Abstract
Vpx is a virion-associated protein of human immunodeficiency virus type 2 (HIV-2) and simian immunodeficiency viruses. The yeast two-hybrid system was used to identify invariant chain (Ii) as a cellular protein that interacts with HIV-2 Vpx. Vpx-Ii interaction was confirmed in cell-free reactions using bacterially expressed glutathione S-transferase fusion proteins and by coimmunoprecipitation in transfected and infected cells. In chronically infected cells expressing Vpx, Ii levels were markedly decreased, presumably due to enhanced degradation. These findings suggest that Vpx may disrupt major histocompatibility complex class II antigen presentation.
Human immunodeficiency virus type 2 (HIV-2), like HIV-1, is a causative agent of AIDS, but it is limited in its geographical distribution primarily to West Africa (29, 41). HIV-2 exhibits lower pathogenicity than HIV-1, as determined by measurements of virus load and rates of progression to clinical immunodeficiency. HIV-1, HIV-2, and the simian immunodeficiency viruses (SIVs) share significant genetic homology. However, vpx, which is present in HIV-2 and most SIVs, is absent from HIV-1. Vpx is a 17-kDa accessory protein which is packaged in the virion in an amount comparable to that of the Gag proteins, as a result of an interaction with the C-terminal p6 portion of the Gag polyprotein (18, 36, 45). The presence of Vpx in the virion suggests that it has an important function in the early portion of the viral life cycle. One such function, which has been demonstrated for SIV Vpx, is to direct the nuclear import of the preintegration complex of viral DNA and various cellular and viral proteins in quiescent cells (20). This allows HIV-2 to infect terminally differentiated macrophages, which serve as an important reservoir for the virus (30).
Vpx can localize to multiple subcellular compartments. When Vpx is expressed with Gag, it is targeted to the plasma membrane and incorporated into budding virus particles (45). In the absence of Gag, Vpx can localize to the nucleus, consistent with its nuclear targeting function (13, 36a). In addition, Vpx is found in some cells in a perinuclear distribution (45). The varied subcellular localizations of Vpx suggest that this protein may serve multiple distinct functions. In order to define these Vpx functions, this study sought to identify cellular proteins which interact with Vpx.
Vpx binds to Ii in the yeast two-hybrid assay.
In order to identify Vpx-interacting proteins, Vpx was used in a two-hybrid screen of a human cDNA library (2). To express the Gal4 DNA binding domain-Vpx fusion protein, pTM-Vpx (21) was digested with NcoI and BamHI, and the vpx DNA fragment was ligated into NcoI- and BamHI-digested pAS1-CYH to generate pAS1-Vpx. The human cDNA library in the pACT2 vector was constructed from Jurkat cells and was a generous gift from Stephen Elledge (Baylor College of Medicine). Saccharomyces cerevisiae strain Y190 expressing pAS-1 Vpx was transformed using the lithium acetate method (5) with 100 μg of DNA from the pACT2 human B-cell cDNA library. Approximately 1.1 × 106 double transformants were assayed by selection for histidine, leucine, and tryptophan prototrophy. β-Galactosidase activity was assessed on nitrocellulose filter replicas of yeast transformants (9), and three colonies that expressed high levels of β-galactosidase activity were obtained. The pACT2 clone from each of these colonies was isolated on selective medium and mated to strain Y187, containing nonspecific cDNA fused to the GAL4 DNA binding domain. The three positive clones did not interact with several nonspecific baits, including HIV-1 Tat, HIV-1 Rev, p53, SNF1, and laminin. The pACT2 clone from each of the three positive colonies was rescued by electroporation into competent HB101 cells. Restriction enzyme analysis of the library cDNA inserts suggested that two of these three pACT2 plasmids contained overlapping cDNA sequences. DNA sequence analysis, performed by the dideoxynucleotide chain termination method (United States Biochemical), and a search of GenBank with a BLAST search using the National Center for Biotechnology Information website revealed that the third clone did not correspond to a previously submitted nucleotide sequence. The two related clones contained sequences encoding C-terminal residues 87 to 216 and 134 to 216 of human invariant chain (Ii) (Fig. 1). The ability of these two independently derived clones to strongly interact with Vpx, but not nonspecific proteins, suggested that the Ii interaction with Vpx is significant.
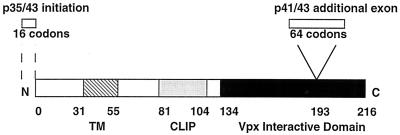
FIG. 1.
Schematic structure of the four isoforms of Ii. Alternative initiation generates p35 and p43, whereas alternative splicing generates p41 and p43. The minimal region found to interact with Vpx in the yeast two-hybrid screen is in black. The transmembrane (TM) and CLIP domains are also indicated. Numbers of residues for each domain are indicated.
Ii is a type II transmembrane protein (Fig. 1). Ii has multiple isoforms, p33, p35, p41, and p43, derived by alternative splicing (p41 and p43) or alternative translational initiation (p35 and p43) (Fig. 1) (35, 43). All four isoforms include the Vpx-interactive domain, and this domain overlaps with the trimerization domain of Ii (6). Ii forms a trimer in the endoplasmic reticulum, wherein each subunit of Ii binds to major histocompatibility complex (MHC) class II α-β dimer, thereby forming a nonameric complex (39). The α and β chains are efficiently transported through the Golgi apparatus and into the MHC class II compartment (MIIC), where peptide loading occurs by displacing the CLIP domain of Ii (Fig. 1) (25, 28). In the absence of the Ii chaperone, α and β chains of MHC class II accumulate in the endoplasmic reticulum (ER) and demonstrate increased binding activity for endogenous peptides (7, 27, 34).
Vpx binds to the C-terminal 83 residues of Ii in vitro.
The interaction between Vpx and Ii was confirmed using recombinant glutathione S-transferase (GST) fusion proteins. Sequences encoding the smallest Vpx-interactive domain of Ii, residues 134 to 216, were isolated by digestion of the pACT2 plasmid with XhoI, blunt ended with the Klenow fragment of DNA polymerase I, and ligated into the SmaI site of pGEX-2T (Pharmacia). Production of the fusion protein and subsequent purification of glutathione-Sepharose beads were performed using standard techniques (42).
Metabolically labeled Vpx was generated in BSC40 cells using the vaccinia virus expression system, as described previously (36). Cells which were 90% confluent on a 100-mm-diameter tissue culture plate were infected at a multiplicity of infection of 10 for 1 h with the vaccinia virus vTF7-3, expressing T7 polymerase (33). The cells were then transfected with 10 μg of pTM-Vpx DNA using Lipofectin (Gibco). Four hours after transfection, the cells were labeled in cysteine- and methionine-free medium containing 50 μCi of Tran35S-label per ml. Twenty hours after transfection, the cells were lysed in 10 mM Tris-Cl (pH 7.5)–0.15 M NaCl–1% Triton X-100–1 mM EDTA (lysis buffer) and clarified by centrifugation. One-tenth of the cellular lysate was added to GST or GST-Ii bound to glutathione-Sepharose beads and rotated for 1 h at 4°C. After extensive washing in lysis buffer, protein was eluted from the beads by boiling for 3 min in Laemmli buffer (24). Bound proteins were detected by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by autoradiography.
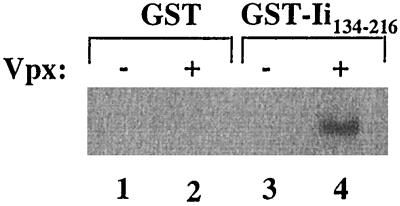
Vaccinia virus-expressed Vpx specifically bound to GST-Ii134–216 but not to GST alone (Fig. 2). An additional GST fusion protein was generated with the full-length p33 Ii protein, which also specifically interacted with Vpx (data not shown). Furthermore, bacterially expressed Vpx was capable of binding to GST-Ii134–216 (data not shown).
FIG. 2.
Specific binding of Vpx to GST-Ii134–316 fusion protein. Equal amounts of cellular lysate from metabolically labeled Vpx-expressing BSC40 cells were incubated with GST (lane 2) or GST-Ii134–216 (lane 4) bound to glutathione-Sepharose beads. Bound proteins were subjected to SDS-PAGE and autoradiography.
Vpx binds to Ii in transfected cells.
In order to determine if the interaction of Vpx with Ii occurs in mammalian cells, Vpx was expressed in a vaccinia virus expression system in HeLa-CIITA cells, which constitutively express endogenous Ii as a result of stable transfection and expression of the class II transactivator (CIITA). Alternatively, Vpx and Ii expression plasmids were expressed in BSC40 cells infected with vTF7-3, as described above. cDNAs encoding the p33 and p35/33 forms of Ii were under the control of the T7 promoter and were designated pAR.33 and pAR.35/33, respectively (4). The cells were metabolically labeled, and cell lysates were harvested, as described above. Lysates were immunoprecipitated with 2 μl of PIN.1 antiserum (39), followed by the addition of protein G beads (Sigma). Precipitates were analyzed by SDS-PAGE and autoradiography.
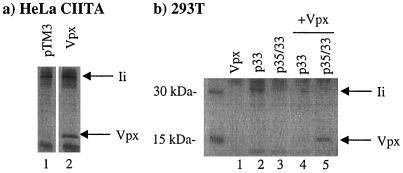
Using HeLa-CIITA cells transfected with the control vector pTM3 (33), anti-Ii immunoprecipitates revealed a band of approximately 33 kDa (Fig. 3a, lane 1). In contrast, in HeLa-CIITA cells expressing Vpx, anti-Ii immunoprecipitates revealed bands of 33 and 17 kDa (Fig. 3a, lane 2). In HeLa cells, a GFP-Vpx fusion protein was localized in the nucleus and, to a lesser extent, in the cytoplasm (36a). In contrast, in HeLa-CIITA cells, GFP-Vpx localized primarily in a perinuclear cytoplasmic compartment (data not shown).
FIG. 3.
Vpx interacts with Ii in transiently transfected cells. The indicated proteins were expressed in HeLa-CIITA cells (a) or BSC40 cells (b) using the vaccinia virus expression system. Metabolically labeled proteins from the cell lysates were immunoprecipitated using PIN.1 antiserum and subjected to SDS-PAGE and autoradiography.
With BSC40 cells expressing only the p33 form or both the p35 and p33 forms of Ii, anti-Ii immunoprecipitates revealed a band of 33 to 35 kDa (Fig. 3b, lanes 2 and 3). In contrast, anti-Ii immunoprecipitates from BSC40 cells expressing only Vpx revealed no specific bands (Fig. 3b, lane 1). When Vpx was coexpressed in BSC40 cells with the p33 or p35 and p33 forms of Ii, anti-Ii immunoprecipitates revealed bands of 33 to 35 kDa as well as the 17-kDa Vpx protein (Fig. 3b, lanes 4 and 5). It is notable that more Vpx could be coimmunoprecipitated from BSC40 cells expressing p35 and p33 forms of Ii (Fig. 3b, lane 5), than from BSC40 cells expressing only the p33 form of Ii (Fig. 3b, lane 4). The p35 form of Ii includes 16 additional amino acids at the N terminus that serve as an ER retention signal (4).
Vpx also coimmunoprecipitated with the p43 and p41 forms of Ii (data not shown). Furthermore, a deletion of residues 20 to 40 of Vpx, a region that is predicted to form an amphipathic helix, abrogates the interaction with Ii (data not shown). Specificity of this interaction was further demonstrated by the inability of Ii to bind to HIV-1 Vpu (data not shown). These experiments indicate that Vpx interacts with multiple isoforms of Ii in mammalian cells and that this binding requires residues 20 to 40 of Vpx.
Vpx interacts with Ii in HIV-2-infected cells.
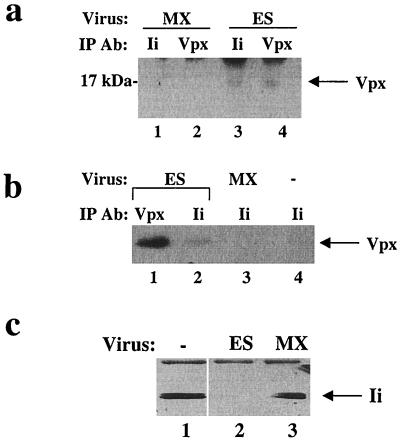
CEMx174 cells were infected for 7 to 14 days with the wild-type HIV-2 (ES) or an isogenic virus lacking Vpx expression (MX), using 100 ng of p27 antigen as determined by enzyme-linked immunosorbent assay (Coulter). The pES proviral clone was derived from the functional HIV-2 ROD-derived clone, pSE, after digestion with SalI to remove a flanking cellular sequence (22). MX, previously designated pMX1+62, includes mutations at the initiator codon of vpx as well as the second methionine codon and does not produce a stable Vpx protein. Metabolic labeling and cell lysis were performed as described above. Immunoprecipitations were performed with either the monoclonal anti-Ii antibody PIN.1 (39) or the polyclonal anti-Ii antiserum (40). Precipitated proteins were immunoblotted with anti-Vpx antiserum (21) or anti-Ii antibody PIN.1 (39). Immunoprecipitation of Ii from ES-infected cells resulted in coprecipitation of Vpx (Fig. 4a, lanes 3 and 4). In contrast, immunoprecipitation of Ii from MX-infected cells resulted in no detectable Vpx protein (Fig. 4a, lanes 1 and 2). Ii was detected in anti-Ii immunoprecipitates from both ES- and MX-infected cells (data not shown).
FIG. 4.
Ii interacts with Vpx in HIV-2-infected cells. (a) CEMx174 cells were infected with MX or ES virus. Cell lysates were immunoprecipitated (IP) with PIN.1 or polyclonal antiserum (Ab), followed by SDS-PAGE and immunoblotting with Vpx antiserum. (b) T2 cells were infected with MX or ES virus, and immunoprecipitated with polyclonal Ii or Vpx antiserum, and subjected to SDS-PAGE and immunoblotting with polyclonal Vpx antiserum. (c) T2 cells, chronically infected with ES or MX, were immunoprecipitated with polyclonal Ii antiserum. Immunoprecipitated proteins were subjected to SDS-PAGE followed by immunoblotting using PIN.1 antiserum.
T2 cells, a variant of CEMx174 cells which express Ii but not MHC class II (1), were also utilized for infection experiments. Immunoprecipitation of Ii from T2 cells infected with ES for 7 to 14 days resulted in coprecipitation of Vpx (Fig. 4b, lanes 1 and 2). In contrast, no specific bands could be visualized on anti-Vpx immunoblots of anti-Ii immunoprecipitates from MX-infected T2 cells (Fig. 4b, lane 3) or uninfected T2 cells (Fig. 4b, lane 4). Ii was detected in anti-Ii immunoprecipitates from ES- and MX-infected cells and uninfected T2 cells (data not shown).
In addition to the experiments described above, chronically infected T2 cells were also utilized. In this case, anti-Ii immunoprecipitates and immunoblots from uninfected cells and MX-infected cells revealed a band of 33 to 35 kDa (Fig. 4c, lanes 1 and 3). However, anti-Ii immunoprecipitates and immunoblots from ES-infected cells revealed no detectable Ii protein. Similar results were obtained with productively infected primary human macrophages (data not shown). This finding suggested that Vpx interaction with Ii may enhance Ii degradation.
A novel mechanism of immune evasion resulting from Vpx interaction with Ii.
The ability of CD4+ T cells to recognize exogenously derived antigens is dependent upon their efficient cell surface presentation by antigen-presenting cells in association with MHC class IIα and β chains. This in turn relies upon the association of Ii with the α and β chains in the ER, Golgi, and MIIC compartments. Without the chaperone activity of Ii, class II molecules aggregate in the ER as a result of tight binding to other ER-resident chaperones that lack the targeting signals found in the cytosolic tail of Ii (3, 8). Class II molecules that escape through the secretory pathway to the cell surface bind to endogenous rather than exogenous peptides. Thus, an important function of Ii is the occupation of the class II peptide groove during trafficking, such that endogenous peptides cannot be bound. Once in the MIIC compartment, Ii is displaced from this binding site as a result of sequential cleavage of Ii by cellular proteases, such as cathepsin S (38). The CLIP peptide, consisting of luminal residues 81 to 104, is the final product of Ii proteolysis, which is in direct association with the class II peptide binding groove (11). This fragment is then displaced by HLA-DM in order for exogenous peptides to bind to MHC class II.
Viruses have evolved a variety of strategies to interfere with normal cellular processes critical for host immune surveillance. There are several examples of viral gene products which inhibit MHC class I antigen presentation (31). For example, HIV-1 Vpu and Nef accessory proteins inhibit MHC class I expression during processing in the ER or through endocytosis, respectively (16, 23). Adenovirus E3-19K protein binds and arrests MHC class I molecules in the ER (10), whereas herpes simplex virus type 1-infected-cell protein 47 inhibits the transport of peptides into the ER by the transporters associated with antigen presentation (15, 19, 46). Cytomegaloviruses (CMVs) have multiple genes to interfere with the MHC class I pathway of antigen presentation.
Previously described examples of viral down-regulation of MHC class II involve inhibition of transcription (17, 32). For example, CMV represses CIITA mRNA expression, resulting in a defect in MHC class II mRNA synthesis (26). Human CMV also inhibits class II trafficking to the cell surface indirectly, through global effects on the secretory pathway (12, 44). The present study is the first report of a viral protein that is able to interact with the class II chaperone Ii, presumably leading to a decrease in its availability for α and β chain association. The minimal region for Ii binding to Vpx is residues 134 to 216, a region important for Ii trimerization. Further studies are needed to address whether binding to Vpx inhibits oligomerization and results in decreased stability of Ii. Protein-protein interactions are often mediated through amphipathic helical domains, so it is interesting that residues 118 to 208 of Ii are helical in structure (37) and that the amphipathic helix of Vpx appears to be important for Ii binding.
Dendritic cells, such as Langerhans cells, which are found within mucous membranes throughout the body, are one of the first cell types to encounter microbial pathogens. Upon such encounters, these cells then migrate to lymphoid organs, presenting exogenously derived antigens in association with MHC class II molecules in order to activate circulating T cells. It has been demonstrated that dendritic cells found at mucosal surfaces are infected with HIV-1 (14). Although similar studies have not yet been done with HIV-2, it is likely that these cells are also infected with this lentivirus. This represents one cell type wherein Vpx may interfere with normal immune function in vivo. In addition, HIV-2 productively infects human macrophages, cells which play an important role in antigen presentation. Efficient macrophage infection is critical for SIVSM dissemination in vivo, and Vpx is important for establishment of this infection (20). Further work is needed to address the downstream implications of this Vpx-Ii interaction for effective antigen presentation. However, these studies elucidate a unique interaction between a viral protein and component of the MHC class II pathway and may provide further insight into a novel mechanism utilized by HIV-2 to alter normal host immune function.
Acknowledgments
We thank Timothy Schaif for polyclonal Ii antibody and for helpful discussion, and we thank Ted Hansen, Charles Rice, and Douglas Dean for critical input.
This work was supported by Public Health Service grants AI36071 and AI34736 and Training Grant AI07172.
REFERENCES
- 1.Albert L J, Densin L K, Ghumman B, Bangia N, Cresswell P, Watts T H. Quantitative defect in staphylococcal enterotoxin A binding and presentation by HLA-DM-deficient T2.Ak cells corrected by transfection of HLA-DM genes. Cell Immunol. 1998;183:42–51. doi: 10.1006/cimm.1997.1236. [DOI] [PubMed] [Google Scholar]
- 2.Bai C, Elledge S J. Gene identification using the yeast two-hybrid system. Methods Enzymol. 1996;273:331–347. doi: 10.1016/s0076-6879(96)73029-x. [DOI] [PubMed] [Google Scholar]
- 3.Bakke O, Dobberstein B. MHC class II-associated invariant chain contains a sorting signal for endosomal compartments. Cell. 1990;63:707–716. doi: 10.1016/0092-8674(90)90137-4. [DOI] [PubMed] [Google Scholar]
- 4.Balasubramanian A, Lamb C A, Cresswell P. Transport properties of free and MHC class II-associated oligomers containing different isoforms of human invariant chain. Int Immunol. 1993;6:439–451. doi: 10.1093/intimm/6.3.439. [DOI] [PubMed] [Google Scholar]
- 5.Becker D, Guarente L. Guide to yeast genetics and molecular biology. San Diego, Calif: Academic Press; 1991. [Google Scholar]
- 6.Bertolino P, Staschewski M, Trescol-Biemont M-C, Freisewinkel I M, Schenck K, Chretien I, Forquet F, Gerlier D, Rabourdin-Combe C, Koch N. Deletion of a C-terminal sequence of the class II-associated invariant chain abrogates invariant chains oligomer formation and class II antigen presentation. J Immunol. 1995;154:5620–5629. [PubMed] [Google Scholar]
- 7.Bodmer H, Viville S, Benoist C, Mathis D. Diversity of endogenous epitopes bound to mHC class II molecules limited by invariant chain. Science. 1994;263:1284–1286. doi: 10.1126/science.7510069. [DOI] [PubMed] [Google Scholar]
- 8.Bonnerot C, Marks M S, Cosson P, Robertson E J, Bikoff E K, Germain R N, Bonifacino J S. Association with BiP and aggregation of class II MHC molecules synthesized in the absence of invariant chain. EMBO J. 1994;13:934–944. doi: 10.1002/j.1460-2075.1994.tb06338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breedon L, Nasmyth K. Regulation of the yeast HO gene. Cold Spring Harbor Symp Quant Biol. 1985;50:643–650. doi: 10.1101/sqb.1985.050.01.078. [DOI] [PubMed] [Google Scholar]
- 10.Burgert H G, Kvist S. An adenovirus type 2 glycoprotein blocks cell surface expression of human histocompatibility class I antigens. Cell. 1985;41:987–997. doi: 10.1016/s0092-8674(85)80079-9. [DOI] [PubMed] [Google Scholar]
- 11.Cresswell P. Invariant chain structure and MHC class II function. Cell. 1996;84:505–507. doi: 10.1016/s0092-8674(00)81025-9. [DOI] [PubMed] [Google Scholar]
- 12.Fish K N, Britt W, Nelson J A. A novel mechanism for persistence of human cytomegalovirus in macrophages. J Virol. 1996;70:1855–1862. doi: 10.1128/jvi.70.3.1855-1862.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fletcher T M, Brichacek B, Sharova N, Newman M A, Stivahtis G, Sharp P M, Emerman M, Hahn B H, Stevenson M. Nuclear import and cell cycle arrest of the HIV-1 vpr protein are encoded by two separate genes in HIV-2/SIVsm. EMBO J. 1996;15:6155–6165. [PMC free article] [PubMed] [Google Scholar]
- 14.Frankel S S, Wenig B M, Burke A P. Replication of HIV-1 in dendritic cell-derived syncytia at the mucosal surface of the adenoid. Science. 1996;272:115–117. doi: 10.1126/science.272.5258.115. [DOI] [PubMed] [Google Scholar]
- 15.Fruh K, Ahn K, Djaballah H, Sempe P, vanEndert P M, Tampe R, Peterson P A, Yang Y. A viral inhibitor of peptide transporters for antigen presentation. Nature. 1995;375:415–418. doi: 10.1038/375415a0. [DOI] [PubMed] [Google Scholar]
- 16.Greenberg M E, Iafrate A J, Skowronski J. The SH3 domain-binding surface and an acidic motif in HIV-1 nef regulate trafficking of class I MHC complexes. EMBO J. 1998;17:2777–2789. doi: 10.1093/emboj/17.10.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heise M T, Connick M, Virgin H W. Murine cytomegalovirus inhibits interferon gamma-induced antigen presentation to CD4 T cells by macrophages via regulation of expression of major histocompatibility complex class II-associated genes. J Exp Med. 1998;187:1–10. doi: 10.1084/jem.187.7.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henderson L E, Sowder R C, Copeland T D, Beneviste R E, Oroszlan S. Isolation and characterization of a novel protein (X-ORF product) from SIV and HIV-2. Science. 1988;241:199–201. doi: 10.1126/science.3388031. [DOI] [PubMed] [Google Scholar]
- 19.Hill A, Jugovic P, York I, Russ G, Bennink J, Yewdell J, Ploegh H, Johnson D. Herpes simplex virus turns off TAP to evade host immunity. Nature. 1995;375:411–415. doi: 10.1038/375411a0. [DOI] [PubMed] [Google Scholar]
- 20.Hirsch V M, Sharkey M E, Brown C R, Brichacek B, Goldstein S, Wakefield J, Byrum R, Elkins W R, Hahn B H, Lifson J D, Stevenson M. Vpx is required for dissemination and pathogenesis of SIVsmPBj: evidence of macrophage-dependent viral amplification. Nat Med. 1998;4:1401–1408. doi: 10.1038/3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horton R, Spearman P, Ratner L. HIV-2 viral protein X association with the gag p27 capsid protein. Virology. 1994;199:453–457. doi: 10.1006/viro.1994.1144. [DOI] [PubMed] [Google Scholar]
- 22.Hu W, Vander Heyden N, Ratner L. Analysis of the function of viral protein X (VPX) of HIV-2. Virology. 1989;173:624–630. doi: 10.1016/0042-6822(89)90574-6. [DOI] [PubMed] [Google Scholar]
- 23.Kerkau T, Bacik I, Bennink J R, Yewdell J W, Hunig T, Schimpl A, Schubert U. The human immunodeficiency virus type 1 (HIV-1) Vpu protein interferes with an early step in the biosynthesis of major histocompatibility complex (MHC) class I molecules. J Exp Med. 1997;185:1295–1305. doi: 10.1084/jem.185.7.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 25.Lamb C A, Yewdell J W, Bennink J R, Cresswell P. Invariant chain targets HLA class II molecules to acidic endosomes containing internalized influenza virus. Proc Natl Acad Sci USA. 1991;88:5998–6002. doi: 10.1073/pnas.88.14.5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.LeRoy E, Muhlethaler-Mottet A, Davrinche C, Mach B, Davignon J-L. Escape of human cytomegalovirus from HLA-DR-restricted CD4+ T-cell response is mediated by repression of gamma interferon-induced class II transactivator expression. J Virol. 1999;73:6582–6589. doi: 10.1128/jvi.73.8.6582-6589.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lightsone L, Hargreaves R, Bobek G, Peterson M, Aichinger G, Lombardi G, Lechler R. In the absence of the invariant chain, HLA-DR molecules display a distinct array of peptides which is influenced by the presence or absence of HLA-DM. Proc Natl Acad Sci USA. 1997;94:5772–5777. doi: 10.1073/pnas.94.11.5772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lotteau V, Teyton L, Peleraux A, Nilsson T, Karlsson L, Schmid S L, Quaranta V, Peterson P A. Intracellular transport of class II MHC molecules directed by invariant chain. Nature. 1990;348:600–605. doi: 10.1038/348600a0. [DOI] [PubMed] [Google Scholar]
- 29.Marlink R, Kanki P, Thior I, Travers K, Eisen G, Siby T, Traore I, Hsieh C-C, Dia M C, Gueye E-H, Hellinger J, Gueye-Ndiaye A, Sankale J-L, Ndoye I, Mboup S, Essex E. Reduced rate of disease development after HIV-2 infection as compared to HIV-1. Science. 1994;265:1587–1590. doi: 10.1126/science.7915856. [DOI] [PubMed] [Google Scholar]
- 30.Meltzer M S, Gendelman H E. Mononuclear phagocytes as targets, tissue reservoirs, and immunoregulatory cells in human immunodeficiency virus disease. Curr Top Microbiol Immunol. 1992;181:239–263. doi: 10.1007/978-3-642-77377-8_9. [DOI] [PubMed] [Google Scholar]
- 31.Miller D M, Sedmak D D. Viral effects on antigen presentation. Curr Opin Immunol. 1999;11:94–99. doi: 10.1016/s0952-7915(99)80017-x. [DOI] [PubMed] [Google Scholar]
- 32.Miller D M, Rahill B M, Boss J M, Lairmore M D, Durbin J E, Waldman W J, Sedmak D D. Human cytomegalovirus inhibits major histocompatibility complex class II expression by disruption of the Jak/Stat pathway. J Exp Med. 1998;187:675–683. doi: 10.1084/jem.187.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moss B E, Mizukami T, Alexander W A, Fuerst T R. New expression vectors. Nature. 1990;348:91–92. doi: 10.1038/348091a0. [DOI] [PubMed] [Google Scholar]
- 34.Newcomb J R, Cresswell P. Characterization of endogenous peptides bound to purified HLA-DR molecules and their absence from invariant chain-associated alpha beta dimers. J Immunol. 1993;150:499–507. [PubMed] [Google Scholar]
- 35.O'Sullivan D M, Noonan D, Quaranta V. Four Ia invariant chain forms derived from a single gene by alternate splicing and alternative initiation of transcription/translation. J Exp Med. 1987;166:444–460. doi: 10.1084/jem.166.2.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pancio H A, Ratner L. Human immunodeficiency virus type 2 Vpx-Gag interaction. J Virol. 1998;72:5271–5275. doi: 10.1128/jvi.72.6.5271-5275.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36a.Pancio H A, Vander Heyden N, Ratner L. The C-terminal proline-rich tail of human immunodeficiency virus type 2 Vpx is necessary for nuclear localization of the viral preintegration complex in nondividing cells. J Virol. 2000;74:6162–6167. doi: 10.1128/jvi.74.13.6162-6167.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park S-J, Sadegh-Nasseri S, Wiley D C. Invariant chain made in Escherichia coli has an exposed N-terminal segment that blocks antigen binding to HLA-DR1 and a trimeric C-terminal segment that binds empty HLA-DR1. Proc Natl Acad Sci USA. 1995;92:11289–11293. doi: 10.1073/pnas.92.24.11289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riese R J, Wolf P R, Bromme D, Natkin L R, Villadangos J A, Ploegh H L, Chapman H A. Essential role for cathepsin S in MHC class II-associated invariant chain processing and peptide loading. Immunity. 1996;4:357–365. doi: 10.1016/s1074-7613(00)80249-6. [DOI] [PubMed] [Google Scholar]
- 39.Roche P A, Marks M S, Cresswell P. Formation of a nine-subunit complex by HLA class II glycoproteins and the invariant chain. Nature. 1991;354:392–394. doi: 10.1038/354392a0. [DOI] [PubMed] [Google Scholar]
- 40.Schaiff W T, Hruska K A, Bono C, Shuman S, Schwartz B D. Invariant chain influences post-translational processing of HLA-DR molecules. J Immunol. 1991;147:603–608. [PubMed] [Google Scholar]
- 41.Simon F, Matheron S, Tamalet C, Loussert-Ajaka I, Bartczak S, Pepin J M, Dhiver C, Gamba E, Elbim C, Gastaut J A, Saimot A G, Brun-Vezinet F. Cellular and plasma viral load in patients infected with HIV-2. AIDS. 1993;7:1411–1417. doi: 10.1097/00002030-199311000-00002. [DOI] [PubMed] [Google Scholar]
- 42.Smith D B, Johnson K S. Single-step purification of polypeptides expressed in Escherichia coli as fusion with glutathione S-transferase. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 43.Strubin M, Bete C, Mach B. Alternative splicing and alternative initiation of translation explain the four forms of Ia antigen-associated invariant chain. EMBO J. 1986;5:3483–3488. doi: 10.1002/j.1460-2075.1986.tb04673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tong X, Boll W, Kirchhausen T, Howley P M. Interaction of the bovine papillomavirus E6 protein with the clathrin adapter complex AP-1. J Virol. 1998;72:476–482. doi: 10.1128/jvi.72.1.476-482.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu X, Conway J A, Kim J, Kappes J C. Localization of the Vpx packaging signal within the C terminus of the human immunodeficiency virus type 2 Gag precursor protein. J Virol. 1994;68:6161–6169. doi: 10.1128/jvi.68.10.6161-6169.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.York I A, Roop C, Andrews D W, Riddell S R, Graham F L, Johnson D C. A cytosolic herpes simplex virus protein inhibits antigen presentation to CD8+ T lymphocytes. Cell. 1994;77:525–535. doi: 10.1016/0092-8674(94)90215-1. [DOI] [PubMed] [Google Scholar]