Abstract
Chronic sleep disturbance affects daily functioning, leading to decreased concentration, fatigue, and higher healthcare costs. Traditional insomnia medications are often associated with adverse side effects. This study investigated the efficacy of a novel compound derived from Rhodiola rosea and Nelumbo nucifera extracts (named RNE) in improving sleep quality with fewer side effects. The study included individuals between the ages of 20 and 65 with subthreshold insomnia and evaluated the effects of RNE on sleep, fatigue, and quality of life. Participants took 750 mg of RNE daily at bed-time for two weeks. The study used the Insomnia Severity Index (ISI), the Pittsburgh Sleep Quality Index (PSQI), a sleep diary, the Fatigue Severity Scale (FSS), and the Short Form 36 Health Survey (SF-36) for assessments. Of the 20 participants, 13 completed the study and showed significant improvements in sleep quality. The results showed improvements in ISI and PSQI scores, a 57% reduction in wake-time after sleep onset, and improved sleep efficiency. Although FSS scores remained unchanged, significant improvements were seen in SF-36 physical and mental health scores. The results suggest that RNE is an effective, low-risk option for sleep disturbance, significantly improving sleep quality and overall wellbeing without significant side effects.
Keywords: sleep disturbance, Rhodiola rosea, Nelumbo nucifera, natural product, dietary supplement, ISI
1. Introduction
Sleep disturbance is a common symptom, but chronic exposure results in a suboptimal health status [1]. Chronic sleep disturbances can result in the low performance of activities during the day, including decreased concentration and lethargy, leading to a poor quality of life and an increased burden of medical use and costs. As sleep is crucial for health, sleep deficits are associated with various diseases. Sleep deficiency is an indicator or a risk factor for neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease [2,3,4]. In addition, sleep difficulty has a bidirectional relationship with psychiatric diseases such as depression and anxiety [5,6]. The association between sleep disturbance and other health conditions, including obesity, pain, and male infertility, has been investigated [7,8,9].
In the United States, 16.3% of adults diagnosed with sleep disorders and 12.7% of adults with sleep problems reported using sleep aids [10]. Currently, a pharmacological approach accounts for the most considerable portion of insomnia treatments, with most of the drugs, including sedatives or hypnotics, being synthetic chemicals [11,12]. Consequently, the market for sleeping pills has grown; the dependence on sleeping pills has also risen. Zolpidem, the most commonly used medication for insomnia, has been recognized for its fast action, but it can cause side effects such as increased respiratory arousal, decreased attention, hallucinations, and suicidality, posing problems for both patients and society [13]. Therefore, there is a need for new pharmacological approaches such as using natural resources, which are generally considered to have fewer side effects, and the demand for sleep-enhancing supplements is increasing.
Rhodiola rosea Linn., commonly referred to as roseroot, has been approved by the Korean Food and Drug Administration as a supplement to improve stress-induced fatigue. Rosavin, a compound found in R. rosea, was reported to have potential therapeutic benefits for stress-related conditions and depression [14]. The study examined 3% rosavin and 1% salidroside combinations in animal models, showing anti-depressant, adaptogenic, and anxiolytic effects by reducing immobility and resting times while increasing active behaviors [15]. The combination reduced CRF-induced anorexia by lowering postsynaptic serotonergic receptor stimulation, indicating anti-anorexia and anti-stress effects [16]. Combined with eleutherosides, schisandrins, salidroside, and tyrosol, rosavin enhanced stress tolerance in BALB/c mice [17]. Nelumbo nucifera Gaertn., also known as lotus, is a medicinal herb and its seeds have been traditionally used in East Asia to treat people suffering from sleep disturbances. Neferine, a key phytochemical extracted from the seeds of N. nucifera, has demonstrated neuroprotective effects, including sleep induction [18]. It synergizes with thiopental to enhance sleep and exhibits sedative and anxiolytic effects similar to diazepam without affecting motor coordination [19]. Neferine also shows anti-depressant-like effects through serotonin receptor interactions [20]. Its anxiolytic/sedative effect has been confirmed in an animal model, suggesting that the seeds could be effective in treating sleep disorders caused by anxiety or depression [21].
This study investigated an extract prepared using a mixture of R. rosea roots and N. nucifera seeds extract (named RNE) as a possible new agent that could improve sleep quality without the side effects found in other insomnia medications. We examined changes in sleep quality, fatigue, and quality of life after RNE administration in adults with subthreshold insomnia.
2. Materials and Methods
This clinical trial was designed as a pre-post study. The study was ethically and scientifically conducted in accordance with the Korean Good Clinical Practice and the Helsinki Declaration. The study protocol was approved by the Kyung Hee University Korean Medical Hospital Institute Review Board (KOMCIRB-170320-HR-009). The study was performed from 27 June 2017 to 26 September 2017. All participants were provided explanations about the study and enrolled after voluntarily providing written informed consent.
2.1. Participants
We recruited adults aged between 20 and 65 years with subthreshold sleep disturbances. A sample size of 20 was planned as it was a pilot study. We included participants who voluntarily consented to participate and signed the informed consent form. We screened participants and collected clinical information from those who met the following inclusion criteria: (1) males or females aged 20 to 65 years old; (2) normal daily life, but with objective or subjective sleeping problems; (3) an Insomnia Severity Index (ISI) score of 8–14; (4) volunteering to participate in the clinical trial and agreeing to give informed consent. The exclusion criteria were individuals who (1) had been clinically diagnosed as having sleep disorders such as sleep apnea syndrome or restless leg syndrome; (2) were under treatment for alcohol/substance abuse or psychosis; (3) were unable to sustain a regular sleep schedule due to irregular work times, shifts, or night duties; (4) had a past history of an allergic reaction after the administration of herbal medicine; (5) were pregnant or lactating females or females at risk of becoming pregnant due to inappropriate contraception; (6) had participated in other clinical trials examining different medication within the previous month; (7) were expected to miss the scheduled study visits due to trips abroad or any expected long-term absence during the trial period; (8) were currently taking medication or supplements for sleep; (9) had a habit of consuming more than the standard recommendation of alcohol more than three times a week; (10) had a habit of drinking more than three cups of coffee a day; (11) had an abnormal laboratory finding; and (12) were deemed inappropriate for the clinical trial by the researchers.
2.2. Intervention
The raw materials of ethanol extract of R. rosea and ethanol extract of N. nucifera were purchased from Hyundai Bioland (Ansan, Republic of Korea). We standardized R. rosea and N. nucifera with their active substances, rosavin and neferine, respectively. The investigational product was prepared as capsules containing an extract (375 mg) prepared using the roots of R. rosea and seeds of N. nucifera at a 2:1 ratio by weight. The investigational products were encapsulated with the mixture in capsules at the Good Manufacturing Practices certified facility of Suheung Co., Ltd. (Cheongju, Republic of Korea). The participants were asked to orally ingest 2 capsules/day (750 mg/day) at bed-time for 2 weeks. If compliance was less than 70%, the participant was eliminated from the study.
2.3. Outcome Measures
The outcomes were measured at the baseline and 1 and 2 weeks after RNE administration. The primary outcomes were the differences in ISI and Pittsburgh Sleep Quality Index (PSQI) scores, while the secondary outcomes were the differences in the sleep diary, Fatigue Severity Scale (FSS), and Short Form 36 Health Survey (SF-36) scores.
2.3.1. Insomnia Severity Index (ISI)
The ISI is a subjective self-report measurement tool that comprises a 5-point scale, with 7 items assessing the severity of sleep onset, sleep maintenance, early morning awakening problems, sleep dissatisfaction, interference of sleep difficulties with day-time functioning, noticeability of sleep problems by others, and related distress [22]. A score of 8–14 is classified as subthreshold insomnia.
2.3.2. Pittsburgh Sleep Quality Index (PSQI)
The PSQI, developed by Buysse, is an efficient tool to measure the quality of sleep and sleep disturbance [23]. This tool uses a self-report questionnaire comprising 19 questions and 7 components (component 1: subjective sleep quality; 2: sleep latency; 3: sleep duration; 4: habitual sleep efficiency (SE); 5: sleep disturbance; 6: use of sleeping medication; and 7: day-time dysfunction). Each component includes related questions, with scores ranging from 0 to 3. A total score > 5 indicates poor sleep.
2.3.3. Sleep Diary
A sleep diary was used to examine the overall aspect of sleep. Sleep-onset latency (SOL), wake-time after sleep onset (WASO), total sleep time (TST), and SE were calculated; sleep and day-time satisfaction data (e.g., day-time exercise, caffeine use, and alcohol use) were also collected. The sleep diary, containing records of sleep patterns since the last visit, was analyzed. The sleep diaries of weeks 1 and 2 were compared using the average SOL, WASO, TST, and SE scores during that week.
2.3.4. Fatigue Severity Scale (FSS)
The FSS assesses day-time fatigue caused by insomnia. This tool is a commonly used self-report questionnaire that evaluates self-perceived feelings related to fatigue [24]. A higher score indicates higher fatigue.
2.3.5. Short Form (36) Health Survey (SF-36)
The SF-36 was used to assess changes in the quality of life and diverse aspects of function [25]. Eight aspects were evaluated: physical functioning (PF), role limitations due to physical problems (RP), bodily pain (BP), general health (GH), vitality (VT), social functioning (SF), role limitations due to emotional problems (RE), and mental health (MH). Changes in the score of each aspect were compared. A higher score indicated a better quality of life.
2.4. Safety
The participants visited every week and were checked for vital signs, compliance with the intervention, and adverse events. Blood urea nitrogen, creatinine, aspartate transaminase (AST), and alanine aminotransferase (ALT) levels were estimated at the last visit.
2.5. Statistical Analysis
An intent-to-treat (ITT) analysis was used; if not applicable, a per-protocol analysis was used instead. Missing data were assumed using the last observation carried forward (LOCF) method for the ITT analysis. As the sample size was too small to achieve normality, the Friedman test and the Wilcoxon signed-rank test were used, if applicable. A statistical significance was set at p < 0.05 and a 95% confidence interval. Analyses were performed using IBM SPSS Statistics 22 (IBM Corp., Armonk, NY, USA).
3. Results
3.1. Participants
In total, 6 males (30%) and 14 females (70%) were included in this study. The average age was 33.40 ± 14.27 years. Of the participants. 12 (60%) were social drinkers, 4 (20%) smoked, and 15 (75%) drank approximately 1 cup of coffee a day on an average (Table 1).
Table 1.
Sociodemographic characteristics and sleep status of the included participants at screening (n = 20).
| Parameter | |
|---|---|
| Age (years) | 33.40 ± 14.27 |
| 19 ≤ age < 30 | 11 (55.0%) |
| 30 ≤ age < 40 | 4 (20.0%) |
| 40 ≤ age < 50 | 1 (5.0%) |
| 50 ≤ age < 60 | 2 (10.0%) |
| 60 ≤ age < 65 | 2 (10.0%) |
| Sex | |
| Female | 14 (70.0%) |
| Male | 6 (30.0%) |
| Education status (years) | 15.95 ± 1.39 |
| High school | 10 (50.0%) |
| ≥College | 10 (50.0%) |
| Height (cm) | 162.56 ± 8.41 |
| Weight (kg) | 60.52 ± 13.50 |
| BMI | 22.81 ± 4.28 |
| Social drinking | 12 (60.0%) |
| Smoking | 4 (20.0%) |
| Drinking coffee | 15 (75.0%) |
| Coffee (cups/day) | 1.01 ± 0.80 |
| ISI | 12.85 ± 1.39 |
| Difficulties in initiating sleep | 20 (100.0%) |
| Frequency (days/week) | 4.63 ± 1.79 |
| Difficulties in maintaining sleep | 12 (60.0%) |
| Frequency (days/week) | 4.67 ± 2.76 |
| Early morning awakening | 11 (55.0%) |
| Frequency (days/week) | 2.92 ± 2.22 |
| Day-time sleepiness | 17 (85.0%) |
| Frequency (days/week) | 4.75 ± 2.01 |
| Day-time symptoms | 20 (100.0%) |
| Treatment for insomnia | 4 (20.0%) |
| Medication | 4 (20.0%) |
| Cognitive behavioral therapy | 2 (10.0%) |
| Alcohol and substance abuse | 0 (0.0%) |
Notes: Values are means ± SD or n (%) of subjects.
The sleep statuses of the participants at screening are shown in Table 1. The average ISI score was 12.85 ± 1.39. Each participant had difficulties initiating sleep and the average frequency was 4.63 ± 1.79 days/week. Twelve participants (60%) had difficulties maintaining sleep and the average frequency was 4.67 ± 2.76 days/week. Although 11 individuals (55%) woke early in the morning, with an average frequency of 2.92 ± 2.22 days/week, 17 (85.0%) felt sleepy in the day-time, averaging 4.75 ± 2.01 days/week. Some experienced day-time discomfort due to sleep disturbances, including fatigue, sleepiness, eye strain, headache, grogginess, and memory decline. One participant took a sleeping pill, three used a sleep aid or supplement, and two had used cognitive behavioral therapy in the past. None of the participants included in the study were under any treatment a month before the trial or had a history of alcohol or substance abuse.
In all, 13 participants completed the study. Six individuals withdrew their consent to participate and one dropped out because of low compliance.
3.2. Sleep
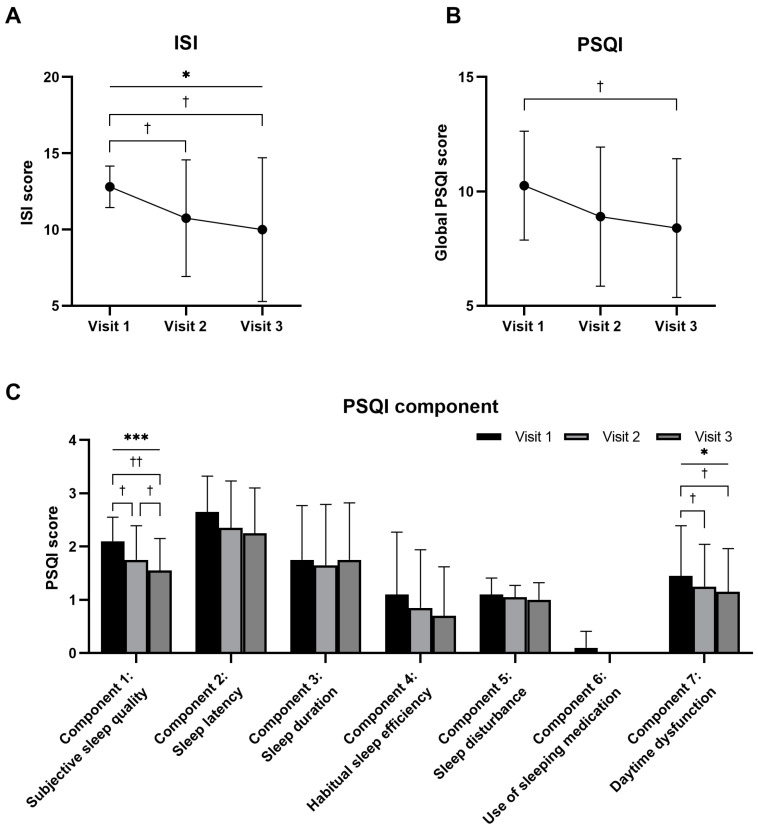
The ISI scores for visits 1, 2, and 3 were 12.80 ± 1.36, 10.75 ± 3.82, and 10.00 ± 4.71, respectively. Based on Friedman’s test, these changes were significant over time (p = 0.0180). Statistically significant differences were also observed between visits 1 and 2, and visits 1 and 3 (visit 1 vs. visit 2, p = 0.0299; visit 2 vs. visit 3, p = 0.2009; visit 1 vs. visit 3, p = 0.0191) (Figure 1A).
Figure 1.
Comparison of Insomnia Severity Index (ISI) (A) and Pittsburgh Sleep Quality Index (PSQI) (B,C) scores for every visit after oral administration of a mixture of Rhodiola rosea and Nelumbo nucifera extracts (named RNE) (n = 20). Notes: Intent-to-treat analysis. Statistical significance evaluated using the Friedman test: * p < 0.05; *** p < 0.001. Statistical significance evaluated using the Wilcoxon signed-rank test: † p < 0.05; †† p < 0.01.
The PSQI scores for visits 1, 2, and 3 were 10.25 ± 2.38, 8.90 ± 3.04, and 8.40 ± 3.03, respectively. Although the change over time was not statistically significant (p = 0.0675), there was a statistically significant difference between visits 1 and 3 (visit 1 vs. visit 2, p = 0.0547; visit 2 vs. visit 3, p = 0.1878; visit 1 vs. visit 3, p = 0.0209) (Figure 1B). Components 1 (subjective sleep quality) and 7 (day-time dysfunction) also showed significant differences over time. In contrast, components 2 (sleep latency), 3 (sleep duration), 4 (habitual SE), 5 (sleep disturbance), and 6 (use of sleeping medication) did not show statistically significant differences between the three visits (Figure 1C).
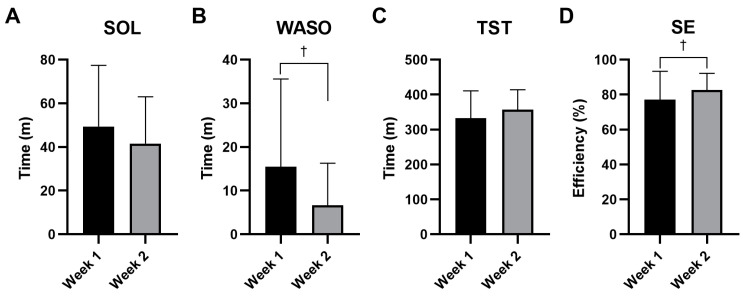
As the sleep diary measured the sleep status of weeks 1 and 2, it was difficult to obtain the baseline values. Therefore, we were unable to implement LOCF for the participants who dropped out after visit 1 and a per-protocol analysis was performed. The SOL, which was 49.37 ± 28.08 min for week 1, decreased to 41.53 ± 21.51 min for week 2; however, the difference was not statistically significant (p = 0.2213) (Figure 2A). In contrast, WASO was 15.47 ± 20.09 min for week 1 and statistically significantly decreased to 6.68 ± 9.60 min for week 2 (p = 0.0159) (Figure 2B). TST was 332.47 ± 78.08 min for week 1; this increased to 357.45 ± 56.51 min for week 2, but the increase was not statistically significant (p = 0.2787) (Figure 2C). SE was 77.05 ± 16.34% for week 1 and significantly increased to 82.65 ± 9.55% for week 2 (p = 0.0392) (Figure 2D).
Figure 2.
Comparison of sleep diary data for each week after oral administration of a mixture of Rhodiola rosea and Nelumbo nucifera extracts (named RNE) (n = 13). Sleep-onset latency (SOL) (A), wake-time after sleep onset (WASO) (B), total sleep time (TST) (C), sleep efficiency (SE) (D) were analyzed. Notes: Per-protocol analysis. Statistical significance evaluated using the Wilcoxon signed-rank test: † p < 0.05. Abbreviations: SOL: sleep-onset latency; WASO: wake-time after sleep onset; TST: total sleep time; SE: sleep efficiency.
3.3. Fatigue
The FSS assesses fatigue during the day-time; the higher the score, the greater the fatigue. The FSS scores for visits 1, 2, and 3 were 37.65 ± 8.67, 38.50 ± 9.24, and 37.15 ± 7.29, respectively. The change in FSS total score over time was not statistically significant (p = 0.2757) and there were no statistically significant differences between the visits (visit 1 vs. 2, p = 0.5747; visit 2 vs. 3, p = 0.1546; visit 1 vs. 3, p = 0.7264) (Table 2).
Table 2.
Comparison of Fatigue Severity Scale (FSS) scores for every visit after oral administration of a mixture of Rhodiola rosea and Nelumbo nucifera extracts (named RNE) (n = 20).
| Visit 1 | Visit 2 | Visit 3 | p-Values | |
|---|---|---|---|---|
| FSS | 37.65 ± 8.67 | 38.50 ± 9.24 | 37.15 ± 7.29 | X2 = 2.5769, p = 0.2757 * |
| V1 vs. V2: Z = −0.5611, p = 0.5747 † V2 vs. V3: Z = −1.4236, p = 0.1546 † V1 vs. V3: Z = −0.3499, p = 0.7264 † |
Notes: Values are means ± SD. ITT analysis. * Statistical significance evaluated using the Friedman test. † Statistical significance assessed using the Wilcoxon signed-rank test. Abbreviations: V: visit.
3.4. Quality of Life
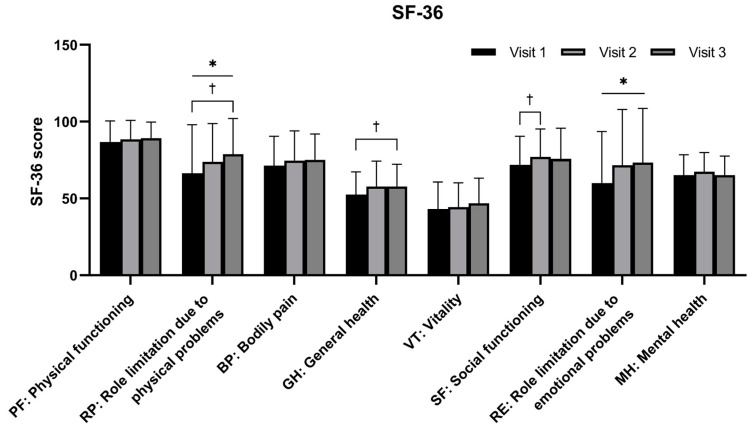
RP and RE showed statistically significant changes in scores over time (p = 0.0361 and 0.0457, respectively). Although the score change over time was not statistically significant, significant changes were seen in GH between visits 1 and 3 (p = 0.0296) and in SF between visits 1 and 2 (p = 0.0461). There were no statistically significant differences in PF, BP, VT, or MH between the three visits (Figure 3).
Figure 3.
Comparison of Short Form (36) Health Survey (SF-36) scores for every visit after oral administration of a mixture of Rhodiola rosea and Nelumbo nucifera extracts (named RNE) (n = 20). Notes: Intent-to-treat analysis. Statistical significance evaluated using the Friedman test: * p < 0.05. Statistical significance evaluated using the Wilcoxon signed-rank test: † p < 0.05.
3.5. Change in Sleep According to Age Group
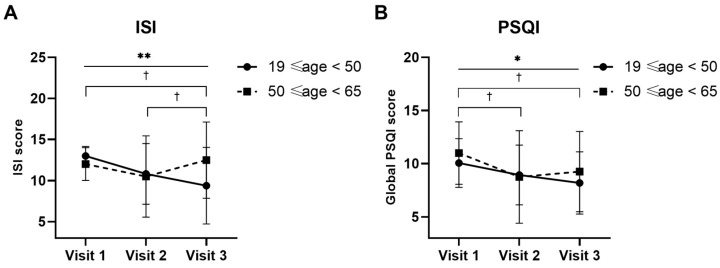
The ISI and PSQI scores were analyzed according to age to observe the effectiveness of treatment on sleep, with the aim of excluding the effect of age. The ISI scores for the age group 19 ≤ age < 50 for visits 1, 2, and 3 were 13.00 ± 1.16, 10.81 ± 3.69, and 9.38 ± 4.66, respectively. The results of Friedman’s test indicated that these changes were statistically significant over time (p = 0.0015). Additionally, statistically significant differences were also observed between visits 2 and 3, and visits 1 and 3 (visit 1 vs. visit 2, p = 0.0522; visit 2 vs. visit 3, p = 0.0171; visit 1 vs. visit 3, p = 0.0125) (Figure 4A). The ISI scores for participants aged 50 to 65 for visits 1, 2, and 3 were 12.00 ± 2.00, 10.50 ± 4.93, and 12.50 ± 4.66, respectively. There was no significant difference between visits (Friedman’s test, p = 0.2921; Wilcoxon signed-rank test, visit 1 vs. visit 2, p = 0.5930; visit 2 vs. visit 3, p = 0.1088; visit 1 vs. visit 3, p = 1.0000) (Figure 4A).
Figure 4.
Comparison of Insomnia Severity Index (ISI) (A) and Pittsburgh Sleep Quality Index (PSQI) (B) scores for every visit after oral administration of a mixture of Rhodiola rosea and Nelumbo nucifera extracts (named RNE) in two age groups, 19 ≤ age < 50 (n = 16) and 50 ≤ age < 65 (n = 4). Notes: Intent-to-treat analysis. Statistical significance evaluated using the Friedman test and is indicated as follows: * p < 0.05; ** p < 0.01 in the age group of 19 ≤ age < 50. Statistical significance also evaluated using the Wilcoxon signed-rank test and is indicated as follows: † p < 0.05 in the age group of 19 ≤ age < 50.
The PSQI scores for the age group 19 ≤ age < 50 for visits 1, 2, and 3 were 10.06 ± 2.29, 8.94 ± 2.82, and 8.19 ± 2.93, respectively. The change over time was statistically significant (p = 0.0419). There was a statistically significant difference between visits 1 and 2, and visits 1 and 3 (visit 1 vs. visit 2, p = 0.0474; visit 2 vs. visit 3, p = 0.1064; visit 1 vs. visit 3, p = 0.0216). The PSQI scores for the age group 50 ≤ age < 65 for visits 1, 2, and 3 were 11.00 ± 2.94, 8.75 ± 4.35, and 9.25 ± 3.78, respectively. There was no statistically significant difference between visits and the score for visit 3 slightly increased, similar to the ISI results (Friedman’s test, p = 0.0675; Wilcoxon signed-rank test, visit 1 vs. visit 2, p = 0.4142; visit 2 vs. visit 3, p = 0.1573; visit 1 vs. visit 3, p = 0.5292) (Figure 4B).
3.6. Adverse Events
The overall compliance was 72.66 ± 40.16%. Adverse events with a possible causal relationship to the intervention included constipation (n = 2) and a dry mouth (n = 1). No clinically significant changes were observed in the other safety variables, including vital signs and laboratory test results.
4. Discussion
The current study evaluated the efficacy and safety of RNE in adults with subthreshold sleep disturbances. The subjective and objective sleep status improved after two weeks of administration and no serious adverse events were encountered. However, although RNE could enhance the physical and emotional levels related to the quality of life, it did not significantly relieve fatigue.
This study showed that RNE has the potential to improve overall sleep quality. Subjective evaluation indices for sleep, including the ISI score, PSQI total score, and component 1, improved over time. In the per-protocol analysis, participants who adhered to the trial almost recovered to a normal range of sleep parameters (see Tables S1–S4, which depict the sociodemographic characteristics and sleep status as well as the various scores evaluated after RNE administration under the per-protocol analysis). The ISI score decreased from 12.69 to 8.15, approaching the 8-point cut-off value of the ISI. The PSQI decreased from 10.62 to 7.46, close to a normal score of 5 points (Table S2). It was also noted that SE, the indicator of the objective parameters for sleep quality, significantly improved, as documented in the SE of the sleep diary and component 4 of the PSQI. The SE of the sleep diary was 82.65% in the second week, which is close to 85% and the normal range of sleep. Considering the definitions of ‘>85%’ (0 points) and ‘75–84%’ (1 point) in component 4 of the PSQI, the decrease from 1.31 to 0.54 was consistent with the SE in the sleep diary. Considering the characteristics of sleep that the participants could confront every night, the analysis of those who fully completed the trial should be referred to subsidiarily.
Both the subjective and objective outcome measures of sleep were alleviated after the administration of RNE. The increased subjective sleep quality and SE indicate that RNE probably positively affects sleep disturbance. Along with the subjective quality, the time parameters of sleep also improved. In the sleep diary, SOL decreased by 8 min, WASO decreased by 9 min, and TST increased by 25 min. A similar pattern was observed in the corresponding items in components 2 and 3 of the PSQI. As it is speculated that subjective sleep quality is related to slow-wave sleep and sleep maintenance [26], satisfaction with sleep could also be attributed to a reduction in WASO. This reduction was comparable with other treatment research, where cognitive behavioral therapy decreased SOL and WASO from 60–70 min to 35 min, and melatonin reduced SOL by 7.2 min [27].
RNE contributed to improvements in GH and sleep but did not change fatigue, as revealed by the FSS and VT scores in the SF-36. However, changes in component 7 of the PSQI and other items in the SF-36 reflected an improvement in day-time symptoms. Although the feeling of fatigue or energy loss persisted, there was an overall improvement in both physical and mental factors. A reduction in RE and MH showed that RNE could ameliorate mental aspects such as depression and anxiety. In Korea, R. rosea has been approved as a functional health food with the health claim ‘may help relieve fatigue by stress’ [28]. The seeds of N. nucifera have been traditionally used as anxiolytics in Korean medicine and their effects have been examined in previous studies [19,29]. Although PF did not show a significant difference, its absolute values were the highest among the SF-36 items. Considering that PF is known to have a high ceiling effect [30,31], the physical factors represented by PF, RP, and GH generally increased. This implies that RNE could assist physical recovery. Nevertheless, the relationship between the recovery of physical factors and improved sleep quality from RNE needs to be further explored.
The analysis of the ISI and PSQI scores according to age groups was conducted to observe the effectiveness of the treatment on sleep whilst excluding the potential confounding effect of age. For the younger age group (19 ≤ age < 50), the ISI scores showed a significant improvement over time. The PSQI scores also showed a significant improvement, which did not significantly change over time in the results of total participants. Conversely, for the older age group (50 ≤ age < 65), both the ISI and PSQI scores did not show a significant change between visits. These findings indicate that RNE could significantly improve both insomnia severity and sleep quality in younger adults (19 ≤ age < 50), but might not have a significant effect in older adults (50 ≤ age < 65). These results highlight the importance of considering age when evaluating the efficacy of sleep treatments and suggest that younger individuals may respond more favorably to RNE supplementation to improve sleep-related issues. Analyzing the sleep duration of 730,187 participants revealed a significant difference in sleep patterns at the ages of 33 and 53 years [32]. SOL increases between the late teens and 20s, remains consistent from age 30 to around 50 years, and then steadily increases after age 50. WASO shows a consistent 10 min increase per decade from ages 30 to 60, with minimal changes observed beyond 60 years. In contrast to other sleep parameters, which tend to stabilize after the age of 60, sleep efficiency continues to gradually decline with advancing age [33]. Nevertheless, due to the limited number of elderly participants, further research with larger sample sizes in the older age group may be necessary to fully comprehend the potential advantages of RNE for different age demographics.
Constipation and dry mouth were expected adverse events. The causal relationships need to be considered: one case of constipation improved after menstruation during the period of administration and the case of dry mouth resolved before the intake period ended. Therefore, it may be considered that no serious adverse events were observed during this study.
RNE both reduced the latency to sleep and increased sleep duration; its sleep-promoting, anxiolytic, and anti-convulsant effects were confirmed using mice with pentobarbital-induced sleep. Its plausible mechanism is its impact upon GABAergic and serotonergic systems. The 2:1 ratio of RNE was determined by comparing the efficacy of each ratio between R. rosea and N. nucifera on pentobarbital-induced sleep in mice [34]. The administration of 250 mg/kg R. rosea and N. nucifera single extracts improved sleep duration by 10.1% and 13.1%, respectively, compared with a saline-treated control group. A mixture of these extracts at ratios of 1:1, 2:1, and 4:1 at 250 mg/kg showed greater improvements of 14.6%, 16%, and 15.2%, respectively. For SOL, at a concentration of 500 mg/kg, single extracts of R. rosea and N. nucifera showed improvements of 19.3% and 16.8%, respectively. In comparison, a mixture of these extracts at ratios of 1:1, 2:1, and 4:1 at 500 mg/kg showed greater improvements of 22.5%, 26.7%, and 24.8%, respectively. Of these samples, the 2:1 ratio showed the most significant improvements in both sleep duration and sleep-onset latency [34]. The mechanism of RNE was studied in a preclinical setting. The administration of RNE to ICR mice for six days did not result in a statistically significant increase in melatonin levels, although there was a tendency for melatonin levels to rise with an increase in a dose-dependent manner. In contrast, serum serotonin levels significantly increased at doses of 250, 500, and 1000 mg/kg compared with the control group. Furthermore, the binding of antagonists to GABA, serotonin 2A, and serotonin 2C receptors was inhibited, indicating the potential mechanisms through which these extracts exert their effects [34]. These results were similar to those of previous studies. R. rosea itself has been reported to improve sleep-related parameters such as latency to sleep onset and sleep duration in animals, protecting them from learning and memory deficits due to sleep deprivation [35,36,37]. Its mechanism on its protective effect on sleep is suggested to result from changes in serotonergic and GABAergic immune-related mechanisms, providing protection from oxidative stress and neuron injury [35]. N. nucifera has been documented to increase nonrapid eye movement sleep and change subjective night-time activity, sleep bouts, and sleep time by regulating GABAergic receptors [38,39]. Procyanidin B2 and neferine, a compound from N. nucifera, improve sleep latency and sleep duration [40]. R. rosea also improved depression and anxiety and demonstrated an enhanced resistance to stress in clinical trials [41,42,43,44], while N. nucifera was also reported to have anti-depressant and anxiolytic effects in animal models. Both these herbs may alleviate sleep disturbances derived from depressive or anxiety disorders [19,21,45].
Efforts to search for effective and safe sleep aids from natural products continue. The effects of Valeriana officinalis (valerian), Piper methysticum (kava), Matricaria recutita (German chamomile), Passiflora Incarnata (passionflower), Hypericum perforation (St. John’s Wort), and Panax ginseng (Korea Red Ginseng) have been reported in clinical studies and animal experiments to date [46]. Valeriana officinalis (valerian) improved sleep in adults diagnosed with insomnia and middle-aged adults with mild sleep complaints in various clinical trials [47,48,49,50]. Although the sleep-promoting effects of these various natural products have been reported, with the exception of a few plants, their effects were insignificant or their underlying mechanisms were unclear. The results of RNE suggest new possibilities for natural products as a sleep-improving supplement.
This study had several limitations. The effect of RNE was tested in a pilot study with a small number of participants. The dropout rate was high as the participants who suffered from disturbances every day readily declined to continue in the study. Some of the measures in the per-protocol analysis, such as sleep disturbance and habitual SE in the PSQI, showed a statistical significance. If the intervention did not quickly show efficacy, the participants were likely to decline further participation after the first dose. In contrast, a significant change was observed among the participants who persisted in the study. As the PSQI is designed to evaluate the status of sleep in the previous 4 weeks, this feature may have interfered with the suggested explanation of the study outcomes.
5. Conclusions
A dose of 750 mg/day of RNE improved sleep quality in individuals with subthreshold insomnia. It significantly changed the total ISI and PSQI scores and components over time and showed improvements in WASO and SE. In addition, RNE improved physical and mental health. Clinically significant adverse events and safety-related problems were not observed. These results suggest that RNE may be considered as a preventive or therapeutic agent to improve sleep disturbances. Further clinical trials on the long-term efficacy and safety of RNE administration are warranted.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu16121867/s1, The material includes Tables S1–S4, which depict detailed data, including the sociodemographic characteristics and sleep status at screening, the ISI and PSQI indices, and the FSS and SF-36 for the three visits following the administration of RNE to the 13 participants who completed the trial (per-protocol analysis). The statistical analysis is depicted for the parameters included in the study.
Author Contributions
Conceptualization, W.K.L., H.J. and H.J.C.; methodology, S.-H.C.; validation, S.-H.C.; formal analysis, Y.K.; investigation, Y.K.; resources, S.-H.C.; data curation, Y.K.; writing—original draft preparation, Y.K.; writing—review and editing, Y.K., W.K.L. and H.J.; supervision, W.K.L., H.J.C., M.-K.L. and S.-H.C.; project administration, W.K.L., H.J.C., M.-K.L. and S.-H.C.; funding acquisition, W.K.L., H.J.C. and M.-K.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Kyung Hee University Korean Medical Hospital Institute Review Board (KOMCIRB-170320-HR-009).
Informed Consent Statement
All participants were provided explanations about the study and enrolled after voluntarily providing written informed consent.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
W.K.L., H.J. and M.-K.L. are employees of LG H&H. However, the design and analysis of the results were independently performed by Y.K. and S.-H.C., who are affiliated to Kyung Hee University. The other authors declare that they have no conflicts of interest regarding the publication of this paper.
Funding Statement
This work was supported by LG Household & Health Care (LG H&H) Co., Ltd.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Conklin A.I., Yao C.A., Richardson C.G. Chronic Sleep Disturbance, Not Chronic Sleep Deprivation, Is Associated with Self-Rated Health in Adolescents. Prev. Med. 2019;124:11–16. doi: 10.1016/j.ypmed.2019.04.014. [DOI] [PubMed] [Google Scholar]
- 2.Irwin M.R., Vitiello M.V. Implications of Sleep Disturbance and Inflammation for Alzheimer’s Disease Dementia. Lancet Neurol. 2019;18:296–306. doi: 10.1016/S1474-4422(18)30450-2. [DOI] [PubMed] [Google Scholar]
- 3.Wennberg A.M.V., Wu M.N., Rosenberg P.B., Spira A.P. Sleep Disturbance, Cognitive Decline, and Dementia: A Review. Semin. Neurol. 2017;37:395–406. doi: 10.1055/s-0037-1604351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bohnen N.I., Hu M.T.M. Sleep Disturbance as Potential Risk and Progression Factor for Parkinson’s Disease. J. Park. Dis. 2019;9:603–614. doi: 10.3233/JPD-191627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freeman D., Sheaves B., Waite F., Harvey A.G., Harrison P.J. Sleep Disturbance and Psychiatric Disorders. Lancet Psychiatry. 2020;7:628–637. doi: 10.1016/S2215-0366(20)30136-X. [DOI] [PubMed] [Google Scholar]
- 6.Fang H., Tu S., Sheng J., Shao A. Depression in Sleep Disturbance: A Review on a Bidirectional Relationship, Mechanisms and Treatment. J. Cell. Mol. Med. 2019;23:2324–2332. doi: 10.1111/jcmm.14170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palnitkar G., Phillips C.L., Hoyos C.M., Marren A.J., Bowman M.C., Yee B.J. Linking Sleep Disturbance to Idiopathic Male Infertility. Sleep Med. Rev. 2018;42:149–159. doi: 10.1016/j.smrv.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Muscogiuri G., Barrea L., Annunziata G., Somma C.D., Laudisio D., Colao A., Savastano S. Obesity and Sleep Disturbance: The Chicken or the Egg? Crit. Rev. Food Sci. Nutr. 2019;59:2158–2165. doi: 10.1080/10408398.2018.1506979. [DOI] [PubMed] [Google Scholar]
- 9.Andersen M.L., Araujo P., Frange C., Tufik S. Sleep Disturbance and Pain: A Tale of Two Common Problems. Chest. 2018;154:1249–1259. doi: 10.1016/j.chest.2018.07.019. [DOI] [PubMed] [Google Scholar]
- 10.Chong Y., Fryer C.D., Gu Q. Prescription Sleep Aid Use among Adults: United States, 2005–2010. National Center for Health Statistics; Hyattsville, MD, USA: 2013. pp. 1–8. NCHS Data Brief. [PubMed] [Google Scholar]
- 11.Agarwal S.D., Landon B.E. Patterns in Outpatient Benzodiazepine Prescribing in the United States. JAMA Netw. Open. 2019;2:187399. doi: 10.1001/jamanetworkopen.2018.7399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davies J., Rae T.C., Montagu L. Long-Term Benzodiazepine and Z-Drugs Use in England: A Survey of General Practice. Br. J. Gen. Pract. 2017;67:609–613. doi: 10.3399/bjgp17X691865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edinoff A.N., Wu N., Ghaffar Y.T., Prejean R., Gremillion R., Cogburn M., Chami A.A., Kaye A.M., Kaye A.D. Zolpidem: Efficacy and Side Effects for Insomnia. Health Psychol. Res. 2021;9:24927. doi: 10.52965/001c.24927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aktar M.A., Bhuia M.S., Chowdhury R., Hasan R., Rakib A.I., Al Hasan M.S., Sonia F.A., Islam M.T. Therapeutic Promises of Bioactive Rosavin: A Comprehensive Review with Mechanistic Insight. Chem. Biodivers. 2024:e202400286. doi: 10.1002/cbdv.202400286. [DOI] [PubMed] [Google Scholar]
- 15.Perfumi M., Mattioli L. Adaptogenic and Central Nervous System Effects of Single Doses of 3% Rosavin and 1% Salidroside Rhodiola rosea L. Extract in Mice. Phytother. Res. 2007;21:37–43. doi: 10.1002/PTR.2013. [DOI] [PubMed] [Google Scholar]
- 16.Mattioli L., Perfumi M. Rhodiola rosea L. Extract Reduces Stress- and CRF-Induced Anorexia in Rats. J. Psychopharmacol. 2007;21:742–750. doi: 10.1177/0269881106074064. [DOI] [PubMed] [Google Scholar]
- 17.Panossian A., Wikman G., Kaur P., Asea A. Adaptogens Exert a Stress-Protective Effect by Modulation of Expression of Molecular Chaperones. Phytomedicine. 2009;16:617–622. doi: 10.1016/j.phymed.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Bharathi Priya L., Huang C.-Y., Hu R.-M., Balasubramanian B., Baskaran R. An Updated Review on Pharmacological Properties of Neferine—A Bisbenzylisoquinoline Alkaloid from Nelumbo Nucifera. J. Food Biochem. 2021;45:e13986. doi: 10.1111/jfbc.13986. [DOI] [PubMed] [Google Scholar]
- 19.Sugimoto Y., Furutani S., Itoh A., Tanahashi T., Nakajima H., Oshiro H., Sun S., Yamada J. Effects of Extracts and Neferine from the Embryo of Nelumbo Nucifera Seeds on the Central Nervous System. Phytomedicine. 2008;15:1117–1124. doi: 10.1016/j.phymed.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Sugimoto Y., Furutani S., Nishimura K., Itoh A., Tanahashi T., Nakajima H., Oshiro H., Sun S., Yamada J. Antidepressant-like Effects of Neferine in the Forced Swimming Test Involve the serotonin1A (5-HT1A) Receptor in Mice. Eur. J. Pharmacol. 2010;634:62–67. doi: 10.1016/j.ejphar.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 21.Rajput M.A., Khan R.A. Phytochemical Screening, Acute Toxicity, Anxiolytic and Antidepressant Activities of the Nelumbo Nucifera Fruit. Metab. Brain Dis. 2017;32:743–749. doi: 10.1007/s11011-017-9963-x. [DOI] [PubMed] [Google Scholar]
- 22.Bastien C.H., Vallières A., Morin C.M. Validation of the Insomnia Severity Index as an Outcome Measure for Insomnia Research. Sleep Med. 2001;2:297–307. doi: 10.1016/S1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 23.Buysse D.J., Reynolds C.F., Monk T.H., Berman S.R., Kupfer D.J. The Pittsburgh Sleep Quality Index: A New Instrument for Psychiatric Practice and Research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 24.Krupp L.B., LaRocca N.G., Muir-Nash J., Steinberg A.D. The Fatigue Severity Scale: Application to Patients With Multiple Sclerosis and Systemic Lupus Erythematosus. Arch. Neurol. 1989;46:1121–1123. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 25.Ware J., Snow K., Kosinski M., Gandek B. SF36 Health Survey: Manual and Interpretation Guide. Volume 30 Quality Metric, Inc.; Lincoln, RI, USA: 1993. [Google Scholar]
- 26.Keklund G., Akerstedt T. Objective Components of Individual Differences in Subjective Sleep Quality. J. Sleep Res. 1997;6:217–220. doi: 10.1111/j.1365-2869.1997.00217.x. [DOI] [PubMed] [Google Scholar]
- 27.Morin C.M., Benca R. Chronic Insomnia. Lancet. 2012;379:1129–1141. doi: 10.1016/S0140-6736(11)60750-2. [DOI] [PubMed] [Google Scholar]
- 28.Ministry of Food and Drug Safety . Health Functional Food Code. Ministry of Food and Drug Safety; Cheongju-si, Republic of Korea: 2021. [Google Scholar]
- 29.Kulkarni M.P., Juvekar A.R. Attenuation of Acute and Chronic Restraint Stress-Induced Perturbations in Experimental Animals by Nelumbo Nucifera Gaertn. Indian. J. Pharm. Sci. 2008;70:327–332. doi: 10.4103/0250-474X.42982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montazeri A., Goshtasebi A., Vahdaninia M., Gandek B. The Short Form Health Survey (SF-36): Translation and Validation Study of the Iranian Version. Qual. Life Res. 2005;14:875–882. doi: 10.1007/s11136-004-1014-5. [DOI] [PubMed] [Google Scholar]
- 31.Gandek B., Ware Jr J.E., Aaronson N.K., Alonso J., Apolone G., Bjorner J., Brazier J., Bullinger M., Fukuhara S., Kaasa S. Tests of Data Quality, Scaling Assumptions, and Reliability of the SF-36 in Eleven Countries: Results from the IQOLA Project. J. Clin. Epidemiol. 1998;51:1149–1158. doi: 10.1016/S0895-4356(98)00106-1. [DOI] [PubMed] [Google Scholar]
- 32.Coutrot A., Lazar A.S., Richards M., Manley E., Wiener J.M., Dalton R.C., Hornberger M., Spiers H.J. Reported Sleep Duration Reveals Segmentation of the Adult Life-Course into Three Phases. Nat. Commun. 2022;13:7697. doi: 10.1038/s41467-022-34624-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li J., Vitiello M.V., Gooneratne N.S. Sleep in Normal Aging. Sleep Med. Clin. 2022;17:161–171. doi: 10.1016/j.jsmc.2022.02.007. [DOI] [PubMed] [Google Scholar]
- 34.Jeong H., Lee W.K., Lee M.-K., Kim J.-B., Kim N.-H., Choi H.-J., Cho S.-H., Kim E.-J., Jung W.-Y., Oh H.-A., et al. Composition for improving of sleep disorder comprising Rhodiola rosea and seed of Nelumbo nucifera. 10-2531387. KR Patent. 2023 May 8;
- 35.Hao Y.F., Luo T., Lu Z.Y., Shen C.Y., Jiang J.G. Targets and Underlying Mechanisms Related to the Sedative and Hypnotic Activities of Saponins from Rhodiola rosea L. (crassulaceae) Food Funct. 2021;12:10589–10601. doi: 10.1039/d1fo01178b. [DOI] [PubMed] [Google Scholar]
- 36.Zhang X., Zheng H., Ma Z., Shi L., Zeng S. Effects of Rhodiola rosea on Learning and Memory in Rats after Sleep Deprivation and the Dissection of Its Mechanism. Chin. J. Clin. Pharmacol. Ther. 2012;17:634. [Google Scholar]
- 37.Tokunaga S., Takeda Y., Niimoto T., Nishida N., Kubo T., Ohno T., Matsuura Y., Kawahara Y., Shinomiya K., Kamei C. Effect of Valerian Extract Preparation (BIM) on the Sleep-Wake Cycle in Rats. Biol. Pharm. Bull. 2007;30:363–366. doi: 10.1248/bpb.30.363. [DOI] [PubMed] [Google Scholar]
- 38.Jo K., Choi H.S., Jeon S., Ahn C.W., Suh H.J. Nelumbo nucifera Seed Extract Promotes Sleep in Drosophila melanogaster. Biol. Pharm. Bull. 2018;41:399–408. doi: 10.1248/bpb.b17-00763. [DOI] [PubMed] [Google Scholar]
- 39.Jo K., Kim S., Hong K.B., Suh H.J. Nelumbo nucifera promotes non-rapid eye movement sleep by regulating GABAergic receptors in rat model. J. Ethnopharmacol. 2021;267:113511. doi: 10.1016/j.jep.2020.113511. [DOI] [PubMed] [Google Scholar]
- 40.Xiao H.B., Wang Y.S., Liang L., Lu X., Sun Z.L. Procyanidin B2 from Lotus Seedpod Regulate NO/ADMA/DDAH Pathway to Treat Insomnia in Rats. Fundam. Clin. Pharmacol. 2019;33:549–557. doi: 10.1111/fcp.12462. [DOI] [PubMed] [Google Scholar]
- 41.Mao J.J., Xie S.X., Zee J., Soeller I., Li Q.S., Rockwell K., Amsterdam J.D. Rhodiola rosea versus Sertraline for Major Depressive Disorder: A Randomized Placebo-Controlled Trial. Phytomedicine. 2015;22:394–399. doi: 10.1016/j.phymed.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cropley M., Banks A.P., Boyle J. The Effects of Rhodiola rosea L. Extract on Anxiety, Stress, Cognition and Other Mood Symptoms. Phytother. Res. 2015;29:1934–1939. doi: 10.1002/ptr.5486. [DOI] [PubMed] [Google Scholar]
- 43.Bystritsky A., Kerwin L., Feusner J.D. A Pilot Study of Rhodiola rosea (Rhodax) for Generalized Anxiety Disorder (GAD) J. Altern. Complement. Med. 2008;14:175–180. doi: 10.1089/acm.2007.7117. [DOI] [PubMed] [Google Scholar]
- 44.Darbinyan V., Aslanyan G., Amroyan E., Gabrielyan E., Malmstrom C., Panossian A. Clinical Trial of Rhodiola rosea L. Extract SHR-5 in the Treatment of Mild to Moderate Depression. Nord. J. Psychiatry. 2007;61:343–348. doi: 10.1080/08039480701643290. [DOI] [PubMed] [Google Scholar]
- 45.Prasad D.K., Alva H., Shetty M. Evaluation of Colour Stability of Provisional Restorative Materials Exposed to Different Mouth Rinses at Varying Time Intervals: An in Vitro Study. J. Indian. Prosthodont. Soc. 2014;14:85–92. doi: 10.1007/s13191-013-0276-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feizi F., Namazi N., Rahimi R., Ayati M.H. Medicinal Plants for Management of Insomnia: A Systematic Review of Animal and Human Studies. Galen. Med. J. 2019;8:e1085. doi: 10.31661/gmj.v8i0.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oxman A.D., Flottorp S., Håvelsrud K., Fretheim A., Odgaard-Jensen J., Austvoll-Dahlgren A., Carling C., Pallesen S., Bjorvatn B. A Televised, Web-Based Randomised Trial of an Herbal Remedy (Valerian) for Insomnia. PLoS ONE. 2007;2:e1040. doi: 10.1371/journal.pone.0001040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coxeter P.D., Schluter P.J., Eastwood H.L., Nikles C.J., Glasziou P.P. Valerian Does Not Appear to Reduce Symptoms for Patients with Chronic Insomnia in General Practice Using a Series of Randomised N-of-1 Trials. Complement. Ther. Med. 2003;11:215–222. doi: 10.1016/s0965-2299(03)00122-5. [DOI] [PubMed] [Google Scholar]
- 49.Donath F., Quispe S., Diefenbach K., Maurer A., Fietze I., Roots I. Critical Evaluation of the Effect of Valerian Extract on Sleep Structure and Sleep Quality. Pharmacopsychiatry. 2000;33:47–53. doi: 10.1055/s-2000-7972. [DOI] [PubMed] [Google Scholar]
- 50.Diaper A., Hindmarch I. A Double-Blind, Placebo-Controlled Investigation of the Effects of Two Doses of a Valerian Preparation on the Sleep, Cognitive and Psychomotor Function of Sleep-Disturbed Older Adults. Phytother. Res. 2004;18:831–836. doi: 10.1002/ptr.1574. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.