Abstract
Locked nucleic acids (LNAs) are a subtype of antisense oligonucleotides (ASOs) that are characterized by a bridge within the sugar moiety. LNAs owe their robustness to this chemical modification, which as the name suggests, locks it in one conformation. This perspective includes two components: a general overview on ASOs from one side and on delivery issues focusing on lipid nanoparticles (LNPs) on the other side. Throughout, a screening of the ongoing clinical trials involving ASOs is given, as well as a take on the versatility and challenges of using LNAs. Finally, we highlight the potential of LNPs as carriers for the successful delivery of LNAs.
Keywords: MT: Oligonucleotides: Therapies and Applications, lipid nanoparticles, antisense oligonucleotides, locked nucleic acids, gene delivery, genetic medicine, targeting
Graphical abstract
This review provides a comprehensive overview of antisense oligonucleotides (ASOs) and their clinical trial landscape. Peer and colleagues focus on locked nucleic acids, exploring their multifaceted applications and unique attributes. In addition, Peer and colleagues examine the role of lipid nanoparticles in the realm of ASOs, offering insights into future prospects.
Introduction
Ever since the discovery of DNA as the heredity vector in 1940, our understating of nucleic acids has been growing, allowing us to harness them as therapeutic agents. Although most therapies in the market today are small molecules, the top-down approach in their development makes their discovery a tedious task with a very low chance of success. A staggering number of 10,000 molecules need to be tested for one of them to make it to the market.1 In contrast, oligonucleotides are normally developed in a bottom-up approach, where once the mechanism of a disease or the agents causing it is known, a specific sequence can be tailored to execute the needed effect. Furthermore, the development of several oligonucleotide drugs, e.g., Onpattro (Patisiran, for familial amyloid polyneuropathy) and Defibrotide (for veno-occlusive disease), paved the way for the regulatory rules and conduct of this area.2
As part of developing several oligonucleotide therapeutic approaches, in 1978, Paul Zamecnik et al. published papers in which ODN (oligodeoxynucleotide; synthetic DNA molecules) were used to inhibit the translation of the E1 start region of human papillomavirus by stimulation of RNAse H, demonstrating antiviral activity in vivo. These sequences would be later called antisense oligonucleotides (ASOs).3 Though there was enthusiasm around their discovery, the field was forsaken for a while due to the challenges it posed. However, with the development of chemical modifications, the elucidation of the mechanism of action, and general advancement in gene therapy, we are now witnessing the renaissance of ASOs.
In this perspective, we will concentrate on locked nucleic acids (LNA), a specific subtype of ASOs, which were introduced in the late 1990s.4 The chemical modification that makes an LNA monomer is a methylene bridge between the second oxygen and the 4th carbon in the sugar moiety. We will also highlight the potential future of this field, adding possible improvements.5
ASO generations
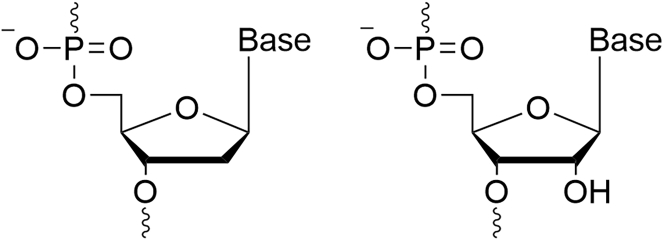
Nucleic acid-based therapy agents are analogs of RNA and DNA (Figure 1) that were created to solve the challenge of instability among these agents. ASOs were designed to bind their RNA target via a Watson-Crick interaction.6
Figure 1.
Chemical structures of natural oligonucleotides
A structure of a deoxyribonucleic acid (DNA) nucleotide (left) and a ribonucleic acid (RNA) nucleotide (right).
The stability, specificity, and potency of ASOs have been developed gradually over time so that the new chemistry tackles the problems of its predecessor. According to these chemical modifications, ASOs are classified into three generations.
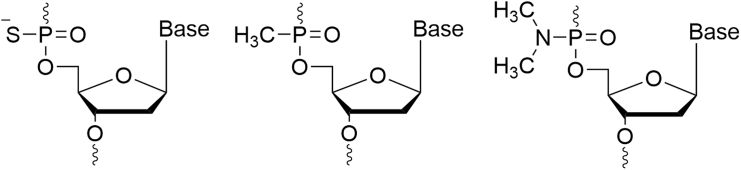
First-generation ASOs
In the first generation of ASOs, a chemical modification was added in the phosphate backbone. Namely, they are obtained by the replacement of one of the non-bridging oxygen atoms in the phosphodiester bond by a sulfur (phosphorothioates), a methyl (methyl-phosphonates), or an amine group (phosphoramidates) (Figure 2). These modifications made a major leap in the field of nucleic acid-based therapy, by increasing their stability and extending their half-life from minutes to days. Furthermore, this generation has the unique ability to recruit RNAse H once meeting its target mRNA.7,8,9 RNAse H will then degrade the mRNA, eventually leading to the downregulation of the corresponding protein. This chemical modification remains the most clinically used.10
Figure 2.
Chemical structures of first-generation ASOs
A structure of a phosphorothioate, methyl-phosphonate, and phosphoramidate nucleotide, respectively.
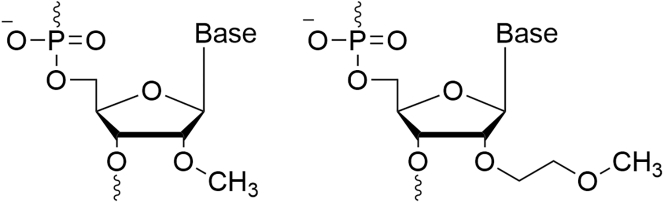
Second-generation ASOs
In the case of the second-generation ASOs, the chemical modification is done in the 2° position of the sugar moiety, yielding RNA analogs. The most common of these modifications are 2-O-methoxyethyl (2°-MOE), and 2-O-methyl (2°-OME) (Figure 3).7 These modifications grant this generation a higher binding affinity while lowering the toxicity and immunogenicity. Unlike the first generation, the agents of this group lack the ability to recruit RNAse H and thus cannot lead to RNA degradation. Nevertheless, protein downregulation may be obtained by this group through sterically blocking the mRNA-ribosome interaction. This group also has the capacity for splice modulation, leading to the inclusion of excluded exons to repair the mRNA and restore the functionality of the target protein.10 Most of the ASOs that are approved for clinical use today use the 2-MOE technology.8,11,12
Figure 3.
Chemical structures of second-generation ASOs
A structure of a 2-O-methyl (2°-OME) nucleotide (left) and a 2-O-methoxyethyl (2°-MOE) nucleotide (right).
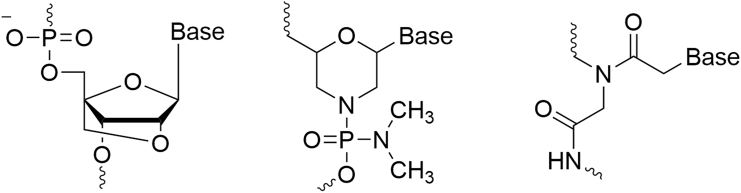
Third generation and the development of locked nucleic acids
While first-generation chemical modifications are in the phosphate backbone, and second-generation modifications are done in the second position in the sugar moiety, yielding RNA analogs, the third generation includes versatile chemical modifications done within the furanose ring, yielding a group of diverse nucleotide analogs (Figure 4). Among this generation are LNAs, phosphorodiamidate morpholino, and peptide nucleic acid. While all 3 are characterized by high potency, LNAs are particularly potent and robust among them.13
Figure 4.
Chemical structures of third-generation ASOs
Structures of a locked nucleic acid (LNA), phosphorodiamidate morpholino (PMO), and peptide nucleic acid (PNA) nucleotide, left to right, respectively.
LNAs were first described by Obika et al. (1997) and Singh, Koshkin, Wengel, and Nielsen (1998) and consist of a bridging between the second and fourth carbons of the furanose ring via an oxymethylene link.14 As the name suggests, the introduced chemical modification locks this RNA analog in one conformation. This change reduces the entropy of LNA hybridization with their target DNA/RNA, or in other words, it increases the chance for such a duplex to form.15 Eventually, the synthesis of LNAs introduced a leap in the affinity of ASOs to their targets.
As mentioned before, the chemical modifications done in each generation grant its monomers a unique feature. Ita and Obika et al. have referred to this concept as “unit chemistry.”16 We can think of the different monomers as LEGO bricks that synergistically complement each other when combined, creating different entities with different abilities.
Clinical trials of ASOs
Many clinical trials were conducted to evaluate the potential therapeutic benefits, as well as the safety profile of ASOs in treating various diseases, including genetic disorders, cancer, and neurological conditions. Although the development of ASOs has faced challenges, so far 10 drugs of this therapeutic class have been approved for marketing for many indications including Duchenne muscular dystrophy (DMD); this includes Fomivirsen, an agent withdrawn due to a reduction in the number of CMV cases following the development of highly active antiretroviral therapy.17,18 The field has continued to show steady progress over the years, with oligonucleotides being under clinical development for approximately three decades, demonstrating the enduring interest and potential of this therapeutic approach (Table 1).19 Most of the ASOs that are approved for clinical use today are second-generation 2-MOE chemical modifications.
Table 1.
Clinical trials of ASOs
| Company | Candidate | Route | Biological target | Condition or disease | Clinical stage | Latest update |
|---|---|---|---|---|---|---|
| Genzyme Corporation | ISIS-301012/Kynamro/Mipomersen | subcutaneous injection | Apolipoprotein B-100 | hyperlipoproteinemia type IIa | marketed | preclinical for atherosclerosis was discontinued |
| Isis Pharmaceuticals | Fomivirsen/ISIS 2922/Vitravene | intravitreal injection | CMV protein IE2 (blocks translation of UL123 gene) | cytomegalovirus infections | market withdrawal | market withdrawal |
| Sarepta Therapeutics | Eteplirsen/AVI-4658 PMO/Exondys 51 | intravenous infusion | Exon 51 of Dystrophin | Duchenne muscular dystrophy | marketed | marketed |
| Genzyme Corporation | Nusinersen/ISIS-396443/Spinraza | intrathecal injection by lumbar puncture | SMN2 gene | spinal muscular atrophy | marketed | marketed |
| Sarepta Therapeutics | Golodirsen/SRP 4053 | intravenous infusion | Exon 53 of dystrophin | Duchenne muscular dystrophy | marketed | marketed |
| National Center of Neurology and Psychiatry | Viltolarsen/Biltrarsen/NCNP-01 | intravenous infusion | Exon 53 of dystrophin | Duchenne muscular dystrophy | marketed | marketed |
| Santaris Pharma | SPC 4955 | subcutaneous injection | Apolipoprotein B-100 (ApoB) | hypercholesterolaemia | phase I | completed |
| eFFECTOR Therapeutics | Zotatifin/eFT 226 | intravenous/subcutaneous | Eukaryotic Initiation Factor-4A (EIF4A1) | COVID 2019 infections | phase I | favorable safety results and positive trends in multiple antiviral activity measures |
| Acasti Pharma | GTX-102 | oral spray | Paternal UBE3A | ataxia telangiectasia | phase I | pharmacokinetic (PK) study for GTX-102 met all outcome measures |
| University of Texas Southwestern Medical Center/Viridian Therapeutics | MRG-110 (Anti-Mir92a; S95010) | local intradermal injection | Microrna-92 | heart failure, ischemia, wounds | phase I | well-tolerated safety profile; efficacy will be evaluated for heart failure and ischemia |
| University of Texas M. D. Anderson Cancer Center/Bio-Path Holdings | BP1002 | intravenous infusion (liposomal) | Proto-oncogene protein c-Bcl-2 | acute myeloid leukemia, advanced lymphoid malignancies | phase I | ongoing |
| Texas A&M University/Ultragenyx Pharmaceutical | GTX-102 | intrathecal (it) injection | Paternal UBE3A | Angelman syndrome | phase I/II | recruiting |
| University of Texas M. D. Anderson Cancer Center/Bio-Path Holdings | Prexigebersen (BP1001) | intravenous infusion (liposomal) | Grb2 | cancer | phase I/II | updated efficacy and safety data from the phase II trial in acute myeloid leukemia (AML) was released |
| Ionis Pharmaceuticals | ISIS-GCGRRx (ISIS 449884) | subcutaneous injection | Glucagon Receptor (GCGR), | type 2 diabetes | phase II | positive data from the phase II |
| GSK | GSK3389404 (IONIS HBV LRx) | subcutaneous injection | Hbv RNA | hepatitis B | phase II | acceptable safety, but no efficacious dosing regimen was identified |
| University of Marburg/Sterna biologicals | SB-010 | inhalation | GATA3 Transcription Factor | allergic asthma; chronic obstructive pulmonary disease; ulcerative colitis | phase II | completed |
| University of Marburg/Sterna biologicals | SB-011 | topical (water/oil/water emulsion) emulsion | GATA3 Transcription Factor | mild to moderate atopic dermatitis | phase II | completed |
| University of Marburg/Sterna biologicals | SB-012 | intrarectally | GATA3 Transcription Factor | ulcerative colitis | phase II | completed |
| Ionis Pharmaceuticals | Sapablursen (ISIS 702843, IONIS-TMPRSS6-LRx) | subcutaneous injection | Transmembrane Protease Serine 6 (TMPRSS6) | beta thalassemia, polycythemia vera | phase II | development of sapablursen in beta thalassemia was discontinued, while that of polycythemia vera is on going |
| Sarepta Therapeutics | Vesleteplirsen/SRP 5051 | intravenous infusion | Exon 51 of Dystrophin | Duchenne muscular dystrophy | phase II | ongoing |
| AstraZeneca/Ionis Pharmaceuticals | Danvatirsen (AZD9150, IONIS-STAT3-2.5Rx) | intravenous infusion | Stat3 | cancer | phase II | promising data in combination with durvalumab |
| eFFECTOR Therapeutics | Zotatifin/eFT 226 | intravenous | Eukaryotic Initiation Factor-4A (EIF4A1) | breast cancer; non-small cell lung cancer | phase II | safe and well-tolerated, with encouraging signals of efficacy |
| Ionis Pharmaceuticals | QR 1123 (IONIS-RHO-2.5Rx; ISIS-GSK5-2.5Rx) | intravitreal (ivt) injection | Rhodopsin | retinitis pigmentosa | phase II | suspended |
| Geron Corporation/Janssen Biotech | Imetelstat (GRN 140719; GRN 163; GRN 163-L) | intravenous infusion | RNA Template of Telomerase Reverse Transcriptase. | cancer | phase II/III | commercial launch announced May 2023 |
| BioMarin Nederland | Drisapersen (GSK2402968) | subcutaneous injection | Exon 51 of Dystrophin mRNA. | muscular dystrophies | phase II/III | discontinued (FDA disapproval) |
| Ionis Pharmaceuticals | Tominersen/IONIS-HTTRx; ISIS-443139; RG 6042 | intrathecal injection | Huntingtin (Htt) Protein |
Huntington’s disease | phase II/III | ongoing |
| Ionis Pharmaceuticals/Roche | IONIS-FB-LRX | subcutaneous injection | Complement Factor B (CFB) | primary Iga nephropathy/geographic atrophy | phase II/III | phase II showed promising clinical data |
| Ionis Pharmaceuticals | Olezarsen (AKCEA-APOCIII-LRx; ISIS 678354) | subcutaneous injection | ApoC-III | hypertriglyceridemia/cardiovascular diseases | phase III | granted a fast-track designation |
| Ludwig Institute for Cancer Research/Biogen/Ionis Pharmaceuticals | Tofersen (BIIB067) | intrathecal injection | Superoxide Dismutase 1 (SOD1) | amyotrophic lateral sclerosis (ALS) | phase III | approved |
| Atlantic Healthcare | Alicaforsen (ISIS 2302) | enema/intravenous infusion | Intercellular Adhesion Molecule-1 (ICAM-1) | ulcerative colitis/Crohn’s disease/pouchitis | phase III | approved |
| Sarepta Therapeutics | Casimersen/AMONDYS 45; SRP-4045 | intravenous infusion | Exon 45 of Dystrophin | Duchenne muscular dystrophy | phase III | interim results show casimersen safe, efficacious |
| Akcea Therapeutics/Ionis Pharmaceuticals | Inotersen (GSK 2998728) | subcutaneous injection | Transthyretin (TTR) | amyloidosis | phase III | marketed |
| Ionis Pharmaceuticals | Donidalorsen (IONIS-PKK-LRX) | subcutaneous injection | Prekallikrein (PKK) | hereditary angioedema | phase III | was found to reduce the number of monthly swelling attacks |
| Ionis Pharmaceuticals | Eplontersen (AKCEA-TTR-LRx) | subcutaneous injection | Transthyretin (TTR) | hereditary transthyretin-mediated amyloid polyneuropathy (hattr-pn) | phase III/pre-registration | completed |
| Korea Institute of Science and Technology/ISIS Pharmaceuticals | ISIS 353512 (ISIS CRPRx) | subcutaneous injection | C-Reactive Proteins (Crp) | inflammatory diseases | phase I | discontinued |
| Enzon Pharmaceuticals | RG 6061/EZN-2968/RO-7070179 | intravenous infusion | Hypoxia-Inducible Factor-1 Alpha | liver cancer; lymphoma; solid tumors | phase I | discontinued |
| Santaris Pharma | SPC 5001 | subcutaneous injection | Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) | hypercholesterolemia | phase I | discontinued |
| Ionis Pharmaceuticals | Tadnersen (BIIB078) | intrathecal injection | Chromosome 9 Open Reading Frame 72 (C9orf72) | amyotrophic lateral sclerosis | phase I | discontinued due to unmet secondary efficacy endpoints and no clinical benefit |
| National Institute of Allergy and Infectious Diseases/Sarepta Therapeutics | radavirsen (AVI-7100) | intravenous infusion | Matrix 1 & 2 Segment Of Influenza A | influenza a virus infections | phase I | discontinued |
| Santaris Pharma | RG 6061 (EZN-2968; RO-7070179; SPC-2968) | intravenous infusion | Hypoxia-Inducible Factor-1 Alpha (HIF-1α) | liver cancer; lymphoma; solid tumors | phase I | discontinued |
| Santaris Pharma | EZN-4176 | intravenous infusion | Androgen Receptor (Ar) | bone cancer; prostate cancer | phase I | discontinued |
| Georgetown University | LErafAON-ETU | intravitreal injection (liposome entrapped) | C-Raf | solid tumors | phase I | discontinued |
| Santaris Pharma | SPC 2996 | intravenous infusion | B cell Lymphoma 2 (Bcl-2) | chronic lymphocytic leukemia | phase I/II | discontinued |
| Santaris Pharma | Miravirsen/SPC-3649 | subcutaneous injection | Mirn122 | chronic hepatitis C | phase I/II | discontinued |
| AstraZenec/Regulus Therapeutics | RG 125 (AZD 4076) | subcutaneous injection | Microrna-103/107 | type 2 diabetic with nonalcoholic fatty liver disease | phase I/II | discontinued |
| Daiichi Sankyo Inc | Renadirsen (DS-5141b) | subcutaneous injection | Dystrophin Exon 45 | Duchenne muscular dystrophy | phase I/II | discontinued |
| Temple University | G 4460 (c-myb ASO G4460; INX 3001; LR3001) | intravenous infusion/parenteral: aso treated bone marrow | Proto Oncogene Proteins C Myb | chronic myeloid leukemia; leukemia | phase I/II | discontinued |
| WaVe life Sciences | Rovanersen/BMTR5150; WV-1092; WVE-120101 | intrathecal injection | Huntington (HTT) | Huntington’s disease | phase I/II | terminated |
| Idera Pharmaceuticals | AEG 35156 | intravenous infusion | X-Linked Inhibitor Of Apoptosis (XIAP) | cancer | phase I/II | terminated; failed to reach endpoints; neurotoxicity |
| WaVe life Sciences | Lexanersen/WV-2603; WVE-120102 | intrathecal injection | Huntington (HTT) | Huntington’s cisease | phase I/II | termination due to lack of efficacy |
| ProQR Therapeutics | Sepofarsen (EW9I3PJC3R; QR-110) | intravitreal (ivt) injection | Cep290 Protein | Leber congenital amaurosis | phase I/II | termination due to sponsor’s decision |
| Ionis Pharmaceuticals | Vupanorsen (IONIS ANGPTL3-LRx/AKCEA-ANGPTL3- LRX/ISIS 703802) | subcutaneous injection | Angiopoietin-Like 3 (Angptl3) | dyslipidemia/nonalcoholic fatty liver disease/diabetes mellitus, type 2 | phase II | discontinuation for hypertriglyceridaemia |
| University of Nebraska Medical Center | Cenersen (Aezea; EL625; EL831; OL(1)p53) | intravenous infusion | Exon 10 In P53 | myelodysplastic syndromes; acute myeloid leukemia; chronic lymphocytic leukemia | phase II | Discontinued. |
| Regulus Therapeutics; Sanofi | Lademirsen (RG 012; RG 456070; SAR 339375) | subcutaneous injection | Microrna-21 | Alport syndrome | phase II | discontinued |
| Isis Pharmaceuticals | AZD 4785 (IONIS-KRAS-2.5Rx) | intravenous infusion | Kras | non-small cell lung cancer, advanced solid tumors | phase II | discontinued |
| Isis Pharmaceuticals | IONIS AR 2.5Rx (AZD 5312; ISIS ARRX; ISIS AZ1Rx; ISIS-560131) | intravenous infusion | Androgen Receptor (Ar) | prostate cancer; solid tumors | phase II | discontinued |
| Isis Pharmaceuticals | ISIS-FXIRx (ISIS 416858) | subcutaneous injection | Factor XI | end-stage renal disease (ESRD)/venous thromboembolism | phase II | discontinued |
| Ionis Pharmaceuticals | Cimdelirsen (IONIS-GHR-LRx; ISIS 766720) | subcutaneous injection | Growth Hormone Receptor (Ghr) | acromegaly | phase II | discontinued |
| Isis Pharmaceuticals | ISIS 5132 (CGP 69846A) | intravenous infusion | C-Raf | breast cancer/ovarian cancer | phase II | discontinued |
| Ionis Pharmaceuticals/University of British Columbia | Apatorsen (ISIS-306053; OGX-427) | intravenous infusion | Heat Shock Protein Hsp27 | cancer | phase II | discontinued |
| Isis Pharmaceuticals | Aprinocarsen (Affinitac; Aprenocarsen sodium; CGP 64128A; ISIS 3521) | intravenous infusion | Protein Kinase C (PKC) | cancer | phase II | discontinued, N/A |
| University of Pennsylvania | Oblimersen (Augmerosen; BCL-2 ASO - Genta; Genasense) | intravenous infusion | B cell Lymphoma 2 (Bcl-2) | cancer | phase II/III | discontinued |
| Prosensa | Drisapersen (GSK2402968) | subcutaneous injection | Exon 51 Of Dystrophin Mrna | muscular dystrophies | phase II/III | discontinued (FDA disapproval) |
| ProQR Therapeutics | Ultevursen (QR-421) | intravitreal (ivt) injection | Exon 13 Of The USH2A Gene | Usher syndromes/retinitis pigmentosa | phase II/III | termination of phase II in Usher syndromes due to the sponsor’s decision; termination of phase II/III trial in retinitis pigmentosa |
| University of British Columbia | Custirsen (ISIS 112989; OGX-011; TV-1011) | intravenous infusion | Clusterin | cancer | phase III | discontinued; did not meet endpoint of significantly extending overall survival |
| WaVe life Sciences | Suvodirsen/WV-3473; WVE-210201 | intravenous | Exon 51 Of Distrophin | Duchenne muscular dystrophy | phase II/III | terminated |
In the case of ASOs, the drug development is disease target centered: i.e., once a biological target is linked to the pathophysiology of a disease, a sequence is tailored in a specific manner to that target, considering the features that can be achieved using different chemical modifications. In the case of small molecules, however, the development is molecule centered, as it starts from the discovery of a compound, after which the chemical properties, features, and biological effects are elucidated. After these tests, the molecule can be matched to a specific disease target and enter preclinical and clinical development. This methodical difference significantly shortens the discovery stage of gene therapy compared to the classic small molecule development. It is estimated that out of 10,000 molecules, only 250 make it to preclinical development, and only 1 is eventually marketed (0.4% from preclinical to marketing).20 According to available information, thus far, 446 ASOs agents have been assessed pre-clinically, out of which 8 are marketed (∼1.8%), with some of the studies summarized in Table 1.17 Despite the higher rate in comparison to small molecules, we believe that ASOs are not reaching their full potential, partially since smart delivery systems are not being harnessed. As an example, clinical studies of ASOs revealed an association with increases of hepatic enzymes, thrombocytopenia, and proteinuria, which are believed to be a consequence of systemic exposure, as well as off-target-sequence-related effects.21 Both problems may be mitigated via a targeted delivery in a cell-specific manner. One example is a drug developed for acute myeloid leukemia (AML) and tested on other types of cancers: Prexigebersen. The chemically modified DNA is incorporated inside a neutrally charged lipid bilayer, creating lipid nanoparticles that reach the target cells without toxic effects.
In conclusion, ASOs represent a therapeutic strategy with significant potential in treating a wide range of diseases. While progress has been made in clinical trials and in understanding their mechanisms of action, further research is needed to address existing challenges and optimize the efficacy and safety of ASO-based therapies, with an emphasis and encouragement to employ delivery systems to achieve those goals.
Incorporating LNAs for versatile uses
LNAs brought a set of remarkable features into the ASOs field. Their most prominent and important feature is their stable conformation, making them very potent compounds with high affinity. In line with the LEGO bricks approach, the incorporation of LNAs in existing oligonucleotide technologies yielded novel biotechnological and therapeutic applications.
The incorporation of LNAs with DNA nucleotides has yielded what is termed antisense LNA. This can be divided into mixmers and gapmers; in the former, LNA units are dispersed across the sequence, while in the latter, there is a central gap of DNA between two over-hinged LNAs.10 The LNA involvement gives the sequence a long half-life, with high potency, while the native DNA allows for RNAse H recruitment once hybridized with complementary RNA.22
The conformational lock within the furanose ring facilitates and favors the binding of LNAs to their complementary sequence. Accordingly, when incorporated with siRNAs, they ensure the loading of the sequence into the RNA-induced silencing complex.23
One of the most interesting applications in which LNAs are employed is pre-mRNA splice switching. Pre-mRNA maturation is a process that takes place in the nucleus and involves a range of ribonucleoproteins comprising a “spliceosome.”24 The creation of different mRNA variants is obtained by sequences around introns and exons, which either enhance or silence spliceosomes. Accordingly, in cases where faulty splicing occurs, splice-switching oligonucleotides (SSOs) may be utilized to tilt the balance back to normal. This process, however, requires a high level of precision; well-defined sequences need to be excluded/included, without degrading the pre-mRNA, which makes LNAs a prominent candidate for the mission, since they lack the ability to recruit RNAse H on one hand and are very potent and precise on the other.23 Roberts et al. used an LNA SSO (LNA SSO-654) to demonstrate the prevention of aberrant splicing of EGFP in transgenic mice. SSOs including LNAs rather than 2′OMe fully modified ASOs were more efficient.25
Indeed, this versatility has thus far proven to be effective in various preclinical and clinical studies; for example, Tassone et al. demonstrated a good safety profile and a promising anti-tumoral activity in a first-in-human, phase 1, open-label, dose-escalation study. In the study, 17 patients received a 13-mer LNA with a phosphorothioate (PS)-modified backbone that inhibits microRNA-221 (LNA-i-miR-221).26 Another target that has been tested in the context of LNAs is miR-21, an oncomiR that plays a pivotal role in many cancers. Javanmard et al. have demonstrated preclinical anticancer activity using an LNA sequence to treat melanoma-bearing male C57BL/6 mice.27 Further highlighting their versatility, it should be noted that LNAs’ efficacy is not limited to cancer. For example, a work by Bockstahler et al. has demonstrated anti-inflammatory activity in A/J mice suffering from (induced) autoimmune myocarditis, ultimately reducing cardiac inflammation and fibrosis.28 The various successes in the works mentioned above, as well as the various clinical trials found in Table 1, show the versatile nature of LNAs in multiple indications, from cancer and inflammatory autoimmunity to genetic diseases such as DMD.
Toxicity
The driving force for chemical modification throughout the generations was to improve the therapeutic window of ASOs; namely, the effective dose should be well below the dose that causes significant adverse events. In the context of adverse events of ASOs, adverse events are divided into two categories: hybridization-dependent off-target effects and non-hybridization-dependent off-target effects.16
If we take the phosphorothioate backbones of the first-generation ASOs as an example, while efficient, they seem to be immunostimulatory, exerting a proinflammatory effect through nucleic acid-sensing TLR receptors among other suggested mechanisms. This adverse effect falls under the definition of non-hybridization-dependent off-target effects, as it is not a result of an ASO-target Watson/Crick base-pairing but rather linked to the high dose in which phosphorothioates should be administered.16,29
Using LNAs with their conformational lock, as well as their short length, makes them very potent compared to other ASOs, which allow them to be administered in lower doses. Unfortunately, most research shows that this comes at the expense of increased hybridization-dependent off-target effects, in which a sequence can bind sequences other than its intended target. This seems to correlate with the short length of the LNAs. Swayze et al. tested different corresponding sequences of ASOs, using MOE versus LNA chemical modification as the sole variation between each correspondent pair. Their results demonstrate a superior efficacy of LNAs in vivo compared to MOEs in the downregulation of TNFRSF1A-associated via death domain mRNA in the livers of Balb/c mice. However, all LNA treatment arms showed a significant increase in transaminases, to an extent where the high-dose group of the most potent sequence had to be sacrificed following a 25% loss of body weight because of hepatotoxicity. Hepatotoxicity in MOE arms was lower according to all the parameters.30
Delivering the ASOs via lipid nanoparticles
As shown in Table 1, the majority of ASO clinical trials have utilized systemic intravenous administration, which means that they will be evenly dispersed throughout tissues and cells, necessitating a high dosage to achieve adequate concentration at the disease site. Consequently, as higher doses of ASOs are more likely to induce adverse effects, this method of general, unguided administration increases the chances for undesired side effects.31,32
Getting to the site of action with adequate quantity is just one of the challenges for ASOs. Effective treatment relies also on sufficient cellular uptake as well as successful exit from subcellular compartments and accurate hybridization to the target sequence. The cellular uptake of unmodified ASOs is typically poor, with only a fraction of ASO molecules managing to enter the cell, often showing less than 1% of the molecules arriving to the cytoplasm.33,34
Even when tested in controlled conditions (in vitro), the specific mechanisms of cellular uptake depend on various factors including chemistry, molecule length, conformation, and concentration, as well as cell type and cell culture conditions or environment among others. For instance, the PS group generally enhances cell uptake but diminishes RNA target affinity, a drawback that can be mitigated by 2′-sugar substituents.33
Despite the chemical modifications that are meant to solve issues of delivery and targeting, challenges such as limited efficacy, off-target effects, and toxicity persist as barriers to broader adoption in clinical practice.33,34
Accordingly, drug delivery systems were created as a method to control the release and the behavior of a therapeutic agent in the body. To date, there is a versatile arsenal of nano-scale delivery systems available. The ADME of these systems may be tailored to the purpose, via modifying the system’s physicochemical properties, with the most important factors being the nanoparticle size, charge, hydrophobicity, and morphology.35 In addition to the intrinsic factors, additional features may be added and fine-tuned to reach a certain goal; for instance, PEGylation of nanoparticles increases their circulation time by lowering their uptake by the mononuclear cells of the immune system.36
Vehicles have been recently implemented in the context of ASO delivery. Among the prominent examples of this are Prexigebersen/BP1001 and BP1002 (Bio-Path Holding). Prexigebersen, a neutral liposomal formulation of an ASO against Grb2 mRNA, is currently being evaluated for its efficacy in combination with chemotherapy in untreated, elderly AML patients as well as in relapsed or refractory AML patients, while BP1002, a neutral liposomal formulation of ASO against Bcl-2, is currently in phase 1 clinical trials in patients with advanced lymphoid malignancies (NCT04072458) and patients with refractory/relapsed AML (NCT05190471), and these are ongoing.37
In addition to the above, many delivery techniques were and are tested, showing potential results such as bio-reducible LNPs that can both achieve a good performance in encapsulating the ASO and in delivering it to intracellular sites of action, as well as to target organs.38
Despite those efforts, we believe that drug delivery systems are not sufficiently employed to benefit the delivery and therapeutic efficacy of ASOs. One category of potentially suitable carriers that has been rising to fame in recent years are lipid nanoparticles (LNPs), which as of today are the most advanced non-viral nucleic acid delivery vehicles. The building blocks of LNPs include 3 helper lipids in the form of PEG-lipids, phospholipids, and cholesterol, and an amino ionizable cationic lipid.39,40 The ionizable cationic lipid was a game changer in the field of nanoparticles due its ability to change its charge in different environments. While in acidic pH the ionizable lipid is positively charged, which increases both payload encapsulation efficiency and intracellular delivery, it is neutrally charged in physiological pH, greatly lowering its immunogenicity in the blood.41,42 Vast libraries of ionizable lipids have been and are still being developed, opening different avenues of targeting, with some being cell specific and others nucleic acid specific.39,40
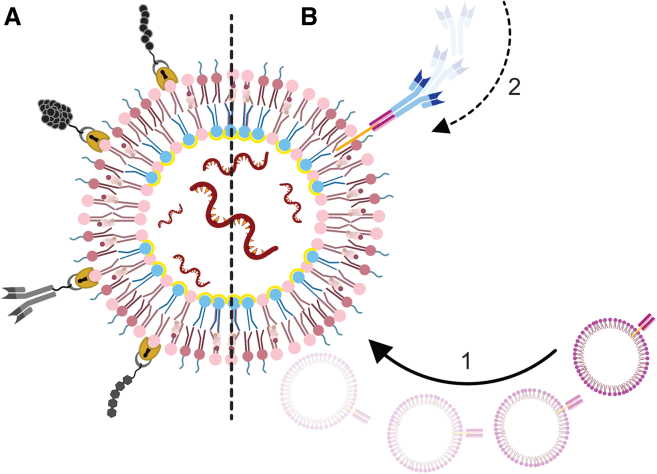
To achieve efficient treatment, LNPs should first reach the cell population of interest, after which they should be internalized to finally release their cargo into the cell cytosol. Over the years, many targeting options and techniques have been developed (e.g., antibodies and ligands) to direct the particles to unload their payload at the disease site or to a specific cell subset. One example of such a system is the ASSET, a self-assembled modular platform developed at our lab, which is a ready-to-use LNP system on which an unlimited repertoire of targeting moieties can be linked, eliminating the need for extensive calibration (Figure 5).43
Figure 5.
Targeting options and techniques for LNPs
(A) Peptides, proteins, antibodies, and carbohydrates (top to bottom respectively) may be incorporated through a covalent bound to a surface lipid. Alternatively, (B) a system developed in our lab (ASSET): (1) membrane-anchored-scFv vesicles are fused with nucleic acid-containing LNPs, yielding LNPs that can (2) non-covalently bind the Fc constant region of any rat IgG2a.
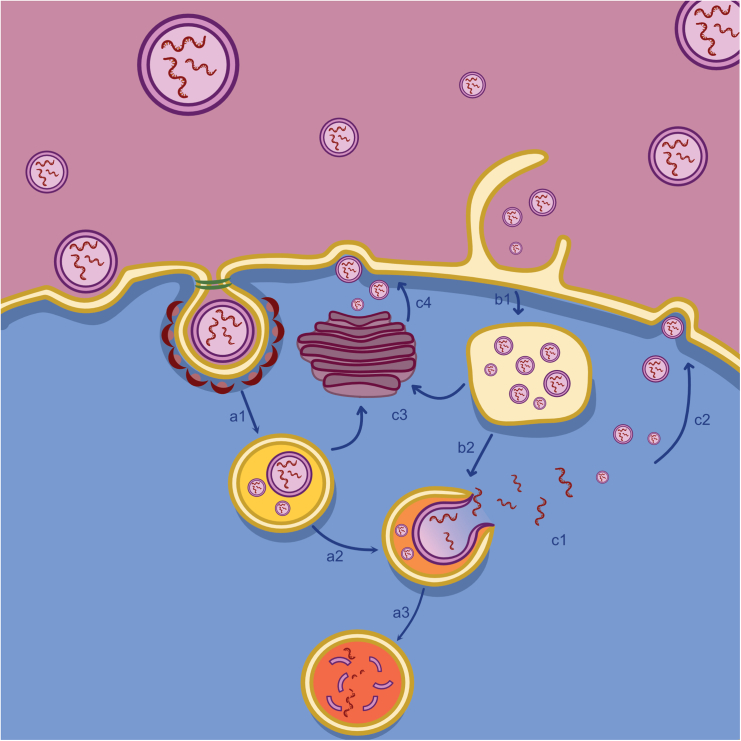
After arriving at the target, LNPs can be internalized through dynamin-dependent pathway, clathrin-dependent pathway, macropinocytosis, or direct fusion with the cell membrane.44 Eventually the internalization mechanism depends on many factors, including size, ionizable lipid of choice, and helper lipids.45 Finally, once inside the endosome, the ionizable cationic lipid becomes positively charged due to the low pH in the endosome, and this initiates the destabilization of the endosome with the LNP-endosomal membrane adhesion, fusion, and final disruption facilitated by cholesterol and helper lipids through phase transition of the LNPs (Figure 6).46
Figure 6.
Possible pathways of LNP internalization
(A1) Internalization may start with clathrin-coated vesicles that mature into an early endosome; (A2) endosomal escape of the cargo (nucleic acid) may occur in the next step where an early endosome matures into a late endosome; alternatively, (A3) the late endosome might mature into a lysosome, where the cargo is likely to be destroyed. LNPs may also be internalized (B1) via macropinocytosis, where (B2) endosomal escape might occur at a later stage from the late endosome. LNPs can exit the cell through vesicle-related and non-vesicle-related routes.47,48
Just 3 years ago, the world witnessed the unprecedented approval of LNPs as the carrier system for the delivery of mRNA vaccines against the COVID-19 virus. Even though ASOs are far smaller than the mRNA used in the approved COVID-19 vaccines, LNPs have also been proven in the past to be efficient carriers for far smaller molecules such as siRNA, as LNPs delivering siRNA were clinically approved even before the COVID-19 vaccines in the form of Patisiran, a transthyretin-targeting siRNA encapsulated in an LNP. Therefore, it is logical to assume that LNPs can also efficiently encapsulate and deliver ASOs in vivo, potentially reducing the required dosage for efficacy and thus the adverse side effects. The mentioned approval of the COVID-19 vaccine drove a much-needed advancement in the research and characterization in the field of LNPs, opening new avenues for their implementation, and we believe that delivering ASOs can be one new approach.
Future outlook
In this perspective, we have discussed the use of ASOs with an emphasis on LNAs. What seems to be apparent is that the chemistry and sequence of ASOs dictate not only the function but also the ADME profile of the molecules. Despite the unprecedented potency of LNAs compared to other ASOs, several studies have found that this potency leads to higher incidents of hybridization-dependent off-target effects. Although the dose needed to obtain an effect is lower in the case of LNAs as noted by some studies, their lack of specificity will cause off-target effects in undesired organs, while not delivering an adequate effective dose to the intended site of action. This issue might be lowered to a degree by their incorporation into LNPs. Due to their physicochemical properties, LNPs tend to have a more specific accumulation profile, and accordingly, a lower systemic dose of LNAs will be needed to exert the needed effect at the relevant site of disease. For example, in the case of cancer or inflammatory disease, the enhanced permeability and retention may be used as passive targeting to deliver a concentrated dose of LNAs. Alternatively, LNPs may be decorated with an active targeting moiety, delivering the payload, LNAs in this case, to a further specific site.
Diving closely into cellular distribution, it was shown that when LNAs are endocytosed, they will persist in an “inactive” state as long as they are in the endosome, which acts as their trap, and will be “activated” only once shed into the cytoplasm.23,49 We hypothesize that the usage of LNPs as a carrier of LNAs might help in increasing the percentage of “activated” LNAs out of a given dose, particularly LNPs that are fabricated using ionizable lipids. These ionizable lipids become positively charged in the acidic environment of the endosome, which facilitates electrostatic interactions, leading to their fusion with the negatively charged endosomal membrane.
Collectively, we believe that the introduction of LNPs will lower the required dose by tens of folds compared to naked LNAs and may ultimately open new therapeutic modalities.
Acknowledgments
D.P. acknowledges the support from The EXPERT project (European Union’s Horizon 2020 research and innovation program, under grant agreement # 825828), ISF grant (2012/20), and the Lewis Trust for Blood Cancer.
S.Q. thanks the Neubauer Family Foundation for her Ph.D. fellowship.
Author contributions
S.Q. wrote the review. S.Q., D.B., and I.H.-H. contributed to the revision. G.S.N. created the figures of the chemical structures. D.P. supervised the writing and editing of the article.
Declaration of interests
D.P. receives licensing fees (to patents on which he was an inventor) from, invested in, consults (or on scientific advisory boards or boards of directors) for, lectured (and received a fee), or conducts sponsored research at TAU for the following entities: ART Biosciences, BioNtech SE, Earli Inc., Kernal Biologics, Geneditor Biologics, Newphase Ltd., NeoVac Ltd., Roche, SirTLabs Corporation, and Teva Pharmaceuticals Inc.
References
- 1.Gallego V., Naveiro R., Roca C., Ríos Insua D., Campillo N.E. AI in drug development: a multidisciplinary perspective. Mol. Divers. 2021;25:1461–1479. doi: 10.1007/s11030-021-10266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yin W., Rogge M. Targeting RNA: A Transformative Therapeutic Strategy. Clin. Transl. Sci. 2019;12:98–112. doi: 10.1111/cts.12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agrawal S. The Evolution of Antisense Oligonucleotide Chemistry—A Personal Journey. Biomedicines. 2021;9 doi: 10.3390/biomedicines9050503. https://www.mdpi.com/2227-9059/9/5/503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hummelshoj L., Ryder L.P., Madsen H.O., Poulsen L.K. Locked nucleic acid inhibits amplification of contaminating DNA in real-time PCR. Biotechniques. 2005;38:605–610. doi: 10.2144/05384RR01. [DOI] [PubMed] [Google Scholar]
- 5.Obika S., Nanbu D., Hari Y., Morio K.i., In Y., Ishida T., Imanishi T. Synthesis of 2′-O,4′-C-methyleneuridine and -cytidine. Novel bicyclic nucleosides having a fixed C3, -endo sugar puckering. Tetrahedron Lett. 1997;38:8735–8738. doi: 10.1016/S0040-4039(97)10322-7. https://www.sciencedirect.com/science/article/pii/S0040403997103227 [DOI] [Google Scholar]
- 6.Bennett C.F. Therapeutic Antisense Oligonucleotides Are Coming of Age. Annu. Rev. Med. 2019;70:307–321. doi: 10.1146/annurev-med-041217-010829. [DOI] [PubMed] [Google Scholar]
- 7.Hagedorn P.H., Persson R., Funder E.D., Albæk N., Diemer S.L., Hansen D.J., Møller M.R., Papargyri N., Christiansen H., Hansen B.R., et al. Locked nucleic acid: modality, diversity, and drug discovery. Drug Discov. Today. 2018;23:101–114. doi: 10.1016/j.drudis.2017.09.018. [DOI] [PubMed] [Google Scholar]
- 8.Quemener A.M., Bachelot L., Forestier A., Donnou-Fournet E., Gilot D., Galibert M.D. The powerful world of antisense oligonucleotides: From bench to bedside. Wiley Interdiscip. Rev. RNA. 2020;11 doi: 10.1002/wrna.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao S., Dagnaes-Hansen F., Nielsen E.J.B., Wengel J., Besenbacher F., Howard K.A., Kjems J. The Effect of Chemical Modification and Nanoparticle Formulation on Stability and Biodistribution of siRNA in Mice. Mol. Ther. 2009;17:1225–1233. doi: 10.1038/mt.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dhuri K., Bechtold C., Quijano E., Pham H., Gupta A., Vikram A., Bahal R. Antisense oligonucleotides: An emerging area in drug discovery and development. J. Clin. Med. 2020;9:2004–2024. doi: 10.3390/JCM9062004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.CHMP. Assessment report - Tegsedi ; International non-proprietary name: inotersen; Procedure No. EMEA/H/C/004782/0000. 2018. www.ema.europa.eu/contact
- 12.CHMP Assessment report - Kynamro Solution for injection 189mg; International non-proprietary name: mipomersen; Procedure No. EMEA/H/C/002429/0000. 2013. www.ema.europa.eu
- 13.DeVos S.L., Miller T.M. Antisense Oligonucleotides: Treating Neurodegeneration at the Level of RNA. Neurotherapeutics. 2013;10:486–497. doi: 10.1007/s13311-013-0194-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petersen M., Nielsen C.B., Nielsen K.E., Jensen G.A., Bondensgaard K., Singh S.K., Rajwanshi V.K., Koshkin A.A., Dahl B.M., Wengel J., Jacobsen J.P. The conformations of locked nucleic acids (LNA) J. Mol. Recognit. 2000;13:44–53. doi: 10.1002/(SICI)1099-1352(200001/02)13:1<44::AID-JMR486>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 15.Koshkin A.A., Nielsen P., Meldgaard M., Rajwanshi V.K., Singh S.K., Wengel J. LNA (Locked Nucleic Acid): An RNA Mimic Forming Exceedingly Stable LNA:LNA Duplexes. J. Am. Chem. Soc. 1998;120:13252–13253. doi: 10.1021/ja9822862. [DOI] [Google Scholar]
- 16.Ito K.R., Obika S. In: Comprehensive Medicinal Chemistry III. Chackalamannil S., Rotella D., Ward S.E., editors. Elsevier; 2017. Recent Advances in Medicinal Chemistry of Antisense Oligonucleotides; pp. 216–232. [DOI] [Google Scholar]
- 17.Springer AdisInsight. 2023. https://adisinsight.springer.com/
- 18.Knox C., Wilson M., Klinger C.M., Franklin M., Oler E., Wilson A., Pon A., Cox J., Chin N.E.L., Strawbridge S.A., et al. DrugBank 6.0: the DrugBank Knowledgebase for 2024. Nucleic Acids Res. 2024;52:D1265–D1275. doi: 10.1093/nar/gkad976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stein C.A., Castanotto D. FDA-Approved Oligonucleotide Therapies in 2017. Mol. Ther. 2017;25:1069–1075. doi: 10.1016/j.ymthe.2017.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matthews H., Hanison J., Nirmalan N. “Omics”-informed drug and biomarker discovery: Opportunities, challenges and future perspectives. Proteomes. 2016;4 doi: 10.3390/proteomes4030028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoy S.M. Nusinersen: A Review in 5q Spinal Muscular Atrophy. CNS Drugs. 2021;35:1317–1328. doi: 10.1007/s40263-021-00878-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Furdon P.J., Dominski Z., Kole R. RNase H cleavage of RNA hybridized to oligonucleotides containing methylphosphonate, phosphorothioate and phosphodiester bonds. Nucleic Acids Res. 1989;17:9193–9204. doi: 10.1093/nar/17.22.9193. http://europepmc.org/abstract/MED/2555787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lundin K.E., Højland T., Hansen B.R., Persson R., Bramsen J.B., Kjems J., Koch T., Wengel J., Smith C.I.E. Biological Activity and Biotechnological Aspects of Locked Nucleic Acids. Adv. Genet. 2013;82:47–107. doi: 10.1016/B978-0-12-407676-1.00002-0. [DOI] [PubMed] [Google Scholar]
- 24.Wahl M.C., Will C.L., Lührmann R. The Spliceosome: Design Principles of a Dynamic RNP Machine. Cell. 2009;136:701–718. doi: 10.1016/j.cell.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 25.Roberts J., Palma E., Sazani P., Ørum H., Cho M., Kole R. Efficient and Persistent Splice Switching by Systemically Delivered LNA Oligonucleotides in Mice. Mol. Ther. 2006;14:471–475. doi: 10.1016/j.ymthe.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 26.Tassone P., Di Martino M.T., Arbitrio M., Fiorillo L., Staropoli N., Ciliberto D., Cordua A., Scionti F., Bertucci B., Salvino A., et al. Safety and activity of the first-in-class locked nucleic acid (LNA) miR-221 selective inhibitor in refractory advanced cancer patients: a first-in-human, phase 1, open-label, dose-escalation study. J. Hematol. Oncol. 2023;16 doi: 10.1186/s13045-023-01468-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Javanmard S.H., Vaseghi G., Ghasemi A., Rafiee L., Ferns G.A., Esfahani H.N., Nedaeinia R. Therapeutic inhibition of microRNA-21 (miR-21) using locked-nucleic acid (LNA)-anti-miR and its effects on the biological behaviors of melanoma cancer cells in preclinical studies. Cancer Cell Int. 2020;20 doi: 10.1186/s12935-020-01394-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bockstahler M., Salbach C., Müller A.M., Kübler A., Müller O.J., Katus H.A., Frey N., Kaya Z. LNA oligonucleotide mediates an anti-inflammatory effect in autoimmune myocarditis via targeting lactate dehydrogenase B. Immunology. 2022;165:158–170. doi: 10.1111/imm.13421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frazier K.S. Antisense Oligonucleotide Therapies:The Promise and the Challenges from a Toxicologic Pathologist’s Perspective. Toxicol. Pathol. 2015;43:78–89. doi: 10.1177/0192623314551840. [DOI] [PubMed] [Google Scholar]
- 30.Swayze E.E., Siwkowski A.M., Wancewicz E.V., Migawa M.T., Wyrzykiewicz T.K., Hung G., Monia B.P., Bennett C.F. Antisense oligonucleotides containing locked nucleic acid improve potency but cause significant hepatotoxicity in animals. Nucleic Acids Res. 2007;35:687–700. doi: 10.1093/nar/gkl1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Godfrey C., Desviat L.R., Smedsrød B., Piétri-Rouxel F., Denti M.A., Disterer P., Lorain S., Nogales-Gadea G., Sardone V., Anwar R., et al. Delivery is key: lessons learnt from developing splice-switching antisense therapies. EMBO Mol. Med. 2017;9:545–557. doi: 10.15252/emmm.201607199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scharner J., Aznarez I. Clinical Applications of Single-Stranded Oligonucleotides: Current Landscape of Approved and In-Development Therapeutics. Mol. Ther. 2021;29:540–554. doi: 10.1016/j.ymthe.2020.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baker Y.R., Thorpe C., Chen J., Poller L.M., Cox L., Kumar P., Lim W.F., Lie L., McClorey G., Epple S., et al. An LNA-amide modification that enhances the cell uptake and activity of phosphorothioate exon-skipping oligonucleotides. Nat. Commun. 2022;13 doi: 10.1038/s41467-022-31636-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akhtar S., Hughes M.D., Khan A., Bibby M., Hussain M., Nawaz Q., Double J., Sayyed P. The delivery of antisense therapeutics. Adv. Drug Deliv. Rev. 2000;44:3–21. doi: 10.1016/S0169-409X(00)00080-6. https://www.sciencedirect.com/science/article/pii/S0169409X00000806 [DOI] [PubMed] [Google Scholar]
- 35.Borel T., Sabliov C.M. Nanodelivery of Bioactive Components for Food Applications: Types of Delivery Systems, Properties, and Their Effect on ADME Profiles and Toxicity of Nanoparticles. Annu. Rev. Food Sci. Technol. 2014;5:197–213. doi: 10.1146/annurev-food-030713-092354. [DOI] [PubMed] [Google Scholar]
- 36.Fan W., Peng H., Yu Z., Wang L., He H., Ma Y., Qi J., Lu Y., Wu W. The long-circulating effect of pegylated nanoparticles revisited via simultaneous monitoring of both the drug payloads and nanocarriers. Acta Pharm. Sin. B. 2022;12:2479–2493. doi: 10.1016/j.apsb.2021.11.016. https://www.sciencedirect.com/science/article/pii/S2211383521004548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bio-Path Holdings. PREXIGEBERSEN (LIPOSOMAL GRB2 ANTISENSE) FOR ACUTE MYELOID LEUKEMIA (AML). https://www.biopathholdings.com/pipeline/prexigebersen/.
- 38.Yang L., Ma F., Liu F., Chen J., Zhao X., Xu Q. Efficient Delivery of Antisense Oligonucleotides Using Bioreducible Lipid Nanoparticles In Vitro and In Vivo. Mol. Ther. Nucleic Acids. 2020;19:1357–1367. doi: 10.1016/j.omtn.2020.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramishetti S., Hazan-Halevy I., Palakuri R., Chatterjee S., Naidu Gonna S., Dammes N., Freilich I., Kolik Shmuel L., Danino D., Peer D. A Combinatorial Library of Lipid Nanoparticles for RNA Delivery to Leukocytes. Adv. Mater. 2020;32 doi: 10.1002/adma.201906128. [DOI] [PubMed] [Google Scholar]
- 40.Naidu G.S., Yong S.-B., Ramishetti S., Rampado R., Sharma P., Ezra A., Goldsmith M., Hazan-Halevy I., Chatterjee S., Aitha A., Peer D. A Combinatorial Library of Lipid Nanoparticles for Cell Type-Specific mRNA Delivery. Adv. Sci. 2023;10 doi: 10.1002/advs.202301929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han X., Zhang H., Butowska K., Swingle K.L., Alameh M.-G., Weissman D., Mitchell M.J. An ionizable lipid toolbox for RNA delivery. Nat. Commun. 2021;12:7233. doi: 10.1038/s41467-021-27493-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uebbing L., Ziller A., Siewert C., Schroer M.A., Blanchet C.E., Svergun D.I., Ramishetti S., Peer D., Sahin U., Haas H., Langguth P. Investigation of pH-Responsiveness inside Lipid Nanoparticles for Parenteral mRNA Application Using Small-Angle X-ray Scattering. Langmuir. 2020;36:13331–13341. doi: 10.1021/acs.langmuir.0c02446. [DOI] [PubMed] [Google Scholar]
- 43.Kedmi R., Veiga N., Ramishetti S., Goldsmith M., Rosenblum D., Dammes N., Hazan-Halevy I., Nahary L., Leviatan-Ben-Arye S., Harlev M., et al. A modular platform for targeted RNAi therapeutics. Nat. Nanotechnol. 2018;13:214–219. doi: 10.1038/s41565-017-0043-5. [DOI] [PubMed] [Google Scholar]
- 44.Sahay G., Querbes W., Alabi C., Eltoukhy A., Sarkar S., Zurenko C., Karagiannis E., Love K., Chen D., Zoncu R., et al. Efficiency of siRNA delivery by lipid nanoparticles is limited by endocytic recycling. Nat. Biotechnol. 2013;31:653–658. doi: 10.1038/nbt.2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Evers M.J.W., Kulkarni J.A., van der Meel R., Cullis P.R., Vader P., Schiffelers R.M. State-of-the-Art Design and Rapid-Mixing Production Techniques of Lipid Nanoparticles for Nucleic Acid Delivery. Small Methods. 2018;2 doi: 10.1002/smtd.201700375. [DOI] [Google Scholar]
- 46.Ramezanpour M., Tieleman D.P. Computational Insights into the Role of Cholesterol in Inverted Hexagonal Phase Stabilization and Endosomal Drug Release. Langmuir. 2022;38:7462–7471. doi: 10.1021/acs.langmuir.2c00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oh N., Park J.H. Endocytosis and exocytosis of nanoparticles in mammalian cells. Int. J. Nanomedicine. 2014;9:51–63. doi: 10.2147/IJN.S26592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Delehedde C., Even L., Midoux P., Pichon C., Perche F. Intracellular routing and recognition of lipid-based mRNA nanoparticles. Pharmaceutics. 2021;13 doi: 10.3390/pharmaceutics13070945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eckstein F. The versatility of oligonucleotides as potential therapeutics. Expert Opin. Biol. Ther. 2007;7:1021–1034. doi: 10.1517/14712598.7.7.1021. [DOI] [PubMed] [Google Scholar]