Abstract
The importance of essential oils and their components in the industrial sector is attributed to their chemical characteristics and their application in the development of products in the areas of cosmetology, food, and pharmaceuticals. However, the pharmacological properties of this class of natural products have been extensively investigated and indicate their applicability for obtaining new drugs. Therefore, this review discusses the use of these oils as starting materials to synthesize more complex molecules and products with greater commercial value and clinic potential. Furthermore, the antiulcer, cardiovascular, and antidiabetic mechanisms of action are discussed. The main mechanistic aspects of the chemopreventive properties of oils against cancer are also presented. The data highlight essential oils and their derivatives as a strategic chemical group in the search for effective therapeutic agents against various diseases.
Keywords: essential oil, cardiovascular, antidiabetes, antiulcer, cancer chemopreventive, organic synthesis, natural products, metabolites, medicinal plants, volatiles
1. The Transformation of Readily Available Essential Oil Constituents into High-Value Products
Essential oils have been used by humanity for thousands of years, basically due to their very pleasant aromas, flavors, and relevant pharmacological properties. This has led to the creation of the flavor and fragrance industry and a significant participation in the pharmaceutical industry. The major modification observed over the last two centuries is the change from the direct utilization of the essential oils and their major constituents to new chemical products obtained by their synthetic transformations. The principal essential oils usually present a major component, associated with a characteristic aroma, and they are potentially a great starting material for more complex compounds of highly increased commercial value. In this review, we describe and discuss some major essential oils, their principal components, and their synthetic transformations into more highly valued products [1,2]. Clearly, essential oils are an important part of the renewable biomass already in use.
Essential oils, as obtained from nature by classical methods, are generally composed of very complex mixtures of terpenoids and phenylpropanoids, both groups being of scientific and commercial interest. The major constituents are monoterpenes with 10 carbon atoms, in various carbon skeleton arrangements, with none or few functional groups, but usually available in both enantiomeric forms. Organic chemists describe these compounds as members of the chiral pool, an extremely important gift from nature that allows us to synthesize much more complex molecules, both of academic and industrial interest, in their pure enantiomeric forms [3]. As is well accepted, odor, flavor, and pharmacological properties are all directly related to their enantiomeric purity.
At this point, we should mention organic synthesis as being the science that allows the transformation of these relatively simple essential oil constituents into the desired finished products. A parallel situation exists in nature where simple molecules are transformed into much more complex molecules, and where nature is the supreme artist in this activity. Synthetic transformation is a sequence of constructive reactions in which the starting material molecule is structurally modified in the direction of the desired and proposed final molecules [4] (Figure 1).
Figure 1.
Organic synthesis, the constructive process.
This process is interactive, involving planning known as retrosynthetic analysis and then constructive execution of the strategy, with consequent modifications both of the plan and the execution as determined by the ongoing experimental results obtained [5].
The latest experimental revolution is the introduction of a new enabling technology for synthetic execution. The traditional equipment set-up is the batch reactor made of glass or metal, in which a defined and limited quantity of starting materials are transformed into products. We are now using the continuous flow technology [6,7] in which the starting materials are pressurized into a continuous flow and then pass through the requisite reactors, and other required equipment, leading then to the collector. This technology can be run even on a 24 h per day routine, and obviously multiplied up with parallel systems. This technology is being transplanted from the research environment into the pharmaceutical industry with increasing intensity and success.
We shall focus on Brazilian essential oils, which represent a major world market but are also similar to those of other major third world producers; that is, mainly exportation of the crude essential oils but relatively little local synthetic exploration.
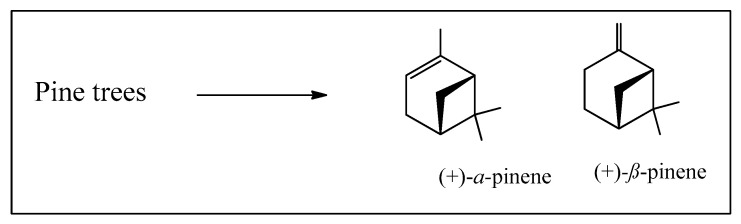
We will start with pine trees and their essential oils, historically known as turpentine, now substituted by petrochemically derived solvents in the paint industry. Pine trees are excellent examples of industrial excellence, as they furnish wood and thus paper, and as a secondary product, the essential oil is obtained from other parts of the tree. It is difficult to provide accurate and reliable amounts of the different essential oils being produced around the world. However, we will estimate turpentine oil production as being of the order of 400,000 tons/year worldwide. Certainly, the other major essential oils are being produced on much more modest scales. Pinus elliottii Engelm. (slash pine) has been successfully adapted to many parts of southern Brazil and, together with Pinus taeda L., is our main source of pinenes (Figure 2).
Figure 2.
Pine trees and the pinenes.
These essential oils are mainly composed of α- and β-pinenes, present in varying percentages, and one of the enantiomeric forms depending upon the species (Figure 2). What can we do with them apart from using their excellent dissolving properties [8,9]? We have decided to carefully select examples of industrial transformations, which involve large-scale production while using relatively simple chemical processes. However, we will not detail further the major industrial operation which is the production of diverse polymers, including “natural” rubbers.
The major transformation of the pinenes is in the production of α-terpineol for its fragrance qualities and antimicrobial activity, already executed under continuous flow conditions [10]. A personal affirmation is that a natural chemical is identical to its synthetic version, only differing in their respective impurities, availabilities, and price.
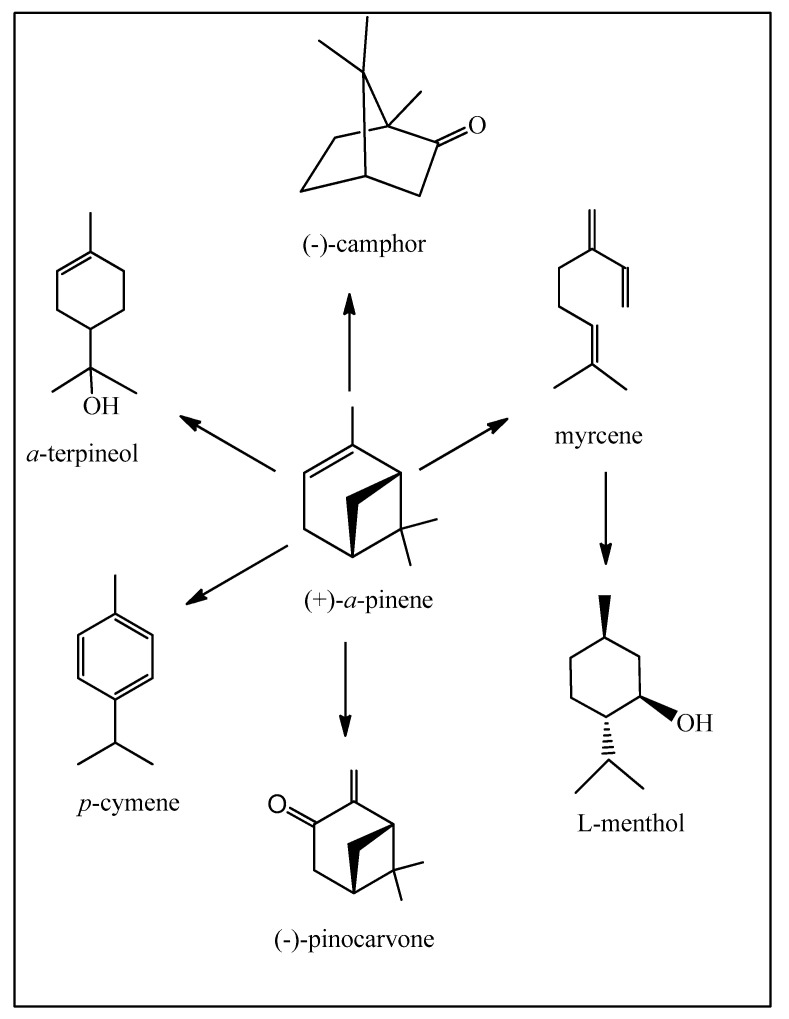
As a general rule we will not detail the synthetic transformations or specify the individual reactions performed but simply present the overall process from starting materials to products. The pinenes are also used to produce camphor, para-cymene, and myrcene (for transformation into L-menthol). These compounds have important fragrance and pharmacological properties. para-Cymene can be envisaged as a green solvent which can substitute the petrochemically derived solvents benzene, toluene, and the xylenes (Figure 3). The photo-oxidation of α-pinene to pinocarvone can be executed under continuous flow conditions [11,12].
Figure 3.
The pinenes to α-terpineol, camphor, p-cymene, pinocarvone, and myrcene (for L-menthol).
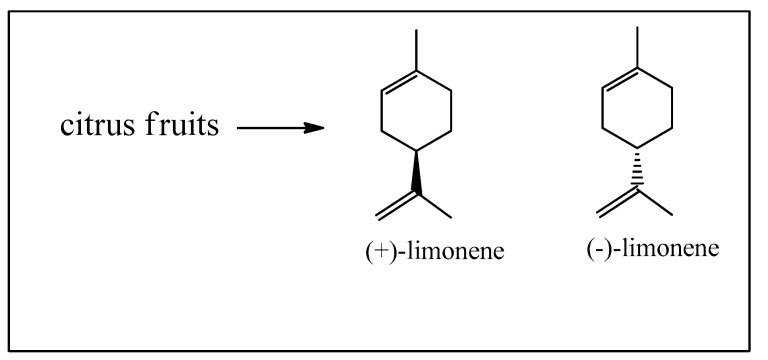
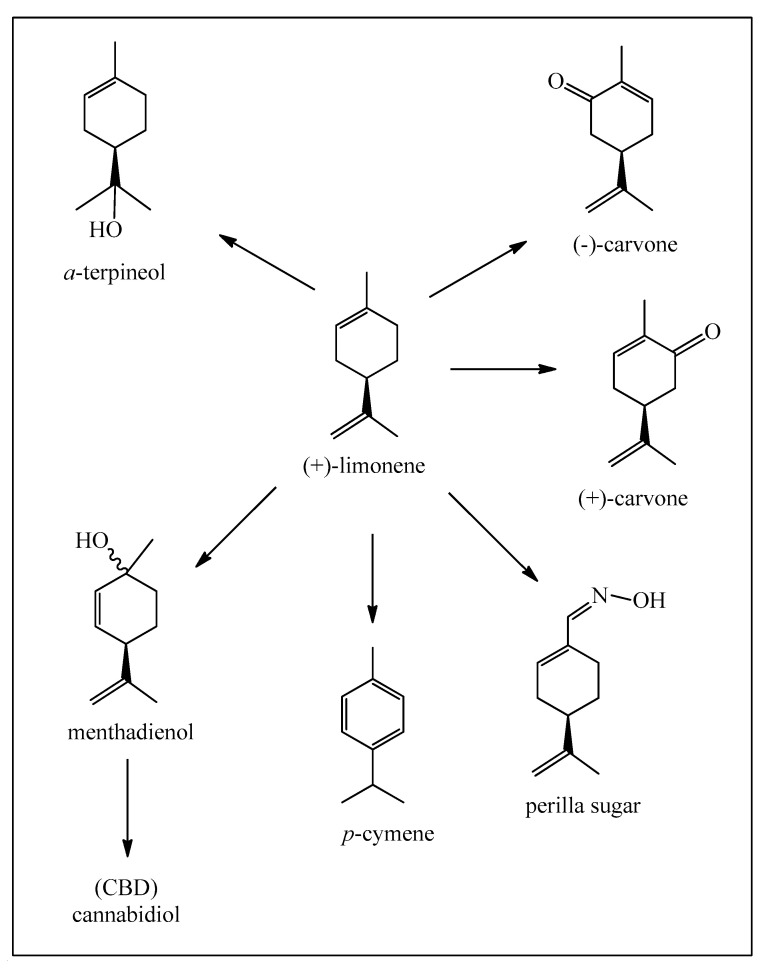
The second major starting materials are the enantiomeric limonenes, produced from citrus fruit essential oils obtained from the peel, as a secondary product to the immense citrus fruit juice market (Figure 4) [13].
Figure 4.
Citrus fruits and the limonenes.
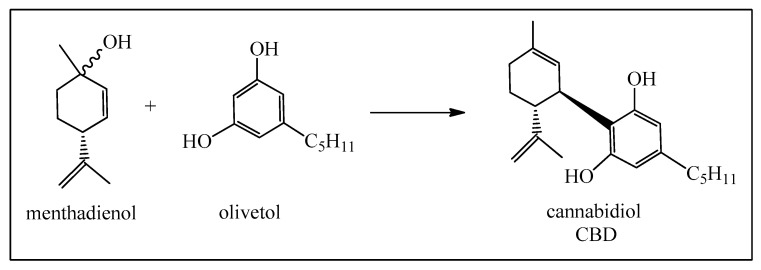
These compounds have a much simpler molecular structure than the pinenes but are also utilized in the production of α-terpineol. Besides important polymer production, limonenes are also used for the synthesis of para-cymene and enantiomeric carvones. Limonenes can be selectively oxidized at the isolated methyl group, leading finally to the oxime of perillaldehyde, usually known as perilla sugar, an excellent sweetener. Limonene can also be photo-oxidized to the p-menthadienol epimers (Figure 5) [14], and then directly transformed into cannabidiol (CBD) as shown in Figure 6 [15,16]. At this point, we should point out that the legalized and highly recommended cannabidiol (CBD) can be synthesized easily from limonene and also from α-pinene, citral, and isopulegol. A great advantage is that synthetic cannabinoids do not contain the psychoactive tetrahydrocannabinol (Δ9-THC), as opposed to mixtures isolated from Cannabis sativa L. which do and therefore are subjected to very strict legal controls.
Figure 5.
The limonenes to α-terpineol, para-cymene, perilla sugar, and the carvones; menthadienol and CBD.
Figure 6.
Menthadienols plus olivetol lead to cannabidiol.
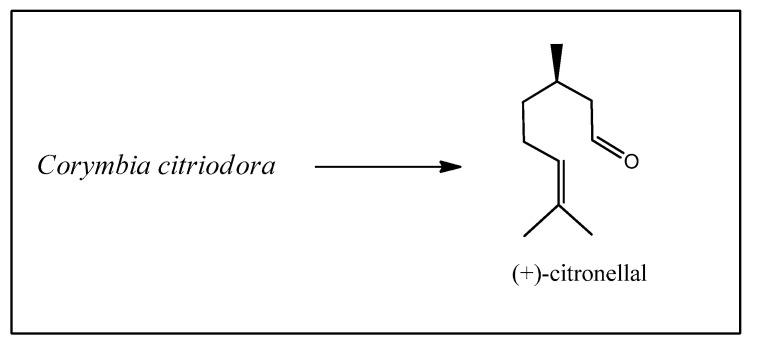
The next starting material is citronellal, obtained from the essential oil of Corymbia citriodora (Hook.) K.D. Hill & L.A.S. Johnson, a Eucalyptus species widely grown in Brazil for paper and pulp production (Figure 7) [17].
Figure 7.
Corymbia citriodora and citronellal.
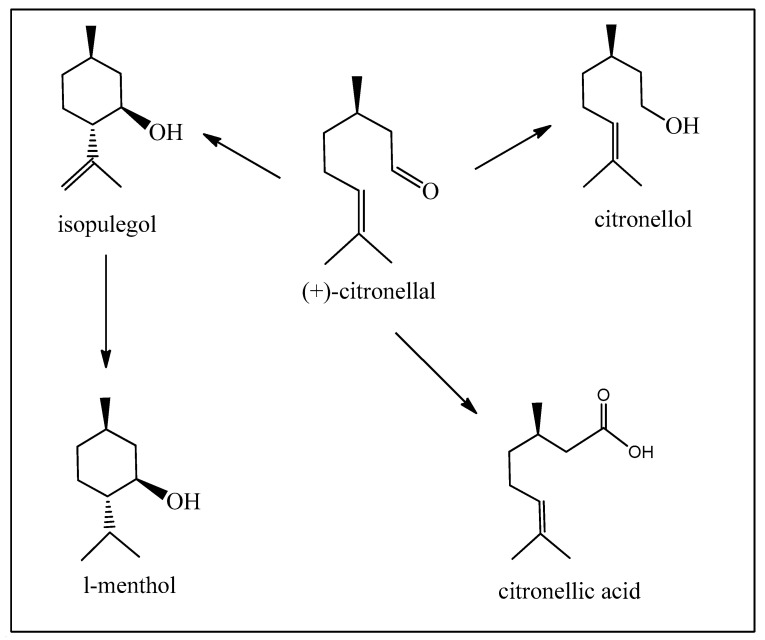
Citronellal should be the best starting material for obtaining L-menthol, a compound with desirable flavor and fragrance with local anesthetic and cooling properties. However, L-menthol is presently obtained from Mentha piperita L. essential oil, and the major part is produced by other synthetic procedures on a reasonably large industrial scale (Figure 8). The most important and acclaimed synthesis starts from the pinenes, which was developed by the company Takasago, and is responsible for the major quantities available worldwide (see Figure 3) [18,19,20]. This is basically due to the greater availability of pinenes.
Figure 8.
Citronellal derivatives; L-menthol from citronellal by way of isopulegol.
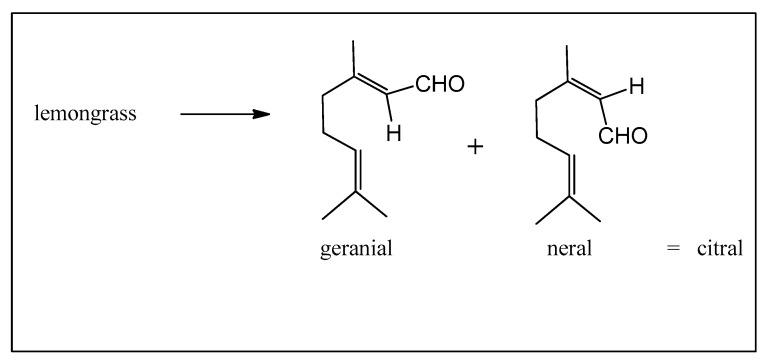
Citral, a variable mixture of geranial and neral, is available in several essential oils including lemongrass (Cymbopogon genus), widely grown in Brazil as a home remedy (Figure 9).
Figure 9.
Citral, geranial, and neral from lemongrass.
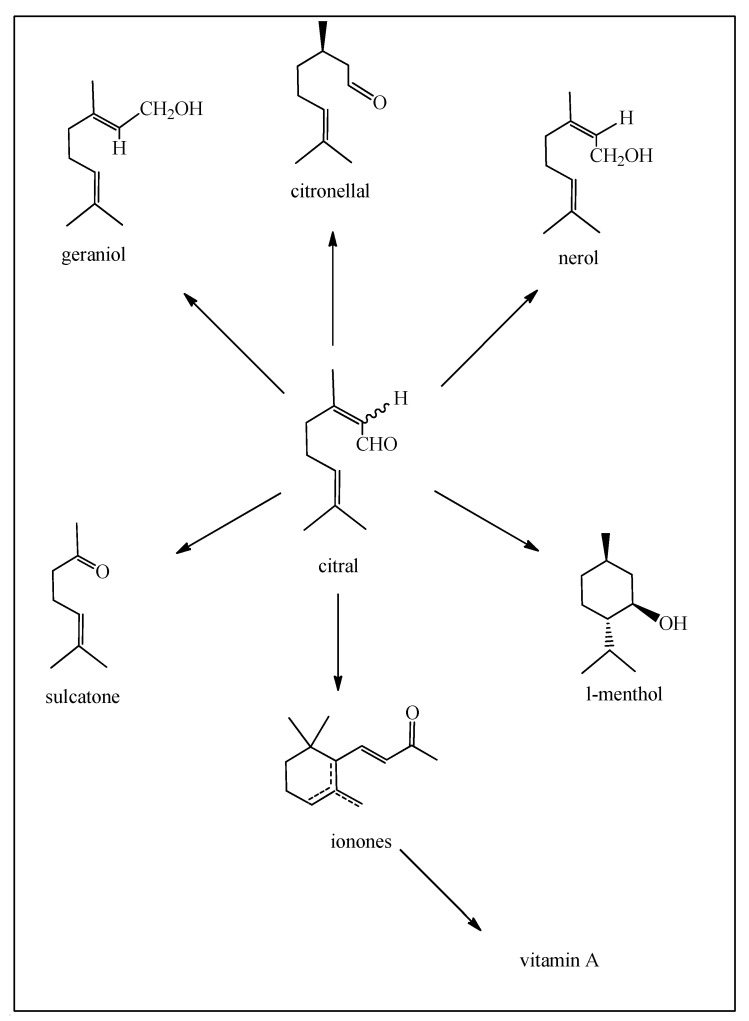
Citral can be used for the production of L-menthol [18,21], pseudo-ionone [22], the ionones, and then on to vitamin A (Figure 10), and also for certain certified cannabinoids.
Figure 10.
Citral-derived products.
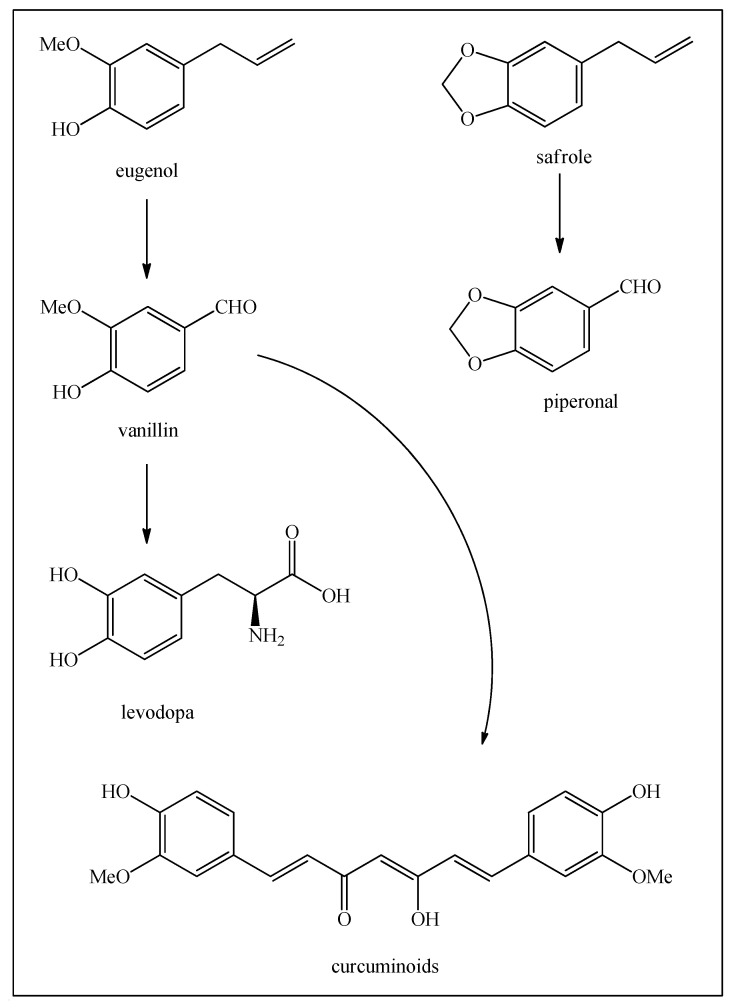
Of the phenylpropanoids, eugenol (found in many herbs, including cloves and cinnamon) is certainly the most useful at the moment, being transformed into vanillin, and is also available on a much larger scale from sugarcane lignins. Vanillin is then the starting material in the syntheses of levodopa and the curcuminoids (Figure 11) [23].
Figure 11.
Eugenol to vanillin, levodopa, and curcuminoids.
In a different synthetic strategy, essential oil constituents can be envisaged as starting materials in chemoenzymatic syntheses [24,25,26,27], also known as biocatalysis, in which (modified) enzymes are called into play. This is especially interesting when organic syntheses are shown to be less effective. The reason for this is usually the lack of selectivity in classical chemical reactions, with the formation of several products in lower yields, and demanding extensive mixture separations. The chemoenzymatic alternative, when possible, affords much higher selectivity and thus higher yields and easier product purification. At the present moment, much academic activity is clearly demonstrating the enormous potential of this alternative. We can imagine, in the very near future, syntheses involving one or more chemoenzymatic reactions together with more classical chemical reactions.
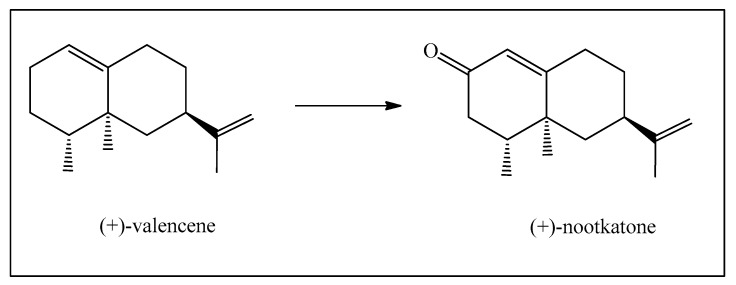
An excellent example is the transformation of valencene, isolated from certain citric fruit essential oils, into the fragrance nootkatone with a very marked grapefruit-like aroma. Although valencene obtained from the essential oil is not very pure and is now becoming relatively expensive, nootkatone is a highly prized product (Figure 12) [28].
Figure 12.
Valencene to nootkatone.
We should emphasize that all these readily available starting materials have been extensively used in elaborate syntheses to obtain highly complex molecules of more limited commercial interest. Here, we have concentrated on simpler synthetic procedures leading to high volume and commercially relevant products. The novelties reside in the new technological processes, principally continuous flow instead of batches, and chemoenzymatic and biocatalytic reactions substituting more conventional reagents.
2. Mechanisms of Pharmacological Action of Essential Oils
2.1. Mechanisms of Antiulcer Action of Essential Oils
Peptic ulcer disease (PUD) occurs in the stomach (gastric ulcer) and the first portion of the small intestine (duodenal ulcer). Signs and symptoms can include abdominal pain, upper gastrointestinal bleeding, gastric outlet obstruction, and perforation [29]. Among all complications, peptic ulcer bleeding is one of the most common clinical diseases [30] and its morbidity has not decreased [31]. The wounds caused by PUD can penetrate the gastric mucosa and reach the muscular coat, subsequently forming a cavity characterized by different stages of necrosis, neutrophil infiltration, blood flow reduction, increased oxidative stress, and acute and chronic inflammation [32,33].
PUD is a source of significant morbidity and mortality worldwide, with much of the burden in low-income and middle-income countries [34]. The prevalence of PUD has been estimated at around 5–10% [35] and it affects 4 million people worldwide annually [36]. Disequilibrium between defensive factors (bicarbonate production, mucus secretion, prostaglandins (PGs), nitric oxide (NO), and antioxidants) and aggressive factors (oxidative stress and free radicals, acid and pepsin, fall in the gastric blood flow, H. pylori infection, and non-steroidal anti-inflammatory drugs (NSAIDs)) in the gastric mucosa is reported to be a fundamental mechanism involved in the pathogenesis of disease peptic ulcer disease [30]
The decrease in the incidence of peptic ulceration in the last 30 years is partly due to the substantial progress in the pharmacological management of dyspeptic symptoms as well as to a significant understanding of its pathophysiology [37]. Management of PUD entails reducing the production of gastric acids and includes antiacids (sodium bicarbonate, aluminum hydroxide), H2 receptor antagonists (cimetidine, ranitidine, famotidine), proton pump inhibitors (omeprazole, lansoprazole, pantoprazole), and cytoprotective agents (sucralfate, bismuth or prostaglandin analogs). In the case of a gastric ulcer associated with H. pylori, treatment consists of a combination of antibiotics, such as amoxicillin or clarithromycin, and acid secretion inhibitors [38].
Despite good therapeutic efficacy, these agents often cause severe side effects, such as osteoporosis, gynecomastia, hypoacidity, hypergastrinemia, iron and vitamin B12 deficiencies, thrombocytopenia, and cardiovascular disease risks, that limit their clinical use [39,40,41,42,43,44].
For these reasons, research for the development of new gastroprotective agents that are safer for long-term use has attracted interest. Given this, natural products (medicinal/herbal plants and extracts) are considered attractive sources for potential new drugs and have shown promising results for the treatment of gastric ulcers [45,46,47]. The efficacy of herbal medicines is comparable or superior to synthetic drugs with fewer adverse effects and they have been proven as one of the best strategies for the disease management of ulcers [48,49].
2.1.1. Antioxidant Activity
Hyptis martiusii Benth. (Lamiaceae) is an aromatic plant found in northeastern Brazil. The leaves of this plant are used against diseases of the stomach and intestine [50]. Caldas et al. [51] characterized the mechanisms of action involved in the gastroprotection of the essential oil of H. martiusii (EOHM) using an ethanol-induced gastric ulcer model in rats. Treatment with EOHM (400 mg/kg) decreased the rate of lipid peroxidation by significantly diminishing the production of malondialdehyde and reversing the reduction in levels of sulfhydryl groups in the mucosa in ethanol-induced gastric mucosal lesions in rats. In an in vitro study of radical scavenging activity (DPPH assay), the free radical scavenging ability of the EOHM showing that the EOHM exhibited no significant relative ability to promote the capture of the DPPH radical at any of the concentrations tested. Similarly, 1,8-cineole, a major component of the essential oil, was unable to promote the capture of DPPH.
In a new study with 1,8-cineole, also called eucalyptol, Caldas et al. [52] showed that oral administration of this monoterpene (100 mg/kg) was able to revert the reduction of the levels of sulfhydryl groups in the gastric mucosa by 62%, restoring the antioxidant system to base levels in the gastric ulcer model induced by ethanol in Wistar rats. The monoterpene decreased the rate of lipid peroxidation and myeloperoxidase activity (MPO) by 55.3% and 59.4%, respectively, by diminishing the production of malondialdehyde by ethanol when compared to the injured control group. Acute administration of ethanol to rodents produces gastric mucosal damage that involves intracellular oxidative stress, intracellular thiol groups, and microcirculation disturbances [53]. These results suggest the involvement of an antioxidant mechanism in the gastroprotective activity of 1,8-cineole and that compound action is related to the cytoprotection effect of the essential oil of H. martiusii.
Syzygium aromaticum L., known as clove, is a dried flower bud belonging to the Myrtaceae, rich in volatile compounds and antioxidants such as eugenol and α-humulene. Clove essential oil (CEO) is traditionally used in many disorders. The literature evidenced several remarkable properties, such as treatment for tooth infections and toothache [54] and antiangiogenic [55], antioxidant [56], and anti-inflammatory activities [57]. Recently, Hobani et al. [58] reported that eugenol (5 and 10 mg/kg, p.o.) exhibited a reduction of gastric damage by ethanol-induced gastric ulceration. The extent of inhibition was 83.55 and 41.06%, respectively. In addition, eugenol protected the NP-SH and GSH levels, and it reduced the gastric tissue MDA level. Alcohol causes depletion of the gastric wall mucosal barrier via ROS formation and an increase in permeability [59]. In turn, ROS generation can cause oxidative stress, with the main source of ROS being infiltrating inflammatory cells, such as neutrophils that react with lipids to produce lipid peroxidation [60]. This study demonstrates that eugenol has gastroprotective properties against ethanol-induced ulcers, probably via improvement of cellular antioxidant defense. These results are in line with the works carried out by Barboza et al. [61] and Jung et al. [62] who demonstrated that eugenol exerts an action on oxidative stress and exhibits antioxidant activity and a protective effect against gastric damage, suggesting that eugenol has therapeutic potential for the treatment of inflammatory conditions such as gastritis.
2.1.2. Mucosal Proliferation
Pogostemonis Herba is a known traditional Chinese medicinal herb in Southeast Asia. It originates from the dried aerial part of Pogostemon cablin (Blanco) Benth. (Labiatae). Clinically, Pogostemonis Herba has been used by traditional Chinese physicians to treat a large number of medical conditions, for example, common cold, diarrhea, and H. pylori-related gastritis [63]. According to the literature, among the majority components of P. cablin (Blanco) Benth. essential oil (Lamiaceae) are patchouli alcohol, β-patchoulene, and pogostone, which have anti-inflammatory and gastroprotective activities [64,65,66].
Patchouli alcohol is a tricyclic sesquiterpene and also the major active ingredient from the essential oil of Pogostemonis Herba. This terpene has exhibited diverse pharmacological activities, for instance, enhancing cognition in mice with memory impairment induced by scopolamine and anti-inflammatory and anti-influenza virus activities in vitro and vivo [67]. A study developed by Zheng et al. [68] investigated the antiulcerogenic properties of patchouli alcohol (PA) against various experimental gastrointestinal ulcerations in rats. Animal groups pretreated with PA (10, 20, and 40 mg/kg mg/kg, p.o.) exhibited a dose-dependent reduction of gastric damage by ethanol-induced gastric ulceration. The extent of inhibition for the doses employed was 49.20%, 63.89%, and 71.85%, respectively. The experimental model of ethanol-induced gastric ulcers is often employed to screen antiulcer compounds [64]. Ethanol damages the gastric mucosa by diverse routes, including the generation of oxygen-derived free radicals, lipid peroxidation, depletion of mucus, the reduction of antioxidant defense, and decreased prostaglandin level [69].
To verify the participation of mucus secretion in the gastroprotective effect of PA, the pylorus ligation-induced ulcer rat model was used. This model undergoes hypersecretion which leads to the accumulation of gastric acid in the stomach lumen and promotes the generation of oxidative free radicals [70], resulting in autodigestion of gastric mucosa and breakdown of the mucosal barrier [71]. The mucus layer is the primary defense of the gastric mucosa. It acts as a physical barrier against the aggressive effect of gastric juice [72]. Increased mucus secretion can increase the buffering of acids in gastric juice and can reduce mucosal damage mediated by oxygen free radicals [73]. Agents that increase the secretion of mucus are effective in preventing the ulcers induced by this method [74]. The authors showed that the amount of adhered gastric mucus was augmented (p < 0.05) by pretreatment with PA compared to the control group. In line with this view, it could be said that PA protects the gastric mucosa through enhanced mucus secretion and potentially plays an important role in gastric mucosal protection.
2.1.3. H+/K+ ATPase Activity
Cymbopogon citratus (DC) Stapf, belonging to the family Poaceae, popularly known as lemongrass, is an aromatic plant used in Brazilian popular medicine for the treatment of gastric and nervous disorders [75]. The essential oil from C. citratus (EOCC) is mostly composed of the monoterpenes citral, nerol, and geraniol [76]. Venzon et al. [77] showed that EOCC, citral, and geraniol at doses of 1–100 mg/kg (p.o.) exerted marked protection of the gastric mucosa in an acute ethanol-induced ulcer model. In addition, it was demonstrated that the essential oil of C. citratus and citral at 100 μg/mL were able to inhibit, in vitro, the activity of the H+/K+ ATPase by 28.26% and 44.36%, respectively. However, the geraniol did not inhibit this enzyme. The gastric H+/K+ ATPase, a member of the P2-type ATPase family, found in gastric parietal cells, is known to transport H+ against a concentration gradient, leading to acid secretion [78]. It has been well-established in the literature that effective proton–potassium ATPase inhibitors (PPIs) are potential antiulcerative agents since they interfere with the cascade of events of gastric ulcerations [79]. PPIs play a crucial role in the management of gastroesophageal reflux disease, Barrett’s esophagus, and in the treatment of Helicobacter pylori infection [80]. Based on the findings, it is possible to infer that the inhibition of the effect of the proton pump exerted by EOCC and citral may be related to their gastroprotective effect and that is not an essential mechanism involved in the gastric healing effects promoted by geraniol.
2.1.4. Nitric Oxide
Nitric oxide (NO), a potent vasodilator, appears to be a major regulator of blood flow and gastric microcirculation [81]. NO has been shown to protect against ethanol-induced gastric lesions, whereas inhibition of NO synthesis (NOS) has been demonstrated to increase the susceptibility of the stomach to ethanol injury [82].
Croton rhamnifolioides Pax & Hoffm. (Euphorbiaceae) is an aromatic plant species used in folk medicine to treat inflammation and ulcers [83,84]. A previous study identified 57 compounds in the essential oil of C. rhamnifolioides, whose major constituents were sesquicineole, α-phellandrene, 1,8-cineole, and (E)-caryophyllene [85]. Martins et al. [86] investigated the anti-inflammatory activity of the essential oil from C. rhamnifolioides leaves (OEFC) and 1,8-cineole, its major constituent. OEFC (25 mg/kg) and 1,8-cineole (10.33 mg/kg) reduced edema in several models of inflammation (histamine, arachidonic acid, carrageenan, croton oil, 1% dextran, and granuloma), indicating regulatory action on the release of inflammatory mediators. Intending to investigate the gastroprotective properties of this plant, Vidal et al. [87] evaluated C. rhamnifolioides essential oil (OECC) using the absolute ethanol, acidified ethanol, or indomethacin gastric lesion model in mice. Animals that were pretreated orally with OECC (200 mg/kg) showed a significant reduction in lesions. Administration of L-arginine (600 mg/kg, i.p.), a substrate for NOS, also reduced the lesion area, and the pretreatment of L-NAME (20 mg/kg, i.p.), an inhibitor of nitric oxide synthase, reversed both protective effects of L-arginine and OECC, suggesting the likely participation of nitric oxide in the gastroprotective activity of OECC. In agreement with this study, Lima-Accioly et al. [88] demonstrated that the gastroprotective effect of an essential oil obtained from Croton nepetaefolius is associated with the same mechanism.
Nitric oxide (NO) appears to be a key mediator of gastrointestinal mucosal defense mechanisms, such as gastric blood flow and gastric microcirculation [89]. Nitric oxide synthase (NOS)-derived NO causes vascular dilation by stimulating soluble guanylyl cyclase and increasing cGMP in the smooth muscle cells. Therefore, nitric oxide plays an important role in the gastric mucosa against ethanol-induced gastric lesions, and conversely, inhibition of NO synthesis increases the susceptibility of the stomach to ethanol injury [90]. In conclusion, when OECC is administered orally it exerts its gastroprotective activity by mechanisms that involve at least the participation of nitric oxide. 1,8-Cineole, the major component in OECC (18.32%), may be one of the components responsible for the observed effect [52].
2.1.5. Prostaglandin E2 Levels
Citrus belongs to the family Rutaceae and is one of the main fruit tree crops grown throughout the world. Citrus essential oil is an important biologically active product from citrus peel. The citrus EO is composed of tens to hundreds of various compounds [91]. Citrus lemon Burm. f. (Rutaceae) is the third most important cultivated citrus species with a production of 7.3 million tons around the world annually [92]. The phytochemical analysis of Citrus lemon essential oil (CLEO) showed that its main compounds are two monoterpenes, limonene, and β-pinene [93].
Limonene, a monocyclic monoterpene, is a major constituent in several citrus oils (orange, lemon, lime, and grapefruit). It is effective in relieving heartburn and gastroesophageal reflux disorder. In animal studies, this monoterpene has also demonstrated chemoprotective activity for several types of cancer [94]. β-Pinene is a bicyclic hydrocarbon found in many essential oils from plants. This monoterpene has antimicrobial, anti-inflammatory, gastroprotective, and cytoprotective activities [95].
For establishing the gastroprotective action mechanism of CLEO and its main compounds, limonene and pinene, Rozza et al. [96] evaluated whether the gastroprotective effect from CLEO is due to its main compound limonene or the synergic activity of all components of this oil. To this end, three experimental models were used to investigate the bioactivity of the products: gastric ulcers induced by ethanol and indomethacin and their anti-Helicobacter pylori activity in vitro. The results indicate bioactive CLEO (250 mg/kg) and limonene (177 mg/kg) in gastroprotection (p < 0.05) in ethanol- and indomethacin-induced gastric ulcer models, while β-pinene (33 mg/kg) did not exert effective gastroprotection (53.26% and 37%, respectively).
Indomethacin (IND) induces gastric mucosal damage by ROS through a significant increase in membrane lipid peroxidation [97]. In addition, IND blocks the gastroprotective effects of prostaglandin E2 (PGE2) that augments mucus and bicarbonate secretions as well as gastric blood supply [98]. Thus, the involvement of PGE2 in the gastroprotective effect of CLEO and limonene was evaluated using the indomethacin-induced ulcer model. The results showed that the gastroprotective effect of CLEO (250 mg/kg, p.o.) includes the maintenance of PGE2 levels with indomethacin administration (30 mg/kg). Meanwhile, in the limonene group, the results indicate that the gastroprotective effect against indomethacin is not related to PGE2 level maintenance. Thus, data analysis shows that maintenance of PGE2 levels is observed in the gastroprotective mechanism of CLEO, but it was not suggested in the limonene mechanism.
Patchouli, known as Pogostemon cablin Benth. and belonging to the family Lamiaceae, is an important aromatic plant from Southeast Asia. Various bioactive compounds have been identified in patchouli and, among the compounds, pogostone is of great importance [99,100]. An earlier study by Chen et al. [101] reported the strong potential of pogostone in an indomethacin-induced gastric ulcer model in rats. Pogostone (10, 20, and 40 mg/kg) demonstrated inhibitory activity with the highest inhibition rate at a dose of 40 mg/kg (82.2%). Furthermore, a lower but still significant level of gastroprotection was obtained with 20 and 10 mg/kg pogostone resulting in an average gastric ulcer inhibitory rate of 56.6% and 47.5%, respectively. Pogostone (10, 20, and 40 mg/kg) also promoted the increment of gastric mucosal PGE2 levels (58.6, 64.9, and 67.6 ng/g) in a dose-related manner and significantly increased both COX-1 and COX-2 expressions. Additionally, pogostone (10, 20, and 40 mg/kg) significantly augmented endogenous SOD, GSH, and CAT activities, diminishing the constituent MDA level in the gastric mucosa in a dose-dependent manner. From their results, it was concluded that pogostone displayed gastroprotective action by enhancing gastric mucosal defensive factors which is rooted in the modulation of PGE2 levels and enhancement of the cellular antioxidant mechanism. Therefore, it could be a good therapeutic agent for the treatment of gastric ulcers.
2.1.6. Reduction of Bacterial Colonization: Helicobacter pylori
Geraniol is an acyclic isoprenoid monoterpene found in essential oils of several aromatic plants, such as Cinnamomum tenuipilum Kosterm (Lauraceae) and Cymbopogon citratus (DC) Stapf [77]. Furthermore, this monoterpene has been shown to exhibit important pharmacological properties, including antioxidant, anti-inflammatory, antimicrobial, and antitumor activities [102].
Recently, Bhattamisra et al. [103] investigated the antiulcer and anti-Helicobacter pylori activity of geraniol in an experimental chronic gastric ulcer model (acetic acid ulcers induced by submucosal injection of acetic acid). Treatment with geraniol at doses of 15 and 30 mg/kg (i.d.) resulted in fewer dilated blood vessels and hemorrhagic streaks on the gastric mucosal surface, producing a significant reduction in the ulcer index. The results showed that geraniol attenuated (p < 0.05) the extent of damage in the stomach induced by acetic acid by 42% and 52%, respectively.
In the rapid urease test (RUT), geraniol (15 and 30 mg/kg) showed a 17% and 33% reduction in H. pylori-positive antral samples, respectively. These results were supported by the histopathological data. Treatment with geraniol (30 mg/kg) reduced inflammation (p < 0.05) with a reduction in lesion scores and decreased bacterial load in the gastric mucosa in comparison to ulcer control in the H. pylori group. The RUT is an indirect test of the presence of H. pylori based on the presence of urease in the gastric mucosa [104]. The sensitivity of the RUT is high and has been reported to vary between approximately 80% and 100% with specificity between 97% and 99% [105]. Based on these findings, Bergonzelli et al. [106] determined that geraniol had a minimal inhibitory concentration (MIC) of 2 mg/L and inhibited 92% of H. pylori growth.
The antiulcer and anti-H. pylori actions of geraniol may involve direct gastroprotective effects, as well as an antibacterial action against H. pylori. It is important to highlight the report of the great healing and gastroprotective activity of several monoterpenes. However, they do not have anti-H. pylori action (in vitro). Therefore, geraniol has potential use in the treatment of peptic ulcers associated with H. pylori.
2.1.7. Inflammation: Role of Proinflammatory Cytokines
The acetic-acid-induced gastrointestinal ulcer model is similar to human ulcers in terms of location, severity, and chronicity. This model has proven suitable for investigating the effect of treatment on the healing process of chronic gastrointestinal ulcers [107] and it has been used to screen antisecretory and cytoprotective drugs [108]. Gastric ulcer occurs due to changes in multiple factors including prostaglandins, growth factor, nitric oxide, cytokine, microcirculation, and mucus adhesion [109]. After a gastric ulcer is induced by acetic acid, gastric inflammation increases interaction between leukocytes and endothelial cells characterized by the migration of macrophages in the ulcer area. The migrated macrophages then release proinflammatory cytokines such as TNF-α and interleukin-1β (IL-1β) [110,111]. TNF-α and IL-1β are considered parameters of the systemic inflammatory reaction and represent two important factors in the pathogenesis of gastric ulcers, contributing to many forms of gastric mucosal damage [112,113].
Gallesia integrifolia (Spreng.) Harms (Phytolaccaceae), a tree popularly known as the “garlic plant”, is a native and endemic plant of Brazil [114]. In traditional medicine, the bark of this species is utilized to prepare teas for treating ulcers, cough, flu, pneumonia, vermin, gonorrhea, prostate tumors, and rheumatism [115,116].
Arunachalama et al. [117] evaluated the gastric antiulcer action of the essential oil of the inner stem bark of G. integrifolia (EOGI). In this work, the authors described the protective effect of oral treatment of EOGI on gastric lesions induced by several necrotizing agents and found that EOGI (5, 20, or 80 mg/kg, p.o.) significantly reduced the levels of TNF-α and IL-1β in mice subjected to gastric ulcer induced by 99.5% acetic acid (20 mL). These results suggest that the ulcer healing effect of EOGI on gastric mucosal injury is probably related to a decrease in the TNF-α and IL-1 beta levels in inflammatory tissue.
Chen et al. [66] evaluated the antiulcerogenic potential of pogostone using ethanol-induced gastric ulcers in rats as an experimental model. Pogostone (10, 20, and 40 mg/kg) exhibited a dose-dependent protective effect against ethanol gastric lesions (44.87, 76.84, and 95.05%, respectively) and restored the depletion of NP-SH and increased PGE2 levels. Moreover, pogostone at a dose of 20 and 40 mg/kg was able to reduce the levels of the proinflammatory cytokines TNF-α and IL-6, however, the low dose did not achieve statistical significance. On the other hand, pogostone at all tested doses increased the anti-inflammatory factor IL-10 in a dose-dependent manner. In conclusion, the results indicate a cytoprotective role of pogostone affording gastroprotection against gastric damage induced by ethanol, which is possibly mediated, in part, by endogenous prostaglandins, enhancement of antioxidant activity, and reduction of the secretions of proinflammatory mediators, including the high level of anti-inflammatory cytokine in rats exposed to ethanol.
β-Patchoulene (β-PAE) and patchoulene epoxide (PAO), obtained from the essential oil of Pogostemon cablin (Blanco) Benth., were evaluated concerning the protective effect against ulcers produced by indomethacin and in ethanol-induced gastric ulcer models, respectively. Wua et al. [118] demonstrated that β-PAE (10, 20, and 40 mg/kg) reduced gastric damage in the order of 33.84%, 61.53%, and 78.40%, respectively. The histopathological analysis confirmed that β-PAE-treated groups displayed less mucosal damage by indomethacin according to the decreasing ulcer area. The authors also showed that β-PAE exerted an antiulcer effect by inhibiting TNF-α-activated, NF-κB, and JNK signaling pathways. Moreover, Liang et al. [119] evidenced that pretreatment with PAO (10, 20, and 40 mg/kg) was also effective in reducing ethanol-induced gastric injuries by 67.23%, 82.15%, and 84.93%, respectively. Additionally, PAO was able to reverse the increase in the levels of proinflammatory cytokines (TNF-α and IL-1β) induced by ethanol, also modulating the expression of NF-κB-pathway-related proteins, including p-IκBα, IκBα, p-p65, and p65. Taken together, these results show that β-PAE and PAO have a broad spectrum of gastroprotective activity that overcomes the harmful actions of indomethacin or ethanol on the gastric mucosa and reveal the potential use of these compounds as therapeutic agents in the treatment of ethanol- or NSAID-associated gastropathy.
2.1.8. Cell Proliferation
Epidermal growth factor (EGF) is a protein responsible for activating mesenchymal and epithelial cells, stimulating epidermal proliferation and repair after injury [120]. Previous studies have demonstrated that EGF is involved in gastric ulcer healing in experimental gastric and duodenal ulcers in animal models, for example, cold restraint stress [121] and acetic acid [122]. This polypeptide inhibits gastric acid secretion, increases mucus release, and is active in upper and lower gastrointestinal lesions, stimulating cells involved in the healing process [123,124].
The vascular endothelial growth factor (VEGF) participates in the protection of the gastric mucosa, increasing vascular permeability and reducing the area of hemorrhagic lesions [123]. In addition, this peptide can contribute to the development of the angiogenic response that regulates the reconstruction of microvessels and connective tissue cells, contributing restoration of the mucosal architecture, thereby promoting ulcer healing [124,125]. The production of VEGF, occurring in the normal gastric mucosa, is significantly enhanced in the gastric mucosa after injury induced by alcohol [126], dexamethasone [127], or acetic acid [128]. Hence, these growth factors (EGF and VEGF) stimulate with variable power all the cellular elements needed to cure ulcers.
Recently, Bueno et al. [129] investigated the mechanisms of the essential oil from Baccharis trimera (EOBT) in gastroprotection against acute gastric ulcer lesions caused by absolute ethanol and a chronic model of gastric lesions induced by acetic acid in rats. This study showed that oral pretreatment with EOBT (100 and 200 mg/kg) decreased gastric injury by 94% and 98%, respectively, in ethanol-induced gastric ulcers. In the gastric lesions induced by acetic acid, EOBT (100 mg/kg) was effective in healing gastric ulcers after 10 (65.5%) and 14 days (61%) of treatment. However, EOBT (100 mg/kg) only once a day for seven days was not able to heal gastric lesions, despite reducing the lesion area (46.7%). To determine whether vascular endothelial growth factor (VEGF) and epidermal growth factor (EGF) are involved in the gastroprotection of EOBT, gastric lesions induced by acetic acid were assessed. The data showed that there was no change in EGF expression in the stomach of rats treated for 14 days with EOBT (100 mg/kg). While in the gastric mucosa of rats treated with the essential oil, there was a significant increase in VEGF expression when compared to the control group. Taken together, these data suggest that the expression of VEGF but not EGF can favor the acceleration of ulcer healing by EOBT determined at the late stages of this healing process. The analysis of the chemical composition of EOBT indicated that it contains carquejyl acetate, ledol, and carquejol as the major components, which may contribute to the biological properties of this essential oil.
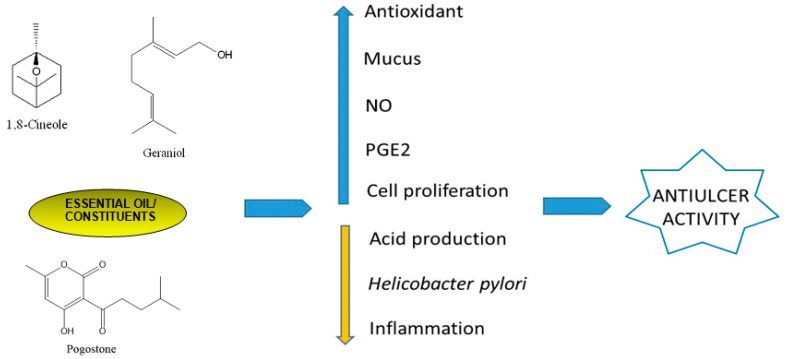
In conclusion, essential oils have been the target of many investigations due to their pharmacological properties, such as the antiulcer effect on models of gastric ulcers. antiulcer effect. The antiulcer activity of these natural products can be attributed to several mechanisms, e.g., antioxidant, inhibition of acid secretion, increase in mucus content, and activity against H. pylori (Figure 13).
Figure 13.
Mechanisms of action proposed for essential oils and constituents with antiulcer activity.
2.2. Mechanisms of Chemopreventive Action of Essential Oils
Cancer is a disease responsible for a high number of deaths annually and is considered a serious public health problem. For example, in 2022, around 20 million new cases of cancer emerged, and 9.7 million deaths caused by this disease were recorded. It is estimated that one in five people of both sexes develops cancer during their lifetime. Also alarming is the proportion of deaths: around one in nine for men and one in twelve for women [130]. The risk factors include family history, tobacco/alcohol use, obesity, infection, and dietary factors, such as low consumption of fruit/vegetables and high consumption of heat-processed meat and red meat. Performing physical activity regularly can also be included as a beneficial recommendation in reducing the occurrence of cancer and other diseases [130,131].
In the second half of the last century, more than 600,000 compounds were evaluated for their anticancer activities. However, only about 40 drugs were viable for clinical use [132,133]. There are several challenges to be overcome in searching for new pharmacotherapeutic approaches and chemical agents with anticancer and/or chemopreventive properties. Therefore, the search for new alternative treatments for medicinal and preventive use must be a priority.
Cancer chemoprevention is established as the use of natural, synthetic, or biological agents to restrict, reverse, or prevent the early stages of carcinogenesis or the advancement of premalignant cells into invasive disease. These agents are known as blockers and suppressors and act by interrupting multiple pathways and processes of tumor development, such as detoxification of electrophilic reactive substances and elimination of free radicals, reduction of cellular uptake, and metabolic activation of procarcinogens, in addition to stimulation of repair pathways [132,134].
Many natural agents with chemopreventive properties are found in foods and are present in the diet, including β-carotene [135], retinol [132], and lycopene [136], which are not constituents of essential oils. These compounds contain isoprene units in their chemical structures, similar to those found in antitumor monoterpenes from essential oils [137,138]. Therefore, the inclusion of foods rich in these essential oil components in the daily diet could be an interesting strategy to prevent or inhibit the progress of the early stages of cancer.
Among essential oil components, isoprene derivatives can be highlighted as promising cancer chemopreventive and antitumoral agents [139,140,141,142]. The protective effects of this class of bioactive compounds have been shown in different models of carcinogenesis in rodents [143]. In addition, the inhibitory effects of isoprene derivatives have been extensively shown in several human cancer cell lines [142]. Monoterpenes such as cyclic D-limonene [144] and acyclic geraniol [145,146] and sesquiterpenes such as acyclic farnesol [147] are examples of promising isoprene derivatives for their cancer chemopreventive and antitumoral potential.
Initial cancer chemopreventive and antitumoral mechanistic focus was directed towards isoprene derivatives’ inhibitory effects on 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase activity [140,142]. This is the main enzyme regulating cholesterol biosynthesis in mammalian cells through the mevalonate pathway [148]. It is particularly important for normal cell proliferation as it provides cholesterol, which is a component of cellular membranes, as well as farnesyl and geranylgeranyl pyrophosphates that activate proto-oncogenes including RAS and RHO [148]. This isoprenylation process is key for their binding to the plasma membrane and cell signaling [149]. Different cancer cells including hepatic and mammary ones present higher HMG-CoA reductase activity due to loss of its cholesterol-mediated negative feedback control, favoring cell cycle progression [142,150,151]. Both mammal and plant cells share the initial steps of the mevalonate pathway, which originates cholesterol in the former and the thousands of isoprene derivatives in the latter [152]. Interestingly, while cholesterol transcriptionally inhibits HMG-CoA reductase, essential oils’ isoprene derivatives posttranscriptionally inhibit this enzyme [153,154]. Of note, cancer cells are especially sensitive to isoprene derivatives’ HMGCoA reductase negative feedback regulation, highlighting this enzyme as a key molecular target for cancer chemoprevention and treatment [142]. Importantly, in recent years, other molecular pathways and mechanisms implied in this class of cancer chemopreventive and antitumoral agents have been identified [144,145,147].
D-Limonene is a cyclic monoterpene found in the essential oils of citrus fruit peels including oranges, lemons, limes, and tangerines [155]. Inhibition of proto-oncogene prenylation is one of its main anticancer molecular mechanisms [156,157]. This can be accomplished through posttranscriptional inhibition of HMG-CoA reductase or farnesyl protein transferases [157,158]. Rodent skin carcinogenesis chemoprevention by D-limonene was associated with Ras signaling, as well as anti-inflammatory, antioxidant, and proapoptotic actions through increased Bax and decreased Bcl-2 expression [159]. Accumulating evidence shows that the monoterpene’s main anticancer cellular mechanisms include induction of apoptosis [144,160]. D-Limonene inhibition of lung cancer growth in vitro and in vivo involved induction of apoptosis, which was related to increased levels of cleaved PARP and Bax and decreased levels of Bcl-2, suggesting that the mitochondria-mediated intrinsic death pathway could be involved in cell death induction [161]. In addition, in this study, D-limonene autophagy induction, which was accompanied by increased atg5 expression, could be relevant for the apoptosis effects [161]. Increased Bax and caspase-3 and decreased Bcl-2 expression were associated with G2/M arrest and apoptosis induction in T24 bladder cancer cells [162]. A D-limonene-rich blood orange (Citrus sinensis (L) Osbeck) volatile oil was shown to dose-dependently inhibit cell proliferation and induce apoptosis, as well as to inhibit angiogenesis in SW480 and HT-29 human colon cancer cells [163]. These effects involved dose-dependent induction of Bax/Bcl-2 and inhibition of vascular endothelial growth factor (VEGF) expression [163]. Suppression of the PI3K/Akt pathway was suggested to be related to D-limonene apoptosis induction in LS174T human colon cancer cells [164]. Furthermore, MAP38 and ERK pathways are relevant ways of mediating D-limonene apoptosis induction in BW5147 murine lymphoma cells [165].
Geraniol is an acyclic monoterpene found in the essential oils of several aromatic plants such as lavender, citronella, and lemongrass [166,167]. It presented chemopreventive activities in rodent models of mammary [168], liver [152,169], renal [146], and tongue [170] carcinogenesis. In addition, it presented inhibitory effects in several cancer cell lines [145,146]. Geraniol’s cancer protective effects involve HMG-CoA-reductase-related [168,171] or independent effects [152,172]. In addition, geraniol has been shown to modulate oxidative stress [170], inflammation [173], cell proliferation [174], and apoptosis [152]. In vivo chemopreventive activities against tongue carcinogenesis by geraniol involved inhibition of phase I enzymes and induction of phase II antioxidant and carcinogen detoxifying enzymes via activation of transcription factor Nrf-2 [170]. In addition, apoptosis induction by geraniol during rodent carcinogenesis was accompanied by modulation of different molecular targets including Kim-1, NF-κB, PCNA, and p53 [146]. In vitro and in vivo growth inhibitory effects by geraniol against A549 human lung adenocarcinoma cells involved inhibition of cell proliferation and induction of apoptosis [175]. These protective effects of geraniol involved decreased activity of HMG-CoA reductase that was accompanied by decreased Ras binding to the plasma membrane [175]. On the other hand, hepatocarcinogenesis chemoprevention by geraniol involved inhibition of RhoA binding to the plasma membrane without decreasing HMG-CoA reductase, suggesting that the monoterpene could have inhibited the oncogene prenylation through geranylgeranyl transferase inhibition [169]. In MCF-7 human breast cancer cells, geraniol treatment elicited G1 and G2/M growth arrest and cell proliferation inhibition [172]. These effects were accompanied by inhibition of CDK 2 activity and expression of cyclins D1, E, and A, and CDKs 2 and 4, although inhibition of the mevalonate pathway through HMG-CoA reductase inhibition did not exert a relevant role [172]. Interestingly, inhibition of Hep-2 human hepatic cancer cell proliferation by geraniol involved inhibition of the mevalonate pathway at a step after lanosterol synthesis, suggesting that the monoterpene effects on cholesterol synthesis can be independent of HMG-CoA reductase modulation [174]. In addition, geraniol’s antiproliferative effects were accompanied by inhibition of protein prenylation [174]. Other relevant geraniol molecular targets in cancer cells include E2F8A [176] and HSP90 [177]. A systematic computational-based approach via network pharmacology proposed 38 potential geraniol molecular targets, several of which are involved in cancer pathways (estrogen receptors, G protein subunits, caspases, MAP kinases, ornithine decarboxylase 1, among others) [178]. This reinforces geraniol as a multi-targeted anticancer agent that seems to act through several molecular pathways, a feature that increases its effectiveness for cancer control [179]. Of note, geraniol was recently shown to upregulate PTEN through the downregulation of mir-21 in an in vivo breast cancer model [180]. This suggests that the monoterpene could also modulate epigenetic processes that are key for cancer prevention.
Farnesol is an acyclic sesquiterpene found in the essential oils of several aromatic plants such as lemongrass, citronella, rose, and musk [147]. It has presented chemopreventive activities in rodent models of pancreatic [181], liver [141,182], colon [183], and skin [184] carcinogenesis. In addition, it presented inhibitory effects in several cancer cell lines [147,185]. Farnesol’s cancer protective effects involve HMGCoA-reductase-related [152,186] or independent effects [183]. In addition, farnesol has been shown to modulate oxidative stress [187], inflammation [182], cell proliferation [152], and apoptosis [188]. Farnesol’s protective effects against chemical hepatocarcinogenesis were associated with the induction of SOD, GPX, and CAT antioxidant enzymes as well as inhibition of COX-2 and TNF-α proinflammatory markers [182]. Similar effects on these antioxidant enzymes were reported after farnesol treatment in rats submitted to colon [189] and lung [190] carcinogenesis. Skin carcinogenesis prevention by sesquiterpene involves inhibition of oxidative stress and inflammation, as well as reduction of cell proliferation and induction of apoptosis [184]. These protective effects were related to the inhibition of COX-2 and of the Ras/Raf/p-ERK1/2 pathway and to an increase in the proportion of Bax/Bcl-2. These results indicate that farnesol acts by interfering with multiple signaling pathways [184]. Apoptosis induction by farnesol in DU145 human prostate cancer cells involved PI3K/Akt and mitogen-activated protein kinase signaling pathways [191]. Similarly, HeLa human cervical cancer cells treated with farnesol also exhibited increased apoptosis and downregulation of the PI3K/Akt pathway [192]. Furthermore, other molecular targets have been implied in farnesol’s proapoptotic effects in cancer cells including PPARγ [193], STAT-3 [194], and the TF4-ATF3-CHOP cascade of ER stress [195].
2.3. Mechanisms of Cardiovascular Action of Essential Oils
Cardiovascular diseases (CVDs) have a major impact on global health as shown in the World Health Organization (WHO) reports, accounting for 31% of total deaths worldwide. It is expected that by 2030, 20% of the world’s population over 65 will have 40% of CVD deaths. Hypertension is more prevalent in developed countries, affecting approximately 45% of the general population, due to incorrect lifestyle and behavioral habits such as diets, alcohol abuse, physical inactivity, and stress [196]. Hypertension is characterized by a blood pressure greater than 130/80 mm Hg, according to the American College of Cardiology (ACC)/American Heart Association (AHA) guidelines and is strongly correlated with a high incidence of mortality, as it is the most direct causal risk factor for cardiovascular disease (CVD) leading to stroke and ischemic heart disease [197].
Anantha et al. [198] emphasized that blood pressure is a result of cardiac output and systemic vascular resistance. Vascular tone may be elevated due to increased α-adrenoceptor stimulation or increased peptides such as angiotensins or endothelins. The renin–angiotensin system is involved at least in some forms of hypertension (e.g., renovascular hypertension) and is suppressed in the presence of primary hyperaldosteronism. In this context, Takimoto-Ohnishi and Murakami [199] reported that the renin–angiotensin system (RAS) is a regulatory cascade that plays major physiological roles in blood pressure regulation and electrolyte homeostasis. The homeostasis of body fluids and sodium is controlled by the renin–angiotensin system. Angiontensin II is the most potent hormone of this system, as it plays an important role in regulating vascular tone, cardiac function, and renal sodium reabsorption [200].
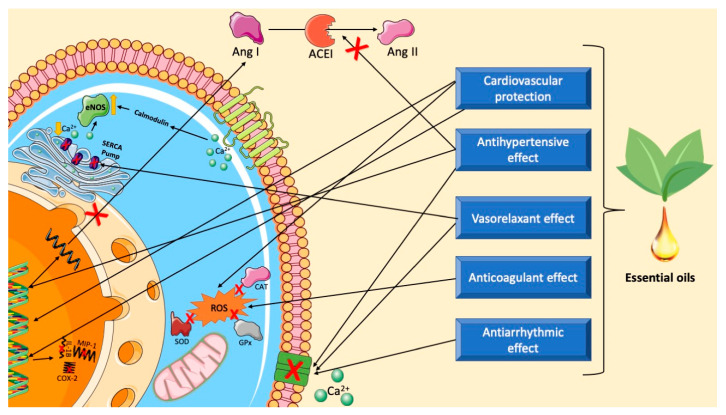
Currently, there are several groups of drugs widely used in the treatment of hypertension, such as diuretics, beta-blockers, angiotensin-converting enzyme (ACE) inhibitors, Ca2+ channel blockers, angiotensin II receptor antagonists, and renin inhibitors. However, some of the substances in these drug groups have significant side effects, mainly on the central nervous system [201]. As a result, studies indicate the effectiveness of compounds present in essential oils in reducing blood pressure and heart rate [202]. Figure 14 and Table 1 summarize some mechanisms underlying antihypertensive effects of essential oils.
Figure 14.
Pharmacological effects and potential targets of essential oils from plants against systemic arterial hypertension and implications thereof. Legend: Ang I—Angiotensin I; ACEI—Angiotensin I-converting enzyme inhibitor; Ang II—Angiotensin II; eNOS—endothelial nitric oxide synthase; SERCA—sarcoplasmic/endoplasmic reticulum Ca2+—ATPase; MIP-1—macrophage inflammatory protein-1; COX-2—cycloxigenase-2; IL-1β—interleukin-1β; CAT—catalase; ROS—reactive oxygen species; SOD—superoxide dismutase; GPx—glutathione peroxidase.
Table 1.
Essential oils from plant species and their pharmacological mechanisms on hypertension.
| Plant Species (Family) | Major Compounds from Essential Oils | Pharmacological Action | References |
|---|---|---|---|
| Seseli pallasii Besser (Apiaceae) | α-Pinene (42.7–48.2%) | Vasorelaxant and ACE-inhibiting effects | [203] |
|
Aframomum melegueta (Roscoe) K. Schum. and Aframomum daniellii (Hook.f.) K. Schum. (Zingiberaceae) |
Eugenol A. melegueta: 82.2% A. daniellii: 51.1% |
EO inhibited angiotensin I-converting enzyme activity | [204] |
|
Pogostemon elsholtzioides Benth. (Lamiaceae) |
Curzerene: 46.1% | Involvement of nitric oxide synthase and K+ channel activation | [205] |
| Alpinia zerumbet (Pers.) B.L.Burtt & R.M.Sm. (Zingiberaceae) | 1,8-Cineole (24.2%), terpinen-4-ol (20.4%), and p-cymene (15.7%) | Vasodilator effect mediated by inhibition of Ca2+ influx and release from intracellular storage, as well as an activation of the NOS/sGC pathway | [206] |
| Trachyspermum ammi Sprague (Apiaceae) | Thymol (38.1%), gamma-terpinene (33.3%), and p-cymene (23.1%) | Vasorelaxant effect by inhibition of extracellular Ca2+ influx via calcium channels | [207] |
| Artemisia campestris L. (Asteraceae) | Spathulenol: 10.1% | Vasorelaxation induced by AcEO via L-type calcium channels | [208] |
| Lippia alba (Mill.) N.E.Br. (Verbenaceae) | Citral | Vasorelaxant effect in isolated aorta, via three hypothesized mechanisms: blockade of Ca2+ influx or changes in calcium binding protein sensitization and/or intracellular calcium storage | [209] |
| Chrysopogon zizanioides (L.) Roberty (Poaceae) | Khusimol (8.2%), β-vetivenene (8.2%), β-funebrene (5.1%), β-vetispirene (4.8%), β-vetivone (4.7%), δ-selinene (4%), (E)-isovalencenol (3.3%), α-vetivone (3.3%), β-calacorene (3%), vetivonic acid (2.9%), and vetiselinenol (2.8%). | The root essential oil of C. zizanioides possesses a vasorelaxant effect through the muscarinic pathway as well as acts as a calcium channel blocker | [210] |
| Rosa damascena Mill. (Rosaceae) | 2-Phenyl-ethyl | Vasorelaxation by activation of large-conductance Ca2+-activated K+ (BKCa) channels | [211] |
2.3.1. Inhibition of Angiotensin Converting Enzyme (ACE)
Suručić et al. [203] described that Seseli pallasii Besser (Apiaceae) essential oil relaxed isolated endothelium intact mesenteric arteries’ rings precontracted with phenylephrine with IC50 = 3.10 nl/mL (IC50 = 2.70 μg/mL). The S. pallasii essential oil was found to exhibit a dose-dependent ACE inhibitory activity with an IC50 value of 0.33 mg/mL. The results suggested that a combination of vasorelaxing and ACE inhibitory effects of the S. pallasii essential oil might have potential therapeutic significance in hypertension.
Adefegha and Oboh [204] compared the action of captopril and essential oils of the Zingiberaceae family in hypertensive rats, and both inhibited angiotensin I converting enzyme activity, with essential oils having the highest ACE inhibitory activity, and this was attributed to the major constituent from Aframomum melegueta K. Schum. (alligator pepper) and Aframomum daniellii K. Schum. (African or “false” cardamom), eugenol.
The essential oil from Blepharocalyx salicifolius (Kunth) O. Berg (Myrtaceae family) showed 34 compounds. The sesquiterpenes were the largest fraction of 55.9%. The main component was spathulenol with 11.6%, which caused an antihypertensive effect with a decrease in systolic blood pressure (SBP) and diastolic blood pressure (DBP) (12% and 23%, respectively) and a decrease in cardiac ACE activity of 41.5% in spontaneously hypertensive rats [212].
2.3.2. Modulation NO/cGMP Pathway
The essential oil from Pogostemon elsholtzioides Benth. (Lamiaceae) induced dose-dependent vasodilation in precontracted aortic rings and a vasorelaxant effect, attributed to the major constituents, such as curzerene (46.10%), benzophenone, α-cadinol, and germacrone. There was also an antihypertensive effect, observed in hypertensive rats that had a systolic pressure of 100 mmHg and a diastolic pressure of 64.77 mmHg at the beginning of the experiment. After administration of EO, there was a decrease to 84.18 mmHg of systolic pressure and 21.69 mmHg of diastolic pressure. The vasorelaxant effect in rat aortic rings involves the activation of both nitric oxide synthase and K+ channels, which indicates the possible role of the NO/cGMP/PKG pathway in this effect [205].
Alpinia zerumbet has demonstrated antihypertensive and vasodilator effects. Rocha et al. [206] pointed out that the essential oils of Alpinia zerumbet showed an endothelium-independent vasorelaxant effect in second-order branches of the mesenteric artery. There was a vasodilator effect mediated by the inhibition of Ca2+ influx and release from intracellular storage, as well as an activation of the NOS/sGC pathway, being significant for the treatment of hypertension.
2.3.3. Modulation of Ion Channels
Another interesting experiment showed that the essential oils of Trachyspermum ammi (Linn.) Sprague (Apiaceae) seeds caused a completely endothelium-independent vasorelaxation effect and extracellular Ca2+ influx, attributed to the main constituents: thymol (38.1%), gamma-terpinene (33.3%), and p-cymene (23.1%) [207]. Jamhiri et al. [213] stated that thymol has a strong antioxidant potential that causes cardioprotective effects and prevents left ventricular hypertrophy by increasing serum antioxidant capacity.
Dib et al. [208] showed that Artemisia campestris L. (Asteraceae) essential oil (AcEO) had a vasorelaxation effect, acting through L-type calcium channels and SERCA pumps to reduce intracellular calcium, and, consequently, triggers sustained vasodilation. The maximal antioxidant effect was obtained with the dose of 2 mg/mL of AcEO. A dose of 1 mg/mL showed a maximum antiplatelet effect of 49.73% ± 9.54 and 48.20% ± 8.49, respectively, on thrombin and ADP. The main components of this oil are spathulenol, β-eudesmol, and p-cymene.
The essential oil from Lippia alba (Mill.) N.E. Brown (Verbenaceae), known as lemon balm, is a strong vasorelaxant in the isolated aorta, probably due to the presence of the citral compound that causes blockage of Ca2+ influx across the cell membrane or changes in calcium binding protein sensitization and/or intracellular calcium storage [209].
The cumulative addition of essential oil nanoemulsion (EONE) of Chrysopogon zizanioides (L.) Roberty (Poaceae) produced a vasorelaxant effect in thoracic aortic rings of spontaneously hypertensive rats. This essential oil possesses a vasorelaxant effect through the muscarinic pathway as well as acts as a calcium channel blocker. The study shows that C. zizanioides root EO has the potential for further investigation as a possible antihypertensive drug [210].
2-Phenyl-ethyl alcohol, an isolated component of Rosa damascena Mill (Rosaceae) essential oil, exhibited a potent vasorelaxation effect in the mesenteric artery and aorta of hypertensive rats. The activation of calcium-sensitive potassium channels might be the putative mechanism of the vasorelaxant effect [211].
2.4. Antidiabetes Action Mechanisms of Essential Oil
Diabetes mellitus is a chronic disease that affects around 3% of the worldwide population, with a prospect of increasing by 2030, and its prevalence has increased given the aging population. In 2015, the International Diabetes Federation (IDF) estimated that 1 in 11 adults aged 20 to 79 years had type 2 diabetes mellitus. Diabetes mellitus ranks ninth among diseases that cause loss of healthy life years [214]. About 422 million people have DM and 1.6 million deaths are directly attributed to the disease each year [215]. Likewise, an increase in the number of deaths related to DM between 1990 and 2019, from 1,278,866 to 2,988,924, has been reported. For disability-adjusted life years (DALYs), growth was observed from 28,586,671 in 1990 to 70,888,154 in 2019.
Diabetes mellitus (DM) is a metabolic disorder of multiple etiologies, which is mainly characterized by persistent elevation of the level of glucose in the blood (hyperglycemia), with disturbances in the metabolism of carbohydrates, lipids, and proteins, resulting from a deficiency in insulin secretion, its action, or both [216,217,218].
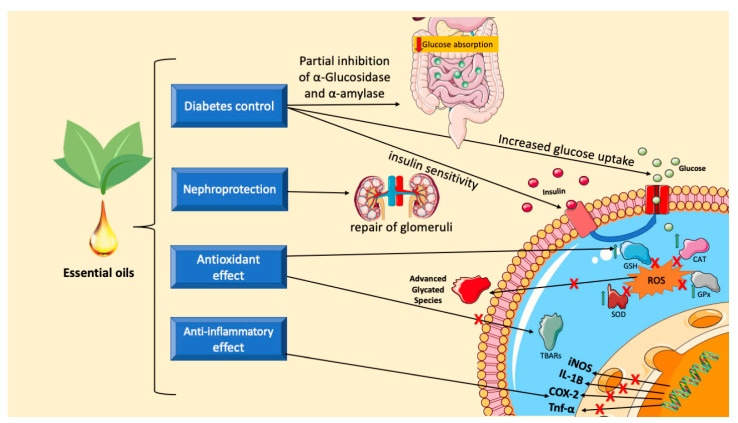
Essential oils have a complex chemical composition that allows them to reach multiple physiological structures and metabolic processes of antidiabetic aspects (see Figure 15 and Table 2), with several mechanisms of action described [219]. Therefore, these products and their components may have antiglycemic actions and potential use as pharmacologically active chemical agents capable of reducing the harmful effects of diabetes mellitus on the health of people affected by the disease. Additional investigations must be carried out, including clinical studies, to verify the viability of these compounds as candidates for antidiabetic drugs.
Figure 15.
Pharmacological effects and potential targets of essential oils from plants against diabetes mellitus. Legend: iNOS—inducible nitric oxide synthase; COX-2—cycloxigenase-2; IL-1β—interleukin-1β; TNF-α—tumor necrosis factor-α; CAT—catalase; ROS—reactive oxygen species; SOD—superoxide dismutase; GSH—reduced gluthatione; GPx—glutathione peroxidase; TBARS—thiobarbituric acid reactive substances.
Table 2.
Essential oils from plant species and their pharmacological mechanisms on diabetes.
| Plant Species (Family) | Major Compounds from Essential Oils | Pharmacological Action | References |
|---|---|---|---|
|
Citrus × sinensis (L.) Osbeck (Rutaceae) and Citrus limon (L.) Burm. f. (Rutaceae) |
D-Limonene C. sinensis: 92.14% C. limon: 53.07% |
The EO inhibited α-amylase and α-glucosidase activities | [220,221] |
| 62 species from the families: Lauraceae, Myristicaceae, Myrtaceae, Oleaceae, Pinaceae, Piperaceae, Poaceae, Rosaceae, Rutaceae, Santalaceae, Verbenaceae, and Zingiberaceae | - | In vitro α-amylase inhibitory potentials were obtained after the evaluation of Eucalyptus radiata, Laurus nobilis, and Myristica fragrans EOs. | [222] |
| Syzygirum aromaticum L. (Myrtaceae) | Eugenol | There was a significant decline in blood glucose levels, total cholesterol, xanthine oxidase, antioxidant activities, and it was a potent α-amylase inhibitor | [223] |
| Ocimum basilicum L. (Lamiaceae) | Linalool, methyl estragole, methyl cinnamate, and methyl chavicol | Ocimum basilicum essential oil had a strong α-amylase inhibitory activity | [224] |
| Serevenia buxifolia (Poir.) Ten. (Rutaceae) | β-caryophyllene (32.5%) and elixene (9.8%) | Antidiabetic potential by inhibiting the enzymes α-amylase and α-glucosidase | [225] |
| Kaempferia galanga (L.) (Zingiberaceae) | Ethyl p-methoxycinnamate: 66.39% | Antidiabetic activity using α-amylase inhibitory activity assay | [226] |
| Dracocephalum heterophyllum Benth. (Lamiaceae) | - | Antidiabetic potential by inhibiting the enzymes α-amylase and α-glucosidase | [227] |
|
Mentha suaveolens “Variegata” (Labiatae) (MSEO); Lavandula stoechas L. (Lamiaceae) (LSEO); Ammi visnaga (L.) Lam. (Apiaceae) (AVEO) |
MSEO: fenchone (29.77%) and camphor (24.90%) LSEO: piperitenone oxide (74.55%) AVEO: linalool (38.24%) |
Antidiabetic action by inhibiting the enzymes α-amylase and α-glucosidase | [228] |
| Hypericum scabrum L. (Clusiaceae) | - | The administration of Hypericum scabrum L. essential oil caused an increase in the level of GSH, GPx, SOD, and CAT activities and there was a decrease in the levels of MDA | [229] |
2.4.1. Inhibition of α-Amylase and α-Glucosidase
Oboh et al. [220] investigated Citrus sinensis L. Osbeck (orange) and Citrus limon (L.) Burm. f. (lemon) essential oils and their interactions with α-amylase, α-glucosidase, and ACE activities. The results showed that the EO inhibited α-amylase and α-glucosidase activities. The components of EO from orange and lemon peels contained mostly monoterpene hydrocarbons, oxygenated monoterpenes, and sesquiterpenes. Limonene was the main constituent, 92.14% in orange and 53.07% in lemon. In a study carried out with D-limonene administered to streptozotocin-induced diabetic rats, it was demonstrated that this monoterpene alters several biochemical parameters associated with diabetes and, therefore, can contribute to preventing clinical complications arising from this disease [230]. In this way, the antienzymatic actions of Citrus sinensis L. Osbeck and Citrus limon (L.) Burm. f. essential oils must be related to the antidiabetic activity of their majority constituent, limonene.
A study by Lekshmi et al. [231] showed that turmeric essential oil (Curcuma longa L.—Zingiberaceae) has a protective effect against type 2 diabetes through different mechanisms involving a hypoglycemic effect attributed to upregulation of insulin sensitivity and lower cellular glucose uptake. The major constituent of Curcuma longa EO is ar-turmerone, which can inhibit the activities of α-amylase (IC50 = 24.5 μg/mL) and α-glucosidase (IC50 = 0.28 μg/mL), two key enzymes in glucose digestion. This biological activity was also evidenced in Mentha viridis Linn (Lamiaceae) essential oil, which showed a significant ability to inhibit α-amylase (IC50 = 101.72 μg/mL) and α-glucosidase (IC50 = 86.93 μg/mL) [232].
In a profile study of 62 essential oils from different plant species and botanical families, hypoglycemic activity was evidenced in an enzymatic assay of α-amylase and chemical characterization. The different plants belonged to the following families: Lauraceae, Myristicaceae, Myrtaceae, Oleaceae, Pinaceae, Piperaceae, Poaceae, Rosaceae, Rutaceae, Santalaceae, Verbenaceae, and Zingiberacea. In vitro α-amylase inhibitory potentials were obtained after evaluation of Eucalyptus radiata A. Cunn, Laurus nobilis L. (Lauraceae), and Myristica fragrans Houtt. (Myristicaceae) EOs. These products exhibited inhibitory capacities comparable to those of the positive control (acarbose) [222].
In the study conducted by Radünz et al. [221], among all the EOs evaluated, thyme EO had the most significant inhibition of α-glucosidase (98.9%), followed by sweet orange EO (Citrus sinensis L. Osbeck, Rutaceae) which showed the most potent inhibitory effect on α-amylase (95.4%). The inhibitory effects of thyme and orange EOs were attributed to their main components, thymol and D-limonene, respectively. Incomplete enzyme inhibition and medium- and high-range inhibition for α-amylase and α-glucosidase, respectively, are interesting for clinical treatment as they allow diabetes control without compromising nutrients or glucose absorption in the small intestine.
Nait-Irahal et al. [223] analyzed the effect of intraperitoneal administration of clove essential oil (Syzygirum aromaticum L., Myrtaceae) in diabetic rats. A significant reduction in blood glucose, total cholesterol, and xanthine oxidase levels and potent inhibition of the α-amylase enzyme were observed. In another study, the essential oil from Ocimum basilicum L. (Lamiaceae) demonstrated strong α-amylase inhibitory activity (IC50 = 74.13 μg/mL). The essential oil is mainly composed of linalool, methyl estragole, methyl cinnamate, and methyl chavicol [224]. Linalool has many pharmacological actions. There are even reports of the nephroprotective action of this monoterpene in diabetic rats [233]. These data highlight the therapeutic potential of this natural product for the treatment of diabetes.
Serevenia buxifolia (Poir.) Ten. (Rutaceae) is a perennial citrus plant whose essential oil has antidiabetic potential, with IC50 values of 87.8 and 134.9 μg/mL against α-amylase and α-glucosidase, respectively. The biological action was concentration-dependent. This oil contains 33 components, such as β-caryophyllene (32.5%) and elixene (9.8%) [225]. There are several reports of the antidiabetic action of β-caryophyllene. This sesquiterpene was administered orally in a dose-dependent manner to diabetic rats, in combination with the hypoglycemic drug glibenclamide (600 μg/kg body weight), for 45 days. There was a significant reduction in glucose due to the increase in plasma insulin levels and the actions of carbohydrate metabolic enzymes were almost normalized [234]. In another study, this sesquiterpene had an insulinotropic and antidiabetic action when combined with L-arginine. The experiments were carried out in type 2 diabetic rats and a decrease in glucose was observed. Additionally, there was a reduction in lipid levels and oxidative stress [235]. So, scientific reports demonstrate the high potential of this natural product for restoring glucose homeostasis.
Kaempferia galanga (L.) is a plant with aromatic rhizomes and belongs to the Zingiberaceae family. Its essential oil has a high concentration of ethyl p-methoxycinnamate and shows α-amylase inhibitory activity with an IC50 value of 18.50 μg/mL. Acarbose, an antidiabetic drug, had IC50 = 20.39 μg/mL [226]. Chander et al. [227] also found excellent in vitro action of Dracocephalum heterophyllum Benth. essential oil (Lamiaceae) in inhibiting the enzymes α-amylase and α-glucosidase. β-Citronellol (31.5–83.7%) is the major constituent in samples of this oil. The antidiabetic action of citronellol has been demonstrated in an experimental model using streptozotocin-induced diabetic rats. This monoterpene was administered to animals orally (50 mg/kg) for 30 days and attenuated hyperglycemia via the action of strategic carbohydrate metabolic enzymes [236].
The essential oils of the Moroccan medicinal plants Mentha suaveolens “Variegata” (Labiatae), Lavandula stoechas L. (Lamiaceae), and Ammi visnaga (L.) Lam. (Apiaceae) were evaluated for their in vitro ability to inhibit the enzymes α-amylase and α-glucosidase. The study by El Hachlafi et al. [228] highlighted the outstanding biological action of the three plant species, whose essential oils were able to repress the activities of the enzymes in low concentrations compared to the standard drug used (acarbose). Mentha suaveolens essential oil mainly contains the constituents fenchone (29.77%) and camphor (24.90%). These monoterpenes are also the main components of Lavandula stoechas L. essential oil (fenchone (31.81%) and camphor (29.60%)). The three products were tested against the enzymes α-amylase and α-glucosidase. They showed inhibitory activity with IC50 of 106.73, 104.19, and 76.92 μg/mL against α-amylase and 98.54, 69.03, and 105.26 μg/mL in assays using α-glucosidase [237]. Therefore, these data indicate that fenchone and camphor should contribute to the antidiabetic action of Mentha suaveolens essential oil.
2.4.2. Oxidative Stress and Inflammation-Related Mechanisms
The essential oil from Hypericum scabrum L. (Clusiaceae) has a protective and healing effect on wounds in streptozotocin-induced diabetic rats. The administration of Hypericum scabrum L. essential oil caused an increase in the level of GSH, GPx, SOD, and CAT activities and there was a decrease in the levels of MDA. H. scabrum L. essential oil may help reduce lipid peroxidation, oxidative stress, and the associated complications that go along with them, in addition to playing a beneficial role in the management of diabetic wounds [229]. Gandhi et al. [238] discussed in a data analysis that constituents of essential oils, including linalool, cinnamaldehyde, zerumbone, myrtenol, thujone, α-terpineol, geraniol, citral, eugenol, thymol, carvacrol, citronellol, and thymoquinone, have antidiabetic properties through modulation of metabolic pathways associated with glucose metabolism. For example, these constituents of essential oils can reduce the expression of TNF-α, IL-4, iNOS, and COX-2, as well as regulate signaling molecules, such as AMPK, caspase-3, NF-κB, and Nrf2/ HO-1.
3. Conclusions
The synthetic routes presented to obtain bioactive derivatives demonstrate the importance of the constituents of these oils as starting materials in the synthesis of strategic products for several sectors, particularly for the pharmaceutical sector. The interference of these oils in various metabolic pathways, inhibiting or activating metabolites related to organic disorders associated with diseases such as diabetes, hypertension, cancer, and ulcers, suggests the promising use of these products as prototypes for new drugs. Additional studies are necessary to advance the understanding of the pharmacological activities and toxicological aspects aiming to establish the therapeutic potential of these components and their synthetic derivatives in a clinical stage.
Acknowledgments
This research was supported by National Council for Scientific and Technological Development (CNPq) and Coordination for the Improvement of Higher Education Personnel (CAPES).
Abbreviations
| CAT | Catalase |
| cGMP | Cyclic guanosine monophosphate |
| COX-1 | Cyclooxygenase 1 |
| COX-2 | Cyclooxygenase 2 |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| GSH | Glutathione |
| H+/K+ ATPase | Hydrogen potassium ATPase |
| IL-1β | Interleukin 1β |
| IL-6 | Interleukin 6 |
| IL-10 | Interleukin 10 |
| L-NAME | N (G)-nitro-L-arginine methyl ester |
| MDA | Malondialdehyde |
| MIC | Minimum inhibitory concentration |
| MPO | Myeloperoxidase |
| NF-κB | Nuclear factor kappa B |
| NO | Nitric oxide |
| NOS | Nitric oxide synthase |
| NP-SH | Non-protein Sulfhydryl |
| NSAIDs | Non-steroidal anti-inflammatory drugs |
| PGE2 | Prostaglandin E2 |
| p-IκBα and IκBα | Inhibitors of the transcription factor NF-κB |
| p-p65 and p65 | NF-κB subunits |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| TNF-α | Tumor necrosis factor alpha |
Author Contributions
Writing—review and editing, supervision, funding acquisition, D.P.d.S.; Methodology, writing—original draft preparation, F.d.A.O., D.D.R.A., C.d.O.B., T.P.O., T.J.B., and A.B.S.D.; Writing—review and editing, D.V.d.F., F.d.A.O., and D.D.R.A. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Swift K.A. Catalytic transformations of the major terpene feedstocks. Top. Cat. 2004;27:143–155. doi: 10.1023/B:TOCA.0000013549.60930.da. [DOI] [Google Scholar]
- 2.Schwab W., Fuchs C., Huang F.C. Transformation of terpenes into fine chemicals. Eur. J. Lipid Sci. Technol. 2013;115:3–8. doi: 10.1002/ejlt.201200157. [DOI] [Google Scholar]
- 3.Brill Z.G., Condakes M.L., Ting C.P., Maimone T.J. Navigating the Chiral Pool in the Total Synthesis of Complex Terpene Natural Products. Chem. Rev. 2017;117:11753–11795. doi: 10.1021/acs.chemrev.6b00834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brocksom T.J., Desidera A.L., de Carvalho Alves L., Thiago de Oliveira K. The New Directions of Organic Synthesis. Curr. Org. Synth. 2015;12:496–522. doi: 10.2174/157017941205150821121222. [DOI] [Google Scholar]
- 5.Corey E., Cheng X.-M. The Logic of Chemical Synthesis. Wiley-Interscience; New York, NY, USA: 1995. p. 436. [Google Scholar]
- 6.De Souza J.M., Galaverna R., de Souza A.A.N., Brocksom T.J., Pastre J.C., de Souza R.O.M.A., de Olveira K.T. Impact of continuous flow chemistry in the synthesis of natural products and active pharmaceutical ingredients. An. Acad. Bras. Cienc. 2018;90:1131–1174. doi: 10.1590/0001-3765201820170778. [DOI] [PubMed] [Google Scholar]
- 7.De Souza A.A.N., Paez E.B.A., de Assis F.F., Brocksom T.J., de Oliveira K.T. Improved Synthesis of Bioactive Molecules Through Flow Chemistry. Top. Med. Chem. 2021;38:317–372. [Google Scholar]
- 8.Nyamwihura R.J., Ogungbe I.V. The pinene scaffold: Its occurrence, chemistry, synthetic utility, and pharmacological importance. RSC Adv. 2022;12:11346–11375. doi: 10.1039/D2RA00423B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sagorin G., Cazeils E., Basset J.-F., Reiter M. From Pine to Perfume. Chimia. 2021;75:780–787. doi: 10.2533/chimia.2021.780. [DOI] [PubMed] [Google Scholar]
- 10.de Carvalho B.L.C., Aguillon A.R., Leão R.A.C., de Souza R.O.M.A. Two-step continuous flow synthesis of a-terpineol. Tetrahedron Lett. 2021;80:153318. doi: 10.1016/j.tetlet.2021.153318. [DOI] [Google Scholar]
- 11.Rosa G.H.S., Santos T.I.S., Brocksom T.J., de Oliveira K.T. Diverse continuous photooxygenation reactions of (+) and (−)-α-pinenes to the corresponding pinocarvones or trans-pinocarveols. React. Chem. Eng. 2023;8:790–797. doi: 10.1039/D2RE00385F. [DOI] [Google Scholar]
- 12.Park C.Y., Kim Y.J., Lim H.J., Park J.H., Kim M.J., Seo S.W., Park C.P. Continuous flow photooxygenation of monoterpenes. RSC Adv. 2015;5:4233–4237. doi: 10.1039/C4RA12965B. [DOI] [Google Scholar]
- 13.Ciriminna R., Lomeli-Rodriguez M., Carà P.D., Lopez-Sanchez J.A., Pagliaro M. Limonene: A versatile chemical of the bioeconomy. Chem. Commun. 2014;50:15288–15296. doi: 10.1039/C4CC06147K. [DOI] [PubMed] [Google Scholar]
- 14.Aguillon A.R., Leão R.A.C., de Oliveira K.T., Brocksom T.J., Miranda L.S.M., de Souza R.O.M.A. Process Intensification for Obtaining a Cannabidiol Intermediate by Photo-oxygenation of Limonene under Continuous-Flow Conditions. Org. Process. Res. Dev. 2020;24:2017–2024. doi: 10.1021/acs.oprd.0c00131. [DOI] [Google Scholar]
- 15.Maiocchi A., Barbieri J., Fasano V., Passarella D. Stereoselective Synthetic Strategies to (-)-Cannabidiol. Chem. Sel. 2022;7:e202202400. doi: 10.1002/slct.202202400. [DOI] [Google Scholar]
- 16.Aguillón A.R., Leão R.A.C., Miranda L.S.M., de Souza R.O.M.A. Cannabidiol Discovery and Synthesis, a Target-Oriented Analysis in Drug Production Process. Chem. Eur. J. 2021;27:5577–5600. doi: 10.1002/chem.202002887. [DOI] [PubMed] [Google Scholar]
- 17.Lenardão E.J., Botteselle G.V., de Azambuja F., Perin G., Jacob R.G. Citronellal as key compound in organic synthesis. Tetrahedron. 2007;63:6671–6712. doi: 10.1016/j.tet.2007.03.159. [DOI] [Google Scholar]
- 18.Mäki-Arvela P., Simakova I., Vajglová Z., Murzin D.Y. One-Pot Synthesis of Menthol from Citral and Citronellal Over Heterogeneous Catalysts. Catal. Surv. Asia. 2023;27:2–19. doi: 10.1007/s10563-022-09376-6. [DOI] [Google Scholar]
- 19.Emura M., Matsuda H. A Green and Sustainable Approach: Celebrating the 30th Anniversary of the Asymmetric l-Menthol Process. Chem. Biodiversity. 2014;11:1688–1699. doi: 10.1002/cbdv.201400063. [DOI] [PubMed] [Google Scholar]
- 20.Dylong D., Hausoul P.J.C., Palkovits R., Eisenacher M. Synthesis of (−)-menthol: Industrial synthesis routes and recent development. Flavour. Fragr. J. 2022;37:195–209. doi: 10.1002/ffj.3699. [DOI] [Google Scholar]
- 21.Simakova I.L., Vajglová Z., Martínez-Klimov M., Eränen K., Peurla M., Mäki-Arvela P., Murzin D.Y. One-Pot Synthesis of Menthol from Citral over Ni/H-β-38 Extrudates Containing Bentonite Clay Binder in Batch and Continuous Reactors. Org. Process. Res. Dev. 2023;27:295–310. doi: 10.1021/acs.oprd.2c00337. [DOI] [Google Scholar]
- 22.Zhou F., Liu H., Wen Z., Zhang B., Chen G. Toward the Efficient Synthesis of Pseudoionone from Citral in a Continuous-Flow Microreactor. Ind. Eng. Chem. Res. 2018;57:11288–11298. doi: 10.1021/acs.iecr.8b02367. [DOI] [Google Scholar]
- 23.Carmona-Vargas C.C., Alves L.D.C., Brocksom T.J., de Oliveira K.T. Combining batch and continuous flow setups in the end-to-end synthesis of naturally occurring curcuminoids. React. Chem. Eng. 2017;2:366–374. doi: 10.1039/C6RE00207B. [DOI] [Google Scholar]
- 24.de Carvalho C.C.C.R., da Fonseca M.M.R. Biotransformation of terpenes. Biotechnol. Adv. 2006;24:134–142. doi: 10.1016/j.biotechadv.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 25.Marmulla R., Harder J. Microbial monoterpene transformations—A review. Front. Microbiol. 2014;5:346. doi: 10.3389/fmicb.2014.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soares-Castro P., Soares F., Santos P.M. Current Advances in the Bacterial Toolbox for the Biotechnological Production of Monoterpene-Based Aroma Compounds. Molecules. 2021;26:91. doi: 10.3390/molecules26010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Souza Sevalho E., Paulino B.N., de Souza A.Q.L., de Souza A.D.L. Fungal biotransformation of limonene and pinene for aroma production. Braz. J. Chem. Eng. 2023;40:1–21. doi: 10.1007/s43153-022-00239-1. [DOI] [Google Scholar]
- 28.Wriessnegger T., Augustin P., Engleder M., Leitner E., Müller M., Kaluzna I., Schürmann M., Mink D., Zellnig G., Schwab H., et al. Production of the sesquiterpenoid (+)-nootkatone by metabolic engineering of Pichia pastoris. Metab. Eng. 2014;24:8–29. doi: 10.1016/j.ymben.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 29.Kavitt R.T., Lipowska A.M., Anyane-Yebo A., Gralnek I.M. Diagnosis and treatment of peptic ulcer disease. Am. J. Med. 2019;132:447–456. doi: 10.1016/j.amjmed.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 30.Malfertheiner P., Chan F.K., McColl K.E. Among all complications, peptic ulcer bleeding is one of the common clinical diseases Peptic ulcer disease. Lancet. 2009;374:1449–1461. doi: 10.1016/S0140-6736(09)60938-7. [DOI] [PubMed] [Google Scholar]
- 31.Bardhan K.D., Williamson M., Royston C., Lyon C. Admission rates for peptic ulcer in the trent region, UK, 1972–2000. changing pattern, a changing disease? Dig. Liver Dis. 2004;36:577–588. doi: 10.1016/j.dld.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 32.Proctor M.J., Deans C. Oesophagus and stomach. Complications of peptic ulcers. Surgery. 2014;32:599–607. doi: 10.1016/j.mpsur.2014.09.005. [DOI] [Google Scholar]
- 33.Mehdi S.-R., Fokou P.V.T., Sharopov F., Martorell M., Ademiluyi A.O., Rajkovic J., Salehi B., Martins N., Iriti M., Javad S.-R. Antiulcer Agents: From plant extracts to phytochemicals in healing promotion. Molecules. 2018;23:1751. doi: 10.3390/molecules23071751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Søreide K., Thorsen K., Harrison E.M., Søreide J.A. Perforated peptic ulcer. Lancet. 2015;386:1288–1298. doi: 10.1016/S0140-6736(15)00276-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lanas A., Chan F.K.L. Peptic ulcer disease. Lancet. 2017;390:613–624. doi: 10.1016/S0140-6736(16)32404-7. [DOI] [PubMed] [Google Scholar]
- 36.Zelickson M.S., Bronder C.M., Johnson B.L., Camunas J.A., Smith D.E., Rawlinson D., Von S., Stone H.H., Taylor S.M. Helicobacter pylori is not the predominant etiology for peptic ulcers requiring operation. Am. Surg. 2011;77:1054–1060. doi: 10.1177/000313481107700827. [DOI] [PubMed] [Google Scholar]
- 37.Yuan Y., Padol I.T., Hunt R.H. Peptic ulcer disease today. Nat. Clin. Pract. Gastroenterol. Hepatol. 2006;3:80–89. doi: 10.1038/ncpgasthep0393. [DOI] [PubMed] [Google Scholar]
- 38.Chey W.D., Leontiadis G.I., Howden C.W., Moss S.F. ACG Clinical guideline: Treatment of Helicobacter pylori infection. Am. J. Gastroenterol. 2017;112:212–239. doi: 10.1038/ajg.2016.563. [DOI] [PubMed] [Google Scholar]
- 39.Cekin A.H., Sahinturk Y., Akbay Harmandar F., Uyar S., Yolcular B.O., Cekin Y. Use of probiotics as an adjuvant to sequential H. pylori eradication therapy: Impact on eradication rates, treatment resistance, treatment-related side effects, and patient compliance. Turk. J. Gastroenterol. 2017;28:3–11. doi: 10.5152/tjg.2016.0278. [DOI] [PubMed] [Google Scholar]
- 40.Tran-Duy A., Spaetgens B., Hoes A.W., de Wit N.J., Stehouwer C.D. Use of proton pump inhibitors and risks of fundic gland polyps and gastric cancer: Systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2016;14:1706–1719. doi: 10.1016/j.cgh.2016.05.018. [DOI] [PubMed] [Google Scholar]
- 41.Waldum H.L., Fossmark R. Proton pump inhibitors and gastric cancer: A long expected side effect finally reported also in man. Gut. 2018;67:199–200. doi: 10.1136/gutjnl-2017-315629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koyyada A. Long-term use of proton pump inhibitors as a risk factor for various adverse manifestations. Therapies. 2021;76:13–21. doi: 10.1016/j.therap.2020.06.019. [DOI] [PubMed] [Google Scholar]
- 43.Manolis A.A., Massaad J., Cai Q., Wehbi M. Proton pump inhibitors and cardiovascular adverse effects: Real or surreal worries? Eur. J. Int. Med. 2020;72:15–26. doi: 10.1016/j.ejim.2019.11.017. [DOI] [PubMed] [Google Scholar]
- 44.Dacha S., Razvi M., Massaad J., Cai Q., Wehbi M. Hypergastrinemia. Gastroenterology. 2015;3:201–208. doi: 10.1093/gastro/gov004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gohar A.A., Zaki A.A. Assessment of some herbal drugs for prophylaxis of peptic ulcer. Iran. J. Pharm. Res. 2014;13:1081–1086. [PMC free article] [PubMed] [Google Scholar]
- 46.Adinortey M.B., Ansah C., Adinortey C.A., McGiboney J., Nyarko A. In vitro H+/K+-ATPase inhibition, antiradical effects of a flavonoid-rich fraction of dissotisrotundifolia, and in silico pass prediction of its isolated compounds. J. Nat. Sci. Biol. Med. 2018;9:47–53. doi: 10.4103/jnsbm.JNSBM_104_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Awaad A.S., El-Meligy R.M., Soliman G.A. Natural products in treatment of ulcerative colitis and peptic ulcer. J. Saudi Chem. Soc. 2013;17:101–124. doi: 10.1016/j.jscs.2012.03.002. [DOI] [Google Scholar]
- 48.Bi W.P., Man H.B., Man M.Q. Efficacy and safety of herbal medicines in treating gastric ulcer: A review. World J. Gastroenterol. 2014;20:17020–17028. doi: 10.3748/wjg.v20.i45.17020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singh A.K., Singh S.K., Singh P.P., Srivastava A.K., Pandey K.D., Kumar A., Yadav H. Biotechnological aspects of plants metabolites in the treatment of ulcer: A new prospective. Biotechnol. Rep. 2018;18:e00256. doi: 10.1016/j.btre.2018.e00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Agra M.F., Silva K.N., Basılio I.J.L.D., Franca P.F., Barbosa-Filho J.M. Survey of medicinal plants used in the region northeast of Brazil. Braz. J. Pharmacog. 2008;18:472–508. doi: 10.1590/S0102-695X2008000300023. [DOI] [Google Scholar]
- 51.Caldas G.F.R., Oliveira A.R.S., Araujo A.V., Quixabeira D.C.A., Silva-Neto J.C., Costa-Silva J.R., Menezes I.R.A., Ferreira F., Leite A.C.L., Costa J.G.M., et al. Gastroprotective and ulcer healing effects of essential oil of Hyptis martiusii Benth. (Lamiaceae) PLoS ONE. 2014;9:e84400. doi: 10.1371/journal.pone.0084400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Caldas G.F.R., Oliveira A.R.S., Araújo A.V., Lafayette S.S.L., Albuquerque G.S., Silva-Neto J.C., Costa-Silva J.R., Ferreira F., Costa J.G.M., Wanderley A.G. Gastroprotective effect of the monoterpene 1,8-cineole (eucalyptol) PLoS ONE. 2015;10:e0134558. doi: 10.1371/journal.pone.0134558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Robert A. Cytoprotection by prostaglandins. Gastroenterology. 1979;77:761–767. doi: 10.1016/0016-5085(79)90235-X. [DOI] [PubMed] [Google Scholar]
- 54.Martínez-Herrera A., Pozos-Guillén A., Ruiz-Rodríguez S., Garrocho-Rangel A., Vértiz-Hernández A., Escobar-García D.M. Effect of 4-Allyl-1-hydroxy-2-methoxybenzene (eugenol) on inflammatory and apoptosis processes in dental pulp fibroblasts. Mediat. Inflamm. 2016;2016:9371403. doi: 10.1155/2016/9371403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aisha A., Nassar Z., Siddiqui M., Abu-Salah K., Alrokayan S., Ismail Z., Abdul Majid A. Evaluation of antiangiogenic, cytotoxic and antioxidant effects of Syzygium aromaticum L. extracts. Asian J. Biol. Sci. 2011;4:282–290. doi: 10.3923/ajbs.2011.282.290. [DOI] [Google Scholar]
- 56.Bakour M., Soulo N., Hammas N., Fatemi H., Aboulghazi A., Taroq A., Abdellaoui A., Al-waili N., Lyoussi B. The antioxidant content and protective effect of argan oil and Syzygium aromaticum essential oil in hydrogen peroxide-induced biochemical and histological changes. Int. J. Mol. Sci. 2018;19:610. doi: 10.3390/ijms19020610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Elbestawy M.K.M., El-Sherbiny G.M., Moghannem S.A. Antibacterial, antibiofilm and anti-inflammatory activities of eugenol clove essential oil against resistant Helicobacter pylori. Molecules. 2023;28:2448. doi: 10.3390/molecules28062448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hobani Y.H., Mohan S., Shaheen E., Abdelhaleem A., Ahmad M.F., Bhatia S., Abou-Elhamd A.S. Gastroprotective effect of low dose Eugenol in experimental rats against ethanol induced toxicity: Involvement of antiinflammatory and antioxidant mechanism. J. Ethnopharmacol. 2022;289:115055. doi: 10.1016/j.jep.2022.115055. [DOI] [PubMed] [Google Scholar]
- 59.Abdel-Salam O.M.E., Czimmer J., Debreceni A., Szolcsanyi J., Mozsik G. Gastric mucosal integrity: Gastric mucosal blood flow and microcirculation. An overview. J. Physiol. Paris. 2001;95:105–127. doi: 10.1016/S0928-4257(01)00015-8. [DOI] [PubMed] [Google Scholar]
- 60.Kwiecieñ S., Brzozowski T., Konturek P.C.H., Konturek S.J. The role of reactive oxygen species in action of nitric oxide-donors on stress-induced gastric mucosal lesions. J. Physiol. Pharmacol. 2002;53:761–773. [PubMed] [Google Scholar]
- 61.Barboza J.N., Bezerra Filho C.S.M., Silva R.O., Medeiros J.V.R., Sousa D.P. An overview on the anti-inflammatory potential and antioxidant profile of eugenol. Oxid. Med. Cell. Longev. 2018;2018:3957262. doi: 10.1155/2018/3957262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jung J., Lee J.H., Bae K.H., Jeong C.S. Anti-gastric actions of eugenol and cinnamic acid isolated from Cinnamomi Ramulus. Yakugaku Zasshi. 2011;131:1103–1110. doi: 10.1248/yakushi.131.1103. [DOI] [PubMed] [Google Scholar]
- 63.Zhao Z., Lu J., Leung K., Chan C.L., Jiang Z.H. Determination of patchoulic alcohol in Herba Pogostemonis by GC–MS–MS. Chem. Pharm. Bull. 2005;53:856–860. doi: 10.1248/cpb.53.856. [DOI] [PubMed] [Google Scholar]
- 64.Liu Z.H., Liang J.L., Wu J.Z., Chen H.B., Zhang Z.B., Yang H.M., Chen L.P., Chen H.M., Su Z.R., Li Y.C. Transformation of patchouli alcohol to beta-patchoulene by gastric juice: Beta-patchoulene is more effective in preventing ethanolinduced gastric injury. Sc. Rep. 2017;7:5591. doi: 10.1038/s41598-017-05996-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang B.Z., Chen X.Y., Chen H.B., Wang L., Liang J.L., Luo D.D., Liu Y.H., Yang H.M., Li Y.C., Xie J.H. Anti-inflammatory activity of beta-patchoulene isolated from patchouli oil in mice. Eur. J. Pharmacol. 2016;781:229–238. doi: 10.1016/j.ejphar.2016.04.028. [DOI] [PubMed] [Google Scholar]
- 66.Chen H.M., Liao H.J., Liu Y.H., Zheng Y.F., Wu X.L., Su Z.Q., Zhang X., Lai Z.Q., Lai X.P., Xiu Z.X. Protective effects of pogostone from Pogostemonis Herba against ethanol-induced gastric ulcer in rats. Fitoterapia. 2015;100:110–117. doi: 10.1016/j.fitote.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 67.Xu F., Yang Q., Wu L., Qi R., Wu Y., Li Y., Tang L., Guo D., Liu B. Investigation of inclusion complex of patchouli alcohol with β-cyclodextrin. PLoS ONE. 2017;12:e0169578. doi: 10.1371/journal.pone.0169578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zheng Y.F., Xie J.H., Xu Y.F., Liang Y.Z., Mo Z.Z., Jiang W.W., Chen X.Y., Liu Y.H., Yu X.D., Huang O., et al. Gastroprotective effect and mechanism of patchouli alcohol against ethanol, indomethacin and stress-induced ulcer in rats. Chem. Biol. Interact. 2014;222:27–36. doi: 10.1016/j.cbi.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 69.Zhao W., Zhu F., Shen W., Fu A., Zheng L., Yan Z., Zhao L., Fu G. Protective effects of DIDS against ethanol-induced gastric mucosal injury in rats. Acta Biochim. Biophys. Sin. 2009;41:301–308. doi: 10.1093/abbs/gmp014. [DOI] [PubMed] [Google Scholar]
- 70.Huilgol S.V., Kumar V.H. Evaluation of antiulcerogenic potential of antioxidant α-tocopherol in pylorus-ligated albino rats. J. Basic Clin. Physiol. Pharmacol. 2014;25:81–85. doi: 10.1515/jbcpp-2013-0035. [DOI] [PubMed] [Google Scholar]
- 71.Vats S., Gupta T. Evaluation of bioactive compounds and antioxidant potential of hydroethanolic extract of Moringa oleifera Lam. from Rajasthan, India. Physiol. Mol. Biol. Plants. 2017;23:239–248. doi: 10.1007/s12298-016-0407-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bi L.C., Kaunitz J.D. Gastroduodenal mucosal defense: An integrated protective response. Curr. Opin. Gastroen. 2003;19:526–532. doi: 10.1097/00001574-200311000-00003. [DOI] [PubMed] [Google Scholar]
- 73.Repetto M.G., LlesuyBraz S.F. Antioxidant properties of natural compounds used in popular medicine for gastric ulcers. J. Med. Biol. Res. 2002;35:523–534. doi: 10.1590/S0100-879X2002000500003. [DOI] [PubMed] [Google Scholar]
- 74.Ahmad A., Gupta G., Afzal M., Kazmi I., Anwar F. Antiulcer and antioxidante activities of a new steroid from Morus alba. Life Sci. 2013;92:202–210. doi: 10.1016/j.lfs.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 75.Barbosa L.C.A., Pereira U.A., Martinazzo A.P., Maltha C.R.A., Teixeira R.T., Melo E.C. Evaluation of the chemical composition of brazilian commercial Cymbopogon citratus (D.C.) Stapf samples. Molecules. 2008;13:1864–1874. doi: 10.3390/molecules13081864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Avoseh O., Oyedeji O., Rungqu P., Nkeh-Chungag B., Oyedeji A. Cymbopogon species; ethnopharmacology, phytochemistry and the pharmacological importance. Molecules. 2015;20:7438–7453. doi: 10.3390/molecules20057438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Venzon L., Mariano L.N.B., Somensi L.B., Boeing T., Souza P., Wagner T.M., Andrade S.F., Nesello L.A.N., Silva L.M. Essential oil of Cymbopogon citratus (lemongrass) and geraniol, but not citral, promote gastric healing activity in mice. Biomed. Pharmacother. 2018;98:118–124. doi: 10.1016/j.biopha.2017.12.020. [DOI] [PubMed] [Google Scholar]
- 78.Shin J.M., Munson K., Vagin O., Sachs G. The gastric HK-ATPase: Structure, function, and inhibition. Pflüg. Arch. Europ. J. Phy. 2009;457:609–622. doi: 10.1007/s00424-008-0495-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Naik Y., Jayaram S., Nayaka M.A.H., Lakshman, Dharmesh S.M. Gastroprotective effect of swallow root (Decalepis hamiltonii) extract: Possible involvement of H+–K+–ATPase inhibition and antioxidative mechanism. J. Ethnopharmacol. 2007;112:173–179. doi: 10.1016/j.jep.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 80.Khan M.A., Howden C.W. The role of proton pump inhibitors in the management of upper gastrointestinal disorders. Gastroenterol. Hepatol. 2018;14:169–175. [PMC free article] [PubMed] [Google Scholar]
- 81.Wallace J.L. Nitric oxide, aspirin-triggered lipoxins and NOaspirin in gastric protection. Inflamm. Allergy Drug Targets. 2006;5:133–137. doi: 10.2174/187152806776383116. [DOI] [PubMed] [Google Scholar]
- 82.Kawano S., Tsuji S. Role of mucosal blood flow: A conceptional review in gastrointestinal injury and protection. J. Gastrointest. Hepatol. 2000;15:D1–D6. doi: 10.1046/j.1440-1746.2000.02142.x. [DOI] [PubMed] [Google Scholar]
- 83.Oliveira R.L.C., Neto E.M.F.L., Araújo E.L., Albuquerque U.P. Conservation priorities and population structure of woody medicinal plants in an area of caatinga vegetation (Pernambuco State, NE Brazil) Environ. Monit. Assess. 2007;132:189–206. doi: 10.1007/s10661-006-9528-7. [DOI] [PubMed] [Google Scholar]
- 84.Randau K.P., Florêncio D.C., Ferreira C.P., Xavier H.S. Pharmacognostic study of Croton rhamnifolius HBK and Croton rhamnifolioides Pax & Hoffm. (Euphorbiaceae) Rev. Bras. Farmacogn. 2004;14:89–96. doi: 10.1590/S0102-695X2004000200001. [DOI] [Google Scholar]
- 85.Santos G.K.N., Dutra K.A., Lira C.S., Lima B.N., Napoleão T.H., Paiva P.M.G., Maranhão C.A., Brandão S.S.F., Navarro D.M.A.F. Effects of Croton rhamnifolioides essential oil on Aedes aegypti oviposition, larval toxicity and trypsin activity. Molecules. 2014;19:16573–16587. doi: 10.3390/molecules191016573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Martins A.O.B.P.B., Rodrigues L.B., Cesário F.R.A.S., Oliveira M.R.C., Tintino C.D.M., Castro F.F., Alcântara I.S., Fernandes M.N.M., Albuquerque T.R., Silva M.S.A., et al. Anti-Inflammatory and Physicochemical Characterization of the Croton rhamnifolioides Essential Oil Inclusion Complex in β-Cyclodextrin. Biology. 2020;9:114. doi: 10.3390/biology9060114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vidal C.S., Martins A.O.B.P.B., Silva A.A., Oliveira M.R.C., Ribeiro-Filho J., Albuquerque T.R., Coutinho H.D.M., Almeida J.R.G.S., Quintans Junior L.J., Menezes I.R.A. Gastroprotective effect and mechanism of action of Croton rhamnifolioides essential oil in mice. Biomed. Pharmacother. 2017;89:47–55. doi: 10.1016/j.biopha.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 88.Lima-Accioly P.M., Lavor-Porto P.R., Cavalcante F.S., Magalhães P.J.C., Lahlou S., Morais S.M., Leal-Cardoso J.H. Essential oil of Croton nepetaefolius and its main constituent, 1, 8-cineole, block excitability of rat sciatic nerve in vitro. Clin. Exp. Pharmacol. Physiol. 2006;33:1158–1163. doi: 10.1111/j.1440-1681.2006.04494.x. [DOI] [PubMed] [Google Scholar]
- 89.Whittle B.J.R., Lopez-Belmonte J., Moncada S. Regulation of gastric mucosal integrity by endogenous nitric oxide: Interactions with prostanoids and sensory neuropeptides in the rat. Br. J. Pharmacol. 1990;99:607–611. doi: 10.1111/j.1476-5381.1990.tb12977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kwiecien S., Pawlik M.W., Brzozowski T., Konturek P.C., Sliwowski Z., Pawlik W.W., Konturek S.J. Nitricoxide (NO)-releasing aspirin and (NO) donors in protection of gastric mucosa against stress. J. Physiol. Pharmacol. 2008;59:103–115. [PubMed] [Google Scholar]
- 91.Sharma K., Mahato N., Cho M.H., Lee Y.R. Converting citrus wastes into value-added products: Economic and environmently friendly approaches. Nutrition. 2017;34:29–46. doi: 10.1016/j.nut.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 92.M’hiri N., Ghali R., Nasr I.B., Boudhrioua N. Effect of different drying processes on functional properties of industrial lemon by product. Process. Saf. Environ. Prot. 2018;116:450–460. doi: 10.1016/j.psep.2018.03.004. [DOI] [Google Scholar]
- 93.Hsouna A.B., Halima N.B., Smaoui S., Hamdi N. Citrus lemon essential oil: Chemical composition, antioxidant and antimicrobial activities with its preservative effect against Listeria monocytogenes inoculated inminced beef meat. Lipids Health Dis. 2017;16:146. doi: 10.1186/s12944-017-0487-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sun J. D-Limonene: Safety and clinical applications. Altern. Med. Rev. 2007;12:3. [PubMed] [Google Scholar]
- 95.Salehi B., Upadhyay S., Orhan I.E., Jugran A.K., Jayaweera S.L.D., Dias D.A., Sharopov F., Taheri Y., Martins N., Baghalpour N., et al. Review therapeutic potential of α- and β-pinene: A miracle gift of nature. Biomolecules. 2019;9:738. doi: 10.3390/biom9110738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rozza A.L., Moraes T.M., Kushimab H., Tanimoto A., Marques M.O.M., Bauab T.M., Hiruma-Lima C.A., Pellizzona C.L. Gastroprotective mechanisms of Citrus lemon (Rutaceae) essential oil and its majority compounds limonene and beta-pinene: Involvement of heat-shock protein-70, vasoactive intestinal peptide, glutathione, sulfhydryl compounds, nitric oxide and prostaglandin E2. Chem. Biol. Interact. 2011;189:82–89. doi: 10.1016/j.cbi.2010.09.031. [DOI] [PubMed] [Google Scholar]
- 97.Yoshikawa T., Naito Y., Kishi A., Tomii T., Kaneko T., Iinuma S., Ichikawa H., Yasuda M., Takahashi S., Kondo M. Role of active oxygen, lipid peroxidation and antioxidants in the pathogenesis of gastric mucosal injury induced by indomethacin in rats. Gut. 1993;34:732–737. doi: 10.1136/gut.34.6.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Asako H., Kubes P., Wallace J., Gaginella T., Wolf R.E., Granger D.N. Indomethacin-induced leukocyte adhesion in mesenteric venules: Role of lipoxygenase products. Am. J. Physiol.-Gastrointest. Liver Physiol. 1992;262:G903–G908. doi: 10.1152/ajpgi.1992.262.5.G903. [DOI] [PubMed] [Google Scholar]
- 99.Guan L., Quan L.H., Xu L.Z., Cong P.Z. Chemical constituents of Pogostemon cablin (Blanco) Benth. Planta Med. 1998;64:464–466. [PubMed] [Google Scholar]
- 100.Luo J., Feng Y., Guo X., Li X. GC-MS analysis of volatile oil of Herba Pogostemonis collected from Gaoyao county. Zhong Yao Cai. 1999;22:25–28. [PubMed] [Google Scholar]
- 101.Chen X.Y., Chen H.M., Liu Y.H., Zhang Z.B., Zheng Y.F., Su Z.Q., Zhang X., Xie J.H., Liang Y.Z., Fu L.D., et al. The gastroprotective effect of pogostone from Pogostemonis Herba against indomethacin-induced gastric ulcer in rats. Exp. Biol. Med. 2016;241:193–204. doi: 10.1177/1535370215600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lei Y., Fu P., Jun X., Cheng P. Pharmacological Properties of Geraniol—A Review. Planta Med. 2019;85:48–55. doi: 10.1055/a-0750-6907. [DOI] [PubMed] [Google Scholar]
- 103.Bhattamisra S.K., Kuean C.H., Chieh B.L., Yan V.L.Y., Lee C.K., Hooi L.P., Shyan L.P., Liew Y.K., Candasamy M., Sahu P.S. Antibacterial activity of geraniol in combination with standard antibiotics against Staphylococcus aureus, Escherichia coli and Helicobacter pylori. Nat. Prod. Commun. 2018;13:791–793. doi: 10.1177/1934578X1801300701. [DOI] [Google Scholar]
- 104.Noh C.K., Lee G.H., Park J.W., Roh J.R., Han J.H., Lee E., Park B., Lim S.G., Shin S.J., Cheong J.Y., et al. Diagnostic accuracy of “sweeping” method compared to conventional sampling in rapid urease test for Helicobacter pylori detection in atrophic mucosa. Sci. Rep. 2020;10:18483. doi: 10.1038/s41598-020-75528-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Uotani T., Graham D.Y. Diagnosis of Helicobacter pylori using the rapid urease test. Ann. Transl. Med. 2015;3:9. doi: 10.3978/j.issn.2305-5839.2014.12.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bergonzelli G.E., Donnicola D., Porta N., Corthésy-Theulaz I.E. Essential oils as components of a diet-based approach to management of Helicobacter infection. Antimicrob. Agents Chemother. 2003;47:3240–3246. doi: 10.1128/aac.47.10.3240-3246.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Takagi K., Okabe S., Saziki R. A new method for the production of chronic gastric ulcer in rats and the effect of several drugs on its healing. Jpn. J. Pharmacol. 1969;19:418–426. doi: 10.1254/jjp.19.418. [DOI] [PubMed] [Google Scholar]
- 108.Okabe S., Amagase K. An Overview of acetic acid ulcer models—The history and state of the art of peptic ulcer research. Biol. Pharm. Bull. 2005;28:1321–1341. doi: 10.1248/bpb.28.1321. [DOI] [PubMed] [Google Scholar]
- 109.Kobayashi T., Ohta Y., Yoshino J., Nakazawa S. Teprenone promotes the healing of acetic acid-induced chronic gastric ulcers in rats by inhibiting neutrophil infiltration and lipid peroxidation in ulcerated gastric tissues. Pharmacol. Res. 2001;43:23–30. doi: 10.1006/phrs.2000.0748. [DOI] [PubMed] [Google Scholar]
- 110.Huang X.R., Hui C.W.C., Chen Y.X., Chun B., Wong Y., Fung P.C.W., Metz C., Cho C.H., Hui W.M., Bucala R., et al. Macrophage migration inhibitory factor is an important mediator in the pathogenesis of gastric inflammation in rats. Gastroenterology. 2001;21:619–630. doi: 10.1053/gast.2001.27205. [DOI] [PubMed] [Google Scholar]
- 111.Watanabe T., Higuchi K., Tominaga K., Fujiwara Y., Arakawa T. Acid regulates inflammatory response in a rat model of induction of gastric ulcer recurrence by interleukin 1β. Gut. 2001;48:774–781. doi: 10.1136/gut.48.6.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Martin G.R., Wallace J.L. Gastrointestinal inflammation: A central component of mucosal defense and repair. Exp. Biol. Med. 2006;231:130–137. doi: 10.1177/153537020623100202. [DOI] [PubMed] [Google Scholar]
- 113.Liu Z., Luo Y., Cheng Y., Zou D., Zeng A., Yang C., Xu J., Zhan H. Gastrin attenuates ischemia-reperfusion-induced intestinal injury in rats. Exp. Biol. Med. 2016;241:873–881. doi: 10.1177/1535370216630179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Akisue M.K., Akisue G., Oliveira F.C.C. Pharmacognostic characterization of pau d’alho Gallesia integrifolia (Spreng.) Harms. Rev. Bras. Farmacogn. 1986;1:166–182. doi: 10.1590/S0102-695X1986000200007. [DOI] [Google Scholar]
- 115.Grandtner M.M., Chevrette J. Dictionary of Trees. 1st ed. Academic Press; London, UK: 2013. [Google Scholar]
- 116.Lorenzi H. Árvores Brasileiras: Manual de Identificação e Cultivo de Plantas Arbóreas Nativas do Brasil. 4th ed. Instituto Plantarum de Estudos da Flora Ltda; São Paulo, Brazil: 2002. [Google Scholar]
- 117.Arunachalama K., Baloguna S.O., Pavana E., Almeida G.V.B., Oliveira R.G., Wagner T., Cechinel Filho V., Martins D.T.O. Chemical characterization, toxicology and mechanism of gastric antiulcer action of essential oil from Gallesia integrifolia (Spreng.) Harms in the in vitro and in vivo experimental models. Biomed. Pharmacother. 2017;94:292–306. doi: 10.1016/j.biopha.2017.07.064. [DOI] [PubMed] [Google Scholar]
- 118.Wua J.Z., Liu Y.-H., Liang J.L., Huang Q.H., Dou Y.X., Nie J., Zhuo J.Y., Wu X., Chend J.N., Sub Z.R., et al. Protective role of β-patchoulene from Pogostemon cablin against indomethacin-induced gastric ulcer in rats: Involvement of anti-inflammation and angiogenesis. Phytomedicine. 2018;39:111–118. doi: 10.1016/j.phymed.2017.12.024. [DOI] [PubMed] [Google Scholar]
- 119.Liang J., Dou Y., Wu X., Li H., Wu J., Huang Q., Luo D., Yi T., Liu Y., Su Z., et al. Prophylactic efficacy of patchoulene epoxide against ethanol-induced gastric ulcer in rats: Influence on oxidative stress, inflammation and apoptosis. Chem. Biol. Interact. 2018;283:30–37. doi: 10.1016/j.cbi.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 120.Tarnawski A.S. Cellular and molecular mechanisms of gastrointestinal ulcer healing. Dig. Dis. Sci. 2005;50:S24–S33. doi: 10.1007/s10620-005-2803-6. [DOI] [PubMed] [Google Scholar]
- 121.Çelebi N., Turkyilmaz A., Gonul B., Ozogu C. Effects of epidermal growth factor microemulsion formulationon the healing of stress-induced gastric ulcers in rats. J. Control. Release. 2002;83:197–210. doi: 10.1016/S0168-3659(02)00198-0. [DOI] [PubMed] [Google Scholar]
- 122.Tarnawski A., Stachura J., Durbin T., Sarfeh I.J., Gergely H. Increased expression of epidermal growth factor receptor during gastric ulcer healing in rats. Gastroenterology. 1992;102:695–698. doi: 10.1016/0016-5085(92)90123-G. [DOI] [PubMed] [Google Scholar]
- 123.Milani S., Calabro A. Role of growth factors and their receptors in gastric ulcer healing. Microsc. Res. Tech. 2001;53:360–371. doi: 10.1002/jemt.1104. [DOI] [PubMed] [Google Scholar]
- 124.Szabo S., Vincze A. Growth factors in ulcer healing: Lessons from recent studies. J. Physiol. 2000;94:77–81. doi: 10.1016/S0928-4257(00)00146-7. [DOI] [PubMed] [Google Scholar]
- 125.Jones M.K., Kawanaka H., Baatar D., Szabo I.L., Tsugawa K., Pai R., Koh G.Y., Kim I., Sarfeh I.J., Tarnawski A.S. Gene therapy for gastric ulcers with single local injection of naked DNA encoding VEGF and angiopoietin-1. Gastroenterology. 2001;121:1040–1047. doi: 10.1053/gast.2001.29308. [DOI] [PubMed] [Google Scholar]
- 126.Tarnawski A., Sekhon S., Ichikawa Y., Sarfeh I.J. Vascular endotelial growth factor (VEGF) enhances angiogenesis in injured gastric mucosa and accelerates healing of ethanol-induced erosions. Gastroenterology. 1998;114:A307. doi: 10.1016/S0016-5085(98)81247-X. [DOI] [Google Scholar]
- 127.Luo J.C., Shin V.Y., Liu E.S.L., Ye Y.N., Wu W.K.K., So W.H.L., Chang F.Y., Cho C.H. Dexamethasone delays ulcer healing by inhibition of angiogenesis in rat stomachs. Eur. J. Pharmacol. 2004;485:275–281. doi: 10.1016/j.ejphar.2003.11.038. [DOI] [PubMed] [Google Scholar]
- 128.Suzuki N., Takahashi S., Okabe S. Relationship between vascular endothelial growth factor and angiogenesis in spontaneous and indomethacin-delayed healing of acetic acid-induced gastric ulcers in rats. J. Physiol. Pharmacol. 1998;49:515–527. [PubMed] [Google Scholar]
- 129.Bueno G., Rico S.L.C., Perico L.L., Ohara R., Rodrigues V.P., Emílio-Silva M.T., Assunçao R., Rocha L.R.M., Nunes D.S., Besten M.A., et al. The essential oil from Baccharis trimera (Less.) DC improves gastric ulcer healing in rats through modulation of VEGF and MMP-2 activity. J. Ethnopharmacol. 2021;271:113832. doi: 10.1016/j.jep.2021.113832. [DOI] [PubMed] [Google Scholar]
- 130.Bulanda S., Lau K., Nowak A., Łyko-Morawska D., Kotylak A., Janoszka B. The Risk of Oral Cancer and the High Consumption of Thermally Processed Meat Containing Mutagenic and Carcinogenic Compounds. Nutrients. 2024;16:1084. doi: 10.3390/nu16071084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bray F., Laversanne M., Sung H., Ferlay J., Siegel R.L., Soerjomataram I., Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024;74:229–263. doi: 10.3322/caac.21834. [DOI] [PubMed] [Google Scholar]
- 132.Xie X., Li Y., Lian S., Lu Y., Jia L. Cancer metastasis chemoprevention prevents circulating tumour cells from germination. Signal Transduct Target Ther. 2022;7:341. doi: 10.1038/s41392-022-01174-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Narang A.S., Desai D.S. Anticancer Drug Development—Unique Aspects of Pharmaceutical Development. Pharm. Perspect. Cancer Ther. 2009:49–52. doi: 10.1007/978-1-4419-0131-6_2. [DOI] [Google Scholar]
- 134.Di Sotto A., Mancinelli R., Gullì M., Eufemi M., Mammola C.L., Mazzanti G., Di Giacomo S. Chemopreventive Potential of Caryophyllane Sesquiterpenes: An Overview of Preliminary Evidence. Cancers. 2020;12:3034. doi: 10.3390/cancers12103034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kamal N., Ilowefah M.A., Hilles A.R., Anua N.A., Awin T., Alshwyeh H.A., Aldosary S.K., Jambocus N.G.S., Alosaimi A.A., Rahman A., et al. Genesis and Mechanism of Some Cancer Types and an Overview on the Role of Diet and Nutrition in Cancer Prevention. Molecules. 2022;27:1794. doi: 10.3390/molecules27061794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Puah B.-P., Jalil J., Attiq A., Kamisah Y. New Insights into Molecular Mechanism behind Anti-Cancer Activities of Lycopene. Molecules. 2021;26:3888. doi: 10.3390/molecules26133888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Machado T.Q., da Fonseca A.C.C., Duarte A.B.S., Robbs B.K., de Sousa D.P. A Narrative Review of the Antitumor Activity of Monoterpenes from Essential Oils: An Update. Biomed Res. Int. 2022;2022:6317201. doi: 10.1155/2022/6317201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sobral M.V., Xavier A.L., Lima T.C., de Sousa D.P. Antitumor activity of monoterpenes found in essential oils. Sci. World J. 2014;2014:953451. doi: 10.1155/2014/953451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.de Sousa D.P., Damasceno R.O.S., Amorati R., Elshabrawy H.A., de Castro R.D., Bezerra D.P., Nunes V.R.V., Gomes R.C., Lima T.C. Essential Oils: Chemistry and Pharmacological Activities. Biomolecules. 2023;13:1144. doi: 10.3390/biom13071144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Elson C.E. Suppression of mevalonate pathway activities by dietary isoprenoids: Protective roles in cancer and cardiovascular disease. J. Nutr. 1995;125:1666S–1672S. doi: 10.1093/jn/125.suppl_6.1666S. [DOI] [PubMed] [Google Scholar]
- 141.Ong T.P., Cardozo M.T., de Conti A., Moreno F.S. Chemoprevention of hepatocarcinogenesis with dietary isoprenic derivatives: Cellular and molecular aspects. Curr. Cancer Drug Targets. 2012;12:1173–1190. doi: 10.2174/156800912803987986. [DOI] [PubMed] [Google Scholar]
- 142.Mo H., Elson C.E. Studies of the isoprenoid-mediated inhibition of mevalonate synthesis applied to cancer chemotherapy and chemoprevention. Exp. Biol. Med. 2004;229:567–585. doi: 10.1177/153537020422900701. [DOI] [PubMed] [Google Scholar]
- 143.Mo H., Jeter R., Bachmann A., Yount S.T., Shen C.L., Yeganehjoo H. The Potential of Isoprenoids in Adjuvant Cancer Therapy to Reduce Adverse Effects of Statins. Front. Pharmacol. 2019;9:1515. doi: 10.3389/fphar.2018.01515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.de Vasconcelos C.B.J., de Carvalho F.O., de Vasconcelos C., Meneses D., Calixto F.A.F., Santana H.S.R., Almeida I.B., de Aquino L.A.G., de Souza A.A.A., Serafini M.R. Mechanism of Action of Limonene in Tumor Cells: A Systematic Review and Meta-Analysis. Curr. Pharm. Des. 2021;27:2956–2965. doi: 10.2174/1381612826666201026152902. [DOI] [PubMed] [Google Scholar]
- 145.Silva G.D.S.E., Marques J.N.J., Linhares E.P.M., Bonora C.M., Costa É.T., Saraiva M.F. Review of anticancer activity of monoterpenoids: Geraniol, nerol, geranial and neral. Chem. Biol. Interact. 2022;362:109994. doi: 10.1016/j.cbi.2022.109994. [DOI] [PubMed] [Google Scholar]
- 146.Ahmad S.T., Arjumand W., Seth A., Nafees S., Rashid S., Ali N., Sultana S. Preclinical renal cancer chemopreventive efficacy of geraniol by modulation of multiple molecular pathways. Toxicology. 2011;290:69–81. doi: 10.1016/j.tox.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 147.Jung Y.Y., Hwang S.T., Sethi G., Fan L., Arfuso F., Ahn K.S. Potential Anti-Inflammatory and Anti-Cancer Properties of Farnesol. Molecules. 2018;23:2827. doi: 10.3390/molecules23112827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Goldstein J.L., Brown M.S. Regulation of the mevalonate pathway. Nature. 1990;343:425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 149.Winter-Vann A.M., Casey P.J. Post-prenylation-processing enzymes as new targets in oncogenesis. Nat. Rev. Cancer. 2005;5:405–412. doi: 10.1038/nrc1612. [DOI] [PubMed] [Google Scholar]
- 150.Gregg R.G., Davidson M., Wilce P.A. Cholesterol synthesis and HMG CoA reductase activity during hepatocarcinogenesis in rats. Int. J. Biochem. 1986;18:389–393. doi: 10.1016/0020-711x(86)90046-7. [DOI] [PubMed] [Google Scholar]
- 151.Bathaie S.Z., Ashrafi M., Azizian M., Tamanoi F. Mevalonate Pathway and Human Cancers. Curr. Mol. Pharmacol. 2017;10:77–85. doi: 10.2174/1874467209666160112123205. [DOI] [PubMed] [Google Scholar]
- 152.Ong T.P., Heidor R., de Conti A., Dagli M.L., Moreno F.S. Farnesol and geraniol chemopreventive activities during the initial phases of hepatocarcinogenesis involve similar actions on cell proliferation and DNA damage, but distinct actions on apoptosis, plasma cholesterol and HMGCoA reductase. Carcinogenesis. 2006;27:1194–1203. doi: 10.1093/carcin/bgi291. [DOI] [PubMed] [Google Scholar]
- 153.Peffley D.M., Gayen A.K. Plant-derived monoterpenes suppress hamster kidney cell 3-hydroxy-3-methylglutaryl coenzyme a reductase synthesis at the post-transcriptional level. J. Nutr. 2003;133:38–44. doi: 10.1093/jn/133.1.38. [DOI] [PubMed] [Google Scholar]
- 154.Moreno F.S., Rossiello M.R., Manjeshwar S., Nath R., Rao P.M., Rajalakshmi S., Sarma D.S. Effect of beta-carotene on the expression of 3-hydroxy-3-methylglutaryl coenzyme A reductase in rat liver. Cancer Lett. 1995;96:201–208. doi: 10.1016/0304-3835(95)03933-n. [DOI] [PubMed] [Google Scholar]
- 155.Chebet J.J., Ehiri J.E., McClelland D.J., Taren D., Hakim I.A. Effect of d-limonene and its derivatives on breast cancer in human trials: A scoping review and narrative synthesis. BMC Cancer. 2021;21:1–11. doi: 10.1186/s12885-021-08639-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Crowell P.L., Chang R.R., Ren Z., Elson C.E., Gould M.N. Selective inhibition of isoprenylation of 21–26 kDa proteins by anticarcinogen D-limonene and its metabolites. J. Biol. Chem. 1991;266:17679–17685. doi: 10.1016/S0021-9258(19)47425-5. [DOI] [PubMed] [Google Scholar]
- 157.Chen X., Yano Y., Hasuma T., Yoshimata T., Yinna W., Otani S. Inhibition of farnesyl protein transferase and P21ras memebrane association by d-limonene in human pancreas tumor cells in vitro. Chin. Med. Sci. J. 1999;14:138–144. [PubMed] [Google Scholar]
- 158.Kawata S., Nagase T., Yamasaki E., Ishiguro H., Matsuzawa Y. Modulation of the mevalonate pathway and cell growth by pravastatin and d-limonene in a human hepatoma cell line (Hep G2) Br. J. Cancer. 1994;69:1015–1020. doi: 10.1038/bjc.1994.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chaudhary S.C., Siddiqui M.S., Athar M., Alam M.S. D-Limonene modulates inflammation, oxidative stress and Ras-ERK pathway to inhibit murine skin tumorigenesis. Hum. Exp. Toxicol. 2012;31:798–811. doi: 10.1177/0960327111434948. [DOI] [PubMed] [Google Scholar]
- 160.Mukhtar Y.M., Adu-Frimpong M., Xu X., Yu J. Biochemical significance of limonene and its metabolites: Future prospects for designing and developing highly potent anticancer drugs. Biosci. Rep. 2018;38:BSR20181253. doi: 10.1042/BSR20181253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yu X., Lin H., Wang Y., Lv W., Zhang S., Qian Y., Deng X., Feng N., Yu H., Qian B. d-limonene exhibits antitumor activity by inducing autophagy and apoptosis in lung cancer. Onco Targets Ther. 2018;11:1833–1847. doi: 10.2147/OTT.S155716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ye Z., Liang Z., Mi Q., Guo Y. Limonene terpenoid obstructs human bladder cancer cell (T24 cell line) growth by inducing cellular apoptosis, caspase activation, G2/M phase cell cycle arrest and stops cancer metastasis. J. BUON. 2020;25:280–285. [PubMed] [Google Scholar]
- 163.Chidambara K.N.M., Jayaprakasha G.K., Patil B.S. D-limonene rich volatile oil from blood oranges inhibits angiogenesis, metastasis and cell death in human colon cancer cells. Life Sci. 2012;91:429–439. doi: 10.1016/j.lfs.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 164.Jia S.S., Xi G.P., Zhang M., Chen Y.B., Lei B., Dong X.S., Yang Y.M. Induction of apoptosis by D-limonene is mediated by inactivation of Akt in LS174T human colon cancer cells. Oncol. Rep. 2013;29:349–354. doi: 10.3892/or.2012.2093. [DOI] [PubMed] [Google Scholar]
- 165.Manuele M.G., Barreiro M.L.A., Davicino R., Ferraro G., Cremaschi G., Anesini C. Limonene exerts antiproliferative effects and increases nitric oxide levels on a lymphoma cell line by dual mechanism of the ERK pathway: Relationship with oxidative stress. Cancer Investig. 2010;28:135–145. doi: 10.3109/07357900903179583. [DOI] [PubMed] [Google Scholar]
- 166.Sharmeen J.B., Mahomoodally F.M., Zengin G., Maggi F. Essential Oils as Natural Sources of Fragrance Compounds for Cosmetics and Cosmeceuticals. Molecules. 2021;26:666. doi: 10.3390/molecules26030666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mori N., Usuki T. Extraction of essential oils from tea tree (Melaleuca alternifolia) and lemon grass (Cymbopogon citratus) using betaine-based deep eutectic solvent (DES) Phytochem. Anal. 2022;33:831–837. doi: 10.1002/pca.3132. [DOI] [PubMed] [Google Scholar]
- 168.Yu S.G., Hildebrandt L.A., Elson C.E. Geraniol, an inhibitor of mevalonate biosynthesis, suppresses the growth of hepatomas and melanomas transplanted to rats and mice. J. Nutr. 1995;125:2763–2767. doi: 10.1093/jn/125.11.2763. [DOI] [PubMed] [Google Scholar]
- 169.Cardozo M.T., de Conti A., Ong T.P., Scolastici C., Purgatto E., Horst M.A., Bassoli B.K., Moreno F.S. Chemopreventive effects of β-ionone and geraniol during rat hepatocarcinogenesis promotion: Distinct actions on cell proliferation, apoptosis, HMGCoA reductase, and RhoA. J. Nutr. Biochem. 2011;22:130–135. doi: 10.1016/j.jnutbio.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 170.Madankumar A., Jayakumar S., Gokuladhas K., Rajan B., Raghunandhakumar S., Asokkumar S., Devaki T. Geraniol modulates tongue and hepatic phase I and phase II conjugation activities and may contribute directly to the chemopreventive activity against experimental oral carcinogenesis. Eur. J. Pharmacol. 2013;705:148–155. doi: 10.1016/j.ejphar.2013.02.048. [DOI] [PubMed] [Google Scholar]
- 171.Crespo R., Montero S.V., Abba M.C., de Bravo M.G., Polo M.P. Transcriptional and posttranscriptional inhibition of HMGCR and PC biosynthesis by geraniol in 2 Hep-G2 cell proliferation linked pathways. Biochem. Cell Biol. 2013;91:131–139. doi: 10.1139/bcb-2012-0076. [DOI] [PubMed] [Google Scholar]
- 172.Duncan R.E., Lau D., El-Sohemy A., Archer M.C. Geraniol and beta-ionone inhibit proliferation, cell cycle progression, and cyclin-dependent kinase 2 activity in MCF-7 breast cancer cells independent of effects on HMG-CoA reductase activity. Biochem. Pharmacol. 2004;68:1739–1747. doi: 10.1016/j.bcp.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 173.Vuch J., Marcuzzi A., Zanin V., Crovella S. Comments to the editor concerning the paper entitled “Preclinical renal cancer chemopreventive efficacy of geraniol by modulation of multiple molecular pathways” Shiekh Tanveer Ahmad et al. Toxicology. 2012;293:123–124. doi: 10.1016/j.tox.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 174.Polo M.P., de Bravo M.G. Effect of geraniol on fatty-acid and mevalonate metabolism in the human hepatoma cell line Hep G2. Biochem. Cell Biol. 2006;84:102–111. doi: 10.1139/o05-160. [DOI] [PubMed] [Google Scholar]
- 175.Galle M., Crespo R., Kladniew B.R., Villegas S.M., Polo M., de Bravo M.G. Suppression by geraniol of the growth of A549 human lung adenocarcinoma cells and inhibition of the mevalonate pathway in culture and in vivo: Potential use in cancer chemotherapy. Nutr. Cancer. 2014;66:888–895. doi: 10.1080/01635581.2014.916320. [DOI] [PubMed] [Google Scholar]
- 176.Lee S., Park Y.R., Kim S.H., Park E.J., Kang M.J., So I., Chun J.N., Jeon J.H. Geraniol suppresses prostate cancer growth through down-regulation of E2F8. Cancer Med. 2016;5:2899–2908. doi: 10.1002/cam4.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Gaonkar R., Singh J., Chauhan A., Avti P.K., Hegde G. Geraniol and Citral as potential therapeutic agents targeting the HSP90 activity: An in silico and experimental approach. Phytochemistry. 2022;195:113058. doi: 10.1016/j.phytochem.2021.113058. [DOI] [PubMed] [Google Scholar]
- 178.Zhang Y.F., Huang Y., Ni Y.H., Xu Z.M. Systematic elucidation of the mechanism of geraniol via network pharmacology. Drug. Des. Dev. Ther. 2019;13:1069–1075. doi: 10.2147/DDDT.S189088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Cho M., So I., Chun J.N., Jeon J.H. The antitumor effects of geraniol: Modulation of cancer hallmark pathways (Review) Int. J. Oncol. 2016;48:1772–1782. doi: 10.3892/ijo.2016.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.El-Ganainy S.O., Shehata A.M., El-Mallah A., Abdallah D., El-Din M.M.M. Geraniol suppresses tumour growth and enhances chemosensitivity of 5-fluorouracil on breast carcinoma in mice: Involvement of miR-21/PTEN signalling. J. Pharm. Pharmacol. 2023;75:1130–1139. doi: 10.1093/jpp/rgad060. [DOI] [PubMed] [Google Scholar]
- 181.Burke Y.D., Stark M.J., Roach S.L., Sen S.E., Crowell P.L. Inhibition of pancreatic cancer growth by the dietary isoprenoids farnesol and geraniol. Lipids. 1997;32:151–156. doi: 10.1007/s11745-997-0019-y. [DOI] [PubMed] [Google Scholar]
- 182.Balaraman G., Sundaram J., Mari A., Krishnan P., Salam S., Subramaniam N., Sirajduddin I., Thiruvengadam D. Farnesol alleviates diethyl nitrosamine induced inflammation and protects experimental rat hepatocellular carcinoma. Environ. Toxicol. 2021;36:2467–2474. doi: 10.1002/tox.23359. [DOI] [PubMed] [Google Scholar]
- 183.Rao C.V., Newmark H.L., Reddy B.S. Chemopreventive effect of farnesol and lanosterol on colon carcinogenesis. Cancer Detect. Prev. 2002;26:419–425. doi: 10.1016/s0361-090x(02)00119-8. [DOI] [PubMed] [Google Scholar]
- 184.Chaudhary S.C., Alam M.S., Siddiqui M.S., Athar M. Chemopreventive effect of farnesol on DMBA/TPA-induced skin tumorigenesis: Involvement of inflammation, Ras-ERK pathway and apoptosis. Life Sci. 2009;85:196–205. doi: 10.1016/j.lfs.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 185.Delmondes G.A., Bezerra D.S., Dias D.Q., Borges A.S., Araújo I.M., Lins G.C., Bandeira P.F.R., Barbosa R., Coutinho H.D.M., Felipe C.F.B., et al. Toxicological and pharmacologic effects of farnesol (C15H26O): A descriptive systematic review. Food Chem. Toxicol. 2019;129:169–200. doi: 10.1016/j.fct.2019.04.037. [DOI] [PubMed] [Google Scholar]
- 186.Meigs T.E., Simoni R.D. Farnesol as a regulator of HMG-CoA reductase degradation: Characterization and role of farnesyl pyrophosphatase. Arch. Biochem. Biophys. 1997;345:1–9. doi: 10.1006/abbi.1997.0200. [DOI] [PubMed] [Google Scholar]
- 187.Vinholes J., Gonçalves P., Martel F., Coimbra M.A., Rocha S.M. Assessment of the antioxidant and antiproliferative effects of sesquiterpenic compounds in in vitro Caco-2 cell models. Food Chem. 2014;156:204–211. doi: 10.1016/j.foodchem.2014.01.106. [DOI] [PubMed] [Google Scholar]
- 188.Chagas C.E., Vieira A., Ong T.P., Moreno F.S. Farnesol inhibits cell proliferation and induces apoptosis after partial hepatectomy in rats. Acta Cir. Bras. 2009;24:377–382. doi: 10.1590/s0102-86502009000500007. [DOI] [PubMed] [Google Scholar]
- 189.Khan R., Sultana S. Farnesol attenuates 1,2-dimethylhydrazine induced oxidative stress, inflammation and apoptotic responses in the colon of Wistar rats. Chem. Biol. Interact. 2011;192:193–200. doi: 10.1016/j.cbi.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 190.Qamar W., Sultana S. Farnesol ameliorates massive inflammation, oxidative stress and lung injury induced by intratracheal instillation of cigarette smoke extract in rats: An initial step in lung chemoprevention. Chem. Biol. Interact. 2008;176:79–87. doi: 10.1016/j.cbi.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 191.Park J.S., Kwon J.K., Kim H.R., Kim H.J., Kim B.S., Jung J.Y. Farnesol induces apoptosis of DU145 prostate cancer cells through the PI3K/Akt and MAPK pathways. Int. J. Mol. Med. 2014;33:1169–1176. doi: 10.3892/ijmm.2014.1679. [DOI] [PubMed] [Google Scholar]
- 192.Wang Y.L., Liu H.F., Shi X.J., Wang Y. Antiproliferative activity of Farnesol in HeLa cervical cancer cells is mediated via apoptosis induction, loss of mitochondrial membrane potential (ΛΨm) and PI3K/Akt signalling pathway. J. BUON. 2018;23:752–757. [PubMed] [Google Scholar]
- 193.Au-Yeung K.K., Liu P.L., Chan C., Wu W.Y., Lee S.S., Ko J.K. Herbal isoprenols induce apoptosis in human colon cancer cells through transcriptional activation of PPARgamma. Cancer Investig. 2008;26:708–717. doi: 10.1080/07357900801898656. [DOI] [PubMed] [Google Scholar]
- 194.Lee J.H., Kim C., Kim S.H., Sethi G., Ahn K.S. Farnesol inhibits tumor growth and enhances the anticancer effects of bortezomib in multiple myeloma xenograft mouse model through the modulation of STAT3 signaling pathway. Cancer Lett. 2015;360:280–293. doi: 10.1016/j.canlet.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 195.Joo J.H., Ueda E., Bortner C.D., Yang X.P., Liao G., Jetten A.M. Farnesol activates the intrinsic pathway of apoptosis and the ATF4-ATF3-CHOP cascade of ER stress in human T lymphoblastic leukemia Molt4 cells. Biochem. Pharmacol. 2015;97:256–268. doi: 10.1016/j.bcp.2015.08.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Alves-Silva J.M., Zuzarte M., Girão H., Salgueiro L. The Role of Essential Oils and Their Main Compounds in the Management of Cardiovascular Disease Risk Factors. Molecules. 2021;26:3506. doi: 10.3390/molecules26123506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Mohammed M.K., Mohammed L.K.M.H.M. Cardamom as a blood pressure lowering natural food supplement in patients with grade one hypertension. Hypertension. 2020;1:2. doi: 10.1002/ptr.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Anantha T., Ramesh M., Mounika B., Bhuvaneswari K., Nama S. Review on Hypertension. Int. J. Curr. Trends Pharm. Res. 2013;1:88–96. [Google Scholar]
- 199.Takimoto-Ohnishi E., Murakami K. Renin–angiotensin system research: From molecules to the whole body. J. Physiol. Sci. 2019;69:581–587. doi: 10.1007/s12576-019-00679-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Shigemura N., Takai S., Hirose F., Yoshida R., Sanematsu K., Ninomiya Y. Expression of Renin-Angiotensin System Components in the Taste Organ of Mice. Nutrients. 2019;11:2251. doi: 10.3390/nu11092251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Brazilian Society of Hypertension (SBH) VI Brazilian Guidelines of Hypertension-DBH VI. Rev. Bras. Hipertens. 2010;17:11–17. [Google Scholar]
- 202.Osaili T.M., Dhanasekaran D.K., Zeb F., Faris M.E., Naja F., Radwan H., Cheikh Ismail L., Hasan H., Hashim M., Obaid R.S. A Status Review on Health-Promoting Properties and Global Regulation of Essential Oils. Molecules. 2023;28:1809. doi: 10.3390/molecules28041809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Suručić R., Kundaković T., Lakušić B., Drakul D., Milovanović S.R., Kovačević N. Variations in Chemical Composition, Vasorelaxant and Angiotensin I-Converting Enzyme Inhibitory Activities of Essential Oil from Aerial Parts of Seseli pallasii Besser (Apiaceae) Chem. Biodivers. 2017;14:e1600407. doi: 10.1002/cbdv.201600407. [DOI] [PubMed] [Google Scholar]
- 204.Adefegha S.A., Olasehinde T.A., Oboh G. Essential Oil Composition, Antioxidant, Antidiabetic and Antihypertensive Properties of Two Aframomum Species. J. Oleo Sci. 2017;66:51–63. doi: 10.5650/jos.ess16029. [DOI] [PubMed] [Google Scholar]
- 205.Shiva A.K., Jeyaprakash K., Chellappan D.R., Murugan R. Vasorelaxant and cardiovascular properties of the essential oil of Pogostemon elsholtzioides. J. Ethnopharmacol. 2017;199:86–90. doi: 10.1016/j.jep.2017.01.036. [DOI] [PubMed] [Google Scholar]
- 206.Rocha D.G., Holanda T.M., Braz H.L.B., de Moraes J.A.S., Marinho A.D., Maia P.H.F., de Moraes M.E.A., Fechine-Jamacaru F.V., Filho M.O.M. Vasorelaxant effect of Alpinia zerumbet’s essential oil on rat resistance artery involves blocking of calcium mobilization. Fitoterapia. 2023;169:105623. doi: 10.1016/j.fitote.2023.105623. [DOI] [PubMed] [Google Scholar]
- 207.Zadeh G.S., Panahi N. Endothelium-independent vasorelaxant activity of Trachyspermum ammi essential oil on rat aorta. Clin. Exp. Hypertens. 2017;39:133–138. doi: 10.1080/10641963.2016.1235178. [DOI] [PubMed] [Google Scholar]
- 208.Dib I., Fauconnier M.L., Sindic M., Belmekki F., Assaidi A., Berrabah M., Mekhfi H., Aziz M., Legssyer A., Bnouham M., et al. Chemical composition, vasorelaxant, antioxidant and antiplatelet effects of essential oil of Artemisia campestris L. from Oriental Morocco. BMC Complement. Altern. Med. 2017;17:82. doi: 10.1186/s12906-017-1598-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Da Silva R.E.R., De Morais L.P., Silva A.A., Bastos C.M.S., Pereira-Gonçalves Á., Kerntopf M.R., Menezes I.R.A., Leal-Cardoso J.H., Barbosa R. Vasorelaxant effect of the Lippia alba essential oil and its major constituent, citral, on the contractility of isolated rat aorta. Biomed. Pharmacother. 2018;108:792–798. doi: 10.1016/j.biopha.2018.09.073. [DOI] [PubMed] [Google Scholar]
- 210.Sivakumar L., Chellappan D.R., Sriramavaratharajan V., Murugan R. Root essential oil of Chrysopogon zizanioides relaxes rat isolated thoracic aorta—An ex vivo approach. Z. Naturforsch C J. Biosci. 2020;76:161–168. doi: 10.1515/znc-2020-0143. [DOI] [PubMed] [Google Scholar]
- 211.Singh M.K., Savita K., Singh S., Mishra D., Rani P., Chanda D., Verma R.S. Vasorelaxant property of 2-phenyl ethyl alcohol isolated from the spent floral distillate of damask rose (Rosa damascena Mill.) and its possible mechanism. J. Ethnopharmacol. 2023;313:116603. doi: 10.1016/j.jep.2023.116603. [DOI] [PubMed] [Google Scholar]
- 212.Moreira C.M., Fernandes M.B., Santos K.T., Schneider L.A., Da Silva S.E.B., Sant’Anna L.S., Paula F.R. Effects of essential oil of Blepharocalyx salicifolius on cardiovascular function of rats. FASEB J. 2018;32:715–717. doi: 10.1096/fasebj.2018.32.1_supplement.715.17. [DOI] [Google Scholar]
- 213.Jamhiri M., Hafizibarjin Z., Ghobadi M., Moradi A., Safari F. Effect of Thymol on Serum Antioxidant Capacity of Rats Following Myocardial Hypertrophy. J. Arak Uni. Med. Sci. 2017;20:10–19. [Google Scholar]
- 214.Muzy J., Campos M.R., Emmerick I., Silva R.S.D., Schramm J.M.A. Prevalência de diabetes mellitus e suas complicações e caracterização das lacunas na atenção à saúde a partir da triangulação de pesquisas [Prevalence of diabetes mellitus and its complications and characterization of healthcare gaps based on triangulation of studies] Cad. Saude Publica. 2021;37:e00076120. doi: 10.1590/0102-311X00076120. [DOI] [PubMed] [Google Scholar]
- 215.World Health Organization (WHO) Diabetes. World Health Organization; Geneva, Switzerland: 2020. [(accessed on 10 February 2024)]. Available online: https://www.who.int/health-topics/diabetes#tab=tab_1. [Google Scholar]
- 216.Praveena S., Sujatha P., Sameera K. Trace elements in diabetes mellitus. J. Clin. Diagn. Res. 2013;7:1863–1865. doi: 10.7860/JCDR/2013/5464.3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.IDF . International Diabetes Federation. Diabetes Atlas. 7th ed. International Diabetes Federation; Brussels, Belgium: 2015. [Google Scholar]
- 218.American Diabetes Association (ADA) Classification and diagnosis of diabetes: Standards of medical care in diabetes-2018. Diabetes Care. 2018;41:13–27. doi: 10.2337/dc18-S002. [DOI] [Google Scholar]
- 219.Heghes S.C., Filip L., Vostinaru O., Mogosan C., Miere D., Iuga C.A., Moldovan M. Essential oil-bearing plants from Balkan Peninsula: Promising sources for new drug candidates for the prevention and treatment of diabetes mellitus and dyslipidemia. Front. Pharmacol. 2020;11:989. doi: 10.3389/fphar.2020.00989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Oboh G., Olasehinde T.A., Ademosun A.O. Inhibition of enzymes linked to type-2 diabetes and hypertension by essential oils from peels of orange and lemon. Int. J. Food Prop. 2017;20:S586–S594. doi: 10.1080/10942912.2017.1303709. [DOI] [Google Scholar]
- 221.Radünz M., Camargo T.M., dos Santos Hackbart H.C., Alves P.I.C., Radünz A.L., Gandra E.A., Zavareze E.D.R. Chemical composition and in vitro antioxidant and antihyperglycemic activities of clove, thyme, oregano, and sweet orange essential oils. LWT. 2021;138:110632. doi: 10.1016/j.lwt.2020.110632. [DOI] [Google Scholar]
- 222.Capetti F., Cagliero C., Marengo A., Bicchi C., Rubiolo P., Sgorbini B. Bio-Guided Fractionation Driven by In Vitro α-Amylase Inhibition Assays of Essential Oils Bearing Specialized Metabolites with Potential Hypoglycemic Activity. Plants. 2020;9:1242. doi: 10.3390/plants9091242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Nait-Irahal I., Darif D., Guenaou I., Hmimid F., Azzahra Lahlou F., Ez-Zahra Ousaid F., Abdou-Allah F., Aitsi L., Akarid K., Bourhim N. Therapeutic Potential of Clove Essential Oil in Diabetes: Modulation of Pro-Inflammatory Mediators, Oxidative Stress and Metabolic Enzyme Activities. Chem. Biodivers. 2023;20:e202201169. doi: 10.1002/cbdv.202201169. [DOI] [PubMed] [Google Scholar]
- 224.Eid A.M., Jaradat N., Shraim N., Hawash M., Issa L., Shakhsher M., Nawahda N., Hanbali A., Barahmeh N., Taha B., et al. Assessment of anticancer, antimicrobial, antidiabetic, anti-obesity and antioxidant activity of Ocimum Basilicum seeds essential oil from Palestine. BMC Complement. Med. Ther. 2023;23:221. doi: 10.1186/s12906-023-04058-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Bui A.V., Pham T.V., Nguyen K.N., Nguyen N.T., Huynh K.D., Dang V.S., Ruml T., Truong D.H. Chemical compositions and biological activities of Serevenia buxifolia essential oil leaves cultivated in Vietnam (Thua Thien Hue) Food Sci. Nutr. 2023;11:4060–4072. doi: 10.1002/fsn3.3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Begum C.T., Gogoi R., Sarma N., Pandey S.K., Lal M. Novel ethyl p-methoxy cinnamate rich Kaempferia galanga (L.) essential oil and its pharmacological applications: Special emphasis on anticholinesterase, anti-tyrosinase, α-amylase inhibitory, and genotoxic efficiencies. Peer J. 2023;11:e14606. doi: 10.7717/peerj.14606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Chander R., Maurya A.K., Kumar K., Kumari S., Kumar R., Agnihotri V.K. In vitro antidiabetic and antimicrobial activity of Dracocephalum heterophyllum Benth. essential oil from different sites of North-western Himalayas India. Nat. Prod. Res. 2023;37:1002–1005. doi: 10.1080/14786419.2022.2096603. [DOI] [PubMed] [Google Scholar]
- 228.El Hachlafi N., Benkhaira N., Al-Mijalli S.H., Mrabti H.N., Abdnim R., Abdallah E.M., Jeddi M., Bnouham M., Lee L.H., Ardianto C., et al. Phytochemical analysis and evaluation of antimicrobial, antioxidant, and antidiabetic activities of essential oils from Moroccan medicinal plants: Mentha suaveolens, Lavandula stoechas, and Ammi visnaga. Biomed. Pharmacother. 2023;164:114937. doi: 10.1016/j.biopha.2023.114937. [DOI] [PubMed] [Google Scholar]
- 229.Ibaokurgil F., Yildirim B.A., Yildirim S. Effects of Hypericum scabrum L. essential oil on wound healing in streptozotocin-induced diabetic rats. Cutan. Ocul. Toxicol. 2022;41:137–144. doi: 10.1080/15569527.2022.2052890. [DOI] [PubMed] [Google Scholar]
- 230.Bacanlı M., Anlar H.G., Aydın S., Çal T., Arı N., Ündeğer Bucurgat Ü., Başaran A.A., Başaran N. d-limonene ameliorates diabetes and its complications in streptozotocin-induced diabetic rats. Food Chem. Toxicol. 2017;110:434–442. doi: 10.1016/j.fct.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 231.Lekshmi P.C., Arimboor R., Indulekha P.S., Menon A.N. Turmeric (Curcuma longa L.) volatile oil inhibits key enzymes linked to type 2 diabetes. Int. J. Food Sci. Nutr. 2012;63:832–834. doi: 10.3109/09637486.2011.607156. [DOI] [PubMed] [Google Scholar]
- 232.Bouyahya A., Lagrouh F., El Omari N., Bourais I., El Jemli M., Marmouzi I., Salhic N., Abbesc F.M.E., Belmehdid O., Dakkaa N., et al. Essential oils of Mentha viridis rich phenolic compounds show important antioxidant, antidiabetic, dermatoprotective, antidermatophyte and antibacterial properties. Biocatal. Agric. Biotechnol. 2020;23:101471. doi: 10.1016/j.bcab.2019.101471. [DOI] [Google Scholar]
- 233.Deepa B., Anuradha C.V. Effects of linalool on inflammation, matrix accumulation and podocyte loss in kidney of streptozotocin-induced diabetic rats. Toxicol. Mech. Methods. 2013;23:223–234. doi: 10.3109/15376516.2012.743638. [DOI] [PubMed] [Google Scholar]
- 234.Basha R.H., Sankaranarayanan C. β-Caryophyllene, a natural sesquiterpene, modulates carbohydrate metabolism in streptozotocin-induced diabetic rats. Acta Histochem. 2014;116:1469–1479. doi: 10.1016/j.acthis.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 235.Kumawat V.S., Kaur G. Insulinotropic and antidiabetic effects of β-caryophyllene with l-arginine in type 2 diabetic rats. J. Food Biochem. 2020;44:e13156. doi: 10.1111/jfbc.13156. [DOI] [PubMed] [Google Scholar]
- 236.Srinivasan S., Muruganathan U. Antidiabetic efficacy of citronellol, a citrus monoterpene by ameliorating the hepatic key enzymes of carbohydrate metabolism in streptozotocin-induced diabetic rats. Chem. Biol. Interact. 2016;250:38–46. doi: 10.1016/j.cbi.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 237.El Omari N., Balahbib A., Bakrim S., Benali T., Ullah R., Alotaibi A., Naceiri E.l., Mrabti H., Goh B.H., Ong S.K., et al. Fenchone and camphor: Main natural compounds from Lavandula stoechas L., expediting multiple in vitro biological activities. Heliyon. 2023;9:e21222. doi: 10.1016/j.heliyon.2023.e21222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Gandhi G.R., Hillary V.E., Antony P.J., Zhong L.L.D., Yogesh D., Krishnakumar N.M., Ceasar S.A., Gan R.Y. A systematic review on anti-diabetic plant essential oil compounds: Dietary sources, effects, molecular mechanisms, and safety. Crit. Rev. Food Sci. Nutr. 2023:1–20. doi: 10.1080/10408398.2023.2170320. [DOI] [PubMed] [Google Scholar]