Atrial fibrillation is a common arrhythmia, especially among elderly people. Restoration of sinus rhythm by cardioversion or drug treatment improves symptoms, cardiac output, and exercise tolerance. Unfortunately, the recurrence rate in successfully treated patients is high. Research into the underlying electrophysiological mechanisms that cause atrial fibrillation has fuelled the development of new therapeutic approaches. This article describes the advances in understanding how the arrhythmia is generated and discusses the therapeutic potential of radiofrequency ablation.
Methods
We obtained information from several sources. We used Medline and BIDS to identify key papers, and we searched abstracts from the meetings of the American College of Cardiology, American Heart Association, and North American Society of Pacing and Electrophysiology between 1995 and 2000 for additional information on evolving technologies.
Atrial fibrillation
The prevalence of atrial fibrillation in the adult population is 0.5%, rising to 10% among people aged over 75 years.1 It is associated with a 5-6 fold increase in the incidence of stroke.2 A 70 year old person with atrial fibrillation thus has an annual risk of stroke or transient cerebral ischaemic attack of 5%.
Atrial fibrillation may be paroxysmal, persistent, or permanent. Paroxysmal atrial fibrillation is characterised by repeated, self terminating episodes of arrhythmia. This can progress to either persistent atrial fibrillation, in which an intervention such as direct current cardioversion may restore sinus rhythm, or permanent atrial fibrillation, which is resistant to cardioversion. Many patients with atrial fibrillation are asymptomatic, especially if their heart rate is not rapid. If symptoms do occur their severity is determined by the underlying state of the heart and the patient's heart rate at rest and during exercise.
Restoration of sinus rhythm with direct current or pharmacological cardioversion improves symptoms, cardiac output, and exercise tolerance and is initially successful in up to 90% of patients, but the rate of recurrence after a year can be as high as 60%.3,4 The risk of recurrence is determined both by mechanical factors (such as valve disease) and electrophysiological triggers (such as atrial ectopy). As a result, considerable effort has been directed at identifying the electrophysiological mechanisms that cause atrial fibrillation and at new therapeutic approaches that target these mechanisms.
Anticipated developments
Radiofrequency focal ablation will become the treatment of choice for patients with structurally normal hearts and paroxysmal atrial fibrillation
The “ablate and pace” strategy, ablation of the His bundle and implantation of a pacemaker, will be more widely used when drugs that block the atrioventricular node provide inadequate heart rate control
Linear ablation techniques will evolve but have only limited application in selected patients with permanent atrial fibrillation
Electrophysiological mechanisms that cause atrial fibrillation
Atrial fibrillation is the result of both substrate and trigger. The substrate is most often a pathology that affects the atria, such as hypertensive heart disease (associated with increased stress on the atrial wall).5 The trigger often consists of an ectopic atrial electrical focus.6 Although this focus can arise anywhere in the atria, left atrial foci at or within the pulmonary vein orifices are often responsible for episodes of atrial fibrillation in patients with otherwise normal hearts.
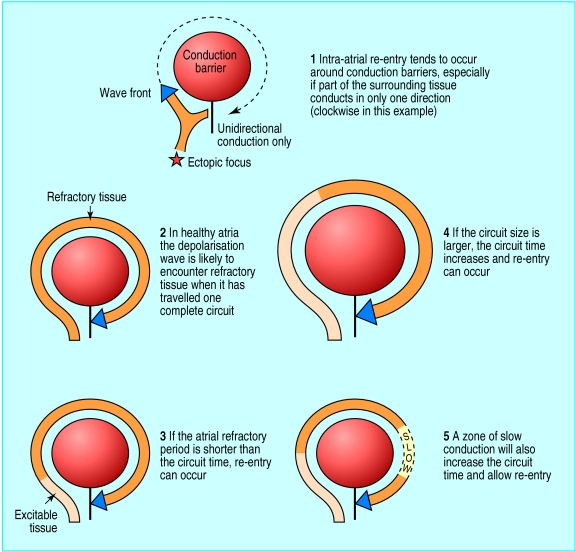
Once initiated, the arrhythmia is maintained by the mechanism of re-entry.7 Macro re-entry occurs when a continuous loop of atrial depolarisation is set up around an anatomical or functional conduction barrier (such as a vein orifice or zone of diseased atrial tissue). It occurs especially when conduction propagates in only one direction around the barrier (fig 1). During atrial fibrillation many such re-entry circuits are established in the atria. These sustain themselves more readily in diseased or enlarged atria.
Figure 1.
Electrical re-entry, the mechanism responsible for initiating and maintaining atrial fibrillation
Over time, the prospect of spontaneous return to sinus rhythm diminishes because of maladaptive changes that occur in atrial tissue. These include shortening of the refractory period of atrial myocytes, which encourages macro re-entry because myocytes are excitable for a greater proportion of each cycle (fig 1). This process is known as electrical remodelling.8
Current management of atrial fibrillation
Current management focuses on two issues—preventing stroke and treating the arrhythmia. Guidelines for preventing stroke are published elsewhere.9 Arrhythmia is managed by the sinus rhythm strategy or the rate control strategy. The sinus rhythm strategy strives to restore and maintain sinus rhythm with cardioversion or antiarrhythmic drugs, or both. Drugs such as sotalol, propafenone, and amiodarone are used to preserve sinus rhythm, but these can be proarrhythmic or may not be tolerated.
The rate control strategy accepts that atrial fibrillation is likely to persist and uses atrioventricular node blocking drugs such as digoxin or β blockers to limit the heart rate. The risk of stroke and some of the haemodynamic consequences of atrial fibrillation persist, and heart rate control can be difficult to achieve both at rest and during exercise. The rate control option tends to be used for patients with substantial structural heart disease or when the sinus rhythm strategy has failed. Despite their disadvantages, these treatments provide satisfactory symptom control for most patients with atrial fibrillation.
New non-pharmacological treatments
Atrial fibrillation can be treated with physical as well as pharmacological interventions. Internal cardioversion administered by catheter is effective in three quarters of patients who are resistant to external cardioversion, but it is still associated with a high recurrence rate.10 Atrial pacing can prevent atrial fibrillation by inhibiting ectopic activity and preventing excessive bradycardia. Trials are in progress to evaluate pacemakers that detect trends in heart rate and ectopy known to be associated with paroxysms of atrial fibrillation and that initiate single site or multisite pacing in response to these changes.11,12 Implantable atrial defibrillators detect episodes of atrial fibrillation at their onset and deliver an internal direct current shock.13
Cardioversion is administered before electrical remodelling occurs but can be unpleasant for the patient. These devices are reserved for patients with symptomatic, drug resistant, paroxysmal atrial fibrillation. For patients with persistent or permanent atrial fibrillation, surgical techniques have been developed that prevent re-entry by means of linear atrial incisions that act as electrical barriers.14–16
Radiofrequency ablation
Except for atrial surgery, none of the above treatments directly and permanently targets the trigger or substrate for atrial fibrillation. There is therefore considerable interest in recent developments in catheter ablation, which target these mechanisms and which may offer a cure for selected patients with this condition.
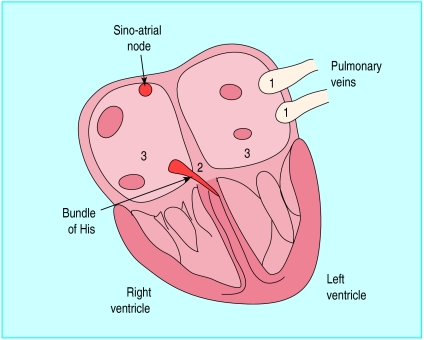
Radiofrequency ablation is the targeted cautery of cardiac tissue by local application of radiofrequency energy. Target zones are identified during an electrophysiological study, in which a series of catheters are placed in the heart (fig 2). Accessory pathways or other critical parts of arrhythmia circuits are identified by recording the cardiac electrical activation sequence. Once a target zone is identified, ablation is performed by heating the interface between catheter and endocardium until cell death occurs, at temperatures over 47°C.
Figure 2.
Target sites for different radiofrequency ablation approaches in treating atrial fibrillation: 1 pulmonary vein ectopic foci, 2 proximal bundle of His, 3 atrial walls for linear ablation
Ablation has become the treatment of choice for most patients with supraventricular tachycardia due to accessory pathways or to atrioventricular nodal re-entry, and is becoming widely used for treating atrial flutter.16 Until recently, ablation has had only a limited role in treating atrial fibrillation itself, but better understanding of the mechanisms causing such arrhythmia has stimulated the development of new ablation techniques that target both substrate and trigger. The role of ablation differs for each of the presentations of atrial fibrillation.
Paroxysmal atrial fibrillation
Some patients with self terminating paroxysms of atrial fibrillation have their arrhythmia initiated by rapid repetitive discharges from foci within one or more of the pulmonary veins or, occasionally, the superior vena cava.6,17–19 These structures contain sleeves of atrial tissue that extend a variable distance from the atria themselves. The culprit foci often lie several centimetres inside the vein, and spontaneous ectopic activity arising therein propagates into the atria, initiating and sometimes maintaining atrial fibrillation.
It is possible to locate these foci during an electrophysiological study. As they are usually in the pulmonary veins, the interatrial septum has to be punctured to allow catheters to pass into the left atrium and pulmonary veins from the right atrium. Radiofrequency ablation can be used either to destroy the culprit foci or to isolate them electrically from the atria. This technique is highly effective in the short term, but initial reports suggest high recurrence rates, possibly because of the occurrence of multiple foci in different pulmonary veins or because of inadequate isolation of culprit foci.19 The procedure may be complicated by pulmonary vein stenosis, a potentially serious complication that can lead to pulmonary hypertension and right sided cardiac failure. An early report cited a 5% incidence of pulmonary vein stenosis, although relatively high radiofrequency energies were used, often in sites deep within the veins.19
New techniques are being evaluated with the goal of achieving complete and rapid electrical isolation of the pulmonary veins from adjacent atrial tissue. It is possible that this anatomical approach will provide better results than simply ablating in the region of the foci themselves.
Persistent atrial fibrillation
Persistent atrial fibrillation usually affects patients with structural or ischaemic heart disease. Atrial disease tends to maintain atrial fibrillation once it occurs. Sinus rhythm can be restored with electrical or pharmacological cardioversion, but fibrillation tends to recur because of the persistence of triggering foci and underlying atrial disease. Focal ablation in this setting is less successful for the above reasons and possibly because of the presence of multiple triggering foci within the atria and pulmonary veins. Hybrid ablation strategies that target both the trigger, through focal ablation, and the substrate, by linear ablation and compartmentalisation (see below), are under development.20
Although restoration of sinus rhythm is the goal for these patients, it cannot always be achieved or sustained. In resistant cases ablation can be used to control heart rate in patients who develop an inappropriate tachycardia during exercise or for whom drugs are ineffective or have unacceptable side effects. The region of the compact atrioventricular node or His bundle is ablated to achieve complete atrioventricular block; a permanent pacemaker is then implanted to control heart rate.21–23 This is known as the “ablate and pace” strategy. Modern pacemakers are capable of increasing the ventricular rate appropriately in response to exercise during atrial fibrillation. In patients with paroxysmal atrial fibrillation a mode switching pacemaker is used, which, as well as controlling the heart rate during paroxysms, maintains synchrony of atrial and ventricular contraction during sinus rhythm. Regularising the ventricular rhythm during atrial fibrillation also improves symptoms and haemodynamic performance.21,22 Concerns have been expressed about an association between total atrioventricular nodal ablation and death due to bradycardia-related torsades de pointes ventricular tachycardia. This risk can be virtually eliminated with pacing at 80-90 beats/min in the month after ablation.24
Permanent atrial fibrillation
For permanent atrial fibrillation, the principal objective, other than effective anticoagulation to prevent stroke, is control of heart rate. This can usually be achieved with atrioventricular node blocking drugs. When drugs are ineffective, poorly tolerated, or cause symptomatic bradycardia, “ablate and pace” treatment is indicated. Anticoagulation is required afterwards because the atria continue to fibrillate and the risk of systemic embolism persists.
Recognition that atrial fibrillation is maintained by the presence of macro re-entry circuits, which occupy a critical amount of space in the atria, has prompted the development of treatments that prevent re-entry and thus prevent atrial fibrillation. The aim of these treatments is to create zones that block electrical conduction to prevent the propagation of these circuits. The surgical maze procedure is one such treatment, and both the rate of successful treatment and the rate of operative complication have driven the development of catheters that can produce contiguous, linear lesions that can compartmentalise the atria without the need for surgery.14 Early results indicate that linear ablation requires further refinement, but it may have a future role in the management of persistent and permanent atrial fibrillation.20
Which patients are suitable for ablation?
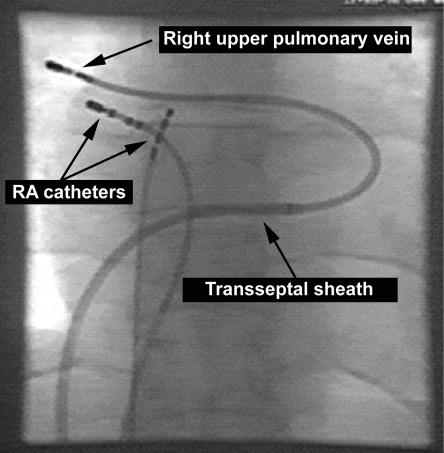
At present, radiofrequency ablation is not a first line treatment for atrial fibrillation, but it should be considered for certain patients. For patients with paroxysmal atrial fibrillation, focal ablation may be an option if antiarrhythmic drugs are ineffective or not tolerated. Patients who have frequent atrial ectopic beats with a consistent P wave morphology indicating a single ectopic focus are most suited to focal ablation (fig 3). This procedure is new and has not yet been adopted by all regional electrophysiology centres.
Figure 3.
Radiograph showing catheters positioned within the heart for focal pulmonary vein ablation. The tip of the catheter in the right upper pulmonary vein is outside the cardiac silhouette
For persistent and permanent atrial fibrillation, ablation of the His bundle and implantation of a pacemaker is a good option if satisfactory control of heart rate cannot be achieved with atrioventricular node blocking drugs, or if side effects occur. This procedure can also be used for paroxysmal atrial fibrillation in patients not suited to focal ablation. Although promising, linear ablation is still an experimental treatment and requires further evaluation before it can be recommended in clinical practice.
Footnotes
Competing interests: None declared.
References
- 1.Domanski MJ. The epidemiology of atrial fibrillation. Coron Artery Dis. 1995;6:95. doi: 10.1097/00019501-199502000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Wolf PA, Dawber TR, Emerson T, Jr, Kannel WB. Epidemiologic assessment of chronic atrial fibrillation and risk of stroke: the Framingham study. Neurology. 1978;28:973–977. doi: 10.1212/wnl.28.10.973. [DOI] [PubMed] [Google Scholar]
- 3.Arnold AZ, Mick MJ, Mazurek RP, Loop FD, Trohman RG. Role of prophylactic anticoagulation for direct current cardioversion in patients with atrial fibrillation or atrial flutter. J Am Coll Cardiol. 1992;19:851–855. doi: 10.1016/0735-1097(92)90530-z. [DOI] [PubMed] [Google Scholar]
- 4.Tieleman RG, Van Gelder IC, Crijins HJGM, de Kam PJ, Van den Berg MP, Haaksma J, et al. Early recurrence of atrial fibrillation after electrical cardioversion: a result of fibrillation-induced electrical remodelling of the atria? J Am Coll Cardiol. 1998;31:167–173. doi: 10.1016/s0735-1097(97)00455-5. [DOI] [PubMed] [Google Scholar]
- 5.Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population based cohort. The Framingham study. JAMA. 1994;271:840–844. [PubMed] [Google Scholar]
- 6.Haïssaguerre M, Jaïs P, Shah DP, Takahashi A, Hocini M, Quiniou G, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 7.Moe GK. On the multiple wavelet hypothesis of atrial fibrillation. Arch Int Pharmacodyn. 1962;140:183–188. [Google Scholar]
- 8.Goette A, Honeycutt C, Langberg JJ. Electrical remodelling in atrial fibrillation: time course and mechanisms. Circulation. 1996;94:2968–2974. doi: 10.1161/01.cir.94.11.2968. [DOI] [PubMed] [Google Scholar]
- 9.Scottish Intercollegiate Guidelines Network. Antithrombotic therapy. A national clinical guideline. Edinburgh: SIGN; 1999. [Google Scholar]
- 10.Coumel P, Frioucourt P, Mugica J, Attuel P. Long-term prevention of vagal arrhythmias by atrial pacing at 90 beats per minute. PACE Pacing Clin Electrophysiol. 1983;6:552–560. doi: 10.1111/j.1540-8159.1983.tb05295.x. [DOI] [PubMed] [Google Scholar]
- 11.Delfaut P, Prakash A, Giorgberidze I. The role of pacemaker therapy in the prevention of atrial fibrillation. Semin Intervent Cardiol. 1997;2:219–225. [PubMed] [Google Scholar]
- 12.Saksena S, Prakash A, Mangeon L, Varanasi S, Kolettis T, Mathew P, et al. Clinical efficacy and safety of atrial defibrillation using biphasic shocks and current non-thoracotomy endocardial lead configurations. Am J Cardiol. 1995;76:913–921. doi: 10.1016/s0002-9149(99)80261-6. [DOI] [PubMed] [Google Scholar]
- 13.Cox JL, Boineau JP, Schuessler RB, Kater KM, Lappas DG. Five year experience with the maze procedure for atrial fibrillation. Ann Thorac Surg. 1993;56:814–824. doi: 10.1016/0003-4975(93)90338-i. [DOI] [PubMed] [Google Scholar]
- 14.Cox JL, Ad N, Palazzo T. Impact of the maze procedure on the stroke rate in patients with atrial fibrillation. J Thorac Cardiovasc Surg. 1999;118:833–840. doi: 10.1016/s0022-5223(99)70052-8. [DOI] [PubMed] [Google Scholar]
- 15.Cosio FG, Lopez-Gil M, Goicolea A, Arribas F, Barroso JL. Radiofrequency ablation of the inferior vena cava-tricuspid valve isthmus in common atrial flutter. Am J Cardiol. 1993;71:705–709. doi: 10.1016/0002-9149(93)91014-9. [DOI] [PubMed] [Google Scholar]
- 16.Nabar A, Rodriguez ML, Timmermans C, Van den Dool A, Smeets JL, Wellens HJ. Effect of right atrial isthmus ablation in the occurrence of atrial fibrillation: observations in four patient groups having type 2 atrial flutter with or without associated atrial fibrillation. Circulation. 1999;99:1441–1445. doi: 10.1161/01.cir.99.11.1441. [DOI] [PubMed] [Google Scholar]
- 17.Tsai CF, Tai CT, Hseih MH, Lin WS, Yu WC, Ueng KC, et al. Initiation of atrial fibrillation by ectopic beats originating from the superior vena cava. Electrophysiological characteristics and results of radiofrequency ablation. Circulation. 2000;102:67–74. doi: 10.1161/01.cir.102.1.67. [DOI] [PubMed] [Google Scholar]
- 18.Tsai CF, Chen SA, Tai CT, Chiou CW, Prakash VS, Yu WC, et al. Bezold-Jarisch-like reflex during radiofrequency ablation of the pulmonary vein tissues in patients with paroxysmal focal atrial fibrillation. J Cardiovasc Electrophysiol. 1999;10:27–35. doi: 10.1111/j.1540-8167.1999.tb00638.x. [DOI] [PubMed] [Google Scholar]
- 19.Robbins IM, Colvin EV, Doyle TP, Kemp EW, Loyd JE, McMahon WS, et al. Pulmonary vein stenosis after catheter ablation of atrial fibrillation. Circulation. 1998;98:1769–1775. doi: 10.1161/01.cir.98.17.1769. [DOI] [PubMed] [Google Scholar]
- 20.Maloney JD, Milner L, Barold S, Czerska B, Markel M. Two-staged biatrial linear and focal ablation to restore sinus rhythm in patients with refractory chronic atrial fibrillation: procedure experience and follow-up beyond 1 year. PACE Pacing Clin Electrophysiol. 1998;21:2527–2532. doi: 10.1111/j.1540-8159.1998.tb01213.x. [DOI] [PubMed] [Google Scholar]
- 21.Fitzpatrick AS, Kourouyan HD, Siu A, Lee R, Lesh MD, Epstein LM, et al. Quality of life and outcomes after radiofrequency His bundle catheter ablation and permanent pacemaker implantation: impact of treatment in paroxysmal and established atrial fibrillation. Am Heart J. 1996;131:499–507. doi: 10.1016/s0002-8703(96)90528-1. [DOI] [PubMed] [Google Scholar]
- 22.Natale A, Zimerman L, Tomassni G, Kearney M, Kent V, Brandon MJ, et al. Impact on ventricular function and quality of life of transcatheter ablation of the atrioventricular junction in chronic atrial fibrillation with a normal ventricular response. Am J Cardiol. 1994;74:242–246. doi: 10.1016/s0002-9149(97)89296-x. [DOI] [PubMed] [Google Scholar]
- 23.Haïssaguerre M, Jaïs P, Shah DC, Gencel L, Pradeau V, Garrigues S, et al. Right and left atrial radiofrequency catheter therapy of paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 1996;7:1132–1144. doi: 10.1111/j.1540-8167.1996.tb00492.x. [DOI] [PubMed] [Google Scholar]
- 24.Wood MA, Brown-Mahoney C, Kay N, Ellenbogen KA. Clinical outcomes after ablation and pacing therapy for atrial fibrillation: a meta-analysis. Circulation. 2000;101:1138–1144. doi: 10.1161/01.cir.101.10.1138. [DOI] [PubMed] [Google Scholar]