Abstract
It is becoming increasingly apparent that commensal skin bacteria have an important role in wound healing and infection progression. However, the precise mechanisms underpinning many of these probiotic interactions remain to be fully uncovered. In this work, we demonstrate that the common skin commensal Cutibacterium acnes can limit the pathogenicity of the prevalent wound pathogen Pseudomonas aeruginosa in vivo. We show that this impact on pathogenicity is independent of any effect on growth, but occurs through a significant downregulation of the Type Three Secretion System (T3SS), the primary toxin secretion system utilised by P. aeruginosa in eukaryotic infection. We also show a downregulation in glucose acquisition systems, a known regulator of the T3SS, suggesting that glucose availability in a wound can influence infection progression. C. acnes is well known as a glucose fermenting organism, and we demonstrate that topically supplementing a wound with glucose reverses the probiotic effects of C. acnes. This suggests that introducing carbon source competition within the wound microenvironment may be an effective way to prevent or limit wound infection.
Subject terms: Bacteriology, Biofilms, Pathogens
Introduction
The skin is a key barrier that provides physical protection against environmental insults and opportunistic pathogens. Damage to this barrier significantly increases the likelihood of infection, which can lead to serious acute complications such as bacteraemia and sepsis if untreated1,2. If treatment of the infected wound with frontline therapeutics fails, wound infections can become chronic, leading to further treatment failures and prolonged hospital stays. It has been estimated that around 1–2% of the general population in developed countries suffer from chronic wounds across all age groups3,4. This creates major pressure on the economy but also has a significant detrimental effect on the patient’s quality of life and wellbeing4,5. The most commonly isolated bacterial pathogens from wounds are Staphylococcus aureus, Pseudomonas aeruginosa, Klebsiella pneumoniae, Enterobacter spp, Enterococcus faecalis and Acinetobacter baumannii6–8. These bacteria belong to the ESKAPE pathogens, a watchlist of pathogens that are rapidly gaining antibiotic resistance. These pathogens also feature on the World Health Organisations Priority List for which there is an urgent need to develop alternative treatment strategies9–13.
The skin microbiome is one of the largest bacterial communities in the human body and plays an important role in the maintenance of the skin barrier and in regulating wound healing12,14,15. Amongst the commensal skin microbiota, several commensal species can occasionally lead to opportunistic infection including Staphylococcus epidermidis, Streptococcus mitis, Cutibacterium (formerly Propionibacterium) acnes, Corynebacterium spp and Acinetobacter johnsonii16–18. Some true pathogens are also part of the skin microbiome, including S. aureus, P. aeruginosa and Streptococcus pyogenes; however, colonisation of intact skin with these pathogens tends to be asymptomatic and transient19,20.
Disruption of skin integrity provides an opportunity for commensal skin bacteria and environmental microorganisms to colonise the wound bed soon after the injury21. Wound beds are a nutrient-rich environment that provides access to deeper tissues for microorganisms to colonise and thrive22,23. Early wound colonisation by commensal non-pathogenic bacteria has been described as beneficial due to the principle of competitive exclusion in which some species out-compete each other for the space and nutrients available in a wound24. Lactobacillus reuteri has been shown to inhibit S. aureus adherence via competitive exclusion in a keratinocyte co-culture model25. Additionally, vancomycin-induced skin microbiota dysbiosis in mice was reported to delay wound healing, implying that the skin microbiota is important in the process of wound healing26. Furthermore, wounds that displayed a dysbiotic skin microbiome were reported to exhibit increased inflammation and delayed healing in comparison to healthy microbiome wounds in rats27.
C. acnes is a commensal organism but can cause acne vulgaris and various opportunistic infections, e.g. surgical site infections28,29. C. acnes strains are categorised into several phylotypes depending on the expression of putative virulence factors, i.e., IA1 is typically associated with acneic skin, whereas IA2, IB, IC and II are associated with healthy skin30,31. Phylotype I has been associated with moderate-to-severe acne, whereas all other phylotypes are either isolated from soft tissue infections or normal skin microbiota and are considered true commensals32,33. C. acnes has been reported to inhibit several pathogenic bacteria via the production of secreted chemicals, such as short-chain fatty acids (SCFAs), propionic acid and bacteriocins. For example, short-chain fatty acids produced by C. acnes have been reported to inhibit biofilm formation and decrease the bioburden of S. aureus in wounds34,35. C. acnes isolates have also been shown to alter S. aureus antibiotic susceptibility and prevent the maturation of S. aureus biofilms which increased their susceptibility to antimicrobial treatments36. C. acnes produces bacteriocins that have an antibiotic effect on S. epidermidis37. Production of propionic acid by C. acnes has been shown to kill S. aureus and lower the pH of the wound38,39. Intriguingly, no antibacterial effects against Gram-negative bacteria have been reported. In the present study we investigate the antivirulence effects of C. acnes isolates on the common wound pathogen P. aeruginosa. We propose that this effect is achieved via carbon-source competition within the wound, thus introducing the concept of carbon-source competition within the wound as a potential alternative treatment strategy for P. aeruginosa wound infections.
Results
C. acnes strains decrease P. aeruginosa PA14 induced mortality in burn infection in G. mellonella
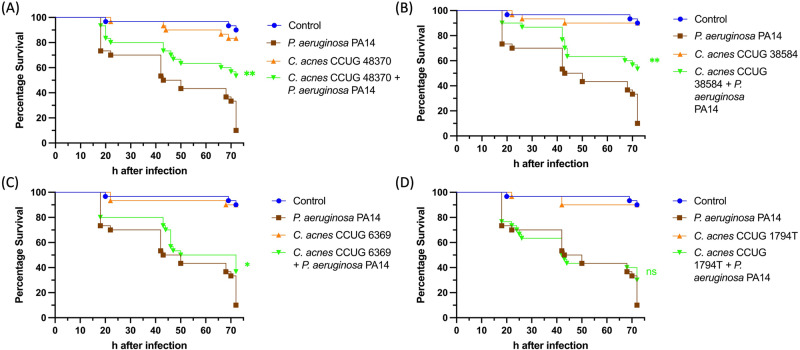
Specific species of commensal skin bacteria, including C. acnes have been identified as pioneer colonisers of the wound bed that can promote healing18,40. To explore the capacity of C. acnes to limit infection progression, the invertebrate burn wound model G. mellonella was chosen. This model has been extensively validated as a robust tool to study burn wound infection and unlike other in vivo burn wound models it can be used to screen for novel wound therapeutics including probiotic bacteria41–44. A panel of C. acnes strains comprised of C. acnes CCUG 1794T, CCUG 38584, CCUG 48370, CCUG 6369 was selected to assay in this model45 (Supplementary Table 1). After establishing burn wounds on G. mellonella larvae, the wounds were seeded with each of the C. acnes strains. None of the C. acnes strains had a significant impact on larval survival confirming their status as commensals. To assess their impact on the pathogenicity of the burn wound isolate P. aeruginosa PA14, the G. mellonella burn wound assays were repeated with P. aeruginosa PA14 being applied to the wound immediately after C. acnes seeding. Wounds without C. acnes added but infected with P. aeruginosa PA14 were used as a positive control. Administration of P. aeruginosa PA14 alone led to 90% mortality after 72 h as expected. Larvae treated with C. acnes CCUG 48370 and C. acnes CCUG 38584 prior to infection with P. aeruginosa PA14 exhibited a significant 43% increase in survival, while larvae treated with C. acnes CCUG 6369 displayed a 20% increase in survival (Fig. 1 ABC). Larvae treated with C. acnes CCUG 1794T prior to infection exhibited no significant decrease in mortality, demonstrating that this reduced virulence effect was strain specific (Fig. 1 D).
Fig. 1. Kaplan–Meier Survival curves of in vivo P. aeruginosa PA14 burn infection using the G. mellonella burn wound infection model.
A P. aeruginosa PA14 vs. C. acnes CCUG 48370, Log rank p value < 0.005. B P. aeruginosa PA14 vs. C. acnes CCUG 38584, Log rank p value < 0.005. C P. aeruginosa PA14 vs. C. acnes CCUG 6369, Log rank p value < 0.05. D P. aeruginosa PA14 vs. C. acnes CCUG 1794T, p value > 0.05. The specimens were monitored for 72 h starting at 18 h; n = 30 as three biological replicates of 10 larvae per experimental group. Log-rank (Mantel–Cox) test was performed to assess the statistical significance with Bonferroni correction, ns = p value > 0.05, **p value < 0.005.
C. acnes decreases P. aeruginosa biofilm formation but does not impact growth
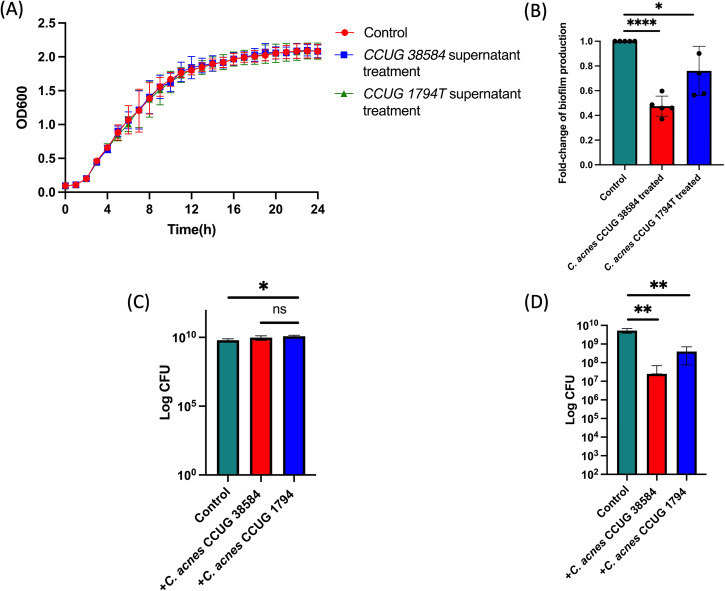
C. acnes is known to be able to secrete a range of antibacterial compounds18,46. To explore if C. acnes was limiting P. aeruginosa growth and whether this was influencing infection progression, the growth of P. aeruginosa was monitored in the presence of the supernatant of C. acnes CCUG 38584, a strain that had a major impact on infection progression and C. acnes CCUG 1794T, a strain that did not have a significant impact on infection progression (Fig. 2A). Growth curves of P. aeruginosa PA14 obtained in media supplemented with 50% supernatant were not significantly different from those obtained in the absence of supernatant indicating that C. acnes had no impact on P. aeruginosa growth.
Fig. 2. Impact of C. acnes on P. aeruginosa growth and biofilm formation.
A Growth curve of P. aeruginosa PA14 liquid cultures in the presence of C. acnes strains supernatants (50%) showing no significant difference in the growth rates between exposures. Two-way ANOVA with multiple comparisons was performed to assess statistical significance. P value > 0.05 was determined as insignificant (ns). B The effect on biofilm formation of P. aeruginosa PA14 in the presence of C. acnes strains supernatants after 24 h. C. acnes CCUG 38584 supernatant treatment exhibited a 52.5% reduction of the biofilm production in P. aeruginosa PA14, whereas C. acnes CCUG 1794T exhibited a less significant 24% reduction in comparison to the control treatment. Unpaired Student’s t test (two tailed) was performed to assess statistical significance. ****p value < 0.0001, *p value < 0.05. Error Bars are Standard Deviation. C Growth of P. aeruginosa PA14 in an artificial sebum model in the presence of C. acnes CCUG 1794T and CCUG 38584 cultures. No negative effects on growth were observed. Unpaired Student’s t test (two tailed) was performed to assess statistical significance *p value < 0.05. Error Bars are Standard Deviation. D Biofilm formation of P. aeruginosa PA14 in an artificial sebum model in the presence of C. acnes CCUG 1794T and CCUG 38584 cultures after 48 h. Unpaired Student’s t test (two tailed) was performed to assess statistical significance **p value < 0.01. Error Bars are Standard Deviation.
C. acnes has previously been shown to impact biofilm formation in Gram-positive bacteria including S. aureus and S. epidermidis; however, the specific strains tested did not affect P. aeruginosa PAO1 biofilm formation34,36. To explore the effect of C. acnes on biofilm formation by P. aeruginosa PA14, a biofilm assay was conducted in media supplemented with 50% C. acnes supernatant. The presence of the supernatant of C. acnes CCUG 38584 led to a significant reduction in biofilm formation by 52.5% in PA14 (Fig. 2B). The supernatant of C. acnes CCUG 1794T also significantly reduced biofilm formation, albeit to a lesser extent (24% inhibition) (Fig. 2B).
To more accurately replicate the conditions observed in a human wound and to explore if contact-dependent antagonistic mechanisms could be influencing P. aeruginosa growth, P. aeruginosa was co-cultured with C. acnes CCUG 38584 and C. acnes CCUG 1794T in an artificial sebum model (Fig. 2C). Co-culture with C. acnes strains had no negative effect on growth of PA14 however in agreement with our previous assays, biofilm formation was inhibited during co-culture, with C. acnes CCUG 38584 again having a greater effect than C. acnes CCUG 1794T (Fig. 2D). The lack of an inhibiting effect of C. acnes strains on P. aeruginosa PA14 growth suggests that the therapeutic effect of C. acnes is not due to restriction of growth of P. aeruginosa PA14. However, the reduction of the biofilm formation by P. aeruginosa PA14 in the presence of C. acnes could be a contributing factor to the reduction in mortality in vivo.
C. acnes CCUG 38584 significantly alters the expression of important virulence and metabolism pathways of P. aeruginosa
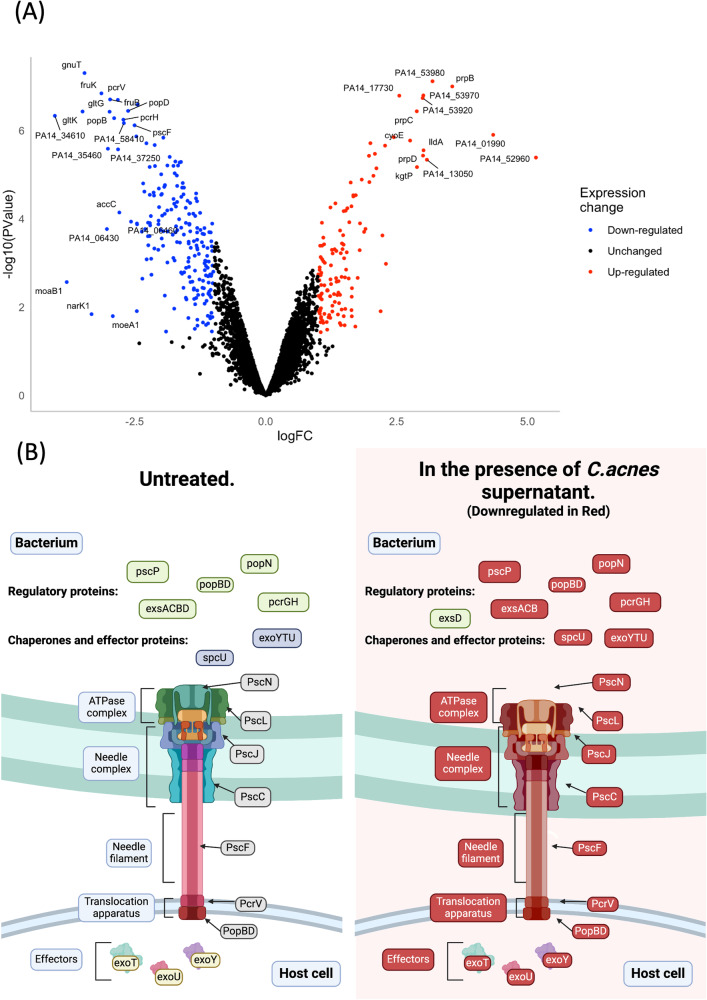
To explore the P. aeruginosa transcriptional response to exposure to C. acnes supernatant and potentially elucidate the cause of the antivirulence effect, RNA-seq analysis was performed on P. aeruginosa PA14 grown in the presence of 50% C. acnes CCUG 38584 supernatant. The samples were grown to mid-exponential phase before RNA was extracted and RNA-Seq performed. As a result, 334 P. aeruginosa genes were differentially expressed in the presence of supernatant of C. acnes CCUG 38584 compared to control samples of cells grown in 50% uninoculated C. acnes growth medium (Fig. 3A). The differential gene expression analysis provided several insights into the mechanisms that could be responsible for the reduced virulence observed in the aforementioned experiments as there were several important virulence factors encoding genes downregulated. Specifically, almost all of T3SS encoding genes were significantly downregulated, including all three exotoxins (exoT, exoU, and exoY) and the master regulator of the T3SS, exsA (Figs. 3A and 3B)47. The T3SS is a crucial virulence factor against eukaryotic cells and P. aeruginosa mutants lacking the T3SS displayed a significantly reduced virulence in several animal models including in the G. mellonella burn wound model41,48. Significant down-regulation of most of the genes encoding for the T3SS indicates that this effect is most likely responsible for the reduced virulence seen in the G. mellonella model. The evidence that the master regulator of the T3SS was also downregulated indicates that the specific mechanism of downregulation most likely lies upstream of this component of the T3SS regulatory pathway. These findings were further supported by the KEGG pathway analysis performed on the downregulated genes, which showed that bacterial secretion system encoding genes was one of the most represented gene groups (Supplementary Tables 2.1, 2.2, Supplementary Fig. 2.1, 2.2).
Fig. 3. The influence of C. acnes supernatant on P. aeruginosa gene expression.
A Volcano plot representing results of differential gene expression analysis of P. aeruginosa PA14 in the presence of C. acnes CCUG 38584 versus TSB control. Quantified genes were differentially expressed when Log-fold change > |1| and p value < 0.05. Genes which exhibited Log-fold change > |2.5| and p value < 0.05 are labelled. B A schematic representation of the T3SS in P. aeruginosa with the individual protein components labelled. In the presence of C. acnes CCUG 38584 supernatant, most of the key genes encoding for this secretion system are downregulated and the expression of this system is suppressed. Created with BioRender.com.
Certain environmental conditions are known to influence the expression of the T3SS, most notably calcium concentration, glucose availability and metabolic stress in general49–51. Within the RNA-Seq data set several of the downregulated genes are associated with the glucose uptake in P. aeruginosa. gltF-gltG-gltK which encodes the primary ABC transporter responsible for glucose membrane transport were significantly downregulated52,53. This is in agreement with previous data demonstrating the down-regulation of these uptake systems in low glucose conditions54,55. gnuT and PA14_37250 genes involved in glucose and lactate uptake were also significantly downregulated56. C. acnes is a glucose fermenting microorganism57,58. This suggests that C. acnes could be outcompeting P. aeruginosa for glucose within the wound microenvironment, therefore having a negative impact on pathogenicity without impacting P. aeruginosa growth, as P. aeruginosa can readily switch between carbon sources with little effect on overall growth59. In addition, glucose is known to enhance P. aeruginosa biofilm formation suggesting a potential mechanism for the reduced biofilm phenotype60.
The effects of C. acnes CCUG 38584 on P. aeruginosa PA14 are glucose availability dependent
The hypothesis that competition for glucose is responsible for the reduced pathogenicity of P. aeruginosa in a wound seeded with C. acnes does not explain why some strains of C. acnes appear to have a greater effect on pathogenicity than others, unless the rate of glucose fermentation varied significantly between C. acnes strains. In order to test this hypthesis, the C. acnes strain with the highest (C. acnes CCUG 38584) and the lowest antivirulence effect (C. acnes CCUG 1794T) were selected and glucose concentration was monitored over time (Fig. 4A). C. acnes CCUG 38584 depleted glucose from the media at a faster rate than C. acnes CCUG 1794T with a significant difference observed at 48 h, 60 h and 72 h. There was no significant difference observed in the growth of these two strains confirming that the observed probiotic effects were not due to a reduced growth rate between the strains (Supplementary Figure 1). This aligns with our previous findings of the antivirulence effect variability between these two strains and supports the glucose dependant therapeutic effect hypothesis.
Fig. 4. The role of glucose in the C. acnes antivirulence effect.
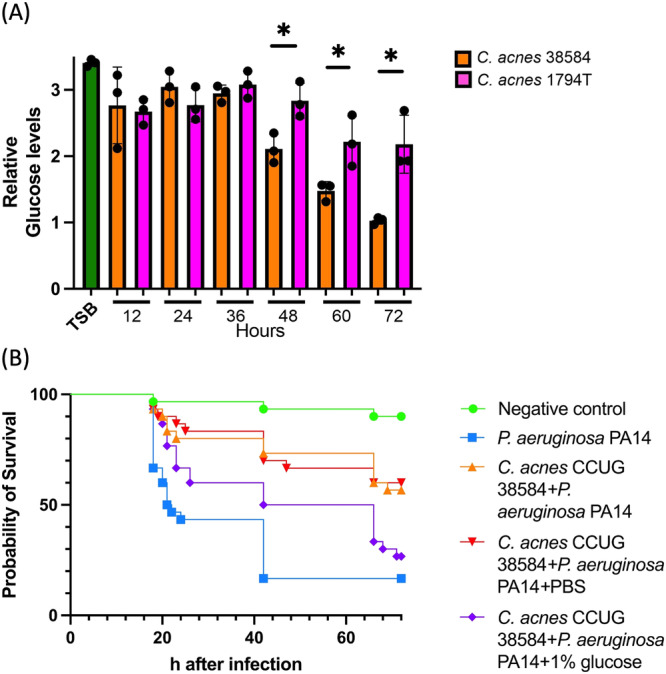
A Glucose content quantification in the supernatants of C. acnes CCUG 38584 and CCUG 1794T, demonstrating a declining glucose concentration over the 72 h observation of C. acnes growth. There was no significant difference between the C. acnes strains glucose consumption observed until the 48 h mark. At 48 h, 60 h and 72 h CCUG 38584 depleted glucose in the media significantly more than C. acnes CCUG 1794T, unpaired Student’s t test p value < 0.05, three biological replicates with two advanced technical replicates were analysed. Error Bars are Standard Deviation. B Survival curve of in vivo P. aeruginosa PA14 burn infection with C. acnes CCUG 38584 treatment with topically supplemented glucose, n = 30, 3 biological replicates with 10 larvae per experimental group. Log-rank (Mantel-Cox) test was performed to assess the statistical significance with Bonferroni-correction. CCUG 38584 + PA14 + PBS versus CCUG 38584 + PA14 + 1% glucose, p value < 0.05.
To further challenge the implicated importance of glucose scavenging in the C. acnes therapeutic effect, it was hypothesised that it should be possible to mitigate the effect through the exogenous application of glucose to the co-colonised wound. The G. mellonella survival assay was repeated with topical supplementation with a 1% glucose solution. Seeding of the wound with C. acnes CCUG 38584 prior to infection with P. aeruginosa PA14 resulted in the anticipated increase in survival. However, groups topically supplemented with 1% glucose had an increased virulence comparable to that seen with a P. aeruginosa PA14 only infection, while topical supplementation with a no-glucose control solution had no negative impact on the C. acnes therapeutic effect (Fig. 4B). This supports the hypothesis that glucose competition is one of the primary mechanisms involved in the P. aeruginosa PA14 in vivo mortality reduction by C. acnes.
Discussion
Wound care and management is a serious health issue worldwide, an issue that will only be exacerbated in the coming decades as a result of the growing ageing population2,18. One of the most commonly isolated wound pathogens is P. aeruginosa61. P. aeruginosa infected wounds are notoriously challenging to treat due to a wide range of virulence factors that it utilises, its metabolic flexibility and its capacity to overcome antibiotic challenge62,63. Therefore, there is an urgent need for alternative treatments for this pathogen.
The skin-gut microbiota axis effect on skin homoeostasis and skin wound healing has been firmly established64,65. Oral gut probiotic therapy has been reported to modulate immune response both locally and systemically66–68. In humans, probiotic supplementation of the gut has been reported to reduce inflammation, reverse liver injury and improve wound healing in chronic diabetic ulcers69–72. The positive effect of probiotic supplementation of the gut microbiota has also been shown to improve wound healing in rats and human intestinal myofibroblasts73,74. Topical applications of several commensal bacterial species have also been shown to influence wound infection and healing, such as Lactobacillus spp and S. epidermidis42,75–78. In the present study, we demonstrated that seeding a wound with C. acnes prior to the topical infection by P. aeruginosa PA14 significantly improves the survival of G. mellonella larvae (Fig. 1). One of the primary reasons for the reduction in in vivo mortality could be a reduction in the bioburden of P. aeruginosa. However, when tested, there was no effect of the supernatant or co-culture on the growth of P. aeruginosa, which implied that bacteriocins and acid production by C. acnes or direct contact mediated interspecies competition are unlikely to be responsible for antivirulence effect on P. aeruginosa in vivo. Furthermore, C. acnes supernatant and co-culture with C. acnes significantly decreases P. aeruginosa biofilm production (Fig. 2). Biofilm formation is one of the most important P. aeruginosa PA14 virulence factors, which allows it to evade the host immune system and establish persistent wound infections79. The reduction of biofilm formation of P. aeruginosa in the presence of C. acnes supernatant could also be a contributing factor to the reduction of in vivo mortality80.
In order to gain further insight into the underlying mechanism of the C. acnes effect on P. aeruginosa, differential gene expression analysis was performed on P. aeruginosa in the presence of C. acnes cell-free supernatant. Among the differentially expressed genes, the expression of genes encoding for the T3SS, the primary eukaryotic toxin delivery system of P. aeruginosa, was significantly downregulated. This likely explains the significant reduction in virulence as the T3SS is known to play a key role in P. aeruginosa pathogenicity and disrupting it has been previously shown in the model to significantly decrease pathogenicity41,48. Additionally, several genes associated with glucose acquisition were down-regulated including genes ecoding the high-affinity glucose transporter GltFGK and glucose uptake response regulator GltR as well as porin protein-encoding genes such as oprB. Several studies have demonstrated the link between glucose availability and the activity of the T3SS, with a disruption in glucose acquisition systems leading to a repression of the T3SS49,51,54,55,81. In addition to that, biofilm formation in P. aeruginosa has been reported to be glucose dependent, which would explain the reduced biofilm formation observed60.
Glucose is one of the preferred carbon sources for many microorganisms and as such would be a high-value commodity in the wound microenvironment82. C. acnes preferred carbon sources are glycerol and glucose which are readily available in the wound mircoenvironment57,58,83. This is particularly important in a burn wound setting as trauma-induced insulin resistance is known to significantly increase the levels of glucose in the wound microenvironment84. We demonstrate that the C. acnes strain CCUG 38584 which exhibited therapeutic effects against P. aeruginosa ferments glucose at a higher rate than the non-therapeutic CCUG 1794T, which implies that C. acnes CCUG 38584 is a more potent glucose scavenger in the wound (Fig. 4A). P. aeruginosa has a remarkable carbon source plasticity and can readily switch between carbon sources with limited effect on growth, explaining why co-culture with C. acnes, depleting the media of glucose, has no impact on P. aeruginosa growth59. Furthermore, the therapeutic effect of C. acnes CCUG 38584 was reversed upon topical supplementation of the wound with glucose (Fig. 4B). This supports the hypothesis that carbon-source competition between commensal bacteria and wound pathogens may be a potent alternative wound treatment vector as well as a prophylaxis of wound infections.
A limitation of this study is that it was performed in the G. mellonella burn wound model. However, this model has previously been shown to recapitulate many of the hallmarks of human burn wound trauma and is robust model for the screening and development of novel wound therapeutics41–44. It is also worth noting that performing the screening and characterisation described in this study in a mouse or rabbit burn wound model would be extremely difficult due to the numbers of animals required and the severity of the procedures involved. Future work will focus on further validating our findings in higher eukaryotic models such as mice and exploring the impact of C. acnes on other wound types such as diabetic ulcers.
In conclusion, this study has identified C. acnes as a potential wound probiotic, which was capable of reducing P. aeruginosa virulence in vivo and limiting biofilm formation. Moreover, it demonstrates a proof of principle that competitive exclusion by carbon-source competition could be an effective platform on which to develop alternative therapeutic avenues for phophylaxis and treatment of wound infections.
Methods
Bacterial strains growth assay
P. aeruginosa (PA14) and C. acnes (CCUG 38584, CCUG 1794T, CCUG 6369, CCUG 48370) were used. P. aeruginosa PA14 is a wound isolate and is a reference strain85. C. acnes CCUG 38584 belongs to phylotype I and its isolation origin is unknown86,87. C. acnes CCUG 1794T is a human facial acne isolate, phylotype IA157. C. acnes CCUG 6369 belongs to phylotype II and was isolated from a human subcutaneous abscess88. C. acnes CCUG 48370 is a vaginal discharge isolate and its phylotype is unknown45. Strain information is also available in Supplementary Table 1. P. aeruginosa was grown in LB Broth (Miller, Fisher BioReagents BP1426-2) overnight at 37 °C at 180 rpm and on LB Agar (Miller, Fisher BioReagents Microbiology Media) plates overnight at 37 °C. C. acnes was grown in tryptic soy broth (TSB, NutriSelect Plus) and on blood agar plates for 48–72 h at 37 °C. Blood agar plates were prepared by supplementing tryptic soy agar (NutriSelect Plus) with defibrinated horse blood (Thermo Scientific Oxoid, SR0050C) in a ratio of 19:1. C. acnes was grown anaerobically in a hermetic chamber with an AnaeroGen 2.5 L Sachet (Thermo Scientific Oxoid).
Growth and biofilm assay
Overnight cultures of P. aeruginosa PA14 were adjusted to OD = 0.1 at 600 nm. OD-adjusted bacteria were then washed by centrifugation at 8000 rpm for 3 mins, disposing of the supernatant and resuspending the bacterial pellet in fresh media 3 times. Washed culture was pipetted into a 96-well plate with 200 µl for positive control and 100 µl for the experimental conditions. PA14 was tested against 100 µl TSB broth and 100 µl of C. acnes strains cell-free supernatant. C. acnes strains supernatant was obtained by centrifuging the 48–72 h cultures for 10 mins at 5000 rpm and filter sterilising the supernatant with 0.2 nm filter. The set-up 96-well plate was incubated overnight in the Clariostar plate reader at 37 °C at 200 rpm to obtain a growth measurement. Upon the completion of the growth curve, the 96-well plate was taken out of the plate reader and the contents of each well were pipetted out. Wells were washed with 250 µl of distilled water 3 times. After washing, wells were stained with 220 µl of 1% crystal violet solution for 10 mins. Wells were then washed with 250 µl of distilled water to get rid of excess stain 5 times. The 96-well plate was then left to dry for 2 h. After the plate dried, 200 µl of 99% ethanol was added to each well and left to de-stain for 3 h. The de-stained well absorbance was read at 600 nm in the Clariostar plate reader.
P. aeruginosa cellular co-culture with C. acnes
P. aeruginosa strains PA14, and C. acnes strains CCUG 1794 and C. acnes CCUG 38584 were grown (either alone or in combination) in an artifical sebum model which was prepared and inoculated as previously described89. Following incubation at 37 °C under aerobic conditions (for 24 h and 48 h), the number of CFU was quantified by plating on Difco Pseudomonas Isolation Agar (PIA; BD Diagnostics) and Reinforced Clostridual Medium (RCM; Oxoid). PIA plates were incubated aerobically and RCM plates anaerobically (both at 37 °C). Biofilm quantification by plating was performed as previously described, artifical sebum was placed into tubes with 10 mL PS, the sessile cells were removed by three cycles of vortexing and sonication (Branson 3510; Branson Ultrasonics Corp., Danbury, CT) and the number of CFU/biofilm was determined by plating the resulting suspensions of Difco Pseudomonas Isolation Agar (PIA; BD Diagnostics).
Animal acquisition and preparation
Galleria mellonella were obtained from LiveFood UK Ltd. (Somerset, United Kingdom). Only 6th instar larvae were used for experiments, which is the life stage at which they do not require feeding and weigh approximately 200 mg. Prior to use, larvae were stored at 4 °C. Before the experiment, larvae were sorted into Petri dishes lined with filter paper (Whatman, Fisher, United Kingdom). 10 caterpillars per dish were allowed and then stored at 4 °C until use.
In vivo burn infection and treatment assay
70% ethanol was used to sterilise the larval body surface. Petri dishes were left open in a sterile environment to allow for the ethanol to evaporate after sterilisation. The burn was induced by using a heated steel element embedded in insulating material to consistently achieve a burn area of approximately 2 mm2. The burn instrument was heated in the middle flame of the Bunsen burner until it was red/white-hot and applied to the middle segment of G. mellonella back for 4 s. The burn wound was colonised with C. acnes, by picking up a few colonies from the blood agar plate with a pipette tip and gently brushing it against the burn wound surface. Immediately afterwards, 10 µl of overnight culture of PA14 grown to ~OD600 = 3 was applied topically to the wound. Any larva that showed a major haemolymph loss or protruding insides after the procedure was immediately euthanized by placing it at −20 °C for at least 20 mins to minimise suffering. Post-procedure the invertebrates were incubated at 37 °C for 72 h to monitor. Mortality was recorded upon the complete loss of motility and melanisation of the larval body41.
For glucose treatment experiments, 1 h post P. aeruginosa PA14 inoculation into the wound, wounds were treated with 10 µl of either 1% glucose solution or PBS. Any larva that showed a major haemolymph loss or protruding insides after the procedure was immediately euthanized by placing it at −20 °C for at least 20 mins to minimise suffering. Post-procedure the invertebrates were incubated at 37 °C for 72 h to monitor. Mortality was recorded upon the complete loss of motility and melanisation of the larval body41.
RNA extraction and sequencing
P. aeruginosa PA14 was grown in the overnight tube at 37 °C at 180 rpm. Cultures were then adjusted to OD = 0.1 with LB and added in proportion 1:1 to TSB or the supernatant of C. acnes CCUG 38584 to be grown to OD600 = 0.6–0.7 at 37 °C at 180 rpm. Upon reaching the desired optical density, 1 ml of the cultures was aliquoted and spun down at 5000 rpm for 10 mins, the supernatant was discarded, and the pellets were resuspended in the RNAlater buffer (ThermoFischer). The resuspended cells were stored in the buffer at 4 °C overnight. RNA extraction procedure was performed with RNeasy Mini Kit (Qiagen). Extracted RNA was quantified using Nanodrop. The quality of the extraction was assessed with Bioanalyser. Extracted RNA samples were stored at −20 °C until shipment to the sequencing facility. The cDNA synthesis and Illumina sequencing were performed by Microbial Genome Sequencing Centre (MiGS). Samples were DNase treated with Invitrogen DNase (RNase free). Library preparation was performed using Illumina’s Stranded Total RNA Prep Ligation with Ribo-Zero Plus kit and IDT for Illumina indices. Sequencing was performed on a NextSeq2000 giving 2x50bp reads.
Raw fastq files were obtained from the sequencing facility. Adapter and quality trimming were performed by MiGS using bcl2fastq. Reads and their quality was assessed with fastqc and visualised through multiqc90,91. Read mapping was performed with hisat2 with ‘--very-sensitive’ parameter92. Read quantification was performed with featureCounts available within the Subread package93. Read normalisation was performed using the edgeR package in R with the Trimmed Mean of M values algorithm94. Differential expression analysis was performed using edgeR’s Quasi-Linear F-Test (qlfTest) functionality against treatment groups. All quantified genes were subset by log-fold change (logFC) > |1| and p-value < 0.05 to create a list of differentially expressed genes. All quantified genes were visualised in a volcano plot in ggplot2 R package. An additional table demonstrating the subset of genes with (logFC) > |2| and p-value < 0.05 can be found in Supplementary Table 3. The differentially expressed genes were additionally analysed with KEGG pathway analysis (FUNAGE-Pro) and the results were made available in the supplementary materials (Supplementary Tables 2.1, 2.2, Supplementary Figs. 2.1, 2.2).
Glucose content quantification of C. acnes supernatants
C. acnes strains were grown in 15 ml of tryptic soy broth (TSB, NutriSelect Plus) in a 50 ml Falcon tube for 72 h at 37 °C. C. acnes were grown anaerobically in a hermetic chamber with an AnaeroGen™ 2.5 L Sachet (Thermo Scientific™ Oxoid™). Every 12 h 1 ml of the culture was pipetted out into an Eppendorf tube and frozen at −20 °C. At 0 h 1 ml of TSB was aliquoted and frozen as well. Once the 72 h sample was collected, the glucose content was measured with Glucose Colorimetric kit (Thermo-Fischer Scientific) in accordance with the protocol provided in the kit. Samples with the highest OD at 24 h were removed from the pool.
Statistical analysis
For survival curves, the Log Rank statistical test was used to determine the significance of the findings and the values were corrected for multiple comparisons using the Bonferroni correction. Unpaired Student’s t test was performed on glucose content and biofilm formation data. Two-way ANOVA was performed for the growth curve statistical significance assessment. For the gene differential expression p values were generated by using the Quasi-linear F-test.
Supplementary information
Acknowledgements
R.R.M.C. is supported by the British Society for Antimicrobial Chemotherapy BSAC-2018-0095. R.R.M.C. and E.M. are supported by the NC3Rs PhD Studentship NC/V001582/1. R.R.M.C. is supported by a Biotechnology and Biological Sciences Research Council New Investigator Award BB/V007823/1 and Medical Research Council Grant MR/Y001354/1. R.R.M.C. is also supported by the Academy of Medical Sciences/the Wellcome Trust/the Government Department of Business, Energy and Industrial Strategy/the British Heart Foundation/Diabetes UK Springboard Award [SBF006\1040].
Author contributions
E.M., L.E. and P.R. executed all experiments. E.M., L.E. and P.R., T.C. and R.R.M.C. wrote the manuscript. RRMC and TC conceived the study and assisted in data processing.
Data availability
The RNA-seq datasets produced in this study are available at the National Center for Biotechnology Information Gene Expression Omnibus public database under accession number GSE236405.
Competing interests
The authors declare no non-financial competing interests but Brunel University London has filed a priority patent and PCT covering the therapeutic use of C. acnes (GB2214521.3).
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41522-024-00518-4.
References
- 1.Esmail, M. A. M., Abdulghany H. M. & Khairy R. M. Prevalence of multidrug-resistant enterococcus faecalis in hospital-acquired surgical wound infections and bacteremia: concomitant analysis of antimicrobial resistance genes. infectious diseases: research and treatment. Infect. Dis.12, 1178633719882929 (2019). [DOI] [PMC free article] [PubMed]
- 2.Qureshi, M. A., Khatoon, F. & Ahmed, S. (2015) An overview on wounds their issues and natural remedies for wound healing. Biochem. Physiol.4, 165 (2015).
- 3.Nussbaum SR, et al. An economic evaluation of the impact, cost, and medicare policy implications of chronic nonhealing wounds. Value Health. 2018;21:27–32. doi: 10.1016/j.jval.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 4.Guest JF, et al. Health economic burden that wounds impose on the National Health Service in the UK. BMJ Open. 2015;5:e009283. doi: 10.1136/bmjopen-2015-009283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phillips CJ, et al. Estimating the costs associated with the management of patients with chronic wounds using linked routine data. Int. Wound J. 2016;13:1193–1197. doi: 10.1111/iwj.12443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pfalzgraff A, Brandenburg K, Weindl G. Antimicrobial peptides and their therapeutic potential for bacterial skin infections and wounds. Front. Pharmacol. 2018;9:281. doi: 10.3389/fphar.2018.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Azzopardi EA, Azzopardi SM, Boyce DE, Dickson WA. Emerging gram-negative infections in burn wounds. J. Burn Care Res. 2011;32:570–576. doi: 10.1097/BCR.0b013e31822ac7e6. [DOI] [PubMed] [Google Scholar]
- 8.Amissah NA, et al. Methicillin resistant Staphylococcus aureus transmission in a ghanaian burn unit: the importance of active surveillance in resource-limited settings. Front. Microbiol. 2017;8:1906. doi: 10.3389/fmicb.2017.01906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lambert M, et al. Clinical outcomes of health-care-associated infections and antimicrobial resistance in patients admitted to European intensive-care units: a cohort study. Lancet Infect. Dis. 2011;11:30–38. doi: 10.1016/S1473-3099(10)70258-9. [DOI] [PubMed] [Google Scholar]
- 10.Serra R, et al. Chronic wound infections: The role of Pseudomonas aeruginosa and Staphylococcus aureus. Expert Rev. Anti-infective Ther. 2015;13:605–613. doi: 10.1586/14787210.2015.1023291. [DOI] [PubMed] [Google Scholar]
- 11.Haghi F, et al. Diversity of virulence genes in multidrug resistant Pseudomonas aeruginosa isolated from burn wound infections. Microb. Pathogenesis. 2018;115:251–256. doi: 10.1016/j.micpath.2017.12.052. [DOI] [PubMed] [Google Scholar]
- 12.Egert, M., Simmering, R. (2016). The Microbiota of the Human Skin. In: Schwiertz, A. (eds) Microbiota of the Human Body. Advances in Experimental Medicine and Biology, vol 902. Springer, Cham. 10.1007/978-3-319-31248-4_5 [DOI] [PubMed]
- 13.Tacconelli E, et al. ‘Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis’. Lancet Infect. Dis. 2018;18:318–327. doi: 10.1016/S1473-3099(17)30753-3. [DOI] [PubMed] [Google Scholar]
- 14.Chehoud C, et al. ‘Complement modulates the cutaneous microbiome and inflammatory milieu’. Proc. Natl Acad. Sci. 2013;110:15061–15066. doi: 10.1073/pnas.1307855110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grice EA. ‘The skin microbiome: potential for novel diagnostic and therapeutic approaches to cutaneous disease’. Semin. Cutan. Med. Surg. 2014;33:98. doi: 10.12788/j.sder.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reid G, et al. ‘Microbiota restoration: natural and supplemented recovery of human microbial communities’. Nat. Rev. Microbiol. 2010;9:27–38. doi: 10.1038/nrmicro2473. [DOI] [PubMed] [Google Scholar]
- 17.Gao Z, Tseng C, Pei Z, Blaser MJ. Molecular analysis of human forearm superficial skin bacterial biota. Proc. Natl Acad. Sci. 2007;104:2927–2932. doi: 10.1073/pnas.0607077104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plichta JK, et al. Cutaneous burn injury promotes shifts in the bacterial microbiome in autologous donor skin: implications for skin grafting outcomes. Shock. 2017;48:441. doi: 10.1097/SHK.0000000000000874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cogen AL, Nizet V, Gallo RL. Skin microbiota: a source of disease or defence? Br. J. Dermatol. 2008;158:442–455. doi: 10.1111/j.1365-2133.2008.08437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Costello EK, et al. Bacterial community variation in human body habitats across space and time. Science. 2009;326:1694–1697. doi: 10.1126/science.1177486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ladhani HA, Yowler CJ, Claridge JA. Burn wound colonization, infection, and sepsis. Surg. Infect. 2021;22:44–48. doi: 10.1089/sur.2020.346. [DOI] [PubMed] [Google Scholar]
- 22.Pastar I, et al. Interactions of methicillin resistant Staphylococcus aureus USA300 and Pseudomonas aeruginosa in polymicrobial wound infection. PLoS ONE. 2013;8:e56846. doi: 10.1371/journal.pone.0056846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomic-Canic M, et al. Skin Microbiota and its Interplay with Wound Healing. Am. J. Clin. Dermatol. 2020;21:36–43. doi: 10.1007/s40257-020-00536-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szabó K, et al. Factors shaping the composition of the cutaneous microbiota. Br. J. Dermatol. 2017;176:344–351. doi: 10.1111/bjd.14967. [DOI] [PubMed] [Google Scholar]
- 25.Prince T, Mcbain AJ, O’neill CA. Lactobacillus reuteri protects epidermal keratinocytes from staphylococcus aureus-induced cell death by competitive exclusion. Appl. Environ. Microbiol. 2012;78:5119–5126. doi: 10.1128/AEM.00595-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang M, et al. Oral antibiotic treatment induces skin microbiota dysbiosis and influences wound healing. Microb. Ecol. 2015;69:415–421. doi: 10.1007/s00248-014-0504-4. [DOI] [PubMed] [Google Scholar]
- 27.Kunimitsu M, Nakagami G, Minematsu T, Koudounas S, Sanada H. An in vivo critically colonised wound model with dysbiotic wound microbiota. Int. wound J. 2023;20:648–658. doi: 10.1111/iwj.13906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dréno B, et al. Cutibacterium acnes (Propionibacterium acnes) and acne vulgaris: a brief look at the latest updates. J. Eur. Acad. Dermatol. Venereol. 2018;32:5–14. doi: 10.1111/jdv.15043. [DOI] [PubMed] [Google Scholar]
- 29.Byrd AL, Belkaid Y, Segre JA. The human skin microbiome. Nat. Rev. Microbiol. 2018;16:143–155. doi: 10.1038/nrmicro.2017.157. [DOI] [PubMed] [Google Scholar]
- 30.Dagnelie M, et al. Cutibacterium acnes phylotypes diversity loss: a trigger for skin inflammatory process. J. Eur. Acad. Dermatol. Venereol. 2019;33:2340–2348. doi: 10.1111/jdv.15795. [DOI] [PubMed] [Google Scholar]
- 31.O’Neill, A. M., et al. Genetic and functional analyses of cutibacterium acnes isolates reveal the association of a linear plasmid with skin inflammation. J. Invest. Dermatol.144, 116-124.e4 (2024). [DOI] [PMC free article] [PubMed]
- 32.McDowell A, et al. An expanded multilocus sequence typing scheme for propionibacterium acnes: investigation of ‘pathogenic’, ‘commensal’ and antibiotic resistant strains. PLoS ONE. 2012;7:e41480. doi: 10.1371/journal.pone.0041480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barnard E, Nagy I, Hunyadkürti J, Patrick S, McDowell A. Multiplex touchdown PCR for rapid typing of the opportunistic pathogen Propionibacterium acnes. J. Clin. Microbiol. 2015;53:1149–1155. doi: 10.1128/JCM.02460-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakamura K, et al. Short chain fatty acids produced by Cutibacterium acnes inhibit biofilm formation by Staphylococcus epidermidis. Sci. Rep. 2020;10:21237. doi: 10.1038/s41598-020-77790-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang TY, Herr DR, Huang C, Jiang Y. Amplification of probiotic bacteria in the skin microbiome to combat Staphylococcus aureus infection. Microbiol. Aust. 2020;41:61–64. doi: 10.1071/MA20018. [DOI] [Google Scholar]
- 36.Abbott C, Grout E, Morris T, Brown HL. Cutibacterium acnes biofilm forming clinical isolates modify the formation and structure of Staphylococcus aureus biofilms, increasing their susceptibility to antibiotics. Anaerobe. 2022;76:102580. doi: 10.1016/j.anaerobe.2022.102580. [DOI] [PubMed] [Google Scholar]
- 37.Claesen J, et al. A cutibacterium acnes antibiotic modulates human skin microbiota composition in hair follicles. Sci. Transl. Med. 2020;12:eaay5445. doi: 10.1126/scitranslmed.aay5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shu M, et al. Fermentation of Propionibacterium acnes, a commensal bacterium in the human skin microbiome, as skin probiotics against methicillin-resistant Staphylococcus aureus. PLoS ONE. 2013;8:e55380. doi: 10.1371/journal.pone.0055380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y, et al. Propionic acid and its esterified derivative suppress the growth of methicillin-resistant Staphylococcus aureus USA300. Beneficial Microbes. 2014;5:161. doi: 10.3920/BM2013.0031. [DOI] [PubMed] [Google Scholar]
- 40.Fournière M, Latire T, Souak D, Feuilloley MGJ, Bedoux G. Staphylococcus epidermidis and cutibacterium acnes: two major sentinels of skin microbiota and the influence of cosmetics. Microorg. (Basel) 2020;8:1–31. doi: 10.3390/microorganisms8111752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maslova, E. et al. An invertebrate burn wound model that recapitulates the hallmarks of burn trauma and infection seen in mammalian models. Front. Microbiol.11, 998 (2020). [DOI] [PMC free article] [PubMed]
- 42.Maslova E, Osman S, McCarthy RR. Using the Galleria mellonella burn wound and infection model to identify and characterize potential wound probiotics. Microbiology (Reading) 2023;169:001350. doi: 10.1099/mic.0.001350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Santos AL, et al. Light-activated molecular machines are fast-acting broad-spectrum antibacterials that target the membrane. Sci. Adv. 2022;8:eabm2055. doi: 10.1126/sciadv.abm2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blasio, S. D. et al. Bolaamphiphile analogues of 12-bis-THA Cl2 are potent antimicrobial therapeutics with distinct mechanisms of action against bacterial, mycobacterial, and fungal pathogens. mSphere8, e0050822 (2023). [DOI] [PMC free article] [PubMed]
- 45.Unemo M, Friberg Ö, Enquist E, Källman J, Söderquist B. Genetic homogeneity/heterogeneity of Propionibacterium acnes isolated from patients during cardiothoracic reoperation. Anaerobe. 2007;13:121–126. doi: 10.1016/j.anaerobe.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 46.Ahle CM, Feidenhans C, Brüggemann H. Cutibacterium acnes . Trends Microbiol. 2023;31:419–420. doi: 10.1016/j.tim.2022.10.006. [DOI] [PubMed] [Google Scholar]
- 47.Horna G, Ruiz J. Type 3 secretion system of Pseudomonas aeruginosa. Microbiol. Res. 2021;246:126719. doi: 10.1016/j.micres.2021.126719. [DOI] [PubMed] [Google Scholar]
- 48.HOLDER IA, NEELY AN, FRANK DW. Type III secretion/intoxication system important in virulence of Pseudomonas aeruginosa infections in burns. Burns. 2001;27:129–130. doi: 10.1016/S0305-4179(00)00142-X. [DOI] [PubMed] [Google Scholar]
- 49.O’Callaghan J, et al. A novel host-responsive sensor mediates virulence and type III secretion during Pseudomonas aeruginosa―host cell interactions. Microbiology (Reading) 2012;158:1057–1070. doi: 10.1099/mic.0.056127-0. [DOI] [PubMed] [Google Scholar]
- 50.Rietsch A, Mekalanos JJ. Metabolic regulation of type III secretion gene expression in Pseudomonas aeruginosa. Mol. Microbiol. 2006;59:807–820. doi: 10.1111/j.1365-2958.2005.04990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rietsch A, Wolfgang MC, Mekalanos JJ. Effect of metabolic imbalance on expression of type III secretion genes in Pseudomonas aeruginosa. Infect. Immun. 2004;72:1383–1390. doi: 10.1128/IAI.72.3.1383-1390.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Adewoye LO, Worobec EA. Identification and characterization of the gltK gene encoding a membrane-associated glucose transport protein of pseudomonas aeruginosa. Gene. 2000;253:323–330. doi: 10.1016/S0378-1119(00)00285-7. [DOI] [PubMed] [Google Scholar]
- 53.Falchi FA, et al. Sanguinarine inhibits the 2-ketogluconate pathway of glucose utilization in Pseudomonas aeruginosa. Front. Microbiol. 2021;12:744458. doi: 10.3389/fmicb.2021.744458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Camus L, et al. Trophic cooperation promotes bacterial survival of Staphylococcus aureus and Pseudomonas aeruginosa. ISME J. 2020;14:3093–3105. doi: 10.1038/s41396-020-00741-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Raneri M, et al. Pseudomonas aeruginosa mutants defective in glucose uptake have pleiotropic phenotype and altered virulence in non-mammal infection models. Sci. Rep. 2018;8:16912–15. doi: 10.1038/s41598-018-35087-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tognon M, Köhler T, Luscher A, van Delden C. Transcriptional profiling of Pseudomonas aeruginosa and Staphylococcus aureus during in vitro co-culture. BMC Genomics. 2019;20:30. doi: 10.1186/s12864-018-5398-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dekio I, et al. Dissecting the taxonomic heterogeneity within Propionibacterium acnes: proposal for Propionibacterium acnes subsp. acnes subsp. nov. and Propionibacterium acnes subsp. elongatum subsp. nov. Int. J. Syst. Evolut. Microbiol. 2015;65:4776–4787. doi: 10.1099/ijsem.0.000648. [DOI] [PubMed] [Google Scholar]
- 58.Platsidaki E, Dessinioti C. Recent advances in understanding Propionibacterium acnes (Cutibacterium acnes) in acne [version 1; peer review: 2 approved] F1000 Res. 2018;7:1953. doi: 10.12688/f1000research.15659.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dolan, S. K. et al. Contextual flexibility in pseudomonas aeruginosa central carbon metabolism during growth in single carbon sources. mBio11, e02684-19 (2020). [DOI] [PMC free article] [PubMed]
- 60.She P, et al. Effects of exogenous glucose on Pseudomonas aeruginosa biofilm formation and antibiotic resistance. MicrobiologyOpen (Weinh.) 2019;8:e933. doi: 10.1002/mbo3.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Khosravi AD, Hoveizavi H, Mohammadian A, Farahani A, Jenabi A. Genotyping of multidrug-resistant strains of Pseudomonas Aeruginosa isolated from burn and wound infections by ERIC-PCR. Acta Cirurgica Brasileira. 2016;31:206–211. doi: 10.1590/S0102-865020160030000009. [DOI] [PubMed] [Google Scholar]
- 62.Gonzalez, M. R. et al. Effect of human burn wound exudate on Pseudomonas aeruginosa virulence. mSphere1, e00111-15 (2016). [DOI] [PMC free article] [PubMed]
- 63.Asokan GV, Sanad H, Ahmed E, Ramadhan T. WHO global priority pathogens list: a bibliometric analysis of medline-pubmed for knowledge mobilization to infection prevention and control practices in Bahrain. Oman Med. J. 2019;34:184–193. doi: 10.5001/omj.2019.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lukic J, et al. Probiotics or pro‐healers: the role of beneficial bacteria in tissue repair. Wound Repair Regeneration. 2017;25:912–922. doi: 10.1111/wrr.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Patel BK, Patel KH, Huang RY, Lee CN, Moochhala SM. The gut-skin microbiota axis and its role in diabetic wound healing—a review based on current literature. Int. J. Mol. Sci. 2022;23:2375. doi: 10.3390/ijms23042375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guillemard E, Tondu F, Lacoin F, Schrezenmeir J. Consumption of a fermented dairy product containing the probiotic Lactobacillus casei DN-114 001 reduces the duration of respiratory infections in the elderly in a randomised controlled trial. Br. J. Nutr. 2010;103:58–68. doi: 10.1017/S0007114509991395. [DOI] [PubMed] [Google Scholar]
- 67.Varian BJ, et al. Microbial lysate upregulates host oxytocin. Brain, Behav., Immun. 2017;61:36–49. doi: 10.1016/j.bbi.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zakostelska Z, et al. Intestinal microbiota promotes psoriasis-like skin inflammation by enhancing Th17 response. PLoS ONE. 2016;11:e0159539. doi: 10.1371/journal.pone.0159539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kirpich IA, et al. Probiotics restore bowel flora and improve liver enzymes in human alcohol-induced liver injury: a pilot study. Alcohol. 2008;42:675–682. doi: 10.1016/j.alcohol.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lamprecht M, et al. Probiotic supplementation affects markers of intestinal barrier, oxidation, and inflammation in trained men; a randomized, double-blinded, placebo-controlled trial. J. Int. Soc. Sports Nutr. 2012;9:45. doi: 10.1186/1550-2783-9-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mohseni S, et al. The beneficial effects of probiotic administration on wound healing and metabolic status in patients with diabetic foot ulcer: A randomized, double‐blind, placebo‐controlled trial. Diabetes/Metab. Res. Rev. 2018;34:e2970. doi: 10.1002/dmrr.2970. [DOI] [PubMed] [Google Scholar]
- 72.Tembhre, M. K., Chawla, M. K., Berthiaume, F. & Kumar, S. in Probiotic Research in Therapeutics (Beri, K., Deol, P. K. & Sandhu, S. K. (eds). (Springer). 10.1007/978-981-16-5628-6_8.
- 73.Campos, L. F. et al. Effects of probiotics supplementation on skin wound healing in diabetic rats. Arq. Bras. Cir. Dig.33, e1498 (2020). [DOI] [PMC free article] [PubMed]
- 74.Tarapatzi G, et al. The probiotic strains bifidοbacterium lactis, Lactobacillus acidophilus, Lactiplantibacillus plantarum and Saccharomyces boulardii regulate wound healing and chemokine responses in human intestinal subepithelial myofibroblasts. Pharmaceuticals (Basel) 2022;15:1293. doi: 10.3390/ph15101293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sugimoto S, et al. Staphylococcus epidermidis Esp degrades specific proteins associated with Staphylococcus aureus biofilm formation and host-pathogen interaction. J. Bacteriol. 2013;195:1645–1655. doi: 10.1128/JB.01672-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Satish L, Gallo PH, Johnson S, Yates CC, Kathju S. Local probiotic therapy with lactobacillus plantarum mitigates scar formation in rabbits after burn injury and infection. Surg. Infect. 2017;18:119. doi: 10.1089/sur.2016.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brachkova MI, et al. Alginate films containing Lactobacillus plantarum as wound dressing for prevention of burn infection. J. Hosp. Infect. 2011;79:375–377. doi: 10.1016/j.jhin.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 78.Stanbro J, et al. Topical delivery of lactobacillus culture supernatant increases survival and wound resolution in traumatic Acinetobacter baumannii Infections. Probiotics Antimicrobial Proteins. 2020;12:809–818. doi: 10.1007/s12602-019-09603-z. [DOI] [PubMed] [Google Scholar]
- 79.Ghanbarzadeh Corehtash Z, Khorshidi A, Firoozeh F, Akbari H, Mahmoudi Aznaveh A. Biofilm formation and virulence factors among pseudomonas aeruginosa isolated from burn patients. Jundishapur J. Microbiol. 2015;8:e22345. doi: 10.5812/jjm.22345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Seth AK, et al. Quantitative comparison and analysis of species-specific wound biofilm virulence using an in vivo, rabbit-ear model. J. Am. Coll. Surg. 2012;215:388–399. doi: 10.1016/j.jamcollsurg.2012.05.028. [DOI] [PubMed] [Google Scholar]
- 81.Wolfgang MC, Lee VT, Gilmore ME, Lory S. Coordinate regulation of bacterial virulence genes by a novel adenylate cyclase-dependent signaling pathway. Dev. Cell. 2003;4:253–263. doi: 10.1016/S1534-5807(03)00019-4. [DOI] [PubMed] [Google Scholar]
- 82.Jeckelmann J, Erni B. Transporters of glucose and other carbohydrates in bacteria. Pflügers Arch. 2020;472:1129–1153. doi: 10.1007/s00424-020-02379-0. [DOI] [PubMed] [Google Scholar]
- 83.Huang TY, Jiang YE, Scott DA. Culturable bacteria in the entire acne lesion and short-chain fatty acid metabolites of Cutibacterium acnes and Staphylococcus epidermidis isolates. Biochem. Biophys. Res. Commun. 2022;622:45–49. doi: 10.1016/j.bbrc.2022.06.068. [DOI] [PubMed] [Google Scholar]
- 84.Berlanga-Acosta J, et al. Burn injury insulin resistance and central nervous system complications: a review. Burns Open. 2020;4:41–52. doi: 10.1016/j.burnso.2020.02.001. [DOI] [Google Scholar]
- 85.Mikkelsen H, McMullan R, Filloux A. The Pseudomonas aeruginosa reference strain PA14 displays increased virulence due to a mutation in ladS. PloS ONE. 2011;6:e29113. doi: 10.1371/journal.pone.0029113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Webster GF, Cummins CS. Use of bacteriophage typing to distinguish Propionibacterium acne types I and II. J. Clin. Microbiol. 1978;7:84–90. doi: 10.1128/jcm.7.1.84-90.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Davidsson S, et al. Multilocus sequence typing and repetitive-sequence-based PCR (DiversiLab) for molecular epidemiological characterization of Propionibacterium acnes isolates of heterogeneous origin. Anaerobe. 2012;18:392–399. doi: 10.1016/j.anaerobe.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 88.McDowell A, Barnard E, Liu J, Li H, Patrick S. Proposal to reclassify Propionibacterium acnes type I as Propionibacterium acnes subsp. acnes subsp. nov. and Propionibacterium acnes type II as Propionibacterium acnes subsp. defendens subsp. nov. Int. J. Syst. Evolut. Microbiol. 2016;66:5358–5365. doi: 10.1099/ijsem.0.001521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brackman G, et al. Dressings loaded with cyclodextrin-hamamelitannin complexes increase Staphylococcus aureus susceptibility toward antibiotics both in single as well as in mixed biofilm communities. Macromol. Biosci. 2016;16:859–869. doi: 10.1002/mabi.201500437. [DOI] [PubMed] [Google Scholar]
- 90.Andrews, S. (2010). FastQC: a quality control tool for high throughput sequence data [Online]. Available online at: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/.
- 91.Ewels P, Magnusson M, Lundin S, Käller M. MultiQC: summarize analysis results for multiple tools and samples in a single report. Bioinformatics. 2016;32:3047–3048. doi: 10.1093/bioinformatics/btw354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kim D, Thawng CN, Lee K, Wellington EMH, Cha C. A novel sulfonamide resistance mechanism by two-component flavin-dependent monooxygenase system in sulfonamide-degrading actinobacteria. Environ. Int. 2019;127:206–215. doi: 10.1016/j.envint.2019.03.046. [DOI] [PubMed] [Google Scholar]
- 93.Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30:923–930. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 94.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The RNA-seq datasets produced in this study are available at the National Center for Biotechnology Information Gene Expression Omnibus public database under accession number GSE236405.