Abstract
Purpose
To perform a systematic review and meta-analysis to assess the effect of enzyme replacement therapy on cardiac MRI parameters in patients with Fabry disease.
Materials and Methods
A systematic literature search was conducted from January 1, 2000, through January 1, 2024, in PubMed, ClinicalTrials.gov, Embase, and Cochrane Library databases. Study outcomes were changes in the following parameters: (a) left ventricular wall mass (LVM), measured in grams; (b) LVM indexed to body mass index, measured in grams per meters squared; (c) maximum left ventricular wall thickness (MLVWT), measured in millimeters; (d) late gadolinium enhancement (LGE) extent, measured in percentage of LVM; and (e) native T1 mapping, measured in milliseconds. A random-effects meta-analysis of the pooled mean differences between baseline and follow-up parameters was conducted. The study protocol was registered in PROSPERO (CRD42022336223).
Results
The final analysis included 11 studies of a total of 445 patients with Fabry disease (mean age ± SD, 41 years ± 11; 277 male, 168 female). Between baseline and follow-up cardiac MRI, the following did not change: T1 mapping (mean difference, 6 msec [95% CI: −2, 15]; two studies, 70 patients, I2 = 88%) and LVM indexed (mean difference, −1 g/m2 [95% CI: −6, 3]; four studies, 290 patients, I2 = 81%). The following measures minimally decreased: LVM (mean difference, −18 g [95% CI: −33, −3]; seven studies, 107 patients, I2 = 96%) and MLVWT (mean difference, −1 mm [95% CI: −2, −0.02]; six studies, 151 patients, I2 = 90%). LGE extent increased (mean difference, 1% [95% CI: 1, 1]; three studies, 114 patients, I2 = 85%).
Conclusion
In patients with Fabry disease, enzyme replacement therapy was associated with stabilization of LVM, MLVWT, and T1 mapping values, whereas LGE extent mildly increased.
Keywords: Fabry Disease, Enzyme Replacement Therapy (ERT), Cardiac MRI, Late Gadolinium Enhancement (LGE)
Supplemental material is available for this article.
© RSNA, 2024
Keywords: Fabry Disease, Enzyme Replacement Therapy (ERT), Cardiac MRI, Late Gadolinium Enhancement (LGE)
Summary
Patients with Fabry disease undergoing enzyme replacement therapy showed stabilization of left ventricular mass and native T1 mapping, whereas the extent of late gadolinium enhancement slightly increased.
Key Points
■ In this systematic review and meta-analysis of 445 patients with Fabry disease who underwent baseline and follow-up cardiac MRI after enzyme replacement therapy, pooled analysis revealed no changes in native T1 mapping (mean difference, 6 msec [95% CI: −2, 15]), slightly decreased left ventricular mass (mean difference, −18 g [95% CI: −33, −3]), and increased late gadolinium enhancement extent (mean difference, 1% [95% CI: 1, 1]) between time points.
■ There was no evidence of effect modification according to presence of late gadolinium enhancement on left ventricular mass changes (mean difference, 12; 95% CI: −36, 60; P = .62).
■ Prospective studies are needed to evaluate the clinical benefits of enzyme replacement therapy according to baseline cardiac MRI findings.
Introduction
Fabry disease is a progressive, X-linked-inherited disorder secondary to reduced or absent activity of the lysosomal enzyme α-galactosidase A, resulting in an intracellular accumulation of globotriaosylceramide (Gb3) in a wide range of organs (1). Cardiac involvement is the leading cause of death (2,3). The hallmarks of Fabry disease cardiomyopathy are the myocardial accumulation of Gb3 in the early stages, left ventricular hypertrophy (LVH), myocardial fibrosis, and inflammation in more advanced stages (4–6). Cardiac MRI is the main imaging tool to noninvasively assess and stage cardiac involvement in Fabry disease because it can precisely measure left ventricular mass (LVM) and maximum left ventricular wall thickness (MLVWT) (7) as well as depict indirect signs of myocardial Gb3 accumulation and fibrosis through reduced native T1 mapping values (8,9) and late gadolinium enhancement (LGE) (10). The introduction of Fabry disease–specific enzyme replacement therapy (ERT) has changed the natural history of Fabry disease by delaying, halting, or reverting Gb3 accumulation in various organs (11). Early ERT seems essential because initiation of therapy after irreversible organ damage might be ineffective (3,12–15). Pharmacologic chaperone therapy (PCT) is a recent promising alternative to ERT (16,17). However, because of the low incidence of Fabry disease, studies addressing the effect of ERT or PCT on morpho-functional, structural, and tissue-related features of cardiac involvement in the disease have been limited by small sample sizes (18–30). Therefore, there is no robust evidence regarding the effects of ERT on cardiac MRI parameters. The current systematic review and meta-analysis was performed to assess the effect of ERT and/or PCT on cardiac MRI findings in patients with Fabry disease.
Materials and Methods
This study was performed in accordance with the checklist of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement (31) (Table S1). The study protocol was registered in the PROSPERO database (CRD42022336223).
Search Strategy
Two independent investigators (S.F., six published meta-analyses; K.S., no published meta-analyses) performed a systematic review of the literature exploring the effect of ERT and/or PCT on cardiac MRI parameters in patients with Fabry disease. The PubMed, ClinicalTrials.gov, Embase, and Cochrane Library databases were searched using the following Medical Subject Heading terms: “Fabry disease” and “Enzyme Replacement Therapy” in various combinations with other keywords in free-text format: “Cardiac MRI,” “Cardiac Magnetic Resonance,” “Cardiovascular Magnetic Resonance,” “CMR,” “ERT,” and “Chaperone.” The date parameters were January 1, 2000, through January 1, 2024. Articles were initially screened by title and abstract content. In addition, backward snowballing (ie, a review of references from identified articles and pertinent reviews) was used to identify any additional citations.
Study Eligibility
Full-length original publications in peer-reviewed journals that assessed patients with Fabry disease undergoing baseline cardiac MRI and, after treatment with ERT and/or PCT, follow-up cardiac MRI, were retrieved. We excluded studies that included the following: (a) patients without a genetically confirmed diagnosis of Fabry disease; (b) patients with Fabry disease who were younger than 18 years of age; and (c) patients undergoing follow-up cardiac MRI earlier than 1 month from baseline. We also excluded reviews, editorials, case reports, experimental studies, and conference abstracts. No language or sample size restrictions were applied. Each eligible article meeting the inclusion criteria was reviewed by two independent reviewers (S.F. and K.S.). Disagreements were resolved by consensus or in combination with a third investigator (G.G., 36 published meta-analyses).
Data Extraction
The following descriptive data were independently extracted by two investigators (S.F. and K.S.) using a standardized data extraction form: (a) study: first author, year of publication, number of patients; (b) sociodemographic and clinical factors: age, sex, body surface area, cardiovascular risk factors; and (c) cardiac MRI parameters: left ventricular ejection fraction, left ventricular end-diastolic volume, left ventricular end-systolic volume, right ventricular ejection fraction, right ventricular end-diastolic volume, right ventricular end-systolic volume, MLVWT, LVM, left atrial volume, right atrial volume, native T1 mapping, and LGE presence and extent. Cardiac MRI parameters were extracted from baseline and follow-up examinations.
Quality of Evidence and Grading of Evidence
Two researchers (S.F. and K.S.) independently assessed the quality of the included studies and risk of bias according to the ROBINS-I tool (32). The certainty of evidence for the effect of ERT on cardiac MRI parameters was evaluated by implementing the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) Working Group system (33). In brief, we took into account the five GRADE Working Group considerations (risk of bias, consistency of effect, imprecision, indirectness, and publication bias) and adjudicated the certainty of the body of evidence separately for each cardiac MRI parameter evaluated. We combined quality of evidence across all fields and defined four categories (high, moderate, low, or very low).
Data Synthesis and Statistical Analysis
Mean differences between baseline and follow-up cardiac MRI parameters were used as summary statistics for study outcomes. SDs for mean differences, if not reported in the original studies, were extracted from CIs, P values from parametric tests of changes, and using the correlation coefficient of changes from the baseline measurements, as described in the Cochrane handbook (34). When no information for the variability of the mean difference was given, the correlation coefficient (r2) between the two time points was retrieved for imputing the SD of the mean change. Subsequently, in case of missing correlations, the imputation of SD was based on correlations from other included studies with the largest possible number of patients and similar follow-up times (34). The study end points represented within-group changes of the following cardiac MRI parameters: LVM (in grams); LVM indexed for body surface area (in grams per meters squared); MLVWT (in millimeters), LGE (percentage of LVM), and native T1 myocardial mapping (in milliseconds) between baseline and follow-up cardiac MRI. Changes in LGE extent were evaluated in patients with Fabry disease showing LGE at baseline cardiac MRI.
We implemented the Sidik and Jonkman (35) heterogeneity estimator for the between-studies variance instead of the standard DerSimonian and Laird estimator, which often underperforms with a small number of studies (36). Heterogeneity among studies was assessed using the Cochrane Q test and the I2 statistic to distinguish whether a random or fixed-effects method should be implemented. When significant heterogeneity was observed, a random-effects model was used.
For studies including a control group of patients with Fabry disease not undergoing ERT, we retrieved the difference in mean cardiac MRI changes between follow-up and baseline for treated (a) and untreated (b) patients. We then subtracted (b) from (a) to quantify the difference in cardiac MRI changes between treated and control patients. We determined measures of dispersion for these differences, and a separate meta-analysis with these studies was conducted using both a fixed and random-effects model.
Publication bias was assessed using the Egger test, with significant bias defined as a P value less than .1. When publication bias was adjudicated as significant, the “trim and fill” method was used to examine whether hypothetical missing studies would change the derived estimates.
We also sought to quantify the effect of specific covariates on the pooled estimates for LVM and MLVWT changes by ERT by implementing exploratory meta-regression analyses. Confounders assessed in the meta-regression analyses included mean age, the percentage of male patients (to conceptualize the effect of sex), follow-up times in months, and presence of LGE as binary variable. Meta-regression analysis was not performed for changes in LGE extent because of the limited number of retrieved studies (37). Funnel and Baujat plots were produced for the detection of outlying or influential studies.
Statistical analysis was performed with R software, version 4.2.1 (2022–06–23), and STATA package, version 11.1 (Stata). All tests were two-tailed. Statistical significance was set at a P value less than .05.
Results
Literature Search
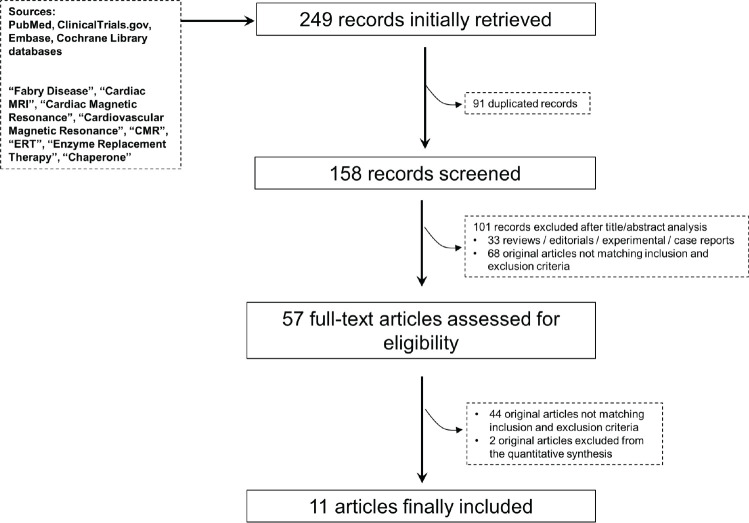
The flowchart of the meta-analysis (Fig 1) displays the literature search results. Initially, 249 articles were identified, and 192 (duplicated and nonrelevant articles) were subsequently excluded through screening of the title and abstract. Fifty-seven articles were evaluated as full-text articles. Eleven were deemed eligible for quantitative analysis of ERT effects on cardiac MRI parameters (18–28). Native T1 values were derived from regions of interest drawn in the septum (24), in the mid left ventricular wall (25) or not further specified (26). Two studies were deemed eligible for quantitative analysis of PCT effects on cardiac MRI parameters (29,30). Thus, a meta-analysis was not feasible in this group of patients.
Figure 1:
Study screening flow diagram.
Study Characteristics
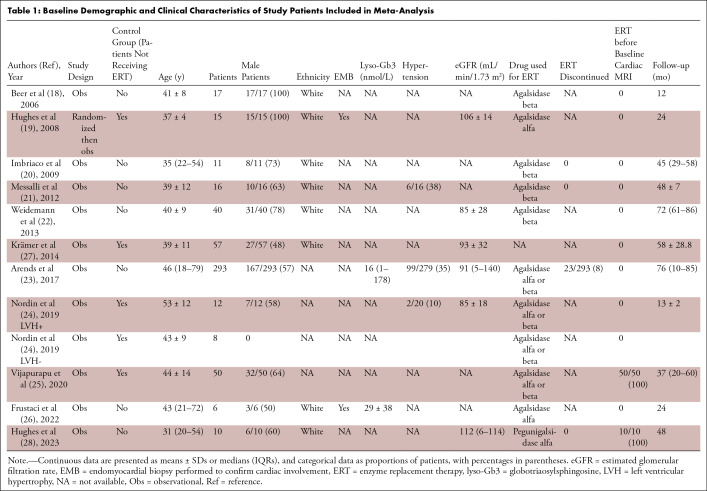
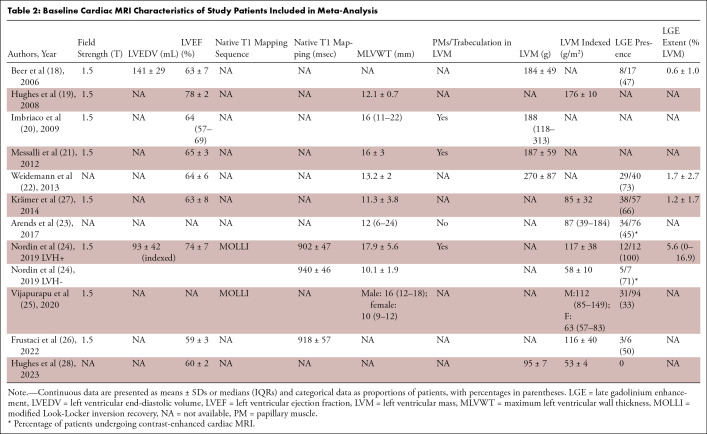
Eleven studies including 445 patients with Fabry disease (277 [62%] male, 168 [38%] female) treated with ERT and undergoing baseline and follow-up cardiac MRI were selected for quantitative analysis (Tables 1 and 2). The mean age ± SD of the included patients was 41 years ± 11, and the median follow-up time was 45 (IQR, 24–58) months. At baseline, LVM ranged from 95 to 270 g, LVM indexed ranged from 53 to 176 g/m2, and MLVWT ranged from 10 to 18 mm. T1 values ranged from 902 to 940 msec and LGE extent, from 0.6% to 5.6%. The largest population included 203 patients (23) and the smallest, six (26). Data regarding changes between baseline and follow-up cardiac MRI of LVM were reported in seven studies (18–22,26,28), LVM indexed in four studies (23,24,26,28), MLVWT in six studies (19–22,24,27), native T1 mapping in two studies (24,25), and LGE extent in three studies (18,22,27). General and baseline cardiac MRI characteristics of the studies included in the present meta-analysis are shown in Tables 1 and 2. The risk of bias was moderate for most included studies, except for two studies that had serious risk of bias (18,26) and one that had low risk of bias (19). The complete assessment with the ROBINS-I tool is shown in Table S2.
Table 1:
Baseline Demographic and Clinical Characteristics of Study Patients Included in Meta-Analysis
Table 2:
Baseline Cardiac MRI Characteristics of Study Patients Included in Meta-Analysis
Meta-Analyses
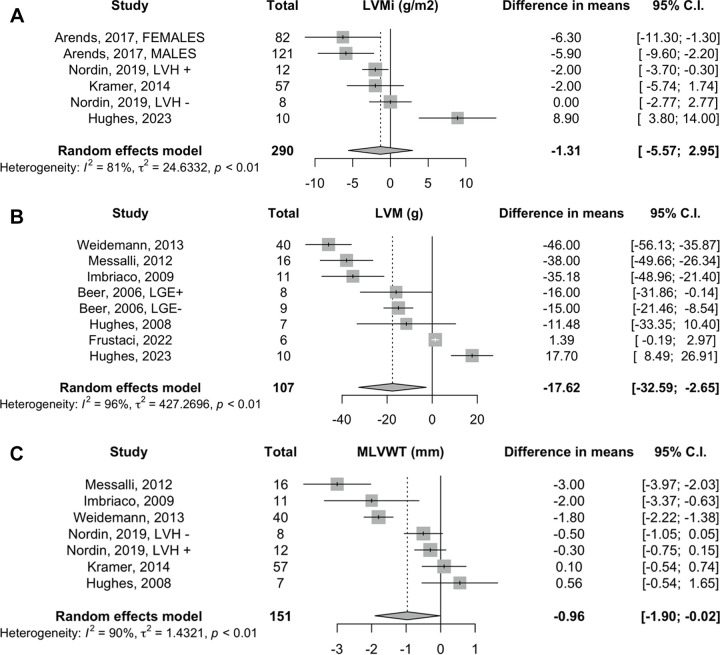
Effect of ERT on LVM and MLVWT.— Analysis for LVM indexed showed no changes with the random-effects model, with a mean difference of −1 g/m2 (95% CI: −6, 3; four studies, 290 patients, I2 = 81%) (Fig 2A). The results derived by the fixed-effects model are reported in Appendix S1 (Fig S1). ERT was associated with minimal LVM decrease (mean difference, −18 g [95% CI: −33, −3]; seven studies, 107 patients, I2 = 96%) (Fig 2B). After removal of studies on LVM with imputed SDs of the mean difference (18,21,22), the random-effects pooled estimate did not retain statistical significance (mean difference, −6 g [95% CI: −28, 16]; four studies, 34 patients, I2 = 93%) (Fig S2). ERT was associated with minimal decrease in MLVWT (mean difference, −1 mm [95% CI: −2, −0.02]; six studies, 151 patients, I2 = 90%) (Fig 2C). However, upon exclusion of the studies with imputed SD of the mean difference (21,22,27), the combined estimate of ERT on MLVWT did not reach statistical significance (mean difference, −0.5 mm [95% CI: −1.5, 0.5]; three studies, 38 patients, I2 = 64%), and the heterogeneity was reduced by 26% (I2; P = .40) (Fig S3).
Figure 2:
Effect of enzyme replacement therapy on left ventricular mass indexed (LVMi), left ventricular mass (LVM), and maximum left ventricular wall thickness (MLVWT). Forest plots: meta-analyses on (A) left ventricular mass indexed (in grams per millimeter squared), (B) left ventricular mass (in grams), and (C) maximum left ventricular wall thickness (in millimeters) with Sidik and Jonkman correction. Effect sizes: differences in means between baseline and follow-up measurements. LGE = late gadolinium enhancement, LVH = left ventricular hypertrophy.
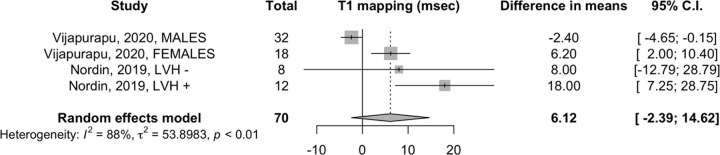
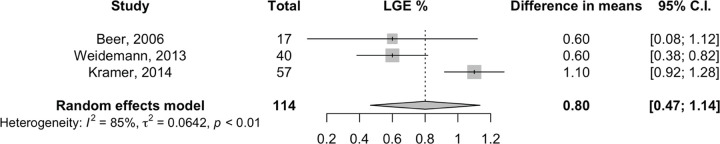
Effect of ERT on native T1 mapping and LGE extent.— Analysis for native T1 mapping showed no changes, with a mean difference of 6 msec (95% CI: −2, 15; two studies, 70 patients, I2 = 88%), with the random-effects model (Fig 3). In patients exhibiting LGE at baseline cardiac MRI, we found a minimal increase of LGE extent equal to a mean difference of 0.8% of LVM (95% CI: 0.5, 1.1; three studies, 114 patients, I2 = 85%) with the random-effects model (Fig 4).
Figure 3:
Effect of enzyme replacement therapy on T1 mapping. Forest plot: meta-analysis of native T1 mapping (in milliseconds) with Sidik and Jonkman correction. Effect size: difference in means between baseline and follow-up measurements. LVH = left ventricular hypertrophy.
Figure 4:
Effect of enzyme replacement therapy on extent of late gadolinium enhancement (LGE). Forest plot: meta-analysis of extent of late gadolinium enhancement (LGE; percentage of left ventricular mass) with Sidik and Jonkman correction. Effect size: difference in means between baseline and follow-up measurements.
Patients receiving ERT versus controls.— Four studies (19,24,25,27) included a control group of patients with Fabry disease not undergoing ERT. Among them, one study (27) reported on LGE extent changes and two (24,25) reported on T1 changes over time in treated and untreated patients with Fabry disease.
An exploratory meta-analysis assessing specific cardiac MRI changes in treated versus untreated patients with Fabry disease was feasible only for LVM indexed, the changes for which in both groups were provided by three studies (19,24,27). A random-effects model showed a beneficial effect of ERT on LVM indexed, although this result did not reach statistical significance (mean difference, −8 g/m2 [95% CI: −18, 1]) (Fig S4). The results derived by the fixed-effects model are reported in Appendix S1 (Fig S4).
Meta-Regression Analyses
None of the parameters of the meta-regression model on LVM-indexed data reached statistical significance. Application of the same model to MLVWT revealed a significant effect modification according to follow-up time, with a 0.07-mm decrease (95% CI: −0.1, −0.03) in the effect size of ERT on MLVWT (Tables S3 and S4; Figs S5 and S6). In addition, LGE presence increased the effect size by 3 (95% CI: 1, 5). There was no evidence of ERT effect modification on MLVWT changes according to male sex (0.4 mm [95% CI: −2, 2]) (Table S4).
Publication Bias and Grading of Evidence
No publication bias was detected for LVM indexed by the Egger test (P = .80) (Table S5). Furthermore, there was no visual evidence of asymmetry on the funnel plot (Fig S7). Regarding LVM, there was borderline evidence of small-study bias (Egger test P = .08) (Table S5), and we identified an outlying study by Frustaci et al (26) on the funnel plot (Fig S8) and Baujat plot (Fig S9). After we removed this study from the analysis, the mean difference remained significant and was determined at −21 g (95% CI: −37, −4; six studies, 101 patients, I2 = 94%) (Fig S10). However, when the “trim and fill” method was used, the pooled estimate did not retain statistical significance, with a mean difference of −6 g (95% CI: −25, 14; nine studies, 163 patients, I2= 97%) (Fig S11). Studies assessing MLVWT, native T1 mapping values, and LGE showed no significant bias by Egger tests (Table S5). In addition, funnel plots for other cardiac MRI parameters did not provide any visual evidence that could indicate small study bias (Figs S12–S14).
According to the GRADE Working Group system (33), the level of certainty for the association between ERT and cardiac MRI was low in all parameters but LGE extent and T1 mapping, which were adjudicated to be very low (Table S6).
Discussion
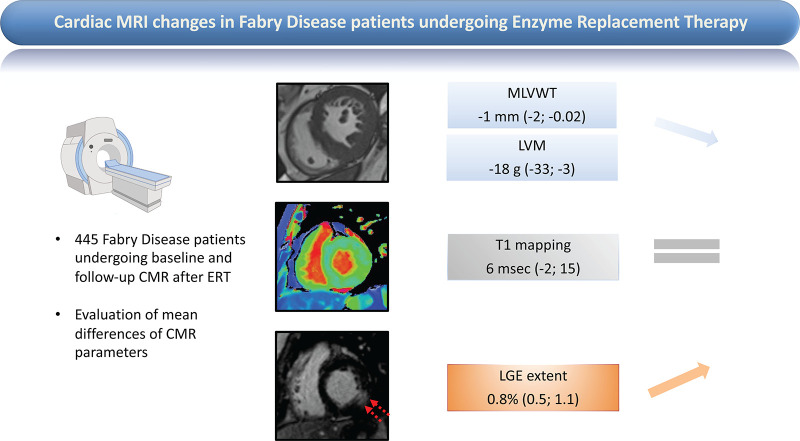
The present meta-analysis provides pooled adjusted estimates on cardiac MRI parameter changes in 445 patients with Fabry disease after ERT. We found a minimal decrease in LVM (mean difference, −18 g [95% CI: −33, −3]) and MLVWT (mean difference, −1 mm [95% CI: −2, −0.02]) whereas LGE slightly increased (mean difference, 1% [95% CI: 1, 1]), with no modification of T1 relaxation time (mean difference, 6 msec [95% CI: −2, 15]) (Fig 5).
Figure 5:
Effects of enzyme replacement therapy (ERT) on cardiac MRI parameters in patients with Fabry disease. Changes in maximum left ventricular wall thickness (MLVWT), left ventricular mass (LVM), native T1 mapping, and extent of late gadolinium enhancement (LGE) are reported in patients with Fabry disease undergoing baseline and follow-up cardiac MRI after ERT. Three basal short-axis cardiac MRI scans of patients with Fabry disease are shown (left): cine steady-state-free precession (top left), precontrast shortened modified Look-Locker inversion recovery (center left), and postcontrast inversion recovery fast gradient echo (bottom left); the images are used to measure, respectively, MLVWT/LVM, native T1 mapping, and extent of LGE (bottom left, red arrows). Cardiac MRI changes under ERT are reported as pooled mean differences (95% CIs) for each parameter (right).
Cardiac involvement is the main cause of impaired quality of life and death in patients with Fabry disease (4). Cardiac MRI allows staging of cardiomyopathy in Fabry disease (4,5,13): (a) early Gb3 accumulation yielding T1 relaxation time lowering; (b) T1 lowering associated with LVH; and (c) fibrosis or inflammation (ie, LGE starting in the basal posterior wall) associated with LVH and pseudonormalization of native T1 values in the areas of fibrosis but not in remote areas. This scenario can differ in some patients with Fabry disease, particularly female patients, who may show LGE before LVH (38). Notably, LVH, low T1 values, and LGE at cardiac MRI have all been associated with adverse clinical outcomes (4,13,39).
ERT has the potential to slow or revert Fabry disease organ involvement (2). In a double-masked study, Eng et al (40) demonstrated that ERT resulted in histologic clearance of the deposits of Gb3 from cardiac cells. This phenomenon aligns with LVM and MLVWT regression after ERT, which was initially observed in echocardiography-based studies (41,42). Notably, cardiac MRI is more accurate than echocardiography in measuring LVM and MLVWT. Cardiac MRI does not depend on patients’ acoustic window in measuring MLVWT; rather, it directly quantifies LVM, regardless of mathematical assumptions (43), and it considers left ventricular trabeculae and papillary muscles, which are typically abnormally increased in Fabry disease (7).
We found discordance among studies in the direction of the effect size of ERT on LVM changes. This aspect underscores the necessity of a meta-analysis to derive pooled inference, especially given the absence of large studies that would affect clinical practices. Overall, our results show mass stabilization during ERT over time of almost 4 years. In fact, the analyses on LVM, LVM indexed, or MLVWT between baseline and follow-up cardiac MRI indicated no changes or minimal reduction of these parameters, excluding an increase of mass indexes. Notably, preventing LVH development or progression is considered a main therapeutic goal in patients with Fabry disease (44).
In our analysis, the thinning of left ventricular wall thickness observed in late Fabry disease stages characterized by myocardial fibrosis (45) was unlikely to influence our results on LVM because the changes in this parameter were not influenced by the presence of LGE, although the latter affected MLVWT changes. Accordingly, our findings indicate that the effect of ERT on mass stabilization might persist throughout the progressive stages of Fabry disease cardiomyopathy. Three studies reported data on LVM-indexed changes in patients treated with ERT versus untreated patients (19,24,27). An exploratory meta-analysis conducted on these studies with a fixed-effects pooled estimate showed a favorable effect of ERT in significantly reducing LVM indexed in treated patients as compared with an increase of LVM indexed in untreated patients. In contrast, Vijapurapu et al (25) and Hughes et al (28) observed an increase in LVM in treated patients with Fabry disease. Notably, these studies included patients already receiving ERT before study initiation. This limitation might have prevented the authors from considering an effect of ERT on LVM occurred before study enrollment. The results in this subgroup of patients compared with a control group align with previous echocardiographic findings (46) and with the main results of our study in supporting an effective role of ERT in LVM stabilization in patients with Fabry disease, excluding LVM increase during therapy.
Unfortunately, we could not assess whether these results translate into clinical patient benefits because of a lack of data in the available literature. LVH characterizes advanced stages of Fabry disease cardiomyopathy and has been consistently associated with poorer clinical outcomes related to heart failure and malignant arrhythmias (4). By demonstrating an association between ERT and stabilization in LVM, independently from the presence of underlying myocardial fibrosis, our data suggest a favorable effect in preventing LVH; still, dedicated studies remain necessary to test whether the effects of ERT on LVM translate into clinically relevant benefits.
Low native T1 values represent myocardial Gb3 accumulation, which occurs in 85% of patients with Fabry disease with LVH and up to 59% of LVH-negative patients. Native T1 values are lower in patients with LVH than those without LVH (6). Thus, it is believed that myocardial storage occurs early but moves forward and evolves in more advanced stages (5,47,48). In our analysis, native T1 mapping values did not significantly change between baseline and follow-up cardiac MRI. Thus, ERT might be effective in attenuating or stopping T1 lowering, although it might not be effective in reverting T1 alterations. This discrepancy could be related to two main factors. First, additional mechanisms of action on top of Gb3 clearance by ERT might prevent LVH, including hindering downstream alterations in myocardial perfusion secondary to Gb3 storage. In fact, the sole role of ERT on Gb3 cell clearance might be secondary in preventing LVH because it mainly concerns endothelial cells, which have a small contribution to mass (24). Second, the ERT effects on T1 that we measured may have been affected by the T1 value increase in patients with LVH (24). In these patients, an increased value of T1 at follow-up (ie, pseudonormalization) might occur, given the development of replacement myocardial fibrosis (4). Discrepancies in myocardial regions evaluated for T1 mapping analysis among studies might also contribute to discordant ERT effects on T1 values compared with other cardiac MRI parameters, which will have to be addressed explicitly by dedicated prospective studies with a homogeneous methodologic approach.
LGE in Fabry disease was initially interpreted as an increase in extracellular space secondary to replacement myocardial fibrosis; the current belief is that it also underlies active inflammation with troponin release (4,24,47). According to our analysis, ERT was ineffective in decreasing or halting LGE in patients showing it at baseline. Remarkably, our study design precludes us from inferring any effect of ERT in preventing LGE. Our results align with current recommendations to start ERT early (15) because LGE is more common in advanced stages (4,5,49). Accordingly, a phase 4 ERT study focusing on renal function showed the highest benefit in patients starting with better renal function (50). Once a substantial amount of myocardial fibrosis is present, a self-maintaining irreversible vicious cycle might prevent any influences of ERT from reverting or stopping underlying mechanisms promoting its growth and expansion, which may follow independent and parallel pathways to those regulating LVM response to ERT (51). Our findings, showing LGE increase in patients with LGE, call for prospective studies assessing the effect of ERT on cardiovascular outcomes in those patients. At the same time, the lack of efficacy of ERT in LGE regression encourages novel and alternative treatments for the same purpose (51). Emerging cardiac MRI parameters sensitive to cardiac involvement in Fabry disease, including extracellular volume (6) and native T2 mapping (24), might be used along with LGE to refine and tailor therapy for patients with Fabry disease.
Our meta-analysis had several limitations. First, the absence of a control group not undergoing ERT in all but four (19,24,25,27) of the included studies hinders comparisons of the evolution of cardiac MRI parameters in treated and untreated patients. Second, given the lack of dedicated studies, it is unknown whether the observed variations in cardiac MRI parameters translate into improved cardiovascular outcomes for treated patients. Regarding LGE extent, only one study (24) measured it as grams per meters squared. LGE extent was mainly evaluated as changes in percentage of LVM; however, in doing so, changes in LVM can potentially affect this measurement independently from variation of myocardial fibrosis. One study (23) did not clearly specify whether follow-up cardiac MRI was performed using the same MRI scanner and acquisition protocol as the baseline examination, opening to potential discrepancies in cardiac MRI parameters highly sensible to equipment characteristics (ie, T1 mapping). Frustaci et al (26) reported no changes in T1 mapping between baseline and follow-up cardiac MRI, so we assumed that the values at baseline and follow-up were identical. However, minor changes in T1 values are likely due to the intra- and interobserver variability of the method. Overall, we included observational studies with an intrinsic risk for selection bias that can detect associations but not ascribe causality. Moreover, some studies included in the meta-analysis presented significant heterogeneity concerning patient samples. We sought to explore heterogeneity by meta-regression models on baseline confounders. Still, because of a limited number of synthesized studies, meta-regression results should be cautiously interpreted (52); a prespecified number of confounders based on biologic plausibility and availability of summary data were tested for effect modification, but only linear meta-regression analyses were conducted. Moreover, the patients included in the study of Vijapurapu et al (25) and Hughes et al (28) had already been treated with ERT before baseline cardiac MRI, and 23 of 293 (8%) patients in the study of Arends et al (23) were assessed at follow-up cardiac MRI after discontinuation of ERT. These limitations might account for the potential underestimation of the effects of ERT on cardiac remodeling.
Finally, cardiac phenotype is likely not the only variable affecting ERT response; sex, ERT-specific drug, duration, and induced antibodies might influence ERT effects on cardiac involvement in Fabry disease (4). These phenomena, together with novel cardiac MRI markers of Fabry disease cardiomyopathy (ie, T2 mapping, global longitudinal strain, and left atrial sizes and function) (24,53–55), could not be adjudicated as eligible for meta-analysis given the scarcity of data available. Future investigations that include imaging and clinical follow-up are needed for a more granular framework for predicting ERT response, allowing physicians to tailor the best treatment for each individual patient.
In conclusion, ERT in patients with Fabry disease was associated with stabilization of LVM and T1 mapping values, whereas LGE extent slightly increased. These results should be cautiously interpreted in view of significant heterogeneity across investigations and the low level of certainty for the association between ERT and cardiac MRI parameter changes. Longitudinal studies are needed to assess the effect of ERT on cardiovascular outcomes.
S.F. and E.K. contributed equally to this work.
M.P. and G.G. are co–senior authors.
Authors declared no funding for this work.
Disclosures of conflicts of interest: S.F. No relevant relationships. E.K. No relevant relationships. A.S. No relevant relationships. A.C. Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Amicus Therapeutics and Sanofi Genzyme (direct payment to the undersigned). K.S. No relevant relationships. P.G.M. Consulting fees from Perspectum Diagnostics. G.M. No relevant relationships. M.L. No relevant relationships. G.C. No relevant relationships. M.F. No relevant relationships. M.P. Consulting fees from Sanofi, Takeda, Pfizer, and Bristol Myers Squibb; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Sanofi, Takeda, Pfizer, and Bristol Myers Squibb; support for meetings and/or travel from Sanofi, Takeda, Pfizer, and Bristol Myers Squibb. G.G. Honoraria from Pfizer; support from Pfizer to attend meetings.
Abbreviations:
- ERT
- enzyme replacement therapy
- Gb3
- globotriaosylceramide
- GRADE
- Grades of Recommendation, Assessment, Development and Evaluation
- LGE
- late gadolinium enhancement
- LVH
- left ventricular hypertrophy
- LVM
- left ventricular mass
- MLVWT
- maximum left ventricular wall thickness
- PCT
- pharmacologic chaperone therapy
References
- 1. Brady RO , Gal AE , Bradley RM , Martensson E , Warshaw AL , Laster L . Enzymatic defect in Fabry's disease. Ceramidetrihexosidase deficiency . N Engl J Med 1967. ; 276 ( 21 ): 1163 – 1167 . [DOI] [PubMed] [Google Scholar]
- 2. Ortiz A , Abiose A , Bichet DG , et al . Time to treatment benefit for adult patients with Fabry disease receiving agalsidase β: data from the Fabry Registry . J Med Genet 2016. ; 53 ( 7 ): 495 – 502 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Linhart A , Germain DP , Olivotto I , et al . An expert consensus document on the management of cardiovascular manifestations of Fabry disease . Eur J Heart Fail 2020. ; 22 ( 7 ): 1076 – 1096 . [DOI] [PubMed] [Google Scholar]
- 4. Pieroni M , Moon JC , Arbustini E , et al . Cardiac involvement in fabry disease: JACC review topic of the week . J Am Coll Cardiol 2021. ; 77 ( 7 ): 922 – 936 . [DOI] [PubMed] [Google Scholar]
- 5. Nordin S , Kozor R , Medina-Menacho K , et al . Proposed stages of myocardial phenotype development in Fabry disease . JACC Cardiovasc Imaging 2019. ; 12 ( 8 Pt 2 ): 1673 – 1683 . [DOI] [PubMed] [Google Scholar]
- 6. Figliozzi S , Camporeale A , Boveri S , et al . ECG-based score estimates the probability to detect Fabry disease cardiac involvement . Int J Cardiol 2021. ; 339 : 110 – 117 . [DOI] [PubMed] [Google Scholar]
- 7. Kozor R , Callaghan F , Tchan M , Hamilton-Craig C , Figtree GA , Grieve SM . A disproportionate contribution of papillary muscles and trabeculations to total left ventricular mass makes choice of cardiovascular magnetic resonance analysis technique critical in Fabry disease . J Cardiovasc Magn Reson 2015. ; 17 ( 1 ): 22 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sado DM , White SK , Piechnik SK , et al . Identification and assessment of Anderson-Fabry disease by cardiovascular magnetic resonance noncontrast myocardial T1 mapping . Circ Cardiovasc Imaging 2013. ; 6 ( 3 ): 392 – 398 . [DOI] [PubMed] [Google Scholar]
- 9. Pica S , Sado DM , Maestrini V , et al . Reproducibility of native myocardial T1 mapping in the assessment of Fabry disease and its role in early detection of cardiac involvement by cardiovascular magnetic resonance . J Cardiovasc Magn Reson 2014. ; 16 ( 1 ): 99 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moon JC , Sheppard M , Reed E , Lee P , Elliott PM , Pennell DJ . The histological basis of late gadolinium enhancement cardiovascular magnetic resonance in a patient with Anderson-Fabry disease . J Cardiovasc Magn Reson 2006. ; 8 ( 3 ): 479 – 482 . [DOI] [PubMed] [Google Scholar]
- 11. Schiffmann R , Kopp JB , Austin HA 3rd , et al . Enzyme replacement therapy in Fabry disease: a randomized controlled trial . JAMA 2001. ; 285 ( 21 ): 2743 – 2749 . [DOI] [PubMed] [Google Scholar]
- 12. Ortiz A , Germain DP , Desnick RJ , et al . Fabry disease revisited: management and treatment recommendations for adult patients . Mol Genet Metab 2018. ; 123 ( 4 ): 416 – 427 . [DOI] [PubMed] [Google Scholar]
- 13. Perry R , Shah R , Saiedi M , et al . The role of cardiac imaging in the diagnosis and management of Anderson-Fabry disease . JACC Cardiovasc Imaging 2019. ; 12 ( 7 Pt 1 ): 1230 – 1242 . [Published correction appears in JACC Cardiovasc Imaging 2019;12(9):1903.] [DOI] [PubMed] [Google Scholar]
- 14. Germain DP , Elliott PM , Falissard B , et al . The effect of enzyme replacement therapy on clinical outcomes in male patients with Fabry disease: a systematic literature review by a European panel of experts . Mol Genet Metab Rep 2019. ; 19 : 100454 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Biegstraaten M , Arngrímsson R , Barbey F , et al . Recommendations for initiation and cessation of enzyme replacement therapy in patients with Fabry disease: the European Fabry Working Group consensus document . Orphanet J Rare Dis 2015. ; 10 ( 1 ): 36 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Germain DP , Hughes DA , Nicholls K , et al . Treatment of Fabry's disease with the pharmacologic chaperone migalastat . N Engl J Med 2016. ; 375 ( 6 ): 545 – 555 . [DOI] [PubMed] [Google Scholar]
- 17. Ishii S . Pharmacological chaperone therapy for Fabry disease . Proc Jpn Acad, Ser B, Phys Biol Sci 2012. ; 88 ( 1 ): 18 – 30 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Beer M , Weidemann F , Breunig F , et al . Impact of enzyme replacement therapy on cardiac morphology and function and late enhancement in Fabry's cardiomyopathy . Am J Cardiol 2006. ; 97 ( 10 ): 1515 – 1518 . [DOI] [PubMed] [Google Scholar]
- 19. Hughes DA , Elliott PM , Shah J , et al . Effects of enzyme replacement therapy on the cardiomyopathy of Anderson-Fabry disease: a randomised, double-blind, placebo-controlled clinical trial of agalsidase alfa . Heart 2008. ; 94 ( 2 ): 153 – 158 . [DOI] [PubMed] [Google Scholar]
- 20. Imbriaco M , Pisani A , Spinelli L , et al . Effects of enzyme-replacement therapy in patients with Anderson-Fabry disease: a prospective long-term cardiac magnetic resonance imaging study . Heart 2009. ; 95 ( 13 ): 1103 – 1107 . [DOI] [PubMed] [Google Scholar]
- 21. Messalli G , Imbriaco M , Avitabile G , et al . Role of cardiac MRI in evaluating patients with Anderson-Fabry disease: assessing cardiac effects of long-term enzyme replacement therapy [in Italian] . Radiol Med (Torino) 2012. ; 117 ( 1 ): 19 – 28 . [DOI] [PubMed] [Google Scholar]
- 22. Weidemann F , Niemann M , Störk S , et al . Long-term outcome of enzyme-replacement therapy in advanced Fabry disease: evidence for disease progression towards serious complications . J Intern Med 2013. ; 274 ( 4 ): 331 – 341 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Arends M , Biegstraaten M , Hughes DA , et al . Retrospective study of long-term outcomes of enzyme replacement therapy in Fabry disease: analysis of prognostic factors . PLoS One 2017. ; 12 ( 8 ): e0182379 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nordin S , Kozor R , Vijapurapu R , et al . Myocardial storage, inflammation, and cardiac phenotype in Fabry disease after one year of enzyme replacement therapy . Circ Cardiovasc Imaging 2019. ; 12 ( 12 ): e009430 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vijapurapu R , Baig S , Nordin S , et al . Longitudinal assessment of cardiac involvement in Fabry disease using cardiovascular magnetic resonance imaging . JACC Cardiovasc Imaging 2020. ; 13 ( 8 ): 1850 – 1852 . [DOI] [PubMed] [Google Scholar]
- 26. Frustaci A , Najafian B , Donato G , et al . Divergent impact of enzyme replacement therapy on human cardiomyocytes and enterocytes affected by Fabry disease: correlation with mannose-6-phosphate receptor expression . J Clin Med 2022. ; 11 ( 5 ): 1344 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Krämer J , Niemann M , Störk S , et al . Relation of burden of myocardial fibrosis to malignant ventricular arrhythmias and outcomes in Fabry disease . Am J Cardiol 2014. ; 114 ( 6 ): 895 – 900 . [DOI] [PubMed] [Google Scholar]
- 28. Hughes D , Gonzalez D , Maegawa G , et al . Long-term safety and efficacy of pegunigalsidase alfa: A multicenter 6-year study in adult patients with Fabry disease . Genet Med 2023. ; 25 ( 12 ): 100968 . [DOI] [PubMed] [Google Scholar]
- 29. Gatterer C , Beitzke D , Graf S , et al . Long-term monitoring of cardiac involvement under migalastat treatment using magnetic resonance tomography in Fabry disease . Life (Basel) 2023. ; 13 ( 5 ): 1213 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Camporeale A , Bandera F , Pieroni M , et al . Effect of Migalastat on cArdiac InvOlvement in FabRry DiseAse: MAIORA study . J Med Genet 2023. ; 60 ( 9 ): 850 – 858 . [DOI] [PubMed] [Google Scholar]
- 31. Page MJ , McKenzie JE , Bossuyt PM , et al . The PRISMA 2020 statement: an updated guideline for reporting systematic reviews . BMJ 2021. ; 372 ( 71 ): n71 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sterne JA , Hernán MA , Reeves BC , et al . ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions . BMJ 2016. ; 355 : i4919 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Guyatt GH , Oxman AD , Vist GE , et al. ; GRADE Working Group . GRADE: an emerging consensus on rating quality of evidence and strength of recommendations . BMJ 2008. ; 336 ( 7650 ): 924 – 926 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Higgins JPT , Li T , Deeks JJ . Chapter 6: Choosing effect measures and computing estimates of effect . Cochrane Handbook for Systematic Reviews of Interventions version 6.4 . https://training.cochrane.org/handbook/current/chapter-06. Updated August 2023. Accessed January 14, 2024.
- 35. Sidik K , Jonkman JN . Simple heterogeneity variance estimation for meta‐analysis . Journal of the Royal Statistical Society Series C . 2005. ; 54 ( 2 ): 367 – 384 . [Google Scholar]
- 36. Veroniki AA , Jackson D , Viechtbauer W , et al . Methods to estimate the between-study variance and its uncertainty in meta-analysis . Res Synth Methods 2016. ; 7 ( 1 ): 55 – 79 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pateras K , Nikolakopoulos S , Roes K . Data-generating models of dichotomous outcomes: heterogeneity in simulation studies for a random-effects meta-analysis . Stat Med 2018. ; 37 ( 7 ): 1115 – 1124 . [DOI] [PubMed] [Google Scholar]
- 38. Niemann M , Herrmann S , Hu K , et al . Differences in Fabry cardiomyopathy between female and male patients: consequences for diagnostic assessment . JACC Cardiovasc Imaging 2011. ; 4 ( 6 ): 592 – 601 . [DOI] [PubMed] [Google Scholar]
- 39. Camporeale A , Pieroni M , Pieruzzi F , et al . Predictors of clinical evolution in prehypertrophic Fabry disease . Circ Cardiovasc Imaging 2019. ; 12 ( 4 ): e008424 . [DOI] [PubMed] [Google Scholar]
- 40. Eng CM , Guffon N , Wilcox WR , et al. ; International Collaborative Fabry Disease Study Group . Safety and efficacy of recombinant human alpha-galactosidase A replacement therapy in Fabry's disease . N Engl J Med 2001. ; 345 ( 1 ): 9 – 16 . [DOI] [PubMed] [Google Scholar]
- 41. Beck M , Ricci R , Widmer U , et al . Fabry disease: overall effects of agalsidase alfa treatment . Eur J Clin Invest 2004. ; 34 ( 12 ): 838 – 844 . [DOI] [PubMed] [Google Scholar]
- 42. Weidemann F , Breunig F , Beer M , et al . Improvement of cardiac function during enzyme replacement therapy in patients with Fabry disease: a prospective strain rate imaging study . Circulation 2003. ; 108 ( 11 ): 1299 – 1301 . [DOI] [PubMed] [Google Scholar]
- 43. Moon JC , Prasad SK . Cardiovascular magnetic resonance and the evaluation of heart failure . Curr Cardiol Rep 2005. ; 7 ( 1 ): 39 – 44 . [DOI] [PubMed] [Google Scholar]
- 44. Wanner C , Arad M , Baron R , et al . European expert consensus statement on therapeutic goals in Fabry disease . Mol Genet Metab 2018. ; 124 ( 3 ): 189 – 203 . [DOI] [PubMed] [Google Scholar]
- 45. Hasegawa H , Takano H , Shindo S , et al . Images in cardiovascular medicine. Transition from left ventricular hypertrophy to massive fibrosis in the cardiac variant of Fabry disease . Circulation 2006. ; 113 ( 16 ): e720 – e721 . [DOI] [PubMed] [Google Scholar]
- 46. Germain DP , Weidemann F , Abiose A , et al. ; Fabry Registry . Analysis of left ventricular mass in untreated men and in men treated with agalsidase-β: data from the Fabry Registry . Genet Med 2013. ; 15 ( 12 ): 958 – 965 . [DOI] [PubMed] [Google Scholar]
- 47. Moon JC , Sachdev B , Elkington AG , et al . Gadolinium enhanced cardiovascular magnetic resonance in Anderson-Fabry disease. Evidence for a disease specific abnormality of the myocardial interstitium . Eur Heart J 2003. ; 24 ( 23 ): 2151 – 2155 . [DOI] [PubMed] [Google Scholar]
- 48. Nordin S , Kozor R , Baig S , et al . Cardiac phenotype of prehypertrophic Fabry disease . Circ Cardiovasc Imaging 2018. ; 11 ( 6 ): e007168 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Weidemann F , Niemann M , Breunig F , et al . Long-term effects of enzyme replacement therapy on fabry cardiomyopathy: evidence for a better outcome with early treatment . Circulation 2009. ; 119 ( 4 ): 524 – 529 . [DOI] [PubMed] [Google Scholar]
- 50. Banikazemi M , Bultas J , Waldek S , et al. ; Fabry Disease Clinical Trial Study Group . Agalsidase-beta therapy for advanced Fabry disease: a randomized trial . Ann Intern Med 2007. ; 146 ( 2 ): 77 – 86 . [DOI] [PubMed] [Google Scholar]
- 51. Weidemann F , Sanchez-Niño MD , Politei J , et al . Fibrosis: a key feature of Fabry disease with potential therapeutic implications . Orphanet J Rare Dis 2013. ; 8 ( 1 ): 116 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Thompson SG , Higgins JPT . How should meta-regression analyses be undertaken and interpreted? Stat Med 2002. ; 21 ( 11 ): 1559 – 1573 . [DOI] [PubMed] [Google Scholar]
- 53. Spinelli L , Giugliano G , Imbriaco M , et al . Left ventricular radial strain impairment precedes hypertrophy in Anderson-Fabry disease . Int J Cardiovasc Imaging 2020. ; 36 ( 8 ): 1465 – 1476 . [DOI] [PubMed] [Google Scholar]
- 54. Bernardini A , Camporeale A , Pieroni M , et al . Atrial dysfunction assessed by cardiac magnetic resonance as an early marker of Fabry cardiomyopathy . JACC Cardiovasc Imaging 2020. ; 13 ( 10 ): 2262 – 2264 . [DOI] [PubMed] [Google Scholar]
- 55. Lillo R , Graziani F , Panaioli E , et al . Right ventricular strain in Anderson-Fabry disease . Int J Cardiol 2021. ; 330 : 84 – 90 . [DOI] [PubMed] [Google Scholar]