Abstract
In this study, we have investigated the influence of antigen targeting after DNA vaccination upon the induction of cellular immune responses against human immunodeficiency virus type 1 (HIV-1) Gag. In addition to the standard version of HIV-1 Gag, we constructed Gag expression vectors that encode a secreted (Sc-Gag) and a cytoplasmic (Cy-Gag) Gag molecule. Although all three HIV-1 Gag expression vectors induced detectable humoral and cellular immune responses, after intramuscular injection the DNA vector encoding the Sc-Gag generated the highest primary cytotoxic T-lymphocyte (CTL) and T-helper responses. Mice immunized with one of the HIV-1 Gag DNA vectors (but not with the control vector pcDNA3.1) developed a protective immune response against infection with recombinant vaccinia virus expressing HIV-1 Gag, and this response persisted for 125 days. The magnitude of the protection correlated with the levels of Gag-specific ex vivo CTL activity and the number of CD8+ T cells producing gamma interferon. The DNA vector encoding the Sc-Gag induced higher levels of protection and greater secondary CTL responses than did the DNA vector encoding Cy-Gag.
Cellular immune responses against human immunodeficiency virus type 1 (HIV-1) and the related simian immunodeficiency virus (SIV) have been shown to play an important role in controlling HIV-1 and SIV infection and in delaying disease progression. Containment of primary HIV-1 infection in infected individuals correlates with the emergence of virus-specific cytotoxic T-lymphocyte (CTL) responses (4, 14, 26). In chronically infected individuals, a high-frequency CTL response against HIV-1 is also correlated with a low viral load and slow disease progression (24, 25). An HIV-1-specific CTL response has also been demonstrated in certain highly exposed seronegative individuals (2, 15, 32). Also, strong HIV-specific proliferative responses, which may be critical for the maintenance of CTL responses, have been identified in long-term nonprogressors (31, 35).
HIV-1 Gag is one of the most conserved viral proteins. Broad, cross-clade CTL responses recognizing conserved epitopes in HIV-1 Gag have been detected in HIV-1-infected people (11, 21), and the development of a safe and effective HIV-1 vaccine may depend on the induction of effective CTL and/or T-helper responses against conserved HIV-1 proteins such as Gag. DNA vaccines have been shown to induce efficient cellular immune responses and protection against a variety of viral, bacterial, and parasitic pathogens in animal models. However, DNA vaccines that could induce potent cellular immune responses against HIV-1 Gag are not yet available.
We have recently demonstrated that by destroying inhibitory sequences in the coding region of HIV-1 gag, we could significantly increase Gag protein expression in primate as well as in mouse cells (27, 34, 36, 37) and dramatically enhance immune responses induced by DNA vaccine (27). Since this new Gag expression vector is Rev/RRE-independent and species-independent, it provides a feasible approach to systematically evaluating the strategies that could lead to the maximum induction of cellular immune responses against HIV Gag molecules in animal models.
Intramuscular (i.m.) administration of a DNA vaccine represents a simple and effective means of inducing both humoral and cellular immune responses (10). There are three potential pathways responsible for antigen presentation after i.m. injection of DNA. First, muscle cells could take up the DNA, express the encoded protein antigen, and present it to immune cells. Recent data suggest that this pathway is rather unlikely in vivo (40). Second, dendritic cells attracted to the site of injection may take up the DNA, express the encoded protein, and present it to T and B cells. Third, muscle cells may take up the DNA and express the protein antigen, with the antigen then being transmitted to dendritic cells for presentation. If the second possibility is the case, a protein that is synthesized and degraded in the cytoplasm of dendritic cells would be an excellent target for major histocompatibility complex (MHC) class I presentation and induction of CTL responses. Alternatively, if the third scenario were true, a protein synthesized in the muscle cells that could be targeted efficiently to dendritic cells would induce the best CTL response.
To distinguish among these different possibilities, we have now constructed and compared three different forms of HIV-1 Gag DNA vaccine vectors for the induction of immune responses. These different forms of Gag include (i) a standard Gag (St-Gag) that assembles into particles, which are efficiently released from cells and become surrounded by host-cell-derived lipid membrane acquired during virus budding; (ii) a cytoplasmic form of Gag (Cy-Gag) that fails to target the plasma membrane and therefore remains in the cytoplasm; and (iii) a secreted form of Gag (Sc-Gag) that is synthesized on the cytoplasmic face of the rough endoplasmic reticulum (ER), transported through the ER and Golgi apparatus, and released as a secreted protein (i.e., not surrounded by a lipid membrane). When these forms of Gag were administered to mice as DNA vaccine, we found that the DNA vector encoding the Sc-Gag generated better primary CTL and T-helper responses than did the DNA vector encoding Cy-Gag. Furthermore, the DNA vector encoding the Sc-Gag also generated a higher level of secondary CTL responses than did the DNA vector encoding Cy-Gag after DNA priming and recombinant vaccinia virus-Gag boost. Vaccinia virus titers were notably reduced in the ovaries of mice immunized with Gag DNA vaccine more than 125 days before infection, as compared to the titer in mice that received only the control DNA vector. These data indicate that CD8+ T-cell memory elicited by DNA vaccination is functionally relevant and provides protective immunity in this system. Again, the DNA vector encoding the Sc-Gag provided better protection against recombinant vaccinia virus-Gag than did the DNA vector encoding Cy-Gag.
MATERIALS AND METHODS
Construction of HIV-1 Gag expression vectors.
The standard Gag expression vector (pSt-GAG) used in these experiments was pGAGINS which has been previously described (27, 34). The expression vector for the secreted form of Gag (pSc-GAG) was created by fusing the HIV-1 gag sequence with the first 21 amino acids (aa) of human tissue plasminogen activator (t-PA). The sense oligonucleotide (5′ CTA GAA TGG ATG CAA TGA AGA GAG GGC TCT GCT GTG TGC TGC TGC TGT GTG GAG CAG TCT TCG TTT CGG 3′) was annealed with the antisense oligonucleotide (5′ CTA GCC GAA ACG AAG ACT GCT CCA CAC AGC AGC AGC ACA CAG CAG AGC CCT CTC TTC ATT GCA TCC ATT 3′) and was inserted into the XbaI site of pGAGINS that was in frame with the gag gene. The cytoplasmic form of the Gag expression vector (pCy-GAG) was created by insertion of an oligonucleotide linker that destroyed the myristylation signal in the HIV-1 Gag molecule. The sense oligonucleotide (5′ CTA GAA TGG CTG CGA GAG 3′) and the antisense oligonucleotide (5′ CTA GCT CTC GCA GCC ATT 3′) were annealed and inserted into pGAGINS by using the XbaI site. The correct plasmids were identified by DNA sequencing.
Antibodies and synthetic peptide.
HIV-1-positive human serum was obtained from an HIV-1-infected subject in Baltimore, Md. Alkaline phosphatase (AP)-conjugated goat anti-human immunoglobulin G (IgG) and AP-conjugated goat anti-mouse IgG were purchased from Jackson ImmunoResearch Laboratories, Inc. (West Grove, Pa.). AP-conjugated antibodies recognizing mouse IgG1, IgG2a, and IgG2b were obtained from Southern Biotechnology, Inc. (Birmingham, Ala.). HIV-1 Gag p24 peptide (p7g, aa 199 AMQMLKETI 207) was synthesized on an Applied Biosystems (Berkeley, Calif.) model 433A peptide synthesizer. Peptide stock solutions were prepared in phosphate-buffered saline (PBS) and were diluted in culture medium to give the appropriate concentration.
Cell lines and transfections.
COS-7 cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 100 U of penicillin per ml, and 100 mg of streptomycin per ml and were passaged upon confluence. p815 cells were maintained in Dulbecco's modified Eagle's medium supplemented with 15% fetal bovine serum, 100 U of penicillin per ml, and 100 mg of streptomycin per ml (complete medium). Cell culture reagents were obtained from Life Technologies (Gaithersburg, Md.). COS-7 cells were transiently transfected by the DEAE-dextran method as previously described (17).
Immunoblotting.
Three days after transfection, cell lysates were prepared as previously described (16). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis was carried out by using standard methods. Proteins were transferred to nitrocellulose filters (Schleicher and Schuell) as previously described (27). The blots were stained by using HIV-1-positive human serum in a PBS solution with 1% nonfat dried milk. The secondary antibody was an alkaline phosphatase-conjugated anti-human antibody (Jackson ImmunoResearch), and staining was carried out with a solution containing 5-bromo-4-chloro-3-indolylphosphate (BCIP) and nitroblue tetrazolium prepared from chemicals obtained from Sigma. Quantitation of Western blots was carried out with the NIH Image Program, version 1.52. Western blots were scanned by using an Eagle Eye, and digital image analysis was carried out with NIH Image.
Vaccination of mice.
Plasmids were propagated in bacterial strain JM109 and were purified with a QIAGEN (Chatsworth, Calif.) Endo-free Maxiprep Kit. The plasmids were resuspended at 1 mg/ml in sterile normal saline solution and were stored at −20°C until the day of immunization. Female BALB/c mice, 6 to 8 weeks old (purchased from Charles River Laboratories, Wilmington, Mass.), were divided into four groups of five mice each, and the mice were each immunized with a total of 100 μg of DNA by i.m. injection with 50 μl of plasmid DNA in separate sites in both side quadriceps, followed by two i.m. booster vaccinations at 2-week intervals as previously described (27).
For immunization with DNA plus recombinant vaccinia viruses, mice received three injections of 50 μg each over 3 weeks (weeks 0, 1, and 2). At 125 days after the last DNA immunization, mice were intraperitoneally (i.p.) inoculated with 107 PFU of recombinant vaccinia viruses containing the HIV-1 gag gene (vP1287, catalog no. 3542; NIH AIDS Research and Reference Reagent Program). All animals used in this study were maintained at the Johns Hopkins University, Baltimore, Md., under the supervision of University Laboratory Animal Resources.
Measurement of anti-Gag antibody titers in vaccinated mice.
BALB/c mice were injected three times i.m. with 100 μg of plasmid DNA each injection at weeks 0, 2, and 4. Anti-Gag antibodies were measured at weeks 3, 4, and 6. Sera were collected from each mouse, and sera within each treatment group were pooled and analyzed by immunoblotting by using purified HIV-1 virions as previously described (27). AP-conjugated anti-mouse IgG, IgG1, IgG2a, or IgG2b, as appropriate, was used as a secondary antibody.
Lymphocyte proliferation assay.
At week 6 (2 weeks after the last DNA inoculation), animals were sacrificed. Lymphocytes from harvested mouse spleens were prepared by Ficoll-Hypaque (Pharmacia, Piscataway, N.J.) density gradient centrifugation. The isolated cells were resuspended at 2 × 106 cells/ml in RPMI 1640. A 100-μl aliquot containing 2 × 105 cells was immediately added to each well of a 96-well microtiter round-bottom plate. Recombinant p24 protein (100 μl at 20 μg/ml, kindly provided by David Schwartz) was added to each well in sextuplicate. The cells were incubated at 37°C in 5% CO2 for 3 days. As a positive control, cells were also stimulated with 1 μg of anti-CD3 antibody (Southern Biotechnology) per ml. Tritiated thymidine (1 μCi) was then added to each well, and the cells were incubated for 8 to 12 h at 37°C. The cells in the plate were washed and harvested, and the amount of incorporated tritiated thymidine was measured in a beta plate reader. The results were expressed as stimulation indices (the ratio of the counts per minute obtained with versus without antigen).
In vitro cytokine assay.
Mouse spleen cells were cultured with p24 antigens in parallel with the proliferation assays. Culture supernatants were collected 3 days after addition of antigen, and the concentrations of interleukin-4 (IL-4) and gamma interferon (IFN-γ) were determined by enzyme-linked immunosorbent assay (ELISA) by using commercial kits (Endogen, Cambridge, Mass.). For these assays, the limit of detection was 5 pg of IL-4 or IFN-γ per ml.
CTL assay.
At week 6 (2 weeks after the final DNA inoculation), animals were sacrificed. Spleens were removed from the immunized mice (five per group) and from naive mice and were compressed through sterile nylon mesh with a rubber stopper and then washed twice with RPMI 1640. Splenic mononuclear cells were isolated by centrifugation through Ficoll-Hypaque (Pharmacia) discontinuous density gradients. The harvested cells were centrifuged for 10 min in a Sorvall H-1000B rotor at 1,000 rpm (200 × g), were washed three times with RPMI 1640, and were resuspended in complete culture medium (RPMI 1640 medium supplemented with 10% fetal calf serum, 50 μM 2-mercaptoethanol, 2 mM l-glutamine, 100 U of penicillin per ml, and 100 μg of streptomycin per ml) for the in vitro restimulation. Cell viability was determined by trypan blue exclusion. The stimulator cells (107/ml), harvested from naive mice, were pulsed with 10 μg of MHC class I-restricted p24 peptide (p7g, aa 199 AMQMLKETI 207) per ml for 1 h at 37°C and then irradiated at 3,000 rads. The cells were pelleted and washed four to five times with RPMI medium. The effector cells (4 × 107 cells) were incubated with stimulator cells at an effector-stimulator ratio of 5:1 for 6 days in a T-25 flask with 10 ml of culture medium containing 10 U of IL-2 per ml.
To measure the specific lysis of these target cells, we used the lactate dehydrogenase (LDH) release assay. This assay yields results similar to those obtained with the standard chromium release assays but does not require the use of radioisotopes. In 96-well round-bottom plates, target cells were incubated with effector cells at various effector-target ratios for 4 h in phenol red-free RPMI 1640 containing 3% fetal calf serum, 2 mM l-glutamine, 100 U of penicillin per ml, and 100 μg of streptomycin per ml. The target cells (p815, 107 cells/ml) were incubated with 10 μg of MHC class I-restricted p24 peptide (aa 199 AMQMLKETI 207) per ml for 1 h at 37°C and then washed three times with assay medium. Fifty microliters of the supernatant per well was then transferred to 96-well plates, and lysis was determined by measuring LDH release by using the Cytotox 96 assay kit (Promega Corp., Madison, Wis.). The released LDH converts the added substrate tetrazolium salt into a red formazan product, and the amount of color is proportional to the number of lysed cells. The absorbance values from supernatants were recorded at 490 nm on an ELISA microplate reader. The percentage of specific lysis of the peptide-pulsed p815 target cells for a given effector cell sample was calculated by the following formula: specific lysis = (optical density [OD] of experimental LDH release − OD of effector cell spontaneous LDH release − OD of target cell spontaneous LDH release)/(maximum target cell LDH release − OD of target cell spontaneous LDH release) × 100%. All determinations were performed in triplicate.
Ex vivo CTL responses.
Female BALB/c mice (10 per group), 6 to 8 weeks old, were immunized by i.m. injection with 50 μg of plasmid DNA (one injection per week) for 3 consecutive weeks. Immunized mice were infected i.p. with recombinant vaccinia virus encoding HIV-1 Gag (vP1287, 107 PFU per mouse) at 125 days after the last DNA vaccination. Five days later, the mice were sacrificed and the class I-restricted, Gag-specific CTL activity in fresh spleen cells isolated from recombinant vaccinia virus-challenged mice was measured without in vitro restimulation by using HIV-1 p24 peptide (aa AMQMLKETI)-pulsed p815 as target cells.
Intracellular cytokine staining and flow cytometry analysis.
Splenocytes from naive or vaccinated groups of mice were incubated with or without the p24 peptide (aa AMQMLKETI). The p24 peptide was added at a concentration of 2 μg/ml for 24 h, and Golgistop (Pharmingen, San Diego, Calif.) was added 6 h before the cells were harvested. The cells were then washed once in fluorescence-activated cell sorter buffer and stained with phycoerythrin-conjugated monoclonal rat anti-mouse CD8 antibody (Pharmingen). Intracellular cytokine staining was also carried out by using the Cytofix/Cytoperm kit as suggested by the manufacturer (Pharmingen). Fluorescein isothiocyanate-conjugated anti-IFN-γ antibodies and the immunoglobulin isotype control antibody (rat IgG1) were all purchased from Pharmingen. Analysis was done on a Becton Dickinson FACScan with CELLQuest Software (Becton Dickinson Immunocytometry Systems, Mountain View, Calif.).
Cytokine ELISPOT assay.
The ELISPOT assay described by Miyahira et al. and Murali-Krishna et al. was modified to detect HIV-1 Gag-specific CD8+ T cells (22, 23). Ninety-six-well filtration plates (Millipore, Bedford, Mass.) were coated overnight at 4°C with 50 μl (10 μg/ml) of anti-mouse IFN-γ (R46A2; Pharmingen) in sterile PBS. The plates were blocked for 2 h at 37°C with sterile RPMI 1640 containing 10% fetal calf serum and 1% bovine serum albumin and were washed three times with sterile PBS. Various dilutions of splenocytes in 200 μl of complete medium with or without MHC class I-restricted p24 peptide (aa AMQMLKETI) were placed in each well and incubated at 37°C for 24 h. Plates were washed with PBS containing 0.025% Tween-20 and were overlaid with 50 μl (5 μg/ml) of biotinylated anti-mouse IFN-γ (XMG1.2; Pharmingen). The plates were washed six times with PBS containing 0.025% Tween-20 and were treated with 1.25 μg of avidin-conjugated alkaline phosphatase (Sigma) per ml for 2 h at room temperature. After a final wash with PBS, IFN-γ spot-forming cells were detected by the addition of BCIP-nitroblue tetrazolium solution (Sigma) and were counted with a stereomicroscope.
Vaccinia virus titer in the ovaries of challenged mice.
At 125 days after the final DNA vaccination, mice (10 per group) were challenged i.p. with 107 PFU of vaccinia virus expressing HIV-1 Gag (vP1287). Five days after the challenge, the mice were sacrificed, and the ovaries were removed, homogenized, sonicated, and assayed for vP1287 titer by plating serial 10-fold dilutions on a plate of BSC-1 indicator cells. After 2 days of culture, the medium was removed, the BSC-1 cell monolayer was stained with 0.1% crystal violet (Sigma) for 5 min, and the number of plaques per well was counted.
RESULTS
Construction and expression of various HIV-1 Gag expression vectors.
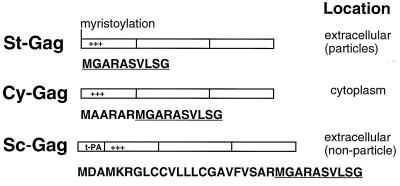
The Gag molecule is the only HIV-1 viral structural protein required for assembly of virus-like particles. It is designed to interact with itself, to target the plasma membrane, to interact with the lipid membrane, and to assemble and bud from the cell surface. The vector pSt-GAG expresses St-Gag molecules and has been previously described (27). For these studies, a vector expressing Cy-Gag, pCy-GAG, was generated by insertion mutations that destroyed the myristylation signal of the Gag molecule. Myristylation and a cluster of positively charged amino acids in Gag are a bipartite plasma membrane-targeting signal for HIV-1 Gag molecules (5, 12, 16, 42). To generate the vector expressing Sc-Gag, pSc-GAG, the first 21 amino acids from t-PA were fused with the N terminus of Gag (Fig. 1). The first 21 amino acids of t-PA target protein synthesis to the ER and lead to the secretion of the fusion protein.
FIG. 1.
Schematic representation of HIV-1 Gag expression vectors. Cytomegalovirus promoter and bovine growth hormone polyadenylation signal flank HIV-1 Gag coding sequences (not shown). The first 10 amino acids of HIV-1 Gag (underlined) and the inserted amino acids before Gag are shown below each construct.
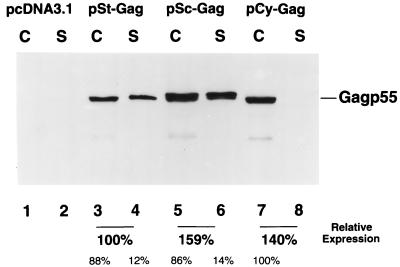
Protein expression by the various HIV-1 Gag expression vectors was evaluated in transfected COS-7 cells. When cell lysates from the transfected COS-7 cells were analyzed by immunoblotting by using an HIV-1-positive human serum, Gag protein was detected in transfected COS-7 cells with the pSt-GAG, pSc-GAG, and pCy-GAG constructs (Fig. 2, lanes 3, 5, and 7). As expected, Gag protein was not detected in the control pcDNA3.1-transfected COS-7 cells (Fig. 2, lane 1). Gag protein in the pelletable form was also detected in the culture supernatants from cells transfected with pSt-GAG (lane 4) and pSc-GAG (lane 6), but not with pCy-GAG-transfected COS-7 cells (Fig. 2). No pelletable form of Gag p55 was detected in the culture supernatants of pcDNA3.1- (lane 2) or pCy-GAG-transfected (lane 8) COS-7 cells (Fig. 2). Gag molecules in the supernatants of pSt-GAG- and pSc-GAG-transfected COS-7 cells were also analyzed by 15 to 60% linear sucrose density equilibrium gradients as described previously (16). Although virus-like particles were detected for St-Gag in the fractions of sucrose gradients corresponding to the density of wild-type HIV-1 virions, no virus-like particles were detected in the same fractions of sucrose gradients prepared with Sc-Gag (data not shown). These data indicate that targeting the HIV-1 Gag molecule to various subcellular compartments was achieved by modifying the St-Gag DNA vaccine vector.
FIG. 2.
HIV-1 Gag expression in transfected COS cells with various DNA constructs. Cell lysates (lanes C) and Sc-Gag (lanes S) in the supernatants from pcDNA3.1-, pSt-GAG-, pSc-GAG-, and pCy-GAG-transfected cells were prepared and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to nitrocellulose filters, and analyzed by immunoblotting with an HIV-1-positive human serum. The Gag expression levels in pSt-GAG-, pSc-GAG-, and pCy-GAG-transfected cells and supernatants were quantified as described in Materials and Methods. The total amount of St-Gag was assigned as 100%. The relative amounts for Sc-Gag and Cy-Gag are indicated. The distributions of Gag in the cell lysates and in the supernatants are also indicated.
Anti-Gag antibody responses in mice immunized with naked DNA vaccine.
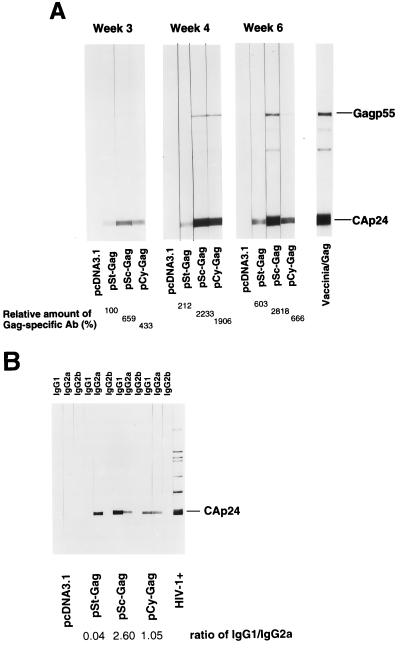
BALB/c mice were i.m. immunized with one of the various DNA vectors three times (at weeks 0, 2, and 4). Anti-Gag antibodies in sera collected from the mice at weeks 3, 4, and 6 were analyzed by immunoblotting by using purified mature HIV-1 virions as previously described (27). Mice immunized with all three Gag expression vectors showed anti-Gag antibody responses at weeks 3, 4, and 6; the anti-Gag antibodies reacted predominantly with CAp24 (Fig. 3A). No anti-Gag antibodies were detected in mice immunized with pcDNA3.1. pSt-GAG generated a predominant IgG2a antibody response and pSc-GAG and pCy-GAG generated both IgG1 and IgG2a antibodies (Fig. 3B), although the IgG1 antibody responses seemed to be stronger than the IgG2a antibody response in the pSc-GAG-immunized mice (Fig. 3B). No IgG2b anti-Gag antibody was detected in any group of mice.
FIG. 3.
BALB/c mice were i.m. injected three times with 100 μg of one of the plasmid DNAs at weeks 0, 2, and 4. (A) Anti-HIV-1 Gag antibody responses were measured at weeks 3, 4, and 6. Sera were collected from each group of mice injected with one of the various DNA constructs, and the sera were pooled and analyzed by immunoblotting by using purified mature HIV-1 virions. Vaccinia/Gag, sera from mice infected with recombinant vaccinia virus-Gag. The intensity of the Gag-specific antibody response for pSt-GAG-immunized mice at week 3 was designated as 100%. (B) Gag-specific IgG subclass responses in DNA-immunized mice were assayed by using alkaline phosphatase-conjugated anti-mouse IgG1, IgG2a, or IgG2b as secondary antibody. The relative intensities of the IgG1 and IgG2a responses were quantified as described in Materials and Methods. The ratio of IgG1 to IgG2a is indicated.
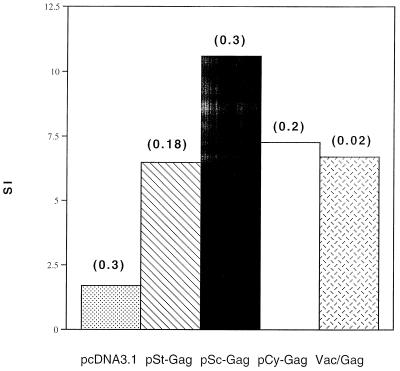
T-cell-proliferative response and cytokine production by splenocytes from DNA-vaccinated mice.
BALB/c mice were injected i.m. with plasmid DNA at weeks 0, 2, and 4. Splenocytes from the immunized mice were harvested at week 6, were pooled, and were incubated for standard in vitro lymphocyte proliferation assays with recombinant p24 protein. As shown in Fig. 4, Sc-Gag generated a better Gag-specific T-cell-proliferative response than did St-Gag or Cy-Gag. Sc-Gag also induced a higher T-cell-proliferative response than did the recombinant vaccinia virus expressing the HIV-1 Gag protein.
FIG. 4.
Lymphocyte proliferation assay. BALB/c mice were immunized as outlined in the legend to Fig. 3. T-cell-proliferation responses were measured at week 6. Splenocytes from each mouse group were harvested, pooled, and tested in a standard [3H]thymidine uptake assay by using purified recombinant p24 as antigen. The number above each bar represents standard deviation.
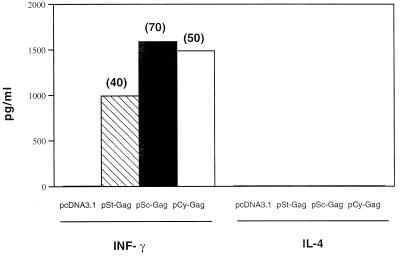
IFN-γ and IL-4 levels were measured by ELISA in supernatants of p24 protein-stimulated spleen cells. The cytokine pattern found in the supernatants of spleen cells after stimulation with recombinant p24 antigens was clearly that of type 1 cytokine, characterized by elevated levels of IFN-γ. The levels of IL-4 were below the level of detection (5 pg/ml) (Fig. 5), although supernatant from control anti-CD3 antibody-stimulated splenocyte cultures contained detectable levels of secreted IL-4 (data not shown).
FIG. 5.
In vitro cytokine production. BALB/c mice were immunized as in Fig. 3. Splenocytes from each mouse group were harvested at week 6, were pooled, and were cultured at 200,000 per well in 96-well plates stimulated with recombinant p24 as antigen. Culture supernatants were collected before adding the tritiated thymidine, and concentrations of IL-4 and IFN-γ were determined by ELISA by using commercial kits (Endogen). For these assays, the limit of detection was 5 pg of IL-4 or IFN-γ per ml. The number above each bar represents standard deviation.
CTL responses.
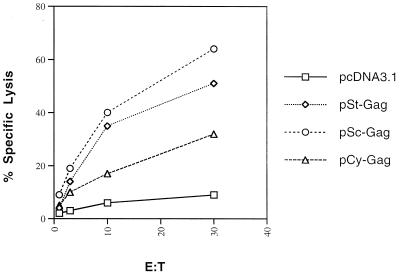
Mice were immunized with various DNA constructs as described above. At week 6, splenocytes from the mice in each group were harvested and pooled, and the CTL responses specific to HIV-1 Gag were measured following antigen restimulation in vitro. The group of vaccinated mice that received pSc-GAG developed a higher level of HIV-1 Gag-specific lytic activity than those that received pCy-GAG (Fig. 6). It seems that retaining more HIV-1 Gag in the cytoplasm did not generate better MHC class I peptides for the induction of CTL when the mice were immunized i.m. with DNA.
FIG. 6.
BALB/c mice were immunized as in Fig. 3. Anti-HIV-1 Gag CTL responses were measured at week 6. Splenocytes from the mice in each group were harvested and pooled, and the CTL responses specific to HIV-1 p24 peptide were measured following antigen restimulation in vitro. The target cells (p815) were pulsed with p24 peptide.
Ex vivo secondary CTL response.
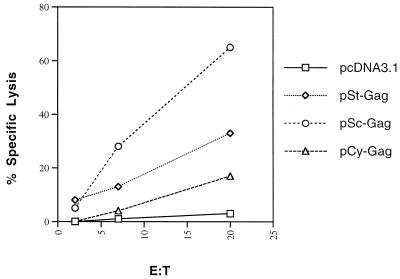
Recent data suggest that DNA vaccination is an excellent strategy for priming cellular immune responses. We were also interested in determining which form of DNA vector can induce higher priming. We immunized the mice three times with various HIV-1 Gag DNA expression vectors, and 125 days after the last DNA injection they were infected i.p. with a recombinant vaccinia virus vector encoding HIV-1 Gag (vP1287, 107 PFU per mouse). Five days later, the mice (three per group) were sacrificed and the MHC class I-restricted, Gag-specific CTL activity in fresh spleen cells was measured without in vitro restimulation. Spleen cells from mice immunized with control vector and challenged with vP1287 showed only background activity, with less than 10% cytolytic activity (Fig. 7). Spleen cells from mice immunized with all three Gag expression vectors showed significant cytolytic activity after challenge with vP1287 (Fig. 7). pSc-GAG generated the highest Gag-specific CTL activity, and pCy-GAG generated the lowest Gag-specific CTL activity (Fig. 7). Since significant CTL responses were not detected in mice immunized with control DNA vector 5 days after challenge with vP1287, the CTL response observed in mice immunized with Gag expression vectors 5 days after challenge with vP1287 must have come from memory T cells.
FIG. 7.
BALB/c mice were i.m. injected three times with 50 μg of plasmid DNA at weeks 0, 1, and 2. At 125 days after the last DNA injection, mice were i.p. challenged with a recombinant vaccinia virus vector encoding HIV-1 Gag (107 PFU per mouse). Anti-HIV-1 Gag CTL responses were measured five days later. Splenocytes from the mice in each group were harvested and pooled, and the CTL responses specific to the HIV-1 p24 peptide (aa AMQMLKETI) were measured by using target cells (p815) pulsed with p24 peptide without in vitro restimulation.
Intracellular cytokine staining and flow cytometry analysis and ELISPOT assay.
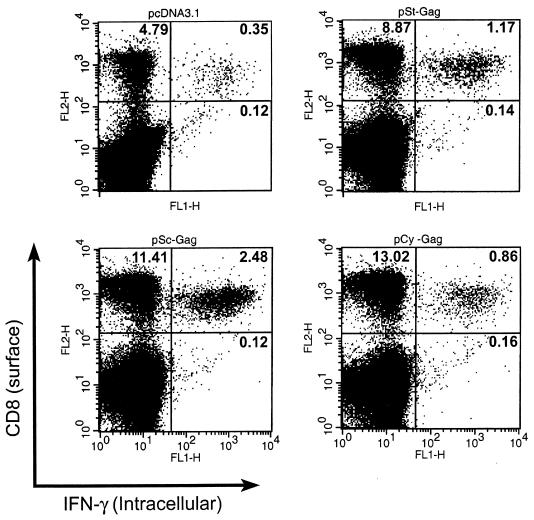
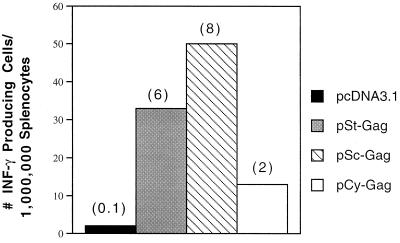
CD8+ T lymphocytes are one of the most crucial components of antiviral effector cells. We therefore assessed the number of HIV-1 Gag-specific CD8+ T cells expressing IFN-γ in the spleens of mice immunized with DNA vector and challenged with vP1287. These cells were measured by brief stimulation in vitro for 24 h with MHC class I-restricted HIV p24 peptide (aa AMQMLKETI), followed by staining for CD8 and intracellular IFN-γ or ELISPOT assays. Intracellular cytokine staining and ELISPOT are sensitive functional assays used to measure the IFN-γ production at the single-cell level. We observed a high level of Gag-specific CD8+ effector cells in the spleens of mice immunized with the pSc-GAG plasmid (2.5% of the total splenocytes and 18% of the total CD8+ T cells) within 5 days of vP1287 challenge (Fig. 8). In contrast, less than 0.4% of the total splenocytes were CD8+ T cells expressing IFN-γ in control vector-immunized mice. The level of Gag-specific CD8+ effector cells in the spleens of mice immunized with pCy-GAG plasmid (0.8% of the total splenocytes and 7% of the total CD8+ T cells) was lower than that obtained with pSc-GAG. The background level in mice immunized with pcDNA3.1 and challenged with recombinant vaccinia virus expressing HIV-1 Gag was low, suggesting that Gag-specific CD8+ IFN-γ-producing cells were generated from memory T cells.
FIG. 8.
Intracellular cytokine staining and flow cytometry analysis. Mice were immunized as outlined in the legend to Fig. 7 and were i.p. challenged with a recombinant vaccinia virus vector encoding HIV-1 Gag (107 PFU per mouse) at 125 days after the final DNA vaccination. Splenocytes were harvested 5 days after the recombinant vaccinia virus challenge. Splenocytes from vaccinia virus-challenged mice were cultured in vitro with p24 peptide (aa AMQMLKETI) for 24 h and were stained for both CD8 and intracellular IFN-γ. Numbers indicate the percentage of cells in each quadrant.
The results of the ELISPOT assay (Fig. 9) paralleled those obtained for intracellular cytokine staining and flow cytometry analysis. The background level of Gag-specific CD8+ effector cells secreting IFN-γ in the spleens of mice immunized with the control vector was low (Fig. 9). We observed a higher level of Gag-specific CD8+ effector cells in the spleens of mice immunized with pSc-GAG plasmid than in those immunized with the pCy-GAG plasmid (Fig. 9) within 5 days of vP1287 challenge.
FIG. 9.
ELISPOT assay. Mice were immunized as outlined in the legend to Fig. 7 and were i.p. challenged with a recombinant vaccinia virus vector encoding HIV-1 Gag (107 PFU per mouse) at 125 days after the final DNA vaccination. Splenocytes were harvested 5 days after the recombinant vaccinia virus challenge. The spot numbers are the means of the triplicates. Error bars indicate the standard deviations from triplicated cultures.
Vaccinia virus titer in the ovaries of immunized mice.
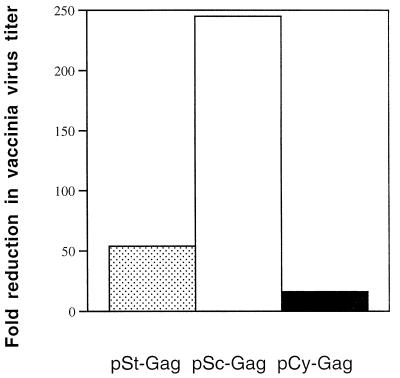
Finally, we addressed the question of whether the immune responses induced by HIV-1 Gag DNA vaccines were protective, and we also assessed the stability of long-term memory in DNA vaccination by using recombinant vaccinia virus expressing HIV-1 Gag (vP1287). We chose the i.p. route for these experiments because this particular vaccinia virus has been found to replicate best in the ovaries (3). On day 125 after the last DNA immunization, we challenged the immunized mice by i.p. infusion with vP1287. Five days after the challenge, the mice were sacrificed, the ovaries were removed, and the vP1287 titer in the ovaries was determined. Mice immunized with the pSc-GAG vaccine showed a 245-fold reduction in the average viral titer in the ovaries after an i.p. challenge with vP1287, compared to the titer in control vector-immunized animals (Fig. 10). In contrast, mice immunized with the pCy-GAG vaccine showed only a 15-fold reduction in viral titer in the ovaries (Fig. 10). These results suggest that mice immunized with HIV-1 Gag DNA vectors developed anti-Gag cellular immune responses which could protect the mice against infection with vaccinia virus expressing HIV-1 Gag.
FIG. 10.
Studies of vaccinia virus titer in the ovaries of immunized mice. At 125 days after the final DNA vaccination, mice were challenged i.p. with 107 PFU of vaccinia virus expressing HIV-1 Gag. Five days after the challenge, mice were sacrificed, and ovaries were removed, homogenized, and sonicated. The supernatants of the homogenates were assayed for virus titer on BSC-1 indicator cells. Results were expressed as fold of reduction in vaccinia virus titer in Gag-DNA-vaccinated mice versus the titer in control mice.
DISCUSSION
We have previously demonstrated that silent-site mutations of multiple inhibitory sequences in the coding region of HIV-1 gag make the expression of Gag proteins Rev and RRE independent and species independent (34). This approach has allowed us to induce strong humoral and cellular immune responses in mice and to create a small animal model (mice) for systematically evaluating various DNA vaccination strategies (27). In the present study, we have addressed the question of whether targeting HIV-1 Gag to various subcellular compartments could influence the induction of immune responses in DNA-immunized mice. In the wild-type virus, expression of HIV-1 Gag protein alone leads to efficient targeting of the Gag molecules to the plasma membrane and the assembly of virus-like particles, which are released after budding from the plasma membrane. By destroying the myristylation signal of HIV-1 Gag, we created mutant Gag proteins that are not targeted efficiently to the plasma membrane and remain primarily in the cytoplasm (16, 42). We have also created Sc-Gag molecules by the addition of the t-PA signal peptide sequence to the N terminus of the HIV-1 Gag molecule. This sequence provides a signal for translocation of the secreted protein into the lumen of the ER, for transport through the ER and Golgi apparatus, and for release in the form of Sc-Gag molecules.
Our results demonstrate that targeting the HIV-1 Gag molecules to different subcellular compartments does indeed influence both the humoral and cellular immune responses that are elicited by i.m. DNA vaccination. The Sc-Gag antigen elicited a higher T-cell-proliferative response than did Cy-Gag antigen. Somewhat surprisingly, the Sc-Gag antigen also induced a stronger CTL response than did the Cy-Gag antigen. After immunization with DNA vectors expressing Gag antigens, mice developed more potent and rapid CTL responses against Gag upon challenge with recombinant HIV-1 Gag-vaccinia virus than did mice receiving only control DNA vectors. The secondary CTL response developed against the DNA vector containing Sc-Gag antigen was also higher than that in mice which received DNA vector expressing the Cy-Gag antigen.
There have been several reports regarding the use of t-PA signal peptides in DNA vaccines. In the case of HIV-1 Env DNA vaccine (20), replacing the authentic signal peptide of gp160 with that of t-PA was intended to overcome the Rev/RRE requirement for Env protein expression (6). Replacing the signal peptide sequences of mycobacterial proteins with that of t-PA in DNA vectors has been shown to correlate with more protection against tuberculous challenge in mice, although CTL responses were not measured (19). DNA vectors containing fusion of t-PA peptide with Plasmodium vivax antigens did not significantly increase antibody production in mice, and cellular immune responses were not evaluated (30). Whether the t-PA signal peptide can enhance the induction of immune responses for cytoplasmic antigens in general by means of a DNA vaccine strategy requires further investigation.
The mechanism underlying the induction of immune responses after i.m. DNA vaccination is still largely unclear. It has been shown that bone-marrow-derived antigen-presenting cells are critical for the induction of immune responses after i.m. DNA injection, suggesting that muscle cells are not responsible for the priming of immune responses (40). It is possible that dendritic cells or other antigen-presenting cells are directly transfected by the DNA after i.m. injection, and endogenously synthesized antigens are presented (1, 7, 8, 39). Alternatively, muscle cells may take up the DNA, express the protein antigen, and transfer the antigen to antigen-presenting cells (9, 40). If the former possibility is the predominant pathway for antigen presentation, one would expect that Cy-Gag molecules will have a better chance than Sc-Gag molecules to be processed by cytoplasmic proteasomes and presented on MHC class I molecules. Since the Sc-Gag molecules induced better CTL responses than did the Cy-Gag molecules in our system, it appears that the alternative pathway (uptake by muscle cells) is likely to be more efficient. Muscle cells may take up injected DNA and express protein antigens that are subsequently transferred to migratory dendritic cells. In this case, a secreted antigen may be transferred more efficiently than a cytoplasmic antigen.
The enhanced CTL responses induced by the DNA vector containing Sc-Gag molecules, compared to a DNA vector expressing Cy-Gag molecules, also correlated with better protection against subsequent infection with a recombinant vaccinia virus expressing HIV-1 Gag in immunized mice. HIV-1 Gag CTL responses induced by the DNA vaccine may kill recombinant vaccinia virus-Gag-infected cells before more progeny viruses can be produced, therefore reducing the viral load in the ovaries of the mice. Mice immunized with DNA vector containing Sc-Gag molecules showed a 245-fold reduction in recombinant vaccinia virus titer when compared to the results for mice immunized with the control DNA vector. On the other hand, mice immunized with a DNA vector containing Cy-Gag molecules showed only a 15-fold reduction in recombinant vaccinia virus titer compared to that for mice immunized with the control DNA vector.
Our strategy of DNA vaccination followed by recombinant vaccinia virus-Gag challenge also allowed us to establish a novel physiological method for measuring the memory CD8+ cytotoxic activity in DNA-vaccinated mice without in vitro restimulation. We have also found a good correlation between memory CTL activity and the number of Gag-specific, IFN-γ-producing CD8+ T cells identified by intracellular cytokine staining and ELISPOT. This in vivo challenge and stimulation system can be applied to other antigens in which the MHC class I epitopes are unclear and can extend the evaluation of relative long-term potencies among several vaccine strategies.
A recent study has shown that altering the cellular location of glycoprotein D (gD) from bovine herpesvirus 1 by DNA vaccine modulates humoral immune response. Although both the secreted and cytosolic forms of gD induced an IgG2a antibody response, the secreted form of gD induced a stronger IgG1 response than IgG2a response (18). We have observed similar results for Sc-Gag and Cy-Gag in this study. On the other hand, St-Gag, which is competent for forming virus-like particles, induced a predominantly IgG2a antibody response. Our data is consistent with the idea that location of antigens after DNA immunization could influence the type and potency of humoral immune responses.
Effective HIV-1 antigen-specific CD4+ T-helper responses have been shown to correlate with control of virus infection (31). These responses are thought to make a critical contribution to the maintenance of effective immunity in chronic viral infection by enhancing the CTL response or increasing production of antiviral cytokines or chemokines. We have now evaluated the induction of T-helper responses after DNA immunization in mice by measuring T-cell proliferative and cytokine responses after recombinant p24 stimulation in vitro. It is encouraging to find that Sc-Gag induced a higher T-cell-proliferative response than did the vaccinia virus vector expressing HIV-1 Gag. A better T-helper response may also be partially responsible for the enhanced CTL responses induced by the DNA vector expressing Sc-Gag molecules. Furthermore, not only will vaccine strategies that induce CD4+ T-helper cells in the memory stage shorten the time of antiviral response after infection and therefore give the host an upper hand, but it has also been shown that memory CD4+ T cells are more resistant to primary CCR5-tropic virus infection than naive CD4+ T cells when activated (28).
Although DNA vaccine alone has been shown to protect against pathogenic challenges in small animals (41), its performance in primates has been generally disappointing. DNA vaccine, even with repeated boosting, induces only moderate immune responses when compared to live-attenuated virus or recombinant virus vaccines. However, recent studies have demonstrated that heterologous priming-boosting immunization regimens using DNA plus recombinant modified vaccinia virus Ankara vectors can induce strong cellular immune responses and protection against malaria in mice (33, 38) and SIVmac (13, 29) in monkey models. Although T-cell immune responses induced by DNA immunization are moderate, they are highly focused upon a few specific epitopes, because of the small number of other epitopes expressed by this antigen delivery system. A boost with a recombinant vaccinia virus expressing the same antigen presumably stimulates this population of primed memory T cells. Our data showed that pSc-GAG induced higher memory T-cell responses than other Gag expression vectors as measured by ex vivo CTL activity, higher number of CD8+ IFN-γ-producing cells after stimulation with MHC class I-restricted HIV-1 Gag-specific peptide, and greater protection against recombinant vaccinia virus-Gag infection. These Gag expression vectors may be useful for further evaluation of heterologous priming and boosting with DNA plus viral vector in inducing protective cellular immune responses. Similar strategies could be considered for nonhuman primate models where SIV or simian/human immunodeficiency virus challenge can be evaluated.
ACKNOWLEDGMENTS
We thank C. H. Chen, T. L. Tian, and T. C. Wu from the Johns Hopkins Hospital Department of Pathology for technical assistance. We are grateful to David Schwartz and Richard Markham for helpful discussions. The following reagents were obtained through the AIDS Research Reagents Program, Division of AIDS, NIAID, and NIH: recombinant vaccinia virus-Gag (vP1287, catalog no. 3542) and purified p24Gag (catalog no. 382).
This work was supported by National Institutes of Health grants AI-33862 and AI-42624 to X.-F.Y.
REFERENCES
- 1.Akbari O, Panjwani N, Garcia S, Tascon R, Lowrie D, Stockinger B. DNA vaccination: transfection and activation of dendritic cells as key events for immunity. J Exp Med. 1999;189:169–178. doi: 10.1084/jem.189.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aldhous M C, Watret K C, Mok J Y, Bird A G, Froebel K S. Cytotoxic T lymphocyte activity and CD8 subpopulations in children at risk of HIV infection. Clin Exp Immunol. 1994;97:61–67. doi: 10.1111/j.1365-2249.1994.tb06580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belyakov I M, Derby M A, Ahlers J D, Kelsall B L, Earl P, Moss B, Strober W, Berzofsky J A. Mucosal immunization with HIV-1 peptide vaccine induces mucosal and systemic cytotoxic T lymphocytes and protective immunity in mice against intrarectal recombinant HIV-vaccinia challenge. Proc Natl Acad Sci USA. 1998;95:1709–1714. doi: 10.1073/pnas.95.4.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borrow P, Lewicki H, Hahn B H, Shaw G M, Oldstone M B. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994;68:6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bryant M, Ratner L. Myristoylation-dependent replication and assembly of human immunodeficiency virus 1. Proc Natl Acad Sci USA. 1990;87:523–527. doi: 10.1073/pnas.87.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chapman B S, Thayer R M, Vincent K A, Haigwood N L. Effect of intron A from human cytomegalovirus (Towne) immediate-early gene on heterologous expression in mammalian cells. Nucleic Acids Res. 1991;19:3979–3986. doi: 10.1093/nar/19.14.3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chattergoon M A, Robinson T M, Boyer J D, Weiner D B. Specific immune induction following DNA-based immunization through in vivo transfection and activation of macrophages/antigen-presenting cells. J Immunol. 1998;160:5707–5718. [PubMed] [Google Scholar]
- 8.Condon C, Watkins S C, Celluzzi C M, Thompson K, Falo L D., Jr DNA-based immunization by in vivo transfection of dendritic cells. Nat Med. 1996;2:1122–1128. doi: 10.1038/nm1096-1122. [DOI] [PubMed] [Google Scholar]
- 9.Corr M, von Damm A, Lee D J, Tighe H. In vivo priming by DNA injection occurs predominantly by antigen transfer. J Immunol. 1999;163:4721–4727. [PubMed] [Google Scholar]
- 10.Donnelly J J, Ulmer J B, Shiver J W, Liu M A. DNA vaccines. Annu Rev Immunol. 1997;15:617–648. doi: 10.1146/annurev.immunol.15.1.617. [DOI] [PubMed] [Google Scholar]
- 11.Durali D, Morvan J, Letourneur F, Schmitt D, Guegan N, Dalod M, Saragosti S, Sicard D, Levy J P, Gomard E. Cross-reactions between the cytotoxic T-lymphocyte responses of human immunodeficiency virus-infected African and European patients. J Virol. 1998;72:3547–3553. doi: 10.1128/jvi.72.5.3547-3553.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gottlinger H G, Sodroski J G, Haseltine W A. Role of capsid precursor processing and myristoylation in morphogenesis and infectivity of human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1989;86:5781–5785. doi: 10.1073/pnas.86.15.5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanke T, Samuel R V, Blanchard T J, Neumann V C, Allen T M, Boyson J E, Sharpe S A, Cook N, Smith G L, Watkins D I, Cranage M P, McMichael A J. Effective induction of simian immunodeficiency virus-specific cytotoxic T lymphocytes in macaques by using a multiepitope gene and DNA prime-modified vaccinia virus Ankara boost vaccination regimen. J Virol. 1999;73:7524–7532. doi: 10.1128/jvi.73.9.7524-7532.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koup R A, Safrit J T, Cao Y, Andrews C A, McLeod G, Borkowsky W, Farthing C, Ho D D. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Langlade-Demoyen P, Ngo-Giang-Huong N, Ferchal F, Oksenhendler E. Human immunodeficiency virus (HIV) nef-specific cytotoxic T lymphocytes in noninfected heterosexual contact of HIV-infected patients. J Clin Investig. 1994;93:1293–1297. doi: 10.1172/JCI117085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee Y-M, Tian C-J, Yu X-F. A bipartite membrane-binding signal in the human immunodeficiency virus type-1 matrix protein is required for the proteolytic processing of Gag precursors in a cell type-dependent manner. J Virol. 1998;72:9061–9068. doi: 10.1128/jvi.72.11.9061-9068.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee Y-M, Tang X B, Cimakasky L M, Hildreth J E, Yu X F. Mutations in the matrix protein of human immunodeficiency virus type 1 inhibit surface expression and virion incorporation of viral envelope glycoproteins in CD4+ T lymphocytes. J Virol. 1997;71:1443–1452. doi: 10.1128/jvi.71.2.1443-1452.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewis P J, van Drunen Littel-van den Hurk S, Babiuk L A. Altering the cellular location of an antigen expressed by a DNA-based vaccine modulates the immune response. J Virol. 1999;73:10214–10223. doi: 10.1128/jvi.73.12.10214-10223.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Z, Howard A, Kelley C, Delogu G, Collins F, Morris S. Immunogenicity of DNA vaccines expressing tuberculosis proteins fused to tissue plasminogen activator signal sequences. Infect Immun. 1999;67:4780–4786. doi: 10.1128/iai.67.9.4780-4786.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu S, Santoro J C, Fuller D H, Haynes J R, Robinson H L. Use of DNAs expressing HIV-1 Env and noninfectious HIV-1 particles to raise antibody responses in mice. Virology. 1995;209:147–154. doi: 10.1006/viro.1995.1238. [DOI] [PubMed] [Google Scholar]
- 21.McAdam S, Kaleebu P, Krausa P, Goulder P, French N, Collin B, Blanchard T, Whitworth J, McMichael A, Gotch F. Cross-clade recognition of p55 by cytotoxic T lymphocytes in HIV-1 infection. AIDS. 1998;12:571–579. doi: 10.1097/00002030-199806000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Miyahira Y, Murata K, Rodriguez D, Rodriguez J R, Esteban M, Rodrigues M M, Zavala F. Quantification of antigen specific CD8+ T cells using an ELISPOT assay. J Immunol Methods. 1995;181:45–54. doi: 10.1016/0022-1759(94)00327-s. [DOI] [PubMed] [Google Scholar]
- 23.Murali-Krishna K, Altman J D, Suresh M, Sourdive D J, Zajac A J, Miller J D, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 24.Musey L, Hughes J, Schacker T, Shea T, Corey L, McElrath M J. Cytotoxic-T-cell responses, viral load, and disease progression in early human immunodeficiency virus type 1 infection. N Engl J Med. 1997;337:1267–1274. doi: 10.1056/NEJM199710303371803. [DOI] [PubMed] [Google Scholar]
- 25.Ogg G S, Jin X, Bonhoeffer S, Dunbar P R, Nowak M A, Monard S, Segal J P, Cao Y, Rowland-Jones S L, Cerundolo V, Hurley A, Markowitz M, Ho D D, Nixon D F, McMichael A J. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science. 1998;279:2103–2106. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- 26.Pantaleo G, Demarest J F, Soudeyns H, Graziosi C, Denis F, Adelsberger J W, Borrow P, Saag M S, Shaw G M, Sekaly R P, et al. Major expansion of CD8+ T cells with a predominant V beta usage during the primary immune response to HIV. Nature. 1994;370:463–467. doi: 10.1038/370463a0. [DOI] [PubMed] [Google Scholar]
- 27.Qiu J T, Song R, Dettenhofer M, Tian C, August T, Felber B K, Pavlakis G N, Yu X F. Evaluation of novel human immunodeficiency virus type 1 Gag DNA vaccines for protein expression in mammalian cells and induction of immune responses. J Virol. 1999;73:9145–9152. doi: 10.1128/jvi.73.11.9145-9152.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riley J L, Levine B L, Craighead N, Francomano T, Kim D, Carroll R G, June C H. Naive and memory CD4 T cells differ in their susceptibilities to human immunodeficiency virus type 1 infection following CD28 costimulation: implications for transmission and pathogenesis. J Virol. 1998;72:8273–8280. doi: 10.1128/jvi.72.10.8273-8280.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robinson H L, Montefiori D C, Johnson R P, Manson K H, Kalish M L, Lifson J D, Rizvi T A, Lu S, Hu S L, Mazzara G P, Panicali D L, Herndon J G, Glickman R, Candido M A, Lydy S L, Wyand M S, McClure H M. Neutralizing antibody-independent containment of immunodeficiency virus challenges by DNA priming and recombinant pox virus booster immunizations. Nat Med. 1999;5:526–534. doi: 10.1038/8406. [DOI] [PubMed] [Google Scholar]
- 30.Rogers W O, Gowda K, Hoffman S L. Construction and immunogenicity of DNA vaccine plasmids encoding four Plasmodium vivax candidate vaccine antigens. Vaccine. 1999;17:3136–3144. doi: 10.1016/s0264-410x(99)00146-2. [DOI] [PubMed] [Google Scholar]
- 31.Rosenberg E S, Billingsley J M, Caliendo A M, Boswell S L, Sax P E, Kalams S A, Walker B D. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278:1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 32.Rowland-Jones S L, Nixon D F, Aldhous M C, Gotch F, Ariyoshi K, Hallam N, Kroll J S, Froebel K, McMichael A. HIV-specific cytotoxic T-cell activity in an HIV-exposed but uninfected infant. Lancet. 1993;341:860–861. doi: 10.1016/0140-6736(93)93063-7. [DOI] [PubMed] [Google Scholar]
- 33.Schneider J, Gilbert S C, Blanchard T J, Hanke T, Robson K J, Hannan C M, Becker M, Sinden R, Smith G L, Hill A V. Enhanced immunogenicity for CD8+ T cell induction and complete protective efficacy of malaria DNA vaccination by boosting with modified vaccinia virus Ankara. Nat Med. 1998;4:397–402. doi: 10.1038/nm0498-397. [DOI] [PubMed] [Google Scholar]
- 34.Schneider R, Campbell M, Nasioulas G, Felber B K, Pavlakis G N. Inactivation of the human immunodeficiency virus type 1 inhibitory elements allows Rev-independent expression of Gag and Gag/protease and particle formation. J Virol. 1997;71:4892–4903. doi: 10.1128/jvi.71.7.4892-4903.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwartz D, Sharma U, Busch M, Weinhold K, Matthews T, Lieberman J, Birx D, Farzedagen H, Margolick J, Quinn T, et al. Absence of recoverable infectious virus and unique immune responses in an asymptomatic HIV+ long-term survivor. AIDS Res Hum Retrovir. 1994;10:1703–1711. doi: 10.1089/aid.1994.10.1703. [DOI] [PubMed] [Google Scholar]
- 36.Schwartz S, Campbell M, Nasioulas G, Harrison J, Felber B K, Pavlakis G N. Mutational inactivation of an inhibitory sequence in human immunodeficiency virus type 1 results in Rev-independent gag expression. J Virol. 1992;66:7176–7182. doi: 10.1128/jvi.66.12.7176-7182.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwartz S, Felber B K, Pavlakis G N. Distinct RNA sequences in the gag region of human immunodeficiency virus type 1 decrease RNA stability and inhibit expression in the absence of Rev protein. J Virol. 1992;66:150–159. doi: 10.1128/jvi.66.1.150-159.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sedegah M, Jones T R, Kaur M, Hedstrom R, Hobart P, Tine J A, Hoffman S L. Boosting with recombinant vaccinia increases immunogenicity and protective efficacy of malaria DNA vaccine. Proc Natl Acad Sci USA. 1998;95:7648–7653. doi: 10.1073/pnas.95.13.7648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Torres C A, Iwasaki A, Barber B H, Robinson H L. Differential dependence on target site tissue for gene gun and intramuscular DNA immunizations. J Immunol. 1997;158:4529–4532. [PubMed] [Google Scholar]
- 40.Ulmer J B, Deck R R, Dewitt C M, Donnhly J I, Liu M A. Generation of MHC class I-restricted cytotoxic T lymphocytes by expression of a viral protein in muscle cells: antigen presentation by non-muscle cells. Immunology. 1996;89:59–67. doi: 10.1046/j.1365-2567.1996.d01-718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ulmer J B, Donnelly J J, Parker S E, Rhodes G H, Felgner P L, Dwarki V J, Gromkowski S H, Deck R R, DeWitt C M, Friedman A, et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259:1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 42.Yuan X, Yu X, Lee T H, Essex M. Mutations in the N-terminal region of human immunodeficiency virus type 1 matrix protein block intracellular transport of the Gag precursor. J Virol. 1993;67:6387–6394. doi: 10.1128/jvi.67.11.6387-6394.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]