Abstract
Clostridium perfringens is an important opportunistic microorganism in commercial poultry production that is implicated in necrotic enteritis (NE) outbreaks. This disease poses a severe financial burden on the global poultry industry, causing estimated annual losses of $6 billion globally. The ban on in-feed antibiotic growth promoters has spurred investigations into approaches of alternatives to antibiotics, among which Bacillus probiotics have demonstrated varying degrees of effectiveness against NE. However, the precise mechanisms underlying Bacillus-mediated beneficial effects on host responses in NE remain to be further elucidated. In this manuscript, we conducted in vitro and genomic mining analysis to investigate anti-C. perfringens activity observed in the supernatants derived from 2 Bacillus amyloliquefaciens strains (FS1092 and BaD747). Both strains demonstrated potent anti-C. perfringens activities in in vitro studies. An analysis of genomes from 15 B. amyloliquefaciens, 11 B. velezensis, and 2 B. subtilis strains has revealed an intriguing clustering pattern among strains known to possess anti-C. perfringens activities. Furthermore, our investigation has identified 7 potential antimicrobial compounds, predicted as secondary metabolites through antiSMASH genomic mining within the published genomes of B. amyloliquefaciens species. Based on in vitro analysis, BaD747 may have the potential as a probiotic in the control of NE. These findings not only enhance our understanding of B. amyloliquefaciens's action against C. perfringens but also provide a scientific rationale for the development of novel antimicrobial therapeutic agents against NE.
Key words: Bacillus amyloliquefaciens, Clostridium perfringens, inhibition, antimicrobial compound
INTRODUCTION
Necrotic enteritis (NE) is a debilitating disease in broiler chickens, manifesting in both clinical (high mortality) and subclinical (poor performance in growth and higher feed conversion) forms (Lee and Lillehoj 2016, Tsiouris 2016). Clostridium perfringens is an important opportunistic pathogen implicated in NE outbreaks in commercial poultry production (Lee and Lillehoj 2021). NE ranks as one of the most economically devastating bacterial enteric ailments, causing annual losses exceeding 6 billion US dollars for the global poultry industry (Wade and Keyburn 2015). NE is a multifactorial disease, necessitating several predisposing and co-factors for an outbreak, including co-infection with coccidiosis, dietary factors (the use of fishmeal and cereal-based diets may lead to increased digesta viscosity and intestinal mucus), immunosuppression, and poor management practices such as high stocking density, ammonia exposure, and heat stress (Prescott et al. 2016). Additionally, the presence of the critical NE B-like toxin (netB) gene in C. perfringens Type G strains is a key contributing factor (Timbermontet et al. 2009, Prescott et al. 2016). The rise in NE incidence is closely linked to the voluntary reduction or removal of antibiotic growth promoters from feed (Cooper and Songer 2009). Therefore, exploring alternatives to antibiotics is paramount to reducing the growing NE problem.
Extensive efforts have been directed toward developing antibiotic alternatives to safeguard poultry health and performance (Gadde et al. 2017a). These alternatives include probiotics, prebiotics, synbiotics, organic acids, enzymes, antimicrobial peptides, hyperimmune egg antibodies, bacteriophages, clay, and metals. Among them, Bacillus-based direct-fed microbials have gained prominence in maintaining or restoring the intestinal health of poultry with increasing regulation since the ban on antimicrobial growth promoters. This is mainly owing to their capability to confer gut health benefits and survive the rigorous conditions during chicken feed preparation (Gadde et al. 2017b, Grant et al. 2018, Khalique et al. 2020). These probiotics exert their effects by outcompeting pathogenic bacteria for nutrients, producing natural antimicrobial peptide compounds, and modulating the gut microbiota to promote beneficial microorganisms in the gastrointestinal tract, thus contributing to various immunological benefits (Neveling and Dicks 2021).
Bacillus amyloliquefaciens (Ba), a gram-positive bacterium found in soil and formerly categorized as B. subtilis subvariant, is commonly employed as a biocontrol agent for enhancing plant growth and controlling plant diseases (Zhang et al. 2022a). In recent years, it has garnered much attention as a potential probiotic in food animal agriculture, including chickens (Latorre et al. 2015, de Oliveira et al. 2019, Shini et al. 2020, Zhang et al. 2022b). In chickens, the gut microbiome plays a pivotal role in overall health and productivity, in which an imbalance may lead to various health issues, including digestive problems, reduced nutrient absorption, and heightened susceptibility to diseases. B. amyloliquefaciens has been demonstrated to effectively ameliorate subclinical NE, thereby enhancing gut health by increasing gut microbiota diversity and reducing the abundance of harmful bacteria (Zhang et al. 2022a). Additionally, it has been shown to improve nutrient absorption, leading to enhanced feed efficiency and overall performance (Latorre et al. 2015, de Oliveira et al. 2019, Shini et al. 2020).
However, the precise mechanisms by which B. amyloliquefaciens promotes performance remain to be further defined. This study aims to predict antimicrobial secondary metabolites or their corresponding peptides derived from B. amyloliquefaciens strains by comprehensive genomic analysis and test their in vitro activities of antimicrobial compounds that can inhibit the growth of pathogenic C. perfringens.
MATERIALS AND METHODS
Bacillus Bacteria and Cultures
B. amyloliquefaciens strain FS1092 and D747 (EMFSL lab, ARS, USDA, Beltsville, MD, USA), along with Bacillus subtilis 168 (ATCC, Manassas, VA), were cultivated in Tryptic Soy Broth media (TSB, Sigma-Aldrich, St. Louis, MO) at 28°C. C. perfringens strains Del1 and LLY_TpeL17 were initially isolated from NE-afflicted chicken farms (Li et al. 2017, Gu et al. 2019). The Del1 and LLY_TpeL17 strain stocks were cultured in Tryptose Sulfite Cycloserine medium (TSC, Perfringens Agar Base, Oxoid, Nepean, Ontario, Canada) with C. perfringens selective supplement (D-cycloserine 0.4 mg/mL, Oxoid). Subsequently, the C. perfringens strains were anaerobically grown at 37°C in chopped meat glucose (CMG) medium (Anaerobe Systems, Morgan Hill, CA), followed by BYC medium [(3.7% brain heart infusion medium (BD Bacto, Sparks, MD), 0.5% yeast extract (Fisher Scientific, Hampton, NH), 0.05% L-cysteine (Sigma-Aldrich, St. Louis, MO)]. The complete genome sequences for strain FS1092 are available from a previous study (Gonzalez-Escalona et al., 2020), but the genome sequence for strain Ba D747 is unpublished.
Inhibition of C. perfringens Growth by B. amyloliquefaciens Cell-Free Supernatant in Liquid Culture and Well Diffusion Assay
The cell-free supernatants from B. amyloliquefaciens and B. subtilis cultures were acquired by centrifugation for 5 min at 6,000 x g after culturing 24, 48, and 72 h, followed by filtration through a 0.2-micron pore-size filter (Millipore, St. Louis, MO). The supernatants were stored at 4°C until use.
To determine if the cell-free supernatant from the above bacteria could inhibit the C. perfringens growth, the overnight culture from C. perfringens Del1 and LLY-TpeL17 strains were diluted in BYC broth, and the cell-free culture supernatant from Ba D747 or TSB broth was added into freshly diluted bacterial culture in 1:10 ratio, and cultivated overnight anaerobically. The optical density at 590 nm (OD590) values was recorded for the bacteria growth, and inhibition capability by Ba D747 was determined by comparison with the TSB control.
To assess the antimicrobial activity of B. amyloliquefaciens cell-free supernatant against C. perfringens strains and optimal collection times of cell-free supernatant, the agar well diffusion method was employed. TSC broth with 1.5% agar (20 mL) was poured into a sterile round petri dish (90 mm diameter, Biologix Inc, Lenexa, Kansas, MO). After agar solidification, 10 mL of autoclaved TSC agar mixed with 50 µL overnight BYC cultures of C. perfringens Del1 and LLY-TpeL17 was poured on the previous agar layer. Wells were created using pipettor tips of 6-mm diameter, and each well was loaded with 100 µL of cell-free supernatant from each B. amyloliquefaciens isolates Ba D747, FS1092 or Bs 168, or supernatant collected at 3 different time points. The plates were refrigerated at 4°C for 3 to 4 h to allow the supernatant to be absorbed by the agar. Each test was performed in triplicates. Subsequently, the plates were anaerobically incubated at 37°C overnight. Clear inhibition zones devoid of C. perfringens growth around the sample-dropping wells were observed against the background of full bacterial growth on TSC agar plates, and the diameters of these zones were measured. Once the optimal collection time of supernatant was determined, such supernatant would be used for further inhibition testing from different bacterial sources.
Computational Genomics and Gene Analysis of B. amyloliquefaciens, Bacillus velezensis, and B. subtilis strains
Detailed genome identification and sequence analysis information for B. amyloliquefaciens, related B. velezensis, and B. subtilis strains is shown in Table 1. Genomic data for the study were retrieved from GenBank. A phylogenetic tree was constructed based on the similarities and differences in DNA, projected RNA, or protein sequences among these organisms. This tree serves as a visual representation of the evolutionary relationships among different species or taxonomic groups (Pearson et al. 2009). To examine the patterns of gene presence and absence across the 14 B. amyloliquefaciens strains, we employed the Roary tool, known for its efficiency in constructing comprehensive pan genomes from prokaryotic samples and delineating both core and accessory gene sets (Page et al. 2015, Costa et al. 2020). In this procedure, the genomes of each of the 28 strains listed in Table 1 underwent initial annotation using the Prokka procedure (Seemann 2014). Subsequently, Roary was employed to generate a pan-genome from the gff files produced by Prokka. To establish a core gene alignment, MAFFT with specific options was utilized (Katoh and Standley 2013). Ultimately, the “gene_presence_absence.Rtab” and “accessory_ binary_ genes. fa. newick” output files served as the basis for assessing presence-absence patterns and conducting phylogenetic tree analyses across all strains. Prediction of the secondary/specialized metabolite biosynthetic gene clusters (SM BGCs) was performed using the program “antibiotics and secondary metabolite analysis shell—antiSMASH” in microbial genome mining tasks (Blin et al., 2023).
Table 1.
Genomes of B. amyloliquefaciens and closely related species strains used for gene mining in this study.
| Bacterial species | Strain | Accession# |
|---|---|---|
| Bacillus amyloliquefaciens | B15 | CP130445.1 |
| B25 | CP065159.1 | |
| BA11 | CP071042.1 | |
| BA40 | CP018152.1 | |
| C6.7 | LN999829.1 | |
| CAU B946 | CP079834.1 | |
| D747 | Unpublished | |
| EA19 | HE617159.1 | |
| ELA1901024 | CP075547.1 | |
| FS1092 | JALMGL010000001.1 | |
| GXU-1 | JALMGM010000001.1 | |
| H57 | LMUC01000001-LMUC01000016 | |
| LM2303 | CP038028 | |
| SN16-1 | CP021505.1 | |
| SRCM101367 | CP014783.1 | |
| Bacillus velezensis | 19573-3 | CP067043.1 |
| BV5 | ASM2453958v1 | |
| BZR 277 | CP064845 | |
| BZR 86 | CP064846 | |
| DTU001 | CP035533.1 | |
| Hx05 | CP040672.1 | |
| LF01 | CP058216 | |
| M75 | CP016395.1 | |
| SRCM101368 | CP031694 | |
| WRN014 | CP041361.1 | |
| ZL918 | CP021338.1 | |
| Bacillus subtilis | CP009684.1 | 168 |
| AL009126.3 | B-1 |
Statistical Analysis
The OD590 values of bacterial cultures or the diameters of the inhibition zones were subjected to analysis using the GLM procedure of SAS v9.4 for Windows (Cary, NC). Statistically significant differences were defined at p ≤ 0.05, and all data were presented as mean ± standard deviation for each treatment.
RESULTS
Inhibition of C. perfringens Growth
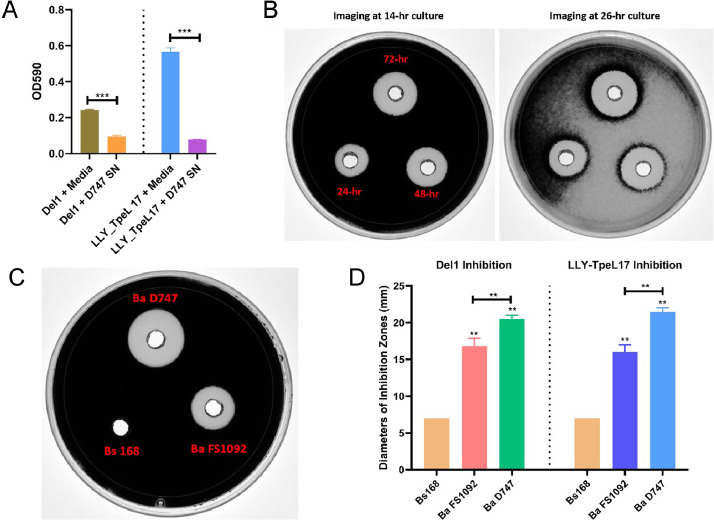
This study aimed to investigate whether these B. amyloliquefaciens PS1092 and D747 strains could also suppress the growth of the very pathogenic C. perfringens strains, isolated from the NE-afflicted chicken gut. When B. amyloliquefaciens D747 supernatant was added to the diluted overnight C. perfringens culture at a 1:10 dilution, it exhibited robust inhibition activity against C. perfringens LLY_TpeL17 strain, with highly significant differences (P ≤ 0.001) (Figure 1A).
Figure 1.
The growth inhibition of Clostridium perfringens by cell-free supernatant from Bacillus amyloliquefaciens D747 in liquid culture (1A), collected at 3 times of points by Bacillus amyloliquefaciens strain D747 (1B) or by culture supernatant from various sources (1C, 1D) in agar well diffusion assay. In 1A, the cell-free supernatants from Ba D747 24-h culture were added into C. perfringens cultures of Del1 and LLY_TpeL17 (TpeL17) strains, and OD590 was measured after the overnight culturing anaerobically. In 1b, the C. perfringens LLY_TpeL17 strain was seeded in the TSC agar plate, and the cell-free supernatant collected from 3-time points of cultures from B. amyloliquefaciens D747 was then loaded on the plate well, and images were taken after 14-h and 26-h culture. In 1C, 3 culture supernatant samples from B. subtilis 168 strain, B. amyloliquefaciens D747, and FS1092 strains were loaded on the wells of C. perfringens LLY_TpeL17-seed TSC agar plate. The inhibition zone diameters were measured and analyzed for both C. perfringens Del1 and LLY_TpeL17 strains (1D). The horizon line represents the base level of the empty well diameter. All the data were expressed as mean ± standard deviation for each treatment. The symbols of ** and *** represent statistical differences at P ≤ 0.01, and P ≤ 0.001, respectively.
Next, we determined the optimal time for collecting supernatants from bacterial cultures. Figure 1B illustrates the inhibition zones of Ba D747 cell-free supernatant on the growth of the C. perfringens LLY_TpeL17 strain. The diameters of the clear inhibition zones were measured for the supernatants collected from 24, 48, and 72-h cultures, resulting in measurements of 14.5, 18.5, and 19.5 mm, respectively. Notably, the diameter of the inhibition zone for the supernatant collected at the 48-h (18.5 mm) nearly approached that of the 72-h collection (19.5 mm), but was significantly larger than the 24-h collection (14.5 mm). Consequently, the 48-h culture supernatant was selected for our subsequent inhibition studies.
Interestingly, C. perfringens colonies initially appeared black on TSC agar with selective supplement (observed at 14-h culture), but their color gradually faded during extended incubation periods (observed at 26-h culture, as shown in Figure 1B).
Figure 1C demonstrates the inhibition zones produced by supernatants from 3 different bacterial cultures on the growth of C. perfringens. Ba D747 exhibited superior anti-C. perfringens activity compared to FS1092, displaying significant differences (p ≤ 0.01) as evidenced by the larger inhibition zone diameter against both C. perfringens Del1 and LLY_TpeL17 strains. In contrast, the supernatant from Bs168 did not show inhibition ability, as no clear visible inhibition zone was observed (Figures 1C and 1D).
Genomic Analysis of B. amyloliquefaciens, Bacillus velezensis and B. subtilis Strains
Given the robust anti-C. perfringens activity displayed by these 2 B. amyloliquefaciens strains, it becomes intriguing to delve into the potential gene clusters responsible for the biosynthesis of antimicrobial substances they may generate. The genomic information is publicly available for Ba FS1092 (Genbank Accession#: CP038028), while it is unpublished for Ba D747. Consequently, the genome of Ba FS1092 was mainly utilized as a reference to perform sequence similarity searches against available complete genome sequences of other B. amyloliquefaciens strains and closely related species in Genbank. In total, 27 complete genomes were analyzed within this study, comprising 14 B. amyloliquefaciens, 11 B. velezensis, and 2 B. subtilis strains.
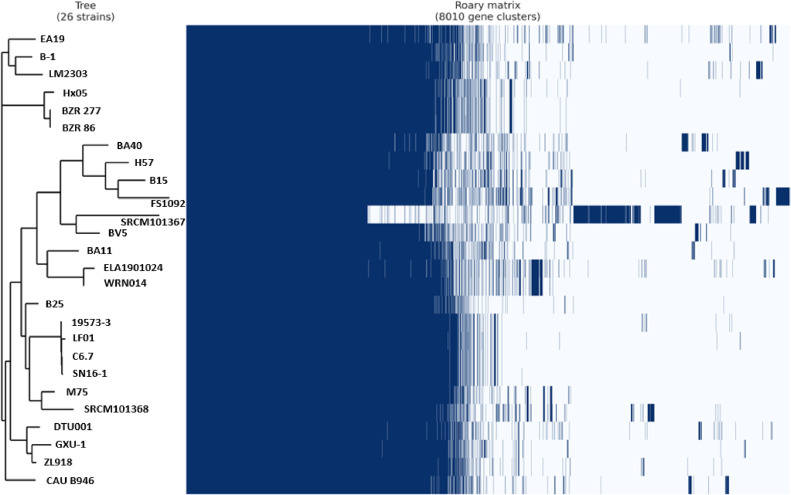
The resulting phylogenetic tree for B. amyloliquefaciens strains revealed that FS1092 clustered with other Ba strains known for their published anti-C. perfringens activities, such as Ba40 and H57 (see Figure 2). Figure 2 also illustrates the Roary matrix for B. amyloliquefaciens strains, a tool used for constructing large-scale pan-genomes from prokaryotic samples by identifying both core and accessory genes. Notably, Ba FS1092 may possess some unique genes, as indicated in Table 2 (also refer to Supplementary data). Among these genes, sdpC was also found to belong to a cluster of genes in B. subtilis responsible for encoding a peptide toxin known as SDP. The SDP toxin appeared to induce autolysis by disrupting the proton motive force during the early stages of sporulation (Lamsa et al. 2012).
Figure 2.
Pangenome analysis for 26 Bacillus genomes (Table 1) using Roary. The left side represents evolutionary relationships among 26 strains based on their core genomes. The right side represents the matrix where conserved core genes and a variable set of accessory genes were either present or absent.
Table 2.
Unique genes among 14 Bacillus amyloliquefaciens strains.
| Genes | Annotation | B15 | BA40 | FS1092 | H57 | SRCM101267 | B25 | B946 | BA11 | C6.7 | EA19 | ELA1901024 | GXU-1 | LM2303 | SN16-1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| thrZ | Threonine–tRNA ligase 2 | Y | Y | Y | Y | N | N | N | N | N | N | N | N | N | N |
| sdpC | Sporulation delaying protein C | Y | Y | Y | Y | Y | N | N | N | N | N | N | N | N | N |
| group_1017 | hypothetical protein | Y | Y | Y | Y | Y | N | N | N | N | N | N | N | N | N |
| nasA | Nitrate transporter | Y | Y | Y | Y | N | N | N | N | N | N | N | N | N | N |
| nasB | Assimilatory nitrate reductase electron transfer subunit | Y | Y | Y | Y | N | N | N | N | N | N | N | N | N | N |
| nasC | Assimilatory nitrate reductase catalytic subunit | Y | Y | Y | Y | N | N | N | N | N | N | N | N | N | N |
| group_1758 | Tryptophan RNA-binding attenuator protein inhibitory protein | Y | Y | Y | Y | N | N | N | N | N | N | N | N | N | N |
| group_1847 | hypothetical protein | Y | Y | Y | Y | Y | N | N | N | N | N | N | N | N | N |
| group_1848 | HTH-type transcriptional regulatory protein GabR | Y | Y | Y | Y | N | N | N | N | N | N | N | N | N | N |
| group_2020 | hypothetical protein | Y | Y | Y | Y | N | N | N | N | N | N | N | N | N | N |
| norG_1 | HTH-type transcriptional regulator NorG | Y | Y | Y | Y | N | N | N | N | N | N | N | N | N | N |
| argO | Arginine exporter protein ArgO | Y | Y | Y | Y | N | N | N | N | N | N | N | N | N | N |
| group_2017 | hypothetical protein | Y | Y | Y | Y | N | N | N | N | N | N | N | N | N | N |
| group_1943 | hypothetical protein | Y | Y | Y | Y | N | N | N | N | N | N | N | N | N | N |
| group_272 | hypothetical protein | Y | Y | Y | Y | Y | N | N | N | N | N | N | N | N | N |
| group_3429 | hypothetical protein | Y | Y | Y | Y | N | N | N | N | N | N | N | N | N | N |
| group_1163 | Thiol-disulfide oxidoreductase YkuV | Y | Y | Y | Y | N | N | N | N | N | N | N | N | N | N |
Y, presence; N, absence.
Microorganisms possess the capability to synthesize small bioactive compounds as part of their secondary metabolism, which serve critical roles in various bioactivities, including their applications in medicine and agriculture, particularly in antimicrobial contexts. To facilitate the identification of natural product biosynthetic pathways and the prediction of these bioactive metabolites, scientists have developed specialized tools like the antiSMASH software. This software enables the exploration of microbial genomes through genome sequencing and mining, with a particular focus on secondary/specialized metabolite biosynthetic gene clusters (SM BGCs) (Blin et al. 2023). Table 3 provides a comprehensive list of antimicrobial compounds predicted through the genome mining of SM BGCs using the antiSMASH tool. In general, these analyses indicate that most B. amyloliquefaciences strains are capable of producing 7 antimicrobial compounds, namely Bacillaene, Bacillibactin, Bacilysin, Deficidin, Fengycin, Macrolactin H (with a high degree of similarity, close to 100%), and Surfactin (with similarity ranging from 78% to 86%). The pilot study of recent genome sequencing of Ba D747 indicated that it may produce an additional antimicrobial compound Thermoactinoamide. On the other hand, Plantazolicin is predicted to be produced by fewer than half of the Ba strains. In comparison, the commonly studied B. subtilis 168 strain, often employed in protease-deficient mutant research, is predicted to produce Bacillaene, Bacillibactin, Bacilysin, Deficidin, Fengycin, and Surfactin.
Table 3.
Secondary metabolites predicted by the antiSMASH database.
| Antibiotics produced |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bacterial strain | Accession# | Bacillaene | Bacillibactin | Bacilysin | Difficidin | Fengycin | Macrolactin H | Plantazolicin | Surfactin | Subtilin | Thermoactinoamide |
| Ba B15 | CP014783.1 | 100% | 100% | 100% | 100% | 100% | 100% | 86% | |||
| Ba B25 | LN999829.1 | 100% | 100% | 100% | 100% | 100% | 100% | 78% | |||
| Ba BA11 | JALMGL010000001.1 | 100% | 80% | 100% | |||||||
| Ba BA40 | JALMGM010000001.1 | 100% | 100% | 100% | 46% | 80% | 100% | 82% | |||
| Ba C6.7 | CP130445.1 | 100% | 100% | 100% | 100% | 100% | 100% | 86% | |||
| Ba CAU B946 | HE617159.1 | 100% | 100% | 100% | 93% | 100% | 100% | 83% | 82% | ||
| Ba D747 | Unpublished | 100% | 100% | 100% | 53% | 86% | 90% | 43% | 100% | ||
| Ba EA19 | CP079834.1 | 100% | 100% | 100% | 93% | 100% | 100% | 100% | 82% | ||
| Ba ELA1901024 | CP071042.1 | 100% | 100% | 100% | 100% | 100% | 100% | 82% | |||
| Ba FS1092 | CP038028 | 100% | 100% | 100% | 100% | 100% | 82% | 100% | |||
| Ba GXU-1 | CP065159.1 | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 82% | ||
| Ba H57 | LMUC01000001- | 100% | 100% | 100% | 100% | 86% | 100% | 82% | |||
| LMUC01000016 | |||||||||||
| Ba LM2303 | CP018152.1 | 100% | 100% | 100% | 100% | 100% | 100% | 91% | 82% | ||
| Ba SN16-1 | CP075547.1 | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 82% | ||
| Ba SRCM101367 | CP021505.1 | 100% | 100% | 100% | 93% | 82% | |||||
| Bs 168 | AL009126.3 | 100% | 100% | 100% | 100% | 82% | |||||
Bs 168 also produces pulcherriminic acid, sporulation killing factor, sublancin 168, and subtilosin A (100% Similarity).
Ba BA40 also produces Amylocyclicin and Mersacidin (100% Similarity).
Ba EA19 also produces Bacillothiazol (100% similarity).
Ba D747 also produces Plipastatin (38% similarity).
DISCUSSION
Probiotics offer an effective alternative to antibiotics by nurturing beneficial bacteria in the gastrointestinal tract. They promote nutrient absorption, bolster production traits, and fortify immunity (El-Hack et al. 2020). In this study, we investigated the inhibitory effects of B. amyloliquefaciens strains, on pathogenic C. perfringens Del1 and LLY-TpeL17 strains, known for carrying the netB gene. This gene encodes a pivotal virulence factor, a pore-forming toxin, which contributes to the pathogenesis of necrotic enteritis in broiler chickens (Keyburn et al. 2010). The agar well diffusion study unveiled that culture supernatants from both Ba D747 and FS1092 strains effectively curtailed the growth of C. perfringens Del1 and LLY-TpeL17 strains, with Ba D747 demonstrating superior inhibitory activity. It is worth noting that very pathogenic LLY_TpeL17 harbors not only the netB gene but also the tpeL gene which encodes a large clostridial cytotoxin that is linked to exacerbating the severity of necrotic enteritis in chickens (Coursodon et al. 2012, Prescott et al. 2016).
Since genomic information for Ba D747 was unpublished yet, we resorted to a BLAST alignment analysis that placed D747 and FS1092 in proximity to other Ba strains with documented C. perfringens inhibitory activities in the phylogenetic tree. These strains include H57, BA11, and BA40. In genomic mining analysis, Ba FS1092 was predicted as a potential producer of a range of secondary antimicrobial compounds, including Subtilin. Subtilin, initially isolated from B. subtilis, is a neutral metalloprotease and an alkaline serine protease with a preference for targeting gram-positive microorganisms (Stein et al. 2005).
Antimicrobial compounds are substances that can kill or inhibit the growth of specific microorganisms, including bacteria, viruses, fungi, and parasites. These compounds demonstrate broad applications in the treatment of infectious diseases and food preservation. In our study, the majority of B. amyloliquefaciens strains produced secondary antimicrobial compounds such as Bacillaene, Bacillibactin, Bacilysin, Deficidin, Fengycin, Macrolactin H, and Surfactin. Each of these compounds may potentially suppress the growth of C. perfringens at varying degrees. For instance, Bacillaene, originally isolated from B. subtilis, inhibits prokaryotic protein synthesis through mechanisms that remain unclear (Rabbee and Baek, 2020). Bacillibactin, a catecholic iron siderophore, plays a pivotal role in facilitating Fe(III) acquisition and is suggested to passively inhibit microbial pathogens (Li et al. 2014; Rabbee and Baek, 2020). Bacilysin acts as an antibiotic that relies on peptide transporters for entry into target cells, disrupting the biosynthetic pathway of bacterial peptidoglycan or fungal mannoprotein (Rabbee and Baek, 2020). Fengycin, composed of a cyclic octapeptide, is believed to induce cell death in the target organism by compromising cell membrane integrity and altering cell permeability (Rabbee and Baek, 2020). Macrolactin H, a product of polyketide biosynthesis, inhibits bacterial peptide deformylase (Schneider et al. 2007). Deficidin, originally isolated from Bacillus subtilis, possesses potent in vitro antibacterial activity by affecting the cell wall (Zimmerman et al. 1987). Surfactin, characterized by its amphiphilic structure, functions as a biosurfactant molecule with antimicrobial activity by damaging bacterial cell membranes (Rabbee and Baek, 2020). The additional compound Thermoactinoamide projected to be produced by Ba D747 is a new cyclic hexapeptide, originally extracted from the thermophilic bacterium Thermoactinomyces vulgaris strain ISCAR 2354 in Iceland, which can inhibit the growth of Staphylococcus aureus ATCC 6538 (Teta et al. 2017). The lower levels of similarities to some antimicrobial compounds for Ba D747 may result from the partial genome sequencing of this strain with the Illumina sequencing approach.
B. subtilis 168 exhibited no inhibitory effect on C. perfringens, in contrast to Ba FS1092 and D747. A comparison of the antimicrobial compounds produced by B. subtilis 168 and FS1092 revealed 4 shared (Bacillibactin, Bacilysin, Fengycin, Surfactin) and 2 unique (Deficidin and Macrolactin H) compounds for B. amyloliquefaciens FS1092. The presence of Deficidin and Macrolactin H may play pivotal roles in Ba FS1092′s inhibitory activities against gram-positive C. perfringens strains, although other factors or variations in compound concentration might also contribute to differences in C. perfringens inhibition.
Apart from the production of antimicrobial compounds, B. amyloliquefaciens could outcompete C. perfringens for nutrients and other resources within the gut environment. Studies involving murine models have demonstrated that the Ba40 strain was able to outperform C. perfringens by adhering to gut epithelium and proliferating (Zhao et al. 2016, Jiang et al. 2022). Additionally, B. amyloliquefaciens stimulates the host's immune response against C. perfringens, reducing the release of pro-inflammatory cytokines (Zhao et al. 2016, Jiang et al. 2022). By producing immunomodulatory compounds such as exopolysaccharides, B. amyloliquefaciens activates the host's immune system, enhancing its resistance to bacterial infections (Sung et al. 2022). In essence, the inhibitory effects of B. amyloliquefaciens on C. perfringens could result from a combination of direct and indirect mechanisms, working synergistically to curtail C. perfringens colonization and proliferation in the gut, thereby promoting gut health in chickens.
Interestingly, the inhibitory activity from Ba D747 bacterial culture against pathogenic CP netB+tpeL+ LLY-TpeL17 strain was influenced by both culture temperature (28°C and 42°C) and shaking speed (80 rpm and 225 rpm) (data not shown). Supernatants collected from 48-h bacterial cultures at 28°C (225 rpm), and 42°C (80 rpm) demonstrated robust anti-CP activity, whereas those from 42°C (225 rpm) cultures did not. The mechanism remains unclear. Assumingly, the optimal growth temperature range for many Bacillus strains is around 30°C to 37°C. At temperatures below this range (such as 28°C), bacterial growth may slow down, allowing more time for the production and accumulation of inhibitory compounds in the culture supernatant. Conversely, at temperatures above the optimal range (such as 42°C) at full aeration (high speed 225 rpm), overgrowth may occur and the synthesis and secretion of inhibitory compounds may be downregulated in response to temperature and aeration changes, potentially reducing the overall production of inhibitory substances.
While these findings hold promise for the use of B. amyloliquefaciens as an inhibitory bioagent against C. perfringens, further research is imperative to evaluate its safety and efficacy in vivo. For example, in one study, administration of lyophilized vegetative B. amyloliquefaciens cells with feed did not demonstrate a significant protective effect against necrotic enteritis in an extremely severe experimental broiler NE model, despite the clear in vitro inhibitory activity of Ba supernatant against C. perfringens strains (Geeraerts et al. 2016). One plausible explanation could be that the established infection model was exceptionally severe, as indicated by a lesion score exceeding 3.0 in the challenge control group. Under such circumstances, the beneficial effects of Ba may have been overshadowed by the severity of the infection. Currently, the lack of comprehensive genomic information on this Ba strain limits the prediction of antibiotic compound activity through genomic mining.
B. amyloliquefaciens also exhibits potential as a vaccine vector that delivers foreign antigens to the immune system and elicits a protective immune response. B. amyloliquefaciens possesses several attributes that make it an attractive candidate for vaccine vector development, including spore formation, resilience in challenging environmental conditions, and the ability to produce immunomodulatory compounds. Genetically modified protease-deficient strains of B. amyloliquefaciens were developed as a host of efficient and stable expression vectors (Wang et al. 2019). Extensive research is required to construct Ba-specific shuttle vectors and express key antigen targets, either in plasmid-based expression systems or on the spore surface display, targeting pathogenic C. perfringens in vitro. Subsequent safety and efficacy evaluations in vivo are essential steps in this endeavor.
While direct-fed microbials (DFM) producing antimicrobials may offer benefits in animal health and performance, it is essential to consider their potential implications for antimicrobial resistance, as conventional antibiotics growth promoters, in terms of indirect selective pressures on the microbial populations in the animal's gut, horizontal gene transfer, cross-resistance, and microbial ecological disruption. Some antimicrobial peptides (AMP), for example, Pediocin A produced by Pediococcus pentaceus FBB61 and Sublacin produced by B. subtilis 168, have demonstrated antimicrobial activity against C. perfringens type A infection in poultry (Grilli et al. 2009; Wang et al. 2015). However, excessive exposure of pathogens to antimicrobial peptides may lead to the development of AMP-resistant strains (Abreu et al. 2023). Very little information is available on whether antimicrobial substances produced by DFMs generate antimicrobial resistance. Further research is needed to better understand these potential risks and to develop strategies to mitigate them while maximizing the benefits of using DFMs in food animal production.
In summary, our study highlights the potent anti-C. perfringens activity of 2 B. amyloliquefaciens strains, FS1092 and D747, as demonstrated in in vitro studies. Particularly, D747 exhibited superior activity compared to FS1092. Genomic mining analysis reveals that Ba strains with anti-C. perfringens activities tend to cluster together. Moreover, Ba strains are predicted to produce 7 major secondary antimicrobial metabolites with broad antimicrobial potential for applications in medicine and agriculture. Based on in vitro analysis, BaD747 may have the potential as a probiotic in the control of NE. While further research is required to fully understand the potential benefits of B. amyloliquefaciens for animal health, these initial findings highlight these bacterial strains as promising probiotics with a wide range of potential advantages.
DISCLOSURES
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
This article is derived from the subject data funded in part by the Agricultural Research Service, USDA. The sponsors had no role in the writing process of the article.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2024.103871.
Appendix. Supplementary materials
REFERENCES
- Abreu R., Semedo-Lemsaddek T., Cunha E., Tavares L., Oliveira M. Antimicrobial drug resistance in poultry production: current status and innovative strategies for bacterial control. Microorganisms. 2023;11:953. doi: 10.3390/microorganisms11040953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blin K., Shaw S., Augustijn H.E., Reitz Z.L., Biermann F., Alanjary M., Fetter A., Terlouw B.R., Metcalf W.W., Helfrich E.J.N, van Wezel G.P., Medema M.H., Weber T. AntiSMASH 7.0: new and improved predictions for detection, regulation, chemical structures and visualization. Nucleic Acids Res. 2023;51(W1):W46–W50. doi: 10.1093/nar/gkad344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper K.K., Songer J.G. Necrotic enteritis in chickens: a paradigm of enteric infection by Clostridium perfringens type A. Anaerobe. 2009;15:55–60. doi: 10.1016/j.anaerobe.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Costa S.S., Guimaraes L.C., Silva A., Soares S.C., Barauna R.A. First steps in the analysis of prokaryotic pan-genomes. Bioinform Biol Insights. 2020;14 doi: 10.1177/1177932220938064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coursodon C.F., Glock R.D., Moore K.L., Cooper K.K., Songer J.G. TpeL-producing strains of Clostridium perfringens type A are highly virulent for broiler chicks. Anaerobe. 2012;18:117–121. doi: 10.1016/j.anaerobe.2011.10.001. [DOI] [PubMed] [Google Scholar]
- de Oliveira M.J.K., Sakomura N.K., de Paula Dorigam J.C., Doranalli K., Soares L., Viana G.D.S. Bacillus amyloliquefaciens CECT 5940 alone or in combination with antibiotic growth promoters improves performance in broilers under enteric pathogen challenge. Poult Sci. 2019;98:4391–4400. doi: 10.3382/ps/pez223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hack M.E.A., El-Saadony M.T., Shafi M.E., Qattan S.Y.A., Batiha G.E., Khafaga A.F., Abdel-Moneim A.E., Alagawany M. Probiotics in poultry feed: a comprehensive review. J. Anim. Physiol. Anim. Nutr. (Berl) 2020;104:1835–1850. doi: 10.1111/jpn.13454. [DOI] [PubMed] [Google Scholar]
- Gadde U., Kim W.H., Oh S.T., Lillehoj H.S. Alternatives to antibiotics for maximizing growth performance and feed efficiency in poultry: a review. Anim Health Res Rev. 2017;18:26–45. doi: 10.1017/S1466252316000207. [DOI] [PubMed] [Google Scholar]
- Gadde U., Oh S., Lee Y., Davis E., Zimmerman N., Rehberger T., Lillehoj H.S. The effects of direct-fed microbial supplementation, as an alternative to antibiotics, on growth performance, intestinal immune status, and epithelial barrier gene expression in broiler chickens. Probiotics Antimicrobial Proteins. 2017;9:397–405. doi: 10.1007/s12602-017-9275-9. [DOI] [PubMed] [Google Scholar]
- Geeraerts S., Delezie E., Ducatelle R., Haesebrouck F., Devreese B., Van Immerseel F. Vegetative Bacillus amyloliquefaciens cells do not confer protection against necrotic enteritis in broilers despite high antibacterial activity of its supernatant against Clostridium perfringens in vitro. Br. Poult. Sci. 2016;57:324–329. doi: 10.1080/00071668.2016.1169246. [DOI] [PubMed] [Google Scholar]
- Grant A., Gay C.G., Lillehoj H.S. Bacillus spp. as direct-fed microbial antibiotic alternatives to enhance growth, immunity, and gut health in poultry. Avian Pathol. 2018;47:339–351. doi: 10.1080/03079457.2018.1464117. [DOI] [PubMed] [Google Scholar]
- Grilli E., Messina M., Catelli E., Morlacchini M., Piva A. Pediocin a improves growth performance of broilers challenged with Clostridium perfringens. Poult. Sci. 2009;88:2152–2158. doi: 10.3382/ps.2009-00160. [DOI] [PubMed] [Google Scholar]
- Gu C., Lillehoj H.S., Sun Z., Lee Y., Zhao H., Xianyu Z., Yan X., Wang Y., Lin S., Liu L. Characterization of virulent netB+/tpeL+ Clostridium perfringens strains from necrotic enteritis–affected broiler chicken farms. Avian Dis. 2019;63:461–467. doi: 10.1637/11973-092018-Reg.1. [DOI] [PubMed] [Google Scholar]
- Gu G., Gonzalez-Escalona N., Zheng J., Bolten S., Luo Y., Mafiz A., Leon M.S., Nou X. Genome sequences of Brevundimonas naejangsanensis strain FS1091 and Bacillus amyloliquefaciens strain FS1092, isolated from a fresh-cut-produce-processing plant. Microbiol Resour Announc. 2020;9(4):e01448–19. doi: 10.1128/MRA.01448-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z., Su W., Yang M., Li W., Gong T., Zhang Y., Wen C., Wang X., Wang Y., Jin M., Lu Z. Screening of bacteria inhibiting clostridium perfringens and assessment of their beneficial effects in vitro and in vivo with whole genome sequencing analysis. Microorganisms. 2022;10 doi: 10.3390/microorganisms10102056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K., Standley D.M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyburn A.L., Bannam T.L., Moore R.J., Rood J.I. NetB, a pore-forming toxin from necrotic enteritis strains of Clostridium perfringens. Toxins (Basel) 2010;2:1913–1927. doi: 10.3390/toxins2071913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalique A., Zeng D., Shoaib M., Wang H., Qing X., Rajput D.S., Pan K., Ni X. Probiotics mitigating subclinical necrotic enteritis (SNE) as potential alternatives to antibiotics in poultry. AMB Express. 2020;10:50. doi: 10.1186/s13568-020-00989-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamsa A., Liu W.T., Dorrestein P.C., Pogliano K. The Bacillus subtilis cannibalism toxin SDP collapses the proton motive force and induces autolysis. Mol Microbiol. 2012;84:486–500. doi: 10.1111/j.1365-2958.2012.08038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latorre J.D., Hernandez-Velasco X., Kuttappan V.A., Wolfenden R.E., Vicente J.L., Wolfenden A.D., Bielke L.R., Prado-Rebolledo O.F., Morales E., Hargis B.M., Tellez G. Selection of Bacillus spp. for cellulase and xylanase production as direct-fed microbials to reduce digesta viscosity and Clostridium perfringens proliferation using an in vitro digestive model in different poultry diets. Front. Vet. Sci. 2015;2:25. doi: 10.3389/fvets.2015.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.-W., Lillehoj H.S. An update on direct-fed microbials in broiler chickens in post-antibiotic era. Anim. Prod. Sci. 2016;57:1575–1581. [Google Scholar]
- Lee K.-W., Lillehoj H.S. Role of Clostridium perfringens necrotic enteritis B-like toxin in disease pathogenesis. Vaccines. 2021;10:61. doi: 10.3390/vaccines10010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Li Q., Xu Z., Zhang N., Shen Q., Zhang R. Responses of beneficial Bacillus amyloliquefaciens SQR9 to different soilborne fungal pathogens through the alteration of antifungal compounds production. Front. Microbiol. 2014;5:636. doi: 10.3389/fmicb.2014.00636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Lillehoj H.S., Gadde U.D., Ritter D., Oh S. Characterization of Clostridium perfringens strains isolated from healthy and necrotic enteritis-afflicted broiler chickens. Avian Dis. 2017;61:178–185. doi: 10.1637/11507-093016-Reg.1. [DOI] [PubMed] [Google Scholar]
- Neveling D.P., Dicks L.M.T. Probiotics: an antibiotic replacement strategy for healthy broilers and productive rearing. Probiotics Antimicrob Proteins. 2021;13:1–11. doi: 10.1007/s12602-020-09640-z. [DOI] [PubMed] [Google Scholar]
- Page A.J., Cummins C.A., Hunt M., Wong V.K., Reuter S., Holden M.T., Fookes M., Falush D., Keane J.A., Parkhill J. Roary: rapid large-scale prokaryote pan genome analysis. Bioinformatics. 2015;31:3691–3693. doi: 10.1093/bioinformatics/btv421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson T., Okinaka R.T., Foster J.T., Keim P. Phylogenetic understanding of clonal populations in an era of whole genome sequencing. Infect Genet Evol. 2009;9:1010–1019. doi: 10.1016/j.meegid.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Prescott J.F., Smyth J.A., Shojadoost B., Vince A. Experimental reproduction of necrotic enteritis in chickens: a review. Avian Pathol. 2016;45:317–322. doi: 10.1080/03079457.2016.1141345. [DOI] [PubMed] [Google Scholar]
- Rabbee M.A., Baek K.H. Antimicrobial activities of lipopeptides and polyketides of Bacillus velezensis for agricultural applications. Molecules. 2020,;25 doi: 10.3390/molecules25214973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider K., Chen X.H., Vater J., Franke P., Nicholson G., Borriss R., Sussmuth R.D. Macrolactin is the polyketide biosynthesis product of the pks2 cluster of Bacillus amyloliquefaciens FZB42. J. Nat. Prod. 2007;70:1417–1423. doi: 10.1021/np070070k. [DOI] [PubMed] [Google Scholar]
- Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. 2014;30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- Shini S., Zhang D., Aland R.C., Li X., Dart P.J., Callaghan M.J., Speight R.E., Bryden W.L. Probiotic Bacillus amyloliquefaciens H57 ameliorates subclinical necrotic enteritis in broiler chicks by maintaining intestinal mucosal integrity and improving feed efficiency. Poult. Sci. 2020;99:4278–4293. doi: 10.1016/j.psj.2020.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein T., Heinzmann S., Dusterhus S., Borchert S., Entian K.D. Expression and functional analysis of the subtilin immunity genes spaIFEG in the subtilin-sensitive host Bacillus subtilis MO1099. J. Bacteriol. 2005;187:822–828. doi: 10.1128/JB.187.3.822-828.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung W.W., Lin Y.Y., Huang S.D., Cheng H.L. Exopolysaccharides of Bacillus amyloliquefaciens Amy-1 mitigate inflammation by inhibiting ERK1/2 and NF-kappaB pathways and activating p38/Nrf2 pathway. Int. J. Mol. Sci. 2022;23 doi: 10.3390/ijms231810237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teta R., Marteinsson V.T., Longeon A., Klonowski A.M., Groben R., Bourguet-Kondracki M.L., Costantino V., Mangoni A. Thermoactinoamide A, an antibiotic lipophilic cyclopeptide from the icelandic thermophilic bacterium thermoactinomyces vulgaris. J. Nat. Prod. 2017;80:2530–2535. doi: 10.1021/acs.jnatprod.7b00560. [DOI] [PubMed] [Google Scholar]
- Timbermont L., Lanckriet A., Gholamiandehkordi A.R., Pasmans F., Martel A., Haesebrouck F., Ducatelle R., Van Immerseel F. Origin of Clostridium perfringens isolates determines the ability to induce necrotic enteritis in broilers. Comp. Immunol. Microbiol. Infect. Dis. 2009;32:503–512. doi: 10.1016/j.cimid.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Tsiouris V. Poultry management: a useful tool for the control of necrotic enteritis in poultry. Avian Pathol. 2016;45:323–325. doi: 10.1080/03079457.2016.1154502. [DOI] [PubMed] [Google Scholar]
- Wade B., Keyburn A. The true cost of necrotic enteritis. World Poult. 2015;31:16–17. [Google Scholar]
- Wang H., Zhang X., Qiu J., Wang K., Meng K., Luo H., Su X., Ma R., Huang H., Yao B. Development of Bacillus amyloliquefaciens as a high-level recombinant protein expression system. J. Ind. Microbiol Biotechnol. 2019;46:113–123. doi: 10.1007/s10295-018-2089-2. [DOI] [PubMed] [Google Scholar]
- Wang S., Zeng X., Wang Q., Zhu J., Peng Q., Hou C., Thacker P., Qiao S. The Antimicrobial peptide sublancin ameliorates necrotic enteritis induced by Clostridium perfringens in broilers. Am. Soc. Anim. Sci. 2015;93:4750–4760. doi: 10.2527/jas.2015-9284. [DOI] [PubMed] [Google Scholar]
- Zhang H., Zhou Y., Xu H., Liang C., Zhai Z. Bacillus amyloliquefaciens BLCC1-0238 alone or in combination with mannan-oligosaccharides alleviates subclinical necrotic enteritis in broilers. Probiotics Antimicrob. Proteins. 2022;14:158–168. doi: 10.1007/s12602-021-09853-w. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Li Y., Liang S., Zheng W., Chen X., Liu J., Wang A. Study on the preparation and effect of tomato seedling disease biocontrol compound seed-coating agent. Life (Basel) 2022;12 doi: 10.3390/life12060849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P.Y., Li H.L., Mohammadi M., Kim I.H. Effect of dietary lactulose supplementation on growth performance, nutrient digestibility, meat quality, relative organ weight, and excreta microflora in broilers. Poult. Sci. 2016;95:84–89. doi: 10.3382/ps/pev324. [DOI] [PubMed] [Google Scholar]
- Zimmerman S.B., Schwartz C.D., Monaghan R.L., Pelak B.A., Weissberger B., Gilfillan E.C., Mochales S., Hernandez S., Currie S.A., Tejera and E., et al. Difficidin and oxydifficidin: novel broad spectrum antibacterial antibiotics produced by Bacillus subtilis. I. Production, taxonomy and antibacterial activity. J. Antibiot (Tokyo) 1987;40:1677–1681. doi: 10.7164/antibiotics.40.1677. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.