Abstract
Background
Vasoplegia after cardiac surgery is associated with adverse outcomes. However, the clinical effects of vasoplegia and the significance of its duration after continuous-flow left ventricular assist device (CF-LVAD) implantation are less known.
Objectives
This study aimed to identify predictors of and outcomes from transient vs prolonged vasoplegia after CF-LVAD implantation.
Methods
The study was a retrospective review of consecutive patients who underwent CF-LVAD implantation between January 1, 2005, and December 31, 2017. Vasoplegia was defined as the presence of all of the following: mean arterial pressure ≤65 mm Hg, vasopressor (epinephrine, norepinephrine, vasopressin, or dopamine) use for >6 hours within the first 24 hours postoperatively, cardiac index ≥2.2 L/min/m2 and systemic vascular resistance <800 dyne/s/cm5, and vasodilatory shock not attributable to other causes. Prolonged vasoplegia was defined as that lasting 12 to 24 hours; transient vasoplegia was that lasting 6 to <12 hours. Patient characteristics, outcomes, and risk factors were analyzed.
Results
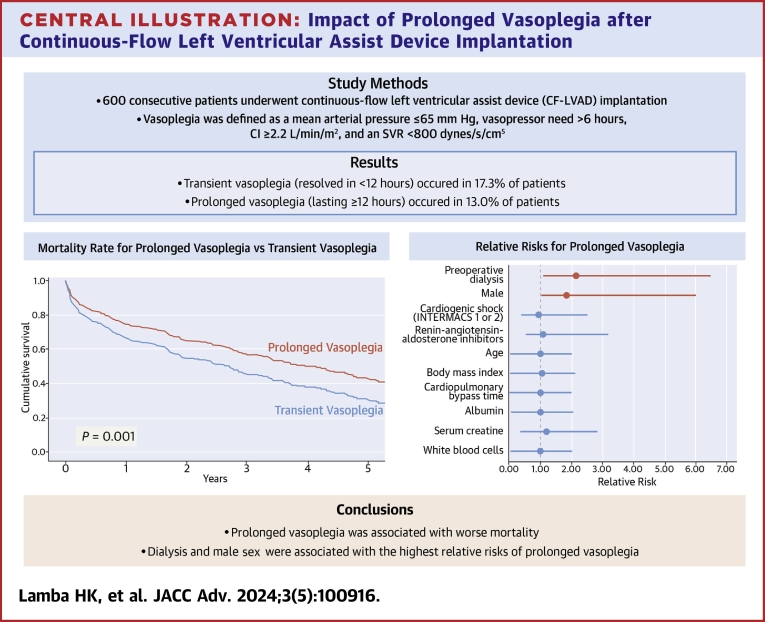
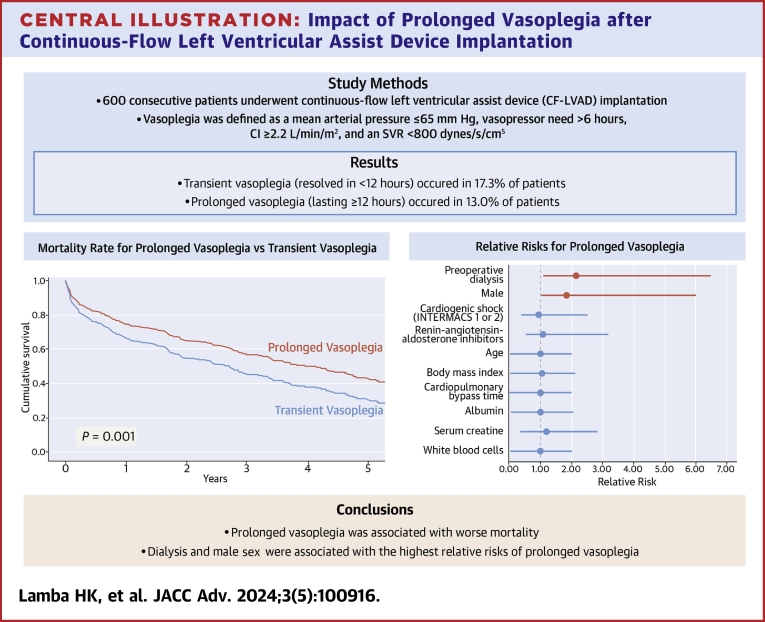
Of the 600 patients who underwent CF-LVAD implantation during the study period, 182 (30.3%) developed vasoplegia. Mean patient age was similar between the vasoplegia and no-vasoplegia groups. Prolonged vasoplegia (n = 78; 13.0%), compared with transient vasoplegia (n = 104; 17.3%), was associated with greater 30-day mortality (16.7% vs 5.8%; P = 0.02). Risk factors for prolonged vasoplegia included preoperative dialysis and elevated body mass index.
Conclusions
Compared with vasoplegia overall, prolonged vasoplegia was associated with worse survival after CF-LVAD implantation. Treatment to avoid or minimize progression to prolonged vasoplegia may be warranted.
Key words: health care, heart-assist devices, outcome assessment, postoperative complications, risk factors
Central Illustration
Vasoplegia (vasoplegic shock) is characterized by high cardiac output with low systemic vascular resistance (SVR), resulting in refractory hypotension. Thought to arise as a severe systemic inflammatory response, vasoplegia is most frequently encountered in patients with sepsis, but it is also common after cardiac surgery and cardiopulmonary bypass. Depending on how vasoplegia is defined, its reported incidence ranges from 5% to nearly 50% among patients undergoing heart transplant,1, 2, 3, 4, 5, 6 general cardiac surgery,7, 8, 9 or left ventricular assist device implantation.2,4,10 These studies consistently associate vasoplegia with poor outcomes, especially early morbidity and death.1, 2, 3, 4, 5, 6, 7, 8, 9 Nonetheless, little consensus exists on an appropriate definition of vasoplegia, and the literature is widely heterogeneous regarding risk factors and treatment strategies for vasoplegia in patients undergoing continuous-flow left ventricular assist device (CF-LVAD) implantation (Supplemental Table 1).2,4,10
We conducted a study to characterize vasoplegia in the CF-LVAD population, investigate the impact of vasoplegia on postoperative outcomes, and identify predictors of vasoplegia development. Objective definitions of postoperative vasoplegia were used to more accurately identify the patient cohort. Also, we characterized the duration of vasoplegia by identifying patients with transient vasoplegia and prolonged vasoplegia. We hypothesized that patients who developed vasoplegia, especially prolonged vasoplegia, after CF-LVAD implantation would have more adverse outcomes and poorer survival.
Methods
Data were retrospectively reviewed from consecutive adults (age ≥18 years) who underwent primary CF-LVAD implantation at our institution between January 1, 2005, and December 31, 2017. The study was approved by the Baylor College of Medicine Institutional Review Board.
Baseline patient characteristics were collected from clinical records, as were hourly hemodynamic data and vasopressor use during the first postoperative 24 hours. Patients who received multiple forms of temporary mechanical circulatory support (MCS) in the index admission before CF-LVAD implantation were counted for each type of MCS, but only once to calculate overall MCS use. With this approach, the sum of the individual MCS devices exceeds the total MCS use.
Postoperative complications were determined by chart review and included early hospital readmission (within 30 days of discharge from index admission), right ventricular failure (RVF), acute kidney injury (AKI), neurological dysfunction, and infection. Perioperative (within 30 days of CF-LVAD implantation) stroke was identified from the presence of symptoms, results from cerebral computed tomography imaging, and confirmatory neurology consultation. Postoperative acute renal failure was defined as a 3-fold increase in creatinine level from baseline or need for postoperative renal replacement therapy during the index admission.
Postoperative vasoplegia was defined as a severe hypotensive state characterized by: 1) mean arterial pressure (MAP) ≤65 mm Hg; 2) treatment with a vasopressor (epinephrine, norepinephrine, vasopressin, or dopamine) for >6 hours within the first 24 hours postoperatively; 3) cardiac index ≥2.2 L/min/m2 (calculated from measurements obtained with an indwelling pulmonary artery catheter) and SVR <800 dyne/s/cm5; and 4) vasodilatory shock not attributable to infection, cardiogenic shock, RVF, or any other cause. Patients taking milrinone regularly were not classified as having vasoplegia.
In accordance with Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) descriptions,11 we defined RVF as prolonged signs and symptoms of RV dysfunction: central venous pressure (CVP) >18 mm Hg with a cardiac index <2.0 L/min/m2 in the absence of increased left atrial filling pressure or pulmonary capillary wedge pressure >18 mm Hg, cardiac tamponade, ventricular arrhythmias, and/or pneumothorax requiring either right VAD implantation or inhaled nitric oxide or inotropic therapy for ≥14 days after CF-LVAD implantation.
To avoid the confounding effect of postoperative epinephrine use on the dosage of other vasopressors, we categorized preoperative epinephrine as an inotrope and postoperative epinephrine as a vasopressor, reflecting our institutional practice pattern. Usage and dosage of specific vasopressors was determined by the treating clinicians. Our general hemodynamic targets are a MAP of approximately 80 mm Hg and a CVP of around 10 mm Hg, consistent with recommendations from the International Society for Heart and Lung Transplantation.12
When indicated, renal replacement therapy was used with continuous venovenous hemodialysis with an effluent flow rate of 25 mL/kg/h and a blood flow rate of 200 to 300 mL/min.13 Clinical decision-making was multispecialty, with daily rounding by a comprehensive team that included cardiovascular surgeons, heart failure cardiologists, critical care specialists, and pharmacists.
The definitions of vasoplegia in previous studies and the current study are listed in Supplemental Table 1 for comparison. Postoperative vasoplegia was further classified as transient or prolonged. Our focus on vasoplegia was limited to the first 24 hours postoperatively; thus, we defined transient vasoplegia as lasting 6 to <12 hours and prolonged vasoplegia as lasting 12 to 24 hours.
Primary end points were development of postoperative vasoplegia and development of prolonged vasoplegia. Secondary end points included postoperative adverse outcomes, hospital length of stay, early readmission, and survival. Patients were followed until transplant, death, or the end of the observation period.
Statistical analysis
Categorical variables are shown as number and percentage and compared by using the Pearson chi-square test or Fisher exact test, as appropriate. Continuous variables are described as mean ± SD and compared by using unpaired t-tests (for normally distributed data) or Wilcoxon rank sum tests (for non-normally distributed data).
Risk factor models were developed for binary outcome variables, including postoperative and prolonged vasoplegia. The covariates in each model included known risk factors (Supplemental Table 1) that were available in our data set as well as additional variables that were selected from all available patient characteristics (age, sex, comorbidities, laboratory values, hemodynamic values, and medication history) by performing a modified Poisson analysis with robust selection. For each binary response and the covariates, a modified Poisson regression analysis with robust standard errors14,15 was performed to identify risk factors and estimate relative risk (RR).
Cox proportional survival analysis was performed with adjustment for factors previously associated with all-cause mortality in LVAD recipients (age, INTERMACS profile 1 or 2, preoperative dialysis) and for sex. P < 0.05 was considered statistically significant. Missing data were considered missing at random and were not imputed. Schoenfeld residual plots (not reported here) were performed for each covariate to ensure there was no strong evidence of violating the proportional hazards assumption.
The statistical analyses were performed using IBM SPSS Statistics for Macintosh, version 25 (SPSS Inc).
Results
In all, 600 patients underwent implantation of a CF-LVAD device during the study period. At least 90% of the data were available for all variables except preoperative CVP (48.6% complete) and mean right atrial pressure (78.5% complete). The median follow-up was 3.2 years.
Vasoplegia vs no vasoplegia
Of the 600 patients, 182 (30.3%) developed postoperative vasoplegia within the first 24 hours. Two patients with vasoplegia had CVP >18 mm Hg but did not meet the prespecified definition of RVF. Within the postoperative vasoplegia group, 104 patients (57.1%; 17.3% of entire sample) had transient vasoplegia that resolved within 12 hours, and 78 patients (42.9%; 13.0% of entire sample) developed prolonged vasoplegia that lasted ≥12 hours.
Patients with postoperative vasoplegia were more likely to be male (89.6% vs 74.9%; P < 0.001) and had a higher body mass index (BMI) (31.7 ± 20.4 kg/m2 vs 27.7 ± 6.4 kg/m2; P = 0.01) than patients who did not develop vasoplegia (Table 1). They were also more likely to have had preoperative stroke (20.3% vs 12.9%; P = 0.047) and preoperative dialysis (8.8% vs 4.3%; P = 0.03). Furthermore, patients with postoperative vasoplegia had a higher neutrophil:lymphocyte ratio (6.7 ± 6.4 vs 5.6 ± 5.7; P = 0.04) and a higher mean Model for End-Stage Liver Disease (MELD) score (14.9 ± 6.0 vs 13.0 ± 5.3; P < 0.001) than the no-vasoplegia patients. The type of CF-LVAD used did not differ between groups. The vasoplegia group had a nonsignificantly longer mean cardiopulmonary bypass time (88.6 ± 51.9 minutes vs 81.3 ± 51.3 minutes; P = 0.10) (Table 1).
Table 1.
Baseline Characteristics of Patients Who Developed Postoperative Vasoplegia Compared With Patients Who Did Not
| Vasoplegia (n = 182) | No Vasoplegia (n = 418) | P Value | |
|---|---|---|---|
| Demographic data | |||
| Male | 163 (89.6) | 313 (74.9) | <0.001 |
| Age, y | 53.8 ± 13.0 | 55.2 ± 13.5 | 0.20 |
| BMI, kg/m2 | 31.7 ± 20.4 | 27.7 ± 6.4 | 0.01 |
| Ischemic cardiomyopathy | 82 (45.1) | 197 (47.1) | 0.60 |
| Bridge to transplant | 91 (50.0) | 227 (54.3) | 0.60 |
| Past medical history | |||
| Diabetes mellitus | 81 (44.5) | 183 (43.8) | 0.90 |
| Hypertension | 118 (64.8) | 257 (61.5) | 0.40 |
| COPD | 24 (13.2) | 63 (15.1) | 0.50 |
| Stroke | 37 (20.3) | 54 (12.9) | 0.047 |
| Dialysis | 16 (8.8) | 18 (4.3) | 0.03 |
| Previous cardiac surgery | 68 (37.4) | 143 (34.2) | 0.50 |
| Previous mechanical circulatory support | 80 (44.0) | 188 (45.0) | 0.60 |
| Intra-aortic balloon pump | 70 (38.5) | 160 (38.3) | 0.90 |
| Transseptal LVAD (TandemHeart) | 19 (10.4) | 37 (8.9) | 0.60 |
| Transaortic LVAD (Impella) | 15 (8.2) | 26 (6.2) | 0.40 |
| VA-ECMO | 5 (2.7) | 11 (2.6) | 0.90 |
| INTERMACS 1 or 2 | 84 (46.2) | 190 (45.5) | 0.80 |
| Preoperative mean MELD score | 14.9 ± 6.0 | 13.0 ± 5.3 | <0.001 |
| Preoperative medication use | |||
| ACE inhibitors | 94 (51.6) | 244 (58.4) | 0.10 |
| Angiotensin II receptor blockers | 36 (19.8) | 68 (16.3) | 0.30 |
| Aldosterone antagonists | 109 (59.9) | 256 (61.2) | 0.80 |
| Calcium channel blockers | 15 (8.2) | 34 (8.1) | >0.99 |
| Inotropes | 144 (79.1) | 335 (80.1) | 0.80 |
| Vasopressors | 29 (15.9) | 67 (16.0) | >0.99 |
| Beta-blockers | 125 (68.7) | 306 (73.2) | 0.30 |
| Nitrates | 80 (44.0) | 199 (47.6) | 0.40 |
| Aspirin | 137 (75.3) | 314 (75.1) | >0.99 |
| Preoperative laboratory values | |||
| Serum creatinine, mg/dL | 1.5 ± 0.8 | 1.4 ± 0.8 | 0.20 |
| Neutrophil:lymphocyte ratio | 6.7 ± 6.4 | 5.6 ± 5.7 | 0.04 |
| Aspartate aminotransferase, U/L | 58.9 ± 72.7 | 73.7 ± 211.0 | 0.40 |
| Alanine aminotransferase, U/L | 65.2 ± 121.7 | 80.9 ± 200.3 | 0.30 |
| Serum albumin, g/dL | 5.6 ± 7.5 | 4.6 ± 5.3 | 0.10 |
| Total bilirubin, mg/dL | 6.8 ± 44.5 | 1.7 ± 3.2 | 0.10 |
| Preoperative hemodynamic data | |||
| CVP, mm Hg | 13.3 ± 7.4 | 11.4 ± 6.9 | 0.04 |
| Pulmonary vascular resistance, WU | 3.8 ± 4.2 | 3.7 ± 3.0 | 0.80 |
| Pulmonary artery pulsatility index | 3.3 ± 4.4 | 4.0 ± 4.1 | 0.20 |
| Mean RAP/PCWP | 0.52 ± 0.3 | 0.48 ± 0.3 | 0.30 |
| Mean RAP/PCWP >0.54 | 56 (30.8) | 113 (27.0) | 0.40 |
| CVP/PCWP | 0.55 ± 0.3 | 0.5 ± 0.4 | 0.40 |
| Intraoperative characteristics | |||
| Concomitant procedures | 49 (26.9) | 119 (28.5) | 0.90 |
| LVAD type | 0.20 | ||
| HeartMate II | 150 (82.4) | 307 (73.4) | |
| HeartWare | 29 (15.9) | 97 (23.2) | |
| Other | 3 (1.6) | 14 (3.3) | |
| Cardiopulmonary bypass time, min | 88.6 ± 51.9 | 81.3 ± 51.3 | 0.10 |
Values are n (%) or mean ± SD.
ACE = angiotensin-converting enzyme; BMI = body mass index; COPD = chronic obstructive pulmonary disease; CVP = central venous pressure; INTERMACS = Interagency Registry for Mechanically Assisted Circulatory Support; LVAD = left ventricular assist device; MELD = Model for End-Stage Liver Disease; PCWP = pulmonary capillary wedge pressure; RAP = right atrial pressure; VA-ECMO = venoarterial extracorporeal membrane oxygenation.
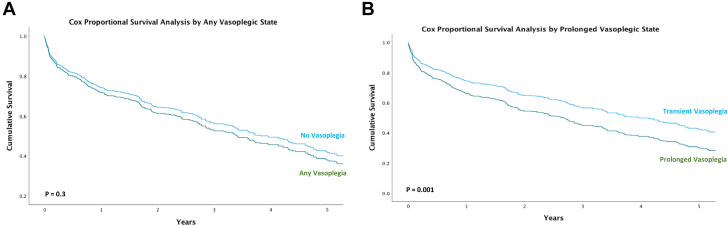
The frequency of adverse events did not differ between the vasoplegia and no-vasoplegia groups (Table 2). Mortality was similar at 30 days (9.9% vasoplegia vs 6.9% no-vasoplegia; P = 0.20) but was nonsignificantly higher in the vasoplegia cohort at 60 days (14.8% vs 10.0%; P = 0.09) and 90 days (18.1% vs 12.7%; P = 0.08). Cox proportional survival estimates indicated similar all-cause survival in the any-vasoplegia and no-vasoplegia groups (P = 0.40; 95% CI: 0.9-1.4) after adjustment for age, INTERMACS profiles 1 and 2, preoperative dialysis, and sex.
Table 2.
Postoperative Adverse Events in Patients Who Developed Postoperative Vasoplegia Compared With Patients Who Did Not
| Vasoplegia (n = 182) | No Vasoplegia (n = 418) | P Value | |
|---|---|---|---|
| Acute kidney injury | 35 (19.2) | 77 (18.4) | 0.90 |
| Renal replacement therapy | 27 (14.8) | 68 (16.3) | 0.80 |
| Stroke | 40 (22.0) | 106 (25.4) | 0.40 |
| Reoperation for bleeding | 51 (28.0) | 93 (22.2) | 0.10 |
| Right heart failure | 6 (3.3) | 21 (5.0) | 0.30 |
| Early right ventricular assist device | 2 (1.1) | 10 (2.4) | 0.30 |
| Tracheostomy | 27 (14.8) | 60 (14.4) | 0.90 |
| Sepsis | 62 (34.1) | 131 (31.3) | 0.50 |
| Readmission | 29 (15.9) | 88 (21.1) | 0.20 |
| Length of stay (d) | 43.9 ± 48.7 | 44.9 ± 68.0 | 0.90 |
| Mortality | |||
| 30-d | 18 (9.9) | 29 (6.9) | 0.20 |
| 60-d | 27 (14.8) | 42 (10.0) | 0.09 |
| 90-d | 33 (18.1) | 53 (12.7) | 0.08 |
| 1-y | 62 (34.1) | 128 (30.6) | 0.40 |
| Overall | 86 (47.3) | 206 (49.3) | 0.60 |
Values are n (%) or mean ± SD.
Prolonged vasoplegia vs transient vasoplegia
Compared with the transient-vasoplegia group, the prolonged-vasoplegia group had higher baseline serum creatinine levels (1.6 ± 0.9 vs 1.3 ± 0.6; P = 0.01) and a higher mean MELD (16.5 ± 6.2 vs 13.8 ± 5.7; P = 0.003) and more often had preoperative dialysis (14.1% vs 4.8%; P = 0.03) (Table 3). Preoperatively, the prolonged-vasoplegia group was less likely than the transient-vasoplegia group to be treated with angiotensin-converting enzyme inhibitors (ACEIs) (43.6% vs 57.7%; P = 0.06). No other differences in preoperative medication use were observed.
Table 3.
Baseline Characteristics of Patients Who Developed Transient Vasoplegia Compared With Prolonged Postoperative Vasoplegia
| Transient Vasoplegia (n = 104) | Prolonged Vasoplegia (n = 78) | P Value | |
|---|---|---|---|
| Demographic data | |||
| Male | 93 (89.4) | 70 (89.7) | 0.70 |
| Age | 53.3 ± 13.1 | 54.4 ± 12.9 | 0.60 |
| BMI, kg/m2 | 31.7 ± 26.2 | 31.7 ± 7.4 | 0.10 |
| Ischemic cardiomyopathy | 44 (42.3) | 38 (48.7) | 0.70 |
| Bridge to transplant | 59 (56.7) | 32 (41.0) | 0.20 |
| Past medical history | |||
| Diabetes mellitus | 48 (46.2) | 33 (42.3) | 0.60 |
| Hypertension | 66 (63.5) | 52 (66.7) | 0.40 |
| COPD | 14 (13.5) | 10 (12.8) | 0.90 |
| Stroke | 19 (18.3) | 18 (23.1) | 0.50 |
| Dialysis | 5 (4.8) | 11 (14.1) | 0.03 |
| Previous cardiac surgery | 37 (35.6) | 31 (39.7) | 0.50 |
| Previous mechanical circulatory support | 42 (40.4) | 38 (48.7) | 0.20 |
| Intra-aortic balloon pump | 40 (38.5) | 46 (59.0) | 0.90 |
| Transseptal LVAD (TandemHeart) | 17 (16.3) | 7 (9.0) | 0.60 |
| Transaortic LVAD (Impella) | 6 (5.8) | 9 (11.5) | 0.20 |
| VA-ECMO | 3 (2.9) | 2 (2.6) | 0.90 |
| INTERMACS 1 or 2 | 46 (44.2) | 38 (48.7) | 0.60 |
| Preoperative mean MELD score | 13.8 ± 5.7 | 16.5 ± 6.2 | 0.003 |
| Preoperative medication use | |||
| ACE inhibitors | 60 (57.7) | 34 (43.6) | 0.06 |
| Angiotensin II receptor blockers | 20 (19.2) | 16 (20.5) | 0.80 |
| Aldosterone antagonists | 59 (56.7) | 50 (64.1) | 0.50 |
| Calcium channel blockers | 9 (8.7) | 6 (7.7) | 0.80 |
| Inotropes | 81 (77.9) | 63 (80.8) | 0.30 |
| Vasopressors | 15 (14.4) | 14 (17.9) | 0.40 |
| Beta-blockers | 66 (63.5) | 59 (75.6) | 0.20 |
| Nitrates | 44 (42.3) | 36 (46.2) | 0.60 |
| Aspirin | 81 (77.9) | 56 (71.8) | 0.30 |
| Preoperative laboratory values | |||
| Serum creatinine, mg/dL | 1.3 ± 0.6 | 1.6 ± 0.9 | 0.01 |
| Neutrophil:lymphocyte ratio | 6.3 ± 6.7 | 7.4 ± 6.1 | 0.30 |
| Aspartate aminotransferase, U/L | 55.6 ± 72.3 | 63.2 ± 73.6 | 0.80 |
| Alanine aminotransferase, U/L | 66.8 ± 138.6 | 63.1 ± 96.0 | 0.90 |
| Serum albumin, g/dL | 6.1 ± 8.3 | 4.9 ± 6.1 | 0.20 |
| Total bilirubin, mg/dL | 8.6 ± 57.8 | 4.3 ± 14.0 | 0.80 |
| Preoperative hemodynamic data | |||
| CVP, mm Hg | 13.4 ± 7.8 | 13.1 ± 6.8 | 0.80 |
| Pulmonary vascular resistance, WU | 3.7 ± 4.6 | 3.9 ± 3.7 | 0.80 |
| Pulmonary artery pulsatility index | 3.4 ± 5.2 | 3.2 ± 3.0 | 0.90 |
| Mean RAP/PCWP | 0.5 ± 0.2 | 0.54 ± 0.3 | 0.40 |
| Mean RAP/PCWP >0.54 | 31 (29.8) | 25 (32.1) | 0.80 |
| CVP/PCWP | 0.52 ± 0.3 | 0.58 ± 0.4 | 0.50 |
| Intraoperative characteristics | |||
| Concomitant procedures | 27 (26.0) | 22 (28.2) | 0.40 |
| LVAD type | 0.30 | ||
| HeartMate II | 84 (80.8) | 66 (84.6) | |
| HeartWare | 17 (16.3) | 12 (15.4) | |
| Other | 3 (2.9) | 0 (0.0) | |
| Cardiopulmonary bypass time, min | 84.5 ± 54.1 | 94.2 ± 48.5 | 0.20 |
Values are n (%) or mean ± SD. Transient vasoplegia was defined as that lasting 6 to <12 hours; prolonged vasoplegia was defined as that lasting 12 to 24 hours.
ACE = angiotensin-converting enzyme; BMI = body mass index; COPD = chronic obstructive pulmonary disease; CVP = central venous pressure; INTERMACS = Interagency Registry for Mechanically Assisted Circulatory Support; LVAD = left ventricular assist device; MELD = Model for End-Stage Liver Disease; PCWP = pulmonary capillary wedge pressure; RAP = right atrial pressure; VA-ECMO = venoarterial extracorporeal membrane oxygenation.
Patients with prolonged vasoplegia had higher rates of postoperative AKI (33.3% vs 18.3%; P = 0.02) and new postoperative renal replacement therapy (28.2% vs 13.5%; P = 0.01) than patients with transient vasoplegia (Table 4). Patients with prolonged vasoplegia also had higher 30-day (16.7% vs 5.8%; P = 0.02), 60-day (21.8% vs 9.6%; P = 0.03), and 90-day mortality (24.4% vs 13.5%; P = 0.053). Cox proportional survival estimates indicated worse all-cause overall survival in the prolonged-vasoplegia group (P = 0.03; 95% CI: 1.03-1.9) after adjustment for age, INTERMACS profiles 1 and 2, preoperative dialysis, and sex (Figure 1).
Table 4.
Postoperative Clinical Outcomes in Patients Who Developed Transient Vasoplegia Compared With Prolonged Postoperative Vasoplegia
| Transient Vasoplegia (n = 104) | Prolonged Vasoplegia (n = 78) | P Value | |
|---|---|---|---|
| Acute kidney injury | 19 (18.3) | 26 (33.3) | 0.02 |
| Renal replacement therapy | 14 (13.5) | 22 (28.2) | 0.01 |
| Stroke | 24 (23.1) | 26 (33.3) | 0.40 |
| Early stroke | 4 (3.8) | 3 (3.8) | 0.80 |
| Reoperation for bleeding | 26 (25.0) | 25 (32.1) | 0.40 |
| Right heart failure | 1 (1.0) | 5 (6.4) | 0.10 |
| Early right ventricular assist device | 0 (0) | 2 (2.6) | 0.20 |
| Tracheostomy | 15 (14.4) | 12 (15.4) | >0.99 |
| Sepsis | 34 (32.7) | 28 (35.9) | 0.80 |
| Readmission | 15 (14.4) | 14 (17.9) | 0.50 |
| Length of stay (d) | 42.2 ± 47.1 | 46.2 ± 50.9 | 0.60 |
| Mortality | |||
| 30-d | 6 (5.8) | 13 (16.7) | 0.02 |
| 60-d | 10 (9.6) | 17 (21.8) | 0.03 |
| 90-d | 14 (13.5) | 19 (24.4) | 0.053 |
| 1-y | 26 (29.8) | 27 (39.7) | 0.20 |
| Overall | 55 (52.9) | 55 (70.5) | 0.10 |
Values are n (%) or mean ± SD. Transient vasoplegia was defined as that lasting 6 to <12 hours; prolonged vasoplegia was defined as that lasting 12 to 24 hours.
Figure 1.
Cox Proportional Survival Analysis, by Vasoplegic State
(A) Any vasoplegia (green) vs no vasoplegia (blue), P = 0.30. (B) Prolonged vasoplegia (green) vs transient vasoplegia (blue), P = 0.001.
Modified Poisson analysis revealed 4 risk factors for the development of postoperative vasoplegia (Table 5): male sex (RR: 1.99; 95% CI: 1.18-2.99; P = 0.0006), BMI (RR: 1.05; 95% CI: 1.03-1.07; P < 0.001), preoperative dialysis (odds ratio [OR]: 1.69; 95% CI: 1.09-2.65; P = 0.02), and preoperative MELD (OR: 1.04; 95% CI: 1.004-1.08; P = 0.03). In addition, 2 risk factors for prolonged vasoplegia were identified: the need for preoperative dialysis (OR: 2.39; 95% CI: 1.18-4.83, P = 0.02) and BMI (OR: 1.06; 95% CI: 1.03-1.1; P < 0.001).
Table 5.
Risk Factors for Any Vasoplegia and Prolonged Vasoplegia After CF-LVAD Implantation
| RR | 95% CI | P Value | |
|---|---|---|---|
| Risk factors for any vasoplegia | |||
| Sex | 1.99 | 0.82-3.23 | 0.006 |
| Preoperative dialysis | 1.69 | 1.09-2.65 | 0.02 |
| Cardiogenic shock (INTERMACS 1 or 2) | 0.91 | 0.66-1.25 | 0.55 |
| Vasopressors | 1.15 | 0.80-1.65 | 0.46 |
| Beta-blockers | 0.8 | 0.60-1.07 | 0.13 |
| Nitrates | 0.92 | 0.70-1.22 | 0.59 |
| Ischemic cardiomyopathy | 0.96 | 0.70-1.32 | 0.85 |
| Renin-angiotensin-aldosterone inhibitor use | 0.83 | 0.57-1.23 | 0.33 |
| Age, y | 0.99 | 0.97-1.01 | 0.89 |
| Body mass index, kg/m2 | 1.05 | 1.03-1.07 | <0.001 |
| mRAP/PCWP | 1.12 | 0.72-1.74 | 0.63 |
| Cardiopulmonary bypass time, min | 1.001 | 0.99-1.004 | 0.29 |
| Albumin, g/dl | 1.001 | 0.98-1.02 | 0.94 |
| White blood cell count, 109/L | 0.99 | 0.97-1.03 | 0.85 |
| Neutrophil:lymphocyte ratio | 1.02 | 0.99-1.04 | 0.08 |
| Serum creatinine, mg/dL | 0.77 | 0.58-1.03 | 0.08 |
| Destination therapy | 2.3 | 0.52-10.2 | 0.30 |
| Model for End-Stage Liver Disease | 1.04 | 1.004-1.08 | 0.03 |
| Diabetes | 0.93 | 0.70-1.25 | 0.63 |
| Risk factors for prolonged vasoplegia | |||
| Sex | 1.96 | 0.85-4.48 | 0.11 |
| Preoperative dialysis | 2.39 | 1.18-4.83 | 0.02 |
| Cardiogenic shock (INTERMACS 1 or 2) | 0.94 | 0.56-1.57 | 0.81 |
| Pressors | 1.34 | 0.76-2.36 | 0.31 |
| Beta-blockers | 0.76 | 0.46-1.23 | 0.31 |
| Nitrates | 0.89 | 0.55-1.43 | 0.62 |
| Ischemic cardiomyopathy | 1.21 | 0.68-2.13 | 0.52 |
| Renin-angiotensin-aldosterone inhibitor use | 0.99 | 0.50-1.99 | 0.99 |
| Age, y | 1.01 | 0.99-1.03 | 0.65 |
| Body mass index, kg/m2 | 1.06 | 1.03-1.09 | <0.001 |
| mRAP/PCWP | 1.3 | 0.65-2.60 | 0.45 |
| Cardiopulmonary bypass time, min | 1.003 | 0.99-1.01 | 0.13 |
| Albumin, g/dl | 0.99 | 0.95-1.04 | 0.81 |
| White blood cell count, 109/L | 0.97 | 0.91-1.04 | 0.41 |
| Neutrophil:lymphocyte ratio | 1.01 | 0.98-1.05 | 0.42 |
| Serum creatinine | 0.94 | 0.60-1.48 | 0.8 |
| Destination therapy | 1.8 | 0.43-8.7 | 0.63 |
| Model for End-Stage Liver Disease | 1.05 | 0.99-1.1 | 0.09 |
| Diabetes | 0.77 | 0.48-1.23 | 0.27 |
CF-LVAD = continuous-flow left ventricular assist device; INTERMACS = Interagency Registry for Mechanically Assisted Circulatory Support; mRAP/PCWP = mean right atrial pressure/pulmonary capillary wedge pressure; RR = relative risk.
Discussion
In our cohort of CF-LVAD patients, 30% of patients developed postoperative vasoplegia, and 13% developed vasoplegia that lasted at least 12 hours. The main finding was that although development of postoperative vasoplegia was not associated with adverse outcomes, prolonged vasoplegia was associated with greater early mortality. Prolonged vasoplegia was also associated with greater likelihood of postoperative AKI (Central Illustration).
Central Illustration.
Impact of Prolonged Vasoplegia after Continuous-Flow Left Ventricular Assist Device Implantation
Patients who developed vasoplegia were grouped into those who had vasoplegia for <12 hours (transient) or ≥12 hours (prolonged). Survival in patients with transient vasoplegia was comparable to survival in those with no vasoplegia; prolonged vasoplegia was associated with worse survival than no or transient vasoplegia. CI = cardiac index; INTERMACS = Interagency Registry for Mechanically Assisted Circulatory Support; SVR = systemic vascular resistance.
The general consensus among previous reports of vasoplegia after cardiac surgery is that vasoplegia is associated with higher mortality rates.1, 2, 3, 4, 5, 6, 7, 8, 9, 10 However, to our knowledge, few studies have focused specifically on the CF-LVAD population to capture the evolution of vasoplegia over time or have used immediate postoperative hemodynamic parameters to identify patients with postoperative vasoplegia. Our cohort of 600 patients compares favorably with the number of CF-LVAD patients in 4 previous studies of postoperative vasoplegia (Supplemental Table 1),2,4,10,16 which had samples ranging from 33 to 252 patients.
One challenge in interpreting the vasoplegia literature is heterogeneity among definitions, as highlighted in Supplemental Table 1. Cardiac index was used fairly consistent across the 4 studies, the threshold varying from 2.2 to 2.5 L/min/m2. In contrast, SVR varied significantly across the studies. Tecson et al2 and van Vessem et al10 each had no SVR requirement in their definitions of vasoplegia, whereas de Waal et al4 and Swan et al16 included either a MAP or an SVR threshold, rather than using both, to identify patients with vasoplegia. This may be problematic because the combination of SVR and a low MAP, as used in the current analysis, is essential for differentiating vasoplegic shock from shock of other causes. For example, in the recent study by Swan et al,16 38% of the patients with vasoplegia had concomitant RVF.
Interestingly, although the other studies were strict about the number or doses of vasopressors required to identify patients with vasoplegia, the incidence of vasoplegia observed in our study (30%) was similar to those reported by van Vessem et al10 (29%) and De Waal et al4 (33%) but much lower than those reported by Tecson et al2 (50%) and Swan et al16 (61%). Again, this difference may have been due to the inconsistent use of SVR and MAP in those other studies to capture patients with true vasoplegia. Swan et al,16 who reported the highest incidence of vasoplegia (61%), used a comparatively liberal definition of vasoplegia: an elevated MAP or SVR or a normal cardiac index, with norepinephrine equivalent requirements >0.1 μg/kg/min for at least 15 minutes. In contrast, to comprehensively identify patients with vasoplegia during the first 24 hours after CF-LVAD implantation, we incorporated a rigorous definition that required all hemodynamic thresholds to be met and included a strict vasopressor prerequisite. Our goal was to minimize other confounding causes, such as RVF, hemorrhagic shock, and hypovolemic shock.
In our cohort, postoperative vasoplegia itself was not associated with early or long-term mortality, whereas prolonged vasoplegia was associated with both. Thus, studies that define vasoplegia according to hemodynamic parameters or vasopressors administered at the time of intensive care unit arrival may overestimate the incidence of vasoplegia or may actually be identifying transient vasoplegia, which has less clinical significance than prolonged vasoplegia. In addition, whereas others have investigated vasoplegia 48 to 72 hours after implantation, multiple investigators have shown that the first 12 to 24 hours—the focus of our study—is the critical period for developing vasoplegia after CF-LVAD implantation.4,16
Identifying patients at risk for prolonged vasoplegia may allow early risk stratification and intervention. We found that BMI, MELD score, and preoperative renal status were directly associated with whether a patient developed both postoperative vasoplegia in general and prolonged vasoplegia in particular. We did not find an association between preoperative use of ACEIs or angiotensin receptor blockers (ARBs) and the development of vasoplegia after CF-LVAD implantation; whereas other investigators10 have found an association between ACEIs or ARBs and postimplantation vasoplegia, most others did not find an association, including Tecson et al2 after CF-LVAD implantation and Patarroyo et al1 and Chan et al5 after heart transplant. In our analysis, 56% of patients received ACEIs and 17% received ARBs preoperatively; we typically hold these medications in patients presenting with higher-acuity INTERMACS profiles because of hemodynamic considerations.
Preoperative dialysis was a risk factor for any vasoplegia and for prolonged vasoplegia. Similarly, de Waal et al4 and Chan et al5 found that worsening renal function, as indicated by either the use of dialysis or increasing serum creatinine, was an independent predictor of vasoplegia development.4,5 Indeed, renal failure was the only preoperative risk factor for developing vasoplegia (OR: 1.47) identified by Dayan et al17 in a meta-analysis of 10 articles and more than 30,000 patients after cardiac surgery. It is not entirely clear whether the vasoplegia is exacerbating the AKI, the worsening AKI is prolonging the vasoplegia, or the renal replacement therapy is contributing to the ongoing vasopressor needs; in all likelihood, they are interrelated.18 Intraoperative strategies for reducing AKI in these patients include targeted perfusion strategies, such as goal-directed therapy, in addition to meticulous surgical technique to reduce the need for transfusions.19
Efforts to reduce postoperative AKI requiring renal replacement therapy after LVAD placement are critical, as AKI development is associated with a significant increase in mortality risk.20 Although the exact pathophysiology of AKI remains unclear, a pivotal role has been identified for renally excreted inflammatory markers, including copeptin and bradykinin, during the developmental stages of vasoplegia.21,22 The persistence of these inflammatory substances may perpetuate the vasodilatory state in patients with abnormal renal function. Our results indicating an association between obesity and prolonged vasoplegia may be related to pro-inflammatory adipokines.23 Similar to results from a major randomized trial in cardiac surgery24 and 2 other LVAD reports,2,10 we did not find an association between preoperative use of ARBs and the development of vasoplegia after CF-LVAD implantation. Moreover, although we had no information on the use of angiotensin receptor-neprilysin inhibitors in this patient cohort, others have not found an association between preoperative angiotensin receptor-neprilysin inhibitor use and postoperative vasoplegia after CF-LVAD implantation.25
The MELD score also has been validated in multiple reports as a prognostic indicator for patients undergoing CF-LVAD implantation.26,27 Similar to van Vessem et al,10 we found a direct association between MELD score and vasoplegia risk. Other investigators also have found higher mortality rates after LVAD implantation in patients with MELD scores ≥14.28,29 An elevated preoperative MELD score may reflect an elevated inflammatory state. Vasodilatory states involve higher levels of pro-inflammatory factors, including nitric oxide, interleukins (ILs) such as IL-1 and IL-6, tumor necrosis factor-α, and copeptin, and are exacerbated by receiving more blood transfusions.22,30,31 In states of worsening liver function, Kupffer cells, which are specialized hepatic macrophages, sometimes release inflammatory endotoxins and cytokines, including IL-6.32,33 Furthermore, increased nitric oxide biosynthesis, which is commonly caused by hepatic failure, has been observed in portal-hypertensive states.34 Higher MELD score can also be a reflection of congestive hepatopathy.35 Because patients with elevated MELD scores are at higher risk for developing any vasoplegia and prolonged vasoplegia, further investigation into therapeutic interventions that could benefit these patients by mitigating that risk are warranted. Lastly, because CF-LVADs lack pulsatility, they may intrinsically increase the incidence of vasoplegia.36
Transient vasoplegia was not associated with increased mortality in our study, whereas the progression to prolonged vasoplegia within the 12- to 24-hour window was associated with adverse outcomes. Therefore, early treatment of vasoplegia may offer a potential therapeutic target to prevent the development of prolonged vasoplegia.
When vasoplegia is severe, high doses of multiple vasopressors may be insufficient. In our practice, we begin by avoiding medications associated with vasoplegia (eg, propofol, milrinone37). Our current pharmacological strategy after norepinephrine and vasopressin administration exceeds a norepinephrine equivalent dosage of 0.3 μg/kg/min is to initiate angiotensin II.38,39 Once a MAP of 70 mm Hg is achieved and the other vasopressors are down-titrated, the angiotensin II is weaned off. We are mindful of maintaining a renal perfusion pressure (MAP-CVP) ≥60 mm Hg and adjusting hemodynamic targets accordingly to reduce the risk for AKI.40 Patients at higher risk for developing postoperative prolonged vasoplegia may benefit from earlier administration of angiotensin II or other therapeutic adjuncts to achieve a target MAP. Increasing data suggest that angiotensin II benefits patients with vasoplegic shock who need renal replacement therapy for AKI.41 We will occasionally use rescue measures, including glucocorticoids and mineralocorticoids, methylene blue, or hydroxycobalamin.42,43 In addition, lactic acidosis is treated aggressively with sodium bicarbonate or, in severe cases, with renal replacement therapy as needed.
Study Limitations
This study was susceptible to the limitations of any single-institution retrospective report, despite having a larger sample size than the other CF-LVAD vasoplegia studies in the current literature. First, it is difficult to isolate vasoplegic shock completely, as there may have been an element of hemorrhagic shock or RVF in some patients. Moreover, some patients who presented with prolonged vasoplegia in the first 24 hours may have developed RVF later. Three of the other LVAD vasoplegia studies did not exclude patients with RVF,2,10,16 and 1 excluded patients who subsequently needed a right ventricular assist device.4 We recognize that vasoplegic shock may coexist with RVF in some patients or can lead to RVF in the early perioperative period. We attempted to minimize the confounding from RVF by including the high cardiac output and low SVR requirements characteristic of vasoplegia but not RVF. Moreover, chart abstraction was performed for every patient categorized as having vasoplegia, in order to confirm that concomitant RVF was not present.
Second, we did not have access to frequency of administration for adjunct therapies, such as glucocorticoids, methylene blue, and hydroxocobalamin, which are often used in the operating room to treat vasoplegia. However, the focus of this study was the development of vasoplegia and differences in outcomes with prolonged vasoplegia, as opposed to the efficacy of therapeutic strategies to mitigate the time course or severity of postoperative vasoplegia. These are areas we aim to investigate in future studies. Along those lines, although we had a substantial list of preoperative medications (Tables 2 and 4), we did not have data on the use of thyroid medications or on thyroid conditions in general. Third, our institution had not yet approved angiotensin II use during the study period; in our current practice, it is part of our vasopressor escalation algorithm. Fourth, we focused our analysis on the first 24 hours after surgery, but it is intuitive that patients who had prolonged vasoplegia beyond 24 hours probably had worse outcomes than those whose prolonged vasoplegia had resolved. Additionally, whereas this study was conducted before the HeartMate 3 (Abbott Laboratories) era, and earlier-generation CF-LVAD devices were used, the primary focus was early development of vasoplegia in the first 24 hours after surgery. Nearly a quarter of the patients in a study by Swan et al16 had a HeartMate 3, and no association was found between vasoplegia and type of LVAD. Therefore, while it is doubtful the specific device type significantly influences the incidence of prolonged vasoplegia, future studies with a higher proportion of HeartMate 3 recipients will be needed to support that claim. We believe that the lessons learned from this study are instructive for the current generation of CF-LVAD implants. Finally, in our analysis, we mitigated the effect of potential confounders by using multivariate analysis and domain knowledge; however, as is the case with all observational studies, we acknowledge that we cannot entirely eliminate the possibility of unmeasured or residual confounding.
Conclusions
Vasoplegia develops after CF-LVAD implantation in almost one-third of cases. Earlier recognition of this occurrence and identification of patients at higher risk for developing vasoplegia, including those with a higher MELD score, elevated BMI, or renal dysfunction, may allow for targeted therapeutic interventions to mitigate patients’ risk of developing prolonged postoperative vasoplegia. Further investigation into early administration of aggressive treatments to mitigate the development of prolonged vasoplegia and reduce early mortality rates is warranted. Thoughtful patient selection and preoperative optimization should continue to be a focus.
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE: Recognizing risk factors for prolonged vasoplegia can allow earlier identification of higher-risk patients and earlier initiation of therapeutic interventions. Prolonged vasoplegia is strongly associated with the development of AKI. Targeted strategies to reduce renal injury (preoperatively, intraoperatively, and postoperatively) are worthwhile.
TRANSLATIONAL OUTLOOK: This study represents a large series of patients who developed vasoplegia after left ventricular assist device implantation. Earlier identification of patients at higher risk for developing vasoplegia may allow for earlier intervention with pharmacological adjuncts, either preoperatively or intraoperatively, to curb the development of vasoplegia. Future directions include randomized trials using novel vasopressors or other adjuncts before vasoplegia develops to determine if prolonged postoperative vasoplegia can be mitigated.
Funding support and author disclosures
Dr Nair is a consultant for Abbott; and has received speaking fees from Abbott and Impulse Dynamics. Dr Frazier has received travel support from BiVACOR Inc. Dr Loor has received institutional grant support to Baylor College of Medicine from Abiomed, Transmedics, Abbott, and Maquet; and is a consultant for Abiomed & TransMedics, and is a scientific advisor for TransMedics. Dr Shafii has received institutional grant support to Baylor College of Medicine from Abbott and Evaheart, Inc. Dr Chatterjee has served on advisory boards for Edwards Lifesciences, La Jolla Pharmaceutical Company, Baxter Pharmaceuticals, and Eagle Pharmaceuticals, Inc. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors thank Jeanie F. Woodruff, BS, ELS, and Stephen N. Palmer, PhD, ELS, of the Department of Scientific Publications at The Texas Heart Institute for their editorial contributions.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For a supplemental table, please see the online version of this paper.
Supplementary data
References
- 1.Patarroyo M., Simbaqueba C., Shrestha K., et al. Pre-operative risk factors and clinical outcomes associated with vasoplegia in recipients of orthotopic heart transplantation in the contemporary era. J Heart Lung Transplant. 2012;31:282–287. doi: 10.1016/j.healun.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 2.Tecson K.M., Lima B., Lee A.Y., et al. Determinants and outcomes of vasoplegia following left ventricular assist device implantation. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.117.008377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Byrne J.G., Leacche M., Paul S., et al. Risk factors and outcomes for 'vasoplegia syndrome' following cardiac transplantation. Eur J Cardio Thorac Surg. 2004;25:327–332. doi: 10.1016/j.ejcts.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 4.de Waal E.E.C., van Zaane B., van der Schoot M.M., et al. Vasoplegia after implantation of a continuous flow left ventricular assist device: incidence, outcomes and predictors. BMC Anesthesiol. 2018;18:185. doi: 10.1186/s12871-018-0645-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan J.L., Kobashigawa J.A., Aintablian T.L., et al. Characterizing predictors and severity of vasoplegia syndrome after heart transplantation. Ann Thorac Surg. 2018;105:770–777. doi: 10.1016/j.athoracsur.2017.09.039. [DOI] [PubMed] [Google Scholar]
- 6.Chemmalakuzhy J., Costanzo M.R., Meyer P., et al. Hypotension, acidosis, and vasodilatation syndrome post-heart transplant: prognostic variables and outcomes. J Heart Lung Transplant. 2001;20:1075–1083. doi: 10.1016/s1053-2498(01)00299-6. [DOI] [PubMed] [Google Scholar]
- 7.Levin M.A., Lin H.M., Castillo J.G., Adams D.H., Reich D.L., Fischer G.W. Early on-cardiopulmonary bypass hypotension and other factors associated with vasoplegic syndrome. Circulation. 2009;120:1664–1671. doi: 10.1161/CIRCULATIONAHA.108.814533. [DOI] [PubMed] [Google Scholar]
- 8.Mekontso-Dessap A., Houël R., Soustelle C., Kirsch M., Thébert D., Loisance D.Y. Risk factors for post-cardiopulmonary bypass vasoplegia in patients with preserved left ventricular function. Ann Thorac Surg. 2001;71:1428–1432. doi: 10.1016/s0003-4975(01)02486-9. [DOI] [PubMed] [Google Scholar]
- 9.Argenziano M., Chen J.M., Choudhri A.F., et al. Management of vasodilatory shock after cardiac surgery: identification of predisposing factors and use of a novel pressor agent. J Thorac Cardiovasc Surg. 1998;116:973–980. doi: 10.1016/S0022-5223(98)70049-2. [DOI] [PubMed] [Google Scholar]
- 10.van Vessem M.E., Palmen M., Couperus L.E., et al. Incidence and predictors of vasoplegia after heart failure surgery. Eur J Cardio Thorac Surg. 2017;51:532–538. doi: 10.1093/ejcts/ezw316. [DOI] [PubMed] [Google Scholar]
- 11.Society of Thoracic Surgeons Appendix M. STS INTERMACS users' guide. https://www.uab.edu/medicine/intermacs/images/Intermacs-Users-Guide-v5.0-2018-06-28.pdf
- 12.Feldman D., Pamboukian S.V., Teuteberg J.J., et al. The 2013 International Society for Heart and Lung Transplantation guidelines for mechanical circulatory support: executive summary. J Heart Lung Transplant. 2013;32:157–187. doi: 10.1016/j.healun.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 13.See E.J., Bellomo R. How I prescribe continuous renal replacement therapy. Crit Care. 2021;25:1–3. doi: 10.1186/s13054-020-03448-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen W., Qian L., Shi J., Franklin M. Comparing performance between log-binomial and robust Poisson regression models for estimating risk ratios under model misspecification. BMC Med Res Methodol. 2018;18:63. doi: 10.1186/s12874-018-0519-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallis J.A., Turner E.L. Relative measures of association for binary outcomes: challenges and recommendations for the global health researcher. Ann Glob Health. 2019;85:137. doi: 10.5334/aogh.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swan J.T., Iso T., Rizk E., et al. Defining vasoplegia following durable, continuous flow left ventricular assist device implantation. ASAIO J. 2022;68:46–55. doi: 10.1097/MAT.0000000000001419. [DOI] [PubMed] [Google Scholar]
- 17.Dayan V., Cal R., Giangrossi F. Risk factors for vasoplegia after cardiac surgery: a meta-analysis. Interact Cardiovasc Thorac Surg. 2019;28:838–844. doi: 10.1093/icvts/ivy352. [DOI] [PubMed] [Google Scholar]
- 18.Villa G., Husain-Syed F., Saitta T., et al. Hemodynamic instability during acute kidney injury and acute renal replacement therapy: pathophysiology and clinical implications. Blood Purif. 2021;50:729–739. doi: 10.1159/000513942. [DOI] [PubMed] [Google Scholar]
- 19.Ranucci M., Johnson I., Willcox T., et al. Goal-directed perfusion to reduce acute kidney injury: a randomized trial. J Thorac Cardiovasc Surg. 2018;156:1918–1927.e2. doi: 10.1016/j.jtcvs.2018.04.045. [DOI] [PubMed] [Google Scholar]
- 20.Parikh U., Lamba H., Ajmal M., et al. Predictors of renal replacement therapy in patients with continuous flow left ventricular assist devices. J Artif Organs. 2021;24:207–216. doi: 10.1007/s10047-020-01239-z. [DOI] [PubMed] [Google Scholar]
- 21.Gavras I. Bradykinin-mediated effects of ACE inhibition. Kidney Int. 1992;42:1020–1029. doi: 10.1038/ki.1992.383. [DOI] [PubMed] [Google Scholar]
- 22.Colson P.H., Bernard C., Struck J., Morgenthaler N.G., Albat B., Guillon G. Post cardiac surgery vasoplegia is associated with high preoperative copeptin plasma concentration. Crit Care. 2011;15:R255. doi: 10.1186/cc10516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ouchi N., Parker J.L., Lugus J.J., Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Diepen S., Norris C.M., Zheng Y., et al. Comparison of angiotensin-converting enzyme inhibitor and angiotensin receptor blocker management strategies before cardiac surgery: a pilot randomized controlled registry trial. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.118.009917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haider L., Hugon-Vallet E., Constantin J.P., Riad Z., Sebbag L., Mewton N. ARNI pre-operative use and vasoplegic syndrome in patients undergoing heart transplantation or left ventricular assist device surgery. Med Sci. 2021;10:2. doi: 10.3390/medsci10010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deo S.V., Daly R.C., Altarabsheh S.E., et al. Predictive value of the model for end-stage liver disease score in patients undergoing left ventricular assist device implantation. ASAIO J. 2013;59:57–62. doi: 10.1097/MAT.0b013e31827c0c77. [DOI] [PubMed] [Google Scholar]
- 27.Yang J.A., Kato T.S., Shulman B.P., et al. Liver dysfunction as a predictor of outcomes in patients with advanced heart failure requiring ventricular assist device support: use of the Model of End-Stage Liver Disease (MELD) and MELD eXcluding INR (MELD-XI) scoring system. J Heart Lung Transplant. 2012;31:601–610. doi: 10.1016/j.healun.2012.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonde P., Ku N.C., Genovese E.A., et al. Model for End-stage Liver Disease score predicts adverse events related to ventricular assist device therapy. Ann Thorac Surg. 2012;93:1541–1547. doi: 10.1016/j.athoracsur.2012.02.008. Discussion 1547-1548. [DOI] [PubMed] [Google Scholar]
- 29.Critsinelis A., Kurihara C., Volkovicher N., et al. Model of End-Stage Liver Disease-eXcluding International Normalized Ratio (MELD-XI) scoring system to predict outcomes in patients who undergo left ventricular assist device implantation. Ann Thorac Surg. 2018;106:513–519. doi: 10.1016/j.athoracsur.2018.02.082. [DOI] [PubMed] [Google Scholar]
- 30.Baker T.A., Romero J., Bach H.H., et al. Systemic release of cytokines and heat shock proteins in porcine models of polytrauma and hemorrhage. Crit Care Med. 2012;40:876–885. doi: 10.1097/CCM.0b013e318232e314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fransen E., Maessen J., Dentener M., Senden N., Buurman W. Impact of blood transfusions on inflammatory mediator release in patients undergoing cardiac surgery. Chest. 1999;116:1233–1239. doi: 10.1378/chest.116.5.1233. [DOI] [PubMed] [Google Scholar]
- 32.Masai T., Sawa Y., Ohtake S., et al. Hepatic dysfunction after left ventricular mechanical assist in patients with end-stage heart failure: role of inflammatory response and hepatic microcirculation. Ann Thorac Surg. 2002;73:549–555. doi: 10.1016/s0003-4975(01)03510-x. [DOI] [PubMed] [Google Scholar]
- 33.Callery M.P., Kamei T., Flye M.W. Endotoxin stimulates interleukin-6 production by human Kupffer cells. Circ Shock. 1992;37:185–188. [PubMed] [Google Scholar]
- 34.Sogni P., Moreau R., Gadano A., Lebrec D. The role of nitric oxide in the hyperdynamic circulatory syndrome associated with portal hypertension. J Hepatol. 1995;23:218–224. doi: 10.1016/0168-8278(95)80339-4. [DOI] [PubMed] [Google Scholar]
- 35.Xanthopoulos A., Starling R.C., Kitai T., Triposkiadis F. Heart failure and liver disease: cardiohepatic interactions. J Am Coll Cardiol HF. 2019;7:87–97. doi: 10.1016/j.jchf.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 36.Witman M.A., Garten R.S., Gifford J.R., et al. Further peripheral vascular dysfunction in heart failure patients with a continuous-flow left ventricular assist device: the role of pulsatility. J Am Coll Cardiol HF. 2015;3:703–711. doi: 10.1016/j.jchf.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chatterjee S. Management of vasoplegic shock in left ventricular assist device insertion procedures. Tex Heart Inst J. 2023;50 doi: 10.14503/THIJ-23-8172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khanna A., English S.W., Wang X.S., et al. Angiotensin II for the treatment of vasodilatory shock. N Engl J Med. 2017;377:419–430. doi: 10.1056/NEJMoa1704154. [DOI] [PubMed] [Google Scholar]
- 39.Klijian A., Khanna A.K., Reddy V.S., et al. Treatment with angiotensin II is associated with rapid blood pressure response and vasopressor sparing in patients with vasoplegia after cardiac surgery: a post-hoc analysis of Angiotensin II for the Treatment of High-Output Shock (ATHOS-3) study. J Cardiothorac Vasc Anesth. 2021;35:51–58. doi: 10.1053/j.jvca.2020.08.001. [DOI] [PubMed] [Google Scholar]
- 40.Ostermann M., Hall A., Crichton S. Low mean perfusion pressure is a risk factor for progression of acute kidney injury in critically ill patients - a retrospective analysis. BMC Nephrol. 2017;18:151. doi: 10.1186/s12882-017-0568-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tumlin J.A., Murugan R., Deane A.M., et al. Outcomes in patients with vasodilatory shock and renal replacement therapy treated with intravenous angiotensin II. Crit Care Med. 2018;46:949–957. doi: 10.1097/CCM.0000000000003092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Busse L.W., Barker N., Petersen C. Vasoplegic syndrome following cardiothoracic surgery—review of pathophysiology and update of treatment options. Crit Care. 2020;24:36. doi: 10.1186/s13054-020-2743-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suero O.R., Park Y., Wieruszewski P.M., Chatterjee S. Management of vasoplegic shock in the cardiovascular intensive care unit after cardiac surgery. Crit Care Clin. 2023;40:73–88. doi: 10.1016/j.ccc.2023.06.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.