Abstract
Resistance training not only can improve or maintain muscle mass and strength, but also has favorable physiological and clinical effects on cardiovascular disease and risk factors. This scientific statement is an update of the previous (2007) American Heart Association scientific statement regarding resistance training and cardiovascular disease. Since 2007, accumulating evidence suggests resistance training is a safe and effective approach for improving cardiovascular health in adults with and without cardiovascular disease. This scientific statement summarizes the benefits of resistance training alone or in combination with aerobic training for improving traditional and nontraditional cardiovascular disease risk factors. We also address the utility of resistance training for promoting cardiovascular health in varied healthy and clinical populations. Because less than one-third of US adults report participating in the recommended 2 days per week of resistance training activities, this scientific statement provides practical strategies for the promotion and prescription of resistance training.
Keywords: AHA Scientific Statements, cardiovascular diseases, exercise, resistance training, risk factors
Resistance training ([RT] exercise that evokes muscular contraction against an external force) improves or maintains muscle mass and strength, and has beneficial physiological and clinical effects on cardiovascular disease (CVD) and CVD risk factors.1,2 Epidemiological evidence suggests that RT is associated with a lower risk of all-cause mortality and CVD morbidity and mortality.2 Adults who participate in RT have ≈15% lower risk of all-cause mortality and 17% lower risk of CVD, compared with adults who report no RT. Approximately 30 to 60 minutes per week of RT is associated with the maximum risk reduction for all-cause mortality and incident CVD.2 Given the expanded evidence supporting the use of RT to combat CVD, we updated the previous American Heart Association (AHA) 2007 scientific statement on the topic.3 This updated scientific statement synthesizes newer evidence regarding the effect of RT on both traditional (eg, blood pressure, lipids) and nontraditional (eg, arterial stiffness, physical functioning, depression) CVD risk factors. One of the 8 components in AHA Life’s Essential 8 is a focus on physical activity and “moving more,” through both aerobic and muscle-strengthening activities.4 The effects of RT in adults with and without CVD and the benefits associated with combination (aerobic+resistance) training are discussed. Despite the well-documented benefits, only 28% of US adults report participating in 2 days per week of RT as recommended by the 2018 Federal Physical Activity Guidelines.5 This scientific statement is intended to provide a summary of cardiovascular-related benefits of RT tailored to clinicians and public health promotion. In addition to reviewing the benefits for performing RT, this scientific statement addresses the promotion, prescription, and safety considerations for RT engagement.
HEALTH BENEFITS OF RT
Traditional CVD Risk Factors
Resistance training can improve traditional CVD risk factors, including blood pressure (BP), glycemia, lipids, and body composition. Included evidence is based largely on randomized controlled trials of medium length (2–6 months); few data were available for trials >6 months. Most trials implemented programs of moderate- to high-intensity (40%–80% of maximum effort) RT on 2 to 3 days per week.
RT and Resting BP
RT can reduce resting BP in healthy adults,6–8 in those with prehypertension, hypertension, and elevated cardiometabolic risk.6,7,9 Several proposed mechanisms responsible for these benefits include improvements in endothelial function, vasodilatory capacity, and vascular conductance.10 Among healthy young adults (≤40 years of age), RT can elicit small, but significant reductions in diastolic BP (−1 mm Hg).6 For middle-aged and older healthy adults (>40 years), RT results in larger reductions in systolic BP (−4 mm Hg) and diastolic BP (−2 mm Hg).7 Effects of RT are more pronounced for both systolic and diastolic BP in those with prehypertension9 (−3 mm Hg systolic BP; −3 mm Hg diastolic BP) and hypertension8 (−6 mm Hg systolic BP; −5 mm Hg diastolic BP) compared with normotensive individuals. The listed evidence suggests that the decreases in resting systolic BP are similar when comparing RT with antihypertensive medications.6
RT and Glycemia
RT is associated with improvements in glycemia and insulin resistance across varied populations.7,11–13 Several proposed beneficial mechanisms of RT include improved insulin sensitivity, increased GLUT4 translocation in skeletal muscle, and increased energy expenditure both during and after exercise.14 In observational studies, regular participation in RT is associated with a 17% lower incidence of diabetes compared with no participation in RT.2 The dose-response association appears nonlinear with a progressively lower risk of diabetes associated with participation in up to 60 minutes per week of RT, followed by a continued, more gradual decrease beyond this threshold duration.2
RT interventions may reduce fasting glucose by 2 to 5 mg/dL among older adults,7 and among those with prediabetes12 and type 2 diabetes, as well,13,15 but not in young and healthy participants.7 Among older patients with type 2 diabetes, RT was associated with a 0.34% decline in hemoglobin A1c.13 Patients with more recent documented type 2 diabetes (<6 years) and those with higher hemoglobin A1c at baseline (≥7.5%) demonstrated greater decreases in hemoglobin A1c after RT.15
RT and Lipid Profiles
There is a favorable, although modest, effect of RT on total cholesterol, triglycerides, and high-density lipoprotein cholesterol.7,16 Resistance training interventions result in improvements in high-density lipoprotein cholesterol (+2 to +12 mg/dL), total cholesterol (−8 mg/dL), and triglycerides (−7 to −13 mg/dL).7,16 The effect of RT on lipids and lipoproteins may be less pronounced in younger adults (<40 years of age), corresponding to significant, although small, improvements in high-density lipoprotein cholesterol only (+2 mg/dL).7 Evidence for an effect on low-density lipoprotein cholesterol is less consistent. A meta-analysis of 46 studies, including varied populations with and without elevated cardiometabolic risk, reported a significant decrease of approximately −10 mg/dL in low-density lipoprotein cholesterol.16 In contrast, another meta-analysis found no significant reductions in low-density lipoprotein cholesterol in older adults without elevated cardiometabolic risk, whereas older adults with elevated cardiometabolic risk showed significant decreases in low-density lipoprotein cholesterol (−13.4 mg/dL) after RT.7
RT, Body Composition, and Weight
RT appears to have a beneficial effect on lean body mass and fat mass.17,18 Among adults who are overweight or obese, RT alone is associated with increased lean body mass (0.8 kg), decreased body fat percentage (−1.6%), and decreased whole-body fat mass (−1.0 kg) compared with nontraining controls.17 RT alone is unlikely to produce clinically significant weight loss.19 RT improves body composition by reducing body fat stores, increasing or maintaining muscle mass, and increasing resting metabolic rate; it may attenuate weight gain over time.20
In summary, more recent data suggest that RT has significant and favorable effects on traditional CVD risk factors, including resting BP, glycemia, lipids and lipoproteins, and body composition. Benefits tend to be greater in older adults and those with elevated cardiometabolic risk factors.
RT and Nontraditional CVD Risk Factors
Accumulating research since 2007 has identified potential mechanisms, beyond the favorable effect on conventional CVD risk factors by which RT may reduce CVD risk (Table 1). Resistance training appears to confer small to moderate beneficial increases in cardiorespiratory fitness through mechanisms such as increased leg strength, improvements in oxidative enzymes, and increased type II muscle fibers.21 Although RT often has modest benefits for cardiorespiratory fitness, this can still be clinically meaningful given the well-established benefits of even moderate levels of cardiorespiratory fitness for lower risk of cardiovascular events and mortality in adults with and without cardiovascular disease.22 Higher volumes of RT are most beneficial to elicit changes in fitness.21 RT appears to favorably influence endothelial function,7,23 whereas the effects of RT on arterial stiffness and inflammatory makers are less consistent, ranging from null to beneficial associations.7,16,24,25 Limited evidence from studies with small sample sizes suggests there are beneficial effects of RT on fibrinolysis.26 A 2022 AHA presidential advisory statement introduced the Essential 8, adding sleep health as a new component and emphasizing the foundational factor of psychological health and well-being for preserving and optimizing cardiovascular health.4 Recent evidence suggests that RT is associated with enhanced sleep quality,27 and reduced symptoms of depression and anxiety and improved quality of life, as well.28–30
Table 1.
Associations of Resistance Training With Nontraditional Cardiovascular Risk Factors
| Nontraditional risk factor | Association | Summary |
|---|---|---|
| Cardiorespiratory fitness | ↑ or ↔ | Small or moderate improvements in fitness in adults with and without CVD (+1 to 3 mL·kg−1·min−1 in Vo2max).6,17 For people with coronary heart disease, similar improvements in Vo2max shown with RT (17%) as with aerobic training (21%)18 |
| Arterial stiffness | ↔, ↑, or ↓ | Low-intensity to moderate-intensity RT favorably associated with lower central (−0.7±1.4 m/s) and peripheral (−1.3±1.07 m/s) PWV.20 Effects of high-intensity RT are inconsistent, identifying studies with positive and negative associations with PWV.20 |
| Inflammation (CRP) | ↓ or ↔ | RT lowers CRP by −0.26 to −0.37 mg/L in adults overall.6,13 RT lowers CRP in adults with elevated cardiometabolic risk by −2.47 mg/L.6 Among 3 studies of adults with overweight or obesity, associations for CRP coincided with fat mass reduction.21 |
| Fibrinolysis and coagulation | ↑ fibrinolysis ↔ coagulation | Higher volume and intensity RT associated with a greater fibrinolytic response and platelet activity, although on the basis of limited evidence in only apparently healthy young adults.22 Among patients with coronary artery disease, a single RT session was associated with improvements in the fibrinolytic response without elevating potential thrombotic markers.22 |
| Endothelial function | ↑ | Improvements of ≈2%–3% (flow-mediated dilation) in adults with and without cardiometabolic conditions.6,23 |
| Depression and anxiety | ↓ | Moderate-effect sizes in reduction in depressive symptoms (ES=0.66).24 Small-to-moderate effect in reductions in anxiety (ES=0.33).25 |
| Quality of life | ↑ | Positive effect on mental health–related QoL measures, including total Mental Component (ES=0.54), Mental health (ES=0.64), and Vitality (ES=0.39).26 Positive effect on physical health–related QoL measures, including total Physical Component (ES=0.50), Bodily pain (ES=0.81), General health (ES=0.57), and Physical functioning (ES=0.40).26 |
| Sleep | ↑ sleep quality | Moderate-effect sizes in better sleep outcomes, with the strongest beneficial associations for sleep quality. Associations are less consistent for sleep duration.27 |
CRP indicates C-reactive protein; CVD, cardiovascular disease; ES, effect size; PWV, pulse wave velocity; QoL, quality of life; and RT, resistance training. ↑ represents direct association; ↓ represents inverse association; ↔ represents no association.
Resistance Versus Aerobic Versus Combined Training and CVD Risk Factors
A common yet understudied question is “What type of exercise is most effective for preventing CVD?” Several large observational studies suggest that, although no statistically significant differences were found between RT versus aerobic training (AT) alone, combining RT and AT (combination training [CT]) resulted in slightly larger reductions in some CVD risk factors, including obesity,31 diabetes,32 and hypercholesterolemia.33 CT appears to have stronger associations than either AT or RT alone with all-cause and CVD mortality.2,34 Compared with adults reporting no activity, individuals participating in CT have a 40% to 46% lower risk of all-cause and CVD mortality.2 In contrast, RT or AT alone is associated with an 18% to 29% lower risk of all-cause and CVD mortality compared with no activity.2
Several meta-analyses, based primarily on interventions ranging 2 to 6 months, summarized the comparative effectiveness of RT, AT, and CT on CVD risk factors (Table 2). Overall, despite variations in study design and populations, RT, AT, and CT appear to similarly improve BP and lipids.35–37 CT appears to be more effective for improving body composition and glycemic control especially in patients with type 2 diabetes.12,19,37–39 RT is potentially a viable alternative to AT and may provide independent and additive benefits to AT for improving CVD risk factors. However, additional well-designed large randomized controlled trials with long-term (≥6 months) interventions directly comparing RT, AT, and CT are needed.
Table 2.
Associations of Resistance, Aerobic, and Combined Training With Traditional CVD Risk Factors
| Magnitude of benefit | Conclusion | Summary of evidence | |||
|---|---|---|---|---|---|

|

|
|
|||
| Blood pressure | + | + | + | RT, AT, and CT have similar favorable, small to moderate effects on both systolic and diastolic BP | Systolic BP significantly reduced after RT (−1.8 mm Hg) and AT (−3.5 mm Hg), but insignificantly after CT (−1.4 mm Hg). Diastolic BP significantly reduced after RT (−3.2 mm Hg), AT (−2.5 mm Hg), and CT (−2.2 mm Hg).32 No significant differences between training types. |
| Lipid profile | + | + | + | RT, AT, and CT have similar favorable small to moderate effects on lipids | RT, AT, and CT improve lipid profile (eg, triglyceride, HDL and LDL cholesterol) by 4%–5%. No significant differences between training types.33,34 |
| Glycemic control | + | ++ | +++ | All modes have benefits. CT may have the strongest associations followed by AT, then RT. | In patients with type 2 diabetes, CT lowered HbA1c by 0.17% more than AT, and AT lowered HbA1c by 0.20% more than RT.35 In patients with prediabetes, CT and AT are superior to RT in reducing HbA1c and CT is most effective in controlling fasting blood glucose levels.10 |
| Body weight: Weight loss | 0 | + | + | AT and CT have small to moderate effects on weight loss. CT may be most beneficial for weight maintenance. | Greater reductions in body weight in CT (−2.0 kg) and AT (−1.2 kg), compared with RT.34 |
| Weight maintenance | 0 | + | ++ | When used in combination with AT, RT may help assist with weight loss or maintenance by increasing resting metabolic rate, fat oxidation, and lean mass.36 | |
| Body composition: Lean mass | ++ | + | +++ | RT is more beneficial for lean mass gains than AT. AT is more beneficial for fat mass loss than RT. CT provides the greatest benefits for both fat and lean mass. |
Lean body mass improves more in CT (+0.9 kg) and RT (+1.3 kg), compared with AT.34 |
| Fat mass | 0 | ++ | +++ | Greater reductions in fat mass in CT (−1.9 kg) and AT (−1.2 kg), compared with RT.34 CT is also superior to AT or RT for reducing subcutaneous abdominal fat.37 |
|
+ small to moderate benefit; ++ moderate benefit; +++ moderate to large benefit; 0 no effect.  = resistance training;
= resistance training;  = aerobic training; and
= aerobic training; and  = combined training. AT indicates aerobic training; BP, blood pressure; CT, combination training; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; LDL, low-density lipoprotein; and RT, resistance training.
= combined training. AT indicates aerobic training; BP, blood pressure; CT, combination training; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; LDL, low-density lipoprotein; and RT, resistance training.
Individuals who do not meet the aerobic physical activity guidelines are less likely to participate in RT than those who are aerobically active. For example, only 3.6% of adults who report no aerobic activity do RT, compared with 43.5% of adults who are aerobically active and participate in RT.5 Physical inactivity is an established CVD risk factor. RT may be a viable strategy to support cardiovascular health among those who are otherwise aerobically inactive. As demonstrated in this scientific statement on RT’s independent cardiovascular benefits, RT programs targeted toward populations with low levels of aerobic activity may improve cardiorespiratory fitness, physical function, and cardiovascular health through RT alone.
RT BENEFITS AND CONSIDERATIONS FOR SPECIFIC POPULATIONS
In addition to the aforementioned cardiovascular benefits of RT, there are additional benefits for specific populations with or at high risk of CVD. In some populations, RT can provide unique benefits, yet requires specific considerations. We updated the 2007 summary of evidence on women, patients with heart failure (HF), and older adults. In addition, we summarize the evidence for RT among pregnant and postpartum women, and examples of other chronic conditions including people living with peripheral artery disease (PAD), human immunodeficiency virus (HIV), Alzheimer disease and related dementias (ADRD), and chronic kidney disease (CKD).
Women
RT interventions can improve muscular strength and endurance, body composition, and CVD risk factors in women of all ages.20,40,41 CVD and osteoporosis have a bidirectional relationship and shared common risk factors.42 Significant deterioration in bone mineral density over time, a particular concern in postmenopausal women, may be partially prevented with RT.16 RT improves bone mineral density in the femoral neck and the lumbar spine in pre- and postmenopausal women.43,44 RT, when combined with other weight-bearing, high-impact, aerobic activities (eg, jumping rope, tennis), appears most beneficial for bone health in women.43,45
Most women do not regularly engage in RT. According to 2018 data, only 24% of US women engaged in RT ≥2 days per week.5 To maximize adherence, RT programs should proactively address common barriers and motivations specific to women. In the limited research on this topic, some strategies, such as group-based training, social support, family-friendly, or home-based workout options requiring minimal or no equipment, have been used to increase RT participation in women.46 However, given the low RT participation rates and the minimal evidence, this highlights an area for future research on RT program adoption and maintenance among women.
Pregnant and Postpartum Women
Resistance training, in general, is safe and recommended during pregnancy and the postpartum period.47 In pregnant women with no contraindications, RT can be an integral component of an exercise program and does not appear to have adverse effects on maternal or fetal health during pregnancy.48 Maternal benefits are most favorable for CT versus RT or AT alone, with the strongest evidence pertaining to improvements in cardiorespiratory fitness and urinary incontinence.49 Limited high-quality trials of RT-only have addressed maternal CVD risk factors. One report suggested that RT may reduce the need for insulin therapy in women with gestational diabetes and support healthy gestational weight gain.48 Among postpartum women enrolled in an 18-week supervised RT intervention, there were small to moderate reductions in postnatal depression scores and favorable changes in body composition.50
Pregnancy-related symptoms, safety concerns, lack of information, and inadequate social support are common barriers to exercise during pregnancy.51 Motivational counseling using the “5 A’s” (ask, advise, assess, assist, and arrange), has been proposed to promote exercise during pregnancy and the postpartum period.47 Before recommending a RT routine, health care professionals should conduct an evaluation to rule out contraindications (eg, preterm labor, preeclampsia, severe anemia).47 RT programs should follow pregnancy-specific guidelines, given the physiological alterations during pregnancy, including joint instability, postural changes, and increases in body temperature.48
Older Adults
The number of adults meeting the muscle-strengthening Physical Activity Guidelines declines across the life course, with older adults ≥65 years of age having the lowest proportions participating in RT. For example, 34% of adults 25 to 34 years of age versus 19% of adults ≥65 years of age reported participating in RT ≥2 times per week.5 Resistance training can slow the rate of aging-related declines in muscle mass, power, strength, and function in healthy older adults and those with chronic conditions.20 In healthy older adults, RT improves muscle strength and power and results in increased mobility, physical function, and cardiorespiratory fitness.1,20 ln older adult populations with frailty, sarcopenia, or osteoarthritis, and in institutionalized older adults (mean age ≥80 years), gains in strength after RT meaningfully improved physical function.12,52–55 RT can benefit muscle mass during aging. Greater skeletal muscle mass in older adults is independently associated with clinical and functional end points such as better physical performance, mobility, and the prevention of injurious falls.56 A key component of the Physical Activity Guidelines for older adults is a focus on balance, to address the major health concern of falls.57 Falls in older adults are a leading cause of chronic disability and loss of independence. RT can reduce the risk of falls and injury from falls (eg, fractures) in older adults. RT programs that incorporate balance-challenge exercises (eg, feet closer together, minimal hand support) may be the most effective in fall prevention.20
RT interventions in older adults with known or suspected CVD demonstrated improvements in risk factors, including glucose tolerance, lipids and lipoproteins, insulin resistance, and resting BP.15,58,59 A dose-response association has been demonstrated; higher training volumes (2–3 sets per exercise) and intensity (55%–80% of 1 repetition maximum [RM]), compared with lower volume (1 set per exercise) and intensity (<55% of 1-RM) resulted in greater reductions in total body fat mass and waist circumference55,60 and enhanced muscle quality, mass, strength, and functional status.20,54,60,61 Modifications to RT exercises should be considered on the basis of the health status and the presence of chronic health conditions.20 Older adults can benefit from participating in RT to attenuate age-related declines in physical capacity and prolong functional independence.20,62
People With HF
Exercise training improves cardiovascular fitness or functional capacity in patients with HF.63–65 The underlying mechanisms for improvements may differ between HF with reduced ejection fraction and HF with preserved ejection fraction. Although there was initial reluctance in applying RT to patients with HF, due to disproportionate increases in the rate-pressure product and systemic vascular resistance and concomitant decreases in left ventricular ejection fraction, these perceptions have been refuted.64
RT significantly improves lower and upper extremity strength and endurance, cardiorespiratory fitness , 6-minute walk distance (+49.9 m), and quality of life.63,64 Clinicians may consider prescribing RT when AT is deemed inappropriate or unviable, because RT alone can elicit meaningful benefits.64 RT can be considered an initial strategy in patients with HF who are deconditioned to a point where AT can be difficult to initiate.66 Combining RT with AT in clinically stable patients with HF is safe and may provide independent and additive benefits, including improved capacity for occupational and leisure-time activities, muscle strength and endurance, cardiorespiratory fitness, and quality of life.
People With PAD
Lower extremity PAD, characterized by atherosclerotic blockages of lower extremity arteries, is associated with reduced lower extremity muscle mass and strength, and greater walking impairment secondary to intermittent claudication, compared with people without PAD.67 A recent AHA scientific statement on optimal exercise programs concluded that, although consistent walking is first-line therapy for improving walking impairment in PAD, lower extremity RT provides an alternative therapeutic intervention in people with PAD.68 RT alone can obviate the ischemic leg symptoms associated with walking exercise for PAD, and therefore may be more acceptable and less difficult than walking.69–71 RT with and without AT has favorable effects on selected walking measures and lower extremity strength.70 RT programs are associated with a 49.4-m improvement in 6-minute walk performance and a 0.67 standardized mean difference improvement in peak walking distance compared with control nonexercise groups.70 Yet, RT was less effective for 6-minute walk or treadmill walking distance compared with supervised walking programs.70 Moderate- to high-intensity RT is associated with more pronounced improvement in walking performance compared with light-intensity RT.71 The effects of RT on vascular outcomes, such a blood flow, blood pressure, and functional capacity, have been investigated in only a few studies with mixed results, suggesting areas for future research.70,71 In summary, although less potent than supervised walking, RT may provide an alternative with substantial benefits in prolonging walking performance in patients with PAD.
People Living With HIV
People living with HIV experience comorbidities (eg, CVD, sarcopenia, frailty) earlier and more frequently than those without HIV and common treatment regimens for HIV may further exacerbate cardiovascular risk.72–74 People living with HIV face unique challenges to engaging in, and responding to RT, including mitochondrial dysfunction, altered proteostasis, muscle wasting, lipodystrophy, and cardiopulmonary deconditioning.75 In general, RT is deemed safe and recommended for this population.75 RT alone or combined with aerobic exercise improves strength, physical function, cardiorespiratory fitness, and cardiovascular health.76–78 People living with HIV initiating RT may get additional benefits doing so under the supervision of a trained exercise professional.75
People Living With ADRD
ADRD affects millions of Americans and is a leading cause of morbidity and mortality in older adults. ADRD shares many of the same risk factors as CVD, including hypertension, diabetes, and physical inactivity.79 RT can evoke functional brain changes, reduce white matter atrophy, and is associated with smaller white matter lesions.80,81 Although RT can improve cognitive function in those with ADRD, the baseline level of cognitive impairment may influence responses to RT and bring about additional safety considerations. Those with moderate-to-severe symptoms of cognitive impairment may not derive the same benefits as those with mild symptoms.82 Degree of impairment, in general, should not prevent individuals with ADRD from engaging in RT. To maximize safety, it is necessary to tailor the prescribed RT regimen, setting, supervision, and equipment to the individual’s cognitive function.
People Living With CKD
CKD is a risk factor for CVD morbidity and mortality. People with CKD experience significant reductions in functional capacity, muscle wasting, and muscular dysfunction.83 RT for patients with CKD at all stages, including those undergoing dialysis, can be effective in increasing muscle mass, reducing intramuscular fat, improving muscle metabolism, increasing strength and functional capacity, and improving quality of life.83,84 RT is safe and well tolerated in this patient population.83–85 Individuals with CKD have an increased risk of bone fractures and tendon ruptures and a higher prevalence of diabetes, and these risks should be considered when developing a RT program.83
PRESCRIPTION OF RT
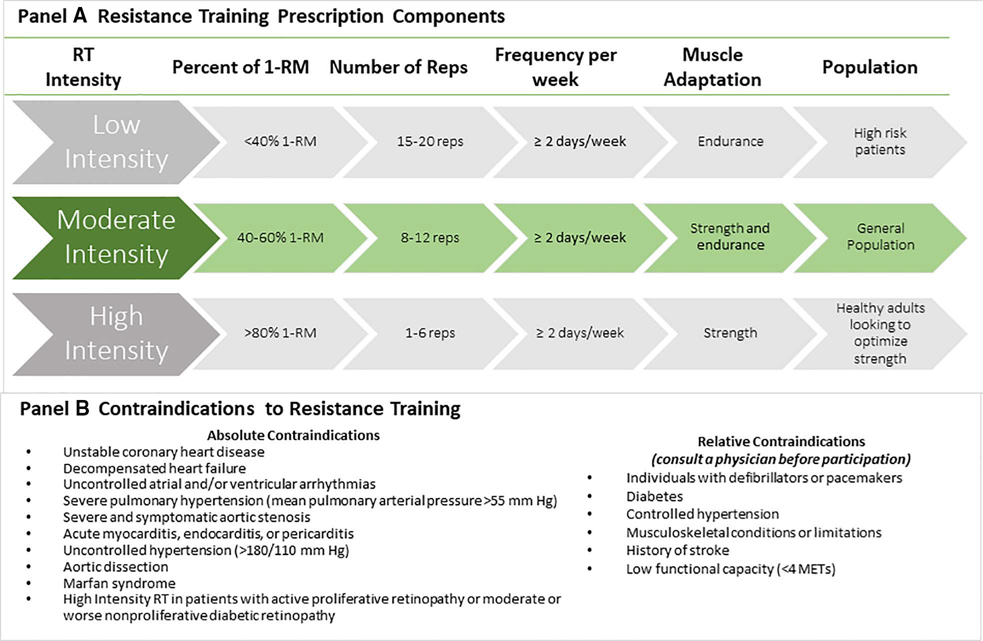
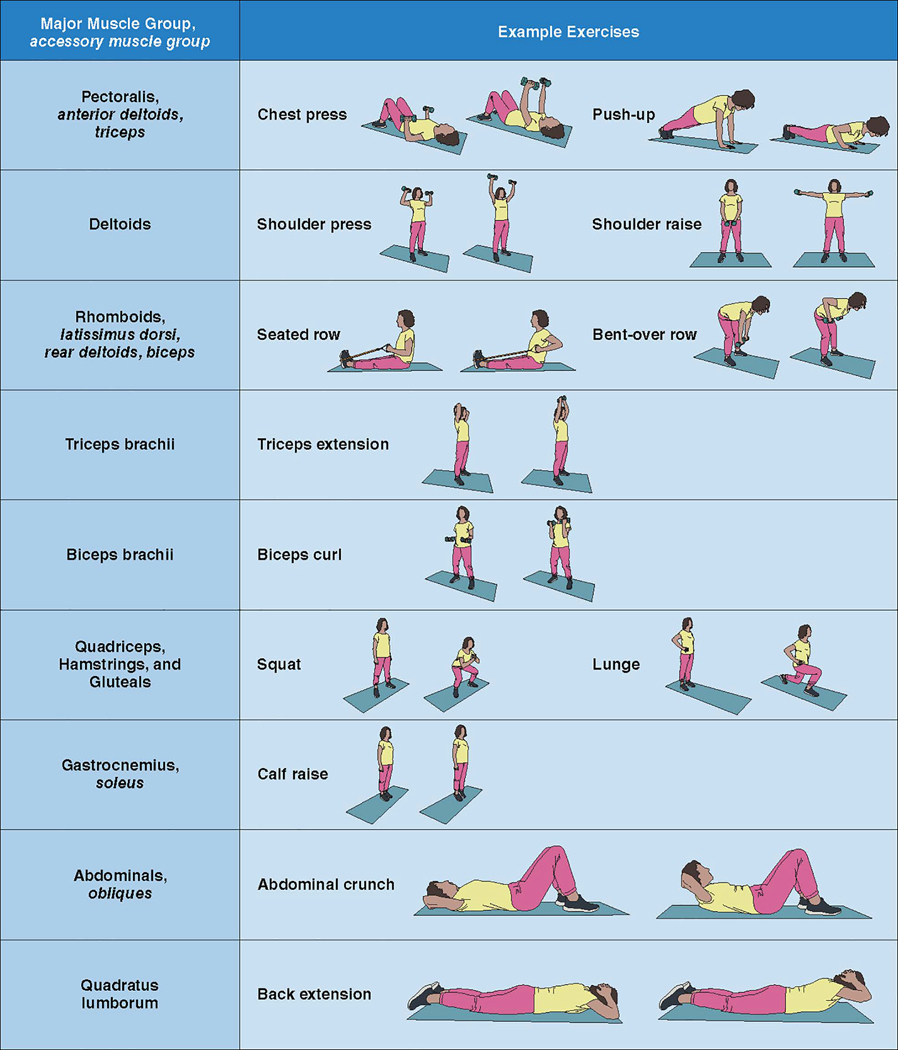
Figure 1 shows the general guidelines for RT in apparently healthy adults and clinical populations. RT can include free weights (ie, dumbbells), body weight (ie, push-ups, squats), machine weights, and resistance bands. For healthy adults, regimens of 8 to 10 different exercises involving major muscle groups (Figure 2), each exercise performed in 1 to 3 sets of moderate-intensity loads that permits 8 to 12 repetitions per set to volitional fatigue, ≥2 times per week, is effective for achieving muscular and cardiovascular benefits.1,20,57,86 Planned rest days between sessions can allow for appropriate neuromuscular adaptations.87,88 For clinical populations, modifications to lower intensity loads with higher repetitions can minimize risk while still providing health benefits. Body weight training can be as effective as training with weight machines or free weights.
Figure 1. Prescription and safety considerations for resistance training.
A, Resistance training prescription components. Muscular strength is the ability of a muscle to generate force, and muscular endurance is the ability of a muscle to perform repeated contractions or maintain a contraction for a prolonged period of time. Lower repetitions (reps) with a heavier weight may better optimize muscular strength. Higher repetitions with a lighter weight may better enhance muscular endurance. Using weight loads that permit 8 to 12 repetitions, in general, will facilitate improvements in both muscular strength and endurance, provide cardiovascular health benefits, and be safe for the general population. B, Contraindications to resistance training. Before initiating an RT program, patients should consult with a physician for absolute and relative contraindications. METS indicates metabolic equivalents of task; 1-RM, 1-repetition maximum; and RT, resistance training.
Figure 2. Major muscle groups and example exercises.
The name of the major muscle group involved in each exercise is in standard font. Accessory muscle groups involved in exercises are shown in italics. It is not necessary to perform all exercises in each resistance training session. Some compound exercises target >1 major muscle group. Eight to 10 exercises can be selected so that each major muscle group is exercised. Exercises can be completed using machines, free weights, elastic bands, or body weight.
RT Program Progression
When beginning a new RT program, an initial intensity should correspond to 40% to 60% of 1-RM, then gradually increasing the resistance, number of sets, or frequency of training over time.86–88 This progressive overload is key to maintenance or continued improvements in muscle adaptation and strength over time.1,20,89 The “2 for 2” rule can be applied: when an individual can achieve 2 more repetitions of a given exercise in 2 consecutive RT sessions, weight can be increased 2% to 10%.1 After 6 months of regular RT, individuals free from contraindications can use a wider range of repetitions and heavier weights (ie, >80% 1-RM), with longer rest intervals between sets of exercises.1,20 Programs should be periodized, meaning that the RT program undulates intensities and volumes to maximize gains and help avoid injury.1
Safety of RT
Signs or symptoms of myocardial ischemia, ventricular arrhythmias, and abnormal hemodynamic responses occur less frequently during submaximal and maximal resistance versus aerobic exercise.89 The lower heart rate and higher myocardial perfusion pressure that predictably accompany resistance exercise may explain this phenomenon.89 In studies of healthy adults, low-risk cardiac patients, individuals with controlled hypertension, those with a history of stroke, and recipients of organ transplants, no significant cardiovascular events were reported during RT and 1-RM strength testing.3 On the basis of limited data, a review of exercise randomized controlled trials in adults with coronary heart disease concluded that RT has a lower rate of cardiovascular complications compared with AT.89 In this review, across 23 trials reporting on adverse events (n=1174 total participants), there were 63 nonfatal cardiovascular-related complications during AT training and testing, whereas only 1 occurred during RT training and none during RT testing.89 None of the events led to study termination, extended hospitalization, or death. However, one-third of the studies in this review did not include adverse event information, emphasizing the need for better reporting in studies.89
After cardiac surgery through median sternotomy, AT has been prioritized for cardiac rehabilitation over RT due to the perturbation of sternal precautions.90 Progressive unweighted upper limb and trunk RT, ensuring the movements are pain free and upper limbs are kept close, have been shown to be safe and effective.90 A meta-analysis of 7 trials demonstrated RT alone or with AT can improve physical and functional recovery, such as cardiorespiratory fitness.90 However, future research is needed to determine optimal timing and progression of RT after a median sternotomy.
Initial Evaluation for Contraindications
Practitioners can initially evaluate the safety of RT participation using the same contraindications as the endurance component of adult fitness or exercise-based cardiac rehabilitation programs (Figure 1). Current statements and guidelines also recommend avoidance of intense exercise in selected patients with inherited cardiomyopathies.3 Although patients with hypertrophic cardiomyopathy are advised to avoid RT, an AHA statement regarding physical activity participation for young patients with genetic cardiovascular diseases suggested that low-intensity RT with machines might be permissible.91 RT programs of even lower relative intensities (eg, 20% of 1-RM) can safely improve strength after an acute coronary event.3 Therefore, some programs have adopted a more flexible approach for high-risk patients or those with absolute contraindications to traditional RT. Patients can safely perform modified approaches such as weight-bearing calisthenics, rubber band or spring devices, pulley weights, or light dumbbells or wrist weights. As with AT, adverse signs and symptoms (eg, dizziness, excessive dyspnea, chest pain and pressure, palpitations) require immediate medical evaluation, and patients should discontinue participating in RT until obtaining further medical clearance.86
Relative contraindications may apply to clinical subpopulations in which they should seek physician consultation and medical clearance before starting an RT program (Figure 1). Individuals with implanted pacemakers or defibrillators should consult with their physicians before engaging in upper-body RT.20 Repetitive-motion activities such as RT can result in pacing lead fractures and dislodgment. In the absence of absolute contraindications (Figure 1), patients with type 2 diabetes can participate in RT.11,20,92 Patients should monitor glucose levels before and after RT sessions to prevent exercise-induced hypoglycemia.20 Caution is advised for individuals with diabetic neuropathy because of greater susceptibility to orthostatic hypotension and musculoskeletal injury due to impaired sensory awareness and attenuated pain perception.3 High-intensity RT is contraindicated in patients with active proliferative retinopathy or moderate to severe nonproliferative diabetic retinopathy because it may trigger vitreous hemorrhage and retinal detachment.92 Individuals with musculoskeletal limitations, advanced arthritic conditions, severe osteoporosis and neuropathies, or previous stroke may benefit from low- to moderate-intensity RT.20 Machines are likely safer than free weights for these patients, and the guidance of an exercise professional may provide enhanced benefit. Patients with controlled hypertension can safely participate in low- to moderate-intensity RT with proper breathing techniques.20 Medications can affect hemodynamics, ECG changes, and exercise capacity, and therefore, should be considered when designing RT prescriptions.86 For example, patients taking antihypertensive medications should incorporate extended cooldowns to prevent hypotension.
DISCUSSION
RT benefits cardiovascular health through avenues such as lowering BP, improving cholesterol, and improving insulin sensitivity. Controlling traditional and nontraditional risk factors decreases the risk of CVD and overall mortality. In observational studies, the dose-response association of RT with mortality and CVD is curvilinear. The greatest reduction in risk occurs between those performing no RT versus modest amounts of RT, the maximal benefit occurs at 30 to 60 minutes per week, and a lower risk compared with no RT remained until 130 to 140 minutes per day.2 The evidence on higher levels is sparse and limits conclusions on the benefits or risks of high volumes of RT. Clinical trials also support similar benefits with modest training regimens of 2 sessions per week as enough to elicit benefits.
RT supports musculoskeletal and cardiovascular health for individuals across the adult life span. RT can benefit a wide range of populations living with chronic conditions who are at elevated CVD risk. This scientific statement focuses on RT in adult populations; however, RT can be initiated earlier than adulthood. The Physical Activity Guidelines recommend that children and adolescents 6 to 17 years of age participate in muscle-strengthening activities on at least 3 days as part of the recommended 1 hour per day of moderate to vigorous physical activity.57 Youth athletes and nonathletes can experience positive outcomes from a well-supervised RT program, emphasizing proper technique.93 Benefits range from lowering risks of injury, improved fitness, to better physical literacy, which may support continuing RT when entering adulthood.93
Adequate intake of protein is necessary to fully realize the benefits of RT. Protein ingestion before or after a bout of RT stimulates muscle protein synthesis for building or maintaining muscle mass.94 These benefits are important to support preserving muscle mass or delaying muscle loss during aging of all adults. A balanced diet through the consumption of whole foods with overall protein intake on the basis of the Acceptable Macronutrient Distribution Range is adequate to support muscle health benefits.94
Population participation rates are lower for RT than AT.5 To promote RT, it is important to proactively address the unique barriers of this training modality, such as equipment availability, perceived complexity, and how to safely and effectively perform RT.82 Most RT programs are delivered face-to-face by exercise professionals, to demonstrate and supervise techniques for individuals new to RT.95 However, the growth of mobile applications, online videos, and video conferencing may expand reach and reduce cost for RT interventions, particularly among adults with no contraindications who require minimal supervision. Exercises requiring minimal equipment (eg, elastic bands) to no equipment (eg, body weight exercises) can reduce complexity when developing home-based and digital RT programs. Research regarding strategies that minimize complexity and equipment to address RT adoption and maintenance while providing sustained health benefits remain limited. Additional trials are needed to clarify the optimal RT prescriptions and behavioral change strategies in heterogeneous populations.95
There are disparities in RT participation across demographic groups. Populations of older age, female sex, non-White race and ethnicity, and lower socioeconomic status are significantly less likely to participate in RT.5,96 RT promotion should be appropriately tailored to specific populations and consider a wide range of factors influencing RT participation. In comparison with research on determinants of aerobic activity participation, there is considerably less research on RT participation from a socioecologic approach. To address the low rates of RT and disparities in RT participation, it is vital to pursue implementation science, identify the intrapersonal (eg, intentions, self-efficacy), interpersonal (eg, social norms, social support), and environmental factors (eg, recreational facilities and access, neighborhood design), and to create feasible programs that can support the adoption and maintenance of RT.
CONCLUSION
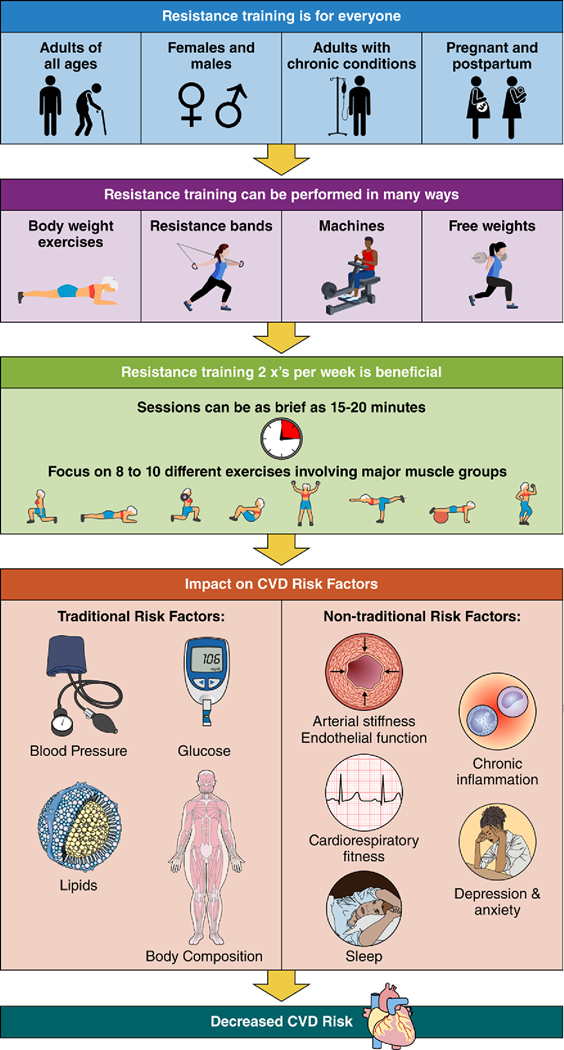
Evidence describing the benefits of RT on traditional and nontraditional CVD risk factors has grown substantially since the 2007 AHA scientific statement. RT programs need not be time-consuming to be efficacious, requiring only 30 to 60 minutes per week (Figure 3). In general, a single set of 8 to 12 repetitions to volitional fatigue, using moderate weight loads of 40% to 60% of 1-RM, for 8 to 10 different exercises involving major muscle groups, performed twice per week are highly effective. Well-designed randomized controlled trials with long-term (≥6 months) interventions incorporating evidence-based behavior change and maintenance techniques are warranted and likely to reveal strategies for improved implementation of RT in clinical and nonclinical settings. Recent evidence clearly demonstrates that RT is a safe, effective, and essential component of the overall physical activity regimen for CVD risk reduction.
Figure 3. Summary of resistance exercise training.
CVD indicates cardiovascular disease.
Footnotes
ARTICLE INFORMATION
The American Heart Association makes every effort to avoid any actual or potential conflicts of interest that may arise as a result of an outside relationship or a personal, professional, or business interest of a member of the writing panel. Specifically, all members of the writing group are required to complete and submit a Disclosure Questionnaire showing all such relationships that might be perceived as real or potential conflicts of interest.
This statement was approved by the American Heart Association Science Advisory and Coordinating Committee on September 6, 2023, and the American Heart Association Executive Committee on September 18, 2023. A copy of the document is available at https://professional.heart.org/statements by using either “Search for Guidelines & Statements” or the “Browse by Topic” area. To purchase additional reprints, Meredith.Edelman@wolterskluwer.com
The American Heart Association requests that this document be cited as follows: Paluch AE, Boyer WR, Franklin BA, Laddu D, Lobelo F, Lee D, McDermott MM, Swift DL, Webel AR, Lane A; on behalf the American Heart Association Council on Lifestyle and Cardiometabolic Health; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Epidemiology and Prevention; and Council on Peripheral Vascular Disease. Resistance exercise training in individuals with and without cardiovascular disease: 2023 update: a scientific statement from the American Heart Association. Circulation. 2023;148:e•••–e•••. doi: 10.1161/CIR.0000000000001189
The expert peer review of AHA-commissioned documents (eg, scientific statements, clinical practice guidelines, systematic reviews) is conducted by the AHA Office of Science Operations. For more on AHA statements and guidelines development, visit https://professional.heart.org/statements. Select the “Guidelines & Statements” drop-down menu, then click “Publication Development.”
Permissions: Multiple copies, modification, alteration, enhancement, and distribution of this document are not permitted without the express permission of the American Heart Association. Instructions for obtaining permission are located at https://www.heart.org/permissions. A link to the “Copyright Permissions Request Form” appears in the second paragraph (https://www.heart.org/en/about-us/statements-and-policies/copyright-request-form).
Disclosures
| Writing group member | Employment | Research grant | Other research support | Speakers’ bureau/honoraria | Expert witness | Ownership interest | Consultant/advisory board | Other |
|---|---|---|---|---|---|---|---|---|
| Amanda E. Paluch | University of Massachusetts Amherst | None | None | None | None | None | None | None |
| Abbi Lane | University of Michigan | Nurasource (co- investigator on grant that ended in 2022)†; American Heart Association (career development award – no cost extension)†; NHLBI (R01)† | None | None | None | None | None | None |
| William R. Boyer | California Baptist University | None | None | None | None | None | None | None |
| Barry A. Franklin | William Beaumont Hospital | None | None | None | None | None | None | None |
| Deepika Laddu | Arbor Research Collaborative for Health | NIH/NHLBI (K01 career development grant award: 1K01HL148503-01)† | None | None | None | None | None | None |
| Duck-chul Lee | Iowa State University | NIH (NHLBI; PI of funded R01HL133069 grant)†; NIH (NIDDK; co-investigator of funded R21DK131429 grant)†; NIH (NHLBI; PI of pending R01HL171098 grant, if funded)†; NIH (NIMH; co-investigator of funded R61MH129407 grant)† | None | None | None | None | None | None |
| Felipe Lobelo | Emory University Rollins School of Public Health | None | None | None | None | None | None | None |
| Mary M. McDermott | Northwestern University, Feinberg School of Medicine | ArtAssist (other research support)†; Mars (other research support)*; ReserveAge (other support)*; Chromadex (other support)*; Helixmith (grant and other research support)† | None | None | None | None | None | None |
| Damon L. Swift | University of Virginia | None | None | None | None | None | None | None |
| Allison R. Webel | University of Washington School of Nursing | NIH (they have ~5 million in NIH grants paid to her university)† | None | None | None | None | None | None |
| Reviewer | Employment | Research grant | Other research support | Speakers’ bureau/honoraria | Expert witness | Ownership interest | Consultant/advisory board | Other |
|---|---|---|---|---|---|---|---|---|
| Kevin S. Heffernan | Syracuse University | None | None | None | None | None | None | None |
| Carlos Iribarren | Kaiser Permanente of Northern California | None | None | None | None | None | None | None |
| Judith G. Regensteiner | University of Colorado Denver School of Medicine | None | None | None | None | None | None | None |
| Kara M. Whitaker | University of Iowa | None | None | None | None | None | None | None |
| Payman Zamani | University of Pennsylvania | Amgen†; | None | Pfizer* | None | None | Vyaire* | None |
REFERENCES
- 1.American College of Sports Medicine. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2009;41:687–708. doi: 10.1249/MSS.0b013e3181915670 [DOI] [PubMed] [Google Scholar]
- 2.Momma H, Kawakami R, Honda T, Sawada SS. Muscle-strengthening activities are associated with lower risk and mortality in major non-communicable diseases: a systematic review and meta-analysis of cohort studies. Br J Sports Med. 2022;56:755–763. doi: 10.1136/bjsports-2021-105061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams MA, Haskell WL, Ades PA, Amsterdam EA, Bittner V, Franklin BA, Gulanick M, Laing ST, Stewart KJ; American Heart Association Council on Clinical Cardiology. Resistance exercise in individuals with and without cardiovascular disease: 2007 update: a scientific statement from the American Heart Association Council on Clinical Cardiology and Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2007;116:572–584. doi: 10.1161/CIRCULATIONAHA.107.185214 [DOI] [PubMed] [Google Scholar]
- 4.Lloyd-Jones DM, Allen NB, Anderson CAM, Black T, Brewer LC, Foraker RE, Grandner MA, Lavretsky H, Perak AM, Sharma G, et al. ; on behalf of American Heart Association. Life’s Essential 8: updating and enhancing the American Heart Association’s Construct of Cardiovascular Health: a presidential advisory from the American Heart Association. Circulation. 2022;146:e18–e43. doi: 10.1161/CIR.0000000000001078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hyde ET, Whitfield GP, Omura JD, Fulton JE, Carlson SA. Trends in meeting the physical activity guidelines: muscle-strengthening alone and combined with aerobic activity, United States, 1998–2018. J Phys Act Health. 2021;18:S37–S44. doi: 10.1123/jpah.2021-0077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naci H, Salcher-Konrad M, Dias S, Blum MR, Sahoo SA, Nunan D, Ioannidis JPA. How does exercise treatment compare with antihypertensive medications? A network meta-analysis of 391 randomised controlled trials assessing exercise and medication effects on systolic blood pressure. Br J Sports Med. 2019;53:859–869. doi: 10.1136/bjsports-2018-099921 [DOI] [PubMed] [Google Scholar]
- 7.Ashton RE, Tew GA, Aning JJ, Gilbert SE, Lewis L, Saxton JM. Effects of short-term, medium-term and long-term resistance exercise training on cardiometabolic health outcomes in adults: systematic review with meta-analysis. Br J Sports Med. 2020;54:341–348. doi: 10.1136/bjsports-2017-098970 [DOI] [PubMed] [Google Scholar]
- 8.Loaiza-Betancur AF, Pérez Bedoya E, Montoya Dávila J, Chulvi-Medrano I. Effect of isometric resistance training on blood pressure values in a group of normotensive participants: a systematic review and meta-analysis. Sports Health. 2020;12:256–262. doi: 10.1177/1941738120908070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacDonald HV, Johnson BT, Huedo-Medina TB, Livingston J, Forsyth KC, Kraemer WJ, Farinatti PT, Pescatello LS. Dynamic resistance training as stand-alone antihypertensive lifestyle therapy: a meta-analysis. J Am Heart Assoc. 2016;5:e003231. doi: 10.1161/JAHA.116.003231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fecchio RY, Brito LC, Peçanha T, de Moraes Forjaz CL. Potential mechanisms behind the blood pressure-lowering effect of dynamic resistance training. Curr Hypertens Rep. 2021;23:35. doi: 10.1007/s11906-021-01154-5 [DOI] [PubMed] [Google Scholar]
- 11.Brown EC, Franklin BA, Regensteiner JG, Stewart KJ. Effects of single bout resistance exercise on glucose levels, insulin action, and cardiovascular risk in type 2 diabetes: a narrative review. J Diabetes Complications. 2020;34:107610. doi: 10.1016/j.jdiacomp.2020.107610 [DOI] [PubMed] [Google Scholar]
- 12.Huang L, Fang Y, Tang L. Comparisons of different exercise interventions on glycemic control and insulin resistance in prediabetes: a network meta-analysis. BMC Endocr Disord. 2021;21:181. doi: 10.1186/s12902-021-00846-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jansson AK, Chan LX, Lubans DR, Duncan MJ, Plotnikoff RC. Effect of resistance training on HbA1c in adults with type 2 diabetes mellitus and the moderating effect of changes in muscular strength: a systematic review and meta-analysis. BMJ Open Diabetes Res Care. 2022;10:e002595. doi: 10.1136/bmjdrc-2021-002595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strasser B, Pesta D. Resistance training for diabetes prevention and therapy: experimental findings and molecular mechanisms. Biomed Res Int. 2013;2013:805217. doi: 10.1155/2013/805217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishiguro H, Kodama S, Horikawa C, Fujihara K, Hirose AS, Hirasawa R, Yachi Y, Ohara N, Shimano H, Hanyu O, et al. In search of the ideal resistance training program to improve glycemic control and its indication for patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Sports Med. 2016;46:67–77. doi: 10.1007/s40279-015-0379-7 [DOI] [PubMed] [Google Scholar]
- 16.Costa RR, Buttelli ACK, Vieira AF, Coconcelli L, Magalhães RL, Delevatti RS, Kruel LFM. Effect of strength training on lipid and inflammatory outcomes: systematic review with meta-analysis and meta-regression. J Phys Act Health. 2019;16:477–491. doi: 10.1123/jpah.2018-0317 [DOI] [PubMed] [Google Scholar]
- 17.Lopez P, Taaffe DR, Galvão DA, Newton RU, Nonemacher ER, Wendt VM, Bassanesi RN, Turella DJP, Rech A. Resistance training effectiveness on body composition and body weight outcomes in individuals with overweight and obesity across the lifespan: a systematic review and meta-analysis. Obes Rev. 2022;23:e13428. doi: 10.1111/obr.13428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wewege MA, Desai I, Honey C, Coorie B, Jones MD, Clifford BK, Leake HB, Hagstrom AD. The effect of resistance training in healthy adults on body fat percentage, fat mass and visceral fat: a systematic review and meta-analysis. Sports Med. 2022;52:287–300. doi: 10.1007/s40279-021-01562-2 [DOI] [PubMed] [Google Scholar]
- 19.Swift DL, McGee JE, Earnest CP, Carlisle E, Nygard M, Johannsen NM. The effects of exercise and physical activity on weight loss and maintenance. Prog Cardiovasc Dis. 2018;61:206–213. doi: 10.1016/j.pcad.2018.07.014 [DOI] [PubMed] [Google Scholar]
- 20.Fragala MS, Cadore EL, Dorgo S, Izquierdo M, Kraemer WJ, Peterson MD, Ryan ED. Resistance training for older adults: position statement from the National Strength and Conditioning Association. J Strength Cond Res. 2019;33:2019–2052. doi: 10.1519/JSC.0000000000003230 [DOI] [PubMed] [Google Scholar]
- 21.Cornelissen VA, Fagard RH, Coeckelberghs E, Vanhees L. Impact of resistance training on blood pressure and other cardiovascular risk factors: a meta-analysis of randomized, controlled trials. Hypertension. 2011;58:950–958. doi: 10.1161/HYPERTENSIONAHA.111.177071 [DOI] [PubMed] [Google Scholar]
- 22.Ross R, Blair SN, Arena R, Church TS, Després JP, Franklin BA, Haskell WL, Kaminsky LA, Levine BD, Lavie CJ, et al. ; on behalf of the American Heart Association Physical Activity Committee of the Council on Lifestyle and Cardiometabolic Health, Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Cardiovascular and Stroke Nursing; Council on Functional Genomics and Translational Biology; Stroke Council. Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation. 2016;134:e653–e699. doi: 10.1161/CIR.0000000000000461 [DOI] [PubMed] [Google Scholar]
- 23.Silva J, Menêses AL, Parmenter BJ, Ritti-Dias RM, Farah BQ. Ef-fects of resistance training on endothelial function: a systematic review and meta-analysis. Atherosclerosis. 2021;333:91–99. doi: 10.1016/j.atherosclerosis.2021.07.009 [DOI] [PubMed] [Google Scholar]
- 24.Jurik R, Żebrowska A, Stastny P. Effect of an acute resistance training bout and long-term resistance training program on arterial stiffness: a systematic review and meta-analysis. J Clin Med. 2021;10:3492. doi: 10.3390/jcm10163492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gonzalo-Encabo P, Maldonado G, Valadés D, Ferragut C, Pérez-López A. The role of exercise training on low-grade systemic inflammation in adults with overweight and obesity: a systematic review. Int J Environ Res Public Health. 2021;18:13258. doi: 10.3390/ijerph182413258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nascimento Dda C, Neto FR, de Santana FS, da Silva RA, Dos Santos-Neto L, Balsamo S. The interactions between hemostasis and resistance training: a review. Int J Gen Med. 2012;5:249–254. doi: 10.2147/IJGM.S29197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kovacevic A, Mavros Y, Heisz JJ, Fiatarone Singh MA. The effect of resistance exercise on sleep: a systematic review of randomized controlled trials. Sleep Med Rev. 2018;39:52–68. doi: 10.1016/j.smrv.2017.07.002 [DOI] [PubMed] [Google Scholar]
- 28.Gordon AM, Schneider JA, Chinnan A, Charles JR. Efficacy of a hand-arm bimanual intensive therapy (HABIT) in children with hemiplegic cerebral palsy: a randomized control trial. Dev Med Child Neurol. 2007;49:830–838. doi: 10.1111/j.1469-8749.2007.00830.x [DOI] [PubMed] [Google Scholar]
- 29.Gordon BR, McDowell CP, Hallgren M, Meyer JD, Lyons M, Herring MP. Association of efficacy of resistance exercise training with depressive symptoms: meta-analysis and meta-regression analysis of randomized clinical trials. JAMA Psychiatry. 2018;75:566–576. doi: 10.1001/jamapsychiatry.2018.0572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hart PD, Buck DJ. The effect of resistance training on health-related quality of life in older adults: systematic review and meta-analysis. Health Promot Perspect. 2019;9:1–12. doi: 10.15171/hpp.2019.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brellenthin AG, Lee DC, Bennie JA, Sui X, Blair SN. Resistance exercise, alone and in combination with aerobic exercise, and obesity in Dallas, Texas, US: a prospective cohort study. PLoS Med. 2021;18:e1003687. doi: 10.1371/journal.pmed.1003687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shiroma EJ, Cook NR, Manson JE, Moorthy MV, Buring JE, Rimm EB, Lee IM. Strength training and the risk of type 2 diabetes and cardiovascular disease. Med Sci Sports Exerc. 2017;49:40–46. doi: 10.1249/MSS.0000000000001063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bakker EA, Lee DC, Sui X, Eijsvogels TMH, Ortega FB, Lee IM, Lavie CJ, Blair SN. Association of resistance exercise with the incidence of hypercholesterolemia in men. Mayo Clin Proc. 2018;93:419–428. doi: 10.1016/j.mayocp.2017.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao M, Veeranki SP, Magnussen CG, Xi B. Recommended physical activity and all cause and cause specific mortality in US adults: prospective cohort study. BMJ. 2020;370:m2031. doi: 10.1136/bmj.m2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cornelissen VA, Smart NA. Exercise training for blood pressure: a systematic review and meta-analysis. J Am Heart Assoc. 2013;2:e004473. doi: 10.1161/JAHA.112.004473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mann S, Beedie C, Jimenez A. Differential effects of aerobic exercise, resistance training and combined exercise modalities on cholesterol and the lipid profile: review, synthesis and recommendations. Sports Med. 2014;44:211–221. doi: 10.1007/s40279-013-0110-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwingshackl L, Dias S, Strasser B, Hoffmann G. Impact of different training modalities on anthropometric and metabolic characteristics in overweight/obese subjects: a systematic review and network meta-analysis. PLoS One. 2013;8:e82853. doi: 10.1371/journal.pone.0082853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwingshackl L, Missbach B, Dias S, König J, Hoffmann G. Impact of different training modalities on glycaemic control and blood lipids in patients with type 2 diabetes: a systematic review and network meta-analysis. Diabetologia. 2014;57:1789–1797. doi: 10.1007/s00125-014-3303-z [DOI] [PubMed] [Google Scholar]
- 39.Yarizadeh H, Eftekhar R, Anjom-Shoae J, Speakman JR, Djafarian K. The effect of aerobic and resistance training and combined exercise modalities on subcutaneous abdominal fat: a systematic review and meta-analysis of randomized clinical trials. Adv Nutr. 2021;12:179–196. doi: 10.1093/advances/nmaa090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramos-Campo DJ, Andreu-Caravaca L, Carrasco-Poyatos M, Benito PJ, Rubio-Arias J. Effects of circuit resistance training on body composition, strength, and cardiorespiratory fitness in middle-aged and older women: a systematic review and meta-analysis. J Aging Phys Act. 2021;30:725–728. doi: 10.1123/japa.2021-0204 [DOI] [PubMed] [Google Scholar]
- 41.Thomas E, Gentile A, Lakicevic N, Moro T, Bellafiore M, Paoli A, Drid P, Palma A, Bianco A. The effect of resistance training programs on lean body mass in postmenopausal and elderly women: a meta-analysis of observational studies. Aging Clin Exp Res. 2021;33:2941–2952. doi: 10.1007/s40520-021-01853-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lampropoulos CE, Papaioannou I, D’Cruz DP. Osteoporosis: a risk factor for cardiovascular disease? Nat Rev Rheumatol. 2012;8:587–598. doi: 10.1038/nrrheum.2012.120 [DOI] [PubMed] [Google Scholar]
- 43.Kistler-Fischbacher M, Weeks BK, Beck BR. The effect of exercise intensity on bone in postmenopausal women (part 2): a meta-analysis. Bone. 2021;143:115697. doi: 10.1016/j.bone.2020.115697 [DOI] [PubMed] [Google Scholar]
- 44.Kelley GA, Kelley KS, Kohrt WM. Exercise and bone mineral density in premenopausal women: a meta-analysis of randomized controlled trials. Int J Endocrinol. 2013;2013:741639. doi: 10.1155/2013/741639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martyn-St James M, Carroll S. Effects of different impact exercise modalities on bone mineral density in premenopausal women: a meta-analysis. J Bone Miner Metab. 2010;28:251–267. doi: 10.1007/s00774-009-0139-6 [DOI] [PubMed] [Google Scholar]
- 46.Vasudevan A, Ford E. Motivational factors and barriers towards initiating and maintaining strength training in women: a systematic review and meta-synthesis. Prev Sci. 2022;23:674–695. doi: 10.1007/s11121-021-01328-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Physical activity and exercise during pregnancy and the postpartum period: ACOG Committee Opinion, Number 804. Obstet Gynecol. 2020;135:e178–e188. doi: 10.1097/AOG.0000000000003772 [DOI] [PubMed] [Google Scholar]
- 48.Schoenfeld B Resistance training during pregnancy: safe and effective program design. Strength Cond J. 2011;33:67–75. doi: 10.1519/ssc.0b013e31822ec2d8 [DOI] [Google Scholar]
- 49.Perales M, Santos-Lozano A, Ruiz JR, Lucia A, Barakat R. Benefits of aerobic or resistance training during pregnancy on maternal health and perinatal outcomes: a systematic review. Early Hum Dev. 2016;94:43–48. doi: 10.1016/j.earlhumdev.2016.01.004 [DOI] [PubMed] [Google Scholar]
- 50.LeCheminant JD, Hinman T, Pratt KB, Earl N, Bailey BW, Thackeray R, Tucker LA. Effect of resistance training on body composition, self-efficacy, depression, and activity in postpartum women. Scand J Med Sci Sports. 2014;24:414–421. doi: 10.1111/j.1600-0838.2012.01490.x [DOI] [PubMed] [Google Scholar]
- 51.Coll CV, Domingues MR, Gonçalves H, Bertoldi AD. Perceived barriers to leisure-time physical activity during pregnancy: a literature review of quantitative and qualitative evidence. J Sci Med Sport. 2017;20:17–25. doi: 10.1016/j.jsams.2016.06.007 [DOI] [PubMed] [Google Scholar]
- 52.Valenzuela T Efficacy of progressive resistance training interventions in older adults in nursing homes: a systematic review. J Am Med Dir Assoc. 2012;13:418–428. doi: 10.1016/j.jamda.2011.11.001 [DOI] [PubMed] [Google Scholar]
- 53.Talar K, Hernández-Belmonte A, Vetrovsky T, Steffl M, Kałamacka E, Courel-Ibáñez J. Benefits of resistance training in early and late stages of frailty and sarcopenia: a systematic review and meta-analysis of randomized controlled studies. J Clin Med. 2021;10:1630. doi: 10.3390/jcm10081630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu CJ, Latham NK. Progressive resistance strength training for im-proving physical function in older adults. Cochrane Database Syst Rev. 2009;2009:CD002759. doi: 10.1002/14651858.CD002759.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen N, He X, Feng Y, Ainsworth BE, Liu Y. Effects of resistance training in healthy older people with sarcopenia: a systematic review and meta-analysis of randomized controlled trials. Eur Rev Aging Phys Act. 2021;18:23. doi: 10.1186/s11556-021-00277-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cawthon PM, Orwoll ES, Peters KE, Ensrud KE, Cauley JA, Kado DM, Stefanick ML, Shikany JM, Strotmeyer ES, Glynn NW, et al. ; Osteoporotic Fractures in Men (MrOS) Study Research Group. Strong relation between muscle mass determined by D3-creatine dilution, physical performance, and incidence of falls and mobility limitations in a prospective cohort of older men. J Gerontol A Biol Sci Med Sci. 2019;74:844–852. doi: 10.1093/gerona/gly129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, George SM, Olson RD. The physical activity guidelines for Americans. JAMA. 2018;320:2020–2028. doi: 10.1001/jama.2018.14854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McLeod KA, Jones MD, Thom JM, Parmenter BJ. Resistance training and high-intensity interval training improve cardiometabolic health in high risk older adults: a systematic review and meta-analysis. Int J Sports Med. 2022;43:206–218. doi: 10.1055/a-1560-6183 [DOI] [PubMed] [Google Scholar]
- 59.Dos Santos Araujo JE, Nunes Macedo F, Sales Barreto A, Viana Dos Santos MR, Antoniolli AR, Quintans-Junior LJ. Effects of resistance and combined training on vascular function in type 2 diabetes: a systematic review of randomized controlled trials. Rev Diabet Stud. 2019;15:16–25. doi: 10.1900/RDS.2019.15.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Borde R, Hortobágyi T, Granacher U. Dose-response relationships of resistance training in healthy old adults: a systematic review and meta-analysis. Sports Med. 2015;45:1693–1720. doi: 10.1007/s40279-015-0385-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Steib S, Schoene D, Pfeifer K. Dose-response relationship of resistance training in older adults: a meta-analysis. Med Sci Sports Exerc. 2010;42:902–914. doi: 10.1249/MSS.0b013e3181c34465 [DOI] [PubMed] [Google Scholar]
- 62.Forman DE, Arena R, Boxer R, Dolansky MA, Eng JJ, Fleg JL, Haykowsky M, Jahangir A, Kaminsky LA, Kitzman DW, et al. ; on behalf of the American Heart Association Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; and Stroke Council. Prioritizing functional capacity as a principal end point for therapies oriented to older adults with cardiovascular disease: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2017;135:e894–e918. doi: 10.1161/CIR.0000000000000483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Giuliano C, Karahalios A, Neil C, Allen J, Levinger I. The effects of resistance training on muscle strength, quality of life and aerobic capacity in patients with chronic heart failure: A meta-analysis. Int J Cardiol. 2017;227:413–423. doi: 10.1016/j.ijcard.2016.11.023 [DOI] [PubMed] [Google Scholar]
- 64.Fisher S, Smart NA, Pearson MJ. Resistance training in heart failure patients: a systematic review and meta-analysis. Heart Fail Rev. 2021;27:1665–1682. doi: 10.1007/s10741-021-10169-8 [DOI] [PubMed] [Google Scholar]
- 65.Fukuta H, Goto T, Wakami K, Kamiya T, Ohte N. Effects of exercise training on cardiac function, exercise capacity, and quality of life in heart failure with preserved ejection fraction: a meta-analysis of randomized controlled trials. Heart Fail Rev. 2019;24:535–547. doi: 10.1007/s10741-019-09774-5 [DOI] [PubMed] [Google Scholar]
- 66.Conraads VM, Beckers PJ. Exercise training in heart failure: practical guidance. Heart. 2010;96:2025–2031. doi: 10.1136/hrt.2009.183889 [DOI] [PubMed] [Google Scholar]
- 67.McDermott MM, Ferrucci L, Gonzalez-Freire M, Kosmac K, Leeuwenburgh C, Peterson CA, Saini S, Sufit R. Skeletal muscle pathology in peripheral artery disease: a brief review. Arterioscler Thromb Vasc Biol. 2020;40:2577–2585. doi: 10.1161/ATVBAHA.120.313831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Treat-Jacobson D, McDermott MM, Bronas UG, Campia U, Collins TC, Criqui MH, Gardner AW, Hiatt WR, Regensteiner JG, Rich K; on behalf of the American Heart Association Council on Peripheral Vascular Disease; Council on Quality of Care and Outcomes Research; and Council on Cardiovascular and Stroke Nursing. Optimal exercise programs for patients with peripheral artery disease: a scientific statement from the American Heart Association. Circulation. 2019;139:e10–e33. doi: 10.1161/CIR.0000000000000623 [DOI] [PubMed] [Google Scholar]
- 69.Polonsky TS, McDermott MM. Lower extremity peripheral artery disease without chronic limb-threatening ischemia: a review. JAMA. 2021;325:2188–2198. doi: 10.1001/jama.2021.2126 [DOI] [PubMed] [Google Scholar]
- 70.Blears EE, Elias JK, Tapking C, Porter C, Rontoyanni VG. Supervised resistance training on functional capacity, muscle strength and vascular function in peripheral artery disease: an updated systematic review and meta-analysis. J Clin Med. 2021;10:2193. doi: 10.3390/jcm10102193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Parmenter BJ, Mavros Y, Ritti Dias R, King S, Fiatarone Singh M. Resistance training as a treatment for older persons with peripheral artery disease: a systematic review and meta-analysis. Br J Sports Med. 2020;54:452–461. doi: 10.1136/bjsports-2018-100205 [DOI] [PubMed] [Google Scholar]
- 72.Webel AR, Schexnayder J, Cioe PA, Zuñiga JA. A review of chronic comorbidities in adults living with HIV: state of the science. J Assoc Nurses AIDS Care. 2021;32:322–346. doi: 10.1097/JNC.0000000000000240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Levett TJ, Cresswell FV, Malik MA, Fisher M, Wright J. Systematic review of prevalence and predictors of frailty in individuals with human immunodeficiency virus. J Am Geriatr Soc. 2016;64:1006–1014. doi: 10.1111/jgs.14101 [DOI] [PubMed] [Google Scholar]
- 74.Oliveira VHF, Borsari AL, Webel AR, Erlandson KM, Deminice R. Sarcopenia in people living with the human immunodeficiency virus: a systematic review and meta-analysis. Eur J Clin Nutr. 2020;74:1009–1021. doi: 10.1038/s41430-020-0637-0 [DOI] [PubMed] [Google Scholar]
- 75.Montoya JL, Jankowski CM, O’Brien KK, Webel AR, Oursler KK, Henry BL, Moore DJ, Erlandson KM. Evidence-informed practical recommendations for increasing physical activity among persons living with HIV. AIDS. 2019;33:931–939. doi: 10.1097/QAD.0000000000002137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zanetti HR, Lopes LTP, Gonçalves A, Soares VL, Soares WF, Hernandez AV, Tse G, Liu T, Biondi-Zoccai G, Roever L, et al. Effects of resistance training on muscle strength, body composition and immune-inflammatory markers in people living with HIV: a systematic review and meta-analysis of randomized controlled trials. HIV Res Clin Pract. 2021;22:119–127. [PubMed] [Google Scholar]
- 77.Pérez Chaparro CGA, Zech P, Schuch F, Wolfarth B, Rapp M, Heiβel A. Effects of aerobic and resistance exercise alone or combined on strength and hormone outcomes for people living with HIV. A meta-analysis. PLoS One. 2018;13:e0203384. doi: 10.1371/journal.pone.0203384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Voigt N, Cho H, Schnall R. supervised physical activity and improved functional capacity among adults living with HIV: a systematic review. J Assoc Nurses AIDS Care. 2018;29:667–680. doi: 10.1016/j.jana.2018.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, Boehme AK, Buxton AE, Carson AP, Commodore-Mensah Y, et al. ; on behalf of the American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2022 update: a report from the American Heart Association. Circulation. 2022;145:e153–e639. doi: 10.1161/CIR.0000000000001052 [DOI] [PubMed] [Google Scholar]
- 80.Li Z, Peng X, Xiang W, Han J, Li K. The effect of resistance training on cognitive function in the older adults: a systematic review of randomized clinical trials. Aging Clin Exp Res. 2018;30:1259–1273. doi: 10.1007/s40520-018-0998-6 [DOI] [PubMed] [Google Scholar]
- 81.Herold F, Törpel A, Schega L, Müller NG. Functional and/or structural brain changes in response to resistance exercises and resistance training lead to cognitive improvements - a systematic review. Eur Rev Aging Phys Act. 2019;16:10. doi: 10.1186/s11556-019-0217-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen KH, Chen HH, Li L, Lin HC, Chen CL, Chen NC. The impact of exercise on patients with dementia: a 2-year follow-up. Medicine (Baltimore). 2020;99:e20597. doi: 10.1097/MD.0000000000020597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ronai P, Sorace P. Resistance training for persons with chronic kidney disease. Strength Cond J. 2008;30:28–30. doi: 10.1519/ssc.0b013e318174bb97 [DOI] [Google Scholar]
- 84.Clyne N, Anding-Rost K. Exercise training in chronic kidney disease: effects, expectations and adherence. Clin Kidney J. 2021;14:ii3–ii14. doi: 10.1093/ckj/sfab012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Deligiannis A, D’Alessandro C, Cupisti A. Exercise training in dialysis patients: impact on cardiovascular and skeletal muscle health. Clin Kidney J. 2021;14:ii25–ii33. doi: 10.1093/ckj/sfaa273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liguori G, American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. 11th ed. Wolters Kluwer; 2022. [Google Scholar]
- 87.Grgic J, Schoenfeld BJ, Latella C. Resistance training frequency and skeletal muscle hypertrophy: a review of available evidence. J Sci Med Sport. 2019;22:361–370. doi: 10.1016/j.jsams.2018.09.223 [DOI] [PubMed] [Google Scholar]
- 88.Grgic J, Schoenfeld BJ, Skrepnik M, Davies TB, Mikulic P. Effects of rest interval duration in resistance training on measures of muscular strength: a systematic review. Sports Med. 2018;48:137–151. doi: 10.1007/s40279-017-0788-x [DOI] [PubMed] [Google Scholar]
- 89.Hollings M, Mavros Y, Freeston J, Fiatarone Singh M. The effect of progressive resistance training on aerobic fitness and strength in adults with coronary heart disease: a systematic review and meta-analysis of randomised controlled trials. Eur J Prev Cardiol. 2017;24:1242–1259. doi: 10.1177/2047487317713329 [DOI] [PubMed] [Google Scholar]
- 90.Pengelly J, Pengelly M, Lin K-Y, Royse C, Royse A, Bryant A, Williams G, El-Ansary D. Resistance training following median sternotomy: a systematic review and meta-analysis. Heart Lung Circ. 2019;28:1549–1559. doi: 10.1016/j.hlc.2019.05.097 [DOI] [PubMed] [Google Scholar]
- 91.Maron BJ, Chaitman BR, Ackerman MJ, Bayés de Luna A, Corrado D, Crosson JE, Deal BJ, Driscoll DJ, Estes NA 3rd, Araújo CG, et al. ; for the Working Groups of the American Heart Association Committee on Exercise, Cardiac Rehabilitation, and Prevention; Councils on Clinical Cardiology and Cardiovascular Disease in the Young. Recommendations for physical activity and recreational sports participation for young patients with genetic cardiovascular diseases. Circulation. 2004;109:2807–2816. doi: 10.1161/01.CIR.0000128363.85581.E1 [DOI] [PubMed] [Google Scholar]
- 92.Colberg SR, Sigal RJ, Yardley JE, Riddell MC, Dunstan DW, Dempsey PC, Horton ES, Castorino K, Tate DF. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39:2065–2079. doi: 10.2337/dc16-1728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stricker PR, Faigenbaum AD, McCambridge TM; Council on Sports Medicine and Fitness. Resistance training for children and adolescents. Pediatrics. 2020;145:e20201011. doi: 10.1542/peds.2020-1011 [DOI] [PubMed] [Google Scholar]
- 94.Jäger R, Kerksick CM, Campbell BI, Cribb PJ, Wells SD, Skwiat TM, Purpura M, Ziegenfuss TN, Ferrando AA, Arent SM, et al. International Society of Sports Nutrition position stand: protein and exercise. J Int Soc Sports Nutr. 2017;14:20. doi: 10.1186/s12970-017-0177-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ma JK, Leese J, Therrien S, Hoens AM, Tsui K, Li LC. A scoping review of interventions to improve strength training participation. PLoS One. 2022;17:e0263218. doi: 10.1371/journal.pone.0263218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bennie JA, De Cocker K, Teychenne MJ, Brown WJ, Biddle SJH. The epidemiology of aerobic physical activity and muscle-strengthening activity guideline adherence among 383,928 US adults. Int J Behav Nutr Phys Act. 2019;16:34. doi: 10.1186/s12966-019-0797-2 [DOI] [PMC free article] [PubMed] [Google Scholar]