Abstract
Introduction
Spinal muscular atrophy (SMA) is a rare, autosomal recessive, neuromuscular disease that leads to progressive muscular weakness and atrophy. Nusinersen, an antisense oligonucleotide, was approved for SMA in China in February 2019. We report interim results from a post-marketing surveillance phase 4 study, PANDA (NCT04419233), that collects data on the safety, efficacy, and pharmacokinetics of nusinersen in children with SMA in routine clinical practice in China.
Methods
Participants enrolled in PANDA will be observed for 2 years following nusinersen treatment initiation. The primary endpoint is the incidence of adverse events (AEs)/serious AEs (SAEs) during the treatment period. Efficacy assessments include World Health Organization (WHO) Motor Milestones assessment, the Hammersmith Infant Neurological Examination (HINE), and ventilation support. Plasma and cerebrospinal fluid (CSF) concentrations of nusinersen are measured at each dose visit.
Results
Fifty participants were enrolled as of the January 4, 2023, data cutoff: 10 with infantile-onset (≤ 6 months) and 40 with later-onset (> 6 months) SMA. All 50 participants have received at least one dose of nusinersen; 6 have completed the study. AEs were experienced by 45 (90%) participants and were mostly mild/moderate; no AEs led to nusinersen discontinuation or study withdrawal. Eleven participants experienced SAEs, most commonly pneumonia (n = 9); none were considered related to study treatment. Stability or gain of WHO motor milestone was observed and mean HINE-2 scores improved in both subgroups throughout the study. No serious respiratory events occurred, and no permanent ventilation support was initiated during the study. Pre-dose nusinersen CSF concentrations increased steadily through the loading-dose period, with no accumulation in plasma after multiple doses.
Conclusion
Nusinersen was generally well tolerated with an acceptable overall safety profile, consistent with the known safety of nusinersen. Efficacy, safety, and nusinersen exposure are consistent with prior observations. These results support continuing PANDA and evaluation of nusinersen in Chinese participants with SMA.
Trial Registration
ClinicalTrials.gov identifier, NCT04419233.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-024-02852-7.
Keywords: Efficacy, Nusinersen, Pharmacokinetics, Phase IV, Safety, Spinal muscular atrophy
Key Summary
| Nusinersen therapy has demonstrated clinically meaningful benefits with improved motor skills, respiratory function, survival, and other outcomes in infants, children, and adults with spinal muscular atrophy (SMA). |
| We report interim safety, efficacy, and pharmacokinetics results from PANDA (NCT04419233), a phase 4 post-marketing surveillance study of people with SMA treated with nusinersen in routine clinical practice in China. |
| Fifty participants were enrolled in PANDA as of January 4, 2023, all receiving at least one dose of nusinersen. |
| Adverse events (AEs, primary endpoint) were experienced by 45 (90%) participants and were mostly mild/moderate; no AEs led to nusinersen discontinuation or study withdrawal. |
| Nusinersen was generally well tolerated with an acceptable overall safety profile, consistent with the known safety of nusinersen; efficacy, safety, and nusinersen exposure are consistent with prior observations. |
Introduction
Spinal muscular atrophy (SMA) is an autosomal recessive neuromuscular disease characterized by the degeneration of motor neurons in the spinal cord, which leads to progressive muscular weakness and atrophy [1, 2]. The pathologic cause of SMA is disruption of the survival motor neuron 1 (SMN1) gene, which leads to insufficient levels of full-length survival motor neuron (SMN) protein [2, 3]. In mainland China, the incidence of SMA is reported as approximately 14 per 100,000 live births or 1338 births annually [4, 5]. Some clinical characteristics of people in China with SMA may potentially vary from those of other ethnic groups, and there is a need for additional studies on SMA's natural history and treatment in the Chinese population [6, 7].
Historically, the natural history of SMA includes four major recognized phenotypes that are dependent on age of onset and achieved motor abilities [1, 8–10]. An uncommon and unusually severe clinical phenotype, sometimes classified as type 0, has been recognized, with respiratory insufficiency present at birth and death typically occurring within weeks of birth [11]. Type I SMA has a disease onset within the first 6 months of life; these children are never able to sit or walk and usually die from respiratory failure by 2 years of age; people with Type II are able to sit but never walk unaided and may have respiratory involvement and reduced survival, with symptoms presenting between 6 and 18 months of age; people with Type III are able to sit and walk but may become severely and increasingly disabled; people with Type IV typically have disease onset after the age of 18 years and have normal life expectancies. 5q SMA includes Types I, II, III, and IV.
Nusinersen is an antisense oligonucleotide administered intrathecally by lumbar puncture (LP), which increases SMN protein expression in individuals with SMA [12, 13]. Nusinersen therapy has demonstrated clinically meaningful benefits with improved motor skills, respiratory function, survival, and other outcomes in infants, children, and adults [12–21]. Nusinersen therapy is well tolerated, and the adverse event (AE) profile is consistent with events typically seen in individuals with SMA or in the context of LP procedures [22]. Nusinersen is approved in the USA, throughout the European Union, and in other countries, including regulatory approval in China in February 2019 and in Hong Kong in September 2018. We report interim safety, efficacy, and pharmacokinetics (PK) results from a phase 4 post-marketing surveillance (PMS) study of people with SMA treated with nusinersen in routine clinical practice in China.
Methods
Study Design and Treatment
PANDA (NCT04419233) is a prospective, 2-year, multicenter PMS study conducted to collect safety, efficacy, and PK data from people with SMA treated with nusinersen in routine clinical practice in China. The study was designed based on a request by the National Medical Products Administration in China as a post-marketing requirement. All participants are to receive 12 mg nusinersen according to the approved prescribing information in China (5 ml doses per administration: four loading doses on Days 0, 14, 28, and 63, and maintenance doses administered once every 4 months thereafter). Participants are observed for 2 years following treatment initiation.
This study was performed in accordance with Title 21, United States Code of Federal Regulations (CFR) Parts 50, 54, 56, and 312 Subpart D; the International Council for Harmonisation Guideline on Good Clinical Practice (GCP) (E6); the European Union Clinical Trial Directive 2001/20/EC or Clinical Trial Regulation 536/2014; the ethical principles outlined in the Declaration of Helsinki; and/or, where applicable, European Directive 2001/20 regarding GCP in the conduct of clinical trials on medicinal products for human use and Directive 2005/28 on GCP for investigational medicinal products for human use. Ethics committee approval of all study documents was obtained prior to the start of the study at the following sites that enrolled participants: Ethics Committee (EC) of Peking University First Hospital, No. 8 Xishiku Street, Xicheng District, Beijing, China; Childrens Hospital of Fudan University, No. 399 Wanyuan Road, Minhang District, Shanghai, China; EC of Beijing Children’s Hospital, No. 56 Nanlishi Road, Xicheng District, Beijing, China; EC of Shenzhen Children’s Hospital, No. 7019, Yitian Road, Shenzhen City, Guangdong Province, China; EC of Guangzhou Woman and Children’s Medical Center, No. 9 Jinsui Road, Zhujiang New Town, Tianhe District, Guangzhou City, Guangdong Province, China; Medical Ethics Committee of Xiangya Hospital of Central South University, No. 87 Xiangya Road, Kaifu District, Changsha City, Hu’nan Province, China; EC of the First Affiliated Hospital of Fujian Medical University, No. 20 Chazhong Road, Fuzhou City, Fujian Province, China; EC of the First Hospital of Jilin University, No. 1 Xinmin Street, Changchun City, Jilin Province, China; Medical Ethics Committee of the Children’s Hospital of Zhejiang University School of Medicine, No. 3333 Binsheng Road, Binjiang District, Hangzhou City, Zhejiang Province, China; EC of Shengjing Hospital of China Medical University, No. 36 Sanhao Street, Heping District, Shengyang City, Liaoning Province, China; Clinical Trial Ethical Committee of West China Second Hospital, Sichuan University, No. 20 San Duan South Renmin Road, Chengdu City, Sichuan Province, China. Informed consent was obtained from all participants or their legally authorized representative in accordance with local practice and regulations.
Participants
The decision to enroll participants in PANDA, including documentation of 5q SMA diagnosis, was made by the investigator and agreed to by the participant or their parent/legally authorized representative. Participants’ demographic data and medical/SMA disease history (including number of copies of SMN2) were obtained at baseline. Additional information about deletion mutations was captured when the SMN2 copy number was non-zero. Key exclusion criteria included hypersensitivity to nusinersen, inability to comply with study requirements, current/past treatment with nusinersen, or other unspecified reasons that, in the opinion of the investigator or sponsor, make the participant unsuitable for enrollment. Participants were considered lost to follow-up if they repeatedly failed to return for routine study visits and were unable to be contacted after three attempts by site staff.
Study Outcomes and Measurements
The primary objective of PANDA was the evaluation of nusinersen's safety in the post-marketing setting in China. To analyze the incidence of AEs and serious AEs (SAEs) during the treatment period (the primary endpoint), AEs were recorded from the time of the first LP procedure (or, for SAEs, from the time following informed consent) until study completion or discontinuation and coded according to the Medical Dictionary for Regulatory Activities, version 25.1. Investigator responsibilities included reviewing all AEs to determine seriousness and fulfillment of collection criteria, monitoring and recording all SAEs and nonserious AEs, determining the onset, resolution, and relationship to treatment of each SAE and nonserious AE, and reporting SAEs and nonserious AEs to local ethics committees as required by law. Anesthesia/sedation may have been used for the LP procedure per each participating institution's guidelines.
Secondary objectives were to assess the efficacy and PK of nusinersen. Efficacy assessments included World Health Organization (WHO) Motor Milestones, status of ventilatory support (all participants), and the Hammersmith Infant Neurological Examination (HINE) [Sects. 1 and 3 in all participants aged ≤ 24 months; Sect. 2 (HINE-2) in all participants who did not achieve walking alone according to the WHO Motor Milestones Criteria assessment at the time of the first dosing]. HINE-2 motor milestone response was defined as maintenance or improvement of baseline status in at least one motor milestone and a greater number of categories maintained or improved than categories worsened. After a participant was able to walk alone at two consecutive study visits, only the WHO Motor Milestones assessment was performed. Efficacy assessments were performed by site investigators who were trained in the performance of these assessments at the site initiation visit. The assessments were conducted as part of routine medical practice. At each visit, ventilator use within the preceding 7 days was collected. To assess PK, plasma nusinersen levels were collected from all participants pre-dose and from participants who were > 6 weeks of age at 2, 4, 8, and 24 h post-dose on Day 1 and 4 h post-dose on Day 29. Cerebrospinal fluid (CSF) nusinersen samples were collected pre-dose at each dosing visit. CSF concentrations were measured using a validated hybridization electrochemiluminescence assay (hybridization ECL assay) with a lower limit of quantitation (LLOQ) of 0.500 ng/ml.
Statistical Analyses
Safety and efficacy analyses were carried out in the safety population, defined as all participants who received at least one dose of nusinersen as of the January 4, 2023, data cutoff. Those who had an evaluable PK sample following the first injection constituted the PK analysis population. Descriptive summary statistics were calculated for continuous variables; counts and percentage were used for categorical variables. AEs were analyzed based on treatment emergence; an AE that was present prior to receiving the first dose of nusinersen that subsequently worsened in severity or was not present but subsequently appeared was classified as treatment emergent. An AE was considered “related” to the use of nusinersen sodium injection if there was a possibility that the event might have been caused by it, including a positive rechallenge, a reasonable temporal sequence between administration of the product and the event, a known response pattern of the suspected product, improvement following discontinuation or dose reduction, a biologically plausible relationship between the product and the AE, or a lack of an alternative explanation for the AE. Endpoints are summarized by age of onset of SMA symptoms; participants are divided into two subgroups: onset of SMA symptoms at ≤ 6 months of age (infantile onset) and those with onset at > 6 months (later onset).
For analysis of WHO Motor Milestones, the percentage of participants who achieved each milestone is presented at baseline and at last visit, with the two subgroups (infantile and later-onset SMA) further stratified by age at enrollment: (1) infantile-onset SMA: age at enrollment ≤ 2 years and > 2 years; (2) later-onset SMA: age at enrollment ≤ 2 years, > 2 to ≤ 6 years, and > 6 years.
For the HINE, the status at baseline and at each subsequent visit is recorded for each milestone assessed using Sect. 2; the eight milestones are summed to give a total score, which is presented over time. HINE Sect. 2 (HINE-2) was assessed in all participants who did not achieve walking alone according to the WHO motor milestone assessment at the time of the first dosing. Once a participant achieved walking alone according to the WHO motor milestone at two consecutive study visits, HINE-2 would no longer be performed. Baseline scores for Sects. 1 and 3, and individual item scores, were measured over time for each participant; the response on each item over time was summarized. Ventilator use within the preceding 7 days of study visits was summarized. Summary statistics are provided for PK results.
Results
Participants
Fifty participants have enrolled across 11 study sites and received at least one dose of nusinersen, constituting the safety analysis set; 50 have an evaluable PK sample after the first injection (PK analysis set). Results presented here are for the interim safety analysis set unless specified otherwise.
At data cutoff, nine participants have had the opportunity to complete the study (interim analysis set); of these, six (67%) completed the study and three (33%) were withdrawn from the study by their parent/guardian but are still receiving nusinersen. As of the data cutoff date, 13 (26%) participants have discontinued study treatment and/or withdrawn from the study. Of the 13 participants who withdrew from the study, 11 withdrew to pursue treatment closer to home, one withdrew because of dissatisfaction with treatment effect, and one did not have additional details about withdrawal in the case report form. Among the 50 enrolled participants, those in the infantile-onset SMA group (n = 10) had a median (range) age of 2.6 (1–5) years and those in the later-onset SMA group (n = 40) had a median (range) age of 6.6 (1–21) years (Table 1). Mean (range) age at symptom onset was 0.32 (0.1–0.5) years for the infantile-onset group and 1.48 (0.6–10.0) years for the later-onset group. Thirty-two (64%) participants had three SMN2 copies, including seven of ten participants in the infantile-onset SMA group. Eight (16%) participants had missing SMN2 copy number data. All 50 participants have received at least four loading doses of nusinersen by the time of this data cutoff; the median (range) time on study is 14.4 (2.0–22.7) months.
Table 1.
Baseline demographics and disease history
| n (%)a | SMA onset ≤ 6 months (n = 10) |
SMA onset > 6 months (n = 40) |
Overall (N = 50) |
|---|---|---|---|
| Age, median (range), years | 2.6 (1–5) | 6.6 (1–21) | 4.6 (1–21) |
| Age at baseline categories, years | |||
| < 5 | 10 (100) | 16 (40.0) | 26 (52.0) |
| 5 to < 10 | 0 | 16 (40.0) | 16 (32.0) |
| 10 to < 15 | 0 | 5 (12.5) | 5 (10.0) |
| ≥ 15 | 0 | 3 (7.5) | 3 (6.0) |
| Sex | |||
| Male | 4 (40.0) | 18 (45.0) | 22 (44.0) |
| Female | 6 (60.0) | 22 (55.0) | 28 (56.0) |
| Race | |||
| Asian (Chinese) | 10 (100) | 40 (100) | 50 (100) |
| Height, cm | |||
| n | 9 | 37 | 46 |
| Mean (SD) | 87.9 (13.0) | 110.0 (23.8) | 105.7 (23.7) |
| Weight, kg | |||
| n | 10 | 39 | 49 |
| Mean (SD) | 11.6 (3.4) | 21.3 (10.9) | 19.3 (10.6) |
| Age at symptom onset, mean (range), years | 0.32 (0.1–0.5) | 1.48 (0.6–10.0) | 1.25 (0.1–10.0) |
| Time from symptom onset to first study dose, mean (range), years | 2.27 (0.2–4.3) | 5.37 (0.2–20.5) | 4.75 (0.2–20.5) |
| Age at SMA diagnosis, mean (range), years | 1.05 (0.1–4.0) | 3.11 (0.7–16.0) | 2.70 (0.1–16.0) |
| Time from SMA diagnosis to first study dose, mean (range), years | 1.54 (0.0–3.3) | 3.74 (0.0–18.1) | 3.30 (0.0–18.1) |
| SMN2 copy number | |||
| 0 | 0 | 0 | 0 |
| 1 | 0 | 0 | 0 |
| 2 | 2 (20.0) | 5 (12.5) | 7 (14.0) |
| 3 | 7 (70.0) | 25 (62.5) | 32 (64.0) |
| 4 | 0 | 2 (5.0) | 2 (4.0) |
| 5 | 0 | 1 (2.5) | 1 (2.0) |
| Unknown | 1 (10.0) | 7 (17.5) | 8 (16.0) |
SD standard deviation, SMA spinal muscular atrophy, SMN2 survival motor neuron 2
aUnless otherwise noted
Primary Endpoint
Nusinersen was generally well tolerated with an acceptable overall safety profile. AEs were experienced by 45 (90%) participants; these were of mild/moderate intensity in most (n = 40; 80%) participants, and none led to nusinersen discontinuation or study withdrawal (Table 2). The most common AEs were upper respiratory tract infections (26%), pneumonia (22%), and procedural pain (22%). Hematuria was reported in three (6%) participants (Table 3). All three cases were considered mild, and two were judged to be related to nusinersen. Including these two cases of hematuria, a total of eight (16%) participants experienced AEs considered by the investigator as related to treatment; all other related AEs (dizziness, headache, abdominal mass, hepatic function abnormal, post-LP syndrome, procedural vomiting, blood creatinine abnormal) were reported by only one participant each. Thirteen (26%) participants experienced LP-related AEs, none of which were SAEs. The most common LP-related AE was procedural pain, reported in 11 (22%) participants. Eleven participants experienced SAEs (defined as events that were life-threatening or requiring hospitalization, Table 2). The most common was pneumonia (in nine participants); none were considered related to nusinersen. No deaths were reported during the study. Changes and shifts from baseline in laboratory results were not considered clinically significant, and no significant trends were noted between the infantile- and later-onset SMA subgroups. There were also no significant trends reported in vital signs.
Table 2.
Overall summary of safety results
| n (%) | SMA onset ≤ 6 months (n = 10) |
SMA onset > 6 months (n = 40) |
Overall (N = 50) |
|---|---|---|---|
| Any event | 10 (100) | 35 (88) | 45 (90) |
| Severitya | |||
| Mild | 2 (20) | 22 (55) | 24 (48) |
| Moderate | 5 (50) | 11 (28) | 16 (32) |
| Severe | 3 (30) | 2 (5) | 5 (10) |
| Relatedb event | 2 (20) | 6 (15) | 8 (16) |
| Serious event | 7 (70) | 4 (10) | 11 (22) |
| Related serious event | 0 | 0 | 0 |
| Events leading to drug withdrawal | 0 | 0 | 0 |
| Events leading to study withdrawal | 0 | 0 | 0 |
| Fatal events | 0 | 0 | 0 |
SMA spinal muscular atrophy, AEs adverse events
aEach participant counted once at maximum severity
bRelated as assessed by the investigator. These related AEs by preferred terms were: for SMA onset ≤ 6 months: dizziness (n = 1), hematuria (n = 1); for SMA onset > 6 months: headache (n = 1), hematuria (n = 1), abdominal mass (n = 1), hepatic function abnormal (n = 1), post-lumbar puncture syndrome and procedural vomiting (n = 1), blood creatinine abnormal (n = 1)
Table 3.
Incidence of adverse events by preferred term
| SMA onset ≤ 6 months (n = 10) |
SMA onset > 6 months (n = 40) |
Overall (N = 50) |
|
|---|---|---|---|
| Participants with any adverse event, n (%) | 10 (100) | 35 (88) | 45 (90) |
| Upper respiratory tract infection | 2 (20) | 11 (28) | 13 (26) |
| Pneumonia | 6 (60) | 5 (13) | 11 (22) |
| Procedural pain | 3 (30) | 8 (20) | 11 (22) |
| Bronchitis | 1 (10) | 8 (20) | 9 (18) |
| Pyrexia | 0 | 6 (15) | 6 (12) |
| Urinary tract infection | 1 (10) | 4 (10) | 5 (10) |
| Nasopharyngitis | 0 | 4 (10) | 4 (8) |
| Anemia | 1 (10) | 2 (5) | 3 (6) |
| C-reactive protein increased | 1 (10) | 2 (5) | 3 (6) |
| COVID-19 | 0 | 3 (8) | 3 (6) |
| Gastroenteritis | 1 (10) | 2 (5) | 3 (6) |
| Hematuria | 1 (10) | 2 (5) | 3 (6) |
| Hypokalemia | 2 (20) | 1 (3) | 3 (6) |
| Diarrhea | 1 (10) | 1 (3) | 2 (4) |
| Headache | 1 (10) | 1 (3) | 2 (4) |
| Hyponatremia | 1 (10) | 1 (3) | 2 (4) |
| Iron deficiency | 0 | 2 (5) | 2 (4) |
| Neutrophil count decreased | 1 (10) | 1 (3) | 2 (4) |
| Post lumbar puncture syndrome | 0 | 2 (5) | 2 (4) |
| Vomiting | 0 | 2 (5) | 2 (4) |
| Weight decreased | 1 (10) | 1 (3) | 2 (4) |
Events shown are those occurring in at least two participants
SMA spinal muscular atrophy, COVID-19 coronavirus disease 2019
Secondary Endpoints
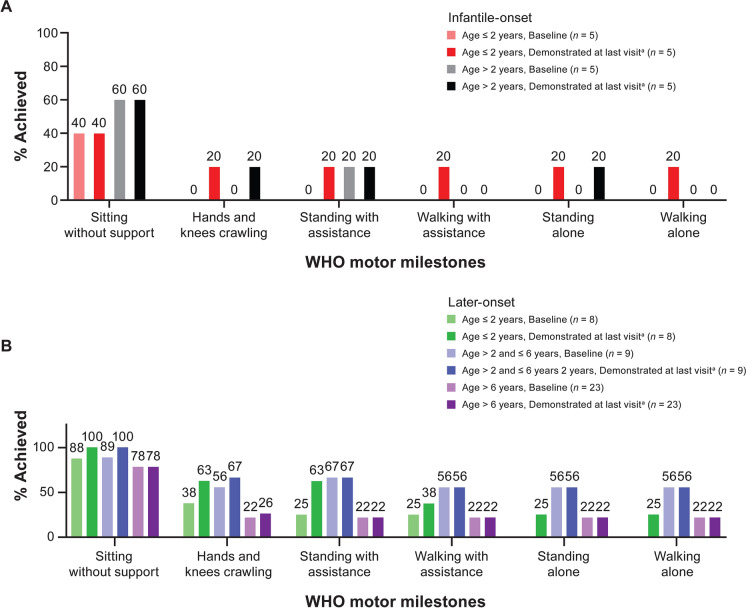
In the infantile-onset group at baseline, two of five participants aged ≤ 2 years and three of five participants aged > 2 years achieved “sitting without support,” and one of five aged > 2 years achieved “standing with assistance” (Fig. 1). Each of the six motor milestones had been achieved by at least one participant in the age ≤ 2 years subgroup. Three participants who were able to sit without support at baseline gained additional milestones that were demonstrated at all subsequent visits, including the last visit. One participant (aged ≤ 2 years subgroup) demonstrated “hands and knees crawling,” “standing with assistance,” “walking with assistance,” “standing alone,” and “walking alone;” one participant (aged > 2 years subgroup) demonstrated “hands and knees crawling;” one (aged > 2 years subgroup) demonstrated “standing alone.” All milestones that were achieved at baseline in the infantile-onset group were maintained as of each participant’s last observed visit.
Fig. 1.
WHO motor milestones at baseline and last visit for A infantile-onset and B later-onset participants. aIncludes motor milestones demonstrated at baseline and motor milestones gained during the study that were demonstrated at the last visit. Age categories are age at study baseline. All milestones that were achieved at baseline were maintained as of each participant’s last observed visit except for one participant in the later-onset group (aged > 6 years at enrollment) who demonstrated sitting without support at baseline and all subsequent visits except the last visit (Day 423). No other milestones have been demonstrated by this participant at baseline or any subsequent visits to date. All milestones that were gained throughout study were demonstrated at all subsequent visits with one exception. One participant in the later-onset group (aged ≤ 2 years at enrollment) demonstrated standing with assistance at Day 183 through Day 663 (last visit) with the exception of non-demonstration of this milestone at the Day 543 visit. SMA spinal muscular atrophy, WHO World Health Organization
In the later-onset group, each of the six motor milestones had been achieved by at least one participant (Fig. 1). Three participants (one in each age subgroup) gained the ability to “sit without support” during the study. Participants who demonstrated motor milestones at baseline also gained additional milestones during the study. In the youngest subgroup (aged ≤ 2 years), four individual participants gained one, two, two, and five milestones, respectively. Single participants in each of the older two subgroups (aged > 2 to ≤ 6 years, > 6 years) gained the motor milestone of “hands and knees crawling.” All milestones that were achieved at baseline in the later-onset group were maintained as of each participant’s last observed visit with the exception of one participant in the oldest subgroup (aged > 6 years at enrollment) who demonstrated “sitting without support” at baseline and all subsequent visits except the last visit (Day 423). No other milestones were demonstrated by this participant at baseline or at any subsequent visits to date. All milestones that were gained throughout study by the later-onset group were demonstrated at all subsequent visits except for one participant (aged ≤ 2 years at enrollment) who demonstrated “standing with assistance” at Day 183 through Day 663 (last visit), with the exception of non-demonstration of this milestone at the Day 543 visit.
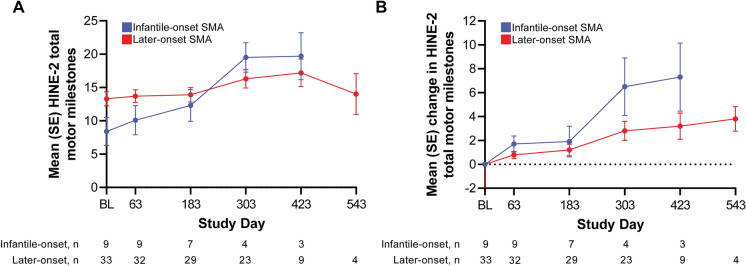
In participants with infantile- and later-onset SMA, gradual improvement was seen in HINE-2 total motor milestone scores over time (Fig. 2). This improvement was also consistent in participants with data through end of study (2 years) (Supplementary Material Fig. S2). Four participants had no evaluable baseline HINE-2 but had evaluable HINE-2 scores post-baseline; these four were not included in the calculations for Fig. 1. Baseline mean HINE-2 scores were 8.4 and 13.3 for the infantile-onset and later-onset groups, respectively. The corresponding mean change from baseline in HINE-2 scores was 7.3 and 3.2 for the infantile-onset and later-onset groups at Day 423.
Fig. 2.
Gradual improvement was observed in mean (SE) HINE Sect. 2 total motor milestone scores, A total motor milestone score, and B change from baseline over time. Data points where participant counts were fewer than three in either group were not included. The decrease in the mean total score on Day 543 was due to the low number of participants who completed that visit (n = 4); Day 543 scores for these four participants were stable or improved from the preceding visit. BL baseline, HINE Hammersmith Infant Neurological Examination, SE standard error, SMA spinal muscular atrophy
The proportion of HINE-2 responders was seven of nine (77.8%) evaluable participants in the infantile-onset SMA subgroup and 30 of 32 (93.8%) evaluable participants in the later-onset SMA subgroup. HINE Sects. 1 and 3 were only assessed in 13 participants at baseline who were aged ≤ 24 months; means in most individual scores have shown stability or improvement. In the infantile-onset SMA subgroup, mean scores for ‘posture: arms’ and ‘behavior: emotional state’ initially decreased but improved at later timepoints (although only one participant was evaluated after Day 183).
For the secondary endpoint of ventilatory support status collected within the preceding 7 days of a study visit, one participant required ventilation 24 h/day at the two study visits (baseline and Day 63) prior to study withdrawal. This participant had tracheostomy placement 4.5 months before the first study dose of nusinersen, was 1 month old at symptom onset and 21 months old at first dose of nusinersen, and had two SMN2 copies. Another participant reported BiPAP (bilevel positive airway pressure) use of 8 h/day at one visit (Day 183); medical history was unremarkable. No other participants reported any ventilator use in the 7 days preceding their study visits. No participants were placed on permanent ventilation or underwent tracheostomy during the study. One participant with a reported history of tracheostomy was noted to have received concomitant medications through a nasogastric tube. There was no other reported evidence of gastrostomy or gastric feeding tubes in medical history, AE listings, or concomitant non-drug treatments or procedures.
Following first dose treatment, there was a steady increase in average pre-dose nusinersen concentrations in CSF through the loading dose period and into the maintenance dose period, approaching steady state toward the 2-year end of the study (Fig. 3). Individual profiles demonstrate that intra- and inter-participant variability of time-dependent CSF trough concentrations (Supplementary Material Fig. S3) is consistent with prior findings [23]. Among the participants whose plasma samples were taken at 2, 4, 8, and 24 h post-dose on Day 1 (n = 23), the geometric means for maximum plasma concentration, time to maximum concentration, and area under the concentration-time curve to 24 h were 196.7 ng/ml, 4.5 h, and 2531.8 h·ng/ml, respectively. There was no accumulation in plasma concentration of nusinersen after multiple doses. Figure 3 shows CSF values from Day 14 onward; some CSF concentrations were non-zero at Day 0 (pre-dose) due to artifacts in measurement. Overall median (5–95th percentiles) Day 14 (pre-dose) and Day 663 (pre-dose) CSF nusinersen levels were 3.9 (0.75–8.7) and 7.63 (4.08–10.2) ng/ml, respectively.
Fig. 3.
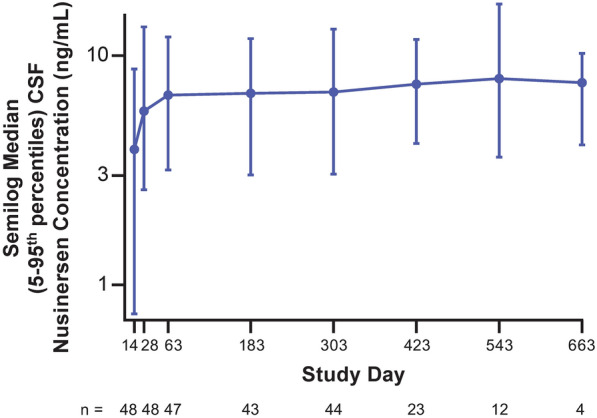
Median (5–95th percentiles) CSF nusinersen concentrations for overall participants (n = 50). CSF cerebrospinal fluid
Discussion
This interim analysis demonstrated that the safety of the approved dosing regimen of nusinersen in people with SMA in China was similar to the established safety profile of nusinersen, with no new trends or AEs identified. No deaths were reported, and no participants experienced AEs that led to discontinuation of study treatment or withdrawal from the study. Most AEs that were considered related to the study treatment or LP procedure were mild or moderate in severity and consistent with the established safety profile of nusinersen [12, 14, 16, 17, 22]. There are relatively few studies [6, 7] in people with SMA in China. PANDA adds to this limited body of evidence. The data are consistent with studies showing efficacy and overall safety of nusinersen in children with type I and II SMA [24, 25] and in improving health-related quality of life [26].
Efficacy outcomes are consistent with previous clinical experience with nusinersen [14, 15, 17, 19, 27]. The WHO Motor Milestones achievement showed stability or improvement in all milestones in both the infantile- and later-onset groups. Mean HINE-2 scores showed improvement in total motor milestone scores, and the majority in both groups were responders at last visit. No participants initiated permanent ventilation or had tracheostomies during the study. However, given the relatively heterogeneous population and limited follow-up in a low number of participants, the interpretation of the efficacy data is relatively limited. Other limitations include lack of a comparator group as an interim analysis of an open-label study, potential practice differences between sites, and broader inclusion criteria compared with other nusinersen studies. However, the heterogeneity of participants in this real-world clinical setting may more closely represent the overall population with SMA.
The steady increase in nusinersen CSF concentrations for all participants throughout the loading dose period and into the maintenance dose period and the finding that trough CSF levels approached a steady state within 24 months follow the pattern seen in population PK modeling [23]. The observed pre-dose CSF concentrations over these last three maintenance doses are within the 5–95% interval predicted using the population PK model for the standard maintenance dose regimen (2.4–11.2 ng/ml, respectively) [23].
Conclusions
The interim analysis of the PANDA data is consistent with previous clinical study experience and supports continuing PANDA and evaluation of nusinersen in people with SMA in China.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the study participants and Prof Feng Gao for his contribution to the study.
Medical Writing/Editorial Assistance.
Biogen provided funding for medical writing support in the development of this paper. Yien Liu, PhD (Excel Scientific Communications, Fairfield, CT, USA), wrote the first draft of the manuscript based on input from authors, and Linda Cirella (Excel Scientific Solutions, Fairfield, CT, USA) copyedited and styled the manuscript per journal requirements.
Author Contributions
Biogen reviewed and provided feedback on the paper. The authors had full editorial control of the paper and provided their final approval of all content. Study concept/design: Michael Monine, Corinne Makepeace, Xin Jin, Richard Foster, Russell Chin, Zdenek Burger. Major role in acquisition of data: Yuwu Jiang, Yi Wang, Hui Xiong, Wenhui Li, Rong Luo, Wenxiong Chen, Fei Yin, Junlan Lü, Jianmin Liang, Wan-Jin Chen, Xinguo Lu, Hua Wang, Jihong Tang. Analysis/interpretation of data: all authors. Drafting/revision of the manuscript for content: all authors.
Funding
Biogen (Cambridge, MA, USA) sponsored this study and was responsible for funding the journal’s Rapid Service and Open Access fees.
Data Availability
Requests for the materials and data supporting this manuscript should be submitted to https://vivli.org/.
Declarations
Conflict of Interest
Yuwu Jiang, Yi Wang, Hui Xiong, Wenhui Li, Rong Luo, Wenxiong Chen, Fei Yin, Junlan Lü, Jianmin Liang, Wan-Jin Chen, Xinguo Lu, Hua Wang, and Jihong Tang: nothing to disclose. Michael Monine, Corinne Makepeace, Richard Foster, Zdenek Berger: employees of Biogen and may hold stock in the company. Xin Jin: employee of Cytel at the time of development. Russell Chin: employee of Biogen at the time of development.
Ethical Approval
This study was performed in accordance with Title 21, United States Code of Federal Regulations (CFR) Parts 50, 54, 56 and 312 Subpart D; the International Council for Harmonisation Guideline on GCP (E6); the European Union Clinical Trial Directive 2001/20/EC or Clinical Trial Regulation 536/2014; and the ethical principles outlined in the Declaration of Helsinki; and/or, where applicable, the European Directive 2001/20 in relation to GCP in the conduct of clinical trials on medicinal products for human use and Directive 2005/28 on GCP for investigational medicinal products for human use. Ethics committee approval of all study documents was obtained prior to the start of the study at the following sites that enrolled participants: EC of Peking University First Hospital, No. 8 Xishiku Street, Xicheng District, Beijing, China; Childrens Hospital of Fudan University, No. 399 Wanyuan Road, Minhang District, Shanghai, China; EC of Beijing Children's Hospital, No. 56 Nanlishi Road, Xicheng District, Beijing, China; EC of Shenzhen Children’s Hospital, No. 7019, Yitian Road, Shenzhen City, Guangdong Province, China; EC of Guangzhou Woman and Children's Medical Center, No. 9 Jinsui Road, Zhujiang New Town, Tianhe District, Guangzhou City, Guangdong Province, China; Medical Ethics Committee of Xiangya Hospital of Central South University, No. 87 Xiangya Road, Kaifu District, Changsha City, Hu'nan Province, China; EC of the First Affiliated Hospital of Fujian Medical University, No. 20 Chazhong Road, Fuzhou City, Fujian Province, China; EC of the First Hospital of Jilin University, No. 1 Xinmin Street, Changchun City, Jilin Province, China; Medical Ethics Committee of the Children's Hospital of Zhejiang University School of Medicine, No. 3333 Binsheng Road, Binjiang District, Hangzhou City, Zhejiang Province, China; EC of Shengjing Hospital of China Medical University, No. 36 Sanhao Street, Heping District, Shengyang City, Liaoning Province, China; Clinical Trial Ethical Committee of West China Second Hospital, Sichuan University, No. 20 San Duan South Renmin Road, Chengdu City, Sichuan Province, China. Informed consent was obtained from all participants or their legally authorized representative in accordance with local practice and regulations.
References
- 1.Darras BT, Monani UR, De Vivo DC. Genetic disorders affecting the motor neuron: spinal muscular atrophy. Swaiman’s Pediatric Neurology. 6. Edinburgh: Elsevier; 2017. [Google Scholar]
- 2.Lunn MR, Wang CH. Spinal muscular atrophy. Lancet. 2008;371:2120–2133. doi: 10.1016/s0140-6736(08)60921-6. [DOI] [PubMed] [Google Scholar]
- 3.Lefebvre S, Bürglen L, Reboullet S, et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. 1995;80:155–165. doi: 10.1016/0092-8674(95)90460-3. [DOI] [PubMed] [Google Scholar]
- 4.Sheng-Yuan Z, Xiong F, Chen YJ, et al. Molecular characterization of SMN copy number derived from carrier screening and from core families with SMA in a Chinese population. Eur J Hum Genet. 2010;18:978–984. doi: 10.1038/ejhg.2010.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Bureau of Statistics in China. National Economy Withstood Pressure and Reached a New Level in 2022. 2023 (updated January 17, 2023). Available from: http://www.stats.gov.cn/english/PressRelease/202301/t20230117_1892094.html (accessed Sep 25, 2023).
- 6.Ge X, Bai J, Lu Y, Qu Y, Song F. The natural history of infant spinal muscular atrophy in China: a study of 237 patients. J Child Neurol. 2012;27:471–477. doi: 10.1177/0883073811420152. [DOI] [PubMed] [Google Scholar]
- 7.Yang YY, Yuan P, Li M, Jiang L, Hong SQ. Natural history of spinal muscular atrophy in children: an analysis of 117 cases. Zhongguo Dang Dai Er Ke Za Zhi. 2021;23:1038–1043. doi: 10.7499/j.issn.1008-8830.2106025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finkel R, Bertini E, Muntoni F, Mercuri E. 209th ENMC International Workshop: outcome measures and clinical trial readiness in spinal muscular atrophy 7–9 November 2014, Heemskerk, The Netherlands. Neuromuscul Disord. 2015;25:593–602. doi: 10.1016/j.nmd.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Aponte Ribero V, Martí Y, Batson S, et al. Systematic literature review of the natural history of spinal muscular atrophy: motor function, scoliosis, and contractures. Neurology. 2023;101:e2103–e2113. doi: 10.1212/wnl.0000000000207878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wijngaarde CA, Stam M, Otto LAM, et al. Population-based analysis of survival in spinal muscular atrophy. Neurology. 2020;94:e1634–e1644. doi: 10.1212/wnl.0000000000009248. [DOI] [PubMed] [Google Scholar]
- 11.Arnold WD, Kassar D, Kissel JT. Spinal muscular atrophy: diagnosis and management in a new therapeutic era. Muscle Nerve. 2015;51:157–167. doi: 10.1002/mus.24497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finkel RS, Chiriboga CA, Vajsar J, et al. Treatment of infantile-onset spinal muscular atrophy with nusinersen: a phase 2, open-label, dose-escalation study. Lancet. 2016;388:3017–3026. doi: 10.1016/s0140-6736(16)31408-8. [DOI] [PubMed] [Google Scholar]
- 13.Chiriboga CA, Swoboda KJ, Darras BT, et al. Results from a phase 1 study of nusinersen (ISIS-SMN(Rx)) in children with spinal muscular atrophy. Neurology. 2016;86:890–897. doi: 10.1212/wnl.0000000000002445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Acsadi G, Crawford TO, Müller-Felber W, et al. Safety and efficacy of nusinersen in spinal muscular atrophy: the EMBRACE study. Muscle Nerve. 2021;63:668–677. doi: 10.1002/mus.27187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Darras BT, Chiriboga CA, Iannaccone ST, et al. Nusinersen in later-onset spinal muscular atrophy: long-term results from the phase 1/2 studies. Neurology. 2019;92:e2492–e2506. doi: 10.1212/wnl.0000000000007527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finkel RS, Chiriboga CA, Vajsar J, et al. Treatment of infantile-onset spinal muscular atrophy with nusinersen: final report of a phase 2, open-label, multicentre, dose-escalation study. Lancet Child Adolesc Health. 2021;5:491–500. doi: 10.1016/S2352-4642(21)00100-0. [DOI] [PubMed] [Google Scholar]
- 17.Finkel RS, Mercuri E, Darras BT, et al. Nusinersen versus sham control in infantile-onset spinal muscular atrophy. N Engl J Med. 2017;377:1723–1732. doi: 10.1056/NEJMoa1702752. [DOI] [PubMed] [Google Scholar]
- 18.Hagenacker T, Wurster CD, Gunther R, et al. Nusinersen in adults with 5q spinal muscular atrophy: a non-interventional, multicentre, observational cohort study. Lancet Neurol. 2020;19:317–325. doi: 10.1016/S1474-4422(20)30037-5. [DOI] [PubMed] [Google Scholar]
- 19.Maggi L, Bello L, Bonanno S, et al. Nusinersen safety and effects on motor function in adult spinal muscular atrophy type 2 and 3. J Neurol Neurosurg Psychiatry. 2020;91:1166–1174. doi: 10.1136/jnnp-2020-323822. [DOI] [PubMed] [Google Scholar]
- 20.Mercuri E, Darras BT, Chiriboga CA, et al. Nusinersen versus sham control in later-onset spinal muscular atrophy. N Engl J Med. 2018;378:625–635. doi: 10.1056/NEJMoa1710504. [DOI] [PubMed] [Google Scholar]
- 21.Walter MC, Wenninger S, Thiele S, et al. Safety and treatment effects of nusinersen in longstanding adult 5q-SMA type 3—a prospective observational study. J Neuromuscul Dis. 2019;6:453–465. doi: 10.3233/JND-190416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Darras BT, Farrar MA, Mercuri E, et al. An integrated safety analysis of infants and children with symptomatic spinal muscular atrophy (SMA) treated with nusinersen in seven clinical trials. CNS Drugs. 2019;33:919–932. doi: 10.1007/s40263-019-00656-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacCannell D, Berger Z, East L, et al. Population pharmacokinetics-based recommendations for a single delayed or missed dose of nusinersen. Neuromuscul Disord. 2021;31:310–318. doi: 10.1016/j.nmd.2021.02.014. [DOI] [PubMed] [Google Scholar]
- 24.Chen L, Liu F, Fang D, Li J. Study on the efficacy, safety, and biomarkers of nusinersen in type II and III spinal muscular atrophy in children. Front Pediatr. 2023;11:1294405. doi: 10.3389/fped.2023.1294405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhuang W, Lu M, Wu Y, et al. Safety concerns with nusinersen, risdiplam, and onasemnogene abeparvovec in spinal muscular atrophy: a real-world pharmacovigilance study. Clin Drug Investig. 2023;43:949–962. doi: 10.1007/s40261-023-01320-4. [DOI] [PubMed] [Google Scholar]
- 26.Duan C, Ai D, Xu Q, Sui B, Zhao K. Assessment of health-related quality of life in patients with spina muscular atrophy in China. Intractable Rare Dis Res. 2022;11:189–195. doi: 10.5582/irdr.2022.01094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tulinius M, Castro D, Finkel RS, et al. Long-term safety and efficacy of nusinersen in infantile-onset spinal muscular atrophy: 5-year interim from SHINE. SMA-Europe - 3rd International Scientific Congress on Spinal Muscular Atrophy; Barcelona, Spain; 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Requests for the materials and data supporting this manuscript should be submitted to https://vivli.org/.