Abstract
Cognitive decline is a significant health concern in our aging society. Here, we used the model organism C. elegans to investigate the impact of the IIS/FOXO pathway on age-related cognitive decline. The daf-2 Insulin/IGF-1 receptor mutant exhibits a significant extension of learning and memory span with age compared to wild-type worms, an effect that is dependent on the DAF-16 transcription factor. To identify possible mechanisms by which aging daf-2 mutants maintain learning and memory with age while wild-type worms lose neuronal function, we carried out neuron-specific transcriptomic analysis in aged animals. We observed downregulation of neuronal genes and upregulation of transcriptional regulation genes in aging wild-type neurons. By contrast, IIS/FOXO pathway mutants exhibit distinct neuronal transcriptomic alterations in response to cognitive aging, including upregulation of stress response genes and downregulation of specific insulin signaling genes. We tested the roles of significantly transcriptionally-changed genes in regulating cognitive functions, identifying novel regulators of learning and memory. In addition to other mechanistic insights, a comparison of the aged vs young daf-2 neuronal transcriptome revealed that a new set of potentially neuroprotective genes is upregulated; instead of simply mimicking a young state, daf-2 may enhance neuronal resilience to accumulation of harm and take a more active approach to combat aging. These findings suggest a potential mechanism for regulating cognitive function with age and offer insights into novel therapeutic targets for age-related cognitive decline.
Research organism: C. elegans
Introduction
The loss of cognitive function is a rising problem in our aging society. A 2008 study estimated that at least 22.2% (about 5.4 million) of individuals over the age of 71 in the United States have at least mild cognitive impairment (Plassman, 2008; Langa and Levine, 2014; Gillis et al., 2019). Furthermore, global dementia cases are predicted to triple from an estimated 57.4 million cases in 2019–152.8 million cases in 2050 (Feigin et al., 2020; Nichols and Vos, 2020). As most industrialized countries are experiencing a rapid increase in the proportion of the aged population, understanding and potentially preventing the underlying issues of neuronal structural and behavioral decline associated with aging is crucial for societal health.
C. elegans is an excellent model system for studying neuronal aging, given its tractable genetics, short lifespan, and simple nervous system (White et al., 1986). Most importantly, C. elegans experiences rapid loss of learning and memory with age (Kauffman et al., 2010): by Day 4 of adulthood, all long-term associative memory ability is lost, and by Day 8, C. elegans cannot carry out associative learning or short-term associative memory (Kauffman et al., 2010) – despite the fact that these worms can still move and chemotaxis perfectly well. That is, with age worms first lose long-term memory ability (by Day 4), then short-term memory and learning ability (Day 6–8), then chemotaxis (Day 10–12), then motility (Day 16) (Kauffman et al., 2010; Hahm et al., 2015). Because learning and memory decline extremely early, we consider worms that are only a week old to already be ‘cognitively aged,’ despite the fact that they can chemotaxis and move well, and will continue to live for another one to two weeks. Therefore, we can examine neurons from these 7–8-day-old adults to explore the causes of these cognitive declines in animals that are otherwise quite healthy. Many human neuronal aging phenotypes and genes of interest for mammalian neuronal function are conserved in C. elegans (Arey and Murphy, 2017), making discoveries in C. elegans possibly applicable to humans.
The Insulin/IGF-1-like signaling (IIS)/FOXO pathway was first discovered to play a role in longevity in C. elegans. The lifespan of daf-2/Insulin/IGF-1 receptor mutants is twice that of wild-type animals (Kenyon et al., 1993), and this lifespan extension requires the downstream Forkhead box O (FOXO) transcription factor DAF-16 (Kenyon et al., 1993). DAF-16/FOXO controls the expression of many genes that contribute to longevity, including stress response, proteostasis, autophagy, antimicrobial, and metabolic genes (Murphy et al., 2003). As a conserved regulator, the IIS/FOXO pathway also regulates longevity in Drosophila, mice, and humans (Clancy et al., 2001; Blüher et al., 2003; Suh et al., 2008; Willcox et al., 2008). In addition to regulating lifespan, the IIS pathway regulates neuronal function via the FOXO transcription factor. In particular, C. elegans IIS/daf-2 mutants display DAF-16-dependent improved learning, short-term memory, and long-term memory (Kauffman et al., 2010). While both young and old daf-2 adult worms display increased learning and memory relative to wild-type, the duration of this extension is not known, and the mechanisms by which daf-2 mutants maintain neuronal function in older worms are not yet understood. Compared to wild-type worms, daf-2 mutants better maintain maximum velocity (Hahm et al., 2015), motility (Liu et al., 2013; Li et al., 2016), neuromuscular junctions, the ability to regenerate axons (Byrne et al., 2014; Lakhina et al., 2019), and neuronal morphology with age (Pan et al., 2011; Tank et al., 2011; Toth et al., 2012). In particular, we previously showed that while daf-2 has lower observed motility on food (Bansal et al., 2015), this apparent is due to its high levels of a food receptor, ODR-10 (Hahm et al., 2015), and its downregulation reveals the much higher mobility of daf-2 animals (Hahm et al., 2015), even on food, in addition to its much higher and maintained maximum velocity with age. Previously, we found that daf-2 worms also extend learning beyond the wild-type’s ability (Kauffman et al., 2010), but the full duration of this extension with age was not known. That is, exactly how late in life daf-2 mutants can still learn and remember, and whether this is proportional to their lifespan extension, was not previously determined.
We previously performed neuron-specific RNA-sequencing in young (Day 1) adult C. elegans and identified neuron-specific targets (Kaletsky et al., 2016); genes upregulated in daf-2 mutant neurons are distinct from those in the whole animal, and we found that these neuronal genes are necessary for the observed improvements in memory and axon regeneration in daf-2 mutant worms. However, whether daf-2 uses the same or different genes in young and old worms to improve and maintain cognitive function with age is unknown. Recent datasets using whole-animal single-cell RNA-seq have been generated for wild-type and daf-2 worms, and these are sufficient for whole-body aging and pseudobulk analyses (Gao et al., 2023; Wang et al., 2022; Roux et al., 2023), but we have found that those data are not deep enough to use specifically for in-depth analysis of neurons, which can be difficult to gather from whole animals. Other data are from larval stages and cannot be extrapolated to aging adults (Taylor et al., 2021). To identify the transcriptional differences in the aging nervous system that might contribute to the loss of neuronal function with age in wild-type worms and the differences responsible for the extended abilities of daf-2 animals, here we performed RNA sequencing on FACS-isolated neurons of aged (Day 8) wild-type and IIS/FOXO mutants. To further investigate the role of the neuronal IIS/FOXO pathway, we identified genes both upregulated by the IIS/FOXO pathway, and genes that are differentially expressed in daf-2 mutants with age. We found that daf-2 differentially-regulated genes in the aged neurons are different from young neurons; in fact, many of these Day 8 daf-2 vs daf-16;daf-2 upregulated genes are stress response and proteolysis genes that may promote neuronal function and health. We then used functional assays to assess the contributions of daf-2-regulated genes to learning and memory. Our results suggest that daf-2’s neuronal targets in older worms are required to maintain neuronal functions with age, suggesting that additional and alternative mechanisms are at work in these aged mutants from their young counterparts.
Results
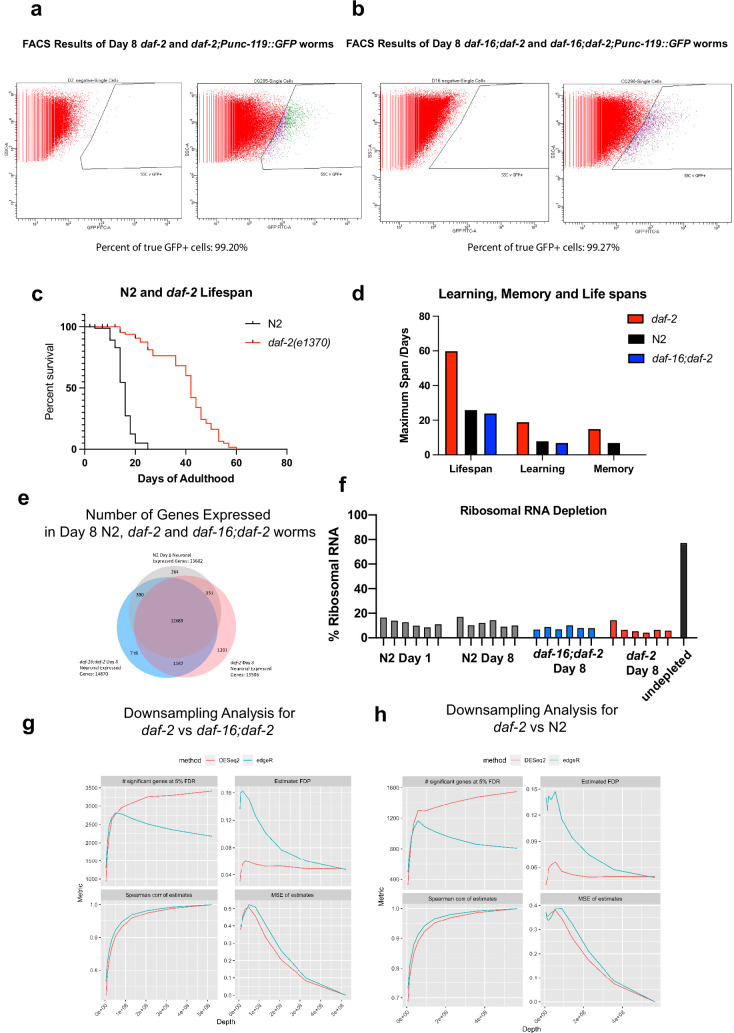
Wild-type neurons lose their neuronal function and identity with age
Previously, we found that cognitive abilities in C. elegans, including learning, short-term memory, and long-term memory, all decline with age (Kauffman et al., 2010). Moreover, neuronal morphology and regeneration ability are also impaired with age (Byrne et al., 2014; Pan et al., 2011; Tank et al., 2011; Toth et al., 2012). However, how these phenotypes are regulated at the molecular level in aging neurons remains to be systematically characterized. Therefore, we were interested in first identifying gene expression changes with age in wild-type neurons to characterize the normal physiological aging process. Before choosing timepoints to assess neuronal transcriptome changes, we carried out associative learning and short-term associative memory assays (Kauffman et al., 2010) as we have previously described (Kauffman et al., 2010; Kauffman et al., 2011; Stein and Murphy, 2012; Stein and Murphy, 2014). Briefly, well-fed worms are starved for 1 hr, then re-fed while exposed to the neutral odorant butanone for 1 hr; a choice assay between butanone and control immediately after training tests associative learning, while a choice assay after 1 hr of recovery on food-only plates tests short-term associative memory (Kauffman et al., 2010). Adult Day 1 worms are fully developed, young, and healthy, while wild-type Day 7–8 worms, although still in their mid-life, have completely lost their learning and short-term memory abilities already by Day 7 (Kauffman et al., 2010; Figure 1a), thus we consider them ‘aged’ for the purposes of understanding loss of cognitive ability. Therefore, we reasoned that a comparison of adult wild-type Day 1 neurons with wild-type neurons that are at least aged Day 7–8 should reveal changes with age that result in loss of cognitive function.
Figure 1. Identifying neuronal aging targets in wild-type (WT) worms using neuron-specific RNA-sequencing.
(a) Wild-type learning and 1 hr memory results on Day 1 and Day 7. Learning and memory results are represented as learning index (LI). Details of the LI calculation are explained in the methods. Learning, n=10, memory, n=5. ****p<0.01. Student’s t-test. (b) PCA plot for Day 1 (orange) and Day 8 (blue) neuronal bulk RNA-seq samples. (c) Volcano plot comparing age-associated differentially-expressed genes in WT neurons. Genes downregulated with age (orange) and upregulated with age (blue) were obtained by neuron-specific RNA sequencing of adult wild-type animals with neuron-specific GFP expression. Adjusted p-value <0.001, log2(Fold-change) >2. n=6 biological replicates per age. 1146 genes were significantly downregulated with age (higher in young neurons) and 2016 genes were upregulated with age (higher in old neurons) (d) Tissue prediction scores for genes higher in young neurons. (e) Gene ontology (GO) terms of genes that decline with age in wild-type neurons. Synaptic and signaling GO terms are enriched in neuronal genes. p-value calculated using hypergeometric distribution probability. (f) Comparison of whole-body higher-in-young genes and neuronal higher-in-young genes. GO Terms and representative genes were performed using g:Profiler software. P-value of overlapping regions were calculated using a hypergeometric calculator. (g) Normalized reads of ins-6, unc-4, mec-7, folt-2, fbf-1, and madd-4, in Day 1 and Day 8 neurons in our dataset. p-adjusted values were calculated from DESeq2 software. Box plots: center line, median; box range, 25-75th percentiles; whiskers denote minimum-maximum values.
Figure 1—figure supplement 1. Aged neuron-specific sequencing.
Figure 1—figure supplement 2. Whole-worm RNA-sequencing identifies whole-body changes during aging.
To identify genes that regulate age-related morphological and functional decline in wild-type neurons, we performed neuron-specific transcriptomic analysis using our previous FACS neuronal isolation method (Kaletsky et al., 2016) on six biological replicates each of Day 1 and Day 8 adult wild-type worms, where 100,000 GFP + cells were collected for each sample (Figure 1—figure supplement 1a–c). Because we previously found that whole-worm analyses mask changes found specifically in neurons (Kaletsky et al., 2016), to complement our aging neuron studies, we also carried out RNA-sequencing analyses of aging whole worms (Figure 1—figure supplement 2a–e), which we found is dominated by changes in the extracellular matrix (Figure 1—figure supplement 2b), stress response/pathogen genes (Figure 1—figure supplement 2c) and the alimentary system (intestine) (Figure 1—figure supplement 2d), overshadowing neuronal changes.
Principal components analysis of the FACS-isolated neuron RNA-seq samples indicated that they are well separated by age (Figure 1b), and downsampling analysis (Robinson and Storey, 2014; Figure 1—figure supplement 1e) suggested that we have sequenced to saturation, with an average of 41,636,463 uniquely-counted reads and detected the expression of 19725 coding and non-coding genes (log10(TPM) >0.5) (Figure 1—figure supplement 1d). Enrichment analysis of genes that are differentially expressed with age (Figure 1c–e) suggested that neuronal sorting and sequencing were successful because the sequenced genes are enriched for neuronal genes such as mec-7, mec-12, and twk-49, as expected, and less enriched for all other major tissues. Tissue enrichment analysis of differentially-expressed genes suggested that aging neurons lose genes most expressed in the nervous system and neurons (Figure 1d), as one might expect. Gene ontology (GO) analysis suggested that genes declining with age in neurons encode proteins important in neuronal function (Figure 1e and f), including synaptic proteins (e.g. srh-59, rab-3, sng-1, sup-1), potassium channels (e.g. egl-23, twk-7, twk-49, ncs-5), and transmembrane transporters (e.g. folt-2, ccb-2, unc-79, exp-1). The decrease in expression of these genes during aging may indicate that neurons are losing their identity and their ability to perform neuronal functions, such as signal transduction and axonal transport, and correlates with the behavioral and morphological declines observed in aging wild-type worms.
Comparing whole-worm sequencing and neuron-specific sequencing (Figure 1f), we found that genes involved in metabolic processes decline with age only in the body, and genes encoding structural proteins, lipid localization, and muscle system processes decline with age in both the body and in neurons, while neurons specifically lose genes that are associated with neuronal function, including synaptic proteins, neuropeptide signaling, and other neuron functions, correlating with neuronal loss of function with age. Together, these results indicate that neurons harbor many unique age-related changes that could be overshadowed in the whole-worm transcriptome but are revealed by neuron-specific sequencing.
Many genes that are more highly expressed in young neurons are known to be specific to a subset of neurons. ins-6, an insulin-like peptide specific to the ASI, ASJ, and AWA neurons (Taylor et al., 2021) that regulates longevity (Artan et al., 2016) and aversive learning (Chen et al., 2013), is significantly downregulated with age (Figure 1g). srd-23, a serpentine receptor located at the AWB neuron cilia (Brear et al., 2014), also decreases expression with age (Figure 1—figure supplement 1f). Furthermore, various genes specific to sensory neurons (txt-12, flp-33), touch neurons (mec-7), and motor neurons (unc-4) decline in expression with age (Figure 1g, Figure 1—figure supplement 1f). Previous studies showed that loss of genes including ins-6 (Chen et al., 2013), mec-7 (Savage et al., 1989), unc-4, folt-2 (Lakhina et al., 2019), madd-4 (Maro et al., 2015), and fbf-1 (Stein and Murphy, 2014) lead to behavioral dysfunction in motility and chemosensory abilities; therefore, the decreased expression of these neuron type-specific genes with age may impact the function of individual neurons and disrupt neural circuit communication, ultimately contributing to the declines in behavior observed during aging (Figure 1g).
As neurons age, genes that increase in expression, while assigned to the nervous system (Figure 2a) are not specific for neuron function; instead, aged wild-type neurons express higher levels of many predicted F-box genes with predicted proteasome E3 activity (e.g. F-box proteins fbxa-158, fbxb-51, pes-2.1, and SKp1-related proteins skr-12, skr-6). Some transcription regulation (e.g. ced-13, tbx-43, nhr-221, end-1), and chromatin structure and function (e.g. his-54, dot-1.2, jmjd-3.2, hil-7, utx-1) genes also increase with age (Figure 2b), even though neurons appear to lose their neuron-specific transcriptional identity with age.
Figure 2. Genes that increase with age cause behavioral defects.
(a) Tissue prediction score for wild-type genes expressed at higher levels in aged worms. (b) Gene ontology (GO) terms of genes expressed higher in aged neurons highlight transcription regulation and proteolysis. GO term analysis was done using Wormcat 2.0. (c) Normalized reads of utx-1 on Day 1 and Day 8. (d) Short-term associative memory (STAM) assay shows that neuron-sensitized adult-only utx-1 knockdown improves 1 hr and 2 hr memory of wild-type worms on Day 2. RNAi was performed using the neuron-RNAi sensitized strain LC108. (e) Normalized reads of ins-19 on Day 1 and Day 8. (f) ins-19 mutation improves learning and memory in STAM on Day 3 of adulthood. (g) Normalized reads of nmgp-1 on Day 1 and Day 8. (h) nmgp-1 neuron-sensitized RNAi knockdown improves memory in STAM on Day 2. RNAi was performed using the neuron-RNAi sensitized strain LC108.P-adj value of normalized count change generated from DEseq2 analysis. (c, e, g) Box plots: center line, median; box range, 25-75th percentiles; whiskers denote minimum-maximum values. Normalized reads and adjusted p-value were calculated using the DESeq2 software. Each dot represents one sequencing replicate. (d, f, h) n=5 plates in each behavioral experiment. Representative result of two biological repeats is shown. *p<0.05. **p<0.01. ***p<0.001. ****p<0.0001. Two-way ANOVA with Tukey’s post-hoc analysis.
One ongoing discussion about changes during aging is how to interpret an increase in expression with age. There are two main models for genes that increase their expression with age and have a resulting impact on function: that they rise with age to compensate for lost function (‘compensatory’) and, therefore, promote function, or that their expression is deleterious to function and only rises with age through dysregulation. If a gene is compensatory, then its knockdown would abrogate learning and memory, even in young animals. If a gene’s function is harmful to neurons, reducing its expression might be beneficial to the worm, even in young animals (Of course, there may be other scenarios in which a gene with multiple functions may be detrimental for some behaviors but beneficial for other physiological roles). To test this hypothesis, we reduced the expression of a small set of highly upregulated candidate genes in categories that might function in a compensatory manner. These include utx-1, a histone demethylase known to play a role in development (Vandamme et al., 2012) and lifespan in worms (Jin et al., 2011; Maures et al., 2011; Guillermo et al., 2021), and whose homolog has been implicated in cognition in mammals (Shaw et al., 2023; Tang et al., 2017); ins-19, an insulin-like peptide; and nmgp-1, a neuronal glycoprotein involved in chemosensation (Fernández et al., 2022). In each case, we see that gene expression is significantly higher in old than in young neurons (Figure 2c, e and g). If a gene increases expression to benefit neurons, we would expect to see no difference in memory in young animals where there is no defect; by contrast, if the increase of a gene is deleterious, we would expect to see an improvement in behavior when knocked down, even in young animals. We performed adult-only neuron-sensitized RNAi knockdown to prevent any possible deleterious effects caused by changes during development, which largely takes place in early larval stages; testing in young adult animals is logical because there is no memory in aged wild-type, so any deleterious effect of knocking down a potentially compensatory gene in an aged would not result in a change. For all behavioral assays, we first prioritized significantly-changed genes with high fold-change, and then those with mammalian homologs.
We found that 48 hr (L4-Day 2) of adult-only knockdown of utx-1 increases 1 hr and 2 hr memory (Figure 2d), the loss-of-function mutation of ins-19 increases both learning and memory (Figure 2f) and the adult-only knock-down of nmgp-1 extends memory at 2 hr (Figure 2h). That is, in each of these cases, reduction of these genes did not impair memory, as loss of a compensatory function would appear; rather, loss of these age-upregulated genes improved wild-type memory. These results indicate that at least some neuronal genes that increase with age can have a negative impact on learning and memory, as demonstrated by the improvement of memory when knocked down, even in young animals. While it is still possible that some upregulated genes may act in a compensatory manner, the simplest model is that at least some are actively deleterious for learning and memory. We previously observed that for genes that play a role in complex behaviors like learning and memory, the loss of single genes can have a large impact on these complex behaviors (Lakhina et al., 2015), unlike the additive roles of longevity-promoting genes (Murphy et al., 2003). Therefore, one mechanism by which wild-type worms lose their learning and memory functions with age is not just by loss of neuronal gene expression, as one might expect, but also by dysregulation of expression of genes that can negatively impact learning and memory.
daf-2 mutants maintain learning and memory with age
We previously found that daf-2 animals have extended motility (Maximum Velocity) that correlates with and predicts their extension of lifespan (Hahm et al., 2015). Additionally, not only do young daf-2 worms have better memory than wild-type worms, but daf-2 mutants also maintain learning and memory better with age (Kauffman et al., 2010; Kaletsky et al., 2016). However, the duration of this improvement was unknown. To determine the proportion of life that worms can learn and remember, we tested wild-type, daf-2, and daf-16;daf-2 worms for their learning and associative memory ability every day until these functions were lost. We found that while wild-type worms lose their learning and short-term memory abilities by Day 7–8 (Figure 1a, Figure 3a and b), learning and memory span were significantly extended in daf-2 mutants (Figure 3a and b); thus, a comparison of daf-2 neurons with wild-type neurons at Day 8 should reveal differences relevant to cognitive aging. The extension of learning and memory is dependent on the FOXO transcription factor DAF-16 (Figure 3a); in fact, while daf-16;daf-2 mutants still have the ability to learn for a few days, these mutants are completely unable to carry out any memory ability, even on Day 1. Thus, learning ability, which is similar in wild-type and daf-2;daf-16 mutants, is mechanistically distinct from short-term memory ability (Stein and Murphy, 2014). daf-2 worms maintained learning ability until Day 19 and short-term (1 hr) memory ability until Day 15, more than twice the duration of wild-type worms, while daf-16;daf-2 worms exhibit no short-term memory ability, even on Day 1 of adulthood (Figure 3b). Our data suggest that the learning span-to-lifespan (Figure 3—figure supplement 1c) and memory span-to-lifespan ratios in daf-2 worms were similar to or slightly higher than that of wild-type worms (Figure 3c and d), indicating that daf-2 mutants maintain cognitive function for at least proportionally as long as wild-type worms do. Thus, daf-2 mutants maintain their higher cognitive quality of life longer than wild-type worms, while daf-16;daf-2 mutants spend their whole lives without memory ability (Figure 3d), in contrast to claims that daf-2 mutants are less healthy than wild-type or daf-16 worms (Bansal et al., 2015). Additionally, it should be noted that because our choice assays distinguish motility function from learning and memory function (Kauffman et al., 2011), the improvements in memory with age shown by daf-2 mutants relative to wild-type are distinct from daf-2’s improvements in motility that we previously showed (Hahm et al., 2015). Therefore, we are interested in these genes that might contribute to the extended cognitive function that daf-2 worms demonstrate.
Figure 3. Identifying neuronal IIS/FOXO targets in aged worms using neuron-specific RNA-sequencing.
(a) daf-2 mutants show better learning maintenance with age compared to N2 and daf-16;daf-2 worms. n=10 plates in each condition. (b) daf-2 mutants show better memory maintenance with age compared to N2 worms. daf-16;daf-2 worms do not have 1 hr memory on Day 1 of adulthood. N=10 plates in each condition. (c–d) daf-2 mutants have a slightly larger learning span/lifespan ratio and memory span/lifespan ratio than N2 (wild-type). Lifespan shown in Figure 3—figure supplement 1c. (e) PCA plot of Day 8 N2, daf-2, and daf-16;daf-2 neuronal RNA sequencing results. (f) Volcano plot of neuronal daf-2-regulated, daf-16-dependent up- and downregulated genes on adult Day 8 (Adjusted p-value < 0.05, log2(Fold-change) >0.5, n=6 biological replicates per strain). 570 genes were significantly upregulated and 814 genes were downregulated in daf-2 neurons compared with daf-16;daf-2. (g) Volcano plot of whole-worm daf-2 vs daf-16;daf-2 differentially-expressed genes during aging. 3154 genes are higher in daf-2, 1289 genes are higher in daf-16;daf-2 (log2[Fold-change(daf-2 vs daf-16;daf-2)]>1.5, p-adjusted <0.01). (h) Comparison of neuronal and whole-worm Day 8 daf-2 differentially-expressed genes (overlap p=3.34E-63, hypergeometric test). Neuron-specific and shared daf-2 upregulated genes with the highest fold changes are labeled.
Figure 3—figure supplement 1. Neuron-specific sequencing of Day 8 daf-2 and daf-16;daf-2 mutants.
Figure 3—figure supplement 2. Whole-worm RNA-sequencing identifies changes in aged daf-2 mutants.
Figure 3—figure supplement 3. DAF-16-dependent and -independent daf-2-regulated genes show different features.
Aging IIS/FOXO neurons express stress-resistance genes to maintain neuronal function with age
To identify genes that may improve memory and slow cognitive aging in long-lived daf-2 mutants, we compared the transcriptional profiles of Day 8 FACS-isolated neurons from daf-2 animals with Day 8 FACS-isolated wild-type and daf-16;daf-2 neurons; by Day 8, wild-type and daf-16;daf-2 worms have already lost their learning and memory ability, but daf-2 worms still maintain their cognitive functions (Figure 3a and b). It should be noted that wild-type worms still have normal chemotaxis and motility at Day 8 (Kauffman et al., 2010), and there is a separation of several days between the loss of cognitive functions and the loss of motility (Kauffman et al., 2010); therefore, comparison of the neuronal transcriptomes of daf-2 with wild-type and daf-16;daf-2 at this age should specifically highlight genes that are required for learning and memory rather than other functions.
The PCA of the daf-2, daf-16;daf-2, and wild-type neuronal Day 8 transcriptomes (Figure 3e) indicates that aged daf-16;daf-2 mutant neurons are similar to aged wild-type neurons, correlating well with their similarly worsened cognitive functions at this age; that is, at a transcriptomic level, aged (Day 8) wild-type neurons and aged daf-16;daf-2 neurons are similar, which is echoed by their shared inability to carry out learning and memory by Day 8 of adulthood (Figure 3ea, b). By contrast, the transcriptomes of aged daf-2 mutant neurons are distinct from both aged wild-type and aged daf-16;daf-2 neuron transcriptomes, just as the cognitive abilities of daf-2 are much greater than wild-type or daf-16;daf-2 at this age. Downsampling analysis shows that our sequencing depth is sufficient to saturate the detectable differential expression (Figure 3—figure supplement 1g, h). We obtained an average of 47,233,119 counted reads per sample (Supplementary file 5) and detected the expression of 16,488 coding and non-coding genes (Figure 3—figure supplement 1e).
We identified 570 upregulated and 814 downregulated genes in Day 8 daf-2 neurons compared to Day 8 daf-16;daf-2 neurons (Figure 3f). A large fraction of the downregulated genes in Day 8 daf-2 vs daf-16;daf-2 neurons are ‘nematode-specific peptide family’ (nspc-) genes of unknown function (Figure 3f). While the daf-2 vs daf-16;daf-2 changes in whole worms largely replicated the results from our previous studies of young animals (Murphy et al., 2003; Tepper et al., 2013; Figure 3g, Supplementary file 6g), comparison of the daf-2 vs daf-16;daf-2 differential transcriptional changes in Day 8 whole worms and Day 8 neurons reveal shared (174 genes) and neuron-specific gene expression changes (396 genes; dod-24, srh-2, lin-42, etc.) (Figure 3h, Figure 3—figure supplement 2c). Not surprisingly, previously identified genes from whole-worm daf-2 vs daf-16;daf-2 and N2 (e.g. sod-3, mtl-1, cpi-1, hsp-12.6, etc.) that play roles in both neurons and other tissues even in Day 1 daf-2 mutants appear in the shared list (Some neuron-specific Day 8 daf-2-upregulated genes have not been reported to be expressed in neurons previously (e.g. spin-2), further suggesting the value of transcriptomic analyses of isolated neurons in mutant backgrounds at this age).
Many genes upregulated in Day 8 daf-2 neurons relative to daf-16;daf-2 are related to stress responses, including heat stress (e.g. hsp-12.6, hsp-12.3, F08H9.4/hsp), oxidative stress (e.g. sod-3), and metal stress genes (e.g. mtl-1); and proteolysis (e.g. cpi-1, cpr-2, and tep-1). The upregulation of these genes may perform neuroprotective functions, as their homologs in mammals have been shown to do (Table 1). Specifically, 36 of the top 100 upregulated genes have identified orthologs or identified domains with known functions, of which 32 of (89%) have functions in promoting neuronal health. These mammalian homologs protect neurons against protein aggregation and harmful metabolites (e.g. cpi-1, alh-2, ttr-41, gpx-5) (Gauthier et al., 2011; Carmichael et al., 2021; Li et al., 2011; Lee et al., 2020; Hambright et al., 2017), maintain synaptic organization and neuronal homeostasis (e.g. dod-24, ptr-19, plep-1) (González-Calvo et al., 2022; Ung et al., 2018; Perland et al., 2016), facilitate neuronal injury repair (e.g. F08H9.4, sod-3) (Huang et al., 2023; Flynn and Melov, 2013), and maintain normal neuronal function (e.g. lgc-28, slc-36.3, lin-42) (Koukouli and Changeux, 2020; Zeiger et al., 2008; Lautrup et al., 2019; Smies et al., 2022). Together, these genes may help maintain daf-2’s neuronal health and protect neurons from accumulation of environmental harm during aging.
Table 1. List of top daf-2 vs daf-16;daf-2 upregulated genes with orthologs that have neuroprotective functions.
| Gene name | Full name | log2(FC) | p-adj | Mammalian ortholog | Ortholog full name | Inferred function |
|---|---|---|---|---|---|---|
| Neuroprotective against Neurodegenerative Diseases | ||||||
| cpi-1 | Cysteine Protease Inhibitor 1 | 2.07 | 7.80E-19 | CST3 | Cystatin C | Protease inhibitor, suppresses AD pathology Gauthier et al., 2011 |
| alh-2 | ALdehyde deHydrogenase 2 | 1.83 | 2.65E-05 | ALDH1A1 | Aldehyde dehydrogenase 1 | Expressed in dopaminergic neurons. Regulates dopamine release in Parkinson’s Disease Carmichael et al., 2021 |
| ttr-41,45,2 | TransThyretin-Related family domain 41,45,2 | 1.68 | 3.98E-06 | Inhibits Aβ fibril formation, and suppresses the AD pathology Li et al., 2011 | ||
| cyp-33B1 | CYtochrome P450 family 33B1 | 1.34 | 2.04E-03 | CYP2J2 | Cytochrome P450 2J2 | Protective against Parkinson’s Disease through altered metabolism Li et al., 2018; Ferguson and Tyndale, 2011 |
| spin-2 | SPINster (Dm lysosomal permease) homolog 2 | 1.27 | 6.20E-04 | SPNS2 | Spinster homolog 2 | Sphingosine-1-phosphate Transporter, neuroprotective in AD Zhong et al., 2019 |
| gpx-5 | Glutathione PeroXidase 5 | 1.27 | 3.99E-04 | GPX3,5,6 | glutathione peroxidase 3,5,6 | Protects again lipid peroxidation, protects against neurodegeneration Lee et al., 2020; Hambright et al., 2017 |
| cpr-2 | Cysteine PRotease related 2 | 1.25 | 5.01E-03 | CTSB | Cathepsin B | Lysosomal Protease, Involved in Aβ and APP protein degradation Cermak et al., 2016 |
| djr-1.2 | DJ-1 (mammalian transcript’l regulator) Related 1.2 | 1.09 | 3.90E-03 | PARK7 | Parkinsonism associated deglycase | Neuroprotective against Parkinson’s Disease; Prevents accumulation of harmful metabolites Heremans et al., 2022 |
| Synaptic Organization Maintenance | ||||||
| dod-24 | Downstream Of DAF-16 (regulated by DAF-16) 24 | 1.93 | 1.39E-07 | Cub-like Domain Containing Protein | Clustering of neurotransmitter receptor proteins González-Calvo et al., 2022 | |
| ptr-19,15 | PaTched Related family 19,15 | 1.21 | 1.72E-05 | PTCHD1,3,4 | Patched domain-containing 1,3,4 | Synaptic organization, autism risk factor Ung et al., 2018; Pastore et al., 2022 |
| hbl-1 | HunchBack Like (fly gap gene-related) 1 | 1.16 | 6.47E-06 | hb | Hunchback (fly) | Regulate synapse number and locomotor circuit function Lee et al., 2022 |
| cutl-4 | CUTiclin-Like 4 | 1.08 | 2.74E-02 | pio | Piopio (fly) | ECM protein for axonal growth and synapse formation Broadie et al., 2011 |
| lron-2 | eLRR (extracellular Leucine-Rich Repeat) ONly 2 | 1.06 | 8.70E-05 | LGI1,2 | Leucine-Rich Glioma Inactivated protein 1 | Modulation of trans-synaptic proteins. Protection against seizure Fels et al., 2021 |
| Neuronal Homeostasis Maintenance | ||||||
| mocs-1 | MOlybdenum Cofactor Sulfurase 1 | 1.05 | 1.17E-04 | MOCOS | Molybdenum cofactor sulfurase | Regulation of redox homeostasis and synaptogenesis. Down in ASD Rontani et al., 2021 |
| plep-1 | PLugged Excretory Pore 1 | 1.12 | 2.92E-03 | MFSD11 | Major facilitator superfamily domain 11 | Putative SLC solute carrier protein, involved in brain energy homeostasis Perland et al., 2016 |
| cky-1 | CKY homolog 1 | 1.08 | 1.58E-04 | NPAS4 | Neuronal PAS Domain Protein 4 | Calcium-dependent transcription factor, neuronal homeostasis maintenance Fu et al., 2020; Shan et al., 2018 |
| Neuronal Injury Repair facilitation | ||||||
| F08H9.4, hsp-12.3,12.6 | small HSP domain-containing protein | 1.94 | 7.33E-06 | HSPB2 | Heat-shock Protein Beta 2 | Facilitates PNS injury regeneration, suppresses inflammation Huang et al., 2023 |
| sod-3 | SOD superoxide dismutase 3 | 1.66 | 3.05E-09 | SOD2 | superoxide dismutase2 | Converts superoxide to the less reactive hydrogen peroxide (H2O2). Protects neurons from injury. Flynn and Melov, 2013 |
| Normal Neuronal Activity Maintenance | ||||||
| lgc-28 | Ligand-Gated ion Channel 28 | 1.38 | 7.29E-04 | CHRNA6,3 | Neuronal acetylcholine receptor subunit alpha-6,3 | Nicotinic receptor. Regulates cognitive functions and addiction Koukouli and Changeux, 2020; Zeiger et al., 2008 |
| F22B7.9 | 1.33 | 8.91E-15 | METTL23 | methyltransferase like 23 | Interacts with GABPA; disruption causes intellectual disability Bernkopf et al., 2014; Reiff et al., 2014 | |
| fat-5 | FATty acid desaturase 5 | 1.31 | 3.40E-03 | SCD5 | StearoylCoA Desaturase-5 | Neuronal Cell Proliferation and Differentiation Sinner et al., 2012 |
| slc-36.3 | SLC (SoLute Carrier) homolog 36.3 | 1.25 | 2.88E-03 | SLC36A4 | Solute Carrier Family36 Member4 | amino acid transporter, transports Trp, involved in kynurenic acid pathway Lautrup et al., 2019 |
| lin-42 | abnormal cell LINeage 42 | 1.15 | 1.66E-04 | PER1,2 | Period 1,2 | Phosphorylates CREB, modulates CREB-mediated memory consolidation Smies et al., 2022 |
| ctsa-1.1 | CaThepSin A homolog 1.1 | 1.07 | 4.97E-05 | CTSA | Lysosomal Ser carboxy-peptidase Cathepsin A | Involved in normal neuronal development De Pasquale et al., 2020; Hsu et al., 2018; |
| gsnl-1 | GelSoliN-Like 1 | 1.06 | 2.93E-04 | AVIL | advillin | Facilitates somatosensory neuron axon regeneration Chuang et al., 2018 |
We found that about a third of the daf-2-upregulated genes were shared between the daf-2 vs daf-16;daf-2 analysis and the daf-2 vs N2 analysis (338 genes) (Figure 3—figure supplement 3). Of the unshared genes, the daf-2-maintained genes that are specific to the daf-2 vs N2 comparison are bZIP transcription factors, including zip-5, zip-4, atf-2, and proteasome components (Figure 3—figure supplement 3D). These results indicate that other transcription factors may participate in regulating daf-2 functions in aged neurons in addition to the daf-16/FOXO transcription factor.
IIS/FOXO transcriptomic changes are necessary for daf-2 mutant’s improved neuronal functions
We were interested not only in the genes that remained upregulated with age, but also in genes that might have increased with age in the high-performing daf-2 mutants. That is, are there genes that increase in expression in daf-2 mutants that are necessary or beneficial for their continued high performance with age? Some of the Day 8 daf-2 vs wild-type or daf-16;daf-2 upregulated genes are also Class 1 DAF-16-dependent genes (Murphy et al., 2003) (sod-3, hsp-12.3, fat-5, and mtl-1, hil-1, and dao-2). However, many more genes were differentially expressed in Day 8 daf-2 vs daf-16;daf-2 neurons from our Day 1 data (Kaletsky et al., 2016; Figure 4a, Figure 3—figure supplement 2d). Of the ‘new’ genes – that is, genes upregulated specifically in neurons of Day 8 vs Day 1 of daf-2 vs daf-16;daf-2 – many have mammalian homologs that have been shown to play neuroprotective roles, by protecting against aggregation proteins and harmful metabolites, maintaining synaptic organization, neuronal homoeostasis, or neuronal activity, or facilitating neuronal injury repair (see Table 1 for specific references).
Figure 4. Neuronal IIS/FOXO aging targets regulate memory decline with age in daf-2 worms.
(a) Comparison of neuronal Day 1 and Day 8 daf-2 vs daf-16;daf-2 upregulated genes. All shared genes and top Day 8-specific daf-2 upregulated genes are labeled. (b) daf-2-regulated fold-change profile of candidate genes. All candidates are upregulated in daf-2 mutants. (c) Description of candidate genes. log2(Fold-change) and p-adjusted values from the daf-2 vs daf-16;daf-2 comparison unless stated otherwise. (d) Candidate gene knockdown effects on Day 6 adult daf-2 learning (0 hr after conditioning). Two candidate genes, dod-24 and F08H9.4, show a significant decrease in learning ability. N=5 plates in each condition, merged results of 3 biological repeats shown. (e) Candidate gene knockdown effects on Day 6 adult daf-2 short-term memory (1 hr after conditioning). C44B7.5, dod-24, F08H9.4, mtl-1, and alh-2 showed significant decreases in memory. n=5 plates in each condition, the representative image of three biological repeats shown. (d-e) RNAi was performed using a neuron-sensitized RNAi strain CQ745: daf-2(e1370) III; vIs69 [pCFJ90(Pmyo-2::mCherry +Punc-119::sid-1)] V.*p<0.05. **p<0.01. ***p<0.001. ****p<0.0001. One-way ANOVA with Dunnet’s post-hoc analysis. Box plots: center line, median; box range, 25-75th percentiles; whiskers denote minimum-maximum values.
If the upregulated genes in aged daf-2 neurons are responsible for the extended memory span of daf-2 mutants, knocking down those genes should block older daf-2 mutants’ memory functions. Therefore, we tested the effect of RNAi knockdown of the top fold-change candidate genes on daf-2’s memory in aged adults. We chose Day 6 for testing because by then, like on Day 8, wild-type worms have already lost their learning and most memory abilities, but daf-2 worms retain normal cognitive functions, and this time point avoids the increased naïve chemotaxis that we observe in older daf-2 animals. As shown in Figure 4b–c, we selected these significantly differentially expressed candidate genes based on their ranking in fold-change. Previously, we have found that the top significantly differentially-expressed genes (by fold-change) are most likely to have strong effects on function, while less differentially-changed genes have less of an effect (Murphy et al., 2003; Kaletsky et al., 2016; Lakhina et al., 2015), therefore, we prioritized genes that are significantly different and the most highly expressed in daf-2 mutants compared to daf-16;daf-2 mutants for subsequent testing (Figure 4c). daf-2 worms, including neurons, are susceptible to RNA interference (Kaletsky et al., 2016; Wang, 2004). Of the eight candidate genes we tested, the reduction of three of them (F08H9.4, mtl-1, and dod-24, originally classified as a Class II gene with proposed immune activity) significantly decreased daf-2’s learning ability on Day 6 (Figure 4d). Those genes plus reduction of two additional genes (C44B7.5 and alh-2) affected 1 hr memory (Figure 4e) in Day 6 daf-2 mutants. That is, knockdown of the heat shock-related gene F08H9.4, the innate immunity gene dod-24, aldehyde dehydrogenase alh-2, and previously uncharacterized gene C44B7.5 are required to some degree for daf-2’s extended memory ability. The reduction of the metal stress gene mtl-1, which is expressed in neurons as well as the rest of the body, had a slight effect on learning and memory.
One caveat of these experiments is that, while we found these genes through the isolation of neurons from aged worms and subsequent RNA-sequencing, the knockdown of the genes and its effects are not necessarily neuron-autonomous; however, alh-2 and F08H9.4 were reported to only be expressed in neurons and the cephalic sheath cell (Kaletsky et al., 2018), and C44B7.5 and dod-24, while expressed more broadly, were not upregulated in daf-2 in the whole-worm analysis (Figure 3f), therefore, their effects are most likely neuron-autonomous. In fact, dod-24 is one of the original Class 2 daf-2-downregulated genes from whole-animal analyses, suggesting that dod-24’s increase in expression is specifically in neurons, therefore, the effect of its knockdown is most likely to be neuron-autonomous.
Together, these data suggest that the specific genes that are differentially regulated in Day 8 daf-2 mutants may aid in slowing neuronal function decline and behavioral changes associated with aging. Furthermore, memory maintenance with age might require additional genes that function in promoting stress resistance and neuronal resilience, which were not previously uncovered in analyses of young animals.
Discussion
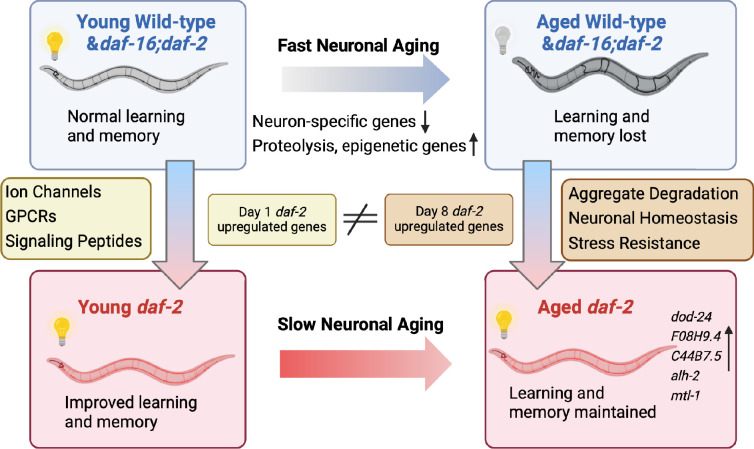
Although it has been shown previously that daf-2 worms maintain various functions with age, how long they can maintain learning and memory with age, and the genes that might be responsible for these extended neuronal functions, have not been previously explored. Here, we have found that daf-2 worms maintain learning and memory abilities proportional with (or even slightly beyond) their degree of lifespan extension, underscoring daf-2’s improved healthspan (Hahm et al., 2015). To understand how memory is lost with age and retained in insulin/IGF-1-like signaling mutants, we have characterized the neuronal transcriptomes of aged wild-type worms and IIS (daf-2) and IIS/FOXO (daf-16;daf-2) mutants (Figure 5). We found that wild-type neuronal aging is characterized by a downregulation of neuronal function genes and an upregulation of proteolysis genes and transcriptional and epigenetic regulators, which together may help explain the loss of neuronal identity and function with age. We also identified the transcriptomic profile accompanying daf-2’s extended learning and memory span. Specifically, daf-2 neurons maintain higher expression of stress response genes and predicted neuronal homeostasis functions (Table 1), which may help make them more resistant to environmental adversities and age-related decline. We also identified genes responsible for wild-type worms’ worsened learning and memory with age.
Figure 5. Aged daf-2 neurons upregulate neuroprotective genes to maintain improved cognitive behaviors.
During normal neuronal aging, neuron-specific genes decrease in expression, while proteolysis and epigenetic regulators are upregulated, resulting in neuron dysfunction and cognitive function loss. In aged daf-2 mutants, upregulation of neuroprotective genes including dod-24, F08H9.4, C44B7.5, alh-2, and mtl-1 contribute to daf-2’s improved cognitive function. The diagram was generated using Biorender, and published using a CC BY-NC-ND license with permission.
© 2024, BioRender Inc
Figure 5 was created using BioRender, and is published under a CC BY-NC-ND 4.0. Further reproductions must adhere to the terms of this license.
Figure 5—figure supplement 1. Comparison with recent sequencing datasets.
By employing a FACS-based neuron-sorting technique, we selectively analyzed adult neuron-function-related genes and investigated their aging process, which is not easily discernible through whole-worm sequencing (Gao et al., 2023; Wang et al., 2022; Roux et al., 2023; Figure 5—figure supplement 1). Sequencing many biological repeats of aging neurons to high depth with ribosomal RNA depletion allowed us to detect a larger number of genes compared to other neuron-related bulk and single-cell sequencing profiles (Wang et al., 2022), providing a deep transcriptomic dataset of aged wild-type, IIS mutant, and IIS/FOXO mutant neurons. Our analysis allowed us to identify differentially expressed genes that are known to be expressed in at a small number of neurons, even for low-abundance genes. Notably, our sequencing results uncovered genes previously not known to be expressed in neurons that remained undetected in other datasets. Moreover, we revealed the involvement of known neuronal genes in the aging process, such as ins-6 and srd-23. We hope that this dataset will become a valuable resource for detecting new candidates in neuronal aging.
For example, dod-24, which we observed to be upregulated in daf-2 neurons and required for daf-2’s extended memory, was downregulated in the daf-2 whole-worm transcriptome (Figure 3—figure supplement 2c). dod-24 has been traditionally classified as a Class II gene that is downregulated in daf-2 worms and upregulated by daf-16 RNAi treatment (Murphy et al., 2003; Tepper et al., 2013). Functionally, it has been shown to be an innate immunity gene upregulated during pathogen infection (Shapira et al., 2006; Eckl et al., 2017; Mack et al., 2022), and its whole-body reduction has been shown to extend the lifespan of wild-type animals (Murphy et al., 2003). However, here we find that dod-24 is beneficial in the nervous system and required for daf-2’s extended learning and memory in aged worms. This intriguing contrast between the whole-worm transcriptome and the neuron-specific transcriptome suggests that some genes may have distinct regulatory roles in the nervous system, necessitating a more precise approach beyond whole-worm transcriptomics.
Using this neuron-specific sequencing profile of aged cells, we identified key pathways that change during neuron aging. Our sequencing of aged neurons uncovered active transcriptomic alterations during aging, resulting in not just transcriptional silencing but also upregulation of various pathways. We found that the Day 8 daf-2 vs daf-16;daf-2 neuronal differentially-expressed genes that we have newly discovered here differ from the neuronal Day 1 daf-2 vs daf-16;daf-2 dataset we previously obtained (Kaletsky et al., 2016). These Day 8 differentially-expressed genes are not canonical neuronal genes, such as receptors and ion channels; instead, there are more metabolic and proteolytic genes whose protein orthologs have been shown to be neuroprotective. All top 50 genes and 90% of the top 100 genes with identified mammalian orthologs have been shown to be essential to neuronal functions in mammals (Table 1). These results indicate that instead of simply mimicking a young state, daf-2 may enhance neuron’s resilience to the accumulation of harm and take a more active approach to combat aging. These changes suggest that daf-2’s extended memory maintenance may require different mechanisms than function in young animals; daf-2 may maintain neuronal function not just by retaining a youthful transcriptome, but also by increasing the expression of genes that promote resilience, such as stress-response genes and proteolysis inhibitors.
In addition to examining aging in wild-type and IIS/FOXO mutants independently, our results further linked the normal aging process to altered gene regulation in the IIS pathway. utx-1, nmgp-1, and ins-19 increase in expression in aged neurons, and we found that their reduction improved memory, indicating that at least some of the genes whose expression rises with age can have a negative impact on normal cognitive functions, rather than acting in a compensatory manner. utx-1 is an H3K27me3 histone demethylase we found to be higher in wild-type aged neurons, but it is also involved in the IIS pathway. The downregulation of utx-1 has been shown to regulate development (Vandamme et al., 2012) and promote longevity (Jin et al., 2011; Maures et al., 2011; Guillermo et al., 2021), and its mammalian homolog has been implicated in regulating cognitive abilities (Shaw et al., 2023; Tang et al., 2017). The longevity response of utx-1 depends on daf-16 (Jin et al., 2011; Maures et al., 2011; Guillermo et al., 2021). The loss of utx-1 decreases methylation on the daf-2 gene, thus increasing DAF-16’s nuclear localization, mimicking a daf-2 mutation (Jin et al., 2011). This example of the crosstalk between normal aging and IIS/FOXO mutants offers valuable insights into modifying the aging process for enhanced longevity and cognitive health.
We found that the insulin-like peptide ins-19 was upregulated in aged neurons and was downregulated in aged daf-2 neurons, and its downregulation in wild-type worms extended memory span. Insulin-like peptides play crucial roles as receptor ligands (in both agonist and antagonist roles) for DAF-2, and we have found them to be downregulated in daf-2 mutants compared with daf-16;daf-2 mutants, possibly creating a feedback loop that dampens the insulin signaling pathway, as was previously shown for ins-7 and ins-18 (Murphy et al., 2003; Murphy et al., 2007). These peptides exhibit diverse functions in development, dauer formation, and longevity (Murphy et al., 2003; Murphy et al., 2007; Thomé-Duret et al., 1998; Kawano et al., 2000; Pierce et al., 2001; Li et al., 2003). Notably, certain insulin-like peptides have been linked to neuronal activities, such as the regulation of aversive learning by the two antagonistic peptides ins-6 and ins-7 (Chen et al., 2013), and reduced long-term learning and memory by ins-22 RNAi (Lakhina et al., 2015). In our study, the expression changes of ins-19 during wild-type aging and in daf-2 mutants provide an example of how longevity mutants can reverse wild-type transcriptional changes during aging, ultimately reducing behavioral and functional decline.
Summary of mechanistic insights
Our analysis of transcriptomes from isolated aged Day 8 neurons of wild-type, daf-2, and daf-16;daf-16 mutants reveal several major mechanistic insights. Specifically, we found that wild-type neurons lose their neuronal identity through a combination of the loss of neuron-specific function genes with age, and the concomitant dysregulated increase in non-neuronal genes with age. Furthermore, at least a fraction of the top-upregulated genes with age can play deleterious roles; that is, they rise with age, and their knockdown improves function, even in young animals. This argues against the idea that all of these genes play a compensatory role with age.
We also found that the knockdown of individual top-ranked genes that function in learning and memory can have a large impact - like removing a cog of a machine. This is in contrast to our earlier findings regarding gene reduction in lifespan, where most cellular longevity processes regulated by DAF-16 activity appear to be additive, and therefore loss of individual major genes downstream of DAF-2 and DAF-16 have at most a 5–10% impact (White et al., 1986; Murphy et al., 2003). Several of these genes we found to be required for daf-2’s age-related improvement in learning and memory - namely dod-24, F08H9.4, C44B7.5, and alh-2 – were previously not associated with memory function. Finally, these genes are distinct from the set of upregulated Day 1 daf-2 vs daf-16;daf-2 genes; how they each individually maintain neuronal function better with age will be interesting to dissect.
Conclusions
Beyond our sequencing analysis, we have established links between genomics, function, and behavior. We also identified several new genes required for daf-2’s age-related improvement in learning and memory, shedding light on their neuron-specific roles. These additional findings further suggest that neuronal sequencing datasets can be used to identify functional candidates and pathways during the aging process. By bridging the gap between transcriptomic landscapes, genetic regulation, and functional outcomes, our study provides a greater understanding of the mechanisms underlying neuronal aging, providing insights into the development of aging interventions.
Methods
Strains and worm cultivation
N2 (wild-type), OH441: otIs45(unc-119::GFP), CQ295: otIs45(unc-119::GFP);daf-2(e1370), CQ296: otIs45(unc-119::GFP);daf-16(mu86);daf-2(e1370), LC108: uIs69 (myo-2p::mCherry +unc-119p::sid-1), CQ705: daf-2(e1370) III, 3 X outcrossed, CQ745: daf-2(e1370) III; vIs69 [pCFJ90(Pmyo-2::mCherry +Punc-119::sid-1)] V, QL188: ins-19(tm5155) II, CX3695: kyIs140(str-2::GFP +lin-15(+)), CQ461: (daf-2(e1370);Pmec-4::mCherry), and CQ501: (daf-2 (e1370);zip-5(gk646);Pmec-4::mCherry). Strains were grown on high-growth media (HGM) plates seeded with E. coli OP50 bacteria using standard methods Brenner, 1974.
Tissue-specific isolation
For neuronal isolation, five plates of fully-grown worms from HG plates were synchronized by hypochlorite treatment, eggs spread on seeded plates to hatch, and at least five plates/replicate were grown to L4 on HGM plates until transferred to HGM plates with FUdR to avoid progeny contamination. This gives us ~6000 healthy Day 8 worms to sort. Neuron isolation and Fluorescent-activated cell sorting were carried out as previously described (Kaletsky et al., 2016; Kaletsky et al., 2018). Briefly, worms were treated with 1000 uL lysis buffer (200 mM DTT, 0.25% SDS, 20 mM HEPES pH 8.0, 3% sucrose) for 6.5 min to break the cuticle. Then worms were washed and resuspended in 500 uL 20 mg/mL pronase from Streptomyces griseus (Sigma-Aldrich). Worms were incubated at room temperature with mechanical disruption by pipetting until no whole-worm bodies were seen, and then ice-cold osmolarity-adjusted L-15 buffer(Gibco) with 2% Fetal Bovine Serum (Gibco) were added to stop the reaction. Prior to sorting, cell suspensions were filtered using a 5 um filter and sorted using a FACSVantage SE w/ DiVa (BD Biosciences; 488 nm excitation, 530/30 nm bandpass filter for GFP detection). Sorting gates were determined by comparing with age-matched, genotype-matched non-fluorescent cell suspension samples. Fluorescent neuron cells were directly sorted into Trizol LS. 100,000 GFP + cells were collected for each sample.
RNA extraction, library generation, and sequencing
We used the standard trizol-chloroform-isopropanol method to extract RNA, then performed RNA cleanup using RNeasy MinElute Cleanup Kit (Qiagen). RNA quality was assessed using the Agilent Bioanalyzer RNA Pico chip, and bioanalyzer RIN >6.0 samples were observed before library generation. 2 ng of RNA was used for library generation using Ovation SoLo RNA-Seq library preparation kit with AnyDeplete Probe Mix- C. elegans (Tecan Genomics) according to the manufacturer’s instructions (Barrett et al., 2021). Library quality and concentration was assessed using an Agilent Bioanalyzer DNA 12000 chip. Samples were multiplexed and sequencing were performed using NovaSeq S1 100nt Flowcell v1.5 (Illumina).
Data processing
FastQC was performed on each sample for quality control analysis. RNA STAR package was used for mapping paired-end reads to the C. elegans genome ce11 (UCSC Feb 2013) using the gene model ws245genes.gtf. Length of the genomic sequence around annotated junctions is chosen as read length –1. 50–70% of reads were uniquely mapped. Reads uniquely mapped to the genome were then counted using htseq-count (mode = union). DESeq2 analysis was then used for read normalization and differential expression analysis on counted reads (Love et al., 2014). Genes with a log10TPM >0.5 were considered as detected and genes with a log2(fold-change) >0.5 and p-adjusted <0.05 are considered differentially expressed in further analysis. Gene ontology analysis were performed using gprofiler (Raudvere et al., 2019) or WormCat 2.0 (Holdorf et al., 2020) and category 2 was selected to show. Tissue query was performed on the top 500 highest fold-change genes, using the worm tissue query website (https://www.worm.princeton.edu; Kaletsky et al., 2018), and only major systems were selected in the analysis.
Learning and memory experiments
We performed Short-Term Associative Memory (STAM) experiments as previously described (Kauffman et al., 2010; Kauffman et al., 2011). Briefly, we used five plates of synchronized adult worms/samples to perform each experiment. One plate of worms was used to test the naïve chemotaxis assay without conditioning, while the other three plates were washed into M9, and washed three additional times to get rid of the bacteria. These worms are starved for 1 hr to prime them for food uptake. Then these worms are transferred to conditioning plates with NGM plates seeded with OP50 and 10% butanone stripes on the lid for 1 hr to perform conditioning. After conditioning, worms are either transferred from the conditioning plate directly to the chemotaxis plates to assess learning, or transferred to the holding plate for 1 hr or 2 hr to assess for memory. After staying on holding plates for 1 hr or 2 hr, worms are then transferred onto chemotaxis plates to assess for short-term memory. Chemotaxis assays were performed by transferring worms onto chemotaxis plates with 1 uL 10% butanone and 1 uL ethanol spots separated by 8 cm on a 10 cm NGM plate. Worms who have reached either the butanone spot or the ethanol spot are paralyzed by the 1 uL 7.5% NaN3 on these spots. For each timepoint, five chemotaxis plates are used to minimize the variation of the outcome. We performed this chemotaxis assay to butanone on naïve and appetitive-trained worms at different time points to assess change in preference to butanone.
Chemotaxis index is calculated as (# of worms at butanone-# of worms at ethanol)/(total # of worms - # of worms at origin).
Learning index is calculated by subtracting the trained chemotaxis index with naïve chemotaxis index.
For learning and memory span assays, we obtained synchronized worms from hypochlorite-treated eggs. Synchronized worms were washed onto 5’-fluorodeoxyuridine (FUdR) at L4 and maintained on FUdR plates by transferring to new plates every 2 days. 1 Day Prior to experiments, worms are washed onto fresh HG plates without FUdR to avoid change in behavior caused by FUdR. To verify that FUdR has no effect on short-term memory, we compared worms with and without FUdR (Figure 5—figure supplement 1d), and found no differences. For utx-1 and nmgp-1 RNAi experiments, synchronized L4 neuron-RNAi sensitized worms were washed onto HGM plates with carbenicillin and IPTG and seeded with HT115 RNAi bacteria containing the RNAi constructs from the Ahringer Library. For daf-2 upregulated candidates’ RNAi experiments, synchronized L4 daf-2 neuron-RNAi-sensitized worms were washed onto HGM plates added with carbenicillin, FUdR, and isopropyl-b-D-thiogalactopyranoside (IPTG) and seeded with HT115 bacteria containing RNAi constructs generated from the Ahringer RNAi Library, then were transferred onto fresh RNAi plates every 2 days until Day 6. 1 Day Prior to experiments, worms are transferred onto plates without FUdR.
Quantitative and statistical analysis
All experimental analysis was performed using Prism 8 software. Two-way ANOVA with Tukey post-hoc tests were used to compare the learning curve between control and experimental groups. One-way ANOVA followed by Dunnet post-hoc tests for multiple comparisons was performed to compare learning or 2 hr memory between various treatment groups and control. Chi-square test was performed to compare the neuron morphology change between young and aged AWC neurons. All GO term analyses were performed using Wormcat 2.0 software with Bonferroni corrected adjusted p-values. Venn diagram overlaps were compared using the hypergeometric test. Differential expression analysis of RNA-seq were performed using DESeq2 algorithm and adjusted p-values were generated with Wald test using Benjamini and Hochberg method (BH-adjusted p-values). Additional statistical details of experiments, including sample size (with n representing the number of chemotaxis assays performed for behavior, RNA collections for RNA-seq, and the number of worms for microscopy), can be found in the methods and figure legends. Regression analyses were performed using sklearn packages. Correlations were calculated using the SciPy packages.
Materials availability
Further information and requests for resources and reagents should be directed to and will be fulfilled by Coleen T. Murphy (ctmurphy@princeton.edu).
Acknowledgements
We thank the Caenorhabditis Genetics Center (CGC) for strains, WormBase (version WS289) for information, Jasmine Ashraf, William Keyes, Yichen Weng, and Titas Sengupta for help with the experiments, R Arey and other Murphy lab members for an early replicate of daf-2 learning and memory with age, members of the Murphy Lab for input on the manuscript, Christina DeCoste, Katherine Rittenbach and the Flow Cytometry Facility for cell sorting assistance, Wei Wang and the Genomics Facility for sequencing assistance, Lance Parsons, Bruce Wang, and Chen Dan for insights on data analysis, and Biorender.com for schematic design. CTM. is the Director of the Simons Collaboration on Plasticity in the Aging Brain (SCPAB), which supported the work, and the Glenn Center for Aging Research at Princeton. YW and SZ are supported by the China Scholarship Council (CSC). KM is supported by the HHMI Gilliam Fellows Program.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Contributor Information
Coleen T Murphy, Email: ctmurphy@princeton.edu.
Scott F Leiser, University of Michigan-Ann Arbor, United States.
Pankaj Kapahi, Buck Institute for Research on Aging, United States.
Funding Information
This paper was supported by the following grants:
Simons Foundation Simons Collaboration on Plasticity and the Aging Brain to Coleen T Murphy.
Howard Hughes Medical Institute Gilliam Fellows Program to Katherine Morillo.
China Scholarship Council to Yifei Weng, Shiyi Zhou.
Additional information
Competing interests
No competing interests declared.
Author contributions
Conceptualization, Formal analysis, Investigation, Visualization, Methodology, Writing - original draft, Writing - review and editing.
Conceptualization, Formal analysis, Investigation, Visualization, Methodology.
Investigation.
Conceptualization, Methodology.
Formal analysis.
Conceptualization, Supervision, Funding acquisition, Writing - original draft, Writing - review and editing.
Additional files
Data availability
Sequencing reads are deposited at NCBI BioProject under accession number PRJNA999305.
The following dataset was generated:
Weng Y, Zhou S, Murphy CT. 2024. Analysis of the Neuron-specific IIS/FOXO Transcriptome in Aged Animals Reveals Regulators of Neuronal and Cognitive Aging. NCBI BioProject. PRJNA999305
References
- Arey RN, Murphy CT. Conserved regulators of cognitive aging: from worms to humans. Behavioural Brain Research. 2017;322:299–310. doi: 10.1016/j.bbr.2016.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artan M, Jeong DE, Lee D, Kim YI, Son HG, Husain Z, Kim J, Altintas O, Kim K, Alcedo J, Lee SJV. Food-derived sensory cues modulate longevity via distinct neuroendocrine insulin-like peptides. Genes & Development. 2016;30:1047–1057. doi: 10.1101/gad.279448.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal A, Zhu LJ, Yen K, Tissenbaum HA. Uncoupling lifespan and healthspan in Caenorhabditis elegans longevity mutants. PNAS. 2015;112:E277–E286. doi: 10.1073/pnas.1412192112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A, McWhirter R, Taylor SR, Weinreb A, Miller DM, Hammarlund M. A head-to-head comparison of ribodepletion and polyA selection approaches for Caenorhabditis elegans low input RNA-sequencing libraries. G3. 2021;11:jkab121. doi: 10.1093/g3journal/jkab121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernkopf M, Webersinke G, Tongsook C, Koyani CN, Rafiq MA, Ayaz M, Müller D, Enzinger C, Aslam M, Naeem F, Schmidt K, Gruber K, Speicher MR, Malle E, Macheroux P, Ayub M, Vincent JB, Windpassinger C, Duba HC. Disruption of the methyltransferase-like 23 gene METTL23 causes mild autosomal recessive intellectual disability. Human Molecular Genetics. 2014;23:4015–4023. doi: 10.1093/hmg/ddu115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blüher M, Kahn BB, Kahn CR. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science. 2003;299:572–574. doi: 10.1126/science.1078223. [DOI] [PubMed] [Google Scholar]
- Brear AG, Yoon J, Wojtyniak M, Sengupta P. Diverse cell type-specific mechanisms localize G Protein-Coupled receptors to Caenorhabditis elegans sensory cilia. Genetics. 2014;197:667–684. doi: 10.1534/genetics.114.161349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:1.71. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadie K, Baumgartner S, Prokop A. Extracellular matrix and its receptors in Drosophila neural development. Developmental Neurobiology. 2011;71:1102–1130. doi: 10.1002/dneu.20935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne AB, Walradt T, Gardner KE, Hubbert A, Reinke V, Hammarlund M. Insulin/IGF1 signaling inhibits age-dependent axon regeneration. Neuron. 2014;81:561–573. doi: 10.1016/j.neuron.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael K, Evans RC, Lopez E, Sun L, Kumar M, Ding J, Khaliq ZM, Cai H. Function and regulation of ALDH1A1-Positive Nigrostriatal dopaminergic neurons in motor control and parkinson’s disease. Frontiers in Neural Circuits. 2021;15:644776. doi: 10.3389/fncir.2021.644776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cermak S, Kosicek M, Mladenovic-Djordjevic A, Smiljanic K, Kanazir S, Hecimovic S. Loss of Cathepsin B and L Leads to Lysosomal Dysfunction, NPC-like cholesterol sequestration and accumulation of the key Alzheimer’s Proteins. PLOS ONE. 2016;11:e0167428. doi: 10.1371/journal.pone.0167428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Hendricks M, Cornils A, Maier W, Alcedo J, Zhang Y. Two insulin-like peptides antagonistically regulate aversive olfactory learning in C. elegans. Neuron. 2013;77:572–585. doi: 10.1016/j.neuron.2012.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang YC, Lee CH, Sun WH, Chen CC. Involvement of advillin in somatosensory neuron subtype-specific axon regeneration and neuropathic pain. PNAS. 2018;115:E8557–E8566. doi: 10.1073/pnas.1716470115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy DJ, Gems D, Harshman LG, Oldham S, Stocker H, Hafen E, Leevers SJ, Partridge L. Extension of life-span by loss of CHICO, a Drosophila Insulin receptor substrate protein. Science. 2001;292:104–106. doi: 10.1126/science.1057991. [DOI] [PubMed] [Google Scholar]
- De Pasquale V, Moles A, Pavone LM. Cathepsins in the pathophysiology of mucopolysaccharidoses: new perspectives for therapy. Cells. 2020;9:979. doi: 10.3390/cells9040979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckl J, Sima S, Marcus K, Lindemann C, Richter K. Hsp90-downregulation influences the heat-shock response, innate immune response and onset of oocyte development in nematodes. PLOS ONE. 2017;12:e0186386. doi: 10.1371/journal.pone.0186386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigin VL, Vos T, Nichols E, Owolabi MO, Carroll WM, Dichgans M, Deuschl G, Parmar P, Brainin M, Murray C. The global burden of neurological disorders: translating evidence into policy. The Lancet Neurology. 2020;19:255–265. doi: 10.1016/S1474-4422(19)30411-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fels E, Muñiz-Castrillo S, Vogrig A, Joubert B, Honnorat J, Pascual O. Role of LGI1 protein in synaptic transmission: From physiology to pathology. Neurobiology of Disease. 2021;160:105537. doi: 10.1016/j.nbd.2021.105537. [DOI] [PubMed] [Google Scholar]
- Ferguson CS, Tyndale RF. Cytochrome P450 enzymes in the brain: emerging evidence of biological significance. Trends in Pharmacological Sciences. 2011;32:708–714. doi: 10.1016/j.tips.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández EM, Cutraro YB, Adams J, Monteleone MC, Hughes KJ, Frasch AC, Vidal‐Gadea AG, Brocco MA. Neuronal membrane glycoprotein (nmgp‐1)gene deficiency affects chemosensation‐related behaviors, dauer exit and egg‐laying in Caenorhabditis elegans. Journal of Neurochemistry. 2022;160:234–255. doi: 10.1111/jnc.15543. [DOI] [PubMed] [Google Scholar]
- Flynn JM, Melov S. SOD2 in mitochondrial dysfunction and neurodegeneration. Free Radical Biology and Medicine. 2013;62:4–12. doi: 10.1016/j.freeradbiomed.2013.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Guo O, Zhen Z, Zhen J. Essential functions of the transcription factor Npas4 in neural circuit development, plasticity, and diseases. Frontiers in Neuroscience. 2020;14:603373. doi: 10.3389/fnins.2020.603373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao SM, Qi Y, Zhang Q, Mohammed AS, Lee YT, Guan Y, Li H, Fu Y, Wang MC. Aging Atlas Reveals Cell-Type-Specific Regulation of Pro-Longevity Strategies. bioRxiv. 2023 doi: 10.1101/2023.02.28.530490. [DOI] [PMC free article] [PubMed]
- Gauthier S, Kaur G, Mi W, Tizon B, Levy E. Protective mechanisms by cystatin C in neurodegenerative diseases. Frontiers in Bioscience. 2011;3:541–554. doi: 10.2741/s170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis C, Mirzaei F, Potashman M, Ikram MA, Maserejian N. The incidence of mild cognitive impairment: A systematic review and data synthesis. Alzheimer’s & Dementia. 2019;11:248–256. doi: 10.1016/j.dadm.2019.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Calvo I, Cizeron M, Bessereau JL, Selimi F. Synapse formation and function across species: ancient Roles for CCP, CUB, and TSP-1 structural domains. Frontiers in Neuroscience. 2022;16:866444. doi: 10.3389/fnins.2022.866444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillermo ARR, Chocian K, Gavriilidis G, Vandamme J, Salcini AE, Mellor J, Woollard A. H3K27 modifiers regulate lifespan in C. elegans in a context-dependent manner. BMC Biology. 2021;19:59. doi: 10.1186/s12915-021-00984-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahm J-H, Kim S, DiLoreto R, Shi C, Lee S-JV, Murphy CT, Nam HG. C. elegans maximum velocity correlates with healthspan and is maintained in worms with an insulin receptor mutation. Nature Communications. 2015;6:1–7. doi: 10.1038/ncomms9919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambright WS, Fonseca RS, Chen L, Na R, Ran Q. Ablation of ferroptosis regulator glutathione peroxidase 4 in forebrain neurons promotes cognitive impairment and neurodegeneration. Redox Biology. 2017;12:8–17. doi: 10.1016/j.redox.2017.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heremans IP, Caligiore F, Gerin I, Bury M, Lutz M, Graff J, Stroobant V, Vertommen D, Teleman AA, Van Schaftingen E, Bommer GT. Parkinson’s disease protein PARK7 prevents metabolite and protein damage caused by a glycolytic metabolite. PNAS. 2022;119:e2111338119. doi: 10.1073/pnas.2111338119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdorf AD, Higgins DP, Hart AC, Boag PR, Pazour GJ, Walhout AJM, Walker AK. WormCat: an online tool for annotation and visualization of Caenorhabditis elegans genome-scale data. Genetics. 2020;214:279–294. doi: 10.1534/genetics.119.302919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu A, Podvin S, Hook V. Lysosomal cathepsin protease gene expression profiles in the human brain during normal development. Journal of Molecular Neuroscience. 2018;65:420–431. doi: 10.1007/s12031-018-1110-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Meng S, Wu B, Shi H, Wang Y, Xiang J, Li J, Shi Z, Wu G, Lyu Y, Jia X, Hu J, Xu Z-X, Gao Y. HSPB2 facilitates neural regeneration through autophagy for sensorimotor recovery after traumatic brain injury. JCI Insight. 2023;8:e168919. doi: 10.1172/jci.insight.168919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C, Li J, Green CD, Yu X, Tang X, Han D, Xian B, Wang D, Huang X, Cao X, Yan Z, Hou L, Liu J, Shukeir N, Khaitovich P, Chen CD, Zhang H, Jenuwein T, Han J-DJ. Histone demethylase UTX-1 regulates C. elegans life span by targeting the insulin/IGF-1 signaling pathway. Cell Metabolism. 2011;14:161–172. doi: 10.1016/j.cmet.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Kaletsky R, Lakhina V, Arey R, Williams A, Landis J, Ashraf J, Murphy CT. The C. elegans adult neuronal IIS/FOXO transcriptome reveals adult phenotype regulators. Nature. 2016;529:92–96. doi: 10.1038/nature16483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaletsky R, Yao V, Williams A, Runnels AM, Tadych A, Zhou S, Troyanskaya OG, Murphy CT. Transcriptome analysis of adult Caenorhabditis elegans cells reveals tissue-specific gene and isoform expression. PLOS Genetics. 2018;14:e1007559. doi: 10.1371/journal.pgen.1007559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman AL, Ashraf JM, Corces-Zimmerman MR, Landis JN, Murphy CT. Insulin signaling and dietary restriction differentially influence the decline of learning and memory with age. PLOS Biology. 2010;8:e1000372. doi: 10.1371/journal.pbio.1000372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman A, Parsons L, Stein G, Wills A, Kaletsky R, Murphy C. C. elegans positive butanone learning, short-term, and long-term associative memory assays. Journal of Visualized Experiments. 2011;1–9:2490. doi: 10.3791/2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano T, Ito Y, Ishiguro M, Takuwa K, Nakajima T, Kimura Y. Molecular cloning and characterization of a new Insulin/IGF-like peptide of the nematode Caenorhabditis elegans. Biochemical and Biophysical Research Communications. 2000;273:431–436. doi: 10.1006/bbrc.2000.2971. [DOI] [PubMed] [Google Scholar]
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- Koukouli F, Changeux JP. Do nicotinic receptors modulate high-order cognitive processing? Trends in Neurosciences. 2020;43:550–564. doi: 10.1016/j.tins.2020.06.001. [DOI] [PubMed] [Google Scholar]
- Lakhina V, Arey RN, Kaletsky R, Kauffman A, Stein G, Keyes W, Xu D, Murphy CT. Genome-wide functional analysis of CREB/Long-term memory-dependent transcription reveals distinct basal and memory gene expression programs. Neuron. 2015;85:330–345. doi: 10.1016/j.neuron.2014.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhina V, McReynolds M, Grimes DT, Rabinowitz JD, Burdine RD, Murphy CT. ZIP-5/bZIP Transcription Factor Regulation of Folate Metabolism Is Critical for Aging Axon Regeneration. bioRxiv. 2019 doi: 10.1101/727719. [DOI]
- Langa KM, Levine DA. The diagnosis and management of mild cognitive impairment. JAMA. 2014;312:2551. doi: 10.1001/jama.2014.13806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautrup S, Sinclair DA, Mattson MP, Fang EF. NAD+ in brain aging and neurodegenerative disorders. Cell Metabolism. 2019;30:630–655. doi: 10.1016/j.cmet.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Ismail T, Kim Y, Chae S, Ryu H-Y, Lee D-S, Kwon TK, Park TJ, Kwon T, Lee H-S. Xenopus gpx3 mediates posterior development by regulating cell death during embryogenesis. Antioxidants. 2020;9:1265. doi: 10.3390/antiox9121265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, Linskens AM, Doe CQ. Hunchback activates Bicoid in Pair1 neurons to regulate synapse number and locomotor circuit function. Current Biology. 2022;32:2430–2441. doi: 10.1016/j.cub.2022.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Kennedy SG, Ruvkun G. DAF-28encodes a C. elegans insulin superfamily member that is regulated by environmental cues and acts in the DAF-2 signaling pathway. Genes & Development. 2003;17:844–858. doi: 10.1101/gad.1066503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Masliah E, Reixach N, Buxbaum JN. Neuronal production of transthyretin in human and murine alzheimer’s disease: is it protective? The Journal of Neuroscience. 2011;31:12483–12490. doi: 10.1523/JNEUROSCI.2417-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L-B, Lei H, Arey RN, Li P, Liu J, Murphy CT, Xu XZS, Shen K. The neuronal kinesin UNC-104/KIF1A Is a key regulator of synaptic aging and insulin signaling-regulated memory. Current Biology. 2016;26:605–615. doi: 10.1016/j.cub.2015.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wu J, Yu X, Na S, Li K, Yang Z, Xie X, Yang J, Yue J. The protective role of brain CYP2J in Parkinson’s Disease Models. Oxidative Medicine and Cellular Longevity. 2018;2018:1–12. doi: 10.1155/2018/2917981. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Liu J, Zhang B, Lei H, Feng Z, Liu J, Hsu A-L, Xu XZS. Functional aging in the nervous system contributes to age-dependent motor activity decline in C. elegans. Cell Metabolism. 2013;18:392–402. doi: 10.1016/j.cmet.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biology. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack HID, Kremer J, Albertini E, Mack EKM, Jansen-Dürr P. Regulation of fatty acid desaturase- and immunity gene-expression by mbk-1/DYRK1A in Caenorhabditis elegans. BMC Genomics. 2022;23:08176-y. doi: 10.1186/s12864-021-08176-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maro GS, Gao S, Olechwier AM, Hung WL, Liu M, Özkan E, Zhen M, Shen K. MADD-4/Punctin and Neurexin Organize C. elegans GABAergic postsynapses through neuroligin. Neuron. 2015;86:1420–1432. doi: 10.1016/j.neuron.2015.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maures TJ, Greer EL, Hauswirth AG, Brunet A. The H3K27 demethylase UTX-1 regulates C. elegans lifespan in a germline-independent, insulin-dependent manner. Aging Cell. 2011;10:980–990. doi: 10.1111/j.1474-9726.2011.00738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy CT, McCarroll SA, Bargmann CI, Fraser A, Kamath RS, Ahringer J, Li H, Kenyon C. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature. 2003;424:277–283. doi: 10.1038/nature01789. [DOI] [PubMed] [Google Scholar]
- Murphy CT, Lee SJ, Kenyon C. Tissue entrainment by feedback regulation of insulin gene expression in the endoderm of Caenorhabditis elegans. PNAS. 2007;104:19046–19050. doi: 10.1073/pnas.0709613104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols E, Vos T. Estimating the global mortality from Alzheimer’s disease and other dementias: A new method and results from the Global Burden of Disease study 2019. Alzheimer’s & Dementia. 2020;16:e042236. doi: 10.1002/alz.042236. [DOI] [Google Scholar]
- Pan CL, Peng CY, Chen CH, McIntire S. Genetic analysis of age-dependent defects of the Caenorhabditis elegans touch receptor neurons. PNAS. 2011;108:9274–9279. doi: 10.1073/pnas.1011711108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastore SF, Ko SY, Frankland PW, Hamel PA, Vincent JB. PTCHD1: identification and neurodevelopmental contributions of an autism spectrum disorder and intellectual disability susceptibility gene. Genes. 2022;13:527. doi: 10.3390/genes13030527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perland E, Lekholm E, Eriksson MM, Bagchi S, Arapi V, Fredriksson R. The putative SLC Transporters Mfsd5 and Mfsd11 are abundantly expressed in the mouse brain and have a potential role in energy homeostasis. PLOS ONE. 2016;11:e0156912. doi: 10.1371/journal.pone.0156912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce SB, Costa M, Wisotzkey R, Devadhar S, Homburger SA, Buchman AR, Ferguson KC, Heller J, Platt DM, Pasquinelli AA, Liu LX, Doberstein SK, Ruvkun G. Regulation of DAF-2 receptor signaling by human insulin andins-1, a member of the unusually large and diverse C. elegans insulin gene family. Genes & Development. 2001;15:672–686. doi: 10.1101/gad.867301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassman BL. Prevalence of cognitive impairment without dementia in the United States. Annals of Internal Medicine. 2008;148:427. doi: 10.7326/0003-4819-148-6-200803180-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudvere U, Kolberg L, Kuzmin I, Arak T, Adler P, Peterson H, Vilo J. g:Profiler: a web server for functional enrichment analysis and conversions of gene lists (2019 update) Nucleic Acids Research. 2019;47:W191–W198. doi: 10.1093/nar/gkz369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiff RE, Ali BR, Baron B, Yu TW, Ben-Salem S, Coulter ME, Schubert CR, Hill RS, Akawi NA, Al-Younes B, Kaya N, Evrony GD, Al-Saffar M, Felie JM, Partlow JN, Sunu CM, Schembri-Wismayer P, Alkuraya FS, Meyer BF, Walsh CA, Al-Gazali L, Mochida GH. METTL23, a transcriptional partner of GABPA, is essential for human cognition. Human Molecular Genetics. 2014;23:3456–3466. doi: 10.1093/hmg/ddu054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DG, Storey JD. subSeq: determining appropriate sequencing depth through efficient read subsampling. Bioinformatics. 2014;30:3424–3426. doi: 10.1093/bioinformatics/btu552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rontani P, Perche O, Greetham L, Jullien N, Gepner B, Féron F, Nivet E, Erard-Garcia M. Impaired expression of the COSMOC/MOCOS gene unit in ASD patient stem cells. Molecular Psychiatry. 2021;26:1606–1618. doi: 10.1038/s41380-020-0728-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux AE, Yuan H, Podshivalova K, Hendrickson D, Kerr R, Kenyon C, Kelley D. Individual cell types in C. elegans age differently and activate distinct cell-protective responses. Cell Reports. 2023;42:112902. doi: 10.1016/j.celrep.2023.112902. [DOI] [PubMed] [Google Scholar]
- Savage C, Hamelin M, Culotti JG, Coulson A, Albertson DG, Chalfie M. mec-7 is a beta-tubulin gene required for the production of 15-protofilament microtubules in Caenorhabditis elegans. Genes & Development. 1989;3:870–881. doi: 10.1101/gad.3.6.870. [DOI] [PubMed] [Google Scholar]
- Shan W, Nagai T, Tanaka M, Itoh N, Furukawa‐Hibi Y, Nabeshima T, Sokabe M, Yamada K. NeuronalPASdomain protein 4 (Npas4) controls neuronal homeostasis in pentylenetetrazole‐induced epilepsy through the induction of Homer1a. Journal of Neurochemistry. 2018;145:19–33. doi: 10.1111/jnc.14274. [DOI] [PubMed] [Google Scholar]
- Shapira M, Hamlin BJ, Rong J, Chen K, Ronen M, Tan M-W. A conserved role for A GATA transcription factor in regulating epithelial innate immune responses. PNAS. 2006;103:14086–14091. doi: 10.1073/pnas.0603424103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw CK, Abdulai-Saiku S, Marino F, Wang D, Davis EJ, Panning B, Dubal DB. X chromosome factor kdm6a enhances cognition independent of its demethylase function in the aging XY MaleBrain. The Journals of Gerontology. 2023;78:938–943. doi: 10.1093/gerona/glad007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinner DI, Kim GJ, Henderson GC, Igal RA. StearoylCoA Desaturase-5: a novel regulator of neuronal cell proliferation and differentiation. PLOS ONE. 2012;7:e39787. doi: 10.1371/journal.pone.0039787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smies CW, Bodinayake KK, Kwapis JL. Time to learn: The role of the molecular circadian clock in learning and memory. Neurobiology of Learning and Memory. 2022;193:107651. doi: 10.1016/j.nlm.2022.107651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein GM, Murphy CT. The intersection of aging, longevity pathways, and learning and memory in C. elegans. Frontiers in Genetics. 2012;3:259. doi: 10.3389/fgene.2012.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein GM, Murphy CTC. C. elegans positive olfactory associative memory is a molecularly conserved behavioral paradigm. Neurobiology of Learning and Memory. 2014;115:86–94. doi: 10.1016/j.nlm.2014.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh Y, Atzmon G, Cho MO, Hwang D, Liu B, Leahy DJ, Barzilai N, Cohen P. Functionally significant insulin-like growth factor I receptor mutations in centenarians. PNAS. 2008;105:3438–3442. doi: 10.1073/pnas.0705467105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang GB, Zeng YQ, Liu PP, Mi TW, Zhang SF, Dai SK, Tang QY, Yang L, Xu YJ, Yan HL, Du HZ, Teng ZQ, Zhou FQ, Liu CM. The Histone H3K27 Demethylase UTX regulates synaptic plasticity and cognitive behaviors in mice. Frontiers in Molecular Neuroscience. 2017;10:00267. doi: 10.3389/fnmol.2017.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tank EMH, Rodgers KE, Kenyon C. Spontaneous age-related neurite branching in Caenorhabditis elegans. Journal of Neuroscience. 2011;31:9279–9288. doi: 10.1523/JNEUROSCI.6606-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SR, Santpere G, Weinreb A, Barrett A, Reilly MB, Xu C, Varol E, Oikonomou P, Glenwinkel L, McWhirter R, Poff A, Basavaraju M, Rafi I, Yemini E, Cook SJ, Abrams A, Vidal B, Cros C, Tavazoie S, Sestan N, Hammarlund M, Hobert O, Miller DM. Molecular topography of an entire nervous system. Cell. 2021;184:4329–4347. doi: 10.1016/j.cell.2021.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper RG, Ashraf J, Kaletsky R, Kleemann G, Murphy CT, Bussemaker HJ. PQM-1 Complements DAF-16 as a key transcriptional regulator of DAF-2-mediated development and longevity. Cell. 2013;154:676–690. doi: 10.1016/j.cell.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomé-Duret V, Aussedat B, Reach G, Gangnerau MN, Lemonnier F, Klein JC, Zhang Y, Hu Y, Wilson GS. Continuous glucose monitoring in the free-moving rat. Metabolism. 1998;47:799–803. doi: 10.1016/S0026-0495(98)90115-9. [DOI] [PubMed] [Google Scholar]
- Toth ML, Melentijevic I, Shah L, Bhatia A, Lu K, Talwar A, Naji H, Ibanez-Ventoso C, Ghose P, Jevince A, Xue J, Herndon LA, Bhanot G, Rongo C, Hall DH, Driscoll M. Neurite sprouting and synapse deterioration in the aging Caenorhabditis elegans Nervous System. Journal of Neuroscience. 2012;32:8778–8790. doi: 10.1523/JNEUROSCI.1494-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ung DC, Iacono G, Méziane H, Blanchard E, Papon M-A, Selten M, van Rhijn J-R, Montjean R, Rucci J, Martin S, Fleet A, Birling M-C, Marouillat S, Roepman R, Selloum M, Lux A, Thépault R-A, Hamel P, Mittal K, Vincent JB, Dorseuil O, Stunnenberg HG, Billuart P, Nadif Kasri N, Hérault Y, Laumonnier F. Ptchd1 deficiency induces excitatory synaptic and cognitive dysfunctions in mouse. Molecular Psychiatry. 2018;23:1356–1367. doi: 10.1038/mp.2017.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandamme J, Lettier G, Sidoli S, Di Schiavi E, Nørregaard Jensen O, Salcini AE. The C. elegans H3K27 Demethylase UTX-1 is essential for normal development, independent of its enzymatic activity. PLOS Genetics. 2012;8:e1002647. doi: 10.1371/journal.pgen.1002647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang The insulin pathway regulates RNAi in C. elegans. Cold Spring Harbor Symposium on Quantitative Biology. 2004;01:429–433. doi: 10.1101/sqb.2004.69.429. [DOI] [Google Scholar]
- Wang X, Jiang Q, Song Y, He Z, Zhang H, Song M, Zhang X, Dai Y, Karalay O, Dieterich C, Antebi A, Wu L, Han JJ, Shen Y. Ageing induces tissue‐specific transcriptomic changes in Caenorhabditis elegans. The EMBO Journal. 2022;41:1–20. doi: 10.15252/embj.2021109633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- Willcox BJ, Donlon TA, He Q, Chen R, Grove JS, Yano K, Masaki KH, Willcox DC, Rodriguez B, Curb JD. FOXO3A genotype is strongly associated with human longevity. PNAS. 2008;105:13987–13992. doi: 10.1073/pnas.0801030105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiger JS, Haberstick BC, Schlaepfer I, Collins AC, Corley RP, Crowley TJ, Hewitt JK, Hopfer CJ, Lessem J, McQueen MB, Rhee SH, Ehringer MA. The neuronal nicotinic receptor subunit genes (CHRNA6 and CHRNB3) are associated with subjective responses to tobacco. Human Molecular Genetics. 2008;17:724–734. doi: 10.1093/hmg/ddm344. [DOI] [PubMed] [Google Scholar]
- Zhong L, Jiang X, Zhu Z, Qin H, Dinkins MB, Kong J, Leanhart S, Wang R, Elsherbini A, Bieberich E, Zhao Y, Wang G. Lipid transporter Spns2 promotes microglia pro-inflammatory activation in response to amyloid-beta peptide. Glia. 2019;67:498–511. doi: 10.1002/glia.23558. [DOI] [PMC free article] [PubMed] [Google Scholar]