Abstract
Background
Recent trial data refute concerns about neurocognitive off-target effects of neprilysin inhibition with sacubitril and suggest benefit in patients with heart failure and ejection fraction >40%. We hypothesized that sacubitril/valsartan is associated with improved cognitive outcomes in patients with heart failure and reduced ejection fraction (HFrEF).
Objectives
The purpose of this study was to compare 3-year cognitive outcomes in patients with HFrEF who receive sacubitril/valsartan vs angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs).
Methods
Retrospective cohort study of: 1) 11,313 adults with HFrEF (International Classification of Diseases-10th Revision-Clinical Modification [ICD-10-CM] codes: I50.2 or I50.4) started on sacubitril/valsartan between 1/1/2015 and 12/31/2019; and 2) 11,313 propensity matched patients receiving ACEI/ARB during that time. Data were obtained from the TriNetX Research Network, encompassing 41 health care organizations in the United States. Primary endpoint was the composite of cognitive decline (ICD-10-CM: R41.8), dementia (ICD-10-CM: F01-F03), and Alzheimer’s disease (ICD-10-CM: G30).
Results
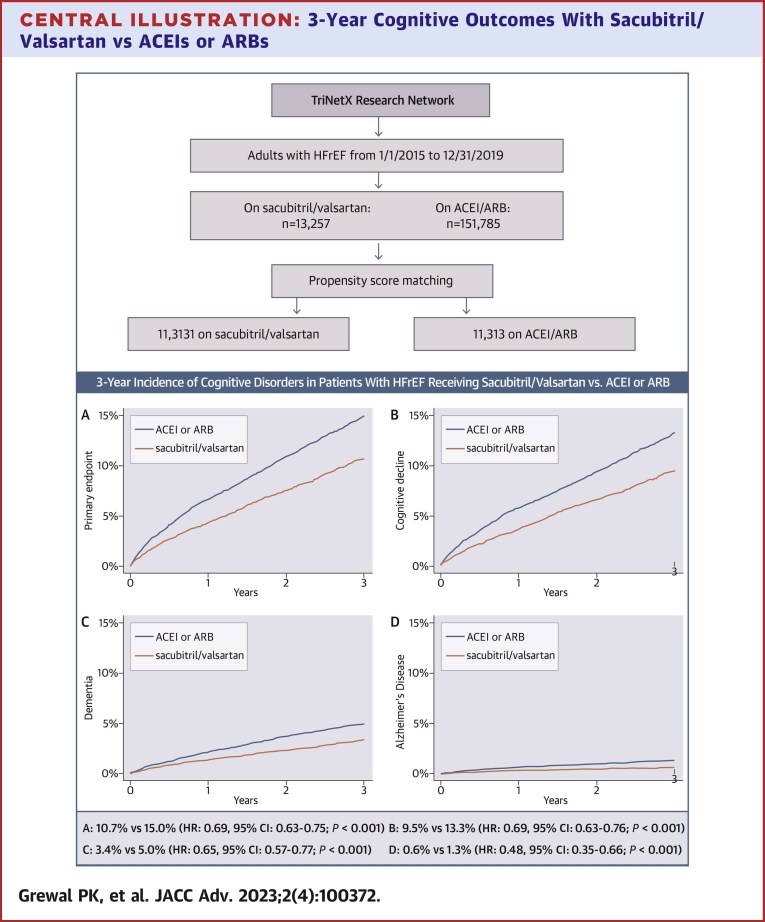
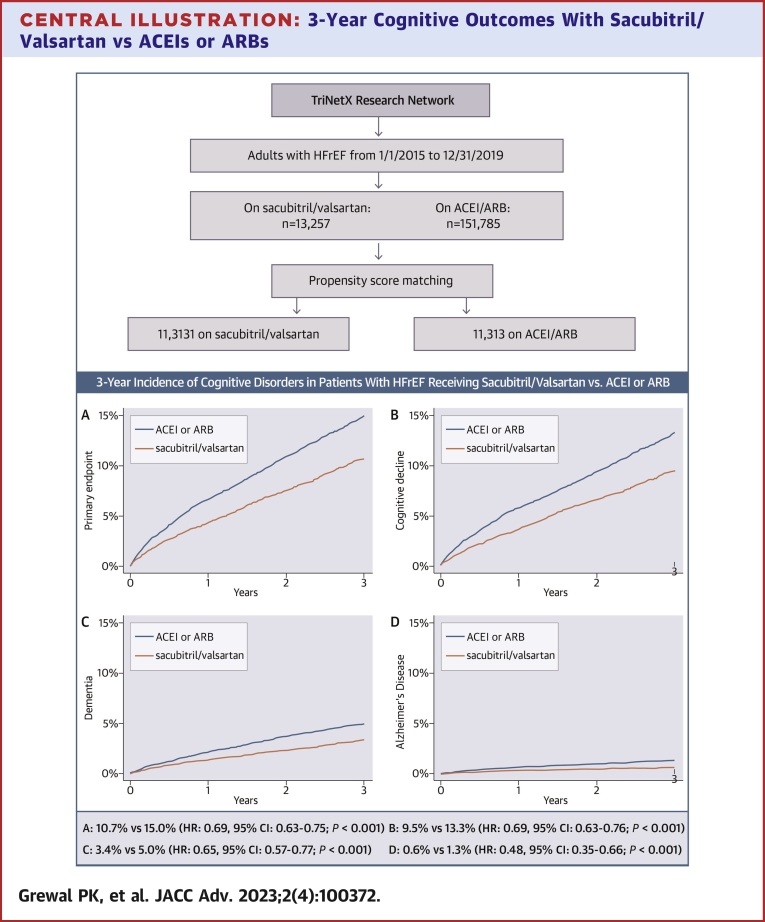
At 3 years, 858 patients on sacubitril/valsartan met the primary endpoint vs 1,209 on ACEI/ARB (3-year incidence: 10.7% vs 15.0%; HR: 0.69; 95% CI: 0.63-0.75; P < 0.001), with consistently lower rates of cognitive decline (9.5% vs 13.3%; HR: 0.69; 95% CI: 0.63-0.76; P < 0.001), dementia (3.4% vs 5.0%; HR: 0.65; 95% CI: 0.57-0.77; P < 0.001), and Alzheimer’s disease (0.6% vs 1.3%; HR: 0.48; 95% CI: 0.35-0.66; P < 0.001) in the sacubitril/valsartan cohort. Results were consistent in matched sex and race subgroups. Three-year mortality was 22.0% on sacubitril/valsartan vs 24.6% on ACEI/ARB (HR: 0.89; 95% CI: 0.84-0.94; P < 0.001).
Conclusions
Sacubitril/valsartan was associated with lower 3-year rates of neurocognitive disorders when compared to ACEI/ARBs in patients with HFrEF.
Key words: Alzheimer’s disease, cognitive decline, dementia, heart failure, neprilysin, sacubitril/valsartan
Central Illustration
Current heart failure (HF) guidelines recommend sacubitril/valsartan as the preferred renin-angiotensin system inhibitor in heart failure with reduced ejection fraction (HFrEF).1,2 Encouraging results in patients with HF and left ventricular ejection fraction (LVEF) >40% have led to expanded U.S. Food and Drug Administration indication and a IIb guideline recommendation for sacubitril/valsartan in this patient group also.2,3
Sacubitril exerts its effects by inhibition of neprilysin, a type 2 integral membrane-bound, zinc-dependent metalloprotease that functions via proteolytic cleavage of peptides in various body systems.4 Renal inhibition of neprilysin prevents degradation of natriuretic peptides, leading to natriuresis and vasodilation, thereby benefiting patients with HF. However, in the central nervous system, neprilysin inhibition prevents enzymatic breakdown of amyloid β,5 which has been associated with Alzheimer’s disease.4 For example, a meta-analysis revealed lower levels of neprilysin expression and activity in patients with Alzheimer’s disease.6 Data from primates and humans have yielded inconclusive findings regarding the clinical relevance of neprilysin activity in the central nervous system.7,8 In the recently presented PERSPECTIVE (Efficacy and Safety of LCZ696 Compared to Valsartan on Cognitive Function in Patients With Chronic Heart Failure and Preserved Ejection Fraction) trial,9 which randomized patients with HF and LVEF >40% to either sacubitril/valsartan or valsartan, there was no difference in cognitive function change after 3 years, and brain amyloid β deposition on positron emission tomography tended to be less with sacubitril/valsartan. This trial also demonstrated that cognitive defects are highly prevalent in patients HF. However, no study to date has investigated the association of sacubitril/valsartan with long-term cognitive outcomes in patients with HFrEF. Of note, reduced cardiac output has been associated with risk for dementia and Alzheimer’s disease in the Framingham Heart Study.10
We hypothesized that the incremental benefit of sacubitril/valsartan over angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs) on functional capacity and cardiac function11 may help prevent cognitive decline in patients with HFrEF, especially in view of the PERSPECTIVE data suggesting that neprilysin inhibition may in fact reduce amyloid β accumulation. In this propensity-matched, retrospective cohort study, we evaluated the incidence of neurocognitive diagnoses, including cognitive decline, dementia, and Alzheimer’s disease, in adults with HFrEF who were switched to (or started) sacubitril/valsartan between 2015 and 2019, without further exposure to ACEIs or ARBs, compared to patients receiving ACEI and/or ARB exclusively during the same period.
Methods
Data source and study design
This is a retrospective, observational, propensity-matched cohort study using data from the TriNetX Analytics Research Network. TriNetX is a global electronic health records network that provides access to anonymous electronic medical records from approximately 88 million patients receiving care at 58 health care organizations. For the present study, data were contributed from 41 healthcare organizations in the United States. TriNetX provides data including demographics, diagnostic and procedural information, and standard measurements (including vital signs, laboratory results, and medications) using standardized coding systems (International Classification of Diseases-10th Revision-Clinical Modification [ICD-10-CM] and Current Procedural Terminology codes for diagnoses and procedures, Logical Observation Identifiers Names and Codes for vital signs and laboratory values, and RxNorm for medications). TriNetX, LLC has received a waiver from Western IRB and complies with the Health Insurance Portability and Accountability Act, as only de-identified data are used,12,13 Data from a final search run on December 21, 2021 were used in this analysis. We followed the REporting of studies Conducted using Observational Routinely collected Data reporting guidelines as a framework12,14 (Supplemental Appendix).
Study population
We identified adult patients (age ≥18 years) with: 1) a diagnosis of HFrEF (ICD-10-CM codes I50.2 or I50.4) between January 1, 2015, and December 31, 2019, from 41 healthcare organizations participating in TriNetX; 2) a second healthcare encounter confirming the diagnosis within a month of the index encounter; 3) initiation of sacubitril/valsartan during the index encounter without subsequent ACEI or ARB prescription; and 4) an additional visit within 1 year of index encounter to ensure up-to-date baseline characteristics. These patients constituted the parent sacubitril/valsartan cohort. Patients with the same diagnostic and healthcare encounter criteria but only receiving ACEI or ARB during the same inception period constituted the parent ACEI/ARB cohort.
Main exposures
The medications in TriNetX are represented at the level of ingredients, coded to RxNorm, and organized by Veterans Administration National Drug File therapeutic classes. These include ACEI (CV800), ARB (CV805), and antihypertensive medications combinations (CV400), which include sacubitril as an ingredient (1656328). Recording of a sacubitril/valsartan combination prescription qualified patients for the sacubitril/valsartan parent cohort. Patients receiving valsartan without sacubitril were included in the ACEI/ARB parent cohort.
Outcomes
The primary endpoint was the incidence of new neurocognitive diagnoses identified through ICD-10-CM codes, defined as the composite of cognitive decline (ICD-10-CM: R41.8), dementia (ICD-10-CM: F01-F03), and Alzheimer’s disease (ICD-10-CM: G30), over 3 years. The follow up window was selected to reduce censoring while maximizing follow up, as the inception period (2015-2019) was relatively recent. The individual components were the secondary endpoints. In exploratory analyses, we examined the incidence of subtypes of dementia (vascular dementia [F01], dementia in other diseases classified elsewhere [F02], and unspecified dementia [F03]).
Statistical analysis
We used the online analytic tools provided by the TriNetX platform to perform 1:1 propensity score matching and generate balanced subsets of the cohorts for over 100 covariates, including the following characteristics: demographics; diseases and medications of circulatory, endocrine, respiratory, genitourinary, musculoskeletal, nervous, and digestive systems; mental, behavioral, and neurodevelopmental disorders; use of vitamin supplements, herbs, and alternative therapies; laboratory values of complete blood count with differential, electrolytes, renal function, iron metabolism, lipid panel, and N-terminal pro-B-type natriuretic peptide levels. The online TriNetX platform performs logistic regression to generate the propensity score based on the predicted probability of a patient belonging to a certain cohort. For each patient in the smaller cohort, the system chooses a match from the larger cohort using the greedy nearest neighbor approach with a caliper of 0.1 pooled standard deviations.15, 16, 17 The order of records is randomized to eliminate bias using a fixed seed during matching, allowing for reproducibility. We repeated the matching process to create matched subgroups of interest: sex (men vs women) and race (White vs Black, as numbers of other race were too small for matching). In a sensitivity analysis, we repeated the matching process and the analysis for all patients with HF, ICD-10-CM: I50, as patients with midrange, preserved, or undocumented ejection fraction may have been receiving sacubitril/valsartan. Outcomes of interest were compared only between matched cohorts and subgroups of cohorts. Mortality was also evaluated in the matched cohorts during the follow-up window (3 years).
The TriNetX platform uses the Kaplan-Meier method to estimate the incidence of the outcome of interest and compares the distribution of the event-free curves with the log-rank test. Patients with any occurrence of any outcome of interest before inception window were excluded from outcome analysis. HRs with 95% CIs are estimated with Cox proportional hazards models using the R survival package v3.2-3. The test for proportionality is based on the scaled Schoenfeld residuals using the same R package. We considered statistically significant differences with a 2-sided P value of 0.05 or less.
Results
Baseline characteristics
We identified 13,257 adults with HFrEF (I50.2 or I50.4) who started receiving sacubitril/valsartan between 2015 and 2019 (without any subsequent exposure to ACEI/ARB) and 151,785 patients who received ACEIs or ARBs exclusively during the same period. The geographic distribution of the parent sacubitril/valsartan cohort was 21.5% Northeast, 10.0% Midwest, 58.4% South, 5.8% West, and 4.4% multiregional (ie, having received care in sites from multiple regions). The corresponding distribution of the parent ACEI/ARB cohort was 29.4% Northeast, 17.4% Midwest, 42.8% South, 8.6% West, and 1.8% multiregional. After propensity score matching for the characteristics described in the methods, the matched cohorts comprised 11,313 patients each. Table 1 summarizes the characteristics of the parent cohorts and the propensity matched cohorts. Patients in the sacubitril/valsartan matched cohort were 65.2 ± 13.7 years of age; 66.3% were men; 67.6% were White, 21.6% Black, and 1.1% Asian; 4.0% were Hispanic; and 89.7% were previously on ACEI/ARB. The most common comorbidities were hypertension (82.3%), ischemic heart disease (71.1%), and dyslipidemia (70.4%), followed by respiratory diseases (65.7%), diabetes (44.9%), and atrial fibrillation (43.2%). All characteristics of the matched ACEI/ARB cohort demonstrated standardized mean differences <0.1 vs the sacubitril/valsartan cohort, except for systolic blood pressure which was marginally lower (121 ± 20 mm Hg vs 123 ± 22 mm Hg; standardized mean difference 0.111) in the sacubitril/valsartan cohort.
Table 1.
Baseline Patient Characteristics for Treatment Cohorts Before Matching and After Matching
| Before Matching |
After Matching |
|||||
|---|---|---|---|---|---|---|
| Sacubitril/Valsartan (n = 13,257) | ACE Inhibitor or ARB (n = 151,785) | SMD | Sacubitril/Valsartan (n = 11,313) | ACE Inhibitor or ARB (n = 11,313) | SMD | |
| Demographics | ||||||
| Age, y | 65.1 ± 13.7 | 67.7 ± 14.2 | 0.187 | 65.2 ± 13.7 | 65.2 ± 14.6 | 0.003 |
| Male | 8,834 (66.6) | 91,208 (60.9) | 0.136 | 7,501 (66.3) | 7,606 (66.1) | 0.005 |
| Race | ||||||
| White | 8,949 (67.5) | 97,232 (64.1) | 0.073 | 7,651 (67.6) | 7,606 (67.2) | 0.009 |
| Black | 2,803 (21.1) | 34,912 (23.0) | 0.045 | 2,441 (21.6) | 2,490 (22.0) | 0.011 |
| Asian | 156 (1.2) | 1,645 (1.1) | 0.009 | 126 (1.1) | 139 (1.2) | 0.011 |
| Hispanic | 531 (4.0) | 10,207 (6.7) | 0.121 | 447 (4.0) | 424 (3.7) | 0.011 |
| Body mass index, kg/m2 | 30.8 ± 6.9 | 30.1 ± 7.2 | 0.099 | 30.8 ± 6.9 | 30.7 ± 7.3 | 0.022 |
| Comorbidities | ||||||
| Hypertension | 10,694 (80.7) | 128,277 (84.5) | 0.102 | 9,316 (82.3) | 9,336 (82.5) | 0.005 |
| Ischemic heart disease | 9,247 (69.7) | 99,572 (65.6) | 0.089 | 8,039 (71.1) | 8,003 (70.7) | 0.007 |
| Atrial fibrillation/flutter | 5,599 (42.2) | 63,009 (41.5) | 0.015 | 4,887 (43.2) | 4,857 (42.9) | 0.005 |
| Pulmonary heart disease | 3,000 (22.6) | 33,678 (22.2) | 0.011 | 2,736 (24.2) | 2,750 (24.3) | 0.003 |
| Dyslipidemia | 9,128 (68.9) | 104,598 (68.9) | 0.001 | 7,967 (70.4) | 7,996 (70.7) | 0.006 |
| Diabetes mellitus | 5,776 (43.6) | 69,723 (45.9) | 0.047 | 5,081 (44.9) | 5,169 (45.7) | 0.016 |
| Respiratory diseases | 8,311 (62.7) | 105,799 (69.7) | 0.149 | 7,427 (65.7) | 7,401 (65.4) | 0.005 |
| Cerebrovascular diseases | 2,660 (20.1) | 38,042 (25.1) | 0.120 | 2,369 (20.9) | 2,358 (20.8) | 0.002 |
| Nicotine dependence | 2,410 (18.2) | 32,193 (21.2) | 0.076 | 2,174 (19.2) | 2,107 (18.6) | 0.015 |
| Other arterial disease | 3,277 (24.7) | 39,075 (25.7) | 0.024 | 2,994 (26.5) | 3,039 (26.9) | 0.009 |
| Mental, behavioral, and neurodevelopmental disorders | 6,064 (45.7) | 79,638 (52.5) | 0.135 | 5,404 (47.8) | 5,404 (47.8) | 0.000 |
| Medication use | ||||||
| Aspirin | 8,177 (61.7) | 94,957 (62.6) | 0.018 | 7,022 (62.1) | 6,884 (60.9) | 0.025 |
| Beta-blockers | 11,205 (84.5) | 119,509 (78.7) | 0.150 | 9,452 (83.6) | 9,415 (83.2) | 0.009 |
| Calcium-channel blockers | 4,237 (32.0) | 62,618 (41.3) | 0.194 | 3,780 (33.4) | 3,798 (33.6) | 0.003 |
| Diuretics | 10,877 (82.0) | 114,766 (75.6) | 0.158 | 9,274 (82.0) | 9,254 (81.8) | 0.005 |
| Antilipemic agents | 8,908 (67.2) | 99,904 (65.8) | 0.029 | 7,583 (67.0) | 7,574 (67.0) | 0.002 |
| Previous use of ACEI | 6,931 (52.3) | 80,194 (52.8) | 0.011 | 6,329 (55.9) | 6,265 (55.4) | 0.011 |
| Previous use of ARB | 5,761 (43.5) | 37,115 (24.5) | 0.410 | 3,824 (33.8) | 3,796 (33.5) | 0.005 |
| Vitamins | 5,020 (37.9) | 66,421 (43.8) | 0.120 | 4,339 (38.4) | 4,281 (37.8) | 0.011 |
| Herbs and alternative therapy | 2,531 (19.1) | 31,503 (20.8) | 0.042 | 2,239 (19.8) | 2,246 (19.9) | 0.002 |
| Vital signs | ||||||
| Systolic BP, mm Hg | 120 ± 19 | 127 ± 22 | 0.288 | 121 ± 20 | 123 ± 22 | 0.111 |
| Diastolic BP, mm Hg | 71 ± 13 | 72 ± 14 | 0.062 | 71 ± 13 | 71 ± 14 | 0.006 |
| Heart rate, beats/min | 76 ± 15 | 77 ± 16 | 0.019 | 76 ± 15 | 76 ± 16 | 0.011 |
| Respiratory rate, beats/min | 17 ± 14 | 17 ± 84 | 0.004 | 17 ± 16 | 16 ± 12 | 0.034 |
| Laboratory | ||||||
| NT-proBNP, pg/mL | 4,535 ± 7,558 | 5,354 ± 9,602 | 0.095 | 4,702 ± 7,709 | 4,551 ± 8,602 | 0.019 |
| Total cholesterol, md/dL | 151 ± 46 | 156 ± 47 | 0.114 | 150 ± 46 | 153 ± 45 | 0.059 |
| Hemoglobin A1c, % | 6.9 ± 2.0 | 6.8 ± 1.9 | 0.060 | 6.9 ± 2.0 | 6.9 ± 1.9 | 0.017 |
| TSH, units/volume | 2.9 ± 14.1 | 2.8 ± 10.7 | 0.007 | 2.7 ± 4.7 | 2.7 ± 4.6 | 0.001 |
| Iron, mcg/dL | 61.6 ± 39.8 | 57.2 ± 41.6 | 0.108 | 60.7 ± 39.1 | 58.4 ± 44.2 | 0.054 |
| Ferritin, ng/mL | 245 ± 500 | 329 ± 1,249 | 0.088 | 248 ± 517 | 285 ± 532 | 0.070 |
| Blood urea nitrogen, mg/dL | 23.5 ± 12.9 | 23.7 ± 14.3 | 0.011 | 23.7 ± 13.2 | 23.7 ± 14 | 0.003 |
| Creatinine, mg/dL | 1.28 ± 1.03 | 1.47 ± 1.98 | 0.119 | 1.29 ± 1.06 | 1.41 ± 1.48 | 0.097 |
Values are mean ± SD or n (%).
ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin II receptor blocker; BP = blood pressure; NT-proBNP = N-terminal pro-B-type natriuretic peptide; SMD = standardized mean difference; TSH = thyroid stimulating hormone.
Incidence of neurocognitive diagnoses
At 3 years, the primary endpoint (incident cognitive decline, dementia, or Alzheimer’s disease) was met by 858 patients in the sacubitril/valsartan vs 1,209 patients in the ACEI/ARB matched cohorts. The corresponding Kaplan-Meier cumulative 3-year incidence was 10.7% vs 15.0%, with a HR of 0.69 (95% CI: 0.63-0.75; P < 0.001), Central Illustration A and Table 2. The 3-year Kaplan-Meier incidence of the secondary endpoints was consistently lower in the sacubitril/valsartan cohort; 9.5% vs 13.3% for cognitive decline (HR: 0.69; 95% CI: 0.63-0.76; P < 0.001), 3.4% vs 5.0% for dementia (HR: 0.65; 95% CI: 0.57-0.77; P < 0.001), and 0.6% vs 1.3% for Alzheimer’s disease (HR: 0.48; 95% CI: 0.35-0.66; P < 0.001), (Central Illustration B to D, Table 2).
Central Illustration.
3-Year Cognitive Outcomes With Sacubitril/Valsartan vs ACEIs or ARBs
Kaplan-Meier estimates for the 3-year cumulative incidence of (A) the primary endpoint (cognitive decline, dementia, or Alzheimer’s disease [whichever occurred first]); (B) cognitive decline; (C) dementia; and (D) Alzheimer’s disease, among patients with heart failure and reduced ejection fraction who started receiving sacubitril/valsartan between 1/1/2015 and 12/31/2019 vs propensity score-matched patients receiving angiotensin-converting enzyme inhibitors or angiotensin receptor blockers with an index encounter during the same timeframe. Patients were captured from the TriNetX database. ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker; HFrEF = heart failure with reduced ejection fraction.
Table 2.
Three-Year Incidence of Neurocognitive Diagnoses Among Patients With Systolic HF (ICD-10-CM: I50.2 or I50.4) Receiving Sacubitril/Valsartan vs Propensity Score-Matched Patients Receiving ACEIs or ARBs
| Sacubitril/Valsartan (n = 11,313)a |
ACEI/ARB (n = 11,313)a |
HR (95% CI) | P Value | |||
|---|---|---|---|---|---|---|
| Events | 3-y K-M Estimate | Events | 3-y K-M Estimate | |||
| Primary endpoint | 858 | 10.7% | 1,209 | 15.0% | 0.69 (0.63-0.75) | <0.001 |
| Cognitive decline | 767 | 9.5% | 1,087 | 13.3% | 0.69 (0.63-0.76) | <0.001 |
| Dementia | 281 | 3.4% | 427 | 5.0% | 0.65 (0.57-0.77) | <0.001 |
| Alzheimer’s disease | 56 | 0.6% | 118 | 1.3% | 0.48 (0.35-0.66) | <0.001 |
ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker; HF = heart failure; ICD-10-CM = International Classification of Diseases-10th Revision-Clinical Modification; K-M = Kaplan-Meier.
Patients with prevalent diagnoses of interest at baseline were excluded from calculation of incidence for each analysis. Outcomes were defined using the following ICD-10-CM codes: dementia (vascular dementia [F01], dementia in other diseases [F02], and unspecified dementia [F03]), Alzheimer’s disease [G30], cognitive decline (other symptoms and signs involving cognitive functions and awareness, R41.8]). The primary endpoint was defined as the composite (first occurrence) of any of these diagnoses.
In an exploratory analysis, we examined the incidence of subtypes of dementia (vascular dementia [F01], dementia in other diseases [F02], and unspecified dementia [F03]). The results were consistent across dementia subtypes (Table 3).
Table 3.
Three-Year Incidence of Dementia Subtype Diagnoses Among Patients With Systolic HF (ICD-10-CM: I50.2 or I50.4) Receiving Sacubitril/Valsartan vs Propensity Score-Matched Patients Receiving ACEIs or ARBs
| Sacubitril/Valsartan (n = 13,323)a |
ACEI/ARB (n = 13,323)a |
HR (95% CI) | P Value | |||
|---|---|---|---|---|---|---|
| Events | 3-y K-M Estimate | Events | 3-y K-M Estimate | |||
| Vascular dementia | 80 | 0.96% | 132 | 1.54% | 0.61 (0.46-0.81) | 0.001 |
| Dementia in other diseases | 81 | 0.96% | 149 | 1.74% | 0.55 (0.42-0.72) | <0.001 |
| Unspecified dementia | 233 | 2.83% | 358 | 4.18% | 0.65 (0.55-0.77) | <0.001 |
ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin II receptor blocker; HF = heart failure; ICD-10-CM = International Classification of Diseases-10th Revision-Clinical Modification; K-M = Kaplan-Meier.
Patients with prevalent diagnoses of interest at baseline were excluded from calculation of incidence for each analysis. Outcomes were defined using the following ICD-10-CM codes: vascular dementia, F01; dementia in other diseases, F02; and unspecified dementia, F03.
Subgroup analyses
In a sacubitril/valsartan subcohort of 7,505 men, the primary endpoint was met by 10.4% at 3 years vs 14.9% among men in a 1:1 matched ACEI/ARB subcohort (HR: 0.68; 95% CI: 0.61-0.75; P < 0.001), whereas the corresponding rates among matched subcohorts of 3,799 women were 11.3% and 14.0%, respectively (HR: 0.77; 95% CI: 0.66-0.90; P = 0.001) (Table 4). The lower rates of the secondary endpoints with sacubitril/valsartan were consistent in men and women, except for rates of Alzheimer’s disease in men, which were not significantly different between treatment groups.
Table 4.
Three-Year Incidence of Neurocognitive Diagnoses Among Patients With Systolic HF Receiving Sacubitril/Valsartan vs Propensity Score-Matched Patients Receiving ACEIs or ARBs in Sex Subgroups
| Sacubitril/Valsartan (n = 7,505)a |
ACEI/ARB (n = 7,505)a |
HR (95% CI) | P Value | |||
|---|---|---|---|---|---|---|
| Events | 3-y K-M Estimate | Events | 3-y K-M Estimate | |||
| Men | ||||||
| Primary endpoint | 556 | 10.4% | 796 | 14.9% | 0.68 (0.61-0.75) | <0.001 |
| Cognitive decline | 490 | 9.2% | 711 | 13.1% | 0.67 (0.60-0.75) | <0.001 |
| Dementia | 179 | 3.2% | 268 | 4.8% | 0.67 (0.55-0.81) | <0.001 |
| Alzheimer | 42 | 0.7% | 56 | 1.0% | 0.76 (0.51-1.13) | 0.177 |
| Sacubitril/Valsartan (n = 3,799)a |
ACEI/ARB (n = 3,799)a |
HR (95% CI) | P Value | |||
|---|---|---|---|---|---|---|
| Events | 3-y K-M Estimate | Events | 3-y K-M Estimate | |||
| Women | ||||||
| Primary endpoint | 301 | 11.3% | 391 | 14.0% | 0.77 (0.66-0.90) | 0.001 |
| Cognitive decline | 276 | 10.1% | 342 | 12.0% | 0.81 (0.70-0.95) | 0.011 |
| Dementia | 100 | 3.7% | 156 | 5.4% | 0.66 (0.52-0.85) | 0.001 |
| Alzheimer’s disease | 14 | 0.5% | 47 | 1.6% | 0.31 (0.17-0.56) | < 0.001 |
ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin II receptor blocker; HF = heart failure; K-M = Kaplan-Meier.
Patients with prevalent diagnoses of interest at baseline were excluded from calculation of incidence for each analysis.
In a sacubitril/valsartan subcohort of 7,629 White patients, the primary endpoint was met by 11.8% at 3 years vs 15.0% among White patients in a 1:1 matched ACEI/ARB subcohort (HR: 0.75; 95% CI: 0.68-0.84; P < 0.001). The corresponding rates among matched subcohorts of 2,423 Black patients were 8.8% and 14.6%, respectively (HR: 0.60; 95% CI: 0.49-0.73; P < 0.001) (Table 5). The lower rates of the secondary endpoints with sacubitril/valsartan were consisted in White and Black patients, except for rates of Alzheimer’s disease in Black patients, which were not significantly different between treatment groups.
Table 5.
3-Year Incidence of Neurocognitive Diagnoses Among Patients With Systolic HF Receiving Sacubitril/Valsartan vs Propensity Score-Matched Patients Receiving ACEIs or ARBs in Racial Subgroups
| Sacubitril/Valsartan (n = 7,629)a |
ACEI/ARB (n = 7,629)a |
HR (95% CI) | P Value | |||
|---|---|---|---|---|---|---|
| Events | 3-y K-M Estimate | Events | 3-y K-M Estimate | |||
| White | ||||||
| Primary endpoint | 631 | 11.8% | 816 | 15.0% | 0.75 (0.68-0.84) | <0.001 |
| Cognitive decline | 556 | 10.4% | 730 | 13.1% | 0.75 (0.67-0.83) | <0.001 |
| Dementia | 223 | 4.0% | 302 | 5.4% | 0.74 (0.62-0.88) | 0.001 |
| Alzheimer’s disease | 47 | 0.8% | 77 | 1.3% | 0.62 (0.43-0.89) | 0.008 |
| Sacubitril/Valsartan (n = 2,423)a |
ACEI/ARB (n = 2,423)a |
HR (95% CI) | P Value | |||
|---|---|---|---|---|---|---|
| Events | 3-y K-M Estimate | Events | 3-y K-M Estimate | |||
| Black | ||||||
| Primary endpoint | 157 | 8.8% | 259 | 14.6% | 0.60 (0.49-0.73) | <0.001 |
| Cognitive decline | 151 | 8.4% | 241 | 13.5% | 0.62 (0.51-0.76) | <0.001 |
| Dementia | 37 | 1.9% | 63 | 3.3% | 0.60 (0.40-0.89) | 0.011 |
| Alzheimer’s disease | 10 | 0.4% | 15 | 0.8% | 0.48 (0.19-1.17) | 0.097 |
ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin II receptor blocker; HF = heart failure; K-M = Kaplan-Meier.
Patients with prevalent diagnoses of interest at baseline were excluded from calculation of incidence for each analysis.
Sensitivity analyses
The results remained consistent when the analysis was expanded to include patients with all forms of HF (ICD-10-CM: I50). In propensity matched cohorts of 13,323 patients each for sacubitril/valsartan and ACEI/ARB treatment groups, the 3-year cumulative incidence of the primary endpoint was 10.5% in the sacubitril/valsartan cohort vs 13.5% in the ACEI/ARB cohort (HR: 0.76; 95% CI: 0.70-0.82; P < 0.001). Similarly, the HR estimates for the secondary end points were consistent across endpoints, albeit the magnitude of association was diminished compared to the HFrEF cohorts (Table 6).
Table 6.
Three-Year Incidence of Neurocognitive Diagnoses Among Patients With Any HF (ICD-10-CM: I50) Receiving Sacubitril/Valsartan vs Propensity Score Matched Patients Receiving ACEIs or ARBs
| Sacubitril/Valsartan (n = 13,323)a |
ACEI/ARB (n = 13,323)a |
HR (95% CI) | P Value | |||
|---|---|---|---|---|---|---|
| Events | 3-y K-M Estimate | Events | 3-y K-M Estimate | |||
| Primary endpoint | 985 | 10.5% | 1,296 | 13.5% | 0.76 (0.70-0.82) | <0.001 |
| Cognitive decline | 865 | 9.1% | 1,149 | 11.8% | 0.76 (0.69-0.82) | <0.001 |
| Dementia | 333 | 3.4% | 446 | 4.4% | 0.76 (0.66-0.88) | <0.001 |
| Alzheimer’s disease | 73 | 0.7% | 122 | 1.2% | 0.61 (0.46-0.82) | 0.001 |
ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin II receptor blocker; HF = heart failure; ICD-10-CM = International Classification of Diseases-10th Revision-Clinical Modification; K-M = Kaplan-Meier.
Patients with prevalent diagnoses of interest at baseline were excluded from calculation of incidence for each analysis. Outcomes were defined using the following ICD-10-CM codes: dementia (vascular dementia [F01], dementia in other diseases [F02], and unspecified dementia [F03]), Alzheimer’s disease [G30], cognitive decline (other symptoms and signs involving cognitive functions and awareness, R41.8]). The primary endpoint was defined as the composite (first occurrence) of any of these diagnoses.
Mortality
Three-year all-cause mortality was significantly lower in the sacubitril/valsartan cohort (2,072 of 11,313 patients, Kaplan-Meier estimate 22.0%) when compared to the ACEI/ARB cohort (2,363 of 11,313 patients, 24.6%) (HR: 0.89; 95% CI: 0.84-0.94; P < 0.001).
Discussion
In this propensity-matched retrospective cohort study, use of sacubitril/valsartan in patients with HFrEF (based on ICD-10-CM codes I50.2 and I50.4), was associated with a lower incidence of neurocognitive disorder diagnoses (cognitive decline, dementia, and Alzheimer’s disease) at 3 years, compared to use of ACEI/ARB. These findings were consistent across the individual neurocognitive disorder diagnoses and in subcohorts of men and women, as well as in White and Black patients. Although the rates of Alzheimer’s disease in men and Black patients were lower with sacubitril/valsartan vs female and White patients, respectively, these differences were not significantly different between the treatment groups, likely due to the small number of Alzheimer events in the subgroups. In line with PARADIGM-HF (Prospective Comparison of angiotensin receptor-neprilysin inhibitor with angiotensin-converting enzyme inhibitor to Determine Impact on Global Mortality and Morbidity in Heart Failure),18 3-year all-cause mortality was lower in the sacubitril/valsartan compared to the ACEI/ARB cohort, suggesting that matching for HFrEF severity was adequate. Although the use of ACEI/ARB prior to the initiation of sacubitril/valsartan may have a carryover effect, cohorts were matched for prior use of ACEI/ARB to control for this confounder. Our study inception period spanned from 2015 to 2019, at the cusp of the PIONEER-HF (Comparison of Sacubitril–Valsartan versus Enalapril on Effect on NT-proBNP in Patients Stabilized from an Acute Heart Failure Episode) trial publication, which established the safety of predischarge use of sacubitril/valsartan in hospitalized patients with HF.19 Therefore, the use of ACEIs/ARBs prior to the initiation of sacubitril/valsartan reflects clinical practice during the inception timeframe, allowing for generalizability of our findings. Our results corroborate and extend those of the recently presented PERSPECTIVE trial in patients with mildly reduced and preserved LVEF,9 by providing large-scale, real-world data on cognitive outcomes in patients with HFrEF. Several points in our study merit further discussion.
Interfering with the enzymatic breakdown of amyloid β in the central nervous system by neprilysin5 has raised concerns about long-tern neurocognitive safety of sacubitril/valsartan. These concerns were founded on the theory associating amyloid β depositions with Alzheimer’s disease.20 Decreased neprilysin expression in the hippocampus of patients with Alzheimer’s disease has been spatially correlated with increased formation of amyloid β plaques.5 Also, in a meta-analysis of 11 studies, the level of neprilysin mRNA in patients with Alzheimer’s disease was lower compared to those without the diagnosis.6 Specific to neprilysin inhibition, daily administration of sacubitril/valsartan in female cynomolgous monkeys resulted in elevations of amyloid β within 2 weeks.7 However, in 43 humans receiving sacubitril/valsartan or placebo daily for 2 weeks, cerebrospinal fluid samples showed that sacubitril/valsartan, compared to placebo, resulted in only elevations in hydrophilic, nonaggregable isoforms of amyloid b, without an increase in aggregable isoforms.8 Of note, the association between amyloid β deposition in the brain and Alzheimer’s disease has been debated recently,15 and journals have expressed concerns about fundamental research supporting the amyloid β theory.16
Systemic inflammatory processes also contribute to the cognitive decline seen in patients with HE in addition to amyloid β deposition.21 Inflammatory cytokines have been implicated in the progression of HF due to shear myocardial stress, myocardial and tissue ischemia, and proinflammatory comorbidities such as diabetes mellitus.21 In a cross-sectional study in patients with HF that examined the association of inflammatory markers, cognitive dysfunction, and severity of HF, cognitive function as assessed by the Montreal Cognitive Assessment score was inversely associated interleukin-6 and C-reactive protein levels, but not tumor necrosis factor, while controlling for covariates including education and psychosocial factors.21 Later studies examining the effect of sacubitril/valsartan on peripheral vascular function and inflammatory markers noted a reduction in interleukin-18 and tumor necrosis factor levels in patients with HFrEF with sacubitril/valsartan.22 Sacubitril/valsartan also reverses cardiac remodeling by decreasing left atrial and ventricular volumes and filling pressures, leading to a reduction in eccentric hypertrophy, increase in ejection fraction, and increase in cardiac output, which in turn can promote better tissue perfusion. Therefore, sacubitril/valsartan may synergistically improve cerebral perfusion and reduce proinflammatory cytokines contributing to the lower rates of neurocognitive disorders in patients with HFrEF.
The comparison of our absolute 3-year risk estimates with previous work is challenging. Accelerated cognitive decline is a long-recognized sequela of HF,23 but data on the exact prevalence and incidence of cognitive impairment in these patients are limited. This is partially because of varying definitions and tools used to identify cognitive impairment and data usually expressed as quantitative decline in cognitive tests and not as dichotomous definitions. In a recent study from Minnesota that used a broad set of International Classification of Diseases-9th Revision-Clinical Modification and ICD-10-CM codes, similar to our study, to identify Alzheimer’s disease and related cognitive disorders,24 the 3-year cumulative incidence of the end point after HF diagnosis was 17.1%, which is strikingly similar to the rate of the composite end point in our ACEI/ARB group. Finally, safety analyses of adverse event reports from PARADIGM-HF and other trials provide an estimate for the incidence of dementia in HFrEF.25 Our findings are consistent with the annual rate of dementia in both PARADIGM-HF arms, which was approximately 1% when a broad definition was applied.25 As our population was unselected and therefore had a higher comorbidity burden compared to clinical trial participants, a slightly higher cognitive event rate is not surprising. This is supported also by the slightly lower mortality benefit observed with sacubitril/valsartan in our study.
The PERSPECTIVE trial enrolled patients with mildly reduced and preserved LVEF,9 with an average age of 72 years. Because of age and higher comorbidity burden, these patients are at higher risk for cognitive decline, a risk-enriched population for the outcome of interest. In fact, 60% of patients had some cognitive impairment at baseline. The results of PERSPECTIVE refuted the concerns outlined above. Although cognitive function declined over 3 years in both arms (sacubitril/valsartan vs valsartan), as measured by a CogTest, there was no difference in change in cognitive function. Brain amyloid β deposition on positron emission tomography scans tended to be less with sacubitril/valsartan, albeit statistical significance was borderline. Although cognitive tests and imaging data were not available in our study, our results complement and extend the PERSPECTIVE findings. We studied patients with HFrEF, a younger HF population (average age in our study was 65 years), who have been shown to have lower cardiac output at rest and exercise compared to patients with preserved ejection fraction.26 Reduced cardiac output has been associated with incident dementia and Alzheimer’s disease in the Framingham Heart Study10 and accelerated cognitive decline in another cohort.27 We observed lower 3-year incidence of neurocognitive diagnoses with sacubitril/valsartan vs ACEI/ARB. Although bias cannot be excluded in an observational study despite careful matching, we believe that the association of sacubitril/valsartan with lower cognitive event rates in patients with HFrEF in our study is plausible. Bias toward less severe HF in the sacubitril/valsartan cohort is unlikely, as sacubitril/valsartan would be used preferentially in advanced HFrEF. Higher competing mortality in the ACEI/ARB group could partially explain the results, but the difference in mortality was considerably smaller. Therefore, we postulate that the association of sacubitril/valsartan with lower cognitive event rates in our study can be explained by its incremental benefits on cardiac function and functional capacity over ACEI/ARB agents,11 in conjunction with the neutral (and potentially beneficial) effect on amyloid β accumulation as shown in PERSPECTIVE. However, serial cardiac imaging or functional test data were not available in our study. Therefore, our data are hypothesis-generating and suggest a favorable effect of sacubitril/valsartan on cognitive outcomes in patients with HFrEF, warranting further prospective investigation.
Study limitations
First, the incidence estimates of new cognitive disorders were based on diagnostic codes and not on quantitative diagnostic tools, eg, a Mini-Cog or a CogState test. Therefore, underestimation or misclassification of the outcome is possible and our absolute risk estimates must be interpreted with caution. On the other hand, because of aligned inception periods and definitions, ascertainment bias is probably balanced between cohorts and therefore the relative risk estimates should be valid. In addition, our results are consistent with a recent study using administrative codes to detect new-onset cognitive disorders in patients with HF.24 Second, the entry criteria for the study included a diagnostic code-based definition for HFrEF and did not include quantitative LVEF. Therefore, patients with borderline or preserved ejection fraction may have been included. However, as the indications for sacubitril/valsartan during the inception period (2015-2019) of this study were restricted to HFrEF (expanded indication was granted by U.S. Food and Drug Administration in early 2021), it is unlikely that patients with mildly reduced or preserved LVEF were overrepresented in the sacubitril/valsartan cohort. Third, in cohorts with high mortality, as in this case, a competing-risks analysis would provide more accurate estimates of absolute and relative risks for more uncommon events. We were not able to conduct this analysis because of limitations of the online analytics platform. This should affect less the more common events, ie, the primary composite end point and cognitive decline, but risks for the less common events, ie, dementia and Alzheimer’s disease, may have been overestimated. Fourth, the number of events in the subgroups and the individual cognitive endpoints was smaller, especially for the Alzheimer endpoint, and therefore, the estimates and the differences thereof between subgroups must be interpreted with utmost caution. Also, the follow-up period was relatively short. Finally, we speculate that the association with fewer neurocognitive disorders in the sacubitril/valsartan group may have been the result of improved cardiac output and/or functional capacity with this agent, other mechanisms, including disruption of blood-brain barrier, cerebral hypoperfusion, oxidative damage, platelet hyperreactivity, brain-endothelium inflammatory activation, systemic inflammation, and adrenergic system alterations are known mechanisms that impair cognition in HF. However, we did not have serial cardiac or brain imaging, functional test, or biomarker data to support these hypotheses.
Conclusions
In a large contemporary health care database from the United States, use of sacubitril/valsartan among patients with HFrEF was associated with lower rates of cognitive events, based on ICD-10-CM diagnostic codes, over a 3-year follow-up period, compared to use of ACEI or ARB, in a propensity score-matched patient population. These results extend the reassuring findings of the detailed, mechanistic PERSPECTIVE trial in patients with mildly reduced and preserved LVEF to patients with HFrEF. However, the efficacy of sacubitril/valsartan for the prevention of cognitive decline in patients with HFrEF and the identification of the pathophysiologic mechanisms that are potentially impacted warrant further prospective investigation.
Funding support and author disclosures
Dr Butler is a consultant to Abbott, Amgen, American Regent, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, CVRx, G3 Pharmaceutical, Impulse Dynamics, Innolife, Janssen, LivaNova, Medtronic, Merck, Novartis, Novo Nordisk, Pfizer, Roche, and Vifor. Dr Goldschmidt has been a speaker for AstraZeneca, Pfizer, and Abbott. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE AND PATIENT CARE: Results from this study suggest that not only use of sacubitril/valsartan is not associated with worse cognitive outcomes, as initially feared on the basis of theoretical concerns about neprilysin inhibition in the brain, but rather offers benefit for this health domain among patients with HFrEF. These results need prospective confirmation, but in the meantime it makes sense to follow the current guideline recommendations and start patients with HF and reduce ejection fraction on sacubitril/valsartan whenever possible.
TRANSLATIONAL OUTLOOK: The hypothesis-generating results from this study warrant a prospective mechanistic study similar to the PERSPECTIVE trial in patients with mildly reduced and preserved ejection fraction. Such a study should include serial measures of cardiac output and functional capacity as, in contrast to the neutral PERSPECTIVE findings, a beneficial effect is suggested in patients with HF and reduced junction fraction, which could potentially be attributed to improved cardiac output and functional capacity.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental appendix, please see the online version of this paper.
Supplemental Appendix
References
- 1.McDonagh T.A., Metra M., Adamo M., et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 2.Heidenreich P.A., Bozkurt B., Aguilar D., et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79:e263–e421. doi: 10.1016/j.jacc.2021.12.012. [DOI] [PubMed] [Google Scholar]
- 3.Solomon S.D., McMurray J.J.V., Anand I.S., et al. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381:1609–1620. doi: 10.1056/NEJMoa1908655. [DOI] [PubMed] [Google Scholar]
- 4.Nalivaeva N.N., Zhuravin I.A., Turner A.J. Neprilysin expression and functions in development, ageing and disease. Mech Ageing Dev. 2020;192 doi: 10.1016/j.mad.2020.111363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carpentier M., Robitaille Y., DesGroseillers L., Boileau G., Marcinkiewicz M. Declining expression of neprilysin in Alzheimer disease vasculature: possible involvement in cerebral amyloid angiopathy. J Neuropathol Exp Neurol. 2002;61:849–856. doi: 10.1093/jnen/61.10.849. [DOI] [PubMed] [Google Scholar]
- 6.Zhang H., Liu D., Wang Y., Huang H., Zhao Y., Zhou H. Meta-analysis of expression and function of neprilysin in Alzheimer's disease. Neurosci Lett. 2017;657:69–76. doi: 10.1016/j.neulet.2017.07.060. [DOI] [PubMed] [Google Scholar]
- 7.Schoenfeld H.A., West T., Verghese P.B., et al. The effect of angiotensin receptor neprilysin inhibitor, sacubitril/valsartan, on central nervous system amyloid-beta concentrations and clearance in the cynomolgus monkey. Toxicol Appl Pharmacol. 2017;323:53–65. doi: 10.1016/j.taap.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 8.Langenickel T.H., Tsubouchi C., Ayalasomayajula S., et al. The effect of LCZ696 (sacubitril/valsartan) on amyloid-beta concentrations in cerebrospinal fluid in healthy subjects. Br J Clin Pharmacol. 2016;81:878–890. doi: 10.1111/bcp.12861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ESC Press Office Neprilysin inhibition does not affect cognitive function in patients with heart failure. https://www.escardio.org/The-ESC/Press-Office/Press-releases/Neprilysin-inhibition-does-not-affect-cognitive-function-in-patients-with-heart-failure
- 10.Jefferson A.L., Beiser A.S., Himali J.J., et al. Low cardiac index is associated with incident dementia and Alzheimer disease: the Framingham Heart Study. Circulation. 2015;131:1333–1339. doi: 10.1161/CIRCULATIONAHA.114.012438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y., Zhou R., Lu C., Chen Q., Xu T., Li D. Effects of the angiotensin-receptor neprilysin inhibitor on cardiac reverse remodeling: meta-analysis. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.119.012272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taquet M., Geddes J.R., Husain M., Luciano S., Harrison P.J. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8:416–427. doi: 10.1016/S2215-0366(21)00084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Topaloglu U., Palchuk M.B. Using a federated network of real-world data to optimize clinical trials operations. JCO Clin Cancer Inform. 2018;2:1–10. doi: 10.1200/CCI.17.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benchimol E.I., Smeeth L., Guttmann A., et al. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med. 2015;12 doi: 10.1371/journal.pmed.1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Austin P.C. A comparison of 12 algorithms for matching on the propensity score. Stat Med. 2014;33:1057–1069. doi: 10.1002/sim.6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Austin P.C. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10:150–161. doi: 10.1002/pst.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y., Cai H., Li C., et al. Optimal caliper width for propensity score matching of three treatment groups: a Monte Carlo study. PLoS One. 2013;8 doi: 10.1371/journal.pone.0081045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McMurray J.J., Packer M., Desai A.S., et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 19.Velazquez E.J., Morrow D.A., DeVore A.D., et al. Angiotensin-neprilysin inhibition in acute decompensated heart failure. N Engl J Med. 2019;380:539–548. doi: 10.1056/NEJMoa1812851. [DOI] [PubMed] [Google Scholar]
- 20.Mawuenyega K.G., Sigurdson W., Ovod V., et al. Decreased clearance of CNS beta-amyloid in Alzheimer's disease. Science. 2010;330:1774. doi: 10.1126/science.1197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Athilingam P., Moynihan J., Chen L., D'Aoust R., Groer M., Kip K. Elevated levels of interleukin 6 and C-reactive protein associated with cognitive impairment in heart failure. Congest Heart Fail. 2013;19:92–98. doi: 10.1111/chf.12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bunsawat K., Ratchford S.M., Alpenglow J.K., et al. Sacubitril-valsartan improves conduit vessel function and functional capacity and reduces inflammation in heart failure with reduced ejection fraction. J Appl Physiol (1985) 2021;130:256–268. doi: 10.1152/japplphysiol.00454.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vishwanath S., Qaderi V., Steves C.J., Reid C.M., Hopper I., Ryan J. Cognitive decline and risk of dementia in individuals with heart failure: a systematic review and meta-analysis. J Card Fail. 2022;8(8):1337–1348. doi: 10.1016/j.cardfail.2021.12.014. [DOI] [PubMed] [Google Scholar]
- 24.Manemann S.M., Knopman D.S., St Sauver J., et al. Alzheimer's disease and related dementias and heart failure: a community study. J Am Geriatr Soc. 2022;70(6):1664–1672. doi: 10.1111/jgs.17752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cannon J.A., Shen L., Jhund P.S., et al. Dementia-related adverse events in PARADIGM-HF and other trials in heart failure with reduced ejection fraction. Eur J Heart Fail. 2017;19:129–137. doi: 10.1002/ejhf.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Namasivayam M., Lau E.S., Zern E.K., et al. Exercise blood pressure in heart failure with preserved and reduced ejection fraction. J Am Coll Cardiol HF. 2022;10:278–286. doi: 10.1016/j.jchf.2022.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bown C.W., Do R., Khan O.A., et al. Lower cardiac output relates to longitudinal cognitive decline in aging adults. Front Psychol. 2020;11 doi: 10.3389/fpsyg.2020.569355. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.