Abstract
Background
Nonsteroidal anti‐inflammatory drugs (NSAIDs) are used to reduce inflammatory pain and swelling in inflammatory bowel disease (IBD) patients with rheumatological manifestations. While these drugs effectively reduce musculoskeletal pain and stiffness, long‐term use is limited by gastrointestinal (GI) adverse effects (AEs) and disease exacerbation. As an alternative to NSAIDs, selective cyclooxygenase 2 (COX‐2) inhibitors were developed to improve GI safety and tolerability. COX‐2 inhibitors include drugs such as celecoxib, rofecoxib, valdecoxib, etoricoxib, and lumiracoxib. Rofecoxib and valdecoxib have been withdrawn from the market worldwide due to safety concerns (most importantly for cardiovascular adverse events) and lumiracoxib has been withdrawn in many countries due to liver toxicity. However, celecoxib and etoricoxib continue to be available for use in many countries. Several studies have examined whether COX‐2 inhibitors can be safely used for the treatment of rheumatological manifestations of IBD with inconsistent results. Some investigators report acceptable safety profiles associated with these drugs while others found that COX‐2 inhibitors are associated with high rates of disease exacerbation.
Objectives
The objective of this systematic review was to evaluate the tolerability and safety of COX‐2 inhibitors used for the treatment of rheumatological manifestations of IBD.
Search methods
We searched the following databases from inception to 19 September 2013: PubMed, EMBASE, MEDLINE and CENTRAL. The search was not limited by language. Additional trials were identified by manually searching the reference lists of relevant papers and conference proceedings and through correspondence with experts and pharmaceutical companies.
Selection criteria
Randomized controlled trials (RCTs) that compared COX‐2 inhibitors to placebo were considered for inclusion. Participants were adult patients with IBD presenting with rheumatological manifestations of at least two weeks duration.
Data collection and analysis
Two authors independently assessed trial eligibility and extracted data. Methodological quality was assessed using the Cochrane risk of bias tool. The primary outcome measure was the proportion of patients with disease exacerbation as defined by the included studies. Secondary outcomes included GI adverse effects, renal toxicity, cardiovascular and thrombotic events. Data were analysed on an intention‐to‐treat basis where patients with missing final outcomes were assumed to have had an exacerbation of IBD. We calculated the risk ratio (RR) and corresponding 95% confidence interval (95% CI) for dichotomous outcomes. The overall quality of the evidence was assessed using the GRADE criteria.
Main results
There were no RCTs that assessed the tolerability or safety of the withdrawn COX‐2 inhibitors rofecoxib, valdecoxib, or lumiracoxib. Two RCTs (n = 381 IBD patients with rheumatological manifestations) were included in the review. One study (n = 159) compared etoricoxib (60 to 120 mg/day) to placebo in IBD patients with quiescent or active ulcerative colitis or Crohn's disease. The other study (n = 222) compared celecoxib (200 mg twice daily) to placebo in patients with quiescent ulcerative colitis. Both studies were judged to be at low risk of bias. The two included studies were not pooled for meta‐analysis due to differences in patient populations and treatment duration. There was no statistically significant difference in exacerbation of IBD between etoricoxib and placebo. After 12 weeks of treatment the IBD exacerbation rate was 17% (14/82) in the etoricoxib group compared to 19% (15/77) in the placebo group (RR 0.88, 95% CI 0.45 to 1.69). A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was low due to very sparse data (29 events). There was no statistically significant difference in exacerbation of ulcerative colitis between celecoxib and placebo. After two weeks of treatment 4% (5/112) of celecoxib patients experienced an exacerbation of ulcerative colitis compared to 6% (7/110) of patients in the placebo group (RR 0.70, 95% CI 0.23 to 2.14). A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was low due to very sparse data (12 events). The study comparing etoricoxib to placebo documented but did not report on AEs. The proportion of patients who experienced AEs was similar in the celecoxib and placebo groups (21% and 17%, respectively, P > 0.20). No patients in either group died or experienced serious adverse events. Eleven percent of patients in the celecoxib and placebo groups experienced GI AEs (RR 0.97, 95% CI 0.46 to 2.07). A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was low due to very sparse data (24 events). GI AEs led to premature withdrawal from the study in 3% of patients in celecoxib and placebo groups respectively. GI AEs included increased stool frequency, rectal bleeding, and inflamed mucosa. No patients experienced any cardiovascular adverse events. Renal toxicity or thrombotic AEs were not reported.
Authors' conclusions
The results for disease exacerbation and AEs between the COX‐2 inhibitors celecoxib and etoricoxib and placebo were uncertain. Thus no definitive conclusions regarding the tolerability and safety of the short term use of celecoxib and etoricoxib in patients with IBD can be drawn. The two included studies suggest that celecoxib and etoricoxib do not exacerbate IBD symptoms. However, it should be noted that both studies had relatively small sample sizes and short follow‐up durations. Clinicians need to continue to weigh the risks and benefits of these drugs when treating patients IBD patients with rheumatological manifestations in order to avoid disease exacerbation and other adverse effects. Further RCTs are needed to determine the tolerability and safety of celecoxib and etoricoxib in these patients.
Keywords: Humans; Anti‐Inflammatory Agents, Non‐Steroidal; Anti‐Inflammatory Agents, Non‐Steroidal/adverse effects; Celecoxib; Colitis, Ulcerative; Colitis, Ulcerative/complications; Colitis, Ulcerative/drug therapy; Crohn Disease; Crohn Disease/complications; Crohn Disease/drug therapy; Cyclooxygenase 2 Inhibitors; Cyclooxygenase 2 Inhibitors/adverse effects; Diclofenac; Diclofenac/adverse effects; Diclofenac/analogs & derivatives; Etoricoxib; Isoxazoles; Isoxazoles/adverse effects; Lactones; Lactones/adverse effects; Pyrazoles; Pyrazoles/adverse effects; Pyridines; Pyridines/adverse effects; Randomized Controlled Trials as Topic; Safety‐Based Drug Withdrawals; Sulfonamides; Sulfonamides/adverse effects; Sulfones; Sulfones/adverse effects
Plain language summary
Tolerability of selective cyclooxygenase 2 (COX‐2) inhibitors used for the treatment of rheumatological manifestations of inflammatory bowel disease
Nonsteroidal anti‐inflammatory drugs (NSAIDs) are used to reduce inflammatory pain and swelling in inflammatory bowel disease (IBD) patients with rheumatological manifestations (e.g. arthritis). While these drugs effectively reduce musculoskeletal pain and stiffness, long‐term use is limited by gastrointestinal (GI) side effects and disease exacerbation (i.e. an increase in the severity of a disease or its signs and symptoms). As an alternative to NSAIDs, selective cyclooxygenase 2 (COX‐2) inhibitors were developed to improve tolerability (i.e. the degree to which the side effects of a drug can be tolerated by a patient). COX‐2 inhibitors include drugs such as celecoxib, rofecoxib, valdecoxib, etoricoxib, and lumiracoxib. Rofecoxib and valdecoxib have been withdrawn from the market worldwide due to safety concerns (most importantly an increased risk of heart attack or stroke) and lumiracoxib has been withdrawn in many countries due to liver toxicity. However, celecoxib and etoricoxib are available for use in many countries. The purpose of this systematic review was to examine the tolerability and safety of COX‐2 inhibitors used for the treatment of rheumatological manifestations of IBD. Safety refers to whether the drug causes any harm and is typically assessed by the number and type of side effects caused by the drug.
This review does not include any studies that assessed the tolerability and safety of the withdrawn COX‐2 inhibitors rofecoxib, valdecoxib, or lumiracoxib.This review identified two studies that included a total of 381 participants with IBD who were experiencing rheumatological manifestations. One study (159 participants) compared etoricoxib (60 to 120 mg/day) to placebo (e.g. a sugar pill) in people with IBD (ulcerative colitis or Crohn's disease) who were in remission (i.e. no disease symptoms) or had active disease (i.e. had symptoms). The other study (222 participants) compared 2 weeks of treatment with celecoxib (200 mg twice daily) to placebo in people with ulcerative colitis who were in remission. The study that compared etoricoxib to placebo found no clear evidence of a difference in the proportion of patients who experienced exacerbation of IBD after 12 weeks of treatment. Although this study documented side effects experienced by the participants these side effects were not reported in the study manuscript. The study that compared celecoxib to placebo found no clear evidence of a difference in the proportion of patients who experienced exacerbation of ulcerative colitis after two weeks of treatment. The proportion of patients who experienced side effects was similar in the celecoxib and placebo groups (21% and 17%, respectively). No patients in either group died or experienced serious side effects. Eleven percent of patients in the celecoxib and placebo groups experienced GI side effects including increased stool frequency, rectal bleeding, and inflamed mucosa. No patients experienced any cardiovascular side effects (i.e. heart attack or stroke). Renal toxicity (i.e. the poisonous effect of a substance on the kidneys) or thrombotic side effects (i.e. the formation of a blood clot inside a blood vessel) were not reported. The results of the two included studies in this review suggest that celecoxib and etoricoxib do not exacerbate IBD symptoms. However, it should be noted that both studies assessed relatively small numbers of patients and were of short duration. Futhermore, the overall quality of the evidence from the studies was rated as low due to lack of precision of the results. No firm conclusions on the tolerability and safety of celecoxib and etoricoxib can be drawn from these studies. Further studies are needed to determine the tolerability and safety of celecoxib and etoricoxib in patients with rheumatological manifestations of inflammatory bowel disease.
Summary of findings
Summary of findings for the main comparison. Etoricoxib versus placebo for treatment of rheumatological manifestations of inflammatory bowel disease.
| Etoricoxib versus placebo for treatment of rheumatological manifestations of IBD | ||||||
| Patient or population: patients with active or quiescent IBD Settings: outpatient Intervention: Etoricoxib versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Etoricoxib versus placebo | |||||
| Exacerbation of IBD | 195 per 10001 | 172 per 1000 (88 to 330) | RR 0.88 (0.45 to 1.69) | 159 (1 study) | ⊕⊕⊝⊝ low2 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; IBD: Inflammatory bowel disease | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Control group risk estimates come from control arm of meta‐analysis, based on included trials 2 Downgraded two levels due to very sparse data (29 events)
Summary of findings 2. Celecoxib versus placebo for treatment of rheumatological manifestations of ulcerative colitis.
| Celecoxib versus placebo for treatment of rheumatological manifestations of ulcerative colitis | ||||||
| Patient or population: patients with quiescent ulcerative colitis Settings: outpatient Intervention: Celecoxib versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Celecoxib versus placebo | |||||
| Exacerbation of ulcerative colitis | 64 per 10001 | 45 per 1000 (15 to 137) | RR 0.70 (0.23 to 2.14) | 222 (1 study) | ⊕⊕⊝⊝ low2 | |
| GI adverse events | 110 per 10001 | 107 per 1000 (51 to 228) | RR 0.97 (0.46 to 2.07) | 221 (1 study) | ⊕⊕⊝⊝ low3 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Control group risk estimates come from control arm of meta‐analysis, based on included trials 2 Downgraded two levels due to very sparse data (12 events) 3 Downgraded two levels due to very sparse data (24 events)
Background
Description of the condition
Ulcerative colitis (UC) and Crohn's disease (CD), the two primary forms of inflammatory bowel disease (IBD), are chronic, relapsing conditions of the intestinal tract that cause abdominal pain, diarrhea and rectal bleeding. More than 3.5 million people in the United States and Europe have been diagnosed with IBD (Andres 1999; Loftus 2004), and convincing epidemiologic evidence suggests that the incidence rate has risen in Asian countries over the past decade (Sood 2007). Despite intensive investigation into the cause of IBD, its pathogenic mechanism remains poorly understood. Patients with IBD often suffer from a variety of extraintestinal manifestations, including four categories of rheumatic disease: peripheral arthritis, spondylitis, sacroiliitis, and a broad group of less common disorders (Greenstein 1976; Rankin 1990). Peripheral arthritis develops in 15 to 20% of IBD patients and most commonly affects large joints in an asymmetrical pattern (Finch 1989; Gravallese 1988). Spondylitis, indistinguishable from idiopathic ankylosing spondylitis, generally affects the spine and pelvis and has been observed in up to 11% of IBD patients (Schorr‐Lesnick 1988). Sacroiliitis occurs in 4 to 18% of IBD patients and is frequently asymptomatic (Gravallese 1988). Rheumatological manifestations of IBD can cause severe and persistent discomfort which consequently reduces patients' quality of life.
Description of the intervention
Conventional nonsteroidal anti‐inflammatory drugs (NSAIDs) are used to reduce inflammatory pain and swelling in IBD patients with rheumatological manifestations. While these drugs effectively reduce musculoskeletal pain and stiffness, long‐term use is limited by gastrointestinal (GI) adverse effects (AEs) ranging in severity from mild dyspepsia to haemorrhage, perforation and death (Griffin 2001; MacDonald 1997). Silverstein 1995 reported that 1 to 2% of patients treated with NSAIDs daily for one year experienced serious NSAID‐induced GI AEs. Several other studies have found that the use of NSAIDs was associated with the onset or clinical exacerbation of IBD (Beaugerie 2004; Bjarnason 1993; Hawkey 2006; Thiéfin 2005). As an alternative to NSAIDs, selective cyclooxygenase 2 (COX‐2) inhibitors were developed to improve GI safety and tolerability.
COX‐2 inhibitors include drugs such as celecoxib, rofecoxib, valdecoxib, etoricoxib, and lumiracoxib. Unfortunately, some COX‐2 inhibitors have been associated with an increased risk of adverse events which led to their withdrawal from the market. Rofecoxib was associated with an increased risk of myocardial infarction and was withdrawn from the world market in 2004 (Horton 2004; Juni 2004). Valdecoxib was associated with an increased risk of cardiovascular adverse events (Furberg 2005; Nussmeier 2005; Ott 2003), and reports of serious skin infections (Atukorala 2013; Byerly 2005; Ziemer 2007), and was withdrawn from the world market in 2005. Lumiracoxib was associated with severe liver injury and has been withdrawn from the market in many countries (Fok 2013; Pillans 2012).
Celecoxib and etoricoxib remain available for use in many countries. There is some evidence that the cardiovascular adverse effects of rofecoxib and valdecoxib may not be a class effect applicable to all COX‐2 selective inhibitors. A meta‐analysis by Matchaba 2005 that included 34,668 patients found no evidence of an increased risk of cardiovascular adverse effects in patients treated with lumiracoxib compared to placebo, naproxen, diclofenac, ibuprofen, celecoxib, or rofecoxib. A recent meta‐analysis by De Vecchis 2014 indicates that etoricoxib is not associated with an increased risk of serious vascular events when compared to placebo or naproxen. Meta‐analyses have been performed to assess the risk of cardiovascular effects associated with celecoxib and the results have been equivocal. Brophy 2005 found no association between celecoxib and an increased risk of cardiovascular adverse effects. De Vecchis 2014 found a small but increased risk of serious vascular events including an elevated risk of non‐fatal acute myocardial infarction in patients treated with celecoxib compared to placebo. However, when celecoxib was compared to naproxen there was no significant benefit in terms of a reduced risk of serious cardiovascular events (De Vecchis 2014). Despite ongoing concerns over the safety of these agents the use of etoricoxib and celecoxib may be appropriate in some patient populations, particularly patients who are unable to tolerate NSAID‐induced GI AEs.
How the intervention might work
COX is a rate‐limiting enzyme that catalyzes the formation of prostanoids from arachidonic acid. There are two isoforms of COX: COX‐1 and COX‐2. The former is involved in the production of prostaglandins, which are essential for the maintenance of normal endocrine and kidney function, gastric mucosal integrity, and coagulation. In contrast, COX‐2 is specifically produced in response to inflammatory and mitogenic stimuli, suggesting that it plays an important role in mediating inflammation (Crofford 2000). Whereas NSAIDs inhibit the activity of both COX isoforms, COX‐2 inhibitors are more selective (Vane 1971), and are associated with fewer GI AEs (Bombardier 2000; Schnitzer 2004).
Why it is important to do this review
Several studies have examined whether COX‐2 inhibitors can be safely used for the treatment of rheumatological manifestations of IBD with inconsistent results. Some investigators report acceptable safety profiles associated with these drugs (Bonner 2001; Reinisch 2003). However, others note that COX‐2 inhibitors are associated with high rates of disease exacerbation (Biancone 2004; Gornet 2002; Matuk 2004). At present there is no published systematic review examining the safety of COX‐2 inhibitors for the treatment of rheumatological complications in patients with IBD.
Objectives
The objective of this systematic review was to evaluate the tolerability and safety of COX‐2 inhibitors (e.g. celecoxib, rofecoxib, valdecoxib, etoricoxib, and lumiracoxib) used for the treatment of rheumatological manifestations of IBD.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs) were considered for inclusion.
Types of participants
Adult patients (age ≥18 years) with IBD (either UC or CD defined by a combination of clinical, radiographic, endoscopic, and histologic criteria) presenting with rheumatological manifestations for a period of ≥ 2 weeks were considered for inclusion.
Types of interventions
Studies comparing COX‐2 inhibitors to placebo were considered for inclusion.
Types of outcome measures
Primary outcomes
The primary outcome was the proportion of patients with disease exacerbation as defined by the included studies.
Secondary outcomes
Secondary outcomes included GI adverse events, renal toxicity, cardiovascular and thrombotic events.
Search methods for identification of studies
Electronic searches
We searched the following databases from inception to 19 September 2013:
1. PubMed; 2. EMBASE; 3. MEDLINE; and 4. CENTRAL.
The search was not limited by language. The electronic search strategies are summarized in Appendix 1.
Searching other resources
Additional trials were identified by manually searching the reference lists of relevant papers and conference proceedings and through correspondence with experts and pharmaceutical companies (i.e. manufacturers of COX‐2 inhibitors).
Data collection and analysis
Selection of studies
Two authors (Xin‐Pu Miao and Hui‐Yan Li) independently reviewed titles and abstracts of references retrieved from the literature search and selected potentially relevant studies. The full‐text versions of provisionally included studies were then assessed by two authors (Xin‐Pu Miao and Hui‐Yan Li) to determine whether the inclusion criteria was satisfied. Differences in opinion were resolved by discussion until consensus was reached. If an agreement failed to be reached, a third author (Qin Ouyang) was consulted.
Data extraction and management
Two authors (Ren‐Wei Hu and Yan Zhang) extracted data from the relevant trials using a data extraction form. Discrepancies were resolved by discussion until consensus was reached. If an agreement failed to be reached, an independent expert gastroenterologist or statistician was consulted. Justification for excluding studies from the review was documented. Data were entered in the Cochrane Collaboration Review Manager software (RevMan 5.3) and checked for accuracy (RevMan 2014).
The types of data that were extracted included:
a. Study characteristics and design;
b. Characteristics of patients;
c. Inclusion and exclusion criteria;
d. Interventions; and
e. Outcomes.
Assessment of risk of bias in included studies
Two authors independently assessed study quality using the Cochrane risk of bias tool (Higgins 2011). Differences in opinion were resolved by discussion until consensus was reached. The assessed factors included:
1) Randomization sequence generation;
2) Allocation concealment;
3) Blinding of participants, personnel and outcome assessment;
4) Incomplete outcome data;
5) Selective reporting; and
6) Other potential sources of bias.
Studies were then judged as being at high, low or unclear risk of bias based on the information provided in the available publications.
The GRADE approach was used to rate the overall quality of evidence for the primary outcomes. RCTs start as high quality evidence, but may be downgraded due to: (1) limitations in design and implementation (risk of bias), (2) indirectness of evidence, (3) inconsistency (unexplained heterogeneity), (4) imprecision (sparse data), and (5) reporting bias (publication bias). The overall quality of evidence for each outcome was determined after considering each of these elements, and categorized as high quality (i.e. further research is very unlikely to change our confidence in the estimate of effect); moderate quality (i.e. further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate); low quality (i.e. further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate); or very low quality (i.e. we are very uncertain about the estimate) (Guyatt 2008; Schünemann 2011).
Measures of treatment effect
RevMan 5.3 was used for data analysis (RevMan 2014). Dichotomous data were analyzed by calculating the risk ratio (RR) and corresponding 95% confidence interval (95% CI).
Dealing with missing data
We planned to contact authors of included studies to obtain missing data. Data were analyzed on an intention‐to‐treat basis where patients with missing final outcomes were assumed to have had an exacerbation of IBD. If for a particular outcome, less than 80% of patients allocated to the treatments completed the trial, we planned to not use these data as they are considered to be too prone to bias. However, for both included studies more than 80% of patients completed the trial.
Assessment of heterogeneity
Heterogeneity across trials was assessed using the Chi2 and I2 statistics (Higgins 2003). A P value < 0.10 was considered statistically significant. If significant heterogeneity was present (i.e. I2≥ 50%) we planned to explore possible explanations.
Assessment of reporting biases
We planned to assess publication bias by means of a funnel plot (Egger 1997), if there were a sufficient number of included studies (i.e. ≥ 10).
Data synthesis
We planned to calculate the pooled RR and corresponding 95% CI for dichotomous outcomes using a fixed‐effect model if patients, treatments and outcomes were sufficiently similar across studies. If significant heterogeneity was detected we planned to use a random‐effects model. If a high degree of heterogeneity was detected (i.e. I2> 75%), data would not be pooled for meta‐analysis.
Subgroup analysis and investigation of heterogeneity
If data permitted, subgroup analyses were planned for different doses of COX‐2 inhibitors.
Sensitivity analysis
If a sufficient number of randomized trials were identified, we planned sensitivity analysis to explore the impact of study quality and the impact of studies with outlying results.
Results
Description of studies
Results of the search
The electronic database search on 19 September 2013 identified 1923 studies. After removal of duplicates, 1853 studies were screened for inclusion. Of these, 14 potentially relevant studies on the use of COX‐2 inhibitors for the treatment of rheumatological manifestations of IBD were identified (See Figure 1). Eight studies were excluded because they were case reports and an additional four studies were excluded because they were non‐randomized trials. Two RCTs involving a total of 381 participants satisfied the inclusion criteria and were included in the review (El Miedany 2006; Sandborn 2006). No RCTs were identified that assessed the tolerability and safety of the withdrawn COX‐2 inhibitors rofecoxib, valdecoxib, or lumiracoxib for the treatment of rheumatological manifestations of inflammatory bowel disease.
1.
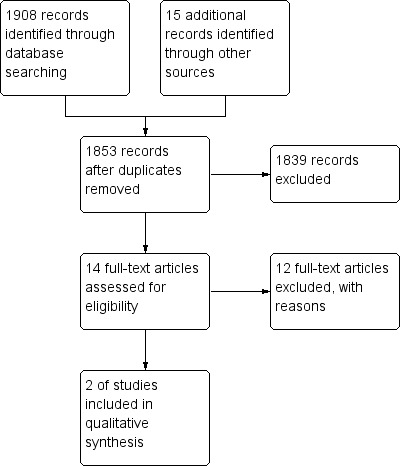
Study flow diagram.
Included studies
1. El Miedany 2006 conducted a randomized, multicenter, double‐blind, placebo‐controlled trial to assess the efficacy and safety of etoricoxib (60 to 120 mg once daily) for the treatment of IBD‐associated arthropathy. Patients with quiescent or active UC or CD and accompanying rheumatological manifestations were randomized to receive etoricoxib (n = 82) or placebo (n = 77) for twelve weeks. Eligibility requirements prevented pregnant patients, smokers (current or former for less than one year) and individuals receiving antibiotics from participating. The primary outcome was change in IBD activity index. The efficacy of etoricoxib as an anti‐inflammatory drug was a secondary outcome and was measured by the progression of the rheumatological condition. Adverse events and bile acid concentrations were also monitored.
2. Sandborn 2006 randomized 222 patients with quiescent UC and a present or past history of nonspecific arthritis, arthralgia or other condition amenable to NSAID therapy to receive celecoxib (n = 112; 200 mg twice daily) or placebo (n = 110) for 14 days in a multicenter double‐blind trial. Patients were excluded if they had a known sulfa allergy or sensitivity to COX inhibitors or both; endoscopic evidence of active UC; a history of gastroduodenal ulcer within one month; received NSAIDs, ulcer medication or antacids within two weeks; received corticosteroids within one month; or required antibiotics, analgesics or corticosteroids during the study. The primary outcome was the proportion of patients with disease exacerbation, as defined by a total Mayo Clinic score of 5 or more at final assessment and an increase in endoscopy score of at least one point. Disease exacerbation in patients missing Mayo Clinic or endoscopic scores due to study withdrawal or termination was assessed during secondary analyses. Adverse events and bile acid concentrations were also monitored.
Excluded studies
Excluded studies and justification for exclusion are reported in the Characteristics of excluded studies tables.
Risk of bias in included studies
Two authors (Xin‐Pu Miao and Hui‐Yan Li) independently assessed risk of bias for each study using the Cochrane risk of bias tool (Higgins 2011). The risk of bias analysis is summarized in (Figure 2).
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
El Miedany 2006 did not report the methods used for randomization and allocation concealment and was rated as unclear for these items. Sandborn 2006 reported a computer‐generated randomization sequence and was rated a low risk for this item. Sandborn 2006 did not describe the method used for allocation concealment and was rated as unclear for this item.
Blinding
El Miedany 2006 reported the use of a matched placebo and was rated as low risk for blinding of participants and personnel. Sandborn 2006 reported that patients and investigators were unaware of treatment assignment and was rated as low risk for blinding of participants and personnel and for outcome assessors. El Miedany 2006 did not describe outcome assessment and this item was rated as unclear.
Incomplete outcome data
Both studies were rated as low risk of bias for incomplete outcome data.
Selective reporting
Both studies were rated as low risk of bias for selective reporting.
Other potential sources of bias
Both included studies were rated as low risk of bias for other potential sources of bias.
Effects of interventions
The two included studies were not pooled for meta‐analysis due to differences in patient populations and treatment duration. El Miedany 2006 enrolled patients with quiescent or active IBD (i.e. both CD and UC) whereas Sandborn 2006 enrolled patients with quiescent UC. El Miedany 2006 treated patients for 12 weeks and Sandborn 2006 treated patients for two weeks.
Exacerbation of IBD
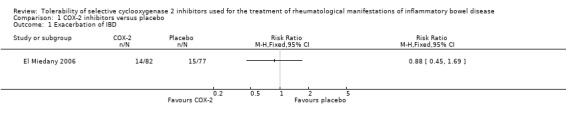
There was no statistically significant difference in exacerbation of IBD between etoricoxib and placebo. After 12 weeks of treatment the IBD exacerbation rate was 17% (14/82) in the etoricoxib group compared to 19% (15/77) in the placebo group (RR 0.88, 95% CI 0.45 to 1.69; See Figure 3). A GRADE analysis indicated that the overall quality of the evidence for this outcome (exacerbation of IBD) was low due to very sparse data (29 events, see Table 1).
3.

Forest plot of comparison: 1 COX‐2 inhibitors versus placebo, outcome: 1.1 No. of IBD exacerbations.
Exacerbation of UC
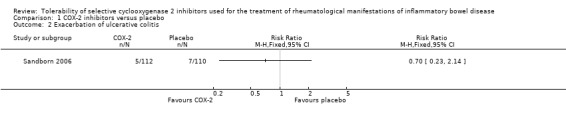
There was no statistically significant difference in exacerbation of UC between celecoxib and placebo. After two weeks of treatment 4% (5/112) of celecoxib patients experienced an exacerbation of UC compared to 6% (7/110) of patients in the placebo group (RR 0.70, 95% CI 0.23 to 2.14; See Figure 4). A GRADE analysis indicated that the overall quality of the evidence for this outcome (exacerbation of UC) was low due to very sparse data (12 events, see Table 2).
4.

Forest plot of comparison: 1 COX‐2 inhibitors versus placebo, outcome: 1.2 Exacerbation of ulcerative colitis.
Safety
El Miedany 2006 documented but did not report AEs.
Sandborn 2006 reported that the proportion of patients who experienced AEs was similar in the celecoxib and placebo groups (21% and 17%, respectively, P > 0.20). No patients in either group died or experienced serious adverse events. Eleven percent of patients in both groups experienced GI AEs (RR 0.97, 95% CI 0.46 to 2.07). A GRADE analysis indicated that the overall quality of the evidence for this outcome was low due to very sparse data (24 events, see Table 2). GI adverse events led to premature withdrawal from the study in 3% of patients in each treatment group. GI AEs included increased stool frequency, rectal bleeding, and inflamed mucosa (e.g. friability, erosions, erythema, absent vascularity, spontaneous bleeding, ulceration). Sandborn 2006 reported that no patients experienced any cardiovascular adverse events. Renal toxicity or thrombotic AEs were not reported.
Discussion
Summary of main results
Two studies compared the tolerability of the COX‐2 inhibitors celecoxib and etoricoxib to placebo in patients with IBD experiencing rheumatological manifestations (El Miedany 2006; Sandborn 2006). El Miedany 2006 treated patients with quiescent or active IBD (n = 159) for 12 weeks and found no clear evidence of a difference in exacerbations of IBD between patients who received etoricoxib or placebo. Sandborn 2006 treated patients with quiescent UC (n = 222) for two weeks and found no clear evidence of a difference in exacerbations of UC between patients who received celecoxib and placebo. El Miedany 2006 did not report on AEs. Sandborn 2006 found no clear evidence of a difference in AEs or GI AEs as the proportion of these events were similar in the treatment and placebo groups.
Overall completeness and applicability of evidence
The results of this review are applicable to patients with IBD who are experiencing rheumatological manifestations. However due to the small number of studies included in this review and differences in patient populations, duration of treatment and length of follow‐up we could not make any definitive conclusions regarding the tolerability and safety of the short term use of celecoxib and etoricoxib in patients with IBD. Neither study was adequately powered to detect an increase in relatively rare but serious adverse events such as renal toxicity, cardiovascular and thrombotic events. Although these outcomes weren't reported in the two included studies no conclusions can be drawn regarding the risk of these adverse events. Furthermore, the tolerability and safety associated with the long‐term use of celecoxib and etoricoxib have not been investigated.
Quality of the evidence
Both studies were judged to be at low risk of bias. However, GRADE analyses indicated that the overall quality of the evidence supporting the primary outcome (disease exacerbation) was low due to very sparse data in both studies (29 events in El Miedany 2006 and 12 events in Sandborn 2006). A GRADE analysis indicated that the overall quality of the evidence supporting the secondary outcome GI AEs was low due to very sparse data (24 events in Sandborn 2006). This indicates that further research will likely have an impact on our confidence in the estimates of effect and is likely to change these estimates.
Potential biases in the review process
We attempted to reduce potential bias in the review process. A comprehensive literature search was performed to identify all applicable studies. Two review authors independently assessed studies for inclusion, extracted data and assessed study quality.
Agreements and disagreements with other studies or reviews
Two studies excluded from this systematic review found that the use of COX‐2 inhibitors in patients with IBD did not elicit disease exacerbation. Reinisch 2003 administered rofecoxib at a dose of up to 25 mg daily to 32 patients with IBD and arthropathy. At day 20 the authors found that 41% of patients treated at a dose of 25 mg/day showed improvement in arthralgia score and functional joint status. Two patients with CD withdrew from the study due to diarrhea and bleeding from a perianal fistula. One patient with indeterminate colitis stopped treatment because of nausea. None of the patients experienced disease exacerbation. Freedman 2002 also confirmed the safety of COX‐2 inhibitors in patients with IBD in a retrospective analysis of patients treated with celecoxib and rofecoxib for periods ranging from one week to 22 months.
In contrast to the positive findings on the use of COX‐2 inhibitors in IBD patients, several studies excluded from this review suggest that these drugs may increase the disease exacerbation in patients with CD or UC (Biancone 2004; Matuk 2004). Biancone 2004 treated patients with inactive IBD (25 CD; 20 UC) and arthralgia with rofecoxib for a period ranging from 3 days to 3 months. The control group consisted of patients with dyspepsia and arthralgia. In the IBD group 71% of patients reported arthralgia relief compared to 70% of patients in the control group. Twenty percent of patients in the IBD group had to discontinue treatment due to GI symptoms compared to 3% of patients with dyspepsia. The authors conclude that while rofecoxib may have effectively treated arthralgia in IBD patients, adverse effects were experienced in almost one‐quarter of this patient group (Biancone 2004). Similarly, Matuk 2004 conducted a retrospective study which examined questionnaire data provided by 33 patents with IBD who received celecoxib, rofecoxib or both medications at different times. All patients experienced IBD exacerbation within six weeks of study initiation. The authors concluded that treatment with COX‐2 medications is associated with disease exacerbation and GI complications in IBD patients (Matuk 2004). Overall, there are sparse available data on the use of COX‐2 inhibitors in patients with IBD.
Authors' conclusions
Implications for practice.
The results for disease exacerbation and AEs between the COX‐2 inhibitors celecoxib and etoricoxib and placebo were uncertain. Thus no definitive conclusions regarding the tolerability and safety of the short term use of celecoxib and etoricoxib in patients with IBD can be drawn. The two included studies suggest that celecoxib and etoricoxib do not exacerbate IBD symptoms. However, it should be noted that both studies had relatively small sample sizes and short follow‐up durations. Clinicians need to continue to weigh the risks and benefits of these drugs when treating IBD patients with rheumatological manifestations in order to avoid disease exacerbation and other adverse effects.
Implications for research.
GRADE analyses indicate that the overall quality of the evidence is low. Further RCTs are needed to determine the tolerability and safety of celecoxib and etoricoxib in patients with rheumatological manifestations of inflammatory bowel disease.
Acknowledgements
Funding for the IBD/FBD Review Group (September 1, 2010 to August 31, 2015) has been provided by the Canadian Institutes of Health Research (CIHR) Knowledge Translation Branch (CON ‐ 105529) and the CIHR Institutes of Nutrition, Metabolism and Diabetes (INMD); and Infection and Immunity (III) and the Ontario Ministry of Health and Long Term Care (HLTC3968FL‐2010‐2235).
Miss Ila Stewart has provided support for the IBD/FBD Review Group through the Olive Stewart Fund.
The authors would like to thank the Cochrane IBD/FBD review group for their assistance with developing the search strategy and the literature search.
Appendices
Appendix 1. Search strategies for electronic databases
COX‐2 Inhibitors in IBD Search Strategy – Sept. 2013
PubMed
#1 (singl* OR doubl* OR tripl* OR trebl* OR blind* OR mask* OR placebo* OR single‐blind* OR double‐blind* OR triple‐blind* OR random* OR (controlled clinical))
#2 ((colitis and ulcerat*) OR Crohn* OR (inflammatory bowel disease*) OR (inflammatory bowel disease) OR IBD)
#3 (#1 AND #2)
#4 (cyclooxygenase‐2 inhibitor* OR cyclooxygenase‐II inhibitor* OR cyclo‐oxygenase‐2 inhibitor* OR cyclo‐oxygenase‐II inhibitor* OR COX2 inhibitor* OR COX‐2 inhibitor* OR COX‐II inhibitor* OR COXII inhibitor* OR cyclooxygenase‐2 inhibitor OR cyclooxygenase‐II inhibitor OR cyclo‐oxygenase‐2 inhibitor OR cyclo‐oxygenase‐II inhibitor OR COX2 inhibitor OR COX‐2 inhibitor OR COX‐II inhibitor OR COXII inhibitor)
#5 (cyclooxygenase‐2 antagonist* OR cyclooxygenase‐II antagonist* OR cyclo‐oxygenase‐2 antagonist* OR cyclo‐oxygenase‐II antagonist* OR COX2 antagonist* OR COX‐2 antagonist* OR COX‐II antagonist* OR COXII antagonist* OR cyclooxygenase‐2 antagonist OR cyclooxygenase‐II antagonist OR cyclo‐oxygenase‐2 antagonist OR cyclo‐oxygenase‐II antagonist OR COX2 antagonist OR COX‐2 antagonist OR COX‐II antagonist OR COXII antagonist)
#6 (prostaglandin synthase inhibitor OR prostaglandin synthase inhibitor* OR prostaglandin synthase antagonist OR prostaglandin synthase antagonist*)
#7 (coxib OR coxibs OR apricoxib OR bardoxolone OR celecoxib OR cimicoxib OR darbufelone OR deracoxib OR etoricoxib OR firocoxib OR flosulide OR (gw 406381) OR iguratimod OR lumiracoxib OR mavacoxib OR meloxicam OR methanesulfonamide OR nimesulide OR parecoxib OR robenacoxib OR tilmacoxib OR valdecoxib OR vedaprofen)
#8 coxibs OR coxibs OR apricoxib OR bardoxolone OR celecoxib OR cimicoxib OR darbufelone OR deracoxib OR etoricoxib OR firocoxib OR flosulide OR (gw 406381) OR iguratimod OR lumiracoxib OR mavacoxib OR meloxicam OR methanesulfonamide OR nimesulide OR parecoxib OR robenacoxib OR tiracoxib OR valdecoxib OR vedaprofen
#9 (#4 OR #5 OR #6 OR #7 OR #8)
#10 (#3 AND #9)
EMBASE
1. random$.tw.
2. factorial$.tw.
3. (crossover$ or cross over$ or cross‐over$).tw.
4. placebo$.tw.
5. single blind.mp.
6. double blind.mp.
7. triple blind.mp.
8. (singl$ adj blind$).tw.
9. (double$ adj blind$).tw.
10. (tripl$ adj blind$).tw.
11. assign$.tw.
12. allocat$.tw.
13. crossover procedure/
14. double blind procedure/
15. single blind procedure/
16. triple blind procedure/
17. randomized controlled trial/
18. or/1‐17
19. (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.)
20. 18 not 19
21. exp Crohn disease/ or crohn*.mp.
22. ulcerative colitis.mp. or exp ulcerative colitis/
23. inflammatory bowel disease.mp. or exp enteritis/
24. IBD.mp.
25. 21 or 22 or 23 or 24
26. 20 and 25
27. exprofecoxib/ or exp cyclooxygenase 2 inhibitor/ or exp prostaglandin synthase inhibitor/ or COX‐2 inhibitor*.mp. or expcelecoxib/
28. (cyclooxygenase‐2 inhibitor* or cyclooxygenase‐II inhibitor* or cyclo‐oxygenase‐2 inhibitor* or cyclo‐oxygenase‐II inhibitor* or COX2 inhibitor* or COX‐2 inhibitor* or COX‐II inhibitor* or COXII inhibitor* or cyclooxygenase‐2 inhibitor or cyclooxygenase‐II inhibitor or cyclo‐oxygenase‐2 inhibitor or cyclo‐oxygenase‐II inhibitor or COX2 inhibitor or COX‐2 inhibitor or COX‐II inhibitor or COXII inhibitor).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
29. (cyclooxygenase‐2 antagonist* or cyclooxygenase‐II antagonist* or cyclo‐oxygenase‐2 antagonist* or cyclo‐oxygenase‐II antagonist* or COX2 antagonist* or COX‐2 antagonist* or COX‐II antagonist* or COXII antagonist* or cyclooxygenase‐2 antagonist or cyclooxygenase‐II antagonist or cyclo‐oxygenase‐2 antagonist or cyclo‐oxygenase‐II antagonist or COX2 antagonist or COX‐2 antagonist or COX‐II antagonist or COXII antagonist).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
30. (prostaglandin synthase inhibitor or prostaglandin synthase inhibitor* or prostaglandin synthase antagonist or prostaglandin synthase antagonist*).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
31. (coxib or coxibs or apricoxib or bardoxolone or celecoxib or cimicoxib or darbufelone or deracoxib or etoricoxib or firocoxib or flosulide or gw 406381 or iguratimod or lumiracoxib or mavacoxib or meloxicam or methanesulfonamide or nimesulide or parecoxib or robenacoxib or tilmacoxib or valdecoxib or vedaprofen).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
32. 27 or 28 or 29 or 30 or 31
33. 26 and 32
MEDLINE
1. random$.tw.
2. factorial$.tw.
3. (crossover$ or cross over$ or cross‐over$).tw.
4. placebo$.tw.
5. single blind.mp.
6. double blind.mp.
7. triple blind.mp.
8. (singl$ adj blind$).tw.
9. (double$ adj blind$).tw.
10. (tripl$ adj blind$).tw.
11. assign$.tw.
12. allocat$.tw.
13. crossover procedure/
14. double blind procedure/
15. single blind procedure/
16. triple blind procedure/
17. randomized controlled trial/
18. or/1‐17
19. (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.)
20. 18 not 19
21. exp Crohn disease/ or crohn*.mp.
22. ulcerative colitis.mp. or exp ulcerative colitis/
23. inflammatory bowel disease.mp. or exp enteritis/
24. IBD.mp.
25. 21 or 22 or 23 or 24
26. 20 and 25
27. exprofecoxib/ or exp cyclooxygenase 2 inhibitor/ or exp prostaglandin synthase inhibitor/ or COX‐2 inhibitor*.mp. or expcelecoxib/
28. (cyclooxygenase‐2 inhibitor* or cyclooxygenase‐II inhibitor* or cyclo‐oxygenase‐2 inhibitor* or cyclo‐oxygenase‐II inhibitor* or COX2 inhibitor* or COX‐2 inhibitor* or COX‐II inhibitor* or COXII inhibitor* or cyclooxygenase‐2 inhibitor or cyclooxygenase‐II inhibitor or cyclo‐oxygenase‐2 inhibitor or cyclo‐oxygenase‐II inhibitor or COX2 inhibitor or COX‐2 inhibitor or COX‐II inhibitor or COXII inhibitor).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
29. (cyclooxygenase‐2 antagonist* or cyclooxygenase‐II antagonist* or cyclo‐oxygenase‐2 antagonist* or cyclo‐oxygenase‐II antagonist* or COX2 antagonist* or COX‐2 antagonist* or COX‐II antagonist* or COXII antagonist* or cyclooxygenase‐2 antagonist or cyclooxygenase‐II antagonist or cyclo‐oxygenase‐2 antagonist or cyclo‐oxygenase‐II antagonist or COX2 antagonist or COX‐2 antagonist or COX‐II antagonist or COXII antagonist).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
30. (prostaglandin synthase inhibitor or prostaglandin synthase inhibitor* or prostaglandin synthase antagonist or prostaglandin synthase antagonist*).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
31. (coxib or coxibs or apricoxib or bardoxolone or celecoxib or cimicoxib or darbufelone or deracoxib or etoricoxib or firocoxib or flosulide or gw 406381 or iguratimod or lumiracoxib or mavacoxib or meloxicam or methanesulfonamide or nimesulide or parecoxib or robenacoxib or tilmacoxib or valdecoxib or vedaprofen).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
32. 27 or 28 or 29 or 30 or 31
33. 26 and 32
CENTRAL
#1 (colitis and ulcerat*) or Crohn* or (inflammatory bowel disease*) or (inflammatory bowel disease) or IBD
#2 cyclooxygenase‐2 inhibitor* or cyclooxygenase‐II inhibitor* or cyclo‐oxygenase‐2 inhibitor* or cyclooxygenase‐II inhibitor* or COX2 inhibitor* or COX‐2 inhibitor* or COX‐II inhibitor* or COXII inhibitor* or cyclooxygenase‐2 inhibitor or cyclooxygenase‐II inhibitor or cyclo‐oxygenase‐2 inhibitor or cyclo‐oxygenase‐IIinhibitor or COX2 inhibitor or COX‐2 inhibitor or COX‐II inhibitor or COXII inhibitor
#3 cyclooxygenase‐2 antagonist* or cyclooxygenase‐II antagonist* or cyclo‐oxygenase‐2 antagonist* or cyclooxygenase‐II antagonist* or COX2 antagonist* or COX‐2 antagonist* or COX‐II antagonist* or COXII antagonist* orcyclooxygenase‐2 antagonist or cyclooxygenase‐II antagonist or cyclo‐oxygenase‐2 antagonist or cyclo‐oxygenase‐IIantagonist or COX2 antagonist or COX‐2 antagonist or COX‐II antagonist or COXII antagonist
#4 prostaglandin synthase inhibitor or prostaglandin synthase inhibitor* or prostaglandin synthase antagonist orprostaglandin synthase antagonist*
#5 coxibs or coxibs or apricoxib or bardoxolone or celecoxib or cimicoxib or darbufelone or deracoxib or etoricoxibor firocoxib or flosulide or (gw 406381) origuratimod or lumiracoxib or mavacoxib or meloxicam ormethanesulfonamide ornimesulide or parecoxib or robenacoxib or tiracoxib or valdecoxib or vedaprofen
#6 #2 or #3 or #4 or #5
#7 #1 and #6
Data and analyses
Comparison 1. COX‐2 inhibitors versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Exacerbation of IBD | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Exacerbation of ulcerative colitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 GI adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
1.1. Analysis.
Comparison 1 COX‐2 inhibitors versus placebo, Outcome 1 Exacerbation of IBD.
1.2. Analysis.
Comparison 1 COX‐2 inhibitors versus placebo, Outcome 2 Exacerbation of ulcerative colitis.
1.3. Analysis.
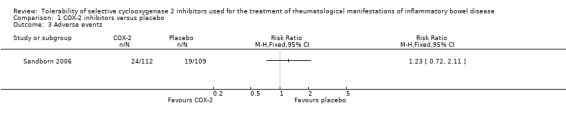
Comparison 1 COX‐2 inhibitors versus placebo, Outcome 3 Adverse events.
1.4. Analysis.
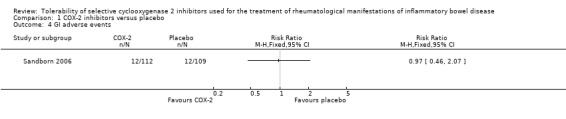
Comparison 1 COX‐2 inhibitors versus placebo, Outcome 4 GI adverse events.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
El Miedany 2006.
| Methods | Randomized, multicenter,double‐blind, placebo‐controlled trial (N = 159) | |
| Participants | Patients (18 to 65 years) with IBD (CD n = 80; UC n = 79) attending rheumatology clinics for treatment of associated rheumatological manifestations Current or former smokers (less than 1 year duration), pregnant women and patients on antibiotics were excluded from the study |
|
| Interventions | Etoricoxib from 60 to 120 mg once daily, according to the rheumatic condition, for 3 months | |
| Outcomes | Exacerbation of IBD | |
| Notes | Change in disease activity was graded as improved, no change or worsened (by one, two, or three classes) The proportion of patients receiving etoricoxib or placebo who experienced exacerbation of their gastrointestinal symptoms was calculated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The control group received a matched placebo tablet once daily |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐outs balanced across intervention groups with similar reasons for withdrawal |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
| Other bias | Low risk | This study appears to be free of other sources of bias |
Sandborn 2006.
| Methods | Randomized, multicenter,double‐blind, placebo‐controlled trial (N = 222) | |
| Participants | Patients (18 to 75 years) with UC in remission and a past or present history of nonspecific arthritis, arthralgia or other condition amenable to NSAID therapy Patients were excluded if they had: active colitis; a history of gastroduodenal ulcer within 1 month; received any NSAIDS, anti‐ulcer medication, or antacids within 2 weeks; received corticosteroids within1 month; needed treatment with antibiotics, analgesics, or corticosteroids during the study; or a known sulfa allergy or hypersensitivity to COX inhibitors |
|
| Interventions | Oral celecoxib at a dose of 200 mg or placebo twice daily for 14 days | |
| Outcomes | Exacerbation of UC | |
| Notes | Disease exacerbation was defined as a total Mayo Clinic score of 5 points or more and an increase in the endoscopic score of 1 point or more | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was performed within each center according to a computer‐generated randomization schedule |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Neither the patients nor the study investigators were aware of the treatment assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The study investigators were not aware of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐outs balanced across intervention groups with similar reasons for withdrawal |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
| Other bias | Low risk | This study appears to be free of other sources of bias |
IBD = Inflammatory bowel disease
CD = Crohn's disease
UC = ulcerative colitis
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Biancone 2004 | Not a randomized controlled trial, open‐label trial with 75 patients, no placebo group |
| Bonner 2001 | Not a randomized controlled trial, case report, no placebo group |
| Charachon A 2003 | Not a randomized controlled trial, case report, no placebo group |
| Freedman 2002 | Not a randomized controlled trial, case report, no placebo group |
| Goh 2002 | Not a randomized controlled trial, case report, no placebo group |
| Gornet 2002 | Not a randomized controlled trial, case report, no placebo group |
| Mahadevant 2002 | Not a randomized controlled trial, case report, no placebo group |
| Matuk 2004 | Not a randomized controlled trial, open study with 33 patients, no placebo group |
| Reinisch 2003 | Not a randomized controlled trial, open‐label study with 32 patients,no placebo group |
| Rey 2005 | Not a randomized controlled trial, case report, no placebo group |
| Takeuchi 2006 | Not a randomized controlled trial, open study with 209 patients, no placebo group |
| Wilcox 2005 | Not a randomized controlled trial, case report, no placebo group |
Characteristics of studies awaiting assessment [ordered by study ID]
NCT00177866.
| Methods | Randomized, double blind, cross‐over trial |
| Participants | Inclusion Criteria: Adults (> 18 to 70 years of age) with a confirmed diagnosis of inactive (CDAI scores < 150) or active Crohn's disease (CDAI scores < 200) at baseline |
| Interventions | Oral celebrex 200 mg or placebo twice daily for 8 weeks, followed by a 1 week "washout period" then oral placebo or celebrex twice daily for 8 weeks |
| Outcomes | Primary outcome: Change in CDAI scores in response to treatment Secondary outcome: Change in SIBDQ scores in response to treatment |
| Notes | This study was terminated because of lack of funding |
CDAI = Crohn's Disease Activity Index
SIBDQ = Short Inflammatory Bowel Disease Questionnaire
Differences between protocol and review
We would like to acknowledge some differences between the protocol and review:
The reviews objective reported in the protocol was, "The objective of this study is to evaluate the tolerability of COX‐2 inhibitors (celecoxib, rofecoxib, valdecoxib, etoricoxib, and lumiracoxib) for the treatment the rheumatological manifestations of IBD..". This was changed to, "The objective of this systematic review was to evaluate the tolerability and safety of COX‐2 inhibitors (e.g. celecoxib, rofecoxib, valdecoxib, etoricoxib, and lumiracoxib) used for the treatment of rheumatological manifestations of IBD" to reflect that fact that several specific adverse events which were described in the published protocol were to be assessed.
The statement, "The primary outcomes must be reported for a study to be included." was removed as part of the inclusion criteria. Please see 'Methodological standards for the conduct of new Cochrane Intervention Reviews (MECIR C8)'.
GRADE was added to the methods section. Please see MECIR C76.
Sources of support
Internal sources
New Source of support, Other.
External sources
No sources of support supplied
Declarations of interest
None known.
New
References
References to studies included in this review
El Miedany 2006 {published data only}
- Miedany Y, Youssef S, Ahmed I, Gaafary M. The gastrointestinal safety and effect on disease activity of etoricoxib, a selective cox‐2 inhibitor in inflammatory bowel diseases. American Journal of Gastroenterology 2006;101(2):311‐7. [DOI] [PubMed] [Google Scholar]
Sandborn 2006 {published data only}
- Sandborn WJ, Stenson WF, Brynskov J, Lorenz RG, Steidle GM, Robbins JL, et al. Safety of celecoxib in patients with ulcerative colitis in remission: a randomized, placebo‐controlled, pilot study. Clinical Gastroenterology and Hepatology 2006;4(2):203‐11. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Biancone 2004 {published data only}
- Biancone L, Tosti C, Geremia A, Fina D, Petruzziello C, Emerenziani S, et al. Rofecoxib and early relapse of inflammatory bowel disease: An open‐label trial. Alimentary Pharmacology and Therapeutics 2004;19(7):755‐64. [DOI] [PubMed] [Google Scholar]
Bonner 2001 {published data only}
- Bonner GF. Exacerbation of inflammatory bowel disease associated with use of celecoxib. American Journal of Gastroenterology 2001;96(4):1306‐8. [DOI] [PubMed] [Google Scholar]
Charachon A 2003 {published data only}
- Charachon A, Petit T, Lamarque D, Soulé JC. Acute ulcerative colitis in a patient treated with rofecoxib who took aspirin as self‐medication. Gastroentérologie Clinique et Biologique. 2003;27(5):511‐3. [PubMed] [Google Scholar]
Freedman 2002 {published data only}
- Freedman GM, Kreitzer JM, Badola R. Rofecoxib‐associated upper gastrointestinal bleed: a case report. Mount Sinai Journal of Medicine 2002;69(1‐2):105‐6. [PubMed] [Google Scholar]
Goh 2002 {published data only}
- Goh J, Wight D, Parkes M, Middleton SJ, Hunter JO. Rofecoxib and cytomegalovirus in acute flare‐up of ulcerative colitis: coprecipitants or coincidence?. American Journal of Gastroenterology 2002;97(4):1061‐2. [DOI] [PubMed] [Google Scholar]
Gornet 2002 {published data only}
- Gornet JM, Hassani Z, Modiglian R, Lémann M. Exacerbation of Crohn's colitis with severe colonic hemorrhage in a patient on rofecoxib. American Journal of Gastroenterology 2002;97(12):3209‐10. [DOI] [PubMed] [Google Scholar]
Mahadevant 2002 {published data only}
- Mahadevan U, Loftus EV Jr, Tremaine WJ, Sandborn WJ. Safety of selective cyclooxygenase‐2 inhibitors in inflammatory bowel disease. American Journal of Gastroenterology 2002;97(4):910‐4. [DOI] [PubMed] [Google Scholar]
Matuk 2004 {published data only}
- Matuk R, Crawford J, Abreu MT, Targan SR, Vasiliauskas EA, Papadakis KA. The spectrum of gastrointestinal toxicity and effect on disease activity of selective cyclooxygenase‐2 inhibitors in patients with inflammatory bowel disease. Inflammatory Bowel Diseases 2004;10(4):352‐6. [DOI] [PubMed] [Google Scholar]
Reinisch 2003 {published data only}
- Reinisch W, Miehsler W, Dejaco C, Harrer M, Waldhoer T, Lichtenberger C, et al. An open‐label trial of the selective cyclo‐oxygenase‐2 inhibitor, rofecoxib, in inflammatory bowel disease‐associated peripheral arthritis and arthralgia. Alimentary Pharmacology and Therapeutics 2003;17(11):1371‐80. [DOI] [PubMed] [Google Scholar]
Rey 2005 {published data only}
- Rey P, Andriamanantena D, Carrère C, Casassus‐Builhè D, Hamant N, Perret JL. Ulcerating haemorrhagic colitis induced by celecoxib. Presse Médicale 2005;34(6):443‐5. [DOI] [PubMed] [Google Scholar]
Takeuchi 2006 {published data only}
- Takeuchi K, Smale S, Premchand P, Maiden L, Sherwood R, Thjodleifsson B, et al. Prevalence and mechanism of nonsteroidal anti‐inflammatory drug‐induced clinical relapse in patients with inflammatory bowel disease. Clinical Gastroenterology and Hepatology 2006;4(2):196‐202. [DOI] [PubMed] [Google Scholar]
Wilcox 2005 {published data only}
- Wilcox GM, Mattia AR. Rofecoxib and inflammatory bowel disease: clinical and pathologic observations. Journal of Clinical Gastroenterology 2005;39(2):142‐3. [PubMed] [Google Scholar]
References to studies awaiting assessment
NCT00177866 {published data only}
Additional references
Andres 1999
- Andres PG, Friedman LS. Epidemiology and the natural course of inflammatory bowel disease. Gastroenterology Clinics of North America 1999;28(2):255‐81. [DOI] [PubMed] [Google Scholar]
Atukorala 2013
- Atukorala I, Hunter DJ. Valdecoxib : the rise and fall of a COX‐2 inhibitor. Expert Opinion on Pharmacotherapy 2013;14(8):1077‐86. [DOI] [PubMed] [Google Scholar]
Beaugerie 2004
- Beaugerie L, Thiéfin G. Gastrointestinal complications related to NSAIDs. Gastroentérologie Clinique et Biologique 2004;28 Spec No 3:C62‐72. [DOI] [PubMed] [Google Scholar]
Bjarnason 1993
- Bjarnason I, Hayllar J, MacPherson AJ, Russell AS. Side effects of nonsteroidal anti‐inflammatory drugs on the small and large intestine in humans. Gastroenterology 1993;104(6):1832‐47. [DOI] [PubMed] [Google Scholar]
Bombardier 2000
- Bombardier C, Laine L, Reicin A, Shapiro D, Burgos‐Vargas R, Davis B, et al. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. New England Journal of Medicine 2000;343(21):1520‐8. [DOI] [PubMed] [Google Scholar]
Brophy 2005
- Brophy JM. Cardiovascular risk associated with celecoxib. New England Journal of Medicine 2005;352(25):2648‐50; author reply 2648‐50. [DOI] [PubMed] [Google Scholar]
Byerly 2005
- Byerly FL, Nelson KC, Granko RP, Morrell DS, Cairns BA. Valdecoxib‐associated acute generalized exanthematous pustulosis. Burns 2005;31(3):383‐7. [DOI] [PubMed] [Google Scholar]
Crofford 2000
- Crofford LJ, Lipsky PE, Brooks P, Abramson SB, Simon LS, Putte LB. Basic biology and clinical application of specific cyclooxygenase‐2 inhibitors. Arthritis and Rheumatism 2000;43(1):4‐13. [DOI] [PubMed] [Google Scholar]
De Vecchis 2014
- Vecchis R, Baldi C, Biase G, Ariano C, Cioppa C, Giasi A, et al. Cardiovascular risk associated with celecoxib or etoricoxib: a meta‐analysis of randomized controlled trials which adopted comparison with placebo or naproxen. Minerva Cardioangiologica 2014 Jul 16. [Epub ahead of print]. [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Finch 1989
- Finch W. Arthritis and the gut. Postgraduate Medicine 1989;86(2):229‐30, 233‐4. [DOI] [PubMed] [Google Scholar]
Fok 2013
- Fok KC, Bell CJ, Read RB, Eckstein RP, Jones BE. Lumiracoxib‐induced cholestatic liver injury. Internal Medicine Journal 2013;43(6):731‐2. [DOI] [PubMed] [Google Scholar]
Furberg 2005
- Furberg CD, Psaty BM, FitzGerald GA. Parecoxib, valdecoxib, and cardiovascular risk. Circulation 2005;111(3):249. [DOI] [PubMed] [Google Scholar]
Gravallese 1988
- Gravallese EM, Kantrowitz FG. Arthritic manifestations of inflammatory bowel disease. American Journal of Gastroenterology 1988;83(7):703‐9. [PubMed] [Google Scholar]
Greenstein 1976
- Greenstein AJ, Janowitz HD, Sachar DB. The extra‐intestinal complications of Crohn's disease and ulcerative colitis: A study of 700 patients. Medicine (Baltimore) 1976;55(5):401‐12. [DOI] [PubMed] [Google Scholar]
Griffin 2001
- Griffin MR, Scheiman JM. Prospects for changing the burden of nonsteroidal anti‐inflammatory drug toxicity. American Journal of Medicine 2001;110(1A):33S‐7S. [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hawkey 2006
- Hawkey CJ. NSAIDs, coxibs, and the intestine. Journal of Cardiovascular Pharmacology 2006;47 Suppl 1:S72‐5. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Horton 2004
- Horton R. Vioxx, the implosion of Merck, and aftershocks at the FDA. Lancet 2004;364(9450):1995‐6. [DOI] [PubMed] [Google Scholar]
Juni 2004
- Jüni P, Nartey L, Reichenbach S, Sterchi R, Dieppe PA, Egger M. Risk of cardiovascular events and rofecoxib: cumulative meta‐analysis. Lancet 2004;364(9450):2021‐9. [DOI] [PubMed] [Google Scholar]
Loftus 2004
- Loftus EV Jr. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology 2004;126(6):1504‐17. [DOI] [PubMed] [Google Scholar]
MacDonald 1997
- MacDonald TM, Morant SV, Robinson GC, Shield MJ, McGilchrist MM, Murray FE, et al. Association of upper gastrointestinal toxicity of non‐steroidal anti‐inflammatory drugs with continued exposure: cohort study. BMJ 1997;315(7119):1333‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Matchaba 2005
- Matchaba P, Gitton X, Krammer G, Ehrsam E, Sloan VS, Olson M, et al. Cardiovascular safety of lumiracoxib: a meta‐analysis of all randomized controlled trials > or =1 week and up to 1 year in duration of patients with osteoarthritis and rheumatoid arthritis. Clinical Therapeutics 2005;27(8):1196‐214. [DOI] [PubMed] [Google Scholar]
Nussmeier 2005
- Nussmeier NA, Whelton AA, Brown MT, Langford RM, Hoeft A, Parlow JL, et al. Complications of the COX‐2 inhibitors parecoxib and valdecoxib after cardiac surgery. New England Journal of Medicine 2005;352(11):1081‐91. [DOI] [PubMed] [Google Scholar]
Ott 2003
- Ott E, Nussmeier NA, Duke PC, Feneck RO, Alston RP, Snabes MC, et al. Efficacy and safety of the cyclooxygenase 2 inhibitors parecoxib and valdecoxib in patients undergoing coronary artery bypass surgery. Journal of Thoracic and Cardiovascular Surgery 2003;125(6):1481‐92. [DOI] [PubMed] [Google Scholar]
Pillans 2012
- Pillans PI, Ghiculescu RA, Lampe G, Wilson R, Wong R, Macdonald GA. Severe acute liver injury associated with lumiracoxib. Journal of Gastroenterology and Hepatology 2012;27(6):1102‐5. [DOI] [PubMed] [Google Scholar]
Rankin 1990
- Rankin GB. Extraintestinal and systemic manifestations of inflammatory bowel disease. Medical Clinics of North America 1990;74(1):39‐50. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schnitzer 2004
- Schnitzer TJ, Burmester GR, Mysler E, Hochberg MC, Doherty M, Ehrsam E, et al. Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), reduction in ulcer complications: randomised controlled trial. Lancet 2004;364(9435):665‐74. [DOI] [PubMed] [Google Scholar]
Schorr‐Lesnick 1988
- Schorr‐Lesnick B, Brandt LJ. Selected rheumatologic and dermatologic manifestations of inflammatory bowel disease. American Journal of Gastroenterology 1988;83(3):216‐23. [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Silverstein 1995
- Silverstein FE, Graham DY, Senior JR, Davies HW, Struthers BJ, Bittman RM, et al. Misoprostol reduces serious gastrointestinal complications in patients with rheumatoid arthritis receiving nonsteroidal anti‐inflammatory drugs. A randomized, double‐blind, placebo‐controlled trial. Annals of Internal Medicine 1995;123(4):241‐9. [DOI] [PubMed] [Google Scholar]
Sood 2007
- Sood A, Midha V. Epidemiology of inflammatory bowel disease in Asia. Indian Journal of Gastroenterology 2007;26(6):285‐9. [PubMed] [Google Scholar]
Thiéfin 2005
- Thiéfin G, Beaugerie L. Toxic effects of nonsteroidal antiinflammatory drugs on the small bowel, colon, and rectum. Joint, Bone, Spine 2005;72(4):286‐94. [DOI] [PubMed] [Google Scholar]
Vane 1971
- Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin‐like drugs. Nature: New Biology 1971;231(25):232‐5. [DOI] [PubMed] [Google Scholar]
Ziemer 2007
- Ziemer M, Wiesend CL, Vetter R, Weiss J, Blaschke S, Norgauer J, et al. Cutaneous adverse reactions to valdecoxib distinct from Stevens‐Johnson syndrome and toxic epidermal necrolysis. Archives of Dermatology 2007;143(6):711‐6. [DOI] [PubMed] [Google Scholar]


