Abstract
Background
Vitamin D deficiency (VDD) is associated with coronary heart disease (CHD) and poor outcomes, but supplementation does not improve prognosis. VDD has been implicated in and may promote greater risk through inflammation and impaired progenitor cell function.
Objectives
The authors examined VDD, high-sensitivity C-reactive protein (hsCRP), circulating progenitor cell (CPC) counts, and outcomes in patients with CHD. They hypothesized that the higher risk with VDD is mediated by inflammation and impaired regenerative capacity.
Methods
A total of 5,452 individuals with CHD in the Emory Cardiovascular Biobank had measurement of 25-hydroxyvitamin D, subsets of whom had hsCRP measurements and CPCs estimated as CD34-expressing mononuclear cell counts. Findings were validated in an independent cohort. 25-hydroxyvitamin D <20 ng/mL was considered VDD. Cox and Fine-Gray models determined associations between marker levels and: 1) all-cause mortality; 2) cardiovascular mortality; and 3) major adverse cardiovascular events, a composite of adverse CHD outcomes.
Results
VDD (43.6% of individuals) was associated with higher adjusted cardiovascular mortality (HR: 1.57, 95% CI: 1.09-2.28). There were significant interactions between VDD and hsCRP and CPC counts in predicting cardiovascular mortality. Individuals with both VDD and elevated hsCRP had the greatest risk (HR: 2.82, 95% CI: 2.16-3.67). Only individuals with both VDD and low CPC counts were at high risk (HR: 2.25, 95% CI: 1.46-3.46). These findings were reproduced in the validation cohort.
Conclusions
VDD predicts adverse outcomes in CHD. Those with VDD, inflammation and/or diminished regenerative capacity are at a significantly greater risk of cardiovascular mortality. Whether targeted supplementation in these high-risk groups improves risk warrants further study.
Key words: cardiovascular outcomes, inflammation, progenitor cells, vitamin D deficiency
Central Illustration
Although vitamin D is best known for its role in calcium homeostasis and bone metabolism, vitamin D deficiency (VDD)—affecting at least 24% of the U.S. population—is also associated with risk factors for cardiovascular disease (CVD) and adverse CVD outcomes.1,2 Despite the large body of epidemiological evidence implicating VDD, vitamin D supplementation trials have largely failed to improve CVD outcomes, and its measurement in higher-risk individuals undergoing angiography was reported to confer little prognostic value.3, 4, 5 These inconsistencies have led to speculation that VDD may simply be an indicator of poor overall health, rather than a mediator of CVD.
Atherosclerosis is a chronic, progressive disease whose drivers include vascular endothelial dysfunction, subendothelial sequestration of cholesterol-rich lipoproteins, and a subsequent inflammatory response that contribute to plaque formation. These injurious processes are mitigated by vascular repair, such that disease progression is modulated by the balance between injury and repair processes.6, 7, 8 Vitamin D plays an important immunomodulatory role in the innate and adaptive immune responses in the context of infectious, inflammatory, and autoimmune diseases.9, 10, 11 It also regulates key processes involved in plaque formation and progression, mediated by endothelial cells, dendritic cells, monocytes, and cholesterol-filled macrophages or “foam cells.”12
High-sensitivity C-reactive protein (hsCRP) is marker of systemic inflammation and an independent predictor of adverse CVD outcomes.13 Epidemiologic studies have reported an inverse relationship between circulating levels of vitamin D [25(OH)D3] and markers of inflammation, including CRP.14 In a cross-sectional analysis of the National Health and Nutrition Examination Surveys (NHANES), participants with both severe VDD and high CRP levels were more likely to report a history of CVD.15
Circulating progenitor cells (CPCs) are bone marrow-derived mononuclear cells that possess the ability to differentiate into several lineages, including hematopoietic subsets distinguished by expression of the CD34 epitope on hematopoietic CD45med cells. Subsets of CD34+ progenitors contribute to vascular repair and regeneration, largely by paracrine mechanisms.7,8 Circulating levels of CPCs and their activity are considered to represent endogenous regenerative potential.16 While we have shown that lower circulating CD34+ cell counts correlate with progressive inflammation, we have also shown that they predict higher mortality in patients with CVD, independent of hsCRP levels.17, 18, 19, 20, 21 Studies in other chronic conditions, such as diabetes, have implicated the vitamin D–vitamin D receptor (VDR) axis in the endothelial regenerative process.22, 23, 24 In vitro, vitamin D improves CPC viability, migration, and colony-forming capacity by releasing vascular endothelial growth factor, guanosine-5′-triphosphatase, matrix metalloproteinases, and increasing nitric oxide production.25,26 It also influences CPC behavior within the injured endothelium by mitigating pro-inflammatory signaling, thus promoting adhesion via vascular endothelial-cadherin junctions and tipping the balance toward cell-mediated regeneration and away from calcification in hypoxia.27,28 Moreover, expression of VDR in CPCs and, consequently, vitamin D’s ability to facilitate these functions is diminished in patients with coronary heart disease (CHD)—especially in those with elevated inflammatory markers.29
Herein, we examined the relationship between VDD, defined as 25-hydroxyvitamin D levels <20 ng/mL, and adverse CHD outcomes while evaluating the influence of inflammation and CPCs.30 Findings were validated in an independent cohort with CHD. We hypothesized that VDD will be associated with adverse outcomes, particularly in individuals with inflammation and diminished regenerative capacity.
Methods
Study population
Participants were enrolled in the Emory Cardiovascular Biobank (EmCAB), an ongoing prospective registry of individuals undergoing cardiac catheterization for known or suspected CHD at 3 Emory Healthcare-affiliated hospitals.31 Individuals ages 20 to 90 years were interviewed to collect demographic information, medical history, CVD risk factor history, medication use, and behavioral habits. Our analysis includes 5,452 participants who had hsCRP and 25-hydroxyvitamin D levels measured at enrollment. A subset (n = 1,523) also had CPC quantitation. Participants with missing biomarker data or presenting with acute myocardial infarction (MI) were excluded.
Sex and race were self-reported. The presence of hypertension, hyperlipidemia, and diabetes was determined by physician diagnosis and/or treatment prescribed in the medical chart. Smoking was classified as nonsmoking or current/past smoking. Blood pressure, weight, and height were measured on enrollment. Body mass index (BMI) was calculated as the weight (kilograms) divided by the square of the height (meters). Serum creatinine measurements at enrollment before cardiac catheterization were obtained using data from routine follow-up clinic visits or hospitalizations within the Emory Healthcare system. The estimated glomerular filtration rate was computed using the Chronic Kidney Disease Epidemiology Collaboration equation. Medications and medical history were obtained by self-report followed by review of relevant medical records.
An independent validation cohort consisted of 535 participants from the Mental Stress Ischemia Prognosis Study (MIPS), a prospective study that recruited patients with stable CHD from 2011 to 2014 at Emory University-affiliated hospitals.32 Demographics and medical history were collected as above. Both studies were approved by the Institutional Review Board at Emory University. All participants provided written informed consent.
Biomarker measurements
Fasting arterial blood samples from the EmCAB cohort were drawn before catheterization and venous samples drawn from MIPS cohort. Samples were stored at −80 °C. Serum hsCRP measurements were determined using sandwich immunoassays by FirstMark, Inc, or by Abbott Laboratories, Inc. Vitamin D levels were measured as serum 25-hydroxyvitamin D levels using the ARCHITECT 25-OH Vitamin D competitive immunoassay by Abbott Laboratories, Inc, with a measurement range of 3.4 to 155.9 ng/mL. Levels <20 ng/mL were defined as VDD.30
Circulating progenitor cells
Flow cytometry was used to quantify CPCs as CD45med mononuclear cells expressing the CD34 epitope from blood samples collected in ethylenediaminetetraacetic acid tubes. Samples were prepared within 24 hours of collection and incubated with fluorochrome-labeled monoclonal antihuman mouse antibodies to identify surface markers expressed on mononuclear cells before quantification using flow cytometry. 300 μL of peripheral blood were incubated with 7 μL of FITC-CD34 and PerCP-CD45 (BD Biosciences) in the dark for 15 minutes. Then 1.5 mL ammonium chloride lysing buffer was added to lyse red blood cells, after which 1.5 mL staining medium (phosphate-buffered saline with 3% heat-inactivated serum and 0.1% sodium azide) was added to stop the lysing reaction. Prior to flow cytometry, 100 μL of AccuCheck Counting Beads (Invitrogen, Cat#:PCB100) were added to act as an internal standard for direct estimation of the concentration of target cell subsets. At least 2.5 million events were acquired from the cytometer. Flow cytometry data were analyzed with Flowjo software (Treestar, Inc) and CPC populations (CD34+/CD45med) were reported as cells/μL. Interobserver variability was tested in twenty samples that were analyzed on 2 occasions by 2 technicians. Percent repeatability coefficient (%) was calculated as the standard deviation of differences between pairs of measurements/mean of measurements × 100. The coefficient was 2.9%.
Outcomes and follow-up
EmCAB participants were followed for primary outcomes including all-cause mortality, cardiovascular mortality, and major adverse cardiovascular events (MACE) over a median 5.3-year period. Mortality and MACE data were available for 5,406 (99.1%) and 5,379 (98.7%) of participants with available 25-hydroxyvitamin D data. Follow-up was conducted by annual phone contact, electronic medical record review, and the social security death index and state records.31 The cause of death was determined through medical record review or by contact with participants’ family member(s), and all-cause death and cardiovascular death were adjudicated by 2 independent, blinded cardiologists. Cardiovascular death was defined as death attributable to ischemic cardiovascular pathology, such as fatal MI, stroke, or sudden death presumed due to cardiovascular cause. MACE was a composite of cardiovascular death, hospitalization for heart failure, nonfatal MI, or nonfatal stroke within 5 years.
MIPS participants were followed for all-cause and cardiovascular mortality over a median 6.4-year period. Mortality data were available for 535 (84.1%) of participants with available 25-hydroxyvitamin D data. Follow-up was conducted by phone contact, medical record review, and the social security death index. Participants were contacted every 6 months for the first 3 years and then at 5 years. First and recurrent events were adjudicated as above.
Statistical analysis
Baseline characteristics of participants in both cohorts were reported as proportions for categorical and means for continuous variables. Differences between the VDD group and either normal or vitamin D insufficient groups were examined using chi-squared tests for categorical and t-tests for continuous variables.
The relationships between hsCRP and CD34+ cells and VDD were examined using linear regression with adjustment for demographic characteristics and risk factors. The relationship between 25-hydroxyvitamin D levels and cardiovascular mortality was initially visualized in restricted cubic splines-based hazard ratio curves with 3 knots, relative to the median of vitamin D, to explore a potential threshold of increasing hazard ratios. Next, differences in event-free survival from all-cause death, cardiovascular death, and MACE between participants with and without VDD were assessed using cumulative incidence function analyses for unadjusted analyses, Cox proportional hazards to adjust for covariates, and Fine-Gray subdistribution hazard models to account for competing risks.33 Covariates included baseline characteristics and behavioral risk factors (age, sex, Black race, BMI, smoking history), clinical risk factors (hypertension, hyperlipidemia, diabetes, >50% obstruction in a major coronary vessel, heart failure, estimated glomerular filtration rate) and medication use (aspirin, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker, statin). Analyses were repeated with adjustment for season at recruitment. To investigate if the relationship between VDD and cardiovascular mortality is modified by inflammation and CD34+ cells, multiplicative interactions were tested between VDD, hsCRP, and CD34+ cells (both were dichotomized using the median). Interactions between VDD and sex, diabetes, hypertension, heart failure, CHD severity, and statin use were also examined.
The c-statistic was used to assess the value of VDD as a covariate in models predicting incident outcomes.34 The baseline model included the same covariates listed above except medication use. Discrimination was also assessed using continuous net reclassification improvement and integrated discrimination improvement measures.35 Analyses were performed using IBM SPSS Statistics Version 28 and R version 4.1.2. P values <0.05 were considered statistically significant.
Results
Characteristics of the cohorts
Baseline characteristics of the EmCAB cohort are summarized in Table 1. Of 5,452 participants, 2,378 (43.6%) had VDD (25-hydroxyvitamin D <20 ng/mL), while 33.3% and 23.1% had insufficient (20-30 ng/mL) or normal (>30 ng/mL) levels, respectively. Individuals with VDD were younger, female, and Black with higher BMI and a higher prevalence of diabetes and prior MI (Table 1).
Table 1.
Baseline Characteristics and Incident Outcomes in the Overall Cohort and by Vitamin D Category
| All (N = 5,452) | Normal (>30 ng/mL) (n = 1,257) | Insufficiency (20-30 ng/mL) (n = 1,817) | Deficiency (<20 ng/mL) (n = 2,378) | |
|---|---|---|---|---|
| Age (y) | 63.4 ± 12.3 | 65.8 ± 12.6 | 64.2 ± 12.0 | 61.4 ± 12.2AB |
| Male | 3,484 (64.0%) | 818 (65.3%) | 1,301 (71.8%) | 1,365 (57.5%)AB |
| Black race | 1,048 (19.2%) | 125 (9.9%) | 236 (13.0%) | 687 (28.9%)AB |
| BMI, kg/m2 | 29.7 ± 6.3 | 28.2 ± 5.6 | 29.3 ± 5.5 | 30.8 ± 6.9AB |
| Current or former smoker | 3,447 (63.2%) | 816 (64.9%) | 1,157 (63.7%) | 1,474 (62.0) |
| History of hypertension | 4,255 (78.5%) | 1,012 (80.7%) | 1,392 (77.1%) | 1,851 (78.4) |
| History of hyperlipidemia | 3,899 (71.9%) | 934 (74.6%) | 1,314 (72.7%) | 1,651 (69.8)A |
| History of diabetes | 1,897 (35.0%) | 398 (31.8%) | 590 (32.7%) | 909 (38.5%)AB |
| CHDc | 1,775 (32.6%) | 378 (30.1%) | 610 (33.6%) | 787 (33.1%) |
| History of MI | 1,116 (20.7%) | 210 (16.9%) | 401 (22.3%) | 505 (21.4%)A |
| History of heart failure | 1,770 (32.5%) | 404 (32.1%) | 577 (31.8%) | 789 (33.2%) |
| History of PCIa | 2,166 (39.7%) | 482 (38.3%) | 753 (41.4%) | 931 (39.2%) |
| History of CABGb | 1,228 (22.5%) | 269 (21.4%) | 444 (24.4%) | 515 (21.7%) |
| eGFR, mL/min/1.73 m2 | 71.9 ± 24.3 | 70.2 ± 21.9 | 71.7 ± 22.5 | 72.8 ± 26.7A |
| Medications | ||||
| ACEI/ARB use | 2,978 (54.6%) | 660 (52.5%) | 1,015 (55.9%) | 1,303 (54.8%) |
| Beta-blocker use | 3,544 (65.0%) | 788 (62.7%) | 1,205 (66.3%) | 1,551 (65.2%) |
| Statin use | 3,843 (70.5%) | 886 (70.5%) | 1,311 (72.2%) | 1,646 (69.2%) |
| Aspirin use | 4,120 (75.6%) | 977 (77.7%) | 1,393 (76.7%) | 1,750 (73.6%)A |
| Plavix use | 2,257 (41.4%) | 473 (37.6%) | 791 (43.5%) | 993 (41.8%)A |
| Biomarkers | ||||
| 25-hydroxyvitamin D (ng/mL) | 23.2 ± 11.1 | 38.5 ± 9.5 | 24.6 ± 2.8 | 14.0 ± 4.0AB |
| hsCRP (mg/L) | 7.8 ± 20.6 | 7.0 ± 18.6 | 6.4 ± 14.8 | 9.3 ± 24.9AB |
| CD34+ cells (cells/μL) | 2.23 ± 5.45 | 1.96 ± 1.43 | 2.43 ± 9.27 | 2.29 ± 2.34 |
| Events | ||||
| All-cause death | 1,480 (27.4%) | 295 (23.6%) | 427 (23.7%) | 758 (32.2%)AB |
| Cardiovascular death | 770 (14.2%) | 156 (12.5%) | 210 (11.7%) | 404 (17.1%)AB |
| MACE | 1,387 (25.7%) | 278 (22.3%) | 447 (24.8%) | 662 (28.1%)A |
| Myocardial infarction | 250 (4.6%) | 49 (3.9%) | 76 (4.2%) | 125 (5.3%) |
Values are mean ± SD or n (%). Letter superscripts denote significant differences (P < 0.05) between the VDD group and either normalA or insufficientB groups.
ACEI/ARB = angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; BMI = body mass index; eGFR = estimated glomerular filtration rate; hsCRP = high-sensitivity C-reactive protein; MACE = major adverse cardiovascular events; MI = myocardial infarction.
Percutaneous coronary intervention.
Coronary artery bypass graft.
Coronary heart disease.
In the validation cohort, 116 participants (21.7%) had VDD, while 30.5% and 47.9% had insufficient and normal levels, respectively. In addition to the characteristics associated with VDD in the EmCAB cohort, VDD in the MIPS cohort was more often present in those with hyperlipidemia and heart failure (Supplemental Table 1).
Relationship between VDD and adverse outcomes
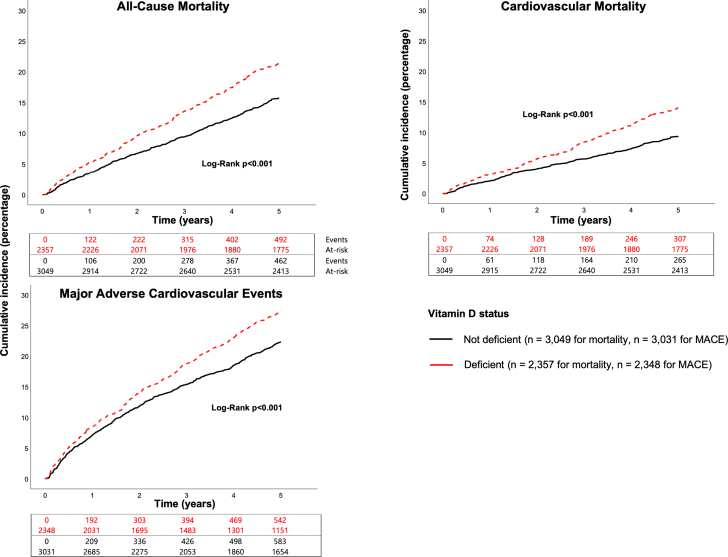
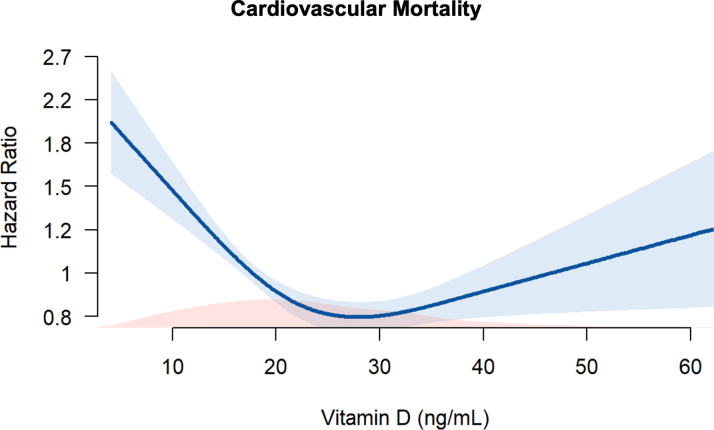
During the follow-up period, there were 955 (17.7%) incident all-cause deaths, 572 (10.6%) cardiovascular deaths, and 1,125 (20.9%) MACE. In unadjusted analyses, individuals with VDD were at significantly higher risk for all adverse outcomes (P < 0.001), with a 41%, 36%, and 30% higher hazard of all-cause mortality, cardiovascular mortality, and MACE, respectively, compared to those without VDD (Figure 1, Table 2). The higher risk with VDD persisted for all adjusted major outcome measures (Table 2). Analyses adjusting for season at the time of recruitment did not attenuate these findings. Spline regression examining the relationship between serum 25-hydroxyvitamin D levels and cardiovascular mortality found increasing hazard ratios at levels <20 ng/mL (Figure 2). Though hazard appeared to be U-shaped, with increasing hazard at levels>35 ng/mL, this was not statistically significant. Sensitivity analyses investigating the association between VDD and cardiovascular mortality for subgroups determined by sex, and presence or absence of diabetes, hypertension, or heart failure, CHD severity and statin use found no significant interactions with these covariates (P > 0.05) (Supplemental Figure 1).
Figure 1.
Cumulative Incidence of Adverse Outcomes According to VDD Status
Incidence of all outcomes was higher (P < 0.001) in VDD individuals. VDD = vitamin D deficiency.
Table 2.
Relationship Between VDD and Adverse Outcomes
| Model | HR/sHR (95% CI) | P Value | |
|---|---|---|---|
| All-cause death (n = 5,033) | Unadjusted | 1.41 (1.24-1.60) | <0.001 |
| 1 | 1.64 (1.43-1.88) | <0.001 | |
| 2 | 1.60 (1.38-1.87) | <0.001 | |
| 3 | 1.60 (1.37-1.86) | <0.001 | |
| Cardiovascular death (n = 5,033) | Unadjusted | 1.36 (0.96-1.94) | 0.083 |
| 1 | 1.64 (1.14-2.37) | 0.008 | |
| 2 | 1.63 (1.13-2.36) | 0.009 | |
| 3 | 1.57 (1.09-2.28) | 0.017 | |
| MACE (n = 4,956) | Unadjusted | 1.30 (0.86-1.97) | 0.21 |
| 1 | 1.60 (1.05-2.45) | 0.030 | |
| 2 | 1.59 (1.03-2.45) | 0.035 | |
| 3 | 1.54 (1.01-2.36) | 0.048 |
Cox (all-cause death) and Fine-Gray models (cardiovascular death, MACE) investigated the relationship between VDD and adverse outcomes.
Model 1 was adjusted for age, sex, Black race, BMI, and smoking history.
Model 2: Model 1 + hypertension, hyperlipidemia, diabetes, CHD, heart failure history, and eGFR.
Model 3: Model 2 + aspirin, ACEI/ARB, and statin use. BoldP values are considered statistically significant (P < 0.05).
MACE = major adverse cardiovascular events; sHR = subdistribution hazard ratio; VDD = vitamin D deficiency.
Figure 2.
Relationship Between Serum Vitamin D Level (ng/mL) and Unadjusted Cardiovascular Mortality in the EmCAB Using Restricted Cubic Splines With 3 Knots
Blue shading indicates 95% CI. Red shading indicates distribution of 25-hydroxyvitamin D levels. EmCAB = Emory Cardiovascular Biobank.
Discrimination testing evaluated the performance of VDD in models predicting all-cause and cardiovascular mortality and MACE. VDD improved prediction of all-cause mortality with a c-statistic of 67.7% (Δ1.4%, 95% CI: 0.1%-2.7%) compared to a model including clinical characteristics and risk factors only (Table 3). Incorporating 25-hydroxyvitamin D levels into all models was associated with a significant net risk reclassification, with a net reclassification improvement of 11.1% (95% CI: 4.9%-17.1%) for all-cause mortality, of 11.8% (95% CI: 4.1%-20.1%) for cardiovascular mortality, and 7.6% (95% CI: 0.8%-14.4%) for MACE (Table 3).
Table 3.
Discrimination Statistics and VDD for Adverse Outcomes
| Model | C-Statistic (%) Without VDD | C-Statistic (%) With VDD | ΔC-Statistic (%) | Net Reclassification Improvement (%) | Integrated Discrimination Improvement (%) | |
|---|---|---|---|---|---|---|
| All-cause death (N = 5,406) | ||||||
| Base covariates | 66.3 (62.8-69.8) | 67.7 (64.2-71.1) | 1.4 (0.1-2.7) | 11.1 (4.9-17.1) | 1.6 (0.5-3.2) | |
| +CD34+ | 66.7 (63.3-70.1) | 68.0 (64.7-71.4) | 1.3 (0.1-2.5) | 11.1 (5.3-17.6) | 1.7 (0.5-3.3) | |
| +hsCRP level | 68.0 (64.6-71.4) | 69.2 (65.8-72.5) | 1.2 (0.1-2.2) | 11.0 (5.2-17.0) | 1.2 (0.2-2.7) | |
| +CD34+ and hsCRP | 68.2 (64.6-71.8) | 69.4 (66.0-72.8) | 1.2 (0.0-2.4) | 11.0 (5.1-17.0) | 1.3 (0.3-2.8) | |
| Cardiovascular death (N = 5,406) | ||||||
| Base covariates | 68.1 (63.7-72.5) | 69.3 (65.2-73.5) | 1.3 (−0.4-2.9) | 11.8 (4.1-20.1) | 1.3 (0.2-3.4) | |
| +CD34+ level | 68.7 (64.3-73.0) | 69.8 (65.5-74.2) | 1.2 (−0.3-2.6) | 11.8 (4.5-19.2) | 1.5 (0.3-3.3) | |
| +hsCRP level | 68.7 (64.7-72.7) | 69.9 (66.0-73.8) | 1.2 (−0.2-2.6) | 11.8 (4.5-19.5) | 1.1 (0.2-2.9) | |
| +CD34+ and hsCRP | 69.1 (64.4-73.9) | 70.2 (65.7-74.7) | 1.1 (−0.4-2.5) | 11.8 (3.6-19.0) | 1.3 (0.2-3.0) | |
Discrimination statistics summarizing predictive ability of VDD for the listed outcomes. Base covariates include those in Model 2 from Table 2. Numbers in parentheses indicate 95% CI.
hsCRP = high-sensitivity C-reactive protein; VDD = vitamin D deficiency.
In the validation cohort, VDD was also associated with all all-cause (HR: 3.41, P = 0.005) and cardiovascular mortality (HR: 4.93, P = 0.010) compared to those without VDD after adjustment for the aforementioned covariates (Supplemental Table 2).
Relationship between VDD, hsCRP, and CD34+ CPCs
Participants with VDD had 33% and 45% higher levels of hsCRP compared to those with vitamin D insufficiency and normal participants, respectively. This difference remained significant after adjustment for demographic, behavioral, and clinical risk factors (P = 0.026). There was no correlation between 25-hydroxyvitamin D levels and CD34+ cell counts, even after covariate adjustment (P = 0.79).
In the validation cohort, participants with VDD also had significantly higher hsCRP levels compared to those with sufficient and insufficient levels (P = 0.019). There was no correlation between 25-hydroxyvitamin D levels and CD34+ cell counts in this cohort (P = 0.90).
Relationship between VDD, inflammation, and outcomes
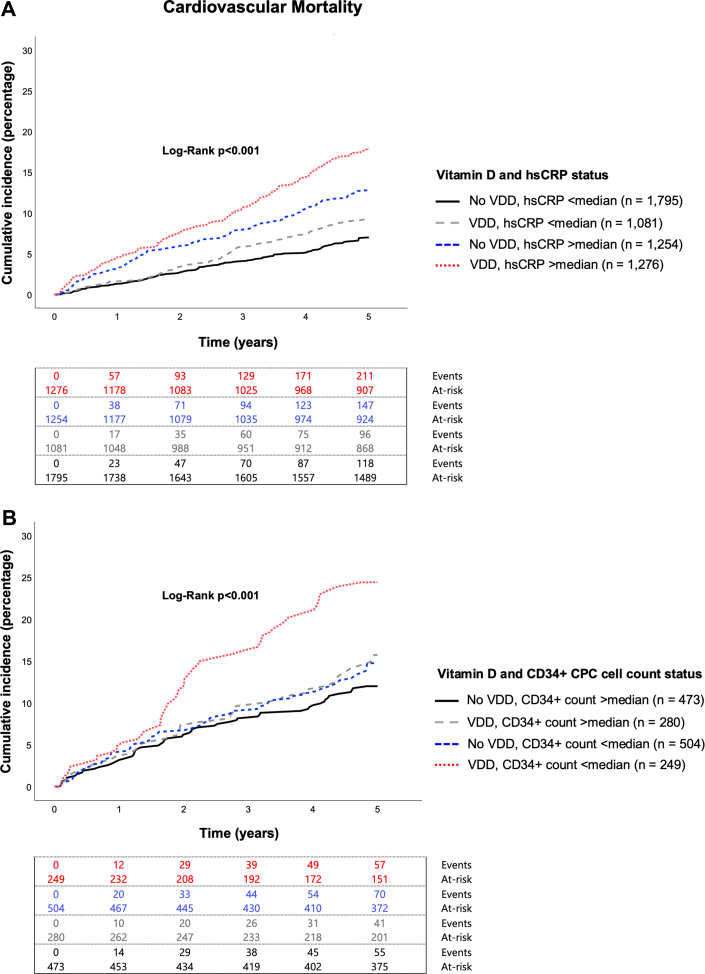
As expected, participants with hsCRP levels above the median (>3 mg/L) experienced a higher incidence of adverse outcomes in both unadjusted (P < 0.001) and adjusted analyses (P < 0.001 for all outcomes) (Supplemental Table 3). In a model including the aforementioned demographic features and risk factors, both hsCRP levels (dichotomous) and VDD were independent predictors of adverse CHD outcomes (P < 0.001 for both covariates across all outcomes). There was a significant VDD by hsCRP interaction for predicting cardiovascular mortality (P = 0.028). When separated into 4 groups according to high and low levels of hsCRP and by the presence or absence of VDD, there was an interdependence between the 2 covariates for prediction of cardiovascular mortality (P < 0.001), with an approximately 3-fold greater hazard of cardiovascular mortality in those with both VDD and elevated hsCRP levels compared to those with normal 25-hydroxyvitamin D and low hsCRP levels (Figure 3A, Table 4). The groups with abnormalities of one of these measures had an intermediate 1.5- to 1.7-fold hazard of cardiovascular mortality (Figure 3A, Table 4). Similar findings were observed for all-cause mortality and MACE. Analyses adjusting for season did not change these findings.
Figure 3.
Cumulative Incidence of Cardiovascular Mortality According to VDD Status, Inflammatory State, and Regenerative Capacity
Cumulative incidence of cardiovascular mortality according to VDD status and (A) hsCRP or (B) CPC counts. Median hsCRP = 3.00 mg/L. Median CD34+ count = 1.6924 cells/μL. CPC = circulating progenitor cell; hsCRP = high-sensitivity C-reactive protein; VDD = vitamin D deficiency.
Table 4.
Relationship Between VDD, Inflammation, CPCs, and Cardiovascular Mortality
| Vitamin D and hsCRP Status | (n = 4,426) | sHR (95% CI) | P Value |
|---|---|---|---|
| No VDD, <median hsCRP | 1,499 | Referent | Referent |
| VDD, <median | 885 | 1.46 (1.07-1.98) | 0.017 |
| No VDD, >median | 1,026 | 1.68 (1.28-2.21) | <0.001 |
| VDD, >median | 1,016 | 2.82 (2.16-3.67) | <0.001 |
| Vitamin D and CD34+ Count Status | (n = 1,309) | ||
|---|---|---|---|
| No VDD, >median CD34+ count | 414 | Referent | Referent |
| VDD, >median | 231 | 1.40 (0.87-2.27) | 0.17 |
| No VDD, <median | 448 | 1.09 (0.74-1.62) | 0.66 |
| VDD, <median | 217 | 2.25 (1.46-3.46) | <0.001 |
| Vitamin D, hsCRP, CD34+ Count | (n = 998) | ||
|---|---|---|---|
| No VDD, normal biomarkers | 264 | Referent | |
| No VDD, either >median hsCRP or <median CPCs | 391 | 0.94 (0.65-1.38) | 0.76 |
| VDD, either >median hsCRP or <median CPCs | 227 | 1.46 (0.96-2.22) | 0.078 |
| VDD, both >median hsCRP and <median CPCs | 116 | 2.66 (1.75-4.05) | <0.001 |
Fine-Gray models for cardiovascular mortality were adjusted for covariates in Model 2 from previous tables. Median hsCRP = 3.00 mg/L. Median CD34+ = 1.6924 cells/L. Interaction P = 0.028 for VDD and hsCRP, interaction P = 0.014 for VDD and CD34+ count. BoldedP-values are considered statistically significant (P < 0.05).
CPC = circulating progenitor cell; hsCRP = high-sensitivity C-reactive protein; sHR = sub-distribution hazard ratio; VDD = vitamin D deficiency.
Discrimination analysis demonstrated that vitamin D status improved prediction of adverse events compared to models including baseline covariates and hsCRP levels. There was also significant improvement in risk reclassification (Table 3).
In the validation cohort (n = 535), participants with hsCRP levels above the lower, cohort-specific median (>1.7 mg/L) were not at a significantly greater risk than those with levels beneath the median (Supplemental Table 4). However, when separated into 4 groups, as above, according to high and low levels of hsCRP and by the presence or absence of VDD, participants with both an elevated hsCRP level and VDD were at the greatest hazard of cardiovascular mortality (HR: 9.2, P = 0.009) (Supplemental Table 5).
Relationship between VDD, CPCs, and outcomes
As previously reported, participants with low CPC counts (CD34+ cells < median) had a higher risk of all-cause and cardiovascular mortality in unadjusted analyses (P = 0.007 and P = 0.017, respectively) (Supplemental Figure 3).18,21 There was a significant VDD by CPCs interaction for predicting cardiovascular mortality (P = 0.014). When separated into 4 groups according to high and low levels of CPCs and by the presence or absence of VDD, only those with VDD and diminished CPC counts were at a significantly higher hazard (HR: 2.25, P < 0.001) compared to the reference group with normal 25-hydroxyvitamin D and normal CPC counts. The groups with abnormalities in one of these 2 measures did not differ significantly from the reference group (Figure 3B, Table 4). Similar findings were observed for all-cause mortality and MACE. Analyses adjusting for season did not attenuate these findings.
Discrimination analyses demonstrated that vitamin D status improved prediction of adverse events compared to models including baseline covariates and CPC counts. There was also significant improvement in risk reclassification (Table 3).
In the validation cohort (n = 439), when separated into 4 groups, as above, according to high and low levels of CPCs and by the presence or absence of VDD, participants with both VDD and diminished CPCs were at the greatest hazard of cardiovascular mortality (HR: 4.27, P = 0.041), while those with abnormalities in only one of the 2 measures did not differ significantly from the reference group (Supplemental Table 5).
Risk stratification by vitamin D status and both inflammation and CPC levels
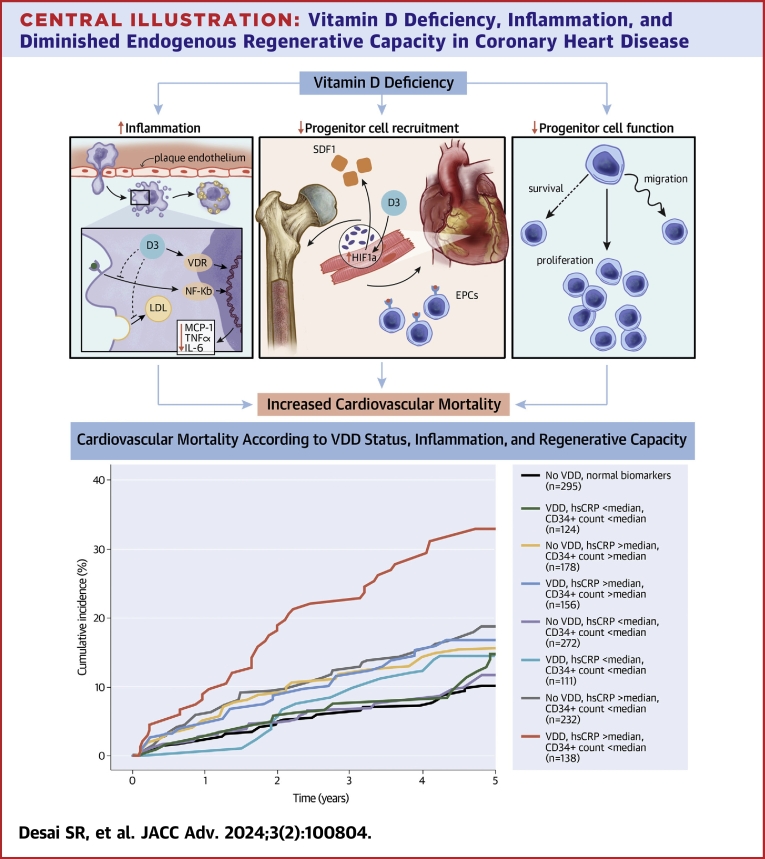
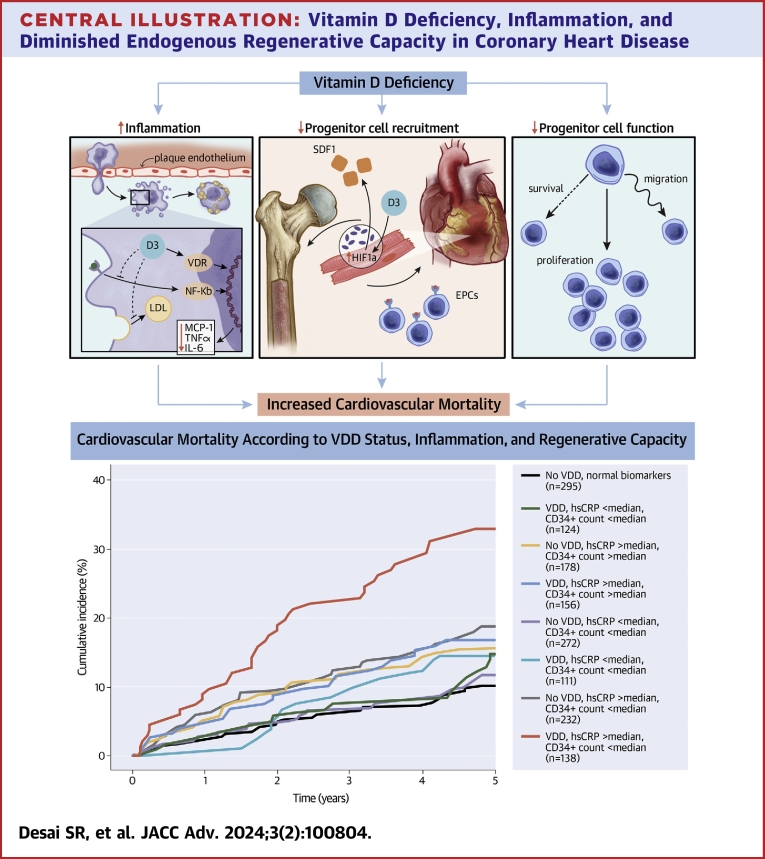
Participants in the EmCAB cohort were divided into groups according to the presence or absence of VDD, elevated hsCRP, and diminished CPC counts for risk stratification. Individuals with all 3 risk factors were at greatest risk (P < 0.001) (Central Illustration). In adjusted comparisons, participants with VDD, high hsCRP, and low CPC levels were at a 2.6-fold higher hazard of cardiovascular mortality (P < 0.001) compared to those without any abnormality (Table 4). Discrimination testing and net reclassification analyses demonstrated improvement when vitamin D status was added to model including risk factors, hsCRP, and CPC levels (Table 3). Similar findings were observed for all-cause mortality and MACE. Analyses adjusting for season did not attenuate these findings.
Central Illustration.
Vitamin D Deficiency, Inflammation, and Diminished Endogenous Regenerative Capacity in Coronary Heart Disease
Vitamin D is implicated in immunomodulation and progenitor cell function. Dysregulation of the inflammatory response and impairment of the regenerative response may explain vitamin D deficiency’s association with increased cardiovascular mortality. Cumulative incidence of cardiovascular mortality according to VDD status, hsCRP, and CPC counts. Median hsCRP = 3.00 mg/L. Median CD34+ count = 1.6924 cells/μL. CPC = circulating progenitor cell; hsCRP = high-sensitivity C-reactive protein; VDD = vitamin D deficiency.
Discussion
In a large prospective study of participants with known or suspected CHD, we demonstrate that VDD is independently associated with an approximately 50 to 60% higher risk of all-cause and cardiovascular mortality and MACE. Moreover, a combination of both VDD and low CPC counts, indicative of impaired regenerative capacity, is associated with a 125% greater risk of cardiovascular mortality compared to those with neither abnormality. In addition, the combination of VDD and high hsCRP, indicative of inflammation, was associated with 180% greater cardiovascular mortality risk compared to those with neither risk factor. These findings were validated in an independent CHD cohort. Together, our observations indicate that a combination of VDD, inflammation, and reduced regenerative capacity identifies the highest risk group with VDD. Our findings imply VDD supplementation in this subgroup may have the greatest likelihood of benefit.
Although randomized controlled vitamin D supplementation trials have failed to improve adverse CVD outcomes in the general population, there is a dearth of studies investigating supplementation in those with established CHD. Studies investigating vitamin D’s relationship with recurrent cardiovascular events have been limited with inconsistent results.3, 4, 5,36, 37, 38 Our study examining the consequences of VDD in nearly 6,000 individuals with known CHD and detailed phenotyping of traditional risk factors, from 2 independent cohorts, constitutes one of the largest observational studies in a high-risk population, with convincing evidence of the independent prognostic value of VDD in predicting adverse cardiovascular events.
Inflammation and VDD
hsCRP is a marker of systemic inflammation, and higher levels despite medical therapy in patients with CHD correlate with worse outcomes.13,39 Our study confirms the inverse correlation between vitamin D and hsCRP. Importantly, our results demonstrate that CHD patients with higher hsCRP are at particularly high risk when they also have VDD and that the converse is also true. This interaction between VDD and inflammation has been observed in the NHANES study of CHD prevalence in the general population, as well as in smaller studies of the CHD population with mixed results, but this is the largest examination of their interrelationship with outcomes in the high-risk CHD cohort referred for cardiac catheterization.40 The symbiotic relationship between vitamin D and immune modulation has been observed in experimental studies, including effects on receptor signaling, regulating transcription, suppressing NF-κB-mediated inflammatory signaling in macrophages, modulating toll-like receptor signaling, and inducing immunotolerant dendritic cells and T-regulatory (Treg) cells by suppressing the inflammatory transcriptome.41 Since hsCRP measurements are widely available, identification of a high-risk group that may benefit from targeted supplementation is clinically feasible.
CPCs and vitamin D
CPCs are considered biomarkers of endogenous regenerative capacity, and low counts are independently predictive of adverse CHD outcomes.18, 19, 20, 21 Although the exact mechanism by which VDD and diminished regenerative capacity increase CVD risk remains unclear, studies of CPCs have found that expression of VDR in CPCs is diminished in patients with CHD and that vitamin D supplementation improves CPC function and promotes CPC-mediated regeneration in the injured endothelium.23,26, 27, 28, 29 Thus, a mechanistic link between VDD and decreased regenerative capacity is plausible, and our analyses demonstrate a synergy between the two that may be leveraged to identify a subset of patients who may benefit from vitamin D supplementation, despite treatment failure in broader populations. Our results demonstrated that only individuals with both VDD and low CPC counts were at high risk. Although CD34+ cell counts are not widely available clinically, the fact that both VDD and low CPC counts are required to identify a high-risk group provides important insights into mechanisms that lead to higher risk with VDD.
CPCs, inflammation, and vitamin D
While we previously identified a relationship between diminished regenerative capacity and progressive inflammation,17 the synergistic relationship between vitamin D, inflammation, and endogenous regenerative capacity with adverse CVD outcomes is a novel finding of this study. The subgroup with VDD and abnormalities in both pathways was at greatest risk, whereas those with abnormalities in either CPCs or inflammation had intermediate risk.
Strengths and limitations
Our study has several strengths. The greater prevalence of VDD among younger Black women in our cohort mirrors observations in the NHANES cohort.1 It is one of the largest to explore the relationship between VDD and outcomes in CHD, and results were validated in another independent cohort. Furthermore, we examined interactions between inflammation and regenerative capacity in this large cohort. Study limitations include its observational nature, preventing conclusions regarding causality between these pathways and outcomes. Given our focus on individuals with CHD, our findings may not be generalizable to the entire population with VDD. The impact of physical activity and diet was not evaluated. The 25-hydroxyvitamin D assay utilized here may underestimate levels of 25-hydroxyvitamin D2 and may thus underestimate levels in those taking D2 supplements. Data on CPC counts were available in a subgroup of participants in both EmCAB and MIPS cohorts and were reproducible. However, the smaller sample size within the validation cohort with available vitamin D, hsCRP, and CPC data, as well as lower hsCRP values within this cohort, limited our ability to study all 3 factors in tandem.
Conclusions
Vitamin D has been implicated as a modulator of inflammation and cofactor in progenitor cell function. VDD, defined as a serum level <20 ng/mL, is associated with higher mortality risk in individuals with CHD, particularly in those with heightened inflammation and diminished regenerative capacity. Whether supplementation would benefit these high-risk groups requires further investigation.
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE: VDD is a well-established risk factor for adverse outcomes in CHD, and vitamin D may mitigate risk through a variety of immunomodulatory and pro-regenerative pathways.
COMPETENCY IN PATIENT CARE: Despite VDD’s association with CHD and adverse outcomes, vitamin D supplementation has not been consistently effective in preventing them.
TRANSLATIONAL OUTLOOK 1: Patients with VDD and CHD appear to be at widely variable risk of adverse outcomes in this large study of high-risk patients referred for coronary angiography.
TRANSLATIONAL OUTLOOK 2: Individuals with VDD may benefit from targeted supplementation based on individualized assessment of underlying inflammation and regenerative capacity.
Funding support and author disclosures
Dr Desai has been supported by grant T32 HL130025. Drs Cheung, Gold, Jain, and Vatsa have been supported by the Abraham J. & Phyllis Katz Foundation (Atlanta, GA). Dr Quyyumi is supported by NIH grants P01HL154996-01A1, R33HL138657-05, U54AG062334-01, P30DK111024-07S2, R61HL154116-01, R01HL109413-07, R01HL166004-01, 15SFCRN23910003, 5P01HL086773-09, 5P01HL101398-05, and 1P20HL113451-04. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors acknowledge previous support from Abbott Laboratories and Virogates for assistance with biomarker measurements.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables and figures, please see the online version of this paper.
Supplementary data
References
- 1.Cui A., Xiao P., Ma Y., et al. Prevalence, trend, and predictor analyses of vitamin D deficiency in the US population, 2001-2018. Front Nutr. 2022;9 doi: 10.3389/fnut.2022.965376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chowdhury R., Kunutsor S., Vitezova A., et al. Vitamin D and risk of cause specific death: systematic review and meta-analysis of observational cohort and randomised intervention studies. BMJ. 2014;348 doi: 10.1136/bmj.g1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbarawi M., Kheiri B., Zayed Y., et al. Vitamin D supplementation and cardiovascular disease risks in more than 83 000 individuals in 21 randomized clinical trials: a meta-analysis. JAMA Cardiol. 2019;4:765–776. doi: 10.1001/jamacardio.2019.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manson J.E., Bassuk S.S., Cook N.R., et al. Vitamin D, marine n-3 fatty acids, and primary prevention of cardiovascular disease current evidence. Circ Res. 2020;126:112–128. doi: 10.1161/CIRCRESAHA.119.314541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerling M.E., James M.T., Wilton S.B., et al. Serum total 25-OH vitamin D adds little prognostic value in patients undergoing coronary catheterization. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.004289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore K.J., Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asahara T., Murohara T., Sullivan A., et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 8.Urbich C., Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 9.White J.H. Vitamin D signaling, infectious diseases, and regulation of innate immunity. Infect Immun. 2008;76:3837–3843. doi: 10.1128/IAI.00353-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krishnan A.V., Feldman D. Molecular pathways mediating the anti-inflammatory effects of calcitriol: implications for prostate cancer chemoprevention and treatment. Endocr Relat Cancer. 2010;17:R19–R38. doi: 10.1677/ERC-09-0139. [DOI] [PubMed] [Google Scholar]
- 11.Olliver M., Spelmink L., Hiew J., Meyer-Hoffert U., Henriques-Normark B., Bergman P. Immunomodulatory effects of vitamin D on innate and adaptive immune responses to Streptococcus pneumoniae. J Infect Dis. 2013;208:1474–1481. doi: 10.1093/infdis/jit355. [DOI] [PubMed] [Google Scholar]
- 12.Kassi E., Adamopoulos C., Basdra E.K., Papavassiliou A.G. Role of vitamin D in atherosclerosis. Circulation. 2013;128:2517–2531. doi: 10.1161/CIRCULATIONAHA.113.002654. [DOI] [PubMed] [Google Scholar]
- 13.Li Y., Zhong X., Cheng G., et al. Hs-CRP and all-cause, cardiovascular, and cancer mortality risk: a meta-analysis. Atherosclerosis. 2017;259:75–82. doi: 10.1016/j.atherosclerosis.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Yang F., Sun M., Sun C., et al. Associations of C-reactive protein with 25-hydroxyvitamin D in 24 specific diseases: a cross-sectional study from NHANES. Sci Rep. 2020;10:5883. doi: 10.1038/s41598-020-62754-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Q., Dai Z., Cao Y., Wang L. Association of C-reactive protein and vitamin D deficiency with cardiovascular disease: a nationwide cross-sectional study from National Health and Nutrition Examination Survey 2007 to 2008. Clin Cardiol. 2019;42:663–669. doi: 10.1002/clc.23189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fadini G.P., Mehta A., Dhindsa D.S., et al. Circulating stem cells and cardiovascular outcomes: from basic science to the clinic. Eur Heart J. 2020;41:4271–4282. doi: 10.1093/eurheartj/ehz923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Almuwaqqat Z., Hwan Kim J., Garcia M., et al. Associations between inflammation, cardiovascular regenerative capacity, and cardiovascular events: a cohort study. Arterioscler Thromb Vasc Biol. 2021;41:2814–2822. doi: 10.1161/ATVBAHA.121.316574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel R.S., Li Q., Ghasemzadeh N., et al. Circulating CD34+ progenitor cells and risk of mortality in a population with coronary artery disease. Circ Res. 2015;116:289–297. doi: 10.1161/CIRCRESAHA.116.304187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samman Tahhan A., Hammadah M., Raad M., et al. Progenitor cells and clinical outcomes in patients with acute coronary syndromes. Circ Res. 2018;122:1565–1575. doi: 10.1161/CIRCRESAHA.118.312821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tahhan A.S., Hammadah M., Sandesara P.B., et al. Progenitor cells and clinical outcomes in patients with heart failure. Circ Heart Fail. 2017;10 doi: 10.1161/CIRCHEARTFAILURE.117.004106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dhindsa D.S., Desai S.R., Jin Q., et al. Circulating progenitor cells and outcomes in patients with coronary artery disease. Int J Cardiol. 2023;373:7–16. doi: 10.1016/j.ijcard.2022.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yiu Y.F., Chan Y.H., Yiu K.H., et al. Vitamin D deficiency is associated with depletion of circulating endothelial progenitor cells and endothelial dysfunction in patients with type 2 diabetes. J Clin Endocrinol Metab. 2011;96:E830–E835. doi: 10.1210/jc.2010-2212. [DOI] [PubMed] [Google Scholar]
- 23.Huang Z., Liu L., Huang S., et al. Vitamin D (1,25-(OH)(2)D(3)) improves endothelial progenitor cells function via enhanced NO secretion in systemic lupus erythematosus. Cardiol Res Pract. 2020;2020 doi: 10.1155/2020/6802562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lo Gullo A., Mandraffino G., Bagnato G., et al. Vitamin D status in rheumatoid arthritis: inflammation, arterial stiffness and circulating progenitor cell number. PLoS One. 2015;10 doi: 10.1371/journal.pone.0134602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hammer Y., Soudry A., Levi A., et al. Effect of vitamin D on endothelial progenitor cells function. PLoS One. 2017;12 doi: 10.1371/journal.pone.0178057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grundmann M., Haidar M., Placzko S., et al. Vitamin D improves the angiogenic properties of endothelial progenitor cells. Am J Physiol Cell Physiol. 2012;303:C954–C962. doi: 10.1152/ajpcell.00030.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schröder-Heurich B., von Hardenberg S., Brodowski L., et al. Vitamin D improves endothelial barrier integrity and counteracts inflammatory effects on endothelial progenitor cells. FASEB J. 2019;33:9142–9153. doi: 10.1096/fj.201802750RR. [DOI] [PubMed] [Google Scholar]
- 28.Hou Y.C., Lu C.L., Zheng C.M., et al. The role of vitamin D in modulating mesenchymal stem cells and endothelial progenitor cells for vascular calcification. Int J Mol Sci. 2020;21:2466. doi: 10.3390/ijms21072466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ai S., He Z., Ding R., et al. Reduced vitamin D receptor on circulating endothelial progenitor cells: a new risk factor of coronary artery diseases. J Atheroscler Thromb. 2018;25:410–421. doi: 10.5551/jat.40808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holick M.F., Binkley N.C., Bischoff-Ferrari H.A., et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 31.Ko Y.A., Hayek S., Sandesara P., Samman Tahhan A., Quyyumi A. Cohort profile: the Emory Cardiovascular Biobank (EmCAB) BMJ Open. 2017;7 doi: 10.1136/bmjopen-2017-018753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hammadah M., Al Mheid I., Wilmot K., et al. The mental stress ischemia prognosis study: objectives, study design, and prevalence of inducible ischemia. Psychosom Med. 2017;79:311–317. doi: 10.1097/PSY.0000000000000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fine J.P., Gray R.J. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 34.Uno H., Cai T., Pencina M.J., D'Agostino R.B., Wei L.J. On the C-statistics for evaluating overall adequacy of risk prediction procedures with censored survival data. Stat Med. 2011;30:1105–1117. doi: 10.1002/sim.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pencina M.J., D'Agostino R.B., Sr., D'Agostino R.B., Jr., Vasan R.S. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. discussion 207-12. [DOI] [PubMed] [Google Scholar]
- 36.Grandi N.C., Breitling L.P., Vossen C.Y., et al. Serum vitamin D and risk of secondary cardiovascular disease events in patients with stable coronary heart disease. Am Heart J. 2010;159:1044–1051. doi: 10.1016/j.ahj.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 37.Wasson L.T., Shimbo D., Rubin M.R., Shaffer J.A., Schwartz J.E., Davidson K.W. Is vitamin D deficiency a risk factor for ischemic heart disease in patients with established cardiovascular disease? 10-Year follow-up of the Nova Scotia Health Survey. Int J Cardiol. 2011;148:387–389. doi: 10.1016/j.ijcard.2010.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Welles C.C., Whooley M.A., Karumanchi S.A., et al. Vitamin D deficiency and cardiovascular events in patients with coronary heart disease: data from the Heart and Soul Study. Am J Epidemiol. 2014;179:1279–1287. doi: 10.1093/aje/kwu059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ridker P.M., MacFadyen J.G., Everett B.M., Libby P., Thuren T., Glynn R.J. Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomised controlled trial. Lancet. 2018;391:319–328. doi: 10.1016/S0140-6736(17)32814-3. [DOI] [PubMed] [Google Scholar]
- 40.Murr C., Pilz S., Grammer T.B., et al. Vitamin D deficiency parallels inflammation and immune activation, the Ludwigshafen Risk and Cardiovascular Health (LURIC) study. Clin Chem Lab Med. 2012;50:2205–2212. doi: 10.1515/cclm-2012-0157. [DOI] [PubMed] [Google Scholar]
- 41.Yin K., Agrawal D.K. Vitamin D and inflammatory diseases. J Inflamm Res. 2014;7:69–87. doi: 10.2147/JIR.S63898. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.