Abstract
Poorly cytopathic or noncytopathic viruses can escape immune surveillance and establish a chronic infection. Here we exploited the strategy of combining antiviral drug treatment with the induction of a neutralizing antibody response to avoid the appearance of neutralization-resistant virus variants. Despite the fact that H25 immunoglobulin transgenic mice infected with lymphocytic choriomeningitis virus mounted an early neutralizing antibody response, the virus escaped from neutralization and persisted. After ribavirin treatment of H25 transgenic mice, the appearance of neutralization-resistant virus was prevented and virus was cleared. Thus, the combination of virus-neutralizing antibodies and chemotherapy efficiently controlled the infection, whereas each defense line alone did not. Similar additive effects may be unexpectedly efficient and beneficial in humans after infections with persistent viruses such as hepatitis C virus and hepatitis B virus and possibly human immunodeficiency virus.
Noncytopathic viruses such as human immunodeficiency virus (HIV), hepatitis B virus (HBV), and hepatitis C virus (HCV) in humans and lymphocytic choriomeningitis virus in mice may establish persistent infections. Therapeutic strategies that allow control or elimination of persisting virus infections by either vaccination or antiviral drug treatment are sought. The nucleoside analog ribavirin has been shown to exhibit a broad spectrum of antiviral activity (45, 47) by blocking the enzyme inosin monophosphate dehydrogenase and suppressing viral RNA synthesis (47). Treatment with ribavirin exhibited some benefit for patients suffering from Lassa fever, Argentine hemorrhagic fever, and hepatitis C (21, 22, 36, 37, 45–47). In experimental animal model infections, ribavirin has also been demonstrated to exhibit broad antiviral activities (7, 26, 28, 29, 53, 54).
The immune response against viruses which can lead to persistent infections is characterized by strong initial cytotoxic T-lymphocyte (CTL) responses followed by poor and delayed virus-neutralizing antibody responses (5, 14, 34, 35, 38, 48, 58, 61). In such infections it has been demonstrated that virus-neutralizing antibodies make a limited contribution to virus clearance (9, 27, 43, 44, 55).
Here we tested whether an early and accelerated virus-neutralizing antibody response or antiviral drug treatment or the combination of both can prevent a chronic infection. In the mouse, the natural host of lymphocytic choriomeningitis virus (LCMV), acute LCMV infection is controlled by CTLs (18, 30, 39, 62). Virus-neutralizing antibodies, which develop late after infection, are crucial for long-term control of LCMV (56) and have an important function in protection against reinfection (8, 57, 60). After infection with a high dose of LCMV strain DOCILE, virus-specific CTLs may be exhausted; this results in a persistent LCMV infection of the host within 10 to 20 days (40). Transfer of immune sera into neonatal mice can contribute to the prevention of persistent infection (9, 10).
In contrast, in the absence of neutralizing-antibody responses, establishment of viral persistence is accelerated (13, 43, 56). H25 transgenic mice, which express the μ heavy chain of the LCMV-neutralizing monoclonal antibody (MAb) KL25, mount an early and accelerated LCMV-neutralizing antibody response comparable to an antibody response after an antiviral vaccination of nontransgenic mice (51). Earlier studies showed that such transgene-encoded virus-neutralizing antibodies enhanced virus clearance after low-dose infection with the intermediately replicating LCMV strain WE. The neutralizing antibodies lowered the viral burden and thereby supported the CTL-mediated virus clearance (51). Similar effects have been observed after transfer of MAbs (9, 50). Here we show that after high-dose infection with the rapidly replicating LCMV strain DOCILE the enhanced virus-neutralizing antibody responses in H25 transgenic mice did not prevent virus persistence, which correlated with antibody escape variants emerging in vivo. However, additional treatment of H25 transgenic mice with the antiviral drug ribavirin together with the early LCMV-neutralizing antibody response prevented selection of LCMV antibody escape variants, and LCMV was cleared from ribavirin-treated H25 transgenic mice. Ribavirin treatment alone, in nontransgenic C57BL/6 mice, did not prevent LCMV persistence. Thus, the additive effect of virus-neutralizing antibodies and antiviral drug treatment prevented persistent virus infection by precluding immune escape of LCMV. These data suggest that similar additive effects may be unexpectedly efficient and beneficial in humans after infections with persistent viruses such as HCV, HBV, and HIV.
MATERIALS AND METHODS
Mice.
H25 transgenic mice expressing the μ heavy chain of the LCMV-neutralizing MAb KL25 produce LCMV-neutralizing immunoglobulin M (IgM) antibodies early after LCMV infection (51). Sex- and age-matched C57BL/6 control mice were purchased from the Institut für Zuchthygiene, University Zurich. Mice were bred under specific-pathogen-free conditions, and experiments were performed under conventional conditions. Mice were treated intraperitoneally (i.p.) with 5 mg of ribavirin (Virazole ribavirin; ICN Farmaceutica, Iztapalapa, Mexico) daily for 2 weeks, and control mice were left untreated.
Virus.
LCMV strain DOCILE was originally provided by J. Pfau, New York, N.Y., and was grown on BHK cells. LCMV antibody escape variants were tested as described previously (52). Briefly, loss of binding to the parental transgenic MAb KL25 was tested by fluorescence-activated cell sorter (FACS) surface staining of LCMV GP expressed on infected MC57G cells. Homogenous cell surface expression of LCMV GP by mutant and wild-type LCMV was confirmed by FACS staining with the MAb WEN1, which recognizes mutant and wild-type LCMV GP to the same extent. Loss of neutralization was tested in an infectious focus reduction assay (11). The presence of LCMV antibody escape mutants was confirmed by reverse transcription-PCR (RT-PCR) Taq cycle sequencing of LCMV GP (Taq Dye Deoxy terminator cycle sequencing kit; Applied Biosystems Inc., Foster City, Calif.; Bio-Rad, Hercules, Calif.). LCMV antibody escape mutants exhibit a characteristic amino acid substitution of the Asn119 of LCMV GP (52).
LCMV titer and neutralization assay.
LCMV titers in tissue homogenates were determined as described previously (11). Anti-LCMV-neutralizing antibody titers were determined by in vitro reduction of infectious foci under nonreducing conditions as described previously (11).
FACS analysis.
FACS analysis was performed on a FACScan from Becton Dickinson (San Diego, Calif.) according to standard procedures. The binding of the transgenic LCMV-neutralizing MAb KL25 (16) and the control LCMV-neutralizing MAb WEN1 (50) to LCMV antibody escape variants was tested on MC57G mouse fibroblasts infected at an initial multiplicity of infection of 0.01 40 h before analysis (52). MAbs KL25 and WEN1 were purified on Staphylococcus aureus protein G (Sepharose fast-flow protein G; Pharmacia, Uppsala, Sweden) and used at a concentration of 10 μg/ml. The binding of KL25 and WEN1 to infected MC57G cells was detected using goat anti-mouse IgG1-fluorescein isothiocyanate (FITC) (Southern Biotechnologies, Birmingham, Ala.) and goat anti-mouse IgG2a-FITC (Southern Biotechnologies), respectively. The hybridoma KL25 was originally used to assemble the transgene for H25 transgenic mice (51, 52), and therefore MAb KL25 shares LCMV-neutralizing specificity with the transgene-encoded antibodies expressed in H25 transgenic mice.
DNA sequence analysis of LCMV GP1.
Total RNA of MC57G cells infected with either wild-type LCMV or the LCMV antibody escape variant for 48 h at an initial multiplicity of infection of 0.01 was isolated according to the method of Chomczynski and Sacchi (17). RT-PCR was performed using LCMV GP1-specific primers as described previously (52). PCR products were sequenced by automated Taq cycle sequencing (Taq Dye Deoxy terminator cycle sequencing kit; Applied Biosystems Inc.; Bio-Rad).
Nucleotide sequence accession numbers.
The sequences of the PCR products are available from the EMBL nucleotide sequence database under accession no. AJ249149 to AJ249159.
RESULTS
Early LCMV-neutralizing antibodies in H25 transgenic mice do not prevent LCMV persistence.
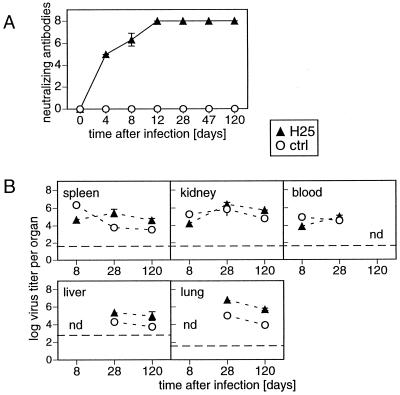
In order to investigate the impact of an early LCMV-neutralizing antibody response on the development of a persisting infection with LCMV, we infected H25 transgenic mice expressing the μ heavy chain of the LCMV-neutralizing MAb KL25 (51) and nontransgenic C57BL/6 control mice intravenously (i.v.) with 5 × 104 PFU of LCMV DOCILE and analyzed neutralizing serum antibody titers and virus titers in different organs. Early after infection by day 4, H25 transgenic mice mounted a strong LCMV-neutralizing antibody response, whereas LCMV-infected control C57BL/6 mice did not exhibit any detectable virus-neutralizing activity (Fig. 1A). However, despite this early LCMV-neutralizing antibody response in H25 transgenic mice, LCMV established a persistent infection. Virus titers similar to those found in nontransgenic C57BL/6 LCMV carrier mice were detected in spleens, kidneys, blood, livers, and lungs of LCMV-infected H25 transgenic mice during the entire observation period of 120 days (Fig. 1B).
FIG. 1.
Early LCMV-neutralizing antibodies of H25 transgenic mice cannot prevent LCMV persistence. H25 transgenic mice and nontransgenic C57BL/6 control (ctrl) mice were infected i.v. with 5 × 104 PFU of LCMV strain DOC. (A) Serum samples were collected at the indicated time points, and LCMV-neutralizing antibody titers were analyzed in an in vitro infectious focus reduction assay. Titer steps represent serial 2-fold dilutions of 10-fold-prediluted sera. (B) Virus titers of organ homogenates were determined by an infectious focus formation assay. Dashed lines, detection limits of the assay. Shown are means and standard deviations for three mice per group from one representative experiment out of three similar experiments. nd, not done.
Selection of LCMV antibody escape variants in H25 transgenic mice.
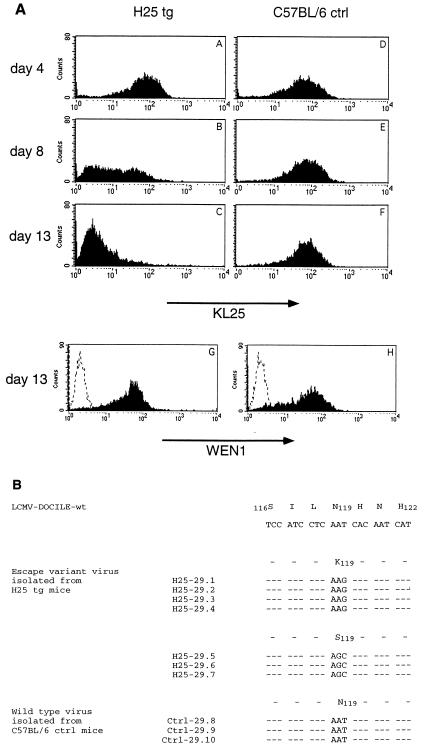
To test whether virus from H25 transgenic mice had escaped the neutralizing-antibody response, virus was collected from blood at days 4, 8, and 13 after infection and used to infect MC57G mouse fibroblasts in vitro. After 40 h of culture, LCMV GP expressed on the surfaces of infected MC57G cells was analyzed by FACS for the binding of MAb KL25. As shown in Fig. 2A, cell surface-expressed LCMV GP of virus isolated from H25 transgenic mice and that isolated from C57BL/6 control mice at day 4 after infection were recognized by MAb KL25 to similar extents (Fig. 2A, graphs A and D). However, MAb KL25 recognized only part of the virus isolated from H25 transgenic mice at day 8 after infection (Fig. 2A, graph B) and no virus isolated from H25 transgenic mice at day 13 after infection (Fig. 2A, graph C), in contrast to results for virus isolated from nontransgenic C57BL/6 mice (Fig. 2A, graphs E and F). Control FACS analysis with MAb WEN1, which recognizes wild-type LCMV GP and the antibody escape variant of LCMV GP to the same extent (52), resulted in comparable staining of the cell cultures infected with day 13 virus isolates, demonstrating equally efficient LCMV GP cell surface expression by wild-type and variant viruses (Fig. 2A, graphs G and H). Similar results were obtained for virus isolated at days 4 and 8 after infection (data not shown). Thus, LCMV which persisted in H25 transgenic mice had escaped the neutralizing-antibody response.
FIG. 2.
Selection of LCMV antibody escape variants in H25 transgenic mice. (A) LCMV was isolated from blood 4, 8, and 13 days after infection of H25 transgenic (tg) mice and of C57BL/6 control (ctrl) mice. After infection of MC57G fibroblasts with the different virus isolates and incubation for 40 h at 37°C and 10% CO2, LCMV GP cell surface expression was analyzed cytofluorometrically with MAbs KL25 (graphs A to F) and WEN1 (graphs G and H; dashed lines represent background staining with goat anti-mouse IgG2a-FITC alone). All samples showed comparable LCMV GP surface expression levels as indicated by similar stainings with the LCMV GP binding control MAb WEN1 (shown are profiles only for day 13 virus isolates in graphs G and H). Stainings with MAb KL25 were comparable with day 4 virus isolates from H25 transgenic mice (graph A) and day 4, 8, and 13 isolates from C57BL/6 control mice (graphs D to F), whereas MAb KL25 stainings were reduced with day 8 and 13 isolates from H25 transgenic mice (graphs B and C), indicating that these virus isolates had escaped the neutralizing-antibody response. Shown is one representative experiment out of three similar experiments. (B) Sequences of LCMV antibody escape variants isolated from blood of H25 transgenic mice (H25-29.1 to H25-29.7) and C57BL/6 control mice (Ctrl-29.8 to Ctrl-29.10) at day 13 after infection are aligned with the wild-type (wt) LCMV DOCILE sequence. Amino acids are numbered as described by Romanowski et al. (49). Dashes, sequence identity. The substitution of Asn119 is indicative of LCMV-neutralizing antibody escape variants (52). The sequence data are available from the EMBL nucleotide sequence database (accession no. AJ249149 to AJ249159).
This is in agreement with previous data demonstrating that LCMV persisting in neonatally infected mice in the presence of neutralizing antibodies are antibody escape variants (52) and with data demonstrating that a MAb neutralizing LCMV strain Armstrong A4 but not strain Armstrong A5 protected mice against infection with Armstrong A4 but not with Armstrong A5 (9). These two viruses had, however, been cloned from a laboratory stock; they were not the result of immune selection.
Generation of LCMV antibody escape variants was confirmed by RT-PCR cloning and Taq cycle sequencing of the LCMV GP: all LCMV isolated from H25 transgenic mice at day 13 after infection exhibited the amino acid substitution of LCMV GP Asn119, which has been shown (52) to be indicative of escape from the KL25 antibody response (Fig. 2B, sequences H25-29.1 to H25-29.7). LCMV isolated from C57BL/6 control mice exhibited wild-type LCMV DOCILE sequences (Fig. 2B, sequences Ctrl-29.8 to Ctrl-29.10).
Additive effect of LCMV-neutralizing antibodies and ribavirin treatment in control of persistent viral infection in vivo.
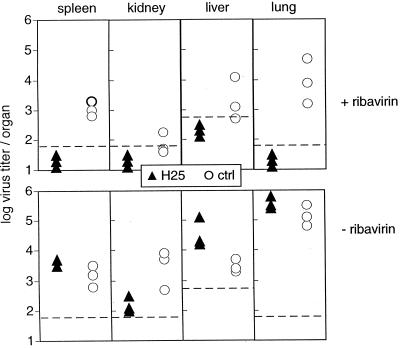
The antiviral drug ribavirin has been shown to interfere with LCMV replication in vitro (23). In order to test the effect of ribavirin on LCMV propagation in vivo, H25 transgenic mice and nontransgenic C57BL/6 mice were infected i.v. with 5 × 104 PFU of LCMV DOCILE and were either treated i.p. with 5 mg of ribavirin daily for 2 weeks or were left untreated. Virus titers in spleen, kidney, liver, and lung were determined 13 days after infection. As shown in Fig. 3, H25 transgenic mice treated with ribavirin had no detectable virus titers in the organs tested. In contrast, ribavirin-treated C57BL/6 control mice lacking virus-neutralizing antibody titers exhibited high virus titers in all organs tested. Both untreated H25 transgenic mice and untreated C57BL/6 control mice had also not cleared LCMV infection, and the virus persisted at least until day 60 after infection (data not shown). Thus, only the additive effect of LCMV-neutralizing antibodies in H25 transgenic mice and ribavirin treatment permitted control of infection and viral clearance.
FIG. 3.
Clearance of LCMV infection from ribavirin-treated H25 transgenic mice. H25 transgenic mice and transgene-negative C57BL/6 control (ctrl) mice were infected i.v. with 5 × 104 PFU of LCMV DOCILE and treated i.p. with 5 mg of ribavirin daily (top) or left untreated (bottom). Virus titers were determined 13 days after infection in an infectious focus formation assay. Dashed lines, detection limits of the assay. Shown are individual values from one representative experiment out of three similar experiments.
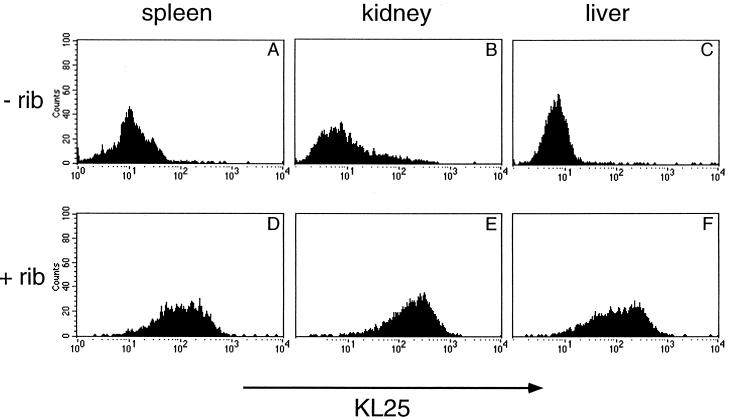
LCMV isolates from spleens, kidneys, and livers of non-ribavirin-treated H25 transgenic mice 11 days after the initial infection were identified as LCMV antibody escape variants. After infection of MC57G cells, surface-expressed LCMV GP did not bind MAb KL25 as revealed by FACS analysis (Fig. 4A to C). In contrast, LCMV isolated from ribavirin-treated H25 transgenic mice at day 11 after infection, i.e., 2 days before virus clearance, consisted of wild-type LCMV still recognized by MAb KL25 (Fig. 4D to F). Thus, ribavirin prevented the emergence of LCMV antibody escape variants in H25 transgenic mice, thereby enabling the neutralizing antibodies to efficiently contribute to the CTL-mediated viral clearance.
FIG. 4.
Prevention of selection of LCMV antibody escape variants by ribavirin treatment of H25 transgenic mice. H25 transgenic mice were infected i.v. with 5 × 104 PFU of LCMV DOCILE and treated i.p. with 5 mg of ribavirin daily (D to F) or left untreated (A to C). Virus isolated at day 11 after infection from spleen, kidney, and liver was grown on MC57G mouse fibroblasts for 40 h, and the binding of MAb KL25 to cell surface-expressed LCMV GP was determined by FACS analysis. Equally efficient LCMV GP surface expression of all infected cultures was confirmed by FACS analysis with the LCMV GP binding control MAb WEN1 (data not shown). LCMV antibody escape variants developed only in untreated H25 transgenic mice. Shown is one representative experiment out of two similar experiments.
DISCUSSION
In the present study, an enhanced and accelerated LCMV-neutralizing antibody response in H25 transgenic mice did not prevent the establishment of a persistent LCMV infection. LCMV escaped the transgene-encoded neutralizing-antibody response by selection of viral antibody escape variants. Escape from the neutralizing-antibody response, however, was prevented by antiviral drug treatment. These data illustrate the subtle balances between virus kinetics and the host immune response and how the balance can be influenced to the advantage of the host by means of an antiviral drug.
Antibodies are effective in antiviral treatment and protection by passive or active vaccination. While very efficient against cytopathic viruses, an isolated antibody response alone often is not sufficient to control noncytopathic or poorly cytopathic viruses (9, 27, 43, 44, 55). For LCMV infection, neutralizing antibodies have been demonstrated to enhance virus clearance mediated mainly by CTLs (9, 51). Preexisting neutralizing antibodies can prevent LCMV persistence (8, 9, 43, 51). However, sometimes an isolated antibody response may be disadvantageous for the host because of the risk of antibody-mediated enhancement of disease (12, 25) or the emergence of viral antibody escape variants (4, 42, 44, 52). In humans, viral antibody escape variants from the cytopathic influenza virus have been described at the population level (20, 32). For the noncytopathic HIV, antibody escape variants have been isolated from infected individuals (4, 42). Likewise, in H25 transgenic mice, noncytopathic LCMV escaped the neutralizing antibody response in vivo within single individuals. Furthermore, there is accumulating evidence, that neutralizing antibody responses against viruses and bacteria, e.g., vesicular stomatitis virus (31), HIV (6, 15, 19, 24, 33, 41, 59), and Haemophilus influenzae (1–3), exhibit very restricted, if not sometimes monoclonal, V-gene usage comparable to the oligoclonal V-gene usage in H25 transgenic mice.
Treatment with the antiviral drug ribavirin as a single agent has been reported to reduce the viral burden of several RNA viruses or at least to diminish clinical symptoms after infection (26, 28, 53, 54). In therapy of human infections, such as Lassa fever and Argentine hemorrhagic fever, all infections with members of the arenavirus family like LCMV, a transient reduction in viral load could be demonstrated (7, 23, 36). Ribavirin is increasingly used in antiviral therapy for hepatitis C but is only effective in a combined treatment with alpha interferon (21, 37, 45, 47). Likewise, in the present study persisting LCMV infection could not be prevented by ribavirin treatment alone. Only the combination with a strong and early virus-neutralizing antibody response efficiently prevented persistent LCMV infection.
The effect of ribavirin on the virus is not absolute and only sterilizing in vitro (23). The main effect of ribavirin in the present study may be to reduce the replication efficiency of LCMV in vivo, thereby rendering the appearance of antibody escape variants considerably less frequent. Although some LCMV antibody escape variants might have been transferred already with the inoculum, the enhanced virus-neutralizing antibodies in ribavirin-treated H25 transgenic mice remained capable of lowering LCMV titer sufficiently so that CTLs were able to control the virus.
In agreement with the present study, virus-specific immune plasma has been shown to exert a beneficial effect of ribavirin on primate survival and control of virus replication after infection with Lassa virus (29). Ribavirin and immune plasma were transferred at the time point of Lassa virus infection. Additive effects were lost if ribavirin and antibodies were transferred later after infection. Possibly, under these circumstances Lassa virus had replicated in vivo and generated antibody escape variants randomly before the time point of ribavirin and antibody transfer. The antibody may have therefore no longer contributed additively to the treatment due to preexistent virus variants.
The present model of antiviral drug treatment in the presence of a strong antiviral antibody response suggests that, if combined, even moderately active antiviral drug treatment plus passive humoral immunotherapy or active vaccination strategies may be efficient against viruses in humans with a tendency to persist, e.g., HBV, HCV, and possibly HIV.
ACKNOWLEDGMENTS
We thank Edit Horvath and Karin Riem for excellent technical assistance, R. Städeli and D. Zimmermann for the generation of DNA sequence data, and P. Aichele for critical reading of the manuscript.
This work was supported by Swiss National Science Foundation grants 31-50884.97 and 31-50900.97 and by the Kanton Zürich.
REFERENCES
- 1.Adderson E E, Shackelford P G, Insel R A, Quinn A, Wilson P M, Carrol W L. Immunoglobulin light chain variable region gene sequences for human antibodies to Haemophilus influenzae type b capsular polysaccharide are dominated by a limited number of V kappa and V lambda segments and VJ combinations. J Clin Investig. 1992;89:729–738. doi: 10.1172/JCI115649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adderson E E, Shackelford P G, Quinn A, Carroll W L. Restricted Ig H chain V gene usage in the human antibody response to Haemophilus influenzae type b capsular polysaccharide. J Immunol. 1991;147:1667–1674. [PubMed] [Google Scholar]
- 3.Adderson E E, Shackelford P G, Quinn A, Wilson P M, Cunningham M W, Insel R A, Carroll W L. Restricted immunoglobulin VH usage and VDJ combinations in the human response to Haemophilus influenzae type b capsular polysaccharide. J Clin Investig. 1993;91:2734–2743. doi: 10.1172/JCI116514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albert J, Abrahamsson B, Nagy K, Aurelius E, Gaines H, Nystrom G, Fenyo E M. Rapid development of isolate-specific neutralizing antibodies after primary HIV-1 infection and consequent emergence of virus variants which resist neutralization by autologous sera. AIDS. 1990;4:107–112. doi: 10.1097/00002030-199002000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Alberti A, Pontisso P, Tagariello G, Cavalletto D, Chemello L, Belussi F. Antibody response to pre-S2 and hepatitis B virus induced liver damage. Lancet. 1988;i:1421–1424. doi: 10.1016/s0140-6736(88)92237-4. [DOI] [PubMed] [Google Scholar]
- 6.Amadori A, Gallo P, Zamarchi R, Veronese M L, DeRossi A, Wolf D, Chieco-Bianchi L. IgG oligoclonal bands in sera of HIV-1 infected patients are mainly directed against HIV-1 determinants. AIDS Res Hum Retroviruses. 1990;6:581–586. doi: 10.1089/aid.1990.6.581. [DOI] [PubMed] [Google Scholar]
- 7.Andrei W, De Clercq E. Molecular approaches for the treatment of hemorrhagic fever virus infections. Antiviral Res. 1993;22:45–75. doi: 10.1016/0166-3542(93)90085-w. [DOI] [PubMed] [Google Scholar]
- 8.Baldridge J R, Buchmeier M J. Mechanisms of antibody-mediated protection against lymphocytic choriomeningitis virus infection: mother-to-baby transfer of humoral protection. J Virol. 1992;66:4252–4257. doi: 10.1128/jvi.66.7.4252-4257.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baldridge J R, McGraw T S, Paoletti A, Buchmeier M J. Antibody prevents the establishment of persistent arenavirus infection in synergy with endogenous T cells. J Virol. 1997;71:755–758. doi: 10.1128/jvi.71.1.755-758.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baldridge J R, Pearce B D, Parekh B S, Buchmeier M J. Teratogenic effects of neonatal arenavirus infection on the developing rat cerebellum are abrogated by passive immunotherapy. Virology. 1993;197:669–677. doi: 10.1006/viro.1993.1642. [DOI] [PubMed] [Google Scholar]
- 11.Battegay M, Cooper S, Althage A, Baenziger J, Hengartner H, Zinkernagel R M. Quantification of lymphocytic choriomeningitis virus with an immunological focus assay in 24 or 96 well plates. J Virol Methods. 1991;33:191–198. doi: 10.1016/0166-0934(91)90018-u. [DOI] [PubMed] [Google Scholar]
- 12.Battegay M, Kyburz D, Hengartner H, Zinkernagel R M. Enhancement of disease by neutralizing antiviral antibodies in the absence of primed antiviral cytotoxic T cells. Eur J Immunol. 1993;23:3236–3241. doi: 10.1002/eji.1830231229. [DOI] [PubMed] [Google Scholar]
- 13.Battegay M, Moskophidis D, Rahemtulla A, Hengartner H, Mak T W, Zinkernagel R M. Enhanced establishment of a virus carrier state in adult CD4+ T-cell-deficient mice. J Virol. 1994;68:4700–4704. doi: 10.1128/jvi.68.7.4700-4704.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Battegay M, Moskophidis D, Waldner H, Bründler M A, Fung-Leung W P, Mak T W, Hengartner H, Zinkernagel R M. Impairment and delay of neutralizing antiviral antibody responses by virus-specific cytotoxic T cells. J Immunol. 1993;151:5408–5415. [PubMed] [Google Scholar]
- 15.Berberian L, Goodglick L, Kipps T J, Braun J. Immunoglobulin VH3 gene products: natural ligands for HIV gp 120. Science. 1993;261:1588–1591. doi: 10.1126/science.7690497. [DOI] [PubMed] [Google Scholar]
- 16.Bruns M, Cihak J, Müller G, Lehmann-Grube F. Lymphocytic choriomeningitis virus. VI. Isolation of a glycoprotein mediating neutralization. Virology. 1983;130:247–251. doi: 10.1016/0042-6822(83)90135-6. [DOI] [PubMed] [Google Scholar]
- 17.Chomczynski P, Sacchi N. Single-step method of RNA isolation by guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 18.Cole G A, Nathanson N, Prendergast R A. Requirement for theta-bearing cells in lymphocytic choriomeningitis virus-induced central nervous system disease. Nature. 1972;238:335–337. doi: 10.1038/238335a0. [DOI] [PubMed] [Google Scholar]
- 19.D'Amelio R, Biselli R, Nisini R, Matricardi P M, Aiuti A, Mezzaroma I, Pinter E, Ponteselli O, Aiuti F. Spectrotype of anti-gp 120 antibodies remains stable during the course of HIV disease. J Acquir Immune Defic Syndr. 1992;5:930–935. [PubMed] [Google Scholar]
- 20.Davenport F M, Minuse E, Hennessy A V, Francis T J. Interpretations of influenza antibody patterns of man. Bull W H O. 1969;41:453–460. [PMC free article] [PubMed] [Google Scholar]
- 21.Davis G L, Esteban-Mur R, Rustgi V, Hoefs J, Gordon S C, Trepo C, Shiffman M L, Zeuzem S, Craxi A, Ling M H, Albrecht J. Interferon alpha-2b alone or in combination with ribavirin for the treatment of relapse of chronic hepatitis C. N Engl J Med. 1998;339:1493–1499. doi: 10.1056/NEJM199811193392102. [DOI] [PubMed] [Google Scholar]
- 22.Enria D A, Briggiler A M, Levis S, Vallejos D, Maiztegui J I, Canonico P G. Preliminary report: tolerance and antiviral effect of ribavirin in patients with Argentine hemorrhagic fever. Antiviral Res. 1987;7:353–359. doi: 10.1016/0166-3542(87)90017-9. [DOI] [PubMed] [Google Scholar]
- 23.Géssner A, Lother H. Homologous interference of lymphocytic choriomeningitis virus involves a ribavirin-susceptible block in virus replication. J Virol. 1989;63:1827–1832. doi: 10.1128/jvi.63.4.1827-1832.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grimaldi L M, Roos R P, Devare S G, Robey W G, Casey J M, Gurney M E, Apatoff B R, Lazzarin D. Restricted heterogeneity of antibody to gp 120 and p24 in AIDS. J Immunol. 1988;141:114–117. [PubMed] [Google Scholar]
- 25.Halstead S B, O'Rourke E J. Dengue viruses and mononuclear phagocytes. I. Infection enhancement by non-neutralizing antibody. J Exp Med. 1977;146:201–217. doi: 10.1084/jem.146.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harvie P, Omar R F, Dusserre N, Desormeaux A, Gourde P, Tremblay M, Beauchamp D, Bergeron M G. Antiviral efficacy and toxicity of ribavirin in murine acquired immunodeficiency syndrome model. J Acquir Immune Defic Syndr. 1996;12:451–461. doi: 10.1097/00042560-199608150-00003. [DOI] [PubMed] [Google Scholar]
- 27.Haynes B F, Pantaleo G, Fauci A S. Toward an understanding of the correlates of protective immunity to HIV infection. Science. 1996;271:324–328. doi: 10.1126/science.271.5247.324. [DOI] [PubMed] [Google Scholar]
- 28.Jahrling P B, Hesse R A, Eddy G A, Johnson K M, Callis R T, Stephen E L. Lassa virus infection of rhesus monkeys: pathogenesis and treatment with ribavirin. J Infect Dis. 1980;141:580–589. doi: 10.1093/infdis/141.5.580. [DOI] [PubMed] [Google Scholar]
- 29.Jahrling P B, Peters C J, Stephen E L. Enhanced treatment of lassa fever by immune plasma combined with ribavirin in cynomolgus monkeys. J Infect Dis. 1984;149:420–427. doi: 10.1093/infdis/149.3.420. [DOI] [PubMed] [Google Scholar]
- 30.Kägi D, Ledermann B, Bürki K, Seiler P, Odermatt B, Olsen K J, Podack E, Zinkernagel R M, Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- 31.Kalinke U, Bucher E M, Ernst B, Oxenius A, Roost H-P, Geley S, Kofler R, Zinkernagel R, Hengartner H. The role of somatic mutation in the generation of the protective humoral immune response against vesicular stomatitis virus. Immunity. 1996;5:1–20. doi: 10.1016/s1074-7613(00)80277-0. [DOI] [PubMed] [Google Scholar]
- 32.Kilbourne E D. Influenza pandemics in perspective. JAMA. 1977;237:1225–1228. [PubMed] [Google Scholar]
- 33.Köhler H, Müller S, Nara P L. Deceptive imprinting in the immune response against HIV-1. Immunol Today. 1994;15:475–478. doi: 10.1016/0167-5699(94)90192-9. [DOI] [PubMed] [Google Scholar]
- 34.Koup R A, Safrit J T, Cao Y, Andrews C A, McLeod G, Borkowsky W, Farthing C, Ho D D. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lemon S M, Thomas D L. Vaccines to prevent viral hepatitis. N Engl J Med. 1997;336:196–204. doi: 10.1056/NEJM199701163360307. [DOI] [PubMed] [Google Scholar]
- 36.McCormick J B, King I J, Webb P A, Scribner C L, Craven R B, Johnson K M, Elliott L H, Belmont-Williams R. Effective therapy with ribavirin. N Engl J Med. 1986;314:20–26. doi: 10.1056/NEJM198601023140104. [DOI] [PubMed] [Google Scholar]
- 37.McHutchinson J G, Gordon S C, Schiff E R, Shiffman M L, Lee W M, Rustgi V K, Goodman Z D, Ling M H, Cort S, Albrecht J K. Interferon alpha-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. N Engl J Med. 1998;339:1485–1492. doi: 10.1056/NEJM199811193392101. [DOI] [PubMed] [Google Scholar]
- 38.Moore J P, Cao Y, Ho D D, Koup R A. Development of the anti-gp120 antibody response during seroconversion to human immunodeficiency virus type 1. J Virol. 1994;68:5142–5155. doi: 10.1128/jvi.68.8.5142-5155.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moskophidis D, Cobbold S P, Waldmann H, Lehmann G F. Mechanism of recovery from acute virus infection: treatment of lymphocytic choriomeningitis virus-infected mice with monoclonal antibodies reveals that Lyt-2+ T lymphocytes mediate clearance of virus and regulate the antiviral antibody response. J Virol. 1987;61:1867–1874. doi: 10.1128/jvi.61.6.1867-1874.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moskophidis D, Lechner F, Pircher H P, Zinkernagel R M. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 1993;362:758–761. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- 41.Müller S, Wang H, Silverman G J, Bramlet G, Haigwood N, Köhler H. B-cell abnormalities in AIDS: stable and clonally-restricted antibody response in HIV-1 infection. Scand J Immunol. 1993;38:327–334. doi: 10.1111/j.1365-3083.1993.tb01734.x. [DOI] [PubMed] [Google Scholar]
- 42.Nowak M A, Anderson R M, McLean A R, Wolfs T F W, Goudsmit J, May R M. Antigenic diversity thresholds and the development of AIDS. Science. 1991;254:963–969. doi: 10.1126/science.1683006. [DOI] [PubMed] [Google Scholar]
- 43.Planz O, Ehl S, Furrer E, Horvath E, Bründler M-A, Hengartner H, Zinkernagel R M. A critical role for neutralizing-antibody-producing B cells, CD4+ T cells, and interferons in persistent and acute infections of mice with lymphocytic choriomeningitis virus: implications for adoptive immunotherapy of virus carriers. Proc Natl Acad Sci USA. 1997;94:6874–6879. doi: 10.1073/pnas.94.13.6874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poignard P, Sabbe R, Picchio G R, Wang M, Gulizia R J, Katinger H, Parren P W H I, Mosier D E, Burton D R. Neutralizing antibodies have limited effects on the control of established HIV-1 infection in vivo. Immunity. 1999;10:431–438. doi: 10.1016/s1074-7613(00)80043-6. [DOI] [PubMed] [Google Scholar]
- 45.Poynard T, Marcellin P, Lee S S, Niederau C, Minuk G S, Ideo G, Bain V, Heathcote J, Zeuzem S, Trepo C, Albrecht J. Randomised trial of interferon α2b plus ribavirin for 48 weeks or for 24 weeks versus interferon α2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. Lancet. 1998;352:1426–1432. doi: 10.1016/s0140-6736(98)07124-4. [DOI] [PubMed] [Google Scholar]
- 46.Reichard O, Andersson J, Schvarcz R, Weiland O. Ribavirin treatment for chronic hepatitis C. Lancet. 1991;337:1058–1061. doi: 10.1016/0140-6736(91)91707-2. [DOI] [PubMed] [Google Scholar]
- 47.Reichard O, Norkrans G, Fryden A, Braconier J H, Sonnerborg A, Weiland O. Randomised, double-blind, placebo-controlled trial of interferon alpha-2b with and without ribavirin for chronic hepatitis C. The Swedish Study Group. Lancet. 1998;351:83–87. doi: 10.1016/s0140-6736(97)06088-1. [DOI] [PubMed] [Google Scholar]
- 48.Robert G M, Brown M, Gallo R C. HTLV-III-neutralizing antibodies in patients with AIDS and AIDS-related complex. Nature. 1985;316:72–74. doi: 10.1038/316072a0. [DOI] [PubMed] [Google Scholar]
- 49.Romanowski V, Matsuura Y, Bishop D H L. Complete sequence of the S RNA of lymphocytic choriomeningitis virus (WE strain) compared to that of Pichinde arenavirus. Virus Res. 1985;3:101–114. doi: 10.1016/0168-1702(85)90001-2. [DOI] [PubMed] [Google Scholar]
- 50.Seiler P, Bründler M-A, Zimmermann C, Weibel D, Bruns M, Hengartner H, Zinkernagel R M. Induction of protective cytotoxic T cell responses in the presence of high titers of virus neutralizing antibodies: implications for passive and active immunization. J Exp Med. 1998;187:649–654. doi: 10.1084/jem.187.4.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seiler P, Kalinke U, Rülicke T, Bucher E M, Böse C, Zinkernagel R M, Hengartner H. Enhanced virus clearance by early inducible lymphocytic choriomeningitis virus-neutralizing antibodies in immunoglobulin-transgenic mice. J Virol. 1998;72:2253–2258. doi: 10.1128/jvi.72.3.2253-2258.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seiler P, Senn B M, Bründler M-A, Zinkernagel R M, Hengartner H, Kalinke U. In vivo selection of neutralization-resistant virus variants, but no evidence for B cell tolerance in lymphocytic choriomeningitis virus-carrier mice expressing a transgenic virus neutralizing antibody. J Immunol. 1999;162:4536–4541. [PubMed] [Google Scholar]
- 53.Sidwell R W, Huffman J H, Barnard D L, Smee D F, Warren R P, Chirigos M A, Kende M, Huggins J. Antiviral and immunomodulating inhibitors of experimentally-induced Punta Toro virus infection. Antiviral Res. 1994;25:105–122. doi: 10.1016/0166-3542(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 54.Smee D F, Gilbert J, Leonhardt J A, Barnett B B, Huggins J H, Sidwell R W. Treatment of lethal Pichinde virus infections in weanling LVG/Lak hamsters with ribavirin, ribamidine, selenazofurin, and ampligen. Antiviral Res. 1993;20:57–70. doi: 10.1016/0166-3542(93)90059-r. [DOI] [PubMed] [Google Scholar]
- 55.Steiner I. Human herpes viruses latent infection in the nervous system. Immunol Rev. 1996;152:157–173. doi: 10.1111/j.1600-065x.1996.tb00915.x. [DOI] [PubMed] [Google Scholar]
- 56.Thomsen A R, Johansen J, Marker O, Christensen J P. Exhaustion of CTL memory and recrudescence of viremia in lymphocytic choriomeningitis virus-infected MHC class II-deficient mice and B cell-deficient mice. J Immunol. 1996;157:3074–3080. [PubMed] [Google Scholar]
- 57.Thomsen A R, Marker O. The complementary roles of cellular and humoral immunity in resistance to re-infection with LCM virus. Immunology. 1988;65:9–15. [PMC free article] [PubMed] [Google Scholar]
- 58.Weiss R A, Clapham P R, Cheingsong P R, Dalgleish A G, Carne C A, Weller I, Tedder R S. Neutralization of human T-lymphotropic virus type III by sera of AIDS and AIDS-risk patients. Nature. 1985;316:69–71. doi: 10.1038/316069a0. [DOI] [PubMed] [Google Scholar]
- 59.Wisnewski A, Cavacini L, Posner M. Human antibody variable region gene usage in HIV-1 infection. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;11:31–38. doi: 10.1097/00042560-199601010-00004. [DOI] [PubMed] [Google Scholar]
- 60.Wright K E, Buchmeier M J. Antiviral antibodies attenuate T-cell-mediated immunopathology following acute lymphocytic choriomeningitis virus infection. J Virol. 1991;65:3001–3006. doi: 10.1128/jvi.65.6.3001-3006.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wright T L, Lau J Y N. Clinical aspects of hepatitis B virus infection. Lancet. 1993;342:1340–1344. doi: 10.1016/0140-6736(93)92250-w. [DOI] [PubMed] [Google Scholar]
- 62.Zinkernagel R M, Leist T P, Hengartner H, Althage A. Susceptibility to lymphocytic choriomeningitis virus isolates correlates directly with early and high cytotoxic T cell activity, as well as with footpad swelling reaction, and all three are regulated by H-2D. J Exp Med. 1985;162:2125–2141. doi: 10.1084/jem.162.6.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]