Abstract
A nonnucleoside reverse transcriptase (RT) inhibitor, GW420867, was tested for postexposure prophylaxis (PEP) in rhesus macaques experimentally infected with 100 50% tissue culture infective doses of a chimeric simian/human immunodeficiency virus (SHIV) containing the RT gene of HIV-1 (SHIV-RT). Animals were either mock treated, or treated for 4 weeks starting at 8 or 24 h postinfection (p.i.) with GW420867. While such therapy led to undetectable plasma viremia in three of six monkeys, a transient plasma viremia was noted in the other three treated animals at 2 to 4 weeks following cessation of therapy. Following this transient viremia all drug-treated animals showed low or undetectable levels of plasma viremia up to the last sample examined at 90 weeks p.i. Despite low and/or undetectable viremia, virus-specific cytotoxic T lymphocyte and viral Env-specific proliferative responses were seen in the peripheral blood mononuclear cells of both mock- and drug-treated animals as early as 3 weeks p.i. Such virus-specific cellular responses, however, were better maintained in the drug-treated animals than the mock-treated animals. In contrast to the virus-specific cellular response, the magnitude and kinetics of virus specific humoral responses appeared to correlate with the detection of viremia. These data support the view that a short-term PEP with GW420867 permits the generation and maintenance of long-lasting virus-specific cell-mediated immune responses while markedly reducing viral loads to undetectable levels for a prolonged period of time (90 weeks) and leads to long-term disease protection. This model provides a unique means to define mechanisms and correlates of disease protection.
A dramatic decrease in viral load coincident with a recovery of CD4+ T-cell counts has been noted in human immunodeficiency virus (HIV)-infected patients following highly active antiretroviral therapy (HAART) (2, 10, 11). However, it has been noted that while such therapy does lead to recovery of CD4+ T-cell levels, it fails to establish effective anti-HIV immune responses, as evidenced by a rebound of viremia which has been noted among HIV-infected individuals in whom HAART was discontinued (31). Of great concern is the finding that latent HIV infection may persist for more than several decades even in successful HAART cases (7, 27, 30). These findings indicate a need for defining therapeutic modalities that will not only reduce viral load but concomitantly allow for the induction of a quality and quantity of immune responses that will continue to maintain low viral loads and gradually either eliminate latent virus-infected cells and/or reduce and/or eliminate the likelihood of disease. However, the precise nature of the host immune response(s) that is capable of containing viral load and/or preventing disease remains to be defined. Some insights have been gained on this issue by the study of the host antivirus-specific immune responses in HIV type 1 (HIV-1)-infected long-term nonprogressors and in nonhuman primates infected with attenuated simian immunodeficiency virus (SIV) viral constructs (13, 15). However, to date, there have been limited if any studies of the use of chemotherapeutic drugs that reduce viral loads yet allow for the development of the quality and quantity of antiviral immune responses outlined above.
Our laboratory has been studying a nonnucleosidic class of the quinoxalines as inhibitors of HIV-1 reverse transcriptase (RT), using HBY 097 as the first clinical candidate (16, 19, 20). A search for a compound with improved pharmacokinetics led to the discovery of HBY 1293A (now GW420867), which allows a single daily dosing to reach and maintain therapeutic levels in humans (data not shown). Toward the goal of determining the efficacy of this drug, we utilized rhesus macaques infected intravenously with a relatively high dose (100 50% tissue culture infective doses [TCID50]) of a pathogenic chimeric simian/human immunodeficiency virus (SHIV) containing the RT gene of HIV-1 (SHIV-RT) (23, 25). Groups of animals were either mock treated (control) or treated for 4 weeks with GW420867 starting at 8 or 24 h postinfection (p.i.). Whereas the mock-treated animals showed typical acute and then persistent productive infection, besides a transient viremia in three of six animals at 2 to 4 weeks postcessation of therapy, all six drug-treated animals showed suppression of viral replication to undetectable levels until the last sample analyzed at 90 weeks p.i. Of interest was the finding that despite such marked reduction in viral loads, the drug-treated animals showed readily detectable antiviral cellular immune responses as early as 3 weeks p.i., which were maintained for a prolonged period, in contrast to fading virus-specific cellular response in the mock-treated animals. Such a model for the first time allows for the use of a chemotherapeutic agent to potentially identify disease-protective immune responses. Results of the studies performed constitute the basis of this report.
MATERIALS AND METHODS
Virus.
The chimeric SHIV-RT consists of a SIVmac239 virus backbone in which the SIV RT gene was replaced by the HIV-1 HxB2 RT gene as previously described (25). This SHIV-RT has been shown to induce AIDS in experimentally infected rhesus monkeys (23, 25). The parent viral stocks were prepared by DNA transfection of proviral DNA into COS-1 cells. The virus stock used in the studies was prepared by propagation of the COS-1 virus stock in phytohemagglutinin-activated rhesus monkey peripheral blood mononuclear cells (PBMC) (14). The p27 antigen concentration of the virus stock was determined by using a commercial SIV Gag antigen enzyme-linked immunosorbent assay (ELISA) kit (Coulter, Tokyo, Japan). The TCID50 of the virus stock was determined utilizing herpesvirus saimiri-transformed cynomolgus CD4+ T cells (1).
GW420867 (formerly HBY 1293A).
A quinoxaline class of the nonnucleoside RT inhibitors [S-3-ethyl-6-fluoro-4-isoproxycarbonyl-3,4-dihydro-quinoxaline-2(1H)-one] was provided initially by Hoechst and Bayer AG (Wuppertal, Germany) and subsequently by Glaxo Wellcome (Greenford, Middlesex, United Kingdom). The compound was dissolved with dimethyl sulfoxide at 1 g per ml, and then aliquots were stored at −20°C. Prior to administration into animals, the compound was diluted with a solvent consisting of polyethylene glycol 400, glycerol, and water (960:60:100).
Animal experiment study plan.
Nine rhesus macaques (male, 2 years old, 2 to 3 kg) were screened and found to be seronegative for SIV, simian T-cell leukemia virus, B virus, and type D retroviruses prior to initiation of the study. All animals were housed in individual cages and maintained according to the rules and regulations of the National Institute of Infectious Diseases and guidelines for experimental animal welfare. GW420867 was administered subcutaneously to the animals at a dose of 15 mg per kg of body weight, twice a day (12-h interval) for a total of 28 days. All animals were infected intravenously with 100 TCID50 of SHIV-RT. The first group was treated with GW420867 starting at 8 h p.i. and then received treatment twice daily at 12-h intervals for 28 days. The second group was treated with GW420867 starting at 24 h p.i. and then received treatment twice daily for 28 days. The third group was administered an equivalent dose of the solvent that was used to dissolve GW420867 starting at 8 h p.i. and then received treatment twice daily for 28 days and served as a control group.
Pharmacokinetics of GW420697.
Initial in vitro and in vivo studies were performed in an effort to define the optimal dosage to be administered to the monkeys. The 50% inhibitory concentration of this drug for SHIV-RT is comparable to the value previously defined for several subtype B field isolates of HIV, which ranged from 0.15 to 1.4 nM utilizing human PBMC cultures (data not shown). The 90% inhibitory concentration of the drug determined using a macaque CD4+ T-cell line (1) was found to be 8 nM (2.24 ng per ml). We estimated that the effective minimal in vivo concentration in blood to achieve for GW420867 would be 50 ng per ml. During our experimental study, in which the monkeys were subcutaneously administered the compound at 15 mg per kg of body weight twice daily for 4 weeks, the drug level maintained in the blood was significantly higher than the calculated minimal effective concentration (data not shown).
Sampling of specimen.
Blood samples were collected from each of the nine animals prior to the initiation of the study (baseline) and then at regular time intervals as specified for each of the assays described herein. Biopsies of inguinal lymph nodes from one animal in the control group and from one animal in each of the two drug treatment groups were performed at 8 weeks p.i.
Plasma viral load.
Viral RNA was purified from 0.2 ml of plasma with a commercial viral RNA isolation kit (Boehringer Mannheim, Tokyo, Japan) and dissolved with 50 μl of the elution buffer included in the kit. Aliquots of the viral RNA were stored at −80°C. Plasma viral RNA load was measured initially by a commercially available RT-PCR kit (Boehringer Mannheim) and with the use of a laboratory-generated competitor RNA which served as an external standard. The values were then confirmed by using the quantitative competitive RT-PCR method previously described (26). The sequence of the gag primers utilized included SG05i (5′-ACTGCTGATTCAAAATGCiAACC-3′) and SG06i (5′-CTACTGGTCTiCTCCAAAGAGAGAATTG-3′), which were designed to amplify most SIVmac and SIVsmm gag sequences. The competitor RNA fragment was generated by amplification of SIVmac239 gag sequences with SG05i and SG06i and cloning of the 559-bp fragment into a pGEM-T vector (Promega, Madison, Wis.). The insert was then digested with StuI and XcmI, blunted, and religated in order to remove a 133-bp fragment. The plasmid was then linearized downstream of the insert using SalI, and a competitor RNA was generated by using T7 RNA polymerase. The competitor RNA was purified by RNase-free DNase I digestion, extraction with phenol-chloroform-isoamyl alcohol (24:24:1), and fractionation with an RNase-free G50 column. The copy number of the purified competitor RNA was determined by optical density at 260 nm (OD260), and aliquots were stored at −80°C until use. Serially diluted (1:5) viral RNA mixed with the competitor RNA (usually 100 or 20 copies) was amplified using a single-step RNA PCR kit with the specific primers and an RNase inhibitor (Takara, Tokyo, Japan). The amplified viral gag RNA and competitor RNA were separated on a 2% agarose gel by electrophoresis and stained with SYBR green (Takara). Measurement of amplified RNA was achieved utilizing a fluorescence imaging analyzer (FLA2000; Fuji Film, Tokyo, Japan), and the viral RNA load was calculated using an Excel spreadsheet (Microsoft, Tokyo, Japan) as previously described (26). The sensitivity of plasma viral RNA detection by this technique was determined to be 1,000 copies per ml of plasma.
Proviral DNA load.
PBMC samples were purified from heparinized blood utilizing standard Ficoll-Hypaque gradient centrifugation. Cellular DNA was purified from 106 cells with a commercial DNA purification kit (Qiagen, Tokyo, Japan). The gag DNA was amplified with a commercial DNA PCR kit with Ex Taq DNA polymerase (Takara). Viral DNA standards were prepared by dilution of the plasmid containing the 5′ half of SIVmac239 (17). The nef sequence of viral DNA was amplified to determine the proviral DNA load in samples which had fewer than 10 copies by nested PCRs using two sets of primer pairs as previously described (5). The first primer pair included F34C (nucleotides [nt] 9065 to 9082) (5′-CCTACCTACAATATGGGT-3′) and F35C (nt 9800 to 9778) (5′-CCTCTGACAGGCCTGACTTGCTT-3′), and the second primer pair included N3 (nt 9182 to 9201) (5′-GAAGATGGATACTCGCAATC-3′) and N4 (nt 9552 to 9533) (5′-TAATCCTGCCAATCTGGTAT-3′). The sensitivity of this technique was determined to be one copy per 100,000 cells. The relative numbers of PBMC and lymph node mononuclear cells (LNMC) harboring proviral DNA was determined by the analysis of DNA from fourfold dilutions of the initial 105 cells run in parallel with the viral DNA standards described above. DNAs from the samples were separated by agarose gel electrophoresis and stained with SYBR green, and quantification was achieved using a fluorescence imaging analyzer (FLA2000, Fuji Film).
Quantitative virus isolation (QVI).
Fourfold dilutions of PBMC (starting with 106 in 0.5 ml) were cocultured with 2.5 × 105 C8166 cells (in 0.5 ml) in duplicate wells of 24-well plates. The cocultures were incubated for 3 to 4 weeks, and individual wells were scored for the presence of syncytia. Culture supernatants were subjected to RNA PCR for the detection of viral RNA to confirm viral replication as previously described (23).
Anti-SIV ELISA.
A 1:100 dilution of each plasma sample in phosphate-buffered saline (pH 7.4) containing a blocking reagent (Dainippon Seiyaku, Osaka, Japan) was assayed for the presence of SIV-specific antibodies using standard ELISA techniques. The 96-well microtiter plates were precoated with a SIVmac239 virion lysate as previously described (14). The OD492 was recorded and utilized as a relative measure of antibody titer.
CTL assay.
The cytotoxic T-lymphocyte (CTL) assay method used has been previously described (30). In brief, PBMC samples stored at −150°C were thawed and cultured in RPMI 1640 medium with concanavalin A (5 μg per ml) at 106 PBMC per ml for 3 days, washed and then maintained for another 3 days in medium supplemented with human interleukin 2 (2 U per ml). These effector cells were then cocultured for 7 days with autologous herpesvirus papio-transformed B-lymphoblastoid cell lines (B-LCL), which were previously either infected with recombinant vaccinia virus (rVV) expressing the SIVmac239 gag-pol, SIVmac239 env, or the parental VV (NYCBH strain) for 16 h at 37°C. The VV constructs were obtained via the courtesy of D. Panicali (Therion Corp., Cambridge, Mass.). For the CTL assay, similarly infected autologous (B-LCL) target cells were labeled with 51Cr and then incubated at 104 cells/well with various concentrations of effector cells for 5 h. Supernatant fluids from each of the target cells (104 cells per well) incubated alone with medium was used to calculate spontaneous release, and supernatant fluid from each of the target cells (104 cells per well) incubated with Triton X-100 was used to calculate 100% release. Each effector-to-target cell combination was performed in triplicate. Specific net lysis was calculated as the percentage of SIV Env- or Gag-Pol-specific lysis minus the percentage of lysis obtained using the control VV (NYCBH)-infected target cells. The control lysis was always <10%.
SIV Env-specific cell proliferation.
The method utilized has been previously described (13). In brief, PBMC samples were thawed and triplicate cultures of PBMC (4 × 105 per well) were incubated with irradiated autologous B-LCL (1 × 105 per well) that had been previously infected (for 16 h) with either VV containing SIVmac239 env or control VV. Cultures were performed in triplicate, and [3H]thymidine was added to each well 8 h prior to harvest on day 5. The incorporation of [3H]thymidine was measured by standard liquid scintillation counting. The standard deviation of the triplicate samples was <10%. The stimulation index was calculated by dividing the mean uptake of [3H]thymidine values of the PBMC cocultured with the VV SIV env-infected autologous cells by the mean uptake of [3H]thymidine values obtained by coculture of an aliquot of the same PBMC with the VV (NYCBH)-infected autologous cells. Because depletion of CD4+ cells from PBMC at 8 weeks p.i. with monoclonal antibody 19thy5D7 (17) in the presence of complement resulted in the loss of the env-specific proliferative response in the positive PBMC specimen, the majority of the cells stimulated with Env proteins were assumed to be CD4+ T cells.
RESULTS
Containment of SHIV infection by short-term post-exposure prophylaxis with GW420867.
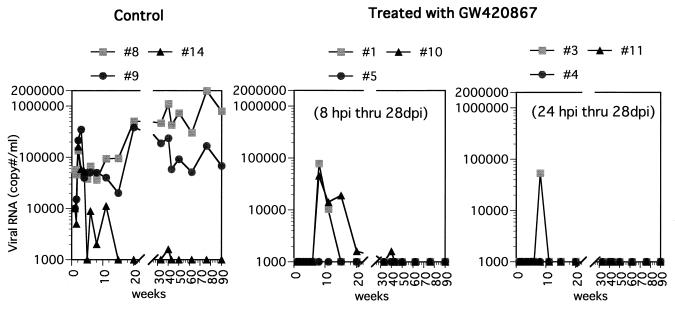
We used GW420867 to treat monkeys that were infected with a high dose (100 TCID50) of SHIV-RT. Treatment was initiated at 8 or 24 h p.i., with the drug or vehicle (in control animals) being administered twice a day for a total of 4 weeks. The effect of GW420867 therapy on plasma and cellular viral load was examined in samples obtained from each of the nine animals at various times p.i. As seen in Fig. 1, all three mock-treated animals showed significant plasma viremia, which reached set points in two of three animals shortly following the acute viremia phase. The third mock-treated animal showed transient plasma viremia following the acute phase but thereafter showed low levels of plasma viremia. In contrast, all six drug-treated animals showed undetectable levels (below the limits of RT-PCR detection, which was determined to be 1,000 copies/ml) of plasma viremia up to 3 weeks postcessation of therapy, regardless of the difference in the time of drug therapy initiation. Thereafter, three of six animals showed transient plasma viremia at about 4 weeks following cessation of drug therapy, which subsequently returned to undetectable levels. Studies of the cellular viral loads as determined by QVI showed a significant number of virus-infected cells in the mock-treated animals at various time points following infection. The frequency of virus replication-competent infected cells decreased with time in the control animals, presumably due to trapping of such cells in regional lymph nodes. In comparison, low but detectable levels of infected cells were noted in three of six drug-treated animals between 6 and 11 weeks p.i., a low level was detected at a single time point in two of six drug-treated animals, and undetectable levels were noted in all the other remaining samples tested (Table 1).
FIG. 1.
Plasma viral RNA in SHIV-RT-infected animals treated with GW420867 or left untreated. Viral RNA levels in the plasma are expressed as copy number per milliliter.
TABLE 1.
QVI
| Wk p.i. | Cellular viral load determined by quantitative virus isolation for animal no.a:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 8b | 9b | 14b | 1c | 5c | 10c | 3d | 4d | 11d | |
| 2 | 16 (+) | 16 (+) | 16 (+) | 0 (−) | 0 (−) | 0 (−) | 0 (−) | 0 (−) | 0 (−) |
| 6 | 16 (+) | 16 (+) | 4 (+) | 4 (+) | 0 (−) | 1 (+) | 16 (+) | 0 (−) | 0 (−) |
| 8 | 64 (+) | 16 (+) | 1 (+) | 16 (+) | 0 (−) | 16 (+) | 16 (+) | 1 (+) | 0 (−) |
| 11 | 16 (+) | 1 (+) | 0 (−) | 4 (+) | 0 (−) | 4 (+) | 16 (+) | 0 (−) | 4 (+) |
| 15 | 4 (+) | 1 (+) | 0 (−) | 0 (−) | 0 (−) | 0 (−) | 0 (−) | 0 (−) | 0 (−) |
Subsequent analyses of samples obtained at 50, 62, 76, and 90 weeks p.i. indicated that plasma viral RNA levels were maintained below 1,000 copies/ml in each of the six drug-treated animals. Results of the proviral DNA analysis (Table 2) correlated with the plasma viral loads. No proviral DNA was detected in PBMC samples at any time during antiviral treatment, while it was readily detectable in samples from the control group early p.i. Also at later time points, a far lower level of infection was noted in the drug-treated group relative to the control group. These data nevertheless demonstrate that despite drug therapy, all the animals in both groups became infected, as evidenced by the detection of SHIV-RT DNA sequences at least at one time point in the PBMC and/or LNMC of each of these animals.
TABLE 2.
SHIV-RT proviral DNA in PBMC and LNMCa
| Sample | Wk p.i. | No. of DNA copies/105 cells for animal no.:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 8b | 9b | 14b | 1c | 5c | 10c | 3d | 4d | 11d | ||
| PBMC | 2 | 300 | 180 | 850 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4 | 340 | 1,600 | 240 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 15 | 150 | 74 | 420 | 1 | 0 | 7 | 16 | 0 | 1 | |
| 40 | 630 | 270 | 25 | 0 | 0 | 1 | 0 | 0 | 0 | |
| 50 | 240 | 340 | 31 | 0 | 0 | 5 | 2 | 0 | 0 | |
| LNMC | 8 | NDe | 1,800 | 310 | ND | 2 | ND | ND | 1 | ND |
PBMC samples from the carrier (control) or GW420867-treated animals obtained at 2, 4, 15, 40, and 50 weeks p.i. and LNMC obtained at 8 weeks p.i. were assayed for SHIV-RT proviral DNA as described in Materials and Methods. The limit of detection was 1 copy/105 cells.
Control.
GW420867 treatment 8 h p.i. through 28 days p.i.
GW420867 treatment 24 h p.i. through 28 days p.i.
ND, biopsy was not done.
Humoral response in SHIV-infected monkeys treated with GW420867.
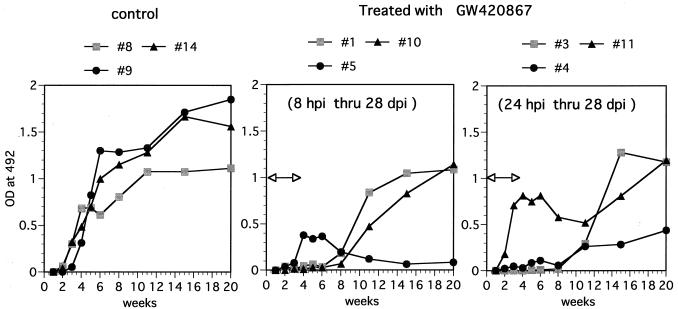
We studied the humoral response against SHIV-RT by using ELISA against SIV virion proteins. Infected but mock-treated (control) animals became seropositive at 4 weeks p.i.; thereafter, high antibody titers were sustained (Fig. 2). In contrast, a varied pattern of anti-SIV humoral response was noted in the drug-treated animals. One drug-treated animal (animal 5) showed a weak but positive transient antibody response (6 to 8 weeks p.i.) which was lost by 10 weeks p.i. despite undetectable viral loads in this animal (Fig. 1 and Table 1). A lymph node biopsy done on this animal showed that this animal was indeed infected but had low levels of proviral DNA (Table 2). Another drug-treated animal (animal 11) showed an initial antiviral antibody response similar to those seen in the control animals but then showed a transient decrease, with a return to high levels by 18 to 20 weeks p.i. This presumably anamnestic response followed the detection of infectious virus in PBMC samples from this animal at 11 weeks p.i. (Table 1). The relatively early seroconversion of drug-treated animals 5 and 11 suggests that the limited viral replication that occurred in these two animals may have led to sufficient availability of antigen to induce antibody responses. Yet another drug-treated animal (animal 4) seroconverted by 11 weeks p.i. but maintained low but significant SIV-specific antibody titers relative to the other remaining three animals. The other three drug-treated animals showed a delay in the kinetics of the humoral antiviral response, with seroconversion at 11 weeks p.i., corresponding to 7 weeks postcessation of drug therapy. In general, therefore, the kinetics of antiviral antibody generation appeared to follow the levels of viremia seen in the respective animals. The antibody titers in the drug-treated animals gradually reached levels approximately similar to those seen in the mock-treated control animals. These data also suggest that there is no correlation between the antiviral humoral responses and protection in these animals. However, such data do not rule out differences in the quality of virus-specific humoral responses that may be the contributing factor in decreased viremia and disease protection.
FIG. 2.
Humoral response against SHIV-RT. Shown are results of antibody ELISA analysis of plasma samples from the control animals and GW420867-treated animals. A 1:100 dilution of each plasma sample in phosphate-buffered saline (pH 7.4) containing a blocking reagent was assayed for SIV-specific antibody by using standard ELISA techniques with 96-well plates precoated with SIVmac239 virion lysate (14). The OD492 was recorded and used as a relative measure of antibody titer. The duration of drug treatment is indicated by the bar with arrowheads.
Cell-mediated immune response in SHIV-infected monkeys treated with GW420867.
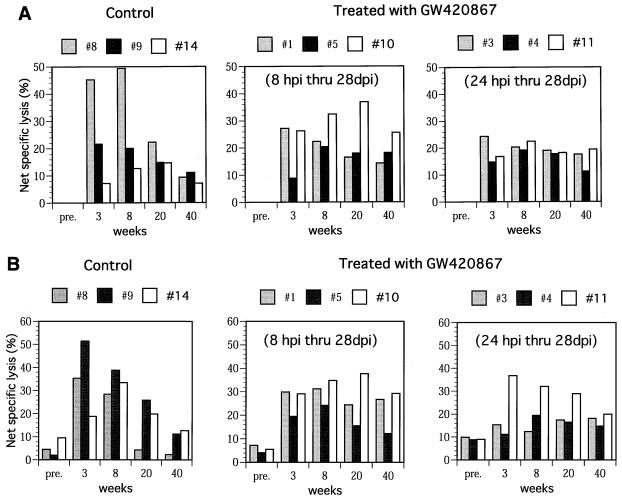
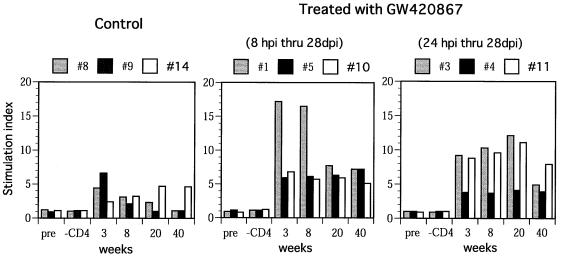
The virus-specific cell-mediated immune responses were also studied in each of the nine animals. Samples assayed included PBMC obtained from the animals at 6 days before infection (baseline) and at 3, 8, 20, and 40 weeks p.i. Significant SIV Gag-Pol (Fig. 3A)- and SIV Env (Fig. 3B)-specific CTL activity was noted in samples obtained at 3 and 8 weeks p.i. from two of three control animals. These levels, however, decreased at 20 and 40 weeks p.i. Interestingly, in one of three control animals (animal 14), there was a delay in the kinetics of CTL activity despite similar levels of acute viremia in this animal (Fig. 1). In contrast to the control animals, all of the drug-treated animals showed significant SIV Gag-Pol- and Env-specific CTL activity in samples from as early as 3 weeks p.i., despite the absence of detectable viral replication during the drug treatment period. More importantly, again in contrast to the control animals, the CTL levels were maintained up to 40 weeks p.i. with minor variations (Fig. 3). It is important to note that viral load by itself does not account for such virus-specific CTL responses, but viral control may be due to other host responses in addition to competent helper T cells. These findings are reminiscent of the data that have documented the maintenance of HIV antigen-specific CTL responses seen in the PBMC of long-term nonprogressors in contrast to those individuals who progress to AIDS (13, 18). In addition to the virus-specific CTL response, the SIV env-specific proliferative responses in these animals were also studied. Results showed that the PBMC from the control animals demonstrated a transient but weak proliferative response at 3 weeks p.i. This response remained low throughout the period of assay in two of the control animals (animals 8 and 9) (Fig. 4) and increased in the remaining animal (animal 14) that showed naturally developed good long-term control of viremia (Fig. 1). In marked contrast, PBMC samples from the drug-treated animals showed significantly higher SIV env-specific proliferative responses as early as 3 weeks p.i., and these responses were maintained throughout the period of assay. In select experiments, depletion of CD4+ T cells prior to the proliferation assay led to marked diminution of the proliferative response, indicating indeed that the proliferative response involved CD4+ T cells in these assays.
FIG. 3.
Virus-specific cellular immunity CTL activity in the PBMC samples of control and GW420867-treated animals was determined by using autologous B-LCL target cells previously infected with a VV construct containing SIVmac gag-pol (A) and a VV construct containing SIVmac env (B). In addition, controls used B-LCL infected with VV wild type (NYCBH strain). The percentage of net specific lysis was calculated for each effector-to-target cell combination, and this value was subtracted from the value obtained with the wild-type VV target cell control. Only results of effector/target ratio of 80:1 are shown. pre., preinfection.
FIG. 4.
In vitro proliferative response of PBMC against SIVmac env-rVV-infected autologous B-LCL. PBMC used for this assay were aliquots of the same specimen used for the CTL assay. The stimulation index was calculated as the mean counts per minute of PBMC cultured with env-rVV divided by the mean counts per minute of PBMC cultured with control VV. pre., preinfection.
DISCUSSION
One of the goals of this study was to investigate whether treatment of monkeys as early as 8 to 24 h p.i. would be able to prevent the establishment of SHIV infection in vivo, as shown in previous studies using (R)-9-(2-phosphonylmethoxypropyl) adenine (PMPA) (24) and zidovudine, respectively (25). Clearly, GW420867 postexposure prophylaxis monotherapy was unable to prevent infection in our protocol, possibly due to the different experimental conditions. Although prevention of infection was not achieved, our results provide four main findings that may prove crucial for the delineation of protective chemotherapeutic interventions in HIV infection and may even provide a rationale for the formulation of an effective vaccine against HIV.
The first finding is that the inhibition of the acute initial viral replication did not appear to restrict the development of virus-specific cell-mediated responses against the virus inoculated, indicating that induction of cell-mediated responses does not require massive antigen production in vivo. Compared to the virus-specific CTL response, the virus-specific humoral response required a higher antigen load, as evidenced by a delay in the kinetics for anti-SIV antibody responses in the drug-treated animals compared to that in the control animals. The second finding is that a delicate balance appeared to be required between very limited viral replication (below detectable levels) and sufficient pathways of antigen presentation to evoke long-lasting disease-protective host antiviral immune functions in the drug-treated animals. This balance appeared to have been preserved by early short-term treatment with this novel RT inhibitor. In addition, very low continuous or intermittent viral replication presumably results in a quality (viral epitope specificity) of virus-specific humoral response that may also play a role in disease protection. Thus, induction of such humoral response during the treatment period correlated with a more-effective restriction of virus replication in the treated animals. The third finding is that antiviral treatment during acute infection appeared to preserve mechanisms responsible for the development and maintenance of an antiviral host defense that included CTL effector function, which was most likely preserved by the continuous support of CD4+ T helper cell function in addition to other as yet undefined mechanisms. Because there was a more significant difference in env-specific proliferative response than in CTL response between the treated groups and the control group, virus-specific CD4+ T cells may play a more important role than CTLs. The fourth finding is that it is possible that there are as yet to be defined additional effector mechanisms other than CTLs that play important roles for the containment of the virus infection. This view is based on the results seen in one of the untreated control animals that suppressed acute viremia despite a relatively low CTL response during primary infection and another control animal that suppressed viremia weakly despite a relatively potent virus-specific CTL response. Chemokines and other cell-free factors have been identified as candidates for such undefined effector mechanisms (4, 8, 22). It is thus possible that virus infection-induced activation of CD4+ and CD8+ T cells may function to secrete high levels of cell-free factors that play a dominant role in these select animals. Thus, orchestrated antiviral host responses consisting of mechanisms that permit low levels of viral replication sufficient to induce and maintain appropriate levels of virus-specific CD4+ T helper responses, which in turn facilitate the generation and maintenance of virus-specific CTL responses, in addition to undefined mechanisms may be the basis for the protective effect observed in the drug-treated animals. Support for this view has been recently documented with the use of a live attenuated SIVΔnef immunization protocol (9). A more detailed characterization of the precise epitopes of the virus that permit the generation of the helper CD4 and cytotoxic T-cell responses coupled with identification of the contributory role, if any, of the other effector immune mechanism(s) in such models, including the one described herein, may provide insights on the nature of the immune responses that constitute effective antiviral immunity and disease protection.
A similar vaccination effect has been noted before in a murine retrovirus model (21). For lentiviruses, SIVΔnef infection of macaques has been described in which low but detectable levels of viral replication were followed by CTL induction during the acute infection period (13). Virus-specific CTL induction in our drug-treated animals appeared similar to that seen in SIVΔnef infected animals, despite apparently even lower levels of virus replication. In addition, although SIVΔnef-induced immunity required 3 to 6 months to be fully protective (28), the data presented herein suggest that vigorous antiviral responses were already present at 4 weeks p.i., when antiviral treatment was discontinued. These differences may suggest that a relationship exists between the strength of the protective immune response and the extent of attenuation of virus as previously reported (6). Alternatively, pathogenic virus may inherently differ from attenuated virus with respect to the influence of virus infection on host immune responses. Thus, the chemotherapy-assisted containment of the pathogenic virus infection may differ quantitatively and qualitatively from the attenuated SIV infection in the context of protection from pathogenic infection. Recent data on a few HIV-1 infected patients for whom HAART was initiated early p.i. support the data reported herein, since discontinuation of HAART in these individuals did not result in viral rebound, suggesting that HAART may be able to induce immune responses capable of containing viral loads (3). In contrast, as previously noted, discontinuation of HAART therapy initiated at later stages of HIV-1 infection did not lead to the induction of immune responses sufficient to contain viral rebound (31).
In the current controversy of early versus late therapy following exposure to HIV, our results strongly suggest that initiation of therapy past the acute viral infection stage may be too late and prevent the optimal development of antiviral response. If human studies come to the same conclusion, early treatment of a (presumed) HIV infection by selected well-tolerated and convenient drugs must become the rule, whenever possible.
ACKNOWLEDGMENTS
We appreciate the generosity of and thank D. L. Panicali (Therion Biologics) for providing the recombinant vaccinia viruses expressing SIVmac239 env, SIVmac251 gag-pol, and the parental virus (NYBCH strain).
This work was supported by AIDS research grants from the Health Sciences Foundation and the Organization for Pharmaceutical Safety and Research in Japan.
REFERENCES
- 1.Akari H, Mori K, Terao K, Otani I, Fukasawa M, Mukai R, Yoshikawa Y. In vitro immortalization of Old World monkey T lymphocytes with Herpesvirus Saimiri. Virology. 1996;218:382–388. doi: 10.1006/viro.1996.0207. [DOI] [PubMed] [Google Scholar]
- 2.Autran B, Carcelain G, Li T S, Blanc C, Mathez D, Tubiana R, Katlama C, Debre P, Leibowitch J. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112–116. doi: 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]
- 3.Balter M. Can immune systems be trained to fight HIV? Science. 1999;286:1470–1471. doi: 10.1126/science.286.5444.1470. [DOI] [PubMed] [Google Scholar]
- 4.Cocchi F, Devico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 5.Daniel M D, Kirchhoff K, Czajak S C, Shegal P K, Desrosiers R C. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science. 1992;258:1938–1941. doi: 10.1126/science.1470917. [DOI] [PubMed] [Google Scholar]
- 6.Desrosiers R C, Lifson J D, Gibbs J S, Czajak S C, Howe A Y, Arthur L O, Johnson R P. Identification of highly attenuated mutants of simian immunodeficiency virus. J Virol. 1998;72:1431–1437. doi: 10.1128/jvi.72.2.1431-1437.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finzi D, Blankson J, Siliciano J D, Margolick J B, Chadwick K, Pierson T, Smith K, Lisziewick J, Lori F, Flexner C, Quinn T C, Chaisson R E, Rosenberg E, Walker B, Gange S, Gallant J, Siliciano R F. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med. 1999;5:512–517. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- 8.Garzino-Demo A, Moss R B, Margolick J B, Cleghorn F, Sill A, Blattner W A, Cocchi F, Carlo D J, Devico A L, Gallo R C. Spontaneous and antigen-induced production of HIV-inhibitory beta-chemokines are associated with AIDS-free status. Proc Natl Acad Sci USA. 1999;96:11986–11991. doi: 10.1073/pnas.96.21.11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gauduin M-C, Glickman R L, Ahmad S, Yilma T, Johnson R P. Immunization with live attenuated simian immunodeficiency virus induces strong type 1 T helper responses and beta-chemokine production. Proc Natl Acad Sci USA. 1999;96:14031–14036. doi: 10.1073/pnas.96.24.14031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gulick R M, Mellors J W, Havlir D, Eron J J, Gonzalez C, McMahon D, Richman D D, Valentine F T, Jonas L, Meibohm A, Emini E A, Chodakewitz J A. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N Engl J Med. 1997;337:734–739. doi: 10.1056/NEJM199709113371102. [DOI] [PubMed] [Google Scholar]
- 11.Hammer S M, Squires K E, Hughes M D, Grimes J M, Demeter L M, Currier J S, Eron J J, Jr, Feinberg J E, Balfour H H, Deyton L R, Chodakewitz J A, Fischl M A. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. N Engl J Med. 1997;337:725–733. doi: 10.1056/NEJM199709113371101. [DOI] [PubMed] [Google Scholar]
- 12.Hiranuma K, Tamaki S, Nishimura Y, Kusuki S, Isogawa M, Kim G, Kaito M, Kuribayashi K, Adachi Y, Yasutomi Y. Helper T cell determinant peptide contributes to induction of cellular immune responses by peptide vaccines against hepatitis C virus. J Gen Virol. 1999;80:187–193. doi: 10.1099/0022-1317-80-1-187. [DOI] [PubMed] [Google Scholar]
- 13.Johnson R P, Glickman R L, Yang J Q, Kaur A, Dion J T, Mulligan M J, Desrosiers R C. Induction of vigorous cytotoxic T-lymphocyte responses by live attenuated simian immunodeficiency virus. J Virol. 1997;71:7711–7718. doi: 10.1128/jvi.71.10.7711-7718.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kestler H W, III, Ringler D J, Mori K, Panicali D L, Sehgal P K, Daniel M D, Desrosiers R C. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell. 1991;65:651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- 15.Klein M R, van Baalen C A, Holwerda A M, Kerkhof Garde S R, Bende R J, Keet I P M, Eeftinck-Schatenkerk J M, Osterhaus A D, Schuitemaker H, Miedema F. Kinetics of Gag-specific cytotoxic T lymphocyte responses during the clinical course of HIV-1 infection: a longitudinal analysis of rapid progressors and long-term asymptomatics. J Exp Med. 1995;181:1365–1372. doi: 10.1084/jem.181.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kleim J P, Bender R, Kirsch R, Meichsner C, Paessens A, Rosner M, Rubsamen-Waigmann H, Kaiser R, Wichers M, Schneweis K E, Winkler I, Riess G. Preclinical evaluation of HBY 097, a new nonnucleoside reverse transcriptase inhibitor of human immunodeficiency virus type 1 replication. Antimicrob Agents Chemother. 1995;39:2253–2257. doi: 10.1128/aac.39.10.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mori K, Ringler D J, Desrosiers R C. Restricted replication of simian immunodeficiency virus strain 239 in macrophages is determined by env but is not due to restricted entry. J Virol. 1993;67:2807–2814. doi: 10.1128/jvi.67.5.2807-2814.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenberg E S, Billingsley J M, Caliendo A M, Boswell S L, Sax P E, Kalams S A, Walker B D. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278:1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 19.Rubsamen-Waigmann H, Huguenel E, Paessens A, Kleim J P, Wainberg M A, Shah A. Second-generation non-nucleosidic reverse transcriptase inhibitor HBY 097 and HIV-1 viral load. Lancet. 1997;349:1517. doi: 10.1016/S0140-6736(97)24021-3. [DOI] [PubMed] [Google Scholar]
- 20.Rubsamen-Waigmann H, Huguenel E, Shah A, Paessens A, Ruoff H J, von Briesen H, Immelmann A, Dietrich U, Wainberg M A. Resistance mutations selected in vivo under therapy with anti-HIV drug HBY 097 may differ from pattern selected in vivo. Antivir Res. 1999;42:15–24. doi: 10.1016/s0166-3542(99)00010-8. [DOI] [PubMed] [Google Scholar]
- 21.Ruprecht R, Gama-Sosa M, Rosas H D. Combination therapy after retroviral inoculation. Lancet. 1988;ii:239–240. doi: 10.1016/s0140-6736(88)91087-2. [DOI] [PubMed] [Google Scholar]
- 22.Tomaras G D, Lacey S F, McDanal C B, Ferrari G, Weinhold K J, Greenberg M L. CD8+ T cell-mediated suppressive activity inhibits HIV-1 after virus entry with kinetics indicating effects on virus gene expression. Proc Natl Acad Sci USA. 2000;97:3503–3508. doi: 10.1073/pnas.070521097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ten Haaft P, Verstrepen B, Ueberla K, Rosenwirth B, Heeney J. A pathogenic threshold of virus load defined in simian immunodeficiency virus- or simian-human immunodeficiency virus-infected macaques. J Virol. 1998;72:10281–10285. doi: 10.1128/jvi.72.12.10281-10285.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsai C C, Follis K E, Sabo A T, Beck W, Grant R F, Bischofberger N, Benveniste R E, Black R. Prevention of SIV infection in macaques in (R)-9-(2-phosphonylmethoxypropyl) adenine. Science. 1995;270:1197–1199. doi: 10.1126/science.270.5239.1197. [DOI] [PubMed] [Google Scholar]
- 25.Uberla K, Stahl-Hennig C, Bottiger D, Matz-Rensing K, Kaup F J, Li J, Haseltine W A, Fleckenstein B, Hunsmann G, Oeberg B, Sodroski J. Animal model for the therapy of acquired immunodeficiency syndrome with reverse transcriptase inhibitors. Proc Natl Acad Sci USA. 1995;92:8210–8214. doi: 10.1073/pnas.92.18.8210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watson W, Ranchalis J, Travis B, McClure J, Sutton W, Johnson P R, Hu S L, Haigwood N L. Plasma viremia in macaques infected with simian immunodeficiency virus: plasma viral load early in infection predicts survival. J Virol. 1997;71:284–290. doi: 10.1128/jvi.71.1.284-290.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong J K, Hezareh M, Günthard H F, Havlir D V, Ignacio C C, Spina C A, Richman D D. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291–1294. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- 28.Wyand M S, Manson K H, Garcia-Moll M, Montefiori D, Desrosiers R C. Vaccine protection by a triple deletion mutant of simian immunodeficiency virus. J Virol. 1996;70:3724–3733. doi: 10.1128/jvi.70.6.3724-3733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yasutomi Y, Reimann K A, Lord C I, Miller M D, Letvin N L. Simian immunodeficiency virus-specific CD8+ lymphocyte response in acutely infected rhesus monkeys. J Virol. 1993;67:1707–1711. doi: 10.1128/jvi.67.3.1707-1711.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang L, Ramratnam B, Tenner-Racz K, He Y, Vesanen M, Lewin S, Talal A, Racz P, Perelson A S, Korber B T, Markowitz M, Ho D D. Quantifying residual HIV-1 replication in patients receiving combination antiretroviral therapy. N Engl J Med. 1999;340:1605–1613. doi: 10.1056/NEJM199905273402101. [DOI] [PubMed] [Google Scholar]
- 31.Zhang L, Lewin S R, Markowitz M, Lin H H, Skulsky E, Karanicolas R, He Y, Jin X, Tuttleton S, Vesanen M, Spiegel H, Kost R, van Lunzen J, Stellbrink H J, Wolinsky S, Borkowsky W, Palumbo P, Kostrikis L G, Ho D D. Measuring recent thymic emigrants in blood of normal and HIV-1-infected individuals before and after effective therapy. J Exp Med. 1999;190:725–732. doi: 10.1084/jem.190.5.725. [DOI] [PMC free article] [PubMed] [Google Scholar]