Abstract
Human immunodeficiency virus type 1 (HIV-1) entry into target cells appears to be triggered when two heptad repeat regions in the ectodomain of gp41 associate, converting the prefusogenic form of gp41 to a fusogenic form. Peptides from these two heptad repeat regions, designated N51 and C43, form a coiled coil consisting of an α-helical trimer of heterodimers which approximates the core of the fusogenic form of gp41. To understand the antigenic structures of gp41 in these two configurations, and to examine the specificity of anti-gp41 antibodies produced by HIV-1-infected individuals, human anti-gp41 monoclonal antibodies (MAbs) were tested for their reactivity against N51, C43, and the complex formed by these peptides. Of 11 MAbs, 7 reacted with the complex but with neither of the parent peptides. These MAbs reacted optimally with the N51-C43 complex prepared at a 1:1 ratio and appeared to recognize the fusogenic form of gp41 in which the two heptad repeat regions are associated to form the coiled coil. The existence of antibodies from HIV-infected humans that exclusively recognize the N51-C43 complex constitutes the first proof that the coiled-coil conformation of gp41 exists in vivo and is immunogenic. Two of the 11 MAbs were specific for the hydrophilic loop region of gp41 and failed to react with either peptide alone or with the peptide complex, while the remaining 2 MAbs reacted with peptide C43. One of these two latter MAbs, 98-6, also reacted well with the equimolar N51-C43 complex, while reactivity with C43 by the other MAb, 2F5, was inhibited by even small amounts of N51, suggesting that the interaction of these peptides occludes or disrupts the epitope recognized by MAb 2F5. MAbs 98-6 and 2F5 are also unusual among the MAbs tested in their ability to neutralize multiple primary HIV isolates, although 2F5 displays more broad and potent activity. The data suggest that anti-gp41 neutralizing activity is associated with specificity for a region in C43 which participates in complex formation with N51.
Over the past few years, the structure of the gp41 transmembrane viral glycoprotein of the type 1 human immunodeficiency virus (HIV) has been elucidated. This protein, made up of ∼345 amino acids (aa), is homologous to the transmembrane domains of several viruses, including those of influenza virus, Moloney murine leukemia virus, and simian immunodeficiency virus (3, 6, 15, 21), and consists of a single chain which is present in an oligomeric form (12, 30) in the envelope of the virion, most likely as a trimeric structure (27, 39). Recent studies of the HIV gp41 sequence, studies of the physical properties of peptides derived from recombinant gp41 (rgp41), and crystallographic studies indicate the following features. The N terminus of the molecule consists of a fusion domain spanning aa 512 to 527 (HIVHXB2R numbering [29] is used), which inserts into the membranes of target cells (26). The fusion domain is followed by a region (aa ∼555 to 590) containing a 4-3 hydrophobic repeat, which is predicted to form a coiled coil (5, 10). This helical region is followed by a hydrophilic, nonhelical loop region characterized by an intrachain disulfide bond, followed by a second set of heptad repeats (aa ∼620 to 670). The transmembrane domain occupies the region between aa 684 and 705, which is followed by the cytoplasmic portion of the molecule, aa 705 to 856.
The ectodomain of gp41 (aa 512 to 683) has received the most scrutiny. Elucidation of its structure has been facilitated by studies of peptides from the two heptad repeat regions. By circular dichroism, these peptides do not display spectral characteristic of α-helices, but when mixed in an equimolar ratio, they form a discrete, extremely stable, α-helical trimer of heterodimers (27, 41). Three peptides representing the N-terminal heptad repeat region form an interior, parallel, homotrimeric coiled-coil core against which three peptides representing the C-terminal heptad repeat region pack in an antiparallel fashion (27). It is thought that this complex represents the core of the fusogenic state of gp41. The native state of gp41, i.e., the prefusogenic form of gp41, is thought to be an oligomer of gp41 in which the N-terminal heptad regions of three or four gp41 molecules self-associate but are prevented from complexing with the C-terminal heptad regions, perhaps by the interaction of gp41 with native gp120 (42). Upon the interaction of gp120 with CD4 and its appropriate coreceptor, in the context of additional molecular interactions between the virus envelope and cell membrane that increase the avidity of the virion for the cell (4, 14, 24), it is thought that gp120 undergoes conformational changes that allow the prefusogenic form of gp41 to assume the fusogenic coiled-coil configuration that brings the viral and cell membranes into juxtaposition (7, 32, 33, 41).
gp41 is an extremely immunogenic glycoprotein, inducing antibodies (Abs) in essentially all HIV-infected individuals, with titers reaching 1:106 (1, 45). While several epitopes have been identified in gp41 (2, 19, 28, 45), two regions seem to be immunodominant. These are the aforementioned nonhelical hydrophilic region containing the intrachain disulfide bond (previously identified as epitope cluster I) and the region containing the C-terminal heptad repeat (previously described as containing epitope cluster II) (45). Abs to cluster I recognize peptides and recombinant proteins containing aa 579 to 604, while Abs to cluster II are generally dependent on conformation to be reactive and require larger fragments of gp41 containing aa ∼644 to 663 (see below and references 2 and 45). While human monoclonal Abs (MAbs) to both clusters I and II can stain infected cells (35, 38, 46) and can mediate Ab-dependent cellular cytotoxicity (ADCC) (38) and complement-dependent virolysis (36), most human MAbs to gp41 do not consistently neutralize either primary isolates or T-cell-line-adapted strains of HIV. Two exceptions to this are human MAb 2F5, which is specific for the ELDKWA epitope at aa 662 to 667 at the C-terminal end of cluster II, and, to a lesser extent, anti-cluster II MAb 98-6 (11, 20, 37).
In order to consolidate the information about the structure of gp41, the conformational changes it undergoes from the prefusogenic form to the fusogenic form, its antigenic and immunogenic characteristics, and the function of anti-gp41 Abs, studies were performed using two peptides representing the N- and C-terminal heptad repeats of the gp41 ectodomain as well as the complex formed by these two peptides. The peptides used in these experiments, N51 (aa 540 to 590) and C43 (aa 624 to 666), were generously provided by David Chan and Peter Kim (Whitehead Institute, Massachusetts Institute of Technology, Cambridge). They were derived by proteolytic digestion with proteinase K of rgp41 which was produced in Escherichia coli transformed with plasmid pHIVHXB2cg (27), and these two major proteolytic fragments were separated and purified as previously described (27). The N51 peptide in isolation is predominantly aggregated, and the C43 peptide displays little secondary structure (27). The N51-C43 complex was prepared in solution by incubation of the two peptides at a 1:1 ratio (unless otherwise specified) for 90 min at 37°C.
rgp41 aa 517 to 757 (rgp41517-757) and a truncated form of rgp41 (rgp41567-647) were both purchased from Intracel (Issaquah, Wash.). Both proteins were produced by E. coli carrying envelope genes from HIVIIIB and are soluble and monomeric according to product information provided by the manufacturer; rgp41517-757 also contains a leader sequence of the 38 C-terminal amino acids from gp120, which permitted its reactivity with Abs both to gp41 and to the C5 region of gp120 (see below).
Eleven human anti-gp41 MAbs were used in this study; each has previously been described (17, 18, 28, 45). The IgG1 recombinant form of MAb 2F5 was obtained from the National Institute for Biological Standards and Control AIDS Reagent Project (Potters Bar, United Kingdom) and from H. Katinger (Institute of Applied Microbiology, Vienna, Austria). The other 10 MAbs were purified from the supernatants of cultured lymphoblastoid cell lines or heterohybridoma by chromatography on protein A-Sephadex. The specificities of these MAbs are described in Table 1. A human MAb to the C5 region of gp120, MAb 670-D (46), and human MAbs to parvovirus B19, 860-55D and 1418 (16), were used as negative controls.
TABLE 1.
Binding of human MAbs to gp41 proteins and peptidesa
| MAb | Specificity | Epitopec (aa) | Reference(s) | Optical densityb with:
|
||||
|---|---|---|---|---|---|---|---|---|
| gp41 (aa 517–757) | Truncated gp41 (aa 567–647) | N51 (aa 540–590) | C43 (aa 624–666) | N51/C43 (1:1) | ||||
| 50-69 | gp41, cluster I | Conformational (579–613) | 17, 45 | 1.51 | 0.88 | 0.07 | 0.07 | 1.71 |
| 181-D | gp41, cluster I | qLLGIWg (591–597) | 45 | 1.53 | 1.10 | 0.06 | 0.06 | 0.06 |
| 246-D | gp41, cluster I | qqLLGIWg (590–597) | 45 | 1.51 | 1.11 | 0.07 | 0.06 | 0.09 |
| 1367 | gp41, cluster I | Conformational (NDd) | 18 | 1.56 | 1.38 | 0.06 | 0.06 | 1.65 |
| 98-6 | gp41, cluster II | Conformational (644–663) | 17, 45 | 1.54 | 0.06 | 0.08 | 1.68 | 1.78 |
| 126-6 | gp41, cluster II | Conformational (644–663) | 45 | 1.53 | 0.06 | 0.06 | 0.07 | 1.68 |
| 167-D | gp41, cluster II | Conformational (644–663) | 45 | 1.52 | 0.07 | 0.07 | 0.11 | 1.67 |
| 1281 | gp41, cluster II | Conformational (ND) | 18 | 1.52 | 0.06 | 0.07 | 0.09 | 1.61 |
| 1342 | gp41, cluster II | Conformational (ND) | 18 | 1.36 | 0.06 | 0.07 | 0.07 | 1.35 |
| 1379 | gp41, cluster II | Conformational (ND) | 18 | 1.36 | 0.06 | 0.07 | 0.08 | 1.55 |
| 2F5 | gp41 | ELDKWA (662–667) | 28 | 1.53 | 0.09 | 0.11 | 1.82 | 0.65 |
| 670-D | gp120, region C5e | PTKAKRR (503–509) | 46 | 1.41 | 0.06 | 0.07 | 0.06 | 0.07 |
| 860-55D | Parvovirus B19 | Conformational | 16 | 0.07 | 0.07 | 0.06 | 0.06 | 0.07 |
Proteins, peptides, and the peptide complex were used at 1.0 μg/ml. All MAbs were used at 1.0 μg/ml.
Boldface values are positive (greater than the mean plus three standard deviations for negative control values).
Capital letters represent the core of the epitope; lowercase letters represent flanking amino acids which probably contribute to the binding energy (45).
ND, not done.
The leader sequence contains the last 38 C-terminal amino acids of gp120 with the epitope for MAb 670-D.
Reactivities of MAbs with proteins, peptides, and peptide complexes were determined by enzyme-linked immunosorbent assay (ELISA). For studies of MAbs with immobilized peptides or proteins, the peptides and proteins were applied to plastic Immulon 2 ELISA plates (Dynex Technologies, Chantilly, Va.) at 1.0 μg/ml. Plates were blocked for 1 h at 37°C with 2% bovine serum albumin in phosphate-buffered saline (PBS) and then washed three times with PBS containing 0.05% Tween 20 before incubation with MAbs. To determine the reactivity of MAbs with the immobilized peptide complex, the N51-C43 complex was formed in solution (see above) and applied to ELISA plates at a final concentration of 1 μg/ml. Binding of MAbs to antigens was detected with alkaline phosphatase-conjugated goat anti-human immunoglobulin G (gamma chain specific), and color was developed with the substrate p-nitrophenyl phosphate. The absorbance of each well was determined at 410 nm using an automated ELISA reader. All reactions were conducted in duplicate, and a positive reaction was defined as one in which the mean absorbance was above the cutoff values, which were equal to the mean plus three standard deviations obtained from reactions with the negative control MAbs.
Using this technique, 11 human anti-gp41 MAbs were tested for their reactivities against two forms of rgp41, against each of the two peptides individually (N51 and C43), and against the complex formed by these peptides at a 1:1 ratio. All of the MAbs reacted with rgp41517-757 (Table 1). Four of these, MAbs 50-69, 181-D, 246-D, and 1367, which were previously described as specific for epitope cluster I, also bound to truncated rgp41567-647, which contains epitope cluster I but not cluster II. Of the four cluster I MAbs, two, 181-D and 246-D, did not recognize either of the peptides or the peptide complex. Indeed, the core epitopes of these two MAbs (aa 591 to 597 and 590 to 597) are not represented in the peptides tested, nor is the new epitope(s) formed by the N51-C43 complex recognized by these two cluster I MAbs. These data reveal for the first time a subdivision of the previously defined cluster I MAbs into those whose epitopes are complex independent or complex dependent. The two cluster I MAbs that recognized the N51-C43 complex, MAbs 50-69 and 1367, did not react with either of the peptides individually. While none of the cluster I MAbs reacted with N51 alone, the presence of N51 on the plate in a reactive form was established by its ability to bind to peptide C43 and form reactive complexes (see Fig. 2).
FIG. 2.
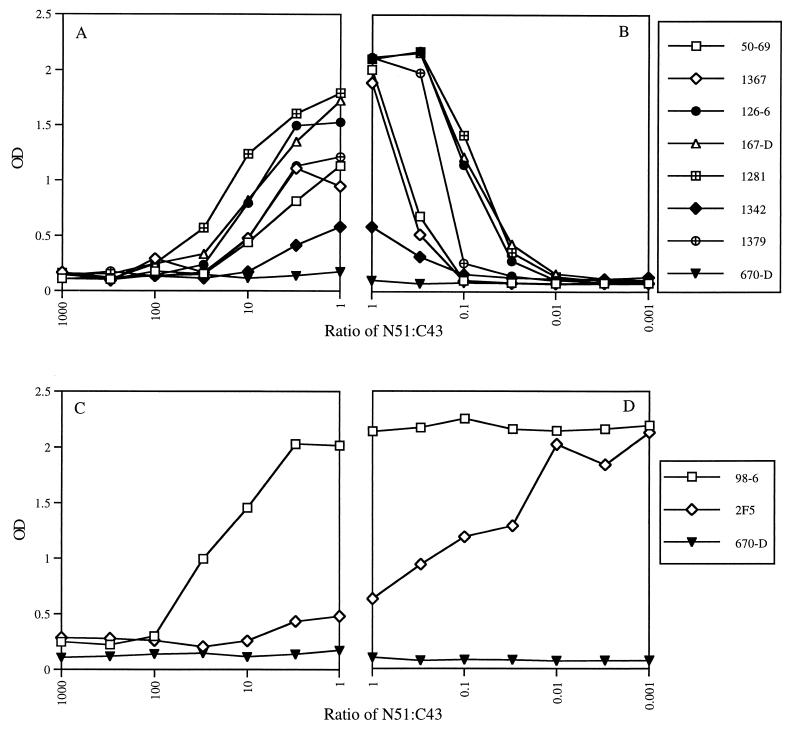
Binding of gp41 MAbs to N51-C43 complexes formed at different ratios. (A and C) Peptide N51 was applied to plates at 1.0 μg/ml and then incubated with peptide C43 at concentrations of between 0.001 and 1.0 μg/ml, resulting in complexes where the ratio of N51 to C43 ranged from 1,000:1 to 1:1. (B and D) Peptide C43 was applied to plates at 1.0 μg/ml and then incubated with peptide N51 at concentrations of between 1.0 and 0.001 μg/ml, resulting in complexes where the ratio ranged from 1:1 to 0.001:1. Each MAb (designated by symbols shown on the right) was tested for binding at a concentration of 1.0 μg/ml.
As expected, the MAbs specific for gp41 epitope cluster II reacted with rgp41517-757, which contains both clusters I and II, but did not react with truncated rgp41567-647, which lacks cluster II (Table 1). Five of these six cluster II MAbs did not react with peptide N51 or C43 but bound strongly to the N51-C43 complex. This demonstrates that, in adopting its three-dimensional structure, this complex acquires the epitope(s) which these conformationally dependent MAbs recognize.
Two MAbs stand out as being distinct: they are MAbs 98-6 and 2F5. Each of these MAbs recognizes peptide C43 (but not N51) and the complex (Table 1). Of the two, MAb 98-6 binds more strongly than MAb 2F5 to the complex prepared at a 1:1 ratio. This result was highly reproducible and suggested a possible difference in the epitopes recognized by these two MAbs.
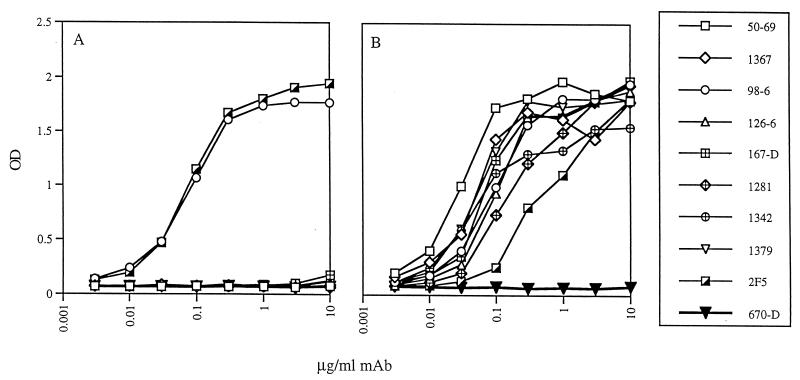
The relative affinities of the MAbs were also determined by ELISA by testing for reactivity against various peptides applied to ELISA plates at 1.0 μg/ml and using MAb concentrations of 0.003 to 10.0 μg/ml. Relative affinity is related to the concentration of MAb giving 50% of maximal binding, which was obtained mathematically by linear interpolation using Quattro Pro software (Corel, Ottawa, Canada). Figure 1 shows the binding curves from a single experiment, and Table 2 lists the average concentration (derived from three experiments) required for each MAb to reach half-maximal binding. The lower the concentration of MAb needed to achieve half-maximal binding, the higher is its relative affinity. Binding experiments with the N51-C43 complex showed a range of over 50-fold in the relative affinities of the nine MAbs tested. However, for MAbs 98-6 and 2F5, while the relative affinities were essentially identical for peptide C43 (with half-maximal binding achieved at 0.06 and 0.08 μg/ml, respectively), MAb 2F5 required a 10-fold-higher concentration than MAb 98-6 to attain half-maximal binding with the N51-C43 complex (Table 2 and Fig. 1).
FIG. 1.
Binding of anti-gp41 MAbs to peptide C43 (A) or to the N51-C43 complex formed in solution at a 1:1 ratio (B). The peptide and the complex were each applied to ELISA plates at 1.0 μg/ml. The anti-gp41 MAbs used are identified on the right; MAb 670-D is specific for the C5 region of gp120 and was used as a negative control. Results are from one of three experiments giving essentially identical results.
TABLE 2.
Binding of anti-gp41 MAbs to gp41 peptides and the N51-C43 complex
| MAb | Concn of MAb (μg/ml) required to achieve half-maximal bindinga with:
|
||
|---|---|---|---|
| N51 | C43 | N51-C43b | |
| 50-69 | —c | — | 0.02 ± 0.004 |
| 1367 | — | — | 0.04 ± 0.004 |
| 98-6 | — | 0.06 ± 0.004 | 0.09 ± 0.008 |
| 126-6 | — | — | 0.08 ± 0.009 |
| 167-D | — | — | 0.06 ± 0.012 |
| 1281 | — | — | 0.13 ± 0.044 |
| 1342 | — | — | 0.04 ± 0.009 |
| 1379 | — | — | 0.04 ± 0.004 |
| 2F5 | — | 0.08 ± 0.005 | 0.97 ± 0.44 |
Results are averages and standard deviations from three experiments.
The peptide complex was formed in solution at a 1:1 ratio and then applied to ELISA plates at a concentration of 1.0 μg/ml.
—, no detectable binding.
Since most of the MAbs showed differences in their abilities to bind to the complex and to either of the individual peptides, it was hypothesized that the formation of the epitope(s) with which these MAbs react might be dependent upon the ratio of the two peptides forming the complex. Therefore, experiments were performed in which MAb binding was studied with complexes in which the N51/C43 ratio varied from 0.001 to 1,000. For this, the following experiments were designed. N51 was applied to ELISA plates at 1 μg/ml, and, after washing, complexes were formed by adding increasing concentrations (0.001 to 1.0 μg/ml) of C43 and incubating for 1.5 h at 37°C; here the N51/C43 ratios ranged from 1,000 to 1 (Fig. 2A and C). In parallel ELISA experiments, C43 was applied to plates at 1 μg/ml, and, after washing, complexes were formed by adding increasing concentrations (0.001 to 1.0 μg/ml) of N51; here the N51/C43 ratios ranged from 1 to 0.001 (Fig. 2B and D). The binding of each MAb, at 1.0 μg/ml, to the resulting peptide complexes was detected as described above. Anti-C5 MAb 670-D was used as a negative control, as it did not bind to the complexes.
Seven of the nine anti-gp41 MAbs capable of binding to the complex bound best when the ratio of N51 to C43 was close to 1:1 (Fig. 2A and B). In contrast, maximum binding of MAb 98-6 occurred when the ratio of N51 to C43 was between 0.001 and 3 (Fig. 2C and D), and indeed, this MAb could bind to C43 in the absence of N51, as shown above (Tables 1 and 2 and Fig. 1A). The suboptimal binding of 98-6 to the complex at ratios of 10 to 1,000 was due to limiting amounts of C43 on the plate, as can also be seen from the dose-response curves for 98-6 and C43 in Fig. 1A. MAb 2F5 showed a still different pattern (Fig. 2C and D). Like MAb 98-6, MAb 2F5 binds to C43 alone; however, it binds maximally to the complex only at ratios of 0.01 to 0.001, i.e., only when extremely small amounts of N51 are present. The decrease in 2F5 binding as increasing amounts of N51 are added for complex formation (from ratios of 0.01 to 100), indicates that as N51 binds to C43, the epitope on C43 recognized by 2F5 becomes occluded or is disrupted.
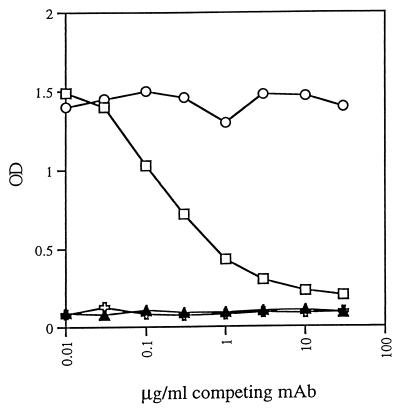
Finally, since all of the experiments described above were performed using a format in which rgp41, peptides, or peptide complexes were presented in solid phase on ELISA plates, experiments were conducted to test if these anti-gp41 MAbs were capable of binding to the peptides or the peptide complex in liquid phase. For this, equimolar amounts of each peptide at 0.1 μg/ml were incubated for 1.5 h at 37°C with a 1:4,000 dilution of biotinylated anti-gp41 MAb 126-6 (126-6*), which reacts only with the N51-C43 complex (Table 1). This peptide–126-6* mixture was then transferred to an ELISA plate previously coated with another anti-gp41 MAb, 50-69, at 2 μg/ml and blocked with 1% bovine serum albumin in PBS. As shown above, MAb 50-69 recognizes an epitope on the N51-C43 complex which is different from that recognized by MAb 126-6 (Table 1). After 1 h at 37°C, the biotin in the captured N51–C43–126-6* product was detected with alkaline phosphatase-labeled streptavidin and p-nitrophenyl phosphate using an automated ELISA reader. To test the specificity of this reaction, 0 to 30 μg of unlabeled MAb 126-6 per ml was included in the initial incubation of N51 plus C43 plus 126-6*. As a negative control for this competition phase of the assay, 0 to 30 μg of unlabeled human anti-parvovirus B19 MAb 1418 per ml was incubated with the N51–C43–126-6* mixture. Negative controls also included incubation of 126-6* with either of the peptides individually, since neither 126-6* nor 50-69 could recognize the individual peptides. As shown in Fig. 3, the labeled MAb bound to the N51-C43 complex in solution, and this N51–C43–126-6* product was subsequently captured by bound MAb 50-69, which is also specific for the N51-C43 complex (recognizing an epitope in the cluster I region [Table 1]). Negative controls in which either N51 or C43 was incubated individually with 126-6* failed to capture the labeled MAb. Additional specificity controls, shown in Fig. 3, indicate that the binding of 126-6* to the N51-C43 complex in solution could be blocked by increasing concentrations of unlabeled 126-6 but not by an unlabeled irrelevant MAb, 1418, which is specific for parvovirus B19. This series of experiments was repeated using biotinylated and unlabeled MAb 167-D, and results essentially identical to those obtained with MAb 126-6 were obtained (data not shown).
FIG. 3.
Binding of biotinylated anti-gp41 MAb 126-6 (126-6*) to N51-C43 complexes in solution. MAb 126-6* was incubated with N51 and C43 in solution, and the N51–C43–126-6* product was captured by MAb 50-69 bound to an ELISA plate (□ and ○). Neither N51 alone ( ) nor C43 alone (▴) bound 126-6*. When unlabeled 126-6 was added to the incubation mixture of N51, C43, and 126-6*, the unlabeled MAb inhibited the binding of 126-6*, showing the specificity of the interaction (□). An irrelevant unlabeled MAb, 1418, to parvovirus B19, did not interfere with the interaction of N51, C43, and 126-6* (○).
) nor C43 alone (▴) bound 126-6*. When unlabeled 126-6 was added to the incubation mixture of N51, C43, and 126-6*, the unlabeled MAb inhibited the binding of 126-6*, showing the specificity of the interaction (□). An irrelevant unlabeled MAb, 1418, to parvovirus B19, did not interfere with the interaction of N51, C43, and 126-6* (○).
These experiments illuminate structural, functional, antigenic, and immunogenic characteristics of gp41, a molecule which performs a critical role in maintaining the conformation and infectivity of the HIV virion. It acts as an anchor for the gp120 envelope glycoprotein and, upon interaction of gp120 with its receptor and coreceptor, undergoes a conformational change to assume a configuration which leads to the fusion of the viral and cell membranes (24). Mutations that affect the formation of the fusogenic form of gp41 inhibit the infectivity of the virus (41–43). As a consequence, the identification and design of reagents which can inhibit the transition of the prefusogenic form to the fusogenic coiled-coil form of gp41 have been actively pursued (7, 13, 43). An extension of this approach suggests that an effective HIV vaccine might be achieved if it could induce Abs that would prevent the formation of fusogenic gp41.
gp41 is known to be extremely immunogenic. Abs to several regions have been identified (2, 19, 22, 45), and human MAbs to various regions of the gp41 ectodomain have been shown to bind to HIV-infected cells (9, 35, 46) and intact virions (36; P. Nyambi and S. Zolla-Pazner, unpublished data) and to mediate complement deposition on virions (36), virolysis (36), ADCC (38), complement-mediated enhancement of infectivity (31), and neutralization (9, 20, 37). Among the several human gp41 MAbs described (2, 9, 17, 28, 45), two with unusual activities are 98-6 and 2F5; MAb 98-6 can mediate ADCC (38) and neutralize several primary isolates when tested in a highly sensitive neutralization assay (20), while MAb 2F5 has broad and potent neutralizing activity for primary HIV isolates from several clades (11, 37).
The majority of anti-gp41 MAbs studied here (7 of 11) did not react with either N51 or C43 individually but reacted with the complex that is formed by these two peptides, which is thought to approximate the core of the fusogenic form of gp41. These human MAbs are similar to a mouse MAb induced by a model gp41-based peptide which folds to form a stable six-helix bundle (23). In the case of the human MAbs, maximal reactivity was noted at an N51/C43 ratio of 1, the ratio that is thought to occur in the α-helical core of the fusogenic form of gp41, consisting of three molecules from the N-terminal heptad repeat region against which are packed in an antiparallel configuration three molecules from the C-terminal heptad repeat region (27, 39). The finding of maximum reactivity against the 1:1 peptide complex suggests that it was the fusogenic form of gp41 which induced these Ab responses in vivo. The data also suggest that, since many of these epitopes are well represented on infected cells and intact virions (9, 35, 36, 46), both infected cells and intact virus particles serve as antigenic stimuli, as opposed to, or in addition to, viral debris (34). The presence of this form of gp41 on virions and infected cells could reflect some spontaneous shedding of gp120 with consequent triggering of the gp41 conformational change. Alternatively, a small proportion of gp41 in the active form may coexist with the prefusogenic form, and after the interaction of gp120 with CD4 and coreceptors, this proportion may shift to a preponderance of the fusogenic form.
MAb 98-6 is an anti-gp41 MAb that is distinct from those which recognize the N51-C43 peptide complex. MAb 98-6 reacts well with both C43 and the N51-C43 complex, and its binding to C43 is not inhibited by N51; indeed, it reacts with the complex whether the N51/C43 ratio is 0.001 or 3. This finding demonstrates that the epitope recognized by MAb 98-6 on peptide C43 is present in both the free and complexed peptide and, therefore, that the epitope that it recognizes is not occluded or disrupted by complex formation. These data also suggest that Abs with this specificity can apparently be induced by gp41 in either its prefusogenic or fusogenic form.
In contrast, MAb 2F5 reacts strongly with C43 but relatively poorly with the complex of N51 and C43 formed with equimolar amounts of each constituent. Indeed, the more N51 is added to C43, the less reactive is the resultant complex with MAb 2F5. This suggests that the epitope recognized by MAb 2F5 on C43 is directly involved with complex formation and is occluded or disrupted as the complex forms. This is consistent with the findings of Sattentau et al. (35), which showed that MAb 2F5 binds well to infected cells but loses its reactivity for cells which are first incubated at 37°C in the presence of soluble CD4. It follows that the inducing antigen must be either a peptide fragment of gp41 lacking the N-terminal heptad repeat, the native prefusogenic form of gp41 present on infected cells and virions, or the gp41 prehairpin intermediate that is thought to be a transitional structure between the prefusogenic and fusogenic forms of gp41 (7). A peptide fragment of gp41 would probably have an extremely short half-life in vivo but could be generated in the cytoplasm of antigen-presenting cells, with subsequent stimulation of T and B cells. Similarly, the prehairpin intermediate probably has such a short half-life that it would not serve as a strong immunogen. The most likely and abundant immunogen for 2F5-like Abs would be the native prefusogenic form of gp41 present on infected cells and virions.
MAb 2F5 neutralizes about 75 to 80% of HIV primary isolates (8, 11, 37). The mean concentration required for 90% neutralization of viruses sensitive to this MAb, tested in several assays, was ∼20 μg/ml, or 125 nM (11). This is comparable to the inhibitory activity of peptide C43, which blocks syncytium formation with a 90% inhibitory dose of ∼140 nM (27), and is similar to the ability of peptide D178 (aa 643 to 678) to block infectivity at a 90% inhibitory dose of 100 to 200 nM (25, 44).
The paucity of human anti-gp41 MAbs that are capable of neutralizing HIV, as opposed to the abundance of gp41 MAbs specific for the fusogenic coiled-coil complex of gp41, may in part reflect the method of selection of the MAb-producing lines rather than the actual proportion of various anti-gp41 MAbs found in vivo. Thus, all human anti-gp41 MAbs described in the literature have been selected on viral lysates or oligomeric forms of gp160 (2, 9, 17, 28, 45). It is probable that the coiled-coil form of gp41 is most prominently displayed under these conditions (40), leading to the preferential selection of MAbs specific for this configuration. Abs reactive to C43, while representing only 2 of the 11 MAbs studied here, are present in essentially all HIV-infected individuals, although Abs that inhibit the ability of N51 to complex with C43 are present in less than 5% of patients' sera that were studied (M. K. Gorny, unpublished data). Thus, Abs with specificity for the particular epitope(s) on C43 involved with formation of the fusogenic gp41 configuration may, indeed, be rather unusual.
Further elucidation of the mechanism of action of anti-gp41 neutralizing MAbs, along with knowledge of the epitopes they target, should permit both the isolation of additional MAbs with neutralizing activity and the design of immunogens that will elicit these Abs. Since these Abs appear to work by blocking a step in the infectious process common to all strains of HIV, immunogens that induce such Abs should be valuable as a vaccine that circumvents the problems inherent in the antigenic variation of HIV.
Acknowledgments
This work was supported in part by NIH grants AI 32424 and HL 59725, by the VA Research Center for AIDS and HIV Infection, and by research funds from the Department of Veterans Affairs.
We acknowledge the provision of peptide reagents from Peter Kim and David Chan and their valuable comments on the experiments described in the manuscript.
REFERENCES
- 1.Barin F, McLane M F, Allan J S, Lee T H, Groopman J E, Essex M. Virus envelope protein of HTLV-III represents major target antigen for antibodies in AIDS patients. Science. 1985;228:1094–1098. doi: 10.1126/science.2986291. [DOI] [PubMed] [Google Scholar]
- 2.Binley J M, Ditzel H J, Barbas III C F, Sullivan N, Sodroski J, Parren P W H I, Burton D R. Human antibody responses to HIV type 1 glycoprotein 41 cloned in phage display libraries suggest three major epitopes are recognized and give evidence for conserved antibody motifs in antigen binding. AIDS Res Hum Retroviruses. 1996;12:911–924. doi: 10.1089/aid.1996.12.911. [DOI] [PubMed] [Google Scholar]
- 3.Blacklow S C, Lu M, Kim P S. A trimeric subdomain of the simian immunodeficiency virus envelope glycoprotein. Biochemistry. 1995;34:14955–14962. doi: 10.1021/bi00046a001. [DOI] [PubMed] [Google Scholar]
- 4.Cantin R, Fortin J-F, Lamontagne G, Tremblay M. The acquisition of host-derived major histocompatibility complex class II glycoproteins by human immunodeficiency virus type 1 accelerates the process of virus entry and infection in human T lymphoid cells. Blood. 1997;90:1091–1100. [PubMed] [Google Scholar]
- 5.Chambers P, Prignle C R, Easton A J. Heptad repeat sequences are located adjacent to hydrophobic regions in several types of virus fusion glycoproteins. J Gen Virol. 1990;71:3075–3080. doi: 10.1099/0022-1317-71-12-3075. [DOI] [PubMed] [Google Scholar]
- 6.Chan D C, Fass D, Berger J M, Kim P S. Core structure of gp41 from the HIV envelope glycoprotein. Cell. 1997;89:263–273. doi: 10.1016/s0092-8674(00)80205-6. [DOI] [PubMed] [Google Scholar]
- 7.Chan D C, Kim P S. HIV entry and its inhibition. Cell. 1998;93:681–684. doi: 10.1016/s0092-8674(00)81430-0. [DOI] [PubMed] [Google Scholar]
- 8.Conley A J, Kessler J A, Boots L J, Tung J-S, Arnold B A, Keller P M, Shaw A R, Emini E A. Neutralization of divergent human immunodeficiency virus type 1 variants and primary isolates by IAM-41-2F5, an anti-gp41 human monoclonal antibody. Proc Natl Acad Sci USA. 1994;91:3348–3352. doi: 10.1073/pnas.91.8.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cotropia J, Ugen K E, Kliks S, Broliden K, Broliden P-A, Hoxie J A, Srikantan V, Williams W V, Weiner D B. A human monoclonal antibody to HIV-1 gp41 with neutralizing activity against diverse laboratory isolates. J AIDS Hum Retroviruses. 1996;12:221–232. doi: 10.1097/00042560-199607000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Delwart E J, Mosialos G, Gilmore T. Retroviral envelope glycoproteins contain a leucine zipper like repeat. AIDS Res Hum Retroviruses. 1990;6:703–706. doi: 10.1089/aid.1990.6.703. [DOI] [PubMed] [Google Scholar]
- 11.D'Souza M P, Livnat D, Bradac J A, Bridges S H the AIDS Clinical Trials Group Antibody Selection Working Group and Collaborating Investigators. Evaluation of monoclonal antibodies to human immunodeficiency virus type 1 primary isolates by neutralization assays: performance criteria for selecting candidate antibodies for clinical trials. J Infect Dis. 1997;175:1056–1062. doi: 10.1086/516443. [DOI] [PubMed] [Google Scholar]
- 12.Earl P L, Doms R W, Moss B. Oligomeric structure of the human immunodeficiency virus type 1 envelope glycoprotein. Proc Natl Acad Sci USA. 1990;87:648–652. doi: 10.1073/pnas.87.2.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eckert D M, Malashkevich V N, Hong L H, Carr P A, Kim P S. Inhibiting HIV-1 Entry: discovery of D-peptide inhibitors that target the gp41 coiled-coil pocket. Cell. 1999;99:103–115. doi: 10.1016/s0092-8674(00)80066-5. [DOI] [PubMed] [Google Scholar]
- 14.Fortin J-F, Cantin R, Lamontagne G, Tremblay M. Host-derived ICAM-1 glycoproteins incorporated on human immunodeficiency virus type 1 are biologically active and enhance viral infectivity. J Virol. 1997;71:3588–3596. doi: 10.1128/jvi.71.5.3588-3596.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallaher W R, Ball J M, Garry R F, Griffin M C, Montelaro R C. A general model for the transmembrane proteins of HIV and other retroviruses. AIDS Res Hum Retroviruses. 1989;5:431–440. doi: 10.1089/aid.1989.5.431. [DOI] [PubMed] [Google Scholar]
- 16.Gigler A, Dorsch S, Hemauer A, Williams C, Kim S, Young N S, Zolla-Pazner S, Wolf H, Gorny M K, Modrow S. Generation of neutralizing human monoclonal antibodies against parvovirus B19 proteins. J Virol. 1999;73:1974–1979. doi: 10.1128/jvi.73.3.1974-1979.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorny M K, Gianakakos V, Sharpe S, Zolla-Pazner S. Generation of human monoclonal antibodies to HIV. Proc Natl Acad Sci USA. 1989;86:1624–1628. doi: 10.1073/pnas.86.5.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorny M K, VanCott T C, Williams C, Revesz K, Zolla-Pazner S. Effects of oligomerization on the epitopes of the human immunodeficiency virus type 1 envelope glycoproteins. Virology. 2000;267:220–228. doi: 10.1006/viro.1999.0095. [DOI] [PubMed] [Google Scholar]
- 19.Goudsmit J, Meloen R H, Brasseur R. Map of sequential B-cell epitopes of the HIV-1 transmembrane protein using human antibodies as probes. Intervirology. 1990;31:327–338. doi: 10.1159/000150169. [DOI] [PubMed] [Google Scholar]
- 20.Hioe C E, Xu S, Chigurupati P, Burda S, Williams C, Gorny M K, Zolla-Pazner S. Neutralization of HIV-1 primary isolates by polyclonal and monoclonal human antibodies. Int Immunol. 1997;9:1281–1290. doi: 10.1093/intimm/9.9.1281. [DOI] [PubMed] [Google Scholar]
- 21.Hughson F M. Enveloped viruses: a common mode of membrane fusion? Curr Biol. 1997;7:R565–R569. doi: 10.1016/s0960-9822(06)00283-1. [DOI] [PubMed] [Google Scholar]
- 22.Javaherian K, Langlois A J, LaRosa G J, Profy A T, Bolognesi D P, Herlihy W C, Putney S D, Matthews T J. Broadly neutralizing antibodies elicited by the hypervariable neutralizing determinant of HIV-1. Science. 1990;250:1590–1593. doi: 10.1126/science.1703322. [DOI] [PubMed] [Google Scholar]
- 23.Jiang S, Lin K, Lu M. A conformation-specific monoclonal antibody reacting with fusion-active gp41 from the human immunodeficiency virus type 1 envelope glycoprotein. J Virol. 1998;72:10213–10217. doi: 10.1128/jvi.72.12.10213-10217.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones P L S J, Korte T, Blumenthal R. Conformational changes in cell surface HIV-1 envelope glycoproteins are triggered by cooperation between cell surface CD4 and co-receptors. J Biol Chem. 1998;273:404–409. doi: 10.1074/jbc.273.1.404. [DOI] [PubMed] [Google Scholar]
- 25.Judice J K, Tom J Y K, Huang W, Wrin T, Vennari J, Petropoulos C J, McDowell R S. Inhibition of HIV type 1 infectivity by constrained alpha-helical peptides: implications for the viral fusion mechanism. Proc Natl Acad Sci USA. 1997;94:13426–13430. doi: 10.1073/pnas.94.25.13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kowalski M, Potz J, Basiripour L, Dorfman T, Goh W C, Terwilliger E, Dayton A, Rosen C, Haseltine W, Sodroski J. Functional regions of the envelope glycoprotein of human immunodeficiency virus type 1. Science. 1987;237:1351–1355. doi: 10.1126/science.3629244. [DOI] [PubMed] [Google Scholar]
- 27.Lu M, Blacklow S C, Kim P S. A trimeric structural domain of the HIV-1 transmembrane glycoprotein. Nat Struct Biol. 1995;2:1075–1082. doi: 10.1038/nsb1295-1075. [DOI] [PubMed] [Google Scholar]
- 28.Muster T, Steindl F, Purtscher M, Trkola A, Klima A, Himmler G, Ruker F, Katinger H. A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J Virol. 1993;67:6642–6647. doi: 10.1128/jvi.67.11.6642-6647.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Myers G, Korber B, Foley B, Smith R F, Jeang K-T, Mellors J W, Wain-Hobson A. Human retroviruses and AIDS. Los Alamos, N.Mex: Theoretical biology and biophysics, Los Alamos National Laboratories; 1996. [Google Scholar]
- 30.Pinter A, Honnen W, Tilley S A, Bona C, Zaghorani H, Gorny M K, Zolla-Pazner S. Oligomeric structure of gp41, the transmembrane protein of HIV. J Virol. 1989;63:2674–2679. doi: 10.1128/jvi.63.6.2674-2679.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robinson W E, Jr, Kawamura T, Gorny M K, Montefiori D C, Mitchell W M, Lake D, Xu J-Y, Matsumoto Y, Sugano T, Masuho Y, Hersh E, Zolla-Pazner S. Human monoclonal antibodies to the human immunodeficiency virus type 1 (HIV-1) transmembrane glycoprotein gp41 enhance HIV-1 infection in vitro. Proc Natl Acad Sci USA. 1990;87:3185–3189. doi: 10.1073/pnas.87.8.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sattentau Q J, Moore J P. Human immunodeficiency virus type 1 neutralization is determined by epitope exposure on the gp120 oligomer. J Exp Med. 1995;182:185–196. doi: 10.1084/jem.182.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sattentau Q J, Moore J P, Vignaux F, Traincard F, Poignard P. Conformational changes induced in the envelope glycoproteins of the human and simian immunodeficiency viruses by soluble receptor binding. J Virol. 1993;67:7383–7393. doi: 10.1128/jvi.67.12.7383-7393.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sattentau Q J, Parren P W H I, Burton D R. HIV-1 antibody debris or virion? Nat Med. 1997;3:366–367. doi: 10.1038/nm0497-366d. [DOI] [PubMed] [Google Scholar]
- 35.Sattentau Q J, Zolla-Pazner S, Poignard P. Epitope exposure on functional, oligomeric HIV-1 gp41 molecules. Virology. 1995;206:713–717. doi: 10.1016/s0042-6822(95)80094-8. [DOI] [PubMed] [Google Scholar]
- 36.Spear G, Takefman D M, Sullivan B L, Landay A L, Zolla-Pazner S. Complement activation by human monoclonal antibodies to human immunodeficiency virus. J Virol. 1992;67:53–59. doi: 10.1128/jvi.67.1.53-59.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trkola A, Pomales A B, Yuan H, Korber B, Maddon P J, Allaway G P, Katinger H, Barbas III C F, Burton D R, Ho D D, Moore J P. Cross-clade neutralization of primary isolates of human immunodeficiency virus type 1 by human monoclonal antibodies and tertrameric CD4-IgG. J Virol. 1995;69:6609–6617. doi: 10.1128/jvi.69.11.6609-6617.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tyler D S, Stanley S D, Zolla-Pazner S, Gorny M K, Shadduck P P, Langlois A J, Matthews T J, Bolognesi D P, Palker T J, Weinhold K J. Identification of sites within gp41 that serve as targets for antibody-dependent cellular cytotoxicity by using human monoclonal antibodies. J Immunol. 1990;145:3276–3282. [PubMed] [Google Scholar]
- 39.Weissenhorn W, Dessen A, Harrison S C, Skehel J J, Wiley D C. Atomic structure of the ectodomain from HIV-1 gp41. Nature. 1997;387:426–430. doi: 10.1038/387426a0. [DOI] [PubMed] [Google Scholar]
- 40.Weissenhorn W, Wharton S A, Calder L J, Earl P L, Moss B, Aliprandis E, Skehal J J, Wiley D C. The ectodomain of HIV-1 env subunit gp41 forms a soluble, alpha-helical, rod-like oligomer in the absence of gp120 and the N-terminal fusion peptide. EMBO J. 1996;15:1507–1514. [PMC free article] [PubMed] [Google Scholar]
- 41.Weng Y, Weiss C D. Mutational analysis of residues in the coiled-coil domain of human immunodeficiency virus type 1 transmembrane protein gp41. J Virol. 1998;72:9676–9682. doi: 10.1128/jvi.72.12.9676-9682.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wild C, Dubay J W, Greenwell T, Baird Jr T, Oas T G, McDanal C, Hunter E, Matthews T. Propensity for a leucine zipper-like domain of human immunodeficiency virus type 1 gp41 to form oligomers correlates with a role in virus-induced fusion rather than assembly of the glycoprotein complex. Proc Natl Acad Sci USA. 1994;91:12676–12680. doi: 10.1073/pnas.91.26.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wild C, Oas T, McDanal C, Bolognesi D, Matthews T. A synthetic peptide inhibitor of human immunodeficiency virus replication: correlation between solution structure and viral inhibition. Proc Natl Acad Sci USA. 1992;89:10537–10541. doi: 10.1073/pnas.89.21.10537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wild C, Shugars D C, Greenwell T K, McDanal C B, Matthews T J. Peptides corresponding to a predictive alpha-helical domain of human immunodeficiency virus type 1 gp41 are potent inhibitors of virus infection. Proc Natl Acad Sci USA. 1994;91:9770–9774. doi: 10.1073/pnas.91.21.9770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu J-Y, Gorny M K, Palker T, Karwowska S, Zolla-Pazner S. Epitope mapping of two immunodominant domains of gp41, the transmembrane protein of human immunodeficiency virus type 1, using ten human monoclonal antibodies. J Virol. 1991;65:4832–4838. doi: 10.1128/jvi.65.9.4832-4838.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zolla-Pazner S, O'Leary J, Burda S, Gorny M K, Kim M, Mascola J, McCutchan F E. Serotyping of primary human immunodeficiency virus type 1 isolates from diverse geographic locations by flow cytometry. J Virol. 1995;69:3807–3815. doi: 10.1128/jvi.69.6.3807-3815.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]