WF, Um KJ, Alhazzani W, et al. : Association of vasopressin plus catecholamine vasopressors vs catecholamines alone with atrial fibrillation in patients with distributive shock: A systematic review and meta-analysis. JAMA 2018; 319:1889–1900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sacha GL, Lam SW, Wang L, et al. : Association of catecholamine dose, lactate, and shock duration at vasopressin initiation with mortality in patients with septic shock. Crit Care Med 2022; 50:614–623 [DOI] [PubMed] [Google Scholar]
- 6.Kwok ES, Howes D: Use of methylene blue in sepsis: A systematic review. J Intensive Care Med 2006; 21:359–363 [DOI] [PubMed] [Google Scholar]
- 7.Puntillo F, Giglio M, Pasqualucci A, et al. : Vasopressor-sparing action of methylene blue in severe sepsis and shock: A narrative review. Adv Ther 2020; 37:3692–3706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Busse LW, Barker N, Petersen C: Vasoplegic syndrome following cardiothoracic surgery-review of pathophysiology and update of treatment options. Crit Care 2020; 24:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jang DH, Donovan S, Nelson LS, et al. : Efficacy of methylene blue in an experimental model of calcium channel blocker-induced shock. Ann Emerg Med 2015; 65:410–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arias-Ortiz J, Vincent JL: Administration of methylene blue in septic shock: Pros and cons. Crit Care 2024; 28:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Page MJ, McKenzie JE, Bossuyt PM, et al. : The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021; 372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sampson M, Shojania KG, McGowan J, et al. : Surveillance search techniques identified the need to update systematic reviews. J Clin Epidemiol 2008; 61:755–762 [DOI] [PubMed] [Google Scholar]
- 13.Bone RC, Balk RA, Cerra FB, et al. : Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992; 101:1644–1655 [DOI] [PubMed] [Google Scholar]
- 14.Friedrich JO, Harhay MO, Angus DC, et al. ; International Forum for Acute Care Trialists (InFACT): Mortality as a measure of treatment effect in clinical trials recruiting critically ill patients. Crit Care Med 2023; 51:222–230 [DOI] [PubMed] [Google Scholar]
- 15.Wan X, Wang W, Liu J, et al. : Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014; 14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DerSimonian R, Laird N: Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177–188 [DOI] [PubMed] [Google Scholar]
- 17.Cumpston M, Li T, Page MJ, et al. : Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for systematic reviews of interventions. Cochrane Database Syst Rev 2019; 10:ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schandelmaier S, Briel M, Varadhan R, et al. : Development of the instrument to assess the credibility of effect modification analyses (ICEMAN) in randomized controlled trials and meta-analyses. CMAJ 2020; 192:E901–E906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guyatt GH, Oxman AD, Vist GE, et al. ; GRADE Working Group: GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336:924–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santesso N, Glenton C, Dahm P, et al. ; GRADE Working Group: GRADE guidelines 26: Informative statements to communicate the findings of systematic reviews of interventions. J Clin Epidemiol 2020; 119:126–135 [DOI] [PubMed] [Google Scholar]
- 21.Ibarra-Estrada M, Kattan E, Aguilera-González P, et al. : Early adjunctive methylene blue in patients with septic shock: A randomized controlled trial. Crit Care 2023; 27:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirov MY, Evgenov OV, Evgenov NV, et al. : Infusion of methylene blue in human septic shock: A pilot, randomized, controlled study. Crit Care Med 2001; 29:1860–1867 [DOI] [PubMed] [Google Scholar]
- 23.Li QS: Application of methylene blue in septic shock. Diet Health 2021; 9:79 [Google Scholar]
- 24.Lu YP, Yu HJ, Liu QY, et al. : Efficacy of continuous intravenous infusion of methylene blue in patients with septic shock. Nat Med J China 2019; 99:4 [Google Scholar]
- 25.Memis D, Karamanlioglu B, Yuksel M, et al. : The influence of methylene blue infusion on cytokine levels during severe sepsis. Anaesth Intensive Care 2002; 30:755–762 [DOI] [PubMed] [Google Scholar]
- 26.Xiong XQ, Jin LD, Wang LR, et al. : Effect of methylene blue on intraoperative oxygen metabolism in patients with septic shock. China J Anesthesiol 2010; 30:4 [Google Scholar]
- 27.Pruna A, Bonaccorso A, Belletti A, et al. : Methylene blue reduces mortality in critically ill and perioperative patients: A meta-analysis of randomized trials. J Cardiothorac Vasc Anesth 2024; 38:268–274 [DOI] [PubMed] [Google Scholar]
- 28.Pasin L, Umbrello M, Greco T, et al. : Methylene blue as a vasopressor: A meta-analysis of randomised trials. Crit Care Resusc 2013; 15:42–48 [PubMed] [Google Scholar]
- 29.Huang X, Yan W, Chen Z, et al. : Effect of methylene blue on outcomes in patients with distributive shock: A meta-analysis of randomised controlled trials. BMJ Open 2024; 14:e080065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gotts JE, Matthay MA: Sepsis: Pathophysiology and clinical management. BMJ 2016; 353:i1585. [DOI] [PubMed] [Google Scholar]
- 31.Keaney JF, Jr, Puyana JC, Francis S, et al. : Methylene blue reverses endotoxin-induced hypotension. Circ Res 1994; 74:1121–1125 [DOI] [PubMed] [Google Scholar]
- 32.Bužga M, Machytka E, Dvořáčková E, et al. : Methylene blue: A controversial diagnostic acid and medication? Toxicol Res (Camb) 2022; 11:711–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adhikari NK, Fowler RA, Bhagwanjee S, et al. : Critical care and the global burden of critical illness in adults. Lancet 2010; 376:1339–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lamontagne F, Day AG, Meade MO, et al. : Pooled analysis of higher versus lower blood pressure targets for vasopressor therapy septic and vasodilatory shock. Intensive Care Med 2018; 44:12–21 [DOI] [PubMed] [Google Scholar]
- 35.Martin GS, Mannino DM, Moss M: The effect of age on the development and outcome of adult sepsis. Crit Care Med 2006; 34:15–21 [DOI] [PubMed] [Google Scholar]