Abstract
Improved treatment outcomes for non-melanoma skin cancers can be achieved if Vitamin D (Vit D) is used as a neoadjuvant prior to photodynamic therapy (PDT). However, the mechanisms for this effect are unclear. Vit D elevates protoporphyrin (PpIX) levels within tumor cells, but also exerts immune-modulatory effects. Here, two murine models, UVB-induced actinic keratoses (AK) and human squamous cell carcinoma (A431) xenografts, were used to analyze the time course of local and systemic immune responses after PDT ± Vit D. Fluorescence immunohistochemistry of tissues and flow analysis (FACS) of blood were employed. In tissue, damage-associated molecular patterns (DAMPs) were increased, and infiltration of neutrophils (Ly6G+), macrophages (F4/80+), and dendritic cells (CD11c+) were observed. In most cases, Vit D alone or PDT alone increased cell recruitment, but Vit D + PDT showed even greater recruitment effects. Similarly for T cells, increased infiltration of total (CD3+), cytotoxic (CD8+) and regulatory (FoxP3+) T-cells was observed after Vit D or PDT, but the increase was even greater with the combination. FACS analysis revealed a variety of interesting changes in circulating immune cell levels. In particular, neutrophils decreased in the blood after Vit D, consistent with migration of neutrophils into AK lesions. Levels of cells expressing the PD-1+ checkpoint receptor were reduced in AKs following Vit D, potentially counteracting PD-1+ elevations seen after PDT alone. In summary, Vit D and ALA-PDT, two treatments with individual immunogenic effects, may be advantageous in combination to improve treatment efficacy and management of AK in the dermatology clinic.
Keywords: Photodynamic therapy, Vitamin D, Non-melanoma skin cancer, Murine model, Immune response
1. Introduction
The incidence of non-melanoma skin cancer (NMSC), including basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) is continuously increasing at the global level [1,2]. NMSCs typically develop in chronically sun-exposed areas of skin after several decades, with the UVB component of sunlight acting as both tumor-initiator and tumor-promoter [3–5]. Actinic keratoses (AK, or pre-SCC) are precancerous lesions that develop after prolonged exposure to sun in Caucasian populations, and carry a significant potential for malignant progression to SCC if undiagnosed and left untreated [6–9]. Some of the most commonly used treatment options available for AKs and NMSC are cryotherapy, topical 5-fluorouracil (5FU), laser therapy, electrocautery, surgery, topical diclofenac, topical imiquimod, and photodynamic therapy (PDT) [10,11]. Amongst those treatment options, PDT offers particular advantages because it is non-invasive, non-scarring, and non-mutagenic. PDT is especially useful for numerous AK lesions that occur in broad areas of field cancerization, typically on the face, scalp, arms, or legs. This is because PDT targets subclinical (invisible) neoplastic lesions as well as visible ones, and due to its non-scarring and non-mutagenic properties can be safely administered as many times as needed to achieve complete lesion clearance [10,11].
Photodynamic therapy (PDT) is a multi-component modality that employs a photosensitizer (PS) and light in the presence of tissue oxygen to kill cancer cells [12–14]. Over the past two decades, PDT has evolved into a popular treatment option for NMSCs in dermatology and dermatologic oncology clinics [7,14–16]. Aminolevulinic acid (ALA)--mediated PDT (ALA-PDT) utilizes ALA as a prodrug which is selectively absorbed by cancer cells and converted into a photosensitizer, protopophyrin IX (PpIX), within mitochondria; PpIX is then activated by exposure to blue or red light [12–14,17,18]. Unfortunately, while PDT is quite effective for AKs, superficial Bowen’s disease (BD), superficial BCC and in -situ SCC lesions, ALA-PDT is less effective for treatment of thicker or larger lesions, mainly due to inefficient ALA absorption, non-uniform distribution of PpIX, and limited penetration of light for activation of PS in deeper tumor areas [14,19,20]. To overcome this limitation, our laboratory has explored combinations of various differentiation-promoting agents, including methotrexate (MTX), 5FU, and vitamin D (Vit D) as a pretreatment (neoadjuvant) with PDT in order to enhance PpIX levels and cell death post PDT (reviewed in [12,14,16]). Vit D may be one of the most interesting and well-studied of these neoadjuvantal agents. Vit D induces cellular differentiation and simultaneously enhances PpIX accumulation and cell death after PDT [21]. In immortalized rat keratinocyte 3D organotypic cultures, pretreatment with Vit D (calcitriol) led to significant increases in PpIX levels followed by phototoxic cell killing when exposed to blue light [22]. In mice, Vit D pretreatment of superficial skin cancers (produced by DMBA/TPA chemical carcinogenesis) or of SCC xenografts tumors (A431 cells implanted subcutaneously) caused enhanced intra-tumoral PpIX levels and resulted in a significant increase in PDT-induced cell death after exposure to light, relative to vehicle treated controls [23]. In humans, to establish proof of principle that a link between cellular differentiation and PDT exists, a clinical trial using topical Vit D and PDT for psoriasis in humans showed that when patients with bilateral psoriasis plaques on their elbows were treated with calcipotriol ointment (treatment) or inert ointment (control) for three days prior to blue light PDT, the Vit D treated plaques attained higher PpIX levels (~130 %) and reduced redness, thickness, itching and scaling relative to the control plaques [24]. Unfortunately, topical Vit D application is hampered by limited penetration, whereas systemic delivery of calcitriol carries a possible risk of hypervitaminosis D and hypercalcemia. To bypass these undesirable side effects, we explored a safer alternative, which is to use the oral/dietary form of Vit D (cholecalciferol; D3), rather than the potent/active hormonal form (calcitriol; 1,25-dihydroxy D3). In a preclinical study using subcutaneous A431 tumors in mice, ingestion of a diet containing either five- or ten-fold higher than normal amounts of cholecalciferol (D3), followed by delivery of systemic ALA, led to significantly enhanced PpIX levels and increased cell death after PDT compared to the tumors from normal diet mice [25]. Since that preclinical study established the efficacy of dietary D3 as a neoadjuvant, the feasibility of oral Vit D as a PDT neoadjuvant was tested in a clinical trial by Bullock et al. [26]. That trial, in patients with AK (pre-SCC) lesions of the face and scalp, established two interesting findings. First, it demonstrated that patients with Vit D deficiency (calcidiol levels <30 ng/dL) show lower clearance rates relative to patients with normal Vit D levels (calcidiol levels >30 ng/dL), in response to PDT. Second, it found that patients receiving oral Vit D pills (10,000 IU), had better lesion clearance rates than patients receiving placebo pills [26]. Overall, the study by Bullock et al. clearly demonstrated that dietary Vit D can be successfully used as a neoadjuvant for PDT [26]. A different research group in Brazil showed that a topical Vit D analog (calcipotriol) can be an effective neoadjuvant for PDT of AKs of the scalp [27]; however, calcipotriol is not approved for this indication in the U.S.
Regarding possible mechanisms of how Vit D might potentiate the efficacy of PDT, attention has turned recently toward understanding immune-modulatory effects of PDT and Vit D. Over the past decade, oncologists have come to realize that some cancer therapies including radiation therapy and PDT, in addition to causing immediate destruction of some tumor cells, also stimulate another type of cell death called immunogenic cell death (ICD), in which partially-damaged cells express increased levels of proteins called damage-associated molecular patterns (DAMPs), which act to induce an anti-tumor immune response [28–31]. PDT in particular generates significant ICD (reviewed in [32]), leading to activation of innate and adaptive arms of the immune system and generating long-term anti-tumor immunity, which could theoretically eliminate tumors completely if the process were optimized [33–36]. Toward that end, we demonstrated that even a mild PDT regimen can generate a significant anti-tumor immune response in a murine model of cutaneous AK, characterized by infiltration of neutrophils, macrophages, and T cells into the lesions, beginning within days and lasting several weeks after PDT [37].
Vitamin D, in addition to its classical roles in bone and muscle health (calcium homeostasis), also has important effects upon the immune system and cancer susceptibility [38]. Vit D deficiency is not only linked to increased incidence of several cancers including NMSCs [38–40] but is also associated with immune dysfunction. The latter was dramatically illustrated by increased morbidity and mortality in Vit D deficient patients during the Covid-19 pandemic [41,42]. Vit D receptors (VDR) are found on many types of cells involved in the immune response, which participate in both immunomodulatory and immunostimulatory effects [39,43]. Particular alleles (single nucleotide variants) of the human Vit D receptor were recently shown to confer increased susceptibility to development of AKs and squamous cell carcinoma in humans [40]. This suggests the involvement of Vit D and the VDR in anti-tumor immunity, as Vit D has been shown to regulate activation of innate immunity and adaptive immunity through direct effects on antigen-presenting cells such as dendritic cells (DC), and T cells [38,39,43].
Given the existing evidence that PDT and Vit D, given individually, can influence anti-tumor immunity in various types of cancer, it seemed pertinent to asked what the immune-modulatory influences Vit D and PDT might be when they are used together in a skin cancer model. In this study, we used a murine model of actinic keratosis to explore how a combination of neoadjuvantal topical Vit D and PDT, two treatment regimens with known immunomodulatory properties when applied individually, stimulates intratumoral recruitment of immune cells into the neoplastic lesions. Additionally, we asked how pretreatment of subcutaneous SCC tumors (human A431 cells in nude mice), using systemic Vit D followed by ALA-PDT, affects innate immune response.
2. Materials and methods
2.1. Cell culture
Human squamous cell carcinoma cells (A431) were purchased from ATCC and cultured in DMEM (supplemented with 10 % fetal bovine serum and penicillin-streptomycin) at 37 °C under 5 % CO2, as per instructions by the vendor.
2.2. UV-induced AK mouse model
Murine model of actinic keratosis (AK) was generated by exposing SKH-1 hairless female mice at 8 weeks of age to UV-irradiation three times weekly. Chronic UV exposure resulted in appearance of AK lesions resembling human actinic keratosis both morphologically and histologically around week 15 [44]. All experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the Cleveland Clinic.
2.3. Subcutaneous SCC (A431) xenograft model
A431 cells (1 × 106) were suspended in injection medium (0.1 ml; Matrigel™ mixed 1:1 with growth medium) and injected bilaterally in the flanks of 8 weeks old female nude mice. Palpable subcutaneous tumors developed at the site of injection after 8–10 days and used for the experiments [23,25].
2.4. Neoadjuvant pretreatment and photodynamic therapy
AK lesions were preconditioned with topical Vit D ointment (3 μg calcitriol/gram; Vectical™), once daily for 3 days. Aquaphor™ (petroleum jelly) was topically applied as vehicle control. Mice with AK lesions were anesthetized with inhalant isoflurane using a vaporizer and ALA solution [20 % in phosphate buffered saline (PBS) with 2 % EDTA and 5 % DMSO] was topically applied and anesthetized mice were immediately placed under Blu-U™ light source (Sun Pharmaceuticals, Mumbai, India; 417 nm, 30 min, 18 Joules/cm2) for PDT using a regimen designed to mimic the clinical protocol currently used for ‘painless PDT’ [45], but adapted here for hairless mice [37]. Mice were sacrificed and lesions harvested at various times post PDT as shown in the figures. Please note: In all figures which show a time course (Figs. 2–7), a label of “VD” on the X-axis means a 3-day treatment (or pretreatment, if followed by PDT) has been performed.
Fig. 2.
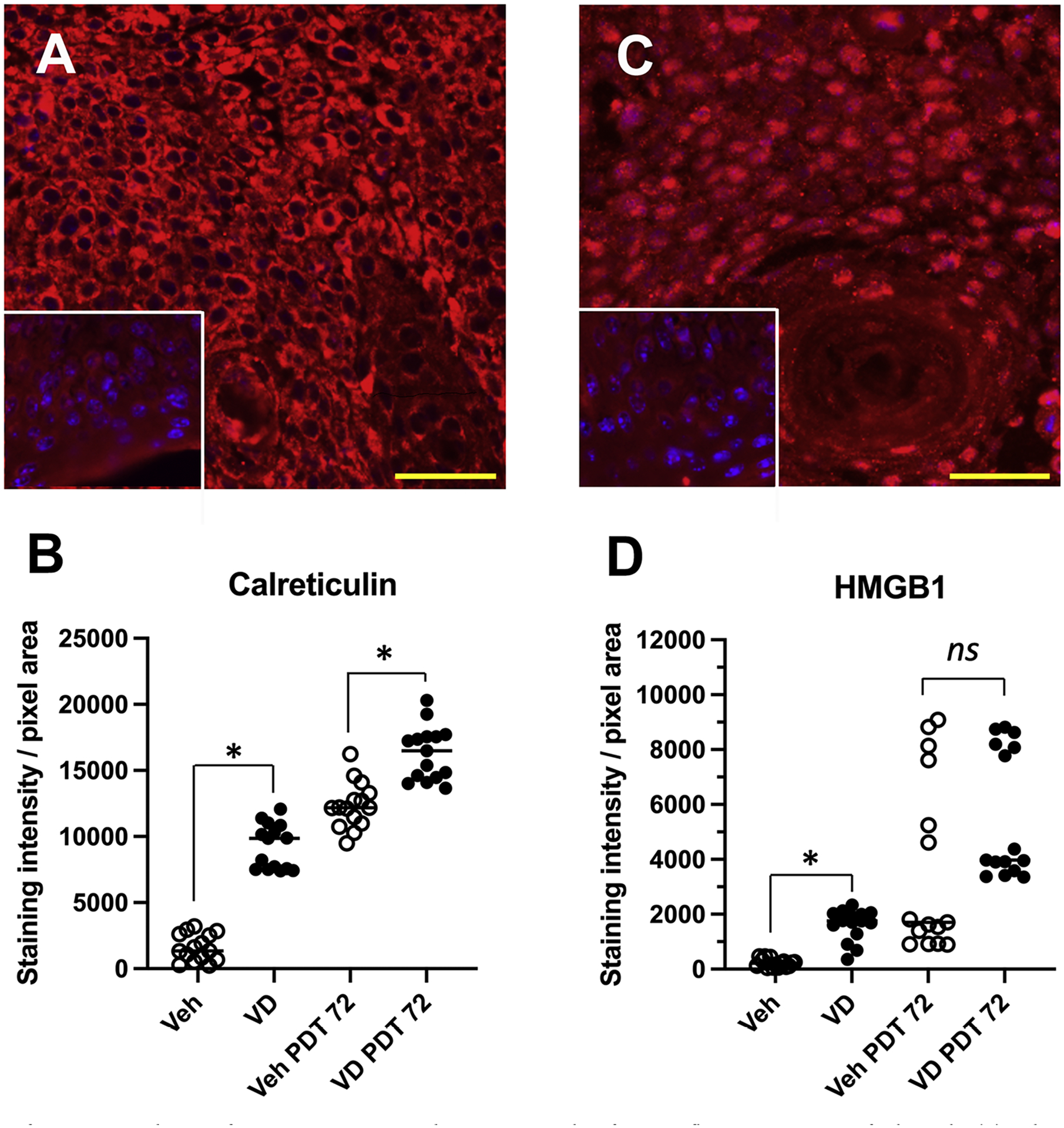
Expression of DAMPS in AK lesions after exposure to Vit D and/or PDT. Examples of immunofluorescent staining of calreticulin (A) and HMGB1 (C), at 72 h after Vit D pretreatment and PDT exposure. Insets, staining controls with no primary antibody. (B, D) Quantification of staining intensity for calreticulin and HMGB1, respectively, from 5 mice (3 lesions/mouse) per experimental condition. Statistical comparisons: Mann-Whitney non-parametric tests, (*), p < 0.0001; ns, not significant. Scale bars, 50 μM.
Fig. 7.
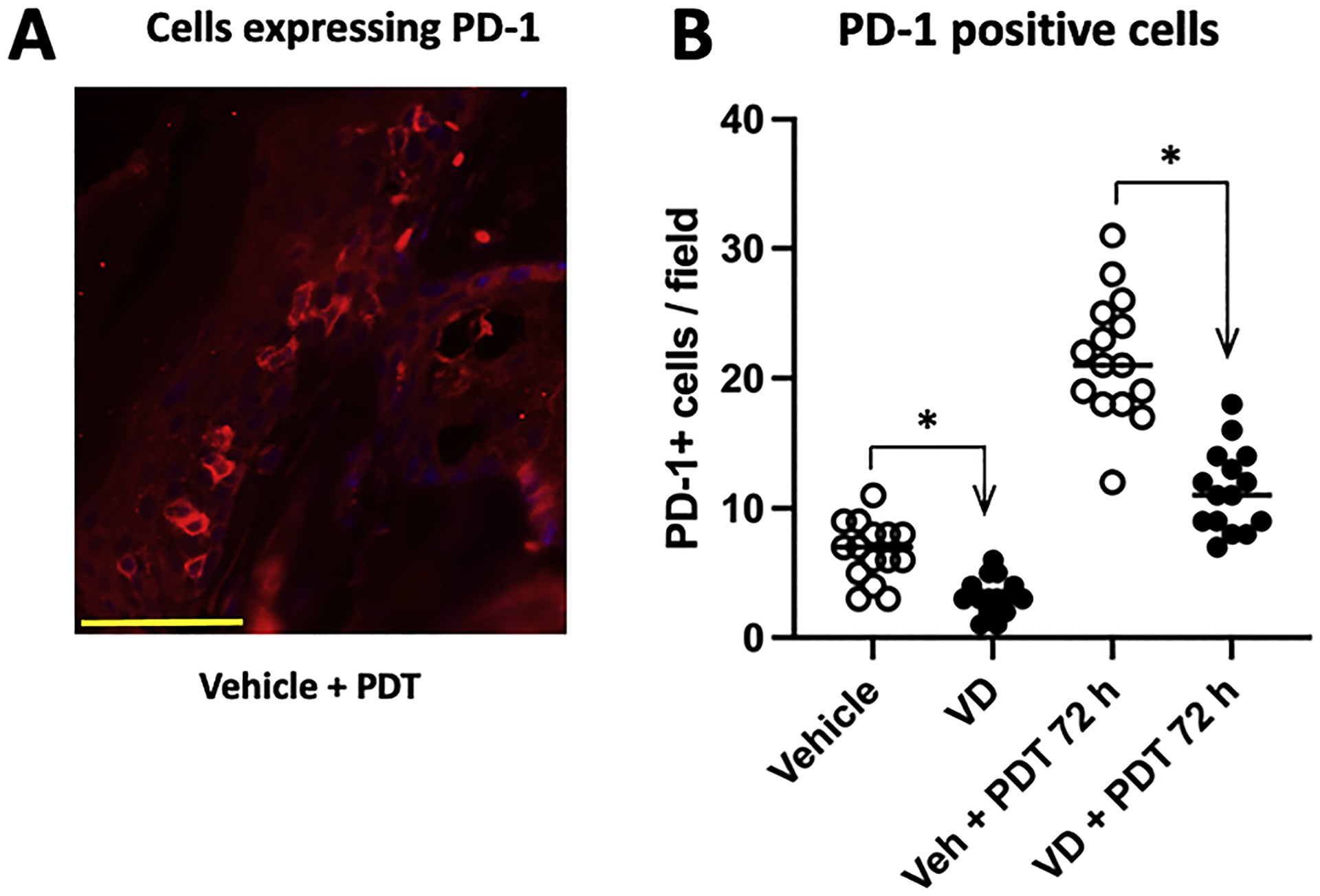
Number of cells expressing PD-1 in AK lesions. AK-bearing mice were either untreated or treated with Vit D alone, PDT alone, or combined Vit d-plus-PDT; lesions were biopsied 72 h post-PDT and the number of cells expressing checkpoint inhibitor receptor PD-1 were scored by IF in AK tissue sections (A). Whereas PDT increased the number of PD-1+ cells in lesions, Vit D (either alone or in combination with PDT) led to a reduction in the number of these cells (B). Data shown for immunofluorescence are from 5 mice (3 images/lesion/mouse) per experimental condition. Differences are significant by Mann-Whitney test, * p < 0.0001. Scale bar, 50 μM.
Mice with A431 tumors received intraperitoneal (IP) injections of Vit D (calcitriol in PBS; 1 ug/Kg body weight) once a day for three consecutive days for neoadjuvant treatment. Mice with A431 tumors (± Vit D pretreatment) received IP injections of ALA (200 mg/kg in PBS), followed by 4 h of incubation and then exposed to 100 J/cm2 of 633 nm light using a LumaCare® xenon source (LumaCare) for PDT. Red light illumination was directed only at the tumor, i.e., the mouse was covered with an opaque foil template containing a hole the same size as the tumor.
The light source was calibrated using a FieldMate® laser power meter (Coherent) [23,25]. Mice were sacrificed and tumors harvested post PDT for immunofluorescence analyses at indicated times in figures.
2.5. Immunofluorescence staining of AK and A431 tumor samples
Formalin-fixed and paraffin embedded (FFPE) sections were processed for immunofluorescence staining for immune cell markers and their relative expression levels were analyzed on a fluorescence microscope. Primary rabbit antibodies to neutrophils (Ly6G), macrophages (F4/80), dendritic cells (CD11c), T cells (CD3, CD8 and FoxP3) and PD1 were from Cell Signaling Technologies (Danvers, MA). The secondary antibody, Cy3-conjugated donkey anti-rabbit IgG was from Jackson ImmunoResearch Laboratories (West Grove, PA). Multiple images (3 fields/tumor) were captured digitally, and the number of positively stained cells were counted and expressed as cells per high power field. Expression levels of DAMPs (total pixel area/field) from digitally captured images were quantitated using ImageJ software [37,46]. Graphs in the figures represent data from three immunofluorescent images from each of five lesions per condition (harvested from five different mice), for each treatment group examined in the time course.
2.6. Fluorescence-activated cell sorting (FACS) and antibodies
Whole blood samples from mice (fresh, not frozen) were blocked with anti-Mouse CD16/CD32 (Clone 93) and subsequently processed for flow cytometry analysis on the BD FACSymphony A5 SORP. The gating scheme used for cell identification is shown in Fig. 1. Analysis was done using FlowJo v10.8 (Treestar, Ashland, OR, USA). Most of the antibodies for immune cell identification were purchased from BD Biosciences, unless otherwise indicated. Antibody panel for analysis included: anti-CD45 (Clone 30-F11), anti-Ly6G (Clone 1A8), anti-CD11c (Clone HL3), anti-NK1.1 (Clone PK136), anti-CD8 (Clone 53–6.7), anti-CD11b (Clone M1/70) anti-CD3e (Clone 500A2), anti-CD335 (NKp46) (Clone 29A1.4), and anti-Ly6C (Clone AL-21). Antibodies for T-reg identification include anti-CD4 (GK1.5), anti-CD25 (Clone PC61), anti-CD127 (Clone-SB/199). Other antibodies used for various classifications include anti-CD154 (CD40 Ligand) (Clone MR1), anti-CD44 (Clone IM7), anti-CD62L (Clone MEL-14), anti-CD69 (Clone H1 2F3), anti-I-A/I-E (BioLegend; Clone M5/114.15.2), anti-CCR2 (Clone 475,301) and anti-CXCR2 (Clone V48–2310). Checkpoint inhibitor antibodies used are anti-PD-1 (Clone J43), anti-PDL1 (eBioscience; Clone MIH5), anti-CD278 (ICOS) (BioLegend; Clone 15F9) and anti-TIM3 (BioLegend; Clone B8.2C12).
Fig. 1.
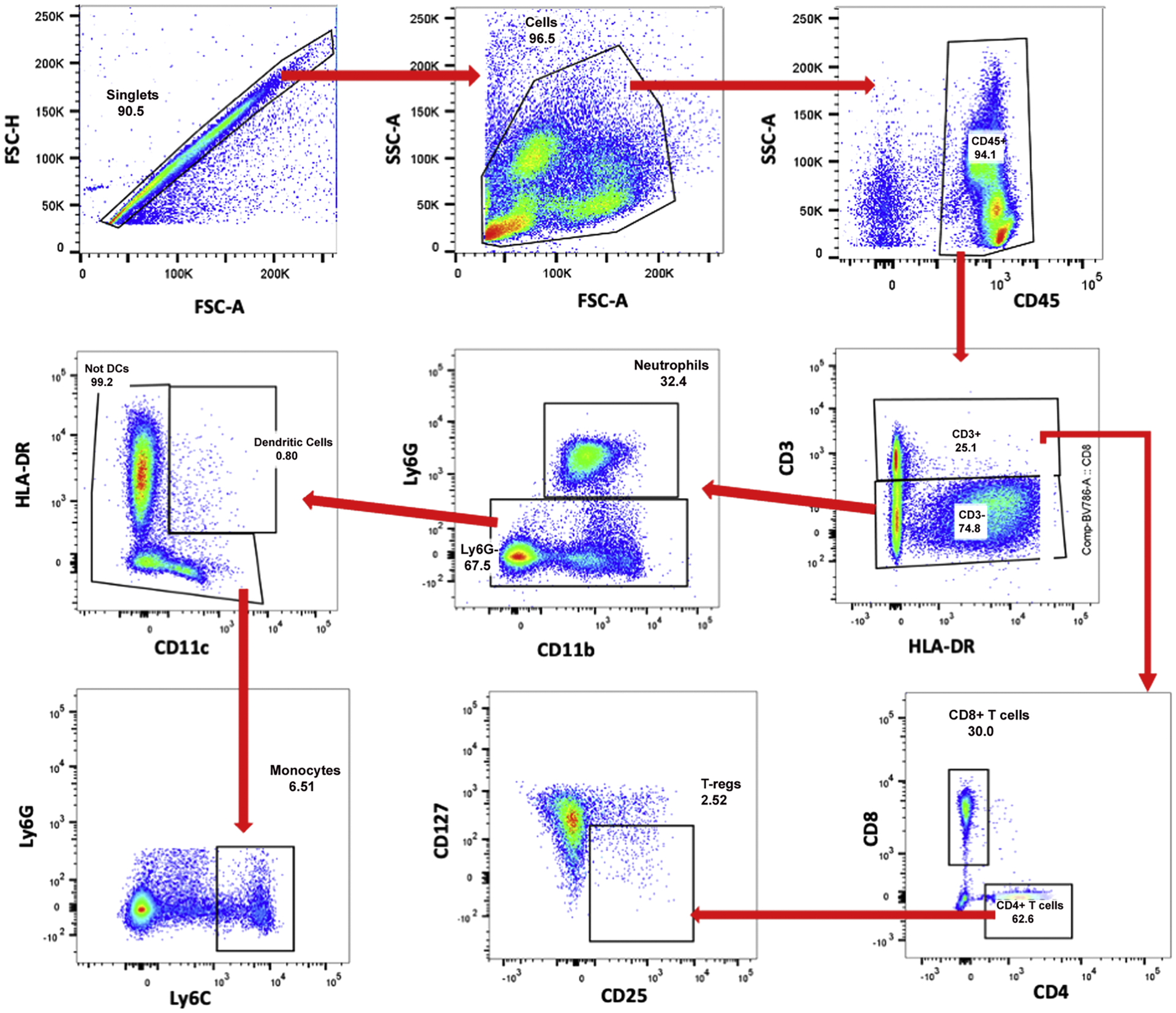
Gating strategy to identify and quantify immunologic cell types from flow cytometry data. Each blood sample was analyzed using the same gating strategy. First, single cells were gated using FSC-A and FSC–H (forward scattering), followed by total cells using FSC-A and SSC-A (side scattering). From total cells, total immune cells (CD45+) were gated. Following this, total T cells (CD3+) and non-T cells (CD3−) were gated. All CD3+ T cells were gated to discriminate CD8 T cells (CD8+) and CD4 T cells (CD4+). From CD4+ T cells, T-regs (CD25+, CD127−) were gated. From CD3− non-T cells, neutrophils (Ly6G+, CD11b+) were gated. From Ly6G− cells, dendritic cells (HLA-DR+, CD11c+) cells were gated. Finally, monocytes (Ly6C+) were gated from non-dendritic cells.
2.7. Statistical analysis
Cell counts per 20X field from immunofluorescence images were analyzed using GraphPad Prism (GraphPad Software, San Diego, CA). Data sets were analyzed by a nonparametric test (Mann-Whitney) for statistical significance. P values < 0.05 were considered statistically significant.
3. Results
To investigate the anti-tumor immune responses triggered by neoadjuvant Vit D (either topical or systemic), combined with ALA-PDT, we designed detailed time course experiments and analyzed the involvement of different immune cell populations in murine models of UV-induced actinic keratosis and subcutaneous SCC (A431 cells).
3.1. Induction of immunogenic cell death (ICD) and enhanced expression of damage-associated molecular patterns (DAMPs) after combination of Vit D and PDT in murine actinic keratosis lesions
To investigate how Vit D affects the expression of DAMPs, we performed immunofluorescence analysis to measure calreticulin (CLR) and high mobility group box 1 HMGB1 expression levels in the AK lesions after treatment with vehicle alone, Vit D alone, PDT alone, or Vit D plus PDT (Fig. 2A and C). Compared to vehicle-only controls, 3 days of Vit D pretreatment resulted in approximately 6- and 8-fold of enhancement in expression levels of CLR and HMGB1, respectively (Fig. 2B and D). Following ALA-PDT, the effect of Vit D pretreatment was still observed at 72 h post-PDT but with expression levels enhanced an additional 1.3-fold and 2.5-fold for CLR and HMGB1, respectively (Fig. 2B and D).
3.2. Additive effect of the combination of Vit D plus PDT regimen on induction of innate immune cell recruitment in murine models of actinic keratosis and of subcutaneous SCC
Mice with AK lesions were topically pretreated with either vehicle or Vit D ointment for 3 days, followed by ALA application and immediate exposure to blue light (ALA-PDT) at time zero. AK lesions were then harvested at the times after PDT indicated in the X-axis labels of the graphs in Figs. 3–7, and tissue sections were analyzed by immunofluorescence using antibodies specific for neutrophils, macrophages and dendritic cells. While neutrophils were absent from untreated AK lesions, a robust infiltration of cells detectable with neutrophil-specific Ly6G antibody was observed in lesions after Vit D pretreatment alone, or at 72 hr after PDT alone or after Vit d-plus-PDT (Fig. 3A). The number of infiltrating neutrophils, already significantly elevated by Vit D pretreatment alone, was nearly double after PDT alone and even higher after Vit d-plus-PDT (Fig. 3B), consistent with an additive effect of Vit D pretreatment upon neutrophil infiltration (Fig. 3B).
Fig. 3.
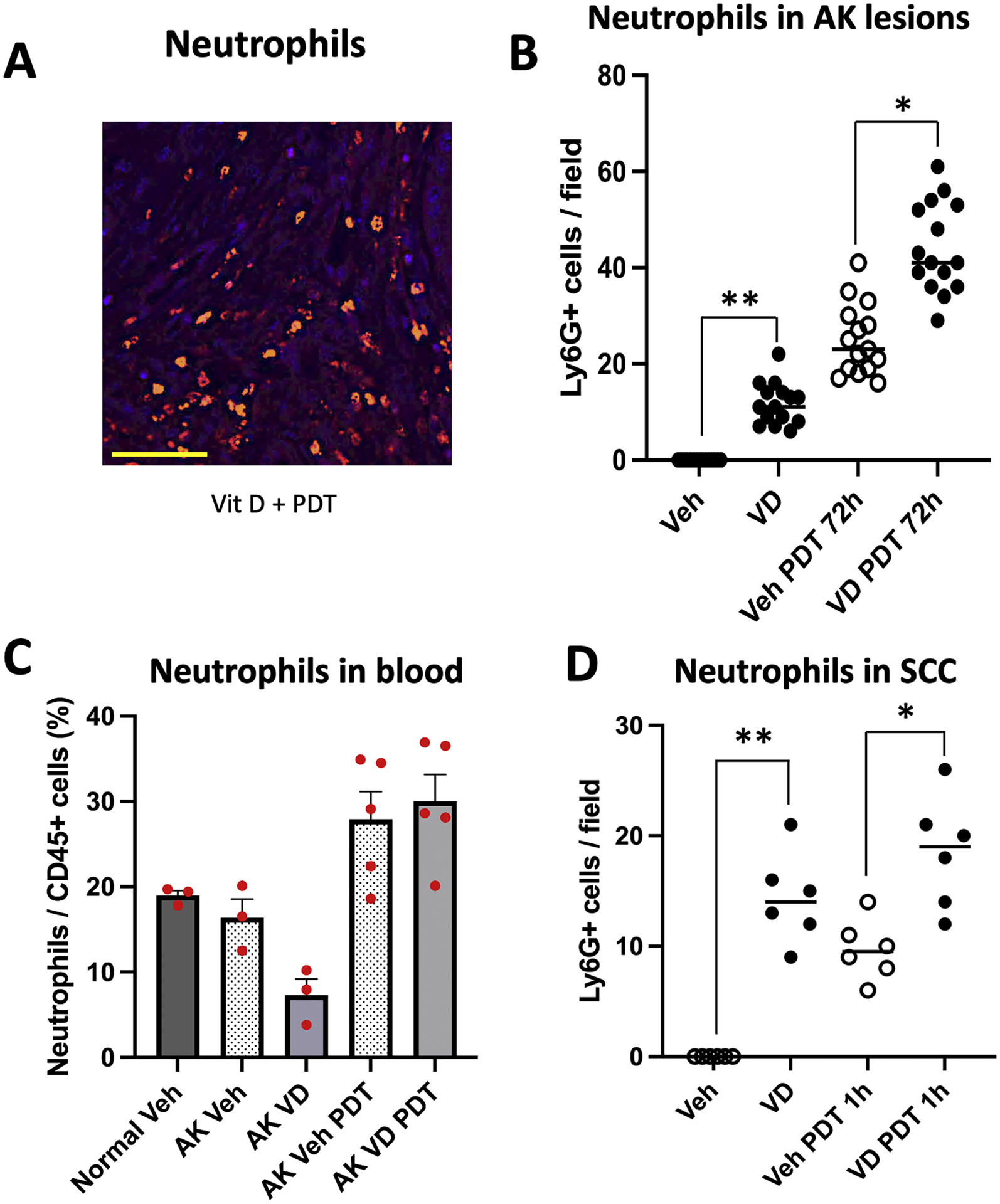
Effects of Vit D and PDT on recruitment of neutrophils into AK and SCC lesions. (A) Examples of immunofluorescent staining (IF) of neutrophils and (B) quantification of numbers of neutrophils per high-power field, in AK lesions. Mice were treated as follows: Veh, vehicle alone. VD, topical Vitamin D pretreatment alone. Veh PDT 72 h, lesions harvested 72 h after PDT alone. VD PDT 72 h, lesions harvested 72 h after PDT combined with Vit D pretreatment. Data shown are from 5 mice (3 images/lesion/mouse) per experimental condition. Differences are significant by nonparametric tests, namely, the Wilcoxon signed-rank test (**, p < 0.0001) and the Mann-Whitney test (*, p< 0.0001). (C) FACS analysis of neutrophils in peripheral blood from the mice with AK, collected 72 h after PDT. Three mice each for normal SKH-1 and SKH-1 mice with vehicle-treated AKs, and 5 mice each for Vit D, PDT and Vit d-PDT treatment conditions were analyzed. Note that differences among different treatment groups were statistically not significant. (D) Neutrophils in subcutaneous A431 SCC tumors harvested 1 hour after PDT. Data shown represent 3 mice (2 tumors/mouse) per experimental condition. Differences are significant, Wilcoxon signed-rank test, (**, p < 0.05), or Mann-Whitney test (*, p< 0.01). Scale bar, 50 μM.
To examine systemic effects of Vit D and PDT, peripheral blood samples of mice were analyzed by FACS to assess changes in the numbers of neutrophils relative to the total population of myeloid (CD45+) cells (Fig. 3C). Compared to naïve (Normal) mice, or to AK-bearing mice exposed to vehicle alone, Vit d-treated mice showed fewer circulating neutrophils which suggests the possibility that relatively more neutrophils exit the blood and enter AK lesions during Vit D pretreatment. After PDT, however, there was a simultaneous increase in neutrophils within AK lesions and in the peripheral blood (Fig. 3B and C). This pattern is consistent with neutrophil responses reported by other groups, who showed that neutrophils are the predominant type of leukocyte recruited early on after PDT. Neutrophils typically enter PDT-treated lesions within minutes, and peak by 24 h post PDT [37,47]. Although, three mice each for flow analysis of normal or vehicle-treated with AKs, and five mice each for Vit D only, ALA-PDT and Vit d-PDT were used, the changes observed with flow analysis of neutrophils in circulation are quite interesting but statistically not significant. The consistency of these trends across multiple cell types seems very suggestive but more replicates will be needed to make more definitive conclusions.
To rule out the possibility that the neutrophil responses described above are unique to superficial AKs, we extended our analysis to a xenograft model of subcutaneous SCC. Human A431 cells were subcutaneously implanted bilaterally in nude mice, and palpable and visible tumor nodules developed within 8–10 days [23,25]. These tumor-bearing mice received systemic Vit D (calcitriol) pretreatment once daily for 3 days. On day 4, mice received systemic ALA for 4 h followed by exposure to red light (PDT). Mice were sacrificed and tumors harvested at different times after PDT. Tissue sections were processed and analyzed by immunofluorescence using antibodies similar to those used earlier for murine AK lesions. Vit D pretreatment alone, or PDT alone, resulted in enhanced infiltration of neutrophils relative to vehicle alone, and there was an additive effect when Vit D pretreatment and PDT were combined (Fig. 3D). Therefore, effects of Vit D and PDT upon neutrophil recruitment appear to be qualitatively similar for superficial AKs (pre-SCC) versus deep SCC tumors.
Next, we examined the presence of tumor-associated macrophages (TAMs) in AK lesions after Vit D and/or PDT. Macrophages expressing F4/80 (a pan macrophage marker) were present at low levels in vehicle-treated AK lesions, but were seen at significantly higher levels (~2-fold) in samples treated with Vit D only for 3 days (Fig. 4A and B). These TAMs increased continuously after PDT for the first 7-days post PDT, and interestingly, these numbers were consistently higher in Vit D-pretreated lesions relative to PDT alone (Fig. 4B). Circulating monocytes (Ly6C+; progenitors for tissue macrophages) were analyzed in blood samples (as described in Fig. 1), but unlike in the case of neutrophils, no clear-cut patterns of change were discernible for monocytes (Fig. 4C). We also looked at TAMs within subcutaneous A431 tumors, and found that the changes after treatment were qualitatively similar to those in superficial AK lesions. Thus, Vit D alone or PDT alone each resulted in an enhanced infiltration (~2-fold) of macrophages, relative to vehicle alone, whereas the effect of combined treatment was greater (~2.5-fold) than either treatment alone (Fig. 4D).
Fig. 4.
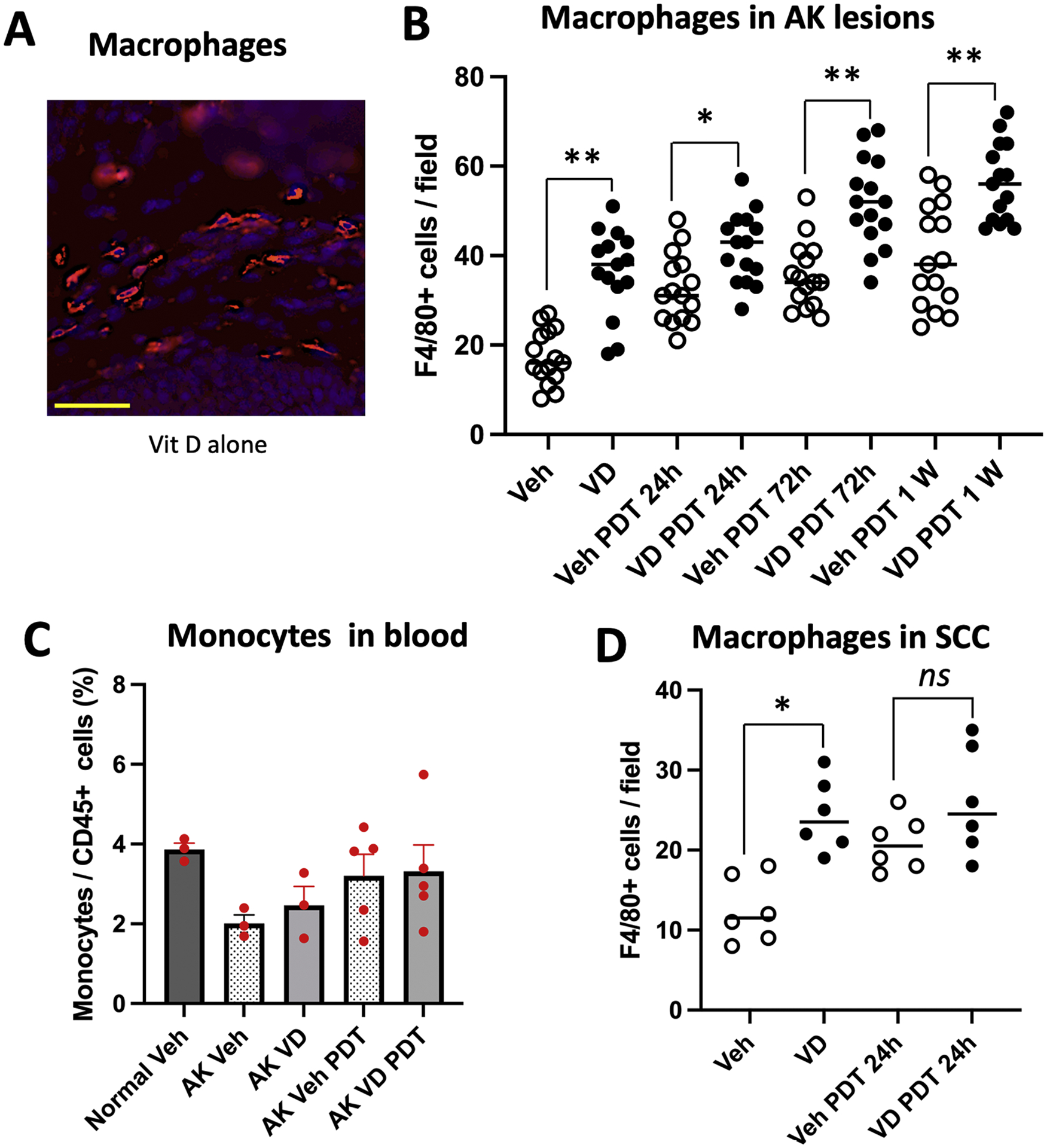
Effects of VD and PDT on recruitment of macrophages into AK and SCC lesions. (A) Representative images, and (B) quantification of macrophages (F4/80 positive cells) in AK lesional tissue, harvested at time zero or at 24 h, 72 h, or 1 week after PDT, with or without Vitamin D pretreatment. Data shown are from 5 mice (3 images/lesion/mouse) per experimental condition. Differences are significant by Mann-Whitney test (*, p<0.005; **, p< 0.0001). (C) Monocytes in peripheral blood from AK-bearing mice at 72 h after PDT; see Fig. 1 for gating scheme used to identify monocytes. Three mice each for normal SKH-1 and SKH-1 mice with vehicle-treated AKs, and 5 mice each for Vit D, PDT and Vit d-PDT treatment conditions were analyzed. (D) Macrophages in subcutaneous A431 SCC tumor, harvested 24 h after PDT. Data shown represent 3 mice (2 tumors/mouse) per experimental condition. Significant difference, Mann-Whitney test (*, p< 0.005); ns, not significant. Scale bar, 50 μM.
Dendritic cells (DCs) are a very important type of antigen presenting cells (APC) in PDT-treated tumors (see Discussion). To investigate the effects of Vit D and PDT on DCs, AK lesions from mice treated with Vit D and/or PDT alone or together were analyzed by immunofluorescence using an antibody specific to CD11c (Fig. 5A). Compared to vehicle, the 3-day pretreatment with Vit D alone resulted in a ~2-fold enhancement of DCs in lesions (Fig. 5B). PDT alone increased DCs in lesions by ~4-fold by 72 h, whereas the combination of Vit D pretreatment and PDT increased that number by an additional ~50 % (Fig. 5B). Amongst circulating myeloid cells, using CD11c and HLA-DR staining (gating scheme in Fig. 1), DC’s comprised a much low percentage than neutrophils and monocytes (Fig. 5C), but did reveal an interesting pattern in which the number of DCs in the blood was decreased in PDT-treated mice. The latter is consistent with migration of DCs from the peripheral blood into PDT-treated lesions, although such a hypothesis will require formal testing in the future. In subcutaneous A431 tumors, just as for neutrophils and macrophages, the response pattern for DCs in subcutaneous A431 tumors was similar to the pattern in the AK lesions where the induction of DC recruitment after Vit d-plus- PDT was greater than after either Vit D or PDT alone (Fig. 5D).
Fig. 5.
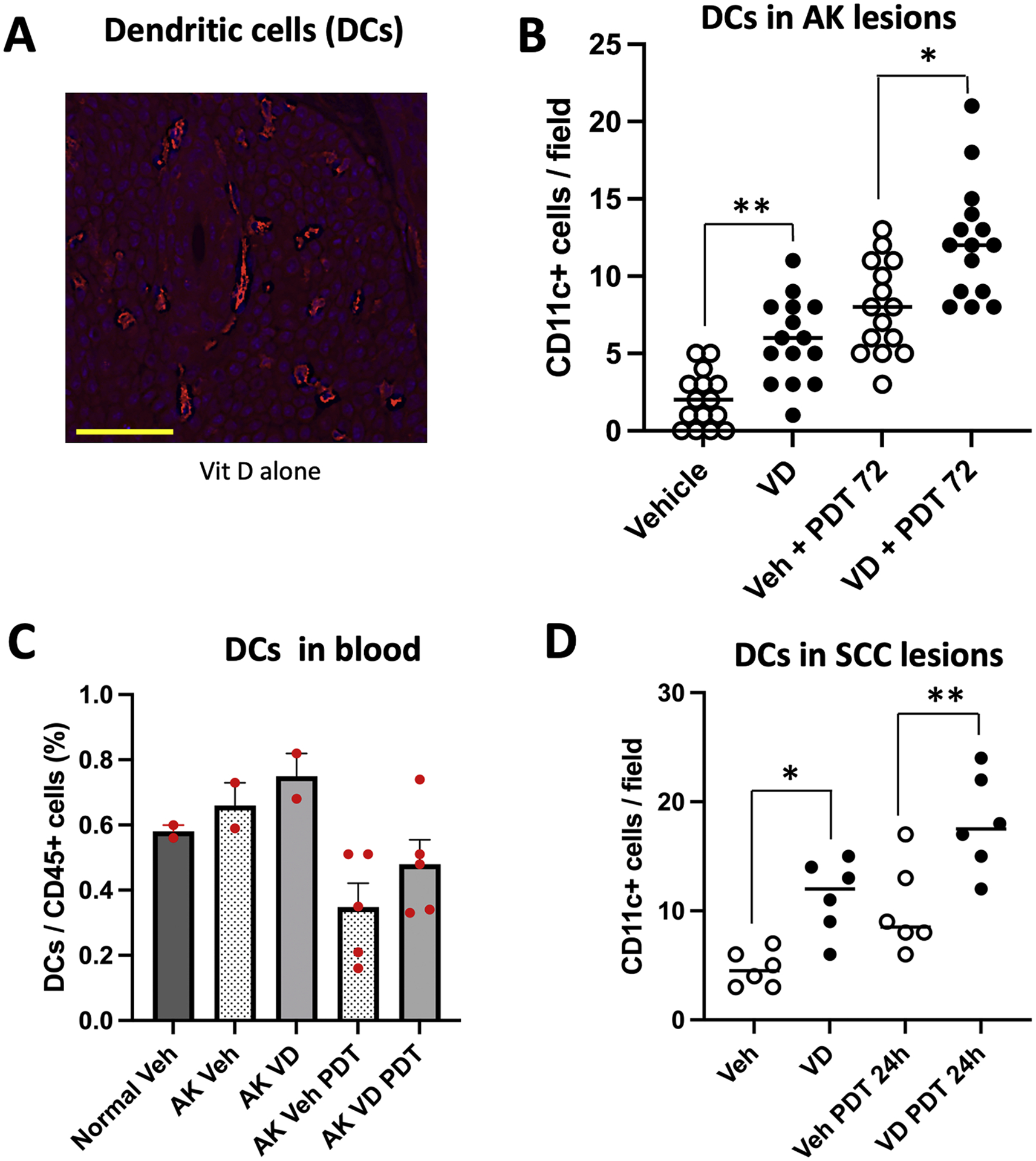
Effects of VD and PDT on recruitment of dendritic cells into AK and SCC lesions. (A) Representative images, and (B) quantification of dendritic cells in AK lesions, either before or 72 h after PDT. Data shown are from 5 mice (3 images/lesion/mouse) per experimental condition. Differences are significant by Mann-Whitney test. (* p< 0.002; ** p<0.0001). (C) Dendritic cells in blood, at 72 h post PDT. Three mice each for normal SKH-1 and SKH-1 mice with vehicle-treated AKs, and 5 mice each for Vit D, PDT and Vit d-PDT treatment conditions were analyzed. (D) Dendritic cells in subcutaneous A431 SCC tumors, at 24 h post PDT. Data shown represent 3 mice (2 tumors/mouse) per experimental condition. Differences are significant by Mann-Whitney test. (* p< 0.01; ** p<0.005). Scale bar, 50 μM.
3.3. Activation of adaptive immune cell recruitment (enhanced infiltration of different T cell populations) in AK lesions after combined Vit D and PDT treatment
PDT-induced activation and differentiation of antigen-presenting immune cells (APCs), such as those discussed above, serve as a link between innate and adaptive arms of immune system because the interaction of APCs with T cells result in proliferation of T cell clones that recognize specific epitopes on the target tumors [48,49]. Here, we analyzed the infiltration of different T cell populations into lesions using antibodies specific for different T cell subtypes. Immunofluorescence staining for CD3, a pan marker for activated T cells (via the T cell receptor), showed that some CD3± T cells were present in vehicle-treated AK lesions (Fig. 6A). Pretreatment with Vit D for 3 days led to an increase (1.5-fold) in overall T-cell infiltration (Fig. 6A). After PDT, a similar trend with increasing numbers of infiltrating CD3+ T cells was observed along the time course (Fig. 6A). Interestingly, an additive effect due to Vit D pretreatment prior to PDT led to higher numbers of infiltrating CD3+T cells was observed at 24 h, 72 h, and 1 week post PDT, with statistical significance at 24 and 72 h (Fig. 6A). The FACS analysis of blood revealed that the numbers of CD3+ cells in circulation were increased at 72 h after PDT, and that these circulating levels were slightly decreased by the combination with Vit D (Fig. 6B). Interestingly, an examination of CD8± (cytotoxic) T-cells (Fig. 6C) and FoxP3± (regulatory) T cells (Fig. 6E) revealed a qualitatively similar pattern, in which the combination of Vit D and PDT always led to higher recruitment into tumors than either treatment alone, and the corresponding cells in circulation were increased after PDT alone, but decreased by exposure Vit D (either alone, or when administered as a neoadjuvant) (Fig. 6D and F). Using the same immunofluorescence methods which successfully identified CD3+, CD8+ and FoxP3+ T cells in AK lesions, we could not observe any CD4+ T cells, possibly due to a technical issue. However, FACS analysis of the mice blood showed a similar trend for CD4± cells, as for the CD3+ T cells in systemic circulation (data not shown).
Fig. 6.
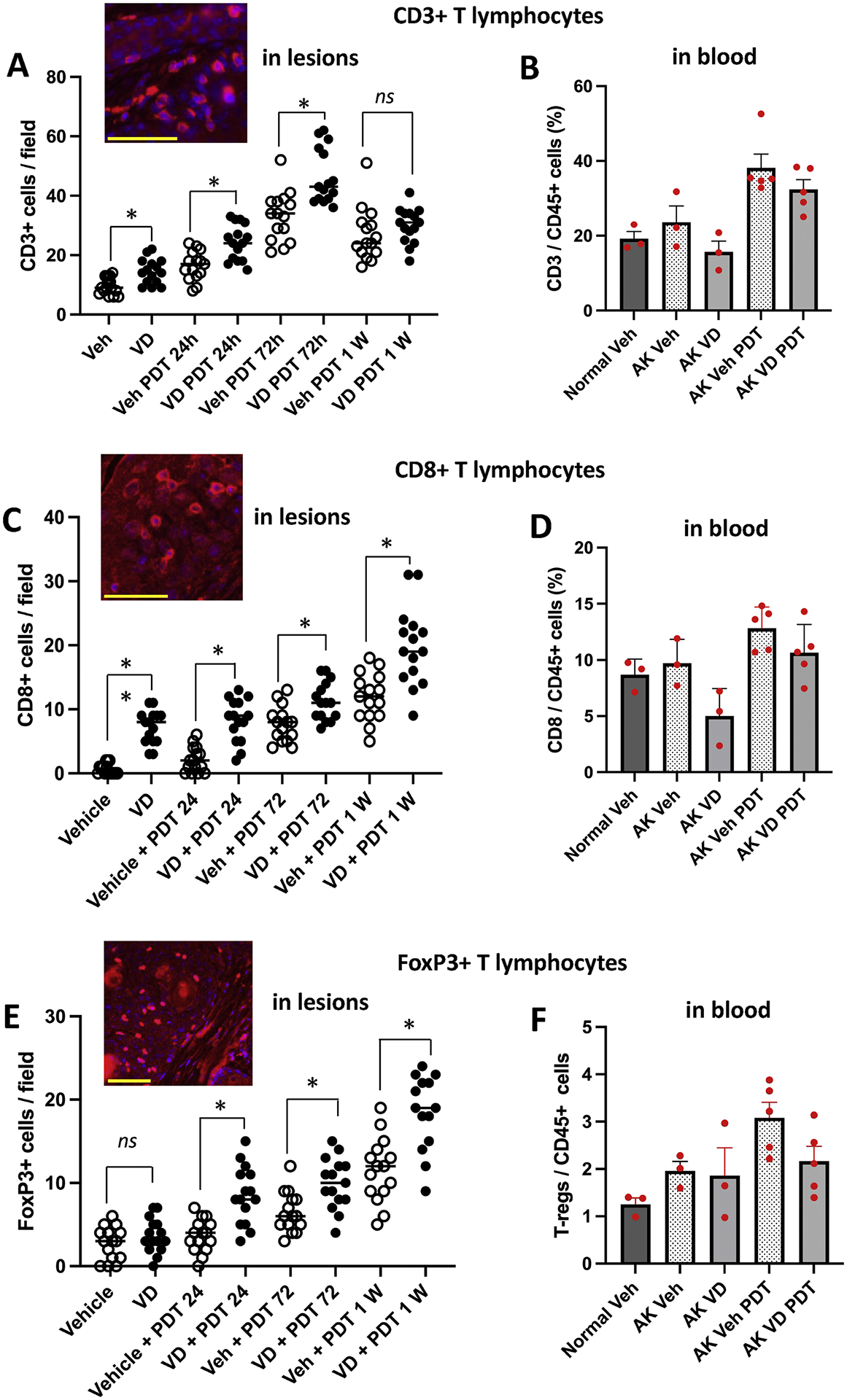
Effects of VD and PDT on recruitment of T cells into AK lesions at specified times after PDT (A, C, E), and in blood at 72 h after PDT (B, D, F). Data shown for immunofluorescence are from 5 mice (3 images/lesion/mouse) per experimental condition. For FACS analysis, three mice each for normal SKH-1 and SKH-1 mice with vehicle-treated AKs, and 5 mice each for Vit D, PDT and Vit d-PDT treatment conditions were analyzed. Insets, representative images of T-cells staining in AK lesional tissue. Changes in cell numbers are statistically different for the following comparisons, by Mann-Whitney test. (A) * p< 0.001; (B) * p< 0.005; ** p<0.0001; (C) * p< 0.005. Scale bars, 50 μM.
In the subcutaneous SCC tumor model (A431 xenografts), we observed changes in CD3+ (total) T cells in mice after Vit D and PDT treatment following a similar trend seen in AK lesions, i.e., enhanced infiltration after PDT and Vit D pretreatment exhibiting an additive effect (data not shown). However, nude mice used for the A431 model are known to possess impaired T cell functions because they lack thymosin, a thymic factor secreted by the thymus, rendering them incapable of differentiation into mature T-cells [50]; therefore further analysis of CD8+ and FoxP3+ T cells was not pursued.
3.4. Vitamin D pretreatment suppresses PDT-induced expression of immune checkpoint marker PD-1 in AK lesions
Tumors often utilize upregulation of inhibitory factors and pathways, often referred to as ‘immune checkpoints’, to grow and survive in and immunosuppressive tumor microenvironment and evade cancer therapies (see Discussion). Here, we investigated the effects of Vit D alone or ALA-PDT and Vit d-PDT on the immune checkpoint markers PD-1, PD-L1 and CTLA-4, using specific antibodies visualized by immunofluorescence. PD-1 positive cells (Fig. 7A) were present in vehicle treated tumors, and their numbers were significantly suppressed by 3 days of Vit D pretreatment (Fig. 7B). The number of PD-1 positive cells per field rose from a low level in vehicle treated tumors to a 4-fold increase at 72 h post PDT (Fig. 7B). Vit d-plus-PDT compared to ALA-PDT alone showed a significant reduction in PD-1 positive cells per field (Fig. 7B). Using a immunofluorescence protocol similar to that which successfully stained PD-1 positive cells, we could not detect any PD-L1 or CTLA-4 positive cells in AK lesions, suggesting that PD-1 may be the predominant immune checkpoint receptor in murine AK lesions. Although PD1+ cells were seen in AK lesions from all treatment groups, due to unknown technical issues, we could not observe any PD-1+ immune cells in circulating blood of any of the mice tested by FACS analysis.
4. Discussion
In the study reported here, we have established that treatment with topical Vit D for 3 days has a profound effect upon the tumor immune landscape of squamous nonmelanoma skin cancer. In both superficial and deep squamous tumors in mice, Vit D stimulates the expression of DAMPs on tumor cells and leads to a significant influx of neutrophils, macrophages, dendritic cells, and T-cells into the neoplastic lesions. The study also confirms and extends our earlier-reported findings [37] which showed that ALA-PDT induces recruitment of these same populations of innate and adaptive immune cells. Here we go further by describing a novel finding that a combination treatment with neoadjuvant Vit D and ALA-PDT leads to enhanced recruitment of these immune cell populations, to an extent greater than with either treatment alone. Also, analyses of the peripheral circulation demonstrated that blood levels of some immune cell types (neutrophils, macrophages T-cells) are increased after PDT, whereas others (DCs) are decreased. Conversely, Vit D appears to decrease the circulating numbers of neutrophils and T-cells, consistent with recruitment out of the blood and into Vit d-treated lesions, although this apparent finding needs to be confirmed via further experimentation.
We now discuss some of the implications of our findings regarding Vit D and ALA-PDT, for each type of molecule and immune cell analyzed in the current study.
Photodynamic therapy is known to induce immunogenic cell death, characterized by synthesis and release of damage-associated molecular patterns (DAMPs) from dying cells which trigger the cascade of anti-tumor immune responses [33,51,52]. DAMPs such as calreticulin (CLR), high mobility group box 1 (HMGB1), Adenosine triphosphate (ATP) and heat shock protein 70 (HSP70), bind to cellular receptors and activate innate immune cells such as antigen presenting cells (APC) e.g., dendritic cells (DCs), macrophages and certain types of T cells involved in processing and presentation of cellular debris as tumor-associated antigens (TAA); these then activate the adaptive arm of anti-tumor immunity [28,29,34,49]. We previously showed that PDT treatment of murine AK lesions resulted in robust upregulation of CLR and HMGB1 and modest upregulation of HSP70, relative to no treatment controls in a time course analyses [37]. Here, we found that treatment with Vit D alone increases CLR and HMGB1 levels to an extent similar to ALA-PDT alone, whereas the combination Vit D and ALA-PDT increases those levels even further.
Neutrophils are a significant population of the white blood cells in humans and the first line of defense of the innate immune system against microbial infections, via mechanisms involving phagocytosis, degranulation and release of structures called neutrophil extracellular traps (NETs) [53]. Both activating and inhibitory roles of Vit D on neutrophil function and activity have been reported. Anti-inflammatory properties of Vit D tend to reduce the production of inflammatory cytokines and ROS and downregulate neutrophil activity and function. In a recent clinical trial involving Covid-19 patients, higher neutrophil counts and neutrophil-lymphocyte ratio (NLR) values were seen in the group of patients with low Vit D levels when compared with the group with normal Vit D [54]. Conversely, Vit D has been shown to promote granulopoiesis and neutrophil generation and function in zebrafish [55] and in vitro treatment of human neutrophils with calcitriol resulted in production of NETs-like structure and expression of TLR7 and secretion of IFN-α as a result of neutrophil activation [53]. Therefore, an induction of neutrophil infiltration and production, observed with Vit D, PDT and Vit d-PDT treatments, may be the result of induction of innate immune responses by these treatments, which is further supported by the induction of DAMPs as shown in Fig. 2. Additionally, the observed positive effects of Vit D on neutrophil infiltration may be specific to the topical application of the active hormonal form (calcitriol) of the Vit D.
Macrophages, or more specifically, Tumor associated macrophages (TAMs) are another class of innate immune cells. TAMs arise via differentiation from monocytes; in an unperturbed tumor microenvironment (TME), the majority of macrophages display an anti-inflammatory (M2) phenotype that favors growth, angiogenesis, immunosuppression, and metastases. Following PDT, the majority of M2 macrophages are replaced by pro-inflammatory (M1) macrophages that secrete immunostimulatory cytokines and promote tumor regression [32,56,57]. Since both monocytes and macrophages express the VDR, and since calcitriol is known to stimulate differentiation of monocytes into macrophages [39,58], an examination of macrophages in AK lesions after Vit D and/or PDT seemed relevant. Our finding here of a ~2-fold elevation of TAMs after PDT, a similar-sized elevation after Vit D alone, and an apparent additive effect after the combination of Vit D and PDT (Fig. 4B), suggests a significant involvement of Vit D regulated pathways in the biology of TAMs that might be exploitable with further scrutiny.
Dendritic cells (DCs) are the predominant antigen presenting cells (APCs) in PDT-treated tumors. After engulfing and processing tumor cell debris produced during immunogenic cell death, APCs present tumor specific antigens to naïve T cells, leading to activation and proliferation of T cells and triggering long-term adaptive immunity. Photofrin-PDT and ALA-PDT have been shown to induce accumulation of CD11c+ and CD1a+ DCs at ~24 h post treatment [59,60]. One of the most relevant effects of Vit D on the immune system is the regulation of monocyte differentiation into immature DCs, and the maturation of DCs to activate naïve T cells and maintain DC survival [61–63]. Since the maturation of DCs is regulated by Vit D, systemic Vit D levels (deficient vs. sufficient) have been linked to the immunologic status of an individual [64]. Overall, the demonstration here of a significant enhancement of the presence of DCs in tumor not only after Vit D alone and PDT alone, but significantly augmented after a combination of the two (Fig. 5), could have mechanistic implications for enhancing the generation of anti-tumor immunity.
T-lymphocytes (T-cells) are key elements of the adaptive immune response, which is primarily responsible for long-term anti-tumor immunity, involves activation of various populations of T-lymphocytes such as CD4+ (helper), CD8+ (cytotoxic) and FoxP3+ (regulatory; Treg) T cells [31,32,35,65]. In most cases, cells of the innate immune system (APCs) serve as a means to activate the adaptive arms of the immune system, because the interaction of APCs with T cells result in proliferation of T cell clones that recognize specific epitopes on the target tumors [48,49]. For Vit D, both indirect and direct mechanisms have been proposed for Vit D effects on T cells. While the indirect pathway involves the stimulation of T cells by APCs, the direct effect of Vit D is dependent on the activation state of T cells as they gain higher VDR concentration after activation [58,64,66].
In this paper, the fact that intralesional T-cells (total CD3+, CD8+ and FoxP3+ subpopulations) become significantly elevated in number after PDT, and are further increased after a Vit d-plus-PDT combination, represents a novel finding that invites further research on the mechanisms leading to these increases and ways to exploit this phenomenon. The observation that FoxP3+ (immunosuppressive T-regs) cells are elevated to the same extent after Vit D or PDT as are CD8+ (cytotoxic anti-tumor) cells, suggest that compensatory (counterbalancing) mechanisms may be at play, and that a further immunologic intervention may be needed to reap the potential benefits of a boost in anti-tumor T-cell populations using Vit D and PDT.
Another novel finding here is that the number of cells expressing the immune checkpoint inhibitor PD-1 is significantly lower after exposure to Vit D. Therapeutic resistance in tumors is mainly attributed to neutralizing anti-tumor immunoregulatory molecules that block the cytotoxic activities of the immune cells, resulting in failure of immunotherapy. A strategy involving immune checkpoint inhibition (ICI) has been developed in the past decade to neutralize these inhibitory signals and help immune cells with tumor recognition and tumoricidal activities. Immune checkpoint molecules are inhibitory receptors present on both immune and tumor cells which inhibit both immune recognition and T cell-mediated tumor destruction [32,67,68]. Some of the most commonly expressed immune checkpoint receptors in NMSCs are PD-1, PD-L1 and CTLA-4 [32,69,70]. Interestingly, regarding the immunomodulatory potential of Vit D, an interaction between ICI efficacy and immune-related adverse events (irAE) has already been reported, with sufficient Vit D levels linked to a better response to ICI therapy and a better prognosis [71]. Our results on suppression of PD-1 by Vit D and its combination with PDT is similar to observations reported in clinical trials with advanced melanoma [72] and non-small cell lung carcinoma (NSCLC) [73]. In a clinical trial with advanced melanoma undergoing anti-PD-1 therapy, Vit D supplementation increased the response rate (56% vs. 36 %) and prolonged progression free survival (11.25 vs. 5.75 months) [72]. In another clinical trial with ICI therapy in advanced lung carcinoma patients, baseline Vit D levels of partial response (PR) patients were significantly higher (19.39 vs. 16.28 ng/ml) than non-PR patients, respectively. Similarly, baseline Vit D levels were higher in grade I irAE patients (20.07 vs. 15.22 ng/ml) than in grade 2/3/4 irAE patients. An assessment of baseline Vit D levels for prognosis of ICI efficacy and prediction of irAE, and a possible supplementation with Vit D to enhance ICI efficacy and reduce moderate to severe itAE was suggested in this study [71]. A correlation with Vit D levels and ICI markers has been reported, with a negative correlation between Vit D levels and PD-1 expression on CD8+ cells in NSCLC patients [73]. Oral Vit D supplementation decreased PD-1 expression, resulting in anti-tumor cytokine production and cytotoxic T cells in NSCLC patients [73].
Overall, our findings in this study will require further consideration and validation. For example, even though PD-1 is associated with immunosuppression, it is also upregulated in cells that are activated, and this regulation is actually done by binding to PDL1. Therefore, our interesting finding of altered PD-1 changes after Vit D and PDT will require further studies before it can be fully understood and harnessed. In summary, the current data show that immune-modulatory effects of Vit D as a neoadjuvant for ALA-PDT are significant deserve further experimental study.
Acknowledgments
This investigation was financially supported by (i) Program Project grant P01 CA084203 and (ii) regular grant R01 CA204158, both from the National Cancer Institute (NCI), National Institutes of Health (NIH), U.S.A. We are grateful to Dr. Tayyaba Hasan (Massachusetts General Hospital) and Dr. Brian Pogue (University of Wisconsin-Madison) for their longstanding collaboration with us through the NIH Program Project grant. We are thankful to Ms. Ava Fan (Cleveland Clinic Lerner College of Medicine) for her help with initial experiments during this investigation.
Footnotes
CRediT authorship contribution statement
Sanjay Anand: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Resources, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Alan Shen: Writing – review & editing, Methodology, Investigation, Formal analysis, Data curation. Cheng-En Cheng: Methodology, Investigation. Jacky Chen: Methodology. Jennifer Powers: Methodology. Pat Rayman: Methodology. Marcela Diaz: Writing – review & editing, Resources, Methodology, Formal analysis. Tayyaba Hasan: Project administration, Funding acquisition, Conceptualization. Edward V Maytin: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Declarations of competing interest
None.
References
- [1].Rogers HW, Weinstock MA, Feldman SR, Coldiron BM, Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012, JAMA Dermatol. 151 (10) (2015) 1081–1086. [DOI] [PubMed] [Google Scholar]
- [2].Siegel RL, Miller KD, Wagle NS, Jemal A, Cancer statistics, 2023, CA Cancer J. Clin 73 (1) (2023) 17–48. [DOI] [PubMed] [Google Scholar]
- [3].Ziegler A, Jonason AS, Leffell DJ, Simon JA, Sharma HW, Kimmelman J, Remington L, Jacks T, Brash DE, Sunburn and p53 in the onset of skin cancer, Nature 372 (6508) (1994) 773–776. [DOI] [PubMed] [Google Scholar]
- [4].Brash DE, Sunlight and the onset of skin cancer, Trends Genet. 13 (10) (1997) 410–414. TIG. [DOI] [PubMed] [Google Scholar]
- [5].Bowden GT, Prevention of non-melanoma skin cancer by targeting ultraviolet-B-light signalling, Nat. Rev. Cancer 4 (1) (2004) 23–35. [DOI] [PubMed] [Google Scholar]
- [6].Morton CA, Topical photodynamic therapy in dermatology, S. Afr. Med. J 91 (8) (2001) 634–637. [PubMed] [Google Scholar]
- [7].Zeitouni NC, Oseroff AR, Shieh S, Photodynamic therapy for nonmelanoma skin cancers. Current review and update, Mol. Immunol 39 (17–18) (2003) 1133–1136. [DOI] [PubMed] [Google Scholar]
- [8].Fahradyan A, Howell AC, Wolfswinkel EM, Tsuha M, Sheth P, Wong AK, Updates on the management of non-melanoma skin cancer (NMSC), Healthcare 5 (4) (2017) (Basel). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dianzani C, Conforti C, Giuffrida R, Corneli P, di Meo N, Farinazzo E, Moret A, Magaton Rizzi G, Zalaudek I, Current therapies for actinic keratosis, Int. J. Dermatol 59 (6) (2020) 677–684. [DOI] [PubMed] [Google Scholar]
- [10].Braathen LR, Morton CA, Basset-Seguin N, Bissonnette R, Gerritsen MJ, Gilaberte Y, Calzavara-Pinton P, Sidoroff A, Wulf HC, Szeimies RM, Photodynamic therapy for skin field cancerization: an international consensus. International society for photodynamic therapy in dermatology, J. Eur. Acad. Dermatol. Venereol 26 (9) (2012) 1063–1066. [DOI] [PubMed] [Google Scholar]
- [11].Lucena SR, Salazar N, Gracia-Cazana T, Zamarron A, Gonzalez S, Juarranz A, Gilaberte Y, Combined treatments with photodynamic therapy for non-melanoma skin cancer, Int. J. Mol. Sci 16 (10) (2015) 25912–25933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ortel B, Shea CR, Calzavara-Pinton P, Molecular mechanisms of photodynamic therapy, Front. Biosci 14 (2009) 4157–4172. [DOI] [PubMed] [Google Scholar]
- [13].Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel D, Korbelik M, Moan J, Mroz P, Nowis D, Piette J, Wilson BC, Golab J, Photodynamic therapy of cancer: an update, CA Cancer J. Clin 61 (4) (2011) 250–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Anand S, Ortel BJ, Pereira SP, Hasan T, Maytin EV, Biomodulatory approaches to photodynamic therapy for solid tumors, Cancer Lett. 326 (1) (2012) 8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Szeimies RM, Hauschild A, Ortland C, Moor AC, Stocker M, Surber C, Photodynamic therapy simplified: nonprepared, moderate-grade actinic keratosis lesions respond equally well to 5-aminolaevulinic acid patch photodynamic therapy as do mild lesions, Br. J. Dermatol 173 (5) (2015) 1277–1279. [DOI] [PubMed] [Google Scholar]
- [16].Maytin EV, Hasan T, Vitamin D and other differentiation-promoting agents as neoadjuvants for photodynamic therapy of cancer, Photochem. Photobiol 96 (3) (2020) 529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kennedy JC, Pottier RH, Pross DC, Photodynamic therapy with endogenous protoporphyrin IX: basic principles and present clinical experience, J. Photochem. Photobiol B 6 (1–2) (1990) 143–148. [DOI] [PubMed] [Google Scholar]
- [18].Zhao B, He YY, Recent advances in the prevention and treatment of skin cancer using photodynamic therapy, Expert Rev. Anticancer Ther 10 (11) (2010) 1797–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Dolmans DE, Fukumura D, Jain RK, Photodynamic therapy for cancer, Nat. Rev. Cancer 3 (5) (2003) 380–387. [DOI] [PubMed] [Google Scholar]
- [20].Ortel B, Calzavara-Pinton P, Advances in photodynamic therapy. A review, G. Ital. Dermatol. Venereol 145 (4) (2010) 461–475. [PubMed] [Google Scholar]
- [21].Ortel B, Sharlin D, O’Donnell D, Sinha AK, Maytin EV, Hasan T, Differentiation enhances aminolevulinic acid-dependent photodynamic treatment of LNCaP prostate cancer cells, Br. J. Cancer 87 (11) (2002) 1321–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sato N, Moore BW, Keevey S, Drazba JA, Hasan T, Maytin EV, Vitamin D enhances ALA-induced protoporphyrin IX production and photodynamic cell death in 3-D organotypic cultures of keratinocytes, J. Invest. Dermatol 127 (4) (2007) 925–934. [DOI] [PubMed] [Google Scholar]
- [23].Anand S, Wilson C, Hasan T, Maytin EV, Vitamin D3 enhances the apoptotic response of epithelial tumors to aminolevulinate-based photodynamic therapy, Cancer Res. 71 (18) (2011) 6040–6050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Maytin EV, Honari G, Khachemoune A, Taylor CR, Ortel B, Pogue BW, Sznycer-Taub N, Hasan T, Vitamin D combined with aminolevulinate (ALA)-mediated photodynamic therapy (PDT) for human psoriasis: a proof-of-principle study, Isr. J. Chem 52 (8–9) (2012) 767–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Anand S, Rollakanti KR, Horst RL, Hasan T, Maytin EV, Combination of oral vitamin D3 with photodynamic therapy enhances tumor cell death in a murine model of cutaneous squamous cell carcinoma, Photochem. Photobiol 90 (5) (2014) 1126–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bullock TA, Negrey J, Hu B, Warren CB, Hasan T, Maytin EV, Significant improvement of facial actinic keratoses after blue light photodynamic therapy with oral vitamin D pretreatment: an interventional cohort-controlled trial, J. Am. Acad. Dermatol (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Torezan L, Grinblat B, Haedersdal M, Valente N, Festa-Neto C, Szeimies RM, A randomized split-scalp study comparing calcipotriol-assisted methyl aminolaevulinate photodynamic therapy (MAL-PDT) with conventional MAL-PDT for the treatment of actinic keratosis, Br. J. Dermatol 179 (4) (2018) 829–835. [DOI] [PubMed] [Google Scholar]
- [28].Garg AD, Nowis D, Golab J, Agostinis P, Photodynamic therapy: illuminating the road from cell death towards anti-tumour immunity, Apoptosis 15 (9) (2010) 1050–1071. [DOI] [PubMed] [Google Scholar]
- [29].Kroemer G, Galluzzi L, Kepp O, Zitvogel L, Immunogenic cell death in cancer therapy, Annu. Rev. Immunol 31 (2013) 51–72. [DOI] [PubMed] [Google Scholar]
- [30].Legrand AJ, Konstantinou M, Goode EF, Meier P, The diversification of cell death and immunity: memento mori, Mol. Cell 76 (2) (2019) 232–242. [DOI] [PubMed] [Google Scholar]
- [31].Falk-Mahapatra R, Gollnick SO, Photodynamic therapy and immunity: an update, Photochem. Photobiol 96 (3) (2020) 550–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Anand S, Chan TA, Hasan T, Maytin EV, Current prospects for treatment of solid tumors via photodynamic, photothermal, or ionizing radiation therapies combined with immune checkpoint inhibition (a review), Pharmaceuticals 14 (5) (2021) (Basel). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Garg AD, Agostinis P, ER stress, autophagy and immunogenic cell death in photodynamic therapy-induced anti-cancer immune responses, Photochem. Photobiol. Sci 13 (3) (2014) 474–487. [DOI] [PubMed] [Google Scholar]
- [34].Yatim N, Cullen S, Albert ML, Dying cells actively regulate adaptive immune responses, Nat. Rev. Immunol 17 (4) (2017) 262–275. [DOI] [PubMed] [Google Scholar]
- [35].Nath S, Obaid G, Hasan T, The course of immune stimulation by photodynamic therapy: bridging fundamentals of photochemically induced immunogenic cell death to the enrichment of T-cell repertoire, Photochem. Photobiol 95 (6) (2019) 1288–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Alzeibak R, Mishchenko TA, Shilyagina NY, Balalaeva IV, Vedunova MV, Krysko DV, Targeting immunogenic cancer cell death by photodynamic therapy: past, present and future, J. Immunother. Cancer 9 (1) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Anand S, Govande M, Yasinchak A, Heusinkveld L, Shakya S, Fairchild RL, Maytin EV, Painless photodynamic therapy triggers innate and adaptive immune responses in a murine model of UV-induced squamous skin pre-cancer, Photochem. Photobiol 97 (3) (2021) 607–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Henn M, Martin-Gorgojo V, Martin-Moreno JM, Vitamin D in cancer prevention: gaps in current knowledge and room for hope, Nutrients 14 (21) (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Starska-Kowarska K, Role of vitamin D in head and neck cancer-immune function, anti-tumour effect, and its impact on patient prognosis, Nutrients 15 (11) (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Bullock TA, Mack JA, Negrey J, Kaw U, Hu B, Anand S, Hasan T, Warren CB, Maytin EV, Significant association of poly-A and Fok1 polymorphic alleles of the vitamin D receptor with vitamin D serum levels and incidence of squamous cutaneous neoplasia, J. Invest. Dermatol 143 (8) (2023) 1538–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Bikle DD, Vitamin D regulation of immune function during covid-19, Rev. Endocr. Metab. Disord 23 (2) (2022) 279–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Bikle DD, Vitamin D and long Covid: is there a role in prevention or treatment? J. Clin. Endocrinol. Metab (2023). [DOI] [PubMed] [Google Scholar]
- [43].Athanassiou L, Mavragani CP, Koutsilieris M, The immunomodulatory properties of vitamin D, Mediterr. J. Rheumatol 33 (1) (2022) 7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Pillon A, Gomes B, Vandenberghe I, Cartron V, Cebe P, Blanchet JC, Sibaud V, Guilbaud N, Audoly L, Lamant L, Kruczynski A, Actinic keratosis modelling in mice: a translational study, PLoS One 12 (6) (2017) e0179991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kaw U, Ilyas M, Bullock T, Rittwage L, Riha M, Vidimos A, Hu B, Warren CB, Maytin EV, A regimen to minimize pain during blue light photodynamic therapy of actinic keratoses: bilaterally controlled, randomized trial of simultaneous versus conventional illumination, J. Am. Acad. Dermatol 82 (4) (2020) 862–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Anand S, Heusinkveld LE, Cheng CE, Lefatshe L, De Silva P, Hasan T, Maytin EV, Combination of 5-fluorouracil with photodynamic therapy: enhancement of innate and adaptive immune responses in a murine model of actinic keratosis(dagger), Photochem. Photobiol 99 (2) (2023) 437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Krosl G, Korbelik M, Dougherty GJ, Induction of immune cell infiltration into murine SCCVII tumour by photofrin-based photodynamic therapy, Br. J. Cancer 71 (3) (1995) 549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Castano AP, Mroz P, Hamblin MR, Photodynamic therapy and anti-tumour immunity, Nat. Rev. Cancer 6 (7) (2006) 535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Yang Y, Hu Y, Wang H, Targeting antitumor immune response for enhancing the efficacy of photodynamic therapy of cancer: recent advances and future perspectives, Oxid. Med. Cell Longev 2016 (2016), 10.1155/2016/5274084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Pelleitier M, Montplaisir S, The nude mouse: a model of deficient T-cell function, Methods Achiev. Exp. Pathol 7 (1975) 149–166. [PubMed] [Google Scholar]
- [51].Garg AD, Krysko DV, Vandenabeele P, Agostinis P, DAMPs and PDT-mediated photo-oxidative stress: exploring the unknown, Photochem. Photobiol. Sci 10 (5) (2011) 670–680. [DOI] [PubMed] [Google Scholar]
- [52].Turubanova VD, Mishchenko TA, Balalaeva IV, Efimova I, Peskova NN, Klapshina LG, Lermontova SA, Bachert C, Krysko O, Vedunova MV, Krysko DV, Novel porphyrazine-based photodynamic anti-cancer therapy induces immunogenic cell death, Sci. Rep 11 (1) (2021) 7205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Agraz-Cibrian JM, Giraldo DM, Urcuqui-Inchima S, 1,25-Dihydroxyvitamin D (3) induces formation of neutrophil extracellular trap-like structures and modulates the transcription of genes whose products are neutrophil extracellular trap-associated proteins: a pilot study, Steroids 141 (2019) 14–22. [DOI] [PubMed] [Google Scholar]
- [54].Pimentel GD, Dela Vega MCM, Pichard C, Low vitamin D levels and increased neutrophil in patients admitted at ICU with COVID-19, Clin. Nutr. ESPEN 44 (2021) 466–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Liao X, Lan Y, Shao R, Liu J, Liang S, Yin Z, Gudmundsson GH, Bergman P, Wan M, Vitamin D enhances neutrophil generation and function in zebrafish (Danio rerio), J. Innate Immun 14 (3) (2022) 229–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Korbelik M, Hamblin MR, The impact of macrophage-cancer cell interaction on the efficacy of photodynamic therapy, Photochem. Photobiol. Sci 14 (8) (2015) 1403–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Mosser DM, Edwards JP, Exploring the full spectrum of macrophage activation, Nat. Rev. Immunol 8 (12) (2008) 958–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Martens PJ, Gysemans C, Verstuyf A, Mathieu AC, Vitamin D’s effect on immune function, Nutrients 12 (5) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Jalili A, Makowski M, Switaj T, Nowis D, Wilczynski GM, Wilczek E, Chorazy-Massalska M, Radzikowska A, Maslinski W, Bialy L, Sienko J, Sieron A, Adamek M, Basak G, Mroz P, Krasnodebski IW, Jakobisiak M, Golab J, Effective photoimmunotherapy of murine colon carcinoma induced by the combination of photodynamic therapy and dendritic cells, Clin. Cancer Res 10 (13) (2004) 4498–4508. [DOI] [PubMed] [Google Scholar]
- [60].Wang H, Li J, Lv T, Tu Q, Huang Z, Wang X, Therapeutic and immune effects of 5-aminolevulinic acid photodynamic therapy on UVB-induced squamous cell carcinomas in hairless mice, Exp. Dermatol 22 (5) (2013) 362–363. [DOI] [PubMed] [Google Scholar]
- [61].Banchereau J, Steinman RM, Dendritic cells and the control of immunity, Nature 392 (6673) (1998) 245–252. [DOI] [PubMed] [Google Scholar]
- [62].Piemonti L, Monti P, Sironi M, Fraticelli P, Leone BE, Dal Cin E, Allavena P, Di Carlo V, Vitamin D3 affects differentiation, maturation, and function of human monocyte-derived dendritic cells, J. Immunol 164 (9) (2000) 4443–4451. [DOI] [PubMed] [Google Scholar]
- [63].Griffin MD, Lutz W, Phan VA, Bachman LA, McKean DJ, Kumar R, Dendritic cell modulation by 1alpha,25 dihydroxyvitamin D3 and its analogs: a vitamin D receptor-dependent pathway that promotes a persistent state of immaturity in vitro and in vivo, Proc. Natl. Acad. Sci. U. S. A 98 (12) (2001) 6800–6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Kamen DL, Tangpricha V, Vitamin D and molecular actions on the immune system: modulation of innate and autoimmunity, J. Mol. Med 88 (5) (2010) 441–450 (Berl). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Hernandez IB, Yu Y, Ossendorp F, Korbelik M, Oliveira S, Preclinical and clinical evidence of immune responses triggered in oncologic photodynamic therapy: clinical recommendations, J. Clin. Med 9 (2) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Baeke F, Korf H, Overbergh L, van Etten E, Verstuyf A, Gysemans C, Mathieu C, Human T lymphocytes are direct targets of 1,25-dihydroxyvitamin D3 in the immune system, J. Steroid Biochem. Mol. Biol 121 (1–2) (2010) 221–227. [DOI] [PubMed] [Google Scholar]
- [67].Guo Y, Wang AY, Novel immune check-point regulators in tolerance maintenance, Front. Immunol 6 (2015) 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Cramer GM, Moon EK, Cengel KA, Busch TM, Photodynamic therapy and immune checkpoint blockade(dagger), Photochem. Photobiol 96 (5) (2020) 954–961. [DOI] [PubMed] [Google Scholar]
- [69].Gubin MM, Zhang X, Schuster H, Caron E, Ward JP, Noguchi T, Ivanova Y, Hundal J, Arthur CD, Krebber WJ, Mulder GE, Toebes M, Vesely MD, Lam SS, Korman AJ, Allison JP, Freeman GJ, Sharpe AH, Pearce EL, Schumacher TN, Aebersold R, Rammensee HG, Melief CJ, Mardis ER, Gillanders WE, Artyomov MN, Schreiber RD, Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens, Nature 515 (7528) (2014) 577–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Pauken KE, Torchia JA, Chaudhri A, Sharpe AH, Freeman GJ, Emerging concepts in PD-1 checkpoint biology, Semin. Immunol 52 (2021) 101480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].You W, Liu X, Tang H, Lu B, Zhou Q, Li Y, Chen M, Zhao J, Xu Y, Wang M, Qian J, Tan B, Vitamin D status is associated with immune checkpoint inhibitor efficacy and immune-related adverse event severity in lung cancer patients: a prospective cohort study, J. Immunother 46 (6) (2023) 236–243. [DOI] [PubMed] [Google Scholar]
- [72].Galus L, Michalak M, Lorenz M, Stoinska-Swiniarek R, Tusien Malecka D, Galus A, Kolenda T, Leporowska E, Mackiewicz J, Vitamin D supplementation increases objective response rate and prolongs progression-free time in patients with advanced melanoma undergoing anti-PD-1 therapy, Cancer 129 (13) (2023) 2047–2055. [DOI] [PubMed] [Google Scholar]
- [73].Li P, Zhu X, Cao G, Wu R, Li K, Yuan W, Chen B, Sun G, Xia X, Zhang H, Wang X, Yin Z, Lu L, Gao Y, 1alpha,25(OH)(2)D(3) reverses exhaustion and enhances antitumor immunity of human cytotoxic T cells, J. Immunother. Cancer 10 (3) (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]


